Abstract
Rationale: It is hypothesized that the metabolic syndrome explains the association between body mass index (BMI) and asthma in adults.
Objectives: Our objective was to longitudinally compare the relative strengths of the associations of the metabolic syndrome and BMI with incident asthma in adults.
Methods: We included 4,619 eligible participants in the Coronary Artery Risk Development in Young Adults (CARDIA) cohort followed over 25 years. Incident asthma was defined by a new self-reported provider asthma diagnosis plus either the presence of asthma symptoms and/or use of asthma medications. Cox proportional hazard analyses were performed.
Measurements and Main Results: Six hundred two subjects (417 women and 185 men) developed incident asthma over 25 years of follow-up. Metabolic syndrome predicted incident asthma among women but not men (unadjusted hazard ratios, 1.50 and 0.98; P = 0.01 and 0.93, respectively). BMI had a similar predictive association among women but not men (unadjusted hazard ratios, 1.19 and 1.04 per 5 units of BMI; P < 0.001 and 0.60, respectively). The association of metabolic syndrome with incident asthma in women was no longer statistically significant after adjustment for BMI (P = 0.44). In contrast, the association of BMI with incident asthma in women remained statistically significant after adjusting for the metabolic syndrome (P = 0.01). In a stepwise model, BMI was a stronger predictor than the metabolic syndrome (P = 0.001).
Conclusions: BMI is a stronger predictor of incident asthma among women than the metabolic syndrome. Other obesity-associated factors that are not a part of the metabolic syndrome may play a role in the BMI–asthma association in women.
Keywords: incident asthma, metabolic syndrome, body mass index
At a Glance Commentary
Scientific Knowledge on the Subject
Excess body mass index (BMI) is an established risk factor for asthma, particularly in women. It is hypothesized that the BMI–asthma association is mediated by the metabolic syndrome.
What This Study Adds to the Field
BMI is a stronger predictor than the metabolic syndrome for incident asthma in women. The metabolic syndrome does not explain the BMI–asthma association. Biomechanical, inflammatory, and metabolic abnormalities associated with obesity that are not a part of the metabolic syndrome may explain the BMI–asthma association.
Obesity and asthma are chronic diseases that have increased in prevalence across the world over the last 2 decades (1, 2). During the period 2009 to 2010, 1 in 12 American adults had asthma, and more than one-third of American adults were obese (3, 4). Obesity, as defined by elevated body mass index (BMI) of 30 kg/m2 or more, is a risk factor for asthma, particularly among women. The basis for this association, however, remains unclear. The metabolic syndrome, as defined by the third Adult Treatment Panel (ATP-III) criteria (5), includes the presence of any three of the following five traits: abdominal adiposity (waist circumference > 102 cm in men and > 88 cm in women), hypertriglyceridemia (≥150 mg/dl or drug treatment for high triglycerides), low high-density lipoprotein (HDL) cholesterol levels (<40 mg/dl in men and <50 mg/dl in women or drug treatment for low HDL), elevated blood pressure (≥130/85 mm Hg or drug treatment for elevated blood pressure), and impaired fasting blood glucose or diabetes mellitus (≥100 mg/dl or antidiabetic drug treatment) (6). Some epidemiologic studies suggest that waist circumference–defined abdominal adiposity, one of the characteristics of the metabolic syndrome, is more strongly associated with prevalent asthma than is BMI-defined obesity (7, 8). This has led to some authors suggesting that the association of BMI-defined obesity with asthma may be mediated by the metabolic syndrome (9–12). It is also not known whether the metabolic syndrome overall or its individual components are stronger predictors for incident asthma than BMI.
Our objectives were to first investigate the sex-specific longitudinal association between the metabolic syndrome (overall and its five individual components) and incident asthma. Our second objective was to compare the relative strengths of the longitudinal associations of the metabolic syndrome and BMI as predictors for incident asthma. Our third objective was to analyze if the longitudinal association of BMI and incident asthma was explained by the metabolic syndrome or the related variable insulin resistance.
