Abstract
Retinoic acid (RA) is required for the successful differentiation and meiotic entry of germ cells in the murine testis. The availability of RA to undifferentiated germ cells begins in a variable, uneven pattern during the first few days after birth and establishes the asynchronous pattern of germ cell differentiation in adulthood. It has been shown that synchronous spermatogenesis can be induced in 2 d postpartum mice, but not in adult mice, by treating vitamin A sufficient males with RA. In this study, neonatal males were treated at different ages with a single dose of RA and spermatogenesis was examined after recovery to adulthood. The failure of exogenous RA to alter asynchrony correlates with the appearance of meiotic preleptotene spermatocytes within the seminiferous epithelium.
Keywords: retinoic acid, synchronous spermatogenesis, asynchronous spermatogenesis, spermatogonia, differentiation, spermatogenic wave, preleptotene spermatocytes
In mice, the cycle of the seminiferous epithelium results in constant sperm production. The cycle initiates with the differentiation of type A undifferentiated spermatogonia into differentiating type A1 spermatogonia. Once triggered to differentiate, it takes approximately 35 d for undifferentiated mouse spermatogonia to mature into spermatozoa.1 The net result is the formation of recurring sets of associated germ cells at different points within the same developmental pathway. These recurring cellular associations are designated as stages that can be visualized when the testis is examined in cross section. There are 12 different stages that have been identified in the adult mouse testis, with the transition of undifferentiated, aligned type A (Aal) spermatogonia to A1 differentiating spermatogonia taking place in Stages VII and VIII.2 Normally, the entry of germ cells into the cycle of the seminiferous epithelium occurs progressively along the longitudinal axis of murine seminiferous tubules, resulting in the asynchronous and continuous production of sperm and the resulting pattern of sperm production can be described as a wave. This mechanism ensures that males constantly produce viable sperm for maximal reproductive success.
There is a growing body of evidence to suggest that the cycle of the seminiferous epithelium is triggered by the active metabolite of vitamin A, retinoic acid (RA). It has been known for many years that RA is required for successful spermatogenesis,3 and RA is also able to act as a potent signaling molecule in many other stem/progenitor cell populations in mammals.4 Animals that are vitamin A-deficient (VAD) are infertile and contain testes completely devoid of successful spermatogenesis that accumulate undifferentiated Aal spermatogonia within the seminiferous epithelium while failing to produce advanced germ cells.5 The apparent VAD-induced block in subsequent germ cell maturation occurs in spermatogonia at the Aal to A1 transition.6,7 Multiple studies have shown that spermatogenesis within a VAD animal can be reinitiated by the injection of exogenous and dietary replenishment of retinoids.8-10 However, rather than sperm production being rescued in a normal manner, the asynchronous spermatogenic wave is lost and germ cell differentiation is initiated and maintained synchronously, resulting in the pulsatile release of sperm to the epididymis occurring only once every 8.6 d.8 The mechanism for this synchronous re-initiation of spermatogenesis in VAD animals appears to be the simultaneous differentiation of most of the accumulated undifferentiated Aal spermatogonia present within the VAD testis.
Until recently, the VAD-rescue paradigm was the only model that enabled the study of synchronized spermatogenesis. This technique allows the study of stage-synchronized spermatogenesis within the adult mouse gonad, and hence, has enhanced our ability to understand the specific molecular events occurring at each different stage of mammalian spermatogenesis. Unfortunately, the production of VAD male mice is costly, time consuming and most animals display significant ill effects, such as muscle tremors, weight loss, lethargy, immunodeficiency, vision impairment, and in some cases, lethality in response to the VAD diet. A recent study demonstrated that treating vitamin A sufficient (VAS) neonatal mice at 2 d postpartum (dpp) with RA also resulted in synchronous spermatogenesis in the adult animal.9 However, treatment of VAS adult males with RA did not result in the synchronization of the seminiferous epithelium. These results suggested that the testis of an adult mouse but not the neonatal mouse is able to prevent inappropriate RA-induced differentiation of spermatogonia. The goal of the current study was to determine at what age this control of differentiation becomes effective within the developing testis.
