Abstract
Purpose
To determine the role of the CCL2/CCR2 axis and inflammatory monocytes (IM; CCR2+/CD14+) as immunotherapeutic targets in the treatment of pancreatic cancer (PC).
Experimental Design
Survival analysis was performed to determine if the prevalence of pre-operative blood monocytes correlates with survival in PC patients following tumor resection. IM prevalence in the blood and bone marrow of PC patients and controls was compared. The immunosuppressive properties of IM and macrophages in the blood and tumors, respectively, of PC patients were assessed. CCL2 expression by human PC tumors was compared to normal pancreas. A novel CCR2 inhibitor (PF-04136309) was tested in an orthotopic model of murine PC.
Results
Monocyte prevalence in the peripheral blood correlates inversely with survival, and low monocyte prevalence is an independent predictor of increased survival in PC patients with resected tumors. IM are increased in the blood and decreased in the bone marrow of PC patients compared to controls. An increased ratio of IM in the blood versus the bone marrow is a novel predictor of decreased patient survival following tumor resection. Human PC produces CCL2, and immunosuppressive CCR2+ macrophages infiltrate these tumors. Patients with tumors that exhibit high CCL2 expression/low CD8 T cell infiltrate have significantly decreased survival. In mice, CCR2 blockade depletes IM and macrophages from the primary tumor and premetastatic liver resulting in enhanced anti-tumor immunity, decreased tumor growth, and reduced metastasis.
Conclusions
IM recruitment is critical to PC progression, and targeting CCR2 may be an effective immunotherapeutic strategy in this disease.
Introduction
Pancreatic ductal adenocarcinoma, often called pancreatic cancer (PC), is an aggressive malignancy with a death rate nearly equal to its incidence and a 5-year survival of less than 5% (1). PC is characterized by a uniquely dense stroma that confers resistance to therapy (2-5). Within this stroma are abundant immunosuppressive myeloid cells which include monocytes/macrophages (2). While it is appreciated that tumor-associated macrophages (TAM) possess important tumor promoting properties in several malignancies (6), the contribution of monocyte mobilization from the bone marrow to the tumor has not been well studied. Here, we investigate the key role of monocyte migration from the bone marrow to the primary tumor and premetastatic site in PC, and demonstrate the efficacy of CCR2 blockade in the treatment of this disease.
Monocytes are produced and stored in the bone marrow, and are CD45+/CD11b+/CD115+/HLA-DR+ in humans and CD45+/CD11b+/CD115+/F4/80+/MHCII− in mice (7). However, monocytes are composed of heterogeneous subsets which include resident monocytes (RM) and inflammatory monocytes (IM) (8). RM are CD16+/CX3CR1high/CD14−/CCR2− in humans and mice (7). RM represent approximately 15% of circulating monocytes in normal healthy humans and 40-50% in mice (9, 10). These cells arise from the differentiation of IM in the periphery where they play a role in steady-state immunosurveillance and inflammation resolution (7, 9) In contrast, IM are CD14+/CCR2+/CD16−/CX3CR1low in humans and Ly6Chi/CCR2+/CD16−/CX3CR1low in mice. These cells are the predominant monocytes in circulation representing approximately 85% of circulating monocytes in normal healthy humans and 50-60% in mice (8, 9). Under physiologic conditions, the CCL2/CCR2 chemokine axis is critical to the mobilization of IM from the bone marrow to the blood as well as their recruitment to sites of inflammation where they extravasate into tissues and differentiate into macrophages or dendritic cells (7). Macrophages can be critical regulators of tumor progression (6); however, these cells are not highly prevalent in normal pancreas and must be selectively recruited during malignant progression (2). As such, monocyte mobilization from the bone marrow is a vital pathway for macrophages to infiltrate tumors in the periphery. Once assimilated, macrophages acquire an immunosuppressive, trophic (alternatively activated or M2) phenotype in the tumor microenvironment and at the premetastatic site (6).
In these studies, we demonstrate that both the prevalence of monocytes in the peripheral blood and the mobilization of IM from the bone marrow are predictive of survival in PC patients. Furthermore, we show that PC utilizes the CCL2/CCR2 axis to favor the mobilization and recruitment of IM from the bone marrow to the primary tumor and premetastatic liver where these cells facilitate tumor growth and metastasis. We identify CCR2 inhibition (CCR2i) using a novel agent (PF-04136309) as an adjunct to standard chemotherapy in PC which blocks IM recruitment resulting in the reduction of tumor growth and metastasis.
Materials and Methods
Analysis of peripheral blood monocyte prevalence and survival with multivariate analysis
All patients (n=483) with PC undergoing pancreaticoduodenectomy at Barnes-Jewish Medical Center between 1997 to 2011 were followed for survival in a prospectively maintained database under an Internal Review Board (IRB) approved protocol. We excluded patients with elevated pre-operative leukocyte counts (>11,000 cells/dl; n=50), patients who did not have pre-operative complete blood counts (CBC) obtained at our institution (n=49), and patients who died within 30 days of surgery (n=7).Patients were stratified into 3 groups based on the percent of blood leukocytes which were monocytes (monocyte prevalence) on pre-operative CBC: low (<6%), normal (≥6 to <11%), and high (≥11%) monocyte groups. Ranges were established such that all patients in the mid group fell within 1 standard deviation (SD) of the mean; thus, patients in the low and high monocyte groups were greater than 1 SD below and above the mean, respectively. Multivariate analysis was carried out using PC patient demographic and pathologic data. Further analytic details are described in the supplementary methods.
Isolation of blood, bone marrow, and tumor from pancreatic cancer patients
Informed consent was obtained on all patients in accordance with institutional Human Studies Committee Protocol. Peripheral blood and bone marrow mononuclear cells were isolated from healthy volunteers and PC patients prior to chemotherapy, radiation, or surgery as has been previously described (11). Human pancreatic adenocarcinomas and normal pancreas were snap frozen in liquid nitrogen or minced, mechanically dissociated, digested in enzyme buffer for 30 min, and filtered.
