Abstract
Background.
Rotavirus is the most common cause of infectious diarrhea in children worldwide. Recent studies have described changes in the burden of all-cause gastroenteritis; however, there are limited data on the clinical and economic impact of rotavirus vaccine on cases of laboratory-confirmed rotavirus disease.
Methods.
We performed a retrospective study of laboratory-confirmed rotavirus disease from July 2003 through June 2010 at a children's hospital and a community hospital in Utah. Demographics and hospital costs for children <5 years with rotavirus symptoms and a positive rotavirus enzyme immunoassay test on a stool specimen were abstracted from electronic medical records. We compared the prevaccine period (2003–2007) with the postvaccine period (2008–2010).
Results.
The overall incidence of rotavirus gastroenteritis declined in the postvaccine period, from 26.6 to 5.2 cases per 10 000 person-years for Salt Lake County residents. The largest decrease in the incidence of rotavirus gastroenteritis was among children <12 months (−87%; 95% confidence interval [CI], 79–93). Older children (12–23 months) also experienced significant decreases (−81%; 95% CI, 72–88), as did those 24–59 months (−61%; 95% CI, 51–71). In 2009, 3 years after rotavirus vaccine introduction, there was a 79% decrease in emergency department visits and a 78% decrease in hospitalizations across both hospitals. The cost of emergency department visits and hospitalizations for rotavirus gastroenteritis decreased by 79% and 72%, respectively, resulting in annual savings of $790 000 at a children's hospital and $140 000 at a community hospital.
Conclusion.
Rotavirus vaccination in infants has dramatically decreased the clinical burden and direct medical costs of rotavirus gastroenteritis in both infants and young children.
Keywords: Immunization, Acute Gastroenteritis, Hospital Costs, Incidence, Pediatrics
Rotavirus is the leading cause of infectious diarrhea in infants and children worldwide [1]. In the United States, almost all children have at least 1 episode of rotaviral diarrhea before their 5th birthday [2]. Before rotavirus vaccine licensure in 2006, rotavirus gastroenteritis was estimated to result in over 400 000 physician visits, more than 200 000 emergency department visits, and 27 000–70 000 hospitalizations annually in the United States [3–6]. Rotavirus gastroenteritis was associated with significant costs to the healthcare system and society, estimated annually at $320 million and almost $900 million, respectively, in the United States [7].
In 2006, a pentavalent oral rotavirus vaccine (RotaTeq; Merck) was licensed in the United States. Two years later, a second live-attenuated rotavirus vaccine (Rotarix; GlaxoSmithKline Biologicals) was licensed. Data from the prelicensure studies estimated rotavirus vaccine efficacy to be 85%–95% for rotavirus hospitalizations [8–11]. Shortly after licensure, the US Advisory Committee on Immunization Practices recommended routine rotavirus vaccination for infants [12]. Early laboratory-based surveillance data in the United States reported a 67% reduction in the number of rotavirus cases in 2007–2008 compared with the 2000–2006 seasons [13]. This decrease occurred when single-dose coverage of the vaccine was estimated at 49% among sentinel sites [14]. Recent studies under conditions of routine use have demonstrated vaccine efficacy rates of 87%–100% against rotavirus gastroenteritis hospitalizations and emergency department visits [15–17].
Many studies describing the burden of rotavirus gastroenteritis during the pre- and postvaccine periods are limited by reliance on indirect methods, including International Classification of Diseases, Ninth Revision, Clinical modification (ICD-9) codes to identify and define cases of rotavirus gastroenteritis [6, 7, 18]. In addition, there are limited data on the economic impact of rotavirus vaccine and its effect upon direct medical costs.
The objectives of our study were to assess the clinical and economic impact of rotavirus vaccination on cases of laboratory-confirmed rotavirus gastroenteritis among children at a tertiary care children's hospital and a community hospital, using actual hospital costs.
METHODS
Human Subjects Protection
The institutional review boards of the University of Utah and Intermountain Healthcare (Intermountain) approved this study.
Setting and Study Design
We performed a retrospective study of laboratory-confirmed rotavirus disease from July 2003 through June 2010 using Intermountain's Enterprise Data Warehouse. We analyzed data from 2 Utah hospitals; (1) Primary Children's Medical Center (PCMC), a 289-bed, free-standing children's hospital that provides primary and tertiary care located in Salt Lake City, Utah, and (2) McKay-Dee Hospital (MCKD), a 352-bed community hospital in Ogden, Utah, with a 26-bed children's ward.
