Abstract
Since the CALUX (Chemically Activated LUciferase gene eXpression) bioassay is a fast and inexpensive tool for the determination of dioxin-like compounds in a large number of samples and requires only small sample volumes, the use of this technique in human biomonitoring programs provides a good alternative to GC-HRMS. In this study, a new CALUX method for the separate analysis of PCDD/Fs and dioxin-like PCBs (dl-PCBs) in small amounts of human milk samples with the new sensitive H1L7.5c1 cell line was used to analyze 84 human milk samples, collected from mothers residing in the Flemish rural communities. The geometric mean CALUX-Bioanalytical Equivalent (CALUX-BEQ) values, reported for the 84 mothers from the study area were 10.4 (95% CI: 9.4–11.4) pg CALUX-BEQ per g lipid or 0.41 (95% CI: 0.37–0.45) pg CALUX-BEQ per g milk for the PCDD/Fs and 1.73 (1.57–1.91) pg CALUX-BEQ per g lipid or 0.07 (95% CI: 0.06–0.08) pg CALUX-BEQ per g milk for the dioxin-like PCBs. Multiple regression analysis showed significant associations between PCDD/Fs and weight change after pregnancy, smoking and consumption of local eggs. One pooled human milk sample was analyzed with both CALUX and GC-HRMS. The ratio of CALUX and GC-HRMS results for this sample were respectively 1.60, 0.58 and 1.23 for the PCDD/Fs, the dl-PCBs and the sum of both fractions, when using the 2005-TEF values. Additionally, also low levels of certain brominated dioxins and furans were detected in the pooled sample with GC-HRMS.
Keywords: PCDD/Fs, dioxin-like PCBs, PBDD/Fs, CALUX, GC-HRMS, human milk
1. Introduction
In the first Flemish Environment and Health survey performed by the Flemish Centre of Expertise on Environment and Health (FLEHS I, 2002–2006) increased concentrations of PCBs, dioxin-like substances and chlorinated pesticides were observed in cord blood of newborns and in blood of 14–15 year-old adolescents and 50–65 year-old adults living in low populated rural communities of East and West Flanders and Flemish Brabant compared to other Flemish regions [1, 2]. Due to the health concern associated with increased body burdens of chlorinated POPs, a follow-up study of the pollutant levels in these rural areas was undertaken. Because POPs are mainly lipophilic, human milk was chosen to assess exposure to these compounds. Human milk is a valuable matrix for human biomonitoring of lipophilic pollutants, since it is a non-invasive sample that is available in sufficient quantities (e.g. compared to cord blood).
For the quantification of PCDD/Fs and/or dioxin-like PCBs in (human) milk samples, both GC-HRMS and CALUX bioassays are used in routine analysis. GC-HRMS analysis usually needs large amounts of human milk and is also quite expensive, while the CALUX bioassay is known as a fast, inexpensive technique that uses small sample volumes. However, so far most validated CALUX methods for quantification of PCDD/Fs and dl-PCBs in human and bovine milk samples have also used relatively large volumes. Some researchers used 60 mL milk [3–5], while others used 20 mL [6] or 10 mL milk [7–9]. Since in this Flemish human milk campaign not only PCDD/Fs and dioxin-like PCB were analyzed, but also other POPs (e.g. pesticides, brominated flame retardants, marker PCBs and perfluorinated compounds), the amount of human milk available for the CALUX bioassay was limited. Therefore, a new, more sensitive method needed to be developed for the separate analysis of PCDD/Fs and dioxin-like PCBs in only 5 mL of (human) milk. The aims of this study were: 1) to develop a new CALUX method for the separate analysis of PCDD/Fs and dl-PCBs in 5 mL of human milk, 2) to compare the concentrations measured in human milk samples from the rural study area to the concentrations measured in Belgian milk samples from former WHO-coordinated studies and from other national and international surveys, 3) to determine the associations between concentrations of PCDD/Fs and dl-PCBs on the one hand and personal characteristics and dietary habits on the other hand and 4) to compare the CALUX results of a pooled milk sample with the GC-HRMS data, obtained from the same sample, 5) to determine the concentration of PBDD/F congeners in human milk from the rural areas.
2. Materials and Methods
2.1 Chemicals and standards
Hexane (for PCDD/Fs and PCBs, minimum 96%), acetone (Pesti-S grade, minimum 99.9%) and toluene (for PCDD/Fs and PCBs, minimum 99.8%) were purchased from Biosolve (The Netherlands). Ethyl acetate pestanal and silica gel 60 for column chromatography were purchased from Sigma-Aldrich (Germany). Sulphuric acid (95%–97%, ACS reagent), Celite 545 (0.02–0.1 mm) and DMSO were obtained from Merck (Germany). Anhydrous sodium sulphate was purchased from Boom (The Netherlands) and X-CARB from XDS (USA). The standard solution of 2,3,7,8 TCDD (50 ng mL−1) was purchased from Campro Scientific (The Netherlands).
2.2 Selection, recruitment of participants and sample collection
The participants were recruited from nine maternities in East and West Flanders. Since the POP levels were meant to be compared with the Belgian results of the WHO human milk surveys from 1987–1988, 1992–1993, 2000–2003 and 2005–2006 [10], the selected mothers had to meet the WHO inclusion criteria for age, parity and single birth and residence time of at least 5 years in the study area. However, because the recruitment turned out to be rather difficult, the selection criteria were broadened [11]. During a period of 14 months (May 2009 until end of June 2010), finally, a total of 84 mothers (30.6% of the selected mothers) participated in the study. At home, the mothers collected the milk two to eight weeks after delivery. At least 50 mL of milk samples had to be collected, after and/or during nursing. The samples were stored at −20 °C until analysis.
After signing the informed consent, the mothers were asked to complete a questionnaire for information about residence during the last 5 years, date and place of birth, mother’s age, weight and length, dietary habits and occupation, smoking and alcohol consumption, fertility and health data, exposure to pollutants indoor or in the workplace, socio-economic status and perception of environmental problems. More details about the personal characteristics of the participants were described by Croes et al., 2012 [11].
