Abstract
The family is one of the primary contexts of child development. Marital and parent–child conflict (family conflict) are common and predict a wide range of negative behavioral and emotional outcomes in children. Thus, an important task for developmental researchers is to identify the processes through which family conflict contributes to children's psychological maladjustment, as well as vulnerability and protective factors in the context of family conflict. In the current paper, we aim to advance a conceptual model that focuses on indices of children's autonomic nervous system (ANS) functioning that increase vulnerability or provide protection against psychological maladjustment in the context of family conflict. In doing so, we provide a selective review that reflects the state of the science linking family conflict, children's ANS activity, and child psychological adjustment, and offer directions and guidance for future research. Our hope is to accelerate research at the intersection of family conflict and ANS functioning to advance understanding of risk and resilience among children.
Many children languish in the context of marital and parent–child conflict, but negative psychological consequences of family conflict are not apparent in other children. In this paper, we advance a conceptual model that focuses on indices of children's autonomic nervous system (ANS) functioning that increase vulnerability or provide protection against psychological maladjustment in the context of family conflict. Our focus on the ANS is based on (a) the compelling history of research on ANS functioning and psychological maladjustment (Beauchaine, 2001), within which conceptual propositions and findings can be contextualized; (b) that ANS parameters can be assessed easily and noninvasively (e.g., see Beauchaine, 2009; Gottesman & Gould, 2003), such as during lab tasks designed to simulate or reflect certain environmental conditions or to evoke certain physiological responses; (c) the evidence that ANS functioning may be responsive to psychosocial intervention (e.g., Raine et al., 2001) and used as a mechanism for change (e.g., biofeedback; Karavidas et al., 2007); (d) the critical role of the ANS as a channel through which information travels to and from the central nervous system; and (e) the considerable body of empirical research demonstrating the moderating role of ANS functioning in the context of family conflict (e.g., El-Sheikh et al., 2009; Katz & Gottman, 1995, 1997), from which we can generate new understanding and hypotheses about sources and sequelae of ANS functioning that moderate the negative effects of family conflict on children.
We discuss the conceptual and empirical foundations of our model that reflect the state of the science regarding links between family conflict, ANS activity, and child adaptation, provide examples of current research, and propose directions for future research. We focus on research directions that are developmentally focused and that promote an integrated conceptualization of psychophysiological functioning in the context of family conflict, with an emphasis on interactions across family conflict and the two branches of the ANS: sympathetic (SNS) and parasympathetic (PNS). By doing so, we hope to accelerate understanding and promote further inquiry into ANS variables that moderate the effects of family conflict on children's psychological adjustment.
Overarching Conceptual Model
Our biopsychosocial conceptual model is situated within a general developmental psychopathology framework that views child maladjustment as an outcome of interactions and transactions among multiple individual and environmental risk factors (Cicchetti, 2006). The model draws from influential perspectives on family conflict (e.g., Cummings & Davies, 1996; Patterson, Reid, & Dishion, 1992), psychophysiology (e.g., Beauchaine, 2001; Berntson, Cacioppo, Quigley, 1991; Porges, 2007), and Biological × Environmental interactions (e.g., Beauchaine, Gatzke-Kopp, & Mead, 2007; Belsky & Pluess, 2009; Boyce & Ellis, 2005).
Our premise is that child psychological outcomes are shaped partly by interactions between family and ANS functioning, such that certain child physiological profiles incur vulnerability or offer protection against family risk. We emphasize that relatively stable individual differences, reflected in ANS measures, can render children more or less susceptible to the negative psychological effects of family conflict. As such, our model is related to and more topic specific than biological sensitivity to context (Boyce & Ellis, 2005) and differential susceptibility to environmental influences (Belsky & Pluess, 2009). Our discussion may be more consistent with differential susceptibility than biological sensitivity because we focus on ANS functioning as a relatively stable individual difference that may moderate the effects of family conflict across a range of severity and chronicity, like the Belsky model, but we certainly recognize that ANS functioning is under some degree of environmental control, as Boyce and Ellis propose. Our model is also distinguished in focus from conceptual frameworks that either link psychophysiological profiles directly with child psychopathology (e.g., Beauchaine, Katkin, Strassberg, & Snarr, 2001), or that emphasize how negative family processes influence emotion and psychophysiology, which in turn contribute to psychological outcomes (e.g., Beauchaine et al., 2007; Katz, 2001). That is, we focus specifically on ANS parameters as moderators of the association between family conflict and child psychological adjustment. Other conceptualizations are either more general in their approach to environmental stress and biological parameters or have implied or specified other types of models (e.g., mediation) linking the familial environment, child biological markers, and psychopathology (e.g., Beauchaine et al., 2007; Belsky & Pluess, 2009; Boyce & Ellis, 2005).
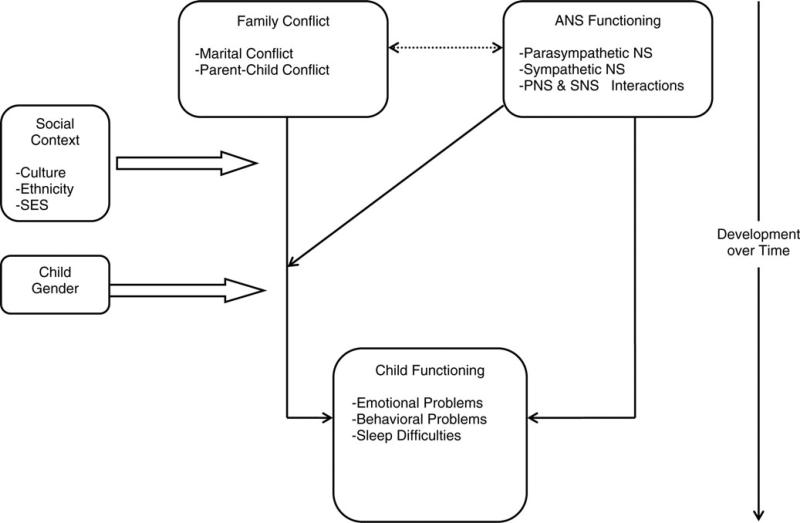
The conceptual model is depicted in Figure 1. Briefly, family conflict is linked with children's physiological and psychological development. ANS functioning may operate as a pathway, or mediator, through which family conflict influences children's psychological adjustment (e.g., Beauchaine et al., 2007; Katz, 2001). More germane to our focus, however, ANS functioning may vary independent of family conflict, and exacerbate or attenuate the impact of family conflict on children's psychological adjustment. In particular, we propose that the moderating influence of the ANS may involve interactions between the SNS and PNS. Biological characteristics (e.g., sex) and social–ecological conditions (e.g., ethnicity, socioeconomic status) are also conceptualized as correlates and moderators of family conflict. It is important to acknowledge that our model focuses on family conflict and the SNS and PNS branches of the ANS, but we also extend discussion to other physiological systems (e.g., hypothalamic–pituitary–adrenal [HPA] axis) as they pertain to interactions with the ANS. We do not specifically address other sources of influence on children's psychological adjustment (e.g., genetic or peer-group factors); as such, the model presented here attempts to explain only part of the variance in children's psychological adjustment.
Figure 1.
The conceptual model. ANS, autonomic nervous system; NS, nervous system; PNS, parasympathetic nervous system; SNS, sympathetic nervous system; SES, socioeconomic status.
Throughout the paper, we raise five potential ways in which ANS functioning may increase risk or provide protection in the context of family conflict. First, baseline levels of ANS activity may affect children's readiness to respond adaptively to experiences of family conflict. For example, moderate baseline levels may facilitate appropriate awareness and active cognitive or behavioral responses, compared to baseline levels that are under- or overactive. Second, ANS activity may affect or reflect children's sensitivity to experiences of family conflict. SNS and PNS levels have actually been linked with sensitivity to aversive and rewarding conditions (Beauchaine, 2001) as well as biological sensitivity to context or differential susceptibility to environmental influence (Belsky & Pluess, 2009; Boyce & Ellis, 2005).
Third, ANS reactivity may influence children's concurrent psychological responses to family conflict, including their emotions (e.g., form, intensity, duration, regulation), cognitions (e.g., attention or encoding, attributions, response evaluations), and behaviors (e.g., social communication, passive hovering, escape–avoidance, aggression); together, these ANS and short-term responses to conflict may exacerbate or attenuate long-term adjustment difficulties. Because children may be aware of their levels of ANS activity (e.g., sweating, increased heart rate), there may be substantial promise in investigating ANS responses as they relate to concurrent psychological responses.
Fourth, each ANS system may influence (e.g., mediate, moderate) the activity of other stress response systems during or following exposure to family conflict. For example, PNS withdrawal may stimulate SNS or HPA activity, or a strong SNS response may override the PNS response and stimulate the HPA axis. Finally, ANS responses to stress may recalibrate the (co)operation of bioregulatory systems (e.g., HPA, immune system, sleep system) over time. For example, repeated activation of one system may contribute to dysfunctions in other systems due to excessive allostatic load, or wear and tear on physiological systems (McEwen, 1998).
Definitional issues
Respiratory sinus arrhythmia (RSA), skin conductance level (SCL), and cardiac preejection period (PEP) are the indices of ANS functioning most commonly studied in the context of family conflict. We focus on these ANS parameters and define them below.
RSA can be used as an estimate of vagal tone or PNS influence on the heart, and change in RSA from baseline to stress can be used to index vagal reactivity. In this paper, we use the terms vagal tone and vagal reactivity when referring to the constructs at a conceptual level; we use the term RSA when reporting study findings. RSA is the rhythmic fluctuation of heart rate during spontaneous breathing and is governed by the myelinated vagus nerve (Porges, 1995). Vagal tone (indexed by resting RSA) reflects the activity of the PNS at rest and perhaps the ability to sustain attention, regulate emotion, and engage in social communication (Porges, 2007; Thayer & Lane, 2009). Vagal reactivity refers to changes in RSA from baseline to challenging conditions, and may be characterized as vagal withdrawal (decreased RSA) or vagal augmentation (increased RSA). Vagal withdrawal accelerates heart rate and increases metabolic output rapidly and incrementally, and may reflect awareness of environmental challenge and mobilization of physiological resources to mount an active coping response (Fox & Calkins, 2003; Porges, 2007). Conversely, vagal augmentation decelerates heart rate in response to challenge and may reflect failure to engage with environmental demands or immobilization responses (Calkins, 1997; Calkins & Dedmon, 2000). Of importance, vagal withdrawal and vagal augmentation occur along a continuum such that greater or lesser degrees of change from baseline conditions occur. RSA has been well validated as a marker of the vagus nerve's influence on the heart; and within a broad range of normal physiological functioning,it is considered a valid measure of vagal tone (Grossman & Taylor, 2007). Pharmacological blockade studies demonstrate that the vagus nerve influences the attenuation or elimination of RSA (Berntson et al., 1997; Hayano et al., 1991; Sherwood, Allen, Obrist, & Langer, 1986).
Evidence for sex differences in RSA is inconclusive. Girls exhibited higher RSA than boys in some studies (Fabes, Eisenberg, Karbon, Troyer, & Switzer, 1994), but findings were reversed in other studies (El-Sheikh, 2005a; Hinnant, Staton, & El-Sheikh, 2010; Salomon, 2005). Furthermore, some studies revealed no sex differences in RSA (Alkon et al., 2003; Calkins, Graziano, & Keane, 2007; Gazelle & Druhen, 2009; Graziano, Keane, & Calkins, 2007; Hessler & Fainsilber Katz, 2007; Suess, Porges, & Plude, 1994). Few studies have examined race differences, although at least one recent study reported higher RSA among African American children compared to European American children (Hinnant et al., 2010).
SCL and SCL reactivity (SCL-R) refer to electrodermal activity and reactivity, respectively. SCL and SCL-R are governed by the activity of sweat glands, which are innervated by the SNS component of the ANS. It is well established that SCL and SCL-R are influenced by SNS activity (Boucein, 1992; Siepmann et al., 2007; Venables & Christie, 1980). The SNS is activated in response to perceived stress or threat, equipping the body for a fight/flight/freeze response. Individuals with heightened SCL and SCL-R may experience high anxiety or fearfulness and exhibit inhibited behavior when faced with perceived threat, often resulting in avoidant (flight) responses (Beauchaine, 2001; Fowles, Kochanska, & Murray, 2000). Note that SCL and RSA tend to be uncorrelated (El-Sheikh et al., 2009). Girls may have higher SCL than boys (e.g., Boucsein, 1992; El-Sheikh, 2007), although findings are relatively mixed (e.g., Venables & Mitchell, 1996). Race differences are more consistent, such that European American children tend to exhibit higher SCL than African American children (e.g., Boucsein, 1992; El-Sheikh, Keiley, & Hinnant, 2010); this finding may be explained by the inverse relation between number of sweat glands and darker skin pigmentation (Boucsein, 1992).
Many of the studies to date on child risk in the context of family conflict have examined skin conductance. Another index of SNS functioning is PEP, which is also termed ventricular ejection time. PEP is the time between when the heart fills with blood and when the blood is ejected out of the heart, and it can be assessed using impedence cardiography (Andreassi, 2007). Lower PEP indicates higher SNS activity and heart rate acceleration (Quigley & Stifter, 2006). PEP is an established measure of SNS activity (Berntson, Cacioppo, & Fieldstone, 1994; Quigley & Stifter, 2006), and the cardiac sympathetic nerves effects on the myocardium are reflected in the shortening or lengthening of preejection period (Imrich et al., 2008; Larkin & Kasprowicz, 1986). Despite the relatively small number of studies that have examined children's PEP, it may be an important index of SNS functioning that reflects trait impulsivity or approach motivation under conditions of reward (Brenner, Beauchaine, & Sylvers, 2005; Richter & Gendolla, 2009), whereas SCL may more specifically reflect inhibition or anxiety (e.g., Beauchaine, Hong, & Marsh, 2008).
