Abstract
Resistance of prostate cancer cells to the next-generation antiandrogen enzalutamide may be mediated by a multitude of survival signaling pathways. In this study, we tested whether increased expression of NF-κB2/p52 induces prostate cancer cell resistance to enzalutamide and whether this response is mediated by aberrant androgen receptor (AR) activation and AR splice variant production. LNCaP cells stably expressing NF-κB2/p52 exhibited higher survival rates than controls when treated with enzalutamide. C4-2B and CWR22Rv1 cells chronically treated with enzalutamide were found to express higher levels of NF-κB2/p52. Downregulation of NF-κB2/p52 in CWR22Rv1 cells chronically treated with enzalutamide rendered them more sensitive to cell growth inhibition by enzalutamide. Analysis of the expression levels of AR splice variants by quantitative reverse transcription PCR and Western blotting revealed that LNCaP cells expressing p52 exhibit higher expression of AR splice variants. Downregulation of expression of NF-κB2/p52 in VCaP and CWR22Rv1 cells by short hairpin RNA abolished expression of splice variants. Downregulation of expression of either full-length AR or the splice variant AR-V7 led to an increase in sensitivity of prostate cancer cells to enzalutamide. These results collectively demonstrate that resistance to enzalutamide may be mediated by NF-κB2/p52 via activation of AR and its splice variants.
Introduction
Localized prostate cancer is dependent on androgens, and the majority of patients respond to androgen ablation. However, virtually every patient will develop castration-resistant prostate cancer (CRPC) and no longer respond to androgen deprivation therapy (ADT). Persistent androgen receptor (AR) activation remains an important player in CRPC progression. CRPC cells often continue to express AR and AR axis genes (1, 2), implying that the AR is active in AR-positive CRPC cells. Such observations form the basis for continued attempts to target the AR axis and for the development of next-generation antiandrogens such as enzalutamide (formerly MDV3100). Enzalutamide binds to the AR with greater affinity than bicalutamide and inhibits its nuclear translocation and expression of its target genes (3). Despite initial success, development of resistance is a contraindication for its use in many patients, and as demographics change, an increasing number of patients are likely to develop resistance to enzalutamide. The mechanisms leading to resistance have been poorly understood, even though a recent report showed that AR splice variants play a major role in development of resistance (4). AR splice variants lack the ligand-binding domain targeted by enzalutamide and variants such as AR-V7 are postulated to be constitutively active. The mechanistic aspects of regulation of variant expression leading to resistance against enzalutamide are unknown. Therefore, an urgent need exists to fully understand the mechanisms of resistance and to devise ways to overcome them.
The classical NF-κB pathway involving the p65/p50 heterodimer has been shown to be constitutively activated in several cancers including prostate cancer (5). The non-canonical NF-κB pathway involves the processing of p100 to NF-κB2/p52 via the recruitment of NF-κB–inducing kinase (NIK) and subsequent activation of IκB kinase α (IKKα). The processing of p100 to p52 is a tightly controlled event in many cells and tissues (6–9). The functional significance of p100 processing has been confirmed by genetic evidence from humans and mice (10). Overproduction of p52 has been observed in several solid tumors including breast and prostate cancers (11, 12). Our previous studies showed that NF-κB2/p52 induces castration-resistant growth in LNCaP cells (13), that several genes involved in processes such as cell growth, proliferation, cell movement are potential targets of NF-κB2/p52 (14), and that NF-κB2/p52 induces aberrant activation of the AR in a ligand-independent manner and thus promotes castration resistance (15).
In this study, we report that NF-κB2/p52 promotes resistance of prostate cancer cells to enzalutamide. We show that increased resistance of prostate cancer cells expressing p52 to enzalutamide may be mediated by induction of AR splice variants (such as AR-V7) and by activation of the AR axis by p52.