Methods
Study Design
This is a longitudinal analysis of the national Coronary Artery Risk Development in Young Adults (CARDIA) dataset over 25 years from 1985 to 2010. This cohort, focusing on cardiovascular disease risk factors, is funded by the NHLBI. During 1985 to 1986, CARDIA randomly recruited 5,115 subjects from the general population in Birmingham, Alabama; Chicago, Illinois; and Minneapolis, Minnesota; and from members of the Kaiser Permanente Medical Care Plan (Oakland, CA). The CARDIA study sample at baseline was balanced by race (52% were African American; 48% were white) and sex (46% were men; 54% were women). Seven follow-up examinations were conducted at years 2, 5, 7, 10, 15, 20, and 25, with 91, 86, 81, 79, 74, 72, and 72% of the cohort returning for follow-up, respectively (13–15).
Demographic characteristics, lifestyle habits (e.g., cigarette smoking and physical activity), and medical history (e.g., history of hypertension or diabetes mellitus) were collected by self-report questionnaires at each examination year. Medications used were verified by direct examination of medication containers by trained study personnel. BMI was calculated by obtaining height and weight measurements in a standardized fashion. Waist circumference was measured at a level midway between the lowest rib and the iliac crest. Seated blood pressure was measured after a 5-minute rest.
Venipuncture was performed after an overnight fast. Patients were asked not to smoke or perform heavy physical activity for 2 hours before their examination visit. Fasting blood glucose was measured by Roche Modular P-hexokinase method. Serum insulin was measured by Elecsys sandwich immunoassay. Triglycerides were measured by UV method and determined enzymatically on the Abbott Spectrum (using Hitachi 917–R1Buffer/4–chlorophenol/enzymes), and HDL cholesterol was measured by Trinder-type method and determined enzymatically after dextran sulfate-magnesium precipitation on the Abbott Spectrum. Homeostasis model assessment of insulin resistance was used as a measure of insulin resistance and was calculated by a standard formula [(fasting glucose in mg/dl × fasting insulin in mg/dL)/22.5]. The assays used for measuring insulin resistance over 25 years were calibrated and corrections applied.
Inclusion and Exclusion Criteria
Among the 5,115 original participants at baseline examination, 300 subjects with preexisting ever-asthma at the Year 0 examination were excluded to allow the study to focus on incident asthma as the outcome. An additional 193 participants were excluded because they did not have any follow-up examination after Year 0. Also excluded were those participants who withdrew consent (n = 1) or had sex change (n = 2).
Dependent and Independent Variables
The primary dependent variable (outcome) was incident asthma at each follow-up visit, as defined by new-onset of a self-reported provider diagnosis of asthma plus either the presence of asthma symptoms in the year preceding the examination and/or use of asthma medications at the time of examination. The specific questions used to define asthma are included in Table E7 of the online supplement.
The primary independent variables (predictors) were the metabolic syndrome (overall and any one of its five component variables) and BMI (as a continuous variable) at each CARDIA visit. The metabolic syndrome was defined as a binary variable by the presence of at least three of five component variables, based on the sex-specific normative values, using the ATP-III criteria (5, 6).
Covariates
Covariates were self-reported and included race as well as time-varying variables such as age, physical activity level, and current smoking status at each CARDIA visit. The following self-reported interaction terms were studied: sex and (for women) menopausal status, to examine the role of serum estrogen on this association. Menopause was assessed in response to the question “Have you gone through menopause or the change of life?” and was available at CARDIA Year 15, 20, and 25 examination visits.
Statistical Analysis
Survival analysis of the time to incident asthma (outcome event) was computed using Cox proportional hazard model. In survival analysis, censoring occurs when information on time to outcome event is not available for all study participants. In our study, censoring occurred if the outcome event did not occur before the end of the observation time or if there was loss to follow-up or death (up to CARDIA 25-yr examination). Time-varying predictors and covariates were included in the multivariable model using Andersen-Gill input format (SAS PROC PHREG). The interaction effects were studied, using formal tests of interaction. A stepwise proportional hazards model was used to determine the relative contributions of the independent variables toward the outcome.