Alpha-amylase has recently begun to be considered as a possible marker of the SNS (Bishop, Walker, Scanlon, Richards, & Rogers, 2006; Chatterton, Vogelsong, Lu, Ellman, & Hudgens, 1996; Granger, Kivlighan, El-Sheikh, Gordis, & Stroud, 2007). However, this is still a point of debate among scholars (Nater et al., 2005, 2006; Rohleder, Nater, Wolf, Ehlert, & Kirschbaum, 2004).
Conceptual issues
Polyvagal theory is exceptionally informative as a conceptual framework for understanding the role of ANS in the context of both stress exposure and psychopathology. Below, we provide a brief overview of polyvagal theory, followed by consideration of Beauchaine's important integration of Gray's motivational theory with Porges’ polyvagal theory.
As a general principle, polyvagal theory (Porges, 2007) posits that vestiges of earlier, less complex stress response systems are available in humans and activated when more contemporary systems fail or become overwhelmed. The first response system to evolve was the dorsal vagal complex, or vegetative vagus, which is distinguished by nonmyelinated vagal motor fibers that originate in the dorsal motor nucleus of the brain. It is proposed that dorsal motor nucleus fibers become active only when innervation from the nucleus ambiguus (NA) branch of the vagus, a more recent evolutionary adaptation (discussed below), is withdrawn. In response to threat, the vegetative vagus minimizes oxygen usage and energy demand by slowing heart rate and reallocating energy throughout the body. Thus, vegetative vagus activity results in subsequent behavioral responses such as freezing in the service of avoidance.
The next evolutionary development is the SNS, which fosters mobilization. To prepare the body for action, the SNS increases cardiac output and sweat gland secretion while simultaneously inhibiting gastrointestinal tract activity. Thus, the body shifts energy from normal homeostatic functions to allow an active behavioral response. The most recent evolutionary development involves the ventral vagal complex, or smart vagus. This complex includes the myelinated vagus and portions of other cranial nerves originating in the NA, which project to various organs in the body. The trigeminal and facial nerves are also commonly considered part of this complex. Collectively, this system influences facial expression, sucking, swallowing, listening, and vocalization, and thus has been described as a social engagement system (Porges, 2007).
In addition, activity of the ventral vagal complex exerts an inhibitory influence on the heart, and its withdrawal stimulates a heart rate increase, independent of sympathetic activity. The myelination of vagal fibers originating in the NA allows for firm control and speed in responding to the environment. Thus, the “vagal brake” can be withdrawn or instated to produce rapid changes in cardiovascular output to meet environmental demands. Furthermore, the ventral vagal complex allows for a metabolically conservative response to the environment by promoting incremental changes in heart rate to support regulated emotional responses (Porges, 2007).
According to the polyvagal theory (Porges, 2007), when confronted with a challenge, mammals automatically respond by first orienting then disengaging the vagal brake, inhibiting parasympathetic influence. This response results in a rapid increase in heart rate that allows the individual to engage attention in the environment, gather information, and/or use appropriate social strategies (such as enlisting complex emotions) to ameliorate the threat. If the challenge diminishes, the vagal brake can quickly reengage to reduce arousal and minimize metabolic expenditure. This ability to transiently engage and disengage with the environment allows for temporary shifts in energy, such as those required for the listening and communication phases of social interaction (Porges, 1998). However, if the stressor is intense or chronic, then the SNS may be activated. SNS activation enables fight/flight/freeze behaviors, but is consequently more metabolically demanding than the initial vagal response. Likewise, if the sympathetic response is not sufficient to meet external challenge, then the dorsal vagal complex may engage, resulting in an immobilization response such as freezing. Although this framework is helpful as a general guideline, the progression does not occur in simple discrete steps; instead, it is characterized by “transitional blends” among systems (Porges, 1998, p. 844). Thus, even when the PNS is adaptively regulating arousal, one or both of the other systems may be activated. Research has firmly established that stress-induced changes in heart rate can be caused by parasympathetic withdrawal, sympathetic engagement, or a combined action of the systems (Cacioppo, Uchino, & Berntson, 1994).
The ANS and its neural substrates serve both regulatory and social engagement functions, according to the polyvagal theory. The regulatory or calming function is explained by the inhibitory influence of the myelinated vagus on the cardiac pacemaker, and the social engagement function stems from the neuroanatomical integration of neural networks that control the vagus and neural networks that control muscles of the face and head, which regulate facial orienting and expression, speaking, and listening (Porges, 2007). The calming and social engagement functions of the ANS may support adaptive responding in the context of family conflict and protect against the development of psychopathology.
Drawing on ideas from polyvagal theory and Gray's motivational theory (Gray, 1987), Beauchaine (2001) explained that regulational functions of the ANS are under PNS control, whereas motivational functions of the ANS are under SNS control. The SNS can be further divided into dimensions that stem from the neural network that governs either behavioral inhibition (BIS) and sensitivity to threat or behavioral activation (BAS) and sensitivity to appetitive stimuli (Gray, 1987). Beauchaine's integrated conceptualization of the PNS, BIS, and BAS draws distinctions between forms of psychopathology associated with different psychophysiological profiles that cannot be drawn based on single physiological systems. For example, he indicated that most forms of psychopathology are characterized by low vagal tone, which may be considered a nonspecific marker of emotion dysregulation. To draw distinctions between different forms of psychopathology (e.g., externalizing, anxiety, depression), markers of the PNS (e.g., RSA), BIS (e.g., SCL), and BAS (e.g., PEP) should be considered. This model has been useful, for example, in distinguishing between adolescents with conduct disorder plus attention-deficit/hyperactivity disorder, who exhibited reduced electrodermal responding and reduced SNS-linked cardiac activity (i.e., lengthened PEP; see also Crowell et al., 2006), and adolescents with attention-deficit/hyperactivity disorder only, who exhibited reduced electrodermal responding but not lengthened PEP (Beauchaine et al., 2001). Research to elucidate the connection between neurophysiological motivational constructs (e.g., BIS, BAS) and peripheral autonomic measures (e.g., SCL, PEP) continues to evolve (e.g., Brenner et al., 2005).
Beauchaine and colleagues (e.g., Beauchaine 2001; Beauchaine et al., 2007; Beauchaine, Klein, Crowell, Derbidge, & Gatzke-Kopp, 2009) have described a developmental model in which inherited impulsivity, marked by low SNS activity and reactivity, may or may not develop into severe conduct problems, depending upon emotion socialization in the family. Early childhood behavior problems may lead to poor PNS (i.e., vagal) and emotion regulation, as well as severe conduct problems in middle childhood, via coercive family processes, in which negative affect and aggressive behavior are negatively reinforced (Patterson et al., 1992). Alternatively, a protective family environment, characterized by consistent positive reinforcement of appropriate behavior and controlled consequences for aggressive behavior, may foster PNS and emotion regulation abilities that buffer impulsive children from developing angry and aggressive behavioral patterns (Beauchaine et al., 2007).
The conceptual frameworks described above demonstrate the value of contemporaneous consideration of the SNS and PNS to fully understand psychological adjustment in the context of family conflict. Next, we review the literature on family conflict and both child adjustment and ANS activity, followed by the literature linking ANS activity with children's maladjustment, and we conclude with research documenting interactions among family conflict and indices of ANS activity, which is the focus of our paper and the subject of most attention.
Literature Review
In the sections that follow, we provide a selective review of research related to the pathways presented in Figure 1. To provide a segue to our main focus, we begin with brief discussions of literature linking family conflict with child adaptation, as well as associations among family conflict, ANS functioning, and child adaptation. Next, we provide more extensive discussion of the newer literature regarding interactions among family conflict and indices of ANS functioning, including multisystem interactions, which is the main focus of this paper.
Family conflict and child adaptation
In this review, we focus on marital conflict and parent–child conflict, broadly construed; other important forms of family conflict, such as sibling conflict, have yet to be investigated in combination with ANS functioning. Because abusive parent–child interactions are different in form and severity from normative forms of family conflict, and may have different associations with either our focal variables (e.g., ANS activity) or different psychopathology processes (e.g., posttraumatic disorders), we do not focus on child abuse. We refer to destructive forms of marital conflict that involve physical aggression, nonverbal or verbal hostility, or threat to the intactness of the family (Cummings, Goeke-Morey, & Papp, 2004). Associations between marital conflict and various dimensions of children's internalizing and externalizing problems have been documented thoroughly, and explained in terms of emotional insecurity (Davies, Harold, Goeke-Morey, & Cummings, 2002), cognitive representations of threat and self-blame (Grych & Fincham, 1990), social learning of aggressive behavior (Bandura, 1977), specific emotions (Crockenberg & Langrock, 2001), and negative spillover into the parent–child relationship (Erel & Burman, 1995). Recent studies have investigated a broader range of health outcomes, finding that children's exposure to marital conflict also increases their risk for sleep disturbances (El-Sheikh, Buckhalt, Mize, & Acebo, 2006) and physical health problems (El-Sheikh, Cummings, Kouros, Elmore-Staton, & Buckhalt, 2008; Troxel & Matthews, 2004).
In addition, numerous studies have linked parent–child conflict with children's externalizing and internalizing problems, and the association appears to be reciprocal (Burt, McGue, Krueger, & Iacono, 2005; Pettit & Arsiwalla, 2008). For example, harsh parenting refers to coercive acts and negative emotional expressions that parents direct toward children, including verbal and physical aggression; the association between harsh parent–child interactions and externalizing behaviors is robust (Gershoff, 2002). Psychological control in the parent–child relationship (e.g., inducing guilt, withdrawing love, invalidating the child's perspective), in contrast, is linked especially with child internalizing symptoms (for a review, see Barber & Harmon, 2002). Despite some evidence for specificity, however, marital conflict and various forms of parent–child conflict have been linked with both internalizing and externalizing problems. Some investigators have suggested that environmental stressors, such as family conflict, operate as nonspecific risk factors in the elicitation of child maladjustment, with physiological arousal and regulation shaping the specific form of maladjustment that emerges (Steinberg & Avenevoli, 2000).
Family conflict and ANS functioning
Several studies offer important evidence for associations linking negative parent–child interactions and marital conflict with patterns of ANS functioning that are generally considered maladaptive. For example, mothers’ negative and controlling parenting behaviors have been linked with lower RSA (Kennedy, Rubin, Hastings, & Maisel, 2004) and lower task-related reductions in RSA (Calkins, Smith, Gill, & Johnson 1998) in young children. Marital conflict also has been linked with lower RSA in infants (Porter, Wouden-Miller, Silva, & Porter, 2003) and lower reductions in RSA during a peer provocation task in middle childhood (Katz, 2007). A recent study reported that adolescents with a history of mental health problems and lower levels of secure-base support from their parent exhibited weaker RSA reductions and RSA recovery during a stress task and reunion with the parent (Willemen, Schuengel, & Koot, 2009).
In addition, El-Sheikh (2005b) reported that aggressive marital conflict was associated with higher baseline SCL (girls and boys) and higher SCL-R reactivity (boys only). These findings are consistent with the allostatic load model, which contends that unremitting exposure to intense stress, failure to habituate to moderate stress, or unchecked activation of stress responses incurs wear and tear on physiological systems (McEwen, 1998). Over time, these physiological systems may become dysregulated and uncoordinated, increasing risk for psychological problems as well as cardiovascular problems and physical illnesses (Gunnar & Vazquez, 2006; McEwen & Lasley, 2002; Repetti, Taylor, & Seeman, 2002).
Employing another measure of SNS activity, Salomon, Matthews, and Allen (2000) reported that higher child- and parent-reported family conflict were associated with a combination of increased PEP and decreased RSA during reactivity tasks in the lab (reaction time, mirror-tracing, social competence interview). It is interesting to note that, across two studies, some form of family conflict has been linked with elevations in SCL responses (El-Sheikh, 2005b) and increases in PEP (Salomon et al., 2000). This pattern of responding across SCL and PEP, representing high activation of the BIS and low activation of the BAS, has been tied to depression in children (Beauchaine, 2001), and the combination of exposure to marital conflict and higher skin conductance reactivity has been linked to internalizing symptoms among girls (El-Sheikh, 2005b). These findings highlight the advantages of investigating multiple physiological markers and distinguishing their roles in emotional and behavioral regulation. Of course, further research is necessary before conclusions can be drawn about specific links between family conflict and different dimensions of the SNS; efforts to discern the role of lab task in physiological responses will be especially important.
ANS functioning and child adaptation
Internalizing problems
A substantial body of literature has linked ANS functioning with children's internalizing problems, such as symptoms of depression and anxiety, shyness, and low self-esteem. For example, Boyce and colleagues (2001) found that lower vagal tone distinguished children with internalizing symptoms from both children with externalizing symptoms and children with neither internalizing nor externalizing symptoms (see also Dietrich et al., 2007), and El-Sheikh and Whitson (2006) reported that lower vagal withdrawal predicted internalizing behaviors among boys. Kagan, Reznick, and Snidman (1988) found that measurements of inhibited children's peripheral physiology implied greater sympathetic arousal (e.g., cardiac accelerations) in comparison to uninhibited children. Inhibited children with the highest heart rates were most likely to remain inhibited over time. Likewise, Schmidt, Fox, Schulkin, and Gold (1999) found that 7-year-old children who were high in temperamental shyness exhibited a greater increase in heart rate during a self-presentation task compared to children low in shyness. Changes in heart rate were probably driven by SNS activity because no differences in PNS activity (i.e., RSA) were observed; note that heart rate is influenced by both the SNS and the PNS. Higher SCLR in response to mildly frightening stimuli also has been linked with self-reported anxiety among adolescents (Weems, Zakem, Costa, Cannon, & Watts, 2005). Thus, the available research has been particularly consistent in demonstrating a positive association between higher SNS activity and children's anxiety symptoms.