Mice, cell lines, and murine pancreatic cancer model
C57BL/6 and CCR2−/− mice (B6.129S4-Ccr2tm1Ifc/J) were purchased from Jackson Laboratories. The murine pancreatic adenocarcinoma cell line KCKO, a metastatic tumor line, was the kind gift of Dr.Pinku Mukherjee (12). Eight to 10 week old mice were anesthetized and injected in the tail of the pancreas with 1×105 KCKO cells suspended in a 1:1 PBS: matrigel mixture. After mice were sacrificed at indicated times, bone marrow was extracted from the femurs and blood collected in heparinized capillary tubes. Blood and bone marrow cells were subjected to RBC lysis (Biolegend) per manufacturer’s protocol. Orthotopic tumor burden was measured by the gross wet weight of the pancreas. Metastatic and disseminated tumors were scored by serial sectioning and gross evaluation, which was validated by tissue pathology.
Chemotherapy and CCR2 inhibitor
PF-04136309 (Pfizer) is a CCR2 kinase antagonist and the details have been published previously (13). Mice were injected subcutaneously with 100 mg/kg of PF-04136309 twice-daily beginning 2 days after tumor implantation. Mice were also injected intravenously with 50 mg/kg of Gemcitabine (Hospira) into the retro-orbital sinus every 4 days. When indicated GEM and PF-04136309 were given in combination without altering dose or schedule of either agent separately.
Flow cytometry
Human and mouse single-cell suspensions were blocked with TruStain FcX™ or anti-CD16/32 antibody respectively (Biolegend) and stained with fluorescent antibodies using standard protocols for flow cytometry. Cells undergoing intracellular staining were permeabilized with eBioscience Permeabilization Buffer according to the manufacturer’s protocol. Antibodies used for human staining are listed in Supplementary Methods.
RNA Isolation and Real Time Polymerase Chain Reaction
Total RNA was isolated by Trizol extraction and reverse transcribed into cDNA. Quantitative real-time PCR (qRT-PCR) was performed using pre-designed TaqMan Gene Expression Assays (Life Technologies) on a 7500 Fast Thermal Cycler (Applied Biosystems). Target gene expression was normalized to GAPDH, HPRT1, or β-actin. The normalized expression levels of genes were analyzed using the 7500 software for 7500 RT PCR system V2.0.6.
T Cell Proliferation Assays
CD14+ cells were isolated from peripheral blood mononuclear cells of PC patients prior to tumor resection and single cell tumor suspensions with the EasySep® Human CD14 Positive Selection Kit per manufacturer’s instructions (Stemcell Technologies™). CD14 depleted PBMCs were labeled with CFSE (Life Technologies) and co-cultured in 96-well round bottom plates (Corning) coated with LEAF™ purified anti-human CD3 (Biolegend, clone OKT3) with varying concentrations of autologous CD14+ cells in complete media supplemented with human Interleukin 2 (National Institute of Health). Cell cultures were harvested after incubating for 72 hours at 37°C and the CFSE dilution of the CD4+ and CD8+ T-cell fractions were analyzed by flow cytometry. The division index (defined as the average number of divisions that a cell present in the starting population has undergone) was calculated using FloJo software.
Immunofluorescence and Immunohistochemistry
Tissue sections from formalin-fixed paraffin-embedded tissue blocks of human PC and normal pancreas were stained as previously described (11). Confocal images were acquired on an Axiovert 100M microscope equipped with a LSM 510 META Confocal Laser Scanning Microscope system (Zeiss). Immunohistochemical Images were acquired at 10× magnification on an Olympus BX51 microscope with a SPOT RT Slider digital camera and software (Diagnostic Instrument, Inc.).See Supplementary Methods for further details.
Pancreatic cancer tissue microarray (TMA) survival analysis
After obtaining IRB approval, TMA studies were conducted on a cohort of 60 previously untreated PC patients who underwent pancreaticoduodenectomy at Barnes-Jewish Hospital. Patients did not receive neo-adjuvant therapy and were typically treated with adjuvant chemotherapy. To construct the TMA, well defined areas of tumor were demarcated and punched (1mm diameter) from paraffin-embedded tumor blocks. An Aperio Scan-Scope XT Slide Scanner (Aperio Technologies) system was used to acquire digital images using a 20× objective. A tumor-specific nuclear algorithm (IHC-MARK) developed in-house (14, 15) was modified to quantify CCL2 and CD8 expression.
Statistical analysis
All data (other than multivariate analyses) was analyzed using Graph Pad Prism version 5.01 (GraphPad Software Inc., La Jolla, CA). Calculating differences in numerical values was performed using Mann-Whitney test for non-parametric data. Fisher’s exact test was used to compare categorical data. p<0.05 defined statistically significant differences.
See Supplementary Methods for additional methodological data
Results
Decreased monocytes in the peripheral blood is associated with better survival in pancreatic cancer patients
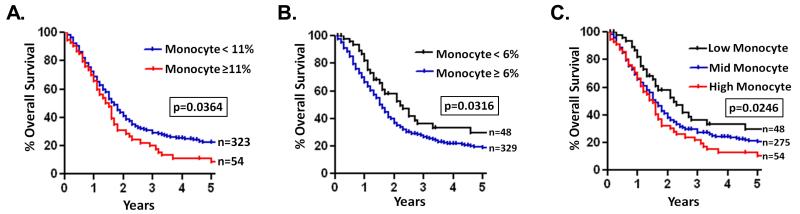
To investigate the importance of monocytes in PC, we examined whether there was a correlation between survival and the prevalence of monocytes in the blood of 377 PC patients. All patients were chemotherapy naïve, diagnosed with local, surgically resectable disease and underwent pancreaticoduodenectomy. Patients were stratified into low (< 6% of leukocytes, >1 SD below mean), mid (≥ 6 to < 11% of leukocytes, within 1 SD of mean), or high (≥ 11% of leukocytes, 1 SD above mean) monocyte groups based on the prevalence of monocytes in their pre-operative complete blood cell count (CBC). There was a significant correlation between the prevalence of pre-operative monocytes in the peripheral blood of PC patients and overall survival (Supplementary Table S1). Patients with high blood monocytes had significantly decreased overall survival compared to the rest of the cohort with a 5-year survival of 11.0% vs 22.1% (p=0.03) (Fig. 1A). Additionally, low blood monocyte count was found to be a prognostic factor for survival in PC patients compared to the rest of the cohort with a 5-year survival of 28.8% vs 20.2% (p=0.04) (Fig. 1B). When comparing patients in the low, mid, and high monocyte groups separately, there was an incremental decrease in survival as blood monocytes increased; 5-year survival was 28.8% vs 20.9% vs 11.0% (mean survival = 35.6 months vs, 27.6 months vs 21.1 months, p=0.02) in the low, mid, and high groups, respectively (Fig. 1C).
