Abstract
Angiosarcoma (AS) is a rare neoplasm of endothelial origin that has limited treatment options and poor five-year survival. As a model for human AS, we studied primary cells and tumorgrafts derived from canine hemangiosarcoma (HSA), which is also an endothelial malignancy with similar presentation and histology. Primary cells isolated from HSA showed constitutive ERK activation. The MEK inhibitor CI-1040 reduced ERK activation and the viability of primary cells derived from visceral, cutaneous, and cardiac HSA in vitro. HSA-derived primary cells were also sensitive to sorafenib, an inhibitor of B-Raf and multi-receptor tyrosine kinases. In vivo, CI-1040 or PD0325901 decreased the growth of cutaneous cell-derived xenografts and cardiac-derived tumorgrafts. Sorafenib decreased tumor size in both in vivo models, although cardiac tumorgrafts were more sensitive. In human AS, we noted that 50% of tumors stained positively for phosphorylated ERK1/2 and that the expression of several MEK-responsive transcription factors was up-regulated. Our data showed that MEK signaling is essential for the growth of HSA in vitro and in vivo and provided evidence that the same pathways are activated in human AS. This indicates that MEK inhibitors may form part of an effective therapeutic strategy for the treatment of canine HSA or human AS, and it highlights the utility of spontaneous canine cancers as a model of human disease.
Keywords: Hemangiosarcoma, Angiosarcoma, MEK, ERK, CI-1040
Introduction
Angiosarcoma (AS) is a malignancy of endothelial origin (1). AS is rare in humans, making up 1–2% of soft-tissue sarcomas (2) and having an estimated incidence of 0.2/100,000 persons per year. Current treatment options are limited to surgery followed by radiation and/or chemotherapy. Despite such treatment, the five-year survival rate is 31% (3). Understanding of the biological pathways driving this disease is crucial for developing novel therapies. To date, angiosarcoma has no clearly defined causative mutation, although activating mutations in key signaling molecules have been reported in AS subtypes. For example, K-Ras2 mutations have been detected in 9 of 24 sporadic hepatic angiosarcomas (4) and also in hepatic AS after occupational exposure to vinyl chloride (5, 6). Ten percent of AS tumors showed activating mutations in KDR, the kinase insert domain receptor (also known as VEGFR2) (7). Also, 25% of a 20-patient study of radiation-induced AS showed a co-amplification of Fms-related tyrosine kinase 4 (Flt4, VEGFR3) and MYC (8). Finally, mutations in phosphatase and tensin homolog (9) and phosphoinositide 3 kinase (10) have been associated with AS.
The rarity of angiosarcoma has restricted the scope of basic and clinical research on this disease. Consequently, attention has turned to experimental models to generate further insight into its biology and treatment, including the study of more common (but related) human tumors, genetically engineered or chemically induced mouse models, and naturally occurring hemangiosarcoma (HSA) in companion dogs (reviewed by (11)). Dogs have a much higher incidence of HSA and a more rapid time course of disease progression than AS in humans. The canine model also offers some unique, advantageous features that distinguish it from other animal models and that open novel experimental opportunities. First, because of selective breeding, genetic variation within canine breeds is very low. Second, because each breed is derived from a small group of founders (most tracing back approximately 150 years), many of the genes associated with polygenic traits are fixed, so that only a few variable genes determine phenotype. This means it will be much easier to identify genetic or biochemical determinants of disease in dogs than in humans. Finally, companion dogs share the same environmental exposures as humans and thus may more accurately reflect the human condition. The ability to identify, recruit, and study cancers within a breed of dog offers new avenues of hope for research in clinical oncology and into the underlying causes of AS (12, 13). To gain insight into the biology of AS, we began a study of naturally occurring canine model of AS, which in dogs is commonly referred to as hemangiosarcoma (HSA).
By analogy to Kaposi sarcoma—another human endothelial malignancy resembling AS, in which constitutive activation of viral G-protein-coupled receptor drives mitogen-activated protein kinase kinase (MEK) activity (14, 15)—we hypothesized that HSA growth and survival was dependent on MEK signaling. To test this concept, we treated tumor-derived canine primary cell isolates and xenograft tumors with agents targeting the Raf-MEK pathway. We found that MEK signaling played an important role in growth and survival of HSA. Our study indicated that MEK inhibitors may form part of an effective strategy for treatment of canine HSA or human AS, and it highlighted the utility of spontaneous canine cancers as a model for human disease.
Methods and Materials
Patient samples
Thirty de-identified, formalin-fixed or optimal cutting temperature medium (OCT) -frozen AS tumor samples were obtained from external sources, including the University of Michigan, the Cooperative Human Tissue Network, and the Ontario Tumor Bank, which is funded by the Ontario Institute for Cancer Research. AS tumor pathology was independently reviewed by pathologists at the Van Andel Research Institute (VARI).
Canine samples
Canine HSA tumor samples were collected from veterinary clinics following a protocol approved by the VARI Institutional Animal Care and Use Committee. Samples were shipped to VARI via overnight courier in ice-chilled phosphate-buffered saline. Upon receipt of a sample, one portion was fixed in formalin, a second was snap-frozen in OCT medium, and the remainder was processed for cell culture as described below. Tumor pathology was reviewed independently by a pathologist specializing in canine oncology.
Cell culture
Tumors from canine HSA of the visceral, cutaneous, and cardiac subtypes were cut into 1-mm3 pieces with a scalpel. The tissue was transferred into an autoclaved flask containing 0.012 g of collagenase (Sigma-Aldrich, St. Louis, MO) in 25 ml of Dulbecco’s Modified Eagle’s Medium (DMEM; Life Technologies, Grand Island, NY) containing 10% heat-inactivated fetal bovine serum (FBS; Life Technologies) and 1% penicillin/streptomycin (pen/strep; Life Technologies). The tissue was incubated overnight at 37 °C with shaking at 85 rpm. Tissue was dissociated by trituration for 3 min using a 25-ml pipet and then centrifuged at 2000 rpm in an Eppendorf 5810 centrifuge using a A-4-62 rotor (Eppendorf, Hamburg, Germany) at room temperature for 5 min. The pellet was re-suspended in 20 ml of medium, triturated for 3 min, and then centrifuged as before; this was repeated twice. The cells were plated on a T75 tissue-culture flask and allowed to adhere overnight before washing to remove non-adherent debris. Cells were allowed to grow, and they then were collected and frozen at the initial and first passages (designated P0 and P1) for long-term storage in a liquid-nitrogen cryovessel.
For serum starvation, cells were grown to 80% confluence. They were then washed twice in PBS and incubated overnight (16 h) in filter-sterilized serum starvation medium (DMEM containing 1% BSA and 1% pen/strep).
Canine endothelial cells were isolated from a nerve sheath tumor (DNSTECs) by separating endothelial cells from tumor and stromal tissues using fluorescence-activated cell sorting (FACS). Canine endothelial cells were gated for CD31(+)/CD45(−)/alphaVbeta3(+) cells using a FACS Aria High Speed Cell Sorter (BD, San Jose, CA).
Madin Darby canine kidney (MDCK) cells (CCL-34) were obtained from the American Tissue Culture Collection (Manassas, VA). Upon receipt these cells were expanded and frozen in multiple aliquots for subsequent use. Upon thawing, MDCK were grown in DMEM containing 10% heat-inactivated FBS and 1% pen/strep. Cells were passaged every 2–3 days. As a rule, cells were passaged no more than 9 times before a new aliquot was thawed for use. In some experiments, MDCK cells were washed with PBS and exposed to ultraviolet radiation using a UV Stratalinker 1800 (Stratagene, La Jolla, CA) for 1 min to activate ERK. Growth medium was added back and cells were allowed to recover for 1 h, after which they were lysed.