Materials and Methods
Cell lines and reagents
LNCaP, CWR22Rv1, and VCaP cells were obtained from the American Type Culture Collection (ATCC). All experiments with cell lines were performed within 6 months of receipt from ATCC or resuscitation after cryopreservation. ATCC uses short tandem repeat (STR) profiling for testing and authentication of cell lines. C4-2B cells were kindly provided and authenticated by Dr. Leland Chung, Cedars-Sinai Medical Center, Los Angeles, CA. Cells were cultured in RPMI containing either 10% complete FBS or 10% charcoal/dextran-stripped FBS (CS-FBS) and penicillin/streptomycin. LNCaP passage numbers less than 20 were used throughout the study. VCaP cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS. NF-κB2/p52 (K-27), AR (441; mouse monoclonal), hemagglutinin (HA), and tubulin antibodies were purchased from Santa Cruz Biotechnologies. Antibodies against AR-V7 splice variant were kindly provided by Dr. Jun Luo (Department of Urology, Johns Hopkins University, Baltimore, MD). All other reagents were of analytical grade and obtained from local suppliers. Sso Fast Eva Green qPCR Supermix was from Bio-Rad.
Generation of stable cell lines
Stable cell lines of LNCaP expressing NF-κB2/p52 (LN-p52) were generated by transfection of plasmids containing the cDNA and selection of clones after application of selective pressure with appropriate antibiotics. LNCaP cells expressing p52 under the control of a tetracycline-inducible cassette (LN/TR/p52) were generated using the ViraPower lentiviral transduction system (Invitrogen).
Cell growth assays
Cells were transfected with plasmids or treated with the indicated reagents, and viable cell numbers were determined at various time points using a Coulter cell counter.
Western blot analysis
Cells were lysed in high-salt buffer containing 50 mmol/L HEPES, pH 7.9, 250 mmol/L NaCl, 1 mmol/L EDTA, 1% NP-40, 1 mmol/L phenylmethylsulfonylfluoride (PMSF), 1 mmol/L Na vanadate, 1 mmol/L NaF, and protease inhibitor cocktail (Roche) as described earlier (16). Total protein was estimated using the Coomassie Protein Assay Reagent (Pierce). Equal amounts of protein were loaded on 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk in PBST (1× PBS + 0.1% Tween-20) and probed with primary antibodies in 1% bovine serum albumin (BSA). The signal was detected by ECL (GE Healthcare) after incubation with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies.
Real-time quantitative reverse transcription PCR
Total RNAs were extracted using TRIzol reagent (Invitrogen). cDNAs were prepared after digestion with RNase-free RQ1 DNase (Promega). The cDNAs were subjected to real-time reverse transcription PCR (RT-PCR) using Sso Fast Eva Green Supermix (Bio-Rad) according to the manufacturer’s instructions and as described previously (15). Each reaction was normalized by coamplification of actin. Triplicates of samples were run on default settings of Bio-Rad CFX-96 real-time cycler.
Clonogenic assays
Anchorage-dependent clonogenic ability assays were performed as described previously (13). Briefly, cells were seeded at low densities (400 cells/dish) in 10-cm culture plates. The plates were incubated at 37°C in media containing either 10% FBS or 10% charcoal-stripped FBS (CS-FBS) and were left undisturbed for 14 days. At the end of the experiment, cells were fixed with methanol, stained with crystal violet, and the numbers of colonies were counted.
Luciferase assays
Cells were transfected with reporters along with plasmids and AR and AR-V7 siRNAs as indicated in the figures. Cell lysates were subjected to luciferase assays with the Luciferase Assay System (Promega).
Statistical analyses
Data are shown as means ± SD. Multiple group comparison was performed by one-way ANOVA followed by the Scheffe procedure for comparison of means. P ≤ 0.05 was considered significant.
