Abstract
We investigated the efficacy on recovery of function following controlled cortical ischemia in the monkey of the investigational cell drug product, CNTO 0007. This drug contains a cellular component, human umbilical tissue-derived cells, in a proprietary thaw and inject formulation. Results demonstrate significantly better recovery of motor function in the treatment group with no difference between groups in the volume or surface area of ischemic damage, suggesting that the cells stimulated plasticity.
Keywords: Monkey, stroke
Introduction
Approximately 795 000 Americans experience a new or recurrent stroke each year (American Heart Association 2012). However, the only experimental therapeutic to have gained FDA (Food and Drug Administration) approval for treatment of stroke in humans is the thrombolytic agent tPA that can dissolve clots and restore blood flow if given within a narrow therapeutic window of a few hours following stroke onset. Nevertheless, in many cases with or without tPA there is significant residual impairment. Attempts to translate to humans treatments that ameliorate these residual deficits in rodents have been singularly unsuccessful (O’Collins et al. 2006). Hence, there is a critical need for a therapeutic agent that facilitates functional recovery following stroke with or without tPA therapy.
Recent studies investigating the use of cell therapies in animal models of ischemic stroke have demonstrated enhancement of neuronal survival, tissue repair, and recovery of function (Grabowski et al. 1993; Chen et al. 2001; Savitz et al. 2002; Hermann and Chopp 2012). Studies have shown that cells isolated either from the Wharton’s jelly of the umbilical cord (Lin et al. 2010) or human umbilical cord blood (Rosenkranz and Meier 2011) secrete neurotrophic factors that are thought to support recovery of function and reorganization. Studies conducted by Chopp and colleagues, demonstrated that intravenous administration of human umbilical tissue-derived cells (hUTC) in a rodent middle cerebral artery occlusion model of stroke, resulted in significant functional recovery when treatment was started at 7 or even 30 days post-stroke (Zhang et al. 2011). The treatment enhanced subventricular zone cell proliferation, synaptogenesis, and vascular density (Zhang et al. 2011).
To investigate the potential restorative ability of the investigational cell drug product, CNTO 0007, that contains hUTC in a proprietary thaw and inject formulation called CSCV4, the present study utilized a non-human primate model of cortical ischemia. This model uses electrophysiological methods to map the hand representation in primary motor cortex and guide placement of a reproducible ischemic lesion limited to the hand representation so that only the function of the contralateral hand is impaired (Moore et al. 2010, 2012). Performance with the opposite unimpaired hand serves as an internal control for motivation or other non-specific factors. The effects of treatment on functional recovery of the impaired hand were quantified.
Methods
Subjects.
A total of 8 young adult male rhesus monkeys (M. mulatta) were used. They ranged from 5 to 10 years of age (approximately equivalent to humans from 15 to 30 years of age; Tigges et al. 1988). Monkeys were obtained from the Caribbean National Primate Research Center. Before entering the study, they received medical examinations that included serum chemistry, hematology, and urine and fecal analysis. Health records were screened to exclude monkeys with a history of malnutrition, chronic illness, diabetes, neurological diseases, or chronic illnesses. All monkeys were given initial magnetic resonance imaging (MRI) scans to ensure there was no occult brain abnormality.
Once enrolled in the study, monkeys were housed in the Laboratory Animal Science Center of Boston University Medical Campus that is AAALAC accredited. Experiments were conducted in accordance with the guidelines of the NIH Committee on Laboratory Animal Resources and with the approval of Institutional Animal Care and Use Committee (IACUC) of the Boston University Medical Campus.
Pre-operative training on fine motor function testing.
Monkeys were trained on the Hand Dexterity Task (HDT) for a total of 15 days (Monday, Wednesday, and Friday each week for 5 weeks). All monkeys in each group received the same amount of pre-operative training on the HDT (15 days). The HDT is a modified version of a Klüver board (Klüver 1935) that requires precise control of the digits, particularly apposition of the finger and thumb, to retrieve small food rewards from two different size wells. Both wells were 1 cm deep and the large well was 25 mm wide and the small well was 18 mm wide. Time to retrieve is recorded by photocells and success is recorded by the tester. The HDT has been used to assess fine motor function of the hand and digits with young and middle-aged rhesus monkeys in this non-human primate model of cortical ischemia (Moore et al. 2010, 2012). A more detailed description of the task and apparatus are described in Moore et al. (2010).
Each test day consisted of 32 trials divided into 16 trials for each hand and further subdivided into 8 trials for each of the two wells. The trials for right and left hands and for each well were presented in a pseudo-random, counterbalanced fashion to eliminate any order effects. Each monkey was given 30 s to complete a trial. If a monkey could not or would not complete an individual trial in 30 s or if a monkey removed its hand from the apparatus without retrieving the reward, the trial was terminated and the monkey was given one additional opportunity to complete that trial. After a second failed attempt, a non-response was recorded, the animal’s difficulties were noted in the study record, and the next trial was initiated.
Hand preference.
At the completion of pre-training on the HDT, free choice trials with both sides of the apparatus baited and accessible to both hands were administered to determine which hand was ‘‘preferred’’ or dominant. This assessment was also compared with the pre-operative acquisition rates for each hand. Based on this assessment, the ischemic lesion was targeted at the hemisphere controlling the dominant hand to ensure that even with impairment, monkeys would be motivated to use the impaired hand during post-operative testing.
Group assignment and blinding.
After the completion of pre-training, based on pre-operative performance animals were randomly assigned (by SPF) in a balanced fashion to receive either the therapeutic treatment or vehicle/placebo. Post-operative administration of cells or vehicle was administered by one of the authors (DLR). All other personnel involved in the study (surgeons, technicians, behavioral testers, etc.) were kept blind to the assignment during surgery and all post-operative testing and assessment including tissue harvest and processing.
Electrophysiological mapping of the motor cortices and lesion production.
To create reproducible cortical ischemic damage and motor deficits, an ischemic lesion was localized to only the representation of the dominant hand in M1. All surgical procedures were carried out under aseptic conditions. Animals were sedated with ketamine hydrochloride (10 mg/ kg) and anesthetized with intravenous sodium pentobarbital (15–25 mg/kg) to effect. Heart rate, respiration, oxygenation, and muscle tonus were monitored to ensure physiological homeostasis and a safe surgical level of anesthesia. The head was stabilized in a stereotactic apparatus, and a midline incision was made followed by reflection of the temporalis muscle. A bone flap over the frontal and parietal lobes was removed in one piece and the dura incised to expose the motor cortex.