Cell Viability Assays
Primary cell isolates were grown in DMEM containing 10% heat-inactivated FBS, 1% pen/strep, and 0.1 mg/ml of endothelial growth supplement (Scientific, Fairlawn, NJ). Cell viability assays were performed with cells between the first and fifth passages (P1 to P5). All assays were performed in triplicate and each assay was independently replicated at least three times. Cells were seeded into 96-well tissue-culture plates in 100 μl of culture medium per well. Treatments began when cells reached 30% confluence. The medium was aspirated off, and 100 μl of fresh medium containing drug was added. Control wells received medium containing the appropriate vehicle. Cells were treated for 72 h with CI-1040 (US Biologics, West Bend, WI), sorafenib-tosylate (LC Laboratory, Woburn, MA), or LY294002 (LC Laboratory) (16–18). Cell viability was determined using the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay (Promega, Madison. WI) according to the manufacturer’s instructions. These assays were measured using a Benchmark Plus microplate spectrophotometer (BIO-RAD, Hercules, CA) at 490 nm and 700 nm reference wavelengths and were normalized against cells treated with medium plus vehicle only. The concentration of compound required to cause 50% inhibition of cell viability (IC50) was calculated by linear regression.
Immunoblotting
Total cell lysates were collected in RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2 mM Na3VO4, 20 mM Na-pyrophosphate, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS, with Complete EDTA-free Protease Tablets [Roche Corp., Palo Alto, CA]) and sonicated three times using a Misonix Sonicator 3000 (Farmingdale, NY). Protein concentrations were determined using the BCA Protein Assay kit (Pierce, Rockford, IL). Cellular lysates were resolved by Novex Pre-Cast Tris-glycine gels (Life Technologies) and then transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, CA). Membranes were blocked with 10% non-fat milk and then incubated with antibodies against phospho-ERK (Thr202/Tyr204) (E10; Cell Signaling, Danvers, MA), ERK (Cell Signaling), alpha-tubulin (Sigma-Aldrich), and phospho-S6 (S235/236) (2F9; Cell Signaling). The membranes were washed three times with TBST (50 mM Tris, 150 mM NaCl, and 0.1% Tween-20) and then incubated with the appropriate HRP-conjugated secondary antibodies (KPL, Gaithersburg, MD) overnight at 4 °C. Washed blots were incubated with Super Signal West Pico Chemiluminescent Substrate (Fisher Scientific) and were exposed to Hyblot CL film (Denville Scientific, South Plainfield, NJ). The film was processed in an X-OMAT 2000A Processor (Kodak, Rochester, NY).
Xenograft and Tumorgraft Experiments
Mice were bred and maintained according to established guidelines and a protocol approved by Van Andel Research Institute’s Institutional Animal Care and Use Committee.
To generate xenografts from canine cutaneous HSA, a primary cell isolate (VCT115) growing in culture was trypsinized and suspended in PBS. One million cells in a volume of 100 μl were injected into five athymic nude mice via intraperitoneal (IP) or subcutaneous routes. Alternatively, canine cardiac HSA tissue from a recently euthanized dog (< 2 h) was cut into 1 mm3 pieces and a piece was implanted subcutaneously into the flank of five athymic nude mice. Mice were monitored 3 times a week by measuring for weight and tumor volume. Tumor volume was calculated from tumor width, height, and depth measured using electronic calipers. Once tumors reach 1000 mm3, the mice were sacrificed, tumors were removed, and pieces (2 mm3) of the explanted tumor were used to propagate the tumor following drug studies.
For the cutaneous HSA CI-1040 study, cutaneous xenografts were implanted into 21 athymic nudes (7 animals for each treatment arm: CI-1040, vehicle, and non-treatment). Treatments began the day after implantation. Mice were treated twice daily by oral gavage of 48 mg/kg of CI-1040 in 10% Cremaphore EL (Sigma-Aldrich), 10% ethanol, and 80% water (19). On treatment day 28 the drug amount was dropped by 20% to 38.4 mg/kg for the duration of the study because of weight loss observed at initial doses. Mice were monitored daily, and their weight and tumor volume were measured 3 times a week. For the cardiac HSA CI-1040 study, we followed a similar procedure except that the full course of treatment was at 38.4 mg/kg. For the cardiac HSA PD0325901 study, PD0325901 (16) treatments began when tumors grew to >50 mm3 by daily oral gavage at 20 mg/Kg (as outlined in (20)).
For the cardiac HSA sorafenib study, cardiac tumorgrafts were implanted into 30 athymic nude mice (10 mice per treatment arm: sorafenib, vehicle, and non-treatment). Treatments began when tumors grew to >50 mm3. Mice were treated once daily by oral gavage of 100 mg/kg sorafenib-tosylate. A 1× dilution with water was prepared daily from a 4× stock of sorafenib in 50:50 of Cremaphore EL:95% ethanol as described in Liu et al. (21). Fresh stock (4X) was made every 3 to 4 days. After 1–2 weeks daily dosing some mice appeared pale and showed evidence of weight loss or bloating. As a consequence, later dosing levels were lowered to 90 mg/kg. Mice were monitored daily, and their weight and tumor volume were measured 3 times a week. At sacrifice, all xenografts and tumorgrafts were harvested, routinely fixed in 10% neutral buffered formalin, and processed. Two-tailed student T-Test determined significance.
Immunohistochemistry
Formalin-fixed, paraffin-embedded (FFPE) tumors were sectioned. Immunostaining was performed on the sections with optimized standard protocols using a Ventana Discovery XL instrument (Ventana Medical Systems, Tucson, AZ) and antibodies against phospho-ERK (Thr202/Tyr204) (20G11; Cell Signaling) and CD31/PECAM-1 (Lab Vision, Kalamazoo, MI). Slides were incubated with HRP-conjugated anti-rabbit IgG secondary antibody (Ventana Medical Systems) and developed with 3–3′-diaminobenzidine (DAB) chromagen substrate. Images were acquired using a Nikon E800 Epifluorescent microscope equipped with a Spot RT3 CCD camera (Diagnostic Instruments, Sterling Heights MI) and Spot Advanced software.
Microarray
RNA was isolated from 18 AS and normal human RNA was isolated from 3 kidney and 2 skeletal muscle OCT frozen sections and 3 samples of frozen whole blood. Twenty-five nanograms of total RNA from each sample was used for amplification, and then was fluorescently labeled using Cy3 and hybridized onto Agilent whole human genome 8×60k gene expression microarrays (Agilent Technologies, Santa Clara, CA) according to Agilent standard procedures. After hybridization for 17 h at 65 °C and 10 rpm, the arrays were washed and scanned with the Agilent G3 high-resolution scanner. Probe features were extracted from the microarray scan data using Feature Extraction software v.10.7.3.1 (Agilent Technologies). Microarray data was read and processed with R/Bioconductor (version 2.15.1/2.16) statistical software environment using the limma package (version 3.12.1). The raw data was within array quantile normalized and probes that mapped to the same gene were combined by averaging. Expression data for MAPK target genes reviewed in Yang et al. (22) were isolated. For each isolated gene, the average expression difference between AS samples (n=18) and the mean of control samples (n=10) was determined. The genes with the highest magnitude of expression differences were isolated and plotted as a heatmap. The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE44115.
