Abstract
Bacterial conjugation is the process by which a conjugative plasmid transfers from donor to recipient bacterium. During this process, single-stranded plasmid DNA is actively and specifically transported from the cytoplasm of the donor, through a large membrane-spanning assembly known as the pore complex, and into the cytoplasm of the recipient. In Gram negative bacteria, construction of the pore requires localization of a subset of structural and catalytically active proteins to the bacterial periplasm. Unlike the cytoplasm, the periplasm contains proteins that promote disulfide bond formation within or between cysteine-containing proteins. To ensure proper protein folding and assembly, bacteria employ periplasmic redox systems for thiol oxidation, disulfide bond/sulfenic acid reduction, and disulfide bond isomerization. Recent data suggest that plasmid-based proteins belonging to the disulfide bond formation family play an integral role in the conjugative process by serving as mediators in folding and/or assembly of pore complex proteins. Here we report the identification of 165 thioredoxin-like family members across 89 different plasmid systems. Using phylogenetic analysis, all but nine family members were categorized into thioredoxin-like subfamilies. In addition, we discuss the diversity, conservation, and putative roles of thioredoxin-like proteins in plasmid systems, which include homologs of DsbA, DsbB, DsbC, DsbD, DsbG, and CcmG from Escherichia coli, TlpA from Bradyrhizobium japonicum, Com1 from Coxiella burnetii, as well as TrbB and TraF from plasmid F, and the absolute conservation of a disulfide isomerase in plasmids containing homologs of the transfer proteins TraH, TraN, and TraU.
Keywords: plasmid, thioredoxin, oxidase, reductase, isomerase, disulfide
1. Introduction
1.1 The mechanism of bacterial conjugation
Self-transmissible conjugative plasmids encode nearly all of the machinery required to promote their horizontal transfer. The much-studied F plasmid is representative of conjugative plasmids. Like all conjugative plasmids, F encodes an array of proteins that combine to carry out DNA transfer. The transfer (tra) region of F plasmid encodes proteins that assemble into the pore complex, aid in the assembly of the pore complex, or play a direct role in the transfer of plasmid DNA (Firth 1996, Frost 1994).
For F and many other plasmids, bacterial conjugation initiates when a bacterium harboring a conjugative plasmid (donor) extends filamentous structures known as pili into the extracellular environment. When one of these pili contacts a non-plasmid-containing bacterium (recipient), the pilus retracts via disassembly, bringing the bacteria together (Curtiss 1969, Firth 1996, Helmuth 1978, Novotny 1968, Ou 1970). After retraction of the pilus, the donor and recipient bacterium form a stable mating pair, and at this point the plasmid DNA is transferred (Achtman 1975, Achtman 1977). Concurrent with pilus extension is the formation of a relaxase-ssDNA complex (Zechner 2000). After formation of the stable mating pair, the relaxase-ssDNA complex is delivered to the pore complex where it is translocated through the pore in an ATP-dependent manner (Gomis-Rüth 2002). After transport into the recipient bacterium, the relaxase ligates the linear, single-stranded copy of plasmid DNA (Dostál 2011, Sherman 1994). With the culmination of second strand synthesis, the recipient cell is considered F positive, as it can now act as a donor in subsequent rounds of bacterial conjugation. For additional information on these topics, the reader is directed to reviews from Firth (Firth 1996), Lawley (Lawley 2003), and Zechner (Zechner 2012).
1.2 Periplasmic transfer proteins
The tra operon of plasmid F encodes 35 different proteins. Ten of these tra genes encode proteins that either fully or partially reside in the bacterial periplasm, several of which contain multiple cysteine residues (Table 1). The E. coli periplasm is home to an array of enzymes that promotes the formation of inter- and intramolecular disulfide linkages. For proteins containing large numbers of cysteines, an enormous number of redox states are theoretically possible (Benham 1993, Cantor 1980, Kauzzmann 1959, Sela 1959). We have calculated the theoretical numbers of redox states and cysteine connectivities for F-encoded periplasmic tra proteins that reside, at least partially, in the periplasm (Table 1). Although these calculations give insight into theoretical complexities, the constraints imposed by the conformations of the folded or folding protein effectively reduces the actual number of redox states a protein can adopt (Bardwell 1993, Martin 1993, Wunderlich 1993). Nevertheless, the conservation of periplasmic redox systems highlights the importance of thioredoxin-like proteins to both the folding and maintenance of disulfide bond-containing proteins in the periplasm.
Table 1.
Periplasmic F plasmid tra proteins containing one or more cysteine residues
| Protein | Localization | No. Cys | theoretical redox states |
cysteine connectivity |
|---|---|---|---|---|
| TraK | P/OM | 1 | 1 | - |
| TraW | P | 1 | 1 | - |
| TraB | P/IM | 2 | 2 | 1 |
| TraF | P | 2 | 2 | 1 |
| TrbB | P | 2 | 2 | 1 |
| TrbC | P | 2 | 2 | 1 |
| TraP | C/IM/P | 6 | 76 | 15 |
| TraH | P/OM | 7 | 232 | 15 |
| TraU | P | 10 | 9496 | 945 |
| TraN | P/OM | 22 | 6.20×1011 | 1.37×1010 |
1.3 Periplasmic redox systems
Gram negative bacteria can express a number of thioredoxin (trx)-like family members in their periplasm (Table 2). These proteins, collectively known as thiol-oxidoreductases, contain a thioredoxin-like fold and a C-X-X-C (C = Cys, X = any residue) redox active site that is responsible for performing specific redox chemistries on protein substrates. One subset of these proteins, the disulfide bond formation (Dsb) family, can be grouped into two primary functional groups (Figure 1). The first of these, the DsbB-dependent family, is responsible for the de novo synthesis of disulfide bonds through direct oxidation of free thiols (Darby 1995, Hiniker 2003, Nakamoto 2004). The second group, the DsbD-dependent family, is responsible for the shuffling of incorrect disulfide bonds in protein substrates (DsbC) or maintenance of single unpaired cysteines (DsbG) (Andersen 1997, Bessette 1999, Depuydt 2009, Missiakas 1994, Rietsch 1996, Shevchik 1994). The distinguishing characteristic of these families is their dependence on one of a pair of inner membrane proteins (DsbB or DsbD) for proper maintenance of their redox states (Bader 1999, Bessette 1999, Rietsch 1997).
Table 2.
Thioredoxin-like families overview
| Family | Prototype Host | Localization | Function |
|---|---|---|---|
| DsbA | E. coli | P | thiol-oxidase; de novo disulfide bond formation |
| DsbB | E. coli | P/IM | redox maintenance of periplasmic thiol-oxidases |
| DsbC | E. coli | P | disulfide bond isomerase, molecular chaperone |
| DsbD | E. coli | P/IM | redox maintenance of periplasmic disulfide bond isomerases and disulfide reductases |
| DsbG | E. coli | P | sulfenate reductase, disulfide bond isomerase, molecular chaperone |
| CcmG | E. coli | P/IM | disulfide reductase, cytochrome c maturation |
| TlpA | B. japonicum | P/IM | unknown, putative disulfide reductase, cytochrome aa3/c maturation |
| Com1 | C. burnetii | P | unknown, putative thiol-oxidase or disulfide bond isomerase |
| TrbB | Plasmid F (E. coli) | P | disulfide bond isomerase |
| TraF | Plasmid F (E. coli) | P | putative molecular chaperone, lacks C-X-X-C active site |
Figure 1.
Mechanisms of DsbB- and DsbD-dependent proteins within the E. coli periplasmic redox system. (A) The periplasmic thiol-oxidase DsbA incorporates a disulfide linkage into a substrate protein, becoming reduced in the process. After reduction, DsbA donates electrons from its active site to the inner membrane protein DsbB in order to reoxidize its active site to reassume its catalytically active state. DsbB will then pass these electrons to ubiquinone (UQ) or menaquinone (MQ) and enter the electron transport chain where they will eventually be passed to terminal electron acceptors. (B) The disulfide bond isomerase DsbC and the disulfide bond isomerase/sulfenate (−SOH) reductase DsbG are both active in their reduced states. Upon isomerization, as shown for DsbC, or sulfenate reduction of substrates, as shown for DsbG, the redox active sites can become oxidized. To reassume their active states, DsbC and DsbG accept electrons from the periplasmic αdomain of the inner membrane protein DsbD. DsbD, in turn, will re-reduce itself by accepting electrons from the cytoplasmic protein thioredoxin A (TrxA), and shuttling them from the cytoplasm, through the inner membrane, and finally into the periplasm.
1.3.1 De novo disulfide bond formation
As a protein containing an N-terminal signal peptide is secreted into the periplasm, DsbA, a 21 kDa monomeric thioredoxin-like family member that is active in its oxidized state (Figure 2), incorporates disulfide bonds between consecutive cysteine residues in the unfolded or partially folded protein substrate (Bardwell 1993, Martin 1993, Wunderlich 1993). In isolated cases, however, DsbA has been shown to promote proper folding of proteins containing nonconsecutive disulfide bonds, as exemplified in studies utilizing RNAse I (Messens 2007). As DsbA oxidizes its substrate, the C-P-H-C (C = Cys, P = Pro, H = His) active site of DsbA is reduced, rendering it inactive. To reoxidize its active site, DsbA donates electrons to the inner membrane protein DsbB (Figure 1) (Bardwell 1993). DsbB is a 20 kDa monomeric protein with four membrane-spanning regions that each contain an essential cysteine (C41, C44, C104, and C130) positioned in the periplasmic space (Inaba 2006, Malojci 2008, Zhou 2008). DsbB accepts electrons from the DsbA active site and donates them to electron acceptors such as ubiquinone (aerobic conditions) or menaquinone (anaerobic conditions) in the inner membrane (Figure 1). Ubiquinone is then oxidized by cytochrome terminal oxidases within the electron transport chain while menaquinone transfers electrons to alternate final electron acceptors such as fumarate or nitrate (Bader 1999, Kobayashi 1997, Kobayashi 1999, Regeimbal 2002). After resolution of the DsbA-DsbB complex, the C-X-X-C active site of DsbA is oxidized, resulting in a recharged and enzymatically functional protein (Bardwell 1993).
Figure 2.
High-resolution structures of prototypical thioredoxin-like family proteins. Shown are cartoon model representations of the structures of thioredoxin-like proteins (clockwise from top-left) DsbA (PDB 1DSB), DsbB (PDB 2K73), and CcmG (PDB 1KNG) from E. coli, TlpA (PDB 1JFU) from B. japonicum, and lastly, DsbG (PDB 1V57) and DsbC (PDB 1EEJ) from E. coli. Shown in red spheres are the redox active cysteines within each protein. Absent are Com1 from C. burnetii, TraF and TrbB from plasmid F, and DsbD from E. coli, for which high resolution structures are not available.
1.3.2 Disulfide bond isomerization
There are estimated to be over 300 different E. coli proteins that localize to the periplasm (Dutton 2008, Hiniker 2004). Of these proteins, only a few, including RNase I, MepA, AppA, and End1, have been found to require a non-consecutive disulfide bond for proper folding and activity (Berkmen 2005, Hiniker 2004). DsbA-catalyzed disulfide bond formation can trap these proteins in non-native states. Additionaly, random oxidation events and environmental stresses can also cause errant bond formation in cysteine-containing periplasmic proteins (Hiniker 2005). To resolve these incorrect bonds, bacteria utilize a secondary pathway composed of the periplasmic disulfide isomerase DsbC and the inner membrane protein DsbD to shuffle disulfide bonds (Berkmen 2005, Hiniker 2004, Rietsch 1996, Shevchik 1994, Zapun 1995).
DsbC, 23 kDa as a monomer, functions as a homodimer (Missiakas 1994, Shevchik 1994). Dimerization results in the formation of a V-shaped binding pocket lined with hydrophobic and uncharged residues (Figure 3) (Banaszak 2004, McCarthy 2000). DsbC is thought to recognize misfolded protein substrates through exposed hydrophobic patches on the protein surface (Darby 1998). Using this method of substrate recognition, DsbC can also promote protein folding as a molecular chaperone for non-cysteine containing proteins (Chen 1999). Each monomer of DsbC contains a C-G-Y-C (C = Cys, G = Gly, Y = Tyr) redox active site, in principle allowing the homodimer to catalyze multiple rounds of disulfide isomerization before becoming fully oxidized. In addition to disulfide isomerase and chaperone activity, dimeric DsbC was also shown to promote substrate reduction at levels similar to TrxA in vitro (Zhao 2003). Interestingly, experiments performed with a single amino acid substitution, DsbC G49R, which disrupts dimerization via electrostatic repulsion in the N-terminal dimerization domain (Bader 2001), or with a truncation mutant lacking a dimerization domain (Sun 2000) reveal that monomeric DsbC retains its disulfide reductase activity, but can no longer catalyze disulfide bond isomerization or function as a molecular chaperone.
Figure 3.
Comparison of reduced forms of DsbC and DsbG homodimers. Space filling models of DsbC (A, pdb 1TJD) and DsbG (B, pdb 1V58) structures displaying hydrophobic and non-charged residues (green), acidic residues (red), basic residues (blue), and the C-X-X-C active sites (yellow). Note the enlongated V-shaped binding cleft of DsbG (43.3A internal, 100.3A external) in comparison to DsbC (27.6A internal, 76.5A external). Additionally, note the presence of acidic residues within the binding cleft of DsbG (B, bottom, red residues), while the DsbC binding cleft (A, bottom) contains only hydrophobic and uncharged residues.