To map the hand representation of the dominant hand, the central and arcuate sulci were visualized and a calibrated photograph was taken and printed. The hand area of M1 was mapped onto this calibrated photograph using electrical stimulation delivered through a small monopolar silver ball electrode placed gently on the surface of the pia and moved systematically in rows from the dorsal aspect of the pre-central gyrus toward the ventral aspect of the sulcus. A surface electrode was used rather than sharp electrode penetration of the cortex (e.g., Nudo and Milliken 1996) in order to avoid creating damage in the motor cortex outside the hand representation (i.e., areas that control other parts of the body).
Stimulation sites were spaced 2 mm apart (anterior to posterior) in rows and each row was separated from the next by 2 mm (ventral to dorsal). Monopolar stimulus pulses of 250 ms duration at amplitudes from 2.0 to 3.0 mA were delivered once every 2 s either singly or in a train of 4 pulses delivered over 40 ms at a rate of 100 Hz. Non-responsive sites were further tested with a 200 Hz train consisting of 4 or 8 pulses of 2 ms duration delivered over 20 or 40 ms, respectively. The intensity of the motor response in the hand and digits was graded on a scale of 1–5 (barely visible to maximal). Specific stimulation sites with the lowest threshold and highest motor response were marked on the calibrated photograph creating a cortical surface map of the hand area which was used to guide the placement of the ischemic lesion (Figure 1). Since the hand representation is known to extend down the rostral bank of the central sulcus, the sulcus was opened down to the fundus along the length of the gyral hand representation by microdissection with a small glass pipette and blunt periosteal elevator taking care to leave the somatosensory areas on the posterior bank intact. The hand area in the sulcus was not electrophysiologically mapped with the electrode to avoid inadvertent damage to the somatosensory areas on the caudal bank of the central sulcus as this mapping requires prolonged retraction. However, we have verified in terminal experiments, the presence of the hand representation on the rostral bank (unpublished).
Figure 1.

Calibrated photograph of the lateral surface of motor and somatosensory cortices showing stimulation sites. Black circles represent sites that when stimulated produced a strong response of hand and/or digits and white circles represent sites that when stimulated produced weak or no response of hand and/or digits. Area of lesion within the sulcus is not shown in this photograph.
Using this map and the adjacent exposed sulcus, ischemic damage was produced by making a small incision in the pia at the dorsal limit of the representation. A small glass suction pipette was then inserted under the pia and used to bluntly transect the small penetrating arterioles as they enter the underlying cortex. Suction and irrigation with sterile saline was sufficient to staunch any bleeding and maintain a clear field all the way down to the fundus of this sulcus. This approach removes the blood supply to the cortex of the hand representation, producing an ischemic lesion of the gray matter that degenerates down to the underlying white matter as illustrated in Figure 2.
Figure 2.

Representative thionin sections through the lesion from one control and one treated animal. Extent of lesion is visible to include surface representation of hand area and rostral bank of central sulcus.
After the lesion was made and any bleeding stopped, the dura was closed, the bone flap was sutured back in place using small burr holes placed in the flap, and the muscle, fascia, and skin were closed in layers. Immediately following surgery, antibiotics and analgesics were administered and the monkeys were kept in an incubator and monitored continuously until anesthesia wore off. They were then returned to their home cage and monitored continuously until fully awake and able to feed and drink. For the next 3–7 days (or as needed) animals were given analgesics and monitored regularly for any signs of infection or complications.
CNTO 0007 infusion.
Intravenous administration of CNTO 0007 was completed between 23 and 24 h following surgery. Sample preparation was conducted using standard tissue culture protocol. Briefly, cells were removed from liquid nitrogen storage, thawed, and then assessed for viability. They were then aliquoted into a sterile syringe and administered intravenously at a concentration of 10 M cells/ml and a rate of 0.5 ml per min using a syringe pump to deliver a total dose 10 M cells/kg. Vehicle was administered at the same volume and rate. The start-time and stop-time of the infusion were recorded. Respiratory rate and oxygen saturation were monitored during the infusion.
Initial cage-side post-operative assessment.
During the initial 2-week post-operative recovery period, each monkey was observed daily in their home cage and both upper limbs were assessed for level of function in terms of muscle tone, strength, tremor, and fine motor function using our Non-Human Primate (NHP) Upper Extremity Motor Dysfunction Scale (Table I). This scale was adapted from Zhang et al. (2000) and the National Institutes of Health Stroke Scale.
Table I.
Non-Human Primate Upper Extremity Motor Dysfunction Scale. Adapted from Zhang et al. 2000 and the National Institutes of Health Stroke Scale.
| #1 | Observed tone in upper limbs | |
| 0 | Normal | |
| 1 | Decreased muscle tone (flaccid, arm hangs limply at side) | |
| 2 | Increased muscle tone (arm is held in flexed position) | |
| #2 | Tremor in upper limbs (when taking food) | |
| 0 | Absent | |
| 1 | Occasionally present | |
| 2 | Present much of the time | |
| 3 | Continuously present | |
| #3 | Fine motor function of hands | |
| 0 | Normal use | |
| 1 | Mild impairment in ability to retrieve food | |
| 2 | Moderate impairment in ability to retrieve food | |
| 3 | Severe impairment in ability to retrieve food | |
| 4 | Unable or refuses to retrieve food | |
| #4 | Functional strength of hand | |
| 0 | Normal–no signs of weakness | |
| 1 | Mild weakness in upper extremity (UE) when reaching or grasping | |
| 2 | Moderate weakness in UE when reaching or grasping | |
| 3 | Severe weakness in UE when reaching or grasping | |
| 4 | Unable or refuses to reach or grasp | |
| #5 | Flexion of digits | |
| 0 | Normal flexion in digits | |
| 1 | Mild impairment in flexion of digits | |
| 2 | Moderate impairment in flexion of digits | |
| 3 | Severe impairment in flexion of digits | |
| 4 | No flexion observed | |
| #6 | Movements of forearm and wrist | |
| 0 | Normal and full movement of wrist | |
| 1 | Mild impairment/weakness/slowness of wrist | |
| 2 | Moderate to severe impairment/weakness/slowness of wrist | |
| 3 | Severe impairment/weakness/slowness of wrist | |
| 4 | Unable to move UE at wrist joint | |
| #7 | Movements of arm and shoulder | |
| 0 | Normal and full movement of arm and shoulder | |
| 1 | Mild impairment/weakness/slowness of arm and shoulder | |
| 2 | Moderate impairment/weakness/slowness of arm and shoulder | |
| 3 | Severe impairment/weakness/slowness of arm and shoulder | |
| 4 | Unable to move UE at arm and/or shoulder joints |
Post-operative testing.