Figure 1. The prevalence of peripheral blood monocytes is prognostic in PC patients.
A, patients were stratified into low (< 6% of leukocytes, > 1 SD below mean), mid (≥ 6 to < 11% of leukocytes, within 1 SD of mean), and high (≥ 11% of leukocytes, > 1 SD above mean) peripheral blood monocyte groups. Kaplan Meier survival curves compare PC patients in high (A) and low (B) blood monocyte groups to the rest of the cohort as well as patients in low, mid, and high blood monocyte groups separately (C). p values are by log-rank (Mantel-Cox) test.
As monocyte prevalence in the blood correlated inversely with survival on univariate analysis, we performed a multivariate analysis using patient demographic and pathologic data to determine if monocyte count was an independent predictor of survival in PC patients. Indeed, low blood monocyte prevalence was independently associated with increased survival on multivariate analysis (hazard ratio=0.58, 95%CI=0.40-0.86). However, the association between high monocyte prevalence and survival was not independent of other pathologic factors. Compared to the rest of the cohort, patients in the high monocyte group had a strong trend towards having a higher incidence of lymph node positive tumors (81.5% vs 68.1%, p=0.066), which is a strong predictor of decreased survival after tumor resection (16). These findings indicate that monocyte prevalence in the peripheral blood is a prognostic indicator for patient survival and thus targeting monocytes might represent an attractive therapeutic strategy.
Inflammatory monocyte mobilization from the bone marrow is prognostic in pancreatic cancer patients
As with other chronic inflammatory conditions, monocytes are elevated in the peripheral blood of patients with solid organ malignancy (17), including PC (Supplementary Fig. S1A). Given the fact that the bone marrow acts as a storage reservoir for monocytes, we hypothesized that IM are being mobilized from the bone marrow to the peripheral blood in human PC (7). Therefore, we analyzed the prevalence of IM and RM in peripheral blood (PBMC) and bone marrow (BMMC) mononuclear cells from non-metastatic PC patients prior to any treatment (i.e. chemotherapy, radiation, or surgical resection) and compared these to normal controls (Fig. 2A). We found that IM were significantly more prevalent in the blood of PC patients (Fig. 2B), however, the prevalence of RM was unchanged (Supplementary Fig. S1B). Consistent with previous reports, IM made up 85% of blood monocytes in healthy individuals (8), and were increased to 92% in PC patients (Supplementary Fig. S1C). Analysis of the bone marrow revealed that the prevalence of IM were significantly decreased in PC patients compared to controls whereas RM were unchanged, suggesting that there is a shift in IM equilibrium from the bone marrow to the peripheral blood during PC (Fig. 2C).
Figure 2. Inflammatory monocyte equilibrium in the peripheral blood and bone marrow is prognostic in pancreatic cancer patients.
A, flow cytometry gating strategies used to define IM (CD115+/CD16−/CX3CR1low/CD14+/CCR2+) and RM (CD115+/CD16+/CX3CR1hi/CD14−/CCR2low) in peripheral blood and bone marrow of PC patients (n=21) and healthy controls (n=11). Graphs compare the prevalence of IM in the blood (B) and bone marrow (C) of PC patients and controls. D, graph compares the blood:bone marrow IM ratios of PC patients with <1 year survival to ≥ 1 year survivors following tumor resection. All graphs show means ± SEM, and p values are by Mann-Whitney test.
Given the finding that patients with elevated peripheral blood monocytes have worse overall survival, we hypothesized that the ratio of IM in the blood versus the bone marrow (blood:bone marrow IM ratio) may serve as a surrogate for IM mobilization which could contribute to PC progression and ultimately decrease survival. This ratio was increased in PC patients compared to normal controls (Supplementary Fig. 1D). Importantly, PC patients who experienced rapid, treatment refractory recurrence and death within the first year following tumor resection had significantly higher blood:bone marrow IM ratios compared to 1-year PC survivors (Fig. 2D). Similarly, PC patients with blood:bone marrow IM ratios ≥ 1.5 had significantly decreased survival (Supplementary Fig. S1D). This suggests that IM mobilization plays a key role in PC patient outcome.
Human PC tumors express CCL2 and are infiltrated by immunosuppressive CCR2+ macrophages
The recruitment of IM in various human inflammatory diseases, such as rheumatoid arthritis,(18), type 1 diabetes(19), and atherosclerosis(20) is mediated by the chemokine CCL2 and its receptor CCR2. Gene expression analysis found that human PC tissue has elevated CCL2 mRNA compared to normal pancreas by both qRT-PCR analysis of surgical specimens and by retrospective analysis of published datasets (21) (Fig. 3A, Supplementary Fig. S2A). Upon further analysis of tumors, CCL2 protein was highly expressed by malignant ducts as well as cells apparently within the stroma, which could represent stromal cells and/or cancer cells in epithelial-mesenchymal transition (Fig. 3A, Supplementary Fig. S2B).
Figure 3. Human pancreatic cancers express CCL2 and are infiltrated by immunosuppressive CCR2+ macrophages.
