Abstract
The effect of the constant illumination on the development of spontaneous tumors in female 129/Sv mice was investigated. Forty-six female 129/Sv mice starting from the age of 2 mo were kept under standard light/dark regimen [12 h light (70 lx):12hr dark; LD, control group], and 46 of 129/Sv mice were kept under constant illumination (24 h a day, 2,500 lx, LL) from the age of 5 mo until to natural death. The exposure to the LL regimen significantly accelerated body weight gain, increased body temperature as well as acceleration of age-related disturbances in estrous function, followed by significant acceleration of the development of the spontaneous uterine tumors in female 129/Sv mice. Total tumor incidence as well as a total number of total or malignant tumors was similar in LL and LD group (p > 0.05). The mice from the LL groups survived less than those from the LD group (χ2 = 8.5; p = 0.00351, log-rank test). According to the estimated parameters of the Cox’s regression model, constant light regimen increased the relative risk of death in female mice compared with the control (LD) group (p = 0.0041). The data demonstrate in the first time that the exposure to constant illumination was followed by the acceleration of aging and spontaneous uterine tumorigenesis in female 129/Sv mice.
Keywords: light at night, tumorigenesis, lifespan, 129/Sv mice
Introduction
The alternation of the day and night seems is a most important regulator of a wide variety of physiological rhythms in living organisms. Exposure to the bright light during the night suppresses the night peak of melatonin—the “hormone of the night.”1 Melatonin is a principal hormone of the pineal gland—the small neuroendocrine gland connected with the brain that mediates information on light from the retina of the eyes to the organism.1,2
Light exposure at night has been found to be related to a number of serious behavior as well as health problems, including cancer. Significant increase in the risk of breast and colorectal cancers was found among women who frequently did not sleep during the period of the night, about 1:30 a.m., when melatonin levels are typically at their highest.3-7 “Melatonin hypothesis” suggests that reduced pineal melatonin production might increase human breast cancer risk, because lower melatonin output would lead to an increase in the level of female sex hormones and would stimulate proliferation of breast tissue.7 Global co-distribution of light at night and cancers of breast, prostate and some others was demonstrated in humans.8-10 Constant illumination probably exerts its detrimental effects on health, tumorigenesis as well as survival via disturbances of the female reproductive cycle.11 The mechanisms involved can be assumed to include the pineal hormone melatonin as well, which, as chemical signal of darkness and controlled by the central circadian clock in N. suprachiasmatici, may play a very central part, since it is suppressed by constant exposure to light and participates in the neuroendocrine control of the female reproductive system. Latest data from experiments in model organisms, gene expression studies and clinical trials imply that dysfunctions of the circadian clock contribute to aging and age-associated pathologies, thereby suggesting a functional link between the circadian clock and age-associated decline of brain functions.12-18 Potential molecular mechanisms underlying this link include the circadian control of physiological processes such as brain metabolism, reactive oxygen species homeostasis, hormone secretion, autophagy and stem cell proliferation.11,16,17
On the basis of “limited evidence in humans for the carcinogenicity of shift-work that involves night work” and “sufficient evidence in experimental animals for the carcinogenicity of light during the daily dark period (biological night),” the International Agency for Research on Cancer (IARC) Working Group concluded that “shift-work that involves circadian disruption is probably carcinogenic to humans” (Group 2A).18-20
In rodents, light at night leads to disruption of the ovulatory cycles followed by hyperplastic processes and tumor development in mammary gland, ovarian and uterine.11,19 The tumor-promoting effect of exposure to the constant illumination regimen was shown on chemical carcinogenesis of mammary gland, liver and nervous system in rats and spontaneous endometrial carcinogenesis in rats.21-27
In this paper, we report for the first time that the exposure to constant illumination was followed by the acceleration of the development of spontaneous uterine hemangiomas and sarcomas in female 129/Sv mice.
Results
The body weight of mice both in groups increased with age, but the body weight gain between the age of 5 and 25 mo was higher in mice of the LL group (51.9%) than that in the LD group (22.6%; p < 0.05) and was significantly higher in the LL regimen as compared with LD regimen from the age of 13 mo to the age of 25 mo (Table 1).
