Background: Pathogenic yersiniae translocate effectors into host cells to interfere with the immune defense.
Results: Yersinia exotoxin CNF-Y enhances effector translocation by activating the GTP-binding protein Rac.
Conclusion: A crucial role of Rac in translocation control and a potential virulence function of CNF-Y is revealed.
Significance: Understanding the cross-talk between bacterial and host cell mechanisms in regulation of effector translocation.
Keywords: Cdc42, Rac, Rho, Rho GTPases, Toxins, Type III Secretion System, CNF-Y, Cytotoxic Necrotizing Factor-Y, Yersinia enterocolitica
Abstract
Pathogenic Yersinia spp. translocate the effectors YopT, YopE, and YopO/YpkA into target cells to inactivate Rho family GTP-binding proteins and block immune responses. Some Yersinia spp. also secrete the Rho protein activator cytotoxic necrotizing factor-Y (CNF-Y), but it has been unclear how the bacteria may benefit from Rho protein activation. We show here that CNF-Y increases Yop translocation in Yersinia enterocolitica-infected cells up to 5-fold. CNF-Y strongly activated RhoA and also delayed in time Rac1 and Cdc42, but when individually expressed, constitutively active mutants of Rac1, but not of RhoA, increased Yop translocation. Consistently, knock-out or knockdown of Rac1 but not of RhoA, -B, or -C inhibited Yersinia effector translocation in CNF-Y-treated and control cells. Activation or knockdown of Cdc42 also affected Yop translocation but much less efficiently than Rac. The increase in Yop translocation induced by CNF-Y was essentially independent of the presence of YopE, YopT, or YopO in the infecting Yersinia strain, indicating that none of the Yops reported to inhibit translocation could reverse the CNF-Y effect. In summary, the CNF-Y activity of Yersinia strongly enhances Yop translocation through activation of Rac.
Introduction
The genus Yersinia comprises three species that are pathogenic for humans. Yersinia pestis is the causative agent of bubonic plague, and the enteropathogenic Yersinia enterocolitica and Yersinia pseudotuberculosis elicit acute enteritis and enteric lymphadenitis (1, 2). After oral ingestion, the enteropathogenic Yersinia spp. reach the distal small intestine, from where a subset invades and crosses the intestinal mucosa mainly via M-cells (3). Once the yersiniae have reached the mucosa-associated lymphatic tissue, they subvert immune cell responses such as phagocytosis and cytokine production and proliferate extracellularly (4, 5). The cornerstone of this phase of infection is the 70-kb virulence plasmid (pYV), which encodes for the Yersinia type III secretion system, and bacterial effector proteins termed Yops that are translocated through the type III secretion system into infected cells (6, 7). Effector translocation is tightly controlled, and Yops are only translocated when there is intimate contact between the bacteria and host cells (8). Interestingly, some of the translocated Yops can inhibit the translocation process in a negative feedback loop type of mechanism (see below) (9–11)
There are at least seven effector Yops, which interfere with major signaling pathways of the host cells (6, 10, 12). YopH is a highly active protein tyrosine phosphatase that dephosphorylates focal adhesion proteins in macrophages and adaptor proteins involved in T- and B-cell signaling (13–17). YopJ/YopP acetylates critical serine and threonine residues in the activation loop of MAPK family kinases (MAPKK) and IκB kinase (IKKβ), thus blocking inflammatory signaling and contributing to induction of apoptosis (18–20). YopM consists mainly of leucine-rich repeats and forms a complex with ribosomal S6 protein kinases (RSKs) and protein kinase C-like kinases (PRKs) that activates these kinases. The biological consequences of activation of these kinases remain as yet unknown (21–25). YopK/YopQ, which exhibits no homology to other known proteins, has been implicated in the control of Yop translocation and prevents inflammasome activation by inhibiting cellular recognition of the type III secretion system (10, 26–29). Three Yops, namely YopE, YopO/YpkA, and YopT, inhibit the activity of small GTP-binding proteins of the Rho family (12). The main function of Rho GTP-binding proteins is regulation of the actin cytoskeleton, and through this, they are involved in a wide range of cellular functions including chemotaxis, phagocytosis, and establishment of polarity (30). Most Rho GTP-binding proteins cycle between an inactive GDP-bound and an active GTP-bound state. The cycling is tightly controlled by three sets of regulatory proteins: guanine nucleotide exchange factors, which catalyze the exchange of bound GDP for GTP; GTPase-activating proteins (GAPs),3 which strongly accelerate the intrinsic GTPase activity; and guanine nucleotide dissociation inhibitors, which extract the GDP-bound form from membranes and keep it in the cytosol (30, 31). The multidomain YopO/YpkA comprises a G-actin-activated serine/threonine kinase module and a module that structurally and functionally mimics a guanine nucleotide dissociation inhibitor, which was reported to bind and inhibit Rac1 (32–35). YopE acts as a GAP for Rho, Rac, and Cdc42 in vitro, and in cellular infection systems in which endogenous Rho family proteins were investigated, it showed a preference for Rac (36, 37). YopT is a cysteine protease that removes the C-terminal isoprenoid moiety of RhoA, Rac1, and Cdc42 in vitro, thereby dislocating these proteins from the plasma membrane and from their guanine nucleotide dissociation inhibitors (38). It is well accepted that by blocking Rho proteins YopE, YopO/YpKA, and YopT can down-regulate chemotactic and phagocytic activities of leukocytes as well as other immune cell functions such as superoxide anion production and IL1β-release (39–41).
YopE was shown to act as a negative regulator of Yop translocation. This notion comes from the finding that Yersinia YopE mutants hypertranslocate the other Yops and that there is an inverse correlation between the amount of translocated YopE and general Yop translocation activity (9, 11). These and further studies brought forward the concept that activation of Rho GTP-binding proteins and the ensuing actin reorganization, which occurs upon adhesion of Yersinia to host cells, support Yop delivery (42, 43). While Yop delivery progresses, the increasing amount of YopE in the host cell cytoplasm is thought to prevent further translocation by down-regulating Rho GTP-binding proteins. Inactivation of Rho (A, B, and C) with bacterial toxins as well as knockdown of Rac1 via siRNA were reported to inhibit Yop translocation (42–44); however, a more comprehensive analysis of the Rho family proteins involved in Yop translocation has not been performed.
Although the Yersinia virulence machinery is very effective at down-regulating Rho GTP-binding proteins, some Yersinia strains also secrete a Rho protein activator termed cytotoxic necrotizing factor (CNF)-Y (45, 46). CNF-Y is a close homolog of cytotoxic necrotizing-factors 1–3 from Escherichia coli, and like these, it deamidates Rho GTP-binding proteins at Gln-63 (RhoA) or Gln-61 (for Rac and Cdc42). Deamidation at this position inhibits the intrinsic and GAP-stimulated GTPase activities of these Rho family proteins and thereby renders them permanently active (47, 48). Although CNF-1 was reported to act equally well on Rho, Rac, and Cdc42 in vitro and in cell cultures, CNF-Y was found to preferentially modify and activate RhoA (49). Presently, there have been no reports of how the activity of CNF-Y may fit into the virulence strategy of Yersinia.
Here, we report that CNF-Y strongly increases the translocation of Yops into Y. enterocolitica-infected cells. The CNF-Y effect appears to be due to activation of mainly Rac and, to a minor extent, Cdc42, but not RhoA, and to the failure of Yops to down-regulate CNF-Y-activated translocation.
EXPERIMENTAL PROCEDURES
Yersinia Strains and Infection Conditions
The Y. enterocolitica and Y. pseudotuberculosis strains used in this study are listed in Table 1. WA-314 was transformed with YopE-β-lactamase (pMK-bla) or YopE-ovalbumin (pMK-ova) plasmids for fluorescence resonance energy transfer (FRET)-based real-time translocation analysis.
TABLE 1.