Results
Prostate cancer cells expressing NF-κB2/p52 are resistant to enzalutamide and bicalutamide
LN-neo (LNCaP cells expressing the empty vector) and LN-p52 cells (LNCaP cells stably expressing p52) were treated with 0 and 20 μmol/L enzalutamide or bicalutamide in media containing either complete FBS or charcoal-stripped FBS, and cell growth was examined after 48 hours. Dimethyl sulfoxide (DMSO) was used as the vehicle control. As shown in Fig. 1A, cells stably expressing p52 exhibited better cell survival ability when exposed to enzalutamide or bicalutamide compared to control LN-neo cells. To confirm these experiments, we treated LN-neo or LN-p52 cells with 0, 20, and 40 μmol/L enzalutamide or bicalutamide and performed clonogenic assays. As shown in Fig. 1B, LN-neo cells were highly sensitive to both enzalutamide and bicalutamide and formed fewer colonies, whereas the number of colonies formed by cells expressing p52 was significantly higher, indicating that NF-κB2/p52 may induce resistance to enzalutamide and bicalutamide in prostate cancer cells. To further confirm these results, we used the tetracycline-inducible system to induce p52 expression in LNCaP cells and tested enzalutamide and bicalutamide sensitivity. We treated LN/TR/Con and LN/TR/p52 cells with 0, 20, and 40 μmol/L enzalutamide or bicalutamide and performed growth assays. As shown in Fig. 1C, induction of expression of p52 by doxycycline significantly enhanced the ability of LN/TR/p52 cells to survive in the presence of enzalutamide or bicalutamide compared to control LN/TR/Con cells. These results collectively demonstrate that prostate cancer cells expressing higher levels of NF-κB2/p52 are more resistant to enzalutamide and bicalutamide compared to cells which do not express p52.
Figure 1.
NF-κB2/p52-expressing prostate cancer cells are resistant to enzalutamide. A, LNCaP cells stably expressing p52 (LN-p52) and control LNCaP cells (LN-neo) were treated with 0 and 20 μmol/L enzalutamide or bicalutamide in media containing either FBS or CS-FBS, and cell numbers were counted after 48 hours. Results are presented as mean ± SD of 3 experiments performed in triplicate. LN-p52 cells exhibited higher survival rates when treated with enzalutamide or bicalutamide than LN-neo cells. B, LN-neo and LN-p52 cells were treated with 0, 20, or 40 μmol/L enzalutamide or bicalutamide, and clonogenic assays were performed. Results are presented as mean ± SD of 2 experiments performed in triplicate. LN-p52 cells formed higher numbers of colonies than LN-neo cells when treated with enzalutamide or bicalutamide. C, LN/TR/p52 cells [expressing p52 under the control of a tetracycline (tet)-inducible promoter] and control LN/TR/Con cells were treated with 0, 20, or 40 μmol/L enzalutamide or 20 μmol/L bicalutamide in the presence or absence of 0.5 μmol/L doxycycline (DOX), and cell numbers were counted after 48 hours. Results are presented as mean ± SD of 3 experiments performed in triplicate. *, P ≤ 0.05. LN/TR/p52 cells displayed higher survival rates when p52 expression was induced with DOX, compared to uninduced LN/TR/p52 cells as well as LN/TR/Con cells.
