Background: Phosphatidylserine (PS) reduces immunogenicity of FVIII by possibly inducing tolerance.
Results: FVIII-PS exposure leads to hypo-responsiveness in hemophilia A mice to FVIII challenge but responds normally to ovalbumin.
Conclusion: Exposure of FVIII in the presence of PS leads to hypo-responsiveness/tolerance.
Significance: An innovative reverse/inverse vaccination could desensitize the patients to antigen.
Keywords: Biotechnology, Blood Coagulation Factors, Dendritic Cells, Factor VIII, Immunosuppression, Phosphatidylserine, GFP-FoxP3, Immunological Tolerance, Regulatory T-cell, Reverse/Inverse Vaccination
Abstract
Administration of recombinant factor VIII (FVIII), an important co-factor in blood clotting cascade, elicits unwanted anti-FVIII antibodies in hemophilia A (HA) patients. Previously, FVIII associated with phosphatidylserine (PS) showed significant reduction in the anti-FVIII antibody response in HA mice. The reduction in the immune response to FVIII-PS could be due either to a failure of the immune system to recognize the antigen (i.e. immunological ignorance) or to an active induction of an antigen-specific nonresponsiveness (i.e. immunological tolerance). If it were a result of tolerance, one would predict that pre-exposure to FVIII-PS would render the mice hypo-responsive to a subsequent FVIII challenge. Here, we have demonstrated that naive HA mice that were pretreated with FVIII-PS showed a significantly reduced FVIII immune response to further challenge with native FVIII and that this decreased responsiveness could be adoptively transferred to other mice. An increase in number of FoxP3-expressing CD4+ regulatory T-cells (Treg) was observed for the FVIII-PS-immunized group as compared with animals that received FVIII alone, suggesting the involvement of Treg in PS-mediated hypo-responsiveness. The PS-mediated reduction in antibody response was reversed by the co-administration of function-blocking anti-TGF-β antibody with FVIII-PS. The decreased response to FVIII induced by FVIII-PS was determined to be antigen-specific because the immune response to another non-cross-reactive antigen (ovalbumin) was not altered. These results are consistent with the notion that FVIII-PS is tolerogenic and suggest that immunization with this tolerogenic form of the protein could be a useful treatment option to minimize immunogenicity of FVIII and other protein-based therapeutics.
Introduction
The advent of recombinant technology is a boon to the development of recombinant therapeutic proteins including the blood clotting factor, factor VIII. Currently, recombinant factor FVIII (FVIII)3 is the first line of therapy for hemophilia A (HA) patients. Unfortunately, a major drawback of the therapy is the generation of anti-protein neutralizing (Nabs) and binding antibodies, which are observed in about 15–30% of the patient population (1). Nabs abrogate the activity of the protein, rendering it less efficacious, whereas other binding antibodies negatively affect the pharmacokinetics of the protein. Any approach to reduce immunogenicity and reverse the inhibitor development would address an unmet medical need.
Our previous studies showed that FVIII complexed with phosphatidylserine (PS) significantly reduced the development of antibody response against FVIII in HA mice (2, 3). The studies aimed at understanding the mechanism of this reduction showed that FVIII-PS down-regulated the expression of CD40 upon exposure to bone marrow-derived dendritic cells (DC) (4). Further, T-cell activation studies wherein the incubation of FVIII-PS exposed DC with FVIII-primed splenic CD4+ T-cells resulted in significant reduction in T-cell proliferation. This was also accompanied by an increase in the secretion of key immunoregulatory TGF-β and IL-10 cytokines and a simultaneous decrease in pro-inflammatory IL-6 and IL-17 cytokines levels in the co-culture. These observations are consistent with the notion that PS could present FVIII to DC in a tolerogenic manner (5–8). Thus, one would expect that pre-exposure to FVIII-PS complex will render a state of immunological hypo-responsiveness toward rechallenge with FVIII. The results presented here demonstrate that pre-exposure to FVIII-PS using a novel reverse/inverse vaccination strategy does induce hypo-responsiveness in the animals.
