Abstract
The human body has developed an elaborate defense system against microbial pathogens and foreign antigens. However, particular microbes have evolved sophisticated mechanisms to evade immune surveillance, allowing persistence within the human host. In an effort to combat such infections, intensive research has focused on the development of effective prophylactic and therapeutic countermeasures to suppress or clear persistent viral infections. To date, popular therapeutic strategies have included the use of live-attenuated microbes, viral vectors and dendritic-cell vaccines aiming to help suppress or clear infection. In recent years, improved DNA vaccines have now re-emerged as a promising candidate for therapeutic intervention due to the development of advanced optimization and delivery technologies. For instance, genetic optimization of synthetic plasmid constructs and their encoded antigens, in vivo electroporation-mediated vaccine delivery, as well as codelivery with molecular adjuvants have collectively enhanced both transgene expression and the elicitation of vaccine-induced immunity. In addition, the development of potent heterologous prime–boost regimens has also provided significant contributions to DNA vaccine immunogenicity. Herein, the authors will focus on these recent improvements to this synthetic platform in relation to their application in combating persistent virus infection.
Keywords: adjuvants, chronic infection, DNA vaccines, electroporation, prime–boost, therapeutic vaccination
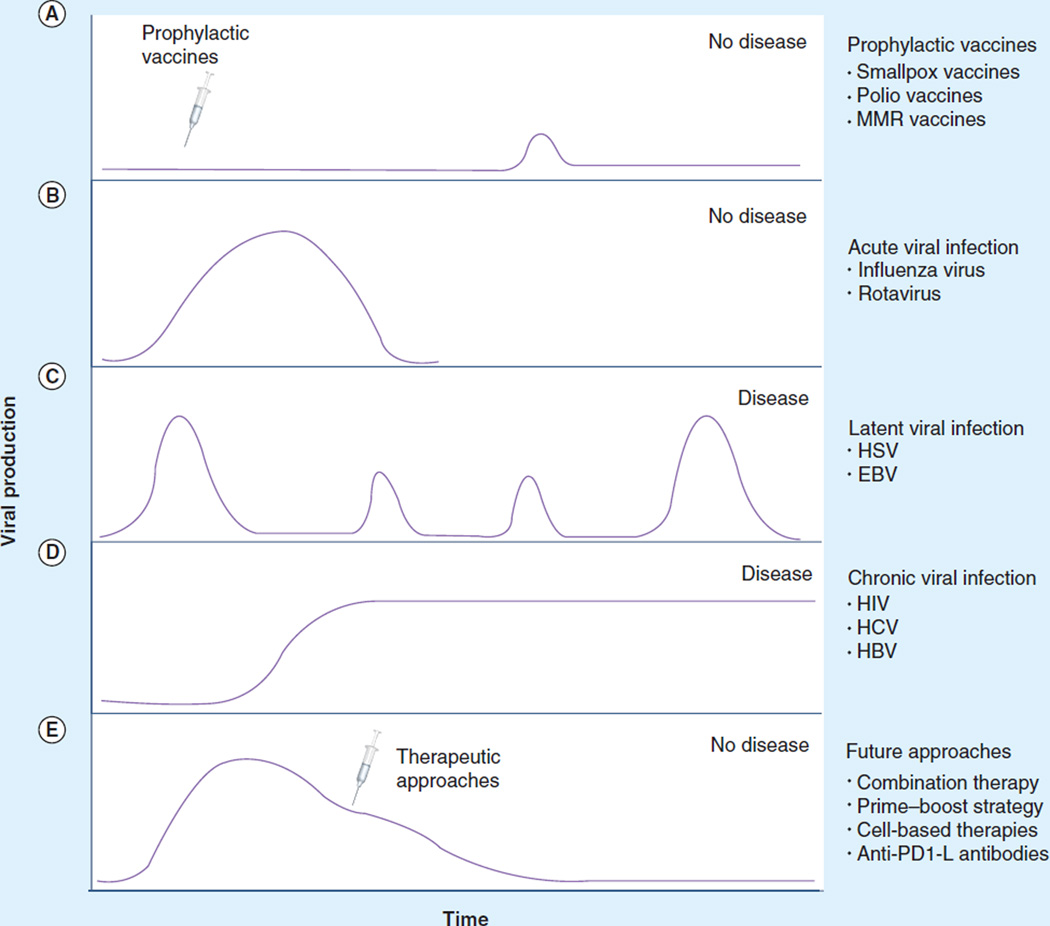
Vaccines represent one of the greatest triumphs of modern medicine. The development of the first vaccine by Edward Jenner in 1796 to prevent infection by the smallpox virus was a watershed moment in the war against microbes. Over the next two centuries, human morbidity and mortality resulting from polio, measles, mumps, rubella, pertussis and diphtheria have dramatically declined by over 95% due to the development of prophylactic vaccines. While these strategies have been exceptionally successful against acute, self-limiting infections (Figure 1A), the development of vaccines effective against many microbes that persist within the human host remains challenging.
Figure 1. Kinetics of viral load during viral infection and after different therapies.
(A) The prevention of viral production and infection when a prophylactic vaccine is administered and establishes effective memory immune responses. (B) The course of an acute infection. Virus-specific T-cell effectors become activated and control the virus infection. After viral clearance, effector cells contract to become memory cells, which are pivotal in preventing reinfection with the same virus. (C) The cycle of a latent persistent viral infection that stays with the host indefinitely. After viral acquisition, virus production ceases; however, because the virus genome is not completely eradicated, the virus can reactivate. Upon the required stimulus for reactivation, the virus can begin producing viral progeny (lytic form of viral cycle), allowing disease to resurface. (D) The natural course of a chronic infection in which incessant viral production exhausts immune responses and disease can no longer be controlled or prevented. Continuous exposure of T cells to viral-specific antigens eventually causes effector cells to become exhausted, leading to deletion of these dysfunctional cells. (E) New therapeutic approaches for chronic viral infections might reconstitute virus-specific immune responses and lead to virus clearance and disease prevention.
Viruses are sophisticated connoisseurs, hijacking their specific host cells and transforming them into virus-producing factories, an obligatory process essential for their survival. They can typically enter the human body via many routes, can be relatively pantropic and express complex evasion mechanisms to thwart virus-specific immune recognition. In general, viral infections result in one of two outcomes: an acute infection where the host is able to effectively eliminate the virus, or a chronic infection where incomplete clearance of the virus by the immune system results in viral persistence (Figure 1C & D) [1]. Successfully controlling and clearing the spread of viruses requires the coordination of multiple immune effector mechanisms. The first line of defense is the innate immune system, which is activated in response to the detection of pathogens binding to pattern-recognition receptors, which then stimulate and mobilize the antiviral activities of innate cells (macrophages, dendritic cells [DCs] and NK cells) to help control viral spread [2]. In some cases, these responses are enough to prevent the spread of the invading pathogen. However, when these mechanisms fail to control the infection, a more versatile line of defense, the adaptive immune response, is initiated. Cells of the adaptive immune system are activated by the innate response, causing their development into effector cells that promote viral clearance. However, several pathogens including HIV, HCV, HBV, HSV and HPV interfere with the effector mechanisms of the adaptive immune response, resulting in the establishment of persistent infection.
Chronic viral infections are typically the result of highly regulated evasion mechanisms employed to circumvent the adaptive immune system. Some of these pathogen-mediated approaches include inhibitors of antigen (Ag) presentation and the elicitation of cellular apoptosis, viral interference with interferon pathways and modulation of cytokine and chemokine activity, which favor pathogen persistence. In addition, in some cases, rapid mutation as facilitated by low-fidelity viral polymerases in response to selective immune pressure, allows viruses to escape immune recognition (e.g., HCV, HIV) [3]. Alternatively, some can escape altogether by establishing latency within host cells (e.g., HSV) [4]. They can also interfere with the function and differentiation of APCs and thereby prevent T- and B-cell activation and expansion [5]. As a result of these strategies and others, the persistence of viral Ag and their prolonged presentation to the immune system contribute to inducing the progressive loss of function of virus-specific T cells. As a result, these T cells become anergic (exhausted) and are ultimately unable to express effector mechanisms critical to control or eliminate the pathogen.
While various therapeutic treatments targeting chronic infections have greatly helped in reducing the severity of disease, they are not 100% curative and may be associated with side effects and be cost prohibitive in some developing nations. For instance, the development of antiretroviral therapy (ART) has had an enormous impact in delaying the onset of AIDS and prolonging the lives of patients infected with HIV. However, the continuous use of ART drug cocktails can lead to undesirable side effects as well as contributing to the emergence of drug-resistant viruses. More importantly, the effective application of ART therapy requires that regimens be highly customized for each patient and is extremely expensive, and therefore not readily available in third world countries due to socioeconomic obstacles [6,7]. As a result, over 90% of HIV-infected individuals worldwide do not benefit from this approach [7,8]. In the case of HBV, currently available therapies such as lamivudine and IFN-α are not fully satisfactory in terms of safety and/or efficacy. Similarly, the current standard of care for chronic HCV is pegylated interferon plus ribavirin, which exhibits a 50% efficacy rate in patients with HCV genotype 1 and is a costly therapy often accompanied by adverse effects [9]. Thus, therapeutic vaccination strategies may help to treat persistent viral infections where drug intervention is limited, and where intracellular pathogens have established mechanisms to escape from host immune system surveillances. The importance of vaccines as therapeutic interventions against chronic diseases can be highlighted by the available Zostavax® vaccine (Merck & Co., NJ, USA) against herpes zoster (known as shingles) and the Gardasil® vaccine (Merck & Co.) that prevents infection against HPV [10,11]. Along these lines, much research has focused on using specific therapeutic vaccination methods in the hope of promoting long-lived immunity to mediate viral control and/or clearance (Figure 1E). Herein, some of these important innovation schemes in regards to improved DNA technology are discussed.
Therapeutic interventions
Live-attenuated vectors
Live-attenuated vectors have been classified as the most effective type of vaccine due to their ability to invade and cause infection that is greatly similar to the native pathogen, but without the associative pathogenic effects [12]. This type of vector is constructed (by modern methods) by attenuating the infectious agent to reduce virulence while retaining microbial viability [13]. Traditionally, one approach of attenuation is achieved by growing the virus in cultured cells of a different species until the virus can no longer grow well in human cells. Such vectors have been shown to be highly effective when used as prophylactic vaccines in nonchronic infections such as measles, mumps, rubella, yellow fever and polio. Despite these achievements, there is the possibility for reversion into virulence due to the viable nature of the vaccine vector, which has been observed for the live-attenuated oral polio vaccine developed by Albert Sabin [14,15] as well as others. Further risks are associated with administering these vectors to immunocompromised individuals who may not be able to effectively respond to the vaccine [12]. Therefore, the development of broad alternative approaches that are not limited by these attributes is an important goal of future vaccine platforms.
Viral vectors
Viral recombinant vector platforms, such as vaccinia virus (VV) or adenoviruses (Ad), are among the most developed and characterized candidates for gene therapy and vaccine applications [16]. VV is the prototypical poxvirus, particularly well known for its role as the successful vaccine vector that helped eradicate the smallpox virus [17]. Based on this success in the 1980s, VV became the first recombinant viral vector platform as an approach to preventing infectious diseases [18]. Since then, research has focused on VV as a vaccine platform for a wide variety of infectious agents as well as for cancer immune therapy [18–26]. Due to its large genome size, recombinant poxviral vectors can carry large amounts of foreign material while retaining their transcriptional and translational capacity. There is also little risk of viral integration into host DNA since replication takes place in the cytoplasm [12].