Prostate cancer cells chronically treated with enzalutamide exhibit higher levels of NF-κB2/p52
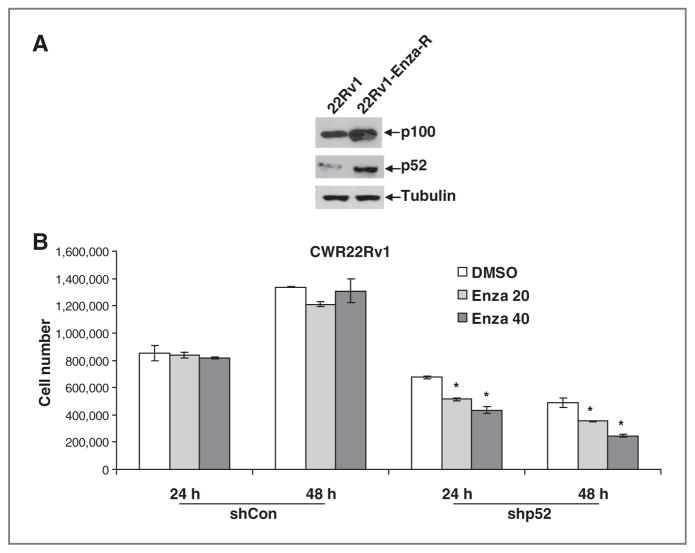
Our previous studies showed that most androgen-dependent prostate cancer cell lines do not express detectable levels of endogenous NF-κB2/p52 (13). Hence, to test whether prostate cancer cells resistant to enzalutamide exhibit higher levels of p52, we treated CWR22Rv1 cells with 5 to 10 μmol/L enzalutamide chronically for more than 10 months. The resultant cells showed higher cell survival rates when treated with enzalutamide. We examined the expression levels of NF-κB2/p52 in these cells by quantitative RT-PCR (qRT-PCR) and Western blotting. As shown in Fig. 2A, CWR22Rv1 cells treated chronically with enzalutamide exhibited higher levels of both precursor p100 as well as p52, indicating that prostate cancer cells resistant to enzalutamide may upregulate the endogenous levels of NF-κB2/p52. To test whether downregulation of p52 resensitizes these cells to enzalutamide, we transfected short hairpin RNAs (shRNA) specific to p52 into CWR22Rv1 cells treated chronically with enzalutamide (expressing higher levels of p52) and examined cell growth after 24 and 48 hours. Downregulation of p52 after transfection was confirmed by qRT-PCR. As shown in Fig. 2B, cells transfected with p52 shRNA were increasingly sensitive to enzalutamide compared to control CWR22Rv1-Enza-R cells, indicating that expression of p52 may be necessary for the survival of cells treated chronically with enzalutamide. These results collectively demonstrate that NF-κB2/p52 may regulate the induction of resistance to enzalutamide in prostate cancer cells.
Figure 2.
Prostate cancer cells treated chronically with enzalutamide upregulate the expression of NF-κB2/p52. A, CWR22Rv1 cells treated chronically with enzalutamide exhibit higher endogenous levels of both p100 and p52. B, CWR22Rv1 cells treated chronically with enzalutamide were transfected with either control shRNAs or shRNAs against NF-κB2/p52 and were treated with 0, 20, or 40 μmol/L enzalutamide. Cell numbers were counted after 24 and 48 hours. Results are presented as mean ± SD of 2 experiments performed in triplicate. *, P ≤ 0.05. Cells transfected with shRNAs against p52 exhibited lower cell survival when treated with enzalutamide.
NF-κB2/p52 enhances expression of AR splice variants
It has been shown that higher levels of AR splice variants may be responsible for the resistance to enzalutamide in prostate cancer (4), hence, we tested whether NF-κB2/p52 regulates the expression of AR splice variants. Total RNAs from LNCaP and C4-2B cells transfected with either empty vector or p52 in media containing either complete or charcoal-stripped FBS (CS-FBS) were analyzed by qRT-PCR for the expression levels of full-length (FL) AR as well as the major splice variant AR-V7. As shown in Fig. 3A, expression of p52 enhanced the expression levels of the splice variant AR-V7 in both FBS and CS-FBS, whereas expression of FL AR remained unchanged in LNCaP cells (left). These results were confirmed by Western blotting using antibodies specific for FL AR and AR-V7 (right). Similar results were observed in C4-2B cells, in which expression of p52 enhanced the expression levels of AR-V7, whereas expression levels of FL AR were unaffected (Fig. 3B). To substantiate these results, we examined expression levels of FL AR and AR-V7 in LN-neo and LN-p52 cells by qRT-PCR and Western blotting and found that expression levels of AR-V7 were elevated in LN-p52 cells compared to LN-neo cells (Fig. 3C). We also analyzed expression levels of AR-V7 in xenografts of LNCaP cells expressing p52 and found that xenografts expressing p52 exhibited significantly higher levels of AR-V7 mRNA compared to control LNCaP cell xenografts (Fig. 3D). These findings show that NF-κB2/p52 may induce upregulation of the expression of AR-V7.