Table 1. Body weight gain dynamics in female 129/Sv mice exposed to various light regimens.
| Body weight (g) at the age of: | |||||||
|---|---|---|---|---|---|---|---|
| Light/dark regimen | 5 mo | 9 mo | 13 mo | 18 mo | 20 mo | 23 mo | 25 mo |
| LD | 23.4 ± 0.3 | 24.8 ± 0.4 | 24.7 ± 0.1 | 26.0 ± 0.5 | 26.6 ± 0.3 | 27.0 ± 0.3 | 28.7 ± 0.4 |
| LL | 23.3 ± 0.3 | 23.1 ± 0.13b | 25.5 ± 0.3a | 29.8 ± 0.3b | 31.6 ± 0.5b | 32.6 ± 0.5b | 35.4 ± 0.8b |
The difference with the LD is significant, ap < 0.05; bp < 0.01, the Student’s t-test.
The body temperature was not changed significantly with age in mice of the LD group, being 37.07 ± 0.066, 37.09 ± 0.05 and 37.66 ± 0.07°C at the ages 6, 10 and 20 mo, respectively. In LL mice, the body temperature was significantly (p < 0.001) higher than these in the control (LD) mice: 37.55 ± 0.036, 38.03 ± 0.07 and 38.15 ± 0.06°C, respectively.
There was no significant difference in the length of estrous cycles between groups exposed to LD or LL regimen since 6th to 13th month of life. At the age of 20 mo, the estrous cycle was longer in the LL mice as compared with the LD mice. (Table 2). At the age of the 20 mo, the relative number of mice with long estrous cycles (> 7 d) was significantly higher in the LL group as compared with the LD group. In the LD group, there was not significant age-related increase the rate of mice with irregular estrous cycles (Table 2). The exposure to the LL regimen significantly accelerated the age-related disturbances in the estrous function in 129/Sv mice. At the age of 20 mo, 68.4% mice in the LL group had irregular estrous cycles, whereas in the LD group only 8.3% (p < 0.05).
Table 2. Parameters of estrous function in female 129/Sv mice exposed to various light regimens.
| Rate of estrous cycles of various length, % | ||||||
|---|---|---|---|---|---|---|
| Age (months) | Number of mice | Length of estrous cycles (days) | < 5 d | 5–7 d | > 7 d | Fraction of mice with irregular estrous cycles (%) |
| LD | ||||||
|---|---|---|---|---|---|---|
| 6 | 46 | 6.4 ± 0.14 | 0 | 76.7 | 23.3 | 8.7 |
| 10 | 46 | 6.5 ± 0.13 | 0 | 76.7 | 23.3 | 10.9 |
| 13 | 45 | 6.3 ± 0.24 | 3 | 73.5 | 23.5 | 20.0 |
| 20 | 36 | 6.1 ± 0.31 | 7.1 | 78.6 | 14.3 | 8.3 |
| LL | ||||||
|---|---|---|---|---|---|---|
| 6 | 46 | 6.7 ± 0.15 | 0 | 82.0 | 18.0 | 15.2 |
| 10 | 46 | 6.8 ± 0.23 | 3.7 | 77.8 | 18.5 | 45.7a |
| 13 | 44 | 6.6 ± 0.39 | 5.6 | 83.5 | 11.1 | 54.5a |
| 20 | 38 | 7.3 ± 0.44* | 0 | 58.3 | 41.7 | 68.4a,b |
a The difference with the LD is significant, p < 0.05; the Fischer exact test. bThe difference with the LL age of 6 mo is significant, р < 0,05.
The estimation in the “open field” test of parameters of locomotor activity revealed the age-related decrease of its indices in 20-mo-old LD mice as compared with the 6-mo-old ones. Thus, relative number of crossed squares decreased by 3.7 times, duration of grooming reaction increased by 8.8 times, duration of feeble reaction increased by 19 times (p < 0.01). The exposure to the LL regimen practically did not modify these parameters (data are not shown).