Externalizing problems
Numerous studies have tested the association between SNS activity and children's externalizing behavior problems. For example, children with disruptive behavior disorder have lower baseline SCL than controls (van Goozen, Matthys, Cohen-Kettenis, Buitelaar, & van Engeland, 2000), and this association persists into adolescence (van Bokhoven, Matthys, van Goozen, & van Engeland, 2005). Some childhood studies report that higher SCLR is associated with children's reactive aggression (Hubbard et al., 2002) and externalizing problems (El-Sheikh, 2005b), but more studies have found that lower SCLR is associated with child externalizing problems (Fung et al., 2005; Herpertz et al., 2005; McBurnett, 1992; Snoek, van Goozen, Matthys, Buitelaar, & van Engeland, 2004; van Goozen, Matthys, Cohen-Kettenis, Gispen-de Wied, & van Engeland, 1998).
According to the results of a recent meta-analysis (Lorber, 2004), individuals with (nonaggressive) conduct problems exhibit lower resting SCL and lower SCL-R to lab tasks, compared to individuals without conduct problems. Longitudinal research is also supportive of the association between SNS underarousal and conduct problems. For example, adult criminals showed significantly lower SCL during middle adolescence compared to adults without a criminal record (Raine, Venables, & Williams, 1990). In addition, in clinical research, Beauchaine and colleagues have shown that preschoolers, elementary-age children, and adolescents with conduct problems exhibit relatively high PEP (or attenuated SNS-linked cardiac activity) at baseline and during reward conditions (Beauchaine et al., 2007; Crowell et al, 2006). In another study, aggressive 8- to 12-year-old boys exhibited no PEP reactivity to reward conditions (see also Hinnant et al., 2010) and lower baseline vagal tone compared to nonaggressive boys, whereas aggressive girls demonstrated greater electrodermal responding than nonaggressive girls (Beauchaine et al., 2008), suggesting a potentially important sex difference in the autonomic correlates of aggression.
Calkins and colleagues (2007) have examined the link between PNS activity and child externalizing behavior. Calkins et al. (2007) found that children with less pronounced reductions in RSA in response to a lab task designed to elicit positive emotion and focused attention also had higher externalizing symptoms at age 5 (see also Calkins et al., 1998). Children with lower baseline RSA at age 2 exhibited a greater decline in externalizing behaviors over time, and these children had lower externalizing behaviors at age 5 compared to children with higher baseline RSA. Of interest, Beauchaine et al. (2007) have also reported that the low RSA among children and adolescents with aggressive forms of disruptive behavior disorders (e.g., oppositional defiant disorder) is not evident during early childhood, suggesting that lower RSA may emerge sometime between early and middle childhood among children with clinical behavior problems. In another longitudinal study, however, the typical declines in child negativity from age 4 to 7 were more pronounced for children with higher baseline RSA, whereas the typical increases in emotion regulation abilities were more pronounced for children with greater reductions in RSA in response to an attention task (Blandon, Calkins, Keane, & O'Brien, 2008).
Thus, an association between increased vagal withdrawal and reduced externalizing behaviors has been documented consistently in nonclinical samples. However, increased vagal withdrawal has also been documented in children with clinically significant levels of externalizing behaviors (e.g., oppositional defiant disorder; conduct disorder) (Beauchaine et al., 2007). Thus, as Beauchaine (2009) suggested, the function of vagal withdrawal may differ in children with and without psychopathology. Another possibility that we have raised is that the risk for psychopathology is particularly high among children who exhibit both increased vagal withdrawal in conjunction with low resting levels of vagal tone (Hinnant & El-Sheikh, 2009). Conversely, children with higher vagal tone or moderate vagal withdrawal may not be at risk for physiological arousal and may actually be well positioned for engagement with the environment and active coping responses (Porges, 2007). Furthermore, the consistent link between lower vagal tone and externalizing behavior in children and adolescents is less clear among infants and toddlers. Higher vagal tone may promote engagement with the social environment (Porges, 2007). Thus, among infants, who are less able to regulate their own emotions, higher vagal tone may illicit emotion socialization from caregivers, thereby reducing behavior problems in the long term, even if early externalizing behaviors are observed (Beauchaine, 2001).
Interactions between family conflict and ANS functioning
Indices of PNS functioning have been examined relatively thoroughly as moderators of marital conflict. In almost all of these studies, higher vagal tone and vagal withdrawal have operated as protective factors in the context of family conflict; that is, the association between marital conflict and child maladjustment is less pronounced among children with higher vagal tone or vagal withdrawal, compared to children with lower vagal tone or vagal augmentation (for an exception, see Obradovic, Bush, Stamperdahl, Adler, & Boyce, 2010). The protective function of higher RSA or greater reductions in RSA during stress or challenge has been evident across cross-sectional (El-Sheikh, Harger, & Whitson, 2001; Katz & Gottman, 1995) and longitudinal studies (El-Sheikh & Whitson, 2006; Katz & Gottman, 1997; Leary & Katz, 2004); across multi-informant (El-Sheikh et al., 2001; El-Sheikh & Whitson, 2006; Katz & Gottman, 1995) and observational (Katz & Gottman, 1995; Leary & Katz, 2004) sources; and across externalizing, internalizing (El-Sheikh et al., 2001; El-Sheikh & Whitson, 2006; Katz & Gottman, 1995, 1997), social (Leary & Katz, 2004), and physical health (El-Sheikh et al., 2001; Whitson & El-Sheikh, 2003) outcomes. Similarly, the protective effects of higher RSA have been documented in the context of parent–child conflict (Whitson & El-Sheikh, 2003) and child maltreatment (Gordis, Feres, Olezeski, Rabkin, & Trickett, 2010). The purported protective effect of higher vagal tone and vagal withdrawal in the context of marital conflict has been attributed to the ways in which these PNS processes may reflect or support emotion regulation and social engagement (Porges, 2007).
Despite the overall evidence for the protective functions of higher vagal tone and increased vagal withdrawal in the context of family conflict, evidence for gender differences is somewhat mixed. For example, in cross-sectional analyses, greater reductions in RSA protected only boys against externalizing and health problems associated with verbal and physical marital conflict, respectively (El-Sheikh et al., 2001). In contrast, in longitudinal analyses, greater reductions in RSA protected only girls against internalizing problems associated with marital conflict (El-Sheikh & Whitson, 2006). Although some sex differences exist across particular studies with particular outcomes, the protective functions of higher vagal tone and increased vagal withdrawal have been replicated among boys and girls.
Several studies have investigated interactions between family conflict and SNS functioning. For example, El-Sheikh (2005b) found a stronger association between marital conflict and both internalizing and externalizing behavior among girls with higher SCL-R, compared to girls with lower SCLR. In longitudinal analyses with the same sample, El-Sheikh, Keller, and Erath (2007) reported that marital conflict predicted increased externalizing problems among boys with lower SCLR and among girls with either lower or higher SCLR (but especially among girls with higher SCLR). Thus, in the context of marital conflict, some existing evidence suggests that higher SCLR may be a stronger vulnerability factor among girls compared to boys (El-Sheikh, 2005b; El-Sheikh et al., 2007), which is consistent with evidence that aggressive girls exhibit higher electrodermal activity than nonaggressive girls (Beauchaine et al., 2008).
Two recent studies provided evidence that externalizing behavior may be high and stable from middle to late childhood among boys with relatively harsh parents and lower SCLR, compared to boys with relatively harsh parents and higher SCLR, whose externalizing behavior may increase during later childhood (age 8 to age 10; Erath, El-Sheikh, & Cummings, 2009; Erath, El-Sheikh, Hinnant, & Cummings, 2010). In these studies, girls and boys with less harsh parents exhibited relatively lower externalizing behavior throughout late childhood irrespective of their SCLR.
Collectively, these studies are relatively consistent in showing that boys with lower SCLR exhibit elevated levels of externalizing problems, particularly in the context of either marital conflict (El-Sheikh et al., 2007) or harsh parenting (Erath et al., 2009, 2010). These findings may be interpreted by integrating learning theories with models of psychophysiology and antisocial behavior. Reduced SCLR may reflect a predisposition toward behavioral disinhibition or punishment insensitivity (Beauchaine, 2001), which may be more common among boys compared to girls. This temperamental characteristic may set the stage for coercive parent–child exchanges, and perhaps increase the likelihood that children will learn aggression from harsh parenting (e.g., observational learning, negative reinforcement) rather than feel punished by it (Dadds & Salmon, 2003; Patterson et al., 1992; Reid, Patterson, & Snyder, 2002).
Evidence has been less consistent regarding the risk for psychological maladjustment among children with higher SCLR in the context of family conflict. In El-Sheikh (2005b) and El-Sheikh et al (2007), higher SCLR was a vulnerability factor for externalizing and internalizing problems in the context of marital conflict among girls. In Erath et al. (2010), however, only boys with higher SCLR and relatively harsh parents exhibited increases in externalizing behavior from middle childhood to late childhood. It is possible that family conflict predicts externalizing behavior over time among children with higher SCLR because their behavior is particularly responsive to environmental stress and contingencies, potentially reflecting susceptibility to environmental influences (Belsky & Pluess, 2009) or biological sensitivity to context (Boyce & Ellis, 2005). Wootton, Frick, Shelton, and Silverthorn (1997) reported that children without callous-unemotional traits, consistent with normal or high SCLR, exhibited elevated conduct problems only in the context of ineffective parenting (see also, Shannon, Beauchaine, Brenner, Neuhaus, and Gatzke-Kopp, 2007). Children who exhibit higher SCLR, who may be more sensitive to aversive circumstances than children with lower SCLR, may be especially likely to react negatively to family conflict or harsh parenting during a developmental period characterized by increasing needs for autonomy from parents. Thus, existing research suggests that the risk associated with SCLR may depend on age, gender, and type of family conflict.
Interactions between the SNS and PNS
Investigations of interactions between family conflict and single indices of autonomic activity (i.e., either SNS or PNS) have provided important insights about risk and resilience in children. However, similar to environmental factors that moderate the influence of one another (e.g., Lindsey, Caldera, & Tankersley, 2009; Pettit, Bates, Dodge, & Meece, 1999), single physiological systems do not operate in isolation. Multiple physiological systems cooperate to serve homeostatic functions under ideal circumstances and to meet individual or environmental demands under challenging conditions (Bauer, Quas, & Boyce, 2002). Because of innate biological vulnerabilities or experiences of intense or chronic stress, however, physiological systems may become uncoordinated or dysregulated, potentially exacerbating the toll of exposure to family stress (Bauer et al., 2002).
Investigators have begun to consider the joint action of the two branches of the ANS, the SNS and PNS, and we expect that further research along these lines will help clarify inconsistencies in the literature on family stress and psychophysiological processes. For example, Beauchaine (2001) explained that low vagal tone is linked with a range of negative child outcomes, including internalizing and externalizing problems; to predict whether low vagal tone will manifest as an externalizing or internalizing problem, the SNS must be considered as well.
Another model, the doctrine of autonomic space, concerns the simultaneous action of the SNS and PNS on target organs that are innervated by both branches of the ANS (e.g., heart; Berntson et al., 1991; Berntson & Cacioppo, 2004). It is important to note that the autonomic space literature refers specifically to SNS-linked cardiac reactivity (e.g., cardiac PEP), which is discussed in further detail below. The autonomic space model does not refer to electrodermal measures of the SNS (i.e., SCL). However, given the much larger body of research linking electrodermal measures (compared to PEP) with family conflict and children's psychological functioning, and the opportunity to test interactions between PNS measures and electrodermal measures in many existing data sets, we believe it is worthwhile to consider whether the effects of SNS activation or inhibition, reflected in electrodermal measures, may be conditional upon PNS activation or inhibition, reflected in measures of RSA. Thus, we consider the synergistic action of the PNS and SNS, like the autonomic space model (El-Sheikh et al., 2009), but acknowledge that our focus on SCL as a marker of SNS activity diverges from the autonomic space model.
According to the autonomic space model (Berntson et al., 1991; Berntson & Cacioppo, 2004), reciprocal activation refers to conditions under which both branches of the ANS promote the same directional response in a target organ or system (e.g., see Table 1). Reciprocal sympathetic activation involves sympathetic activation and parasympathetic inhibition, both of which upregulate physiological processes such as heart rate and cardiovascular output. By comparison, reciprocal parasympathetic activation is characterized by sympathetic inhibition and parasympathetic activation, both of which downregulate similar physiological processes, serving homeostatic and calming functions. Nonreciprocal activation refers to conditions under which branches of the ANS promote opposing responses in target systems. Specifically, coactivation refers to increased sympathetic and parasympathetic action, and coinhibition refers to decreased action of both branches. Because sympathetic and parasympathetic actions serve opposing physiological functions, such parallel, or nonreciprocal, activation may promote opposing physiological outcomes.
Table 1.