As a disadvantage of this platform, recombinant VV can be pathogenic in immunocompromised individuals, which is a major concern when considering its use as a therapeutic vaccine in HIV-infected individuals. Thus, current efforts are directed toward developing a more highly attenuated vector system [27]. Examples of certain highly attenuated, nonreplicating poxvirus strains under development include: the orthopoxviruses, modified vaccinia Ankara (MVA) and NYVAC (derived from the Copenhagen vaccinia strain); and the avipoxviruses, ALVAC and TROVAC (derived from canarypox and fowlpox viruses, respectively). In addition, these attenuated pox vectors have shown effective boosting capabilities in prime–boost regimens. Priming with DNA vaccines and/or viral vector vaccines [23] is being used in a number of studies involving therapeutic vaccination against SIV and HIV infections because of their successful induction of cellular and humoral immunity [28]. During a recent preclinical study, the efficacy of a MVA virus was tested in SIV-infected macaques receiving ART [29]. The authors observed a subset of vaccinated monkeys (five of 12) that had maintained low viral RNA levels below threshold levels had more than 30% CD4+ cells, while the percentage of CD4+ cells in the unvaccinated monkeys (five of six) with higher viral RNA levels had CD4+ cells below 30%. Even though the therapeutic vaccine appeared to benefit several monkeys, there was no control over viral rebound after ART was stopped. While these viruses remain ideal vectors, their construction and production can be complex and expensive, and preparations are not always stable. In addition, prior immunogenicity to the vaccinia vector can limit application of the vaccine to individuals previously vaccinated against smallpox [12]. Nonetheless, great interest remains for such attenuated vectors in vaccine approaches.
Another nonretroviral vector that has been extensively studied as a vaccine platform is Ad. This platform has been developed as a vaccine vector because of its capacity to infect a number of different cell types, its high transduction efficiency, ease of manipulation and its ability to induce strong cellular immune responses [12,30,31]. Currently, there are over 50 adenovirus serotypes, but Ad5 is the most widely studied because of its greater immune potency [32]. Recombinant adenovirus (rAd5) vectors have become attractive vaccines for HIV because they can be administered mucosally. Both SIV [33] and HIV [34] are known to be active in the mucosa. For instance, Mercier and colleagues demonstrated that enteric-coated capsules containing Ad5 vectors expressing HIV-1 Gag and Env peptides stimulated Ag-specific mucosal and systemic immune responses in adult rhesus macaques [35]. While rAd5 has become a promising HIV vaccine candidate due to the induction of strong Ag-specific cellular immunity, their major limitation is the potential for generating antivector immunity upon repeat administration. Furthermore, another limitation is pre-existing immunity to Ad vectors among human populations that are in great need of an HIV vaccine, especially in sub-Saharan Africa where Ad5 seropositive responses are greater than 90% [36,37]. For instance, more recently, the safety and efficacy of rAd5 as a HIV vaccine platform has shown that having established pre-existing anti-Ad5 may enhance or exacerbate the spread of virus as observed in the recent Merck Step trial [38]. However, further testing is warranted. Overall, antivector immunity has proven to impair the reuse of vectors, such as adenovirus or poxvirus-based vaccines [39–41]. This effect potentially limits the ability of these vector platforms for re-administration or to be used for multiple-dose regimens. However, one pivotal approach that investigators are using to get around this issue is discovering and using rare serotype vectors [30]. Overall, continued research is needed to improve the development of safe and effective nonretroviral vector vaccines.
DC vaccines
Therapeutic autologous DCs are currently being tested as personalized vaccine platforms for the control of chronic infection, such as HIV and HBV [42,43]. As potent APCs, DCs are essential for initiating and maintaining virus-specific immunity and have been found to be impaired in patients with persistent infections [44–46]. Therefore, DCs are thought to be ideal biological agents for use in immunotherapeutic strategies aiming to augment T-cell immunity in chronic infections.
DC vaccines are generated by preparing donor monocyte-derived DCs ex vivo and loading them with Ags, and then administering them back into the patient [47]. In 2010, the US FDA approval of Provenge® (Dendreon Corp., WA, USA), an immunotherapy for prostatic cancer that uses the patient’s autologous blood cells stimulated with the disease-related protein prostatic acid phosphatase, demonstrated the possibility of this therapy to potentially be used as a future approach for targeting chronic infections. A recent study interested in polyfunctionality and memory T-cell responses following coculture of autologous lymphocytes found that Gag RNA-loaded DC therapy against HIV-1 induced polyfunctional T cells ex vivo, but a corresponding increase in the phenotype of central and memory T cells was not observed [48]. On the other hand, peptide-pulsed DCs from healthy individuals and DCs isolated from infected individuals have been shown to be potent stimulators of primary and memory HIV/SIV-specific cytotoxic T lymphocytes in vitro [49–52]. Therapeutic vaccination for SIV using the DC vaccine has also revealed a correlation between decreased SIV DNA and RNA levels and increased SIV-specific T-cell responses [53]. Furthermore, a clinical trial reported by Lu et al. found that after administration of three doses of autologous DCs pulsed with whole aldrithiol-2-inactivated autologous virus, plasma viral load decreased by 90% for at least 1 year in eight of the 18 patients [42]. By contrast, a different clinical trial observed a small difference in plasma viral load in HIV-infected patients vaccinated with monocyte-derived DCs pulsed with heat-inactivated autologous virus compared with the control group [54]. These contrasting results may be the effect of different study designs. Even though preliminary results have demonstrated that a virus-pulsed vaccine is a promising strategy for treating patients with chronic infections [42,43,55], the challenge remains of sustaining substantially low viral loads in chronically infected patients. Furthermore, this approach is tempered by the difficulty of developing individualized treatment on a large scale. Thus, similar to the unavailability of HIV antiretrovirals to a majority of infected individuals due to socioeconomic issues, DC-based vaccines will remain focused on patient-specific immune therapy.
DNA vaccines
DNA as a vaccine platform first came into the scientific spotlight in the early 1990s, when it was reported that the delivery of plasmid DNA into the skin or muscle induced an immune response against encoded viral and nonviral Ags [56–59]. Tang et al. were the first to report that delivering a DNA-coated gold microprojectile into the skin of a mouse could elicit antibody (Ab) responses against the delivered Ag [56], but Wang et al. were the first to show immune responses against a chronic viral infection [58]. Subsequently, Liu and colleagues [57] and Robinson et al. [59] both independently reported that injecting plasmid DNA encoding influenza A nucleoprotein intramuscularly generated both humoral and cellular immune responses against influenza virus Ags in mice. In addition, Weiner and colleagues at the University of Pennsylvania (PA, USA) showed that DNA plasmids carrying HIV Ags induced an immune response that mimicked the immunogenicity initiated by live-attenuated viruses, engendering the induction of both humoral and cellular immune responses [60]. The simplicity and elegance of DNA vaccines, which allowed for easy customization through the use of molecular biology and genetic engineering to harness the power of the immune system, generated a great deal of excitement. These findings introduced the potential of DNA as an immunization platform. Since then, researching the effects of this approach on the generation of both cellular and humoral immune response against many chronic viral infections has become the focus of many laboratories.
DNA vaccination has been suggested as an ideal therapeutic strategy due to numerous advantages over competing platforms. For example, DNA vaccines are nonlive and nonreplicating and thus unable to revert into virulent form, unlike live vaccines. Furthermore, DNA vaccines are highly customizable and hence, multiple Ags can be encoded within a single DNA plasmid. This allows for a much greater breadth in the host immune response and better protection as different epitopes within a single pathogen have been shown to elicit different types of immune responses [61]. In addition, optimization of vaccine vectors and encoded Ags such as RNA/codon optimization and Ag consensus has also enhanced expression and cellular/humoral cross-reactivity [62]. Individuals receiving DNA vaccines are unlikely to harbor antiplasmid vector immunity, as seen with adenovirus vectors. For this reason, DNA therapeutic vaccinations can be delivered repeatedly without initiating an immune response against the DNA plasmid [63]. Finally, DNA vaccines are simple and inexpensive to construct, can easily be produced in large quantities, are more temperature-stable than conventional vaccines, and can be easily stored and transported [62]. These advantages may help contribute to the successful delivery and administration of therapeutic vaccines to infected individuals in developing nations.
By delivering DNA via different routes, DNA vaccines can generate a specific type of immune response – cellular versus humoral. For instance, needle injection of DNA mounts a predominately Th1 response while biolistic injections of the same plasmid mainly elicits a Th2 or balanced Th1/Th2 response [64–66]. McCluskie and colleagues demonstrated that the type of Ab response (IgG, IgG1, IgG2a), level of Ab responses and cytotoxic T cell (CTL) activity vary depending on the route of administration in both mice and nonhuman primates (NHPs) as well as the immunization schedule in NHPs [67]. Intranasal versus intramuscular immunization with a DNA–monophosphoryl lipid A vaccine against HIV Type 1 enhanced mucosal Ab responses and systemic cell-mediated immunity in mice [68]. However, the intramuscular vaccine was more advantageous for eliciting Ab responses. The ease of manipulation of DNA vaccines allows for researchers to be selective in the type of immune response against a specific viral infection.
The success of DNA vaccines in preclinical studies quickly lead to clinical trials, and the idea of using DNA to immunize people immediately gained widespread recognition. The first DNA vaccine studies in humans were conducted almost 20 years ago. The goals of the various studies were to evaluate and demonstrate the safety, tolerability and immune potency of the DNA vaccines. In the first Phase I clinical trial, a DNA-based vaccine for HIV-1 infection was evaluated for both therapeutic and prophylactic applications [69]. Soon other DNA vaccine trials would follow, including trials that tested DNA-based vaccines against other HIV Ags, HBV and malaria [70–72]. These introductory studies established that DNA vaccines were tolerable in humans, and that they could enhance T-cell proliferation and CTL activity [73–75], although the immune responses elicited were weaker than expected based on preclinical data. While ‘first-generation’ DNA vaccines failed to demonstrate a robust level of vaccine-specific immunity in humans, exhaustive research has continued to develop new modifications and improvements to the technology to enhance DNA efficacy.
To date, a plethora of approaches have been conducted to improve or augment the immunogenicity elicited by DNA vaccines. These efforts have included: optimization of the vaccine vectors (e.g., RNA/codon optimization) and Ags encoded by the plasmids (e.g., consensus sequences) to enhance Ag expression and cellular/humoral cross-reactivity [62,76]; inclusion of molecular adjuvants to enhance, modulate and skew immune responses; and in vivo electroporation (EP), a promising delivery method that improves the expression and presentation of Ags expressed by DNA vectors [62]. Refer to references [62,77] for a more detailed overview of how DNA vaccines prime immune responses. Finally, the novel protocol of heterologous prime–boost immunization has markedly heightened the immunopotency of DNA vaccination, and as result has sparked great excitement and interest in the DNA platforms to be examined for therapeutic approaches. Although we are far from a complete understanding of how DNA vaccines fully work, recent studies are beginning to shed light on this subject.
Coimmunization with molecular adjuvants
One important finding with regard to DNA-based vaccines is the ability to manipulate the immune response through coadministration of cytokine genes. Genetic molecular adjuvants are normally administered as plasmids encoding a specific cytokine, chemokine or costimulatory molecule. Indeed, the addition of immune-modulatory adjuvants as part of a vaccine cocktail has been demonstrated to boost the adaptive immune response [78]. A number of groups have shown that this response can be modulated both quantitatively and qualitatively through coimmunization with cytokine-expressing plasmids (Table 1). Specifically, it was demonstrated that coimmunization with Th1-type cytokines can enhance cellular immunity and bias the immune response toward a Th1-type (e.g., IL-12) response, while Th2-type (e.g., IL-4) cytokines can boost Ab responses and promote a Th2-type bias [8,79,80]. In choosing an adjuvant that provides a Th1- or Th2-biased response, it is important to consider which type of response may be more helpful in contributing to protection. For example, Leishmania major requires a Th1-type response for effective immunity, while other parasitic and microbial infections require a Th2-type response [81]. This ability to modulate or enhance the immune response in a defined manner has great promise to improve vaccine design and development.
Table 1.