Strains used in this study
| Strain | Relevant characteristic | Sources/References |
|---|---|---|
| Y. enterocolitica | ||
| WA-314 | Wild-type strain; serogroup O8; clinical isolate harboring the virulence plasmid pYVO8 | 66 |
| WA-C | Plasmidless derivative of WA-314 | 66 |
| WA-314ΔE | WA-C harboring pYVΔE | 67 |
| WA-314ΔE/ΔT | WA-C harboring pYVΔE/ΔT | This study |
| WA-314ΔE/ΔT/ΔO | WA-C harboring pYVΔE/ΔT/ΔO | This study |
| WA-314-pMK-bla | Wild-type strain, harboring pMK-bla, encoding for YopE-β-lactamase fusion | This study |
| WA-314-pMK-ova | Wild-type strain, harboring pMK-bla, encoding for YopE-ovalbumin fusion | This study |
| Y. pseudotuberculosis | ||
| YPIII (pIB102) | Wild-type strain with insertional inactivation of YadA | 68, 69 |
In addition, we generated the Y. enterocolitica mutants WA-314ΔE/ΔT and WA-314ΔE/ΔT/ΔO by using the phage recombinases Redα and Redβ as described previously (50). Briefly, mutants were generated by consecutively replacing the coding region of yopT by a chloramphenicol resistance cassette (Cmr) and replacing the coding region of yopO by a spectinomycin resistance cassette (Spr) in the pYVΔE plasmid. Correct replacement of the respective yop genes by the resistance cassettes was verified by PCR and SDS-PAGE of secreted Yop proteins and Western blotting. To rule out any unwanted recombination in the chromosome due to the action of Redα and Redβ, we transferred the mutated plasmids to the previously pYV-cured strain WA-C.
For infection, overnight cultures grown at 27 °C were diluted 1:20 into fresh LB broth and grown for another 1.5 h at 37 °C. Shift of the growth temperature to 37 °C triggers activation of the Yersinia type III secretion machinery for efficient translocation of effectors into the host cell upon cellular contact.
Plasmids and Expression of Proteins
We gratefully acknowledge the gifts of plasmids. The plasmid constructs pMK-bla and pMK-ova were kindly provided by Erwin Bohn (Institute of Medical Microbiology and Hygiene, University of Tuebingen, Tuebingen, Germany); plasmids encoding Myc-Rac1L61, Myc-Cdc42L61, and Myc-RhoAL63 were kindly provided by Dr. Pontus Aspenström (Uppsala University, Uppsala, Sweden); and GST-PAK-CRIB and GST-Rhotekin were kindly provided by Dr. John Collard (Netherlands Cancer Institute, Amsterdam, The Netherlands). The cloning of the GST-CNF-1 expression vector was described previously (51). The gene encoding CNF-Y from Y. pseudotuberculosis YPIII (pIB1) was amplified using PCR primers 5′-CAGGATCCATGAAAAATCAATGGCAA-3′ and 5′-GACTCGAGTATCTTTTCATTTCCCCCT-3′ and cloned into the BamHI and XhoI restriction sites of vector pGEX-2T (Amersham Biosciences, Freiburg, Germany).
For expression of all GST-tagged proteins, overnight bacterial cultures were diluted 1:20 into LB medium containing 100 μg/ml ampicillin and grown at 37 °C to an A600 of 0.6–0.8. Protein expression was induced for 3–4 h with 0.4 mm isopropyl-1-thio-β-d-galactopyranoside. After sonification, GST proteins were purified from bacterial lysates on glutathione-Sepharose 4B beads (GE Healthcare, Munich, Germany). GST-tagged proteins were eluted with reduced glutathione.
Cell Culture and Transfections
Rac1fl/fl mouse embryonic fibroblasts (MEFs) homozygous for a loxP-flanked Rac1 allele (52) were kindly provided by Cord Brakebusch (University of Kopenhagen). Generation and characterization of Rac1-deficient MEFs will be described elsewhere.4 HeLa and Rac1fl/fl or Rac1−/− MEFs were cultured in Dulbecco's modified Eagle's medium containing 10% heat-inactivated fetal calf serum and supplemented with nonessential amino acids solution (Life Technologies).
Plasmid constructs were transfected with the TurboFect transfection kit (Fermentas, St. Leon-Rot, Germany) into HeLa cells as specified by the manufacturer. Constitutively active GTPase constructs were transfected 16 h prior to infection.
GST-CNF-Y, GST-CNF-1, and GST treatment was conducted at a final concentration of 1 μg/ml. Blebbistatin and Y-27632 were obtained from Sigma-Aldrich.
siRNA Knockdown
siRNAs were transfected with Lipofectamine RNAiMAX reagent (Life Technologies) according to the manufacturer's instructions. Specific siRNA against RhoA, -B, and -C and Cdc42 were obtained from Qiagen (Hilden, Germany). A pool of four siRNAs against Rac1 and a pool of nontargeting siRNAs were obtained from Thermo Scientific. Successful knockdown was confirmed by Western blot using the following antibodies: anti-Rac1 (Millipore, Billerica, MA), anti-Cdc42 (BD Biosciences, Heidelberg, Germany), anti-RhoA (Santa Cruz Biotechnology, Heidelberg, Germany), and anti-RhoB and anti-RhoC (Cell Signaling Technology, Danvers, MA).
Detection of Translocated Effectors (Digitonin Lysis Assay)
Translocated effectors were analyzed as described previously (53). In brief, HeLa cells were infected at a ratio of 100 bacteria/cell. Following incubation at 37 °C for different time spans (5–120 min), cells were washed twice with PBS to remove nonadherent bacteria. To remove extracellular effectors, cells were treated with proteinase K (500 mg/ml in PBS) for 20 min at room temperature. Prior to cell lysis, protease activity was blocked by the addition of phenylmethylsulfonyl fluoride (4 mm in PBS). HeLa cells were lysed by the addition of digitonin (1% in PBS) at room temperature for 20 min, with occasional vortexing. Cell debris and attached bacteria were removed from the lysate by centrifugation. The resulting supernatants were analyzed by SDS-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane (Immobilon-P, Millipore, Schwalbach, Germany), and immunoblotted using antisera against YopH and YopE and antibodies against actin (Millipore). Blots were developed with chemiluminescence reagent (SuperSignal West Femto, Pierce Chemical). The protein band intensities were quantified using the ImageJ software, and the values were expressed as the YopH/actin ratio.
RhoGTPase Activity Assays
Activity of Rho GTP-binding proteins was analyzed by GST-PAK-CRIB (for Rac1 and Cdc42) and GST-Rhotekin (for RhoA) pulldown assays. GST-PAK-CRIB and GST-Rhotekin were expressed as described above. HeLa cells were lysed in lysis buffer (GST-PAK-CRIB pulldown: 20 mm Tris, pH 7.5, 150 mm NaCl, 5 mm MgCl, 1% Nonidet P-40, 10% glycerol, and protease inhibitor mixture; alternatively, for additional Rac1 activation time course pulldowns: 50 mm Tris, pH 7.5, 150 mm NaCl, 50 mm MgCl2, 1% Triton X-100, 0.1% SDS, and protease inhibitor mixture; GST-Rhotekin pulldown: 50 mm Tris, pH 7.5, 100 mm NaCl, 2 mm MgCl2, 1% Triton X-100, 10% glycerol, and protease inhibitor mixture) and centrifuged for 10 min at 10,000 × g, and supernatants were incubated for 2 h at 4 °C with GST-PAK-CRIB or GST-Rhotekin prebound to glutathione-Sepharose 4B beads (GE Healthcare). Beads loaded with GTP-bound RhoGTPases were washed three times with PBS buffer, and equal amounts of pulldown and total cell lysates were resolved by SDS-PAGE and immunoblotting as described above. The following antibodies were used: anti-Rac1 (Millipore), anti-Cdc42 (BD Biosciences), anti-RhoA (Santa Cruz Biotechnology), and anti-actin (Millipore).