Study Population
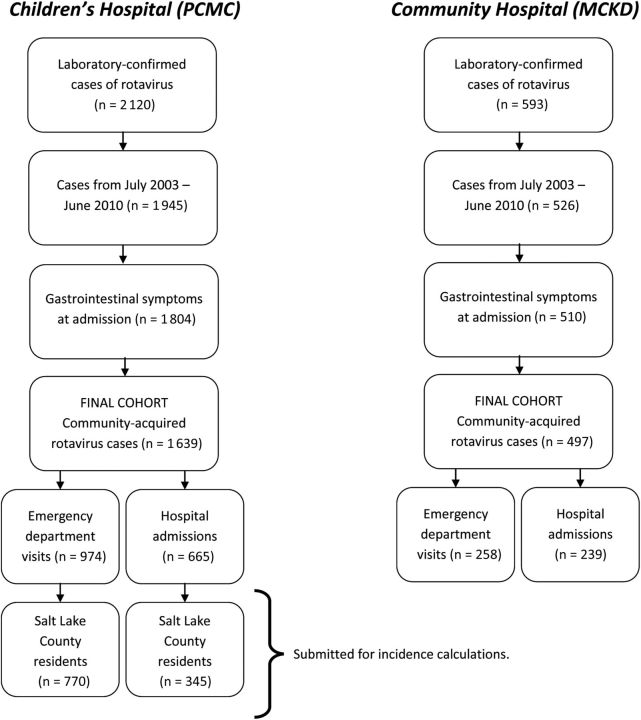
The selection of patients is outlined in Figure 1. We included all children younger than 5 years with nausea, vomiting, or diarrhea cared for at PCMC and MCKD, who had a positive rotavirus enzyme immunoassay (EIA) test on a stool specimen (ImmunoCARD STAT rotavirus; Meridian Bioscience, Cincinnati, OH). Cases of community-acquired rotavirus gastroenteritis were defined as children testing positive for rotavirus within 48 hours of hospital admission. If a child had more than 1 positive EIA test within a 2-week period, it was considered to be a single episode.
Figure 1.
Method for ascertaining emergency department visits and hospitalizations attributable to rotavirus gastroenteritis. Abbreviations: MCKD, McKay-Dee Hospital; PCMC, Primary Children's Medical Center.
Data Collection
Demographics, clinical information, and hospital cost data were abstracted from electronic medical records using Intermountain's Enterprise Data Warehouse. Chronic medical conditions were classified according to an algorithm developed by Feudtner et al [19] for pediatric subjects, which uses ICD-9 coding at hospital discharge to identify comorbidities.
Individual and total direct medical costs were derived from Intermountain's cost-accounting program, standard cost manager, which is a transaction-based micro-costing system that identifies and aggregates the variable and fixed-cost components of hospital services and products according to the date of service [20, 21]. This cost-based accounting system uses time and motion studies to estimate the actual costs of all aspects of hospital care, updated annually, and widely used throughout US hospitals [22, 23]. Costs were converted to 2010 US dollars by applying a yearly consumer price index for medical services.
Definition of Season
A rotavirus season was defined as the time period from July through June of the following year [13]. The prevaccine period was defined as the 4 seasons from July 2003 to June 2007, and the 3 seasons from July 2007 to June 2010 constituted the postvaccine period. The 2006–2007 season was included in the prevaccine period because vaccination coverage rates were low during this season [14].
Rotavirus Vaccine Coverage
Rotavirus vaccination coverage rates were obtained from the US Centers for Disease Control and Prevention's National Immunization Survey (NIS) for 2009 [24] and estimated from the Utah Statewide Immunization Information System.
Statistical Analysis
We calculated the number of rotavirus gastroenteritis cases per 1000 emergency department visits and hospital admissions during the prevaccine and postvaccine periods. To estimate the age-specific incidence for Salt Lake County, we used Salt Lake County residents cared for at PCMC as the numerator and the annual intercensus population estimates for Salt Lake County as the denominator [25]. Rates are reported per 10 000 children per year. Approximately 85% of all pediatric hospitalizations for Salt Lake County residents are at PCMC, and the proportion did not change substantially during the study period (courtesy of Jim Bradshaw, Director of Strategic Planning, Intermountain Healthcare, Salt Lake City, UT). To determine whether changes in testing behavior could have influenced estimated rates of rotavirus, we examined the number of tests ordered by month and the proportion of rotavirus tests that were positive. To explore whether the decrease in confirmed rotavirus cases was reflected in total admissions for age, we identified cases of diarrhea-associated admission using discharge diagnoses as used by Cortes et al [18]. We used ICD-9 codes for acute gastroenteritis, viral enteritis, bacterial enteritis, parasitic enteritis, and diarrhea not otherwise specified and listed as the primary discharge diagnosis or within the first 15 possible discharge diagnoses. We compared mean and median costs per patient episode in the prevaccine and postvaccine periods. Total costs per season were calculated by summing the individual costs per patient evaluated throughout the season and are presented as mean (range). Categorical variables were compared using the χ2 test or Fisher's exact test, as appropriate. Continuous variables were compared with nonparametric Wilcoxon Mann-Whitney tests. Poisson regression was used to evaluate linear trend tests of incidence rates during the pre- and postvaccine periods. A P value <.05 was considered to be significant. Relative incidence rate reductions were calculated as 1- relative risk and are presented with exact 95% CIs; binomial CIs were calculated for the reduction in costs. All statistical analyses were performed using Stata 11.2 (StataCorp LP, College Station, TX).
RESULTS
Rotavirus Vaccine Coverage
The estimated proportion of children in Utah 19–35 months of age who had been completely immunized with rotavirus vaccine in 2009 (defined as ≥ 2 doses of RV1 or ≥ 3 doses of RV5) using NIS data was 44% [24]. Among children younger than 3 years, estimates using the statewide immunization registry were similar: 9% in 2007, 26% in 2008, 40% in 2009, and 44% in 2010 (courtesy of David Foley, Utah Department of Health, Salt Lake City, UT) [26].