The mortality of LD in LL mice was similar until the age of 2 y; however, after this age, mice maintained at LL have increased rate of death (Table 3 and Fig. 2). The longest surviving mouse from the group LL died at the age of 902 d. At this age, 21.7% of mice in the LD groups were alive, and the oldest mouse died 3 mo later.
Table 3. Survival distribution in female 129/Sv mice exposed to various light regimens.
| Number of survivors at the age of: (days) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 300 | 400 | 500a | 550 | 600 | 650 | 700 | 750 | 800 | 850 | 900 | 950 | 1000 |
| LD | 46 | 46 | 43 | 42 | 38 | 34 | 32 | 27 | 22 | 14 | 10 | 2 | 0 |
| LL | 46 | 43 | 41 | 38 | 38 | 34 | 28b | 20b | 13b | 3b | 1a | 0 | 0 |
a The first tumor detected in this interval. bThe difference with the controls of corresponding age is significant, p < 0.05; Fischer’s exact test.

Figure 2. Effect of the exposure to the constant illumination (LL) on survival and tumorigenesis in female 129/Sv mice. Abscissa, age, days. (A) Survival. Ordinate, number of mice, %. (B) Age-dependent tumor rate curves. Ordinate, number of tumor-bearing mice, %.
The mean lifespan of mice, as well as the mean lifespan of the last 10% of survivors median and maximum lifespan were reduced by 8.1–10.3% in the LL group as compared with the LD group (p < 0.001). The aging rate calculated as α in the Gompertz equation was increased, and MRDT was decreased in the LL group in comparison to these parameters for the LD group (p < 0.05) (Table 4). According to the log-rank test, the conditional lifespan distributions of 129/Sv mice (given the animals survived the age of 300 d) subjected to the constant light regimen (LL) differed significantly from the control (LD) group: χ2 = 8.5, p = 0.00351. According to the estimated parameters of the Cox’s regression model, constant light regimen increased the relative risk of death in female mice compared with the control group. Cox’s regression model parameters were β = 0.656; exp(β) = 1.93; se(β) = 0.228; p = 0.0041.
Table 4. Parameters of the lifespan and tumorigenesis in female 129/Sv mice exposed to various light regimens.
| Light regimen | |||
|---|---|---|---|
| Parameters | LD | LL | Ratio |
| Number of mice | 46 | 46 | |
| Mean lifespan (LS), days | 763 ± 21.8 | 701 ± 20.7a | −8.1% |
| Median | 795 | 722 | −9.2% |
| Mean LS of last 10% survivors | 967 ± 10.2 | 867 ± 11.0b | −10.3% |
| Maximum lifespan, days | 997 | 902 | −9.5% |
| Aging rate (α), days−3 | 7.97 (6.74; 8.23) | 9.39 a (8.83; 10.9) | +1.2 times |
| MRDT, days | 87 (84; 103) | 74a (63; 79) | −1.2 times |
| The age of the 1st tumor detection, days | 500 | 442 | |
| Effective number of mice | 45 | 43 | |
| Number of tumor-bearing mice | 37 (86.7%) | 35 (81.4%) | |
| Number of fatal tumor-bearing mice | 31 (68.9%) | 23 (53.5%) | |
| Mean LS of tumor-bearing mice, days | 800 ± 20.6 | 745 ± 17. 4a | −6.9% |
| Localization and type of tumors | |||
| Uterus: | |||
| hemangioma | 6 | 12 | p = 0.065 |
| sarcoma | 30 | 20 | p = 0.079 |
| Malignant lymphoma | 1 | 2 | |
| Lung: | |||
| adenoma | - | 2 | |
| adenocarcinoma | 1 | 1 | |
| Stomach: adenocarcinoma | 1 | - | |
| Bladder: papilloma | - | 1 | |
The difference with the control of the LL: ap < 0.05; bp < 0.001.
The exposure to constant illumination failed to significantly change the total or fatal spontaneous tumor incidence in female 129/Sv mice (Table 4). However, the first tumor in the LL group was detected 2 mo earlier than that in the LD group. At the autopsy, the enlargement of uteri have been observed in the majority of mice (Fig. 2B). Microscopically tumors were classified as hemangionas and sarcomas (Fig. 1).