Figure 3.
NF-κB2/p52 induces higher expression of AR splice variants. Total RNAs from LNCaP (A) and C4-2B (B) cells transfected with empty vector or p52 were analyzed by qRT-PCR for the expression of FL AR and AR-V7 in media containing either FBS or CS-FBS. Expression of p52 enhanced the levels of AR-V7, whereas levels of FL AR remained unchanged. Right, immunoblotting of above lysates with antibodies specific against either FL AR or AR-V7. C, total RNAs from LN-p52 and LN-neo cells were analyzed by qRT-PCR for the expression levels of FL AR or AR-V7. LN-p52 cells showed higher levels of expression of AR-V7 compared to LN-neo cells, whereas FL AR levels were unaffected. Right, immunoblotting of above lysates with antibodies against FL AR or AR-V7. D, expression levels of AR-V7 were enhanced in xenografts from LNCaP cells expressing p52 compared to xenografts from parental LNCaP cells. Results are presented as mean ± SD of 2 experiments performed in triplicate. *, P ≤ 0.05.
Downregulation of NF-κB2/p52 abrogates expression of AR splice variants
Next, we tested whether NF-κB2/p52 was necessary for the enhanced expression of AR splice variants. VCaP and CWR22Rv1 prostate cancer cells express endogenous levels of AR splice variants AR-V1, AR-V5, AR-V7, AR-1/2/2b, and AR-1/2/3/2b. We transfected shRNA specific to p52 into VCaP and CWR22Rv1 cells and examined the expression levels of these splice variants by qRT-PCR using specific primers. As shown in Fig. 4A and B (left), downregulation of p52 reduced the expression levels of most of the splice variants significantly, whereas levels of FL AR remained unaffected. These results were confirmed for AR-V7 expression by Western blotting using antibodies specific against AR-V7 and FL AR in VCaP and CWR22Rv1 cells (Fig. 4A and B, right), indicating that expression of p52 may be necessary for the synthesis of AR splice variants.
Figure 4.
Downregulation of NF-κB2/p52 in prostate cancer (CaP) cells reduces expression of AR splice variants. VCaP (A) and CWR22Rv1 (B) cells were transfected with either control shRNAs or shRNAs against p52 and expression levels of the indicated AR splice variants were analyzed by qRT-PCR. Downregulation of p52 led to a decrease in synthesis of AR splice variants, whereas expression levels of FL AR remained unchanged. Right panels show immunoblots of above lysates with antibodies against FL AR or AR-V7. Results are presented as mean ± SD of 3 experiments performed in triplicate. *, P ≤ 0.05.
Downregulation of FL AR and AR-V7 increases sensitivity of p52-expressing prostate cancer cells to enzalutamide
LNCaP cells stably expressing p52 (LN-p52) exhibit higher levels of AR-V7. We also assessed expression levels of other members of the NF-κB family by Western blotting and found that their levels were not altered (Supplementary Fig. S1), indicating that the effect on AR-V7 expression was mainly due to the expression of NF-κB2/p52. Next, we analyzed expression levels of antiapoptotic proteins such as Bcl-xL, survivin, and cyclin D1 in LN-p52 cells compared to LN-neo cells and found that LN-p52 cells express higher levels of Bcl-xL, survivin, and cyclin D1 (Supplementary Fig. S2), indicating that activation of antiapoptotic genes may play an important role in resistance against enzalutamide. As reported previously, the cells also exhibit aberrant activation of AR in the absence of androgen and exhibit castration-resistant growth (15). In the current study, we also showed that LN-p52 cells are resistant to enzalutamide-induced growth inhibition compared to control LN-neo cells. Hence, to test whether FL AR