Demographics
Characteristics of children with laboratory-confirmed rotavirus disease at PCMC and MCKD from 2003 to 2010 are shown in Table 1. Over the study period, the number of children younger than 5 years with laboratory-confirmed rotavirus disease decreased from a mean of 355 cases per year to 73 cases per year at PCMC (P < .001) and 105 cases per year to 26 cases per year at MCKD (P < .001). There was a slight male predominance at both hospitals that did not change significantly over time. In the postvaccine period, the mean age of children with rotavirus increased from 16 to 21 months (P < .001), and the proportion of children who were 24 months or older increased (P < .001). This was driven by more dramatic decreases in disease among infants targeted for rotavirus immunization. The proportion of children with chronic medical conditions and the length of hospital stay were similar at PCMC and MCKD and did not change with increasing use of rotavirus vaccine.
Table 1.
Characteristics of Children <5 Years With Laboratory-Confirmed Rotavirus Infection at Primary Children's Medical Center and McKay-Dee Hospital, Utah, 2003–2010
| Variable | Children's Hospital (PCMC) |
Community Hospital (MCKD) |
||||
|---|---|---|---|---|---|---|
| Prevaccine (n = 1419) | Postvaccine (n = 220) | P Value | Prevaccine (n = 420) | Postvaccine (n = 77) | P Value | |
| Sex | ||||||
| Male | 813 (57%) | 118 (54%) | NS | 231 (55%) | 46 (60%) | NS |
| Age (months) | ||||||
| Median (IQR) | 13.6 (9.1–19.8) | 18.8 (11.8–28.2) | <.001 | 14.1 (10.4–20.4) | 20.7 (13.8–27.7) | <.001 |
| Age groups | ||||||
| <12 months | 593 (42%) | 58 (26%) | 145 (35%) | 16 (21%) | ||
| 12–23 months | 594 (42%) | 88 (40%) | 202 (48%) | 30 (39%) | ||
| >24 months | 232 (16%) | 74 (34%) | 73 (17%) | 31 (40%) | ||
| Chronic medical condition | ||||||
| Any | 101 (7%) | 17 (8%) | NS | 28 (7%) | 5 (6%) | NS |
| Length of stay (days) | ||||||
| Median (IQR) | 2.0 (1.6–3.6) | 2.4 (1.8–3.6) | NS | 1.8 (1.5–2.5) | 1.8 (1.6–2.0) | NS |
| Emergency department cost per patient ($) | ||||||
| Median (IQR) | 1432 (976–1725) | 1484 (1165–1737) | NS | 511 (95–1356) | 998 (326–1481) | NS |
| Hospitalization cost per patient ($) | ||||||
| Median (IQR) | 4207 (2927–6076) | 4951 (3936–6582) | <.001 | 2700 (2155–3630) | 3232 (2469–3892) | .05 |
Abbreviations: IQR, interquartile range; MKCD, McKay-Dee Hospital; NS, not significant (reported where P values >.05); PCMC, Primary Children's Medical Center.
Testing Patterns
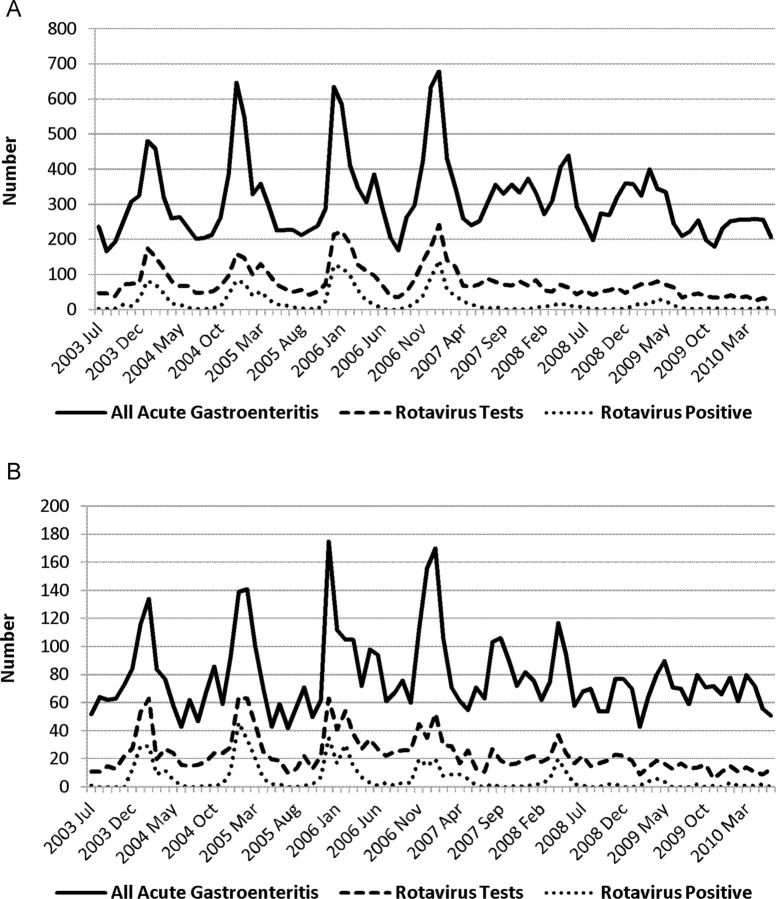
The total number of rotavirus tests ordered by month and the number of positive tests are shown in Figure 2. The number of rotavirus tests ordered decreased in the postvaccine period from a median of 1511 per year to 946 per year (P = .05). The proportion of rotavirus tests that were positive declined from 34% to 12% (P = .03).
