Abstract
Metabolic oligosaccharide engineering (MOE) refers to a technique where non-natural monosaccharide analogs are introduced into living biological systems. Once inside a cell, these compounds intercept a targeted biosynthetic glycosylation pathway and in turn are metabolically incorporated into cell-surface-displayed oligosaccharides where they can modulate a host of biological activities or be exploited as “tags” for bio-orthogonal and chemoselective ligation reactions. Undertaking a MOE experiment can be a daunting task based on the growing repertoire of analogs now available and the ever increasing number of metabolic pathways that can be targeted; therefore, a major emphasis of this article is to describe a general approach for analog design and selection and then provide protocols to ensure safe and efficacious analog usage by cells. Once cell-surface glycans have been successfully remodeled by MOE methodology, the stage is set for probing changes to the myriad cellular responses modulated by these versatile molecules.
Keywords: sialic acid glycoengineering, glycosylation, ManNAc analogs, selectin-based adhesion
INTRODUCTION
Metabolic oligosaccharide engineering—MOE; also referred to as “metabolic glyco-engineering” (Du et al., 2009)—is a technology developed in the early 1990s by Werner Reutter’s group, who discovered that certain structural variants of ManNAc (Kayser et al., 1992) can be taken up by the sialic acid biosynthetic pathway and utilized for oligosaccharide biosynthesis (Fig. 1). As a result, the cell-surface carbohydrates can be endowed with novel structural and chemical features. In theory, the ability to alter the repertoire of sialic acids on the cell surface provides a foundation to manipulate virtually any cellular function modulated by these versatile and multifaceted molecules (Campbell et al., 2007; Aich and Yarema, 2008; Wang et al., 2009; Du and Yarema, 2010). Importantly, the incorporation of bio-orthogonal chemical functionalities such as the ketone (Mahal et al., 1997), azide (Saxon and Bertozzi, 2000), or thiol (Sampathkumar et al., 2006c) groups into the glycocalyx opened the door to the conjugation with complementary probes for applications such as drug and gene delivery (Lee et al., 1999; Mahal et al., 1999).
Figure 1.

Overview of metabolic oligosaccharide engineering (MOE). MOE was pioneered for the sialic acid biosynthetic pathway, which is one of the glycosylation pathways of a cell that can accommodate non-natural analogs. Modifications in this pathway can be introduced as “R1”-modified forms of (A) ManNAc, (B) Neu5Ac, or (C) CMP-Neu5Ac. These metabolic intermediates intercept the sialic acid pathway at various points, are utilized in place of the natural pathway components, and ultimately appear on the cell surface as chemically altered form of Neu5Ac, the most common form of sialic acid found in human cells. Examples of “R1” modifications are shown in Figure 3.
In its first decade, MOE was primarily utilized to modify the cell surface without directly or explicitly altering the biology of the host cell; instead, responses were elicited indirectly (e.g., by changing viral binding; Keppler et al., 2001) or by directing drugs to the modified glycans (Mahal et al., 1997; Nauman and Bertozzi, 2001). Later, as it became evident that MOE can modulate biological responses directly (Schmidt et al., 1998; Büttner et al., 2002; Horstkorte et al., 2004), efforts aimed at using this technology to control cell behavior have intensified. For example, the interrelated abilities of MOE to control cell-substrate binding (Villavicencio-Lorini et al., 2002) and alter gene expression (Kontou et al., 2008; Elmouelhi et al., 2009) are opening new horizons for MOE.
Another fairly recent development of MOE is the extension of this technology beyond the sialic acid pathway to include pathways that process GlcNAc (Khidekel et al., 2004), GalNAc (Hang and Bertozzi, 2001) and fucose (Sawa et al., 2006). As a result, an increased number of glycans found within the glycocalyx can be endowed with novel structural and chemical features. In this article, we focus on ManNAc analogs that target the sialic acid pathway, and describe in detail parameters that require optimizing when adapting this technology for other analogs (as reviewed in detail elsewhere; Aich and Yarema, 2008; Campbell et al., 2007; Du et al., 2009), pathways, and cell types. This set of protocols is intended to serve as a foundation to broadly adapt MOE for any biological application, which theoretically includes any of the vast array of biological processes modulated by cell-surface carbohydrates.
STRATEGIC PLANNING
This article describes the planning process and general procedures required for characterization of cellular metabolism and measurement of biosynthetic incorporation of novel sugar analogs used in MOE experiments. Because of the many analogs now available (see Aich and Yarema, 2008, for a perspective), numerous options exist for MOE experiments, and it is necessary to tailor published protocols for previously untested analogs or cell lines. Accordingly, a perspective of various steps involved in setting up an MOE experiment is provided here with a brief discussion of alternative approaches and common pitfalls to avoid. Overall, as outlined in the flow chart shown in Figure 2, the process begins with the incubation of cells with non-natural analogs (Basic Protocol 1), followed by protocols to assess cell viability by quantifying growth inhibition and cytotoxicity (Basic Protocol 2). Next, the periodate-resorcinol assay, which provides a readout of successful incorporation of analog into the sialic acid pathway, is described (Basic Protocol 3). Protocols are then provided for the quantitative estimation of analog incorporation into cell-surface glycans by using bio-orthogonal ligation reactions and flow cytometry assays (Basic Protocol 4).
Figure 2.
Flow diagram depicting various steps involved in a typical MOE experiment. Procedures that are not described in this unit are denoted with a star; additional information on these steps is provided in the references cited in the main text.
Cell Line Selection
In the early years of MOE, most experiments were performed with human cancer or rodent cell lines, which readily accommodated sugar analog incorporation. More recently, as efforts have turned to applying MOE to healthy cells, it is becoming clear that metabolic incorporation of analogs can vary significantly depending on the glycosylation pathway targeted, the analog used, and even the species involved. Therefore, although MOE technology can be used for virtually any mammalian cell type, the Jurkat line makes an excellent positive control to run in parallel with cell lines of unknown MOE capabilities to ensure the fidelity of reagents and to troubleshoot procedures. Jurkat cells have served as the “workhorse” cell type for MOE (Du et al., 2009) because of several factors. First, they have a relatively modestly sized glycocalyx (~8 nm thick; Freitas, 1999), which ensures that analog uptake will not be inhibited by the thick (up to microns; Cohen et al., 2004) glycocalyx found in some cell types. Conversely, the installed glycans will be easy to detect because they will not be buried under a thick layer of hyaluronic acid or other polysaccharides that comprise a steric barrier to protein-sized probes in some cell types (Weinbaum et al., 2007). Second, Jurkat cells grow in suspension under normal culture conditions, making them trivial to harvest and amenable to time-course evaluations. Suspension culture also avoids potential problems with damage to surface glycoconjugates during the detachment steps required for adherent cell lines. An important caveat for Jurkat cells, however, is that they express truncated O-glycans (Piller et al., 1990), making this line best suited for analyzing analog incorporation into N-glycans.
Analog Selection
Analog selection requires several decisions; the first is to decide what glycan biosynthetic pathway to target. Currently, there are three main monosaccharide targets for surface display (sialic acid, GalNAc, and fucose) and one option for nucleocytoplasmic modifications (O-GlcNAc). These sugars can be experimentally accessed with ManNAc, GalNAc, fucose, and GlcNAc “core” structures shown in Figure 3A. Sialic acid has been the preferred pathway for MOE experiments because there are multiple points to intercept the pathway (e.g., by using ManNAc, Neu5Ac, and CMP-Neu5Ac analogs; see Fig. 1) and the location of this sugar at the outer termini of mammalian glycoconjugates makes it readily accessible for interactions with the microenvironment or exogenously delivered labeling reagents. In practice, ManNAc analogs are preferred over Neu5Ac or CMP-Neu5Ac analogs for MOE experiments largely because of ease of synthesis and the poor cellular uptake properties of CMP-Neu5Ac (a detailed discussion of the relative benefits of ManNAc versus Neu5Ac analogs is provided in Aich and Yarema, 2008 and in Du et al., 2009).
Figure 3.
Analog design features. (A) The “core” structures of ManNAc, GalNAc, fucose, and GlcNAc analogs used in MOE that are further derivatized with R1 and R2 groups. Categories of R1 groups, which are carried through on to the cell surface and displayed as a modify glycan, include those with (B) enlarged hydrocarbon groups, (C) heteroatom (or other) containing functional groups, and (D) direct fluorophores. (E) Examples of R2 groups include a proton (i.e., the -H found on hydroxyl groups) and 2- to 5-carbon short chain fatty acids that are ester-linked to the hydroxyl groups of the monosaccharide analog. Ac and Bu denote acetyl and n-butanoyl substitutions shown in Figure 4 and referenced throughout the main text.
The next decision involves selecting a chemical modification to install into cell-surface glycans; options fall into three main categories: enlarged alkyl hydrocarbon (e.g., alkyl) moieties (Fig. 3B), chemical functional groups (Fig. 3C), or fluorescent tags (Fig. 3D). The first category is exemplified by the initial analogs bearing elongated alkyl chains utilized in the Reutter laboratory’s landmark MOE experiments (Kayser et al., 1992). This group of compounds now includes branched and even ring structures and is ideal for high-flux applications (Kim et al. 2004). One drawback is that the surface incorporation of these analogs cannot be readily quantified. The second category consists of chemical functional groups, many of which are not found in sugars, thereby opening the door to highly versatile bio-orthogonal and chemoselective ligation options for glycan labeling (Lemieux and Bertozzi, 1998). Finally, the third group of fluorophore-bearing analogs is ideal for easy, one-step labeling of surface glycans but, because fluorophores tend to be unwieldy and bulky, they are only incorporated into glycans when introduced as CMP-Neu5Ac analogs (Brossmer and Gross, 1994).
A final design consideration involves the use of short chain fatty acid (SCFA) protecting groups that increase the lipophilicity and cellular uptake of the sugar analog (examples are the “R2” groups shown in Fig. 3E). A benefit of appending ester-linked SCFA to mask the hydrophilic hydroxyl groups of a monosaccharide is that the concentration of analog required to evoke a biological response is dramatically reduced, ranging from ~600-fold for acetyl groups (Jones et al., 2004) to more than 2100-fold for n-butyrate (Kim et al., 2004). This feature is particularly important if experiments will be extended from small-scale cell culture experiments of the type described in this article to animal models or larger-scale biotechnology applications such as the use of analog in recombinant glycoprotein production (Viswanathan et al., 2003; Luchansky et al., 2004). As a potential pitfall, the inclusion of SCFA protecting groups in analog design can substantially alter biological activity (Aich et al., 2008) and, of practical importance, lead to dose-limiting cytotoxcity that depends on the structure of both the “R1” and “R2” groups shown in Figure 3 (Kim et al., 2004). Consequently, as outlined in Basic Protocol 2, empirical determination of the effects of analog on cell viability is required.