A, bar graph compares CCL2 gene expression in human PC (n=11) to normal pancreas (n=10) and representative immunofluorescent confocal images (40×) of human PC stained for cytokeratin 7 (green) and CCL2 (red) with nuclear (Topro) counterstain (blue). B, representative flow cytometry images (gated on CD45+ cells) and immunofluorescent confocal images (40×; CD14 = green, CCR2 = red, and nuclear (Topro) counterstain = blue) of human PC tumors reveal tumor-infiltrating CCR2+ TAM, while bar graph compares tumor-infiltrating TAM to CD8 T cells using flow cytometry. C, representative flow cytometry histogram from T cell suppression assay depicts stimulated, CFSE-labeled CD8 T cells alone (red) or co-cultured at a 1:1 IM (blue) or TAM (green) to lymphocyte ratio after 72 hours, and bar graph compares effect of IM and TAM on T cell division index at various ratios. n=6 experiments. All graphs depict means ± SEM and * denotes p<0.05 by Mann-Whitney test. D, Kaplan-Meier survival curves compare overall survival in PC patients with high CD8 T cell tumor infiltrate/low tumor CCL2 expression to patients with low CD8 T cell infiltrate/high tumor CCL2 expression by tissue microarray (n=60 tumors). p-value is by log-rank (Mantel-Cox) test.
Pancreatic tumors possess a highly immunosuppressive microenvironment in which myeloid cells are crucial (2, 3, 11, 22). There is evidence that IM are recruited to tumors where they differentiate into immunosuppressive TAM (23-25). CCR2+ TAM (CD45+/CD11b+/CD115+/HLA-DR+/CD14+/CCR2+) reside in human PC tumors, and make up roughly 28% of tumor infiltrating leukocytes (Fig. 3B, Supplementary Fig. S2C). However, effector T cells are significantly outnumbered by immunosuppressive TAM as CD8 T cells make up only around 7% of tumor infiltrating leukocytes (Fig. 3B, Supplementary Fig. S2C). We sought to determine if IM are immunosuppressive in the peripheral blood of PC patients or whether tumor infiltration is a prerequisite. We isolated both IM and TAM from the fresh blood and tumors, respectively, of individual PC patients (Supplementary Fig. S2D), and compared their abilities to suppress autologous effector T cell proliferation. We observed that while TAM were markedly immunosuppressive, IM were not (Fig. 3C). This suggests that the tumor microenvironment changes the phenotype of IM following infiltration.
PC patients having tumors with high TAM:CD8 T cell ratios have a poor prognosis (15). Similarly, patients having tumors which express high CCL2 with a low CD8 T cell infiltrate (upon univariate analysis of a tissue microarray) also have significantly reduced survival compared to patients with tumors expressing low CCL2 and having high CD8 T cells (Fig. 3D). This further demonstrates the critical tumor cell-stroma interplay that is characteristic of PC (5).
CCR2 mediates inflammatory monocyte mobilization in murine PC
To determine if targeting IM recruitment through CCR2 signaling inhibition could prevent IM mobilization from the bone marrow in PC, we utilized a murine model. KCKO is a metastatic cell line derived from a genetically-engineered, spontaneous murine PC model (LSL-KRASG12D × p48-Cre) (12). Like human PC, KCKO tumors express increased CCL2 compared to normal murine pancreas (Supplementary Fig. S3A). Also, KCKO does not express CCR2 in vivo. We injected KCKO orthotopically into the pancreas of wild type (WT) mice to study the effects of PC on IM mobilization from the bone marrow. Mimicking our observations in PC patients, tumor bearing mice displayed significant increases in circulating IM while these cells were decreased in the bone marrow (Fig. 4A). Signaling through CCR2 is crucial to monocyte egress from the bone marrow (26, 27). We observed that tumor bearing WT mice treated with a CCR2 antagonist (PF-04136309) and tumor bearing CCR2−/− mice exhibit a significant decrease in circulating IM (Figs. 4A,B, Supplementary Fig. S3B). Additionally, these mice had an increased prevalence of IM in the bone marrow compared to vehicle-treated tumor bearing mice, suggesting that these cells are retained in the bone marrow (Figs. 4A,C, Supplementary Fig. S3C). Furthermore, CCR2 blockade reverses the blood:bone marrow IM ratio in tumor bearing mice which we have shown to be prognostically important in PC patients (Fig. 4D).
Figure 4. CCR2 mediates inflammatory monocyte mobilization from the bone marrow to the blood in murine pancreatic cancer.
A, representative flow cytometry images demonstrate changes in IM prevalence in the blood and bone marrow of control, vehicle-treated, and CCR2i-treated tumor-bearing WT mice after 28 days. Bar graphs show the prevalence of IM in the blood (B) and bone marrow (C) as well as the blood:bone marrow IM ratios (D) of control, vehicle-treated, CCR2−/−, and CCR2i-treated tumor bearing WT mice. All graphs depict means ± SEM and horizontal bars denote statistically significant differences between groups defined as p<0.05 by Mann-Whitney test.
CCR2 inhibition promotes antitumor immunity in murine pancreatic cancer
Like human PC, KCKO implanted in the pancreas of mice is characterized by a dense stromal infiltrate with a predominance of immunosuppressive myeloid cells (Supplementary Fig. S4A). We found that tumor bearing WT mice treated with CCR2i and tumor bearing CCR2−/− mice displayed marked decreases in tumor-infiltrating IM and macrophages (CD45+/CD11b+/F4/80+/Ly6Clow/MHCII+) (Fig. 5A). In contrast, tumor infiltrating effector T cells were increased with a concomitant decrease in regulatory T cells (CD45+/CD4+/CD25+/FoxP3+) (Figs. 5B) suggesting an enhanced anti-tumor immune response. Tumors from WT mice treated with CCR2i and from CCR2−/− mice exhibited a shift from a TH2 to a TH1 gene expression profile, characterized by decreased Arg1, TGF-β, IL-1β, IL-6, and IL-10 with an increase in IFN-gamma (Fig. 5C) (28). Additionally, mRNA expression of the monocyte/macrophage recruitment mediators, CCL2 and macrophage colony stimulating factor (M-CSF), were both increased in tumors from CCR2i treated mice and from CCR2−/− mice by qRT-PCR (Supplementary Figs. S4B-C). This may suggest a regulatory feedback mechanism between tumors and infiltrating macrophages; specifically, tumors deprived of TAM may attempt to recruit additional macrophages by both upregulating CCL2 production and adapting to use alternative mechanisms, such as M-CSF production.
Figure 5. CCR2 blockade promotes anti-tumor immunity and impairs tumor growth.