After oxidation of its redox active site, DsbC interacts with and directly accepts electrons from the inner membrane protein DsbD (Figure 1) (Bessette 1999, Missiakas 1995, Rietsch 1997). DsbD is a 59 kDa monomeric inner membrane protein that consists of three domains: an N-terminal periplasmic αdomain (DsbDα) (Haebel 2002), an inner membrane spanning central β domain (DsbDβ) that possesses 8 transmembrane regions (Cho 2007), and a C-terminal periplasmic γdomain (DsbDγ) (Rozhkova 2004). DsbD accepts electrons from cytoplasmic thioredoxin, and shuttles them sequentially through a well-defined cascade that includes cysteine pairs within each of its three domains. Electrons from cytoplasmic thioredoxin are initially passed to a cysteine pair in DsbDβ, then to DsbDγ, and finally to DsbDα,where they are directly exchanged with DsbC after formation of a transient, but specific, DsbC/DsbD complex (Cho 2007, Katzen 2000, Katzen 2003, Stewart 1999). A recent bioinformatics analysis has revealed that the DsbD-like family can be further subdivided into three classes, which include DsbD-like proteins, CcdA-like proteins, and ScsB-like proteins (Cho 2012a, Cho 2012b). In comparison to the DsbD-like proteins described above, the N-terminal alpha domain of ScsB-like proteins is considerably different. As the N-terminal alpha domain facilitates substrate interactions, this difference suggests that although functionally similar, DsbD-like and ScsB-like families possess unique sets of substrates. Alternatively, CcdA-like proteins differ significantly from both their DsbD-like and ScsB-like relatives, lacking both the alpha and gamma periplasmic domains and possess only six transmembrane regions in comparison to the eight transmembrane regions found in the DsbD-like and ScsB-like families (Cho 2012b).
1.3.3 Maintenance of free thiols
DsbD is also responsible for the redox maintenance of the periplasmic thioredoxin-like family member DsbG. DsbG is a 26 kDa monomer and, like DsbC, exists as a functional V-shaped homodimer (Figure 3) (Heras 2004). DsbG has a C-P-Y-C (C = Cys, P = Pro, Y = Tyr) redox active site in each of its monomers, both of which are catalytically functional. DsbG was originally proposed to be a secondary disulfide isomerase in bacterial systems due to its ability to catalyze disulfide isomerization in vitro, albeit at lower rates than DsbC (Andersen 1997, Bessette 1999, Shao 2000). Structural comparisons of DsbC and DsbG revealed significant differences in their V-shaped binding pockets (Figure 3) (Heras 2004). While the binding pocket of DsbC is lined with uncharged and hydrophobic residues, the pocket of DsbG, which is wider than that of DsbC, contains several acidic residues, suggesting that DsbG is more likely to interact with folded protein substrates. Recent studies by Depuydt, et al., (Depuydt 2009) revealed that DsbG preferentially interacts with the periplasmic proteins YbiS, ErfK, and YnhG, each of which are periplasmic L,D-transpeptidases containing a single unpaired cysteine in their native structure. In vivo, unpaired cysteines can be oxidized by radical oxidative species to form sulfenic acids (-SOH), while further oxidation leads to irreversible formation of sulfinic (-SO2H) and/or sulfonic (-SO3H) acids (Hamann 2002, Poole 2004). Depuydt, et al. were able to convincingly demonstrate that while DsbG can act as a disulfide bond isomerase, its primary responsibility is to maintain the reduced state of free thiols within the bacterial periplasm.
1.4 Cytochrome maturation proteins
A secondary set of thioredoxin-like proteins in the bacterial periplasm function in cytochrome maturation. Although cytochrome maturation and disulfide bond formation and maintenance are two distinct processes, both appear to rely on the same inner membrane proteins for redox state maintenance (Katzen 2000).
CcmG is a 21 kDa inner-membrane associated periplasmic thioredoxin-like protein that belongs to the cytochrome c maturation (Ccm) family within the cytochrome c biogenesis system I in E. coli (Figure 2) (Fabianek 1998). This system is responsible for the posttranslational attachment of heme to the C-X-X-C-H (C = Cys, X = any residue, H = His) motif of apocytochrome c in the bacterial periplasm (Stevens 2011). Although the specific details of the pathway for heme attachment are unclear, previous studies have demonstrated that CcmG: (1) exhibits specific reductase activity within the cytochrome c biogenesis system (Fabianek 1998, Fabianek 1999), (2) is maintained in its reduced state by the periplasmic redox system protein DsbD (Katzen 2000, Stirnimann 2005), and (3) is able to associate with apocytochrome c in a cysteine-independent manner (Turkarslan 2008). In one model, CcmG directly reduces the C-X-X-C-H motif in apocytochrome c, effectively preparing it for heme attachment (Edeling 2002). In an alternative model, a second Ccm family member, CcmH, acts as an intermediate. In this model, CcmG reduces the C-X-X-C motif of CcmH, which then reduces apocytochrome c (Di Matteo 2007, Meyer 2005). Although CcmH contains a C-X-X-C motif, it lacks a thioredoxin-like fold, and therefore will not be discussed further in this review.
TlpA is a 23 kDa, monomeric, inner-membrane-anchored thioredoxin-like protein localized to the periplasm (Figure 2). TlpA was first discovered due to its role in the maturation of cytochrome aa3 in Bradyrhizobium japonicum (Loferer 1993, Loferer 1995b). More recently, TlpA has been associated with both the biogenesis of cytochrome c and the bacterial oxidative stress response in Agrobacterium tumefaciens (Tanboon 2009). Unlike CcmG, whose reductase activity is specific for the cytochrome maturation system, TlpA is able to directly reduce disulfide bonds in insulin in vitro (Loferer 1995a). Although the precise role and/or molecular targets of TlpA in cytochrome maturation are unknown, biophysical characterization has led to the hypothesis that like CcmG, TlpA is acting as a disulfide reductase (Loferer 1995).
1.5 The Com1-like family
Com1 of the rickettsial pathogen Coxiella burnetti is a 27 kDa, outer-membrane-associated thioredoxin-like family member (Hendrix 1993). Initially, Com1-like proteins were considered to be a prototype for a subgroup of the DsbA-like family, but this classification has recently been challenged (Jameson-Lee 2011). Our current knowledge of Com1-like proteins derives largely from studies on the Com1-like protein DsbA2 from Legionella pneumophila. Characterization of DsbA2 and the systems in which it is encoded reveals several important features. First, it was found that the DsbA2 lineage appears to be conserved in, but limited to bacteria that express a type IV secretion system. Moreover, bacteria encoding DsbA2 lack DsbC-like proteins. Finally, it was noted that a BLAST search utilizing DsbA2 as the query did not capture DsbA (Jameson-Lee 2011). This evidence suggests that Com1-like proteins may not be a DsbA-like subgroup. Interestingly, when we analyzed the Com1 protein sequence using the Conserved Domain Database (CDD) (Marchler-Bauer 2011) we found that Com1 appeared to contain a DsbA-like thioredoxin-like fold, but overall was more reminiscent of the DsbG-like family. To date there have been no published functional or structural studies of Com1-like proteins, leaving us with little insight to the functional role of the Com1-like family of proteins.
2. Previous studies on thioredoxin-like proteins in conjugative plasmids
The first thioredoxin-like protein identified in a conjugative system was the 181-residue periplasmic protein TrbB, found during the sequence analysis of genes in the F tra operon (Wu 1987). Subsequent experiments revealed no obvious difference in mating efficiency for a donor harboring a trbB−plasmid, and the protein was deemed nonessential (Kathir 1991). Recently, however, Elton, et al. reported that in a dsbC− background, a donor cell harboring a trbB− plasmid exhibits a small but significant 10-fold decrease in mating efficiency that can be restored by in trans complementation with either DsbC or TrbB (Elton 2005). These authors also used a bioinformatics approach to identify TrbB-like proteins in several plasmids. Furthermore, they identified several plasmids that contain homologs of the putative disulfide isomerase DsbCR27, a DsbC-like protein encoded in the IncH plasmid R27 (Sherburne 2000), and TraF, an essential periplasmic thioredoxin-like family member encoded in plasmid F that lacks a C-X-X-C redox motif (Frost 1994, Wu 1988). Interestingly, TrbB-like and DsbC-like proteins were not found within the same conjugative systems, suggesting these two proteins may be functionally similar. TraF, which shares homology with TrbB (Elton 2005), was later found to associate with an outer membrane complex consisting of several tra proteins, including TraV and TraH, which may anchor TraF to the complex (Arutyunov 2010). Attempts to introduce a C-X-X-C active site into TraF based on homology studies with C-X-X-C-containing TraF-like family members resulted in a protein that was able to rescue mating in a traF−plasmid, but unable to complement DTT hypersensitivity, suggesting it lacks DsbC-like activity (Elton 2005, Missiakas 1994).
Following this, we examined the activity of TrbB from plasmid F, showing that it can catalyze both disulfide bond isomerization and reduction in substrates not involved in conjugation, and demonstrating that it can partially rescue mating of plasmid R27 in a dsbC− / dsbCR27− background (Hemmis 2011). In addition, we found that, like DsbC, the TrbB redox active site (C-P-Y-C, where C= Cys, P = Pro, Y = Tyr) is maintained in the reduced state in vivo by DsbD. In the absence of the bacterially encoded DsbD, TrbB accumulates in an oxidized and inactive state.
Secondary structure comparisons between TrbB and DsbC reveal that TrbB lacks a conserved N-terminal dimerization domain (residues 1–61 in DsbC) and a helical subdomain (residues 123 to 166 in DsbC), both features of DsbC-like and DsbG-like families (Hemmis, manuscript in preparation; Heras 2004, McCarthy 2000). Analytical ultracentrifugation (Hemmis 2011) and size exclusion chromatography (Hemmis, unpublished data) each show that TrbB exists as a 17.7 kDa monomer, suggesting that unlike the dimeric DsbC and DsbG, TrbB may function as a monomer. Additional work has revealed a direct interaction between TrbB and the periplasmic αdomain of DsbD, consistent with a mechanism in which electrons are transferred directly between the bacterial and plasmid redox systems (Hemmis, manuscript in preparation). This discovery, along with structural characterization of the CcmG/DsbDαcomplex (Stirnimann 2005), reveals that dimerization is not necessary for facilitating a direct interaction with DsbD, in contrast to earlier models (Goldstone 2001, Haebel 2002, Inaba 2006).
A model for TrbB function was developed based upon the experimental evidence from these studies (Figure 4). In this model, a functionally active TrbB protein, in its reduced state, will form a disulfide linked enzyme-substrate complex with its substrate. For plasmid F, this substrate may be TraH, TraN, TraP or TraU, each of which contains at least six cysteine residues (Table 1). Depending on the origin of the secondary attacking cysteine, the disulfide bond will either be directly incorporated into the substrate protein (isomerization pathway), or the substrate will become completely reduced (reductase pathway). In the case of complete reduction, subsequent formation of the new disulfide bond requires a separate oxidation event that is facilitated by either DsbA or a reactive oxygen species. It is possible that a single disulfide isomerization event will fail to yield a properly folded substrate; therefore multiple rounds of disulfide reduction/oxidation may be required for the substrate to assume its native state. A similar mechanism has been previously described for the disulfide reductase TrxP from Bacteriodes fragilis (Shouldice 2010).
Figure 4.
Proposed mechanism of TrbB function on a substrate containing three cysteines. Reduced TrbB recognizes a substrate and forms a transient enzyme:substrate complex by means of a direct attack from the N-terminal active site cysteine (C81) of TrbB. Upon formation of the complex, TrbB is thought to catalyze isomerization by one of two pathways, depending on the origin of the resolving cysteine. (1) When the C-terminal cysteine in the TrbB active site (C84) acts as the resolving cysteine, the substrate becomes fully reduced and TrbB becomes fully oxidized. This mechanism then requires a separate oxidation event to re-oxidize the substrate. This event most likely occurs as a second interaction with DsbA or a reactive oxygen species. Oxidized TrbB will then interact with and accept electrons from the inner membrane protein DsbD, effectively recharging it for additional rounds of catalysis. (2) Alternatively, when the resolving cysteine originates from the substrate protein, a new disulfide linkage is directly introduced, and TrbB is released in its reduced state. In this mechanism, TrbB remains active and can immediately catalyze additional rounds of disulfide isomerization. As the folding of the substrate is not directed by the disulfide isomerase, it is possible that a non-native disulfide bond may be reintroduced, making additional rounds of disulfide isomerization necessary.
Plasmid F also encodes two periplasmic proteins, TraK and TraW, which contain only a single cysteine residue. A yeast-two-hybrid performed by Harris and Silvermen (Harris 2004) identified a putative TraW/TrbB interaction. Based on this observation, we can speculate that in addition to disulfide isomerization, TrbB may also participate in maintaining these free thiols, much like DsbG in the bacterial redox system.
3. Identification of thioredoxin-like proteins in conjugative systems
Proteins annotated as disulfide isomerases, thiol-disulfide interchange proteins, and protein disulfide oxidoreductases were previously identified in enterobacteria and analyzed phylogenetically (Hemmis 2011). These studies revealed that TrbB-like proteins cluster independently and distinctly from all other dsb families. In addition, we found that each of the proteins clustered into the TrbB-like family are either encoded in a conjugative plasmid or within a genomic island on a bacterial chromosome corresponding to a plasmid transfer operon, commonly referred to as integrative and conjugative elements (ICEs). These results, combined with information derived from the biophysical characterization of TrbB, led us to conclude that TrbB is the prototype of a previously unrecognized family of plasmid-based disulfide bond isomerases.