The study design was approved by the medical-ethical committee of the University of Antwerp on 10th of July 2009.
2.3 Analytical procedure for quantification of PCDD/Fs and dl-PCBs
PCDD/Fs and dl-PCBs were analyzed in all 84 individual samples using the CALUX bioassay. From each of the 84 individual samples, 10 mL human milk of the initial sample was taken to compose a pooled sample. This pooled sample was used to follow up the time trend of PCDD/F and dl-PCB concentrations that were measured during the former WHO-coordinated human milk surveys [10]. GC-HRMS analysis of pollutants in the pooled sample was done by the WHO reference lab (State Institute for Chemical and Veterinary Analysis of Food (CVUA), Freiburg, Germany), according to the method described by Malisch and van Leeuwen (2002) [12], Kotz et al. (2005) [13] and Hui et al. (2008) [14]. CALUX analysis was performed by the Department of Analytical and Environmental Chemistry at the Free University of Brussels (VUB, Brussels, Belgium). For the determination of dioxin-like compounds in human milk samples, the same protocol as for extraction and clean up of human serum samples was used [15], except for a higher amount of acid silica due to the higher lipid content of human breast milk. Briefly, 5 mL human milk samples were extracted with an acetone/hexane mixture and filtered upon a pre-conditioned celite column. After extraction, the amount of fat was weighted and the extract was redissolved in 5 mL hexane and cleaned up on a pre-conditioned multi-layer silica column coupled in series with a carbon column. The silica gel column (25 mL) was filled from bottom to top with glass wool, 1.9 g (1.3 cc) sodium sulphate, 6.0 g (2 × 4.3 cc) of 33% (w/w) sulphuric acid silica gel and 1.9 g (1.3 cc) sodium sulphate. The carbon column (10 mL) was filled with glass wool, 0.7 g (0.5 cc) sodium sulphate, 0.34 g (1 cc) X-CARB and 0.7 g (0.5 cc) sodium sulphate. The dioxin-like PCBs and the PCDD/Fs were eluted separately from the carbon column and redissolved in a defined volume hexane (1.5 mL and 2 mL for the PCB and PCDD/F fractions, respectively). Concentration-response analysis using pooled milk sample extracts allowed determination of an optimal dilution factor to facilitate screening analysis and to minimize sample volumes needed for analysis. For the dl-PCB fraction a final dilution factor of 1.5 was used, while dilution factors 2.5 and 4 were used for the PCDD/F fraction. The target compounds were analyzed using the enhanced recombinant mouse hepatoma CALUX cell line (H1L7.5c1) which had been stably transfected with an Ah receptor-responsive firefly luciferase reporter gene plasmid (pGudLuc7.5) that contains 20 dioxin-responsive elements [16, 17]. The results were expressed as CALUX bioanalytical equivalents (BEQs), using the inverse prediction method on a Hill-shaped TCDD calibration curve. The final result was expressed in pg BEQ per g fat and in pg BEQ per g milk.
2.4 Statistics
Means, medians, ranges and geometric means were calculated using SAS 9.2 and Statistica 10.0. The geometric means were obtained after back transformation of the mean values of the Ln transformed variables. To determine the factors that influence the PCDD/F and dl-PCB levels in the human milk samples, univariate regression relationships were first calculated. Covariates with a p-value lower than 0.25 were entered in a multiple regression analysis (stepwise selection). Only variables with a p-value less than 0.10 were retained in the multiple regression models. For samples below the Limit Of Quantification (LOQ), half of the LOQ was used. Age, body mass index (BMI) and smoking habits were included as fixed confounders for PCDD/Fs and dl-PCBs in the multiple regression models. For age and BMI, a continuous variable was used as confounding factor, while for smoking the categorical variable current-, ex- or non smoker was used in regression analysis. Univariate and multiple regression analyses were performed on Ln-transformed PCDD/F and dl-PCB values, expressed in pg BEQ per g lipid weight.
3. Results and discussion
3.1 Determination of CALUX-BEQs for PCDD/Fs and dl-PCBs in the individual human milk samples and regression analysis to identify predictors of the measured levels
Five millilitre milk samples were analyzed with the CALUX bioassay for all 84 mothers participating in the study. All samples were above the LOQ (0.1 pg/well or 0.69 pg BEQ per g lipid for the dl-PCBs and 1.15 pg BEQ per g lipid for the PCDD/Fs), for both the PCDD/Fs and the dl-PCBs. The geometric mean PCDD/F and dioxin-like PCB concentrations (raw data) in the total study population were respectively 10.4 (95 % CI: 9.4–11.4) pg CALUX-BEQ per g lipid for the PCDD/Fs and 1.73 (1.57–1.91) pg CALUX-BEQ per g lipid for the dioxin-like PCBs When expressed in wet weight, mean concentrations of 0.41 (95% CI: 0.37–0.45) pg CALUX-BEQ per g milk and 0.07 (95 % CI: 0.06–0.08) pg CALUX-BEQ per g milk were found for the PCDD/Fs and dioxin-like PCBs, respectively (Table 1).
Table 1.
Overview of the concentrations of PCDD/F’s and dl-PCBs, measured in human milk samples from different countries. The results were expressed in pg BEQ per g lipid (CALUX) and/or in pg WHO-TEQ per g lipid (GC-HRMS).