Initial Evaluation of Analog Effects on Cell Viability
Depending on the cell line and the analog under evaluation, biological responses can be realized at concentrations as low as 5 μM (Elmouelhi et al., 2009) for SCFA-derivatized analogs, with the dynamic range reaching 75 mM or higher for free-hydroxyl compounds (Yarema et al. 1998). The upper limit for SCFA-derivatized analogs is usually determined by the dose-limiting toxicity (DLT) and can vary significantly (from 25 to >700 μM) depending on cell line (Yarema et al., 1998), cell density (Jones et al., 2004), rate of delivery, and N-acyl substituent (Kim et al., 2004). Therefore, to narrow the concentration range before undertaking highly complex procedures, such as the flow cytometry-based quantification of surface glycan display outlined in Basic Protocol 4, one (or both) of two rapid screening type assays are recommended to measure cell viability (Basic Protocol 2) and/or metabolic flux (Basic Protocol 3). In general, the effects of novel analogs on cell viability should be evaluated over a range of concentrations (typically 0 to 250 to 500 μM for SCFA-derivatized analogs) as a first step in any MOE experiment.
Detailed Characterization of Analog Metabolism and Glycan Modification
Metabolic flux can be confirmed by colorimetric assays
Analogs sometimes have no deleterious effects during pilot in vitro testing and do not noticeably affect cell viability endpoints tested in Basic Protocol 2. In this case, the analog may lack cytotoxicity or, alternatively, simply may not have been successfully taken up by the cell. Because monosaccharide analogs have chemical properties similar to many components of sugar-rich cell culture media, efforts to probe cellular uptake by measuring residual analog levels in the medium have proved cumbersome (Jones et al., 2004). Therefore, in the absence of radiolabeled metabolic precursors (which are usable but prohibitively expensive; Jacobs et al., 2001), colorimetric techniques can provide a ready means for quantitative estimation of analog flux into intracellular glycosylation pathways. Colorimetric methods exist for quantifying flux into several glycosylation pathways (e.g., glucose, King and Garner, 1947, for various monosaccharides; Walborg and Christensson, 1965), but the periodate-resorcinol assay is particularly valuable because it selectively detects sialic acids while avoiding competing signal from other monosaccharides (Jourdian et al., 1971). Our laboratory typically uses a modified version of the periodate-resorcinol method (described in Basic Protocol 3) that involves scaling the reaction from the initial 4.0-ml sample volumes to 100-μl volumes amenable to quantification in 96-well plates.
The periodate-resorcinol assay involves two major steps: (1) periodate oxidation in which a periodate ion reacts with two hydroxyl groups of vicinal diols, breaking the C-C bond to form two aldehyde groups (Tiziani et al., 2003), and (2) heating the sample with resorcinol to improve sensitivity (up to a few micrograms of sialic acid; Sussich and Cesaro, 2000). It is particularly valuable to gauge flux of analogs—such as ManNProp or ManNBut—that do not have bio-orthogonal functional groups that can be exploited ready determination of the surface display of non-natural glycans (as described for ketones and thiols in Basic Protocol 4). Certain analogs, however (e.g., ManNHex or ManNLev), do not measurably increase intracellular metabolite levels (Kim et al., 2004), although they do successfully transit the pathway and modify surface glycans (Yarema et al., 1998). For these “low-flux” analogs, colorimetric assays will not be informative (comparison with panels of “high-flux” analogs appropriate for the periodate assay are provided in previous publications, e.g., Aich et al., 2008; Kim et al., 2004). Despite these limitations, the combination of cell viability and flux assays reliably provide a narrowed concentration range for select analogs (e.g., 75 to 150 μM instead of 5 to 1500 μM) to use in subsequent assays.
Quantitative estimation of non-natural analog incorporation into surface glycans
Another method of determining analog uptake and metabolism is to directly characterize non-natural glycan display on the cell surface. This approach is more thorough than the colorimetric assay described in Basic Protocol 3 because it measures flux all the way through a metabolic pathway to the cell surface rather than just quantifying pathway intermediates. One method to accomplish this objective is to stain non-natural sugars on the cell surface with complementary antibodies (Lemieux and Bertozzi, 2001; Chefalo et al., 2004); this approach is limited, however, by a lack of antibodies (or lectins) with specificity for most non-natural monosaccharides used in MOE. Therefore, in Basic Protocol 4, a quantitation technique is described that uses chemoselective ligation reactions to detect the subset of analogs that install unique chemical functional groups (some of which are shown in Fig. 3C) into cell-surface-displayed glycans.
There is an ever increasing repertoire of bio-orthogonal reactions—based on the incorporation of chemical functional groups not naturally found in the glycocalyx—that fit into this category. Here, the discussion is limited to cells incubated with either a ketone-(Ac4ManNLev, peracetylated N-levulinoyl mannosamine; Chang et al., 2007) or thiol-bearing analog (Ac5ManNTGc; Sampathkumar et al., 2006c), but the general methodology can be readily adapted to additional functional groups that include such as azides (Saxon and Bertozzi, 2000), alkynes (Sawa et al., 2006), diazirines (Tanaka and Kohler, 2008; Bond et al., 2009), and aryl azides (Han et al., 2005). ManNLev and ManNTGc were selected for description here because they have several properties worth highlighting. For example, both analogs transit the sialic acid biosynthetic pathway and install high levels of modified glycans (estimated at up to 20 million) on the cell surface but do not lead to large increases in intermediary metabolites that are easily detected by the periodate-resorcinol assay described in Basic Protocol 3. Also, in both cases, the new functional groups displayed in the form of modified glycans can be targeted using chemoselective ligands conjugated to biotin that can then be conjugated to FITC-avidin for quantification using flow cytometry; specifically, cells displaying ketone groups can be chemoselectively ligated to hydrazides to form an acyl hydrazine (Yarema et al., 1998), whereas cells bearing thiols can be chemoselectively ligated to maleimide to form thio-ether groups (Sampathkumar et al., 2006c). Due to their chemical properties and the slightly oxidizing conditions in the extracellular milieu in normal tissue culture conditions, an additional nuance exists for glycan-displayed thiols that is worth noting insofar as a majority of cell-surface thiols form cis-disulfides (Sampathkumar et al., 2006b). Therefore, to better estimate the total level of surface sugar analog display, this protocol introduces the mild reducing agent tris-carboxyethyl)phosphine (TCEP), which reduces disulfide bonds before chemoselective ligation of thiols is performed.
Additional options for characterization of surface glycans in MOE cells
In cases where analog incorporation does not result in the display of novel bioorthogonal functional groups on the cell surface, and in the absence of an appropriate antibody or lectin for detection of the non-natural glycan, high-performance liquid chromatography (HPLC) can be used to estimate metabolic incorporation of analog provided that an authentic sample of the surface glycan is available (e.g., Sia5Prop for cells treated with ManNProp; Gagiannis et al., 2007). For the more rigorous and definitive characterization, mass spectrometry can be used to identify metabolically glycoengineered oligosaccharides (Schilling et al., 2001). Finally, the MOE process has the potential to enact unanticipated changes to surface glycans, which can be probed via emergent lectin array technologies (e.g., see Pilobello et al. 2005).
NOTE: A limited number of analogs used in MOE are commercially available; these include ManNAc (from many suppliers), Ac4ManNAc (New Zealand Pharmaceutics), Ac4ManNLev (Invitrogen), Ac4ManNAz, Ac4GalNAz, and Ac4GlcNAc; each of these three azido analogs is available from both Invitrogen and Sigma-Aldrich. Other analogs can be prepared following published protocols (e.g., see Sampathkumar et al., 2006d) that contain recipe-type instructions for synthesizing Ac5ManNGc and Ac5ManNTGc.
NOTE: Monosaccharide analogs stored in dried powder form at −20°C are stable for extended time periods (at least several months up to a few years). Stock solutions are commonly prepared by dissolving the SCFA-derivatized analogs (e.g., 1, 3, 4, or 5, Fig. 4) in ethanol (EtOH) or DMSO at a concentration of 10 mM and storing at 4°C; although sugar analogs are stable in this form over a several-month period, it is recommended to prepare modest-sized “stock” solutions that can be used within a few (6 to 8) weeks. Analogs are typically stored in ethanol for two reasons—the first is that SCFA-derivatized monosaccharides have limited solubility (often 1.0 mM or less, which does not pose a problem for biological assays usually performed at <500 μM)—and the second is to maintain sterility over extended storage periods; DMSO is an alternative solvent vehicle, but is used more often for in vivo studies than in cell culture. Typically, when the solvent level is kept below 1.0% (v/v), neither ethanol nor DMSO has measurable effects on endpoints commonly probed in MOE experiments. Nevertheless, the preferred protocol is to avoid any solvent exposure by allowing evaporation before cells are added or, when this is not possible, to ensure that the same volume is added to each sample.
Figure 4.
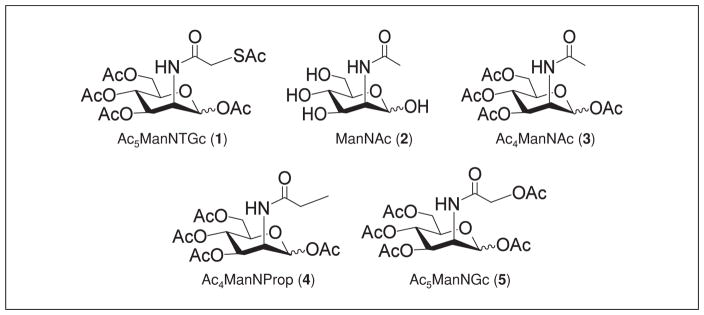
Chemical structures of ManNAc analogs used in this unit.
