Abstract
We investigated the hypothesis that immobilizing TGF-β1 within fibrin hydrogels may act in synergy with cyclic mechanical stimulation to enhance the properties of vascular grafts. To this end, we engineered a fusion TGF-β1 protein that can covalently anchor to fibrin during polymerization upon the action of factor XIII. We also developed a 24-well based bioreactor in which vascular constructs can be mechanically stimulated by distending the silastic mandrel in the middle of each well. TGF-β1 was either conjugated to fibrin or supplied in the culture medium and the fibrin based constructs were cultured statically for a week followed by cyclic distention for another week. The tissues were examined for myogenic differentiation, vascular reactivity, mechanical properties and ECM content. Our results showed that some aspects of vascular function were differentially affected by growth factor presentation vs. pulsatile force application, while others were synergistically enhanced by both. Overall, this two-prong biomimetic approach improved ECM secretion, vascular reactivity and mechanical properties of vascular constructs. These findings may be applied in other tissue engineering applications such as cartilage, tendon or cardiac regeneration where growth factors TGF-β1 and mechano-stimulation play critical roles.
INTRODUCTION
Numerous biomaterials have been examined as scaffolds for engineering vascular grafts. They include biodegradable synthetic polymers [1, 2], elastomers [3], naturally-derived proteins [4–11], decellularized tissues [12, 13] and cell sheet matrices [14, 15]. In addition to searching for suitable scaffold materials, several groups added biomimetic chemical cues, including small peptides or growth factors to enhance TEV patency and remodeling. Insulin and transforming growth factor-β1 (TGF-β1) supplements were shown to have synergistic effects on fibrin-based vascular grafts, increasing ECM protein deposition and vascular contractility [10, 16]. Platelet-derived growth factor-BB (PDGF-BB) and TGF-β1 synergistically enhanced ECM production [17], while hyaluronan oligomers (HA-o) and TGF-β1 synergistically enhanced tropo- and matrix elastin expression [18]. However, simple supplementation of growth factors into the culture medium may generate diffusion gradients that in turn may produce heterogeneities in cell growth or differentiation across the tissue, ultimately affecting function. In addition, these soluble signals are not available to the cells after graft implantation in vivo.
These challenges may be overcome by development of controlled release strategies that chemically or physically immobilize the proteins [19, 20] or embed chemically encapsulated complexes into the scaffolds [20, 21]. The biomimetic cues can then become available either passively by continuous release from the matrix or by active release through the action of cells which are either embedded in the matrix or migrate into the scaffold from the host. Ultimately, these signals could influence cell phenotype and construct properties [22, 23]. Furthermore, tissue constructs decorated with biomimetic cues may continue to receive such signals even after implantation in vivo bypassing issues related to targeted protein delivery, instability and circulation clearance [24]. Our group employed SMC from various stem cell sources including bone marrow and hair follicles to engineer fibrin-based vascular grafts that were implanted into an ovine animal model [9–11, 25–27]. We also developed a fusion protein between TGF-β1 and the α2-PI peptide leader sequence to covalently conjugate TGF-β1 within fibrin hydrogels through the action of Factor XIII [19]. Interestingly, immobilized TGF-β1 prolonged SMAD2 phosphorylation and enhanced vascular contractility of mesenchymal stem cells (MSCs) embedded in the vascular wall.
Many tissues in the body, including the vasculature, experience continuous mechanical stimulation that plays an important role in the development and maintenance of cell phenotype and tissue function [28, 29]. It has long been recognized that mechanical preconditioning that mimics the mechanical microenvironment in vivo is critical in engineering tissues with the desired properties including cell alignment, mechanical strength, vascular contractility and ECM deposition [30–36]. In addition, mechanical stimulation was shown to influence MSC fate decisions increasing smooth muscle differentiation [37] and the ratio of myogenic over adipogenic gene expression [38, 39].
In this study, we hypothesized that conjugation of TGF-β1 in combination with application of pulsatile force may synergistically enhance the properties of vascular grafts. To test this hypothesis, we designed a multi-well device to study vascular constructs under pulsatile force in a high-throughput manner in the presence or absence of fibrin immobilized TGF-β1. Using this system we investigated the aspects of cellular and tissue function that might be affected by the mode of TGF-β1 presentation, pulsatile pressure or their combined action. These include smooth muscle protein expression, vascular contractility, mechanical properties, and collagen and elastin synthesis in the vascular wall. We provide evidence supporting the notion that mimicking both the mechanical and biochemical tissue microenvironments can synergistically enhance the function of bioengineered vascular grafts.
MATERIALS AND METHODS
Manufacturing of the 24-well pulsation plate
We developed a biomechanical 24-well plate made from polycarbonate and assembled by stainless steel parts so that it is autoclavable (Fig. 1 (b)–(d)). Each polycarbonate part was designed with FeatureCam software (Salt Lake City, UT) and milled on an automatic milling machine (HAAS CNC, Oxnard, CA). A flexible silastic mandrel (Helix Medical, Carpinteria, CA) was placed in the middle of each well. To determine the relationship between pressure and % distention of the silastic mandrel a laser micrometer (Tissue Growth Technologies, Minneapolis, MN) was used to measure the mandrel diameter under different pressures.
Figure 1.
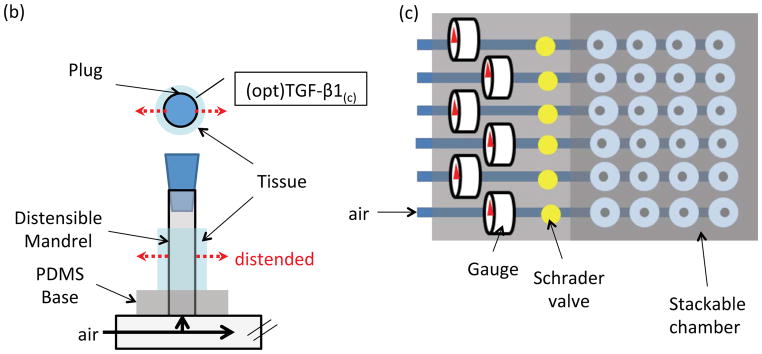
TGF-β1 conjugation strategy and mechanical pulsation plate: (a) Schematic of FXIII mediated peptide/TGF-β1 protein immobilization into fibrin hydrogel. In the single fibrinogen molecule, E represents the E-domain; D represents the D-domains. (b) Schematic of each well: fibrin gel was polymerized around a distensible mandrel in the middle of each well. Compressed air was used to distend the mandrel as shown by the dashed arrows. A polycarbonate plug was used to seal the mandrel tubing. (c) Schematic of the plate. Four wells in a row were connected to a common air source and pulsed under identical conditions. Each plate had six independent rows, each pulsed under a different condition. (d) Distention controlling module: the compressed air was passed through a 0.22 μm filter before entering the system (not shown). A needle valve controlled the amount of air to achieve a certain pressure that was monitored by a pressure gauge. A custom-made electronic controller generating square-function signals was used to control the 3-way solenoid valve that determined the pulsation pattern (time on/time off). (e) Distention-pressure relationship was established by measuring the mandrel diameter as a function of pressure using a laser micrometer (second-order polynomial regression, R2>0.99).