EXPERIMENTAL PROCEDURES
Materials
Excipient-free, recombinant human full-length factor VIII was a generous gift from Western New York Hemophilia Foundation, Buffalo, NY. Brain phosphatidylserine, dimyristoyl phosphatidylcholine, and dimyristoyl phosphatidylglycerol were purchased from Avanti Lipids (Alabaster, AL). Function-blocking anti-TGF-β antibody was purchased from R&D systems (Minneapolis, MN). High purity dexamethasone was purchased from Sigma-Aldrich. Sterile syringes, needles, and isoflurane were purchased from Butler Schein (Dublin, OH). Endosafe® endotoxin kit was purchased from Charles River Laboratories International Inc. (Wilmington, MA). EndoGrade ovalbumin was purchased from Hyglos GmbH.
Exon 16-deleted, transgenic, factor VIII knock-out mice were used for all the studies, unless mentioned otherwise. Green fluorescent protein (GFP)-tagged FoxP3 knock-in FVIII−/− mice were exclusively used for regulatory T-cell (Treg) study. The animals were handled and surgical procedures were performed as per the protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the University at Buffalo.
Methods
Preparation of Protein-Lipid Complex
Liposomes were prepared, sized, and associated with FVIII as per the method described by Ramani et al. (2). The complexes were tested for endotoxin level by using the Endosafe Endochrome-K endotoxin assay kit (Charles River Laboratories International), and endotoxin negative samples were used for in vivo studies.
Pre-exposure to FVIII-Lipid Complexes
A total of 39 naive hemophilic mice were divided into five groups with each group containing 7–8 animals. The animals were administered with once-a-week subcutaneous injections of 1 μg (∼5 IU) of free FVIII or FVIII-PS or FVIII-PC or FVIII-PG or FVIII + dexamethasone (Dex) (henceforth, the FVIII-lipid or FVIII+Dex preparations are abbreviated as FVIII-PS/PC/PG/Dex) for 4 consecutive weeks. Frequent administration of a low dose of Dex (200 ng/injection) was preferred to avoid severe immunosuppression of lymphocyte activity. On the 6th week, all animals were rechallenged aggressively with four weekly subcutaneous administrations of 1 μg of free FVIII. On the 11th week, the animals were sacrificed, and blood was collected in 10% acid citrate dextrose, centrifuged, and plasma was isolated. The base-line anti-FVIII titer values before the start of FVIII rechallenge were determined by immunizing animals with four weekly injections of 1 μg of free FVIII or FVIII-PS/PC/PG/Dex.
Effect of PS on Other Concomitantly Administered Foreign Antigens
Twenty naive hemophilic mice were equally divided into four groups and administered with 1 μg of free FVIII or FVIII-PS/PC complexes once a week for 4 consecutive weeks via the subcutaneous route. The fourth group received four weekly immunizations of 1 μg of ovalbumin (Ova) only and served as a control group. The FVIII- or FVIII-PS/PC-immunized animals were also co-administered with four weekly injections of 1 μg of Ova at a different anatomical site than the FVIII- or FVIII-lipid-administered site. On the 6th week, all animals were sacrificed, and plasma was collected as described.
CD4+CD25+ T-cell Adoptive Transfer Study
Naive hemophilic mice were equally divided into four groups. Each group received four weekly injections of 10 IU of free FVIII (ADVATE®, Baxter, Deerfield, IL; 1,500 IU/vial; activity 1 μg = ∼7 IU of FVIII) or FVIII-PS/PG via the subcutaneous route. The fourth group was kept untreated and served as the naive control group. Two weeks after the last injection, all animals were sacrificed, and their spleens were collected and homogenized. Total lymphocyte count for each spleen cell suspension was determined by using the BC 2800 Vet auto hematology analyzer (Mindray, Mahwah, NJ) instrument and subjected to CD4+CD25+ T-cell isolation using a CD4+CD25+ T-cell isolation kit (Miltenyi Biotec, Auburn, CA).
Approximately 0.1 × 106 CD4+CD25+ T-cells were adoptively transferred into corresponding individual naive HA mice. After a 48-h wait period, all the recipient animals were immunized aggressively with four weekly subcutaneous injections of 1 μg of free FVIII/injection. Two weeks after the last injection, all animals were sacrificed, and plasma samples were collected as described.
