Abstract
Background
Arteriovenous grafts (AVGs) are prone to neointimal hyperplasia leading to AVG failure. We hypothesized that pre-existing pathologic abnormalities of the vessels used to create AVG (including venous intimal hyperplasia, arterial intimal hyperplasia, arterial medial fibrosis, and arterial calcification) are associated with inferior AVG survival.
Study Design
Prospective observational study.
Setting & Participants
Patients with chronic kidney disease undergoing placement of a new AVG at a large medical center who had vascular specimens obtained at the time of surgery (n=76)
Predictor
Maximal intimal thickness of the arterial and venous intima, arterial medial fibrosis, and arterial medial calcification.
Outcome & Measurements
Unassisted primary AVG survival (time to first intervention) and frequency of AVG interventions.
Results
55 patients (72%) underwent interventions and 148 graft interventions occurred during 89.9 years of follow-up (1.65 interventions per graft-year). Unassisted primary AVG survival was not significantly associated with arterial intimal thickness (HR, 0.72; 95% CI, 0.40-1.27; p=0.3), venous intimal thickness (HR, 0.64; 95% CI, 0.37-1.10; p=0.1), severe arterial medial fibrosis (HR, 0.58; 95% CI, 0.32-1.06; p=0.6), or severe arterial calcification (HR, 0.68; 95% CI, 0.37-1.31; p=0.3). The frequency of AVG interventions per year was inversely associated with arterial intimal thickness (relative risk [RR], 1.99; 95% CI, 1.16-3.42; p<0.001 for thickness <10 vs >25 μm); venous intimal thickness (RR, 2.11; 95% CI, 1.39-3.20; p<0.001 for thickness <5 vs >10 μm); arterial medial fibrosis (RR, 3.17; 95% CI, 1.96-5.13; p<0.001 for fibrosis <70% vs ≥70%), and arterial calcification (RR, 2.12; 95% CI, 1.31-3.43; p=0.001 for <10% vs ≥10% calcification).
Limitations
Single center study. Study may be underpowered to demonstrate differences in unassisted primary AVG survival.
Conclusions
Pre-existing vascular pathologic abnormalities in CKD patients may not be significantly associated with unassisted primary AVG survival. However, vascular intimal hyperplasia, arterial medial fibrosis, and arterial calcification may be associated with a decreased frequency of AVG interventions.
Although the use of arteriovenous fistulas (AVFs) for vascular access in the U.S. has increased substantially during the past few years, approximately 25% of hemodialysis patients continue to use arteriovenous grafts (AVGs)1. The major disadvantages of AVGs are their relatively short cumulative survival (median of ~2 years) and frequent stenosis and thrombosis, requiring salvage procedures to uphold their patency for dialysis2. Previous research has not identified specific demographic or clinical features associated with AVG survival 3. Both experimental models and human studies have implicated neointimal hyperplasia at the graft-vein or graft-artery anastomosis in the pathogenesis of AVG stenosis 4,5. This observation raises the possibility that pathologic abnormalities present in the native artery or vein to which an AVG is anastomosed may predispose the patient to accelerated neointimal hyperplasia, and thereby lead to early AVG failure. Specifically, preexisting arterial or venous intimal hyperplasia may predispose to accelerated neointimal hyperplasia after vascular access creation. Likewise, arteries with substantial medial fibrosis or calcification may be stiff and thus produce excessive shear stress after vascular access creation, thereby promoting the development of neointimal hyperplasia and subsequent AVG failure.
A limited number of studies have evaluated preexisting arterial abnormalities in patients with chronic kidney disease. These studies demonstrated arterial medial fibrosis, calcification and intimal hyperplasia 6,7. Pre-existing arterial intimal hyperplasia was associated with decreased AVF survival in one report 7, whereas arterial medial fibrosis was not associated with AVF non-maturation in another study 6. There are contradictory published data on the presence of intimal hyperplasia in the native veins of CKD patients, with some investigators describing frequent and severe intimal hyperplasia 8,9, and others not observing it 6. To our knowledge, there are no published data on whether pre-existing vascular abnormalities in CKD patients are associated with AVG outcomes. The goal of our pilot study was to quantify pre-existing arterial and venous intimal thickness, arterial medial fibrosis, and arterial calcification in the vessels used to create an AVG, and to evaluate whether these pathologic abnormalities are associated with unassisted primary AVG survival. As a secondary endpoint, we evaluated the association between these vascular abnormalities and the frequency of interventions required to maintain AVG patency for dialysis.