Selected cytokine adjuvants coadministered with DNA vaccines to target chronic viral infections.
| Molecular adjuvant | Infection | Model(s) | Ref |
|---|---|---|---|
| IL-2 | HIV-1 | NHPs | [78,96,162] |
| IL-7 | HSV | Mice | [161] |
| IL-7 | HCV | NHPs | [163] |
| IL-12 | HIV | NHPs, mice | [95,96,164,165] |
| IL-12 | HBV | Humans | [166,167] |
| IL-12 | HSV | Mice | [168,169] |
| IL-15 | HIV-1 | NHPs, mice | [88,96] |
| IL-18 | HIV | NHPs, mice | [165,170] |
| IL-18 | HSV | Mice | [171,172] |
| IL-23 | HCV | Mice | [163] |
| IL-28B | HIV | NHPs, mice | [92–94] |
| GM-CSF | HCV | Mice | [163] |
| GM-CSF | HIV | Mice | [86,95,96] |
| IFN-γ | HBV | Mice | [173] |
NHP: Nonhuman primate.
The inclusion of different cytokines is actively being studied as a way to induce and shape both innate and adaptive immune responses. For example, two of the most studied and tested adjuvants are IL-12 and IL-15. The former is a significant cytokine produced by DCs to stimulate NK cells and T-cell activity [82,83]. IL-12 also supports the differentiation of Ag-specific CD4 T cells to produce Th1 cytokines, and also prompts the expansion of Ag-specific CD8 T cells to express cytotoxic molecules such as granzyme B, perforin and IFN-γ. IL-15, another important cytokine, primarily influences memory CD8 T cells to proliferate and enhances the persistence of these memory T cells [83–85]. Kim et al. were the first to investigate the role of codelivery of IL-12 with DNA vaccines, observing an increase in specific CTL responses when mice where coimmunized with a HIV-1 DNA vaccine and an IL-12 cassette [86]. On the other hand, Halwani and colleagues were the first to demonstrate that codelivery of plasmid IL-12 along with a SIV DNA vaccine significantly enhanced its ability to induce SIV-specific cellular immune responses, and a boost with SIV DNA + IL-15 also successfully triggered T-cell memory subsets in NHPs [80]. Additionally, this group found a significantly lower expression of PD-1 on CD4 and CD8 T cells of macaques immunized with SIV DNA + IL-15, indicating that the use of these cytokine adjuvants can downregulate PD-1 expression levels on exhausted T cells, thereby inducing their activation and increasing the magnitude and functionality of Ag-specific T cells [87–89]. Similarly and more recently, IL-28, a cytokine that belongs to the interferon III/λ (IFN-γ) family of cytokines [90,91], has also been shown to play a role in the adaptive immune response. Its inclusion as an immunoadjuvant during small animal and NHP vaccination led to augmented Ag-specific Th1-biased responses, as well as an increased cytotoxic potential in CD8 T cells [92–94]. Furthermore, the adjuvant can augment long-lived memory T cells [94] and decrease the levels of regulatory T cells [92]. Finally, GM-CSF, a cytokine secreted white blood cell growth factor, has also been used as a molecular adjuvant and has been shown to enhance both cellular and humoral responses in mice and NHPs [95–98]. For instance, Robinson and colleagues demonstrated that coadministration of GM-CSF enhanced neutralizing HIV-1-specific Ab production, which showed improved control of a simian–human hybrid virus challenge [99]. Therefore, having a comprehensive understanding of the immune system and its components is critical not only for understanding host–pathogen interactions during infection, but also for improving the efficacy of DNA vaccines to enhance the potency of the immune response to help protect from deadly viral pathogens.
EP delivery
DNA vaccines have been traditionally associated with low transfection efficiency (or inefficient uptake of the plasmids by cells) at the site of vaccination, resulting in poor immunogenicity. However, several delivery methods have markedly increased transfection efficiency after DNA immunization. The use of transcutaneous needles allows for the direct delivery of DNA to the Langerhans cells (APCs of the skin), resulting in a much improved immune response in small animals [100]. Delivery of naked plasmid using different strategies such as gene guns [101], jet injectors and tattoo perforating needles [102], ultrasound [103] and microprojection arrays [104] have also had similar success. However, one of the most promising improved methods of DNA vaccine delivery to date has been EP.
EP is a simple, direct approach that involves the application of short electrical pulses to the vaccine delivery site. This transiently increases cell membrane permeability, allowing for increased plasmid uptake and increased expression in the target tissue of multiple animal models and humans [62,105–108]. As a result, plasmid DNA has a higher transfection efficacy and is thought to increase immunogenicity of EP-mediated DNA vaccination in both small (mice, guinea pigs and rabbits) and large animal models (pigs, rhesus macaques and chimpanzees) [107–113]. In fact, DNA injection followed by EP has been shown to give ten- to 100-fold or more increased transgene expression levels [108,114,115], as well as an increased number of nuclei containing injected plasmids [116]. EP has also been shown to increase vaccine potency by activating APCs and initiating danger signals as well as local inflammation, thus recruiting immune cells to the site of injection [117]. For instance, Liu and colleagues specifically showed that an HIV-1 Env DNA vaccine with EP resulted in effector and central memory CD8 T-cell responses upon re-exposure to Ag [117]. More importantly, they observed a recruitment of large cellular inflammatory infiltrates to the site of inoculation after DNA vaccination with EP. The increased number of APCs made a substantial contribution to the enhanced immunogenicity of the DNA vaccine. In addition, a lower dosage of DNA delivered with EP gives a stronger immune response compared to intramuscular injection of DNA alone. More recently, studies have demonstrated that the combination of DNA and EP does not increase DNA persistence within the host tissue nor DNA integration into the host’s genome, eliminating additional concerns about this part of the DNA technology [118,119]. DNA vaccination in combination with EP is a novel, safe and effective strategy that elicits a strong, broad and long-lasting humoral and cellular immune response. EP is advantageous as a vaccine delivery approach because it can broadly activate CD8 CTLs, which eliminate cells infected with intracellular pathogens. Thus, EP has been successfully used to enhance both cellular and humoral responses in small animal models and humans [26,107,108,120]. More recently, Bagarazzi et al. have published data on EP administration with a DNA HPV vaccine that opens exciting new avenues for this combined technology approach to treat or protect against many human pathogens [121]. They reported that a therapeutic DNA vaccine coadministered with EP on its own in humans could produce long-lived CD8 T cells with cytolytic activity [121]. The results also show that DNA delivered by EP is safe and tolerable [121]. Therefore, EP may allow DNA delivery method choice to increase the cellular expression and immunogenicity of DNA plasmids as a potential therapeutic approach to combat persistent infections, making it much more useful in therapeutic strategies.
Heterologous prime–boost
Following the discouraging results of ‘first-generation’ DNA vaccines to elicit strong immunogenicity in clinical trials, recombinant viral-based vaccines soon fell into the scientific limelight of the vaccine research field. Highly attenuated live recombinant poxviruses, such as the vaccinia-based vector NYVAC [19,122,123], the canarypox-based vector [21,124] or MVA [125–127], have been demonstrated to be safe and immunogenic platforms in NHPs. Although the viral platforms induced strong Ag-specific cellular responses, their major limitation is the potential to induce immunity against the vector itself. This has been proven to impair the reuse of vectors, such as Ad- or poxvirus-based vaccines [39–41]. Specifically, VV and Ad immunity has been reported to diminish the breadth of immune response induced by these vectors [36,128]. Therefore, this effect potentially limits the ability to use multiple-dose regimens of homologous vectors. A potential strategy to overcome these issues was heterologous prime–boost protocols that used DNA vaccines for priming the immune response and a recombinant viral vaccine to boost the response. This approach would be beneficial for recombinant viral vectors, as antivector immune responses can prevent the readministration of the homologous vectors.
A potential strategy to overcome the hurdles of induced antivector immunity was first suggested by studies using the murine model of malaria, whereby DNA priming followed by NYVAC or MVA boost induced higher CD8 T-cell responses than either platform given alone and resulted in protection against malaria infection (Plasmodium yeolii and Plasmodium berghei, respectively) [129,130]. Specifically, Schneider et al., using a malaria vaccine, reported that priming with DNA/MVA led to enhanced immunity and greater protective efficacy than that achieved with either vaccine preparation alone [130]. This finding was quickly extended to other DNA/recombinant vector combinations like poxvirus, NYVAC and rAd. Additional trials using DNA priming followed by MVA boost induced cell-mediated immune responses in NHPs [125,131] and decreased viremia following exposure to SIV [132,133] or HIV-1 [134]. Preclinical studies in macaques further demonstrated that DNA priming and recombinant modified vaccinia Ankara (rMVA) boosting elicited high virus-specific CD4 and CD8 T cells and controlled SIV and HIV challenge [135]. Heterologous DNA prime–boost immunization approaches soon became popular for their ability to elicit a robust level of vaccine-specific cellular and humoral immunity. The use of heterologous platforms to initially prime and subsequently boost an immune response has been demonstrated to be effective for eliciting Ab responses [136] and for their attractive ability to augment T-cell responses [125,137,138], which may be effective in combating persistent infections like HIV, HSV, HBV and HCV. Thus, vaccines using DNA for priming and recombinant vectors for boosting showed great promise in preclinical models, and these results led to DNA vaccination in human clinical trials.
In a study involving the vaccination of malarial DNA sequence encoding full-length Plasmodium falciparum Ag TRAP followed by intradermal delivery of recombinant modified MVA, Hill and colleagues performed the first human evaluation of a heterologous prime–boost vaccine [139]. The DNA-MVA combination reported successful safety and a strong cellular immune response that provided partial protection against malaria, extending the promise that such vaccines might work in humans. Therefore, the ability of DNA to induce both strong cellular and humoral responses illustrated its potential as a vaccine platform to treat persistent infections. The concept of using heterologous prime–boost immunization to induce a strong T-cell response with an Ab response has recently been shown by Catanzaro et al. [74] and Graham et al. [75] in a Phase I trial that used a prime–rAd5 boost approach, which demonstrated that the strategy was capable of eliciting both cellular and humoral immune responses against HIV-specific Ags. Currently, a focused Phase II study led by the NIH vaccine research center is evaluating the HIV-1 DNA prime–rAd5 boost regimen for its efficacy in controlling or reducing viral loads in study participants who become infected postvaccination [201]. Together, these data illustrate the potential and flexibility of DNA as a potent immunization strategy for inducing both Ab and T-cell responses, making it a possible therapeutic approach for eliminating chronic pathogens like HIV, HCV or HSV.
One possible reason for the success of using a heterologous prime–boost regimen is that they induce responses that differ from those induced by repeated dosing of either component alone. Specifically, a report by Cox et al. showed that the cellular responses to a HIV vaccine expressing Gag, Pol and Nef in a heterologous DNA/rAd5 regimen induced a greater Gag-specific CD4 T-cell response than that induced by the homologous rAD5/rAd5 regimen in humans [140]. Using a similar Phase I clinical trial Robinson et al. showed that DNA prime with a rAd5 boost resulted in a greater than 1000-fold increase in Abs and a greater than fivefold increase in T-cell responses against HIV-specific Ags compared with the platforms alone [141]. Furthermore, studies by Schneider et al. [142] and Robinson et al. [143] using heterologous DNA/poxvirus prime–boost immunization strategies interestingly showed that T-cell responses generated with the heterologous DNA/poxvirus strategy produce immune responses ten-times higher than either platform given separately [144]. Similar to DNA prime–viral vector boost HIV-1 vaccines, protein boost can also be given after the DNA prime [145,146]. The DNA prime–protein boost approach has also been proven to be effective in NHPs [147] and, more significantly, in humans [148]. Lu and colleagues have demonstrated that a heterologous DNA prime–protein boost approach can elicit HIV-1 Ag specific and polyfunctional T-cell immune responses [148] and more recently has been shown to be effective in eliciting not only high levels of Ag-specific HIV-1 Abs, but also at improving the quality of Ag-specific Ab responses [148]. Taken together, these studies established that heterologous DNA prime–boost immunizations elicit immune responses of greater breadth than can be achieved by priming and boosting with the same vector. Overall, this strategy has helped to reinvigorate efforts to further improve/construct DNA vaccines for treatment of chronic infections.