or AR-V7 plays a role in the p52-induced resistance to enzalutamide, we transfected siRNAs specific against either FL AR or AR-V7 into LN-neo and LN-p52 cells and monitored cell growth in response to enzalutamide. As shown in Fig. 5A, downregulation of either FL AR or AR-V7 reduced growth of control LN-neo cells by ~20%, and enzalutamide itself reduced growth of LN-neo cells by ~50%. No additional reduction of growth was observed in LN-neo cells when FL AR or AR-V7 was downregulated in the presence of enzalutamide, showing that inhibition of either FL AR or AR-V7 had no effect on the sensitivity of LN-neo cells to enzalutamide. In LN-p52 cells which express higher levels of AR-V7, downregulation of either FL AR or AR-V7 reduced growth by ~50% in the presence of enzalutamide, thus resensitizing LN-p52 cells to enzalutamide. In other words, LN-p52 cells are more sensitive to enzalutamide when expression of either FL AR or AR-V7 was inhibited. These results suggest that resistance of LN-p52 cells to enzalutamide is mediated by alterations in the AR signaling pathway and demonstrate that activation of the AR axis by p52 plays an important role in the p52-induced resistance to enzalutamide. To confirm these results and test whether downregulation of FL AR or AR-V7 modulates p52-induced AR activation, we co-transfected a luciferase reporter containing the enhancer and promoter regions of PSA (PSA-E/P-Luc) along with p52 and siRNAs against FL AR or AR-V7 into VCaP and CWR22Rv1 cells. The cells were treated with either vehicle or 20 μmol/L enzalutamide and luciferase assays performed. VCaP and CWR22Rv1 cells express higher endogenous levels of p52, and our previous studies show that p52 induces ligand-independent activation of AR (13, 15). As shown in Fig. 5B and as shown in our previous studies (15), p52 induces activation of AR-mediated target gene transcription, which was abolished by downregulation of either FL AR or AR-V7. p52-induced activation of AR was unaffected by enzalutamide treatment. Treatment with enzalutamide further enhanced the suppressive effect of siRNAs against FL AR or AR-V7 on p52-induced AR-mediated target gene transcription. These results demonstrate that activation of AR signaling is necessary for the p52-induced resistance against enzalutamide. Similar results were obtained in CWR22Rv1 cells (Fig. 5C), showing that the interplay between FL AR, AR-V7 and NF-κB2/p52 may be critical in the development of resistance to enzalutamide in prostate cancer cells. These results implicate the activation of the AR signaling axis by p52 via FL AR and its splice variants as being responsible for the induction of resistance against enzalutamide.
Figure 5.
Downregulation of FL AR and AR-V7 increases sensitivity of p52-expressing prostate cancer (CaP) cells to enzalutamide. A, LN-p52 and LN-neo cells were transfected with siRNAs specific to either FL AR or AR-V7 and were treated with 0 or 20 μmol/L enzalutamide. Cell numbers were counted after 48 hours. Results are presented as mean ± SD of 3 experiments performed in triplicate. Downregulation of either FL AR or AR-V7 increased sensitivity of LN-p52 cells to enzalutamide. VCaP (B) and CWR22Rv1 (C) cells were transfected with PSA-E/P-Luc reporter, empty vector, or p52 together with siRNAs against FL AR or AR-V7. Cells were treated with 0 or 40 μmol/L enzalutamide, and luciferase assays were performed after 48 hours. Results are presented as mean ± SD of 2 experiments performed in triplicate. *, P ≤ 0.05. Downregulation of either FL AR or AR-V7 suppressed p52-induced activation of AR in both VCaP and CWR22Rv1 cells.