BASIC PROTOCOL 1. INCUBATION OF CELLS WITH SUGAR ANALOGS
The first step in MOE is the incubation of cells with sugar analogs. The protocol described herein is for the incubation of Jurkat cells with the thiol-bearing ManNAc analog Ac5ManNTGc (1 in Fig. 4). Although an exhaustive bevy of analog controls are not always required, in initial experiments it is recommended to compare the effects of a target analog (in this case Ac5ManNTGc) with appropriate control analogs. For example, ManNAc (2) serves as a control for increased flux through the sialic acid pathway and Ac4ManNAc (3) for increased flux plus any “off-target” responses engendered by the acetyl protecting groups, either from their scaffold-dependent effects (Elmouelhi et al., 2009) or from SCFA-specific responses (e.g., HDACi activity; Sampathkumar et al. 2006a) after removal by cellular esterases. Taking a step towards increased complexity, Ac4ManNProp (4) and/or Ac5ManNGc (5) would be appropriate controls for an elongated N-acyl side chain reported to enhance signaling propensities (Kontou et al., 2008) or receptor binding properties (Collins et al., 2000) of these molecules, but without the unique chemical properties of the thiol installed by Ac5ManNTGc at this position.
Materials
Stock solutions (10 mM in ethanol or DMSO) of sugar analogs shown in Figure 4 [Ac5ManNTGc (1), Ac4ManNAc (3), Ac4ManNProp (4), and Ac4ManNGc (5)], or other analogs of choice; if a free hydroxyl monosaccharide (e.g., ManNAc (2) or ManNLev; Mahal et al., 1997) is used in any experiment, a 500 mM stock solution should be prepared in PBS or serum-free culture medium and filter sterilized
Jurkat clone E6-1 (ATCC, cat. no. TIB-152; see Support Protocol 1 for culture and harvesting)
Complete RPMI 1640 medium (see recipe)
Ethanol (200 proof; Pharmco)
6-, 12-, or 24-well tissue culture plates
15- or 50-ml polypropylene centrifuge tubes
Z2 Coulter particle count and size analyzer (Beckman Coulter) or hemacytometer for counting cells
Additional reagents and equipment for harvesting Jurkat cells (Support Protocol 1)
-
Label sterile 24-well tissue culture plates, e.g., with the concentrations of SCFA-derivatized analogs shown in Table 1 or higher millimolar concentrations of free hydroxyl analogs reported elsewhere (Yarema et al., 1998), in triplicate for each concentration. Add the appropriate volume of a 10 mM stock solution of 1 to give the desired final concentrations when 0.5 ml of cells are added later (in step 4).
For volumes of ≤ 1 or 2 μl, even if a pipettor capable of measuring 0.2 to 2 μl is available, it is advisable to use 10× volumes of a 1.0 mM “stock” solution of analog to avoid pipetting errors, because the surface tension of ethanol differs from aqueous solutions sufficiently that it is difficult to add such small volumes accurately and reproducibly.When adherent cells are being tested, the same protocol described for Jurkat cells in steps 1 to 5 can be followed, where the analog is added to the culture plate before the cells. Alternatively, the appropriate amount of analog can be added to the cell culture medium after the cells are plated (steps 3 to 4); this procedure minimizes the deleterious effects of cytotoxic analogs. In this case, the total amount of ethanol added to each well should be kept constant (e.g., by separately adding the appropriate amount shown in the “optional” line of Table 1). Leave tissue culture plates in sterile hood with their lids open for ~15 min to allow the ethanol to evaporate.
While ethanol is evaporating, harvest cells as described in Support Protocol 1 for Jurkat cells, or using usual methods for an alternate cell line of choice.
-
Adjust Jurkat cell density to 5.0 × 105 cells per ml complete RPMI medium for a 48-hr experiment (or 2.5 × 105 cells per ml for a 72-hr experiment) and mix thoroughly to obtain a homogeneous single cell suspension. Add 0.5 ml of cell suspension to each well and incubate cells at 37°C.
When using cell lines that deviate significantly from the growth rate of Jurkat cells, which have a doubling time of ~1 day (~26 hr), the initial cell density should be adjusted to ensure that approximately the same number of cells are present at the end of the experiment for each condition tested.Generally, as long as cells are in a range that supports robust growth (e.g., from 2.0 × 105 to 2.0 ×106 cells per ml for the Jurkat line), cell density does not significantly influence analog metabolism (Jones et al., 2004) but as a cautionary note, very low cell densities (e.g., <1.0 × 105 cells/ml) exacerbate analog toxicity and should be avoided. -
After 48 or 72 hr (or other intervals for time-dependency experiments; see step 6), thoroughly mix to obtain a single-cell suspension by gently pipetting up and down several times with a 1.0-ml pipet. Count an aliquot of the culture to determine cell density. Cells can then be used for toxicity, labeling, or functional assays.
Typically, the surface display of modified glycans reaches maximal levels after 2 or 3 days of incubation with SCFA-derivatized analogs (e.g., Ac4ManNLev), whereas free hydroxyl monosaccharides (e.g., ManNLev) may require up to 5 days to achieve saturating surface display. The shorter time period is advantageous because cells do not need to be passaged (which they do for a 4-day or longer incubation) and it is also generally long enough to observe growth inhibition and early indications of analog-induced cytotoxicity and apoptosis. -
Study analog incorporation as a function of time.
Several options are available. For short time intervals (e.g., <48 hr), we suggest preparing separate plates as described in step 1 and simply harvesting each one at a specified time. For time points <24 hr, a higher initial cell density (e.g., 1.0 × 106 cell/ml) may be required to ensure a sufficient number of cells for analysis. For multi-day time points, because Jurkat and other human cancer cell lines double in ~1 day, one option is to prepare larger volume cultures (e.g., 1.0 ml) and remove 0.5 ml each day for analysis. The volume is replenished with 0.5 ml of fresh medium and the appropriate volume of analog can be added to maintain the initial final concentration; in this case, when the analog is added directly to the culture rather than allowed to evaporate as described in step 2, the “optional” amount of ethanol (e.g., as shown in line 3 of Table 1) should be added to maintain the same amount of solvent in each well.
Table 1.
Final Concentrations of Analogs in Culture Medium after 0.5 ml of Cells are Added
| Final concentration of analog (1, 3, 4, 5; μM) | 0 | 5.0 | 10 | 20 | 40 | 80 | 160 | 320 |
| Stock solution of 10 mM 1, 3, 4, or 5 added (μl) | 0 | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 | 8.0 | 16 |
| Ethanol added (μl) (optional, see text) | 16 | 15.75 | 15.5 | 15 | 14 | 12 | 8.0 | 0 |
SUPPORT PROTOCOL 1. ROUTINE GROWTH AND MAINTENANCE OF JURKAT CELLS
This protocol describes routine methods to maintain Jurkat cells, a lymphocyte line derived from human acute T cell leukemia (clone E6-1 are often referred to as “wild-type” Jurkat cells). In general, no special procedures are needed for preparation of cells for MOE experiments; therefore, the usual methods used to culture the cell line of choice can be followed to prepare cells for use in Basic Protocol 1, above.
Materials
Jurkat clone E6-1 (ATCC, cat. no. TIB-152)
Complete RPMI 1640 medium (see recipe)
Z2 Coulter particle count and size analyzer (Beckman Coulter Inc) or hemacytometer for counting cells
75-cm2 tissue culture flask (Sarstedt, cat. no. 83.1813)
37 °C, 5% CO2, humidified incubator
15- or 50-ml polypropylene centrifuge tubes
-
Add Jurkat cells to the appropriate amount of complete RPMI 1640 medium to attain a cell density of ~1.0–2.5 × 105 cells/ml (e.g., 1.5 × 106 cells in 15 ml in a 75-cm2 flask) and place the flask or culture dish in incubator.
The volume of initial culture medium and/or the number of cells used should be adjusted depending on the number of cells required in step 5 (for use in Basic Protocol 1). -
Incubate up to 4 days.
The cells should reach a density of ≤ 2.0 × 106 cells/ml (if the cells in step 1 are freshly thawed, cell growth to saturating cell density may take up to 1 week).Jurkat cells are suspension cells and tend to form small, loosely associated clusters while growing in suspension; the cells should be dissociated, which can be accomplished by gently pipetting them up and down several times, before counting to ensure accuracy. In cases where the pH indicator in the culture medium has not changed color indicating accumulation of metabolic waste products, and where the cells will be diluted significantly in subsequent use, steps 3 and 4 may not be necessary. Transfer the cell suspension into a 15- or 50-ml centrifuge tube and centrifuge 3.5 min at 1300 × g, room temperature. Carefully discard supernatant and resuspend cells in 10 ml of prewarmed (37°C) complete RPMI medium.
Count the cell density using either an electronic cell counter or a hemacytometer; again, be sure to disperse cell clumps by gently pipetting up and down several times before counting.
Replate an aliquot of the cells as described in step 1 and place the stock flask in incubator; the remainder of the harvested cells (typically 85% to 95% of the total number of cells initially present) can be used in MOE protocols.
BASIC PROTOCOL 2. CELL VIABILITY ASSAYS
SCFA-monosaccharide analogs often have a negative impact on cell viability; these deleterious effects vary greatly, ranging from transient growth inhibition to—at high enough concentrations—the rapid onset of apoptosis. Cytotoxicity depends on analog structure. For example, Jurkat cells incubated with acetylated ManNAc analogs experience cyto-toxicity not observed with free hydroxyl monosaccharides, and the degree of toxicity is influenced by the N-acyl modification (Kim et al., 2004); thus, both the “R1” and “R2” modifications shown in Figure 3 affect cell viability. Cell viability can be evaluated with various levels of sophistication and thoroughness. At the simplest, cell counts obtained in Basic Protocol 1 identify analogs that inhibit cell growth (which can be quantified as an IC50 value that gives an inhibitory concentration that reduces cell counts to 50% of control values) after short-term analog exposure (e.g., 2, 3, or 5 day). Because actual cell death through analog exposure is slow over concentration ranges used in most MOE experiments, additional analyses must be done to determine the viability of cells in samples that contain fewer cells than the negative control (i.e., without analog). Reduced cell numbers could be due to transient growth inhibition from which the cells can recover and resume robust growth, or the remaining cells could be fated to die over the next several days (Sampathkumar et al., 2006a). If this issue needs resolution, trypan blue (or other live/dead assays) can be used to detect if dead cells are already present; standard assays for apoptosis can be run to determine if programmed cell death has been set in motion, or the MTT assay can be used to evaluate the metabolic state of the cells. A straightforward and reliable way to determine lethality, however, is to continue to monitor cell counts over a 2-week period (Sampathkumar et al., 2006a); in general, IC50 data obtained from 2-, 3-, or 5-day cell counts or LD50 data from 15-day experiments are adequate to determine safe levels of analog to use for subsequent metabolic uptake (e.g., Basic Protocol 3) and labeling (Basic Protocol 4) experiments.