Bar graphs compare tumor myeloid infiltrate (A) as well as the prevalence of tumor infiltrating T lymphocytes (B) and immune gene expression (C) in WT tumor-bearing mice treated with vehicle, GEM, CCR2i, and CCR2i+GEM combination after 28 days. D, tumor growth curves and graph compare effect of CCR2 blockade on subcutaneous and orthotopic (at 28 days) KCKO tumor growth. Note: CCR2−/− tumors were significantly smaller than CCR2i-treated tumors at days 25 and 28 on growth curve, p< 0.05. All graphs depict means +/− SEM. Horizontal bars or * denotes p<0.05 whereas ** denotes p<0.01 by Mann-Whitney test.
During tumorigenesis, granulocytes acquire immunosuppressive properties and promote tumor growth (22, 29, 30). These granulocytes are often referred to as granulocytic myeloid-derived suppressor cells (G-MDSC), and their importance has previously been demonstrated in human and murine PC (11, 31). Gemcitabine (GEM) is a standard chemotherapeutic agent used in human PC (32). GEM targets rapidly dividing tumor cells, but also selectively depletes granulocytes (CD45+/CD11b+/Gr1hi/Ly6G+) (33). By contrast, monocytes/macrophages are more resistant to the effects of GEM, and we have found that IM persist in the blood of PC patients treated with chemotherapy (Supplementary Fig. S5). We observed a complementary effect of GEM on granulocyte depletion in the tumors of CCR2i treated mice, as CCR2i alone resulted in a slight increase in granulocytes (Fig. 5A). Alternatively, GEM alone led to an increase in TAM, which CCR2i effectively depleted in the tumors of mice treated with the combination of these two agents (Fig. 5A). CCR2−/− mice and CCR2i treated WT mice displayed significantly reduced tumor growth of both subcutaneous and orthotopic tumors (Fig. 5D). An additive decrease in tumor growth was also observed when GEM was given in combination with CCR2i (Fig. 5D).
CCR2 inhibition prevents liver metastasis in murine PC
IM and macrophages are believed to play a crucial role in the establishment of metastasis (6). CCL2 has been demonstrated to play an important role in the recruitment of metastasis-associated macrophages (MAM; CD11b+/F4/80+/Ly6Clow/CCR2+) to the premetastatic lung in an experimental model of breast cancer (34). In PC, the liver is the most common site of distant metastasis (16). We observed a marked increase in CCL2 mRNA expression by qRT-PCR with a concomitant increase in IM and macrophages in the premetastatic livers of WT mice bearing orthotopic PC tumors (Figs. 6A-C, Supplementary Fig. S6). However, CCR2i efficiently blocked the recruitment of IM and macrophages to premetastatic livers whereas GEM did not (Figs. 6B-D, Supplementary Fig. S6). Twenty-eight days post implantation, this was associated with a significant decrease in liver metastasis compared to vehicle or GEM only treated mice (Fig. 6E). Strikingly, none of the 15 mice treated with the combination of GEM and CCR2i acquired hepatic metastasis compared to 15 of 20 vehicle-treated mice who developed liver metastasis (Fig. 6D). This suggests that targeting CCR2 can prevent liver metastasis in PC.
Figure 6. CCR2 inhibition reduces inflammatory monocytes and metastasis-associated macrophages in premetastatic livers and impairs hepatic metastasis in murine pancreatic cancer.
A, graph compares CCL2 expression using qRT-PCR in baseline (Day 0), premetastatic (Day 6) and metastatic (Day 28) livers of WT mice. B, representative immunofluorescence images (20×) of livers 9 days post-injection from control (matrigel only, MG) mice, vehicle-treated tumor bearing mice, and CCR2i-treated tumor bearing mice for F4/80 (green) expression with a nuclear (Topro) stain (blue). C, graphs depict macrophage and IM prevalence in the livers of MG control or tumor bearing mice treated with vehicle, CCR2i, GEM, or CCR2i+GEM combination by flow cytometry. All flow cytometry and qRT-PCR performed on grossly normal liver (i.e. excluded metastatic liver deposits). All graphs depict means ± SEM and horizontal bars denote statistically significant differences between groups defined as p<0.05 by Mann-Whitney test. D, graph demonstrates the incidence of liver metastasis after 28 days in tumor bearing mice treated with vehicle, GEM, CCR2i, and CCR2i+GEM combination as well as CCR2−/− mice. n = 13-20 mice per group. Horizontal bars denote p<0.05 by Fisher’s exact test.
Discussion
Monocyte mobilization from the bone marrow has been poorly characterized in human solid organ malignancy. We have identified an important pathophysiologic process in PC patients that has both prognostic and therapeutic implications. Current PC staging systems, such as the American Joint Committee on Cancer TNM staging, fail to identify those patients who quickly recur and die from systemic progression of their disease following removal of their tumors (pancreatectomy)(16). We found that survival decreases as the prevalence of blood monocytes increases in PC patients, and that a low prevalence of blood monocytes is an independent predictor of improved survival. Additionally, an increased blood:bone marrow IM ratio predicts which PC patients experience rapid recurrence and death following pancreatectomy. Perhaps, using monocytes in the blood and bone marrow as a biomarker can assist in therapeutic decision-making by indicating which patients would benefit from early aggressive systemic treatment, such as chemotherapy or immunotherapy, rather than initial local treatment, such as surgery. Furthermore, PC patients with evidence of increased monocyte mobilization from the bone marrow may be the ideal candidates in which to utilize anti-monocyte/macrophage therapies.
Tumors are sites of chronic inflammation and monocytes/macrophages influence outcome (35). Immunosuppressive myeloid cells are the predominant tumor-infiltrating leukocytes in PC – not T cells (3, 11, 22). Tumors depend on the stroma for survival and spread, and monocyte/macrophages are critical. TAM arise from monocytes, and these cells suppress anti-tumor immunity (36) as well as directly promote tumor growth (15), invasiveness (37), angiogenesis (38), and chemoresistance (14). TAM within the tumor microenvironment have been shown to correlate with decreased patient survival in several human malignancies, including human PC (39-41). However, we introduce the novel concept that there is a shift in IM equilibrium from the bone marrow to the blood which is also predictive of survival in PC patients.