Autonomic nervous system profiles
| Profile | SNS Activity | PNS Activity | Net Effect on Physiological Arousal |
|---|---|---|---|
| Reciprocal sympathetic | Activation (high SCL or SCL-R) | Inhibition (low RSA or RSA withdrawal) | Increase |
| Reciprocal parasympathetic | Inhibition (low SCL or SCL-R) | Activation (high RSA or RSA augmentation) | Decrease |
| Coactivation | Activation (high SCL or SCL-R) | Activation (high RSA or RSA augmentation) | Ambiguous |
| Coinhibition | Inhibition (low SCL or SCL-R) | Inhibition (low RSA or RSA withdrawal) | Ambiguous |
Note: SNS, sympathetic nervous system; PNS, parasympathetic nervous system; SCL, skin conductance level; RSA, respiratory sinus arrhythmia.
Berntson et al. (1991) explain that modes of reciprocal activation can produce strong, unidirectional changes in the system under autonomic influence. Thus, reciprocal sympathetic activation may be well-suited for adjustments to challenge or stress, particularly when the necessary coping response is well defined, whereas reciprocal parasympathetic activation may be most appropriate for situations in which a calm physiological state is beneficial. Modes of nonreciprocal activation, in contrast, yield a more ambivalent physiological response because the action of ANS branches is in opposition. In the case of coactivation or coinhibition, it is actually possible that little or no change in the state of the target system would occur if the relative activation of sympathetic and parasympathetic branches was equivalent. Thus, nonreciprocal modes may operate to preserve the baseline functional state of an organ or system in situations without challenge or stress. It is also possible that nonreciprocal activation occurs when the optimal behavioral response in a novel or challenging situation is unclear to the individual (Berntson et al., 1991).
Studies that have utilized the autonomic space framework have found that reciprocal sympathetic activation is a normative response to stress among children and adolescents (Salomon et al., 2000). However, the most common response profile may vary developmentally (Alkon et al., 2003), and children with externalizing behavior problems often exhibit coinhibition in response to laboratory challenges (Beauchaine et al., 2007; Boyce et al, 2001). In a series of studies informed by Berntson and colleagues (1991), as well as models reviewed above (Beauchaine, 2001; Porges, 2007), SNS × PNS interactions were examined in the context of marital conflict as predictors of children's externalizing behaviors, as described below (El-Sheikh et al., 2009).
Interactions among the SNS, PNS, and family conflict
A core principle of developmental psychopathology is that child outcomes are best understood in terms of transactions among multiple individual and environmental variables (Masten, 2006). Likewise, a critical next step is to apply integrated conceptualizations of SNS and PNS responses to stressful environmental contexts that evoke ANS reactivity. As such, interactions between SCL and RSA were investigated as moderators of the relation between marital conflict and child externalizing behavior (El-Sheikh et al., 2009).
Analyses revealed that opposing action of the PNS and SNS (i.e., coactivation and coinhibition in autonomic space terms) operated as a vulnerability factor for externalizing behavior in the context of marital conflict, whereas reciprocal action of the PNS and SNS (i.e., reciprocal sympathetic activation and reciprocal parasympathetic activation in autonomic space terms) operated as a protective factor (El-Sheikh et al., 2009). This pattern of findings emerged consistently in three studies with multimethod and multi-informant designs, including mother, father, and child reports of marital conflict; mother, father, and teacher reports of various child externalizing problems, and physiological data on child responses to different laboratory stress tasks. Of note, Gordis et al. (2010) also reported that coinhibition, reflected in low levels of both basal RSA and SCL reactivity, and coactivation, reflected in high levels of both basal RSA and SCL reactivity, exacerbated the association between child maltreatment and aggressive behavior among girls. On the basis of these results, we offered the following tentative assertions about the functions of autonomic profiles in the context of marital conflict (El-Sheikh et al., 2009).
Coactivation may indicate that the PNS response is insufficient for managing a stressor (as reflected in vagal augmentation rather than vagal withdrawal), prompting activation of a significant SNS response. As such, coactivation may reflect physiological overarousal, given the potential SNS “override” of the PNS response (Porges, 1995, 2001), and thus it is possible that coactivation enables dysregulated, fight/flight/freeze responses to conflict as well as child involvement in marital conflict. Such high emotional reactivity may set the stage for coercive exchanges between parents and their children, in which children are negatively reinforced for aggressive attempts to end conflict (Patterson, 2002). High emotional reactivity may also contribute to increased child involvement and exposure to conflict, and thereby enhance sensitization to conflict (Cummings & Davies, 1994).
Coinhibition may reflect an ambivalent physiological response in which the PNS equips the child for action by withdrawing its inhibitory influence, whereas the sympathetic system, conversely, fails to produce the metabolic output needed for an active behavioral or emotional response. Potentially, such a physiological response may promote passive vigilance, which could result in increased exposure to marital conflict and limited efforts to reduce exposure, such as by communicating upset feelings to parents. It is possible that coinhibition of ANS branches is more characteristic of children with underaroused antisocial behavior (Raine, 2002), or callous–unemotional traits (Frick & Ellis, 1999). For example, low vagal tone may reflect poor emotion regulation, and diminished SNS arousal may suggest fearlessness, failure of avoidance learning, or punishment insensitivity (Raine, 2002).
Reciprocal sympathetic activation may reflect an efficient parasympathetic response to stress (as reflected in vagal withdrawal) and a corresponding (moderate) sympathetic response to meet metabolic demands. In the context of marital conflict, reciprocal sympathetic activation may indicate appropriate concern or anger, yet also promotes constructive attemptsto address worries with adults or attempts to reduce exposure to conflict. In contrast, reciprocal parasympathetic activation and sympathetic inhibition may occur when marital conflict is not interpreted as especially threatening, and is managed efficiently and effectively through vagal withdrawal, without resorting to SNS activation. This type of ANS response pattern may reflect effective self-soothing in the context of marital conflict.
Although our work diverges from the autonomic space model (Berntson et al., 1991) in that we examined SCL versus cardiac measures of SNS activity, our findings and those of Gordis et al. (2010) are consistent with this body of work in that SCL appeared to operate like PEP in conjunction with PNS activity to predict child behavior. Although both SCL and PEP are influenced by the SNS, it is important for future research to further support the application of electrodermal measures to the autonomic space model. In addition, although the autonomic space literature focuses on physiological reactivity, we examined all combinations of SCL and RSA at baseline and in response to laboratory tasks and demonstrated their effects (see also Gordis et al., 2010).
Likewise, our findings can be interpreted in the context of our expansion on polyvagal theory propositions (Porges, 2007). Specifically, although Porges did not focus on interactions among the PNS, SNS, and dorsal vagal systems per se, he proposed that responses across these systems in the face of stress do not proceed in a linear fashion and are often characterized by blending of physiological activity across these systems. Findings (El-Sheikh et al., 2009; Gordis et al., 2010) suggest that reciprocal modes of ANS responding may indicate that more evolutionarily advanced response strategies have been effective and sufficient, whereas coactivation and coinhibition may suggest a breakdown in regulation, in which either the PNS or SNS system fails to perform its adaptive function in response to stress.
Our findings are also consistent with Beauchaine's and colleagues’ (2001, 2007) research, which suggests that children with clinical levels of externalizing problems are likely characterized by coinhibition, or reduced activity of both the para-sympathetic and sympathetic branches. Findings reported in the Monograph (El-Sheikh et al., 2009) build on Beauchaine's work and extend it, particularly by showing that certain patterns of SNS and PNS activity can operate as vulnerability or protective factors in the context of marital conflict. It is important to note that neither the polyvagal theory nor autonomic space model deal specifically with exposure to family stress. Our model is most similar to that of Beauchaine and colleagues in its integration of family stress, autonomic activity, and psychopathology, although we focus more on interactions between family risk and the two branches of the ANS in the prediction of psychopathology.
Future Directions
In this section we highlight future research directionsthat would accelerate understanding of ANS variables that transmit, moderate, or reflect the effects of family conflict on children. The types of studies discussed below would significantly advance basic knowledge and applied efforts, by testing novel hypotheses, and by clarifying hypotheses that have been tested in numerous studies with results that are not entirely consistent. For example, whereas several studies have provided evidence that vagal withdrawal operates as a protective factor in the context of marital conflict (El-Sheikh et al., 2001; El-Sheikh & Whitson, 2006; Katz & Gottman, 1997; Leary & Katz, 2004) or parental problem drinking (El-Sheikh, 2001, 2005c), some theory (biological sensitivity to context; Boyce & Ellis, 2005) and empirical work (Obradović, Bush, & Boyce, 2009) suggest that vagal withdrawal may contribute to particularly negative outcomes in the context of family adversity. We anticipate that such inconsistencies, which are not uncommon in the psychophysiological literature, may be resolved at least in part by further investigation of developmental differences in ANS effects, nonlinear effects of ANS functioning, interactions among multiple physiological systems, and differential effects of ANS functioning depending on specific environmental risks, specific laboratory tasks, and specific child outcomes. Each of these propositions will be discussed next.
Developmental progressions
The vast majority of research on children's ANS functioning and psychological adaptation in the context of family conflict has employed cross-sectional or short-term longitudinal designs with two data collection points. Multiyear studies that cross developmental periods and transitions (e.g., transition to elementary school, puberty) are necessary to illuminate the direction of associations, the malleability of ANS functioning, and the potential of developmental change in the intervening role of ANS functioning (e.g., mediation, moderation). To this point, investigators have put forth several intriguing developmental models that warrant further empirical examination.
For example, according to the biological sensitivity to context model (e.g., Boyce & Ellis, 2005; Ellis & Boyce, 2006), children may develop heightened sensitivity to their family context, reflected in high ANS reactivity, under conditions of nurturance and support or under conditions of stress and adversity (for a complementary theory, see differential susceptibility to environmental influence; Belsky, 2005). In the former case, high ANS reactivity is an evolutionarily adaptive response that maximizes exposure to enriching qualities of the environment, whereas in the latter case, high reactivity is an adaptive response (at least in the short term) that promotes vigilance to environmental hazards. In addition, as reviewed above, Beauchaine and colleagues (e.g., Beauchaine, 2001; Beauchaine et al., 2007) have discussed a developmental model in which inherited impulsivity, marked by low SNS activity and reactivity, may or may not develop into severe conduct problems depending upon family processes (e.g., coercive parent–child interactions) that either support or undermine emotional and physiological regulation.
Although the specific hypotheses of the developmental models discussed above require further longitudinal investigation, some empirical support exists for the proposition that ANS activity is responsive to early environmental influence. For example, Gottman, Katz, and Hooven (1996) reported that parental emotion coaching predicted higher baseline RSA during middle childhood. A growing body of studies has linked responsive and synchronous parenting with infants’ higher RSA (fora review, see Propper & Moore, 2006), and concordance between mothers’ and children's vagal withdrawal to environmental challenges appears to increase from infancy through early childhood (Bornstein & Suess, 2000). Prevention and intervention studies also support the malleability of ANS functioning. For instance, educational, social–emotional, and health enrichment in early childhood has been linked with adaptive SNS arousal in response to laboratory tasks (i.e., increased amplitude and speed of electrodermal responding) during preadolescence in a controlled, experimental trial (Raine et al., 2001). Habitual exercise and other lifestyle factors have also been linked with changes in vagal tone over time in adults (Rennie et al., 2003; Thayer & Lane, 2007). Preliminary evidence also supports the utility of acupuncture to increase vagal tone in depressed women, which in turn results in decreased depression (Chambers & Allen, 2002). In addition, RSA may be alterable using biofeedback (Hatch, Borcherding, & German, 1992) or medication (Porges, 1976).
Psychophysiological response patterns probably retain some malleability throughout the lifespan, as suggested by the studies described above, but research suggests that ANS responses become more stable over time. In particular, evidence has emerged for moderate stability in baseline and reactivity levels of SNS and PNS activity in middle to late childhood (Bornstein & Suess, 2000; Calkins & Keane, 2004; Doussard-Roosevelt, Montgomery, & Porges, 2003; El-Sheikh, 2005a, 2007); lower levels of stability are observed for reactivity than resting SNS and PNS measures. As such, one possibility is that, following a period of greater susceptibility to family and general environmental influence, ANS reactivity to stressors becomes relatively stable around late childhood or early adolescence, at which time it can be considered an individual difference variable that exacerbates or ameliorates the risk for adjustment problems in the context of family conflict (El-Sheikh, 2001; El-Sheikh et al., 2007). Our proposition that family factors may exert influences on children's ANS reactivity more strongly in infancy and early childhood, and then primarily function to interact with physiological patterns in late childhood and adolescence, requires furtherempirical investigation. This hypothesis is similar to Barlow's (2000) conceptualization of the development of internalizing disorders in which individual vulnerabilities associated with internalizing symptoms are fostered by environmental stressors early in life (i.e., mediation model), yet go on to amplify environmental stressors later in life (i.e., moderation model).
To understand developmental changes in the intervening role (e.g., mediation or moderation) of ANS activity, we must also understand its normative developmental course. Few studies have examined the developmental course of ANS activity, but existing research suggests some important directions for future research. There is evidence that baseline RSA increases from infancy through middle childhood (Propper & Moore, 2006). However, Salomon (2005) found that greater reductions in RSA during lab stress protocols in prepubertal 8- to 10-year-olds predicted lower resting RSA approximately 3 years later, controlling for earlier resting RSA. Salomon suggested that repeated vagal withdrawal to stress may alter the set point for resting vagal tone, as the PNS adapts to anticipate continued homeostatic disturbances in the future. As such, under conditions of chronic stress in which children repeatedly withdraw vagal tone to increase vigilance and active coping, resting vagal tone may become conditioned to anticipate circumstances that require vigilance and coping, and thus decline over time.