Figure 2.
Patterns of acute gastroenteritis testing, rotavirus testing, and positivity among children <5 years in Utah, 2003–2010. A, Primary Children's Medical Center; B, McKay-Dee Hospital.
Incidence of Rotavirus Gastroenteritis in Salt Lake County
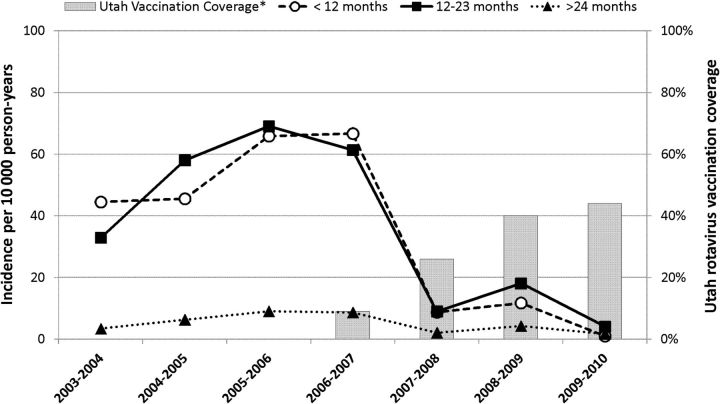
The incidence of hospitalization for laboratory-confirmed rotavirus gastroenteritis among children <5 years in Salt Lake County declined from 26.6 per 10 000 person-years in the prevaccine period to 5.2 per 10 000 person-years in the postvaccine period (−80%; 95% CI, 77–84). The greatest decrease in the incidence of rotavirus gastroenteritis was among children <12 months (55.7 to 7.2 per 10 000; −87%; 95% CI, 82–91). The incidence decreased somewhat less among children 12–23 months (55.4 to 10.4 per 10 000; −81%; 95% CI, 75–86) and > 24 months (6.9 to 2.7 per 10 000; −61%; 95% CI, 45–72) (Figure 3).
Figure 3.
Incidence of rotavirus hospitalizations among Salt Lake County resident children <5 years, by age group, 2003–2010.
Complete rotavirus vaccination coverage, defined as ≥2 doses of RV1 or ≥3 doses of RV5.
Emergency Department Visits
Children's Hospital (PCMC)
The rate of visits for rotavirus disease emergency department visits among children younger than 5 years decreased from 9.9 per 1000 visits in the prevaccine period to 1.7 per 1000 in the postvaccine period (82% reduction; 95% CI, 78–85) (Table 2). Emergency department costs per patient remained stable (Wilcoxon Mann-Whitney test, P > .05). The total seasonal costs attributed to emergency department visits declined from a mean of $330 044 in the prevaccine period to $67 100 in 2007–2008, $103 360 in 2008–2009, and $32 575 in 2009–2010. This represented an average savings of $262 366 per year in direct medical costs.
Table 2.
Clinical and Economic Burden of Rotavirus Gastroenteritis Emergency Department Visits and Hospital Admissions at Primary Children's Medical Center and McKay-Dee Hospital Among Children <5 Years, 2003–2010
| Season | Children's Hospital (PCMC) |
Community Hospital (MCKD) |
||||||
|---|---|---|---|---|---|---|---|---|
| Emergency Department Visits |
Hospital Admissions |
Emergency Department Visits |
Hospital Admissions |
|||||
| Rate/1000a | Annual Cost | Rate/1000a | Annual Cost | Rate/1000a | Annual Cost | Rate/1000a | Annual Cost | |
| Prevaccine | ||||||||
| 2003–2004 | 6.3 | $160 628 | 16.8 | $487 961 | 5.5 | $12 244 | 13.5 | $151 462 |
| 2004–2005 | 8.9 | $240 453 | 22.2 | $577 197 | 10.6 | $47 790 | 13.7 | $154 693 |
| 2005–2006 | 12.4 | $355 758 | 24.1 | $793 239 | 10.5 | $50 508 | 11.5 | $143 866 |
| 2006–2007 | 12.0 | $332 597 | 17.5 | $558 922 | 10.1 | $46 320 | 7.9 | $122 515 |
| Postvaccine | ||||||||
| 2007–2008 | 1.5 | $40 801 | 4.7 | $194 247 | 3.9 | $23 042 | 4.5 | $66 957 |
| Reductionb | 85% (79%–90%) | 85% | 77% (68%–84%) | 68% | 57% (35%–73%) | 41% | 61% (39%–76%) | 53% |
| 2008–2009 | 3.0 | $86 055 | 6.5 | $245 669 | 2.1 | $9380 | 1.4 | $23 001 |
| Reductionb | 70% (61%–77%) | 68% | 68% (57%–76%) | 59% | 77% (60%–88%) | 76% | 88% (74%–95%) | 84% |
| 2009–2010 | 0.8 | $21 552 | 2.4 | $86 939 | 0.8 | $6786 | 1.3 | $24 966 |
| Reductionb | 92% (87%–95%) | 92% | 88% (81%–93%) | 86% | 91% (79%–97%) | 83% | 89% (76%–-96%) | 83% |
Abbreviations: MKCD, McKay-Dee Hospital; PCMC, Primary Children's Medical Center.