Preparation of vascular constructs
Fibrin tissue equivalents were prepared as described previously [9, 11, 19, 25, 27, 40, 41]. Briefly, human dermal fibroblasts derived from neonatal foreskin were suspended in fibrinogen and mixed with another solution containing thrombin, Ca2+, Factor XIII and fusion TGF-β1 that was produced as described previously [19], yielding a final concentration of 2.5 mg/mL fibrinogen, 2.5 U/mL thrombin, 0.1 PEU/mL Factor XIII, and 2.5 mM Ca2+ with 50 ng/mL fusion TGF-β1. Control tissues did not contain the fusion TGF-β1 protein. Each construct containing 106 cells per mL was polymerized around the distensible tubes in the middle of a well in the 24-well pulsation plate. The wells and the mandrels were pre-coated with 2% BSA for at least 20 min. After polymerization for 1 h at 37°C, the gels were detached from the walls and incubated in 3 mL of DMEM containing 10% FBS supplemented with 2 μg/mL insulin, 300 mM ascorbic acid phosphate, and 2 mg/mL 3-amino-n-caproic acid (and 2ng/mL TGF-β1 if no conjugated TGF-β1 in fibrin). The media were replaced in the following day and every other day thereafter. After one week of static culture, some samples were periodically distended (0.3s/1.7s distended/rest, 5.5% distension) for a week while the others were kept under static condition.
Vasoreactivity and mechanical Properties
After two weeks, the tissues were photographed using a digital camera (UVP, Upland, CA) and the dimensions of each cylindrical tissue equivalent were measured using Image J. Then the tissues were released from the mandrel, mounted in an isolated tissue bath and incubated in Krebs-Ringer solution. The tissues were continuously bubbled with 94% O2, 6%CO2 to obtain a pH of 7.4, a PCO2 of 38 mmHg, and a PO2 of 500 mmHg at 37°C environment. Each construct was mounted on two stainless steel hooks through the lumen: one was fixed, and another was connected to a force transducer (ADInstrument, Cororado Springs, CO). Tissues were equilibrated at a basal tension of 1.0 g and constant length for 30–60 min until reaching equilibrium. Subsequently, the thromboxane A2 mimetic U46619 (1mM, Sigma, St. Louis, MO), Endothelin-1 (20nM, Sigma, St. Louis, MO), and potassium chloride (KCl, 118 mM) were added to the water bath in sequence after extensive washes to remove the previous vasoagonist and isometric contraction was recorded using a PowerLab data acquisition unit and analyzed with Chart5 (ADInstruments, Colorado Springs, CO).
The mechanical properties of the tissue constructs were measured using Instron model 3343 with 5N static load cell (Canton, MA). The tissues were subjected to tensile stress at 18mm/min until failure. The stress and strain were monitored and analyzed by the Bluehill3 software.
Western Blot
After two weeks in culture, vascular tissue equivalents were cut into small pieces and homogenized in lysis buffer. The lysates were subjected to WB analysis as described previously [19, 42] using the following antibodies that were diluted in 5% BSA in TBST buffer: mouse anti-human smooth muscle α-actin (ACTA2, 1:1000; Sigma, St. Louis, MO), rabbit anti-human myosin heavy chain (MYH11, 1:500; Biomedical Technologies, Stoughton, MA) or rabbit anti-elastin (1:1000; Abcam, Cambridge, MA)
Collagen quantification
Collagen quantification was based on the hydroxyproline assay described previously [43, 44]. Briefly, the tissues were lyophilized, and then hydrolyzed in 6N HCl overnight at 110°C. Next day, the hydrolysate was lyophilized and dissolved in assay buffer (5% monohydrate citric acid, 12% trihydrate sodium acetate, 3.4% sodium hydroxide and 1.2% (v/v) glacial acetic acid, pH at 6.5). After removing debris by centrifugation, 100 μL of each supernatant was loaded to a well in a 96-well plate followed by incubation with 50 μL of chloramine-T (62mM, Alfa Aesar, Ward Hill MA) for 20 min at RT. Subsequently, 50 μL of Ehrlich’s solution (Sigma, St. Louis, MO) was added to each well and incubated at 65°C for 20 min. The OD550 was measured using a microplate reader (Synergy 4, BioTek, Winooski, VT) and converted to hydroxyproline concentration using a standard curve (0 to 50 mg/ml of L-hydroxyproline, Sigma) and finally to collagen concentration using the conversion factor 7.46 g collagen per g hydroxyproline [44].
Immunostaining
Immunostaining was performed as described previously [45]. Briefly, cells, after four days in either growth medium (GM: DMEM containing 10% MSC-FBS supplemented with 2ng/mL bFGF) or differentiation medium (DM: DMEM containing 10%MSC-FBS supplemented with 2ng/mL TGF-β1) were fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 in PBS. Subsequently, they were blocked with 10% goat serum in PBS for at least two hours and continuously incubated at 4°C overnight with the following antibodies that were diluted in blocking buffer: mouse anti-human smooth muscle α-actin (ACTA2, 1:200; Sigma, St. Louis, MO), mouse anti-human calponin (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-human myosin heavy chain (MYH11, 1:100; Biomedical Technologies, Stoughton, MA,). The following day, the cells were incubated with Alexa Fluor 488 or 594-conjugated goat anti-mouse or anti-rabbit secondary antibody (1:200; 1 hr at RT; Life Technologies, Grand Island, NY), counter-stained with Hoechst nuclear dye (1:400 in PBS; 10 min; Sigma, St. Louis, MO), and overlaid with mounting medium (Electron Microcopy Sciences, Hatfield, PA). Cells that were incubated with only secondary antibody served as negative controls.
Tissue samples were fixed in 10% buffered formalin, dehydrated, embedded in paraffin and sectioned into 5 μm pieces. Tissue sections were deparaffinized, retrieved for antigen unmasking (PickCell Laboratories BV, Amsterdam, Netherlands). After Triton X-100 permeabilization and goat serum blocking, the sections were incubated with primary antibodies overnight at 4°C. The antibodies included: anti-human smooth muscle α-actin (1:200; Sigma, St. Louis, MO), anti-human smooth muscle myosin heavy chain (1:250 dilution; Lab Vision, Fremont, CA), and anti-α-elastin (1:50, DBS, Pleasanton, CA).
Samples were imaged with Zeiss Axio Observer.Z1 fluorescence microscope (LSM 510; Zeiss, Oberkochen, Germany) equipped with a digital camera (ORCA-ER C4742-80; Hamamatsu, Bridgewater, NJ) and images were analyzed using the Image J software.
Florescence Intensity Center (FIC)
In order to quantify the uniformity of the α-actin expressing cells in the tissues, we defined FIC:
where, x is the normalized location (0≤x≤1) and I(x) is the florescence intensity at position x.
The intensity profiles were determined with Image J by drawing lines radially across the ring-shape tissue sections. The position, x, was defined as the ratio of the distance between the point of interest on each line and the medium/tissue interface over the entire line length. Four images were acquired per section, each at a position that was selected at random. Four random lines were drawn on each image to determine the fluorescence intensity profiles. The average FIC of each group was derived from three individual tissue sections using the equation above.
Statistical analysis
All experiments were performed three times with triplicate samples for each condition. Pairwise comparison was analyzed by two-tailed Student t-test and the data was considered statistically different when p<0.05.