| Country | Year | N | Age | Pollutant | Geometric mean |
Median | Min. | Max. | Technique |
|---|---|---|---|---|---|---|---|---|---|
| This study |
‘09– ‘10 |
84 | 28.5 (20.6– 35.6) |
PCDD/Fs | 10.4 | 10.1 | 4.1 | 32.7 | UCD- CALUX |
| 84 | dl-PCBs | 1.7 | 1.7 | 0.6 | 8.1 | UCD- CALUX |
|||
| Pooled sample |
PCDD/Fs | 11.1 | UCD- CALUX |
||||||
| Pooled sample |
dl-PCBs | 2.2 | UCD- CALUX |
||||||
| Pooled sample |
PCDD/Fs | 8.4 (TEF 1998) 6.9 (TEF 2005) |
GC- HRMS |
||||||
| Pooled sample |
dl-PCBs | 5.8 (TEF 1998) 3.7 (TEF 2005) |
GC- HRMS |
||||||
| Germany [41] |
‘00– ‘03 |
169 | 19–42 | PCDD/Fs | 13.30 | 1.80 | 34.70 | GC- HRMS |
|
| Dl-PCBs | 13.00 | 1.21 | 50.10 | GC- HRMS |
|||||
| Germany [42] |
2005 | 43 | PCDD/Fs | 9.91 | 3.34 | 26.29 | GC- HRMS |
||
| dl-PCBs | 9.92 | 3.02 | 25.31 | GC- HRMS |
|||||
| Hong- Kong [7] |
‘01– ‘02 |
11 pooled samples |
26.5– 32.6 |
Sum PCDD/Fs, dl-PCBs |
14.5 | BDS- CALUX |
|||
| 11 pooled samples |
Sum PCDD/Fs, dl-PCBs |
12.8 (TEF 1998) 10.5 (TEF 2005) |
GC- HRMS |
||||||
| 3th and 4th WHO study [43] |
2001& 2006 |
19 European countries |
PCDD/Fs | 8.9 (TEF 1998) |
4.4 | 18.8 | GC- HRMS |
||
| dl-PCBs | 9.4 (TEF 1998) |
2.1 | 20.0 | GC- HRMS |
|||||
| Tianjin, China [6] |
‘06– ‘07 |
60 | <30 | PCDD/Fs | 13.1 | 13.2 | 7.4 | 23.6 | XDS- CALUX |
| dl-PCBs | 1.9 | 1.8 | 0.9 | 7.9 | XDS- CALUX |
||||
| Yantai, China [6] |
‘06– ‘07 |
48 | <30 | PCDD/Fs | 9.9 | 9.4 | 6.6 | 17.5 | XDS- CALUX |
| dl-PCBs | 4.4 | 4.8 | 1.0 | 9.1 | XDS- CALUX |
||||
| Japan [9] | ‘01– ‘03 |
49 | 32.4±4.7 | PCDD/Fs | 11.1 (TEF 1998) |
9.8 | 2.4 | 25.0 | GC- HRMS |
| dl-PCBs | 7.8 (TEF 1998) |
6.9 | 2.1 | 20.6 | GC- HRMS |
||||
| Turkey [44] |
2007 | 51 | 20–40 | PCDD/Fs | 7.5 (TEF 1998) |
0.78 | 29.3 | GC- HRMS |
|
| dl-PCBs | 3.1 (TEF 1998) |
GC- HRMS |
|||||||
| Spain [45] |
2007 | 15 | 25–35 | PCDD/Fs | 7.6 | 2.8 | 11.2 | GC- HRMS |
|
| dl-PCBs | 9.0 | 2.8 | 17.6 | GC- HRMS |
|||||
| Latvia [46] |
2004 | 30 | 25.7 (mean) |
PCDD/Fs | 7.6 | GC- HRMS |
|||
| dl-PCBs | 10.1 | GC- HRMS |
For the confounding variables age (years), BMI (kg m−2) and smoking habits (non-, ex- or current smoker), univariate regression analysis showed only statistically significant results for age of the mother. The CALUX-BEQ values of both the PCDD/Fs and dl-PCBs increased with the age of the mother (p=0.001 for PCDD/Fs and p=0.048 for dl-PCBs). A non-significant trend was observed between smoking and the measured CALUX response of the dioxin-like PCBs (p=0.16) and PCDD/Fs (p=0.21). After exclusion of outliers, PCDD/F levels were borderline significantly higher in mothers with lower BMI (p=0.047).
The tested covariates with p-values less than 0.25 were included in the multiple stepwise regression analysis. For the PCDD/Fs, consumption of eggs from local chickens (yes/no; univariate p=0.023), consumption of dairy products (every day/less; univariate p=0.213), consumption of fish (never/once a week/twice a week or more; univariate p=0.141) and change of weight of the mother (stable/lost/gained weight; univariate p=0.002) were included in the final model. For the dl-PCBs, consumption of meat and poultry (every day/less; univariate p=0.041), consumption of eggs from local chickens (yes/no; univariate p=0.204), outside stoking (yes/no; univariate p=0.035) and change of weight of the mother (stable/lost/gained weight; univariate p=0.02) were included in multiple regression analysis.
No associations were found between dl-PCBs and PCDD/Fs on the one hand and the education level of the mother (4 categories), highest education level in the family (4 categories) and household income (continuous variable, euro) on the other hand. Sex of the baby, the number of years living in the study area (continuous variable, years) and parity (categorical variable, first or second child) were also not significantly associated with the concentration of dl-PCBs or PCDD/Fs.
In multiple stepwise regression analysis (taking into account the predefined confounders age, BMI and smoking, and selected covariates) no significant relationships were found for the dl-PCBs. For the PCDD/Fs significant relationships were found with weight change after pregnancy (p=0.003) and consumption of local eggs (p=0.05), while a borderline non-significant relation was found with smoking before pregnancy (p=0.07) and with the age of the mother (p=0.07). An overview of the p-values obtained with multiple regression analysis is given in Table 2, while in Table 3 a detailed overview of the standardized parameter estimates (Beta), 95% confidence intervals (CIs) and p-values is shown for the PCDD/F fraction.
Table 2.
Overview of the p-values for the dl-PCBs and PCDD/Fs (Ln-transformed) after multiple regression analysis.
| p-value | ||
|---|---|---|
| dl-PCBs (pg BEQ/g lipid) |
PCDD/Fs (pg BEQ/g lipid) |
|
| Age of the mother (years) | 0.37 | 0.07 |
| BMI of the mother (kg m−2) | 0.12 | 0.35 |
| Smoking (current, ex, never) | 0.23 | 0.07 |
| Weight change after pregnancy (gained, lost, stable) |
ns | 0.003 |
| Consumption of local eggs (yes/no) | ns | 0.05 |
ns = p >0.10 in the stepwise model and left out in the final regression model.
Table 3.