aRates are reported as the number of rotavirus gastroenteritis infections per 1000 emergency department visits or hospitalizations per 1000 admissions per year among children <5 years of age.
bRelative percentage reduction compared with average 2003–2007 (95% CI).
Community Hospital (MCKD)
The rate of visits for rotavirus disease at MCKD declined from 9.2 per 1000 emergency department visits in the prevaccine period to 2.3 per 1000 visits in the postvaccine period (75% reduction; 95% CI, 65–83) (Table 2). Per patient emergency department costs did not change (P > .05); however, the total seasonal costs for emergency department visits declined from a mean of $41 742 in the prevaccine period to $23 042 in 2007–2008, $10 085 in 2008–2009, and $6786 in 2009–2010. This represented an average savings of $28 438 per year in direct medical costs.
Hospital Admissions
Children's Hospital (PCMC)
The rate of hospital admission for laboratory-confirmed rotavirus gastroenteritis decreased from a mean of 20.1 per 1000 admissions in the prevaccine period to 4.5 per 1000 in the postvaccine period (78% reduction; 95% CI, 72–82) (Table 2). The mean total seasonal cost of rotavirus hospitalizations was $741 101 per year in the prevaccine period. The total direct costs of rotavirus hospitalizations in the postvaccine period decreased to $253 807 in 2007–2008 (−66%; 95% CI, 56–75), $266 735 in 2008–2009 (−64%; 95% CI, 54–73), and $117 751 in 2009–2010 (–84%; 95% CI, 75–91). Hospitalization costs per patient increased by 18% (P < .001); however, the average reduction in total direct costs of hospitalization compared with the prevaccine period was $528 337 per year.
Community Hospital (MCKD)
At MCKD, hospitalizations for rotavirus declined from 11.7 to 2.4 per 1000 admissions (79% reduction; 95% CI, 70–86). The hospitalization cost per patient increased by 20% (P = .05). The mean total cost of rotavirus hospitalizations declined from $154 379 per year in the prevaccine period to $39 414 per year in the postvaccine period (74% reduction; 95% CI, 64–82)—an annual savings of $114 965.
DISCUSSION
In this 7-year study of children younger than 5 years with laboratory-confirmed rotavirus gastroenteritis, we demonstrated significant changes in the epidemiology and economic burden of rotavirus disease. In the first 3 years after rotavirus vaccine introduction, there was a 78% decrease in hospitalizations and a 79% decrease in emergency department visits in both a tertiary children's and community hospital. The decline in the incidence of rotavirus disease resulted in substantially decreased direct healthcare costs for emergency department visits and hospital admissions, resulting in savings of $790 000 per year at a children's hospital and $140 000 per year at a community hospital. This does not take into account the costs of rotavirus vaccine.
To determine the impact and cost effectiveness of rotavirus vaccination in developed countries, it is critical to have accurate assessments of the incidence and of healthcare costs [27]. Since the introduction of rotavirus vaccine, several studies have described the immediate impact on acute gastroenteritis as well as laboratory-confirmed rotavirus gastroenteritis in children [18, 28–31]. Using administrative data, several authors reported a 16%–50% decline in acute gastroenteritis hospitalizations in the United States as early as 2007 and 2008 [32–34]. During the same period, laboratory testing for rotavirus and the proportion of samples testing positive for rotavirus decreased by 67%–69% [13]. Studies have also demonstrated declines of 67%–87% in hospitalizations for rotavirus gastroenteritis as determined by direct (laboratory-confirmed) and indirect methods [31, 33, 35]. Our estimate of an 80% decline in hospitalizations, from 26.6 cases per 10 000 persons to 5.2 cases per 10 000 person-years, in the postvaccine period is consistent with these estimates. The decline in rotavirus gastroenteritis has occurred with increasing but relatively modest complete rotavirus vaccine coverage. Nationally, 56% of children younger than 2 years had received 1 dose and 33% had completed 3 doses of the rotavirus vaccine by 2008 [14]. In Utah in 2009, 44% of Utah children 19–35 months of age had completed the rotavirus vaccination series, similar to the national average [36].