Post-operative testing on the HDT began 2 weeks after surgery and continued for 12 weeks. Testing on the HDT was conducted on Monday, Wednesday, and Friday each week. However, the hand use requirement was altered so that 70% of the trials required the use of the impaired hand while 30% were given to the intact hand. Since the testing apparatus is designed so that the monkey must use only the left or the right hand in the left and right side of the apparatus, respectively, the greater percentage of trials given to the impaired hand ensures that the monkey uses its impaired hand. This forced use of the impaired hand is similar in nature to constraint-induced therapy as used in human rehabilitation strategies which force use of the impaired limbs. At the same time, the 30% of trials given to the unimpaired hand provide sufficient rewards to maintain motivation and sufficient data to demonstrate that effects are not due to generalized changes in motivation or motor function. Each animal was given 30 s to complete a trial as in pre-operative training. Testing continued for 12 weeks, the time estimated for control subjects to achieve asymptotic stable performance.
Grasp pattern assessment.
Performance on the HDT during pre-operative training and post-operative testing were videotaped with fixed placement cameras. A licensed Occupational Therapist (MAP) who specializes in upper extremity recovery following stroke, analyzed the videotapes using our NHP Grasp Assessment Scale (Moore et al. 2012). This scale was adapted from the Eshkol–Wachman Movement Notation (Carr et al. 1985; Whishaw et al. 2002) and the Fugl-Meyer Motor Assessment Scale (Fugl-Meyer et al. 1975). Our scale rates the shaping of the hand and digits for grasp and the pattern of grasp and release to provide a semi-quantitative measure of grasp function. The original scale consisted of six hierarchical stages that include distinctive features (see Moore et al. 2012). However, in order to increase the sensitivity of this scale for detecting recovery of function, the scale was modified to score eight hierarchical stages in half steps for a total of 13 units with the maximum score of 8 reflecting normal grasp patterns (functional pinch between thumb and one individual digit) (see Table II).
Table II.
Modified Non-Human Primate Grasp Assessment Scale.
| 0.0 | Complete absence of active finger movement and makes no attempt to use impaired upper extremity (UE) to test. |
| 1.0 | Attempts to use impaired UE to retrieve food reward but no flexion and extension of digits either singly or as a group. Unsuccessful at retrieving any food rewards. |
| 2.0 | Flexion and extension of digits as a group using less than 15 degrees of digit metacarpophalangeal (MP) flexion. No evidence of the purposeful ‘‘scooping’’ of reward. No movement of thumb towards palm. Unsuccessful at retrieving any food rewards. |
| 3.0 | Flexion and extension of digits as a group using less than 15 degrees of digit MP flexion. Evidence of the development of compensatory ‘‘scooping’’ movement of reward toward palm of hand with multiple fingers and mass action of digits. Successful retrieval of food reward on at least 10% of trials. No movement of thumb towards palm. |
| 3.5 | Flexion and extension of digits as a group using less than 15 degrees of digit MP flexion. Evidence of the development of compensatory ‘‘scooping’’ movement of reward toward palm of hand with multiple fingers and mass action of digits. Successful retrieval of food reward on at least 25% of trials. No movement of thumb towards palm. |
| 4.0 | Flexion and extension of digits as a group using greater than 15 degrees of digit MP flexion. Perform purposeful compensatory ‘‘scooping’’ movement of reward toward palm of hand with multiple fingers and mass action of digits. Successful retrieval of food reward on at least 25% of trials. No movement of thumb towards palm. |
| 4.5 | Flexion and extension of digits as a group using greater than 15 degrees of digit MP flexion. Perform purposeful compensatory ‘‘scooping’’ movement of reward toward palm of hand with multiple fingers and mass action of digits. Successful retrieval of food reward on at least 25% of trials. Begin to observe slight movement of thumb observed during testing session. |
| 5.0 | Flexion and extension of digits as a group using greater than 15 degrees of digit MP flexion. Perform purposeful compensatory ‘‘scooping’’ movement of reward toward palm of hand with multiple fingers and mass action of digits. Slight movement of thumb towards palm with a web space no greater than one digit width. Thumb does not reach opposition. Successful retrieval of food reward on at least 75% of trials. |
| 5.5 | Flexion and extension of digits as a group using greater than 15 degrees of digit MP flexion. Perform purposeful compensatory ‘‘scooping’’ movement of reward toward palm of hand with multiple fingers and mass action of digits. Thumb in opposition. Successful retrieval of food reward on at least 75% of trials. |
| 6.0 | Reaches toward reward with all digits in extension with evidence of isolated individual finer movement on at least 10% of trials per day. Mass movement of digits still predominates. The compensatory ‘‘scooping’’ movement toward palm is used. Successful retrieval of food reward on at least 75% of trials. |
| 6.5 | Reaches toward reward with all digits in extension with evidence of isolated individual digit movement on 25% of trials per day. Mass movement of digits still predominates. The compensatory ‘‘scooping’’ movement toward palm is used. Successful retrieval of food reward on at least 75% of trials. |
| 7.0 | Reaches toward reward with all digits in extension with evidence of isolated individual digit movement on 50% of trials per day. The compensatory ‘‘scooping’’ movement toward palm is used though becoming less predominant. Successful retrieval of food reward on 100% of trials. |
| 7.5 | Reaches toward reward with all digits in extension. Isolated individual finger movement occurs in greater than 50% of trials. The compensatory ‘‘scooping’’ movement toward palm is used though becoming less predominant. Successful retrieval of food reward on 100% of trials. |
| 8.0 | Pre-operative movement pattern of all digits and hand is observed with no evidence of compensatory ‘‘scooping’’ movements. Functional pinch occurs between the thumb and one individual digit, usually the index finger. |
Perfusion and tissue processing.
For perfusion-fixation of the brain, monkeys were deeply anesthetized with IV sodium pentobarbital (25 mg/kg to effect) and were killed by exsanguination during transcardial perfusion of the brain, first for no more than 5 min with 4 °C Krebs buffer at pH 7.4 and then with 8 liters of 4% paraformaldehyde, pH 7.4 over 10 min to completely fix the brain. The brain was then photographed, in situ, with the photograph aligned in the perspective of the cortical map used to create the lesion. Then the brain was blocked, in situ, in the coronal plane to ensure reproducible planes of section during later processing. It was removed from the skull, weighed, and post-fixed overnight in 4% paraformaldehyde for no more than 18 h. It was then transferred to cryoprotectant solution to eliminate freezing artifact (Rosene et al. 1986). Cryoprotected blocks were flash frozen and stored at 80 °C until they were cut on a microtome into interrupted series of coronal sections (eight series of 30 μm thick sections and one 60 mm thick series, spacing between sections within a series of 300 μm). The 60 μm series was immediately mounted on microscope slides and stained with thionin for lesion reconstruction (Figure 2).
Lesion volume and surface area.