Methods
Overview of Study Design
We invited patients with CKD who were scheduled for creation of a new AVG to participate in this prospective observational study, which had received approval from our local institutional review board. Patient recruitment occurred between 9/1/08 and 4/30/11 with follow-up through 9/30/12. The surgeon determined the optimal location of the AVG after clinical evaluation and review of preoperative ultrasound vascular mapping. During AVG creation, the surgeon obtained small specimens of the artery and vein used to perform the vascular anastomoses. A pathologist without knowledge of the patient’s clinical information assessed the histologic abnormalities in the vascular specimens. AVGs were typically cannulated for dialysis 2-3 weeks after their creation. AVG survival was determined prospectively. Finally, we evaluated the association of frequency of AVG interventions with the patient’s pre-existing vascular pathology.
Study Population
Approximately 500 hemodialysis patients receive their medical care under the supervision of clinical nephrologists at the University of Alabama at Birmingham. Almost all patients have their hospitalizations, surgical and radiologic access procedures at University of Alabama at Birmingham Hospital, improving the quality and completeness of follow-up information on vascular access procedures and outcomes. The electronic medical record was used to extract demographic and clinical information on the study patients. Two full time vascular access coordinators employed by the Division of Nephrology maintained a prospective, computerized database of all access procedures and complications 10.
Preoperative Vascular Mapping
Each patient underwent preoperative ultrasound vascular mapping to assess the diameter of the vessels and exclude the presence of stenosis or thrombosis in the draining vein. Arteriovenous fistulas (AVF) were placed preferentially in patients with suitable vascular anatomy. AVGs were created in patients with anatomy unsuitable for AVF creation or those thought to be at high risk for AVF non-maturation. The minimum sonographic criteria for AVG creation were an arterial diameter of 2 mm, a venous diameter of 4 mm, and the absence of stenosis or thrombosis in the draining vein 11. AVGs were placed preferentially in the upper extremity. However, in patients who had exhausted the vasculature of both upper extremities, the AVG was created in the thigh (provided that the patient did not have significant peripheral vascular disease).
Surgical Procedure
Four experienced surgeons created all the AVGs at a single hospital. Of the 83 study patients, 72 (87%) had already initiated dialysis prior to creation of the AVG and were dialyzing with a tunneled dialysis catheter. The polytetrafluoroethylene (PTFE) AVG was placed at one of 3 anatomic locations: forearm, upper arm, or thigh, on the basis of clinical evaluation and the preoperative vascular mapping. The surgeon used an end-to-side anastomosis between the AVG and the vein and an end-to-side anastomosis between the AVG and the artery. At the time of AVG creation, the surgeon obtained partial (elliptical) specimens (~5 mm in length) of the artery and vein from the sites used for the anastomoses. The surgeon was able to obtain the vascular samples without compromising the technical outcome of the surgery in >90% of AVG procedures.
Pathologic Studies of the Vascular Specimens
The arterial and venous specimens obtained at the time of AVG surgery were fixed in 10% formalin and processed for light microscopy. A single pathologist (S.L.), who had no knowledge of the patient’s clinical information or AVG outcome, evaluated all the tissue samples. Hematoxylin and eosin stains were used to assess the maximal thickness of the arterial and venous intima (measured between the endothelium and the internal elastic lamina); trichrome staining was used to assess medial fibrosis; and von Kossa stains to assess vascular micro-calcification 12,13. Specifically, in trichrome staining, collagen stains blue and smooth muscle stains red; with Von Kossa, calcium stains black. Bioquant Image Analysis software was used to quantify the percent of arterial specimen staining blue and the percent of arterial intima and media specimen staining black. Medial fibrosis and calcification were quantified on the arterial specimens, and expressed as a percent of the entire specimen area.