The CARDIA study is reviewed annually by the institutional review boards at each participating institution, and participants sign a new informed consent form at every examination. Institutional review board approval was also obtained from the University of New Mexico.
Results
Among the 5,115 original participants at the baseline examination in the CARDIA cohort, 4,619 eligible subjects were included in the study (Figure 1), including 2,531 women (54.8%) and 2,088 men (45.2%). Of all eligible women participants, 1,578 (62.3%) were premenopausal at CARDIA examination Year 25. Six hundred two eligible subjects developed incident asthma over 25 years of follow-up, including 417 women (69.3%) and 185 men (30.7%).
Figure 1.

Overview of study participants. CARDIA = Coronary Artery Risk Development in Young Adults.
As shown in Table 1, participants who developed incident asthma over 25 years of follow-up were more likely to be women and obese (both BMI-defined obese as well as abdominally obese by waist circumference) and have greater values of BMI, waist circumference, and insulin resistance, and lower physical activity scores than those who did not develop incident asthma. These relationships were seen among women but not men. In addition, women participants who developed asthma were more likely to be current smokers and have the metabolic syndrome and were less likely to be white than women who did not develop incident asthma.
TABLE 1.
CHARACTERISTICS OF STUDY PARTICIPANTS AT CARDIA YEAR 0 EXAMINATION, 1985 TO 1986
| Characteristics | All Incident Asthma (n = 602) (Mean ± SD or %) | Never Asthma (n = 4,017) (Mean ± SD or %) | P Value |
|---|---|---|---|
| Women | 69.3 | 52.7 | <0.001 |
| Age, yr | |||
| All | 24.9 ± 3.6 | 24.9 ± 3.7 | 0.88 |
| Women | 25.1 ± 3.7 | 24.9 ± 3.7 | 0.49 |
| Men | 24.6 ± 3.6 | 24.9 ± 3.6 | 0.39 |
| White race | |||
| All | 46.0 | 50.1 | 0.06 |
| Women | 42.9 | 49.0 | 0.02 |
| Men | 53.0 | 51.5 | 0.70 |
| Current smokers | |||
| All | 31.2 | 29.6 | 0.19 |
| Women | 33.7 | 27.5 | 0.02 |
| Men | 25.5 | 32.1 | 0.10 |
| Physical activity level* | |||
| All | 3.2 ± 1.2 | 3.3 ± 1.1 | 0.005 |
| Women | 3.0 ± 1.2 | 3.1 ± 1.1 | 0.04 |
| Men | 3.6 ± 1.2 | 3.5 ± 1.1 | 0.75 |
| BMI, kg/m2 | |||
| All | 25.3 ± 5.7 | 24.3 ± 4.9 | <0.001 |
| Women | 25.7 ± 6.3 | 24.2 ± 5.6 | <0.001 |
| Men | 24.4 ± 3.7 | 24.4 ± 3.9 | 1.00 |
| Obesity† | |||
| All | 15.4 | 10.9 | 0.002 |
| Women | 18.8 | 13.5 | 0.007 |
| Men | 7.6 | 8.0 | 1.00 |
| Waist circumference, cm | |||
| All | 76.6 ± 13.1 | 73.7 ± 11.4 | <0.001 |
| Women | 76.6 ± 11.4 | 73.7 ± 13.1 | <0.001 |
| Men | 81.4 ± 9.4 | 81.7 ± 9.4 | 0.63 |
| Metabolic syndrome, % | |||
| All | 3.2 | 2.2 | 0.15 |
| Women | 3.8 | 1.7 | 0.01 |
| Men | 1.6 | 2.8 | 0.48 |
| Abdominal adiposity‡ | |||
| All | 12.7 | 6.8 | <0.001 |
| Women | 17.0 | 9.8 | <0.001 |
| Men | 3.3 | 3.4 | 1.0 |
| Elevated blood pressure‡ | |||
| All | 8.0 | 9.1 | 0.40 |
| Women | 6.0 | 5.2 | 0.50 |
| Men | 13.5 | 12.4 | 0.82 |
| Impaired fasting glucose or diabetes‡ | |||
| All | 3.3 | 2.7 | 0.35 |
| Women | 3.2 | 2.3 | 0.29 |
| Men | 3.8 | 3.1 | 0.66 |