The ‘first generation’ DNA vaccines were capable of inducing Ag-specific T-cell responses measuring only a fraction of what their infectious, viral-vectored counterparts could achieve [62]. Since then, numerous methods for enhancing plasmid DNA immunogenicity (e.g., codon optimization, the inclusion of molecular adjuvants, improved formulation and plasmid delivery) and for overcoming some of the other perceived defects of the DNA vaccine approaches have been developed [62,149]. Genetic optimization strategies to increase gene expression, improved RNA structural design, novel formulation and immune adjuvants have augmented immunogenicity [62]. In addition, the novel protocol of heterologous prime–boost immunization has markedly heightened the immunopotency of DNA vaccination [62]. Although DNA prime–boosting has been shown to potentially be a powerful technique for inducing a broad range of immune responses by showing promising levels of immunity in preclinical models, unfortunately they have not yet been well translated in initial clinical studies [73,75,149,150,202]. The lack of translation from animal model to humans demonstrates the need for continued improvement in delivery technology and other optimizations of the important prime–boost strategy.
Vaccination during ART
Combining therapeutic vaccines with ART has been studied as a method to increase viral control and immune response against HIV. A study headed by von Gegerfelt suggested that DNA therapeutic vaccines could provide an additional benefit to ART [63]. This study found that several macaques presented a significant reduction of high-level viremia even after the animals were released from ART. In addition, vaccination combined with ART induced long-lasting, virus-specific immune responses that were observed long after the termination of ART, which is when viral loads began to rebound. Zur Megede and colleagues [151] also recently studied the stability of strong immune responses elicited by DNA vaccination and EP, as well as the long-lasting effects of DNA after termination of drug therapy. Even though the frequencies of Gag-specific CD8 T cells were increased in this study and remained at significantly higher levels until the end of the study, there appeared to be no dramatic effect on maintaining a constant low viral load post-ART cessation. Initially, viral loads remained low after ART termination, but a slow and steady increase in viremia was observed as the virus began to rebound. Explanation of these results is difficult because it is hypothesized that viral rebound leads to increased cytotoxic T-lymphocyte activity and the elimination of the infected cells [63]. Therefore, more research is warranted to determine if the T-cell-based vaccine used in the study was inefficient or if the drug therapy used somehow affected the immune response of CD8 T cells.
Current therapeutic DNA vaccines
The recent advancement of DNA vaccine Ag design and optimization, inclusion of molecular adjuvants and improved delivery methods have greatly enhanced their immunological performance. This is highly reflected by the numerous ongoing clinical trials investigating DNA vaccines for therapeutic applications (Table 2). Although the use of therapeutic vaccines against chronic infections is still in the early stages of research and development, recent studies have shown that the use of DNA vaccines in preclinical and clinical trials is a safe and effective strategy that can provide beneficial effects for individuals with persistent viral infections [75,152,153]. Therefore, such promising results from preclinical studies using DNA vaccines have subsequently advanced these gene-based vaccines into clinical trials [101,154–158]. The most current clinical trials concerned with safety and immunogenicity of therapeutic DNA vaccines against various chronic infections are outlined in Table 2. While all of these current studies have demonstrated tolerance and safety among healthy and infected subjects, many of these vaccines still require additional optimizations to enhance immunogenicity. For instance, although Alvarez-Lajonchere and colleagues demonstrated that viral capsid proteins encapsulating a HCV DNA vaccine might play an important role as adjuvants and delivery vehicles by demonstrating vaccine-induced cellular and humoral responses, the immune response generated by the vaccine did not reduce HCV viral load and future studies with further optimized plasmids will shed more light on the potential of CIGB-230 HCV vaccines [159]. Furthermore, a multiclade HIV DNA vaccine administered intramuscularly with a needle-free device was very safe, but was demonstrated to be not very immunogenic among infected individuals [160]. Similarly, a HSV-2 vaccine administered to healthy subjects presented limited vaccine-specific cellular immune responses [161]; further demonstrating the complexity of chronic infections and the challenge of imitating preclinical results in clinical trials. Nevertheless, Bagarazzi and colleagues published data showing that a DNA/HPV therapeutic vaccine coadministered with EP in humans could elicit robust HPV specific immune responses, both CD4 Th1 and CD8 cytotoxic T cells, in infected individuals, all while showing the vaccine was safe with minimal side effects at different doses tested [121]. These new results establish that DNA vaccines are practical and useful in humans as therapeutic candidates. Nonetheless, the clinical trials are continuing and the results of ongoing clinical trials will be pivotal for providing insight into the continuing progress of this platform and establishing the impact of the technological advances integrated into the next generation of DNA therapeutic vaccines.
Table 2.
Therapeutic DNA vaccines against chronic viral infections in currently active or recently completed clinical trials.
| Vaccine construct |
Study design | Stage | Cellular responses | Humoral responses | Benefits | Limitations |
|---|---|---|---|---|---|---|
| HCV | ||||||
| ChronVac-C®; HCV DNA Vaccine (Tripep AB, Huddinge, Sweden) [203] |
Nonrandomized, Phase I/II; HCV-1 chronically infected subjects. Intramuscular injection followed by EP Subjects enrolled: 12 |
Active | Plans to measure dose- related antiviral immune response |
Plans to measure dose- related antiviral immune response |
Study will evaluate safety and tolerability of ChronVac-C® delivered with EP; plans to measure dose-related effect on viral load |
Clinical trial ongoing |
| CIGB-230 [159,174]; HCV DNA vaccine with rHCV core protein |
Nonrandomized, Phase I; HCV-chronically infected subjects, nonresponders to previous treatment with interferon plus ribavirin [159,174]. Intramuscular injection. Subjects enrolled: 15 |
Completed | 73.3% of subjects exhibited IFN-γ secretory response 1 month after last immunization; 33% developed de novo response against HCV core [159] |
100% of subjects showed IgG antibodies against Core and NS3, predominantly lgG1, 1 month after last immunization; 86.6% had IgG antibodies against E1; CIGB-230 induced de novo neutralizing antibodies in six out of 14 subjects [159] |
Well tolerated and safe; only slight or moderate adverse events associated with vaccine [174]; induced HCV-specific immune response and preliminary improvement in liver histology [159]; CIGB-230 does not prevent induction of immune response against non-HCV Ags [174] |
100% of subjects had detectable HCV RNA by end of study; limited study size and absence of a placebo resulted in an inconclusive correlation between CIGB-230 and improvement in liver histology [174] |
| HIV-1 | ||||||
| PENNVAX-B; HIV-1 DNA vaccine targeting Gag, Pol and Env proteins [204] |
Nonrandomized, Phase I; HIV-1 infected subjects. Intramuscular injection followed by EP. Subjects enrolled: 12 |
Active | Plans to measure magnitude of HIV-specific immune response as determined by ELISpot |
Not determined | Plans to monitor frequency and severity of adverse events as well as local and systemic reactogenicity signs and symptoms, including CD4 and HIV RNA viral load changes |
Clinical trial ongoing |
| HIV-1 multiclade DNA prime–rAd5 boost [175] |
Nonrandomized, Phase I; HIV-uninfected subjects. Intramuscular injection. Subjects enrolled: 14 |
Completed | T-cell response in 100% of subjects; biased toward CD8+; multiple Ag specific (Env>Gag>Pol/Nef); Sustained for >6 months |
Ab response in 100% of subjects; low neutralizing Ab activity |
Heterologous prime–boost regimens induce a range of responses compared to repeated homologous regimens; also focuses the immune response on the recombinant gene product instead of the vector |
A larger study is being conducted to provide a more statistical assessment of vaccine immunogenicity |
| HIV-1 multiclade DNA vaccine [160] |
Randomized, Phase I/II, placebo-controlled, double-blinded; HIV-1 acute/early infected subjects on antiretroviral therapy. Administered intramuscularly using needle-free Biojector 2000™ (Bioject Medical Technologies, Inc., OR, USA). Subjects enrolled: 20 |
Completed | No significant difference in immunogenicity between vaccine and placebo groups observed; after treatment discontinuation, no significant difference in set-point HIV-1 viral loads or CD4 counts |
Not determined | Well-tolerated and safe; no subject restarted therapy due to safety end points |
No significant difference in set-point HIV-1 viral load. It is not clear why vaccine was not immunogenic |
| HSV | ||||||
| HSV-2 DNA vaccine [161] |
Randomized, Phase 1, placebo-controlled, double-blinded; HSV-2 seronegative subjects. Administered intramuscularly using needle-free Biojector device. Subjects enrolled: 62 |
Completed | Vaccine-induced HSV- specific T-cell activity detected in one of four HSV-seronegative subjects in highest dose group |
This vaccine did not elicit an Ab response |
Safe and well tolerated; no dose-limiting toxicities |
Dose too low to elicit a significant cellular response; vaccine design limited Ab responses |
| HBV | ||||||
| pCMV-S2.S; HBV DNA vaccine encoding the pre-S2 and the SHBV domains [205] |
Randomized, Phase I/II, HBV-infected individuals with effective antiviral (NRTI) treatment and a control group. Intramuscular injection. Subjects enrolled: 70 |
Completed | No study results posted. However, a previous Phase I clinical trial with plasmid pCMV-S2.S showed safety and demonstrated a transient activation of T-cell responses in chronic HBV carriers [176] |
No study results yet reported | To determine whether patients with chronic HBV infection treated with analogs (NRTI) can lead to T-cell restoration and delay viral reactivation after NRTI discontinuation |
No study results yet reported |
| Dual-plasmid HBV DNA vaccine; vaccine encodes HBV envelope middle protein (pS2.S)and adjuvant plasmid, pFP, containing hlL-2 and hlFN-γ fused [177] |
Part I: open-label trial (Group 01): monotherapy. Part II: double-blinded, randomized, placebo- controlled (Group 02): DNA vaccine combined therapy (DNA vaccine plus LAM vs LAM). Chronic HBV patients, aged 18–47 years who were HBsAg/ HBeAg-positive were enrolled. Intramuscular injection. Subjects enrolled: 39 |
Completed | Activated IFN-γ-secreting T-cell responses were higher in Group 02 (59.0±49.3 SFCs/106 PBMC) than in Group 01 (24.3±22.3 SFCs/106 PBMC). At week 52, a significant difference in T-cell response was detected between the two groups of the combined vs LAM monotherapy (5 out of 9 [55.6%] vs 0 out of 6[0%];p = 0.03) |
Not determined | Using a dual-plasmid DNA mediated in vivo EP as a therapeutic vaccine in chronic HBV carriers is well tolerated and safe |
Although HBV-specific T-cell response induced by DNA vaccination under LAM chemotherapy showed a correlation with the suppression of viral replication, statistical significance was not reached in this pilot trial. Further clinical trials using this approach are warranted |
| HPV | ||||||
| VGX-3100; HPV DNA vaccine is a mixture of two plasmids encoding optimized consensus E6 and E7 genes of HPV subtypes 16 and 18 [121] |
Open-label, Phase I; 18 female subjects previously treated for cervical intraepithelial neoplasia grade 2 or 3 (CIN2/3). Intramuscular injection via EP using the CELLECTRA™ (Inovio Pharmaceuticals Inc., PA, USA). Subjects enrolled: 18 |
Completed | Study detected increases of Th1-biased cellular immune responses in 78% of subjects by IFN-γ ELISpot against vaccine- induced HPV-16 or HPV-18 E6 or E7. VGX-300 induced functional CD8+ cytotoxic lymphocytes. Killing activity was still detected 6 months after vaccination. Also, 11 of 14 responders exhibited persistent memory T cells, which can be measured 24 weeks after last immunization |
100% of subjects showed antibody to at least two vaccine Ags; 94% reported positivity to three Ags; 56% showed positivity to all four antigens (HPV-16/-18 E6 and E7). End point titers (responders): HPV-16 E7 94%; HPV-18 E7 100%; HPV-16 E6 67%; HPV-18 E6 39% |
Immunization with EP-mediated delivery demonstrated favorable safety and tolerability; no dose-limiting toxicities were noted |
No placebo control. Limited number of participants. Still need to determine if CD8 CTL cells are capable of reducing cancer lesion size |
Ab: Antibody; Ag: Antigen; CTL: Cytotoxic T cell; ELISpot: Enzyme-linked immunosorbent spot; EP: Electroporation; LAM: Lamivudine; NRTI: Nucleoside reverse transcriptase inhibitor; PBMC: Peripheral blood mononuclear cell; rHCV: Recombinant hepatitis C virus.