The implications are intriguing. Several studies have shown that vagal withdrawal in the context of stress or challenge is associated positively with social and emotional regulation, and also operates as a protective factor in the context of family stress. One tentative possibility, however, is that despite the apparently adaptive function of vagal withdrawal in the short term, it could operate as a risk factor for lower resting vagal tone and the associated mental and physical health problems in the long term in the context of high stress exposure.
The altered operation of the HPA axis in the context of chronic stress (e.g., Miller, Chen, & Zhou, 2007) is an interesting parallel to the declining basal levels of PNS activity among children who exhibit vagal withdrawal earlier in development. According to the hypercortisolism hypothesis, chronic stress sensitizes the HPA axis to subsequent stress, such that the HPA axis increasingly generates physiological resources to cope with perceived threat. In turn, sustained elevations in HPA activity produce allostatic load and damage the development and functioning of neural and cognitive systems, resulting in child health problems and psychological maladjustment (Gunnar & Vazquez, 2006; McEwen, 1998; McEwen & Lasley, 2002; Repetti et al., 2002). In contrast, the hypocortisolism hypothesis (Miller et al., 2007) suggests that chronic HPA activation can result in blunting of responses over time, which is also associated with negative psychological outcomes. Likewise, children may become psychologically sensitized to intense marital conflict, such that they begin to interpret even mild stressors in the family as precursors of serious threat, inducing heightened distress and impeding effective coping (Davies & Cummings, 2006). Thus, initially adaptive physiological and psychological responses may become maladaptive over time due to intense and frequent exposure to environmental stress.
These findings (e.g., Salomon, 2005) may be interpreted within the biological sensitivity to context framework (Boyce & Ellis, 2005), which proposes that highly reactive children, who are presumably most susceptible to environmental influence, should demonstrate the best outcomes under positive conditions and the worst outcomes under negative conditions (for a complementary theory, see differential susceptibility to environmental influence; Belsky, 2005). Highly reactive children (e.g., very high vagal withdrawal) may experience particularly negative outcomes under adverse conditions, at least in part, because repeated incidents of stress exposure may contribute to a state of increased reactivity to stressors over time. Clearly, this is an area of research in which many important questions remain unanswered. Further investigation may yield critical information about when in development adjustment problems are most likely to emerge among children who experience chronic or intense family conflict, depending on their ANS profile.
Further examination of individual systems
Nonlinear associations
It may be important to consider the potential difference between high (i.e., extreme) and optimal levels of arousal and reactivity (Eisenberg, Fabes, Guthrie, & Reiser, 2000), or levels of arousal that are best matched with contextual demands. For instance, as noted above, although a growing body of evidence suggests that vagal withdrawal in response to challenge is generally adaptive (e.g., El-Sheikh, 2005c; El-Sheikh et al., 2001, 2007; Graziano et al., 2007; Katz, 2007), there may be a point at which excessive vagal withdrawal contributes to overarousal or dysregulation and impedes effective coping (Beauchaine, 2001). Consistent with this possibility, Ashman, Dawson, and Panagiotides (2008) found the highest levels of vagal withdrawal among children of chronically depressed mothers, intermediate levels of vagal withdrawal among children of nondepressed mothers, and the lowest levels of vagal withdrawal among children of mothers with stable, mild depression. Other studies examining nonlinear associations have found that optimal behavioral and cognitive functioning are linked with moderate (rather than high or low) physiological responses, including cortisol (e.g., Abercrombie, Speck, & Monticelli, 2006; McBurnett, Lahey, Rathouz, & Loeber, 2000), heart rate, and skin conductance (e.g., Gilbert, 1998). More recently, Keller and El-Sheikh (2009) documented a nonlinear association between salivary α-amylase (sAA), a possible marker of SNS activity, and children's externalizing behavior. Moderate levels of sAA were predictive of lower externalizing behaviors 2 years later. Thus, emerging evidence suggests that optimal environments may predict moderate ANS arousal, which in turn predicts optimal behavioral and psychological outcomes. An important direction for future research is to specify the degrees of SNS and PNS activation that promote positive and negative developmental outcomes.
Physiological recovery
Baseline ANS levels and ANS reactivity to stress or challenge have been examined in numerous studies. However, although recognized as an important parameter of vagal functioning (Porges, 2007), ANS recovery (i.e., return to baseline) following stress exposure is surprisingly understudied. Adult studies have linked slower vagal recovery to an increase in cardiovascular disease (Mezzacappa, Kelsey, Katkin, & Sloan, 2001). Gottman and Katz (2002) found that children with higher baseline RSA exhibited faster heart rate recovery from physiological arousal following stressful parent–child interactions. In middle childhood, lower vagal recovery has been linked with maladaptive emotion regulation responses to frustration (Santucci, Silk, Shaw, Gentzler, Fox, & Kovacs, 2008). To our knowledge, however, no additional studies have examined the association between vagal recovery and child or family functioning. Physiological recovery may be an important parameter that moderates the extent to which family conflict exposure produces allostatic load and spills over into other settings and relationships.
Interactions among baseline, reactivity, and recovery
Individual differences in ANS state during resting conditions and changes in ANS levels during and following environmental stimuli are all part of an integrated system of ANS functioning (Porges, 2007). Whereas these dimensions of ANS functioning (i.e., baseline, reactivity, recovery) have typically been examined independently, their effects may be conditional on one another. In other words, it may not be only the starting point (i.e., basal levels) or change (e.g., during stress) in ANS functioning that is important for understanding adaptive ANS function, but a combination of both. For example, vagal withdrawal appears to reflect active environmental engagement and coping, and higher baseline vagal tone may allow a wider dynamic range for vagal withdrawal to occur adaptively (Beauchaine, 2001; Berntson et al., 1991; Porges, 2007). However, large decreases in vagal tone in response to stress may be maladaptive when resting vagal tone is already quite low. Hinnant and El-Sheikh (2009) found that lower levels of RSA in conjunction with greater reductions in RSA during lab challenge predicted elevated child internalizing symptoms 2 years later. The highest levels of externalizing symptoms were predicted for children who demonstrated lower basal RSA in conjunction with RSA augmentation; this finding was replicated in El-Sheikh, Hinnant, and Erath (2011). Further research on the role of at least one physiological parameter—given at least one other parameter—would yield results that better reflect the complexity of the ANS in the context of stress.
Multisystem interactions
In the sections above, we described recent research on SNS × PNS interactions, including ANS interactions contextualized by family conflict. Although our focus in this paper is on interactions between ANS indices and conflict, HPA axis activity is a major component of the stress response system, and the ANS and HPA systems are linked anatomically and functionally (see Benarroch, 1993; Thayer & Lane, 2006, 2009). Thus, HPA activity may modulate the functioning of the ANS and further interact with family conflict in the prediction of psychopathology (Gordis, Granger, Susman, & Trickett, 2008). In this section, we discuss the emerging literature on two-way interactions between the ANS and HPA activity, and call for investigations of ANS × HPA interactions in the family context.
PNS × HPA
The interdependence of the ANS and HPA axis motivates investigation of their interactive effects. The HPA axis, PNS, and SNS are coordinated via the central autonomic network (CAN), which includes hypothalamic, amygdala, and prefrontal components (Benarroch, 1993; Thayer & Lane, 2009). The CAN regulates both PNS activity (directly) and HPA activity (via the amygdala; Thayer, Hall, Sollers, & Fischer, 2006; for more detail regarding the HPA system and its neurological underpinnings, see Gunnar and Vazquez, 2006). Furthermore, PNS activation may inhibit pervasive stress responses, including cortisol excretion, through a negative feedback mechanism (Bueno et al., 1989; Thayer et al., 2006); it is important to note that there are multiple pathways linking the ANS and HPA systems. Higher resting heart rate variability has been linked with lower HPA activity in adults (Thayer & Sternberg, 2006). Likewise, Gunnar, Porter, Wolf, Rigatuso, and Larson (1995) reported an inverse association between vagal tone and cortisol in infants and Blair, Peters, and Granger (2004) and El-Sheikh, Arsiwalla, Hinnant, and Erath (in press) found that children with higher vagal tone had lower basal cortisol. Conversely, PNS (i.e., vagal) withdrawal is typically associated with increased HPA activity, fostering metabolic activity and mobilization behaviors (Porges, 2001, 2003). For example, kindergarten children who exhibited greater vagal withdrawal in response to a negative affect task also showed an increase in cortisol (Doussard-Roosevelt et al., 2003). Thus, consistent evidence has emerged for coordination between PNS and HPA activity, reflected by the inverse association between the activities of these systems. Despite evidence for PNS–HPA coordination via the CAN under normal circumstances, a breakdown in their “functional connectivity” may occur because of individual-level vulnerabilities or adverse circumstances (Thayer & Steinberg, 2006).
In a recent study, we investigated interactions between resting levels of cortisol and vagal tone as predictors of children's anxiety and depression symptoms across two independent samples (El-Sheikh et al., in press; see also Erath & El-Sheikh, 2009). Prior research has linked either higher cortisol or lower vagal tone with children's internalizing symptoms, but to our knowledge, no prior studies have investigated theinteraction between these two markers of the stress response system. The interaction between cortisol and RSA consistently predicted children's internalizing symptoms. In most cases, greater variability in internalizing symptoms was evident at higher versus lower levels of cortisol; whether internalizing symptoms were higher or lower depended on level of RSA. As hypothesized, the highest levels of internalizing symptoms were generally evident among children with elevated cortisol and lower RSA, whereas higher RSA was generally protective against internalizing symptoms among children with elevated cortisol.
Higher cortisol and lower vagal tone should reflect the highest levels of general physiological arousal, a common symptom of internalizing symptoms, especially anxiety (e.g., Weems et al., 2005). Physiological arousal (e.g., elevated heart rate, which usually occurs with lower vagal tone) may operate as an internal cue of threat that draws attention inward and exacerbates self-focused fears among anxious children or diminishes perceived control among depressed children (Vasey & Daleiden, 1996). Elevated baseline levels of physiological arousal (e.g., lower vagal tone, higher cortisol) may also affect readiness to respond to stress or challenge. For example, low resting vagal tone may set the stage for extremely low vagal tone (or physiological overarousal) following vagal withdrawal in the context of a stressor; children with low baseline RSA and reductions in RSA during lab challenges reported elevated internalizing symptoms (Hinnant & El-Sheikh, 2009).
Along with its implications for physiological arousal (e.g., heart rate deceleration), vagal tone is a well-documented physiological index of emotion regulation (Beauchaine, 2001; Porges, 2007). Thus, lower vagal tone may impede adaptive emotion regulation and exacerbate internalizing symptoms in the context of elevated baseline cortisol. Results of our study seemed to parallel Blair et al's. (2004), finding that the combination of higher cortisol levels and lower RSA levels is found among preschool children with both high approach and high avoidance motivations, which itself is consistent with the anxious–solitary profile often associated with psychosocial maladjustment in childhood and early adolescence (Gazelle & Rudolph, 2004; Rubin & Mills, 1988). This physiological or motivational pattern may reflect a mismatch between arousal and the ability to regulate the arousal that comes with social or environmental engagement. Of course, these potential explanations are obviously tentative and assessments of children's affective responses and coping strategies in conjunction with physiological responses are critical for explicating relations between physiological responses and child adjustment.
SNS × HPA
Although it is clear that the HPA and SNS work concurrently to generate physiological changes associated with stress, their optimal coordination in the context of family conflict is not well understood. The degree of coordination, or symmetry, between HPA and SNS activity may be shaped by exposure to environmental stress. Gordis et al. (2008), for example, reported symmetry between cortisol and sAA reactivity to social stressors among comparison youth but not among maltreated youth. Likewise, Schommer, Hellhammer, and Kirschbaum (2003) reported dissociation between adults’ HPA reactivity and SNS reactivity in the context of repeated psychosocial stress. HPA–SNS asymmetry in the context of environmental adversity may be the result of different rates of habituation to stress across the HPA and SNS (Gordis et al., 2008; Schommer et al., 2003). A challenge for future research is to reconcile findings linking HPA–SNS asymmetry with both environmental adversity (e.g., Gordis et al., 2008; Schommer et al., 2003) and reduced behavioral maladjustment (discussed below; El-Sheikh, Erath, Buckhalt, Granger, & Mize, 2008; Gordis, Granger, Susman, & Trickett, 2006).
Bauer and colleagues (2002) proposed that the influences of the HPA and SNS on child behavioral adjustment may be conditional upon one another, and put forth two testable models. First, the “additive” model assumes that the HPA primarily augments (i.e., is redundant with) the SNS, such that symmetrical activity across these systems could result in hypoarousal (low SNS and low HPA activity) or hyperarousal (high SNS and high HPA activity), and thereby leave children susceptible to adjustment problems. Conversely, children would be at the least risk for adjustment problems when both systems are moderately active, or when the activity of the SNS and HPA is asymmetrical (i.e., one is high and the other is low). Second, the “interactive” model rests on the possibility that the HPA functions primarily to suppress (or counteract) SNS activity, such that symmetrical responses would result in medium levels of arousal (e.g., high HPA modulates high SNS activity). Both models assume that a medium level of arousal is optimal, and moderate levels of arousal have been associated with optimal performance or behavioral adjustment in numerous studies, consistent with the inverted “U” model of arousal and performance (e.g., Kagan, Snidman, Arcus, & Reznick, 1994).