Detailed overview of the standardized parameter estimates (Beta), 95% confidence intervals (CIs) and p-values for the PCDD/F fraction (Ln-transformed) in multiple regression analysis.
| PCDD/Fs (pg BEQ per g lipid) |
|||
|---|---|---|---|
| Beta* | 95% CI | p-value | |
| Weight change after pregnancy | |||
| Lost vs Stable | 0.27 | −0.01; 0.55 | 0.06 |
| Gained vs Stable | −0.50 | −0.78; −0.22 | < 0.001 |
| Lost vs Gained | 0.29 | 0.003; 0.57 | 0.047 |
| Consumption of local eggs | |||
| No vs Yes | −0.23 | −0.46; 0.002 | 0.05 |
| Smoking (current, ex, never) | |||
| Never vs Current | −0.13 | −0.39; 0.12 | 0.30 |
| Ex vs Current | 0.28 | 0.04; 0.53 | 0.02 |
| Ex vs Never | 0.32 | 0.05; 0.60 | 0.02 |
the standardized parameter estimate (Beta) gives the strength and direction of the relationship. The exponential function of the standardized parameter estimate (Beta) is the factor of increase/decrease of the concentration of the PCDD/Fs (non-transformed, in pg BEQ per g lipid), when belonging to a certain category (e.g. not consuming instead of consuming local eggs).
The PCDD/F BEQ-levels were higher in ex-smokers compared to current smokers or women who never smoked (Table 3). Higher PCDD/F concentrations in smokers compared to non-smokers can be expected since dioxins are present in cigarette smoke [18, 19]. The higher BEQs found in ex-smokers compared to current smokers seem quite strange, but this could be explained by the fact that smokers seem to have faster PCDD/F elimination rates compared to non-smokers [20]. This means that a higher intake can be compensated by a higher elimination rate. Srogi [21] also showed that dioxin levels were generally lower in mothers who were heavy smokers, while a study from France found that serum concentrations for the sum of PCDD/Fs and dl-PCBs were significantly lower in smokers compared to non-smokers [22]. It was thought that persistent toxicants present in cigarette smoke have also affinity for the Aryl hydrocarbon Receptor (AhR) and thus compete with the dl-compounds for binding to the receptor. When dividing smoking status into active, passive and not smoking, it was found that the measured dioxin-like activities were higher in milk samples from current smokers (active and passive) compared to non-smokers. For the PCDD/Fs BEQ values of 10.72, 11.74 and 9.85 pg BEQ per g lipid were found for respectively active, passive and non-smokers. For the dl-PCBs, BEQ values of 1.93, 1.98 and 1.57 pg BEQ per g lipid were found for respectively active, passive and non-smokers. Since there is no information available on the number of cigarettes smoked a day (for the active smokers) and the time exposed to cigarette smoke (for the passive smokers), the small (non-significant) differences between CALUX levels in active and passive smoker can not be explained. In multiple regression analysis also significant relationships were found between the PCDD/Fs and weight change after pregnancy (p=0.003) and consumption of local eggs (p=0.05). Participants who lost weight after the pregnancy relative to their weight before pregnancy had significant higher values of PCDD/Fs compared to participants who gained weight or participant with stable weight. Weight losses result in a decrease of adipose tissue and thus in a release of POPs into blood and human milk. Participants who never consumed local eggs showed significantly lower PCDD/F BEQ values, expressed per g lipid (p=0.05).
Regression analysis showed no significant association between dioxin-like compounds and parity. It is however expected that the total concentration of these POPs is lower in multiparous breastfeeding mothers compared to primiparous breastfeeding women, since PCDD/Fs and PCBs are transferred to the off-spring during gestation and lactation [23]. Lorber and Philips [24] estimated that maternal PCB/dioxin body burdens decrease 20% to 70% during six months of exclusive breastfeeding. This means that a mother will transfer more POPs to her first child compared to the later born children. In our study, lower CALUX-BEQ values were also found in the human milk sample of mothers giving birth to a second child compared to the milk samples from primiparous women (2.1 versus 1.8 pg BEQ per g lipid with univatiate p=0.20 for dl-PCBs and 12.2 versus 10.5 pg BEQ per g lipid with univartiate p= 0.17 for PCDD/Fs). The concentration of dioxin-like compounds in breast milk will also depend on the time period of lactation, the degree of exposure and time between the pregnancies, but unfortunately this information was not available in our study.
3.2 Comparison between CALUX and GC-HRMS results and contribution of PBDD/Fs
From each of the 84 individual samples, 10 mL milk was taken to compose a pooled sample. To determine the ratio between CALUX and GC-HRMS, the pooled sample was analyzed once with GC-HRMS and twice with CALUX. Table 4 provides an overview of the GC-HRMS and CALUX results for both the PCDD/Fs and the dioxin-like PCBs in the pooled sample. The GC-HRMS TEQ values were calculated with the TEF schemes from 1998 and 2005 [25, 26]. When using the WHO2005-TEQs, the total TEQ for the sum of dioxins, furans and dl-PCBs was 10.7 pg TEQ per g lipid, while by using the WHO1998 TEQ values, a total concentration of 14.3 pg TEQ per g lipid (i.e. 25 % higher) was calculated. As can be seen in Table A.1 (supplementary material), especially the contribution of mono-ortho PCBs and furans to the total TEQ is much lower, when using the WHO2005-TEQ values.
Table 4.
Overview of the GC-HRMS and CALUX results for PCDD/Fs and dioxin-like PCBs in the pooled human milk sample. Both TEF schemes from 1998 and 2005 were used [25, 26].
| pg T(B)EQ/g lipid |
GC-HRMS (TEF 1998) |
GC-HRMS (TEF 2005) |
CALUX sample 1 |
CALUX sample 2 |
Geometric mean CALUX |
Ratio: mean CALUX/GC- HRMS (TEF ‘98/’05) |
|---|---|---|---|---|---|---|
| PCDD/Fs | 8.4 | 6.9 | 10.2 | 12.0 | 11.1 | 1.32/1.60 |
| dl-PCBs | 5.8 | 3.7 | 2.0 | 2.3 | 2.2 | 0.37/0.58 |
| Total dl-compounds | 14.3 | 10.7 | 12.2 | 14.3 | 13.2 | 0.93/1.24 |
In our study, it was seen that CALUX results were higher for the dioxin fraction and lower for the dl-PCB fraction, when compared to GC-HRMS. The CALUX/GC-HRMS ratios were 1.32/1.60 and 0.37/0.58 for the PCDD/Fs and the dl-PCBs, respectively using either the WHO1998 or WHO2005 TEFs. For the sum of the concentration of the two fractions, CALUX/GC-HRMS ratio was closer to 1 (Table 4: 0.93 and 1.24).