RESULTS
Hypothesis and system of study
Recently we engineered a fusion protein of TGF-β1 with a factor XIII recognition peptide and demonstrated that this protein could be conjugated enzymatically into fibrin hydrogels during polymerization (Fig. 1a). In contrast to unmodified TGF-β1, immobilized TGF-β1 prolonged activation of the Smad2/3 pathway and improved the contractile function of vascular grafts [19]. In addition to chemical cues, blood vessels experience constant pulsatile force, which has been shown to improve the function of engineered tissue constructs [46]. Taken together, these results prompted us to hypothesize that the combination of conjugated TGF-β1 (TGF-β1(c)) and application of pulsatile pressure might further enhance the function of engineered vascular grafts.
To address this hypothesis, we designed a 24-well plate based device that allows culture of fibrin-based vascular grafts under mechanical stimulation. The plate has two compartments: an autoclavable 24-well pulsatile plate and an electronic controller module. Each vascular construct is cultured around a flexible silastic mandrel, which can be pneumatically distended (Fig. 1b). Four wells are connected to a common compressed air source so that there are six individual channels in the 24-well plate (Fig. 1c). The pulsation pattern for each 4-well group was independently controlled by a 3-way solenoid valve, while the pneumatic pressure in the plate was adjusted by a needle valve and monitored by a pressure gauge (Fig. 1d). The silastic mandrel distention was determined as a function of pressure using a laser micrometer that measured the mandrel diameter in real time (Fig. 1e). Based on these measurements the tissue constructs were pulsed periodically (0.3s/1.7s distended/rested as reported previously [7]) at 5.5% distension for one week following one week of static culture.
In this study we employed human neonatal fibroblasts, which showed great proliferation capacity as well as the potential to become myofibroblasts [47]. First, we examined whether TGF-β1 could induce smooth muscle differentiation. To this end, the cells were treated with soluble TGF-β1 (2ng/ml) for 4 days and differentiation was examined by immunostaining for SMC proteins, smooth muscle cell α-actin (αSMA or ACTA2), calponin (CNN1), or myosin heavy chain (MYC11 or MHC). As shown in Fig. 2, cells treated with TGF-β1 showed well-organized αSMA, calponin, and MHC fibers as compared to cells cultured in growth medium indicating differentiation into SMC (Fig. 2).
Figure 2.

Human neonatal fibroblasts were seeded at 3,000 cells/cm2 and cultured for four days in growth medium (GM) or in myogenic differentiation medium (DM) before immunostaining for αSMA, calponin or MHC (green). Cell nuclei were counterstained with Hoechst (blue). Scale bar = 20 μm.
Differential effects of TGF-β1 conjugation and pulsatile force on cell distribution and alignment
We hypothesized that conjugation of TGF-β1 within the hydrogel might eliminate concentration gradients that are likely to exist within soluble TGF-β1 (TGF-β1(s)) treated tissues, thereby affecting cells throughout the tissue. To address this hypothesis, cell-containing fibrin was polymerized around mandrels in the presence of 50 ng/mL of TGF-β1(c). As control, TGF-β1(s) was added into the medium at 2ng/ml and the medium was replenished every two days. This growth factor concentration was previously shown to yield optimal mechanical properties and extracellular matrix synthesis of vascular grafts [25, 27, 40].
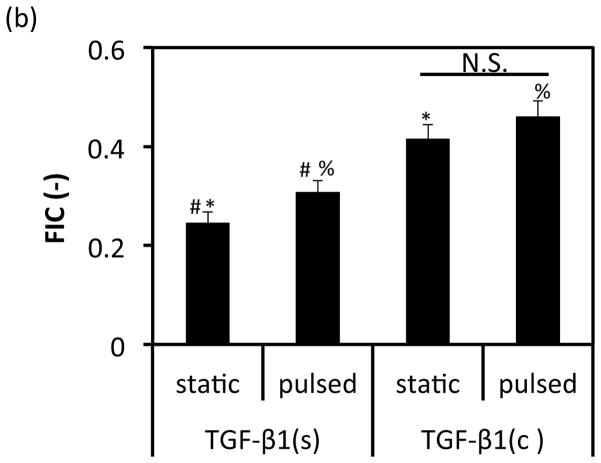
Indeed, immunostaining for αSMA showed that TGF-β1(c) influenced the distribution of αSMA expressing cells (Fig. 3(a)). In static cultures, when TGF-β1(s) was supplied in the medium, there was a clear gradient of αSMA positive cells with the highest αSMA expressing cells at the tissue/liquid interface and the lowest expressing cells closer to the lumen. In contrast, when TGF-β1 was conjugated within the hydrogel, αSMA positive cells distributed uniformly throughout the tissue. Quantitation of cell distribution using the metric of florescence intensity center (FIC) (see Materials and Methods section) confirmed this observation. Specifically, the FICs of TGF-β1(s) treated groups reflected non-uniform cell distribution with more αSMA positive cells at the tissue/liquid interface and fewer close to the lumen. In contrast, the TGF-β1(c) group exhibited FIC close to 0.5, the value representing a perfectly uniform distribution (TGF-β1(s)-static: 0.25±0.02; n=3 vs. TGF-β1(c)-static: 0.42±0.03; n=3, p<0.05; and TGF-β1(s)-pulsed: 0.31±0.02; n=3 vs. TGF-β1(c)-pulsed: 0.46±0.03; n=3, p<0.05) (Fig. 3b).
Figure 3.

Effects of TGF-β1 conjugation and mechanical stimulation on cell distribution and alignment. (a) Immunostaining of vascular constructs for αSMA. Cell nuclei were counterstained with Hoechst (blue). L: depicts the lumen side; Scale bar = 100 μm. (b) Florescence intensity center (FIC) at the indicated conditions. All values represent the mean ± SD of triplicate samples in a representative experiment (n=3). The signs (#, *, %) denote statistical significance (p<0.05) between the indicated samples. N.S.: not significant (p≥0.05).
Application of pulsatile force also improved uniformity in TGF-β1(s) treated tissues to a lesser extent than TGF-β1(c) (TGF-β1(s)-pulsed: 0.31±0.02; n=3 vs. TGF-β1(c)-static: 0.42±0.03; n=3, p<0.05) but had no significant effect within the TGF-β1(c) group (TGF-β1(c)-static: 0.42±0.03 vs. TGF-β1(c)-pulsed: 0.46±0.03; n=3, p>0.05). Finally, pulsation induced circumferential alignment of the cells in TGF-β1(s) and TGF-β1(c) treated tissues, in agreement with previous findings in the literature [30, 31, 33, 34, 48].