| High serum triglycerides‡ | |||
| All | 5.8 | 4.9 | 0.37 |
| Women | 4.1 | 2.6 | 0.10 |
| Men | 9.7 | 7.5 | 0.31 |
| Low serum HDL cholesterol‡ | |||
| All | 28.5 | 25.1 | 0.08 |
| Women | 34.9 | 31.8 | 0.21 |
| Men | 14.1 | 17.7 | 0.26 |
| Insulin resistance (HOMA-IR) | |||
| All | 2.6 ± 1.9 | 2.4 ± 1.6 | 0.03 |
| Women | 2.6 ± 2.0 | 2.4 ± 1.6 | 0.052 |
| Men | 2.4 ± 1.8 | 2.3 ± 1.5 | 0.46 |
| Postmenopausal status in women at Year 25, % women | 43.7 | 36.5 | 0.007 |
Definition of abbreviations: BMI = body mass index; CARDIA = Coronary Artery Risk Development in Young Adults; HDL = high density lipoprotein; HOMA-IR = homeostatic model assessment of insulin resistance.
All data were obtained from CARDIA Year 0 examination except for menopause, which was obtained at Year 25.
Physical activity level from 1 to 5 was obtained from a questionnaire, with 1 being physically inactive, and 5 very active.
Obesity was defined as BMI ≥ 30 kg/m2.
Metabolic syndrome components were defined by the ATP-III criteria: abdominal adiposity (waist circumference > 102 cm in men and > 88 cm in women), hypertriglyceridemia (≥150 mg /dl or drug treatment for high triglycerides), low high-density lipoprotein (HDL) cholesterol levels (<40 mg/dl in men and <50 mg/dl in women or drug treatment for low HDL), elevated blood pressure (≥130/85 mm Hg or drug treatment for elevated blood pressure), and impaired fasting blood glucose or diabetes mellitus (≥100 mg/dl or antidiabetic drug treatment).
Although BMI Explains the Metabolic Syndrome–Asthma Association, the Metabolic Syndrome Does Not Explain the BMI–Asthma Association in Women
In an unadjusted model, the metabolic syndrome, studied as a time-varying variable, predicted incident asthma among women but not men (hazard ratios, 1.50 and 0.98; P = 0.01and 0.93, respectively; sex interaction, P = 0.10; Table 2). The association between the metabolic syndrome and incident asthma among women was no longer significant after adjusting for BMI and other covariates (hazard ratio, 1.16; P = 0.44; fully adjusted model, Table 2).
TABLE 2.
LONGITUDINAL ASSOCIATIONS BETWEEN THE METABOLIC SYNDROME AND INCIDENT ASTHMA IN UNADJUSTED AND ADJUSTED MODELS OVER 25 YEARS
| Statistical Models | All (n =
602) |
Women (n =
417)* |
Men (n =
185) |
Sex Interaction (P Value) | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Unadjusted model | 1.24 (0.95–1.61) | 0.11 | 1.50 (1.10–2.05) | 0.01 | 0.98 (0.60–1.60) | 0.93 | 0.10 |
| Partially adjusted model including BMI (continuous) | 0.95 (0.72–1.25) | 0.72 | 1.05 (0.75–1.49) | 0.76 | 0.92 (0.54–1.57) | 0.75 | 0.18 |
| Fully adjusted model including BMI (continuous) and other covariates† | 1.11 (0.81–1.52) | 0.53 | 1.16 (0.79–1.70) | 0.44 | 0.98 (0.54–1.79) | 0.96 | 0.28 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; HR = hazard ratio.
Adjustment in the statistical models for obesity as a categorical variable, as defined as BMI ≥ 30 kg/m2, instead of the continuous BMI variable resulted in similar results as shown above.
Menopause-specific interaction in women was not significant (P ≥ 0.28) for all analyses.