Results
1. MEK is active in HSA and HSA-derived primary tumor cells
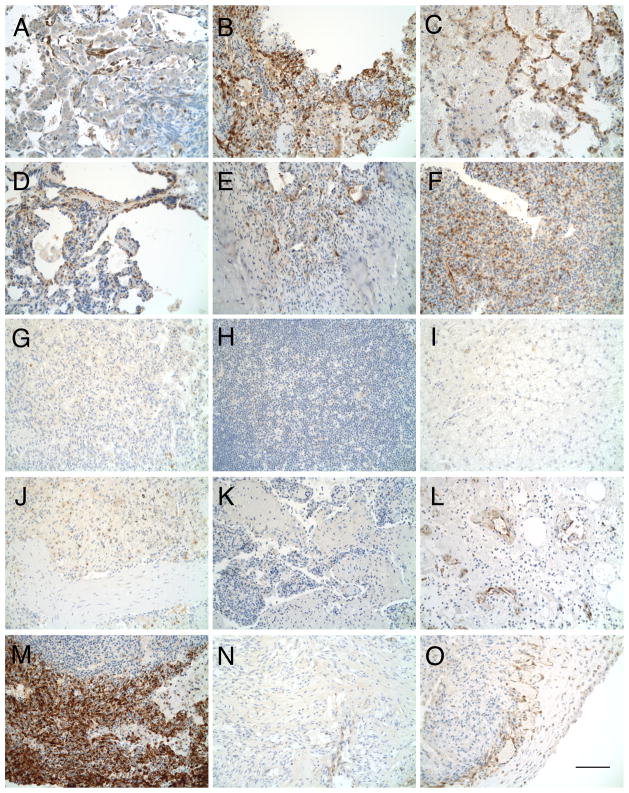
Based on its similarity to Kaposi sarcoma, we hypothesized that HSA was dependent on MEK signaling. To test whether MEK signaling is active in canine HSA, we ran immunohistochemical (IHC) assays on formalin-fixed paraffin-embedded tumor samples using antibodies against phosphorylated ERK1/2, which are direct substrates of MEK 1 and 2. HSA tumors were found to express phospho-ERK1/2, with the majority of the signal present in cells lining irregular blood vessels and areas near the outer portion of the tumor (Figure 1A–O). Some internal focal expression was also seen. Phospho-ERK1/2 was detected in cutaneous, cardiac, and splenic HSA, and ranged from weak (Figure 1L, O), to moderate (Figure 1A, C, D–F), to very strong (Figure 1B, M). In total, 9 out of 15 tumors examined were positive. These results indicated that MEK signaling is a common feature among HSA subtypes.
Figure 1.
HSA primary tumors are pERK positive. pERK-immunostained sections of formalin-fixed HSA visceral (A–K), cutaneous (L–N), and cardiac tumors (O). (A–F, L, M, O) Positive for pERK. (G–K, N) Tissue is negative for pERK. Bar = 100 μm.
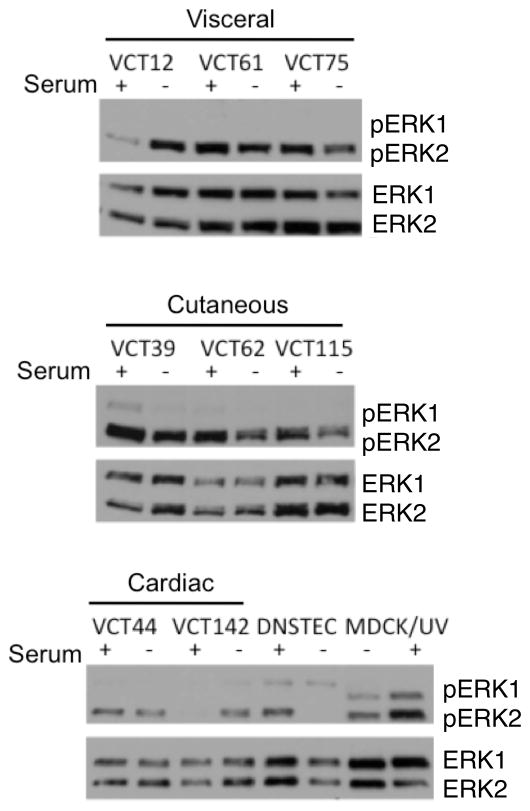
For subsequent testing, we used primary cells derived from tumor samples. To test whether MEK signaling is active in primary cells derived from HSA, we performed immunoblotting of HSA primary cells isolated from cutaneous, visceral, and cardiac tumors before and after serum starvation using antibodies against phospho-ERK and total ERK. DNSTECs were included for comparison, and MDCK cells treated with ultraviolet light were included as a positive control for ERK activation. HSA cells and DNSTECs growing in the presence of 10% serum had levels of phosphorylated ERK2 comparable to those observed in UV-treated MDCK cells (Figure 2). In contrast, ERK1 phosphorylation was low or not detectable relative to UV-treated MDCK cells. Following serum starvation, ERK2 phosphorylation in DNSTECs was undetectable, but the levels of phospho-ERK2 in primary cells derived from HSA remained or increased. These data indicate ERK2 is persistently active in HSA-derived primary cells.
Figure 2.
ERK is constitutively active in HSA-derived cells. Primary cells isolated from visceral, cutaneous, or cardiac HSA were incubated overnight in the presence or absence of serum. Total lysates were collected and immunoblotted against phospho-ERK1/2 and total ERK1/2. DNSTECs were serum-starved and immunoblotted as a negative control. UV-treated MDCK is a positive control for canine ERK1/2 activation.
2. MEK signaling is required for in vitro proliferation of HSA-derived primary tumor cells
To test whether MEK plays a role in the growth and proliferation of HSA, we treated primary cells derived from HSA with the MEK inhibitor CI-1040 and measured the inhibitor’s IC50. HSA primary cell isolates from the three subtypes were treated for 72 h in the presence of a range of CI-1040 concentrations Assays showed that cell viability for all subtypes decreased in a dose-dependent manner, with IC50 values of 2–8 μM. In contrast, DNSTECs were relatively insensitive to CI-1040 and failed to reach 50% growth inhibition even at 10 μM, the highest dose tested (Table 1). Inhibition of cell viability correlated with decreased ERK2 phosphorylation (Supplementary Figure S1A, B). These results indicated that MEK signaling is important for in vitro growth and proliferation of HSA.
Table 1.
Calculated IC50 of HSA primary cell isolates and DNSTEC to small molecule inhibitors
| CI-1040 (μM) | Sorafenib (μM) | LY294002 (μM) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Subtypes | Cell Isolate | IC50 | IC50 | IC50 | |||
|
| |||||||
| Visceral | VCT12 | 2.7 | ±1.5 | 1.7 | ±1.3 | 24.1 | ±1.7 |
| VCT61 | 3.0 | ±0.3 | 3.9 | ±2.9 | 29.7 | ±5.2 | |
| VCT75 | 2.4 | ±0.1 | 1.5 | ±0.6 | 16.2 | ±4.5 | |
|
| |||||||
| Cutaneous | VCT39 | 3.3 | ±1.1 | 2.8 | ±1.4 | 13.1 | ±2.3 |
| VCT62 | 3.6 | ±2.6 | 5.8 | ±2.7 | 8.8 | ±3.4 | |
| VCT115 | 1.7 | ±0.7 | 2.4 | ±0.5 | 9.4 | ±2.9 | |
|
| |||||||
| Cardiac | VCT44 | 7.6 | ±5.6 | 4.0 | ±0.2 | 22.6 | ±4.2 |
| VCT142 | 6.5 | ±4.4 | 2.3 | ±0.3 | 9.2 | ±4.0 | |
|
| |||||||
| DNSTEC | >10 μM | 7.9 | ±3.2 | 14.7 | ±7.9 | ||
To test whether signaling pathways upstream of MEK play a role in the growth and proliferation of HSA, we treated primary cells with sorafenib, a drug targeting B-Raf and receptor tyrosine kinases such as VEGFR2. HSA primary cell isolates from the three subtypes were treated for 72 h in the presence of a range of sorafenib concentrations. We observed that ERK phosphorylation (Supplemental Figure S1C, D) and cell viability for all subtypes decreased in a dose-dependent manner, with IC50 values from 2–6 μM. DNSTECs were only modestly more sensitive to sorafenib, with an IC50 of 8 μM (Table 1). These results indicated that signaling pathways upstream of MEK are important for in vitro growth and proliferation of HSA cells, as well as for other proliferating endothelial cells.
Signaling through Ras activates Raf and PI-3K/AKT signaling. To test for PI-3K/AKT signaling, we treated primary cells derived from HSA with the PI3K inhibitor LY294002. HSA primary cell isolates from the three subtypes were treated for 72 h in the presence of a range of LY294002 concentrations. Cell viability for all subtypes decreased in a dose-dependent manner, with IC50 values of 9–30 μM. Inhibition of cell growth correlated with decreased S6 phosphorylation (Supplemental Figure S1E, F). In contrast, DNSTECs were relatively more sensitive to LY294002, with an IC50 of 15 μM (Table 1). These data indicated PI-3K/AKT signaling is not critical for the in vitro growth and proliferation of primary cells derived from HSA. Collectively these data showed that MEK/ERK specifically, and not any Ras downstream pathway, is important for HSA cell isolate viability.