FRET-based Real-time Translocation Analysis
Methods for detection of translocated effectors by effector-β-lactamase fusions and cephalosporin-derived FRET substrates were described previously and adapted here for real-time analysis by live cell fluorescence microscopy (44, 54, 55). Cells were seeded in 96-well plates (black with clear bottom, Greiner Bio-One) at a density of 2 × 104 cells/well. Prior to infection, cells were treated with DMEM containing CCF4-AM loading solution (CCF4-AM loading kit, Invitrogen, Karlsruhe, Germany; 1 mm CCF4-AM and 2.5 mm probenecid final concentration) for 15 min at room temperature. CCF4-AM is a cell-permeant compound in which the fluorescent dyes coumarin and fluorescein are linked to a cephalosporin core. For infection at a ratio of 200 bacteria/cell, the medium was replaced by an adjusted bacterial suspension in fresh DMEM containing CCF4-AM loading solution. Then the cells were immediately transferred into the environmental chamber of the spinning disk live cell microscope. Intracellular CCF4 hydrolysis (due to translocated YopE-β-lactamase-fusion) was monitored by excitation at 409 nm and detection of green fluorescence emission at 520 nm from the intact CCF4 substrate (FRET) and blue fluorescence emission from the cleaved CCF4 product at 447 nm (coumarin). Images were recorded at intervals of 5 min for 3 h using a spinning-disc confocal system (Improvision, Coventry, UK), equipped with a CSU22 spinning disc (Yokogawa, Tokyo, Japan) fitted on to an Axiovert 200M microscope (Zeiss) with a temperature- and CO2-controlled environmental chamber (Solent Scientific, Segensworth, UK). Images were acquired using a dry immersion EC Plan Neofluar objective (×10 magnification; numerical aperture (NA) 0.3) and a C-9100-2 electron-multiplying charge-coupled device (EM-CCD) camera (Hamamatsu). Analysis of fluorescence intensities was performed with Volocity software version 6.1 (PerkinElmer Life Sciences). Live cell data were quantitatively analyzed by calculation of the fluorescence index Q = (Pcells − Pbackground)/(Scells − Sbackground) where P is the fluorescence intensity of the CCF4 hydrolysis product and S is the fluorescence intensity of the uncleaved CCF4 substrate. This was necessary to compensate for background fluorescence and loss of CCF4 substrate from the cells.
Fluorescence Microscopy
HeLa cells were fixed in 3.7% formaldehyde in PBS for 10 min and permeabilized with 0.1% Triton in PBS for 5 min. Actin was stained with Alexa Fluor 568-labeled phalloidin (Molecular Probes, Karlsruhe, Germany). Coverslips were mounted in Mowiol (Calbiochem, Darmstadt, Germany) containing 0.18% p-phenylenediamine (Sigma-Aldrich, Munich, Germany) as anti-fading reagent. Images of fixed samples were acquired using an Axioplan epifluorescence microscope (Zeiss) equipped with an oil immersion plan Apochromat 63× NA 1.4 objective. Acquisition and processing of images were performed with SPOT 4.6 advanced software (Spot Imaging Solutions, Sterling Heights, MI).
Quantification of Cell-associated Bacteria
Assays were performed in 6-well plates with HeLa cells treated with GST/GST-CNF-Y or expressing Rac1L61. After 1 h of infection with WA-314 (multiplicity of infection 100), cells were thoroughly washed three times with cold PBS to remove nonbinding bacteria and subsequently lysed in lysis buffer (1% Triton in PBS). Cell-associated bacteria were then quantified by serial dilution and culture on LB agar.
RESULTS
CNF-Y Increases Translocation of Yersinia Effectors into Target Cells
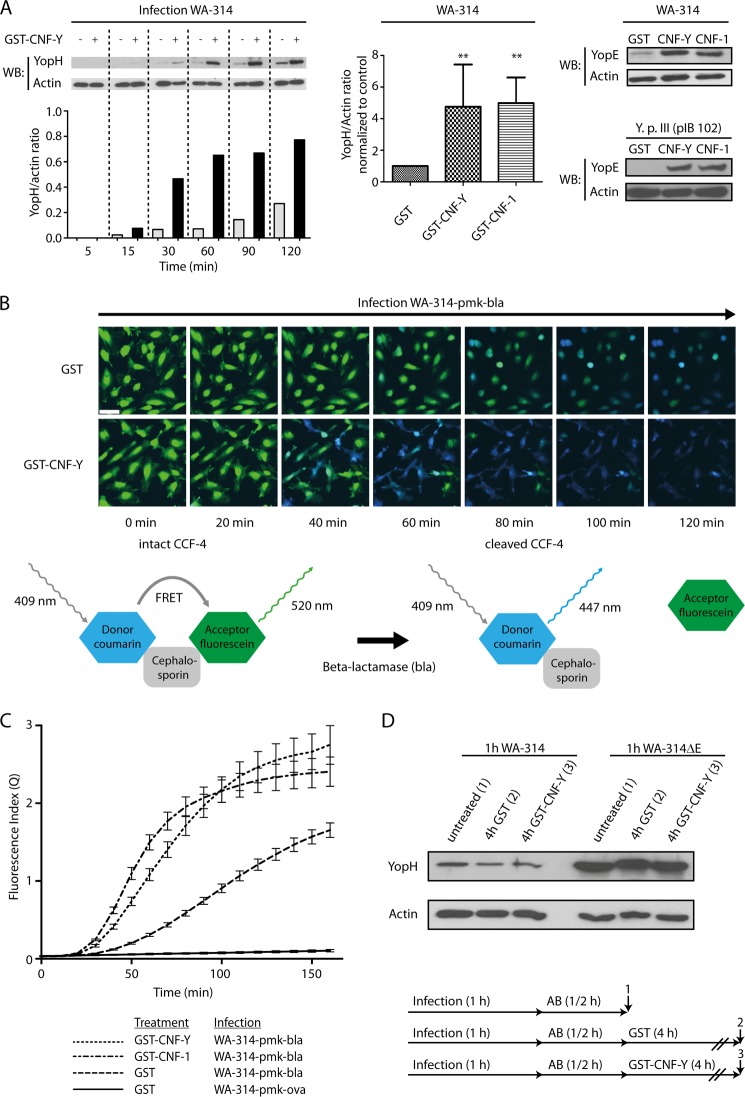
Earlier studies showed that inhibition of Rho or Rac in host cells reduced Yop translocation by Y. enterocolitica and Y. pseudotuberculosis (9, 42–44). To find out whether the converse is also true, namely whether Rho GTP-binding protein activation in host cells stimulates Yop translocation, we employed the toxins CNF-Y of Y. pseudotuberculosis and CNF-1 of E. coli. CNF-1 was reported to modify and permanently activate RhoA, Rac1, and Cdc42 in vitro and in cells, whereas CNF-Y was proposed to have a preference for RhoA in cells (49). HeLa cells were pretreated with these toxins for 2 h before infection with Y. enterocolitica wild type WA-314, and Yop translocation was assessed by selective lysis of the HeLa cells with digitonin and detection of the released (translocated) Yops by Western blot (53). In CNF-Y-treated cells, the amount of translocated YopH rapidly increased within the first 60 min of infection and then leveled off. Untreated cells showed a much slower YopH accumulation rate, which did not reach a maximum up to 120 min of infection (Fig. 1A, left panel; see also Fig. 1C). These different translocation kinetics resulted in about 5-fold higher levels of intracellular YopH in CNF-Y- and CNF-1-treated cells when compared with untreated cells after 60 min of infection (Fig. 1A, middle panel). Similarly increased translocation levels were found for YopE in CNF-Y- and CNF-1-treated cells, showing that the observed phenomenon is not restricted to YopH (Fig. 1A, right panel). Further, CNF-Y and CNF-1 could enhance translocation of effectors into Y. pseudotuberculosis-infected HeLa cells and WA-314-infected mouse embryonic fibroblasts, extending our observations to other Yersinia strains and cell types (Fig. 1A, right panel, and see below). Importantly, control-, CNF-Y-, and CNF-1-treated cells showed a comparable number of adhering WA-314 bacteria, excluding that the increase in Yop translocation was due to increased bacterial adhesion (supplemental Fig. S1A and data not shown).
FIGURE 1.