Covariates include age, race, smoking, and physical activity. Sex was also included in the analysis for all participants.
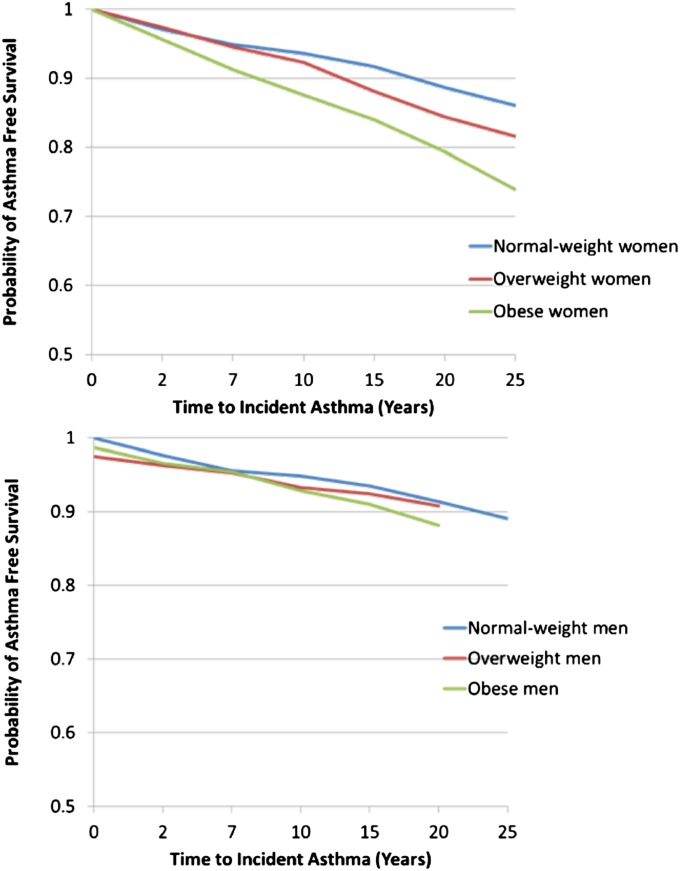
On the other hand, BMI, when studied as a time-varying linear continuous variable in an unadjusted model, was a significant predictor for incident asthma only among women (hazard ratio, 1.19 per 5 units of BMI; P < 0.001; sex interaction, P = 0.04; Table 3). This association among women remained significant even after adjustment for the metabolic syndrome and other covariates (hazard ratio, 1.15 per 5 units of BMI; P < 0.001; Table 3). BMI-defined obese status, studied as a time-varying categorical variable, had a similar predictive association with incident asthma only among women as BMI analyzed as a linear continuous variable (Table E1). For a simple visual presentation of longitudinal data, we summarized this sex-specific finding by using Kaplan-Meier survival curves of normal-weight, overweight, and obese individuals, based on their BMI values averaged over 25 years, in Figure 2. This association between obese category and incident asthma also remained significant after adjustment for the metabolic syndrome (partially-adjusted model, Table E1).
TABLE 3.
LONGITUDINAL ASSOCIATIONS BETWEEN BODY MASS INDEX (ANALYZED AS A CONTINUOUS VARIABLE) AND INCIDENT ASTHMA IN UNADJUSTED AND ADJUSTED MODELS OVER 25 YEARS
| Statistical Models | All (n =
602) |
Women (n =
417)* |
Men (n =
185) |
Sex Interaction P Value | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Unadjusted model | 1.17 (1.11–1.23) | <0.001 | 1.19 (1.12–1.27) | <0.001 | 1.04 (0.90–1.19) | 0.60 | 0.04 |
| Partially-adjusted model including MS | 1.17 (1.11–1.24) | <0.001 | 1.19 (1.11–1.27) | <0.001 | 1.05 (0.90–1.22) | 0.54 | 0.04 |
| Fully-adjusted model including MS and other covariates† | 1.14 (1.06–1.22) | <0.001 | 1.15 (1.06–1.26) | 0.001 | 1.08 (0.92–1.27) | 0.33 | 0.29 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; HR = hazard ratio; MS = metabolic syndrome.