Statistical Analysis
Unassisted primary AVG survival was calculated from its creation to the first intervention (angioplasty, thrombectomy or surgical revision required to restore its patency). We compared clinical and pathologic features between patient subgroups using unpaired t tests or Mann-Whitney test for continuous variables and Chi square analysis for categorical variables. Many of the pathologic measurements were not normally distributed, so the group data were reported as medians and interquartile ranges (IQRs). Cox proportional hazards models were used to calculate unassisted primary AVG survival, with patient follow-up censored at the time of death, kidney transplantation or transfer to an outside dialysis unit. The difference in AVG survival between patient subgroups was evaluated by the log rank test. Cumulative AVG survival was calculated from AVG creation until its permanent failure, regardless of the number of interventions required to maintain patency. We calculated the frequency of percutaneous and surgical interventions required to maintain AVG patency for dialysis, and compared the frequency of interventions in subgroups with different severity of abnormalities using Poisson regression with relative rates. A p value < 0.05 was considered statistically significant.
Results
We enrolled 86 patients receiving a new AVG. Three patients were lost to follow-up shortly after their surgery, such that subsequent AVG outcome could not be ascertained. The surgeon was unable to obtain adequate vascular specimens in 7 patients. Of the remaining 76 patients, there were adequate vascular samples to determine venous intimal thickness in all 76 patients, arterial intimal thickness in 73 patients, arterial medial fibrosis in 68 patients, and calcification in 66 patients. The study population had a mean age of 56 ± 14 (standard deviation) years (range, 23-87 years). African Americans represented 84% of the study patients, reflecting the racial distribution of our dialysis population (Table 1). Females represented 63% of the study population, reflecting the higher use of AVG in women than in men. Diabetes was present in 59%, and hypertension in 95%. A substantial proportion of patients had vascular disease, including coronary artery disease (23%), peripheral vascular disease (8%), and cerebrovascular disease (17%). Congestive heart failure was present in 18% of the study population. Finally, 64% of the AVGs were placed in the upper extremity and 36% in the thigh. An anti-platelet agent (primarily aspirin) was prescribed in 54% of the study patients. Statins, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers were also prescribed in a substantial proportion of the patients.
Table 1. Clinical characteristics of study patients.
| Characteristic | Value | |
|---|---|---|
| Age ≥ 55 y | 47 (57) | |
| Female sex | 52 | 63% |
| Black race | 70 | 84% |
| Comorbid Condition | ||
| Diabetes | 49 | 59% |
| Hypertension | 79 | 95% |
| CAD | 19 | 23% |
| PVD | 7 | 8% |
| CVD | 14 | 17% |
| CHF | 15 | 18% |
| Thigh graft | 30 | 36% |
| Antiplatelet agent | 45 | 54% |
| Aspirin | 43 | 52% |
| Clopidogrel | 7 | 8% |
| Statin | 44 | 53% |
| ACEi/ARB | 52 | 63% |
| CCB | 34 | 41% |
Note: Values are given as number (percentage).
HTN, hypertension; CAD, coronary artery disease; PVD, peripheral vascular disease; CVD, cerebrovascular disease; CHF, congestive heart failure; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker.
The unassisted AVG survival for the study patients was 30% at 1 year and 16% at 3 years. Unassisted primary AVG survival was not significantly associated with patient age, sex, race, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, congestive heart failure, AVG location, surgeon, or use of anti-platelet agents, statins, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, or calcium channel blockers (data not shown). The cumulative AVG survival was 58% at 1 year and 51% at 3 years.