CNF-Y increases Y. enterocolitica effector translocation into target cells. A, left panel, HeLa cells were treated with GST-CNF-Y or GST for 2 h and subsequently infected with WA-314 for 5–120 min as indicated. Cells were lysed with digitonin, and supernatants were analyzed for YopH and actin by Western blotting (WB) and densitometry of respective protein bands. Bars show the YopH/actin ratio of the densitometric values. Middle panel, HeLa cells were treated with GST-, GST-CNF-Y-, or GST-CNF-1 for 2 h and infected for 1 h with WA-314. Bars represent the mean ± S.D. (error bars) of YopH/actin ratio normalized to control (GST-treated cells) of at least three independent experiments. Asterisks indicate significant differences when compared with control (one-way analysis of variance with Dunnett's test; *, p = 0.01–0.05 and **, p = 0.001–0.01). Right panel, GST-, GST-CNF-Y-, or GST-CNF-1-treated HeLa cells were infected with WA-314 or Y. pseudotuberculosis III (pIB 102) for 1 h. A digitonin lysis assay was conducted, and supernatants were analyzed for YopE and actin by Western blotting. B, GST- or GST-CNF-Y-treated HeLa cells were preloaded with CCF4-AM substrate and then infected with WA-314-pMK-bla (translocating YopE-β-lactamase fusion) and WA-314-pMK-ova (translocating YopE-ovalbumin fusion; negative control). Hydrolysis of CCF4 substrate by translocated β-lactamase was monitored by live cell imaging. Excitation at 409 nm resulted in green fluorescence emission (520 nm) of the intact substrate and in blue fluorescence emission (447 nm) of the cleaved hydrolysis product. Depicted merged images (green + blue fluorescence) were taken from representative movies at the indicated time points (see also supplemental Movie S1). GST-CNF-Y-treated cells show more rapid accumulation of the hydrolysis product. C, live cell data were quantitatively analyzed by calculation of the fluorescence index Q = (Pcells − Pbackground)/(Scells − Sbackground) to correct for background fluorescence and loss of CCF-4 substrate from the cells. Graphs represent the mean ± S.D. (error bars) of three independent experiments, each condition sampled in triplicate. D, HeLa cells were infected with WA-314 or WA-314ΔE for 1 h. Infection was then terminated by adding gentamicin (50 μg/ml) and chloramphenicol (20 μg/ml). After 30 min of incubation, GST or GST-CNF-Y was added for 4 h. Digitonin lysates were prepared directly after infection (1) and after GST (2) or GST-CNF-Y (3) treatment. Supernatants were analyzed for YopH and actin by Western blot. Data are representative of three independent experiments.
To verify our results with an independent method, cells were loaded with CCF4, a compound in which the fluorescent dyes coumarin and fluorescein are linked to a cephalosporin core. Excitation of intact CCF4 (409 nm) in cells results in green fluorescence emission (520 nm) due to FRET from coumarin to fluorescein. Hydrolytic cleavage of CCF4 by β-lactamase leads to disruption of FRET, resulting in increased blue fluorescence emission from coumarin (447 nm) (54, 55). The CCF4-loaded cells were infected with WA-314-pmk-bla, a Yersinia strain that translocates a YopE-β-lactamase fusion protein capable of cleaving CCF4. Live cell fluorescence recording showed a low but constant rate of FRET disruption, as evidenced by an increase in blue fluorescence (Fig. 1B). In the CNF-Y-pretreated cells, an accelerated rise of blue fluorescence was detected upon infection with WA-314-pmk-bla, reflecting a greatly enhanced translocation of YopE-β-lactamase (Fig. 1, B and C; see also supplemental Movie S1).
We finally aimed at excluding the possibility that the appearance of higher amounts of YopH in CNF-Y-treated cells was due to enhanced stability of the translocated YopH. To this end, we infected HeLa cells with WA-314 or WA-314ΔE (to obtain higher levels of intracellular YopH) for 1 h. Infection was terminated by the addition of gentamicin and chloramphenicol for 30 min, and only thereafter was GST or GST-CNF-Y added for 4 h. Western blots of cell lysates confirmed that after the addition of antibiotics, no further YopH translocation occurred and clearly demonstrated that the cellular YopH levels were not affected by CNF-Y treatment (Fig. 1D). This indicates that CNF-Y has no effect on the stability of translocated YopH. Taken together, these results clearly demonstrate that treatment of Yersinia-infected host cells with cytotoxic necrotizing factor-Y results in a large increase in Yop translocation.
Active Rac and Cdc42 Stimulate Yersinia Effector Translocation
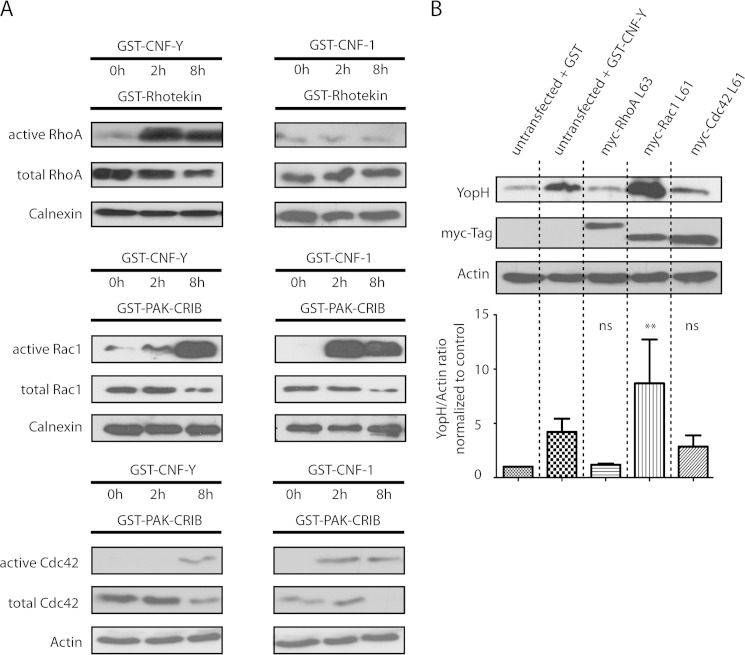
To get an idea which Rho GTP-binding proteins mediate the enhanced Yersinia effector translocation upon CNF-Y treatment of cells, we first performed activity pulldown assays of RhoA, Rac1, and Cdc42. CNF-Y strongly activated RhoA after 2 h, but also Rac1 activity was clearly enhanced at this time point, whereas no Cdc42 activation could be detected (Fig. 2A, see also supplemental Fig. S2 for a tighter time course of Rac activation). After 8 h of CNF-Y treatment, Rac1 was strongly activated and Cdc42 was modestly activated, and their activation levels were very similar to the levels obtained by 2 h of CNF-1 treatment (Fig. 2A). Surprisingly, we could not detect activation of RhoA by CNF-1 under these conditions (Fig. 2A). Thus, besides RhoA, CNF-Y activates Rac1 and Cdc42 in HeLa cells, albeit in a delayed manner when compared with CNF-1.
FIGURE 2.
Profiles and kinetics of RhoA, Rac1, and Cdc42 activation by CNF-Y and CNF-1 and effect of constitutively active RhoA, Rac1, and Cdc42 constructs on Yop translocation. A, HeLa cells were treated with GST-CNF-Y or GST-CNF-1 as indicated. Active RhoA, Rac1, or Cdc42 was precipitated with GST-Rhotekin (RhoA) or GST-PAK-CRIB (Rac1, Cdc42) and analyzed by Western blotting. The input represents ∼4% of the cell lysate used for the respective pulldown at the indicated time point. Immunoblots of pulldown assays and inputs were investigated with the same antibodies. Data are representative of three independent experiments. B, HeLa cells were transfected with the constitutively active constructs Myc-RhoAL63, Myc-Rac1L61, or Myc-Cdc42L61. After 16 h, cells were infected with WA-314 for 1 h, digitonin-lysed, and analyzed by Western blotting. Bars represent the mean ± S.D. (error bars) of YopH/actin ratio normalized to control (GST-treated cells) of three independent experiments. Asterisks indicate significant differences when compared with control (one-way analysis of variance with Dunnett's test; *, p = 0.01–0.05 and **, p = 0.001–0.01). ns, not significant.