3. HSA forms tumorgrafts in nude mice
Because in vitro cell growth does not accurately mimic all aspects of in vivo growth, we made several attempts to grow HSA-derived cells as xenografts or tumorgrafts in athymic nude mice. Unlike other sarcomas we have worked with (data not shown), HSAs do not readily grow in nude mice. Attempts to grow visceral tumors via intraperitoneal or intrasplenic injection and via tumor implants have thus far failed (n = 24). In contrast, when primary cells derived from cutaneous HSA were injected into the dorsal flank of athymic nude mice one of three primary cell cultures developed into tumors. Similarly, grafting of 2 mm3 portions of whole tumor derived from one of two cardiac HSAs into the dorsal flank of athymic nude mice also developed tumors. Once established, both the xenograft and the tumorgraft could be removed and subdivided for reimplantion or frozen for later reimplantation. These two HSA models were used to test the effects of different inhibitors on tumor growth in vivo.
4. The morphology of HSA cutaneous-derived xenograft and cardiac-derived tumorgrafts mimics that of the parental tumors
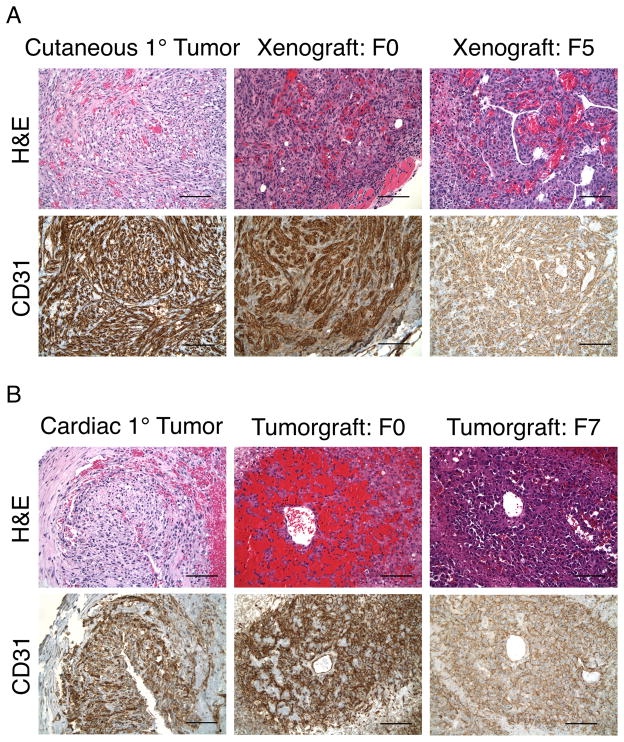
To confirm that xenografts and tumorgrafts retain key properties of HSA, FFPE sections were stained with hemotoxylin & eosin (H&E) or probed with antibodies against CD31 and compared with parental tumors (Figure 3). Cutaneous-derived xenografts generated lesions beginning in the upper dermis and extending deeply into subcutaneous tissue. The xenografts were composed of poorly circumscribed, expansile collections of intersecting fascicles of spindle-shaped tumor cells accompanied by rich admixture of vascular channels, as well as lumina lined by tumor cells having hyperchromatic and pleiomorphic nuclei. Occasional interstitial clusters of neutrophils and mononuclear cells were present. CD31 expression in cutaneous HSA was strong, with diffuse positive staining on the spindle-shaped cells and the vascular lining cells. Overall there was a remarkably strong similarity in light-microscopic patterns among cutaneous lesions pre- and post engraftment at both the histological and IHC levels.
Figure 3.
Morphological and IHC analysis of HSA cutaneous-derived xenografts, cardiac-derived tumorgrafts, and primary HSA. (A) Similarity between primary cutaneous HSA and canine cutaneous-derived xenografts for the F0 and F5 generations. (B) Similarity between primary cardiac HSA with canine cardiac-derived tumorgrafts for the both F0 and F7 generations. Bar = 100 μm.
Cardiac tumorgrafts were characterized by admixtures of highly vascularized neoplastic cells with hyperchromatic and pleiomorphic nuclei presenting as both lumen-lining channels and dispersed clusters of tumor cells. In addition, there were large areas of fibrinoid necrosis, nuclear debris, neutrophils, and scattered mononuclear cells. CD31 expression was strong and diffusely positive on those tumor cells both lining vessels and those in surrounding tissue. Cardiac-derived HSA lesions pre- and post-engraftment also showed a high degree of similarity at the histological and IHC levels. These observations indicate that the morphology of canine tumorgrafts is very similar to that of their parental tumors.
5. Sorafenib and CI-1040 inhibit MAPK activation and xenograft growth
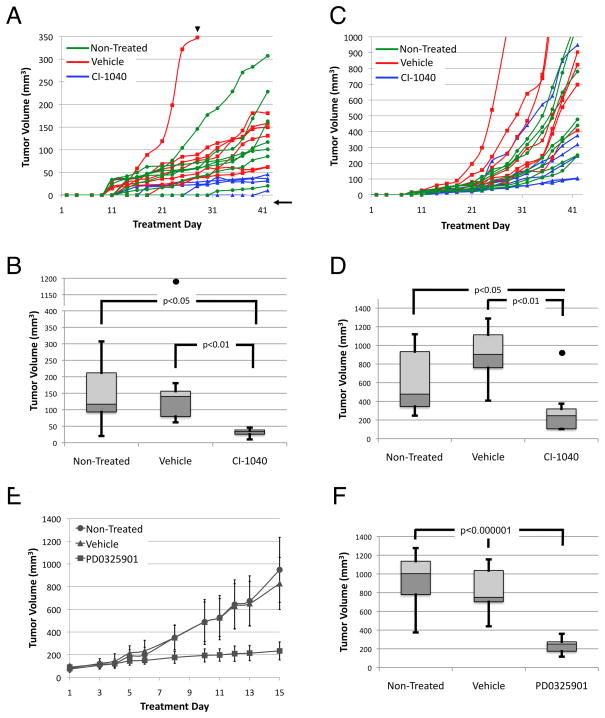
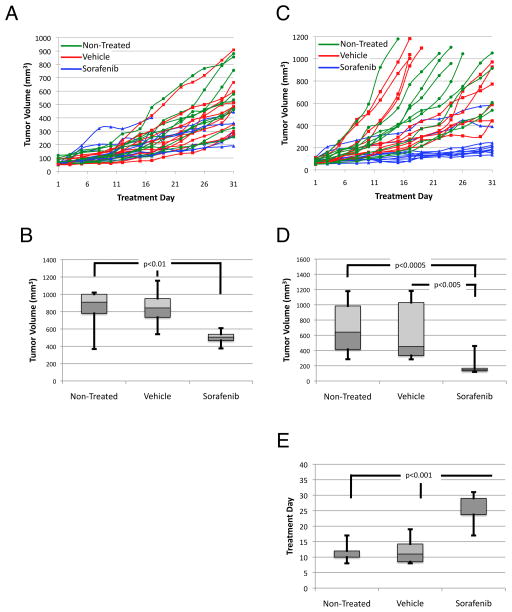
To test whether MEK plays a role in growth of HSA in vivo, we treated HSA tumorgrafts with the MEK inhibitor CI-1040 for 42 days. In the first experiment, HSA cutaneous-derived xenograft fragments less than 50 mm3 in volume were implanted and treatment began the next day. Mice were dosed at 48 mg/kg, but on day 28, the dose of CI-1040 was decreased by 20% to avoid toxicity. Despite this, three treatment mice had to be sacrificed due to significant (>10%) weight loss. Tumor volume was measured three times weekly (Mon., Wed., and Fri.; Figure 4A). At the completion of the experiment, the tumor burden of mice treated with CI-1040 was significantly smaller than that of untreated mice or of mice treated with vehicle alone (Figure 4B). Decreased tumor volume correlated with ERK inhibition as shown by IHC (Supplemental Figure S2A-C). In the second experiment, HSA cardiac tissue-derived tumorgrafts were treated with CI-1040 at 38.3 mg/kg for 42 days beginning one day after implantation. Tumor volume was measured as described above. One mouse had significant weight loss (>10%) and was euthanized after 17 d of treatment. Like cutaneous HSA, cardiac-derived tumorgrafts showed variable rates of growth (Figure 4C). Interestingly, while 5 of 6 treated mice showed a statistically significant reduction in tumor burden compared with non-treated or vehicle-treated mice, one tumorgraft displayed apparent resistance to CI-1040 treatment (Figure 4D). If this resistant tumorgraft is included in the statistical analysis, the tumor volumes of the CI-1040 treated mice are no longer significantly different than tumor volumes of the non-treated group but remain statistically smaller than tumors from vehicle-treated mice. Decreased tumor volume correlated with ERK inhibition as shown by IHC (Supplemental Figure S2D–F). To confirm efficacy of MEK inhibition on established tumors, cardiac-derived tumorgrafts where treated with the more potent MEK inhibitor PD0325901. Treatments were initiated when tumors reached a volume between 50 and 120 mm3. As in CI-1040 treated tumors, PD0325901 significantly decreased tumor growth compared to non-treated and vehicle-treated tumors (Figure 4E, F). These observations indicated cutaneous and cardiac-derived HSA tumorgrafts are sensitive to MEK1/2 inhibition.