No analogous examination of thioredoxin-like families within plasmid systems has been published. To address this issue, we report here our search for plasmid-encoded thioredoxin-like proteins. Annotated genes from plasmids were obtained from GenBank (http://www.ncbi.nlm/nih/gov/GenBank) and grouped into mobility (MOB) families as specified by the relaxase-based classification system proposed by de la Cruz and colleagues (Garcillán-Barcia 2009). Putative thioredoxin-like proteins were identified via a two-step selection process. Proteins were first selected based on the presence of a C-X-X-C motif in their protein sequence. Proteins meeting this criterion were then examined for conserved domains using the CDD (Marchler-Bauer 2011) using an E value cutoff of 1E–20, which should reveal only highly significant matches within the database. Those proteins containing a C-X-X-C motif that were categorized as thioredoxin-like superfamily members were included in our final list (Table S1). This search identified 165 thioredoxin-family members that are encoded in plasmids from five of the seven MOB families. Of these proteins, 60 are encoded in MOBF plasmids, 52 in MOBH plasmids, 41 in MOBP plasmids, 11 in MOBQ plasmids, while only a single thioredoxin-like family member is encoded in a MOBC plasmid (Table 3). Thioredoxin family members were not found in MOBV or MOBHEN plasmids. (The reasons for the absence of thioredoxin-like proteins in MOBv or MOBHEN plasmids are not obvious given the diversity of their hosts. While MOBHEN plasmids have been isolated exclusively from Gram-negative bacteria, including many species potentially pathogenic to humans, MOBV plasmids have been isolated from both Gram-negative and Gram-positive bacteria that occupy various niches.) Among the proteins identified are homologs of DsbA, DsbB, DsbC, DsbG and DsbD from the periplasmic redox system, CcmG and TlpA from cytochrome maturation systems, and TrbB and TraF from plasmid F. With the continuing inclusion of newly discovered plasmid systems, additional sequencing of plasmid genomes, and better annotation of open reading frames, the list of thioredoxin-like proteins in plasmid systems will grow.
Table 3.
Thioredoxin-like proteins within MOB families
| Protein Family | MOBF | MOBH | MOBP | MOBQ | MOBC | MOBV | MOBHEN |
|---|---|---|---|---|---|---|---|
| DsbA | 2 | 1 | 0 | 0 | 0 | 0 | 0 |
| DsbB | 0 | 1 | 5 | 0 | 0 | 0 | 0 |
| DsbC | 5 | 18 | 0 | 0 | 0 | 0 | 0 |
| DsbD | 0 | 2 | 2 | 0 | 0 | 0 | 0 |
| DsbG | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| CcmG | 0 | 0 | 10 | 0 | 0 | 0 | 0 |
| TlpA | 11 | 1 | 0 | 2 | 0 | 0 | 0 |
| Com1 | 9 | 18 | 20 | 0 | 0 | 0 | 0 |
| TrbB | 28 | 0 | 0 | 1 | 0 | 0 | 0 |
| TraF | 5 | 10 | 0 | 0 | 0 | 0 | 0 |
| Other | 0 | 0 | 4 | 8 | 1 | 0 | 0 |
| TOTAL | 60 | 52 | 41 | 11 | 1 | 0 | 0 |
3.1 Size distribution and mobility of trx-like containing plasmids
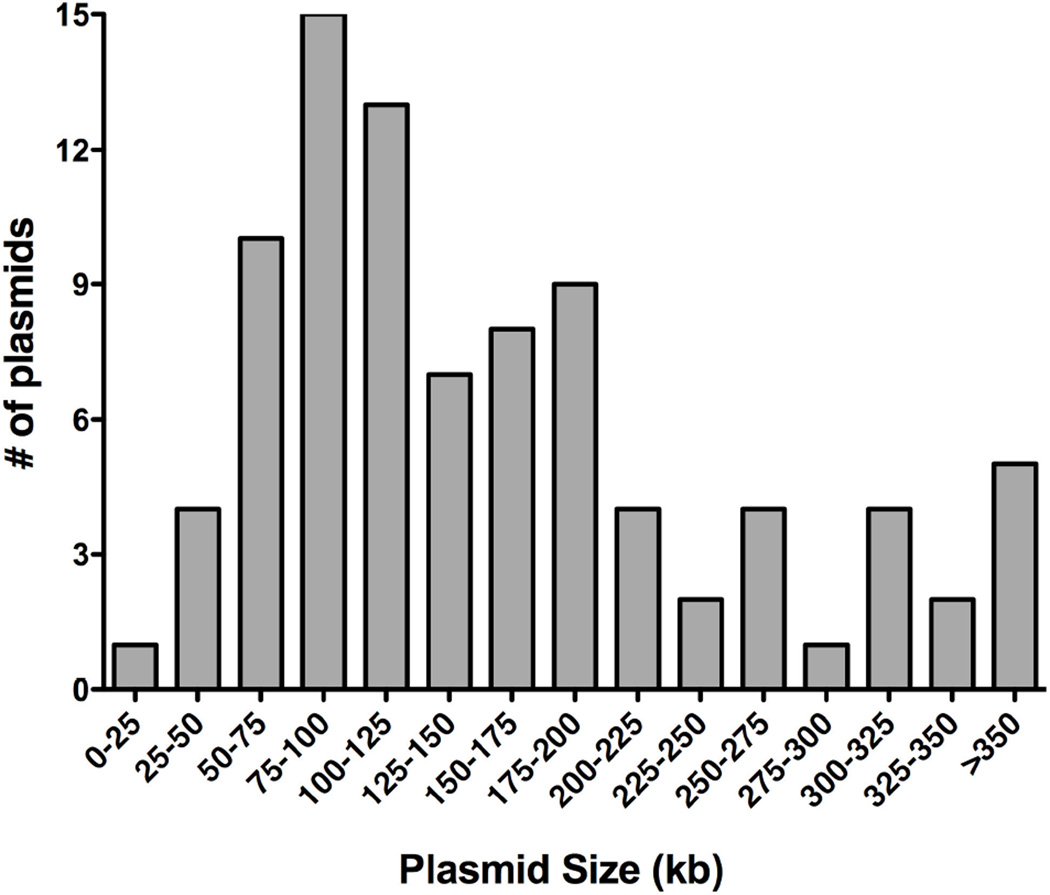
Proteins identified in this study were encoded across 89 different plasmid systems. The size of these plasmids ranges from 9 kbp (pLF5) to 1.68 Mbp (pSymB). A frequency distribution analysis shows a majority of these plasmids are between 90 kbp and 205 kbp in size, with the median size being 126 kbp (Figure 5). The size distribution of plasmids containing thioredoxin-like proteins is similar to that for conjugative plasmids as illustrated by Smillie, et al. (Smillie 2010). A closer inspection shows that 79 of the 89 plasmids (89%) containing thioredoxin-like proteins also encode proteins typical of a type IV secretion system/pore complex, suggesting they are conjugative in nature. To date, however, only 30 of the 89 plasmids (33%) have been shown to transfer experimentally (Ahmer 1999, Boyd 2004, Bröker 2008, Chatterjee 1972, Chen 2003, Chen 2007, Coetzee 1972, Falkow 1962, Furuichi 1984, Gniadkowski 1998, Hedges 1975, Jerke 2008, Johnson 2002, Johnson 2006, Kim 2008, Lederberg 1952, Lee 2008, Maher 1993, Paulsen 2003, Rees 1987, Romine 1999, Shintani 2006, Skyberg 2006, Terawaki 1967, van Kranenburg 2005, Wain 2003, Welch 2007, Zienkiewicz 2007). The association of thioredoxin-like proteins with conjugative plasmids suggests that these proteins confer some advantage on the plasmids, not surprising given the complexity of the periplasm-spanning pore complex found in conjugative plasmids.
Figure 5.
Size distribution of plasmids containing thioredoxin-like proteins. Sizes of the 89 plasmids that encode thioredoxin-like family members were obtained from GenBank and grouped using bin sizes of 25kb.
The distribution of the 165 proteins within each plasmid system was also examined to determine how these proteins were spread across the 89 different plasmid systems. An overwhelming majority (78%) of the plasmids encoded only one or two thioredoxin-like proteins (Table 4). Of the remaining plasmids, pK29, R27, 1(c), 1(d), pUTI89, pSN254, and pSymB encoded three proteins, pHCM1, pMAK1, R478, Rts1, pAPEC-01-R, pAA1, pKMSM01, TC1, and pNF1 encoded four proteins, while pCAR1, pDSHI01, and pDSHI03 each encoded six (Table 4; also see Table S1). Of the 20 different plasmids that encode three or more proteins, ten belong to the MOBH family, while five belong to MOBP, four to MOBF, and one to MOBQ families.
Table 4.
Thioredoxin-like proteins by plasmid systems
| Plasmid | MOB | DsbA | DsbB | DsbC | DsbD | DsbG | CcmG | TlpA | Com1 | TrbB | TraF | Other | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pTEF1 | C | - | - | - | - | - | - | - | - | - | - | 1 | 1 |
| 1(a) | F | - | - | 1 | - | - | - | - | - | - | 1 | - | 2 |
| A | F | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| ColBM | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| F | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| MT | F | - | - | - | - | - | - | - | 1 | 1 | - | - | 2 |
| p1658/97 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pAA1 | F | - | - | - | - | - | - | 3 | 1 | - | - | - | 4 |
| pAOVO01 | F | - | - | 1 | - | - | - | - | - | - | 1 | - | 2 |
| pAPEC-02-ColV | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pAPEC-O2-R | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pAsa5 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pC15-1a | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pC4602-1 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pCAR3 | F | - | - | 1 | - | - | - | - | - | 1 | - | 2 | |
| pED208 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pENTE01 | F | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| pG8786 | F | - | - | - | - | - | - | - | 1 | 1 | - | - | 2 |
| pKB1 | F | - | - | - | - | - | - | 1 | 1 | - | - | - | 2 |
| pKPN3 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pKPN4 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pLEW279b | F | - | - | - | - | - | - | 1 | - | - | - | - | 1 |
| pLPL | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pLPP | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pMAR7 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pMKMS01 | F | - | - | - | - | - | - | 2 | 2 | - | - | - | 4 |
| pNL1 | F | - | - | 1 | - | - | - | - | - | - | 1 | - | 2 |
| pNR1 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pO86A1 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pOU1113 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pPBPR1 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pS18501 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pS19501 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pS19502 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pSF0157 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pSLT | F | 1 | - | - | - | - | - | - | - | 1 | - | - | 2 |
| pSWIT01 | F | - | - | 1 | - | - | - | - | - | - | 1 | - | 2 |
| pUTI89 | F | - | - | - | - | - | - | 1 | 1 | 1 | - | - | 3 |
| pYJ016 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| R100 | F | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| TC1 | F | - | - | - | - | - | - | 3 | 1 | - | - | - | 4 |
| 1(b) | H | 1 | - | - | - | - | - | - | - | - | 1 | - | 2 |
| pAGI2 | H | - | - | - | 1 | 1 | - | 1 | 1 | - | - | - | 4 |
| pAPEC-01-R | H | - | - | 2 | - | - | - | - | 1 | - | 1 | - | 4 |
| pASA4 | H | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| pBVIE02 | H | - | - | - | 1 | - | - | - | - | - | - | - | 1 |
| pCAR1 | H | - | 1 | 1 | - | - | - | - | 3 | - | 1 | - | 6 |
| pHCM1 | H | - | - | 2 | - | - | - | - | 1 | - | 1 | - | 4 |
| pIP1202 | H | - | - | 1 | - | - | - | - | 1 | - | - | - | 2 |
| pK29 | H | - | - | 1 | - | - | - | - | 1 | - | 1 | - | 3 |
| pMAK1 | H | - | - | 2 | - | - | - | - | 1 | - | 1 | - | 4 |
| pMAQU02 | H | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| pP91278 | H | - | - | 1 | - | - | - | - | 1 | - | - | - | 2 |
| pSN254 | H | - | - | 2 | - | - | - | - | 1 | - | - | - | 3 |
| pYR1 | H | - | - | 1 | - | - | - | - | 1 | - | - | - | 2 |
| R27 | H | - | - | 1 | - | - | - | - | 1 | - | 1 | - | 3 |
| R391 | H | - | - | 1 | - | - | - | - | - | - | 1 | - | 2 |
| R478 | H | - | - | 2 | - | - | - | - | 1 | - | 1 | - | 4 |
| Rts1 | H | - | - | 1 | - | - | - | - | 2 | - | 1 | - | 4 |
| 2 | P | - | - | - | - | - | 1 | - | - | - | - | - | 1 |
| 1(c) | P | - | - | - | - | - | 2 | - | 1 | - | - | - | 3 |
| 1(d) | P | - | - | - | - | - | - | - | 3 | - | - | - | 3 |
| 3(b) | P | - | - | - | - | - | 1 | - | - | - | - | - | 1 |
| C | P | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| ColIb-P9 | P | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| pBVIE04 | P | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| pCTX-M3 | P | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| pDC3000A | P | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| pDC3000B | P | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| pDSHI01 | P | - | 2 | - | 1 | - | 1 | - | 2 | - | - | - | 6 |
| pDSHI03 | P | - | 2 | - | 1 | - | 1 | - | 2 | - | - | - | 6 |
| pEL60 | P | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| pFL5 | P | - | 1 | - | - | - | - | - | - | - | - | 1 | 2 |
| pNF1 | P | - | - | - | - | - | 2 | - | 1 | - | - | 1 | 4 |
| pSC138 | P | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| pSERB1 | P | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| pSI-1 | P | - | - | - | - | - | 1 | - | 1 | - | - | - | 2 |
| pTEF2 | P | - | - | - | - | - | - | - | - | - | - | 2 | 2 |
| R64 | P | - | - | - | - | - | - | - | 1 | - | - | - | 1 |
| TC2 | P | - | - | - | - | - | 1 | - | - | - | - | - | 1 |
| 3 | Q | - | - | - | - | - | - | 1 | - | - | - | - | 1 |
| pCAUL01 | Q | - | - | - | - | - | - | - | - | - | - | 1 | 1 |
| pLD-TEX-KL | Q | - | - | - | - | - | - | - | - | 1 | - | - | 1 |
| pSMED01 | Q | - | - | - | - | - | - | - | - | - | - | 1 | 1 |
| pRL11 | Q | - | - | - | - | - | - | - | - | - | - | 1 | 1 |
| pSMED02 | Q | - | - | - | - | - | - | - | - | - | - | 1 | 1 |
| pSymA | Q | - | - | - | - | - | - | - | - | - | - | 1 | 1 |
| pSymB | Q | - | - | - | - | - | - | 1 | - | - | - | 2 | 3 |
| pWCFS103 | Q | - | - | - | - | - | - | - | - | - | - | 1 | 1 |
| TOTAL | 2 | 6 | 23 | 4 | 1 | 10 | 14 | 48 | 29 | 15 | 13 | 165 | |
4. Phylogenetic analysis of trx-like proteins
To determine which thioredoxin-like families are represented in conjugative plasmids, a two-part phylogenetic analysis was performed. First, protein sequence alignments were performed for each of the MOB groups using ClustalW with a pairwise alignment method (Larkin 2007) as implemented in the Mega 5 software package (Tamura 2011). After alignment, Maximum-likelihood phylogenetic trees of each group were built with Mega 5 using a bootstrap number of 100. Trees were built in presence of the prototypical dsb proteins DsbA, DsbB, DsbC, DsbD, and DsbG, the putative dsb protein Com1, the cytochrome maturation proteins CcmG and TlpA, and the F plasmid proteins TraF and TrbB to determine the classification for each of the plasmid-based thioredoxin-like proteins identified in this study. For the final analysis, any of the prototypical proteins listed above that did not group with any plasmid-based proteins were removed.