Next, the Rho GTP-binding proteins found to be activated by CNF-Y were individually expressed as constitutively active mutants in cells, and the effects on Yop translocation were evaluated. These experiments revealed that expression of constitutively active Rac1 (Myc-Rac1L61) strongly increased YopH translocation, even exceeding the effect of CNF-Y treatment (Fig. 2B). Again increased bacterial binding was excluded as reason for the enhanced translocation because equal numbers of cell-associated bacteria were found for untreated and RacL61-expressing cells (supplemental Fig. S1B). Constitutively active Cdc42 (Myc-Cdc42L61) was found to reproducibly induce a moderate increase in YopH translocation, whereas no effect was observed for constitutively active RhoA (Myc-RhoAL63) (Fig. 2B). Thus, these results indicate that CNF-Y effects on Yop translocation are mostly mediated by Rac.
siRNA Knockdown and Knock-out Cell Lines Confirm Relevance of Rac and Cdc42 for Yop Translocation
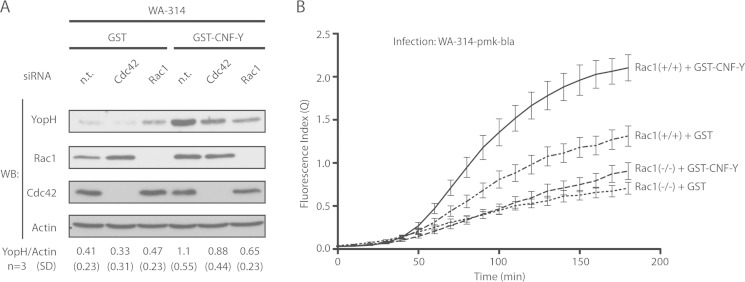
To further verify whether Rac1 and/or Cdc42 regulate Yop translocation, we made use of (i) siRNA knockdown of Rac1 and Cdc42 in HeLa cells and (ii) Rac1 knock-out MEFs. CNF-Y-stimulated translocation of YopH into HeLa cells was reduced by ∼45% when Rac1 was knocked down and by ∼28% when Cdc42 was knocked down in HeLa cells (Fig. 3A). Rac1 and Cdc42 knockdown appeared not to have a significant effect on YopH translocation in GST-treated control cells, but definitive conclusions were difficult because under these assay conditions, translocation efficiency was near the detection limit (Fig. 3A). Next, we employed the real-time YopE-β-lactamase translocation assay described above using mouse embryonic fibroblasts genetically deleted for Rac1 (Rac1−/−) and their parental controls (Rac1fl/fl) (Fig. 3B). Confirming the digitonin lysis assay, CNF-Y-treated Rac1−/− MEFs displayed a pronounced reduction in YopE-β-lactamase translocation when compared with CNF-Y-treated control MEFs (Rac1fl/fl). In addition, this assay revealed that under control conditions (non-CNF-Y-treated), Rac1−/− MEFs showed a markedly reduced Yop translocation rate when compared with control MEFs (Fig. 3B).
FIGURE 3.
Knockdown or knock-out of Rac1 blocks CNF-Y stimulated Yop translocation. A, HeLa cells were transfected with nontargeting (n. t.), Rac1, or Cdc42 siRNA for 72 h and then treated with GST or GST-CNF-Y for 2 h. Subsequently, cells were infected with WA-314 for 1 h and digitonin-lysed, and translocated YopH was analyzed by Western blotting (WB). Mean YopH/actin ratio and S.D. of three independent experiments are stated. B, Rac1−/− and Rac1fl/fl control MEFs were treated for 2 h with GST or GST-CNF-Y and subsequently infected with WA-314-pmk-bla for 3 h. Live cell imaging data were analyzed and quantified as described above. Graphs represent the mean ± S.D. (error bars) of three independent experiments, each condition sampled in triplicate.
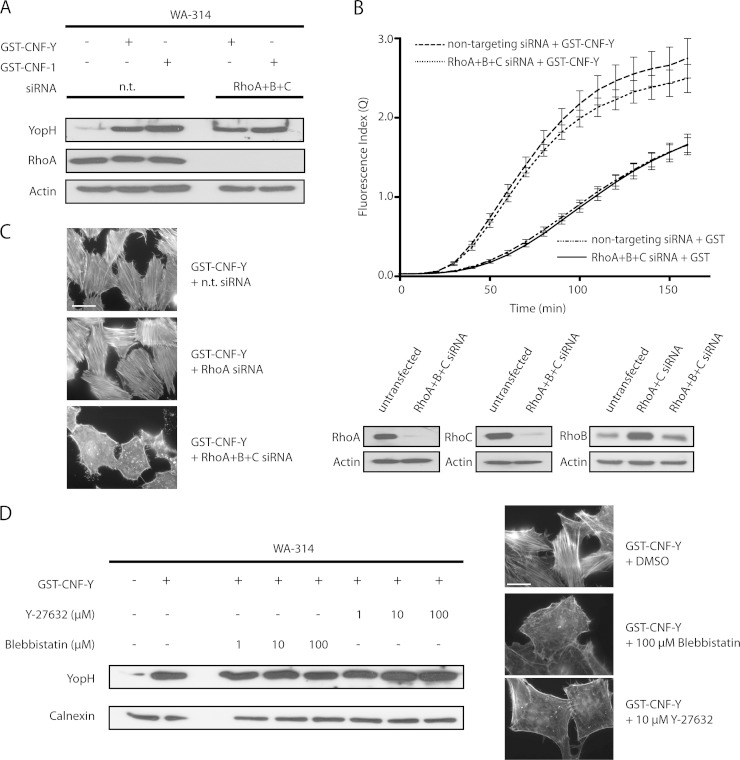
Because we have confirmed that CNF-Y activates RhoA more rapidly than Rac1 and Cdc42, and Rho inhibitors were previously reported to inhibit Yop translocation, we sought to unambiguously establish whether RhoA, -B, or -C is involved in the CNF-Y effect or in Yop translocation by Y. enterocolitica in general (42). To this end, specific siRNAs were employed to knockdown RhoA, -B, and -C individually and in different combinations. RhoA, -B, and -C knockdown was verified by Western blotting (Fig. 4C). In addition, we took advantage of the fact that stress fiber formation is a sensitive and specific indicator of RhoA, -B, and -C activity (56). Interestingly, neither treatment with single siRNAs nor any of the three possible combinations of two siRNAs against RhoA, -B, or -C could prevent CNF-Y-induced stress fiber formation (Fig. 4C and data not shown). Only treatment of cells with a pool of siRNAs against all three Rho isoforms resulted in complete inhibition of stress fiber formation (Fig. 4C). As expected, the siRNA pool strongly reduced the levels of all three Rho isoforms. Interestingly, the endogenously very low RhoB levels were up-regulated by the combination of RhoA and -C siRNAs, explaining their ineffectiveness on stress fiber formation (Fig. 4C). Under these conditions, with no detectable RhoA, -B, or -C activity left, we performed Yop translocation assays. Neither of the two translocation assays allowed us to detect an effect of RhoA, -B, and -C triple knockdown on Yop translocation in CNF-Y-treated or -untreated HeLa cells (Fig. 4, A and B). Furthermore, Y-27632 and blebbistatin, which inhibit the major Rho effectors Rho kinase and myosin II, respectively, did not affect CNF-Y-stimulated YopH translocation, although these inhibitors again had a major impact on stress fiber formation (Fig. 4D). These results strongly suggest that RhoA, -B, and -C are not involved in Y. enterocolitica effector translocation in CNF-Y-treated or -untreated cells. Collectively, we conclude that Rac is the major regulator of Yop translocation in Y. enterocolitica-infected host cells and that its activation is mainly responsible for increased Yop translocation induced by CNF-Y.
FIGURE 4.
RhoA, -B, and -C and downstream targets are dispensable for Y. enterocolitica effector translocation under basal and CNF-stimulated conditions. A, HeLa cells were treated with nontargeting (n. t.) siRNA or with a siRNA pool for 48 h to simultaneously deplete RhoA, -B, and -C, stimulated with GST-CNF-Y or GST-CNF-1 for 2 h, and infected with WA-314 for 1 h. Cells were lysed with digitonin, and supernatants were analyzed for YopH, RhoA, and actin by Western blotting. Data are representative of three independent experiments. B, HeLa cells were treated as above, preloaded with CCF4-AM, and infected with WA-314-pMK-bla for 3 h. Live cell imaging data were analyzed and quantified as described above. Graphs represent the mean ± S.D. (error bars) of three independent experiments, each condition sampled in triplicate. C, left panel, Alexa Fluor 568 phalloidin staining was conducted of nontargeting, RhoA, or RhoA, -B, and -C siRNA-treated HeLa cells stimulated with CNF-Y for 2 h. Abrogation of stress fiber formation was only seen in the RhoA, -B, and -C siRNA-treated cells, verifying the efficient knockdown of RhoA, -B, and -C. Right panel, siRNA knockdown of RhoA, -B, and -C was also confirmed by Western blot. RhoB expression was very low under control conditions, but was found to be up-regulated when RhoA and -C were depleted. In this case, up-regulation was efficiently counteracted by RhoB siRNA. D, HeLa cells were treated with GST or GST-CNF-Y and simultaneously subjected to different concentrations of small inhibitors blebbistatin and Y-27632 or dimethyl sulfoxide (DMSO) alone for 2 h. Evaluation of stress fiber formation was conducted by Alexa Fluor 568 phalloidin staining to determine effectiveness of inhibitors. After infection with WA-314 for 1 h, cells were lysed with digitonin, and supernatants were analyzed for YopH and actin by Western blotting. Data are representative of three independent experiments.