CCR2+ monocytes have been shown to mediate immunosuppression (42) and metastasis (34) in murine cancer models. The CCL2/CCR2 chemokine axis plays an essential role in the recruitment of IM from the bone marrow to peripheral sites of inflammation. Once recruited, the phenotype of monocytes/macrophages depends on the local immune environment (43). Tumors utilize this same pathway to recruit monocytes to the primary tumor where these cells acquire an alternatively activated (M2) phenotype (34, 44). CCL2 expression is also upregulated in the premetastatic liver of mice bearing orthotopic PC tumors. We hypothesize that IM mobilization in PC is a surrogate for macrophage infiltration in the tumor and premetastatic liver. Our experiments in mice support this theory as the ratio of blood:bone marrow IM correlated with the prevalence of macrophages in the primary tumor and premetastatic liver. Targeting TAM in PC is a relatively new strategy (45). We have chosen the approach of depleting macrophages in the tumor and liver by targeting IM with CCR2 blockade, which acts at the level of the bone marrow (26, 27). Unlike many immunotherapeutics, CCR2i does not depend on the delivery of drug to the stroma-dense, poorly vascular tumor microenvironment. On the contrary, CCR2i depends on delivery to the highly vascular bone marrow (26).
In the present study, we revealed that circulating IM were not immunosuppressive in the PC patients examined. However, a monocytic subset of MDSC (Mo-MDSC; CD14+/HLA-DRlow/−) with immunosuppressive properties has been described in the blood of patients with advanced malignancy (46, 47). While we did not observe Mo-MDSC in the blood of the PC patients examined, this could be due to differences in the patient populations studied. We evaluated the blood and bone marrow of surgically resectable, non-metastatic PC patients without evidence of active infection (i.e. pre-op blood leukocytes < 11,000cells/dl) prior to any treatment in order to limit confounding factors which often plague patients with advanced PC, such as infection, biliary obstruction, multiple courses of chemotherapy/radiation, tumor necrosis, and bowel obstruction (16). Admittedly, there is significant overlap between cells defined as Mo-MDSC and IM. All CD14+/CCR2+ cells in the peripheral blood of PC patients in the current study were HLA-DR+ and did not suppress T cell proliferation ex vivo - thereby meeting the definition of IM as opposed to Mo-MDSC (29). It is likely that IM and Mo-MDSC are closely related, and Mo-MDSC may in fact represent a subset of IM as has been suggested (48). There is evidence in patients with GI malignancy that the extent of cell-mediated immune responses (mediated by monocytes/macrophages) is inversely proportional to the stage of disease (49, 50). Perhaps IM in the blood of patients with more advanced malignancy may down-regulate HLA-DR expression and acquire immunosuppressive properties - thereby meeting the definition of Mo-MDSC.
In summary, IM mobilization from the bone marrow predicts survival in human PC, and the CCL2/CCR2 axis plays a critical role in the recruitment of IM to the tumor microenvironment and premetastatic liver. CCR2i may be an ideal compliment to standard chemotherapeutics as this therapy had additive effect on the tumor while dramatically reducing metastasis. Based on the data presented here, we are proceeding with a Phase Ib/II clinical trial using PF-04136309 combined with standard chemotherapy in PC patients with locally advanced, non-metastatic disease (NCT01413022) [ClinicalTrials.gov]. Tumor, blood, and bone marrow are being collected pre- and post-treatment with CCR2i to determine if changing the blood:bone marrow IM ratio can reduce TAM in the tumor, decrease metastasis, and improve patient survival. We believe the work presented here along with our clinical trial will make substantial contributions to the fields of cancer immunotherapy and tumor monocyte/macrophage biology.
Supplementary Material
Translational Relevance.
Pancreatic cancer (PC) is an aggressive malignancy with a dismal prognosis, due in part to a high rate of metastatic dissemination and chemoresistance. Macrophages are predominant in the uniquely dense PC stroma, and these cells enhance tumor growth and metastasis. Inflammatory monocytes (IM; CD14+/CCR2+) depend on CCL2/CCR2 signaling for mobilization from the bone marrow to the blood as well as recruitment to sites of inflammation where they extravasate into tissues and become macrophages. The role of IM in PC as well as the effect of targeting these cells through CCR2 blockade has not been studied. These studies demonstrate that IM recruitment from the bone marrow is prognostically and therapeutically important in PC. This study has laid the foundation for an ongoing Phase Ib trial using CCR2 blockade in combination with chemotherapy in PC patients with locally advanced, non-metastatic disease (NCT01413022).