To reconstruct the volume of the lesion, all sections through the entire extent of the lesion (range of 23–31 sections) were digitized using an EPSON 4490 Scanner to create high-resolution JPEGs of each section. These images were then imported into Image J. To estimate the volume of the lesion, the extent of the lesion on each slide was outlined as well as the total intact gray matter from the depth of the cingulate sulcus on the medial surface to the depth of the lateral sulcus on the lateral surface. An identical assessment of intact gray matter was then done in the opposite intact hemisphere as well. The total volume outlined in each hemisphere was then determined using the Cavalieri estimator (Rosen and Harry 1990) by taking the sum of the areas of each section and multiplying by the distance (0.30 mm) between sections to estimate volume. This allowed us to determine the volume of the lesion by subtracting the volume of the frontal cortex in the damaged hemisphere from the volume in the contralateral intact hemisphere to obtain an estimate of the lesion volume. This approach ensures that lesion volume will not be under- or over-estimated due, respectively, to ‘‘compression’’ of the lesion during in vivo healing or compression or stretching of lesion space during tissue processing.
To determine the surface area of the lesion two approaches were used. First, the histological sections were digitized using the Turboscan system (Objective Imaging, Cambridge, UK) to create photomontages of the entire brain at 4× magnification. The surface length of the lesion on each section was measured in Image J. Again, all surface length measures were totaled and multiplied by 0.30 mm, the distance between sections, to obtain an estimate of the surface area of the lesion. Second, the calibrated photographs of each brain taken immediately after removal from the skull were also imported into Image J and the surface area of the lesion outlined and measured.
Data analysis and statistics
Pre-operative HDT.
The mean time to retrieve a food reward from each of the wells of the HDT with each hand for the last 3 days of pre-operative testing was determined for each monkey. A Student’s t-test was used to compare the performance of control and treated monkeys on these measures.
Post-operative NHP Upper Extremity Motor Dysfunction Scale.
Beginning on post-operative day 1, the degree of motor impairment in the upper extremity was assessed for each monkey in their home cage using our adapted NHP Upper Extremity Motor Dysfunction Scale to assess tone, tremor, fine motor function of the hand, strength of the hand, digit flexion, and movement of the forearm, wrist, arm, and shoulder. Each measure is rated on a 3-, 4-, or 5-point scale of impairment with 0 indicating no impairment (0–2, 0–3, or 0–4; see Table I). The mean rating for each animal on the first three (1–3) and last three (12–14) post-operative days was determined for the measures of fine motor function and strength of the hands. The difference in the mean rating from days 1–3 and days 12–14 was then calculated for each animal, and these difference scores were compared between groups. Separate Student’s t-tests were used to compare the difference scores between groups on the measures of fine motor control and strength of the hands.
Post-operative HDT.
The mean time to retrieve the food reward on the HDT across the 12-week post-operative period was also determined. A Student’s t-test was used to compare the performance of control and treated animals on this measure. In addition, the mean time to retrieve a food reward on the HDT during the initial week of post-operative testing and the final week of testing on the HDT for the control and treated animals was compared using a two-way repeated measures ANOVA with group (control vs. treated) as a between subject variable and time (initial week vs. final week) as a within subjects variable to compare the time to retrieve for the two groups of monkeys.
Post-operative grasp assessment.
The mean number of post-operative days required to return to asymptotic levels of grasp on the HDT with the impaired hand was determined. A return to asymptotic levels of performance was defined as a grasp assessment score at pre-operative levels (score of 8; functional pinch between thumb and one individual digit) for 2 consecutive days for each well. If an animal did not return to pre-operative levels of grasp (score of 8), then the first 2 consecutive days at their highest rating were used as asymptotic performance. A Student’s t-test was used to compare the number of post-operative days required to return to asymptotic levels of grasp on the HDT for the impaired hands of the control and treated monkeys. In addition, the mean grasp assessment rating across the post-operative period for the HDT was also compared using a Mann–Whitney U-test.
Lesion surface area and volume.
A Pearson’s r Correlation was used to determine the presence of a relationship between the surface area measures on the photographs and those calculated on the thionin sections. This analysis revealed a significant relationship between these measures (r = 0.978; p ≤0.05) verifying the accuracy of the two methods to determine the surface area. A Student’s t-test was used to compare the volume of the lesion between the control and treated animals.
Correlations between lesion volume, lesion surface area, and motor function.
Pearson’s r was used to determine the presence of a relationship between the number of post-operative days required to return to pre-operative levels of performance on the HDT, final grasp assessment on the HDT, and final lesion volume.
Results
Pre-operative HDT
The mean time to retrieve a food reward from each of the two different wells (large and small diameter) of the HDT with the dominant hand for the last 3 days of pre-operative testing was determined for each monkey. Separate Student’s t-tests were used to compare the performance of monkeys assigned to the control and treatment groups on each of the two wells. This analysis detected no significant difference between the control and treated monkeys on either well. This confirms the effectiveness of our pre-operative pseudo-random balancing of group assignment.
Post-operative NHP Upper Extremity Motor Dysfunction Assessment Scale
Separate Student’s t-tests revealed a significant difference between the groups in terms of the difference in the mean rating on the measures of fine motor function and strength of the hand from days 1–3 and days 12–14 (p<0.007 and 0.02) with a greater degree of recovery in the treated animals (Figure 3).
Figure 3.
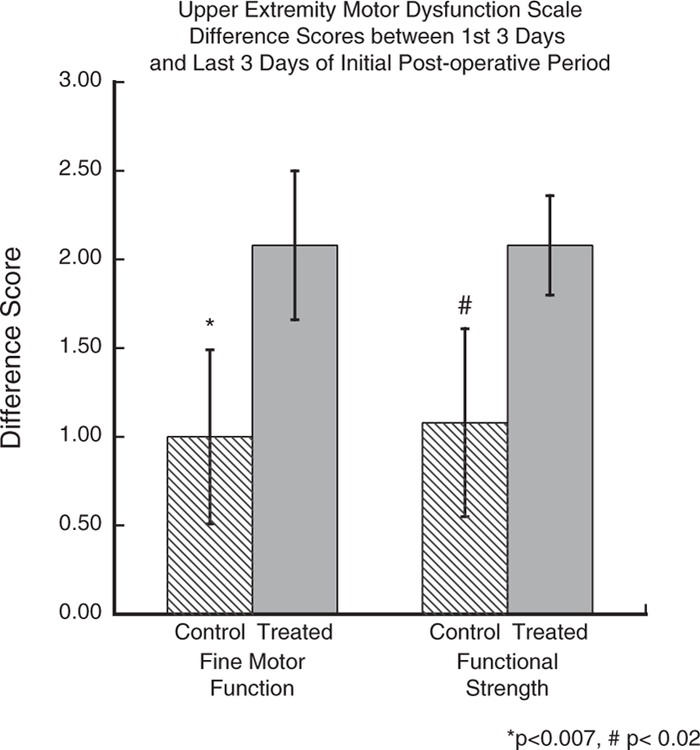
Graph of difference scores on our Non-Human Primate Upper Extremity Motor Dysfunction Scale on measures of fine motor function and of hand strength from the first 3 days (d1–3) and last 3 days (d12–14 of initial post-operative period. Asterisks indicate a significant group difference in recovery of fine motor function (p<0.007) and the # indicates a significant difference in hand strength (p<0.02).