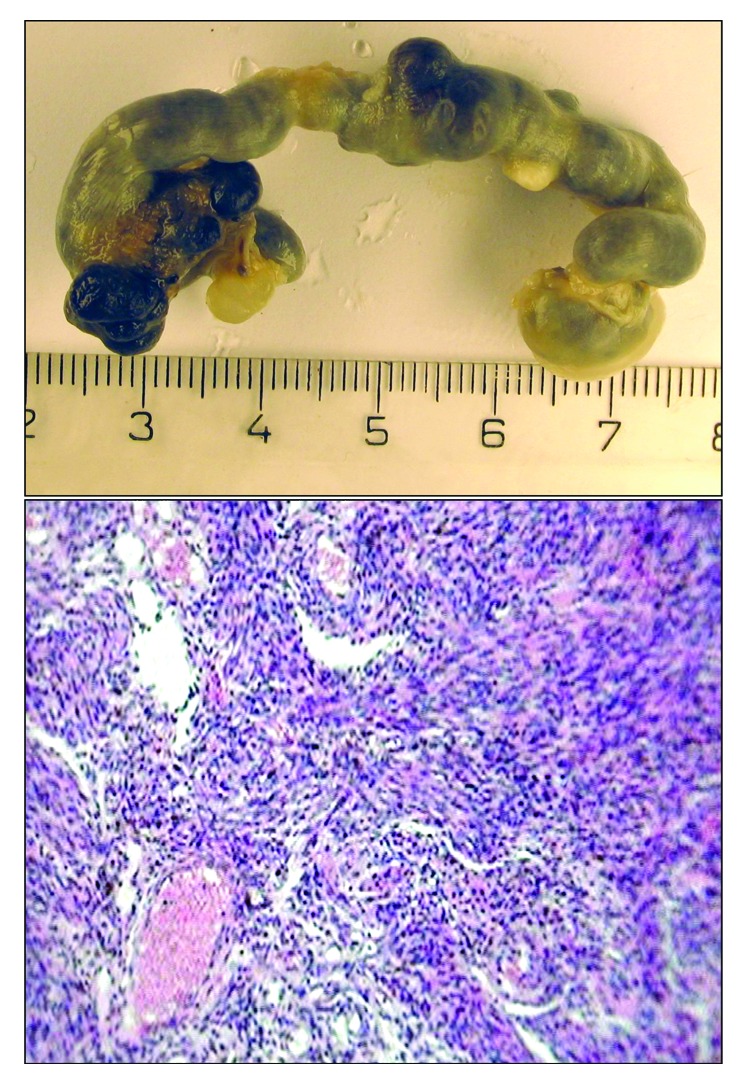
Figure 1. Macro- and microphotograph of uterine sarcoma in 129/Sv mice exposed to constant light regimen (H and E, ×320).
According to the log-rank test the tumor-bearing female mice subjected to the constant light regimen (LL) differed significantly from the respective control (LD) group: group: χ2 = 7.7, p = 0.0055 and for fatal tumor-bearing mice: χ2 = 5.6, p = 0.0183, respectively. According to the estimated parameters of the Cox’s regression model constant light regimen increased the relative risk of death in female tumor-bearing mice compared with the control group. Cox’s regression model parameters were: β = 0.719; exp(β) = 2.05; se(β) = 0.264; p = 0.0065 for total tumor-bearing mice and β = 0.71; exp(β) = 2.03; se(β) = 0.306; p = 0.02.