Treg Study
Immunization studies were conducted in hemophilia A mice model. The animals (n = 3/treatment group) received subcutaneous injections of either free FVIII or FVIII-PS (2 μg of FVIII) every week for 12 weeks. Two weeks after the last injection, the CD4+ T-cells were isolated from spleen of the immunized animal and were stained with FITC conjugated to CD4 antibody and PE-conjugated to anti-FoxP3 antibody. The double positive cells were analyzed using flow cytometry. To account for the spectral overlap of FITC and PE, compensation using singly labeled FITC and PE controls were acquired, and compensation was carried out using FlowJo software.
Role of Treg and TGF-β on PS-mediated Hypo-responsiveness
The role of Treg cells and the regulatory cytokine TGF-β in PS-mediated tolerance was also confirmed using immunogenicity studies conducted in GFP-FoxP3 knock-in FVIII−/− mice, and GFP expression was used as a read-out for population of Tregs. FoxP3-GFP-FVIII−/− mice received four weekly subcutaneous injections of either free FVIII (n = 12) or FVIII-PS (1 μg FVIII/injection) in the presence (n = 10) and in the absence (n = 12) of function-blocking anti-TGF-β antibody. The TGF-β antibody (20 μg/injection; subcutaneous) was administered along with FVIII-PS. Animals were sacrificed on the 6th week, and lymph nodes were isolated and prepared for analysis using flow cytometry. The dot plots generated were analyzed either by Cell Quest software provided by the manufacturer or by FlowJo® software. Further, total lymphocytes were gated based on side scatter versus forward scatter criteria, and the gated regions that contained total lymphocyte count of at least 10% of total lymph node cell count were analyzed for GFP expression. The data were expressed as the percentage of GFP-FoxP3+ cells in the gated total lymphocytes region. The B-cell responses were followed by measuring total anti-FVIII antibody titers in Treg mice.
Determination of Anti-FVIII Nabs and Total Anti-FVIII Antibodies
All plasma samples were analyzed for anti-FVIII Nab titers by activated partial Thromboplastin time assay following Nijmegen's modified Bethesda assay (9) and expressed in Bethesda units/ml. Total anti-FVIII antibody titers were determined by ELISA as described previously (3).
Effect of PS-mediated Hypo-responsiveness on in Vitro Efficacy
Naive hemophilic mice (n = 4 per group) received four weekly injections of 10 IU of free FVIII (ADVATE®, Baxter) or FVIII-PS via the subcutaneous route. One group of animals (n = 4) was left untreated. Two weeks after the last injection, all animals were sacrificed, and their plasma samples were analyzed for clotting time and efficacy. The plasma samples from the above animals were mixed (1:24) with FVIII-deficient human plasma. This mixture was then mixed (1:1) with normal human plasma and incubated at 37 °C for 2 h, and the residual biological activity of FVIII was measured as clotting time using a Coag-A-Mate coagulation analyzer (Organon Teknika Corp., Durham, NC).
Determination of Anti-Ova Antibodies
Total anti-Ova antibody levels in the plasma samples (see “Effect of PS on Other Concomitantly Administered Foreign Antigens”) were measured using a commercially available ELISA kit (Alpha Diagnostics International Inc., San Antonio, TX) and expressed as antibody activity units (titer)/ml (units/ml) as determined from the standard curve. Kilounits/ml represent 1,000 × units/ml.
Statistical Analysis
One-way analysis of variance followed by Tukey's or Dunnett's post hoc analyses was performed using the GraphPad Prism statistical software unless otherwise specified. p < 0.05 was considered as a statistically significant difference. For the Treg study, statistical analysis was carried out using one-tailed paired Student's t test using the Minitab software.