Vascular intimal thickness was determined from the hematoxylin-eosin stains by measuring the maximal thickness of the vascular tissue between the internal elastic lamina and the vascular endothelium (Fig 1). A recent study of radial arteries in apparently health individuals observed that the intimal thickness was <10 μm in 90% of those studied 14. The median vascular intimal thickness in our CKD patients was 12.5 (IQR, 6.1-26.5) μm for the arteries and 6.4 (IQR, 4.4-12.2) μm for the veins (Figure 2). An intimal thickness > 10 μm was present in 56% of the arteries and 33% of the veins. There was no significant association of arterial intimal thickness or venous intimal thickness with patient age, sex, race, diabetes, hypertension, coronary artery disease, peripheral vascular disease, cerebrovascular disease, or congestive heart failure. Venous intimal thickness >10 μm was more common in thigh AVG than upper extremity AVG (64% vs 24%; p=0.001). Arterial intimal thickness was not associated with AVG location. Unassisted primary AVG survival did not differ significantly between patients with and without increased arterial intimal thickness (hazard ratio [HR], 0.72; 95% confidence interval [CI], 0.40-1.27; p=0.3) (Figure 3A). Likewise, unassisted primary AVG survival was similar in patients with and without increased venous intimal thickness (HR, 0.64; 95% CI, 0.37-1.10; p=0.1)(Figure 3B).
Fig 1.
Hematoxylin-eosin stains of arteries and veins used to create arteriovenous grafts. The maximal intimal thickness was measured between the internal elastic lamina (IEL) and the vascular endothelium. A. Artery without intimal hyperplasia (intimal thickness=2 μm). B. Artery with severe intimal hyperplasia (intimal thickness=134 μm). C. Vein without intimal hyperplasia (intimal thickness=2 μm). D. Vein with severe intimal hyperplasia (intimal thickness=82 μm).
Fig 2.
Distribution of arterial and venous intimal thickness, as determined from the Hematoxylin-eosin stains of vessels obtained at the time of arteriovenous grafts. Note the values are not normally distributed.
Fig 3.
Association of unassisted primary arteriovenous graft survival with preexisting arterial (A) and venous (B) intimal hyperplasia.
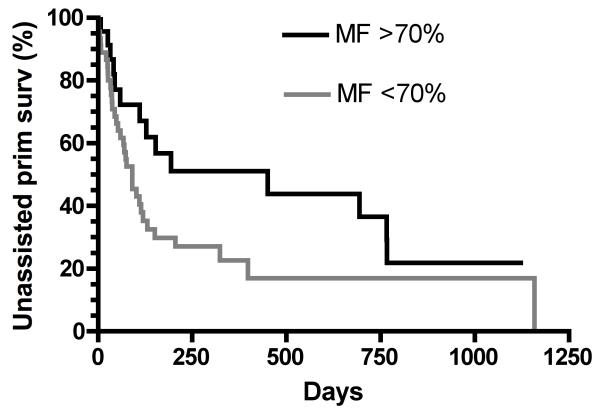
Arterial medial fibrosis was quantified from the trichrome stains (Fig 4A + B). It varied from 49% to 86%, with a normal statistical distribution. Severe medial fibrosis, defined as being ≥70% of the total parenchymal area of the arterial sample, was present in 33% of patients. There was no significant association of medial fibrosis with patient age, sex, race, diabetes, hypertension, coronary artery disease, peripheral vascular disease, cerebrovascular disease, congestive heart failure or AVG location. Unassisted primary AVG survival did not differ significantly between patients with and without severe medial fibrosis (HR, 0.58; 95% CI, 0.32-1.06; p=0.6)(Figure 5).
Fig 4.
Pathologic abnormalities in the arteries obtained during arteriovenous graft creation. A+B, trichrome stain used to quantify medial fibrosis (collagen stains blue); C+D, Von Kossa stain used to quantify arterial calcification (calcium stains black). A. Artery with mild medial fibrosis (49%). B. Artery with severe medial fibrosis (86%). C. Artery with mild calcification (0.4%). D. Artery with severe calcification (47.1%).
Fig 5.
Association of arterial medial fibrosis with unassisted primary arteriovenous graft survival.