Figure 4.
MEK inhibition reduces tumor growth in both cutaneous xenograft and cardiac-derived tumorgraft. (A) Growth curves of individual cutaneous xenografts. Arrowhead represents 20% decrease in drug concentration on treatment day 28. Arrow signifies a tumor estimated to be 10 mm3. (B) Box-and-whisker plot of tumor volume at treatment day 42; the dot represents the fast-growing vehicle outlier. (C) Growth curves of individual cardiac-derived tumorgrafts. (D) Box-and-whisker plot of tumor volume at treatment day 42; the dot represents a CI-1040 refractory tumor. (E) Growth curves of average cardiac-derived tumografts treated with PD0325901, vehicle, or non-treated (n=10). (F) Box-and-whisker plot of tumor volume at treatment day 22. Error bars on growth curves represent standard deviations.
To test whether signaling pathways upstream of MEK play a role in the growth and proliferation of HSA, we treated HSA cutaneous xenografts with sorafenib daily at 100 mg/kg for 42 days. Treatments were initiated approximately 2 to 5 weeks after implantation, when tumors reached a volume between 50 and 120 mm3. Six of 10 mice treated with sorafenib were euthanized before the completion of the experiment due to adverse toxicities including rash, hypothermia, and ascites. At the completion of the experiment, the tumor burden of four remaining mice treated with sorafenib was significantly smaller than that of untreated mice or of mice treated with vehicle alone (Figure 5A, B). Decreased tumor volume correlated with ERK inhibition as shown by IHC (Supplemental Figure S3A–C).
Figure 5.
Sensitivity of cutaneous-derived xenograft and cardiac-derived tumors to sorafenib treatment. (A) Growth curves of individual cutaneous xenografts; 6 of 10 mice developed toxicity and failed to complete the study. (B) Box-and-whisker plot of tumor volume at treatment day 43. (C) Growth curves of individual cardiac tumorgrafts. (D) Box-and-whisker plot of tumor volume at treatment day 22. (D) Box-and-whisker plot representing the treatment day at which tumorgrafts grew to 3 times their treatment-day-1 volume. Error bars on growth curves represent standard deviations.
In the second experiment, cardiac-derived HSA tumorgrafts were treated with sorafenib starting when tumors reached a volume between 50 and 120 mm3. Growth rates were variable. Tumorgrafts implanted in non-treated and vehicle-control mice grew rapidly; several tumors reached a maximum allowed size of 1000 mm3 within three weeks (Figure 5C). These mice were sacrificed and their tumors saved, while the remaining mice continued on therapy. The final volume of these fast-growing tumors was compared with other tumor volumes at the completion of the experiment. Thus tumor volume for non-treated and vehicle control mice shown in Figure 5D is likely lower than it would have been had these tumors been allowed to continue growing for the duration of the experiment along with treated tumors. Despite this, it was apparent that sorafenib significantly inhibited the growth of cardiac-derived HSA tumorgrafts (Figure 5D). Analyzed another way, sorafenib caused a significant reduction in the time taken for tumorgrafts to exceed three times their initial volume on treatment day 1 (Figure 5F); in fact, four sorafenib-treated tumorgrafts never reached three times the initial volume. In addition, two tumors were resistant to sorafenib. Decreased tumor volume correlated with ERK inhibition as shown by IHC (Supplemental Figure S3D–F). These observations indicated cardiac-derived HSA tumorgrafts are sensitive to sorafenib-mediated inhibition of signaling pathways upstream of MEK.
6. Canine HSAs are morphologically and histologically similar to human AS
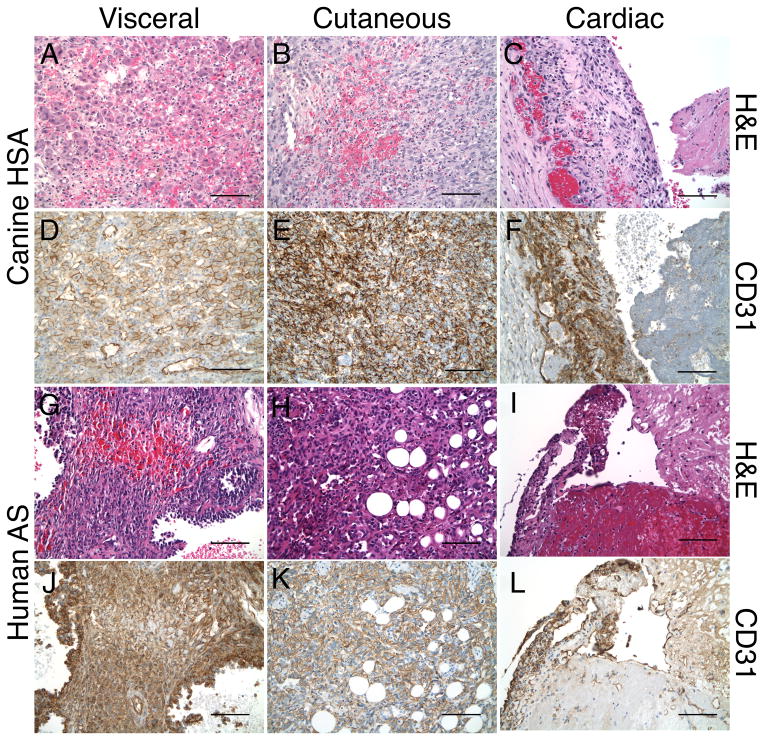
To evaluate the extent of similarity between canine HSA and human AS, we undertook a morphological and immunohistological comparison of the canine visceral, cutaneous, and cardiac HSA subtypes to their human counterparts.
Canine Visceral Hemangiosarcoma
Visceral HSA were highly vascularized infiltrative neoplasms characterized by scattered clusters of cuboidal shaped cells having enlarged hyperchromatic and pleiomorphic nuclei with vesicular chromatin and nucleoli (Figure 6A). The clusters were accompanied by anastamosing cords of cuboidal and spindle shaped cells with occasional multi-nucleation embedded in a highly vascular meshwork including the prominent presence of erythrocytes, pigment-laden macrophages, and large vessels surrounded by plasma cells and lymphocytes. Tumor cells were identified infiltrating the outer walls of large arteries and displayed perineural invasion. In some areas, flattened cells with elongated hyperchromatic nuclei lined aberrant vascular channels. Additional clusters of more epithelioid-appearing tumor cells having enlarged nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm (occasionally containing multiple vacuoles) were also present, as were scattered mitotic figures. Immunohistochemical staining for CD31 revealed strong and diffuse reactivity of endothelial cells lining normal vessels as well as surrounding tumor cells of all morphological subtypes, highlighting the plasma membranes of both normal and neoplastic cells (Figure 6D).
Figure 6.
Morphological and immunohistological similarities of visceral, cutaneous, and cardiac HSA and human AS tumors. (A-F) H&E- and CD31-stained sections of visceral, cutaneous, and cardiac HSA. (G-L) H&E- and CD31-stained sections of visceral, cutaneous, and cardiac AS. Bar = 100 μm.