RESULTS AND DISCUSSION
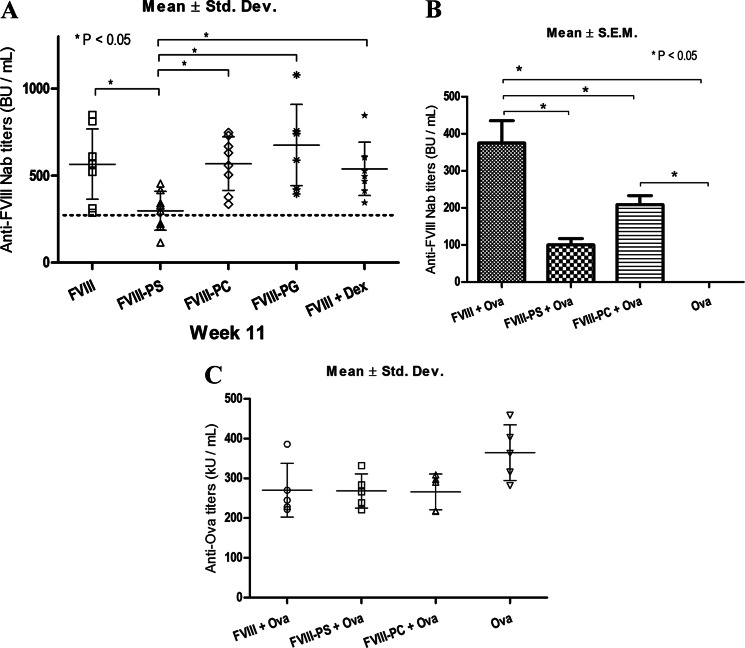
If PS presents FVIII in a tolerogenic manner to DC, pre-exposure should lead to immunological hypo-responsiveness to FVIII rechallenge. Thus, the experimental design involved pre-exposure of HA mice to FVIII-lipid complex, and the antibody response was measured following rechallenge with FVIII. Levels of anti-FVIII Nabs in animals preimmunized with either free FVIII (567 ± 72) or FVIII-PC (570 ± 55 S.E.), FVIII-PG (677 ± 88 S.E.), or FVIII + Dex (539 ± 34 S.E.) showed comparable levels of anti-FVIII Nabs (Fig. 1A). In contrast, animals pretreated with FVIII-PS showed significantly reduced FVIII Nab levels (298 ± 40 S.E.). Further, to determine the rate of progression of immune response after rechallenge, a correlation of the mean anti-FVIII Nab titers measured on the 6th and 11th week for each group was performed (supplemental Table 1). After administration/priming of FVIII or FVIII PS/PC/PG/Dex, the Nab titer levels were measured at the end of the 6th week. Naive HA mice that received free FVIII alone showed high levels of anti-FVIII Nab titers (282 ± 39 S.E.). In comparison, significant reduction in base-line anti-FVIII Nab titers on the 6th week was observed in animals that were immunized with FVIII-PS (93 ± 19 S.E.) or FVIII-PC (111 ± 18 S.E.). This is consistent with our previously observed results where PS significantly reduced FVIII immune response in naive HA mice (2). FVIII complexed with anionic PG liposomes produced Nab levels (195 ± 55 S.E.) statistically comparable with the levels observed in free FVIII-immunized animals. Further, animals that were immunized with FVIII in the presence of low doses of Dex (immunosuppressant) developed relatively minimal levels of anti-FVIII antibodies (39 ± 9 S.E.). However, the Nab-lowering beneficial effect observed with Dex and PC pretreatment on the 6th week did not extend after their administration was stopped (after the 6th week). The data clearly demonstrate that only PS was able to significantly delay the progress of FVIII immune response even after the PS exposure was stopped on the 6th week. Thus, the results indicate that pre-exposure of FVIII-PS leads to hypo-responsiveness toward FVIII rechallenge.
FIGURE 1.
Phosphatidylserine complex induces hypo-responsiveness, but animals respond normally to irrelevant antigen. A, anti-FVIII Nab titer levels on the 11th week. The dotted horizontal line indicates the lowest observed free FVIII titer level. BU, Bethesda units. Std. Dev., standard deviation. B and C, development of anti-FVIII Nab (B) and anti-Ova immune response (C) upon administration of FVIII or FVIII-PS/PC along with ovalbumin. The large asterisks and the stars represent individual data points for the groups FVIII-PG and FVIII + Dex, respectively. kU, kilounits.