Post-operative HDT
A Student’s t-test to compare the mean time to retrieve the food reward on the HDT across the entire 12-week post-operative period revealed that the treatment group was significantly superior to untreated animals on retrieval from the large well (p<0.001) (Figure 4). For the small well there was a trend in the same direction but it only approached significance (p<0.09). The lack of a significant group difference on the time to retrieve from the small well is likely due to the fact that the compensatory scooping grasp developed by the untreated animals appeared to be more effective in scooping the reward out of the smaller diameter well as the animals were able to steady the treat on the side of the well and then scoop it into their palm. In the well with the larger diameter, this compensatory movement was more difficult in the larger space as the reward would ‘‘slip’’ away from the monkey while trying to scoop it out of the well without effective finger–thumb apposition.
Figure 4.

This graph shows the mean time to retrieve rewards across the entire 12-week post-operative testing period for the large well on the HDT. Asterisks indicate a significant group difference in the time required to retrieve rewards (p<0.001).
A two-way repeated measures ANOVA comparing the time to retrieve the reward during the initial week of testing and then during the final week of testing with group (control vs. treated) as a between subject variable and time (initial week vs. final week) as a within subjects variable revealed an overall effect of group [F(1, 6) = 27.296, p = 0.002]. Further, there was a significant effect of time [F(1, 6) = 42.246, p = 0.0006] and a significant group by time interaction [F(1, 15) = 7.88, p = 0.03]. A Bonferroni Post Hoc test revealed that there was a significant difference in performance between the control and treated groups in the initial week of testing with the treated animals having superior performance (p<0.01) (Figure 5).
Figure 5.

This graph illustrates a significant difference in performance between the control and treated groups in the initial week of testing with the treated animals having superior performance (*p<0.01). In addition, there was a significant difference in performance in the control group from the initial week to the final week of testing (#p<0.003). There was not a significant difference in the treated group during this time (p<0.217).
In addition, there was a significant difference in performance in the control group from the initial week to the final week of testing (p<0.003) (Figure 5). There was not a significant difference in the treated group during this time. This is likely due to the fact that the treated animals demonstrated a striking degree of recovery in the initial week of testing reducing the relative difference from performance on the HDT in the final week. Related to this finding, the animals in the treated group reached a post-operative level of performance equal or even greater than their level of performance during pre-operative testing. Whereas no animal in the control group returned to pre-operative levels of performance in terms of time to retrieve. This provides further validation of the potential efficacy of the treatment in this study.
Post-operative HDT grasp assessment
A Student’s t-test was used to compare the mean number of post-operative days required to reach asymptotic levels of grasp for the impaired hand of control and treated monkeys on the large well. This analysis revealed a significant difference between groups on the large well (p<0.01) with treated monkeys showing superior recovery of grasp pattern (Figure 6).
Figure 6.

This graph illustrates the significant difference between groups for the number of post-operative days to attain asymptotic levels of grasp performance on the HDT for the impaired hand on the large well (p<0.01).
In addition, the mean grasp assessment rating across the post-operative period for the HDT was also determined. A Mann–Whitney U-test revealed a significant difference between groups on the large well (p<0.01) (Figure 7).
Figure 7.

This graph demonstrates a significant difference between groups for the mean grasp assessment rating reached across the post-operative period on the HDT until criterion was reached on grasp assessment (p<0.01).
Lesion surface area and volume
Analysis of the surface area and of the volume of the lesions showed no significant group differences on either measure (Table III). Not surprisingly a Pearson’s r Correlation assessing the relationship between lesion volume and the number of post-operative days required to return to pre-operative levels of performance on the HDT as well as with final grasp assessment on the HDT revealed no significant relationship across all subjects or within subjects in either group.
Table III.
Final lesion volumes (mm3), final surface area (mm2), pre-operative mean on the HDT, and mean grasp rating on the HDT during initial and final week of testing for all monkeys.
| Animal | Final lesion volume (mm3) |
Final surface area (histological slides) (mm2) |
Pre-operative HDT–mean of final 3 days of testing (s) |
Mean grasp rating on the HDT–initial week of post-operative testing |
Mean grasp rating on the HDT–final week of post-operative testing |
|---|---|---|---|---|---|
| Control 1 | 86.63 | 51.41 | 1.47 | 6.66 | 7.83 |
| Control 2 | 84.02 | 54.44 | 0.99 | 4.5 | 7.5 |
| Control 3 | 122.94 | 67.93 | 0.92 | 4.17 | 7.5 |
| Control 4 | 58.72 | 43.99 | 1.12 | 4.83 | 7.5 |
| Mean | 88.08 | 54.44 | 1.12 | 5.04 | 7.58 |
| SE | 13.22 | 5.00 | 0.123 | 0.557 | 0.08 |
| Treated 1 | 57.51 | 39.62 | 0.96 | 7.33 | 7.83 |
| Treated 2 | 114.25 | 55.81 | 1.17 | 7.17 | 7.83 |
| Treated 3 | 62.67 | 49.07 | 1.01 | 7.50 | 7.83 |
| Treated 4 | 105.86 | 80.90 | 0.95 | 7.50 | 7.83 |
| Mean | 85.07 | 56.35 | 1.02 | 7.38 | 7.83 |
| SE | 14.56 | 8.83 | 0.05 | 0.07 | 0.00 |
Discussion
Summary
The principal findings of this study are: (1) During the first 14 days after surgery, there was a significant degree of recovery within the treated group on measures of fine motor function and strength of the hand (Figure 3). (2) There was a significant difference between groups for the mean time to retrieve the food reward on the HDT across the 12-week post-operative assessment with the treated group showing a shorter time to retrieve (Figure 4). This is consistent with improved recovery that was particularly evident in the initial week of post-operative testing (Figure 5). (3) In terms of fine motor grasp pattern, as shown in Figure 6, there was a significant group difference in the mean number of post-operative days required to return to asymptotic levels of grasp performance. (4) Finally, in terms of absolute level of grasp pattern, there was a significant effect of treatment on the mean grasp assessment rating across the post-operative period on the HDT (Figure 7). (5) Importantly, there were no group difference in terms of post-mortem lesion volume or lesion surface area and there was no significant correlation between performance on the HDT and lesion volume (Table III). Together these findings provide evidence that cell-based therapy enhanced recovery of fine motor function after ischemic cortical damage in a non-human primate, the rhesus monkey.