HRs are shown per 5 units of BMI in kg/m2. Use of obesity (defined as BMI ≥ 30 kg/m2) as a categorical variable instead of BMI as a continuous variable as a predictor resulted in similar results as above, as shown in Table E1.
Menopause-specific interaction in women was not significant (P ≥ 0.25) for all analyses.
Covariates include age, race, smoking, and physical activity. Sex was also included in the analysis for all participants.
Figure 2.
Kaplan-Meier survival curves for incident asthma of body mass index (BMI) categories among women (top panel) and men (bottom panel). The BMI values of study participants were averaged over 25 years.
Models for components of the metabolic syndrome
Of the five metabolic syndrome components, all studied as time-varying variables, abdominal adiposity, elevated blood pressure, and impaired fasting glucose or diabetes were significantly associated with incident asthma in women in unadjusted models (hazard ratio, 1.58, 1.42, and 1.46; P < 0.001, 0.02, and 0.02, respectively; sex interaction, P = 0.08, 0.52, and 0.02, respectively; Table 4). All three associations were, however, no longer significant when adjusted for BMI (Tables E2, E3, and E4).
TABLE 4.
UNIVARIATE ANALYSES OF THE LONGITUDINAL ASSOCIATIONS BETWEEN VARIOUS COMPONENTS OF THE METABOLIC SYNDROME AND INCIDENT ASTHMA OVER 25 YEARS
| Predictors* | All (n =
602) |
Women (n
= 417)† |
Men (n =
185) |
Sex Interaction P Value | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Abdominal adiposity | 1.63 (1.35–1.97) | <0.001 | 1.58 (1.27–1.95) | <0.001 | 1.06 (0.67–1.66) | 0.82 | 0.08 |
| Elevated blood pressure | 1.24 (0.99–1.54) | 0.06 | 1.42 (1.07–1.88) | 0.02 | 1.26 (0.88–1.81) | 0.20 | 0.52 |
| Impaired fasting glucose or diabetes | 0.96 (0.78–1.33) | 0.76 | 1.46 (1.06–2.02) | 0.02 | 0.81 (0.50–1.29) | 0.37 | 0.02 |
| Hypertriglyceridemia | 1.02 (0.76–1.27) | 0.87 | 1.33 (0.94–1.88) | 0.12 | 1.03 (0.70–1.53) | 0.88 | 0.26 |
| Low HDL cholesterol | 1.13 (0.95–1.35) | 0.17 | 1.12 (0.91–1.37) | 0.28 | 1.0 (0.70–1.39) | 0.95 | 0.43 |
Definition of abbreviations: CI = confidence interval; HDL = high-density lipoprotein; HR = hazard ratio.
Multivariable analyses of significant associations are presented in Tables E2–E4.
Metabolic syndrome components were defined by the Adult Treatment Panel-III criteria.
Menopause-specific interaction in women was not significant (P ≥ 0.19) for all analyses.
There were no significant interactions between menopausal status and the metabolic syndrome (either overall or its components), all variables studied as time varying, on incident asthma. Similarly, there were no significant interactions between menopausal status and BMI on incident asthma.
BMI Is a Stronger Predictor than the Metabolic Syndrome for Incident Asthma in Women
Using time-varying variables in a stepwise model, BMI was a stronger predictor for incident asthma among women than either the metabolic syndrome or its individual components (P ≤ 0.001 for all analyses). BMI was also a stronger predictor for incident asthma among women than either waist circumference or insulin resistance.
Insulin Resistance Does Not Explain the Associations in Women
In Table E6, we adjusted the associations of BMI and of the metabolic syndrome with incident asthma separately for the homeostasis model assessment of insulin resistance, all variables studied as time varying in the model. We found that these associations were not different with and without adjustment for insulin resistance. Therefore, insulin resistance does not explain these associations.