Sequences of plasmid-based thioredoxin family members were also analyzed by the protein sequence clustering program CD-HIT to identify putative protein “clusters” within each MOB group (Huang 2010). Specifically, analyses were performed using the CD-HIT algorithm with a sequence identity cut-off value of 0.4. Protein clusters identified by CD-HIT are labeled in each of the phylogenetic trees (Figures 6–8, hashed lines). Multiple sequence alignments of each cluster have been provided as supplemental material (Figures S1–S15).
Figure 6.
Maximum-likelihood tree of thioredoxin-like proteins within MOBF plasmids. Included in this analysis are 60 plasmid-based thioredoxin-like proteins in addition to the thioredoxin-like prototype proteins TrbB, TraF, TlpA, DsbC, DsbA, and Com1. Proteins cluster into groups consisting of TrbB-like, TraF-like, TlpA-like, DsbC-like, DsbA-like, and Com1-like families (solid lines). Clusters identified by CD-HIT (F1–F9) are shown as dashed lines. Within the TraF-like cluster are two proteins plpl0027 and plpp0035 (denoted by asterisks), which have been proposed to be members of the TrbB-like family.
Figure 8.
Maximum-likelihood phylogenetic analysis of thioredoxin-like proteins within MOBP plasmids. Included in this analysis are 40 plasmid-based thioredoxin-like proteins in addition to the thioredoxin-like prototype proteins Com1, CcmG, DsbB, and DsbD. Proteins cluster into groups consisting of Com1-like, CcmG-like, DsbB-like, DsbD-like families (solid lines), while 4 proteins do not share homology with any prototypical subfamilies. Clusters identified by CD-HIT (P1–P9) are shown as dashed lines.
4.1 MOBf
The MOBF family consists of 114 plasmid systems that are typically conjugative, as opposed to mobilizable, and large. We have identified 60 thioredoxin-like proteins that are encoded across 40 different MOBF plasmids (Table 4; for additional information see Table S1). Phylogenetic analysis of these proteins reveals DsbA-, DsbC-, TrbB-, TraF-, Com1-, and TlpA-like proteins are encoded in at least one MOBF plasmid (Table 3).
The TrbB-like family is the most populated within MOBF plasmids, with a total of 26 different proteins that cluster into two distinct phylogenetic groups (Figure 6). The first group contains proteins homologous to the 19.7 kDa TrbB from plasmid F, while the second contains proteins homologous to the smaller 16.0 kDa TrbB from plasmids pS19501 and pS19502. In addition, we have found seven proteins that belong to the TraF-like family. Further examination of two of these proteins using the CDD reveals an E value of less than 1e–40 for the TrbB-like family, suggesting these two proteins, plpl0027 from pLPL and plpp0035 from pLPP (Figure 6, asterisks), can be considered both TraF- and TrbB-like. A closer inspection of the pLPL and pLPP plasmid genomes reveals that both encode a TraF-like protein that lacks a C-X-X-C motif, which is reminiscent of other TrbB-containing plasmids. For this reason, we propose that plpl0027 and plpp0035 should be primarily considered to be TrbB-like proteins, although they cluster among the TraF-like family. We also identified 11 TlpA-like, nine Com1-like proteins, five DsbC-like, and two DsbA-like proteins in MOBF plasmids (Table 3).
Only 13 of the 40 plasmids from this study encoded multiple thioredoxin-like proteins. Of these plasmids, plasmid pMT encodes TrbB- and Com1-like proteins, plasmid pSLT encodes both a TrbB- and a DsbA-like protein, while plasmids pAOVO01, pCAR3, pNL1, 1(a), and pSWIT01 encode both TraF- and DsbC-like proteins. Plasmids pKB1, pMKMS01, pAA1, and TC1 encode both TlpA- and Com1-like proteins, while pUTI89 encodes TrbB-, DsbA-, and TlpA-like proteins (Table 4; also see Table S1).
4.2 MOBH
MOBH is one of the newer classifications of plasmid systems, and currently includes 25 large, conjugative plasmids. We have identified 52 proteins encoded across 18 of these systems (Table 4; also see Table S1), including DsbA-like, DsbB-like, DsbC-like, DsbD-like, DsbG-like, TraF-like, Com1-like, and TlpA-like family members (Table 3).
Of the 52 thioredoxin-like proteins in MOBH plasmids, 39 are encoded within only eight systems: the plasmids pCAR1, Rts1, pAPEC-01-R, R478, pHCM1, pMAK1, pK29 and R27, and the ICE pAGI2 (Table 4). Each of these plasmids encodes at least one protein belonging to each of the DsbC-, Com1-, and TraF-like families. In some cases, however, the plasmid encodes multiple proteins within the same family. For instance, pCAR1 encodes three different Com1-like proteins, while Rts1 encodes two. Plasmids pAPEC-01-R, R478, pHCM1, and pMAK1 each encode two DsbC-like proteins that cluster into two distinct phylogenetic groups. Closer inspection reveals that the proteins in the first group, which consists of protein clusters H4 and H5 along with DsbC (pCAR1) and Rts1–211 (Rts1), are more distantly related to DsbC than the proteins in the second group, which consists entirely of proteins within cluster H3 (Figure 7). Phyre analysis (Kelley 2009) suggests that the first group (clusters H4 and H5, Rts_211 (Rts1), and DsbC (pCAR1)) possess an elongated N-terminus in comparison to DsbC. Structural predictions using the Phyre algorithm suggest this extension is composed of an additional beta strand-loop-helix within the N-terminal dimerization domain when compared to DsbC from E. coli (data not shown). In addition, the genomic island pAGI2 encodes one protein each within the DsbG-, DsbD-, TlpA-, and Com1-like families. Interestingly, orf-c8 from pAGI2 is the lone DsbG homolog identified in this study.
Figure 7.
Maximum-likelihood tree of thioredoxin-like proteins within MOBH plasmids. Included in this analysis are 52 plasmid-based thioredoxin-like proteins in addition to the thioredoxin-like prototype proteins DsbC, DsbG, DsbB, DsbD, Com1, and TlpA. Proteins cluster into groups consisting of DsbC-like, DsbG-like, TraF-like, DsbA-like, DsbB-like, DsbD-like, Com1-like, and TlpA-like families (solid lines). Clusters identified by CD-HIT (H1–H6) are shown as dashed lines.
Overall, in MOBH plasmids we have identified 18 DsbC-like proteins, 18 Com1-like proteins, 10 TraF-like proteins, two DsbD-like proteins, one DsbA-like protein, one DsbB-like protein, one DsbG-like protein, and one TlpA-like protein (Table 3).
4.3 MOBP
MOBP is an interesting plasmid family in that it contains two subgroups, MOBQ and MOBHEN (Garcillán-Barcia 2009). For these studies, MOBP, MOBQ, and MOBHEN were treated as three distinct phylogenetic groups. Within the 180 plasmids that are strictly considered MOBP, we found 37 thioredoxin-like proteins belonging to the Com1-like, CcmG-like, DsbB-like, and DsbD-like families (Table 3).
Like MOBH, there are several MOBP plasmid systems that encode more than one thioredoxin-like protein, although the trends are much less apparent (Table 4). Of special note, however, are the plasmids pDSHI01 and pDSHI03, which each encode six thioredoxin-like proteins; two Com1-like proteins, two DsbB-like proteins, one CcmG-like protein, and one DsbD-like protein. Phylogenetic analysis (Figure 8) revealed that the two Com1-like proteins and the two DsbBs cluster differently from one another, which may suggest the existence of two specific Com1/DsbB systems that are responsible for unique functions in vivo. Finally, we identified four proteins, EF-B0054 and EF-B0055 from pTEF2, pFL5-05 from pFL5, and pNF1 1020 from pNF1 that, according to the CDD, do not have homology to any specific thioredoxin-like subfamily, bringing the total number of thioredoxin like proteins in MOBP plasmids to 41.
4.4 MOBq
As mentioned above, MOBQ is a subgroup within the MOBP family. MOBQ consists of 21 plasmid systems in which we identified 11 different thioredoxin-like proteins (Table 3). Included in these proteins are the TlpA-like proteins Nham_4643 from plasmid 3 and SMb20213 from pSymB, the TrbB-like pLDTEXKL-p22 from pLD-TEX-KL, and three proteins, pRL11–0200 from pRL11, Smed-5325 from pSMED02, orf28 from pWCFS103, that do not group well with any thioredoxin-like families (data not shown). According to the CDD, however, Smed_5325 seems to have small regions of homology to both TlpA- and DsbD-like families. In addition there are four proteins that have homology to protein families not discussed in this review. Two of these, nuoE2 from pSymA and Smed-3619 from pSMED01 are predicted to be members of the NADH:ubiquinone oxidoreductase family (Nuo)/ Thioredoxin-like [2Fe–2S] Ferredoxin family of proteins. The Thioredoxin-like [2Fe–2S] ferredoxin family is characterized by a four cysteine cluster organized in an approximate C-X10-C-X29-C-X3-C motif that functions in binding of an iron-sulfur cluster (Meyer 2011). Further inspection of the nuoE2 and Smed-3619 sequences, which are 91% identical, reveals that the identified C-X-X-C motif is due to the presence of a fifth cysteine within this conserved four cysteine cluster, resulting in a C-X4-[C-X2-C]-X32-C-X3-C motif in both nuoE2 and Smed-3619. How this extra cysteine affects binding of the iron-sulfur cluster has not been experimentally determined, however, the positioning of the C-X-X-C motif would suggest that neither nuoE2 nor Smed-3619 possess functional redox active sites. The remaining two proteins, Caul-5154 from pCAUL01 and SMb20194 from pSymB are predicted to be members of the Peroxiredoxin-like1 subfamily (cd02969). Peroxiredoxins are typically characterized by a catalytic triad, T-X-X-C-X(x82)-R/S (T = Thr, C = Cys, R = Arg, S = Ser), responsible for its enzymatic activity. The Prx1 subfamily, however, lacks the triad, and only contains only a single conserved cysteine residue. Coincidentally, the conserved cysteine is different than the perixodatic cysteine seen in the catalytic triad (Fomenko 2003).
4.5 MOBC
MOBC is a small plasmid family that was organized by the clustering of 54 different relaxase proteins encoded in conjugative plasmids, mobilizable plasmids, and ICEs. In our studies, we found only a single thioredoxin-like protein in the 22 different MOBC plasmids identified by Garcillan-Barcia, et al. (Garcillán-Barcia 2009) (Table 3). This protein, EFA0039 from plasmid pTEF1, does not cluster well with any of the thioredoxin-like families mentioned in this study (data not shown). A search of the CDD shows homology to a small family of oxidases found only in photosynthetic organisms. This finding is surprising, as pTEF1 was isolated from the commensal gut bacterium Enterococcus faecalis (Paulsen 2003).
5. Trends and Perspectives
5.1 TrbB-containing plasmids
Through phylogenetic analysis, we have found that of the 29 identified TrbB-like family members, 28 are found in MOBF plasmids. Continuing a trend observed earlier, none of these TrbB-like proteins were found on a plasmid encoding a DsbC-like protein. Instead, we found that 28 of the 29 TrbB-containing plasmids also encode TraF-like family members, but these TraF-like proteins lack C-X-X-C motifs in their mature form, and for this reason were not part of our analysis. Conversely, in 14 of the 15 plasmids encoding TraF-like proteins that contain a C-X-X-C motif, we also find DsbC-like family members. Although there is yet no experimental data that allows a comparison of DsbC, TrbB, and TraF functions, the trends revealed in this work raise important questions about the relationship between these proteins.