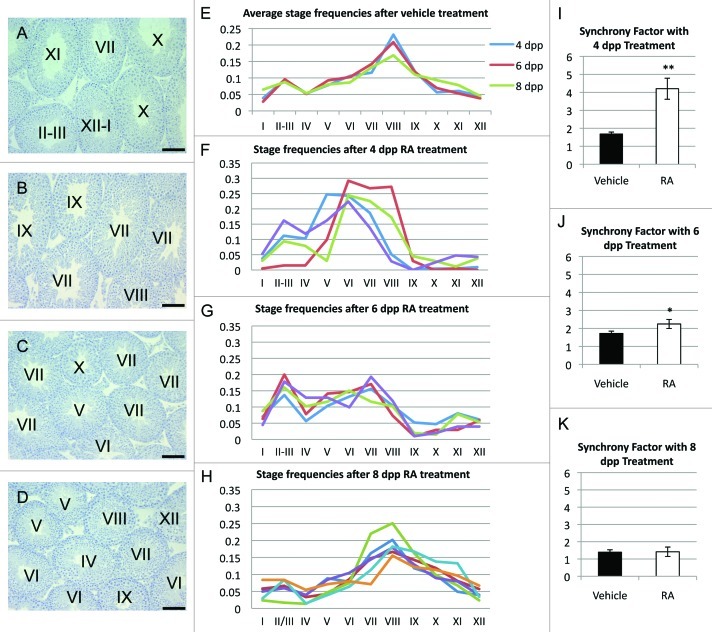
To determine the age at which RA treatment no longer results in synchronous spermatogenesis, murine male pups at 4, 6 and 8 dpp were treated with a single injection of RA and then maintained until they were 65 d of age (Fig. 1). At least 200 tubules from each treated animal were staged and are shown as a fraction of the total stages represented in each sample. In addition, the average synchrony factors were calculated for each sample.10 The synchrony factor is expressed as a fraction of the cycle of the seminiferous epithelium, and is not influenced by the differing duration of the stages of this cycle.
Figure 1. Degrees of synchrony resulting from RA treatment of mice at different neonatal ages. (A–D) Representative cross sections of an adult male testis after vehicle treatment (A), RA treatment at 4 dpp (B), 6 dpp (C) and 8 dpp (D) followed by recovery into adulthood. (E–H) Graphical representation of all 12 stages in cross sections of the seminiferous epithelium after vehicle treatment (E), RA treatment at 4 (F), 6 (G), and 8 (H) dpp and recovery to 65 dpp. (I–K) Resulting synchrony factors with treatment of vehicle or RA at 4 (I), 6 (J), and 8 (K) dpp. * p < 0.05. ** p < 0.005. All error bars represent standard error of the mean. Scale bars represent 100 µm.
The stage frequency profile for the 8 dpp samples were similar to the profile seen for the vehicle controls (Fig. 1E and H) vehicle and 8 dpp samples contained tubule cross sections representing each different stage of the cycle of the seminiferous epithelium and displayed a synchrony factor between 1.4 and 1.8 (Fig. 1K), characteristic of normal asynchronous spermatogenesis in this strain of mice. In contrast, animals treated at 4 dpp exhibited testes with significantly altered stage frequencies (Fig. 1F) and synchrony factors greater than 4 (Fig. 1L), representative of synchronized spermatogenesis. The extent of synchronization in the 4 dpp treated group did not differ significantly from the previously reported data on synchronization in mice treated at 2 dpp.9 Animals treated at 6 dpp showed a perturbation in stage frequencies and synchrony factor when compared with vehicle controls, with approximately double the number of stages represented within Stages II-VI and a significantly lower representation of stages IX-I. However, in agreement with the calculated synchrony factor of less than 2.5, all stages were present in a single testis cross section.
In order to correlate the age at which RA is no longer able to induce synchronous spermatogenesis in VAS neonatal males with the appearance of an advanced germ cell population, detection of meiotic cell types within the seminiferous epithelium was conducted using immunohistochemical detection of nuclear SYCP3. SYCP3 begins to localize within the nucleus upon entry into prophase I of meiosis in preleptotene/leptotene spermatocytes.11 Immunohistochemistry was performed on 4, 6 and 8 dpp testis cross sections of wild-type, non RA-treated testes. At 4 dpp, very few tubule cross-sections contained detectable SYCP3 protein. At 6 dpp, nuclear SYCP3 could be detected in germ cells in approximately half of the tubule cross sections tested, and at 8 dpp, over 80% of seminiferous tubule cross-sections contained nuclear SYCP3-immunopositive cells.