Discussion
In mammals, exposure to bright constant illumination alters the central circadian pacemaker activity of the suprachiasmatic nucleus in the hypothalamus. Constant light exposure or pinealectomy blocks the circadian melatonin signal emanating from the mammalian pineal gland during every 24 h dark period. When introduced during the dark phase, bright light inhibits melatonin production.1,2,11 Artificial increase of the length of light phase of the day (by 2–4 h) was typically followed by the increase in the duration of estrous cycle and in some cases to its disturbances. If the light will be switched on for 24 h per day, the majority of female mice and rats in a short period revealed the persistent estrus syndrome. In physiological circumstances, this syndrome naturally develops at some age (in rats, as usual between 15th and 18th months) and precedes to the anestrus,28-31 being the physiological equivalent of climacteric syndrome and climacteric in women. The ovary of persistent-estrus rats contains follicular cysts, hyperplasia of theca-tissue, whereas the corpora lutea are absent.30,31 Instead of cyclic production of gonadotropins, prolactin, estrogens and progesterone characteristic for normal reproductive period of life, their acyclic production is followed by hyperplastic processes in mammary gland, ovaries and uterus.29,30 We have found that the exposure to the LL regimen leads to the increase in the threshold of sensitivity of the hypothalamus to the feedback inhibition by estrogens in female rats.31 This mechanism is a key mechanism in the aging of reproductive system in female rats as well as in women.31,32 The disturbances in the estrous function developed much more early in 129/Sv mice in the LL group than that in the LD group.
We have observed excess of the body weight in the old female 129/Sv mice maintained at LL regimen as compared with the control LD mice. The obesity, decrease of tolerance to glucose and of the sensitivity to insulin have been observed in rats with persistent estrus.11,33,34 Metabolic syndrome characterized by obesity, hypertriglyceridemia and hypercholesterinemia is observed to decrease the level of high density lipoproteins, blood fibrinolytic activity, arterial hypertension, tolerance to glucose and insulin resistance more frequently.35 The metabolic syndrome is a risk factor not only for cardiovascular diseases but for cancer too.36-38 The inhibition of pineal function due to exposure to continuous light probably facilitates the metabolic syndrome development. The exposure to the LL regimen promoted spontaneous mammary carcinogenesis in female LIO rats33 and in transgenic HER-2/neu FVB/N mice,24 mammary carcinogenesis induced by 7,12-dimethylbenz(a)anthracene or N-nitrosomethylurea in female rats21,22 and colon carcinogenesis induced by 1,2-dimethylhydrazine in rats.39 The exposure to the LL regimen accelerated spontaneous uterine carcinogenesis in BDII rats.25 It was first shown in our present experiments that the constant light illumination promotes the spontaneous development of uterine tumors in 129/Sv mice.
The mechanisms of the protective effect of melatonin on carcinogenesis include the variety of possibilities discussed in several comprehensive reviews and include antioxidant and antiproliferative effects, the increase in apoptosis and inhibitory effect on telomerase activity in tumor cells both in vivo and in vitro, antiestrogenic effect, the decrease IGF-1 and insulin levels, etc.3,19,23,40-45
In conclusion, the results presented in this article demonstrate that exposure to light at night may have an important role in development of not only mammary tumors, but also a wide spectrum of tumors at different localization.
Material and Methods
Animals
Ninety-two 2-mo-old female 129/Sv mice originally provided by The Jackson Laboratory and bred at the animal facility at our Department were randomly subdivided into two groups and kept five per cage in polypropylene cages (30 × 21 × 10 cm) under standard light/dark regimen (12 h light:12 h darkness; LD, control group) of constant light regimen (LL) at a temperature of 22 ± 2°C and received standard laboratory chow47 and tap water ad libitum.
Experimental design
At the LD regimen, mice were exposed from 08:00 to 20:00 h to electric lamps (75 W, 200 V) with the illumination of 70 lx at the bottom of cages at the distance 1.7 min. At the LL regimen mice were exposed to two luminescent lumps LB-40-2 with illumination of 2,600 lx at the bottom of cages at the distance of 1.5 min. The control of the illumination was performed weekly with the luxmeter ТКА PKM. Once a week, all mice were palpated to detect any tumors. All animals were weighed monthly with an electronic balance. Once every 3 mo, vaginal smears taken daily for 2 wk from the animals were cytologically examined to estimate the phases of their estrous functions. In the same period, rectal body temperatures of the mice were measured with an electronic thermometer, TPEM (KMIZ) Once every 3 mo, vaginal smears taken daily for 2 wk from the animals were cytologically examined to estimate the phases of their estrous functions. In the same period, rectal body temperatures of the mice were measured with an electronic thermometer, TPEM (KMIZ). For behavior anomalies parameters of locomotor activity were estimated by the “open field” test in mice of both groups at the age of 6 and 20 mo.47
Pathomorphological examination
All animals were autopsied. All tumors, as well as tissues and organs with suspected tumor development, were excised and fixed in 10% neutral formalin. After routine histological processing, tissues were embedded in paraffin; 5–7 μm thin histological sections were stained with hematoxylin and eosin and were microscopically examined. Tumors were classified according to the “International Agency for Research on Cancer” recommendations.48
Statistics
Experimental results were statistically processed using STATGRAPH. The significance of discrepancies was defined according to the Student’s t-criterion, Fischer’s exact method, χ2 and non-parametric Wilkoxon-Mann-Whitney. Student-Newman-Keuls method was used for all pairwise comparisons. For survival analysis, Cox’s method49 was used for testing two groups. Taron’s life table test50 was used. All reported test values for survival analyses are two-sided.