Cell-based therapies for enhancing recovery
The efficacy of various cell-based treatments for recovery of function has been investigated in animal models of stroke and ischemia where cell therapies can be from exogenous or endogenous sources, can be embryonic, fetal, or adult derived, and be either pluripotential or multi-potential (Chopp et al. 2009; Li and Chopp 2009; Rosenkranz and Meier 2011; Sahota and Savitz 2011). Transplanted embryonic and neural stem cells have been used in rodent models of stroke, and it has been demonstrated that these cells develop into neural precursors, differentiated neurons, and glial cells (Sahota and Savitz 2011). However, both have the significant potential to transform into teratomas and malignant teratocarcinomas (Sahota and Savitz 2011).
Alternatively, transplantation and infusion of human umbilical cord blood cells (hUCB) has also been shown to improve functional outcome in rodent models of stroke (Chen et al. 2001; Brenneman et al. 2010; Rosenkranz and Meier 2011). hUCB contain mesenchymal progenitor cells, endothelial cell precursors, and other immature progenitor cells that have extensive capacity for proliferation (Broxmeyer 1996; Nieda et al. 1997; Erices et al. 2000). Further, hUCB cells can differentiate into neural cells, secrete neurotrophic factors, and produce cytokines such as chemokines, interleukins, and growth factors that may modulate inflammation, apoptosis, angiogenesis, and synaptogenesis (Hess et al. 2002; Newman et al. 2005, 2006; Newcomb et al. 2006; Neuhoff et al. 2007; Rosenkranz and Meier 2011).
Another type of cell-based therapy is bone-marrow-derived stromal cells (BMSC) that are mixed cell populations extracted from adipose tissue, peripheral blood, and muscle (Sng and Lufkin 2012). They contain a mixture of stem and progenitor cells, usually do not form teratomas, and usually do not cause immune rejection issues (Xiong et al. 2011). They have been extensively studied in a variety of rodent models of disease and injury and it has been widely demonstrated that regardless of administration route, BMSC significantly improve function after stroke (Li and Chopp 2009). Specific to the present study though are the findings in rodent models of stroke that intervenous infusion of BMSC survive over a long period of time in recipient animals and promote functional recovery. Whether administered immediately after stroke or 1–4 weeks after stroke (Mahmood et al. 2006; Shen et al. 2007; Chen et al. 2008), BMSC continue to promote recovery of function after 3 months (Mahmood et al. 2005).
Intravenous administration of BMSC in rodent models of intracerebral hemorrhage allows the cells to enter the brain and migrate to the regions around the lesion. This is amplified when the administration is preceded by an infusion of mannitol (Seyfried et al. 2008, 2010). In addition, there is evidence of increased markers of neurogenesis, improved function, and reduced tissue loss in this model following administration of BMSC (Seyfried et al. 2010). Further, Bhasin et al. (2011) demonstrated improved motor function 8 and 24 weeks after intravenous infusion of BMSC in human stroke patients.
It has been hypothesized that BMSC do not replace damaged tissue, but rather secrete growth factors such as brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) and induce intrinsic parenchymal cells to also produce these factors (Chopp and Li 2002; Mahmood et al. 2004, 2005; Sofroniew 2005; Qu et al. 2007; Chopp et al. 2008; Xiong et al. 2011). It has been demonstrated that these factors enhance angiogenesis, synaptogenesis, neurogenesis, and axonal sprouting in the area around the lesion (Mahmood et al. 2005, 2006; Liu et al. 2010; Li et al. 2013).
Human umbilical tissue-derived cells, the cellular component of CNTO 0007, have also been assessed in a rat model of focal ischemia (Zhang et al. 2011). They reported significant improvement in treated subjects compared to controls on performance on the adhesive tape removal test of fine motor function and on a modified neurological severity scale. Similar to the present study, this group also showed that there were no differences in lesion volumes between the treated and control animals. In a related study, hUTC administration 1, 3, or 7 days post-stroke resulted in improved neurological function and increased cell proliferation, vascular density, and synaptogenesis and decreased apoptosis in the peri-lesional region (Yang et al. 2012). Further, using MRI, Jiang et al. (2012) demonstrated that hUTC administration resulted in significant reduction of ventricular volume expansion and improved cerebral blood flow, measures that correlated with histological lesion volume, synaptic density, and functional improvements.
CNTO 0007 in a non-human primate model of ischemia: recovery of grasp and fine motor function
In the present study, the most striking finding was the significant improvement in the time to retrieve and the quality of grasp pattern shown in monkeys treated with CNTO 0007 compared to controls on post-operative assessment of the HDT. While it can be argued that the lesion size in this study was small compared to the lesions that can occur in human stroke patients, and that there was evidence of recovery in the control animals as there is in humans after stroke, this model is effective for the assessment of stroke treatment as the untreated animals do not return to pre-operative levels of performance in terms of time to retrieve or in their grasp patterns. Further, in our previous studies, untreated animals with the identical limited, controlled ischemic lesion in the hand area of M1 do not fully recover fine motor function of the hand but rather develop a compensatory ‘‘scooping’’ motion with their digits and hand without recovery of the finger–thumb pinching action that is their normal behavior (Moore et al. 2012). However, the four treated monkeys in the present study showed complete recovery of the finger–thumb pinching action during the post-operative period and returned to pre-operative levels on the HDT in terms of time to retrieve. Thus, the findings in the present study with non-human primates, while limited by sample size, are in agreement with Zhang et al. (2011) and provide encouragement that this approach could translate to human stroke patients.
Possible mechanisms of recovery
While it does appear that CNTO 0007 did have an effect in recovery of function, particularly in the quality of grasp function, the precise mechanisms underlying this recovery remains unknown. At the cellular level, cortical reorganization following ischemic injury has been found to correlate with dendritic remodeling (Jones and Schallert 1992; Buga et al. 2008), increased levels of the presynaptic growth-associated proteins GAP-43, and synaptophysin (Benowitz and Routtenberg 1997; Stroemer et al. 1998; Carmichael and Chesselet 2002; Carmichael 2006; Buga et al. 2008; Benowitz and Carmichael 2010), axonal sprouting (Carmichael et al. 2005; Li and Carmichael 2006; Buga et al. 2008), and angiogenesis (Krupinski et al. 1994; Beck et al. 2000; Marti et al. 2000; Hayashi et al. 2003; Beck and Plate 2009). In addition, it has been reported that in rodents, focal ischemia results in denervation that stimulates axonal sprouting and synaptogenesis that correlates with functional recovery (Stroemer et al. 1998). Other studies have demonstrated that neurogenesis, synaptogenesis, and vascular density are potential mechanisms underlying recovery following stroke (Zhang et al. 2000; Ward and Cohen 2004; Carmichael 2006; Chopp and Li 2008; Zhang and Chopp 2009).