Materials
Jurkat clone E6-1 (ATCC, cat. no. TIB-152; see Support Protocol 1 for culture and harvesting)
Complete RPMI 1640 medium (see recipe)
Phosphate-buffered saline (PBS), pH 7.4 (Invitrogen, cat. no. 10010-049)
10 mM stock solutions of analog: e.g., Ac4ManNAc (3) plus any additional analogs required for user-specific applications)
Ethanol (EtOH) (200 proof) for controls
24-well tissue culture plates
Z2 Coulter particle count and size analyzer (Beckman Coulter) or hemacytometer for counting cells
Growth inhibition (IC50) and cytotoxicity (LD50) assays
-
Incubate Jurkat cells (1.0 × 105 cells in 0.5 ml) with analog (e.g., 2, 0 to 320 μM), using a 24-well plate as described in Basic Protocol 1, in a 37°C, 5% CO2 incubator for 3 days.
If adherent cells are used, two plates (times 3 replicates) for each condition will be needed because the cells will be trypsinized, counted, and discarded at each of the two time points (days 3 and 5; step 2). Steps 3 to 5 are typically not performed for adherent cells because without trypsinization and passaging they tend to become overconfluent, and with these procedures the manipulations introduce errors that lead to inaccurate results. On day 3, gently pipet Jurkat cells up and down using a 1-ml pipet to dislodge cell clumps. Take two 100-μl aliquots of cell suspension and add each to 10 ml of PBS in a cuvette. Measure and record the cell density using a cell counter. If a cell counter is unavailable, perform a manual cell count using a hemacytometer. Add 1.0 ml of medium to each well and place cells in incubator. Repeat this step on day 5.
On day 7, gently pipet the Jurkat cells to dislodge cell clumps. Remove 1.0 ml of cell suspension and add 1.0 ml of fresh medium. Repeat this step for days 9, 11, and 13.
On day 15, gently pipet the Jurkat cells to dislodge cell clumps. Take a 1.0-ml aliquot and add it to 10 ml of PBS in a cuvette. Measure and record the cell density using a cell counter (or hemacytometer).
Calculate the relative cell number for each analog concentration with respect to the EtOH control for day 3, 5, and 15. Plot the relative cell number with respect to analog concentration for days 2 or 3, 5, and 15, respectively, to determine IC50 or LD50 values, which are the concentrations where relative cell numbers are 50% of untreated control samples.
BASIC PROTOCOL 3. PERIODATE-RESORCINOL ASSAY TO MEASURE ANALOG UPTAKE BY A CELL AND INCORPORATION INTO METABOLIC PATHWAYS
Once cell viability has been analyzed to determine the maximum concentration of glycan analog that can be used without deleteriously affecting cell viability, the next step is to quantify the extent of analog incorporation. The ideal way to measure analog uptake and metabolism is to quantify non-natural glycan display on the cell surface as described in Basic Protocol 4; this approach is preferred to the periodate-resorcinol assay described here in Basic Protocol 3 because it measures flux all the way through a metabolic pathway to the cell surface. However, the detection of non-natural glycans on the cell surface is not trivial and almost always requires more highly sophisticated instrumentation than used for the periodate-resorcinol assay. In brief, the periodate-resorcinol assay is a colorimetric technique that quantifies total and glycoconjugate-bound sialic acids that can provide a rapid quantitative estimation of ManNAc analog incorporation into the sialic acid biosynthetic pathway. It is attractive because it provides a low-cost and technically simple option to monitor cellular uptake and subsequent successful incorporation of “high-flux” analogs into the sialic acid biosynthetic pathway.
Materials
Jurkat clone E6-1 (ATCC, cat. no. TIB-152; see Support Protocol 1 for culture and harvesting)
Complete RPMI 1640 medium (see recipe)
10 mM Ac4ManNAc (3) stock solution plus additional sugar analogs of choice
Ethanol (EtOH) (200 proof; Pharmco) for controls
Phosphate-buffered saline (PBS) pH 7.4 (Invitrogen, cat. no. 10010-049)
0.4 M periodic acid stock solution (see recipe)
6% (w/v) resorcinol (Sigma, cat. no. 108-46-3; store at −20°C)
t-butyl alcohol (2-methyl-propan-2-ol; Sigma, cat. no. 3972-25-6)
10 mM sialic acid stock solution (see recipe)
2.5 mM CuSO4 (see recipe)
Concentrated HCl
Z2 Coulter particle count and size analyzer (Beckman Coulter) or hemacytometer for counting cells
25-cm2 tissue culture flasks
Heat block
96-well microtiter plates
Microplate reader (μQuant, Bio-Tek Instruments)
-
Incubate Jurkat cells (or other line being tested) with sugar analog(s) as described in Basic Protocol 1 but use 25-cm2 tissue culture flasks instead of 24-well plates and seed ~1.0 × 107 cells in 10 ml.
For Jurkat cells, a volume of 10 ml in a 100-mm culture dish will provide a sufficient number of cells to perform the resorcinol assay in triplicate; in some cases a culture size of 2 ml in a 6-well plate or 35-mm culture dish will yield sufficient cells. At a minimum, ~1.0 × 106 cells are recommended for each replicate to obtain reliable results. For adherent cells, a 75-cm2 tissue culture flask will provide enough cells for triplicates.We suggest using a high-flux, low-toxicity analog such as 3 in all experiments as positive control. Over a concentration range of 0 to 250 μM, these compounds are expected to increase levels of total sialic acid by at least 10-fold in most cell lines. -
Harvest the cells after 2 to 3 days.
For Jurkat cells, gently pipet cell suspension up and down using a 1-ml pipet to dislodge cell clumps. If adherent cells are being tested, detach the cells from the culture flask using trypsin. Place the cells in a 15-ml centrifuge tube and centrifuge the cell suspension 5.0 min at 1000 × g, and discard supernatant. Gently resuspend cells in 10 ml PBS. Gently pipet cell suspension up and down using to dislodge cell clumps.
-
Centrifuge cell suspensions in 15-ml centrifuge tubes 3.5 min at 1300 × g, room temperature, and discard the supernatant. Resuspend cells in 5.0 ml of PBS by gentle pipetting. Repeat this step an additional two times to wash the cells.
FBS used in culture media is rich in glycoconjugate-bound sialic acids and must be thoroughly removed to ensure accuracy and reproducibility. Remove two 100-μl aliquots of cells to count the cell density using a cell counter or hemacytometer. Based on the total number of cells in each condition, add the appropriate amount of PBS to obtain cell density of 1.0 × 106 cells/ml. Pipet the cell suspension up and down gently to obtain an homogenous cell suspension.
-
Aliquot 1.0 ml of resuspended cells into three 1.5-ml microcentrifuge tubes (1.0 × 106 cells/tube) to yield three replicates per condition.
In general, at least 1.0 × 106 cells are needed to provide a robust colorimetric signal; an exception is provided by “high-flux” analogs (such as 3 or 4) that increase sialic acid levels by 10-fold or more. In this case, a correspondingly lower number of cells can be used for each replicate. Pellet the cells by centrifugation (as described in step 4) and resuspend in 300 μl of PBS.
Freeze the tubes by placing in a −80°C freezer for ~1 hr (or if available, the tubes can be placed in pulverized dry ice, in which case they will freeze in 5 to 10 min). Thaw the tubes in a 37°C water bath. Repeat for two more freeze/thaw cycles (three times in total).
-
While tubes are undergoing freeze-thaw cycles, prepare the other reagents.
Remove periodic acid stock solution and 6% (w/v) resorcinol solution from storage in a −20°C freezer to thaw at room temperature.
Warm t-butyl alcohol to 25°C (or just above room temperature).
Prewarm heating block to 100°C.
-
Prepare 300 μl sialic acid standards in 1.5-ml microcentrifuge tubes over the concentration ranges shown in Table 2.
Sialic acid standards should be prepared using the same buffer as the samples, which are usually in PBS.
Add 5.0 μl 0.4 M periodic acid to each sample (including standards). Vortex and place on ice for 15 min.
-
Incubate samples for glycoconjugate-bound measurements at 37°C for 80 min.
This step dissociates free sialic acid–periodate complexes such that only “bound” sialic acids (sialic acids that have been converted by cells into glycoconjugates) are detected in the last step. Sialic acids that have only been taken up by the cells but not incorporated into the cells’ metabolic pathways are not detected.To only measure the total sialic acid content (free and bound), omit this step. -
Prepare mixture of resorcinol working reagent as follows:
1.5 ml 6.0% resorcinol
1.5 ml 2.5 mM CuSO4
6.6 ml deionized H2O
5.4 ml concentrated HCl.
Add 500 μl of resorcinol working reagent into each tube, vortex and incubate at 100°C for 10 min using the heating block.
To ensure more uniform heating, fill heat block with water after placing the microcentrifuge tubes in it. When heating, place an empty heat block on top of the microcentrifuge tubes to prevent them from opening. Remove the tubes from the heat block and cool rapidly in room temperature water.
Add 500 μl of t-butyl alcohol to each tube. Mix the tube contents by vortexing. Centrifuge the tubes at maximum speed in a benchtop microcentrifuge for 2 min.
-
Aliquot samples into 96-well plates (two wells for each sample). Measure absorbance at 630 nm.
Absorbance can also be measured by placing samples in a cuvette and measuring using a spectrophotometer. Measurement using a microplate reader allows for smaller-volume samples to be used and provides a semi–high throughput alternative to cuvette-based methods. One disadvantage to the 96-well format is that, unlike a cuvette where path length is always constant, the depth of solution in a well changes the path length and thereby changes the spectroscopic reading. Therefore, it is critical to aliquot precisely the same volume (e.g., 100 μl) into each well; this feature also provides flexibility insofar that if the sample is too dilute to obtain a reading, 200 or 300 μl can be added to the well (conversely, if the sample is too concentrated, 25 or 50 μl can be used).