Discussion
Next-generation antiandrogens such as enzalutamide and inhibitors of androgen synthesis such as abiraterone have revolutionized the standard of care for patients with both early- and late-stage prostate cancer. Despite their successes and continuing widespread use, threat of development of resistance looms large (17, 18). The understanding of mechanisms by which resistance against these agents may develop in prostate cancer cells may be critical for early intervention strategies in the event of development of resistance. Enzalutamide binds to the ligand-binding domain of AR and inhibits its nuclear translocation, DNA binding, and transactivation of target genes (3). In the current study, we show that NF-κB2/p52 may play a crucial role in the development of resistance to enzalutamide and that the interplay between p52 and the AR signaling axis may be one of the underlying mechanisms. Even though enzalutamide and bicalutamide have similar mechanisms of action, clinically patients who progress on bicalutamide may respond to enzalutamide, indicating the existence of different mechanisms of resistance and that NF-κB2/p52 may be one of the many mediators of resistance. Our studies also show that prostate cancer cells treated chronically with enzalutamide may develop resistance against the agent via upregulation of expression of p52. These findings have important implications for therapeutic regimen in which patients are treated for long periods of time with enzalutamide. Further studies are warranted to test whether blocking of cellular signaling pathways in combination with antiandrogens may prove beneficial.
Our current data demonstrate that signaling networks such as p52 and AR interactions can mediate resistance to therapies targeting FL AR, including the next-generation antiandrogen, enzalutamide. The significance of these studies lies in the fact that resistance, either de novo or acquired, is one of the major clinical limitations for new AR inhibitors. The majority of patients who display disease progression on enzalutamide also display increasing prostate-specific antigen (PSA), indicating that enzalutamide-resistant tumors remain driven by persistent AR activity. AR variants are overexpressed in a subset of CRPC metastases and correlate with poor survival (19, 20). As AR splice variants lack the ligand-binding domain, they may be insensitive to inhibition by both bicalutamide and enzalutamide. One of the pioneering studies about enzalutamide showed that it may be effective against cells expressing higher levels of AR splice variants, although this fact remains to be substantiated (21). Even though AR variants have been hypothesized to be independent mediators of castration resistance (4), FL AR may still be necessary and may augment the castration-resistant response (21). Conflicting results have been obtained about the distinct transcriptional programs activated by AR variants and FL AR in prostate cancer cells (4, 22). It is probable that such perceived differences are due to experimental platforms used and do not reflect physiologic deviations. It is also possible that AR variants execute a “tumor-specific” program, which is a part of the broader transcriptional program of the FL AR, and hence are enriched in tumors. This may not necessarily mean that the FL AR is no longer a player in the progression of prostate cancer. It would be more likely that co-operation between FL AR and its splice variants is the driver behind CRPC progression, rather than a distinct and dominant transcriptional program driven by the splice variants alone.
Our earlier studies showed that NF-κB2/p52 promotes castration-resistant progression of prostate cancer by activating the AR in conditions of androgen deprivation (13, 15). In this study, we showed that p52 also induces expression of AR splice variants. Since the mechanism of action of enzalutamide is the inhibition of AR activation, we hypothesized that prostate cancer cells expressing higher levels of p52 may be resistant to enzalutamide. Our current results confirm the hypothesis and point to the role of the interaction between AR and p52 as being one of the critical turns during the progression to castration resistance.
In summary, our study demonstrates a link between persistent activation of the AR by NF-κB2/p52 and development of resistance to enzalutamide in prostate cancer. Future points of interest would be whether overcoming these networks and improving the efficacy of currently available clinical agents represents a viable area of research.
Supplementary Material
Acknowledgments
The authors thank Dr. Jun Luo (Department of Urology, Johns Hopkins University) for the kind gift of AR-V7–specific antibody.
Grant Support
This work was supported in part by NIH CA140468, CA118887, CA109441, and DOD PC080538 (A.C. Gao) and by DOD PC100502 (N. Nadiminty).
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
C.P. Evans has other commercial research support from, honoraria from speakers bureau from, and is a consultant/advisory board member for Medivation/Astellas. No potential conflicts of interest were disclosed by the other authors.