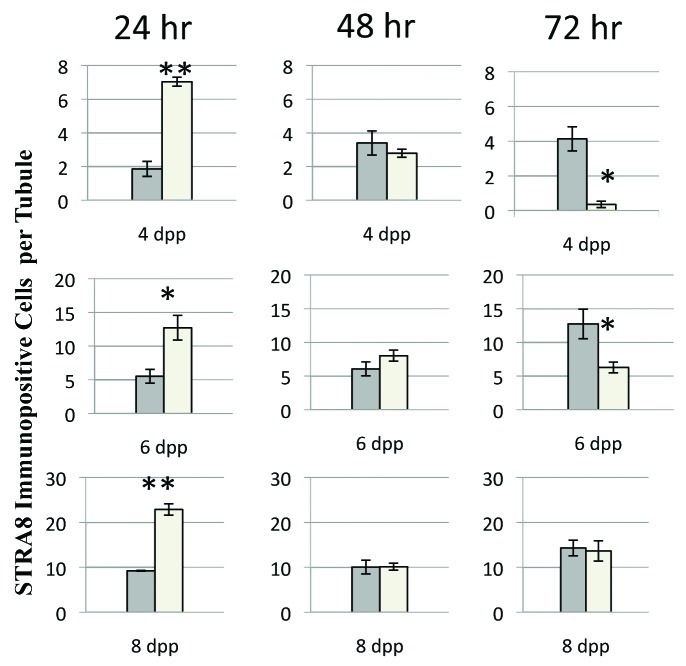
The short-term effects of exogenous RA on germ cell development in this system was examined in testes from mice injected with RA at 4, 6 and 8 dpp and collected 24, 48 and 72 h post-treatment. Stimulated by retinoic acid gene 8 (Stra8) protein is a very sensitive and reliable marker for the short-term action of RA on germ cells and is required for the transition of Aal spermatogonia into A1 spermatogonia.12 At 24 h post-treatment, immunohistochemical detection of STRA8 revealed a significantly higher number of immunopositive cells per tubule in samples treated with RA when compared with vehicle-treated sections in each age group (Fig. 2). By 48 h after treatment there was very little difference in the number of cells positive for STRA8 when RA-treated samples were compared with vehicle controls for each age group. In contrast, by 72 h after treatment, the STRA8 immunopositive cells had nearly disappeared from the testes of the 4 dpp animal samples, were decreased by more than 50% in the testes of the 6 dpp samples and were unchanged in the testes of the 8 dpp samples. This relative number of STRA8 immunopositive cells at each age and time-point correlated with similar changes in the expression of Stra8 transcript, as detected via real time RT-PCR (data not shown).
Figure 2. Quantification of STRA8-immunopositive cells per tubule 24, 48 and 72 h post-RA injection at 4, 6 and 8 dpp, respectively. Filled bars represent data from vehicle treated animals and open bars represent data from RA treated animals. All error bars represent the standard error of the mean. * p < 0.05 ** p < 0.005
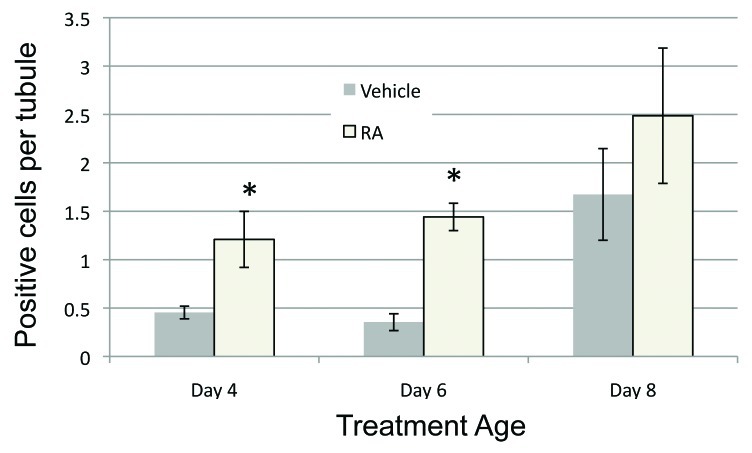
In our previous study we showed that when 2 dpp mice were treated with RA, there was a large increase in the numbers of apoptoic germ cells 48 h post-treatment.9 When testes of 4, 6 and 8 dpp RA-treated mice were analyzed for the presence of apoptotic cells by TUNEL 48 h post-treatment, we observed a similar increase in TUNEL-positive cells when compared with control for the 4 and 6 dpp samples. At 8 dpp, there was a general increase in apoptosis but the number of TUNEL-positive cells in the RA-treated samples was not significantly greater than those counted in the control samples (Fig. 3). Apoptosis in Sertoli cells was not observed at any of the ages examined.