Table 2.
Preparation of Sialic Acid Standardsa
| Volume of 1.0 mM sialic acid stock solution | Volume of PBS | Final sialic acid concentration |
|---|---|---|
| 0 μl | 300 μl | 0 μM |
| 3 μl | 297 μl | 10 μM |
| 6 μl | 294 μl | 20 μM |
| 9 μl | 291 μl | 30 μM |
| 12 μl | 288 μl | 40 μM |
| 15 μl | 285 μl | 50 μM |
Prepare a fresh 1.0 mM stock solution by diluting 50 μl of 10 mM sialic acid (see recipe) in 450 μl of PBS.
BASIC PROTOCOL 4. QUANTITATION OF CELL-SURFACE GLYCOCONJUGATES
The display of non-natural glycans on the cell surface is an inherently inefficient process, especially at steps where analogs enter a cell, as well as bottlenecks within intracellular glycosylation pathways themselves (Viswanathan et al., 2003). Therefore, analog incorporation into glycans is not a linear function of medium concentration (or even intracellular metabolite levels as determined by the periodate-resorcinol assay), but is better estimated through direct quantification of non-natural analogs displayed on the cell surface. The development of non-natural analogs with orthogonal chemistries such as ketones (Yarema et al., 1998), azides (Saxon and Bertozzi, 2000), and thiols (Sampathkumar et al., 2006b) that do not naturally occur in the glycocalyx has greatly facilitated detection and quantification of surface-displayed analogs, as they can be conjugated with fluorescent labels using chemoselective ligation reactions. Commonly, the cell-surface functional groups are reacted with biotin-conjugated chemoselective ligands and subsequently stained using fluorescein-conjugated avidin or streptavidin; quantitative estimation of surface display is then performed using flow cytometry. In this protocol, an approach for the detection of glycan-displayed thiol and ketone functional groups is described.
Materials
Jurkat clone E6-1 (ATCC, cat. no. TIB-152; see Support Protocol 1 for culture and harvesting)
Complete RPMI culture medium (see recipe)
10 mM Ac5ManNTGc (1) stock solution for thiol labeling experiments and/or Ac4ManNLev (Kim et al., 2004) [or 1,3,4-O-Bu3ManNLev (Aich et al., 2008)) stock solution for ketone labeling]
Ethanol (EtOH; 200 proof)
Phosphate-buffered saline (PBS) pH 7.4 (Invitrogen, cat. no. 10010-049)
Tris(2,carboxyethyl)phosphine hydrochloride (TCEP; Sigma, cat. no. C4706)
MB solution (see recipe), freshly prepared
Biotin buffer (see recipe), freshly prepared
5.0 mM biotin hydrazide stock solution (see recipe)
Avidin staining buffer (ASB; see recipe)
Fluorescein isothiocyanate (FITC)-labeled avidin stock solution (see recipe)
Z2 Coulter particle count and size analyzer (Beckman Coulter Inc) or hemacytometer for counting cells
6-well tissue culture plates
Centrifuge
Tubes for flow cytometer (5-ml polystyrene round-bottom tubes, 12 × 75–mm; BD Falcon, BD Biosciences, cat. no. 352054)
Flow cytometer equipped with a 488-nm argon laser (Becton Dickinson FACSCalibur, BD Biosciences)
Incubate cells with sugar analogs
-
1
Incubate Jurkat cells with sugar analog (e.g., 1 for thiol expression or Ac4ManNLev or1,3,4-O-Bu3ManNLev ) as described in Basic Protocol 1, but use 6-well tissue-culture plates (or 35-mm dishes) instead of 24-well plates, and seed ~1.0 × 106 cells in 2.0 ml for each sample to be analyzed.
This volume should provide sufficient number of cells at the end of 2 or 3 days of incubation (i.e., ~ 4.0 × 106 cells in total) to perform the quantitative estimation of surface display in triplicate.When cell availability is limiting, as few as 2 × 105 cells per replicate can be used, or the number of replicates can be reduced to two.Jurkat cell density should be maintained between 0.5 to 2 × 106 cells/ml. Below this density, the cells are more sensitive to analog toxicity, and above this level, their growth rates slow, which may deleteriously affect analog metabolism (Jones et al., 2004).
Prepare cells for labeling of surface functional groups
-
2
Gently pipet cell suspension up and down using a 5.0-ml pipet to dislodge cell clumps. Count an aliquot of cells to determine the cell density.
The cell density—and the total number of cells per sample—is not critically important in these labeling steps. However, if there are too many cells (e.g., >2.0 × 106 per sample), a large pellet is formed that traps reagents during wash steps. By contrast, if there are too few cells (e.g., <0.5 × 106 per sample), the pellet may not be large enough to see during the wash steps and can potentially be lost. -
3
Centrifuge cell suspensions in 15-ml tubes 3.5 min at 1300 × g, room temperature, and discard supernatant.
-
4
Add 3.0 ml of PBS to the cells and resuspend the cells by gentle pipetting. Avoid vortexing, to minimize damage of cells.
-
5
Aliquot 1.0 ml of resuspended cells to three 1.5-ml microcentrifuge tube (there should be a maximum of 2.0 × 106 cells/tube).
This will provide three replicates per condition.
To label for thiol expression
-
6a
Wash the cells twice with PBS. For each wash cycle, centrifuge the cells 3.5 min at 3000 × g, room temperature, discard the supernatant, and gently resuspend cells in 1.0 ml PBS.
-
7a
Prepare a 10 mM solution of TCEP by combining 2.87 mg TCEP with 1.0 ml PBS. Centrifuge the cell suspension 3.5 min at 3000 × g, room temperature, and discard supernatant. Resuspend the cell pellets in 360 μl of PBS and add 40 μl of 10 mM TCEP solution to the samples to yield a final TCEP concentration of 1.0 mM. Lay the samples on the benchtop at room temperature for 1.0 hr. Gently invert the tubes every 15 min to ensure uniform labeling.
It is crucial to mix the reagent immediately upon its addition into the cell suspension, to avoid cell aggregation or cross-linking.The time and concentration required for TCEP treatment has been optimized as a compromise between complete reduction of disulfides (which is estimated to be only 90% to 95% complete under the given conditions) and a loss of cell viability that occurs at longer time periods or higher concentrations of TCEP. To ensure reproducibility between experiments performed on different days, it is recommended to maintain TCEP concentration, time of incubation, and temperature as uniform as possible (note that if room temperature varies from 18° to 25°C over time, this change could measurably affect TCEP reduction of disulfides as well as the labeling efficiency in step 8a).If TCEP treatment for disulfide bond detection is not intended, or to compare TCEP-treated and untreated cells to estimate the degree of disulfide formation under normal culture conditions, add 400 μl of PBS to the cell aliquot(s) and directly proceed to step 8a. -
8a
Add 100 μl of freshly prepared MB solution to the cell suspension and mix immediately with gentle pipetting.
-
9a
Lay the tubes flat on the table at room temperature for 1.0 hr. Flip the tubes every 15 min to ensure uniform labeling.
To label for ketone expression
-
6b
Wash the cells twice with freshly prepared biotin buffer, then twice with PBS. For each wash, centrifuge the cells 2.5 min at 3000 × g, room temperature, discard the supernatant, and gently resuspend cells in 1.0 ml biotin buffer.
-
7b
Centrifuge cells 3.5 min at 3000 × g, room temperature, and resuspend gently in 250 μl biotin buffer.
-
8b
Add 250 μl of 5.0 mM biotin hydrazide stock and mix immediately by gently pipetting up and down.
It is crucial that the cells be properly resuspended before adding biotin hydrazide. Mix the reagent immediately upon its addition into the cell suspension to avoid cell aggregation and cross-linking (note that other precautions described for thiol detection in steps 6a to 9a also apply for ketone labeling here in steps 6b to 9b). -
9b
Lay the tubes flat on the table at room temperature for 2.0 hr. Mix the tubes every 15 min to ensure uniform labeling.
Stain with FITC-avidin
-
10
While the cells are being incubated in the MB (or biotin hydrazide) solution, cool 50 ml of ASB and 50 ml of PBS in an ice bath.
-
11
Centrifuge the tubes from step 9a or b 3.5 min at 3000 × g, room temperature, and discard supernatant. Add 1.0 ml of ice cold ASB to each tube and resuspend gently. Repeat this step two more times. During the third wash, resuspend cells in 100 μl ASB.
-
12
Prepare FITC-avidin working solution by diluting FITC-avidin stock solution 1:250 in PBS. Prepare slightly more than the required amount (200 μl per tube) to avoid insufficient solution for the last tube. Mix well and keep in dark on ice (not more than 12 hr).
-
13
Add 100 μl of FITC-avidin working solution to each tube and mix immediately by gentle pipetting. Incubate tubes on ice out of direct light for 15 min.
It is important to properly resuspend the cells before addition of FITC-avidin. Also, mix the solution immediately after addition to obtain uniform labeling and avoid cell clumping. -
14
Centrifuge the tubes 3.5 min at 3000 × g, room temperature, discard supernatant, and resuspend cells gently in 100 μl of ASB. Repeat step 13.
Inconsistent results may be caused by incomplete removal of unreacted biotin reagents from the cell suspension before staining with FITC-avidin, as FITC-avidin reacts preferentially with the “free” biotin reagents instead of the cell surface. In this protocol, the first addition of FITC-avidin functions to remove any remaining reagents, allowing cells to be more reproducibly stained the second time. -
15
During incubation with FITC-avidin, collect untreated Jurkat cells and place ~1.0 × 106 cells into each of two 1.5-ml microcentrifuge tubes. Wash these cells twice, resuspend in 500 μl PBS, and transfer cell suspension into 5-ml polystyrene tubes. Keep the tubes on ice.
These will be used to adjust flow cytometer settings and define the healthy cell population on the forward scatter–side scatter plot. -
16
Centrifuge the tubes 3.5 min at 3000 × g, room temperature, discard supernatant, and resuspend cells gently in 1.0 ml of ice cold ASB. Wash the cells twice to ensure complete removal of unbound FITC-avidin. For the third wash, resuspend cells in 500 μl PBS and transfer to 5-ml polystyrene tubes and keep on ice out of direct light.