Expert commentary & five-year view
Therapeutic vaccination is, in theory, a very promising strategy for the treatment of chronic viral diseases. Currently, there are no therapeutic vaccines for many viral chronic illnesses such as HIV, HCV, HBV or HPV, which continue to be some of the most life-threatening and rampant diseases globally. Thus, new therapeutic strategies to circumvent these issues and provide effective treatment to a greater population of the world are greatly needed.
In general, because many microbes causing persistent infection have evolved many unique and clever mechanisms to evade, modulate and even exploit the host cell immune responses, it remains a challenge for researchers to develop a highly efficient and safe therapeutic vaccine. A more detailed understanding at the molecular level of host–pathogen interactions and a better definition of immune parameters that correlate with the efficacy of vaccine therapy is needed and should undoubtedly improve the current approaches or lead to new therapeutic vaccines.
Different vaccine platforms have been studied as potential therapeutic vaccines, but the development of DNA-based vaccines in conjunction with EP, and DNA prime–boost approaches have shown to be the most promising lines of attack. In the past several years, only a few therapeutic DNA vaccines against chronic infections have entered clinical trials. Safety and tolerance of these clinical studies are encouraging, but they also highlight the need for a greater depth of understanding in the area of vector biology. Therefore, research is still continuing to explore optimal delivery, dose and optimization techniques of DNA vaccines, as well as adjuvant inclusion and combining other vaccine platforms with DNA vaccines. The results of ongoing clinical trials will be crucially important for revolutionizing the next generation of DNA therapeutic vaccines. Overall, future therapeutic strategies should focus upon mode of administration, Ag selection, adequate immune stimulation and the potential combination of therapeutic vaccines with antiviral drug therapy in order to achieve improved control or clearance of chronic viral infection.
Key issue.
The development of effective vaccines against chronic viral infection that persists within the human host remains a great challenge. However, recent improvements to DNA vaccines and the potential and flexibility of DNA as a potent immunization strategy for inducing both humoral and cellular-mediated immune responses, make them an ideal therapeutic approach for treating or eliminating chronic pathogens like HIV, HCV or HSV.
The inclusion of immune molecular adjuvants with DNA immunization has demonstrated their ability to enhance protective immune responses. The ability to modulate or enhance the immune responses in a defined manner has great promise and is an exciting avenue of improving DNA vaccine design and delivery.
DNA vaccines delivered via electroporation have markedly increased transfection efficiency and immunogenicity of DNA vaccines and may be the delivery method of choice as a potential therapeutic approach to combat persistent infections.
The novel protocol of heterologous prime–boost immunization has markedly heightened the immunopotency of DNA vaccination. The effectiveness of DNA vaccines at priming long-term immune memory and in prime–boost combination strategies has helped to reinvigorate efforts to further improve DNA vaccines for the treatment of chronic infections.
DNA vaccines may have the potential for therapy of HIV, particularly in combination with antiretroviral therapy.
The advancement of DNA vaccine antigen design and optimization to enhance their immunological performance has moved this platform forward into numerous ongoing clinical trials for therapeutic applications. Although the use of DNA therapeutic vaccines against chronic infections is still in the early stages of research, the ongoing clinical trials will be pivotal for providing insight into the continuing progress of this promising platform.
A better definition of the immune parameters that correlate with the efficacy of DNA vaccine therapy is needed and will hopefully facilitate the improvement of the current DNA vaccine approaches.
Acknowledgments
DB Weiner has commercial grant funding, participates in industry collaborations and has received speaking honoraria and fees for consulting. This service includes serving on scientific review committees and advisory boards. Remuneration includes direct payments or stock or stock options and in the interest of disclosure therefore he notes potential conflicts associated with this work with Pfizer, Bristol-Myers Squibb, Inovio, Merck, VGXI, Aldevron, Touchlight, Oncosec, Althea and possibly others. Licensing of technology from his laboratory has created over 100 jobs in the private sector in the biotechlpharma industry. This work was supported in part by U19-AI078675 and RFA08-07-07 (PA Dept Health).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 2003;77(8):4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarikonda G, von Herrath MG. Immunosuppressive mechanisms during viral infectious diseases. Methods Mol. Biol. 2011;677:431–447. doi: 10.1007/978-1-60761-869-0_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahalingam S, Meanger J, Foster PS, Lidbury BA. The viral manipulation of the host cellular and immune environments to enhance propagation and survival: a focus on RNA viruses. J. Leukoc. Biol. 2002;72(3):429–439. [PubMed] [Google Scholar]
- 4.Horst D, Ressing ME, Wiertz EJ. Exploiting human herpesvirus immune evasion for therapeutic gain: potential and pitfalls. Immunol. Cell Biol. 2011;89(3):359–366. doi: 10.1038/icb.2010.129. [DOI] [PubMed] [Google Scholar]
- 5.Oldstone MB. Anatomy of viral persistence. PLoS Pathog. 2009;5(7):e1000523. doi: 10.1371/journal.ppat.1000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisziewicz J, Bakare N, Lori F. Therapeutic vaccination for future management of HIV/AIDS. Vaccine. 2003;21(7–8):620–623. doi: 10.1016/s0264-410x(02)00569-8. [DOI] [PubMed] [Google Scholar]
- 7.Hill A. Optimizing HIV treatment. Curr. Opin. HIV AIDS. 2013;8(1):34–40. doi: 10.1097/COH.0b013e32835b7f28. [DOI] [PubMed] [Google Scholar]
- 8.Barouch DH, Letvin NL. DNA vaccination for HIV-1 and SIV. Intervirology. 2000;43(4–6):282–287. doi: 10.1159/000053995. [DOI] [PubMed] [Google Scholar]
- 9.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon α-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 2002;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 10.Oxman MN, Levin MJ, Johnson GR, et al. Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 2005;352(22):2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 11.Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 12.Bråve A, Ljungberg K, Wahren B, Liu MA. Vaccine delivery methods using viral vectors. Mol. Pharm. 2007;4(1):18–32. doi: 10.1021/mp060098+. [DOI] [PubMed] [Google Scholar]
- 13.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat. Immunol. 2011;12(6):509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu H, Thorley B, Paladin FJ, et al. Circulation of type 1 vaccine-derived poliovirus in the Philippines in 2001. J. Virol. 2004;78(24):13512–13521. doi: 10.1128/JVI.78.24.13512-13521.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kew O, Morris-Glasgow V, Landaverde M, et al. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science. 2002;296(5566):356–359. doi: 10.1126/science.1068284. [DOI] [PubMed] [Google Scholar]
- 16.Limbach KJ, Paoletti E. Non-replicating expression vectors: applications in vaccine development and gene therapy. Epidemiol. Infect. 1996;116(3):241–256. doi: 10.1017/s0950268800052547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esteban M. Attenuated poxvirus vectors MVA and NYVAC as promising vaccine candidates against HIV/AIDS. Hum. Vaccin. 2009;5(12):867–871. doi: 10.4161/hv.9693. [DOI] [PubMed] [Google Scholar]
- 18.Paoletti E, Taylor J, Meignier B, Meric C, Tartaglia J. Highly attenuated poxvirus vectors: NYVAC, ALVAC and TROVAC. Dev. Biol. Stand. 1995;84:159–163. [PubMed] [Google Scholar]
- 19.Hel Z, Venzon D, Poudyal M, et al. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat. Med. 2000;6(10):1140–1146. doi: 10.1038/80481. [DOI] [PubMed] [Google Scholar]
- 20.Bertley FM, Kozlowski PA, Wang SW, et al. Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates. J. Immunol. 2004;172(6):3745–3757. doi: 10.4049/jimmunol.172.6.3745. [DOI] [PubMed] [Google Scholar]
- 21.Pal R, Venzon D, Santra S, et al. Systemic immunization with an ALVAC-HIV-1/ protein boost vaccine strategy protects rhesus macaques from CD4+ T-cell loss and reduces both systemic and mucosal simian–human immunodeficiency virus SHIVKU2 RNA levels. J. Virol. 2006;80(8):3732–3742. doi: 10.1128/JVI.80.8.3732-3742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanke T, Goonetilleke N, McMichael AJ, Dorrell L. Clinical experience with plasmid DNA- and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade A vaccine focusing on T-cell induction. J. Gen. Virol. 2007;88(Pt 1):1–12. doi: 10.1099/vir.0.82493-0. [DOI] [PubMed] [Google Scholar]
- 23.Shimada M, Wang HB, Kondo A, et al. Effect of therapeutic immunization using Ad5/35 and MVA vectors on SIV infection of rhesus monkeys undergoing antiretroviral therapy. Gene Ther. 2009;16(2):218–228. doi: 10.1038/gt.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nájera JL, Gómez CE, García-Arriaza J, Sorzano CO, Esteban M. Insertion of vaccinia virus C7L host range gene into NYVAC-B genome potentiates immune responses against HIV-1 antigens. PLoS ONE. 2010;5(6):e11406. doi: 10.1371/journal.pone.0011406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yilma T, Verardi P, Jones L. Development of safe and efficacious viral vaccines for animals. Grit. Rev. Immunol. 2010;30(3):223–237. doi: 10.1615/critrevimmunol.v30.i3.10. [DOI] [PubMed] [Google Scholar]
- 26.Shedlock DJ, Talbott KT, Wu SJ, et al. Vaccination with synthetic constructs expressing cytomegalovirus immunogens is highly T cell immunogenic in mice. Hum. Vaccin. Immunother. 2012;8(11):1668–1681. doi: 10.4161/hv.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc. Natl Acad. Sci. USA. 1996;93(21):11341–11348. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaccari M, Mattapallil J, Song K, et al. Reduced protection from simian immunodeficiency virus SIVmac251 infection afforded by memory CD8+ T cells induced by vaccination during CD4+ T-cell deficiency. J. Virol. 2008;82(19):9629–9638. doi: 10.1128/JVI.00893-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uberla K, Rosenwirth B, Ten Haaft P, Heeney J, Sutter G, Erfle V. Therapeutic immunization with modified vaccinia virus Ankara (MVA) vaccines in SIV-infected rhesus monkeys undergoing antiretroviral therapy. J. Med. Primatol. 2007;36(1):2–9. doi: 10.1111/j.1600-0684.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 30.Vandenberghe LH, Wilson JM, Gao G. Tailoring the AAV vector capsid for gene therapy. Gene Ther. 2009;16(3):311–319. doi: 10.1038/gt.2008.170. [DOI] [PubMed] [Google Scholar]
- 31.Kron MW, Kreppel F. Adenovirus vectors and subviral particles for protein and peptide delivery. Curr. Gene Ther. 2012;12(5):362–373. doi: 10.2174/156652312802762563. [DOI] [PubMed] [Google Scholar]