As the pre-exposure of FVIII in the presence of PS induces hypo-responsiveness, we propose a novel clinical approach, a reverse/inverse vaccination to reduce unwanted immunogenic response against therapeutic proteins. Unlike conventional vaccination approaches, this approach desensitizes the patient to an antigen, and thus, these patients will be unable to immunologically respond to the protein. During the reverse vaccination strategy, it is desirable that the immunization should not interfere with the ability of the immune system to mount immune responses against other antigens and pathogens. To investigate the antigen specificity and effect of immunization on the systemic immune suppression, another foreign antigen, Ova, was concomitantly administered with either FVIII or FVIII-PS/PC complexes, but at a distant anatomical site. Anti-FVIII Nab titers in animals that were administered with FVIII-PS (100 ± 17 S.E.) were significantly lower than the free FVIII (375 ± 60 S.E.)-immunized group (Fig. 1B). Mice that were immunized with only Ova had no anti-FVIII Nabs. However, all animals showed statistically comparable anti-Ova titers irrespective of the treatment group (Fig. 1C). As the animals responded to Ova by developing comparable titers, the data suggest that FVIII-PS does not render systemic immunosuppressive effects and that the antigen-specific hypo-responsiveness could be achieved by presenting antigen of interest with PS lipid.
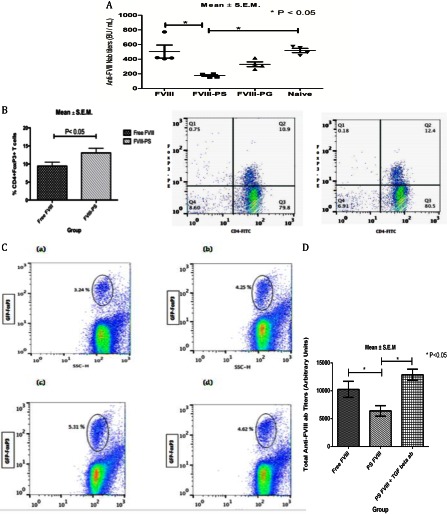
The generation of peripherally induced Tregs can regulate immune response by suppressing effector cells. The involvement of induced Tregs in inducing hypo-responsiveness toward FVIII is supported by our adoptive cell transfer studies. Upon adoptive transfer of CD4+CD25+ T-cells from FVIII- or FVIII-PS/PG-immunized or naive donor mice, recipient mice were challenged with free FVIII, and anti-FVIII Nab titers were measured (Fig. 2A). Mice that received CD4+CD25+ T-cells from FVIII-treated or T-cells from naive (unimmunized) mice elicited robust FVIII immune response (Nab titers of 503 ± 90 S.E. and 519 ± 29 S.E., respectively). In comparison, mice that received CD4+CD25+ T-cells from FVIII-PS-treated donor mice exhibited significantly reduced anti-FVIII Nab titers (177 ± 11 S.E.). However, CD4+CD25+ T-cells transferred from mice treated with FVIII associated with another negative charge PG lipid (FVIII-PG) failed to significantly reduce Nab titers (331 ± 32 S.E.). These results indicate that the FVIII-PS-induced hypo-responsiveness is transferrable and that CD4+CD25+ T-cells may be involved in the generation of this hypo-responsiveness. To further investigate whether induced Tregs are generated upon pre-exposure of FVIII in the presence of PS, the splenocytes derived from immunized animal were analyzed for FoxP3 expression, a biomarker for Treg. An increase in CD4+ FoxP3+ double positive cells was observed for the FVIII-PS treatment group as compared with the FVIII alone treatment group (Fig. 2B). This observation was further confirmed using a Treg hemophilia A tolerance model. GFP-FoxP3 knock-in FVIII−/− mice were immunized with FVIII or FVIII-PS, and GFP expression was used as a read-out for FoxP3 expression and Treg (10, 11) (Fig. 2C). The mean GFP-FoxP3+ cells for the FVIII-PS group as measured by fluorescence was higher than that observed for the free FVIII group, which was comparable with the base-line GFP-FoxP3+ level observed in naive, untreated mice, confirming that exposure of FVIII in the presence of PS increases Treg.
FIGURE 2.