As expected, the highest rates of hospitalization for rotavirus gastroenteritis were in children younger than 24 months of age, and the greatest reductions in disease burden were observed in this age group. In the prevaccine period, children older than 24 months represented 16%–17% of patients treated for rotavirus gastroenteritis in both hospitals, and this increased to 34%–40% during the postvaccine period. Our findings are similar to those of Clark et al [37], who reported an increase in the proportion of children hospitalized with rotavirus gastroenteritis who were older than 18 months after rotavirus vaccine introduction. Of note, we observed a 61% reduction in rotavirus hospitalizations among children 2–4 years of age, from 6.9 per 10 000 to 2.7 per 10 000. The decrease was observed in the first full season after vaccine licensure, even though no children in this age cohort were eligible to receive vaccine. Curns et al [32] observed a similar 50% reduction in gastroenteritis hospitalizations in children 2–4 years old using hospital discharge data from 18 states. Recent data suggests that there has also been a reduction in gastroenteritis hospitalizations among children and adults 5–24 years of age since introduction of pentavalent rotavirus vaccine [38]. This substantial decline in rotavirus gastroenteritis has occurred in the setting of modest vaccine coverage [33, 37, 39]. The large effect observed with modest vaccine coverage combined with the effects in age groups not targeted for vaccine suggests that the indirect impact of rotavirus vaccine has been substantial. This result is encouraging, because modeling studies predicted that the degree of herd protection provided by rotavirus would substantially influence cost effectiveness [40].
An important feature of our study is the ability to directly measure direct healthcare costs, both in a large children's hospital and in a community hospital. The total healthcare costs associated with rotavirus disease decreased significantly after the introduction of rotavirus vaccine in both hospitals. At PCMC, there was a 78% and 83% decrease in total hospitalization and emergency department costs for rotavirus disease, respectively, whereas at MCKD these costs decreased by 79% and 75%, respectively. Our measured cost per hospitalization was similar to estimates of costs derived from charges or payments, methods which can be imprecise. For example, Mast et al [41] used standard methods to estimate costs from hospital-based charges and reported average costs of $3638 for hospital stay and $2350 for short stay hospitalization in children's hospitals in Durham, North Carolina and Cincinnati, Ohio.
Our study was not designed to estimate national costs; however, using the impact in Salt Lake county and the median cost per admission of $4207 at PCMC, we estimate that the introduction of rotavirus vaccine has resulted in annual decrease of 32 510 hospitalizations for community-acquired rotavirus disease in children younger than 5 years and a decrease in direct healthcare costs of $130 million (95% CI, $61 million–395 million) annually. Using MarketScan databases of insurance claims, Cortes [18] estimated that 32 428 hospitalizations were prevented each year, and $139 million in payments were prevented. These estimates are remarkably consistent despite the limitations inherent in each approach, and thus they provide a reasonable estimate of the impact of rotavirus vaccine on healthcare use in the United States.
Our study has several strengths. We limited inclusion to children with laboratory-confirmed rotavirus disease. A number of previous studies have used ICD-9 codes to determine the burden of rotavirus disease from cases of hospitalized acute gastroenteritis [4, 33, 42]. However, the sensitivity of ICD-9 codes in identifying cases of rotavirus disease has been estimated to range from 24% to 47% [43]. Ascertainment of rotavirus disease was fairly complete among hospitalized children at PCMC, because testing of all children with gastroenteritis is required by our infection control committee for the purpose of isolation and cohorting. Differences may exist between children presenting to children's hospitals and community hospitals. We examined both and found that although the overall burden was higher at the children's hospital, the relative impact was similar. Finally, many studies rely on estimating costs from charges [41, 44], which introduces potential errors and bias, but we used a direct cost-accounting system that provided a more accurate measure.
The limitations of our study should be considered. First, we relied on laboratory testing to identify cases of hospitalized rotavirus disease. Although ascertainment among hospitalized children was likely quite complete at PCMC, testing at MCKD may have varied by physician. A substantial proportion of children managed in the emergency department may not have been tested for rotavirus, resulting in an underestimation of the true disease burden. Second, we could not determine the vaccination status of individual children in our study. Because we captured approximately 85% of pediatric hospitalizations among residents of Salt Lake County, our rotavirus incidence estimates are likely conservative. We did not genotype rotavirus isolates, and vaccine efficacy may be influenced by changes in the circulating strain in the community. Third, the median per patient cost for a rotavirus hospitalization increased by 18% and 20% at PCMC and MCKD, respectively. Chang et al [45] observed a 59% increase in the median per patient cost of rotavirus hospitalization in New York state, after the introduction of rotavirus vaccine. We sought to find a possible explanation for these increasing costs and found that the total hospital length of stay and the proportion of children with chronic medical conditions did not change between the prevaccine and postvaccine periods. Fourth, we included the 2006–2007 season in the prevaccine period because this year featured low rotavirus vaccination rates [26]. Furthermore, rotavirus gastroenteritis was diagnosed using a rapid antigen test and not a slower, more sensitive method, such as an enzyme-linked immunosorbent assay. Finally, we could not measure vaccine coverage at the county level.
In conclusion, we demonstrated a significant decrease in rotavirus hospitalizations and emergency department visits in Utah, during a period of increasing rotavirus vaccination in children. The decline in the burden of rotavirus gastroenteritis resulted in substantially decreased healthcare costs, including annual savings of $790 000 at a free-standing children's hospital and $140 000 at a community hospital. The reduction in disease among older children with modest vaccine coverage suggests indirect effects of the vaccine. Continued surveillance is needed to monitor the clinical and economic impact of increasing rotavirus vaccine use.