Synergistic effects of TGF-β1 conjugation and pulsation on MHC expression
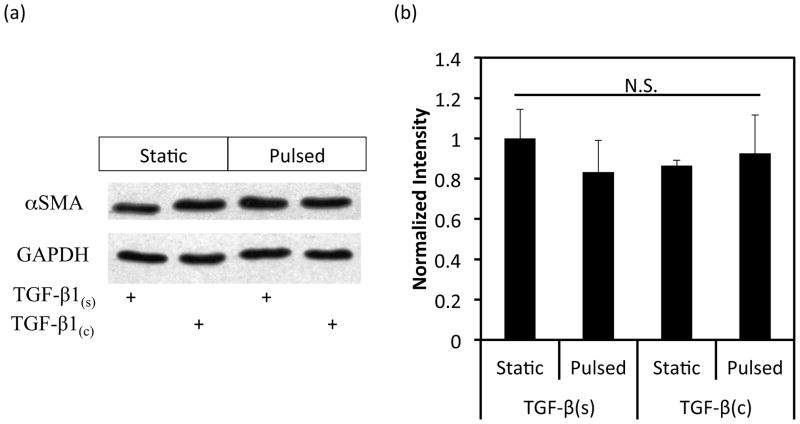
Next we investigated whether TGF-β1 conjugation and pulsation affected expression of early or late SMC marker proteins in 3D tissue constructs using WB. Interestingly, the early SMC marker αSMA was expressed at high level and remained unaffected by TGF-β1(c) or pulsation (Fig. 4a, b). On the other hand, the late SMC marker, myosin heavy chain II (MHC), increased to a similar extent by pulsation or TGF-β1(c) (TGF-b1(s)-pulsed: 1.96±0.32 fold, n=3, p<0.05; TGF-β1(c)-static: 1.80±0.48 fold, n=3, p<0.05 as compared to static-TGF-b1(s)) (Fig. 4c, d). Interestingly, the combination of pulsatile force and TGF-β1(c) enhanced MHC expression even further (3.41±0.76 fold, n=3, p<0.05 as compared to static-TGF-β1(s)). Immunostaining for MHC confirmed the WB measurements and showed that the combination of pulsation and TGF-β1 immobilization resulted in aligned cells that expressed MHC throughout the tissue constructs (Fig. 4e).
Figure 4.
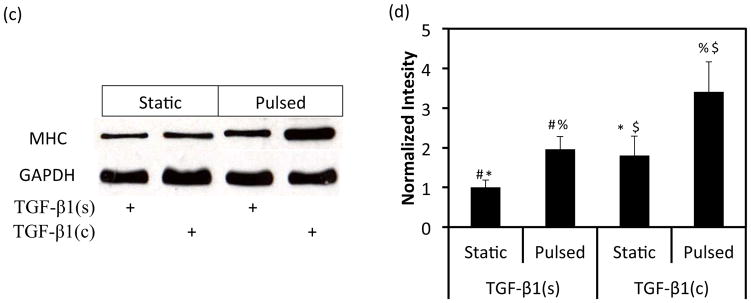
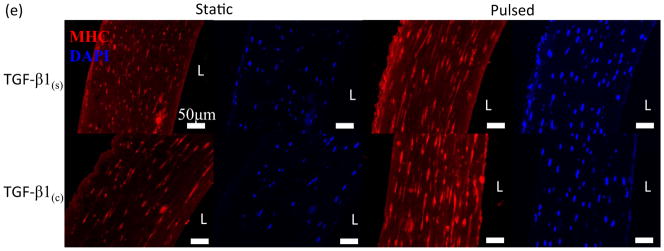
Effects of TGF-β1 conjugation and mechanical stimulation on expression of SMC proteins. (a) WB of TEV lysates for αSMA; GAPDH served as loading control. (b) Quantification of the band intensity in (a) using Image J. The intensity of each αSMA intensity was normalized to that of the corresponding GAPDH and the ratio was normalized to the ratio of the TGF-β1(s)-static group. (c) WB of TEV lysates for MHC; GAPDH served as loading control. (d) Quantification of the band intensity in (c) was done as described in (b). (e) Immunostaining of vascular constructs for MHC (red). Cell nuclei were counterstained with Hoechst (blue). L: depicts the lumen side; Scale bar = 50 μm. (b, d) All values represent the mean ± SD of triplicate samples in a representative experiment (n=3). The symbols (#, *, %, $) denote statistical significance (p<0.05) between the indicated samples. N.S.: not significant (p≥0.05).
The effect of TGF-β1 presentation on vascular contractility
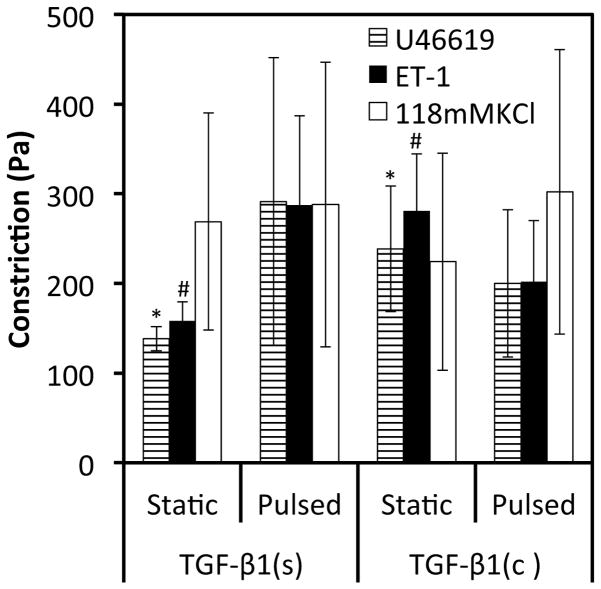
Since vascular contractility is the quintessential property of vascular tissues, we investigated the potential synergistic effects of TGF-β1 conjugation and/or pulsatile force on vascular contractility of fibrin based vascular grafts. All TEV constructs showed significant contraction in response to receptor- or non-receptor mediated agonists (Fig. 5). As compared to the TGF-β1(s) treated group, tissues containing TGF-β1(c) exhibited enhanced reactivity in response to U46619, a thromboxane analog, and to endothelin-1, but not to KCl. Surprisingly, application of pulsatile force did not enhance contractility significantly (n=3; p>0.05 as compared to static-TGF-β1(s) tissues).
Figure 5.
Conjugation of TGF-β1 improved vascular contractility. The force of contraction was measured in response to receptor mediated (U46619 and Endothelin-1) and non-receptor mediated (KCl) vasoconstrictors. All values represent the mean ± SD of triplicate samples in a representative experiment (n=3). The symbols (#, *) denote statistical significance (p<0.05) between the indicated samples.
Synergistic effects of immobilized TGF-β1 and pulsation on mechanical properties of engineered vascular grafts
Next we investigated the potential synergistic effects of TGF-β1 immobilization and pulsatile force application on the mechanical properties of fibrin-based tissue equivalents. For tensile testing, tissue rings were mounted on Instron 3343 and extended at a constant rate of 18 mm/min until they broke. Representative stress-strain curves are shown in Fig. 6a. Under static culture conditions TGF-β1(c) induced a small increase in ultimate tensile stress (UTS, not statistically significant) and Young’s modulus (YM, significant) of the tissue constructs as compared to TGF-β1(s)-static group (UTS: 1.27±0.33 fold, n=3, p>0.05, YM: 1.76±0.51 fold, n=3, p<0.05) (Fig. 6b, c). Interestingly, application of pulsatile force increased both UTS and YM of tissue constructs significantly, regardless of TGF-β1 presentation. Specifically, mechanical stimulation increased UTS by roughly 1.86±0.46 fold (n=3, p<0.05) or 1.70±0.18 fold (n=3, p<0.05) in the presence of TGF-β1(s) or TGF-β1(c), respectively. Similarly, the YM was elevated by 3.62±0.89 (n=3, p<0.05) or 2.22±0.78 (n=3, p<0.05) fold in the presence TGF-β1(s) or TGF-β1(c), respectively. Finally, application of pulsatile pressure decreased tissue ductility (strain at rupture) by 50%, but only for the TGF-β1(s) treated group (Fig. 6d).
Figure 6.