The PS-mediated hypo-responsiveness involves Tregs. A, adoptive Transfer Study: anti-FVIII Nab titers (mean ± S.E.) following adoptive transfer of splenic CD4+CD25+ T-cells from FVIII or FVIII-PS/PG or naive (previously untreated) HA mice into naive, recipient HA mice and rechallenged with free FVIII. BU, Bethesda units. B, bar (left panel) and dot plots (middle panel, FVIII; right panel, FVIII-PS) of the percentage of FoxP3+ cells isolated from splenocytes of hemophilia A mice immunized with FVIII and FVIII-PS. C, dot plots of FoxP3 cells as measured by GFP expression in isolated lymph nodes of Treg hemophilia A mice (GFP knock-in FoxP3 FVIII−/− mice) in naive mice (panel a) and in mice immunized with FVIII (panel b) and FVIII-PS (in the absence (panel c) and in the presence (panel d) of anti-TGF-β). SSC-H denotes the side scatter. D, total anti-FVIII titers measured in Treg hemophilia A mice following immunization with FVIII, FVIII-PS, and FVIII-PS+anti-TGF-β.
Our previous studies using co-culture of DCs (which were exposed either to FVIII or to FVIII-lipid complexes) with FVIII-primed splenic CD4+ T-cells showed that PS down-regulated the expression of CD40 (4). Further, PS significantly reduced T-cell activation. This was accompanied by an increase in the secretion of immune regulatory TGF-β and IL-10 cytokines. At the molecular level, the regulatory cytokine TGF-β is secreted by tolerogenic DC and regulatory T-cells (12–14) and plays an important role in lymphocyte regulation and maintenance of peripheral tolerance (15). Blockade of either TGF-β or its receptors has been shown to lead to lethal inflammation and autoimmunity in mice (16). Furthermore, in the context of FVIII immunity, it has been reported that TGF-β1- and IL-10-conditioned tolerogenic DCs are able to inhibit anti-FVIII antibody response in FVIII−/− mice (17). Hence, to gain an understanding of the molecular mechanism, at least in part, we investigated the role of TGF-β in PS-mediated hypo-responsiveness by immunizing Treg hemophilia A (FVIII−/−) mice with FVIII and FVIII-PS in the presence and in the absence of function-blocking anti-TGF-β antibody. The total anti-FVIII antibody levels in these immunized animals were measured to determine the role of TGF-β on B-cell responses in Treg hemophilia A mice (Fig. 2D). The titer levels for the FVIII-PS-treated group in the absence of anti-TGF-β antibody showed lower titer levels as compared with animals that received FVIII alone, but this reduction in titer levels observed for FVIII-PS is reversed upon administration with anti-TGF-β antibody. In the presence of anti-TGF-β antibody, the titer levels are significantly higher than the FVIII-PS treatment group and are comparable with the FVIII alone treatment group. The administration of function-blocking anti-TGF-β antibody reversed the PS-mediated reduction in antibody titers, confirming the role of regulatory cytokine TGF-β in PS-mediated hypo-responsiveness. In culturing conditions, we found that function-blocking anti-TGF-β and anti-TGF-β receptors reversed the PS-mediated suppression of the T-cell response to FVIII but had no significant effect upon the PS-dependent decrease in IL-6 or IL-17 cytokine levels (data not shown). As TGF-β is a regulatory cytokine acting on multiple cells including FoxP3-expressing Treg and also acts on multifaceted cellular functions, it is very complex to delineate the effects of TGF-β on Treg and its impact on PS-mediated hypo-responsiveness (15) (Fig. 2C, panel d).