Yops Inhibiting Rho GTP-binding Proteins Cannot Block the CNF-Y Effect on Translocation
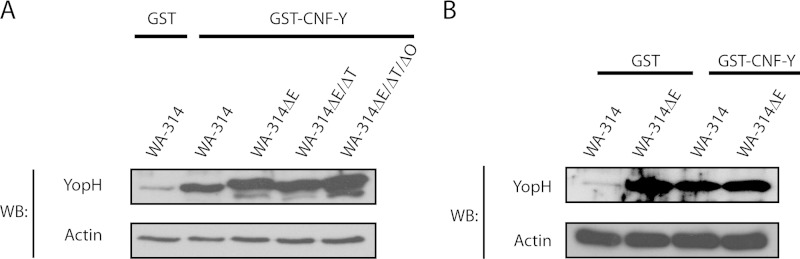
Both YopE and, albeit less well documented, YopT and YopO/YpkA were proposed to block Yop translocation by inhibiting Rho family GTP-binding proteins as part of a negative feedback loop (9, 57). We asked therefore whether these Yops can counteract the stimulatory effect of CNF-Y on Yop translocation. We found that the quantities of YopH translocated by the Y. enterocolitica wild type strain and by strains lacking yopE alone, yopE plus yopT, or yopE plus yopT and yopO (WA-314ΔE, WA-314ΔE/ΔT and WA-314ΔE/ΔT/ΔO) in CNF-Y-pretreated cells were very similar (Fig. 5A). This indicates that YopE, YopT, and YopO are essentially incapable of inhibiting the CNF-Y effect.
FIGURE 5.
YopE/T/O cannot counteract the CNF-Y-mediated hypertranslocation. A, HeLa cells were treated with GST or GST-CNF-Y for 2 h and subsequently infected with WA-314 and Yop mutants WA-314ΔE, WA-314ΔE/ΔT, and WA-314ΔE/ΔT/ΔO for 1 h. Digitonin lysates were analyzed for YopH and actin by Western blotting (WB). Data are representative of three independent experiments. B, GST- and GST-CNF-Y-treated HeLa cells were infected with WA-314 and WA-314ΔE for 1 h. Digitonin lysates were analyzed for YopH and actin by Western blotting. Data are representative of three independent experiments.
Finally, it is interesting to note that the levels of YopH translocation in WA-314ΔE-infected control cells and in WA-314-infected CNF-Y-treated cells were observed to be very similar (Fig. 5B). Because it was reported before that Y. enterocolitica YopE very effectively inhibits Rac1 and this determines feedback inhibition of Yop translocation (9, 37), these data underscore that the negative feedback of Yop translocation brought about by YopE is abrogated by CNF-Y. They also support the central conclusion of this work that Rac plays the central role in the boosting effect of CNF-Y on Yop translocation.
DISCUSSION
Considering the dramatic effects that Yersinia effector Yops can have on host cell function and survival, it is comprehensible that Yop translocation must be tightly controlled. Rho GTP-binding proteins appear to constitute one way of control based on findings that inhibition of Rho and Rac in cells can block Yop translocation (1, 42, 44). Here we provide evidence that the Rho GTP-binding protein activator CNF-Y strongly increases Yop translocation. We found that the CNF-Y effect was almost entirely dependent on Rac and independent of RhoA, -B, and -C. This result was unexpected because first, CNF-Y was reported to prefer RhoA as a substrate over Rac and Cdc42, and second, inhibition of Rho had been shown to affect Yop translocation (42, 44). As described by Hoffmann et al. (49) and confirmed in this study, the CNF-Y effect on the actin cytoskeleton is dominated by stress fibers with only a few membrane ruffles and filopodia present, which suggested Rho activation but no prominent Rac and Cdc42 activation, respectively. However, CNF-Y can modify Rac and Cdc42 in vitro, albeit less effectively than Rho (49), and we demonstrate here that CNF-Y also strongly activates Rac and Cdc42 in cells. CNF-Y activated Rac and Cdc42 to the same extent as RhoA, but significantly delayed in time when compared with CNF-1. Presumably, the lower intrinsic activity of CNF-Y toward Rac1 and Cdc42 requires a longer accumulation time of CNF-Y in cells to fully activate these GTP-binding proteins. In fact, under our experimental conditions, cellular CNF-Y levels still increased between 2 h, when RhoA was already fully activated, and 6 h, when Rac was fully activated (data not shown). In contrast to reduced Yop translocation found in Y. pseudotuberculosis-infected cells treated with the Rho inhibitor C3-transferase from Clostridium botulinum (42), we could not detect an effect of siRNA-mediated knockdown of RhoA, -B, or -C or constitutively active RhoA on Yop translocation by Y. enterocolitica. The reason for this discrepancy is unclear, but Yop translocation might be differentially regulated by Rho GTP-binding proteins dependent on the infecting Yersinia strain. It is also interesting to note that most Y. pseudotuberculosis strains lack YopT, which was reported to preferentially inactivate RhoA in cells (58), whereas CNF-Y, which most efficiently activates RhoA, has so far only been detected in Y. pseudotuberculosis strains. Thus, there might be a specific role for RhoA activation in an aspect of pathogenicity of Y. pseudotuberculosis not shared by Y. enterocolitica.
That Rac activity was found here to be the major determinant of Yop translocation in resting and CNF-Y-stimulated cells fits well with studies demonstrating that translocated YopE is the decisive factor in negative feedback control of translocation (9, 11). YopE was found to preferentially inactivate Rac in different Yersinia infection systems (37, 59), and degradation of ubiquitinated YopE in host cells caused both Rac activation and increased Yop translocation (11). Activation of Rac in host cells by secreted CNF toxins thus may represent another mechanism of how yersiniae promote Yop translocation. Furthermore, deamidation of Rac by CNF-1 not only strongly activates Rac but also renders it resistant toward inactivation by GAPs (47, 48). In fact, our study indicates that neither the RhoGAP YopE nor the YopT and YopO, also reported to act on Rac in some infection systems, could counteract the CNF-Y effect on Yop translocation. Interestingly, cells may have devised a way to overcome this potential deadlock because they can ubiquitinate and degrade CNF-modified/hyperactivated Rac (60, 61). A straightforward question for future work is which downstream effectors of Rac are involved in Yop translocation. Considering that actin depolymerization by cytochalasin D or latrunculin B inhibited Yop translocation (42) and conversely, circular actin enrichments termed “actin halos” were detected around hypertranslocating bacteria on host cells, Rac effectors governing actin polymerization may play a role (42, 43). By activating proteins of the WAVE complex, Rac1 triggers formation of lamellipodia and ruffles, which can engulf invading bacteria (62–64). Enhanced ruffling might cause a larger fraction of the Yersinia surface to come into direct contact with the host plasma membrane, leading to a higher number of needle complexes deposited and concomitant increase in translocation. Alternatively, Rac-dependent actin structures might directly interact with specific components of the type III secretion system, such as the translocation pore, and thus stimulate their activity. Future experiments will have to distinguish between these possibilities.
We also found Cdc42 to play a role in Yop translocation. Cdc42 is known to control cell polarity in various cell types ranging from mammalian neurons to yeast by orchestrating the cytoskeleton, secretory membrane trafficking as well as endocytosis (65). It is unclear at present which of these Cdc42 functions contribute to Yop translocation.
Altogether, CNF-Y can considerably enhance Yop translocation by activation of Rac and Cdc42 in a cell culture model. Whether this is relevant for the Yersinia infection strategy in vivo should be verified in future studies.
Supplementary Material
Acknowledgments
We thank Cord Brakebusch (University of Kopenhagen) for reagents and the Microscopy Imaging Facility (UMIF) for excellent technical assistance. We also thank Stefan Linder for fruitful discussions and Christiane Wiesner for figure preparation.