Based on the foregoing observations, it is hypothesized that cell-based therapies do not replace damaged tissue, but rather secrete growth factors and induce intrinsic parenchymal cells to also produce these factors (Chopp and Li 2002; Mahmood et al. 2004, 2005; Sofroniew 2005; Qu et al. 2007; Chopp et al. 2008; Xiong et al. 2011) enhancing angiogenesis, synaptogenesis, neurogenesis, and axonal sprouting (Mahmood et al. 2005, 2006; Liu et al. 2010; Li et al. 2013). Similarly, Zhang et al. (2011) demonstrated that hUTC enhances synaptogenesis, neural progenitor proliferation, and vascular density and reduces apoptotic cell death following stroke. They suggest that the mechanism of action of the hUTC is expression of neurotrophic factors such as BDNF and bFGF. The findings in the current study of recovery of fine motor grasp provide validation of the efficacy of CNTO 0007 and its cellular component, hUTC, as a treatment for ischemia. Future investigation with this model of the potential paracrine neurotrophic action of hUTC in relationship to recovery of function is necessary to assess this hypothesis.
Acknowledgements
The authors thank Megan McBurnie, Melissa Joblon, Adrian Oblak, Farzad Mortazavi, and Karen Slater for their expert technical assistance with all aspects of this study.
This study was supported by a contract from Advanced Technologies and Regenerative Medicine, LLC (ATRM), RR# 101115-PR, who funded the study and provided CNTO 0007 and a vehicle control.
On 30 December 2012, ATRM merged into DePuy Orthopaedics, Inc.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- American Heart Association. 2012. Heart disease and stroke statistics–2012 update: A report from the American Heart Association. Circulation 125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H, Acker T, Wiessner C, Allegrini PR, Plate KH. 2000. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. Am J Pathol 157(5):1473–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H, Plate KH. 2009. Angiogenesis after cerebral ischemia. Acta Neuropathol 117(5):481–496. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. 1997. GAP-43: An intrinsic determinant of neuronal development and plasticity [Review]. Trends Neurosci 20(2):84–91. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Carmichael ST. 2010. Promoting axonal rewiring to improve outcome after stroke. Neurobiol Dis 37(2):259–266. Epub 2009 Nov 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin A, Srivastava MV, Kumaran SS, Mohanty S, Bhatia R, Bose S, Gaikwad S, Garg A, Airan B. 2011. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra 1(1):93–104. Epub 2011 Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman M, Sharma S, Harting M, Strong R, Cox CS Jr, Aronowski J, Grotta JC, Savitz SI. 2010. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab 30(1):140–149. Epub 2009 Sep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer HE. 1996. Primitive hematopoietic stem and progenitor cells in human umbilical cord blood: An alternative source of transplantable cells. Cancer Treat Res 84:139–148. [DOI] [PubMed] [Google Scholar]
- Buga AM, Dunoiu C, Bălşeanu A, Popa-Wagner A. 2008. Cellular and molecular mechanisms underlying neurorehabilitation after stroke in aged subjects [Review]. Rom J Morphol Embryol 49(3):279–302. [PubMed] [Google Scholar]
- Carmichael ST, Chesselet MF. 2002. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci 22(14):6062–6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. 2005. Growth-associated gene expression after stroke: Evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol 193(2):291–311. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. 2006. Cellular and molecular mechanisms of neural repair after stroke: Making waves [Review]. Ann Neurol 59(5):735–742. [DOI] [PubMed] [Google Scholar]
- Carr JH, Shepherd RB, Nordholm L, Lynne D. 1985. Investigation of a new motor assessment scale for stroke patients. Phys Ther 65: 175–180. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. 2001. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32(4):1005–1011. [DOI] [PubMed] [Google Scholar]
- Chen JR, Cheng GY, Sheu CC, Tseng GF, Wang TJ, Huang YS. 2008. Transplanted bone marrow stromal cells migrate, differentiate and improve motor function in rats with experimentally induced cerebral stroke. J Anat 213(3):249–258. Epub 2008 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, Li Y. 2002. Treatment of neural injury with marrow stromal cells [Review]. Lancet Neurol 1(2):92–100. PMID:12849513. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y, Zhang J. 2008. Plasticity and remodeling of brain [Review]. J Neurol Sci 265(1–2):97–101. Epub 2007 Jul 3. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y. 2008. Treatment of stroke and intracerebral hemorrhage with cellular and pharmacological restorative therapies. Acta Neurochir Suppl 105:79–83. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y, Zhang ZG. 2009. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke 40(3 Suppl):S143–S145. doi: 10.1161/STROKEAHA.108.533141. Epub 2008 Dec 8. PMID:19064763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erices A, Conget P, Minguell JJ. 2000. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 109(1):235–242. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. 1975. The post-stroke hemiplegic patient. Scand J Rehabil Med 7:13–31. [PubMed] [Google Scholar]
- Grabowski M, Brundin P, Johansson BB. 1993. Functional integration of cortical grafts placed in brain infarcts of rats. Ann Neurol 34: 362–368. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Noshita N, Sugawara T, Chan PH. 2003. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab 23(2):166–180. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Chopp M. 2012. Promoting brain remodeling and plasticity for stroke recovery: Therapeutic potential, caveats and consequences for clinical translation. Lancet 11:369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DC, Hill WD, Martin-Studdard A, Carothers J, Brailer J, Carroll J. 2002. Blood into brain after stroke. Trends Mol Med 8(9):452–453. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Thiffault C, Kramer BC, Ding GL, Zhang L, Nejad-Davarani SP, Li L, Arbab AS, Lu M, Navia B, et al. 2012. MRI detects brain reorganization after human umbilical tissue-derived cells (hUTC) treatment of stroke in rat. PLoS ONE 7(8):e42845. doi: 10.1371/journal.pone.0042845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Schallert T. 1992. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Behav Brain Res 51: 156–160. [DOI] [PubMed] [Google Scholar]
- Klüver H 1935. An auto-multi-stimulation reaction board for use with sub-human primates. J Psychol Interdisc Appl 1:123–127. [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang J. 1994. Role of angiogenesis in patients with cerebral ischaemic stroke. Stroke 25: 1794–1798. [DOI] [PubMed] [Google Scholar]
- Li H, Daculsi R, Bareille R, Bourget C, Amedee J. 2013. uPA and MMP-2 were involved in self-assembled network formation in a two dimensional co-culture model of bone marrow stromal cells and endothelial cells. J Cell Biochem 114(3):650–657. [DOI] [PubMed] [Google Scholar]