Figure 3. RA induces cell apoptosis in the neonatal male testis. The average number of TUNEL-positive cells per tubule after vehicle or RA treatment at each treatment age is shown. Filled bars represent data from vehicle treated animals and open bars represent data from RA treated animals. All error bars represent the standard error of the mean. *p < 0.05.
The results of our previous two studies and many other investigations of VAD rats and mice suggest that there are two distinct modes of regulating the spermatogenic wave in rodents. Initially, shortly after birth the periodic appearance of RA-responsive germ cells indicates that either gonocytes or undifferentiated A spermatogonia residing in distinct patches along the tubule progressively transition into A1, STRA8-positive differentiating spermatogonia.12 After 1 cycle (8.6 d in the mouse) the appearance of the preleptotene spermatocytes may reinforce the periodic nature of RA availability and as a result, the wave cannot be altered by exogenous RA in VAS animals. In the absence of advanced germ cells, including most preleptotene spermatocytes, such is the case with neonates or VAD rodents, exogenous retinol or RA re-establishes the cycle in a uniform manner along the tubule but eliminates the spermatogenic wave. Nothing is known about the mechanism by which the presence of advanced germ cells alters the RA response. The mechanism could involve the direct metabolism of RA by germ cells or the presence of advanced germ cells could indirectly affect metabolic or signaling functions in Sertoli cells or it could be a result of Sertoli cell maturation.
In our previous study using 2 dpp mice we demonstrated that exogenous RA resulted in the induction of Stra8 transcript and protein and other differentiation markers in nearly all germ cells by 24 h post-treatment, followed by a dramatic reduction in Stra8 levels and increased apoptosis.9 The synchronous spermatogenesis that resulted from this treatment was delayed from the normal developmental timing by several days and suggested that the re-initiation of spermatogenesis occurred from populations of surviving undifferentiated spermatogonia. Similarly, in this study the STRA8-positive cell number increased for all three age groups 24 h post RA-treatment. These results are consistent with the model we previously presented showing two different pathways to synchronous spermatogenesis.12 In the RA-treated neonate, synchrony occurs because exogenous RA overwhelms the periodic RA availability, many cells undergo apoptosis and recovery of spermatogenesis occurs from undifferentiated spermatogonia. In the VAD model, synchrony results because loss of advanced germ cells eliminates periodic RA availability and germ cells primed to undergo the Aal to A1 transition accumulate in all tubules. RA then releases the block at this transition point and spermatogenesis resumes synchronously without delay in all tubules.
While RA does appear to be sufficient to induce spermatogonial differentiation at 4 dpp and earlier, RA-induced expression of STRA8 does not irreversibly cause a germ cell to undergo differentiation. Immunohistochemical detection of STRA8 revealed that nearly every germ cell within the seminiferous epithelium had detectable amounts of the protein after RA treatment at each age. While increased apoptosis was detectable after RA treatment at both 4 and 6 dpp, the number of apoptotic germ cells does not account for the loss of STRA8-immunopositive cells within the seminiferous epithelium 48 h after injection. It appears that some cells positive for STRA8 must survive but revert to STRA8-negative cells. It is unclear whether such a phenomenon may occur in vivo without significant perturbation of the endogenous RA-signaling cascade in germ cells.
Recently several studies have suggested that RA metabolism is the driving force in establishing the timing of germ cell development and thus the cycling of the seminiferous epithelium. RA metabolism may very well be the clock that determines that every 8.6 d a germ cell undergoes the transition from undifferentiated Aal spermatogonia to differentiating A1 spermatogonia, and after 35 d, eventually into a mature spermatozoon in a precisely recurrent manner. It has been suggested that advanced germ cells, such as round spermatids, are actively involved in the regulation of RA metabolism.13 Our data suggest that there are at least two major control points regulating RA metabolism. According to our previous publication, the initial appearance of patchy areas of RA-positive cells within the developing seminiferous tubules occurs shortly after birth and prior to the appearance of advanced germ cells.12 The second control point then correlates with the appearance of preleptotene spermatocytes, as indicated by this current study. The idea that preleptotene spermatocytes play a role in the availability of RA within the tubule is appealing. The transition of Aal undifferentiated spermatogonia into A1 differentiating spermatogonia could be considered the entry into the cycle of the seminiferous epithelium. The appearance of the preleptotene spermatocytes 8.6 d later could then trigger the reinitation of the Aal to A1 transition and begin the cyclic nature of spermatogenesis.