- 32.Wevers D, Metzger S, Babweteera F, et al. Novel adenoviruses in wild primates: a high level of genetic diversity and evidence of zoonotic transmissions. J. Virol. 2011;85(20):10774–10784. doi: 10.1128/JVI.00810-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;454(7037):1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 34.Schneider T, Jahn HU, Schmidt W, Riecken EO, Zeitz M, Ullrich R. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut. 1995;37(4):524–529. doi: 10.1136/gut.37.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercier GT, Nehete PN, Passeri MF, et al. Oral immunization of rhesus macaques with adenoviral HIV vaccines using enteric-coated capsules. Vaccine. 2007;25(52):8687–8701. doi: 10.1016/j.vaccine.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barouch DH, Pau MG, Custers JH, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anri-Ad5 immunity. J. Immunol. 2004;172(10):6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 37.Nwanegbo E, Vardas E, Gao W, et al. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 2004;11(2):351–357. doi: 10.1128/CDLI.11.2.351-357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buchbinder SP, Mehrotra DV, Duerr A, et al. Step Study Protocol Team. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. •• Illustrates how the HIV Step trial failed to show protective responses and may have increased the risk of HIV infection in subjects with pre-existing Ad5 antibodies
- 39.Murata K, García-Sastre A, Tsuji M, et al. Characterization of in vivo primary and secondary CD8+ T cell responses induced by recombinant influenza and vaccinia viruses. Cell. Immunol. 1996;173(1):96–107. doi: 10.1006/cimm.1996.0255. [DOI] [PubMed] [Google Scholar]
- 40.Tartaglia J, Pincus S, Paoletti E. Poxvirus-based vectors as vaccine candidates. Crit. Rev. Immunol. 1990;10(1):13–30. [PubMed] [Google Scholar]
- 41.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 1995;69(4):2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat. Med. 2004;10(12):1359–1365. doi: 10.1038/nm1147. • Demonstrates that inactivated whole virus-pulsed dendritic cell vaccines could be a promising strategy for treating people with chronic HIV-1 infection
- 43.Chen M, Li YG, Zhang DZ, et al. Therapeutic effect of autologous dendritic cell vaccine on patients with chronic hepatitis B: a clinical study. World J. Gastroenterol. 2005;11(12):1806–1808. doi: 10.3748/wjg.v11.i12.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donaghy H, Pozniak A, Gazzard B, et al. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98(8):2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 45.Pacanowski J, Kahi S, Baillet M, et al. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98(10):3016–3021. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 46.Wang FS, Xing LH, Liu MX, et al. Dysfunction of peripheral blood dendritic cells from patients with chronic hepatitis B virus infection. World J. Gastroenterol. 2001;7(4):537–541. doi: 10.3748/wjg.v7.i4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinman RM. Dendritic cells and vaccines. Proc. (Bayl. Univ. Med. Cent.) 2008;21(1):3–8. doi: 10.1080/08998280.2008.11928346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niu L, Termini JM, Kanagavelu SK, et al. Preclinical evaluation of HIV-1 therapeutic ex vivo dendritic cell vaccines expressing consensus Gag antigens and conserved Gag epitopes. Vaccine. 2011;29(11):2110–2119. doi: 10.1016/j.vaccine.2010.12.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chougnet C, Cohen SS, Kawamura T, et al. Normal immune function of monocyte-derived dendritic cells from HIV-infected individuals: implications for immunotherapy. J. Immunol. 1999;163(3):1666–1673. [PubMed] [Google Scholar]
- 50.Sapp M, Engelmayer J, Larsson M, Granelli-Piperno A, Steinman R, Bhardwaj N. Dendritic cells generated from blood monocytes of HIV-1 patients are not infected and act as competent antigen presenting cells eliciting potent T-cell responses. Immunol. Lett. 1999;66(1–3):121–128. doi: 10.1016/s0165-2478(98)00169-2. [DOI] [PubMed] [Google Scholar]
- 51.Lu W, Achour A, Arlie M, Cao L, Andrieu JM. Enhanced dendritic cell-driven proliferation and anri-HIV activity of CD8(+) T cells by a new phenothiazine derivative, aminoperazine. J. Immunol. 2001;167(5):2929–2935. doi: 10.4049/jimmunol.167.5.2929. [DOI] [PubMed] [Google Scholar]
- 52.Mehlhop E, Villamide LA, Frank I, et al. Enhanced in vitro stimulation of rhesus macaque dendritic cells for activation of SIV-specific T cell responses. J. Immunol. Methods. 2002;260(1–2):219–234. doi: 10.1016/s0022-1759(01)00544-0. [DOI] [PubMed] [Google Scholar]
- 53. Lu W, Wu X, Lu Y, Guo W, Andrieu JM. Therapeutic dendritic-cell vaccine for simian AIDS. Nat. Med. 2003;9(1):27–32. doi: 10.1038/nm806. • Therapeutic dendritic cell vaccines are promising for controlling SIV. This study revealed a correlation between decreased SIV DNA and RNA levels and increased SIV-specific T-cell responses
- 54.García F, Climent N, Assoumou L, et al. DCV2/MANON07- AIDS Vaccine Research Objective Study Group. A therapeutic dendritic cell-based vaccine for HIV-1 infection. J. Infect. Dis. 2011;203(4):473–478. doi: 10.1093/infdis/jiq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Connolly NC, Whiteside TL, Wilson C, Kondragunta V, Rinaldo CR, Riddler SA. Therapeutic immunization with human immunodeficiency virus type 1 (HIV-1) pepride-loaded dendritic cells is safe and induces immunogenicity in HIV-1-infected individuals. Clin. Vaccine Immunol. 2008;15(2):284–292. doi: 10.1128/CVI.00221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356(6365):152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 57.Ulmer JB, Donnelly JJ, Parker SE, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259(5102):1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 58. Wang B, Ugen KE, Srikantan V, et al. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc. Natl Acad. Sci. USA. 1993;90(9):4156–4160. doi: 10.1073/pnas.90.9.4156. • The first use of naked DNA as an HIV-1 vaccine in mice
- 59.Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc. Natl Acad. Sci. USA. 1993;90(24):11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang B, Merva M, Dang K, et al. DNA inoculation induces protective in vivo immune responses against cellular challenge with HIV-1 antigen-expressing cells. AIDS Res. Hum. Retroviruses. 1994;10(Suppl. 2):S35–S41. [PubMed] [Google Scholar]
- 61.Doria-Rose NA, Haigwood NL. DNA vaccine strategies: candidates for immune modulation and immunization regimens. Methods. 2003;31(3):207–216. doi: 10.1016/s1046-2023(03)00135-x. [DOI] [PubMed] [Google Scholar]
- 62.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat. Rev. Genet. 2008;9(10):776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Gegerfelt AS, Rosati M, Alicea C, et al. Long-lasting decrease in viremia in macaques chronically infected with simian immunodeficiency virus SIVmac251 after therapeutic DNA immunization. J. Virol. 2007;81(4):1972–1979. doi: 10.1128/JVI.01990-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feltquate DM, Heaney S, Webster RG, Robinson HL. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 1997;158(5):2278–2284. [PubMed] [Google Scholar]
- 65.Belperron AA, Feltquate D, Fox BA, Horii T, Bzik DJ. Immune responses induced by gene gun or intramuscular injection of DNA vaccines that express immunogenic regions of the serine repeat antigen from. Plasmodium falciparum. Infect. Immun. 1999;67(10):5163–5169. doi: 10.1128/iai.67.10.5163-5169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oliveira SC, Rosinha GM, de-Brito CF, et al. Immunological properties of gene vaccines delivered by different routes. Braz. J. Med. Biol. Res. 1999;32(2):207–214. doi: 10.1590/s0100-879x1999000200009. [DOI] [PubMed] [Google Scholar]
- 67.McCluskie MJ, Brazolot Millan CL, Gramzinski RA, et al. Route and method of delivery of DNA vaccine influence immune responses in mice and non-human primates. Mol. Med. 1999;5(5):287–300. [PMC free article] [PubMed] [Google Scholar]
- 68.Sasaki S, Hamajima K, Fukushima J, et al. Comparison of intranasal and intramuscular immunization against human immunodeficiency virus type 1 with a DNA-monophosphoryl lipid A adjuvant vaccine. Infect. Immun. 1998;66(2):823–826. doi: 10.1128/iai.66.2.823-826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. MacGregor RR, Boyer JD, Ugen KE, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 1998;178(1):92–100. doi: 10.1086/515613. • First Phase I clinical trial showing the safe delivery of a DNA vaccine in HIV-infected subjects
- 70.Kwissa M, von Kampen vK, Zurbriggen R, Glück R, Reimann J, Schirmbeck R. Efficient vaccination by intradermal or intramuscular inoculation of plasmid DNA expressing hepatitis B surface antigen under desmin promoter/enhancer control. Vaccine. 2000;18(22):2337–2344. doi: 10.1016/s0264-410x(00)00030-x. [DOI] [PubMed] [Google Scholar]
- 71. Boyer JD, Wang B, Ugen KE, et al. In vivo protective anti-HIV immune responses in non-human primates through DNA immunization. J. Med. Primatol. 1996;25(3):242–250. doi: 10.1111/j.1600-0684.1996.tb00022.x. • One of the initial studies demonstrating protective HIV immune responses using a HIV-1 DNA vaccine in nonhuman primates (NHPs)
- 72.Wang R, Doolan DL, Le TP, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282(5388):476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 73.Catanzaro AT, Koup RA, Roederer M, et al. Vaccine Research Center 006 Study Team. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J. Infect. Dis. 2006;194(12):1638–1649. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Catanzaro AT, Roederer M, Koup RA, et al. VRC 007 Study Team. Phase I clinical evaluation of asix-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007;25(20):4085–4092. doi: 10.1016/j.vaccine.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 75.Graham BS, Koup RA, Roederer M, et al. Vaccine Research Center 004 Study Team. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J. Infect. Dis. 2006;194(12):1650–1660. doi: 10.1086/509259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization*. Annu. Rev. Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 77.Abdulhaqq SA, Weiner DB. DNA vaccines: developing new strategies to enhance immune responses. Immunol. Res. 2008;42(1–3):219–232. doi: 10.1007/s12026-008-8076-3. [DOI] [PubMed] [Google Scholar]
- 78.Barouch DH, Santra S, Schmitz JE, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290(5491):486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 79.Kim JJ, Trivedi NN, Nottingham LK, et al. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur. J. Immunol. 1998;28(3):1089–1103. doi: 10.1002/(SICI)1521-4141(199803)28:03<1089::AID-IMMU1089>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 80. Halwani R, Boyer JD, Yassine-Diab B, et al. Therapeutic vaccination with simian immunodeficiency virus (SIV)-DNA + IL-12 or IL-15 induces distinct CD8 memory subsets in SIV-infected macaques. J. Immunol. 2008;180(12):7969–7979. doi: 10.4049/jimmunol.180.12.7969. •• Initial study that shows codelivery of IL-12 with a SIV DNA vaccine can enhance SIV-specific cellular immune responses
- 81.Gurunathan S, Prussin C, Sacks DL, Seder RA. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 1998;4(12):1409–1415. doi: 10.1038/4000. [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat. Immunol. 2012;13(8):722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 85.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 1995;154(10):5071–5079. [PubMed] [Google Scholar]
- 86. Kim JJ, Ayyavoo V, Bagarazzi ML, et al. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J. Immunol. 1997;158(2):816–826. • Initial study to show enhanced specific HIV cytotoxic T-cell responses with the codelivery of IL-12 with DNA vaccines in mice
- 87.Boyer JD, Robinson TM, Kutzler MA, et al. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. J. Med. Primatol. 2005;34(5–6):262–270. doi: 10.1111/j.1600-0684.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- 88.Xin KQ, Hamajima K, Sasaki S, et al. IL-15 expression plasmid enhances cell-mediated immunity induced by an HIV-1 DNA vaccine. Vaccine. 1999;17(7–8):858–866. doi: 10.1016/s0264-410x(98)00271-0. [DOI] [PubMed] [Google Scholar]
- 89.Egan MA, Chong SY, Megati S, et al. Priming with plasmid DNAs expressing interleukin-12 and simian immunodeficiency virus gag enhances the immunogenicity and efficacy of an experimental AIDS vaccine based on recombinant vesicular stomatitis virus. AIDS Res. Hum. Retroviruses. 2005;21(7):629–643. doi: 10.1089/aid.2005.21.629. [DOI] [PubMed] [Google Scholar]