Canine Cutaneous Hemangiosarcoma
Cutaneous HSA lesions were non-circumscribed, expansile and infiltrative tumors extending from the upper dermis to include invasion into the underlying skeletal muscle. They comprised highly cellular intersecting fascicles of spindle-shaped cells and vascular channels with elongate pleiomorphic and hyperchromatic nuclei and nucleoli embedded in a collagenous stroma with sparse mononuclear cell infiltration (Figure 6B). The vascular channels were lined by cells of atypical appearance, some of which protruded into the lumens filled with erythrocytes accompanied by scattered mitotic figures. There were focal areas of extravasated erythrocytes, hemosiderin-laden macrophages, and extension into skeletal muscle fibers, which were dissected by the interconnecting vascular channels lined by atypical-appearing tumor cells. CD31 expression was strong and diffusely positive on normal endothelial cells, interstitial tumor cells, and the atypical cells lining vascular channels throughout the neoplasm; the latter were particularly striking in between the bundles of skeletal muscle fibers infiltrated by tumor cells (Figure 6E).
Canine Cardiac Hemangiosarcoma
Cardiac HSA were composed of poorly formed vascular channels lined by hyperchromatic and pleiomorphic nuclei accompanied by inter-anastamosing cords and clusters of tumor cells having elongated hyperchromatic and pleiomorphic nuclei, accompanied by extensive hemorrhage and fibrin deposition (Figure 6C). The surrounding stroma contained hemosiderin-laden macrophages, neutrophils, plasma cells, and lymphocytes with focal zones of necrosis and nuclear debris. CD31 expression was strongly positive among scattered tumor cells lining vascular channels as well as among those tumor cells forming small nests and ill-defined cords (Figure 6F).
Human Visceral Angiosarcoma
Like HSA, visceral AS is a highly vascularized tumor composed of areas of spindle-shaped, diffusely staining, CD31-positive tumor cells lining irregular vascular channels. Also present are necrosis accompanying prominent areas of extravasated erythrocytes and a large area of anastamosing cords of spindle-shaped tumor cells. The tumor cells contain vesicular chromatin with prominent nuclei (Figure 6G, J). The light morphological and histological staining is similar between canine and human visceral AS.
Human Cutaneous Angiosarcoma
Similar to cutaneous HSA cutaneous AS tumors are strongly and diffusely CD31-positive spindle-shaped tumor cells lining irregular vascular channels. Scattered erythrocytes are present, although fewer then in visceral AS (Figure 6H, K).
Human Cardiac Angiosarcoma
Cardiac AS like cardiac HSA are lesions of infiltration of normal tissue by spindle-shaped tumor cells, which are strongly and diffusely CD31-positive. These tumor cells possess hyperchromatic nuclei and are accompanied by areas of hemorrhage (Figure 6I, L).
7. MEK is active in human AS
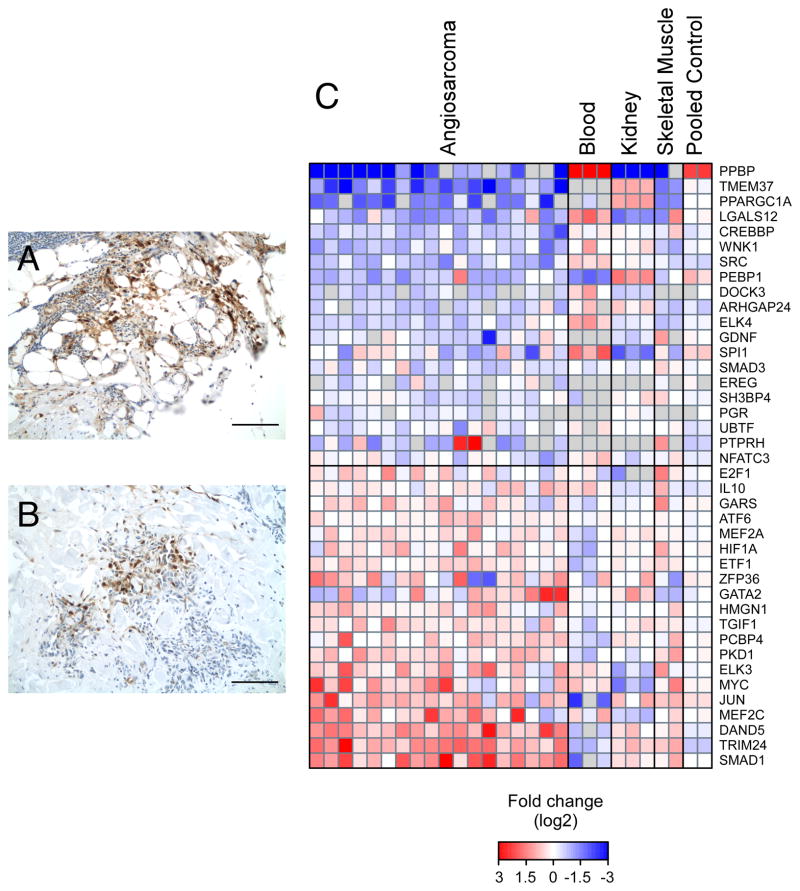
The preceding data indicate human AS and canine HSA share a similar pathology. To determine whether this similarity extends to a molecular level, we used two different approaches to assess MEK activity in human AS. In the first, we immunostained archival FFPE specimens with antibodies against phosphorylated ERK1/2 (Figure 7A, B). Phospho-ERK1/2 was not exclusive to any subtype of AS; it was detected in cutaneous, visceral, and cardiac samples. Phospho-ERK1/2 signal was observed in both the cytoplasm and nucleus of cells lining normal and irregular vascular channels, as well as in intratumoral foci. In total, 9 of 12 tumors tested were positive for phosphorylated ERK1/2. In the second approach, we used OCT-frozen human AS to evaluate the expression of a previously identified panel of MAPK-driven transcription factors (22). We found several MAPK-responsive transcription factors, including SMAD1, TRIM24, MYC, HIF1α, and MEF2C were up-regulated in a panel of angiosarcoma samples relative to RNA isolated from normal human tissue of mesodermal origin (Figure 7C). Myc and HIF1α amplification has been previously noted in human AS (8, 23). To further validate these results, we performed IHC using antibodies against MEF2c. MEF2c showed strong nuclear staining and a weak to moderate diffuse cytoplasmic staining pattern in 4 of 5 human AS examined (Figure S4A). Similar staining was observed 5 of 5 canine HSA examined (Figure S4C). MEF2c signal in AS and HSA samples were above normal kidney signal (Figure S4B, D).
Figure 7.
MEK activity is present in human AS. (A and B) IHC assay shows that phosphorylated ERK1/2 is present in two independent cutaneous AS. (C) The 20 most up-regulated and down-regulated MAPK-responsive transcription factors in AS. RNA isolated from OCT-frozen AS were compared to RNA from normal human tissue (blood, kidney, and skeletal muscle) and pooled normal tissue (Pooled controls 1 and 2). Bar = 100 μm.
Results of these two approaches are consistent with the hypothesis that MEK is active in human AS.
Discussion
Angiosarcoma (AS) is a malignancy of endothelial origin (1). Its rarity has turned attention to experimental models for generating further insights into the disease (11). Studies in engineered rodent models are consistent with a role for MEK signaling in AS. Injection of Moloney mouse sarcoma virus (24, 25), Harvey Sarcoma virus (26, 27), or Kirsten Sarcoma virus (28) into mice or rats induces the formation of angiomatous lesions resembling angiosarcoma or Kaposi sarcoma; these viruses encode mutant, oncogenic forms of cellular oncogenes encoding c-mos, H-ras, and K-ras, respectively. Each of these genes stimulates cellular proliferation and oncogenic transformation by activation of the mitogen-activated protein kinase (MAPK) signaling pathway. Mice engineered to express knock-in mutations (D1226N or Y1228C) in the activation loop of Met develop a high incidence of angiosarcoma with moderately pleiomorphic endothelial cells, cavernous blood vessels, and palisading epithelioid-like cells (29). Met is a tyrosine kinase receptor for the hepatocyte growth factor/scatter factor and is a potent activator of the MAPK signaling pathway, regulating among other things the epithelial-to-mesenchymal transition and metastatic behavior (30).