Acknowledgements
ADD acknowledges funding from NCI cancer center grant P30 CA091842. AWG, DCL, and DGD acknowledge the Siteman Cancer Center Frontier Fund Team Science Award. DCL acknowledges funding from the WU/Pfizer Biomedical Research Grant PW0457. DGD acknowledges support from the Lustgarten Foundation, V Foundation, Edward Mallinckrodt Jr. Award, the Cancer Research Foundation and Siteman Cancer Center Career Development Award. JBM and DES acknowledge funding from NCI grant T32 CA 009621.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. Epub 2010/07/09. doi: caac.20073 [pii] 10.3322/caac.20073. PubMed PMID: 20610543. [DOI] [PubMed] [Google Scholar]
- 2.Clark CE, Beatty GL, Vonderheide RH. Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer. Cancer Lett. 2009;279(1):1–7. doi: 10.1016/j.canlet.2008.09.037. Epub 2008/11/18. doi: 10.1016/j.canlet.2008.09.037. PubMed PMID: 19013709. [DOI] [PubMed] [Google Scholar]
- 3.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67(19):9518–27. doi: 10.1158/0008-5472.CAN-07-0175. Epub 2007/10/03. doi: 67/19/9518 [pii] 10.1158/0008-5472.CAN-07-0175. PubMed PMID: 17909062. [DOI] [PubMed] [Google Scholar]
- 4.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–29. doi: 10.1016/j.ccr.2012.01.007. Epub 2012/03/24. doi: 10.1016/j.ccr.2012.01.007. PubMed PMID: 22439937; PubMed Central PMCID: PMC3371414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim EJ, Simeone DM. Advances in pancreatic cancer. Current opinion in gastroenterology. 2011;27(5):460–6. doi: 10.1097/MOG.0b013e328349e31f. Epub 2011/07/23. doi: 10.1097/MOG.0b013e328349e31f. PubMed PMID: 21778878. [DOI] [PubMed] [Google Scholar]
- 6.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. Epub 2010/04/08. doi: 10.1016/j.cell.2010.03.014. PubMed PMID: 20371344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nature reviews Immunology. 2011;11(11):762–74. doi: 10.1038/nri3070. Epub 2011/10/11. doi: 10.1038/nri3070. PubMed PMID: 21984070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. Epub 2003/07/23. doi: S1074761303001742 [pii]. PubMed PMID: 12871640. [DOI] [PubMed] [Google Scholar]
- 9.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature reviews Immunology. 2005;5(12):953–64. doi: 10.1038/nri1733. Epub 2005/12/03. doi: 10.1038/nri1733. PubMed PMID: 16322748. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler-Heitbrock HW, Strobel M, Kieper D, Fingerle G, Schlunck T, Petersmann I, et al. Differential expression of cytokines in human blood monocyte subpopulations. Blood. 1992;79(2):503–11. Epub 1992/01/15. PubMed PMID: 1370390. [PubMed] [Google Scholar]
- 11.Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer immunology, immunotherapy: CII. 2012;61(9):1373–85. doi: 10.1007/s00262-011-1178-0. Epub 2012/01/05. doi: 10.1007/s00262-011-1178-0. PubMed PMID: 22215137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Besmer DM, Curry JM, Roy LD, Tinder TL, Sahraei M, Schettini J, et al. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Res. 71(13):4432–42. doi: 10.1158/0008-5472.CAN-10-4439. Epub 2011/05/12. doi: 0008-5472.CAN-10-4439 [pii] 10.1158/0008-5472.CAN-10-4439. PubMed PMID: 21558393; PubMed Central PMCID: PMC3129481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue CB, Wang A, Meloni D, Zhang K, Kong L, Feng H, et al. Discovery of INCB3344, a potent, selective and orally bioavailable antagonist of human and murine CCR2. Bioorganic & medicinal chemistry letters. 2010;20(24):7473–8. doi: 10.1016/j.bmcl.2010.10.020. Epub 2010/11/03. doi: 10.1016/j.bmcl.2010.10.020. PubMed PMID: 21036044. [DOI] [PubMed] [Google Scholar]
- 14.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67. doi: 10.1158/2159-8274.CD-10-0028. Epub 2011/11/01. doi: 10.1158/2159-8274.CD-10-0028. PubMed PMID: 22039576; PubMed Central PMCID: PMC3203524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73(3):1128–41. doi: 10.1158/0008-5472.CAN-12-2731. Epub 2012/12/12. doi: 10.1158/0008-5472.CAN-12-2731. PubMed PMID: 23221383; PubMed Central PMCID: PMC3563931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–20. doi: 10.1016/S0140-6736(10)62307-0. Epub 2011/05/31. doi: 10.1016/S0140-6736(10)62307-0. PubMed PMID: 21620466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin GS, Lee BH, Lee S, Chung SY, Kim M, Lim J, et al. Monokine levels in cancer and infection. Annals of clinical and laboratory science. 2003;33(2):149–55. Epub 2003/06/24. PubMed PMID: 12817618. [PubMed] [Google Scholar]
- 18.Koch AE, Kunkel SL, Harlow LA, Johnson B, Evanoff HL, Haines GK, et al. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992;90(3):772–9. doi: 10.1172/JCI115950. Epub 1992/09/01. doi: 10.1172/JCI115950. PubMed PMID: 1522232; PubMed Central PMCID: PMC329929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin AP, Rankin S, Pitchford S, Charo IF, Furtado GC, Lira SA. Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes. 2008;57(11):3025–33. doi: 10.2337/db08-0625. Epub 2008/07/18. doi: 10.2337/db08-0625. PubMed PMID: 18633103; PubMed Central PMCID: PMC2570399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394(6696):894–7. doi: 10.1038/29788. Epub 1998/09/11. doi: 10.1038/29788. PubMed PMID: 9732872. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9(2):166–80. doi: 10.1593/neo.07112. Epub 2007/03/16. PubMed PMID: 17356713; PubMed Central PMCID: PMC1813932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goedegebuure P, Mitchem JB, Porembka MR, Tan MC, Belt BA, Wang-Gillam A, et al. Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr Cancer Drug Targets. 2011;11(6):734–51. doi: 10.2174/156800911796191024. Epub 2011/05/24. PubMed PMID: 21599634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70(14):5728–39. doi: 10.1158/0008-5472.CAN-09-4672. Epub 2010/06/24. doi: 10.1158/0008-5472.CAN-09-4672. PubMed PMID: 20570887. [DOI] [PubMed] [Google Scholar]