- 90.Sheppard P, Kindsvogel W, Xu W, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 2003;4(1):63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 91.Kotenko SV, Gallagher G, Baurin VV, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2003;4(1):69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 92.Morrow MP, Pankhong P, Laddy DJ, et al. Comparative ability of IL-12 and IL-28B to regulate Treg populations and enhance adaptive cellular immunity. Blood. 2009;113(23):5868–5877. doi: 10.1182/blood-2008-11-190520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morrow MP, Yan J, Pankhong P, et al. IL-28B/IFN-lambda 3 drives granzyme B loading and significantly increases CTL killing activity in macaques. Mol. Ther. 2010;18(9):1714–1723. doi: 10.1038/mt.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marrow MP, Yan J, Pankhong P, et al. Unique Th1/Th2 phenotypes induced during priming and memory phases by use of interleukin-12 (IL-12) or IL-28B vaccine adjuvant in rhesus macaques. Clin. Vacc. Immun. 2010b;17:1493–1499. doi: 10.1128/CVI.00181-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim JJ, Simbiri KA, Sin JI, et al. Cytokine molecular adjuvants modulate immune responses induced by DNA vaccine constructs for HIV-1 and SIV. J. Interferon Cytokine Res. 1999;19(1):77–84. doi: 10.1089/107999099314441. [DOI] [PubMed] [Google Scholar]
- 96.Moore AC, Kong WP, Chakrabarti BK, Nabel GJ. Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. J. Virol. 2002;76(1):243–250. doi: 10.1128/JVI.76.1.243-250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lai L, Vödrös D, Kozlowski PA, et al. GM-CSF DNA: an adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology. 2007;369(1):153–167. doi: 10.1016/j.virol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Loudon PT, Yager EJ, Lynch DT, et al. GM-CSF increases mucosal and systemic immunogenicity of an H1N1 influenza DNA vaccine administered into the epidermis of non-human primates. PLoS ONE. 2010;5(6):e11021. doi: 10.1371/journal.pone.0011021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Robinson HL, Montefiori DC, Villinger F, et al. Studies on GM-CSF DNA as an adjuvant for neutralizing Ab elicited by a DNA/MVA immunodeficiency virus vaccine. Virology. 2006;352(2):285–294. doi: 10.1016/j.virol.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 100.Vandervoort J, Ludwig A. Microneedles for transdermal drug delivery: a minireview. Front. Biosci. 2008;13:1711–1715. doi: 10.2741/2794. [DOI] [PubMed] [Google Scholar]
- 101.Bråve A, Ljungberg K, Boberg A, et al. Multigene/multisubtype HIV-1 vaccine induces potent cellular and humoral immune responses by needle-free intradermal delivery. Mol. Ther. 2005;12(6):1197–1205. doi: 10.1016/j.ymthe.2005.06.473. [DOI] [PubMed] [Google Scholar]
- 102.Pokorna D, Rubio I, Müller M. DNA-vaccination via tattooing induces stronger humoral and cellular immune responses than intramuscular delivery supported by molecular adjuvants. Genet. Vaccines Ther. 2008;6(4) doi: 10.1186/1479-0556-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Navot N, Kimmel E, Avtalion RR. Immunisation of fish by bath immersion using ultrasound. Dev. Biol. (Basel) 2005;121:135–142. [PubMed] [Google Scholar]
- 104.Kask AS, Chen X, Marshak JO, et al. DNA vaccine delivery by densely-packed and short microprojection arrays to skin protects against vaginal HSV-2 challenge. Vaccine. 2010;28(47):7483–7491. doi: 10.1016/j.vaccine.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 105.Chattergoon M, Boyer J, Weiner DB. Genetic immunization: a new era in vaccines and immune therapeutics. FASEB J. 1997;11(10):753–763. doi: 10.1096/fasebj.11.10.9271360. [DOI] [PubMed] [Google Scholar]
- 106.Morrow MP, Weiner DB. DNA drugs come of age. Sci. Am. 2010;303(1):48–53. doi: 10.1038/scientificamerican0710-48. [DOI] [PubMed] [Google Scholar]
- 107. Hirao LA, Wu L, Khan AS, Satishchandran A, Draghia-Akli R, Weiner DB. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine. 2008;26(3):440–448. doi: 10.1016/j.vaccine.2007.10.041. •• Demonstrates that delivery of a HIV DNA vaccine with electroporation (EP) enhances immunogenicity in NHPs
- 108. Hirao LA, Wu L, Khan AS, et al. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine. 2008;26(25):3112–3120. doi: 10.1016/j.vaccine.2008.02.036. • Reports that skin EP enhances DNA vaccine immunogenicity compared with naked DNA vaccination
- 109.Widera G, Austin M, Rabussay D, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo . J. Immunol. 2000;164(9):4635–4640. doi: 10.4049/jimmunol.164.9.4635. [DOI] [PubMed] [Google Scholar]
- 110.Babiuk S, Baca-Estrada ME, Foldvari M, et al. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine. 2002;20(27–28):3399–3408. doi: 10.1016/s0264-410x(02)00269-4. [DOI] [PubMed] [Google Scholar]
- 111.Capone S, Zampaglione I, Vitelli A, et al. Modulation of the immune response induced by gene electrotransfer of a hepatitis C virus DNA vaccine in nonhuman primates. J. Immunol. 2006;177(10):7462–7471. doi: 10.4049/jimmunol.177.10.7462. [DOI] [PubMed] [Google Scholar]
- 112. Luckay A, Sidhu MK, Kjeken R, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J. Virol. 2007;81(10):5257–5269. doi: 10.1128/JVI.00055-07. • One of the initial NHP papers demonstrating enhanced DNA vaccine immunogenicity with intramuscular EP delivery
- 113.Simon AJ, Casimiro DR, Finnefrock AC, et al. Enhanced in vivo transgene expression and immunogenicity from plasmid vectors following electrostimulation in rodents and primates. Vaccine. 2008;26(40):5202–5209. doi: 10.1016/j.vaccine.2008.03.058. [DOI] [PubMed] [Google Scholar]
- 114.Jaroszeski MJ, Gilbert R, Nicolau C, Heller R. In vivo gene delivery by electroporation. Adv. Drug Deliv. Rev. 1999;35(1):131–137. doi: 10.1016/s0169-409x(98)00068-4. [DOI] [PubMed] [Google Scholar]
- 115.Somiari S, Glasspool-Malone J, Drabick JJ, et al. Theory and in vivo application of electroporative gene delivery. Mol. Ther. 2000;2(3):178–187. doi: 10.1006/mthe.2000.0124. [DOI] [PubMed] [Google Scholar]
- 116.Cappelletti M, Zampaglione I, Rizzuto G, Ciliberto G, La Monica N, Fattori E. Gene electro-transfer improves transduction by modifying the fate of intramuscular DNA. J. Gene Med. 2003;5(4):324–332. doi: 10.1002/jgm.352. [DOI] [PubMed] [Google Scholar]
- 117.Liu J, Kjeken R, Mathiesen I, Barouch DH. Recruitment of antigen-presenting cells to the site of inoculation and augmentation of human immunodeficiency virus type 1 DNA vaccine immunogenicity by in vivo electroporation. J. Virol. 2008;82(11):5643–5649. doi: 10.1128/JVI.02564-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roos AK, Eriksson F, Timmons JA, et al. Skin electroporation: effects on transgene expression, DNA persistence and local tissue environment. PLoS ONE. 2009;4(9):e7226. doi: 10.1371/journal.pone.0007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bråve A, Gudmundsdotter L, Sandström E, et al. Biodistribution, persistence and lack of integration of a multigene HIV vaccine delivered by needle-free intradermal injection and electroporation. Vaccine. 2010;28(51):8203–8209. doi: 10.1016/j.vaccine.2010.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rosati M, Valentin A, Jalah R, et al. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine. 2008;26(40):5223–5229. doi: 10.1016/j.vaccine.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bagarazzi ML, Yan J, Morrow MP, et al. Immunotherapy against HPV16/18 generates potent Th1 and cytotoxic cellular immune responses. Sci. Transl. Med. 2012;4(155) doi: 10.1126/scitranslmed.3004414. 155ra138. •• First study to report that a therapeutic DNA vaccine coadministered via EP in humans could produce long-lived CD8 T cells with cytolytic activity
- 122.Benson J, Chougnet C, Robert-Guroff M, et al. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIV(mac251): dependence on route of challenge exposure. J. Virol. 1998;72(5):4170–4182. doi: 10.1128/jvi.72.5.4170-4182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abimiku AG, Robert-Guroff M, Benson J, et al. Long-term survival of SIVmac251-infected macaques previously immunized with NYVAC-SIV vaccines. AIDS Res. Hum. Retroviruses. 1997;15:S78. [Google Scholar]
- 124.Franchini G, Robert-Guroff M, Tartaglia J, et al. Highly attenuated HIV type 2 recombinant poxviruses, but not HIV-2 recombinant Salmonella vaccines, induce long-lasting protection in rhesus macaques. AIDS Res. Hum. Retroviruses. 1995;11(8):909–920. doi: 10.1089/aid.1995.11.909. [DOI] [PubMed] [Google Scholar]
- 125.Hanke T, Samuel RV, Blanchard TJ, et al. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 1999;73(9):7524–7532. doi: 10.1128/jvi.73.9.7524-7532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ourmanov I, Bilska M, Hirsch VM, Montefiori DC. Recombinant modified vaccinia virus ankara expressing the surface gp120 of simian immunodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J. Virol. 2000;74(6):2960–2965. doi: 10.1128/jvi.74.6.2960-2965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seth A, Ourmanov I, Kuroda MJ, et al. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc. Natl Acad. Sci. USA. 1998;95(17):10112–10116. doi: 10.1073/pnas.95.17.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cooney EL, McElrath MJ, Corey L, et al. Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a combined vaccine regimen consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proc. Natl Acad. Sci. USA. 1993;90(5):1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sedegah M, Jones TR, Kaur M, et al. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc. Natl Acad. Sci. USA. 1998;95(13):7648–7653. doi: 10.1073/pnas.95.13.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Schneider J, Gilbert SC, Blanchard TJ, et al. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 1998;4(4):397–402. doi: 10.1038/nm0498-397. •• Priming with a DNA/modified vaccinia Ankara malaria vaccine enhanced immunity and greater protective efficacy than that achieved with either platform alone
- 131.Allen TM, Vogel TU, Fuller DH, et al. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 2000;164(9):4968–4978. doi: 10.4049/jimmunol.164.9.4968. [DOI] [PubMed] [Google Scholar]
- 132.Lu S, Arthos J, Montefiori DC, et al. Simian immunodeficiency virus DNA vaccine trial in macaques. J. Virol. 1996;70(6):3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yasutomi Y, Robinson HL, Lu S, et al. Simian immunodeficiency virus-specific cytotoxic T-lymphocyte induction through DNA vaccination of rhesus monkeys. J. Virol. 1996;70(1):678–681. doi: 10.1128/jvi.70.1.678-681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kent SJ, Zhao A, Best SJ, Chandler JD, Boyle DB, Ramshaw IA. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J. Virol. 1998;72(12):10180–10188. doi: 10.1128/jvi.72.12.10180-10188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Amara RR, Villinger F, Altman JD, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292(5514):69–74. doi: 10.1126/science.1058915. • DNA priming and recombinant modified vaccinia Ankara boosting HIV vaccine strategy can induce virus-specific cellular immune responses and control mucosal simian-human immunodeficiency virus challenge
- 136.Letvin NL, Montefiori DC, Yasutomi Y, et al. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc. Natl Acad. Sci. USA. 1997;94(17):9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Leong KH, Ramsay AH, Ramshaw IA, Morin J, et al. Generation of enhanced immune responses by consecutive immunization with DNA and recombinant fowl pox virus. Vaccines. 1995;95:327–331. •• One of the initial studies demonstrating the efficacy of prime-boost immunization