To gain insight into the underlying biology of spontaneous AS, we began a study of naturally occurring HSA in companion dogs. While sharing many features in common with humans AS, HSA has a higher incidence and a more rapid progression. There is substantial variability of HSA among breeds, with larger dogs such as Golden retrievers and German shepherds having a much higher incidence. A health survey of Golden retrievers attributed 62% of all deaths to cancer, with HSA representing 16% of reported cancers (31). Why this tumor is more common in dogs than in humans is uncertain. It is possible that disease-causing genes were overrepresented in the founding populations for these dogs. Another intriguing possibility is that genes promoting susceptibility to HSA may have been co-selected along with deliberately selected traits in the directed evolution of these breeds. Whatever the answer, the genetic uniformity within breeds offers a unique and unbiased opportunity to identify factors promoting AS.
HSA may present in virtually any tissue of the body. While most dogs present with splenic or liver lesions, some breeds show elevated incidence of cutaneous HSA (e.g., Whippets, Italian greyhounds) or cardiac HSA (e.g., Saluki). This is notable because it demonstrates that genetic factors not only influence susceptibility to HSA, but they also determine its location. From a research perspective, this opens new avenues for discovery of the basic mechanisms of the origin and progression of cancer, which could be translated to help our understanding of the causes and treatment of AS in human patients.
In this study we tested the hypothesis that HSA growth is dependent on MEK signaling. Using immunohistochemistry on FFPE HSA samples, we observed isolated cells or clusters of tumor cells expressing phosphorylated ERK1/2 in 60% of samples examined. Furthermore, immunoblotting of lysates made from cultured primary cell isolates derived from canine HSA showed ERK1/2 activity even after serum starvation. These observations indicated MEK is active in canine HSA, but the cause of this aberrant MEK signaling is not known. We have sequenced for reported mutations in several candidate genes including VEGFR and B-Raf, but to date we have not identified any activating mutations. The fact that MEK signaling was present only in isolated cells or clusters of tumor cells indicates ERK1/2 is transiently phosphorylated or that ERK1/2 phosphorylation in subset of cells is sufficient for tumor growth. This is reminiscent of Kaposi sarcoma, another endothelial malignancy, in which proliferating spindle cells release cytokines that drive tumor growth in a paracrine fashion. Consistent with this, elevated levels of VEGF and bFGF, as well as Flt4 (VEGFR3) and FGFR-1, have been detected in association with canine AS (32, 33) and human angiosarcoma (7, 8, 34).
Interestingly, HSA cellular isolates showed a predominance of ERK2 phosphorylation over ERK1. This is consistent with published data indicating ERK2 may play a more prominent role cancer. In a recent study using breast cancer cells von Thun et al. (35) reported knockdown of ERK2 but not ERK1 decreased cell motility in a 3D microenvironment. Similarly, Vantaggiato et al. (36) reported knock down of ERK2 but not ERK1 in NIH 3T3 cells reduces Ras-mediated colony formation. These reports indicate ERK1 and ERK2 may have non-redundant roles in tumor growth and metastasis.
Canine tumor tissues represent a valuable resource for cell-based studies. In our experience of culturing more than 400 canine tumors, approximately 50% will grow in culture for two or more passages. Our success rate in culturing hemangiosarcoma is comparable: we successfully cultured 101 of 203 HSA for two or more passages. We are uncertain why some tumors grow well in culture while others do not. In contrast to parental tumors, 60% of which had ERK1/2-positive cells, cultured HSA uniformly expressed active ERK1/2 even after 24 h of serum starvation. This raises the possibility that in making tumor-derived cell cultures, we may have selected for a subset of tumors with active MEK signaling. Consistent with this, we observed that pharmacologic inhibition of MEK signaling reduced the viability of these cells in vitro. Thus, caution should be used not to extrapolate our in vitro data to represent all HSA. Indeed, an earlier study by Tamburini et al. (37) noted ERK activation in only 1 of 3 cell isolates tested, which is more in line with our observations in parental tumors. In addition, there is a concern that we may have selected for the growth of non-tumor cells in vitro. However, our observations that isolated cell cultures show persistent MEK signaling (Figure 2) and aneuploidy (array CGH data not shown) indicates that tumor-derived cell cultures are a heterogeneous mixture of tumor and tumor-associated stromal cells that retain features in common with their tumor of origin.
Although we have been able to grow cardiac and cutaneous HSA-derived cells as tumorgrafts or xenografts in athymic nude mice, we have been less successful with visceral HSA-derived cells. This is a strong indication that either in vitro cell culture selects for cells with divergent growth abilities or the microenvironment in a mouse is substantially different from that of a dog. Additional growth supplements or modified growth conditions may be required to preserve their ability to grow in vivo. Despite this, those cells or tumors that did grow in vivo had morphology indistinguishable from their parental tumors. This provided us with an opportunity to test the necessity of MEK signaling for HSA growth in vivo.
We observed that whereas both cutaneous HSA xenografts and cardiac HSA tumorgrafts were sensitive to MEK1/2 inhibition by CI-1040, cardiac tumorgrafts were more sensitive to sorafenib (Figure 4, 6). Also, cardiac HSA tumorgrafts were sensitive to a second MEK inhibitor PD0325901 (Figure 4E, F). These results demonstrate that MEK signaling is necessary for growth of HSA in vivo and provide a strong rationale for clinical evaluation of MEK inhibitors, either alone or in combination, for the treatment of HSA. Although, we cannot rule out sorafenib sensitivity is through inhibition of VEGFR2 or other in vivo targets. Our observations that ERK1/2 phosphorylation is detected in human AS and that MEK/ERK-responsive transcription factors are up-regulated in human AS suggest a similar approach for treating patients diagnosed with angiosarcoma. Indeed, in a recent report by Italiano et al. (10) noted ERK phosphorylation in 12 of 39 human angiosarcomas and concluded that inhibition of ERK signaling may be a relevant approach for their treatment. However, a larger cohort of AS samples will be required to definitively translate the importance of MEK signaling from our canine model to AS. Historically, studies with MEK inhibitors in other tumors have for the most part proven ineffective in clinical trials (reviewed by (38)). However, more recent clinical trials using the MEK inhibitor trametinib have shown promising results alone and in combination with a B-Raf inhibitor (reviewed by (39)). Thus, it may be prudent to consider using newer MEK inhibitors such as trametinib or a combination of an earlier MEK inhibitor plus a drug targeting other critical pathways for the treatment of HSA.
In summary, using naturally occurring hemangiosarcoma in companion dogs, we have shown that MEK signaling is essential for growth of hemangiosarcoma in vitro and in vivo. In addition, we have provided evidence indicating the same pathways are activated in human AS. Our study indicates MEK inhibitors may form part of an effective therapeutic strategy for treatment of canine HSA or human AS and highlights the utility of spontaneous canine cancers as a model for human disease.
Supplementary Material
Acknowledgments
This work was supported by grants to N.S. Duesbery by the National Institutes of Health/National Cancer Institute (RC2CA148149), the Animal Cancer Foundation, the American Kennel Club/ Canine Health Foundation, and the Dwight Reed Memorial Foundation.
We thank Alex Blanski, Cathy Weisner, Bree Berghuis, Lisa Turner, and Sok Kean Khoo for technical assistance and Dr. J. Bromberg-White for helpful discussion and comments on the manuscript..
Grant Support
We also acknowledge financial support from the National Institutes of Health/National Cancer Institute (RC2CA148149), the Animal Cancer Foundation, the American Kennel Club/ Canine Health Foundation, and the Dwight Reed Memorial Foundation.