Generally, CALUX measurements are higher for the PCDD/F fraction [3, 5] and lower for the dl-PCB fraction [27] when compared to GC-HRMS. Chou et al. [3] calculated CALUX/GC-HRMS ratios for the PCDD/Fs between 1.9 and 2.4 in cow ‘s milk samples, while Van Overmeire et al. [5] found median CALUX/GC-HRMS ratios of 1.58 and 0.26 for the PCDD/Fs and dl-PCBs, respectively. Carbonnelle et al. [27] suggested a three-fold underestimation of the dl-PCB concentration in different matrices when using a mouse hepatoma CALUX bioassay. Studies in our lab on human serum and atmospheric deposition samples, using the H1L7.5c1 cell line, also yielded higher PCDD/F results with CALUX/GC-HRMS ratios of 2.0 and 1.9 (WHO2005 TEFs), respectively [15, 28]. GC-HRMS results for the dl-PCB fraction in these studies were only based on the non-ortho PCBs (for the serum samples) or on PCB 126 (for the deposition samples) and the CALUX/ GC-HRMS ratio can thus not be compared to the results obtained in the present study.
For the total CALUX response of the sum of PCDD/Fs and dl-PCBs in human breast milk, the CALUX and GC-HRMS data are often in quite good agreement. Hui et al. [7] also found similar results for both techniques when using the TEF 1998 values and an (slight) overestimation for CALUX bioassay compared to GC-HRMS when using the TEF 2005 values (ratio CALUX/GC-HRMS: 1.03 (TEF 1998) and 1.38 (TEF 2005)). The degree of overestimation varied among different geographic origins of the mothers, which could be due to a difference in congener pattern in the different regions (ratios varying between 0.97 and 1.30 (TEF 1998), and between 1.37 and 1.54 (TEF 2005)). Hasegawa et al. [29] reported PCDD/F and dl-PCB levels in fish oil that were respectively three times higher and two times lower with the H1L6.1 mouse CALUX bioassay compared to GC-HRMS. For the sum of dioxins, furans and dl-PCBs similar results were found with both methods. Windal et al. [30] reported similar findings for cod liver oil, sea birds, marine mammals and fishes.
According to Hasegawa et al. [29] and Windal et al. [30] the lower dl-PCB values observed with the CALUX bioassay could be due to the difference between the WHO-TEFs and the CALUX-REPs (Relative Potencies), while the differences obtained for the PCDD/Fs were probably due to other AhR agonists. In our study, the lower CALUX-BEQ values of the PCB fraction in the human milk sample could also be explained by the difference between the GC-HRMS-TEF values and the CALUX-mouse REP values. Since no REP values were available for the H1L7.5 mouse cell line, REP values from the H1L6.1 mouse cell line were used [30, 31]. It is however expected that this would not bias the results, since both mouse cell lines gave similar results for the quantification of human serum samples from the Flemish human biomonitoring study FLEHS II [15] and each of these recombinant CALUX cell lines were developed using the identical original mouse hepatoma cells (hepa1c1c7). When using these CALUX-REP values, a CALUX/GC-HRMS ratio of 1.39 was obtained for the dl-PCBs (Table 5). This small overestimation could be due to the slight differences between REP values of the H1L6.1 and H1L7.5 cell lines (resulting from enhanced sensitivity of the H1L7.5 cells), the uncertainty of the CALUX and GC-HRMS measurements, the uncertainty of the CALUX-REP values (various other reported slightly different REP values for the same cell line) [31, 32] and/or to the presence of other AhR agonists in the extract (e.g. brominated compounds for which no REP values are yet established). For the PCDD/Fs, CALUX/GC-HRMS ratios of 1.32, 1.60 and 1.29 were found when using the WHO-TEF 1998, WHO-TEF 2006 and the CALUX-REP for the H1L6.1 cell line, respectively. The higher ratio when using the 2005 TEF values is mostly due to the lower TEF value of the 2,3,4,7,8-PeCDF congener (TEF 1998: 0.5; TEF 2005: 0.3 and mouse REP 0.58), which was present in high concentrations in the pooled human milk sample.
Table 5.
Overview of the CALUX/GC-HRMS ratio of the PCDD/Fs, dl-PCBs and sum of all dl-compounds, when using different TEF and mouse REP values [29, 30].
| pg T(B)EQ/g lipid | Ratio: mean CALUX/GC-HRMS (TEF 1998) |
Ratio: mean CALUX/GC-HRMS (TEF 2005) |
Ratio: mean CALUX/GC-HRMS (REP mouse H1L6.1) |
|---|---|---|---|
| PCDD/Fs | 1.31 | 1.60 | 1.29 |
| dl-PCBs | 0.37 | 0.58 | 1.39 |
| Total dl-compounds | 0.93 | 1.24 | 1.31 |
For the PCDD/F fraction, the discrepancy between CALUX and GC-HRMS will probably be (at least partially) due to other dioxin and furan analogs (i.e. brominated and mixed brominated/chlorinated dioxins and furans). A wide range of AhR agonists and antagonists have been identified and many are present in the environment. Some of these environmental contaminants, like PXDD/Fs, PBBs and PCNs have been detected in food, human breast milk or human blood samples [33–37]. Concentration levels of these compounds are in most cases quite low, but it is difficult to estimate their contribution to the total BEQ/TEQ, since calibration standards are available for only few congeners (which makes quantification of these compounds difficult) and no official TEF or REP values exist [38].