Mathematical models and estimations
The mathematical model used to describe survival is the Gompertz model with the survival function:
 |
Equation 1 |
where parameters α and β are associated with the aging and initial mortality rate, respectively. Parameters for the model were estimated from data using the maximum likelihood method implemented in the Gauss statistical system.51
Acknowledgments
This paper was supported in part by grant 6383.2012.4 from the President of the Russian Federation. The authors are very thankful to Dr V.N. Nikitina for measuring the light exposure.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24879
References
- 1.Arendt J. Melatonin and the Mammalian Pineal Gland. London: Chapman & Hall, 1995. [Google Scholar]
- 2.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–56. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savvidis C, Koutsilieris M. Circadian rhythm disruption in cancer biology. Mol Med. 2012;18:1249–60. doi: 10.2119/molmed.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93:1563–8. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 5.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Night-shifts work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 2003;5:825–8. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 6.Hansen J, Lassen CF. Nested case-control study of night shift work and breast cancer risk among women in the Danish military. Occup Environ Med. 2012;69:551–6. doi: 10.1136/oemed-2011-100240. [DOI] [PubMed] [Google Scholar]
- 7.Stevens RG. Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. Int J Epidemiol. 2009;38:963–70. doi: 10.1093/ije/dyp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kloog I, Haim A, Stevens RG, Portnov BA. Global co-distribution of light at night (LAN) and cancers of prostate, colon, and lung in men. Chronobiol Int. 2009;26:108–25. doi: 10.1080/07420520802694020. [DOI] [PubMed] [Google Scholar]
- 9.Kloog I, Stevens RG, Haim A, Portnov BA. Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control. 2010;21:2059–68. doi: 10.1007/s10552-010-9624-4. [DOI] [PubMed] [Google Scholar]
- 10.Bartsch C. Light-at-night, cancer and aging. Aging (Albany NY) 2010;2:76–7. doi: 10.18632/aging.100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinogradova I, Anisimov V. Light Regimen, Pineal Preparations, Aging and Lifespan. Experimental Study. Saarbrucken: LAP LAMBERT Academic Publishing. 2012. [Google Scholar]
- 12.Dubrovsky YV, Samsa WE, Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY) 2010;2:936–44. doi: 10.18632/aging.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondratova AA, Dubrovsky YV, Antoch MP, Kondratov RV. Circadian clock proteins control adaptation to novel environment and memory formation. Aging (Albany NY) 2010;2:285–97. doi: 10.18632/aging.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown SA, Schmitt K, Eckert A. Aging and circadian disruption: causes and effects. Aging (Albany NY) 2011;3:813–7. doi: 10.18632/aging.100366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khapre RV, Kondratova AA, Susova O, Kondratov RV. Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle. 2011;10:4162–9. doi: 10.4161/cc.10.23.18381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu EA, Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany NY) 2011;3:479–93. doi: 10.18632/aging.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13:325–35. doi: 10.1038/nrn3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065–6. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 19.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 9. Painting, Firefighting, and Shiftwork. Lyon: IARC. 2010. [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, et al. Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup Environ Med. 2011;68:154–62. doi: 10.1136/oem.2009.053512. [DOI] [PubMed] [Google Scholar]