Materials and Methods
Animals and tissues
All animal experiments were approved by the Washington State University Animal Care and Use Committees and were conducted in accordance with the guiding principles for the care and use of research animals of the National Institutes of Health. A BL/6–129 mouse colony was maintained in a temperature- and humidity-controlled environment with food and water provided ad libitum and were used in these studies. The animals were euthanized by CO2 asphyxiation followed by either decapitation (0 dpp-10 dpp) or cervical dissociation (10 dpp-adult) and their testes collected. Tissues for immunohistochemistry were placed in Bouin’s fixative for 2–5 h (age dependent) or 4% paraformaldehyde for 4 h immediately after collection, then dehydrated through a graded ethanol series and embedded in paraffin. Sections of 4 μm were placed on Superfrost® Plus slides (Menzel-Glaser). Sections from specimens that required more than one plane of examination were separated by at least 50µm. Testis samples for RNA preparation were snap frozen on dry ice immediately after collection and then stored at -80°C until use.
RA treatments
Mice were injected at 4, 6, and 8 dpp with all-trans RA (Sigma-Aldrich) suspended in dimethyl sulfoxide (DMSO) (Sigma-Aldrich). Vehicle control animals were injected with DMSO alone. Animals aged at 4 dpp received 100 µg of all trans-RA, suspended in 10 μl DMSO, via a subcutaneous injection, whereas 6 and 8 dpp male mice received 150 μg of all trans-RA, re-suspended in 10 μl DMSO, via an intraperitoneal injection. For morphological analyses, both RA-treated and vehicle control neonates were allowed to mature to adulthood (65 dpp) to ensure that complete spermatogenesis had been achieved before tissue collection and analysis of staging occurred. For some immunocytochemistry and real time PCR experiments, treated and vehicle control mice were allowed to recover for 24, 48 and 72 h post-treatment before their testes were collected (n = 3 for each time point).
Stage determination
Cross-sections of adult male testes treated with either RA or vehicle were analyzed for the distribution of all 12 stages of the spermatogenic cycle in at least 200 tubules for each sample. The staging analysis was performed on 4 different samples for the 4 and 6 dpp timepoints and on 6 different samples for the 8 dpp timepoint. . By counting the number of stages represented, the degree of spermatogenic “synchrony” can be interpreted by comparison to previously published stage frequencies in several inbred strains of mice. The degree of synchrony can be quantified through the calculation of a synchrony factor, utilizing these frequency values.14
Immunohistochemistry
Immunohistochemical staining was performed following our previously published protocol.12 All incubations were performed at room temperature. Tissue sections were rehydrated using xylene and a graded series of ethanol to de-ionized water. Antigen retrieval was performed in boiling 0.01M sodium citrate solution and followed by an incubation in 3% hydrogen peroxide (Aventor Performance) to quench endogenous peroxidases. The testis sections were then blocked in 10% goat serum and 0.01% bovine serum albumin, diluted in phosphate buffered saline (PBS), followed by overnight incubation with antibodies raised against either synaptonemal complex protein 3 (SYCP3) (Santa Cruz) or STRA8 (produced in-house to recombinant full length protein) diluted in the blocking solution at 1:500 and 1:1000, respectively. The sections were then washed in PBS and incubated with biotinylated secondary goat anti-rabbit (Vector Labs) at a 1:500 dilution. To visualize the secondary antibodies, sections were incubated with streptavidin-conjugated horseradish peroxidase enzyme (Invitrogen) followed by diaminobenzoate substrate (Invitrogen) and quenched in water. Sections were counter-stained using Harris Hematoxylin stain (Sigma-Aldrich) before dehydration and mounting under coverslips in DPX mounting medium (VWR International).