5.2 Com1-like proteins
Com1-like proteins are arguably the least understood of all the thioredoxin-like families, as their in vivo function, structure, and regulation have not been thoroughly characterized. Our analysis has found that although the C-X-X-C active site of Com1-like proteins are embedded within a DsbA-like thioredoxin fold as judged by the CDD, these proteins seem to have both DsbA-like and DsbG-like characteristics. In all, Com1-like proteins were found in 39 different plasmids within the MOBF, MOBH and MOBP families. Six of these 39 plasmid systems encode multiple Com1-like proteins. Specifically, pCAR1 and 1(d) encode three, while Rts1, pMKMS01, pDSH01, and pDSH03 each encode two (Table 4).
A previous analysis of bacteria encoding homologs of the Com1-like protein DsbA2 from Legionella pneumophila revealed an absence of DsbC-like proteins (Jameson-Lee 2011), however, this trend is not upheld in plasmid systems. Of the 24 Com1-like containing plasmids that encode more than one thioredoxin-like protein, 12 possess DsbC-like proteins. Additionally, 8 of these 12 also include a C-X-X-C-containing TraF-like protein.
Although the redox active site of DsbA2 is maintained in a DsbA-like ratio in vivo (60% oxidized: 40% reduced) (Jameson-Lee 2011), its putative dependence on the inner membrane protein DsbB for redox maintenance has not been directly examined. In this study, we identified three plasmids that encode both Com1-like and DsbB-like proteins (pCAR1, pDSHI01, and pDSHI03). Most interesting are plasmids pDSHI01 and pDSHI03, which encode two Com1-like proteins and two DsbB-like proteins, suggesting they may possess two independent Com1/DsbB systems. In each of these plasmids, one of the DsbA/B pairs is encoded sequentially within the plasmid genome, further supporting this hypothesis. There is, however, little evidence for either the in vivo function or substrates of Com1 or DsbA2, hampering speculation on the role of the Com1-like family in conjugative systems.
5.3 Cytochrome biogenesis proteins
Thioredoxin-like proteins belonging to cytochrome biogenesis families have been found in 17 different plasmids. Eight of these contain homologs of CcmG, while nine contain homologs of TlpA. To further understand the presence of these proteins, we searched these 17 plasmid systems for additional cytochrome biogenesis proteins. This search revealed that 16 of the 17 plasmids containing CcmG- or TlpA-like proteins also encoded a cytochrome c biogenesis island or additional cytochrome c maturation proteins (data not shown). In addition, we found that 23 of the 24 proteins within these 16 plasmids resided directly within the biogenesis island, or next to other cytochrome maturation proteins.
The conservation of cytochrome biogenesis proteins within plasmid systems has not yet been investigated. However, the conservation of both CcmG-like and TlpA-like proteins proximal to cytochrome c maturation proteins further supports the role of TlpA-like proteins in the biogenesis of cytochrome c that was first revealed in A. tumefacians.
5.4 Redox state maintenance of thioredoxin-like proteins in plasmids
As thioredoxin-like proteins rely on inner membrane proteins for redox state maintenance in vivo, the presence of proteins such as DsbB and DsbD are essential for the regulation of plasmid-based thioredoxin family members.
Phylogenetic analysis of thioredoxin-like proteins in MOBF plasmids has revealed an overall dearth of DsbB or DsbD-like proteins, which may reflect a dependence on host-encoded proteins for each of these systems, much like the dependence of the F plasmid-based disulfide isomerase TrbB on the E. coli encoded DsbD. In fact, only six of the 89 plasmid systems discussed in this study encode a DsbB or DsbD homolog. One of these, the MOBH plasmid pBVIE02, encodes a DsbD homolog, yet does not possess another thioredoxin-like protein, which raises questions about its primary function. Alternatively, the MOBP plasmids pDSHI01 and pDSHI03 appear to encode a self-contained system, including two possible Com1/DsbB pairs and one CcmG/DsbD pair. The MOBH plasmid pCAR1 encodes a DsbB homolog that may be responsible for the maintenance of its three different Com1-like proteins. The MOBP plasmid pFL5 encodes two sequential thioredoxin-like proteins; the DsbB homolog pFL5-p05 and pFL5-p06, the latter of which does not appear to have homology to any of the prototypical thioredoxin-like families. The sequential positioning of pFL-p05 and pFL-p06, however, may suggest that pFL5-p06 is maintained in the oxidized form by pFL5-p05 in vivo. Finally, the genomic island pAGI2 encodes a DsbD homolog along with Com1-, DsbG-, and TlpA- like family members. One possible explanation for the lack of DsbB and DsbD homologs in plasmids is that bacterially-encoded DsbB and DsbD function as a general oxidase and reductase, respectively, thereby enabling interactions with and subsequent redox state maintenance of proteins encoded within these plasmid systems.
5.5 Potential substrates of trx-like proteins
Of obvious importance to the mechanistic understanding of thioredoxin-like proteins in these systems are the identification of their plasmid encoded substrates. Using a bioinformatics approach, putative substrates within trx-like containing plasmids were selected using two criteria: (1) the presence of two or more cysteine residues and (2) the presence of an N-terminal signal peptide as predicted by the SignalP 4.0 server (Peterson 2011). This approach led to the identification of more than 1000 potential trx-like substrates, including 375 within MOBF plasmids, 241 within MOBH plasmids, 129 within MOBP plasmids, and 298 within MOBQ plasmids. As this number of proteins is quite difficult to analyze, this search was refined to include only those proteins containing 5 or more cysteine residues in their mature state. This additional criterion reduced the overall number of potential substrates to 193. Of these, 114 were encoded in MOBF plasmids, 36 in MOBH plasmids, 17 in MOBP plasmids, and 22 in MOBQ plasmids. We decided to focus further efforts on those proteins encoded by MOBF plasmids, which are summarized in Table 5. Those proteins found within MOBH, MOBP, and MOBQ families have been provided as supplemental information (Table S2). The set of MOBF proteins containing five or more cysteines originate from 36 of the 40 trx-like containing MOBF plasmids discussed in this review. Within the MOBF family, only plasmids A, pAA1, pKB1, and pLEW279b contain no proteins with five or more cysteines.
Table 5.
Periplasmic proteins from MOBF plasmids containing five or more cysteine residues
| Protein | Plasmid | Accession # | Cys # | Plasmid dsb isomerase |
|---|---|---|---|---|
| Bcen2424_6808 | 1(a) | 116687184 | 13 | DsbC |
| Bcen2424_6872 | 1(a) | 116687248 | 8 | DsbC |
| Bcen2424_6920 | 1(a) | 116687295 | 6 | DsbC |
| TraH | 1(a) | 116687190 | 6 | DsbC |
| TraN | 1(a) | 116687185 | 22 | DsbC |
| TraU | 1(a) | 116687182 | 16 | DsbC |
| CoBM53 | ColBM | 157418136 | 12 | TrbB |
| TraN | ColBM | 157418127 | 22 | TrbB |
| TraU | ColBM | 157418125 | 11 | TrbB |
| TraH | F | 9507811 | 6 | TrbB |
| TraN | F | 9507802 | 22 | TrbB |
| TraU | F | 9507800 | 11 | TrbB |
| TraH | MT | 9507811 | 6 | TrbB |
| TraN | MT | 9507802 | 22 | TrbB |
| TraU | MT | 9507800 | 11 | TrbB |
| TraH | p1658/97 | 32460003 | 6 | TrbB |
| TraN | p1658/97 | 32469994 | 22 | TrbB |
| TraU | p1658/97 | 32469992 | 11 | TrbB |
| TraH | pAOVO01 | 121582472 | 6 | DsbC |
| TraN | pAOVO01 | 121582475 | 22 | DsbC |
| TraU | pAOVO01 | 121582478 | 16 | DsbC |
| TraH | pAPEC-02-ColV | 84060759 | 6 | TrbB |
| TraN | pAPEC-02-ColV | 84060766 | 22 | TrbB |
| TraH | pAPEC-02-R | 58000387 | 6 | TrbB |
| TraN | pAPEC-02-R | 58000380 | 22 | TrbB |
| TraU | pAPEC-02-R | 58000378 | 11 | TrbB |
| TraH | pAsa5 | 1453014122 | 6 | TrbB |
| TraN | pAsa5 | 145301419 | 23 | TrbB |
| TraU | pAsa5 | 145301417 | 11 | TrbB |
| TraH | pC15-1a | 41056006 | 6 | TrbB |
| TraN | pC15-1a | 41056998 | 24 | TrbB |
| TraU | pC15-1a | 41056995 | 11 | TrbB |
| TraH | pC4602-1 | 153971630 | 6 | TrbB |
| TraN | pC4602-1 | 153971627 | 34 | TrbB |
| TraU | pC4602-1 | 153971624 | 11 | TrbB |
| TraH | pCAR3 | 113473815 | 6 | DsbC |
| TraN | pCAR3 | 113473812 | 36 | DsbC |
| TraU | pCAR3 | 113473810 | 14 | DsbC |
| TraH | pED208 | 22539465 | 6 | TrbB |
| TraN | pED208 | 22539458 | 23 | TrbB |
| TraU | pED208 | 22539455 | 11 | TrbB |
| Ent638_4232 | pENTE01 | 146284558 | 5 | TrbB* |
| Ent638_4233 | pENTE01 | 146284559 | 6 | TrbB* |
| TraH | pENTE01 | 146284629 | 6 | TrbB* |
| TraN | pENTE01 | 146284624 | 24 | TrbB* |
| TraU | pENTE01 | 146284622 | 9 | TrbB* |
| pG8786_073 | pG8786 | 52788125 | 6 | TrbB |
| TraH | pG8786 | 52788156 | 6 | TrbB |
| TraN | pG8786 | 52788152 | 24 | TrbB |
| TraU | pG8786 | 52788150 | 11 | TrbB |
| TraH | pKPN3 | 229269588 | 6 | TrbB |
| TraN | pKPN3 | 229269580 | 26 | TrbB |
| TraU | pKPN3 | 229269513 | 11 | TrbB |
| TraH | pKPN4 | 152973722 | 6 | TrbB |
| TraN | pKPN4 | 152973714 | 26 | TrbB |
| TraU | pKPN4 | 152973712 | 11 | TrbB |
| plpl0040 | pLPL | 54292946 | 10 | TrbB |
| TraH | pLPL | 54292934 | 6 | TrbB |
| TraN | pLPL | 54292931 | 28 | TrbB |
| TraU | pLPL | 54292929 | 11 | TrbB |
| TraH | pLPP | 54295879 | 6 | TrbB |
| TraN | pLPP | 54295876 | 28 | TrbB |
| TraU | pLPP | 54295874 | 13 | TrbB |
| TraH | pMAR7 | 190014912 | 6 | TrbB |
| TraN | pMAR7 | 190014921 | 22 | TrbB |
| TraU | pMAR7 | 190014923 | 11 | TrbB |
| Mkms_5650 | pMKMS01 | 119855041 | 6 | - |
| TraH | pNL1 | 10956932 | 6 | DsbC |
| TraN | pNL1 | 10956935 | 36 | DsbC |
| TraU | pNL1 | 10956937 | 15 | DsbC |
| TraH | pNR1 | 133756539 | 6 | TrbB |
| TraN | pNR1 | 133756531 | 24 | TrbB |
| TraU | pNR1 | 133756528 | 11 | TrbB |
| TraH | pO86A1 | 116006902 | 6 | TrbB |
| TraN | pO86A1 | 116006894 | 24 | TrbB |
| TraU | pO86A1 | 116006891 | 11 | TrbB |
| pOU1113_32 | pOU1113 | 71559029 | 7 | TrbB |
| TraH | pOU1113 | 71559045 | 6 | TrbB |
| TraN | pOU1113 | 71559050 | 23 | TrbB |
| TraU | pOU1113 | 71559053 | 11 | TrbB |
| TraN | pPBR1 | 47104038 | 30 | TrbB |
| TraU | pPBR1 | 47104036 | 11 | TrbB |
| DNaseI | pS18501 | 152998512 | 6 | TrbB |
| Shew_185_4421 | pS18501 | 152998518 | 5 | TrbB |
| TraH | pS18501 | 152998530 | 6 | TrbB |
| TraN | pS18501 | 152998534 | 28 | TrbB |
| TraU | pS18501 | 152998536 | 10 | TrbB |
| DNaseI | pS19501 | 160872986 | 6 | TrbB |
| TraH | pS19501 | 160872937 | 6 | TrbB |
| TraN | pS19501 | 160872940 | 28 | TrbB |
| TraU | pS19501 | 160872942 | 10 | TrbB |
| DNaseI | pS19502 | 160873009 | 6 | TrbB |
| Sbal195_4604 | pS19502 | 160873014 | 5 | TrbB |
| TraH | pS19502 | 160873029 | 6 | TrbB |
| TraN | pS19502 | 160873032 | 28 | TrbB |
| TraU | pS19502 | 160873034 | 10 | TrbB |
| TraH | pSF0157 | 149930800 | 6 | TrbB |
| TraU | pSF0157 | 149930802 | 11 | TrbB |
| w0012 | pSF0157 | 149930757 | 6 | TrbB |
| w0020 | pSF0157 | 149930775 | 6 | TrbB |
| PSLT014 | pSLT | 17233479 | 6 | TrbB |
| TraH | pSLT | 17233464 | 6 | TrbB |
| TraN | pSLT | 17233459 | 24 | TrbB |
| TraU | pSLT | 17233456 | 11 | TrbB |
| Swit_5220 | pSWIT01 | 148550657 | 6 | DsbC |
| TraH | pSWIT01 | 148550818 | 6 | DsbC |
| TraN | pSWIT01 | 148550815 | 30 | DsbC |
| TraU | pSWIT01 | 148550813 | 15 | DsbC |
| TraH | pUTI89 | 91206377 | 6 | TrbB |
| TraN | pUTI89 | 91206368 | 24 | TrbB |
| TraU | pUTI89 | 91206365 | 11 | TrbB |
| TraH | pY016 | 37595840 | 6 | TrbB |
| TraN | pY016 | 37595836 | 36 | TrbB |
| TraU | pY016 | 37595833 | 11 | TrbB |
| VVP49 | pY016 | 37595870 | 6 | TrbB |
| TraH | R100 | 9507644 | 6 | TrbB |
| TraU | R100 | 9507632 | 11 | TrbB |
| Aaur_pCT10011 | TC1 | 119952502 | 10 | - |
protein contains a C-X-X-S redox active site
Only seven of the 40 MOBF plasmids identified in this review lack a disulfide bond isomerase. These include plasmids A, pAA1, pENTE01, pKB1, pLEW279b, pMKMS01, and TC1. Of the plasmids encoding disulfide bond isomerases, 28 contain TrbB homologs, while five possess DsbC homologs. Our current model proposes that these disulfide bond isomerases aid in the folding and redox state maintenance of a subset of plasmid-encoded disulfide-containing proteins. As would be expected from this hypothesis, our analysis reveals that proteins containing five or more cysteines are predominantly encoded in plasmids containing either TrbB or DsbC (Table 5). In fact, only seven of the 118 proteins identified in MOBF plasmids were encoded in plasmids lacking a disulfide bond isomerase. Of special note is plasmid pENTE01, which encodes five of those seven proteins. A closer inspection of the pENTE01 genome reveals the presence of a TrbB homolog with a serine at C-terminal redox active site position (C-X-X-S). Although it is not a canonical redox active site, this motif has been previously identified in a number of redox enzymes (Fomenko 2002, Fomenko 2003), suggesting that TrbB from pENTE01 may be catalytically active. If this is the case, we then find only two proteins with five or more cysteines, Mkms_5650 from plasmid pMKMS01 and Aaur_pCT10011 from TC1 (Table 5), encoded in a plasmid lacking a disulfide bond isomerase.