At least two recent studies have examined interactions between HPA and SNS systems as predictors of child adjustment. El-Sheikh, Erath, et al. (2008) found that the interaction between baseline HPA (indexed by cortisol level) and SNS activity (indexed by both SCL and sAA) was associated with both externalizing and internalizing problems. The highest levels of externalizing and internalizing problems were found among children with symmetrical HPA and SNS activity, particularly among children with high baseline levels in both domains. Gordis et al. (2006) also found the highest levels of parent-reported aggression among early adolescents with symmetrical HPA and SNS reactivity, yet participants with low SNS reactivity and low cortisol reactivity were most aggressive. The lowest levels of aggression were observed among early adolescents with asymmetrical HPA and SNS reactivity (i.e., high cortisol activity and low SNS activity). Findings from both studies are consistent with the proposition of Bauer et al. (2002), which suggested that redundant actions of the HPA and SNS could result in hyperarousal when both systems are high in activity or hypoarousal when both systems are low in activity.
Differential effects depending on source of stress, lab stimuli, and child outcomes
Source of stress
We have focused on marital and parent–child conflict, in part because there is a relatively well-developed literature on interactions between these sources of family stress and ANS functioning. One important direction for future research, however, is to systematically test potential differences in the effects of marital conflict and parent–child conflict on ANS functioning as well as stressor-specific interactions with ANS functioning. It also will be necessary to extend tests of the current conceptual framework to other sources of significant stress that children experience in the family context.
Such research is underway. For example, Shannon et al. (2007) found that higher RSA conferred partial protection against child depression in the context of maternal melancholic depression. In the context of parental problem drinking, higher RSA (El-Sheikh, 2001) and greater reductions in RSA during lab tasks (El-Sheikh, 2005c) protected children against internalizing, externalizing, and social maladjustment. Greater reductions in RSA during lab tasks protected 3-year-old children against maladaptive behavior associated with prenatal cocaine exposure (Sheinkopf et al., 2007). In addition, Hastings et al. (2008) reported that paternal protective–overcontrol and low support were associated with preschoolers’ internalizing symptoms among children with less pronounced RSA reductions during lab challenges but not among children with greater RSA reductions.
SNS functioning also has been examined as a moderator of other sources of family stress. For example, Shannon et al. (2007) reported that higher SCL-R, an index of SNS activity, conferred partial protection against conduct problems in the context of paternal antisocial behavior; children with lower SCL-R exhibited higher conduct problems at all levels of paternal antisocial behavior. In addition, Cummings, El-Sheikh, Kouros, and Keller (2007) found that higher SCL-R operated as a vulnerability factor for externalizing problems in the context of fathers’ depressive symptoms for girls and boys.
It also will be important to advance understanding of the broader social ecology within which family stress interacts with children's ANS functioning. Accumulation of risk factors contributes to psychological distress, allostatic load, and self-regulatory difficulties (Evans, 2003). Further, according to a multiplicative risk model, risk factors not only add to the impact of other risk factors, but exacerbate the impact of one another (Rutter, 1990).
Lab stimuli
It is possible that the type of laboratory task or stimulus governs whether certain ANS responses will be evoked or moderate child outcomes in the context of family conflict (Hastings et al., 2008). For example, PEP responses may index approach motivation only under conditions of reward (Brenner et al., 2005; Richter & Gendolla, 2009). Children's lab-based responses to simulated family conflict, general interpersonal stressors (e.g., social stress tasks), cognitive challenges (e.g., reaction time tasks), or physical stressors (e.g., cold pressor tasks) may differentially translate into adaptive responses to family conflict in the home. In the context of a cognitive challenge in the lab, Obradović et al. (2009) found a stronger association between marital conflict and externalizing behaviors among children who exhibited greater reductions in RSA; however, in the context of an interpersonal stressor in the lab, there was a stronger association between marital conflict and externalizing behavior among girls who exhibited lower reductions in RSA and lower PEP reactivity. We have also found that children's vagal responding to different lab tasks differentially predict children's externalizing and internalizing symptoms (Hinnant & El-Sheikh, 2009). Systematic comparisons of results across various lab stimuli may generate important insights and clarify inconsistencies in the literature.
Child outcomes
It will be important for future research to further investigate physical health outcomes predicted by family conflict and ANS functioning. Generally, deficiencies in vagal inhibition of sympathetic activation can result in unimpeded energy mobilization and corresponding wear and tear on physiological systems that support mental and physical health, including cardiovascular problems and lower illness resistance (McEwen, 1998; McEwen & Lasley, 2002; Thayer & Lane, 2007). In addition, higher vagal tone and moderate vagal withdrawal in response to stress have been linked with lower body mass index (Masi, Hawkley, Rickett, & Cacioppo, 2007), fewer physical health problems (El-Sheikh et al., 2001; Thayer & Lane, 2007), and less risk for cardiovascular disease (Thayer & Lane, 2007). Physical health has rarely been examined as an outcome of both family and psychophysiological processes (for an exception, see El-Sheikh et al., 2001).
Investigators also have begun to consider associations among ANS functioning and children's sleep. For example, children diagnosed with sleep apnea exhibit greater increases in SNS activity in response to sigh and cold pressor tasks, in comparison to control children; these children also take longer to return to baseline SNS activity following the cold pressor task than control children (O'Brien & Gozal, 2005). El-Sheikh and Buckhalt (2005) found that reduced vagal withdrawal in response to a reaction-time task was correlated with children's greater subjective sleep problems, lower percentage of time in bed asleep, and increased physical activity during the night. Taken together, these findings suggest that children who exhibit less adaptive patterns of ANS functioning are at risk for sleep disruptions; family conflict may moderate these effects.
Conclusions
A substantial and growing body of research supports the following conclusion: the main effects of family conflict on children's psychological problems are often qualified by children's ANS functioning. Some moderation findings have been particularly robust (e.g., higher vagal tone and increased vagal withdrawal as protective factors in the context of marital conflict). Research focused on clarifying the types and intensities of ANS responses that increase or decrease susceptibility to different forms of family conflict would advance multidomain models of child risk and resilience, and help generate new hypotheses about why children are more or less affected by family conflict. This research also may inform prevention and intervention efforts in innovative directions.
Testing such interactive models requires complex data analyses, particularly when developmental changes and interactions among multiple physiological systems and subsystems are considered. A critical challenge for investigators is to manage complex, higher order interactions among family conflict and physiological variables—to test complicated models while maintaining scientific rigor. It is more feasible to examine multiway interactions with large samples and nonredundant physiological parameters; and, careful conceptual rationales and replication across studies can build confidence in moderation findings. Of course, assessments of higher order interactions require increased power for reliable detection of significant effects (Aiken & West, 1991). As such, it may be important to make use of advanced statistical procedures (e.g., latent profile analysis) that account for multiple family and physiological parameters without sacrificing significant statistical power. As investigators overcome methodological challenges and test integrative models, children's psychological adjustment in the context of family conflict will be better understood and perhaps improved as a result of intervention applications.
Acknowledgments
This paper was supported in part by NIH Grants R01 HD046795 and R01-HL093246, NSF Grants 0339115 and 0623936, and Alabama Agricultural Experiment Station/Lindsey Foundation Grant ALA080-001. We gratefully acknowledge contributions made by the staff of the Child Development Lab, especially Lori Staton and Bridget Wingo.
References
- Abercrombie HC, Speck NS, Monticelli RM. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology. 2006;31:187–196. doi: 10.1016/j.psyneuen.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; Newbury Park, CA: 1991. [Google Scholar]
- Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Developmental Psychobiology. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Andreassi JL. Psychophysiology: Human behavior and physiological response. Erlbaum; New York: 2007. [Google Scholar]
- Ashman SB, Dawson G, Panagiotides H. Trajectories of maternal depression over 7 years: Relations with child psychophysiology and behavior and role of contextual risks. Development and Psychopathology. 2008;20:55–77. doi: 10.1017/S0954579408000035. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social learning theory. Prentice–Hall; Englewood Cliffs, NJ: 1977. [Google Scholar]
- Barber BK, Harmon EL. Violating the self: Parental psychological control of children and adolescents. In: Barber BK, editor. Intrusive parenting: How psychological control affects children and adolescents. American Psychological Association; Washington, DC: 2002. pp. 15–52. [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children's behavior: Advantages of a multi-system approach. Journal of Developmental and Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray's Motivational Theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Some difficulties in interpreting psychophysiological research with children [Commentary]. Monographs of the Society for Research in Child Development. 2009;74(1) doi: 10.1111/j.1540-5834.2009.00509.x. Serial No. 292. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Hong J, Marsh P. Sex differences in autonomic correlates of conduct problems and aggression. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:788–796. doi: 10.1097/CHI.0b013e318172ef4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: Discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. Journal of Abnormal Psychology. 2001;110:610–624. doi: 10.1037//0021-843x.110.4.610. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Klein DN, Crowell SE, Derbidge C, Gatzke-Kopp L. Multifinality in the development of personality disorders: A Biology × Sex × Environment interaction model of antisocial and borderline traits. Development and Psychopathology. 2009;21:735–770. doi: 10.1017/S0954579409000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J. Differential susceptibility to rearing influence: An evolutionary hypothesis and some evidence. In: Ellis BJ, Bjorklund DF, editors. Origins of the social mind: Evolutionary psychology and child development. Guilford Press; New York: 2005. pp. 139–163. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clinic Proceedings. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr., Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: Origins, methods, and interpretive Caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT. Heart rate variability: Stress and psychiatric conditions. In: Malik M, Camm AJ, editors. Dynamic electrocardiography. Blackwell/Futura; New York: 2004. pp. 57–64. [Google Scholar]
- Berntson GG, Cacioppo JT, Fieldstone A. Illusions, arithmetic, and the bidirectional modulation of vagal control of the heart. Biological Psychology. 1994;44:1–17. doi: 10.1016/s0301-0511(96)05197-6. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- Bishop NC, Walker GJ, Scanlon GA, Richards S, Rogers E. Salivary IgA responses to prolonged intensive exercise following caffeine ingestion. Medicine and Science in Sports & Exercise. 2006;38:513–519. doi: 10.1249/01.mss.0000187412.47477.ee. [DOI] [PubMed] [Google Scholar]
- Blair C, Peters R, Granger D. Physiological and neuropsycho-logical correlates of approach/withdrawal tendencies in preschool: Further examination of the behavioral inhibition system/behavioral activation system scales for young children. Developmental Psychobiology. 2004;45:113–124. doi: 10.1002/dev.20022. [DOI] [PubMed] [Google Scholar]
- Blandon AY, Calkins SD, Keane SP, O'Brien M. Individual differences in trajectories of emotion regulation processes: The effects of maternal depressive symptomatology and children's physiological regulation. Developmental Psychology. 2008;44:1110–1123. doi: 10.1037/0012-1649.44.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Suess PE. Child and mother cardiac vagal tone: Continuity, stability, and concordance across the first 5 years. Developmental Psychology. 2000;36:54–65. [PubMed] [Google Scholar]
- Boucsein W. Electodermal activity. Plenum Press; New York: 1992. [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Developmental Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ. Autonomic reactivity and psychopathology in middle childhood. British Journal of Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Brenner SL, Beauchaine TP, Sylvers PD. A comparison of psychophysiological and self-report measures of BAS and BIS activation. Psychophysiology. 2005;42:108–115. doi: 10.1111/j.1469-8986.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Bueno L, Gue M, Fargeas MJ, Alvinerie M, Junien JL, Fioramonti J. Vagally mediated inhibition of acoustic stress-induced cortisol release by orally administered kappa-opioid substances in dogs. Endocrinology. 1989;124:1788–1793. doi: 10.1210/endo-124-4-1788. [DOI] [PubMed] [Google Scholar]
- Burt SA, McGue M, Krueger RF, Iacono WG. How are parent–child conflict and childhood externalizing symptoms related over time? Results from a genetically informative cross-lagged study. Development and Psychopathology. 2005;17:145–165. doi: 10.1017/S095457940505008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology. 1994;31:412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31:125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Dedmon SE. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. Journal of Abnormal Child Psychology. 2000;28:103–118. doi: 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology. 2007;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the pre-school period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45:101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Smith CL, Gill KL, Johnson MC. Maternal interactive style across contexts: Relations to emotional, behavioral and physiological regulation during toddlerhood. Social Development. 1998;7:350–369. [Google Scholar]