An overview of the concentrations (in pg per g lipid) for some PBDD/F congeners that were analyzed in the pooled human milk sample with GC-HRMS is presented in Table 6. Although only a few congeners were measured, it is clear that these compounds have a relatively low contribution to the total TEQ/BEQ. When using the same TEF/REP values as for the chlorinated analogs, the TEQ for the PBDD/Fs would be 0.42, 0.34 or 0.41 pg TEQ per g lipid (using medium bound GC-HRMS values, and the WHO-TEFs of 1998 or 2005, or the CALUX-REPs from Brown et al. [31], respectively). This would mean an increase of only 5 % to the total PCDD/F WHO-TEQ value and an adapted CALUX/GC-HRMS ratio of 1.26/1.53/1.24. In a human milk study in Sweden (samples collected in 2002–2003), also low concentration levels of brominated compounds were found. The furan congeners 2,3,7,8-TBDF and 2,3,4,7,8-PeBDF were detected at concentrations of 0.55 pg per g lipid and 0.33 pg per g lipid respectively, which is comparable to the concentrations found in the Flemish pooled milk sample. 1,2,3,7,8-PeBDF was not detected in the Swedish milk samples (Flemish rural sample: 0.2 pg per g lipid), while 1,2,3,4,7,8-/1,2,3,6,7,8-HxBDF was found at a concentration of 3.8 pg per g lipid (Flemish rural sample: below LOQ of 0.3 pg per g lipid) [39 and references herein]. Kotz et al. [13] reported mean upper and lower bound PBDD/F TEQs of 1.08 and 0.32 pg WHO-TEQ per g lipid in human milk samples from the third round of the WHO human milk survey (2001–2002). Dominant PBDD/Fs congeners were 2,3,7,8-TBDF (average concentration 0.7 pg per g lipid, range < 0.1 - 2.7 pg per g lipid) and 2,3,4,7,8-PeBDF (average 0.23 pg per g lipid, range < 0.1 - 1.1 pg per g lipid). Some other congeners, e.g. 2,3,7,8-TBDD (concentrations 0.06 - 0.28 pg per g lipid), 1,2,3,7,8-PeBDD (0.14 - 1.0 pg per g lipid), 1,2,3,7,8-PeBDF and 1,2,3,4,7,8-HxBDF could only be quantified in part of the samples. The sum of all PBDD/F congeners contributed about 12 % to the total WHO-TEQ. In human adipose tissue, PBDD/Fs were found in a Swedish study from 2007. Depending on the TEFs/REPs used for calculating the upper and lower bound TEQ, the PBDD/Fs contributed 1 - 15 % to the total PCDD/F WHO-TEQ [39].
Table 6.
Overview of the concentration levels (in pg per g lipid) of some PBDD/F congeners, measured in the pooled human milk sample.
| Polybrominated dioxins | Concentration (pg per g lipid) |
Polybrominated furans | Concentration (pg per g lipid) |
|---|---|---|---|
| 2,3,7,8-TBDD | 0.05 | 2,3,7,8-TBDF | 0.7 |
| 1,2,3,7,8-PeBDD | <0.07 | 1,2,3,7,8-PeBDF | 0.2 |
| 2,3,4,7,8-PeBDF | 0.4 | ||
| 1,2,3,4,7,8/1,2,3,6,7,8- HxBDD |
<0.07 | 1,2,3,4,7,8/1,2,3,6,7,8- HxBDF |
<0.3 |
| 1,2,3,7,8,9-HxBDD | <0.04 | 1,2,3,7,8,9-HxBDF | na |
| 2,3,4,6,7,8-HxBDF | na | ||
| 1,2,3,4,6,7,8-HpBDD | na | 1,2,3,4,6,7,8-HpBDF | na |
| 1,2,3,4,7,8,9-HpBDF | na | ||
| OBDD | na | OBDF | na |
are concentration levels below the LOQ. These concentrations were set at half the LOQ for TEQ determination. na= result not available.
3.3 Spatial- and time trends of the PCDD/F and dl-PCB concentration levels
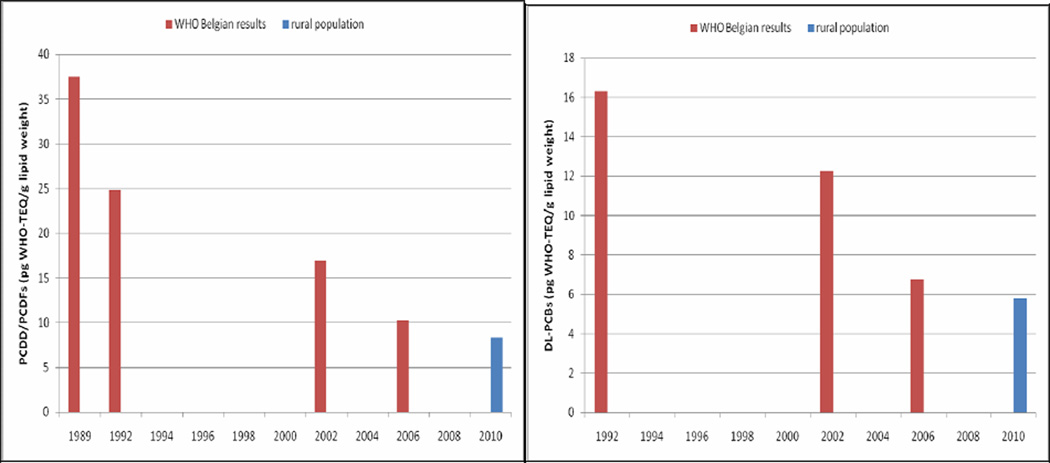
One pooled human milk sample was measured with both CALUX and with GC-HRMS. The GC-HRMS analysis was done in the same lab and following the same protocol as for the WHO human milk surveys, and could therefore be used to study time trends. In the WHO-coordinated human milk surveys (1989–2006) a declining time trend was observed for the Belgian levels of both PCDD/Fs and dl-PCBs. The concentrations of the PCDD/Fs and dl-PCBs measured in the pooled sample of the rural population in 2009–2010 by the WHO reference lab (CVUA, Freiburg, Germany) followed the ongoing declining trend (Figure 1). Furthermore, in all Belgian surveys, it was seen that the concentration of dl-PCBs in human milk was much lower than those of the PCDD/Fs. An overview of the concentrations of the dl-PCBs and PCDD/Fs, measured in different international human biomonitoring studies is presented in Table 1. When comparing these concentrations, one must take into account that the use of TEF 1998 or 2005 values and the selection of CALUX or GC-HRMS as measurement techniques can yield different end results. Therefore, both GC-HRMS (with TEF 1998 and TEF 2005 results) and CALUX results were given for the rural study population to facilitate comparison with international studies. The PCDD/F concentrations in the rural areas of Flanders were comparable to the concentrations found in other European and non-European countries between 2000 and 2007, and to the European mean concentration found in the 3rd and 4th WHO-coordinated human milk survey (8.9 pg WHO-TEQ per g fat, 2001 and 2006, participating countries were Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Finland, Germany, Hungary, Ireland, Luxemburg, The Netherlands, Norway, Romania, Russia, Slovakia, Spain, Sweden and Ukraine). When comparing the Flemish PCDD/F levels, measured in the rural area, to the results from other European countries participating the 4th WHO human milk survey from 2006 (Figure 2), it can be seen that the Belgian levels are still quite high despite the overall declining trend [40]. However, the dl-PCBs levels measured in this study were lower compared to other European and non-European studies.