Another striking observation is the conservation TraH, TraN, and TraU homologs in these systems. Of the 118 proteins identified here, 32 are TraH homologs, 32 are TraN homologs, and 33 are TraU homologs, which together comprise 82% of the identified sequences (Table 5). Moreover, if pENTE01 is to be considered a disulfide bond isomerase containing plasmid, we observe a perfect correlation between the encoding of TraH/TraN/TraU-like proteins and a disulfide bond isomerase, at least within MOBF plasmids. In other words, the 34 plasmids encoding a disulfide bond isomerase are the only plasmids that encode TraH, TraN, or TraU-like proteins. Interestingly, 29 of these 34 plasmids encode all three of the tra proteins. The remaining five each encode two of the three: ColMB encodes TraN and TraU, pAPEC-01-ColV encodes TraH and TraN, pPBR1 encodes TraN and TraU, pSF0157 encodes TraH and TraU, and R100 encodes TraH and TraU (Table 5). Further investigation of these plasmid genomes reveals that three of these plasmids, pAPEC-01-ColV, pSF0157, and R100 encode the third, but the homolog from that respective system is not predicted to have an N-terminal signal peptide. Therefore, only two plasmids, ColBM and pPBR1, which both appear to lack a TraH homolog, do not encode all three. To further establish this correlation, we have found that disulfide bond isomerases are encoded in all but one plasmid containing TraH, TraN, or TraU across all MOB families, with the exception being the MOBH plasmid 1(b) (Figure S2).
Together, these trends suggest that plasmid-based disulfide bond isomerases are indeed responsible for the folding and maintenance of a select subset of plasmid-encoded proteins, and that the core of this subset consists of the transfer proteins TraH, TraN, and TraU. These results also highlight the importance of further examination of the remaining potential substrates we have identified within the MOBP, MOBH, and MOBQ families, for which trends are much less apparent.
5.6 Membrane association via lipid modification
Further analysis of the N-terminal signal peptides of plasmid-encoded thioredoxin like proteins was performed using the LipoP server (Juncker 2003), which analyzes the N-terminal sequence for the presence of a lipobox. A lipobox is a conserved [LVI][ASTVI][GAS]C amino acid sequence within the C-terminal region of a protein’s N-terminal signal peptide (Babu 2006). This motif results in the covalent attachment of a diacylglyceryl moiety onto the free thiol of the conserved cysteine by lipoprotein diacylglyceryl transferase (Babu 2006). After attachment of the diglyceryl moiety, the signal peptide is cleaved N-terminal to the modified cysteine by lipoprotein signal peptidase (Tokunga 1982). In Gram-negative bacteria (and some Gram-positive bacteria), a second round of modification occurs after cleavage, whereby an acyl group will be attached to the free amine of the now N-terminal cysteine by lipoprotein N-acyl transferase (Gupta 1993). Addition of these lipid moieties facilitates association of the modified proteins with the periplasmic face of either the inner or outer membrane (Narita 2004).
Of the 165 proteins discussed in this review, only 14 were predicted to possess a lipobox. Of these, six belong to the TlpA-like family, six to the CcmG-like family, while one each is a member of the TrbB-like and DsbG-like families. In this review we identified only 14 TlpA-like and 10 CcmG-like proteins, which translates to 43% of TlpA-like and 60% of CcmG-like proteins identified in plasmid systems possessing lipoboxes, thus suggesting a critical importance of membrane-association to thioredoxin-like cytochrome maturation family proteins. This trend is not surprising, as previous work has shown both TlpA and CcmG-like proteins to be membrane anchored (see Section 1.4). Alternatively, only 2 of the remaining 141 thioredoxin-like family proteins (1.4%) are predicted to possess lipoboxes, indicating an overall lack of membrane associated thioredoxin-like proteins outside of the cytochrome maturation system.
5.7 Concluding Remarks and Perspectives
Through our examination of thioredoxin-like proteins in mobile plasmids, we have identified 89 different plasmid systems that encode at least one protein within the thioredoxin-like superfamily. Together these plasmid systems encode more than 160 different thioredoxin-like protenis that belong to at least 10 different thioredoxin-like families (DsbA, DsbB, DsbC, DsbD, DsbG, Com1, CcmG, TlpA, TraF, and TrbB).
Based on our analysis, we can draw several conclusions. First, nearly all of the thioredoxin proteins identified are encoded on large, apparently conjugative plasmids. This observation suggests that thioredoxin-like proteins may play an important role in the construction or regulation of the type IV pore complex. In addition, we have found a significant number of cytochrome maturation proteins in these plasmids, suggesting that there is a selective advantage for conjugative plasmids encoding at least part of these systems.
Despite the relative frequency of thioredoxin-like proteins in conjugative plasmids, these proteins are observed in less than one quarter of the conjugative plasmids examined. Clearly these proteins are not generally required for maintenance or transfer of a conjugative plasmid. There are, however, some intriguing trends. We observe no thioredoxin-like proteins in 105 MOBV plasmids and a single thioredoxin-like protein in 22 MOBC plasmids, while 18 of 33 MOBH plasmids encode one to as many as six such proteins. Are the periplasmic proteins encoded by MOBH plasmids more prone to misfolding or oxidation, allowing thioredoxin-like proteins a comparatively greater selective advantage? Do MOBH plasmid hosts frequently inhabit niches that favor plasmids bearing these proteins?
In another example, in MOBF plasmids we frequently observe both a TrbB-like and a TraF-like protein encoded on the same plasmid, however, the TraF-like proteins in these cases lack a C-X-X-C motif. Alternatively, MOBF plasmids encoding TraH, TraN, and TraU homologs that lack TrbB were found, in all cases, to encode both a DsbC-like protein and a TraF-like protein that possesses a complete C-X-X-C redox active site motif. We have yet to observe TrbB-like and DsbC-like proteins encoded together in MOBF plasmids. While the roles of thioredoxin-like protein families have been studied within bacterial systems, to date only three different plasmid-based family members have been investigated experimentally: TrbB and TraF from plasmid F, and DsbC from plasmid R27. Although TrbB and DsbC each exhibit disulfide bond isomerase activity, we have not yet experimentally determined their specific protein targets, nor do we understand the extent of their roles within assembly of the pore complex and/or DNA translocation. In addition, studies have shown that TraF, although lacking a C-X-X-C motif, is an essential protein for pilus extension in the F plasmid system. The in vivo function and protein targets of TraF have not been determined, however it is currently thought to act as a molecular chaperone. In addition to homologs of the canonical dsb family, we have also identified plasmid proteins belonging to the CcmG-like, TlpA-like, and Com1-like families, for which there exists little experimental data.
Although thioredoxin-like proteins appear to play an important part in the conjugative process, there is an overall lack of experimental data addressing the unique and apparently numerous roles of these proteins. For this reason, many important questions remain unanswered. One of the most important is how each of these proteins contributes to the process of bacterial conjugation. Although we speculate that plasmid-based dsb family members are responsible for the correct folding and maintenance of plasmid-encoded proteins, there is no empirical evidence to support this. It has also been hypothesized that plasmid-based dsb proteins could aid in the maintenance of certain host-encoded proteins, or that they are specifically required when bacteria are under particular environmental stresses. Alternatively, these proteins may only be required when the plasmid is in a host that does not possess a complete disulfide bond formation system. We have also identified a large number of proteins belonging to the Com1-like family, whose function is unknown even within bacterial systems. Lastly, we must address why certain plasmids encode cytochrome c biogenesis systems. Are these proteins essential for plasmid transfer or persistence? Are they functionally redundant to proteins within the host-encoded system? For the first time, we have at our disposal a cumulative list of thioredoxin-like proteins conserved within plasmid systems. With continued investigations of the functions of these proteins, great strides can be made into further understanding the intricacies of the conjugative process.