Samples may be kept for up to 3 hr before analysis by flow cytometry.
Analyze by flow cytometry
-
17
Analyze the samples by flow cytometry.
It is advisable to collect at least 10,000 events for each run for more accurate results. Run analysis twice for each sample. -
18
Measure the relative fluorescence content (and hence relative surface display) of cells using the geometric mean in arbitrary units.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps.
Avidin staining buffer (ASB)
25 ml FBS (HyClone, cat. no. SH30071.03)
5.0 ml 10% (w/v) sodium azide (EM Science, cat. no. SX0299-1)
500 ml sterile phosphate-buffered saline (PBS; Invitrogen, cat. no. 10010-049)
-
Store up to 6 to 8 weeks at 4°C
This buffer is used while staining cells for surface display.
Biotin buffer
95 ml PBS (Adjusted to pH 6.5 with concentrated hydrochloric acid)
5.0 ml FBS (HyClone, cat. no. SH30071.03)
Filter sterilize
-
Prepare fresh
This buffer is used to prepare biotin hydrazide solution for labeling surface ketone groups.
Biotin hydrazide stock solution, 5.0 mM
25.834 mg biotin hydrazide (Sigma, cat. no. B-7639)
20 ml PBS (adjusted to pH 6.5 with concentrated hydrochloric acid)
-
Store up to 6 to 8 weeks at 4°C
This solution is used for labeling surface ketone groups. Biotin hydrazide may take up to 1 hr to dissolve. If precipitate forms during storage, do not use.
Complete RPMI 1640 medium
470 ml RPMI 1640 medium (Invitrogen, cat. no. 11875-119)
25 ml fetal bovine serum (FBS; HyClone, cat. no. SH30071.03)
5.0 ml 100× penicillin-streptomycin solution (P/S; Sigma, cat. no. P-0781)
-
Store up to one 1 month at 4°C
This medium is used for culturing Jurkat cells.
CuSO4 solution, 2.5 mM
1.0 ml 40 mM copper sulfate (Sigma, cat. no. 7758-98-7)
15 ml deionized water
Store up to 6 to 8 weeks at 4°C
FITC-avidin stock solution
2.0 mg FITC-labeled avidin (Sigma, cat. no. A-2901; see recipe)
714 μl PBS (Invitrogen, cat. no. 10010-049)
-
Store in dark at 2°C for up to 6 months
Dissolve FITC-labeled avidin completely in PBS.
Dilute stock solution 1:250 in PBS for staining MB labeled cells.
MB solution, 5.0 mM
3.9 mg EZ link maleimide PEO2-biotin (MB; Pierce Biotechnology, cat. no. 21901)
-
1.5 ml PBS (Invitrogen, cat. no. 10010-049)
This solution is used to label surface thiol groups. Dissolve MB in PBS immediately before use.
Periodic acid, 0.4 M
910 mg periodic acid (Sigma, cat. no. 10450-60-9)
10 ml deionized water
Aliquot into ten 1.5-ml microcentrifuge tubes (1.0 ml each)
-
Store at −20°C
This solution is for use in the periodate-resorcinol assay.
Sialic acid stock solution, 10 mM
10 mg N-acetylneuraminic acid (Sigma, cat. no. A-9646)
3.233 ml PBS (Invitrogen, cat. no. 10010-049)
-
Store up to 2 weeks at 4°C
This stock solution is used to establish sialic acid standards in the periodate-resorcinol assay.
COMMENTARY
Background Information
Oligosaccharides on mammalian cell surfaces play critical biological functions, as they contribute to most interactions between the host cell and its environment. These functional roles include numerous biological recognition processes such as viral and bacterial infection, innate and adaptive immunity, and cancer metastasis. Additionally, oligosaccharides, which are commonly conjugated to proteins or lipids to form glycoconjugates, play an essential role in determining the glycoconjugates’ biological activities (Varki, 1993). Accordingly, MOE technology holds great potential for manipulating basic cellular functions for medical applications such as tissue engineering, cancer therapy, and carbohydrate-based medicines. Here, the general steps and protocols for characterization of novel sugar analogs used for MOE are described. Critical parameters for consideration and common problems related to these experiments are discussed below.
Critical Parameters and Troubleshooting
Incubation of cells with sugar analogs
For consistent results and optimal surface labeling, experiments should be performed using healthy and robustly growing cells harvested at subconfluency. Of course, if the goal of the experiment is to probe effects of anomalous metabolic states—such as hypoxia, toxic waste products from overgrowth of cells (such as ammonia, which affects sialic acid metabolism; Zanghi et al. 1998a,b), or pharmacological agents—on glycan production, this caveat does not apply.
The current set of protocols can be adapted to other sugar analogs and cell lines with the conditions (e.g., analog concentrations and incubation time) described herein serving as a reasonable starting point. Variations in the optimal conditions for different cell types and analogs should be expected, but can usually be covered by evaluating cellular responses in the 0 to 250 to 500 μM concentration range for SCFA-derivatized analogs. The current protocol also describes 0.5-ml cultures, which typically provide an ample number of cells for flow cytometry characterization, toxicity and viability assays, or functional characterization. If multiple endpoints are to be measured from a single batch of cells, this protocol can readily be scaled to 1.0 ml in 12-well plates or to 2.0 ml in 6-well plates.
Another potential pitfall is that certain analogs (e.g., ManNProp or ManNAz) experience approximately the same rate of flux in human and rodent cells, whereas others (e.g., ManNLev) supported substantially lower incorporation (e.g., ~20-fold) in rodent cells (Yarema et al., 1998). Finally, to date, the only human cells that we have tested that are relatively refractory to analog metabolism are mesenchymal stem cells and chondrocytes, suggesting that factors such as extracellular matrix secretion by the test cells (both of these lines are copious secretors), which potentially sequester the analog and thereby hinder uptake by a cell, can confound experimental outcomes.
Periodate-resorcinol colorimetric assay
The periodate-resorcinol assay measures glycoconjugate-bound sialic acid when the periodate-oxidation step is performed at 37°C and total sialic acid when the oxidation step is performed on ice (Basic Protocol 3, step 11). Because the oxidation step is highly dependent on temperature, all temperatures stated in the protocol should be carefully controlled to avoid inaccurate results. Another important consideration is that, although conducting the oxidation step at 37°C is generally described as a way to measure “glycoconjugate-bound” sialic acids (Jourdian et al., 1971), we have found that CMP-sialic acid(s) also provides colorimetric signal under these conditions. As a consequence, an increase in “glycoconjugate-bound” signal does not guarantee that cell-surface sialylation has increased (lectin binding analysis can be used to address this issue in more depth), merely that sialic acid has been converted to the nucleotide sugar form within the cell.
Accurate cell enumeration is another critical parameter. Conducting measurements in triplicate minimizes errors and yields results accurate to ±2% (generally expressed as molecules of sialic acid per cell) when cell counting is performed with an electronic cell counter. Moreover, a cell counter can provide cell size information that allows molecules-per-cell data to be easily converted to molar concentrations within a cell. If an electronic cell counter is not available and cell counts are performed with a hemacytometer, our suggestion is to reserve a portion of the cell sample for standard protein assays and express the amount of sialic acid as a function of protein abundance.
Quantification of cell-surface glycoconjugates
Accurate quantitative labeling of surface thiols and ketone groups (Basic Protocol 4) depends on several variables, which include optimal reagent concentrations, incubation times, and reaction temperatures. It is recommended that reagents—e.g., the working solutions of TCEP, MB, biotin hydrazide, and FITC-avidin—be prepared in small amounts on the day of the assay and stored at 4°C or on ice until immediately prior to use. Further optimization may be required when a previously untested cell line is used. For example, certain cell types may be less tolerant to the reaction conditions, thereby necessitating shorter labeling periods to maintain cell viability. Similarly, while the basic methodology described for thiols and ketones can be readily adapted for additional functional groups now available in MOE analogs, customization of reagent concentrations and reaction times is required for each analog, chemoselective ligation partner (and other chemical reagents used), and cell type under test.
When analog cytotoxicity reduces cell growth, the resulting low number of cells at the conclusion of the incubation period can be compensated by adjusting culture conditions appropriately. In general, increased starting densities of cells ameliorate growth inhibition and help maintain cell viability (Jones et al., 2004). When adherent cells are used, trypsinization and re-plating often result in a growth lag and increased sensitivity to sugar analog toxicity, which can significantly reduce the final cell count. One solution is to plate the cells and allow them to resume growth for 12 to 24 hr before introducing sugar analog into the culture medium. This approach may reduce analog uptake efficiency, and higher analog concentrations will be required to achieve targeted levels of surface display (Sampathkumar et al., 2006b).
A common problem, which can be easily detected when samples are run in triplicate, is inconsistent measurement of cell-surface glycan levels. As noted in Basic Protocol 4, step 14, and discussed elsewhere in detail (Yarema 2002), erratic and lower-than-expected signal may be due to the incomplete removal of unreacted biotin reagents from the cell suspension before staining with FITC-avidin. To avoid this problem, samples should be washed at least three times to remove unreacted biotin reagents before FITC-avidin staining is performed. Additionally, staining can be performed twice to aid in the removal of excess biotin reagents. The authors have encountered situations when surface labeling is much higher than expected when adherent cells were used. This outcome can result from detachment reagents (e.g., trypsin), used while harvesting the cells, that damage the membrane and allow nonspecific uptake of labeling reagents. It is therefore recommended that alternate detachment reagents (e.g., nonenzymatic buffers instead of trypsin) or a cell scraper be used for detachment of cells. However, for some cell lines, cell scraping causes mechanical damage to cells, so again, procedures for each cell line may need to be optimized.
Anticipated Results
Cell viability assays
Typically, monosaccharide analogs used in metabolic labeling have either negligible or relatively minor cytotoxicity that does not deleteriously impact cell viability at concentrations required to label cellular glycans. In cases when analogs, such as peracetylated ManNLev, do exhibit noticeable toxicity (Kim et al., 2004), the worst of these effects can be avoided by straightforward procedures such as controlling the rate of analog delivery (Aich et al., 2010) or avoiding very low cell densities (Jones et al., 2004).