- 21.Anisimov VN. The role of pineal gland in breast cancer development. Crit Rev Oncol Hematol. 2003;46:221–34. doi: 10.1016/S1040-8428(03)00021-0. [DOI] [PubMed] [Google Scholar]
- 22.Anisimov VN. Light pollution, reproductive function and cancer risk. Neuro Endocrinol Lett. 2006;27:35–52. [PubMed] [Google Scholar]
- 23.Bartsch C, Barstch H, Peschke E. Light, melatonin and cancer: current results and future perspectives. Biol Rhythm Res. 2009;40:17–35. doi: 10.1080/09291010802066983. [DOI] [Google Scholar]
- 24.Baturin DA, Alimova IN, Anisimov VN, Popovich IG, Zabezhinski MA, Provinciali M, et al. Effect of light regimen and melatonin on the development of spontaneous mammary tumors in HER-2/neu transgenic mice is related to a downregulation of HER-2/neu gene expression. Neuro Endocr Lett. 2001;22:439–45. [PubMed] [Google Scholar]
- 25.Deerberg F, Bartsch C, Pohlmeyer G, Bartsch H. Effect of melatonin and phusiological epiphysectomy on the development of spontaneous endometrial carcinoma in BDII/Han rats. Cancer Biother Radiother. 1997;12:420. [Google Scholar]
- 26.van den Heiligenberg S, Deprés-Brummer P, Barbason H, Claustrat B, Reynes M, Lévi F. The tumor promoting effect of constant light exposure on diethylnitrosamine-induced hepatocarcinogenesis in rats. Life Sci. 1999;64:2523–34. doi: 10.1016/S0024-3205(99)00210-6. [DOI] [PubMed] [Google Scholar]
- 27.Beniashvili DS, Benjamin S, Baturin DA, Anisimov VN. Effect of light/dark regimen on N-nitrosoethylurea-induced transplacental carcinogenesis in rats. Cancer Lett. 2001;163:51–7. doi: 10.1016/S0304-3835(00)00673-X. [DOI] [PubMed] [Google Scholar]
- 28.Anisimov VN. Carcinogenesis and Aging, Vol 2. Boca Raton, FL: CRC Press, Inc. 1987. [Google Scholar]
- 29.Prata Lima MF, Baracat EC, Simões MJ. Effects of melatonin on the ovarian response to pinealectomy or continuous light in female rats: similarity with polycystic ovary syndrome. Braz J Med Biol Res. 2004;37:987–95. doi: 10.1590/S0100-879X2004000700007. [DOI] [PubMed] [Google Scholar]
- 30.Lazarev NI, Ird EA, Smirnova IO. Experimental Models of Endocrine Gynecological Diseases. Moscow: Meditsina 1976. [Google Scholar]
- 31.Dilman VM, Anisimov VN. Hypothalmic mechanisms of ageing and of specific age pathology--I. Sensitivity threshold of hypothalamo-pituitary complex to homeostatic stimuli in the reproductive system. Exp Gerontol. 1979;14:161–74. doi: 10.1016/0531-5565(79)90015-9. [DOI] [PubMed] [Google Scholar]
- 32.Rossmanith WG. Neuroendocrinology of aging in the reproductive system, gonadotropin secretion as an example. In: Follicular Gowrth, Ovulation and Fertilization, Molecular and Clinical Basis. Kumar A, Mukhopadhayay AK, eds. New Dehly: Narosa Publ House:15-25. 2001. [Google Scholar]
- 33.Vinogradova IA, Anisimov VN, Bukalev AV, Semenchenko AV, Zabezhinski MA. Circadian disruption induced by light-at-night accelerates aging and promotes tumorigenesis in rats. Aging (Albany NY) 2009;1:855–65. doi: 10.18632/aging.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinogradova IA. Effect of light regimen on metabolic syndrome development during the aging in rats. Adv Gerontol. 2007;20:70–5. [PubMed] [Google Scholar]