- Chambers AS, Allen JJB. Vagal tone as an indicator of treatment response in major depression. Psychophysiology. 2002;39:861–864. doi: 10.1111/1469-8986.3960861. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Vogelsong KM, Lu Y, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clinical Physiology. 1996;16:433–448. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. Development and Psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Vol. 1. Theory and methods. 2nd ed. Wiley; New York: 2006. pp. 1–23. [Google Scholar]
- Crockenberg S, Langrock A. The role of specific emotions in children's responses to interparental conflict: A test of the model. Journal of Family Psychology. 2001;15:163–182. doi: 10.1037//0893-3200.15.2.163. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Gatzke-Kopp L, Sylvers P, Mead H, Chipman-Chacon J. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. Journal of Abnormal Psychology. 2006;115:174–178. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- Cummings EM, Davies P. Children and marital conflict: The impact of family dispute and resolution. Guilford Press; New York: 1994. [Google Scholar]
- Cummings EM, Davies PT. Emotional security as a regulatory process in normal development and the development of psychopathology. Development and Psychopathology. 1996;8:123–139. [Google Scholar]
- Cummings EM, El-Sheikh M, Kouros CD, Keller PS. Children's skin conductance reactivity as a mechanism of risk in the context of parental depressive symptoms. Journal of Child Psychology and Psychiatry. 2007;48:436–445. doi: 10.1111/j.1469-7610.2006.01713.x. [DOI] [PubMed] [Google Scholar]
- Cummings EM, Goeke-Morey M, Papp L. Everyday marital conflict and child aggression. Journal of Abnormal Child Psychology. 2004;32:191–202. doi: 10.1023/b:jacp.0000019770.13216.be. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Salmon K. Punishment insensitivity and parenting: Temperament and learning as interacting risks for antisocial behavior. Clinical Child and Family Psychology Review. 2003;6:69–86. doi: 10.1023/a:1023762009877. [DOI] [PubMed] [Google Scholar]
- Davies PT, Cummings EM. Interparental discord, family process, and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Vol. 3. Risk, disorder, and adaptation. 2nd ed. Wiley; New York: 2006. pp. 86–128. [Google Scholar]
- Davies PT, Harold GT, Goeke-Morey MC, Cummings EM. Child emotional security and interparental conflict. Monographs of the Society for Research in Child Development. 2002;67(3) Serial No. 270. [PubMed] [Google Scholar]
- Dietrich A, Riese H, Sondeijker FEPL, Greaves-Lord K, van Roon AM, Ormel J, et al. Externalizing and internalizing problems in relation to autonomic function: A population-based study in preadolescents. Journal of American Academy of Child & Adolescent Psychiatry. 2007;46:378–386. doi: 10.1097/CHI.0b013e31802b91ea. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Montgomery LA, Porges SW. Short-term stability of physiological measures in kindergarten children: Respiratory sinus arrhythmia, heart period, and cortisol. Developmental Psychobiology. 2003;43:231–42. doi: 10.1002/dev.10136. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Guthrie IK, Reiser M. Dispositional emotionality and regulation: Their role in predicting quality of social functioning. Journal of Personality & Social Psychology. 2000;78:136–157. doi: 10.1037//0022-3514.78.1.136. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Biological sensitivity to context. Current Directions in Psychological Science. 2006;17:183–187. [Google Scholar]
- El-Sheikh M. Parental drinking problems and children's adjustments: Vagal regulation and emotional reactivity as pathways and moderators of risk. Journal of Abnormal Psychology. 2001;110:499–515. doi: 10.1037//0021-843x.110.4.499. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental and Psychopathology. 2005a;46:66–74. doi: 10.1002/dev.20036. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. The role of emotional responses and physiological reactivity in the marital conflict–child functioning link. Journal of Child Psychology and Psychiatry. 2005b;46:1191–1199. doi: 10.1111/j.1469-7610.2005.00418.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Does poor vagal regulation exacerbate child maladjustment in the context of parental problem drinking? A longitudinal examination. Journal of Abnormal Psychology. 2005c;114:735–741. doi: 10.1037/0021-843X.114.4.735. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M. Children's skin conductance level and reactivity: Are these measures stable over time and across tasks? Developmental Psychobiology. 2007;49:180–186. doi: 10.1002/dev.20171. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Arsiwalla DD, Hinnant JB, Erath SA. Children's internalizing symptoms: The role of interactions between cortisol and respiratory sinus arrhythmia. Physiology & Behavior. doi: 10.1016/j.physbeh.2011.02.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sheikh M, Buckhalt JA. Vagal regulation and emotional intensity predict children's sleep problems. Developmental Psychophysiology. 2005;46:307–317. doi: 10.1002/dev.20066. [DOI] [PubMed] [Google Scholar]
- El Sheikh M, Buckhalt J, Mize J, Acebo C. Marital conflict and disruption of children's sleep. Child Development. 2006;77:31–43. doi: 10.1111/j.1467-8624.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Cummings EM, Kouros CE, Elmore-Staton L, Buck-halt JA. Marital psychological and physical aggression and children’ mental and physical health: Direct, mediated, and moderated effects. Journal of Consulting and Clinical Psychology. 2008;76:138–148. doi: 10.1037/0022-006X.76.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Mize J. Cortisol and children's adjustment: The moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology. 2008;36:601–611. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson S. Exposure to parental conflict and children's adjustment and physical health: The moderating role of vagal tone. Child Development. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Hinnant JB, Erath SA. Developmental trajectories of delinquency symptoms in childhood: The role of marital conflict and autonomic nervous system activity. Journal of Abnormal Psychology. 2011;120:16–32. doi: 10.1037/a0020626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Keiley M, Hinnant JB. Developmental trajectories of skin conductance level in middle childhood: Sex, race, and externalizing behavior problems as predictors of growth. Biological Psychology. 2010;83:116–124. doi: 10.1016/j.biopsycho.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Keller PS, Erath SA. Marital conflict and risk for child maladjustment over time: Skin conductance level reactivity as a vulnerability factor. Journal of Abnormal Child Psychology. 2007;35:715–727. doi: 10.1007/s10802-007-9127-2. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, Staton L. Marital conflict and children's externalizing behavior: Interactions between parasympathetic and sympathetic nervous system activity. Monographs of the Society for Research in Child Development. 2009;74(1) doi: 10.1111/j.1540-5834.2009.00501.x. Serial No. 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Whitson SA. Longitudinal relations between marital conflict and child adjustment: Vagal regulation as a protective factor. Journal of Family Psychology. 2006;20:30–39. doi: 10.1037/0893-3200.20.1.30. [DOI] [PubMed] [Google Scholar]
- Erath SA, El-Sheikh M. Predicting childhood internalizing: HPA PNS interactions.. Paper presented at the biennial meeting of the Society for Research in Child Development; Denver, CO. 2009. [Google Scholar]
- Erath SA, El-Sheikh M, Cummings EM. Harsh parenting and child externalizing behavior: Skin conductance level reactivity as a moderator. Child Development. 2009;80:578–592. doi: 10.1111/j.1467-8624.2009.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erath SA, El-Sheikh M, Hinnant JB, Cummings EM. Skin conductance level reactivity moderates the association between harsh parenting and growth in child externalizing behavior. Developmental Psychology. 2010 doi: 10.1037/a0021909. Advance online publication. doi:10.1037/a0021909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erel O, Burman B. Interrelatedness of marital relations and parent–child relations: A meta-analytic review. Psychological Bulletin. 1995;118:108–132. doi: 10.1037/0033-2909.118.1.108. [DOI] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N, Karbon M, Troyer D, Switzer G. The relations of children's emotion regulation to their vicarious emotional responses and comforting behaviors. Child Development. 1994;65:1678–1693. doi: 10.1111/j.1467-8624.1994.tb00842.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC, Kochanska G, Murray K. Electrodermal activity and temperament in preschool children. Psychophysiology. 2000;37:777–787. [PubMed] [Google Scholar]
- Fox NA, Calkins SD. The development of self-control of emotion: Intrinsic and extrinsic influences. Motivation and Emotion. 2003;27:7–26. [Google Scholar]
- Frick PJ, Ellis M. Callous-unemotional traits and subtypes of conduct disorder. Clinical Child and Family Psychology Review. 1999;2:149–168. doi: 10.1023/a:1021803005547. [DOI] [PubMed] [Google Scholar]
- Fung MT, Raine A, Loeber R, Lynam DR, Steinhauer SR, Venables PH, et al. Reduced electrodermal activity in psychopathy-prone adolescents. Journal of Abnormal Psychology. 2005;114:187–196. doi: 10.1037/0021-843X.114.2.187. [DOI] [PubMed] [Google Scholar]
- Gazelle H, Druhen MJ. Anxious solitude and peer exclusion predict social helplessness, upset affect, and vagal regulation in response to behavioral rejection by a friend. Developmental Psychology. 2009;45:1077–1096. doi: 10.1037/a0016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazelle H, Rudolph KD. Toward and away from the world: Social approach and avoidance trajectories in anxious solitary youth. Child Development. 2004;75:829–849. doi: 10.1111/j.1467-8624.2004.00709.x. [DOI] [PubMed] [Google Scholar]
- Gershoff ET. Corporal punishment by parents and associated child behaviors and experiences: A meta-analytic and theoretical review. Psychological Bulletin. 2002;128:539–579. doi: 10.1037/0033-2909.128.4.539. [DOI] [PubMed] [Google Scholar]
- Gilbert C. Emotional sources of dysfunctional breathing. Journal of Bodywork and Movement Therapies. 1998;2:224–230. [Google Scholar]
- Gordis EB, Feres N, Olezeski CL, Rabkin AN, Trickett PK. Skin conductance reactivity and respiratory sinus arrhythmia among maltreated and comparison youth: Relations with aggressive behavior. Journal of Pediatric Psychology. 2010;35:547–558. doi: 10.1093/jpepsy/jsp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and [alpha]-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Hormones and Behavior. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottman JM, Katz LF. Children's emotional reactions to stressful parent–child interactions. Marriage and Family Review. 2002;34:265–283. [Google Scholar]
- Gottman JM, Katz LF, Hooven C. Parental meta-emotion philosophy and the emotional life of families: Theoretical models and preliminary data. Journal of Family Psychology. 1996;10:243–268. [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis E, Stroud LR. Salivary alpha-amylase in biobehavioral research: Recent developments and applications. Annals of the New York Academy of Sciences. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Gray JA. Perspectives on anxiety and impulsivity: A commentary. Journal of Research in Personality. 1987;21:493–509. [Google Scholar]
- Graziano PA, Keane SP, Calkins SD. Cardiac vagal regulation and early peer status. Child Development. 2007;78:264–278. doi: 10.1111/j.1467-8624.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology. 2007;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Grych JH, Fincham FD. Marital conflict and children's adjustment: A cognitive–contextual framework. Psychological Bulletin. 1990;108:267–290. doi: 10.1037/0033-2909.108.2.267. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Porter FL, Wolf CM, Rigatuso J, Larson MC. Neonatal stress reactivity: Predictions to later emotional temperament. Child Development. 1995;66:1–13. doi: 10.1111/j.1467-8624.1995.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental psychopathology: Vol. 2. Developmental neuroscience. 2nd ed. Wiley; New York: 2006. [Google Scholar]
- Hastings P, Sullivan C, McShane K, Coplan R, Utendale W, Vyncke J. Parental socialization, vagal regulation, and preschoolers’ anxious difficulties: Direct mothers and moderated fathers. Child Development. 2008;79:45–64. doi: 10.1111/j.1467-8624.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- Hatch JP, Borcherding S, German C. Cardiac sympathetic and parasympathetic activity during self-regulation of heart period. Applied Psychophysiology and Biofeedback. 1992;17:89–106. doi: 10.1007/BF01000101. [DOI] [PubMed] [Google Scholar]
- Hayano J, Sakakibara Y, Yamada A, Yamada M, Mukai S, Fujinami T, et al. Accuracy of assessment of cardiac vagal tone by heart rate variability in normal subjects. American Journal of Cardiology. 1991;67:199–204. doi: 10.1016/0002-9149(91)90445-q. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Mueller B, Qunaibi M, Lichterfeld C, Konrad K, Herpertz-Dahlmann B. Response to emotional stimuli in boys with conduct disorder. American Journal of Psychiatry. 2005;162:1100–1107. doi: 10.1176/appi.ajp.162.6.1100. [DOI] [PubMed] [Google Scholar]
- Hessler D, Fainsilber Katz L. Children's emotion regulation: Self-report and physiological response to peer provocation. Developmental Psychology. 2007;43:27–38. doi: 10.1037/0012-1649.43.1.27. [DOI] [PubMed] [Google Scholar]
- Hinnant JB, El-Sheikh M. Children's externalizing and internalizing symptoms over time: The role of individual differences in patterns of vagal responding. Journal of Abnormal Child Psychology. 2009;37:1049–1061. doi: 10.1007/s10802-009-9341-1. [DOI] [PubMed] [Google Scholar]
- Hinnant JB, Staton L, El-Sheikh M. Developmental trajectories of respiratory sinus arrhythmia and pre-ejection period in middle childhood. 2010 doi: 10.1002/dev.20487. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard JA, Smithmyer CM, Ramsden SR, Parker EH, Flanagan KD, Dearing KF, et al. Observational, physiological, and self-reported measures of children's anger: Relations to reactive versus proactive aggression. Child Development. 2002;73:1101–1118. doi: 10.1111/1467-8624.00460. [DOI] [PubMed] [Google Scholar]