Authors’ Contributions
Conception and design: N. Nadiminty, J. Yang, C.P. Evans, A.C. Gao
Development of methodology: N. Nadiminty, A.C. Gao
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): N. Nadiminty, R. Tummala, W. Lou, C.P. Evans, A.C. Gao
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): N. Nadiminty, R. Tummala, C.P. Evans, A.C. Gao
Writing, review, and/or revision of the manuscript: N. Nadiminty, R. Tummala, C.P. Evans, A.C. Gao
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): C. Liu, J. Yang, W. Lou, A.C. Gao
Study supervision: N. Nadiminty, C.P. Evans, A.C. Gao
References
- 1.Hobisch A, Culig Z, Radmayr C, Bartsch G, Klocker H, Hittmair A. Distant metastases from prostatic carcinoma express androgen receptor protein. Cancer Res. 1995;55:3068–72. [PubMed] [Google Scholar]
- 2.van der Kwast TH, Schalken J, Ruizeveld de Winter JA, van Vroonhoven CC, Mulder E, Boersma W, et al. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer. 1991;48:189–93. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]
- 3.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–9. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karin M. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–9. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 7.Xiao G, Fong A, Sun SC. Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J Biol Chem. 2004;279:30099–105. doi: 10.1074/jbc.M401428200. [DOI] [PubMed] [Google Scholar]
- 8.Qing G, Xiao G. Essential role of IkappaB kinase alpha in the constitutive processing of NF-kappaB2 p100. J Biol Chem. 2005;280:9765–8. doi: 10.1074/jbc.C400502200. [DOI] [PubMed] [Google Scholar]
- 9.Qing G, Qu Z, Xiao G. Regulation of NF-kappa B2 p100 processing by its cis-acting domain. J Biol Chem. 2005;280:18–27. doi: 10.1074/jbc.M406619200. [DOI] [PubMed] [Google Scholar]
- 10.Xiao G, Rabson AB, Young W, Qing G, Qu Z. Alternative pathways of NF-kappaB activation: a double-edged sword in health and disease. Cytokine Growth Factor Rev. 2006;17:281–93. doi: 10.1016/j.cytogfr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS., Jr Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene. 2000;19:1123–31. doi: 10.1038/sj.onc.1203412. [DOI] [PubMed] [Google Scholar]
- 12.Lessard L, Karakiewicz PI, Bellon-Gagnon P, Alam-Fahmy M, Ismail HA, Mes-Masson AM, et al. Nuclear localization of nuclear factor-kappaB p65 in primary prostate tumors is highly predictive of pelvic lymph node metastases. Clin Cancer Res. 2006;12:5741–5. doi: 10.1158/1078-0432.CCR-06-0330. [DOI] [PubMed] [Google Scholar]
- 13.Nadiminty N, Chun JY, Lou W, Lin X, Gao AC. NF-κB2/p52 enhances androgen-independent growth of human LNCaP cells via protection from apoptotic cell death and cell cycle arrest induced by androgen-deprivation. Prostate. 2008;68:1725–33. doi: 10.1002/pros.20839. [DOI] [PubMed] [Google Scholar]
- 14.Nadiminty N, Dutt S, Tepper C, Gao AC. Microarray analysis reveals potential target genes of NF-κ2/p52 in LNCaP prostate cancer cells. Prostate. 2010;70:276–87. doi: 10.1002/pros.21062. [DOI] [PubMed] [Google Scholar]
- 15.Nadiminty N, Lou W, Sun M, Chen J, Yue J, Kung HJ, et al. Aberrant activation of the androgen receptor by NF-κB2/p52 in prostate cancer cells. Cancer Res. 2010;70:3309–19. doi: 10.1158/0008-5472.CAN-09-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadiminty N, Lou W, Lee SO, Lin X, Trump DL, Gao AC. Stat3 activation of NF-kappaB p100 processing involves CBP/p300-mediated acetylation. Proc Natl Acad Sci USA. 2006;103:7264–9. doi: 10.1073/pnas.0509808103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornberg E, Ylitalo EB, Crnalic S, Antti H, Stattin P, Widmark A, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6:e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107:16759–65. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu R, Lu C, Mostaghel EA, Yegnasubramanian S, Gurel M, Tannahill C, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–62. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.