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Requena L, Sangueza OP. Cutaneous vascular proliferations. Part III. Malignant neoplasms, other cutaneous neoplasms with significant vascular component, and disorders erroneously considered as vascular neoplasms. J Am Acad Dermatol. 1998;38:143–75. doi: 10.1016/s0190-9622(98)70237-3. [DOI] [PubMed] [Google Scholar]
- 2.Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010;11:983–91. doi: 10.1016/S1470-2045(10)70023-1. [DOI] [PubMed] [Google Scholar]
- 3.Fury MG, Antonescu CR, Van Zee KJ, Brennan MF, Maki RG. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241–7. doi: 10.1097/00130404-200505000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Przygodzki RM, Finkelstein SD, Keohavong P, Zhu D, Bakker A, Swalsky PA, et al. Sporadic and Thorotrast-induced angiosarcomas of the liver manifest frequent and multiple point mutations in K-ras-2. Lab Invest. 1997;76:153–9. [PubMed] [Google Scholar]
- 5.Marion MJ, Froment O, Trepo C. Activation of Ki-ras gene by point mutation in human liver angiosarcoma associated with vinyl-chloride exposure. Molecular Carcinogenesis. 2005;4:450–4. doi: 10.1002/mc.2940040607. [DOI] [PubMed] [Google Scholar]
- 6.Weihrauch M, Bader M, Lehnert G, Koch B, Wittekind C, Wrbitzky R, et al. Mutation analysis of K-ras-2 in liver angiosarcoma and adjacent nonneoplastic liver tissue from patients occupationally exposed to vinyl chloride. Environ Mol Mutagen. 2002;40:36–40. doi: 10.1002/em.10084. [DOI] [PubMed] [Google Scholar]
- 7.Antonescu CR, Yoshida A, Guo T, Chang NE, Zhang L, Agaram NP, et al. KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res. 2009;69:7175–9. doi: 10.1158/0008-5472.CAN-09-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo T, Zhang L, Chang NE, Singer S, Maki RG, Antonescu CR. Consistent MYC and FLT4 gene amplification in radiation-induced angiosarcoma but not in other radiation-associated atypical vascular lesions. Genes Chromosomes Cancer. 2011;50:25–33. doi: 10.1002/gcc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tate G, Suzuki T, Mitsuya T. Mutation of the PTEN gene in a human hepatic angiosarcoma. Cancer Genet Cytogenet. 2007;178:160–2. doi: 10.1016/j.cancergencyto.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Italiano A, Chen CL, Thomas R, Breen M, Bonnet F, Sevenet N, et al. Alterations of the p53 and PIK3CA/AKT/mTOR pathways in angiosarcomas: a pattern distinct from other sarcomas with complex genomics. Cancer. 2012;118:5878–87. doi: 10.1002/cncr.27614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen N, Froman R, Kitchell B, Duesbery N. Angiosarcoma. Clinical and Molecular Aspects. In: Derbel F, editor. Soft Tissue Sarcoma. Rijeka, Croatia: I-Tech Education and Publishing; 2011. pp. 149–74. [Google Scholar]
- 12.Gordon I, Paoloni M, Mazcko C, Khanna C. The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med. 2009;6:e1000161. doi: 10.1371/journal.pmed.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–56. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 14.Depeille P, Young JJ, Boguslawski EA, Berghuis BD, Kort EJ, Resau JH, et al. Anthrax lethal toxin inhibits growth of and vascular endothelial growth factor release from endothelial cells expressing the human herpes virus 8 viral G protein coupled receptor. Clin Cancer Res. 2007;13:5926–34. doi: 10.1158/1078-0432.CCR-07-0732. [DOI] [PubMed] [Google Scholar]
- 15.Sodhi A, Montaner S, Patel V, Gomez-Roman JJ, Li Y, Sausville EA, et al. Akt plays a central role in sarcomagenesis induced by Kaposi’s sarcoma herpesvirus-encoded G protein-coupled receptor. Proc Natl Acad Sci U S A. 2004;101:4821–6. doi: 10.1073/pnas.0400835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett SD, Bridges AJ, Dudley DT, Saltiel AR, Fergus JH, Flamme CM, et al. The discovery of the benzhydroxamate MEK inhibitors CI-1040 and PD 0325901. Bioorg Med Chem Lett. 2008;18:6501–4. doi: 10.1016/j.bmcl.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 17.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–8. [PubMed] [Google Scholar]
- 18.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 19.Sebolt-Leopold JS, Dudley DT, Herrera R, Van Becelaere K, Wiland A, Gowan RC, et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med. 1999;5:810–6. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 20.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–62. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 22.Yang SH, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- 23.Manner J, Radlwimmer B, Hohenberger P, Mossinger K, Kuffer S, Sauer C, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol. 2010;176:34–9. doi: 10.2353/ajpath.2010.090637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoica G, Hoffman J, Yuen PH. Moloney murine sarcoma virus 349 induces Kaposi’s sarcomalike lesions in Balb/c mice. Am J Pathol. 1990;136:933–47. [PMC free article] [PubMed] [Google Scholar]
- 25.Yuen PH, Matherne CM, Molinari-Storey LM. SV7, a molecular clone of Moloney murine sarcoma virus 349, transforms vascular endothelial cells. Am J Pathol. 1991;139:1449–61. [PMC free article] [PubMed] [Google Scholar]
- 26.Chesterman FC, Harvey JJ, Dourmashkin RR, Salaman MH. The pathology of tumors and other lesions induced in rodents by virus derived from a rat with Moloney leukemia. Cancer Res. 1966;26:1759–68. [PubMed] [Google Scholar]
- 27.Harvey JJ. An Unidentified Virus Which Causes the Rapid Production of Tumours in Mice. Nature. 1964;204:1104–5. doi: 10.1038/2041104b0. [DOI] [PubMed] [Google Scholar]
- 28.Pitts OM, Powers JM, Hoffman PM. Vascular neoplasms induced in rodent central nervous system by murine sarcoma viruses. Lab Invest. 1983;49:171–82. [PubMed] [Google Scholar]
- 29.Graveel C, Su Y, Koeman J, Wang LM, Tessarollo L, Fiscella M, et al. Activating Met mutations produce unique tumor profiles in mice with selective duplication of the mutant allele. Proc Natl Acad Sci U S A. 2004;101:17198–203. doi: 10.1073/pnas.0407651101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 31.Glickman LT, Glickman N, Thorpe R. The Golden Retriever Club of America National Health Survey. 1999. [Google Scholar]
- 32.Kato Y, Asano K, Mizutani I, Konno T, Sasaki Y, Kutara K, et al. Gene expressions of canine angiopoietin-1 and -2 in normal tissues and spontaneous tumours. Res Vet Sci. 2006;81:280–6. doi: 10.1016/j.rvsc.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Kodama A, Sakai H, Matsuura S, Murakami M, Murai A, Mori T, et al. Establishment of canine hemangiosarcoma xenograft models expressing endothelial growth factors, their receptors, and angiogenesis-associated homeobox genes. BMC Cancer. 2009;9:363. doi: 10.1186/1471-2407-9-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoica G, Hoffman R, Hawker J, Lawrence M, Kelly K, Meininger C. The role of fibroblast growth factor (FGF) in neoplasms induced by MoMuSV-349. Int J Oncol. 1997;10:205–11. [PubMed] [Google Scholar]
- 35.von Thun A, Birtwistle M, Kalna G, Grindlay J, Strachan D, Kolch W, et al. ERK2 drives tumour cell migration in three-dimensional microenvironments by suppressing expression of Rab17 and liprin-beta2. J Cell Sci. 125:1465–77. doi: 10.1242/jcs.092916. [DOI] [PubMed] [Google Scholar]
- 36.Vantaggiato C, Formentini I, Bondanza A, Bonini C, Naldini L, Brambilla R. ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially. J Biol. 2006;5:14. doi: 10.1186/jbiol38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamburini BA, Phang TL, Fosmire SP, Scott MC, Trapp SC, Duckett MM, et al. Gene expression profiling identifies inflammation and angiogenesis as distinguishing features of canine hemangiosarcoma. BMC Cancer. 10:619. doi: 10.1186/1471-2407-10-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee C-S, Duesbery NS. Highly Selective MEK Inhibitors. Current Enzyme Inhibition. 2010;6:12. [Google Scholar]
- 39.Sullivan RJ, Flaherty KT. Resistance to BRAF-targeted therapy in melanoma. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2012.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.