Periodate-resorcinol colorimetric assays
The periodate-resorcinol assay provides a quantitative measure of the total sialic acid content and the glycoconjugate-bound sialic acid content of cells. When compared to untreated cells, it is common that the total sialic acid content of cells treated with “high-flux” analogs when a natural “core” sugar is used (such as ManNAc), or when one with a modest structural alteration (e.g., ManNProp) is used, can increase by several fold, often 10-fold or more (Kim et al., 2004). Although the assay does not explicitly provide levels of the free monosaccharide form of sialic acid, this endpoint can be easily estimated by subtracting glycoconjugate-bound sialic acids from total levels. Generally, the level of free sialic acid cannot be measured accurately in untreated cells by this assay because it is below the detection limit, which is roughly ~5 μM intracellular levels. Upon analog supplementation, the free sialic acid levels increase dramatically to as high as the millimolar level.
A large increase in intracellular levels of free sialic acid usually does not translate into a corresponding increase in surface display. For example, a 100-fold increase may only result in a 10% increase in sialoglycoconjugate display because of severe bottlenecks in converting the free sugar into the nucleotide sugar donor, transport into the Golgi lumen, and “empty/available” site on nascent glycans. By contrast, as noted earlier, certain analogs do not measurably change total levels of sialic acid in a cell but do transit the pathway as evidenced by appearance on the cell surface when assayed as described in Basic Protocol 4. Overall, it is important to be aware that there is not a linear correspondence between analog uptake and incorporation into a metabolic pathway (measured in Basic Protocol 3) and subsequent surface display (measured in Basic Protocol 4).
Quantitation of cell-surface glycoconjugates
Multiple factors can affect the amount of sugar analogs incorporated and displayed on the cell surface, including the cell type, cell density, incubation time, sugar analog, and metabolic status of the cell (Jones et al., 2004). Also, it should be noted that although the procedure described is highly reproducible, the exact fluorescence intensity value (geometric mean value) obtained from the flow cytometer will vary based on instrument settings. For standard conditions described in this unit, where actively growing Jurkat cells are incubated with 40 μM of 1 for 48 hr, an increase in fluorescence intensity of up to about 10-fold is typically observed.
Time Considerations
Incubation of cells with sugar analogs
The time required to set up Basic Protocol 1 should be less than 2 hr once the sugar analog stock solution has been obtained. Cells are typically incubated with analogs for 2 to 5 days depending on subsequent experiments performed. Note that if adherent cells are used, it is advisable to plate the cells and allow them to recover and grow for 12 to 24 hr before addition of sugar analogs into the culture medium.
Cell viability assays
After initial incubation with sugar analogs (Basic Protocol 1), cell viability assays typically take an additional 2 to 5 days, although, if long-term toxicity is being evaluated, this endpoint requires up to 15 days to complete. Cell counting for suspension cells is rather straightforward and should not take longer than an hour for each time point (day 3, 5, and 15). Dispersion of cell clumps and changing of medium on day 7, 9, 11, and 13 is also straightforward and can be completed in half an hour per time point. If adherent cells are used, growth inhibition assays typically take 5 days to complete, with cell counting being performed on days 3 and 5. Although the cells will require trypsinization and resuspension before counting, this procedure can be completed within an hour per time point.
Periodate-resorcinol assay
In general, after initial incubation with sugar analogs (Basic Protocol 1), the complete assay can be performed in about 6 to 7 hours for 36 to 48 samples. The resuspension, counting, and initial washes followed by three freeze-thaw cycles of the cells should take about 3.5 hr. Reagent preparation can be done concurrently with the freeze-thaw cycles, and therefore does not require additional time. After freeze-thaw cycles are completed, periodic acid oxidation on ice requires about 30 min (to determine total sialic acid content) or about 2 hr (at 37°C) to determine conjugate-bound sialic acid. Finally, incubation with resorcinol working reagent, followed by addition of t-butyl alcohol and measurement of absorbance, should take an additional hour. The total time for a set of periodate-resorcinol assays is 8 to 12 hr.
Quantitation of cell-surface glycoconjugates
After initial incubation with sugar analogs (Basic Protocol 1), the resuspension of cells and conjugation with biotin reagents (TCEP treatment and biotin maleimide for thiol groups or biotin hydrazide for ketone groups) should take about 3 hr for 24 to 36 samples. After these conjugation reactions are complete, washing of cells to remove unreacted biotinylation reagents, followed by staining of cells with FITC-avidin and additional washes to remove unbound reagent, takes another 90 min. Finally, flow cytometry will take up to 2 hr depending on the number of samples. Overall, the entire process can be completed in about 6 to 8 hr.
Acknowledgments
The authors would like to thank the National Institutes of Health (NCI, CA112314-05 and NIBIB, EB005692-04) for financial support.
Literature Cited
- Aich U, Yarema KJ. Metabolic oligosaccharide engineering: Perspectives, applications, and future directions. In: Fraser-Reid B, Tatsuta K, Thiem J, editors. Glycosciences. 2. Springer-Verlag; Berlin, Heidelberg: 2008. pp. 2136–2190. [Google Scholar]
- Aich U, Campbell CT, Elmouelhi N, Weier CA, Sampathkumar SG, Choi SS, Yarema KJ. Regioisomeric SCFA attachment to hexosamines separates metabolic flux from cytotoxcity and MUC1 suppression. ACS Chem Biol. 2008;3:230–240. doi: 10.1021/cb7002708. [DOI] [PubMed] [Google Scholar]
- Aich U, Meledeo MA, Sampathkumar SG, Fu J, Jones MB, Weier CA, Chung SY, Tang BC, Yang M, Hanes J, Yarema KJ. Development of delivery methods for carbohydrate-based drugs: controlled release of biologically-active short chain fatty acid-hexosamine analogs. Glycoconjug J. 2010;27:445–459. doi: 10.1007/s10719-010-9292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond MR, Zhang H, Vu PD, Kohler JJ. Photocrosslinking of glycoconjugates using metabolically incorporated diazirine-containing sugars. Nat Protoc. 2009;4:1044–1063. doi: 10.1038/nprot.2009.85. [DOI] [PubMed] [Google Scholar]
- Brossmer R, Gross HJ. Fluorescent and photoactivatable sialic acids. Meth Enzymol. 1994;247:177–193. doi: 10.1016/s0076-6879(94)47014-6. [DOI] [PubMed] [Google Scholar]
- Büttner B, Kannicht C, Schmidt C, Löster K, Reutter W, Lee HY, Nöhring S, Horstkorte R. Biochemical engineering of cell surface sialic acids stimulates axonal growth. J Neurosci. 2002;22:8869–8875. doi: 10.1523/JNEUROSCI.22-20-08869.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CT, Sampathkumar SG, Weier C, Yarema KJ. Metabolic oligosaccharide engineering: perspectives, applications, and future directions. Mol Biosys. 2007;3:187–194. doi: 10.1039/b614939c. [DOI] [PubMed] [Google Scholar]
- Chang PV, Prescher JA, Hangauer MJ, Bertozzi CR. Imaging cell surface glycans with bioorthogonal chemical reporters. J Am Chem Soc. 2007;129:8400–8401. doi: 10.1021/ja070238o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefalo P, Pan YB, Nagy N, Harding C, Guo ZW. Preparation and immunological studies of protein conjugates of N-acylneuraminic acids. Glycoconjug J. 2004;20:407–414. doi: 10.1023/B:GLYC.0000033997.01760.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Joester D, Geiger B, Addadi L. Spatial and temporal sequence of events in cell adhesion: From molecular recognition to focal adhesion assembly. ChemBioChem. 2004;5:1393–1399. doi: 10.1002/cbic.200400162. [DOI] [PubMed] [Google Scholar]
- Collins BE, Fralich TJ, Itonori S, Ichikawa Y, Schnaar RL. Conversion of cellular sialic acid expression from N-acetyl- to N-glycolylneuraminic acid using a synthetic precursor, N-glycolylmannosamine pentaacetate: Inhibition of myelin-associated glycoprotein binding to neural cells. Glycobiology. 2000;10:11–20. doi: 10.1093/glycob/10.1.11. [DOI] [PubMed] [Google Scholar]
- Du J, Yarema KJ. Carbohydrate engineered cells for regenerative medicine. Adv Drug Deliv Rev. 2010;62:671–682. doi: 10.1016/j.addr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Meledeo MA, Wang Z, Khanna HS, Paruchuri VD, Yarema KJ. Metabolic glycoengineering: Sialic acid and beyond. Glycobiology. 2009;19:1382–1401. doi: 10.1093/glycob/cwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmouelhi N, Aich U, Paruchuri VDP, Meledeo MA, Campbell CT, Wang JJ, Srinivas R, Khanna HS, Yarema KJ. Hexosamine template: A platform for modulating gene expression and for sugar-based drug discovery. J Med Chem. 2009;52:2515–2530. doi: 10.1021/jm801661m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas RA., Jr . Nanomedicine, Volume I: Basic Capabilities. Landes Bioscience; Georgetown, Texas: 1999. [Google Scholar]
- Gagiannis D, Gossrau R, Reutter W, Zimmermann-Kordmann M, Horstkorte R. Engineering the sialic acid in organs of mice using N-propanoylmannosamine. Biochim Biophys Acta. 2007;1770:297–306. doi: 10.1016/j.bbagen.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Han S, Collins BE, Bengtson P, Paulson JC. Homomultimeric complexes of CD22 revealed by in situ photoaffinity protein-glycan crosslinking. Nat Chem Biol. 2005;1:93–97. doi: 10.1038/nchembio713. [DOI] [PubMed] [Google Scholar]