Tolerance induction is an active process that leads to an antigen-specific nonresponsiveness. The data presented here demonstrate an antigen-specific hypo-responsiveness, but not a complete nonresponsiveness to FVIII. This is possibly due to several reasons including the following. (i) The duration and dose of pre-exposure are not sufficient to induce a complete tolerance; (ii) a complete tolerance is established to some epitopes such as C2 domain, which is associated with PS, but tolerance is not induced to all of the possible FVIII epitopes; and (iii) the induction of tolerance is initially complete, but is partially broken by aggressive rechallenge with FVIII. Thus, optimization of several treatment parameters such as dose, duration, and systematic alteration of biophysical properties of protein-lipid complex could lead to the induction of a complete and durable tolerance. It is also important to mention here that residual in vivo FVIII activity was retained, although a delayed progression of anti-FVIII titers was observed from week 6 to week 11 in the FVIII-PS pretreated group. The plasma derived from animals that received multiple injections of FVIII-PS preserved the clotting activity of normal human plasma (36.6 ± 2.98 S.E.) at significantly higher levels as compared with plasma obtained from the FVIII alone treatment group (53.16 ± 3.1 S.E.). Under this experimental condition, inhibitor-free plasma derived from naive animals showed a clotting time of 30.88 ± 0.44 S.E. However, further studies are required to fully capture the effect of PS-induced tolerance on FVIII pharmacodynamics, especially in the presence of anti-FVIII Nabs. In general, regardless of the possible reasons for the partial hypo-responsiveness, pre-exposure of HA mice to FVIII-PS leading to hypo-responsiveness (even after stoppage of PS therapy) is significant and represents a potential for improving upon the therapeutic efficacy of FVIII.
Many strategies have been developed or are currently under active research to improve efficacy of FVIII replacement therapy in patients who have developed inhibitory titers following FVIII treatment. The currently utilized immune tolerance induction strategy in the clinic is to administer very high and frequent doses of FVIII to overload the immune response. Although patients have been shown to benefit from this therapy, immune tolerance induction takes anywhere from months to years to show beneficial effects. Further, the use of high doses of the protein makes the therapy prohibitively expensive, and patients have been shown to relapse even after treatment with this therapy. One approach is the use of immunosuppressive agents, but such strategies have the potentially significant negative effect of compromising the entire immune system. An alternative approach is the use of monoclonal antibodies (18) to eliminate T- and B-cells. However, both these strategies lack and fail to induce lasting FVIII immune tolerance. Newer approaches currently tested at the preclinical stage with successful outcome utilize FVIII gene therapy or delivery of FVIII antigen via administration of apoptotic cells (19, 20). However, the advantage of using reverse vaccination strategy is that a selective and durable reduction in the generation of Nabs to FVIII is achieved rapidly and requires relatively low amounts of the protein in naive recipients. Further, the commercial use of PS as a supplement and the ease of manufacturing to comply with regulatory requirements make it an attractive clinical option to mitigate immunogenicity of FVIII. It is not clear whether PS-mediated hypo-responsiveness is strong enough to reverse an established immune response and restore efficacy in inhibitor patients already treated with FVIII.
In conclusion, a novel reverse vaccination therapy utilizing immunoregulatory effects of PS has the potential to desensitize patients toward therapeutic proteins by inducing tolerance.
Acknowledgments
We thank the staff of the Confocal Microscope and Flow Cytometry Facility in the School of Medicine and Biomedical Sciences at the University at Buffalo. We thank Dr. Krzyzanski for the use of the auto hematology analyzer. We are grateful to Drs. Kazazian and Sarkar of the University of Pennsylvania for providing the factor VIII knock-out mouse model. We are grateful for the GFP-FoxP3 knock-in FVIII−/− mice breeding pair given as a generous gift from Dr. David Scott of the Uniformed Services University of the Health Sciences, and we thank to Dr. Bernstein of the Western New York Hemophilia Foundation for providing albumin-free recombinant factor VIII (ADVATE®). We thank biostatistician Dr. William Greco of University at Buffalo, The State University of New York for independently reviewing our data for statistical significance.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 HL-70227 (to S. V. B.).

This article contains supplemental Table 1.
- FVIII
- factor VIII
- HA
- hemophilia A
- PS
- brain phosphatidylserine
- PC
- dimyristoyl phosphatidylcholine
- PG
- dimyristoyl phosphatidylglycerol
- Dex
- dexamethasone
- Ova
- ovalbumin
- Nab
- neutralizing antibody
- Treg
- regulatory T-cell
- DC
- dendritic cell
- PE
- phycoerythrin.