Supplementary Material
Acknowledgements
This work was supported, in whole or in part, by National Institutes of Health grant GM61017 (to J.F.S.). We also gratefully acknowledge Mohan Bolisetty for his generous help with the phylogenetic analysis presented in this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achtman M, Kennedy N, et al. Cell--cell interactions in conjugating Escherichia coli: role of traT protein in surface exclusion. Proc Natl Acad Sci U S A. 1977;74:5104–5108. doi: 10.1073/pnas.74.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M. Mating aggregates in Escherichia coli conjugation. J Bacteriol. 1975;123:505–515. doi: 10.1128/jb.123.2.505-515.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmer BM, Tran M, et al. The virulence plasmid of Salmonella typhimurium is self-transmissible. J Bacteriol. 1999;181:1364–1368. doi: 10.1128/jb.181.4.1364-1368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen CL, Matthey-Dupraz A, et al. A new Escherichia coli gene, dsbG, encodes a periplasmic protein involved in disulphide bond formation, required for recycling DsbA/DsbB and DsbC redox proteins. Mol Microbiol. 1997;26:121–132. doi: 10.1046/j.1365-2958.1997.5581925.x. [DOI] [PubMed] [Google Scholar]
- Arutyunov D, Arenson B, et al. F plasmid TraF and TraH are components of an outer membrane complex involved in conjugation. J Bacteriol. 2010;192:1730–1734. doi: 10.1128/JB.00726-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu MM, Priya ML, et al. A Database of Bacterial Lipoproteins (DOLOP) with Functional Assignments to Predicted Lipoproteins. J Bacteriol. 2006;188:2761–2773. doi: 10.1128/JB.188.8.2761-2773.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M, Muse W, et al. Oxidative protein folding is driven by the electron transport system. Cell. 1999;98:217–227. doi: 10.1016/s0092-8674(00)81016-8. [DOI] [PubMed] [Google Scholar]
- Bader MW, Hiniker A, et al. Turning a disulfide isomerase into an oxidase: DsbC mutants that imitate DsbA. EMBO J. 2001;20:1555–1562. doi: 10.1093/emboj/20.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszak K, Mechin I, et al. Structure of the reduced disulfide-bond isomerase DsbC from Escherichia coli. Acta Crystallogr D Biol Crystallogr. 2004;60:1747–1752. doi: 10.1107/S0907444904018359. [DOI] [PubMed] [Google Scholar]
- Bardwell JC, Lee JO, et al. A pathway for disulfide bond formation in vivo. Proc Natl Acad Sci U S A. 1993;90:1038–1042. doi: 10.1073/pnas.90.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow M. What antimicrobial resistance has taught us about horizontal gene transfer. Methods Mol Biol. 2009;532:397–411. doi: 10.1007/978-1-60327-853-9_23. [DOI] [PubMed] [Google Scholar]
- Benham CJ, Jafri MS. Disulfide bonding patterns and protein topologies. Protein Sci. 1993;2:41–54. doi: 10.1002/pro.5560020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkmen M, Boyd D, et al. The nonconsecutive disulfide bond of Escherichia coli phytase (AppA) renders it dependent on the protein-disulfide isomerase, DsbC. J Biol Chem. 2005;280:11387–11394. doi: 10.1074/jbc.M411774200. [DOI] [PubMed] [Google Scholar]
- Bessette PH, Aslund F, et al. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc Natl Acad Sci U S A. 1999a;96:13703–13708. doi: 10.1073/pnas.96.24.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessette PH, Cotto JJ, et al. In vivo and in vitro function of the Escherichia coli periplasmic cysteine oxidoreductase DsbG. J Biol Chem. 1999b;274:7784–7792. doi: 10.1074/jbc.274.12.7784. [DOI] [PubMed] [Google Scholar]
- Boyd DA, Tyler S, et al. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob Agents Chemother. 2004;48:3758–3764. doi: 10.1128/AAC.48.10.3758-3764.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröker D, Arenskötter M, et al. Transfer of megaplasmid pKB1 from the rubber-degrading bacterium Gordonia westfalica strain Kb1 to related bacteria and its modification. Appl Microbiol Biotechnol. 2008;77:1317–1327. doi: 10.1007/s00253-007-1262-8. [DOI] [PubMed] [Google Scholar]
- Cabello F, Timmis KN. Plasmids of medical importance. In: Timmis KN, Puhler A, editors. Plasmids of Medical, Environmental and Commercial Importance. Amsterdam: Elsevier/North-Holland Biomedical Press; 1979. pp. 55–70. [Google Scholar]
- Cantor CR, Schimmel P. Biophys Chem. San Francisco: W.H. Freeman; 1980. [Google Scholar]
- Chatterjee AK, Starr MP. Transfer among Erwinia spp. and other enterobacteria of antibiotic resistance carried on R factors. J Bacteriol. 1972;112:576–584. doi: 10.1128/jb.112.1.576-584.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Wu KM, et al. Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genome Res. 2003;13:2577–2587. doi: 10.1101/gr.1295503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Song J, et al. Chaperone activity of DsbC. Journal of Biological Chemistry. 1999;274:19601. doi: 10.1074/jbc.274.28.19601. [DOI] [PubMed] [Google Scholar]
- Chen YT, Lauderdale TL, et al. Sequencing and comparative genomic analysis of pK29, a 269-kilobase conjugative plasmid encoding CMY-8 and CTX-M-3 beta-lactamases in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2007;51:3004–3007. doi: 10.1128/AAC.00167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S-HH, Porat A, et al. Redox-active cysteines of a membrane electron transporter DsbD show dual compartment accessibility. EMBO J. 2007;26:3509–3520. doi: 10.1038/sj.emboj.7601799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Parsonage D, et al. A New Family of Membrane Electron Transporters and Its Substrates, Including a New Cell Envelope Peroxiredoxin, Reveal a Broadened Reductive Capacity of the Oxidative Bacterial Cell Envelope. mBio. 2012a;3:e00291–e00211. doi: 10.1128/mBio.00291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Collet J-F. Many Roles of the Bacterial Envelope Reducing Pathways. Antiox Redox Signal. 2012 doi: 10.1089/ars.2012.4962. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee JN, Datta N, et al. R factors from Proteus rettgeri. J Gen Microbiol. 1972;72:543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Curtiss R. Bacterial conjugation. Annu Rev Microbiol. 1969;23:69–136. doi: 10.1146/annurev.mi.23.100169.000441. [DOI] [PubMed] [Google Scholar]
- Darby NJ, Creighton TE. Catalytic mechanism of DsbA and its comparison with that of protein disulfide isomerase. Biochemistry. 1995;34:3576–3587. doi: 10.1021/bi00011a012. [DOI] [PubMed] [Google Scholar]
- Darby NJ, Raina S, et al. Contributions of substrate binding to the catalytic activity of DsbC. Biochemistry. 1998;37:783–791. doi: 10.1021/bi971888f. [DOI] [PubMed] [Google Scholar]
- Depuydt M, Leonard SE, et al. A periplasmic reducing system protects single cysteine residues from oxidation. Science. 2009;326:1109–1111. doi: 10.1126/science.1179557. [DOI] [PubMed] [Google Scholar]
- Di Matteo A, Gianni S, et al. A strategic protein in cytochrome c maturation: three-dimensional structure of CcmH and binding to apocytochrome c. J Biol Chem. 2007;282:27012–27019. doi: 10.1074/jbc.M702702200. [DOI] [PubMed] [Google Scholar]
- Dostál L, Shao S, et al. Tracking F plasmid TraI relaxase processing reactions provides insight into F plasmid transfer. Nucleic Acids Res. 2011;39:2658–2670. doi: 10.1093/nar/gkq1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton RJ, Boyd D, et al. Bacterial species exhibit diversity in their mechanisms and capacity for protein disulfide bond formation. Proc Natl Acad Sci U S A. 2008;105:11933–11938. doi: 10.1073/pnas.0804621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeling MA, Guddat LW, et al. Structure of CcmG/DsbE at 1.14 A resolution: high-fidelity reducing activity in an indiscriminately oxidizing environment. Structure. 2002;10:973–979. doi: 10.1016/s0969-2126(02)00794-3. [DOI] [PubMed] [Google Scholar]
- Elton TC, Holland SJ, et al. F-like type IV secretion systems encode proteins with thioredoxin folds that are putative DsbC homologues. J Bacteriol. 2005;187:8267–8277. doi: 10.1128/JB.187.24.8267-8277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabianek RA, Hennecke H, et al. The active-site cysteines of the periplasmic thioredoxin-like protein CcmG of Escherichia coli are important but not essential for cytochrome c maturation in vivo. J Bacteriol. 1998;180:1947–1950. doi: 10.1128/jb.180.7.1947-1950.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabianek RA, Hofer T, et al. Characterization of the Escherichia coli CcmH protein reveals new insights into the redox pathway required for cytochrome c maturation. Arch Microbiol. 1999;171:92–100. doi: 10.1007/s002030050683. [DOI] [PubMed] [Google Scholar]
- Falkow S, Baron LS. Episomic element in a strain of Salmonella Typhosa. J Bacteriol. 1962;84:581–589. doi: 10.1128/jb.84.3.581-589.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth N, Ippen-Ihler K, et al. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, DC: ASM Press; 1996. Structure and function of the F factor and mechanism of conjugation; pp. 2377–2401. [Google Scholar]
- Fomenko DE, Gladyshev VN. CxxS: Fold-independent redox motif revealed by genome-wide searches for thiol/disulfide oxidoreductase function. Prot Sci. 2002;11:2285–2296. doi: 10.1110/ps.0218302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomenko DE, Gladyshev VN. Identity and functions of CxxC-derived motifs. Biochem. 2003;38:11214–11225. doi: 10.1021/bi034459s. [DOI] [PubMed] [Google Scholar]
- Frost LS, Ippen-Ihler K, et al. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol Rev. 1994;58:162–210. doi: 10.1128/mr.58.2.162-210.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi T, Komano T, et al. Physical and genetic analyses of the Inc-I alpha plasmid R64. J Bacteriol. 1984;158:997–1004. doi: 10.1128/jb.158.3.997-1004.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcillán-Barcia MP, Francia MV, et al. The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol Rev. 2009;33:657–687. doi: 10.1111/j.1574-6976.2009.00168.x. [DOI] [PubMed] [Google Scholar]
- Gniadkowski M, Schneider I, et al. Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing beta-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob Agents Chemother. 1998;42:827–832. doi: 10.1128/aac.42.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone D, Haebel PW, et al. DsbC activation by the N-terminal domain of DsbD. Proc Natl Acad Sci U S A. 2001;98:9551–9556. doi: 10.1073/pnas.171315498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis-Rüth FX, de la Cruz F, et al. Structure and role of coupling proteins in conjugal DNA transfer. Res Microbiol. 2002;153:199–204. doi: 10.1016/s0923-2508(02)01313-x. [DOI] [PubMed] [Google Scholar]
- Gupta SD, Gan MB, et al. Characterization of a temperature-sensitive mutant of Salmonella typhimurium defective in apolipoprotein N-acyltransferase. J Biol Chem. 1993;268:16551–16556. [PubMed] [Google Scholar]
- Haebel PW, Goldstone D, et al. The disulfide bond isomerase DsbC is activated by an immunoglobulin-fold thiol oxidoreductase: crystal structure of the DsbC-DsbDalpha complex. EMBO J. 2002;21:4774–4784. doi: 10.1093/emboj/cdf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Zhang T, et al. Quantitation of protein sulfinic and sulfonic acid, irreversibly oxidized protein cysteine sites in cellular proteins. Methods Enzymol. 2002;348:146–156. doi: 10.1016/s0076-6879(02)48634-x. [DOI] [PubMed] [Google Scholar]
- Harris RL, Silverman PM. Tra proteins characteristic of F-like type IV secretion systems constitute an interaction group by yeast two-hybrid analysis. J Bacteriol. 2004;186:5480–5485. doi: 10.1128/JB.186.16.5480-5485.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges RW, Rodriguez-Lemoine V, et al. R factors from Serratia marcescens. J Gen Microbiol. 1975;86:88–92. doi: 10.1099/00221287-86-1-88. [DOI] [PubMed] [Google Scholar]
- Helmuth R, Achtman M. Cell-cell interactions in conjugating Escherichia coli: purification of F pili with biological activity. Proc Natl Acad Sci U S A. 1978;75:1237–1241. doi: 10.1073/pnas.75.3.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmis CW, Berkmen M, et al. TrbB from Conjugative Plasmid F Is a Structurally Distinct Disulfide Isomerase That Requires DsbD for Redox State Maintenance. J Bacteriol. 2011;193:4588–4597. doi: 10.1128/JB.00351-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix LR, Mallavia LP, et al. Cloning and sequencing of Coxiella burnetii outer membrane protein gene com1. Infect Immun. 1993;61:470–477. doi: 10.1128/iai.61.2.470-477.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heras B, Edeling MA, et al. Crystal structures of the DsbG disulfide isomerase reveal an unstable disulfide. Proc Natl Acad Sci U S A. 2004;101:8876–8881. doi: 10.1073/pnas.0402769101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiniker A, Bardwell JCA. Disulfide bond isomerization in prokaryotes. Biochemistry. 2003;42:1179–1185. doi: 10.1021/bi027141t. [DOI] [PubMed] [Google Scholar]
- Hiniker A, Bardwell JCA. In vivo substrate specificity of periplasmic disulfide oxidoreductases. J Biol Chem. 2004;279:12967–12973. doi: 10.1074/jbc.M311391200. [DOI] [PubMed] [Google Scholar]
- Hiniker A, Collet J-FF, et al. Copper stress causes an in vivo requirement for the Escherichia coli disulfide isomerase DsbC. J Biol Chem. 2005;280:33785–33791. doi: 10.1074/jbc.M505742200. [DOI] [PubMed] [Google Scholar]
- Huang Y, Niu B, et al. CD-HIT Suite: a web server for clustering and comparing biological sequences. Bioinformatics. 2010;26:680–682. doi: 10.1093/bioinformatics/btq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Murakami S, et al. Crystal structure of the DsbB-DsbA complex reveals a mechanism of disulfide bond generation. Cell. 2006;127:789–801. doi: 10.1016/j.cell.2006.10.034. [DOI] [PubMed] [Google Scholar]
- Jameson-Lee M, Garduño RA, et al. DsbA2 (27 kDa Com1-like protein) of Legionella pneumophila catalyses extracytoplasmic disulphide-bond formation in proteins including the Dot/Icm type IV secretion system. Mol Microbiol. 2011;80:835–852. doi: 10.1111/j.1365-2958.2011.07615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerke K, Nakatsu CH, et al. Comparative analysis of eight Arthrobacter plasmids. Plasmid. 2008;59:73–85. doi: 10.1016/j.plasmid.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Johnson TJ, Giddings CW, et al. Location of increased serum survival gene and selected virulence traits on a conjugative R plasmid in an avian Escherichia coli isolate. Avian Dis. 2002;46:342–352. doi: 10.1637/0005-2086(2002)046[0342:LOISSG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Johnson TJ, Johnson SJ, et al. Complete DNA sequence of a ColBM plasmid from avian pathogenic Escherichia coli suggests that it evolved from closely related ColV virulence plasmids. J Bacteriol. 2006;188:5975–5983. doi: 10.1128/JB.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncker AS, Willenbrock H, et al. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Prot Sci. 2003;12:1652–1662. doi: 10.1110/ps.0303703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathir P, Ippen-Ihler K. Construction and characterization of derivatives carrying insertion mutations in F plasmid transfer region genes, trbA, artA, traQ, and trbB. Plasmid. 1991;26:40–54. doi: 10.1016/0147-619x(91)90035-u. [DOI] [PubMed] [Google Scholar]