- Hang HC, Bertozzi CR. Ketone isosteres of 2-N-acetamidosugars as substrates for metabolic cell surface engineering. J Am Chem Soc. 2001;123:1242–1243. doi: 10.1021/ja002962b. [DOI] [PubMed] [Google Scholar]
- Horstkorte R, Rau K, Laabs S, Danker K, Reutter W. Biochemical engineering of the N-acyl side chain of sialic acid leads to increased calcium influx from intracellular compartments and promotes differentiation of HL60 cells. FEBS Lett. 2004;571:99–102. doi: 10.1016/j.febslet.2004.06.067. [DOI] [PubMed] [Google Scholar]
- Jacobs CL, Goon S, Yarema KJ, Hinderlich S, Hang HC, Chai DH, Bertozzi CR. Substrate specificity of the sialic acid biosynthetic pathway. Biochemistry. 2001;40:12864–12874. doi: 10.1021/bi010862s. [DOI] [PubMed] [Google Scholar]
- Jones MB, Teng H, Rhee JK, Baskaran G, Lahar N, Yarema KJ. Characterization of the cellular uptake and metabolic conversion of acetylated N-acetylmannosamine (ManNAc) analogs to sialic acids. Biotechnol Bioeng. 2004;85:394–405. doi: 10.1002/bit.10901. [DOI] [PubMed] [Google Scholar]
- Jourdian GW, Dean L, Roseman S. The sialic acids. XI A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971;246:430–435. [PubMed] [Google Scholar]
- Kayser H, Zeitler R, Kannicht C, Grunow D, Nuck R, Reutter W. Biosynthesis of a nonphysiological sialic acid in different rat organs, using N-propanoyl-D-hexosamines as precursors. J Biol Chem. 1992;267:16934–16938. [PubMed] [Google Scholar]
- Keppler OT, Horstkorte R, Pawlita M, Schmidt C, Reutter W. Biochemical engineering of the N-acyl side chain of sialic acid: Biological implications. Glycobiology. 2001;11:11R–18R. doi: 10.1093/glycob/11.2.11r. [DOI] [PubMed] [Google Scholar]
- Khidekel N, Ficarro SB, Peters EC, Hsieh-Wilson LC. Exploring the O-GlcNAc proteome: Direct identification of O-GlcNAc-modified proteins from the brain. Proc Natl Acad Sci USA. 2004;101:13132–13137. doi: 10.1073/pnas.0403471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Sampathkumar SG, Jones MB, Rhee JK, Baskaran G, Yarema KJ. Characterization of the metabolic flux and apoptotic effects of O-hydroxyl- and N-acetylmannosamine (ManNAc) analogs in Jurkat (human T-lymphoma-derived) cells. J Biol Chem. 2004;279:18342–18352. doi: 10.1074/jbc.M400205200. [DOI] [PubMed] [Google Scholar]
- King EJ, Garner RJ. The colorimetric determination of glucose. J Clin Pathol. 1947;1:30–33. doi: 10.1136/jcp.1.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontou M, Bauer C, Reutter W, Horstkorte R. Sialic acid metabolism is involved in the regulation of gene expression during neuronal differentiation of PC12 cells. Glycoconjug J. 2008;25:237–244. doi: 10.1007/s10719-008-9104-1. [DOI] [PubMed] [Google Scholar]
- Lee JH, Baker TJ, Mahal LK, Zabner J, Bertozzi CR, Wiemar DF, Welsh MJ. Engineering novel cell surface receptors for virus-mediated gene transfer. J Biol Chem. 1999;274:21878–21884. doi: 10.1074/jbc.274.31.21878. [DOI] [PubMed] [Google Scholar]
- Lemieux GA, Bertozzi CR. Chemose-lective ligation reactions with proteins, oligosaccharides and cells. Trends Biotechnol. 1998;16:506–513. doi: 10.1016/s0167-7799(98)01230-x. [DOI] [PubMed] [Google Scholar]
- Lemieux GA, Bertozzi CR. Modulating cell surface immunoreactivity by metabolic induction of unnatural carbohydrate antigens. Chem Biol. 2001;8:265–275. doi: 10.1016/s1074-5521(01)00008-4. [DOI] [PubMed] [Google Scholar]
- Luchansky SJ, Argade S, Hayes BK, Bertozzi CR. Metabolic functionalization of recombinant glycoproteins. Biochemistry. 2004;43:12358–12366. doi: 10.1021/bi049274f. [DOI] [PubMed] [Google Scholar]
- Mahal LK, Yarema KJ, Bertozzi CR. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science. 1997;276:1125–1128. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- Mahal LK, Yarema KJ, Lemieux GA, Bertozzi CR. Chemical approaches to glycobiology: Engineering cell surface sialic acids for tumor targeting. In: Lee Inoue YC, Troy FA., II, editors. Sialobiology and Other Novel Forms of GlycosylationY. Gakushin Publishing Company; Osaka, Japan: 1999. pp. 273–280. [Google Scholar]
- Nauman DA, Bertozzi CR. Kinetic parameters for small-molecule drug delivery by covalent cell surface targeting. Biochim Biophys Acta. 2001;1568:147–154. doi: 10.1016/s0304-4165(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Piller V, Piller F, Fukuda M. Biosynthesis of truncated O-glycans in the T cell line Jurkat. Localization of O-glycan initiation. J Biol Chem. 1990;265:9264–9271. [PubMed] [Google Scholar]
- Pilobello KT, Krishnamoorthy L, Slawek D, Mahal LK. Development of a lectin microarray for the rapid analysis of protein glycopatterns. ChemBioChem. 2005;6:985–989. doi: 10.1002/cbic.200400403. [DOI] [PubMed] [Google Scholar]
- Sampathkumar SG, Jones MB, Meledeo MA, Campbell CT, Choi SS, Hida K, Gomutputra P, Sheh A, Gilmartin T, Head SR, Yarema KJ. Targeting glycosylation pathways and the cell cycle: Sugar-dependent activity of butyrate-carbohydrate cancer prodrugs. Chem Biol. 2006a;13:1265–1275. doi: 10.1016/j.chembiol.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Sampathkumar SG, Jones MB, Yarema KJ. Metabolic expression of thiol-derivatized sialic acids on the cell surface and their quantitative estimation by flow cytometry. Nat Protoc. 2006b;1:1840–1851. doi: 10.1038/nprot.2006.252. [DOI] [PubMed] [Google Scholar]
- Sampathkumar SG, Li AV, Jones MB, Sun Z, Yarema KJ. Metabolic installation of thiols into sialic acid modulates adhesion and stem cell biology. Nat Chem Biol. 2006c;2:149–152. doi: 10.1038/nchembio770. [DOI] [PubMed] [Google Scholar]
- Sampathkumar SG, Li AV, Yarema KJ. Synthesis of non-natural ManNAc analogs for the expression of thiols on cell surface sialic acids. Nat Protoc. 2006d;1:2377–2385. doi: 10.1038/nprot.2006.319. [DOI] [PubMed] [Google Scholar]
- Sawa M, Hsu TL, Itoh T, Sugiyama M, Hanson SR, Vogt PK, Wong CH. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc Natl Acad Sci USA. 2006;103:12371–12376. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- Schilling B, Goon S, Samuels NM, Gaucher SP, Leary JA, Bertozzi CR, Gibson BW. Biosynthesis of sialylated lipooligosaccharides in Haemophilus ducreyi is dependent on exogenous sialic acid and not mannosamine: Incorporation studies using N-acylmannosamine analogs, N-glycolylneuraminic acid, and 13C-labeled N-acetylneuraminic acid. Biochemistry. 2001;40:12666–12677. doi: 10.1021/bi0107849. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Stehling P, Schnitzer J, Reutter W, Horstkorte R. Biochemical engineering of neural cell surfaces by the synthetic N-propanoyl-substituted neuraminic acid precursor. J Biol Chem. 1998;273:19146–19152. doi: 10.1074/jbc.273.30.19146. [DOI] [PubMed] [Google Scholar]
- Sussich F, Cesaro A. The kinetics of periodate oxidation of carbohydrates: A calorimetric approach. Carbohydr Res. 2000;329:87–95. doi: 10.1016/s0008-6215(00)00158-0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kohler JJ. Photoactivatable crosslinking sugars for capturing glycoprotein interactions. J Am Chem Soc. 2008;130:3278–3279. doi: 10.1021/ja7109772. [DOI] [PubMed] [Google Scholar]
- Tiziani S, Sussich F, Cesàro A. The kinetics of periodate oxidation of carbohydrates 2. Polymeric substrates. Carbohydr Res. 2003;338:1083–1095. doi: 10.1016/s0008-6215(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villavicencio-Lorini P, Laabs S, Danker K, Reutter W, Horstkorte R. Biochemical engineering of the acyl side chain of sialic acids stimulates integrin-dependent adhesion of HL60 cells to fibronectin. J Mol Med. 2002;80:671–677. doi: 10.1007/s00109-002-0382-y. [DOI] [PubMed] [Google Scholar]
- Viswanathan K, Lawrence S, Hinderlich S, Yarema KJ, Lee YC, Betenbaugh M. Engineering sialic acid synthetic ability into insect cells: Identifying metabolic bottlenecks and devising strategies to overcome them. Biochemistry. 2003;42:15215–15225. doi: 10.1021/bi034994s. [DOI] [PubMed] [Google Scholar]
- Walborg EF, Jr, Christensson L. A colorimetric method for the quantitative determination of monosaccharides. Anal Biochem. 1965;13:186–193. doi: 10.1016/0003-2697(65)90188-0. [DOI] [PubMed] [Google Scholar]
- Wang Z, Du J, Che PL, Meledeo MA, Yarema KJ. Hexosamine analogs: From metabolic glycoengineering to drug discovery. Curr Opin Chem Biol. 2009;13:565–572. doi: 10.1016/j.cbpa.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- Yarema KJ. A metabolic substrate-based approach to engineering new chemical reactivity into cellular sialoglycoconjugates. In: Al-Rubeai M, editor. Cell Engineering 3. Glycosylation. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. pp. 171–196. [Google Scholar]
- Yarema KJ, Mahal LK, Bruehl RE, Rodriguez EC, Bertozzi CR. Metabolic delivery of ketone groups to sialic acid residues: Application to cell surface glycoform engineering. J Biol Chem. 1998;273:31168–31179. doi: 10.1074/jbc.273.47.31168. [DOI] [PubMed] [Google Scholar]
- Zanghi JA, Mendoza TP, Knop RH, Miller WM. Ammonia decreases NCAM polysialylation in Chinese hamster ovary and small cell lung cancer cells. J Cell Physiol. 1998a;177:248–263. doi: 10.1002/(SICI)1097-4652(199811)177:2<248::AID-JCP7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Zanghi JA, Mendoza TP, Schmelzer AE, Knop RH, Miller WM. Role of nucleotide sugar pools in the inhibition of NCAM polysialylation by ammonia. Biotechnol Prog. 1998b;14:834–844. doi: 10.1021/bp9800945. [DOI] [PubMed] [Google Scholar]