Acknowledgments
We thank Richard Nelson for his economic advice and statistical support. We also thank Intermountain Healthcare, Salt Lake City, Utah.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the University of Utah, Department of Pediatrics through the Children's Health Research Center and the Pediatric Clinical and Translational Research Scholars Program; the H. A. and Edna Benning Presidential Endowment; and the Primary Children's Medical Center Foundation. This project was further supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (Grant UL1-RR025764).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Parashar UD, Bresee JS, Gentsch JR, Glass RI. Rotavirus. Emerg Infect Dis. 1998;4:561–570. doi: 10.3201/eid0404.980406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles MD, Holman RC, Curns AT, Parashar UD, Glass RI, Bresee JS. Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993-2002. Pediatr Infect Dis J. 2006;25:489–493. doi: 10.1097/01.inf.0000215234.91997.21. [DOI] [PubMed] [Google Scholar]
- 5.Payne DC, Staat MA, Edwards KM, et al. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics. 2008;122:1235–1243. doi: 10.1542/peds.2007-3378. [DOI] [PubMed] [Google Scholar]
- 6.Glass RI, Kilgore PE, Holman RC, et al. The epidemiology of rotavirus diarrhea in the United States: surveillance and estimates of disease burden. J Infect Dis. 1996;174(Suppl 1):S5–S11. doi: 10.1093/infdis/174.supplement_1.s5. [DOI] [PubMed] [Google Scholar]
- 7.Widdowson MA, Meltzer MI, Zhang X, Bresee JS, Parashar UD, Glass RI. Cost-effectiveness and potential impact of rotavirus vaccination in the United States. Pediatrics. 2007;119:684–697. doi: 10.1542/peds.2006-2876. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 9.Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370:1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 10.Vesikari T, Itzler R, Matson DO, et al. Efficacy of a pentavalent rotavirus vaccine in reducing rotavirus-associated health care utilization across three regions (11 countries) Int J Infect Dis. 2007;11(Suppl 2):S29–S35. doi: 10.1016/S1201-9712(07)60019-8. [DOI] [PubMed] [Google Scholar]
- 11.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 12.Prevention of rotavirus disease: updated guidelines for use of rotavirus vaccine. Pediatrics. 2009;123:1412–1420. doi: 10.1542/peds.2009-0466. [DOI] [PubMed] [Google Scholar]
- 13.Tate JE, Panozzo CA, Payne DC, et al. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics. 2009;124:465–471. doi: 10.1542/peds.2008-3528. [DOI] [PubMed] [Google Scholar]
- 14.Rotavirus vaccination coverage and adherence to the Advisory Committee on Immunization Practices (ACIP)-recommended vaccination schedule–United States, February 2006-May 2007. MMWR Morb Mortal Wkly Rep. 2008;57:398–401. [PubMed] [Google Scholar]
- 15.Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics. 2010;125:e208–e213. doi: 10.1542/peds.2009-1246. [DOI] [PubMed] [Google Scholar]
- 16.Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010;125:e199–e207. doi: 10.1542/peds.2009-1021. [DOI] [PubMed] [Google Scholar]
- 17.Staat MA, Payne DC, Donauer S, et al. Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics. 2011;128:e267–e275. doi: 10.1542/peds.2010-3722. [DOI] [PubMed] [Google Scholar]
- 18.Cortes JE, Curns AT, Tate JE, et al. Rotavirus vaccine and health care utilization for diarrhea in US children. N Engl J Med. 2011;365:1108–1117. doi: 10.1056/NEJMoa1000446. [DOI] [PubMed] [Google Scholar]
- 19.Feudtner C, Silveira MJ, Christakis DA. Where do children with complex chronic conditions die? Patterns in Washington State, 1980-1998. Pediatrics. 2002;109:656–660. doi: 10.1542/peds.109.4.656. [DOI] [PubMed] [Google Scholar]
- 20.Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277:301–306. [PubMed] [Google Scholar]
- 21.Harbarth S, Burke JP, Lloyd JF, Evans RS, Pestotnik SL, Samore MH. Clinical and economic outcomes of conventional amphotericin B-associated nephrotoxicity. Clin Infect Dis. 2002;35:e120–e127. doi: 10.1086/344468. [DOI] [PubMed] [Google Scholar]
- 22.Evans RS, Classen DC, Stevens LE, et al. Using a hospital information system to assess the effects of adverse drug events. In: Safran C, editor. New York, NY: McGraw-Hill; 1993. pp. 161–165. 17th Annual Symposium on Computer Applications in Medical Care; 1993. [PMC free article] [PubMed] [Google Scholar]
- 23.Classen DC. Salt Lake City, UT: University of Utah; 1993. Assessing the impact of adverse hospital events on the cost of hospitalizations and other patient outcomes [Thesis] [Google Scholar]