- Imrich R, Eldadah BA, Bentho O, Pechnik S, Sharabi Y, Holmes C, et al. Attenuated pre-ejection period response to tyramine in patients with cardiac sympathetic denervation. Annals of the New York Academy of Sciences. 2008;1148:486–489. doi: 10.1196/annals.1410.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman NC. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N, Arcus D, Reznick JS. Galen's prophecy: Temperament in human nature. Basic Books; New York: 1994. [Google Scholar]
- Karavidas M, Lehrer P, Vaschillo E, Vaschillo B, Marin H, Buyske S, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Applied Psychophysiology and Biofeedback. 2007;32:19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- Katz L. Domestic violence and vagal reactivity to peer provocation. Biological Psychology. 2007;74:154–164. doi: 10.1016/j.biopsycho.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LF. Physiological processes and mediators of the impact of marital conflict on children. In: Grych JH, Finchman FD, editors. Interparental conflict and child development: Theory, research, and applications. Cambridge University Press; New York: 2001. pp. 188–212. [Google Scholar]
- Katz LF, Gottman JM. Vagal tone protects children from marital conflict. Development and Psychopathology. 1995;7:83–92. [Google Scholar]
- Katz LF, Gottman JM. Buffering children from marital conflict and dissolution. Journal of Clinical Child Psychology. 1997;26:157–171. doi: 10.1207/s15374424jccp2602_4. [DOI] [PubMed] [Google Scholar]
- Keller PS, El-Sheikh M. Salivaryalpha-amylase asa longitudinal predictor of children's externalizing symptoms: Respiratory sinus arrhythmia as a moderator of effects. Psychoneuroendocrinology. 2009;34:633–643. doi: 10.1016/j.psyneuen.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Kennedy AE, Rubin KH, Hastings PD, Maisel B. Longitudinal relations between child vagal tone and parenting behavior: 2 to 4 years. Developmental Psychobiology. 2004;45:10–21. doi: 10.1002/dev.20013. [DOI] [PubMed] [Google Scholar]
- Larkin KT, Kasprowicz AL. Validation of a simple method of assessing cardiac preejection period: A potential index of sympathetic nervous system activity. Perception and Motor Skills. 1986;63:295–302. doi: 10.2466/pms.1986.63.1.295. [DOI] [PubMed] [Google Scholar]
- Leary A, Katz LF. Coparenting, family-level processes, and peer outcomes: The moderating role of vagal tone. Development and Psychopathology. 2004;16:593–608. doi: 10.1017/s0954579404004687. [DOI] [PubMed] [Google Scholar]
- Lindsey EW, Caldera YM, Tankersley L. Marital conflict and the quality of young children's peer play behavior: The mediating and moderating role of parent–child emotional reciprocity and attachment security. Journal of Family Psychology. 2009;23:130–145. doi: 10.1037/a0014972. [DOI] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: A meta-analysis. Psychological Bulletin. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Masi CM, Hawkley LC, Rickett EM, Cacioppo JT. Respiratory sinus arrhythmia and diseases of aging: Obesity, diabetes mellitus, and hypertension. Biological Psychology. 2007;74:212–223. doi: 10.1016/j.biopsycho.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS. Developmental psychopathology: Pathways to the future. International Journal of Behavioral Development. 2006;30:47–54. [Google Scholar]
- McBurnett K. Psychobiological approaches to personality and their applications to child psychopathology. Advances in Clinical Child Psychology. 1992;14:107–164. [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allo-static load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Lasley EN. The end of stress. Joseph Henry Press; Washington, DC: 2002. [Google Scholar]
- Mezzacappa ES, Kelsey R, Katkin E, Sloan R. Vagal rebound and recovery from psychological stress. Psychosomatic Medicine. 2001;63:650–657. doi: 10.1097/00006842-200107000-00018. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychology Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, et al. Stress-induced changes in human salivary alpha-amylase activity-associations with adrenergic activity. Psychoneuroendocrinology. 2006;31:49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Gaab J, Berger S, Jud A, Kirschbaum C, et al. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. International Journal of Psychophysiology. 2005;55:333–342. doi: 10.1016/j.ijpsycho.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Obradović J, Bush NR, Boyce WT. The effect of marital conflict and stress reactivity on externalizing varies across different laboratory stressors.. Paper presented at the biennial meeting of the Society for Research in Child Development; Denver, CO. 2009. [Google Scholar]
- Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien LM, Gozal D. Autonomic dysfunction in children with sleep-disordered breathing. Pediatrics. 2005;28:747–752. doi: 10.1093/sleep/28.6.747. [DOI] [PubMed] [Google Scholar]
- Patterson GR. The early development of coercive family processes. In: Reid JB, Patterson GR, Snyder J, editors. Antisocial behavior in children and adolescents: A developmental analysis and model for intervention. American Psychological Association; Washington, DC: 2002. pp. 25–44. [Google Scholar]
- Patterson GR, Reid JB, Dishion TJ. Antisocial boys. Castalia; Eugene, OR: 1992. [Google Scholar]
- Pettit GS, Arsiwalla DD. Commentary on special section on “bidirectional parent child relationships”: The continuing evolution of dynamic, transactional models of parenting and youth behavior problems. Journal of Abnormal Child Psychology. 2008;36:711–718. doi: 10.1007/s10802-008-9242-8. [DOI] [PubMed] [Google Scholar]
- Pettit GS, Bates JE, Dodge KA, Meece DW. The impact of after-school peer contact on early adolescent externalizing problems is moderated by parental monitoring, perceived neighborhood safety, and prior adjustment. Child Development. 1999;70:768–778. doi: 10.1111/1467-8624.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. Peripheral and neurochemical parallels of psychopathology: A psychophysiological model relating autonomic imbalance in hyperactivity, psychopathology, and autism. In: Reese H, editor. Advances in child development and behavior. Vol. 11. Academic Press; New York: 1976. pp. 35–65. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Love: An emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology. 1998;23:837–861. doi: 10.1016/s0306-4530(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: Phylogenetic contributions to social behavior. Physiology & Behavior. 2003;79:503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter CL, Wouden-Miller M, Silva SS, Porter AE. Marital harmony and conflict: Links to infants’ emotional regulation and cardiac vagal tone. Infancy. 2003;4:297–307. [Google Scholar]
- Propper C, Moore GA. The influence of parenting on infant emotionality: A multi-level psychobiological perspective. Developmental Review. 2006;26:427–460. [Google Scholar]
- Quigley KS, Stifter CA. A comparative validation of sympathetic reactivity in children and adults. Psychophysiology. 2006;43:357–365. doi: 10.1111/j.1469-8986.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- Raine A. Annotation: The role of prefrontal deficits, low autonomic arousal, and early health factors in the development of antisocial and aggressive behavior in children. Journal of Child Psychology and Psychiatry. 2002;43:417–434. doi: 10.1111/1469-7610.00034. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Dalais C, Mellingen K, Reynolds C, Med-nick SA. Early educational and health enrichment at age 3–5 years is associated with increased autonomic and central nervous system arousal and orienting at age 11 years: Evidence from the Mauritius Child Health Project. Psychophysiology. 2001;38:254–266. [PubMed] [Google Scholar]
- Raine A, Venables PH, Williams M. Autonomic orienting responses in 15-year-old male subjects and criminal behavior at age 24. American Journal of Psychiatry. 1990;147:933–937. doi: 10.1176/ajp.147.7.933. [DOI] [PubMed] [Google Scholar]
- Reid JB, Patterson GR, Snyder J. Antisocial behavior in children and adolescents: A developmental analysis and model for intervention. American Psychological Association; Washington, DC: 2002. [Google Scholar]
- Rennie KL, Hemingway H, Kumari M, Brunner E, Malik M, Marmot M. Effects of moderate and vigorous physical activity on heart rate variability in a British study of civil servants. American Journal of Epidemiology. 2003;158:135–143. doi: 10.1093/aje/kwg120. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Richter M, Gendolla GHE. The heart contracts to reward: Monetary incentives and preejection period. Psychophysiology. 2009;46:451–457. doi: 10.1111/j.1469-8986.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Nater UM, Wolf JM, Ehlert U, Kirschbaum C. Psychosocial stress-induced activation of salivary alpha-amylase: An indicator of sympathetic activity? Annuals of the New York Academy of Sciences. 2004;1032:258–263. doi: 10.1196/annals.1314.033. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Mills RSL. The many faces of social isolation in childhood. Journal of Consulting and Clinical Psychology. 1988;56:916–924. doi: 10.1037//0022-006x.56.6.916. [DOI] [PubMed] [Google Scholar]
- Rutter M. Psychosocial resilience and protective mechanisms. In: Rolf J, Masten AS, Cicchetti D, Nuechterlein KH, Weintraub S, editors. Risk and protective factors in the development of psychopathology. Cambridge University Press; New York: 1990. pp. 181–214. [Google Scholar]
- Salomon K. Respiratory sinus arrhythmia during stress predicts resting respiratory sinus arrhythmia 3 years later in a pediatric sample. Health Psychology. 2005;24:68–76. doi: 10.1037/0278-6133.24.1.68. [DOI] [PubMed] [Google Scholar]
- Salomon K, Matthews KA, Allen MT. Patterns of sympathetic reactivity in a sample of children and adolescents. Psychophysiology. 2000;37:842–849. [PubMed] [Google Scholar]
- Santucci AK, Silk JS, Shaw DS, Gentzler A, Fox NA, Kovacs M. Vagal tone and temperament as predictors of emotion regulation strategies in young children. Developmental Psychobiology. 2008;50:205–216. doi: 10.1002/dev.20283. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Schulkin J, Gold PW. Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Developmental Psychobiology. 1999;35:119–135. [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus–adrenal–medullary systems to repeated psychosocial stress. Psychosomatic Medicine. 2003;65:450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Shannon KE, Beauchaine TP, Brenner SL, Neuhaus E, Gatzke-Kopp L. Familial and temperamental predictors of resilience in children at risk for conduct disorder and depression. Development and Psychopathology. 2007;19:701–727. doi: 10.1017/S0954579407000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Allen M, Obrist P, Langer A. Evaluation of beta-adrenergic influences on cardiovascular and metabolic adjustments to physical and psychological stress. Psychophysiology. 1986;23:89–104. doi: 10.1111/j.1469-8986.1986.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Sheinkopf SJ, Lagasse LL, Lester BM, Liu J, Seifer R, Bauer CR, et al. Vagal tone as a resilience factor in children with prenatal cocaine exposure. Development and Psychopathology. 2007;19:649–673. doi: 10.1017/S0954579407000338. [DOI] [PubMed] [Google Scholar]
- Siepmann M, Heine B, Kluge A, Ziemssen T, Muck-Weymann M, Kirch W. The effects of lorazepam on skin conductance responses to aversive stimuli in healthy subjects. Clinical Autonomic Research. 2007;17:160–164. doi: 10.1007/s10286-007-0407-2. [DOI] [PubMed] [Google Scholar]
- Snoek H, van Goozen SHM, Matthys W, Buitelaar JK, van Enge-land H. Stress responsivity in children with externalizing behavior disorders. Development and Psychopathology. 2004;16:389–406. doi: 10.1017/s0954579404044578. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Avenevoli S. The role of context in the development of psychopathology: A conceptual framework and some speculative propositions. Child Development. 2000;71:66–74. doi: 10.1111/1467-8624.00119. [DOI] [PubMed] [Google Scholar]
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hall M, Sollers JJ, Fischer JE. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: Evidence for impaired inhibitory control of the HPA axis in heavy drinkers. International Journal of Psychophysiology. 2006;56:244–250. doi: 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biological Psychology. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. Beyond heart rate variability: Vagal regulation of allostatic systems. Annals of the New York Academy of Sciences. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Troxel WM, Matthews KA. What are the costs of marital conflict and dissolution to children's physical health? Clinical Child and Family Psychology Review. 2004;7:29–57. doi: 10.1023/b:ccfp.0000020191.73542.b0. [DOI] [PubMed] [Google Scholar]
- van Bokhoven I, Matthys W, van Goozen SHM, van Engeland H. Prediction of adolescent outcome in children with disruptive behaviour disorders: A study of neurobiological, psychological and family factors. European Child and Adolescent Psychiatry. 2005;14:153–163. doi: 10.1007/s00787-005-0455-x. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Matthys W, Cohen-Kettenis PT, Buitelaar JK, van Engeland H. Hypothalamic–pituitary–adrenal axis and automatic nervous system activity in disruptive children and matched controls. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:1438–1445. doi: 10.1097/00004583-200011000-00019. [DOI] [PubMed] [Google Scholar]
- van Goozen SHM, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional–defiant disorder boys and normal controls. Biological Psychiatry. 1998;43:531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Daleiden EL. Information-processing pathways to cognitive interference in childhood. In: Sarason IG, Pierce G, Sarason B, editors. Cognitive interference: Theory, methods, and findings. Erlbaum; Hillsdale, NJ: 1996. pp. 117–138. [Google Scholar]
- Venables PH, Christie MJ. Electrodermal activity. In: Martin I, Venables PH, editors. Techniques in psychophysiology. Wiley; London: 1980. pp. 3–67. [Google Scholar]
- Venables PH, Mitchell DA. The effects of age, sex and time of testing on skin conductance activity. Biological Psychology. 1996;43:87–101. doi: 10.1016/0301-0511(96)05183-6. [DOI] [PubMed] [Google Scholar]
- Weems CF, Zakem AH, Costa NM, Cannon MF, Watts SE. Physiological response and childhood anxiety: Association with symptoms of anxiety disorders and cognitive bias. Journal of Clinical Child and Adolescent Psychology. 2005;34:712–723. doi: 10.1207/s15374424jccp3404_13. [DOI] [PubMed] [Google Scholar]
- Whitson S, El-Sheikh M. Marital conflict and health: Processes and protective factors. Aggression and Violent Behavior. 2003;8:283–312. [Google Scholar]
- Willemen AM, Schuengel C, Koot HM. Physiological regulation of stress in referred adolescents: The role of the parent–adolescent relationship. Journal of Child Psychology and Psychiatry. 2009;50:482–490. doi: 10.1111/j.1469-7610.2008.01982.x. [DOI] [PubMed] [Google Scholar]
- Wootton JW, Frick PJ, Shelton KK, Silverthorn P. Ineffective parenting and childhood conduct problems: The moderating role of callous-unemotional traits. Journal of Consulting and Clinical Psychology. 1997;65:301–308. doi: 10.1037/0022-006x.65.2.292.b. [DOI] [PubMed] [Google Scholar]