REFERENCES
- 1. Lollar P., Healey J. F., Barrow R. T., Parker E. T. (2001) Factor VIII inhibitors. Adv. Exp. Med. Biol. 489, 65–73 [DOI] [PubMed] [Google Scholar]
- 2. Ramani K., Miclea R. D., Purohit V. S., Mager D. E., Straubinger R. M., Balu-Iyer S. V. (2008) Phosphatidylserine containing liposomes reduce immunogenicity of recombinant human factor VIII (rFVIII) in a murine model of hemophilia A. J. Pharm. Sci. 97, 1386–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Purohit V. S., Ramani K., Sarkar R., Kazazian H. H., Jr., Balasubramanian S. V. (2005) Lower inhibitor development in hemophilia A mice following administration of recombinant factor VIII-O-phospho-l-serine complex. J. Biol. Chem. 280, 17593–17600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gaitonde P., Peng A., Straubinger R. M., Bankert R. B., Balu-Iyer S. V. (2011) Phosphatidylserine reduces immune response against human recombinant factor VIII in hemophilia A mice by regulation of dendritic cell function. Clin. Immunol. 138, 135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Waters B., Lillicrap D. (2009) The molecular mechanisms of immunomodulation and tolerance induction to factor VIII. J Thromb Haemost. 7, 1446–1456 [DOI] [PubMed] [Google Scholar]
- 6. Elgueta R., Benson M. J., de Vries V. C., Wasiuk A., Guo Y., Noelle R. J. (2009) Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229, 152–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quezada S. A., Jarvinen L. Z., Lind E. F., Noelle R. J. (2004) CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22, 307–328 [DOI] [PubMed] [Google Scholar]
- 8. Banchereau J., Steinman R. M. (1998) Dendritic cells and the control of immunity. Nature 392, 245–252 [DOI] [PubMed] [Google Scholar]
- 9. Verbruggen B., Novakova I., Wessels H., Boezeman J., van den Berg M., Mauser-Bunschoten E. (1995) The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb. Haemost. 73, 247–251 [PubMed] [Google Scholar]
- 10. Zhang A. H., Skupsky J., Scott D. W. (2011) Effect of B-cell depletion using anti-CD20 therapy on inhibitory antibody formation to human FVIII in hemophilia A mice. Blood 117, 2223–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fontenot J. D., Rasmussen J. P., Williams L. M., Dooley J. L., Farr A. G., Rudensky A. Y. (2005) Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity 22, 329–341 [DOI] [PubMed] [Google Scholar]
- 12. Maldonado R. A., von Andrian U. H. (2010) How tolerogenic dendritic cells induce regulatory T cells. Adv. Immunol. 108, 111–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steinman R. M., Hawiger D., Nussenzweig M. C. (2003) Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 [DOI] [PubMed] [Google Scholar]
- 14. Horwitz D. A., Zheng S. G., Gray J. D. (2008) Natural and TGF-β-induced Foxp3+CD4+ CD25+ regulatory T cells are not mirror images of each other. Trends Immunol. 29, 429–435 [DOI] [PubMed] [Google Scholar]
- 15. Wan Y. Y., Flavell R. A. (2008) TGF-β and regulatory T cell in immunity and autoimmunity. J. Clin. Immunol. 28, 647–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li M. O., Sanjabi S., Flavell R. A. (2006) Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25, 455–471 [DOI] [PubMed] [Google Scholar]
- 17. Sule G., Suzuki M., Guse K., Cela R., Rodgers J. R., Lee B. (2012) Cytokine-conditioned dendritic cells induce humoral tolerance to protein therapy in mice. Hum. Gene Ther. 23, 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wiestner A., Cho H. J., Asch A. S., Michelis M. A., Zeller J. A., Peerschke E. I., Weksler B. B., Schechter G. P. (2002) Rituximab in the treatment of acquired factor VIII inhibitors. Blood. 100, 3426–3428 [DOI] [PubMed] [Google Scholar]
- 19. Su R. J., Epp A., Feng J., Roy J., Latchman Y., Wu X., Bolgiano D., Josephson N. C. (2011) Suppression of the immune response to FVIII in hemophilia A mice by transgene modified tolerogenic dendritic cells. Mol. Ther. 19, 1896–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skupsky J., Saltis M., Song C., Rossi R., Nelson D., Scott D. W. (2010) Gene therapy for tolerance and vice versa: a case for hemophilia. Curr. Opin. Mol. Ther. 12, 509–518 [PubMed] [Google Scholar]