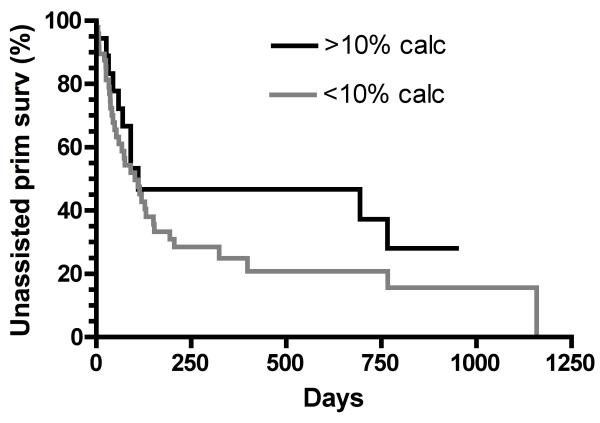
Arterial calcification was quantified from the von Kossa stains, with a wide range between 0% and 48% of the total arterial sample (Fig 4C + D). The sample distribution was skewed, with 73% of arteries having <10% calcification, and 27% having ≥10% calcification. The median arterial calcification was 2.5% (IQR, 0.4%-12.0%) of the parenchymal area. Patients with >10% arterial calcification were more likely than those with <10% arterial calcification to have peripheral vascular disease (28% vs 4%; p=0.006) and congestive heart failure (33% vs 8%; p=0.01), but did not differ in terms of age, sex, race, diabetes, hypertension, or coronary artery disease. The frequency of >10% calcification was similar in femoral arteries and upper extremity arteries (Figure 6). Finally, >10% arterial calcification was more frequent in patients with ≥70% arterial medial fibrosis as compared to those with <70% fibrosis (61% vs 23%; p=0.003). Unassisted primary AVG survival did not differ significantly between patients with arterial calcification ≥10% and <10% (HR, 0.68; 95% CI, 0.37-1.31; p=0.3)(Figure 7).
Fig 6.
Distribution of arterial calcification in arteriovenous grafts placed in the upper extremity and thigh.
Fig 7.
Association of unassisted primary arteriovenous graft survival with preexisting arterial calcification.
The association between each of the four histologic abnormalities (arterial intimal hyperplasia, venous intimal hyperplasia, medial fibrosis, and arterial calcification) and unassisted primary AVG survival is summarized in Table 2. No abnormality was statistically significantly associated with shorter unassisted primary AVG survival. Moreover, cumulative AVG survival was not significantly associated with arterial intimal thickness (HR, 1.45; 95% CI, 0.66-3.14; p=0.4), venous intimal thickness (HR, 0.59; 95% CI, 0.28-1.32; p=0.2), severe arterial medial fibrosis (HR, 0.60; 95% CI, 0.28-1.45; p=0.3), or severe arterial calcification (HR, 0.84; 95% CI, 0.35-2.06; p=0.7).
Table 2. Association of unassisted primary AVG failure with pre-existing vascular abnormalities.
| Variable | Unassisted primary AVG Failure | p-value | |
|---|---|---|---|
| Frequency * | HR (95% CI) | ||
| Arterial intimal thickness | |||
| ≥10 μm | 28/41 (68%) | 0.72 (0.40-1.27) | 0.2 |
| <10 μm | 23/32 (72%) | 1.00 (reference) | -- |
| Venous intimal thickness | |||
| ≥10 μm | 17/25 (68%) | 0.64 (0.37-1.10) | 0.1 |
| <10 μm | 38/51 (74%) | 1.00 (reference) | -- |
| Arterial medial fibrosis | |||
| ≥70% | 14/23 (61%) | 0.58 (0.32-1.06) | 0.6 |
| <70% | 34/45 (76%) | 1.00 (reference) | -- |
| Arterial calcification | |||
| ≥10% | 11/18 (61%) | 0.68 (0.37-1.31) | 0.3 |
| <1 0% | 36/48 (75%) | 1.00 (reference) | -- |
AVG, arteriovenous graft.