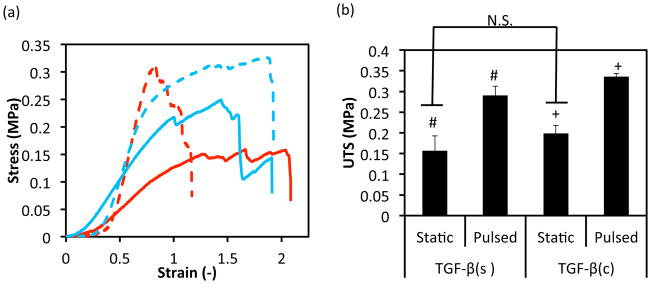

Effects of TGF-β1 conjugation and mechanical stimulation on mechanical properties of vascular grafts. (a) Representative stress-strain curves of TEVs under different treatments. Red lines represent TEVs cultured with soluble TGF-β1(s) and blue lines represent samples with conjugated TGF-β1(c). Solid lines represent samples in static culture for two weeks while dash lines represent TEVs with one week static culture followed by one week under pulsation at 5.5% distention. (b) Ultimate tensile stress (UTS); (c) Young’s moduli; and (d) Strain at rupture. All values represent the mean ± SD of triplicate samples in a representative experiment (n=3). The symbols (#, +) denote statistical significance (p<0.05) between the indicated samples. N.S.: not significant (p≥0.05).
Differential effects of TGF-β1 immobilization and pulsatile strain on ECM composition
Changes in mechanical properties of tissue constructs have been attributed to changes in ECM composition, specifically the collagen and elastin content [49, 50]. Therefore, we investigated the potential synergistic effects of TGF-β1 presentation and application of pulsatile force on collagen and elastin content.
The concentration of collagen was determined by the hydroxyproline assay and was found to increase significantly by application of pulsatile pressure, regardless of the mode of TGF-β1 presentation (Fig. 7a). Specifically, pulsatile force increased the collagen concentration by 1.64±0.47 fold in TGF-β1(s) group (n=3, p<0.05) and by 1.40±0.28 fold in TGF-β1(c) group (n=3, p<0.05). On the other hand, the collagen content was similar for both TGF-β1(s) and TGF-β1(c) static groups (n=3, p>0.05).
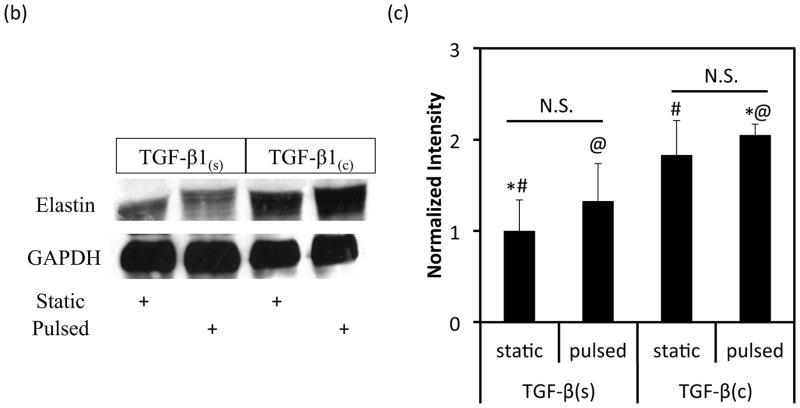
Figure 7.
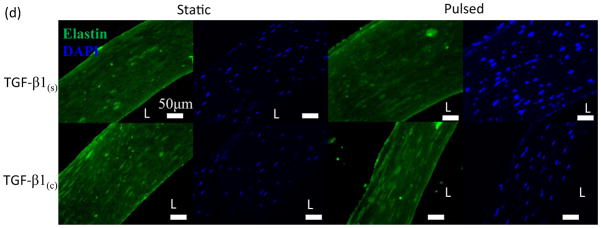
Effects of TGF-β1 conjugation and mechanical stimulation on ECM deposition. (a) Collagen content was measured by the hydroxyproline assay. (b) WB of TEV lysates for elastin; GAPDH served as loading control. (c) Quantification of the bands in (b) using Image J. The intensity of each αSMA intensity normalized to that of the corresponding GAPDH and the ratio was normalized to the ratio of the TGF-β1(s)-static group. (d) Immunostaining of vascular constructs for elastin (green). Cell nuclei were counterstained with Hoechst (blue). Scale bar = 50 μm. (a, c) All values represent the mean ± SD of triplicate samples in a representative experiment (n=3). The symbols (#, +, *, @) denote statistical significance (p<0.05) between the indicated samples. N.S.: not significant (p≥0.05).
Notably, the elastin content was dependent mostly on the mode of TGF-β1 presentation. Western blot with tissue lysates showed that immobilized TGF-β1(c) increased the level of elastin protein significantly (1.83±0.37, n=3, p<0.05 comparing to TGF-β1(s)-static) (Fig. 7b, c). The additional effect of pulsation was small and not statistically significant (1.12±0.2, n=3, p>0.05 compared to TGF-β1(c)-static) (Fig. 7c). Similarly, pulsation had no significant effect on the amount of elastin in tissue constructs that were cultured in the presence of soluble TGF-β1 (1.33±0.41 fold, n=3, p>0.05 comparing to TGF-β1(s)-static). Immunostaining showed higher elastin expression in TGF-β1(c) tissues verifying the WB results (Fig. 7d). Collectively, our results clearly showed that the combination of TGF-β1 immobilization and pulsatile force promoted ECM proteins synthesis and enhanced the mechanical properties of the vascular wall.
DISCUSSION
Fibrin has been used widely for tissue regeneration e.g. tissue engineered blood vessels [51], wound healing [52, 53], or protein and gene delivery [54–56]. The goal of this study was to investigate the synergistic effects of fibrin-bound TGF-β1 and mechanical stimulation on tissue engineered blood vessels (TEVs). Our results showed that these two biomimetic strategies could individually or synergistically enhance the myogenic differentiation, cell orientation, contractility, mechanical properties, and most notably the elastogenic potential of fibrin TEVs. These results suggest that fibrin-immobilized TGF-β1 in combination with cyclic distention may enhance the function of engineered tissues in vitro and potentially also in vivo.
TGF-β1 is involved in numerous cell physiological processes including promotion of smooth muscle differentiation and development of contractile phenotype [57]. In vivo TGF-β1 is secreted as a complex with latency-associated propeptide (LAP) and binds to extracellular matrix through the latent TGF-β binding protein (LTBP) from which it must be released to act on the cells [58]. To mimic this mode of action, we engineered TGF-β1 (TGF-β1(c)) with factor XIII recognition domain that can be enzymatically conjugated to fibrinogen during polymerization into fibrin. The engineered TGF-β1(c) was shown to prolong SMAD2 phosphorylation in 3-D context and increase the contractility of vascular constructs as compared to native TGF-β1 [19]. However, growth factors whether immobilized or soluble act within the mechanical microenvironment of each tissue [59], suggesting that TGF-β1 immobilization may act in synergy with the pulsatile force acting on the vessel wall to promote vascular construct development and function.
To address this hypothesis, we designed a 24-well based bioreactor to generate pulsatile force onto cylindrical constructs each growing around a pneumatically distensible mandrel in the middle of each well. This design provided a relatively high-throughput platform to study the role of mechanical stimulation in tissue-engineered constructs (Fig. 1b–e). Since each tissue construct was cultured in a separate well, it was also possible to investigate the individual or synergistic effects of mechanical cues and biochemical signals in the media or immobilized within the matrix that constitutes the vascular wall. Using this system we investigated the synergistic effects of TGF-β1 matrix immobilization and cyclic mechanical stimulation on the development of vascular constructs.