Detection of apoptosis
Apoptotic detection was performed using the DEADEND TUNEL kit (Promega) as per the manufacturer’s instructions. Sections were counter-stained with DAPI chromatin labeling reagent in Vecta-Shield mounting medium (Vector) and visualized using a Nikon Microphot-FX microscope (Meridian Instrument Co. Inc.). All TUNEL stains were conducted at least twice with consistent results.
Acknowledgments
This research was supported by a Contraceptive Center Grant U54 42454 and by HD 10808 from the NIH to Michael Griswold
Glossary
Abbreviations:
- dpp
days postpartum
- DMSO
dimethyl sulfoxide
- Kit
kit oncogene
- PBS
phosphate-buffered saline
- RA
retinoic acid
- SYCP3
synaptonemal complex protein 3
- Stra8
stimulated by retinoic acid gene 8
- VAD
vitamin A deficient
- VAS
vitamin A sufficient
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/23180
References
- 1.Oakberg EF. A description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat. 1956;99:391–413. doi: 10.1002/aja.1000990303. [DOI] [PubMed] [Google Scholar]
- 2.Russell L. Histologial and histopathological evaluation of the testis. Clearwater, FL: Cache River Press; 1990. [Google Scholar]
- 3.Griswold MD, Hogarth CA, Bowles J, Koopman P. Initiating meiosis: the case for retinoic acid. Biol Reprod. 2012;86:35. doi: 10.1095/biolreprod.111.096610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yousefi B, Azizzadeh F. The histopathalogical effects of retinoic acid on the tissues. Pak J Biol Sci. 2010;13:927–36. doi: 10.3923/pjbs.2010.927.936. [DOI] [PubMed] [Google Scholar]
- 5.McCARTHY PTC, Cerecedo LR. Vitamin A deficiency in the mouse. J Nutr. 1952;46:361–76. doi: 10.1093/jn/46.3.361. [DOI] [PubMed] [Google Scholar]
- 6.Griswold MD, Bishop PD, Kim KH, Ping R, Siiteri JE, Morales C. Function of vitamin A in normal and synchronized seminiferous tubules. Ann N Y Acad Sci. 1989;564:154–72. doi: 10.1111/j.1749-6632.1989.tb25895.x. [DOI] [PubMed] [Google Scholar]
- 7.Van Pelt AM, De Rooij DG. The origin of the synchronization of the seminiferous epithelium in vitamin A-deficient rats after vitamin A replacement. Biol Reprod. 1990;42:677–82. doi: 10.1095/biolreprod42.4.677. [DOI] [PubMed] [Google Scholar]
- 8.Morales CG, Griswold MD. Retinol-induced stage synchronization in seminiferous tubules of the rat. Endocrinology. 1987;121:432–4. doi: 10.1210/endo-121-1-432. [DOI] [PubMed] [Google Scholar]
- 9.Snyder EM, Davis JC, Zhou Q, Evanoff R, Griswold MD. Exposure to retinoic acid in the neonatal but not adult mouse results in synchronous spermatogenesis. Biol Reprod. 2011;84:886–93. doi: 10.1095/biolreprod.110.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Beek ME, Meistrich ML. A method for quantifying synchrony in testes of rats treated with vitamin A deprivation and readministration. Biol Reprod. 1990;42:424–31. doi: 10.1095/biolreprod42.3.424. [DOI] [PubMed] [Google Scholar]
- 11.Yuan L, Liu JG, Zhao J, Brundell E, Daneholt B, Höög C. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol Cell. 2000;5:73–83. doi: 10.1016/S1097-2765(00)80404-9. [DOI] [PubMed] [Google Scholar]
- 12.Snyder EM, Small C, Griswold MD. Retinoic acid availability drives the asynchronous initiation of spermatogonial differentiation in the mouse. Biol Reprod. 2010;83:783–90. doi: 10.1095/biolreprod.110.085811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugimoto R, Nabeshima Y, Yoshida S. Retinoic acid metabolism links the periodical differentiation of germ cells with the cycle of Sertoli cells in mouse seminiferous epithelium. Mech Dev. 2012;128:610–24. doi: 10.1016/j.mod.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Siiteri JE, Karl AF, Linder CC, Griswold MD. Testicular synchrony: evaluation and analysis of different protocols. Biol Reprod. 1992;46:284–9. doi: 10.1095/biolreprod46.2.284. [DOI] [PubMed] [Google Scholar]