Figure 1. WHO-TEQ concentration levels of pooled human milk samples measured by GC-HRMS. Comparison of the Belgian results (1989–2006) with the levels measured in this study. Left: PCDD/Fs; right: dl-PCBs.
Figure 2. Overview of the PCDD/F results of four WHO-coordinated human milk surveys in different European countries, and comparison with the WHO-TEQ found in this study. Measurements were done with GC-HRMS and WHO-TEFs from 1998 were used to calculate the TEQ [39].
4. Conclusions
With a new, more sensitive method, dl-PCBs and PCDD/Fs could be quantified in all 84 human milk samples and in the pooled sample. The dl-PCB levels were, however, for some samples close to the quantification limit and due to the decreasing trend of these POPs in Flanders [41], it is possible that for future experiments higher sample volumes (e.g. 7.5 mL) or more sensitive cell lines will be needed.
Multiple regression analysis on the CALUX data showed significant relationships between PCDD/Fs and weight change after pregnancy, smoking and consumption of local eggs, while no significant relationships were found for the dl-PCBs.
In both the individual samples and the pooled human milk sample from the rural area, lower PCDD/F and dl-PCB concentrations were found compared to the former WHO-coordinated human milk surveys (1987–1988, 1992–1993, 2000–2003 and 2005–2006). The dl-PCB levels in the rural area (2009–2010) were lower compared to other international studies, while the PCDD/Fs were still quite high compared to other European countries, participating the 4th WHO human milk survey from 2006.
When comparing CALUX results with GC-HRMS data, higher PCDD/F concentrations were found, which were probably due to the presence of other AhR-active compounds in the extracts. A wide range of AhR agonists and antagonists have been identified and many are present in the environment. Some of these environmental contaminants, like PXDD/Fs, PBBs and PCNs have been detected in food, human breast milk or human blood samples in low concentrations. In our study, while some polybrominated dioxin and furan congeners were measured in the human milk samples, their contribution to the overall TEQ/BEQ was quite low (only 5%). More research is thus needed to identify and quantify the AhR-active compounds in the extracts that contribute to the overall CALUX response. Establishment of REP values for PCDD/Fs, dl-PCBs, brominated analogs and other AhR-active compounds for the sensitive H1L7.5 cell line will be necessary to get more insight in the results.
Supplementary Material
Acknowledgements
The authors acknowledge all mothers who kindly accepted to participate to this study, as well as the fieldworkers, midwifes, gynecologists, doctors and directors of the participating maternities, whose cooperation was essential for the successful results of this study. The authors also acknowledge A. Kotz for the GC-HRMS analysis of the pooled milk sample.
This study was commissioned, financed and steered by the Environment, Nature and Energy Department of the Flemish government.
The H1L7.5c1 cell line was developed by the University of California-Davis (USA) with funding from a Superfund Research Program grant (ES04699) from the National Institute of Environmental Health Sciences.
References
- 1.Schroijen C, Baeyens W, Schoeters G, Den Hond E, Koppen G, Bruckers L, Nelen V, Van De, Mieroop E, Bilau M, Covaci A, Keune H, Loots I, Kleinjans J, Dhooge W, Van Larebeke N. Chemosphere. 2008;71:1317–1325. doi: 10.1016/j.chemosphere.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Koppen G, Den Hond E, Nelen V, Van De Mieroop E, Bruckers L, Bilau M, Keune H, Van Larebeke N, Covaci A, Van De, Weghe H, Schroijen C, Desager K, Stalpaert M, Baeyens W, Schoeters G. Environ Int. 2009;35:1015–1022. doi: 10.1016/j.envint.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Chou IC, Lee WJ, Wang LC, Chang-Chien GP, Lee WS, Lee H. J Hazard Mater. 2008;154:1166–1172. doi: 10.1016/j.jhazmat.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Van Overmeire I, Van Loco J, Roos P, Carbonnelle S, Goeyens L. Talanta. 2004;63:1241–1247. doi: 10.1016/j.talanta.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Van Overmeire I, Carbonnelle S, Schoeters G, Van Cleuvenbergen R, Windal I, Van Wouwe N, Goeyens L. Organohalogen Compounds. 2003;60:243–246. [Google Scholar]
- 6.Leng JH, Kayama F, Wang PY, Nakamura M, Nakata T, Wang Y. Chemosphere. 2009;75:634–639. doi: 10.1016/j.chemosphere.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Hui L, Hedley A, Nelson E, Malisch R, Wong T, Cowling B. Chemosphere. 2007;69:1287–1294. doi: 10.1016/j.chemosphere.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 8.Nelson EAS, Hui L, Wong T, Hedley A. Environmental science & technology. 2006;40:1432–1438. doi: 10.1021/es052164r. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Nakai K, Matsumura T, Suzuki S, Saito Y, Satoh H. Science of the total Environment. 2008;394:39–51. doi: 10.1016/j.scitotenv.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Colles A, Koppen G, Hanot V, Nelen V, Dewolf MC, Noël E, Malisch R, Kotz A, Kypke K, Biot P. Chemosphere. 2008;73:907–914. doi: 10.1016/j.chemosphere.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Croes K, Colles A, Koppen G, Govarts E, Bruckers L, Van de, Mieroop E, Nelen V, Covaci A, Dirtu AC, Thomsen C, Haug LS, Becher G, Mampaey M, Schoeters G, Van Larebeke N, Baeyens W. Chemosphere. 2012;89:988–994. doi: 10.1016/j.chemosphere.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 12.Malisch R, van Leeuwen F. Organohalogen Compounds. 2002;56:317–320. [Google Scholar]