Conventionally, the delivery of nutrients and growth factors to cells within 3-D engineered tissues is driven by diffusion, yielding concentration gradients within the tissue. Gradients of soluble signals may in turn result in heterogeneous growth or differentiation of cells within the tissue constructs. In addition, fibroblasts compacted fibrin hydrogels to about 5% of the original volume suggesting that the increased fibrin matrix density might result in higher resistance for TGF-β1 diffusion within the tissue. In addition, TGF-β1 is consumed by cells suggesting that the TGF-β1 concentration might decrease more sharply from the tissue/medium interface than what was predicted by Fick’s law [60]. Indeed, we observed a clear ring of αSMA positive cells at the tissue/medium interface in the TGF-β1(s) group. In contrast, αSMA positive cells distributed uniformly throughout the tissues decorated with TGF-β1(c), indicating that the diffusion limitation could be overcome by immobilization of the growth factor in the vascular wall. Pulsation also increased the number of αSMA expressing cells farther from the tissue/medium interface of TGF-β1(s) tissues, albeit to a much lesser extent than TGF-β1(c) (Fig. 3b). One possible explanation might be that pulsatile force caused the micropores of the fibrin hydrogel to enlarge periodically, thereby promoting penetration of nutrients and growth factors deeper into the tissue. However, periodic distention appears to be less effective than TGF-β1 conjugation throughout the wall.
Several studies have recognized that cyclic distension [32, 34, 36, 48, 61] improved the mechanical strength of SMC based tissue constructs. In agreement, cyclic distension significantly increased both UTS and Young’s modulus of fibrin based vascular constructs independent of the mode of TGF-β1 presentation. At the same time, mechanical stimulation increased collagen deposition, which may account for the observed enhanced mechanical properties [32, 34, 62]. Also in agreement with the literature [30, 31, 33, 34, 48, 63] mechanical stimulation induced circumferential cell alignment and this effects was also independent of TGF-β1 presentation. On the other hand, the combination of TGF-β1 immobilization and cyclic pulsation increased expression of the late SMC maturation maker, MHC, which may be attributed – at least in part - to the prolonged activation of the TGF-β1 pathway [19].
Interestingly, cyclic strain did not increase vascular reactivity significantly, in contrast to previous reports with rat SMC in collagen gels [32]. This may be due to differences between human vs. rat cells or differences between collagen vs. fibrin matrix. On the other hand, TGF-β1 immobilization improved tissue vasoconstriction in response to U46619 and Endothelin-1 by approximately 2-fold. Previous work showed that TGF-β1 improved the vascular contractility of fibrin based constructs [10, 26]. We also demonstrated that immobilization of TGF-β1 enhanced reactivity to a higher extent than the soluble factor [19]. Others showed that TGF-β1 increased secretion of the vasoagonist endothelin in lung fibroblasts, ultimately enhancing collagen synthesis and the fibrotic response [64, 65]. It will be interesting to examine whether immobilized TGF-β1 increased expression of endothelin or U46619 receptors to a higher extent than the soluble factor thereby improving the response of tissue constructs to these vasoagonists.
Notably, conjugation of TGF-β1(c) increased expression of elastin significantly, but mechanical stimulation alone had a small effect. TGF-β1 was shown to increase elastin expression by stabilizing the elastin mRNA [66–68] and this effect required the activity of Smads, PKC-δ and p38 [69]. TGF-β1 also increased the mRNA level and enzymatic activity of lysyl oxidase, the enzyme that cross-links collagen and elastin after secretion in the extracellular space [70]. Our previous work showed that immobilized TGF-β1 prolonged Smad2/3 phosphorylation [19], suggesting that one possible explanation for increased elastin expression might be the sustained activity of the Smad pathway. Interestingly, TGF-β1(c) tissues maintained their ductility, which decreased in the TGF-β1(s) group under mechanical stimulation. Although the mechanism remains unknown, increased elastin expression might have contributed to the ductility of TGF-β1(c) constructs.
Overall, the combination of TGF-β1 immobilization and cyclic pulsation eliminated the gradients of differentiation signals leading to enhanced differentiation, uniform cell distribution, and increased circumferential alignment in the vascular wall. It also increased collagen and elastin synthesis leading to enhanced mechanical properties. The differential and synergistic effects of TGF-β1 conjugation and pulsatile stimulation are summarized in Table 1.
Table 1.
Summary of the differential and synergistic effects of cyclic stimulation and the presentation mode of TGF-β1 on TEVs.
| Individual effects on TEVs
|
Synergistic effects on TEVs: TGF-β 1(c)-pulsed | |||
|---|---|---|---|---|
| TGF-β1(s)-pulsed | TGF-β1(c)-static | |||
| Myogenic markers | α-actin expression | N.S. | N.S. | N.S. |
| α-actin distribution | + | ++ | ++ | |
| MHC expression | + | + | ++ | |
|
| ||||
| Reactivity | U46619 | N.S. | + | N.S. |
| Endothelin-1 | N.S. | + | N.S. | |
| KCl | N.S. | N.S. | N.S. | |
|
| ||||
| Mechanical properties | UTS | ++ | N.S. | ++ |
| Young’s modulus | ++ | + | ++ | |
| Strain at rupture | -- | N.S. | N.S. | |
|
| ||||
| ECM proteins | Collagen expression | ++ | N.S. | ++ |
| Elastin expression | N.S. | + | ++ | |
Comparing to TGF-β(s)-static samples:
N.S.: Not Significant
Significantly increased (p<0.05)
Significantly increased (p<0.01)
--: Significantly decreased (p<0.01)
CONCLUSION
In this study, we investigated the synergistic effects of TGF-β1 immobilization and mechanical stimulation on vascular tissue constructs. TGF-β1 immobilization helped to overcome the diffusion limitations hindering access of cells to soluble TGF-β1 yielding tissue constructs with uniform distribution of αSMA+ cells. TGF-β1(c) also increased vascular reactivity to receptor mediated agonists, endothelin-1 and U46619, thereby improving the function of vascular constructs. On the other hand, cyclic mechanical strain enhanced circumferential cell alignment, collagen synthesis and mechanical properties - UTS and Young’s moduli - of tissue constructs independent of the mode of TGF-β1 presentation. The combination of cyclic distention and TGF-β1 immobilization enhanced MHC suggesting that the cells reached a more mature SMC phenotype. Most notably, given the well-recognized difficulty of engineering elastin into the vascular wall, our study provides an elegant solution to this challenge by immobilizing TGF-β1 within the vessel wall. This approach may find applications in engineering other tissues that are affected by TGF-β1 and mechanical stimulation e.g. heart, bladder, cartilage or tendons. Naturally, immobilization of other proteins or peptides affecting stem cell growth or differentiation may further augment the range of tissue engineering and regenerative medicine applications.