- 35.Vinogradova I, Anisimov V. Light Regimen, Pineal Preparations, Aging and Lifespan. Experimental Study. Saarbrucken: LAP LAMBERT Academic Publishing. 2012. [Google Scholar]
- 36.Luchsinger JA. A work in progress: the metabolic syndrome. Sci Aging Knowledge Environ. 2006;2006:pe19. doi: 10.1126/sageke.2006.10.pe19. [DOI] [PubMed] [Google Scholar]
- 37.Dilman VM. Development, Aging and Disease. A New Rationale for an Intervention. Chur: Harwood Academic Publishers. 1994. [Google Scholar]
- 38.Anisimov VN. Metformin for aging and cancer prevention. Aging (Albany NY) 2010;2:760–74. doi: 10.18632/aging.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panchenko AV, Petrischev NN, Kvetnoy IM, Anisimov VN. Colon carcinogenesis in rats maintained at various light regimens. Vopr Onkol. 2008;54:332–6. [PubMed] [Google Scholar]
- 40.Anisimov VN, Popovich IG, Zabezhinski MA, Anisimov SV, Vesnushkin GM, Vinogradova IA. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim Biophys Acta. 2006;1757:573–89. doi: 10.1016/j.bbabio.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Anisimov VN, Popovich IG, Zabezhinski MA. Melatonin and colon carcinogenesis: I. Inhibitory effect of melatonin on development of intestinal tumors induced by 1,2-dimethylhydrazine in rats. Carcinogenesis. 1997;18:1549–53. doi: 10.1093/carcin/18.8.1549. [DOI] [PubMed] [Google Scholar]
- 42.Blask DE. An overview on the neuroendocrine regulation of experimental tumor growth by melatonin and its analogues and the therapeutic use of melatonin in oncology. In: Bartsch C, Bartsch H, Blask DE, Cardinali DP, Hrushesky WJM, Mecke D, eds. The Pineal Gland and Cancer. Neuroimmunoendocrine Mechanisms in Malignancy. Berlin: Springer, pp 309-342. 2001. [Google Scholar]
- 43.Leon-Blanco MM, Guerrero JM, Reiter RJ, Calvo JR, Pozo D. Melatonin inhibits telomerase activity in the MCF-7 tumor cell line both in vivo and in vitro. J Pineal Res. 2003;35:204–11. doi: 10.1034/j.1600-079X.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 44.Reiter RJ. Reactive oxygen species, DNA damage, and carcinogenesis: intervention with melatonin. In: Bartsch C, Bartsch H, Blask DE, Cardinali DP, Hrushesky WJM, Mecke D, eds. The Pineal Gland and Cancer. Neuroimmunoendocrine Mechanisms in Malignancy. Berlin: Springer, pp 442-455. 2001. [Google Scholar]
- 45.Sánchez-Barceló EJ, Cos S, Fernández R, Mediavilla MD. Melatonin and mammary cancer: a short review. Endocr Relat Cancer. 2003;10:153–9. doi: 10.1677/erc.0.0100153. [DOI] [PubMed] [Google Scholar]
- 46.Anisimov VN, Alimova IN, Baturin DA, Popovich IG, Zabezhinski MA, Manton KG, et al. The effect of melatonin treatment regimen on mammary adenocarcinoma development in HER-2/neu transgenic mice. Int J Cancer. 2003;103:300–5. doi: 10.1002/ijc.10827. [DOI] [PubMed] [Google Scholar]
- 47.Anisimov VN, Popovich IG, Zabezhinski MA. Methods of evaluating the effect of pharmacological drugs on aging and lifespan in mice. Methods Mol Biol. 2007;371:227–36. doi: 10.1007/978-1-59745-361-5_17. [DOI] [PubMed] [Google Scholar]
- 48.Turusov VS, Mohr U, eds. Pathology of Tumours in Laboratory Animals. Vol. I. Tumours of the Mouse. 2nd ed. (IARC Sci. Publ. No 111). Lyon: IARC, 1994. [Google Scholar]
- 49.Cox DR, Oakes D. Analysis of Survival Data. London: Chapman & Hall, 1996. [Google Scholar]
- 50.Taron RE. Tests for trend in life table analysis. Biometrika. 1975;62:679–82. doi: 10.1093/biomet/62.3.679. [DOI] [Google Scholar]
- 51.Gauss System and Graphic Manual Maple Valley: Aptech Systems, Inc. , 1994