- Li S, Carmichael ST. 2006. Growth-associated gene and protein expression in the region of axonal sprouting in the aged brain after stroke. Neurobiol Dis 23(2):362–373. [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M. 2009. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett 456(3):120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SZ, Chang YJ, Liu JW, Chang LF, Sun LY, Li YS, Luo GH, Liao CH, Chen PH, Chen TM, et al. 2010. Transplantation of human Wharton’s jelly-derived stem cells alleviates chemically induced liver fibrosis in rats. Cell Transplant 19(11):1451–1463. Epub 2010 Jun 29. PMID:20587139. [DOI] [PubMed] [Google Scholar]
- Liu H, Huang GW, Zhang XM, Ren DL, X Wilson J. 2010. Folic acid supplementation stimulates notch signaling and cell proliferation in embryonic neural stem cells. J Clin Biochem Nutr 47(2):174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Chopp M. 2004. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma 21: 33–39. [DOI] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Qu C, Goussev A, Chopp M. 2005. Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery 57:1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A, Lu D, Qu C, Goussev A, Chopp M. 2006. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J Neurosurg 104(2):272–277. PMID:16509501. [DOI] [PubMed] [Google Scholar]
- Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W. 2000. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol 156(3):965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Pessina MA, Moss MB, Rosene DL. 2010. Assessment of motor function of the hand in aged rhesus monkeys. Somatosens Mot Res 27(3):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Pessina MA, Moss MB, Finklestein SP, Rosene DL. 2012. Recovery from ischemia in the middle-aged brain: A non-human primate model. Neurobiol Aging 33(3):619.e9–619.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff S, Moers J, Rieks M, Grunwald T, Jensen A, Dermietzel R, Meier C. 2007. Proliferation, differentiation, and cytokine secretion of human umbilical cord blood-derived mononuclear cells in vitro. Exp Hematol 35(7):1119–1131. [DOI] [PubMed] [Google Scholar]
- Newcomb JD, Ajmo CT Jr, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE. 2006. Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transplant 15(3):213–223. [DOI] [PubMed] [Google Scholar]
- Newman MB, Willing AE, Manresa JJ, Davis-Sanberg C, Sanberg PR. 2005. Stroke-induced migration of human umbilical cord blood cells: Time course and cytokines. Stem Cells Dev 14(5):576–586. [DOI] [PubMed] [Google Scholar]
- Newman MB, Willing AE, Manresa JJ, Sanberg CD, Sanberg PR. 2006. Cytokines produced by cultured human umbilical cord blood (HUCB) cells: Implications for brain repair. Exp Neurol 199(1):201–208. Epub 2006 May 30. [DOI] [PubMed] [Google Scholar]
- Nieda M, Nicol A, Denning-Kendall P, Sweetenham J, Bradley B, Hows J. 1997. Endothelial cell precursors are normal components of human umbilical cord blood. Br J Haematol 98(3):775–777. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. 1996. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol 75(5):2144–2149. [DOI] [PubMed] [Google Scholar]
- O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 2006. 1,026 experimental treatments in acute stroke. Ann Neurol 59(3):467–477. PMID:16453316. [DOI] [PubMed] [Google Scholar]
- Qu ZB, Liu LX, Wu LF, Jiang HC. 2007. Multiple littoral cell angioma of the spleen: A case report and review of the literature. Onkologie 30: 256–258. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Harry JD. 1990. Brain volume estimation from serial section measurements: A comparison of methodologies. J Neurosci Methods 35(2):115–124. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Roy NJ, Davis BJ. 1986. A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J Histochem Cytochem 34(10):1301–1315. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Meier C. 2011. Umbilical cord blood cell transplantation after brain ischemia—From recovery of function to cellular mechanisms. Ann Anat 193(4):371–379. Epub 2011 Mar 31. [DOI] [PubMed] [Google Scholar]
- Sahota P, Savitz SI. 2011. Investigational therapies for ischemic stroke: Neuroprotection and neurorecovery [Review]. Neurotherapeutics 8(3):434–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz SI, Rosenbaum DM, Dinsmore JH, Wechsler LR, Caplan LR. 2002. Cell transplantation for stroke. Ann Neurol 52:266–275. [DOI] [PubMed] [Google Scholar]
- Seyfried DM, Han Y, Yang D, Ding J, Savant-Bhonsale S, Shukairy MS, Chopp M. 2008. Mannitol enhances delivery of marrow stromal cells to the brain after experimental intracerebral hemorrhage. Brain Res 1224:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried DM, Han Y, Yang D, Ding J, Shen LH, Savant-Bhonsale S, Chopp M. 2010. Localization of bone marrow stromal cells to the injury site after intracerebral hemorrhage in rats. J Neurosurg 112(2):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, Lu M, Raginski K, Vanguri P, Smith A, et al. 2007. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab 27(1):6–13. [DOI] [PubMed] [Google Scholar]
- Sng J, Lufkin T. 2012. Emerging stem cell therapies: Treatment, safety, 0061nd biology. Stem Cells Int 521343 Epub 2012 Aug 2. [DOI] [PMC free article] [PubMed]
- Sofroniew MV. 2005. Reactive astrocytes in neural repair and protection. Neuroscientist 11:400–407. [DOI] [PubMed] [Google Scholar]
- Stroemer RP, Kent TA, Hulsebosch CE. 1998. Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke 29(11):2381–2393. discussion 2393–2395. [DOI] [PubMed] [Google Scholar]
- Tigges J, Gordon TP. McClure HM, Hall EC, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes Regional Primate Research Center. American Journal Primatology 1988;15:263–273. [DOI] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. 2004. Mechanisms underlying recovery of motor function after stroke. Arch Neurol 61:1844–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Suchowersky O, Davis L, Sarna J, Metz G, Pellis S. 2002. Impairment of pronation, supination, and body co-ordination in reach-to-grasp tasks in human Parkinson’s disease (PD) reveals homology to deficits in animal models. Behav Brain Res 133: 165–176. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Meng Y, Zhang Y, Zhang ZG, Morris DC, Chopp M. 2011. Treatment of traumatic brain injury with thymosin β4 in rats. J Neurosurg 114(1):102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Han Y, Zhang J, Seyda A, Chopp M, Seyfried DM. 2012. Therapeutic effect of human umbilical tissue-derived cell treatment in rats with experimental intracerebral hemorrhage. Brain Res 1444: 1–10. Epub 2012 Jan 20. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li Y, Zhang C, Chopp M, Gosiewska A, Hong K. 2011. Delayed administration of human umbilical tissue-derived cells improved neurological functional recovery in a rodent model of focal ischemia. Stroke 42(5):1437–1444. Epub 2011 Apr 14. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. 2000. VEGF enhances angiogenesis and promotes blood–brain barrier leakage in the ischemic brain. J Clin Invest 106:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M. 2009. Neurorestorative therapies for stroke: Underlying mechanisms and translation to the clinic. Lancet Neurol 8(5):491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]