Acknowledgments
This work was supported by grants from the National Heart and Lung Institute (R01 HL086582) and the New York Stem Cell Science Fund (NYSTEM, Contract# C024316) to S.T.A. and D.D.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Niklason LE, Abbott W, Gao J, Klagges B, Hirschi KK, Ulubayram K, et al. Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg. 2001;33:628–38. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 2.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science. 1999;284:489–93. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 3.Wu W, Allen RA, Wang Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat Med. 2012;18:1148–53. doi: 10.1038/nm.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stegemann JP, Hong H, Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol. 2005;98:2321–7. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- 5.Stegemann JP, Nerem RM. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann Biomed Eng. 2003;31:391–402. doi: 10.1114/1.1558031. [DOI] [PubMed] [Google Scholar]
- 6.Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci U S A. 2008;105:6537–42. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isenberg BC, Tranquillo RT. Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann Biomed Eng. 2003;31:937–49. doi: 10.1114/1.1590662. [DOI] [PubMed] [Google Scholar]
- 8.Syedain ZH, Meier LA, Bjork JW, Lee A, Tranquillo RT. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials. 2011;32:714–22. doi: 10.1016/j.biomaterials.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu JY, Swartz DD, Peng HF, Gugino SF, Russell JA, Andreadis ST. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res. 2007;75:618–28. doi: 10.1016/j.cardiores.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Yao L, Swartz DD, Gugino SF, Russell JA, Andreadis ST. Fibrin-based tissue-engineered blood vessels: differential effects of biomaterial and culture parameters on mechanical strength and vascular reactivity. Tissue Eng. 2005;11:991–1003. doi: 10.1089/ten.2005.11.991. [DOI] [PubMed] [Google Scholar]
- 11.Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol. 2005;288:H1451–60. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 12.Peng H, Schlaich EM, Row S, Andreadis ST, Swartz DD. A novel ovine ex vivo arteriovenous shunt model to test vascular implantability. Cells Tissues Organs. 2012;195:108–21. doi: 10.1159/000331415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng HF, Liu JY, Andreadis ST, Swartz DD. Hair follicle-derived smooth muscle cells and small intestinal submucosa for engineering mechanically robust and vasoreactive vascular media. Tissue Eng Part A. 2011;17:981–90. doi: 10.1089/ten.tea.2010.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 15.L’Heureux N, Stoclet JC, Auger FA, Lagaud GJ, Germain L, Andriantsitohaina R. A human tissue-engineered vascular media: a new model for pharmacological studies of contractile responses. FASEB J. 2001;15:515–24. doi: 10.1096/fj.00-0283com. [DOI] [PubMed] [Google Scholar]
- 16.Neidert MR, Lee ES, Oegema TR, Tranquillo RT. Enhanced fibrin remodeling in vitro with TGF-beta1, insulin and plasmin for improved tissue-equivalents. Biomaterials. 2002;23:3717–31. doi: 10.1016/s0142-9612(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 17.Wanjare M, Kuo F, Gerecht S. Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc Res. 2012 doi: 10.1093/cvr/cvs315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gacchina CE, Ramamurthi A. Impact of pre-existing elastic matrix on TGFbeta1 and HA oligomer-induced regenerative elastin repair by rat aortic smooth muscle cells. J Tissue Eng Regen Med. 2011;5:85–96. doi: 10.1002/term.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang MS, Andreadis ST. Engineering fibrin-binding TGF-beta1 for sustained signaling and contractile function of MSC based vascular constructs. Biomaterials. 2011;32:8684–93. doi: 10.1016/j.biomaterials.2011.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang G, Suggs LJ. Matrices and scaffolds for drug delivery in vascular tissue engineering. Adv Drug Deliv Rev. 2007;59:360–73. doi: 10.1016/j.addr.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Arayne MS, Sultana N. Porous nanoparticles in drug delivery systems. Pak J Pharm Sci. 2006;19:158–69. [PubMed] [Google Scholar]
- 22.Zhang G, Drinnan CT, Geuss LR, Suggs LJ. Vascular differentiation of bone marrow stem cells is directed by a tunable three-dimensional matrix. Acta Biomater. 2010;6:3395–403. doi: 10.1016/j.actbio.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Grouf JL, Throm AM, Balestrini JL, Bush KA, Billiar KL. Differential effects of EGF and TGF-beta1 on fibroblast activity in fibrin-based tissue equivalents. Tissue Eng. 2007;13:799–807. doi: 10.1089/ten.2006.0206. [DOI] [PubMed] [Google Scholar]
- 24.Goncalves LM, Epstein SE, Piek JJ. Controlling collateral development: the difficult task of mimicking mother nature. Cardiovasc Res. 2001;49:495–6. doi: 10.1016/s0008-6363(00)00286-8. [DOI] [PubMed] [Google Scholar]
- 25.Han J, Liu JY, Swartz DD, Andreadis ST. Molecular and functional effects of organismal ageing on smooth muscle cells derived from bone marrow mesenchymal stem cells. Cardiovasc Res. 2010;87:147–55. doi: 10.1093/cvr/cvq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao L, Liu J, Andreadis ST. Composite fibrin scaffolds increase mechanical strength and preserve contractility of tissue engineered blood vessels. Pharm Res. 2008;25:1212–21. doi: 10.1007/s11095-007-9499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu JY, Peng HF, Andreadis ST. Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc Res. 2008;79:24–33. doi: 10.1093/cvr/cvn059. [DOI] [PubMed] [Google Scholar]
- 28.Howard J, Grill SW, Bois JS. Turing’s next steps: the mechanochemical basis of morphogenesis. Nat Rev Mol Cell Biol. 2011;12:392–8. doi: 10.1038/nrm3120. [DOI] [PubMed] [Google Scholar]
- 29.Lu D, Kassab GS. Role of shear stress and stretch in vascular mechanobiology. J R Soc Interface. 2011;8:1379–85. doi: 10.1098/rsif.2011.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gassman AA, Kuprys T, Ucuzian AA, Brey E, Matsumura A, Pang Y, et al. Three-dimensional 10% cyclic strain reduces bovine aortic endothelial cell angiogenic sprout length and augments tubulogenesis in tubular fibrin hydrogels. J Tissue Eng Regen Med. 2011;5:375–83. doi: 10.1002/term.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niklason LE, Yeh AT, Calle EA, Bai Y, Valentin A, Humphrey JD. Enabling tools for engineering collagenous tissues integrating bioreactors, intravital imaging, and biomechanical modeling. Proc Natl Acad Sci U S A. 2010;107:3335–9. doi: 10.1073/pnas.0907813106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schutte SC, Chen Z, Brockbank KG, Nerem RM. Cyclic strain improves strength and function of a collagen-based tissue-engineered vascular media. Tissue Eng Part A. 2010;16:3149–57. doi: 10.1089/ten.TEA.2010.0009. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Cen L, Yin S, Liu Q, Liu W, Cao Y, et al. A small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cells. Biomaterials. 2010;31:621–30. doi: 10.1016/j.biomaterials.2009.09.086. [DOI] [PubMed] [Google Scholar]
- 34.Xu ZC, Zhang WJ, Li H, Cui L, Cen L, Zhou GD, et al. Engineering of an elastic large muscular vessel wall with pulsatile stimulation in bioreactor. Biomaterials. 2008;29:1464–72. doi: 10.1016/j.biomaterials.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 35.Seliktar D, Nerem RM, Galis ZS. Mechanical strain-stimulated remodeling of tissue-engineered blood vessel constructs. Tissue Eng. 2003;9:657–66. doi: 10.1089/107632703768247359. [DOI] [PubMed] [Google Scholar]