Given as n/N (%)
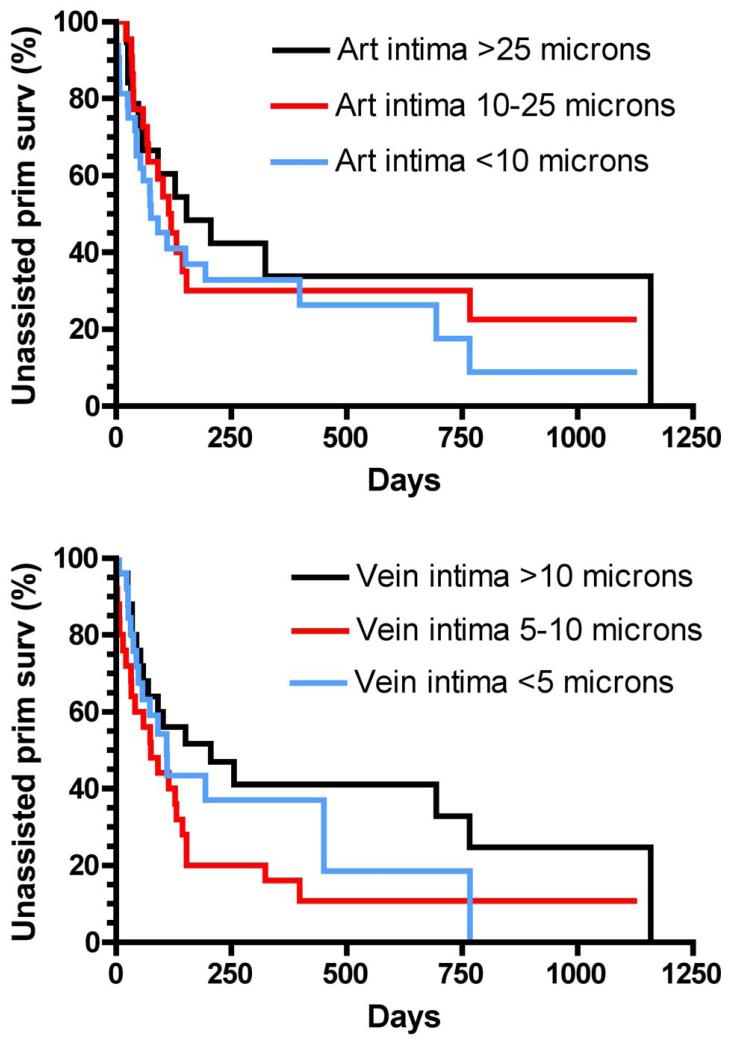
We calculated the frequency of AVG interventions required to maintain long-term patency for dialysis. Overall, there were 148 interventions during 89.9 years of follow-up, yielding an intervention rate of 165 per 100 graft-years. There was an inverse association between the annual frequency of AVG interventions and arterial intimal thickness, with an intervention rate per 100 patient-years of 103, 150, and 205 for arterial intimal thicknesses of >25 μm, 10-25 μm, and <10 μm, respectively (p<0.001) (Table 3). Likewise, the frequency of AVG interventions per 100 patient-years was significantly lower in patients with a higher venous intimal thickness (120, 180, and 254 for intimal thickness >10 μm, 5-10 μm, and <5 μm , respectively; p<0.001). The frequency of AVG interventions per 100 patient-years was substantially lower in patients with more severe medial fibrosis (68 vs 214 for medial fibrosis ≥70% vs <70%; p<0.001). Finally, the frequency of AVG interventions per 100 patient-years was inversely proportionate to arterial calcification (114 vs 240 for patients with calcification ≥10% vs <10%; p=0.001).
Table 3. Association between frequency of graft interventions and preexisting vascular abnormalities.
| Vascular measurement | No. of interventions | Graft-y of follow-up |
Interventions/10 0 patient-y |
RR (95% CI) | p-value |
|---|---|---|---|---|---|
| Arterial intimal thickness | |||||
| >25 μm | 16 | 15.5 | 103 | 1.00 (reference) | -- |
| 10-25 μm | 42 | 28.0 | 150 | 1.45 (0.83-2.65) | 0.2 |
| <10 μm | 72 | 35.1 | 205 | 1.99 (1.16-3.42) | <0.001 |
| Vein intimal thickness | |||||
| >10 μm | 42 | 34.9 | 120 | 1.00 (reference) | -- |
| 5-10 μm | 50 | 27.7 | 180 | 1.50 (0.99-2.27) | 0.05 |
| <5 μm | 47 | 18.5 | 254 | 2.11 (1.39-3.20) | <0.001 |
| Arterial Medial fibrosis | |||||
| ≥70% | 20 | 29.6 | 68 | 1.00 (reference) | -- |
| <70% | 96 | 44.8 | 214 | 3.17 (1.96-5.13) | <0.001 |
| Arterial Calcification | |||||
| ≥10% | 20 | 17.6 | 114 | 1.00 (reference) | -- |
| <10% | 95 | 39.5 | 240 | 2.12 (1.31-3.43) | 0.001 |
RR, relative risk; CI, confidence interval.