- 24.US Centers for Disease Control and Prevention. National Immunization Survey Q-QRN. Available at: http://www.cdc.gov/vaccines/stats-surv/nis/tables/09/tab02_antigen_iap.xls . Accessed December 10, 2011. [Google Scholar]
- 25.Utah Department of Health. Indicator-Based Information System for Public Health (IBIS-PH). Available at: http://ibis.health.utah.gov/home . Accessed November 6, 2011. [Google Scholar]
- 26.Utah Statewide Immunization Information System. Coverage Assessments USIIS. 2011 [Google Scholar]
- 27.Bilcke J, Beutels P. Reviewing the cost effectiveness of rotavirus vaccination: the importance of uncertainty in the choice of data sources. Pharmacoeconomics. 2009;27:281–297. doi: 10.2165/00019053-200927040-00002. [DOI] [PubMed] [Google Scholar]
- 28.Gurgel RG, Bohland AK, Vieira SC, et al. Incidence of rotavirus and all-cause diarrhea in northeast Brazil following the introduction of a national vaccination program. Gastroenterology. 2009;137:1970–1975. doi: 10.1053/j.gastro.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 29.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 30.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 2010;362:299–305. doi: 10.1056/NEJMoa0905211. [DOI] [PubMed] [Google Scholar]
- 31.Anderson EJ, Rupp A, Shulman ST, Wang D, Zheng X, Noskin GA. Impact of rotavirus vaccination on hospital-acquired rotavirus gastroenteritis in children. Pediatrics. 2011;127:e264–e270 doi: 10.1542/peds.2010-1830. [DOI] [PubMed] [Google Scholar]
- 32.Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis. 2010;201:1617–1624. doi: 10.1086/652403. [DOI] [PubMed] [Google Scholar]
- 33.Begue RE, Perrin K. Reduction in gastroenteritis with the use of pentavalent rotavirus vaccine in a primary practice. Pediatrics. 2010;126:e40–e45. doi: 10.1542/peds.2009-2069. [DOI] [PubMed] [Google Scholar]
- 34.Cortese MM, Tate JE, Simonsen L, Edelman L, Parashar UD. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr Infect Dis J. 2010;29:489–494. doi: 10.1097/INF.0b013e3181d95b53. [DOI] [PubMed] [Google Scholar]
- 35.Clark HF, Lawley D, Matthijnssens J, DiNubile MJ, Hodinka RL. Sustained decline in cases of rotavirus gastroenteritis presenting to the Children's Hospital of Philadelphia in the new rotavirus vaccine era. Pediatr Infect Dis J. 2010;29:699–702. doi: 10.1097/INF.0b013e3181d73524. [DOI] [PubMed] [Google Scholar]
- 36.US Centers for Disease Control and Prevention. National Immunization Survey Q-QRN. Available at: http://www.cdc.gov/vaccines/stats-surv/nis/tables/09/tab02_antigen_iap.xls . Accessed December 10, 2011. [Google Scholar]
- 37.Clark HF, Lawley D, Matthijnssens J, DiNubile MJ, Hodinka RL. Sustained decline in cases of rotavirus gastroenteritis presenting to the Children's Hospital of Philadelphia in the new rotavirus vaccine era. Pediatr Infect Dis J. 2010;29:699–702. doi: 10.1097/INF.0b013e3181d73524. [DOI] [PubMed] [Google Scholar]
- 38.Lopman BA, Curns AT, Yen C, Parashar UD. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis. 2011;204:980–986. doi: 10.1093/infdis/jir492. [DOI] [PubMed] [Google Scholar]
- 39.Yen C, Tate JE, Wenk JD, Harris JM, 2nd, Parashar UD. Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics. 2011;127:e9–e15. doi: 10.1542/peds.2010-1393. [DOI] [PubMed] [Google Scholar]
- 40.Shim E, Galvani AP. Impact of transmission dynamics on the cost-effectiveness of rotavirus vaccination. Vaccine. 2009;27:4025–4030. doi: 10.1016/j.vaccine.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Mast TC, Walter EB, Bulotsky M, et al. Burden of childhood rotavirus disease on health systems in the United States. Pediatr Infect Dis J. 2010;29:e19–e25 doi: 10.1097/inf.0b013e3181ca7e2e. [DOI] [PubMed] [Google Scholar]
- 42.Malek MA, Curns AT, Holman RC, et al. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics. 2006;117:1887–1892. doi: 10.1542/peds.2005-2351. [DOI] [PubMed] [Google Scholar]
- 43.Hsu VP, Staat MA, Roberts N, et al. Use of active surveillance to validate international classification of diseases code estimates of rotavirus hospitalizations in children. Pediatrics. 2005;115:78–82. doi: 10.1542/peds.2004-0860. [DOI] [PubMed] [Google Scholar]
- 44.Cortes JE, Curns AT, Tate JE, Parashar UD. Trends in healthcare utilization for diarrhea and rotavirus disease in privately insured US children <5 years of age, 2001-2006. Pediatr Infect Dis J. 2009;28:874–878. doi: 10.1097/INF.0b013e3181a653cd. [DOI] [PubMed] [Google Scholar]
- 45.Chang HG, Smith PF, Tserenpuntsag B, Markey K, Parashar U, Morse DL. Reduction in hospitalizations for diarrhea and rotavirus infections in New York state following introduction of rotavirus vaccine. Vaccine. 2010;28:754–758. doi: 10.1016/j.vaccine.2009.10.075. [DOI] [PubMed] [Google Scholar]