- 24.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116(10):2777–90. doi: 10.1172/JCI28828. Epub 2006/10/04. doi: 10.1172/JCI28828. PubMed PMID: 17016559; PubMed Central PMCID: PMC1578632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83(5):1136–44. doi: 10.1189/jlb.0907611. Epub 2008/02/21. doi: jlb.0907611 [pii] 10.1189/jlb.0907611. PubMed PMID: 18285406. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Cui L, Gonsiorek W, Min SH, Anilkumar G, Rosenblum S, et al. CCR2 and CXCR4 regulate peripheral blood monocyte pharmacodynamics and link to efficacy in experimental autoimmune encephalomyelitis. J Inflamm (Lond) 2009;6:32. doi: 10.1186/1476-9255-6-32. Epub 2009/11/13. doi: 10.1186/1476-9255-6-32. PubMed PMID: 19906300; PubMed Central PMCID: PMC2777898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117(4):902–9. doi: 10.1172/JCI29919. Epub 2007/03/17. doi: 10.1172/JCI29919. PubMed PMID: 17364026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews Immunology. 2008;8(12):958–69. doi: 10.1038/nri2448. Epub 2008/11/26. doi: 10.1038/nri2448. PubMed PMID: 19029990; PubMed Central PMCID: PMC2724991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature reviews Immunology. 2012;12(4):253–68. doi: 10.1038/nri3175. Epub 2012/03/23. doi: 10.1038/nri3175. PubMed PMID: 22437938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews Immunology. 2009;9(3):162–74. doi: 10.1038/nri2506. Epub 2009/02/07. doi: nri2506 [pii] 10.1038/nri2506. PubMed PMID: 19197294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21(6) doi: 10.1016/j.ccr.2012.04.025. Epub 2012/06/16. doi: 10.1016/j.ccr.2012.04.025. PubMed PMID: 22698406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–13. doi: 10.1200/JCO.1997.15.6.2403. Epub 1997/06/01. PubMed PMID: 9196156. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–21. doi: 10.1158/1078-0432.CCR-05-0883. Epub 2005/09/17. doi: 10.1158/1078-0432.CCR-05-0883. PubMed PMID: 16166452. [DOI] [PubMed] [Google Scholar]
- 34.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 475(7355):222–5. doi: 10.1038/nature10138. Epub 2011/06/10. doi: nature10138 [pii] 10.1038/nature10138. PubMed PMID: 21654748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–9. doi: 10.1056/NEJM198612253152606. Epub 1986/12/25. doi: 10.1056/NEJM198612253152606. PubMed PMID: 3537791. [DOI] [PubMed] [Google Scholar]
- 36.Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. European journal of immunology. 2007;37(1):14–6. doi: 10.1002/eji.200636910. Epub 2006/12/22. doi: 10.1002/eji.200636910. PubMed PMID: 17183610. [DOI] [PubMed] [Google Scholar]
- 37.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84(3):623–30. doi: 10.1189/jlb.1107762. Epub 2008/05/10. doi: 10.1189/jlb.1107762. PubMed PMID: 18467655; PubMed Central PMCID: PMC2516896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Palma M, Naldini L. Angiopoietin-2 TIEs up macrophages in tumor angiogenesis. Clin Cancer Res. 2011;17(16):5226–32. doi: 10.1158/1078-0432.CCR-10-0171. Epub 2011/05/18. doi: 10.1158/1078-0432.CCR-10-0171. PubMed PMID: 21576085. [DOI] [PubMed] [Google Scholar]
- 39.Dai F, Liu L, Che G, Yu N, Pu Q, Zhang S, et al. The number and microlocalization of tumor-associated immune cells are associated with patient’s survival time in non-small cell lung cancer. BMC cancer. 2010;10:220. doi: 10.1186/1471-2407-10-220. Epub 2010/05/22. doi: 10.1186/1471-2407-10-220. PubMed PMID: 20487543; PubMed Central PMCID: PMC2880994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujiwara T, Fukushi J, Yamamoto S, Matsumoto Y, Setsu N, Oda Y, et al. Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. The American journal of pathology. 2011;179(3):1157–70. doi: 10.1016/j.ajpath.2011.05.034. Epub 2011/07/21. doi: 10.1016/j.ajpath.2011.05.034. PubMed PMID: 21771572; PubMed Central PMCID: PMC3157220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshikawa K, Mitsunaga S, Kinoshita T, Konishi M, Takahashi S, Gotohda N, et al. Impact of tumor-associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer science. 2012;103(11):2012–20. doi: 10.1111/j.1349-7006.2012.02411.x. Epub 2012/08/31. doi: 10.1111/j.1349-7006.2012.02411.x. PubMed PMID: 22931216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, et al. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell reports. 2012;2(3):628–39. doi: 10.1016/j.celrep.2012.08.006. Epub 2012/09/11. doi: 10.1016/j.celrep.2012.08.006. PubMed PMID: 22959433. [DOI] [PubMed] [Google Scholar]
- 43.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–95. doi: 10.1172/JCI59643. Epub 2012/03/02. doi: 10.1172/JCI59643. PubMed PMID: 22378047; PubMed Central PMCID: PMC3287223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M, et al. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. International journal of cancer Journal international du cancer. 2009;125(6):1276–84. doi: 10.1002/ijc.24378. Epub 2009/05/30. doi: 10.1002/ijc.24378. PubMed PMID: 19479998. [DOI] [PubMed] [Google Scholar]
- 45.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 331(6024):1612–6. doi: 10.1126/science.1198443. Epub 2011/03/26. doi: 331/6024/1612 [pii] 10.1126/science.1198443. PubMed PMID: 21436454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer immunology, immunotherapy: CII. 2011;60(10):1419–30. doi: 10.1007/s00262-011-1028-0. Epub 2011/06/07. doi: 10.1007/s00262-011-1028-0. PubMed PMID: 21644036; PubMed Central PMCID: PMC3176406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, et al. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 2012;72(4):876–86. doi: 10.1158/0008-5472.CAN-11-1792. Epub 2011/12/17. doi: 10.1158/0008-5472.CAN-11-1792. PubMed PMID: 22174368; PubMed Central PMCID: PMC3288305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varga G, Ehrchen J, Tsianakas A, Tenbrock K, Rattenholl A, Seeliger S, et al. Glucocorticoids induce an activated, anti-inflammatory monocyte subset in mice that resembles myeloid-derived suppressor cells. J Leukoc Biol. 2008;84(3):644–50. doi: 10.1189/jlb.1107768. Epub 2008/07/10. doi: 10.1189/jlb.1107768. PubMed PMID: 18611985. [DOI] [PubMed] [Google Scholar]
- 49.Rao B, Wanebo HJ, Pinsky C, Stearns M, Jr., Oettgen HF. Delayed hypersensitivity reactions in patients with carcinoma of the colon and rectum. Surgery, gynecology & obstetrics. 1977;144(5):677–81. Epub 1977/05/01. PubMed PMID: 850850. [PubMed] [Google Scholar]
- 50.Kopersztych S, Rezkallah MT, Miki SS, Naspitz CK, Mendes NF. Cell-mediated immunity in patients with carcinoma: correlation between clinical stage and immunocompetence. Cancer. 1976;38(3):1149–54. doi: 10.1002/1097-0142(197609)38:3<1149::aid-cncr2820380316>3.0.co;2-x. Epub 1976/09/01. PubMed PMID: 1085193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.