- 138.Ramsay AJ, Leong KH, Ramshaw IA. DNA vaccination against virus infection and enhancement of antiviral immunity following consecutive immunization with DNA and viral vectors. Immunol. Cell Biol. 1997;75(4):382–388. doi: 10.1038/icb.1997.60. [DOI] [PubMed] [Google Scholar]
- 139. McConkey SJ, Reece WH, Moorthy VS, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 2003;9(6):729–735. doi: 10.1038/nm881. •• First human evaluation showing safety and strong cellular immune response using heterologous prime-boost vaccine strategy
- 140.Cox KS, Clair JH, Prokop MT, et al. DNA gag/adenovirus type 5 (Ad5) gag and Ad5 gag/Ad5 gag vaccines induce distinct T-cell response profiles. J. Virol. 2008;82(16):8161–8171. doi: 10.1128/JVI.00620-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Robinson HL, Weinhold KJ. Phase 1 clinical trials of the National Institutes of Health Vaccine Research Center HIV/AIDS candidate vaccines. J. Infect. Dis. 2006;194(12):1625–1627. doi: 10.1086/509263. [DOI] [PubMed] [Google Scholar]
- 142.Schneider J, Gilbert SC, Hannan CM, et al. Induction of CD8+ T cells using heterologous prime-boost immunisation strategies. Immunol. Rev. 1999;170:29–38. doi: 10.1111/j.1600-065x.1999.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 143.Robinson HL, Montefiori DC, Johnson RP, Kalish ML, Lydy SL, McClure HM. DNA priming and recombinant pox virus boosters for an AIDS vaccine. Dev. Biol. (Basel) 2000;104:93–100. [PubMed] [Google Scholar]
- 144.Mulligan MJ, Russell ND, Celum C, et al. NIH/NIAID/DAIDS HIV Vaccine Trials Network. Excellent safety and tolerability of the human immunodeficiency virus type 1 pGA2/JS2 plasmid DNA priming vector vaccine in HIV type 1 uninfected adults. AIDS Res. Hum. Retroviruses. 2006;22(7):678–683. doi: 10.1089/aid.2006.22.678. [DOI] [PubMed] [Google Scholar]
- 145.Barnett SW, Rajasekar S, Legg H, et al. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine. 1997;15(8):869–873. doi: 10.1016/s0264-410x(96)00264-2. [DOI] [PubMed] [Google Scholar]
- 146.Richmond JF, Lu S, Santoro JC, et al. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J. Virol. 1998;72(11):9092–9100. doi: 10.1128/jvi.72.11.9092-9100.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Pal R, Kalyanaraman VS, Nair BC, et al. Immunization of rhesus macaques with a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine elicits protective antibody response against simian human immunodeficiency virus of R5 phenotype. Virology. 2006;348(2):341–353. doi: 10.1016/j.virol.2005.12.029. • Illustrates broad envelope antibody responses following a multivalent DNA prime-recombinant R5 envelope boost
- 148. Wang S, Kennedy JS, West K, et al. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008;26(31):3947–3957. doi: 10.1016/j.vaccine.2007.12.060. • Broad antibody responses and poly functional T-cell immune responses induced by a DNA prime-recombinant protein boost HIV vaccine in humans
- 149.Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin. Infect. Dis. 2011;53(3):296–302. doi: 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shedlock DJ, Silvestri G, Weiner DB, et al. Monkeying around with HIV vaccines: using rhesus macaques to define ‘gatekeepers’ for clinical trials. Nat. Rev. Immunol. 2009;9(10):717–728. doi: 10.1038/nri2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.zur Megede J, Sanders-Beer B, Silvera P, et al. A therapeutic SIV DNA vaccine elicits T-cell immune responses, but no sustained control of viremia in SIVmac239-infected rhesus macaques. AIDS Res. Hum. Retroviruses. 2008;24(8):1103–1116. doi: 10.1089/aid.2008.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gorse GJ, Baden LR, Wecker M, et al. HIV Vaccine Trials Network. Safety and immunogenicity of cytotoxic T-lymphocyte poly-epitope, DNA plasmid (EP HIV-1090) vaccine in healthy, human immunodeficiency virus type 1 (HIV-1)-uninfected adults. Vaccine. 2008;26(2):215–223. doi: 10.1016/j.vaccine.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 153.Wilson CC, Newman MJ, Livingston BD, et al. Clinical phase 1 testing of the safety and immunogenicity of an epitope-based DNA vaccine in human immunodeficiency virus type 1-infected subjects receiving highly active antiretroviral therapy. Clin. Vaccine Immunol. 2008;15(6):986–994. doi: 10.1128/CVI.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dueñas-Carrera S, Alvarez-Lajonchere L, César Alvarez-Obregón J, et al. Enhancement of the immune response generated against hepatitis C virus envelope proteins after DNA vaccination with polyprotein-encoding plasmids. Biotechnol. Appl. Biochem. 2002;35(Pt 3):205–212. doi: 10.1042/ba20010089. [DOI] [PubMed] [Google Scholar]
- 155.Fló J. Co-immunization with plasmids coding the full length and a soluble form of glycoprotein D of HSV-2 induces protective cellular and humoral immune response in mice. Vaccine. 2003;21(11–12):1239–1245. doi: 10.1016/s0264-410x(02)00476-0. [DOI] [PubMed] [Google Scholar]
- 156.Alvarez-Lajonchere L, González M, Alvarez-Obregón JC, et al. Hepatitis C virus (HCV) core protein enhances the immunogenicity of a co-delivered DNA vaccine encoding HCV structural antigens in mice. Biotechnol. Appl. Biochem. 2006;44(Pt 1):9–17. doi: 10.1042/BA20050202. [DOI] [PubMed] [Google Scholar]
- 157. Letvin NL, Mascola JR, Sun Y, et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312(5779):1530–1533. doi: 10.1126/science.1124226. • Illustrates a DNA prime–adenovirus boost SIV vaccine in NHP improved SIV challenge outcome
- 158.Meseda CA, Stout RR, Weir JP. Evaluation of a needle-free delivery platform for prime-boost immunization with DNA and modified vaccinia virus ankara vectors expressing herpes simplex virus 2 glycoprotein D. Viral Immunol. 2006;19(2):250–259. doi: 10.1089/vim.2006.19.250. [DOI] [PubMed] [Google Scholar]
- 159. Alvarez-Lajonchere L, Shoukry NH, Grá B, et al. Immunogenicity of CIGB-230, a therapeutic DNA vaccine preparation, in HCV-chronically infected individuals in a Phase I clinical trial. J. Viral Hepat. 2009;16(3):156–167. doi: 10.1111/j.1365-2893.2008.01058.x. •• First report of administration of a DNA vaccine preparation in HCV-infected individuals
- 160.Rosenberg ES, Graham BS, Chan ES, et al. AIDS Clinical Trials Group A5187 Team. Safety and immunogenicity of therapeutic DNA vaccination in individuals treated with antiretroviral therapy during acute/ early HIV-1 infection. PLoS ONE. 2010;5(5):e10555. doi: 10.1371/journal.pone.0010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cattamanchi A, Posavad CM, Wald A, et al. Phase I study of a herpes simplex virus type 2 (HSV-2) DNA vaccine administered to healthy, HSV-2-seronegative adults by a needle-free injection system. Clin. Vaccine Immunol. 2008;15(11):1638–1643. doi: 10.1128/CVI.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sin JI, Kim J, Pachuk C, Weiner DB, Patchuk C. Interleukin 7 can enhance antigen-specific cytotoxic-T-lymphocyte and/or Th2-type immune responses in vivo. Clin. Diagn. Lab. Immunol. 2000;7(5):751–758. doi: 10.1128/cdli.7.5.751-758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hartoonian C, Ebtekar M, Soleimanjahi H, et al. Effect of immunological adjuvants: GM-CSF (granulocyte-monocyte colony stimulating factor) and IL-23 (interleukin-23) on immune responses generated against hepatitis C virus core DNA vaccine. Cytokine. 2009;46(1):43–50. doi: 10.1016/j.cyto.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 164.Chattergoon MA, Saulino V, Shames JP, Stein J, Montaner LJ, Weiner DB. Co-immunization with plasmid IL-12 generates a strong T-cell memory response in mice. Vaccine. 2004;22(13–14):1744–1750. doi: 10.1016/j.vaccine.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 165.Kim JJ, Nottingham LK, Tsai A, et al. Ag-specific humoral and cellular immune responses can be modulated in rhesus macaques through the use of IFN-gamma, IL-12, or IL-18 gene adjuvants. J. Med. Primatol. 1999b;28(4–5):214–223. doi: 10.1111/j.1600-0684.1999.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 166.Yang SH, Lee CG, Park SH, et al. Correlation of antiviral T-cell responses with suppression of viral rebound in chronic hepatitis B carriers: a proof-of-concept study. Gene Ther. 2006;13(14):1110–1117. doi: 10.1038/sj.gt.3302751. [DOI] [PubMed] [Google Scholar]
- 167.Rigopoulou EI, Suri D, Chokshi S, et al. Lamivudine plus interleukin-12 combination therapy in chronic hepatitis B: antiviral and immunological activity. Hepatology. 2005;42(5):1028–1036. doi: 10.1002/hep.20888. [DOI] [PubMed] [Google Scholar]
- 168.Cui FD, Asada H, Jin ML, et al. Cytokine genetic adjuvant facilitates prophylactic intravascular DNA vaccine against acute and latent herpes simplex virus infection in mice. Gene Ther. 2005;12(2):160–168. doi: 10.1038/sj.gt.3302393. [DOI] [PubMed] [Google Scholar]
- 169.Sin JI, Kim JJ, Arnold RL, et al. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J. Immunol. 1999;162(5):2912–2921. [PubMed] [Google Scholar]
- 170.Kim JJ, Yang JS, VanCott TC, et al. Modulation of antigen-specific humoral responses in rhesus macaques by using cytokine cDNAs as DNA vaccine adjuvants. J. Virol. 2000;74(7):3427–3429. doi: 10.1128/jvi.74.7.3427-3429.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee SK, Stack A, Katzowitsch E, Aizawa SI, Suerbaum S, Josenhans C. Helicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5. Microbes Infect. 2003;5(15):1345–1356. doi: 10.1016/j.micinf.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 172.Zhu M, Xu X, Liu H, et al. Enhancement of DNA vaccine potency against herpes simplex virus 1 by co-administration of an interleukin-18 expression plasmid as a genetic adjuvant. J. Med. Microbiol. 2003;52(Pt 3):223–228. doi: 10.1099/jmm.0.04998-0. [DOI] [PubMed] [Google Scholar]
- 173.Chow YH, Chiang BL, Lee YL, et al. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J. Immunol. 1998;160(3):1320–1329. [PubMed] [Google Scholar]
- 174.Castellanos M, Cinza Z, Dorta Z, et al. Immunization with a DNA vaccine candidate in chronic hepatitis C patients is safe, well tolerated and does not impair immune response induction after anti-hepatitis B vaccination. J. Gene Med. 2010;12(1):107–116. doi: 10.1002/jgm.1407. [DOI] [PubMed] [Google Scholar]
- 175.Koup RA, Roederer M, Lamoreaux L, et al. VRC 009 Study Team; VRC 010 Study Team. Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS ONE. 2010;5(2):e9015. doi: 10.1371/journal.pone.0009015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mancini-Bourgine M, Fontaine H, Bréchot C, Pol S, Michel ML. Immunogenicity of a hepatitis B DNA vaccine administered to chronic HBV carriers. Vaccine. 2006;24(21):4482–4489. doi: 10.1016/j.vaccine.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 177.Yang FQ, Yu YY, Wang GQ, et al. A pilot randomized controlled trial of dual-plasmid HBV DNA vaccine mediated by in vivo electroporation in chronic hepatitis B patients under lamivudine chemotherapy. J. Viral Hepat. 2012;19(8):581–593. doi: 10.1111/j.1365-2893.2012.01589.x. [DOI] [PubMed] [Google Scholar]
Websites
- 201.Safety and effectiveness of HIV-1 DNA plasmid vaccine and HIV-1 recombinant adenoviral vector vaccine in HIV-uninfected, circumcised men and male-to-female (MTF) transgender persons who have sex with men. http://clinicaltrials.gov/ct2/show/NCT00865566.
- 202.Kresge KJ. A STEP back? Additional data released from the STEP trial raises questions about whether the vaccine may have increased the risk of HIV infection. IAVI Report (online) 2007 www.iavireport.org/Back-Issues/Pages/IAVI-Report-11(5)-ASTEPBack.aspx.
- 203.Phase I/IIa dose ranging CHRONVAC-C® study in chronic HCV patients. http://clinicaltrials.gov/ct2/show/NCT00563173.
- 204.Study of PENNVAX™-B (Gag, Pol, Env) + electroporation in HIV-1 infected adult participants (HIV-001) http://clinicaltrials.gov/ct2/show/NCT01082692.
- 205.Efficacy and tolerance of naked DNA vaccine in patients with chronic B hepatitis (VAC-ADN) http://clinicaltrials.gov/ct2/show/NCT00536627.