This work was supported in part by a grant from the German Research Council (to K. R.).

This article contains supplemental Movie S1 and Figs. S1 and S2.
Steffen, A., Ladwein, M., Hein, A., Arens, S., Ladwein, K. I., Oelkers, M., Schur, F., Small, J. V., Schwarz, J., Gerhard, R., Faix, J., Stradal, T. E. B., Brakebusch, C., and Rottner, K., J. Cell Sci., in press.
- GAP
- GTPase-activating protein
- CNF
- cytotoxic necrotizing factor
- MEF
- mouse embryonic fibroblast
- PAK
- p21-activated kinase
- CRIB
- Cdc42/Rac interactive binding.
REFERENCES
- 1. Prentice M. B., Rahalison L. (2007) Plague. Lancet 369, 1196–1207 [DOI] [PubMed] [Google Scholar]
- 2. Viboud G. I., Bliska J. B. (2005) Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59, 69–89 [DOI] [PubMed] [Google Scholar]
- 3. Trülzsch K., Oellerich M. F., Heesemann J. (2007) Invasion and dissemination of Yersinia enterocolitica in the mouse infection model. Adv. Exp. Med. Biol. 603, 279–285 [DOI] [PubMed] [Google Scholar]
- 4. Heesemann J., Sing A., Trülzsch K. (2006) Yersinia's stratagem: targeting innate and adaptive immune defense. Curr. Opin. Microbiol. 9, 55–61 [DOI] [PubMed] [Google Scholar]
- 5. Navarro L., Alto N. M., Dixon J. E. (2005) Functions of the Yersinia effector proteins in inhibiting host immune responses. Curr. Opin. Microbiol. 8, 21–27 [DOI] [PubMed] [Google Scholar]
- 6. Cornelis G. R. (2002) The Yersinia Ysc-Yop 'type III' weaponry. Nat. Rev. Mol. Cell Biol. 3, 742–752 [DOI] [PubMed] [Google Scholar]
- 7. Cornelis G. R., Wolf-Watz H. (1997) The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23, 861–867 [DOI] [PubMed] [Google Scholar]
- 8. Pettersson J., Nordfelth R., Dubinina E., Bergman T., Gustafsson M., Magnusson K. E., Wolf-Watz H. (1996) Modulation of virulence factor expression by pathogen target cell contact. Science 273, 1231–1233 [DOI] [PubMed] [Google Scholar]
- 9. Aili M., Isaksson E. L., Carlsson S. E., Wolf-Watz H., Rosqvist R., Francis M. S. (2008) Regulation of Yersinia Yop-effector delivery by translocated YopE. Int. J. Med. Microbiol. 298, 183–192 [DOI] [PubMed] [Google Scholar]
- 10. Dewoody R., Merritt P. M., Houppert A. S., Marketon M. M. (2011) YopK regulates the Yersinia pestis type III secretion system from within host cells. Mol. Microbiol. 79, 1445–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaus K., Hentschke M., Czymmeck N., Novikova L., Trülzsch K., Valentin-Weigand P., Aepfelbacher M., Ruckdeschel K. (2011) Destabilization of YopE by the ubiquitin-proteasome pathway fine-tunes Yop delivery into host cells and facilitates systemic spread of Yersinia enterocolitica in host lymphoid tissue. Infection Immunity 79, 1166–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aepfelbacher M., Trasak C., Ruckdeschel K. (2007) Effector functions of pathogenic Yersinia species. Thromb. Haemost. 98, 521–529 [PubMed] [Google Scholar]
- 13. Black D. S., Bliska J. B. (1997) Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 16, 2730–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Persson C., Carballeira N., Wolf-Watz H., Fällman M. (1997) The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16, 2307–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alonso A., Bottini N., Bruckner S., Rahmouni S., Williams S., Schoenberger S. P., Mustelin T. (2004) Lck dephosphorylation at Tyr-394 and inhibition of T cell antigen receptor signaling by Yersinia phosphatase YopH. J. Biol. Chem. 279, 4922–4928 [DOI] [PubMed] [Google Scholar]
- 16. Gerke C., Falkow S., Chien Y. H. (2005) The adaptor molecules LAT and SLP-76 are specifically targeted by Yersinia to inhibit T cell activation. J. Exp. Med. 201, 361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yao T., Mecsas J., Healy J. I., Falkow S., Chien Y. (1999) Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J. Exp. Med. 190, 1343–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mukherjee S., Keitany G., Li Y., Wang Y., Ball H. L., Goldsmith E. J., Orth K. (2006) Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312, 1211–1214 [DOI] [PubMed] [Google Scholar]
- 19. Mukherjee S., Orth K. (2008) In vitro signaling by MAPK and NFκB pathways inhibited by Yersinia YopJ. Methods Enzymol. 438, 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mittal R., Peak-Chew S. Y., McMahon H. T. (2006) Acetylation of MEK2 and IκB kinase (IKK) activation loop residues by YopJ inhibits signaling. Proc. Natl. Acad. Sci. U.S.A. 103, 18574–18579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hentschke M., Berneking L., Belmar Campos C., Buck F., Ruckdeschel K., Aepfelbacher M. (2010) Yersinia virulence factor YopM induces sustained RSK activation by interfering with dephosphorylation. PLoS One 5, e13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McPhee J. B., Mena P., Bliska J. B. (2010) Delineation of regions of the Yersinia YopM protein required for interaction with the RSK1 and PRK2 host kinases and their requirement for interleukin-10 production and virulence. Infection Immunity 78, 3529–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCoy M. W., Marré M. L., Lesser C. F., Mecsas J. (2010) The C-terminal tail of Yersinia pseudotuberculosis YopM is critical for interacting with RSK1 and for virulence. Infection Immunity 78, 2584–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDonald C., Vacratsis P. O., Bliska J. B., Dixon J. E. (2003) The Yersinia virulence factor YopM forms a novel protein complex with two cellular kinases. J. Biol. Chem. 278, 18514–18523 [DOI] [PubMed] [Google Scholar]
- 25. Evdokimov A. G., Anderson D. E., Routzahn K. M., Waugh D. S. (2001) Unusual molecular architecture of the Yersinia pestis cytotoxin YopM: a leucine-rich repeat protein with the shortest repeating unit. J. Mol. Biol. 312, 807–821 [DOI] [PubMed] [Google Scholar]
- 26. Peters K. N., Anderson D. M. (2012) Modulation of host cell death pathways by Yersinia species and the type III effector YopK. Adv. Exp. Med. Biol. 954, 229–236 [DOI] [PubMed] [Google Scholar]
- 27. Holmström A., Petterson J., Rosqvist R., Håkansson S., Tafazoli F., Fällman M., Magnusson K. E., Wolf-Watz H., Forsberg A. (1997) YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol. Microbiol. 24, 73–91 [DOI] [PubMed] [Google Scholar]
- 28. Holmström A., Rosqvist R., Wolf-Watz H., Forsberg A. (1995) YopK, a novel virulence determinant of Yersinia pseudotuberculosis. Contrib. Microbiol. Immunol. 13, 239–243 [PubMed] [Google Scholar]
- 29. Brodsky I. E., Palm N. W., Sadanand S., Ryndak M. B., Sutterwala F. S., Flavell R. A., Bliska J. B., Medzhitov R. (2010) A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe. 7, 376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jaffe A. B., Hall A. (2005) Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 [DOI] [PubMed] [Google Scholar]
- 31. Heasman S. J., Ridley A. J. (2008) Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 9, 690–701 [DOI] [PubMed] [Google Scholar]
- 32. Prehna G., Ivanov M. I., Bliska J. B., Stebbins C. E. (2006) Yersinia virulence depends on mimicry of host Rho-family nucleotide dissociation inhibitors. Cell 126, 869–880 [DOI] [PubMed] [Google Scholar]
- 33. Juris S. J., Rudolph A. E., Huddler D., Orth K., Dixon J. E. (2000) A distinctive role for the Yersinia protein kinase: actin binding, kinase activation, and cytoskeleton disruption. Proc. Natl. Acad. Sci. U.S.A. 97, 9431–9436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trasak C., Zenner G., Vogel A., Yüksekdag G., Rost R., Haase I., Fischer M., Israel L., Imhof A., Linder S., Schleicher M., Aepfelbacher M. (2007) Yersinia protein kinase YopO is activated by a novel G-actin binding process. J. Biol. Chem. 282, 2268–2277 [DOI] [PubMed] [Google Scholar]