Discussion
Evaluation of the vessels obtained from CKD patients at the time of AVG creation revealed substantial pathologic abnormalities, including frequent increased arterial and venous intimal thickness, arterial medial fibrosis and arterial calcification. We hypothesized that pre-existing increased intimal thickness of the vessels would predispose to accelerated neointimal hyperplasia following AVG creation, thereby leading to AVG failure. However, contrary to our hypothesis, neither increased arterial or venous intimal thickness present in the preoperative tissue specimens was associated with inferior unassisted primary AVG survival. This finding is discrepant from that of a previous study that documented shortened AVF survival in patients with pre-existing arterial intimal hyperplasia7. Two small pilot studies described venous intimal hyperplasia in a high proportion of patients with CKD stages 4-5 undergoing vascular access surgery, but neither one provided a correlation between the histology and vascular access outcomes 8,9.
We had also hypothesized that preexisting arterial medial fibrosis or calcification would make the artery stiffer, thereby increasing shear stress after AVG creation, and contribute to accelerated neointimal hyperplasia and shortened AVG survival. Contrary to this hypothesis, we did not observe a significant association of unassisted primary AVG survival with arterial medial fibrosis or calcification. Moreover, these abnormalities appeared to confer a protective effect against recurrent AVG interventions. One possible explanation is that vessels with pre-existing intimal hyperplasia, medial fibrosis or calcification are relatively resistant to vascular injury resulting from shear stress. In experimental models, compliance mismatch between the vessel and the graft appears to promote intimal hyperplasia 15. Pre-existing vascular abnormalities, by decreasing vascular compliance, may decrease the compliance mismatch in the AVG, thereby attenuating the development of intimal hyperplasia.
It is possible that the pre-existing pathologic abnormalities of the vessels used for AVG anastomosis did affect primary unassisted survival, but this association was obscured by the potent inflammatory stimulus of the AVG provoking development of neointimal hyperplasia. A larger sample size may be required to demonstrate an independent effect of pre-existing vascular pathology on AVG survival. In addition, pharmacologic agents such as fish oil and anti-platelet drugs may prolong unassisted primary AVG survival 16,17. The protective effect of these drugs may obscure a potential relationship between pre-existing vascular pathology and AVG outcomes. However, in the present study, the use of anti-platelet agents was not associated with unassisted primary AVG survival.
The current study has some limitations. First, it represents the experience of a single large medical center, and may not generalize to that at other centers. For example, the vast majority of our patient population was black, and the results may differ in other racial groups. However, blacks are disproportionately represented among hemodialysis patients with AVG 18. Second, it is possible that the small vascular specimens obtained at the time of surgery were not an accurate reflection of the arterial or venous pathology. However, a recent study which obtained a similar size of iliac artery specimens from dialysis patients observed a significant association between calcification measured by the von Kossa stain and by radiography 12. Third, there may be additional pathologic abnormalities in the native arteries or veins that were not detected by the histologic techniques employed in the current study. Finally, this study may have been underpowered to demonstrate differences in unassisted primary AVG survival, as indicated by the wide confidence intervals for some non-significant findings.
In summary, medial fibrosis and calcification are commonly observed in the native arteries used to create AVG in CKD patients. In addition, the arterial and venous intima is frequently thickened. Although none of these histologic findings were significantly associated with unassisted primary AVG survival, all the trends favored longer unassisted primary AVG survival in patients with pre-existing vascular abnormalities. Moreover, there was a highly significant negative correlation between the frequency of AVG interventions and the presence of each of these pathologic abnormalities. Finally, our observations regarding the association between preexisting vascular abnormalities and frequency of AVG interventions do not necessarily generalize to the association in AVFs (for which vein dilation is a key requirement).
Acknowledgements
Michael Taylor, Linda Woodard and Adam Martin provided excellent technical assistance.