- Katzen F, Beckwith J. Transmembrane electron transfer by the membrane protein DsbD occurs via a disulfide bond cascade. Cell. 2000;103:769–779. doi: 10.1016/s0092-8674(00)00180-x. [DOI] [PubMed] [Google Scholar]
- Katzen F, Beckwith J. Role and location of the unusual redox-active cysteines in the hydrophobic domain of the transmembrane electron transporter DsbD. Proc Natl Acad Sci U S A. 2003;100:10471–10476. doi: 10.1073/pnas.1334136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauzmann W. Relative probabilities of isomers in cystine-containing randomly coiled polypeptides. In: Benesch R, Benesch RE, Boyer P, Klotz I, Middlebrook WR, Szent-Gyorgyi A, Schwarz DR, editors. Sulfur in Proteins. New York: Academic Press; 1959. pp. 93–108. [Google Scholar]
- Kelley LA, Sternberg MJE. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Hirono I, et al. Complete DNA sequence and analysis of the transferable multiple-drug resistance plasmids (R Plasmids) from Photobacterium damselae subsp. piscicida isolates collected in Japan and the United States. Antimicrob Agents Chemother. 2008;52:606–611. doi: 10.1128/AAC.01216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Ito K. Respiratory chain strongly oxidizes the CXXC motif of DsbB in the Escherichia coli disulfide bond formation pathway. EMBO J. 1999;18:1192–1198. doi: 10.1093/emboj/18.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Kishigami S, et al. Respiratory chain is required to maintain oxidized states of the DsbA-DsbB disulfide bond formation system in aerobically growing Escherichia coli cells. Proc Natl Acad Sci U S A. 1997;94:11857–11862. doi: 10.1073/pnas.94.22.11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarasamy KK, Toleman MA, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lawley TD, Klimke WA, et al. F factor conjugation is a true type IV secretion system. FEMS Microbiol Lett. 2003;224:1–15. doi: 10.1016/S0378-1097(03)00430-0. [DOI] [PubMed] [Google Scholar]
- Lee CT, Amaro C, et al. A common virulence plasmid in biotype 2 Vibrio vulnificus and its dissemination aided by a conjugal plasmid. J Bacteriol. 2008;190:1638–1648. doi: 10.1128/JB.01484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loferer H, Bott M, et al. Bradyrhizobium japonicum TlpA, a novel membrane-anchored thioredoxin-like protein involved in the biogenesis of cytochrome aa3 and development of symbiosis. EMBO J. 1993;12:3373–3383. doi: 10.1002/j.1460-2075.1993.tb06011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loferer H, Hennecke H. Expression, purification, and functional properties of Bradyrhizobium japonicum TlpA, a thioredoxin-like protein. Eur. J. Biochem. 1995a;223:339–344. doi: 10.1111/j.1432-1033.1994.tb18999.x. [DOI] [PubMed] [Google Scholar]
- Loferer H, Wunderlich M, et al. A bacterial thioredoxin-like protein that is exposed to the periplasm has redox properties comparable with those of cytoplasmic thioredoxins. J Biol Chem. 1995b;270:26178–26183. doi: 10.1074/jbc.270.44.26178. [DOI] [PubMed] [Google Scholar]
- Low KB. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, DC: ASM Press; 1996. Hfr Strains of Escherichia coli K-12; pp. 2402–2408. [Google Scholar]
- Maher D, Taylor DE. Host range and transfer efficiency of incompatibility group HI plasmids. Can J Microbiol. 1993;39:581–587. doi: 10.1139/m93-084. [DOI] [PubMed] [Google Scholar]
- Malojci G, Owen RL, et al. Preparation and structure of the charge-transfer intermediate of the transmembrane redox catalyst DsbB. FEBS Lett. 2008;582:3301–3307. doi: 10.1016/j.febslet.2008.07.063. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Lu S, et al. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:D225–D229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Bardwell JC, et al. Crystal structure of the DsbA protein required for disulphide bond formation in vivo. Nature. 1993;365:464–468. doi: 10.1038/365464a0. [DOI] [PubMed] [Google Scholar]
- McCarthy AA, Haebel PW, et al. Crystal structure of the protein disulfide bond isomerase, DsbC, from Escherichia coli. Nat Struct Biol. 2000;7:196–199. doi: 10.1038/73295. [DOI] [PubMed] [Google Scholar]
- Messens J, Collet J-F, et al. The oxidase DsbA folds a protein with a nonconsecutive disulfide. J Biol Chem. 2007;282:31302–31307. doi: 10.1074/jbc.M705236200. [DOI] [PubMed] [Google Scholar]
- Meyer EH, Giegé P, et al. AtCCMH, an essential component of the c-type cytochrome maturation pathway in Arabidopsis mitochondria, interacts with apocytochrome c. Proc Natl Acad Sci U S A. 2005;102:16113–16118. doi: 10.1073/pnas.0503473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Andrade SL, Einsle O. Thioredoxin-like [2Fe-2S] Ferredoxin. Encyclopedia of Inorganic and Bioinorganic Chemistry. 2011 [Google Scholar]
- Missiakas D, Georgopoulos C, et al. The Escherichia coli dsbC (xprA) gene encodes a periplasmic protein involved in disulfide bond formation. EMBO J. 1994;13:2013–2020. doi: 10.1002/j.1460-2075.1994.tb06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiakas D, Schwager F, et al. Identification and characterization of a new disulfide isomerase-like protein (DsbD) in Escherichia coli. EMBO J. 1995;14:3415–3424. doi: 10.1002/j.1460-2075.1995.tb07347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto H, Bardwell JCA. Catalysis of disulfide bond formation and isomerization in the Escherichia coli periplasm. Biochim Biophys Acta. 2004;1694:111–119. doi: 10.1016/j.bbamcr.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Narita S, Mastuyama S, Tokuda H. Lipoprotein trafficking in Escherichia coli. Arch. Microbiol. 2004;182:1–6. doi: 10.1007/s00203-004-0682-4. [DOI] [PubMed] [Google Scholar]
- National Institute of Allergy and Infectious Diseases. The Problem of Antibiotic Resistance. 2006 Retrieved from http:/www.niaid.nih.gov/factsheets/antimicro.htm on May 1, 2006.
- Neu HC. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- Novotny C, Knight WS, et al. Inhibition of bacterial conjugation by ribonucleic acid and deoxyribonucleic acid male-specific bacteriophages. J Bacteriol. 1968;95:314–326. doi: 10.1128/jb.95.2.314-326.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou JT, Anderson TF. Role of pili in bacterial conjugation. J Bacteriol. 1970;102:648–654. doi: 10.1128/jb.102.3.648-654.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen IT, Banerjei L, et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science. 2003;299:2071–2074. doi: 10.1126/science.1080613. [DOI] [PubMed] [Google Scholar]
- Peterson TN, Brunak S, et al. SignalP 4.0:discriminating signal peptides from transmembrane regions. Nature Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Poole LB, Karplus PA, et al. Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol. 2004;44:325–347. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
- Rees CE, Bradley DE, et al. Organization and regulation of the conjugation genes of IncI1 plasmid colIb-P9. Plasmid. 1987;18:223–236. doi: 10.1016/0147-619x(87)90065-5. [DOI] [PubMed] [Google Scholar]
- Regeimbal J, Bardwell JC. DsbB catalyzes disulfide bond formation de novo. J Biol Chem. 2002;277:32706–32713. doi: 10.1074/jbc.M205433200. [DOI] [PubMed] [Google Scholar]
- Rietsch A, Belin D, et al. An in vivo pathway for disulfide bond isomerization in Escherichia coli. Proc Natl Acad Sci U S A. 1996;93:13048–13053. doi: 10.1073/pnas.93.23.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietsch A, Bessette P, et al. Reduction of the periplasmic disulfide bond isomerase, DsbC, occurs by passage of electrons from cytoplasmic thioredoxin. J Bacteriol. 1997;179:6602–6608. doi: 10.1128/jb.179.21.6602-6608.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romine MF, Stillwell LC, et al. Complete sequence of a 184-kilobase catabolic plasmid from Sphingomonas aromaticivorans F199. J Bacteriol. 1999;181:1585–1602. doi: 10.1128/jb.181.5.1585-1602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhkova A, Stirnimann CU, et al. Structural basis and kinetics of inter- and intramolecular disulfide exchange in the redox catalyst DsbD. EMBO J. 2004;23:1709–1719. doi: 10.1038/sj.emboj.7600178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela M, Lifson S. The reformation of disulfide bridges in proteins. Biochim Biophys Acta. 1959;36:471–478. doi: 10.1016/0006-3002(59)90188-x. [DOI] [PubMed] [Google Scholar]
- Shao F, Bader MW, et al. DsbG, a protein disulfide isomerase with chaperone activity. J Biol Chem. 2000;275:13349–13352. doi: 10.1074/jbc.275.18.13349. [DOI] [PubMed] [Google Scholar]
- Sherburne CK, Lawley TD, et al. The complete DNA sequence and analysis of R27, a large IncHI plasmid from Salmonella typhi that is temperature sensitive for transfer. Nucleic Acids Res. 2000;28:2177–2186. doi: 10.1093/nar/28.10.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman JA, Matson SW. Escherichia coli DNA helicase I catalyzes a sequence-specific cleavage/ligation reaction at the F plasmid origin of transfer. J Biol Chem. 1994;269:26220–26226. [PubMed] [Google Scholar]
- Shevchik VE, Condemine G, et al. Characterization of DsbC, a periplasmic protein of Erwinia chrysanthemi and Escherichia coli with disulfide isomerase activity. EMBO J. 1994;13:2007–2012. doi: 10.1002/j.1460-2075.1994.tb06470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani M, Yano H, et al. Characterization of the replication, maintenance, and transfer features of the IncP-7 plasmid pCAR1, which carries genes involved in carbazole and dioxin degradation. Appl Environ Microbiol. 2006;72:3206–3216. doi: 10.1128/AEM.72.5.3206-3216.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouldice SR, Cho S-HH, et al. In vivo oxidative protein folding can be facilitated by oxidation-reduction cycling. Mol Microbiol. 2010;75:13–28. doi: 10.1111/j.1365-2958.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- Skyberg JA, Johnson TJ, et al. Acquisition of avian pathogenic Escherichia coli plasmids by a commensal E. coli isolate enhances its abilities to kill chicken embryos, grow in human urine, and colonize the murine kidney. Infect Immun. 2006;74:6287–6292. doi: 10.1128/IAI.00363-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie C, Garcillán-Barcia MP, et al. Mobility of plasmids. Microbiol Mol Biol Rev. 2010;74:434–452. doi: 10.1128/MMBR.00020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JM, Mavridou DA, et al. Cytochrome c biogenesis System I. FEBS J. 2011;278:4170. doi: 10.1111/j.1742-4658.2011.08376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart EJ, Katzen F, et al. Six conserved cysteines of the membrane protein DsbD are required for the transfer of electrons from the cytoplasm to the periplasm of Escherichia coli. EMBO J. 1999;18:5963–5971. doi: 10.1093/emboj/18.21.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnimann CU, Rozhkova A, et al. Structural basis and kinetics of DsbD-dependent cytochrome c maturation. Structure. 2005;13:985–993. doi: 10.1016/j.str.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Sun XX, Wang CC. The N-terminal sequence (residues 1–65) is essential for dimerization, activities, and peptide binding of Escherichia coli DsbC. J Biol Chem. 2000;275:22743–22749. doi: 10.1074/jbc.M002406200. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanboon W, Chuchue T, et al. Inactivation of thioredoxin-like gene alters oxidative stress resistance and reduces cytochrome c oxidase activity in Agrobacterium tumefaciens. FEMS Microbiol Lett. 2009;295:110–116. doi: 10.1111/j.1574-6968.2009.01591.x. [DOI] [PubMed] [Google Scholar]
- Terawaki Y, Takayasu H, et al. Thermosensitive replication of a kanamycin resistance factor. J Bacteriol. 1967;94:687–690. doi: 10.1128/jb.94.3.687-690.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunga M, Tokunga H, Wu HC. Post-translational modification and processing of Escherichia coli prolipoprotein in vitro. PNAS. 1982;79:2255–2259. doi: 10.1073/pnas.79.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkarslan S, Sanders C, et al. Compensatory thio-redox interactions between DsbA, CcdA and CcmG unveil the apocytochrome c holdase role of CcmG during cytochrome c maturation. Mol Microbiol. 2008;70:652–666. doi: 10.1111/j.1365-2958.2008.06441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kranenburg R, Golic N, et al. Functional analysis of three plasmids from Lactobacillus plantarum. Appl Environ Microbiol. 2005;71:1223–1230. doi: 10.1128/AEM.71.3.1223-1230.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain J, Diem Nga LT, et al. Molecular analysis of incHI1 antimicrobial resistance plasmids from Salmonella serovar Typhi strains associated with typhoid fever. Antimicrob Agents Chemother. 2003;47:2732–2739. doi: 10.1128/AAC.47.9.2732-2739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Fukasawa T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. I. Transfer of resistance factors by conjugation. J Bacteriol. 1961;81:669–678. doi: 10.1128/jb.81.5.669-678.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch TJ, Fricke WF, et al. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One. 2007;2:e309. doi: 10.1371/journal.pone.0000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JH, Kathir P, et al. The product of the F plasmid transfer operon gene, traF, is a periplasmic protein. J Bacteriol. 1988;170:3633–3639. doi: 10.1128/jb.170.8.3633-3639.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JH, Moore D, et al. Analysis of Escherichia coli K12 F factor transfer genes: traQ, trbA, and trbB. Plasmid. 1987;18:54–69. doi: 10.1016/0147-619x(87)90078-3. [DOI] [PubMed] [Google Scholar]
- Wunderlich M, Otto A, et al. Bacterial protein disulfide isomerase: efficient catalysis of oxidative protein folding at acidic pH. Biochemistry. 1993;32:12251–12256. doi: 10.1021/bi00096a039. [DOI] [PubMed] [Google Scholar]
- Zapun A, Missiakas D, et al. Structural and functional characterization of DsbC, a protein involved in disulfide bond formation in Escherichia coli. Biochemistry. 1995;34:5075–5089. doi: 10.1021/bi00015a019. [DOI] [PubMed] [Google Scholar]
- Zechner EL, de la Cruz F, Eisenbrandt R, Grahn AM, Koraimann G, Lanka E, Muth G, Pansegrau W, Thomas CM, Wilkins BM, Zatyka M. Conjugative-DNA Transfer Processes. In: Thomas CM, editor. The Horizontal Gene Pool. Amsterdam: Harwood Academic Publishers; 2000. pp. 87–174. [Google Scholar]
- Zechner EL, Lang S, et al. Assembly and mechanisms of bacterial type IV secretion machines. Philos Trans R Soc Lond B Biol Sci. 2012;367:1073–1087. doi: 10.1098/rstb.2011.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Peng Y, et al. Dimerization by domain hybridization bestows chaperone and isomerase activities. J Biol Chem. 2003;278:43292–43298. doi: 10.1074/jbc.M306945200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cierpicki T, et al. NMR solution structure of the integral membrane enzyme DsbB: functional insights into DsbB-catalyzed disulfide bond formation. Mol Cell. 2008;31:896–908. doi: 10.1016/j.molcel.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zienkiewicz M, Kern-Zdanowicz I, et al. Mosaic structure of p1658/97, a 125-kilobase plasmid harboring an active amplicon with the extended-spectrum beta-lactamase gene blaSHV-5. Antimicrob Agents Chemother. 2007;51:1164–1171. doi: 10.1128/AAC.00772-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.