- 13.Kotz A, Malisch R, Kypke K, Oehme M. Organohalogen Compounds. 2005;67:1540–1544. [Google Scholar]
- 14.Hui LL, Hedley AJ, Kypke K, Cowling BJ, Nelson EAS, Wong TW, van Leeuwen FXR, Malisch R. Chemosphere. 2008;73:50–55. doi: 10.1016/j.chemosphere.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 15.Croes K, Van Langenhove K, Den Hond E, Bruckers L, Colles A, Koppen G, Loots I, Nelen V, Schoeters G, Nawrot T, Van Larebeke N, Denison M, Vandermarken T, Elskens M, Baeyens W. Talanta. 2011;85:2484–2491. doi: 10.1016/j.talanta.2011.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denison M, He G, D B, Tsutsumi T. Organohalogen Compounds. 2008;70:772–775. [Google Scholar]
- 17.He G, Tsutsumi T, Zhao B, Baston DS, Zhao J, Heath-Pagliuso S, Denison MS. Toxicological Sciences. 2011;123:511–522. doi: 10.1093/toxsci/kfr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muto H, Takizawa Y. Archives of Environmental Health: An International Journal. 1989;44:171–174. doi: 10.1080/00039896.1989.9935882. [DOI] [PubMed] [Google Scholar]
- 19.Pereira MS. Quimica Nova. 2004;27:934–943. [Google Scholar]
- 20.Hays SM, Aylward LL. Regul Toxicol Pharmacol. 2003;37:202–217. doi: 10.1016/s0273-2300(02)00044-2. [DOI] [PubMed] [Google Scholar]
- 21.Srogi K. Environmental Chemistry Letters. 2008;6:1–28. doi: 10.1007/s10311-007-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbotin D. Convention Conseil Régional n°07450095. 2009 [Google Scholar]
- 23.Kiviranta H, Purkunen R, Vartiainen T. Chemosphere. 1999;38:311–323. doi: 10.1016/s0045-6535(98)00192-1. [DOI] [PubMed] [Google Scholar]
- 24.Lorber M, Phillips L. Environmental health perspectives. 2002;110:A325. doi: 10.1289/ehp.021100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. Toxicol Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van den, Berg M, Birnbaum L, Bosveld A, Brunström B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW. Environmental health perspectives. 1998;106:775. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carbonnelle S, Loco JV, Overmeire IV, Windal I, Wouwe NV, Leeuwen SV, Goeyens L. Talanta. 2004;63:1255–1259. doi: 10.1016/j.talanta.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Croes K, Vandermarken T, Van Langenhove K, Elskens M, Desmedt M, Roekens E, Denison MS, Van Larebeke N, Baeyens W. Chemosphere. 2012;88:881–887. doi: 10.1016/j.chemosphere.2012.03.097. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa J, Guruge KS, Seike N, Shirai Y, Yamata T, Nakamura M, Handa H, Yamanaka N, Miyazaki S. Chemosphere. 2007;69:1188–1194. doi: 10.1016/j.chemosphere.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Windal I, Van Wouwe N, Eppe G, Xhrouet C, Debacker V, Baeyens W, De Pauw E, Goeyens L. Environ Sci Technol. 2005;39:1741–1748. doi: 10.1021/es049182d. [DOI] [PubMed] [Google Scholar]
- 31.Brown DJ, Chu MD, Van Overmeire I, Chu A, Clark G. Organohalogen Compounds. 2001;53:211–214. [Google Scholar]
- 32.Samara F, Gullett BK, Harrison RO, Chu A, Clark GC. Environment international. 2009;35:588–593. doi: 10.1016/j.envint.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Gieroń J, Grochowalski A, Chrząszcz R. Chemosphere. 2010;78:1272–1278. doi: 10.1016/j.chemosphere.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 34.Gomara B, Herrero L, Pacepavicius G, Ohta S, Alaee M, Gonzalez MJ. Chemosphere. 2011;83:799–805. doi: 10.1016/j.chemosphere.2011.02.080. [DOI] [PubMed] [Google Scholar]
- 35.Fernandes A, Tlustos C, Rose M, Smith F, Carr M, Panton S. Chemosphere. 2011 doi: 10.1016/j.chemosphere.2011.06.093. [DOI] [PubMed] [Google Scholar]
- 36.Horii Y, Jiang Q, Hanari N, Lam PKS, Yamashita N, Jansing R, Aldous KM, Mauer MP, Eadon GA, Kannan K. Environmental science & technology. 2010;44:5188–5194. doi: 10.1021/es100282d. [DOI] [PubMed] [Google Scholar]
- 37.Alaee M, Pacepavicius G, Reiner E, MacPherson K, Fayez L, Nakao T, Ohta S. Organohalogen Compounds. 2008;70:768–771. [Google Scholar]
- 38.Ohta S, Tokusawa H, Nakao T, Aozasa O, Miyata H, Alaee M. Chemosphere. 2008;73:S31–S38. doi: 10.1016/j.chemosphere.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 39.Ericson Jogsten I, Hagberg J, Lindström G, Bavel B. Chemosphere. 2010;78:113–120. doi: 10.1016/j.chemosphere.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. European Environment and Health Information System (ENHIS) 2009
- 41.Colles A, Koppen G, Van De, Mieroop E, Covaci A, Croes K, Kotz A, Mampaey M, Schoeters G. Organohalogen Compounds. 2011;73:1555–1558. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.