- 35. Groves E., Rittinger K., Amstutz M., Berry S., Holden D. W., Cornelis G. R., Caron E. (2010) Sequestering of Rac by the Yersinia effector YopO blocks Fcγ receptor-mediated phagocytosis. J. Biol. Chem. 285, 4087–4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aepfelbacher M., Roppenser B., Hentschke M., Ruckdeschel K. (2011) Activity modulation of the bacterial Rho GAP YopE: an inspiration for the investigation of mammalian Rho GAPs. Eur. J. Cell Biol. 90, 951–954 [DOI] [PubMed] [Google Scholar]
- 37. Aili M., Isaksson E. L., Hallberg B., Wolf-Watz H., Rosqvist R. (2006) Functional analysis of the YopE GTPase-activating protein (GAP) activity of Yersinia pseudotuberculosis. Cell. Microbiol. 8, 1020–1033 [DOI] [PubMed] [Google Scholar]
- 38. Schmidt G. (2011) Yersinia enterocolitica outer protein T (YopT). Eur. J. Cell Biol. 90, 955–958 [DOI] [PubMed] [Google Scholar]
- 39. Schotte P., Denecker G., Van Den Broeke A., Vandenabeele P., Cornelis G. R., Beyaert R. (2004) Targeting Rac1 by the Yersinia effector protein YopE inhibits caspase-1-mediated maturation and release of interleukin-1β. J. Biol. Chem. 279, 25134–25142 [DOI] [PubMed] [Google Scholar]
- 40. Viboud G. I., Mejía E., Bliska J. B. (2006) Comparison of YopE and YopT activities in counteracting host signalling responses to Yersinia pseudotuberculosis infection. Cell. Microbiol. 8, 1504–1515 [DOI] [PubMed] [Google Scholar]
- 41. Aktories K. (2011) Bacterial protein toxins that modify host regulatory GTPases. Nat. Rev. Microbiol. 9, 487–498 [DOI] [PubMed] [Google Scholar]
- 42. Mejía E., Bliska J. B., Viboud G. I. (2008) Yersinia controls type III effector delivery into host cells by modulating Rho activity. PLoS Pathog. 4, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Viboud G. I., Bliska J. B. (2001) A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. EMBO J. 20, 5373–5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Köberle M., Klein-Günther A., Schütz M., Fritz M., Berchtold S., Tolosa E., Autenrieth I. B., Bohn E. (2009) Yersinia enterocolitica targets cells of the innate and adaptive immune system by injection of Yops in a mouse infection model. PLoS Pathog. 5, e1000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aktories K., Schmidt G. (2003) A new turn in Rho GTPase activation by Escherichia coli cytotoxic necrotizing factors. Trends Microbiol. 11, 152–155 [DOI] [PubMed] [Google Scholar]
- 46. Lockman H. A., Gillespie R. A., Baker B. D., Shakhnovich E. (2002) Yersinia pseudotuberculosis produces a cytotoxic necrotizing factor. Infection Immunity 70, 2708–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Flatau G., Lemichez E., Gauthier M., Chardin P., Paris S., Fiorentini C., Boquet P. (1997) Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387, 729–733 [DOI] [PubMed] [Google Scholar]
- 48. Schmidt G., Sehr P., Wilm M., Selzer J., Mann M., Aktories K. (1997) Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387, 725–729 [DOI] [PubMed] [Google Scholar]
- 49. Hoffmann C., Pop M., Leemhuis J., Schirmer J., Aktories K., Schmidt G. (2004) The Yersinia pseudotuberculosis cytotoxic necrotizing factor (CNFY) selectively activates RhoA. J. Biol. Chem. 279, 16026–16032 [DOI] [PubMed] [Google Scholar]
- 50. Trülzsch K., Sporleder T., Igwe E. I., Rüssmann H., Heesemann J. (2004) Contribution of the major secreted yops of Yersinia enterocolitica O:8 to pathogenicity in the mouse infection model. Infection Immunity 72, 5227–5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Essler M., Linder S., Schell B., Hüfner K., Wiedemann A., Randhahn K., Staddon J. M., Aepfelbacher M. (2003) Cytotoxic necrotizing factor 1 of Escherichia coli stimulates Rho/Rho-kinase-dependent myosin light-chain phosphorylation without inactivating myosin light-chain phosphatase in endothelial cells. Infection Immunity 71, 5188–5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chrostek A., Wu X., Quondamatteo F., Hu R., Sanecka A., Niemann C., Langbein L., Haase I., Brakebusch C. (2006) Rac1 is crucial for hair follicle integrity but is not essential for maintenance of the epidermis. Mol. Cell. Biol. 26, 6957–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nordfelth R., Wolf-Watz H. (2001) YopB of Yersinia enterocolitica is essential for YopE translocation. Infection Immunity 69, 3516–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Charpentier X., Oswald E. (2004) Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 β-lactamase as a new fluorescence-based reporter. J. Bacteriol. 186, 5486–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mills E., Baruch K., Charpentier X., Kobi S., Rosenshine I. (2008) Real-time analysis of effector translocation by the type III secretion system of enteropathogenic Escherichia coli. Cell Host Microbe 3, 104–113 [DOI] [PubMed] [Google Scholar]
- 56. Melendez J., Stengel K., Zhou X., Chauhan B. K., Debidda M., Andreassen P., Lang R. A., Zheng Y. (2011) RhoA GTPase is dispensable for actomyosin regulation but is essential for mitosis in primary mouse embryonic fibroblasts. J. Biol. Chem. 286, 15132–15137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wong K. W., Isberg R. R. (2005) Yersinia pseudotuberculosis spatially controls activation and misregulation of host cell Rac1. PLoS Pathog. 1, e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aepfelbacher M., Trasak C., Wilharm G., Wiedemann A., Trülzsch K., Krauss K., Gierschik P., Heesemann J. (2003) Characterization of YopT effects on Rho GTPases in Yersinia enterocolitica-infected cells. J. Biol. Chem. 278, 33217–33223 [DOI] [PubMed] [Google Scholar]
- 59. Andor A., Trülzsch K., Essler M., Roggenkamp A., Wiedemann A., Heesemann J., Aepfelbacher M. (2001) YopE of Yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells. Cell. Microbiol. 3, 301–310 [DOI] [PubMed] [Google Scholar]
- 60. Doye A., Boyer L., Mettouchi A., Lemichez E. (2006) Ubiquitin-mediated proteasomal degradation of Rho proteins by the CNF1 toxin. Methods Enzymol. 406, 447–456 [DOI] [PubMed] [Google Scholar]
- 61. Lerm M., Pop M., Fritz G., Aktories K., Schmidt G. (2002) Proteasomal degradation of cytotoxic necrotizing factor 1-activated Rac. Infection Immunity 70, 4053–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Humphreys D., Davidson A., Hume P. J., Koronakis V. (2012) Salmonella virulence effector SopE and Host GEF ARNO cooperate to recruit and activate WAVE to trigger bacterial invasion. Cell Host Microbe 11, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Koronakis V., Hume P. J., Humphreys D., Liu T., Hørning O., Jensen O. N., McGhie E. J. (2011) WAVE regulatory complex activation by cooperating GTPases Arf and Rac1. Proc. Natl. Acad. Sci. U.S.A. 108, 14449–14454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Steffen A., Rottner K., Ehinger J., Innocenti M., Scita G., Wehland J., Stradal T. E. (2004) Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. EMBO J. 23, 749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Harris K. P., Tepass U. (2010) Cdc42 and vesicle trafficking in polarized cells. Traffic 11, 1272–1279 [DOI] [PubMed] [Google Scholar]
- 66. Heesemann J., Laufs R. (1983) Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J. Bacteriol. 155, 761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zumbihl R., Aepfelbacher M., Andor A., Jacobi C. A., Ruckdeschel K., Rouot B., Heesemann J. (1999) The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J. Biol. Chem. 274, 29289–29293 [DOI] [PubMed] [Google Scholar]
- 68. Bölin I., Norlander L., Wolf-Watz H. (1982) Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infection Immunity 37, 506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gemski P., Lazere J. R., Casey T., Wohlhieter J. A. (1980) Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infection Immunity 28, 1044–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.