Support: This study was funded by a National Institute of Diabetes and Digestive and Kidney Diseases grant (R01-DK-085027) to Dr Allon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
References
- 1.Lynch JR, Wasse H, Armistead NC, McClellan WM. Achieving the goal of the Fistula First Breakthrough Initiative for prevalent maintenance hemodialysis patients. Am J Kidney Dis. 2011;57:78–89. doi: 10.1053/j.ajkd.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allon M. Current management of vascular access. Clin J Am Soc Nephrol. 2007;2:786–800. doi: 10.2215/CJN.00860207. [DOI] [PubMed] [Google Scholar]
- 3.Allon M, Zhang L, Maya ID, Bray MS, Fernandez JR. Association of factor V gene polymorphism with arteriovenous graft failure. Am J Kidney Dis. 2012;59:682–8. doi: 10.1053/j.ajkd.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy-Chaudhury P, Kelly BS, Miller MA, et al. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59:2325–34. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 5.Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17:1112–27. doi: 10.1681/ASN.2005050615. [DOI] [PubMed] [Google Scholar]
- 6.Allon M, Litovsky S, Young CJ, et al. Medial fibrosis, vascular calcification, intimal hyperplasia and arteriovenous fistula maturation. Am J Kidney Dis. 2011;58:437–43. doi: 10.1053/j.ajkd.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YO, Song HC, Yoon SA, et al. Preexisting intimal hyperplasia of radial artery is associated with early failure of radiocephalic arteriovenous fistula in hemodialysis patients. Am J Kidney Dis. 2003;41:422–8. doi: 10.1053/ajkd.2003.50051. [DOI] [PubMed] [Google Scholar]
- 8.Lee TC, Chauhan V, Krishnamoorthy M, et al. Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant. 2011;26:2264–70. doi: 10.1093/ndt/gfq733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wasse H, Rivera AA, Huang R, et al. Increased plasma chymase concentration and mast cell chymase expression in venous neointimal lesions of patients with CKD and ESRD. Semin Dial. 2011;24:688–93. doi: 10.1111/j.1525-139X.2011.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allon M, Bailey R, Ballard R, et al. A multidisciplinary approach to hemodialysis access: prospective evaluation. Kidney Int. 1998;53:473–9. doi: 10.1046/j.1523-1755.1998.00761.x. [DOI] [PubMed] [Google Scholar]
- 11.Robbin ML, Gallichio ML, Deierhoi MH, Young CJ, Weber TM, Allon M. US vascular mapping before hemodialysis access placement. Radiology. 2000;217:83–8. doi: 10.1148/radiology.217.1.r00oc2883. [DOI] [PubMed] [Google Scholar]
- 12.Schlieper G, Aretz A, Verberckmoes SC, et al. Ultrastructural analysis of vascular calcifications in uremia. J Am Soc Nephrol. 2010;21:689–96. doi: 10.1681/ASN.2009080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizobuchi M, Finch JL, Martin DR, Slatopolsky E. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int. 2007;72:709–15. doi: 10.1038/sj.ki.5002406. [DOI] [PubMed] [Google Scholar]
- 14.Myredal A, Osika W, Gan LM, Friberg P, Johansson M. Increased intima thickness of the radial artery in patients with coronary heart disease. Vascular Medicine. 2010;15:33–7. doi: 10.1177/1358863X09106619. [DOI] [PubMed] [Google Scholar]
- 15.Ballyk PD, Walsh C, Butany J, Ojha M. Compliance mismatch may promote graft-artery intimal hyperplasia by altering suture-line stresses. J Biomechanics. 1998;31:229–37. doi: 10.1016/s0197-3975(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 16.Dixon BS, Beck GJ, Vazquez MA, et al. Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med. 2009;360:2191–201. doi: 10.1056/NEJMoa0805840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lok CE, Moist LM, Hemmelgarn BR, et al. Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts: a randomized cantrolled trial. JAMA. 2012;307:1809–16. doi: 10.1001/jama.2012.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis atients: problems and solutions. Kidney Int. 2002;62:1109–24. doi: 10.1111/j.1523-1755.2002.kid551.x. [DOI] [PubMed] [Google Scholar]