- 36.Kim BS, Nikolovski J, Bonadio J, Mooney DJ. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol. 1999;17:979–83. doi: 10.1038/13671. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi N, Yasu T, Ueba H, Sata M, Hashimoto S, Kuroki M, et al. Mechanical stress promotes the expression of smooth muscle-like properties in marrow stromal cells. Exp Hematol. 2004;32:1238–45. doi: 10.1016/j.exphem.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Khayat G, Rosenzweig DH, Quinn TM. Low frequency mechanical stimulation inhibits adipogenic differentiation of C3H10T1/2 mesenchymal stem cells. Differentiation. 2012;83:179–84. doi: 10.1016/j.diff.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Nikolovski J, Kim BS, Mooney DJ. Cyclic strain inhibits switching of smooth muscle cells to an osteoblast-like phenotype. FASEB J. 2003;17:455–7. doi: 10.1096/fj.02-0459fje. [DOI] [PubMed] [Google Scholar]
- 40.Han J, Mistriotis P, Lei P, Wang D, Liu S, Andreadis ST. Nanog reverses the effects of organismal aging on mesenchymal stem cell proliferation and myogenic differentiation potential. Stem Cells. 2012;30:2746–59. doi: 10.1002/stem.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu JY, Peng HF, Gopinath S, Tian J, Andreadis ST. Derivation of functional smooth muscle cells from multipotent human hair follicle mesenchymal stem cells. Tissue Eng Part A. 2010;16:2553–64. doi: 10.1089/ten.tea.2009.0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bajpai VK, Mistriotis P, Loh YH, Daley GQ, Andreadis ST. Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc Res. 2012;96:391–400. doi: 10.1093/cvr/cvs253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 44.Neuman RE, Logan MA. The determination of hydroxyproline. J Biol Chem. 1950;184:299–306. [PubMed] [Google Scholar]
- 45.Bajpai VK, Mistriotis P, Andreadis ST. Clonal multipotency and effect of long-term in vitro expansion on differentiation potential of human hair follicle derived mesenchymal stem cells. Stem Cell Res. 2012;8:74–84. doi: 10.1016/j.scr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vara DS, Punshon G, Sales KM, Hamilton G, Seifalian AM. Haemodynamic regulation of gene expression in vascular tissue engineering. Curr Vasc Pharmacol. 2011;9:167–87. doi: 10.2174/157016111794519390. [DOI] [PubMed] [Google Scholar]
- 47.Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell. 2003;14:2508–19. doi: 10.1091/mbc.E02-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seliktar D, Black RA, Vito RP, Nerem RM. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann Biomed Eng. 2000;28:351–62. doi: 10.1114/1.275. [DOI] [PubMed] [Google Scholar]
- 49.Gosline J, Lillie M, Carrington E, Guerette P, Ortlepp C, Savage K. Elastic proteins: biological roles and mechanical properties. Philos Trans R Soc Lond B Biol Sci. 2002;357:121–32. doi: 10.1098/rstb.2001.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–58. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grassl ED, Oegema TR, Tranquillo RT. A fibrin-based arterial media equivalent. J Biomed Mater Res A. 2003;66:550–61. doi: 10.1002/jbm.a.10589. [DOI] [PubMed] [Google Scholar]
- 52.Bannasch H, Unterberg T, Fohn M, Weyand B, Horch RE, Stark GB. Cultured keratinocytes in fibrin with decellularised dermis close porcine full-thickness wounds in a single step. Burns. 2008;34:1015–21. doi: 10.1016/j.burns.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Geer DJ, Andreadis ST. A novel role of fibrin in epidermal healing: plasminogen-mediated migration and selective detachment of differentiated keratinocytes. J Invest Dermatol. 2003;121:1210–6. doi: 10.1046/j.1523-1747.2003.12512.x. [DOI] [PubMed] [Google Scholar]
- 54.Padmashali RM, Andreadis ST. Engineering fibrinogen-binding VSV-G envelope for spatially- and cell-controlled lentivirus delivery through fibrin hydrogels. Biomaterials. 2011;32:3330–9. doi: 10.1016/j.biomaterials.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 55.Raut SD, Lei P, Padmashali RM, Andreadis ST. Fibrin-mediated lentivirus gene transfer: implications for lentivirus microarrays. J Control Release. 2010;144:213–20. doi: 10.1016/j.jconrel.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lei P, Padmashali RM, Andreadis ST. Cell-controlled and spatially arrayed gene delivery from fibrin hydrogels. Biomaterials. 2009;30:3790–9. doi: 10.1016/j.biomaterials.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 58.Hyytiainen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci. 2004;41:233–64. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- 59.Riehl BD, Park JH, Kwon IK, Lim JY. Mechanical stretching for tissue engineering: two-dimensional and three-dimensional constructs. Tissue Eng Part B Rev. 2012;18:288–300. doi: 10.1089/ten.teb.2011.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clarke DC, Brown ML, Erickson RA, Shi Y, Liu X. Transforming growth factor beta depletion is the primary determinant of Smad signaling kinetics. Mol Cell Biol. 2009;29:2443–55. doi: 10.1128/MCB.01443-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials. 2004;25:3699–706. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 62.Solan A, Mitchell S, Moses M, Niklason L. Effect of pulse rate on collagen deposition in the tissue-engineered blood vessel. Tissue Eng. 2003;9:579–86. doi: 10.1089/107632703768247287. [DOI] [PubMed] [Google Scholar]
- 63.Rowe SL, Stegemann JP. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules. 2006;7:2942–8. doi: 10.1021/bm0602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi-wen X, Kennedy L, Renzoni EA, Bou-Gharios G, du Bois RM, Black CM, et al. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor beta in human lung fibroblasts. Arthritis Rheum. 2007;56:4189–94. doi: 10.1002/art.23134. [DOI] [PubMed] [Google Scholar]
- 65.Shi-Wen X, Rodriguez-Pascual F, Lamas S, Holmes A, Howat S, Pearson JD, et al. Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol Cell Biol. 2006;26:5518–27. doi: 10.1128/MCB.00625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kahari VM, Olsen DR, Rhudy RW, Carrillo P, Chen YQ, Uitto J. Transforming growth factor-beta up-regulates elastin gene expression in human skin fibroblasts. Evidence for post-transcriptional modulation. Lab Invest. 1992;66:580–8. [PubMed] [Google Scholar]
- 67.Zhang MC, Giro M, Quaglino D, Jr, Davidson JM. Transforming growth factor-beta reverses a posttranscriptional defect in elastin synthesis in a cutis laxa skin fibroblast strain. J Clin Invest. 1995;95:986–94. doi: 10.1172/JCI117808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kucich U, Rosenbloom JC, Abrams WR, Bashir MM, Rosenbloom J. Stabilization of elastin mRNA by TGF-beta: initial characterization of signaling pathway. Am J Respir Cell Mol Biol. 1997;17:10–6. doi: 10.1165/ajrcmb.17.1.2816. [DOI] [PubMed] [Google Scholar]
- 69.Kucich U, Rosenbloom JC, Abrams WR, Rosenbloom J. Transforming growth factor-beta stabilizes elastin mRNA by a pathway requiring active Smads, protein kinase C-delta, and p38. Am J Respir Cell Mol Biol. 2002;26:183–8. doi: 10.1165/ajrcmb.26.2.4666. [DOI] [PubMed] [Google Scholar]
- 70.Hong HH, Uzel MI, Duan C, Sheff MC, Trackman PC. Regulation of lysyl oxidase, collagen, and connective tissue growth factor by TGF-beta1 and detection in human gingiva. Lab Invest. 1999;79:1655–67. [PubMed] [Google Scholar]