Discussion
Although both BMI and the metabolic syndrome predict incident asthma among women, BMI is a stronger predictor than the metabolic syndrome. Furthermore, the metabolic syndrome does not predict incident asthma independent of BMI. On the other hand, the association between BMI and asthma is inadequately explained by the metabolic syndrome (or by insulin resistance), and therefore other factors also must play an important role in this association.
Multiple mechanisms have been proposed to explain the obesity–asthma association. Mechanical effects of obesity on the lung include a reduction in functional residual capacity (16). Breathing at low functional residual capacity can decrease the retractive force on the lung parenchyma, shortening airway smooth muscle and leading to airway narrowing and hyperreactivity among nonobese subjects (17). Inflammatory effects of obesity on the lung involve dysregulation of proinflammatory adipokines (e.g., excess leptin) and antiinflammatory adipokines (e.g., adiponectin deficiency). Excess systemic leptin and low systemic adiponectin are both linked to asthma prevalence, incidence, and severity in specific populations (18–22). Furthermore, several diseases that coexist with obesity have also been linked to asthma prevalence or severity. These diseases include gastroesophageal reflux disease (23), obstructive sleep apnea (24, 25), and depression (26, 27). Obesity-associated insulin resistance has also been shown to be associated with asthma (28–30). Furthermore, obesity may have direct hormonal effects on the airway. Thus, excess adipose tissue is associated with increased estradiol production. Estradiol excess may in turn explain the stronger association between obesity and asthma among women than men (31–33) and among girls with early menarche than late menarche (34). Additional explanations for the greater predilection of obese women than men for asthma include their disproportionately higher leptin and lower adiponectin concentrations in serum, greater metabolic activity of visceral fat despite its relatively lower amount, greater intramuscular fat, and greater contribution of fat than fat-free mass toward the BMI value in women. Although our study showed that associations with incident asthma were present only among women, we did not find significant interaction between menopause and BMI/metabolic syndrome on incident asthma among women, possibly due to the need for a larger sample size to examine such an interaction. Finally, genetic, epigenetic, developmental, and environmental mechanisms have also been postulated to explain the obesity–asthma association.
Some epidemiological studies demonstrate that (waist circumference–defined) abdominal adiposity is more strongly associated with asthma than BMI in adults (7, 8, 35, 36) as well as in children (37). In one of the first prospective studies to associate body fat distribution and asthma risk, a transition from a leaner body silhouette to one that was progressively more android (suggesting predominantly abdominal fat distribution) after menarche was associated with an increased incident asthma risk among French women (38). These findings were supported by a cross-sectional study of California women that showed that abdominal adiposity (waist circumference > 88 cm) was associated with 37% greater odds of asthma prevalence, even among women with a normal BMI value (8). Furthermore, the odds for asthma prevalence were greater among women who had both (BMI-defined) obesity and (waist circumference–defined) abdominal adiposity than women who had obesity but not abdominal adiposity (8). In another large longitudinal study of elderly French subjects, abdominal adiposity (waist circumference ≥ 102 cm in men and ≥ 88 cm in women) was associated with increased prevalent and incident asthma in both men and women, even after adjustment for BMI (39). These studies suggest that abdominal adiposity is a risk factor for asthma among women, independent of BMI. On the other hand, our longitudinal study fails to establish either waist circumference (studied as a continuous variable) or abdominal adiposity (studied as a categorical variable) as stronger predictors than BMI for incident asthma among women.
Abdominal adiposity is only one of the five components of the metabolic syndrome—a combination of medical disorders that, when they occur together, increase the risk for developing systemic inflammatory diseases such as cardiovascular disease and diabetes mellitus. Increased oxidative stress and cellular metabolic derangements are seen in the metabolic syndrome through multiple pathways that in turn may increase airway inflammation, remodeling, and hyperresponsiveness, thus increasing the risk for asthma. One pathway, demonstrated mainly in animal models, involves nitric oxide–arginine metabolism. Arginine bioavailability and endothelial nitric oxide synthase function are reduced in asthma (40, 41). Restoration of normal arginine metabolism by l-arginine supplementation or simvastatin in murine models of asthma is associated with improvement in both airway hyperresponsiveness and the metabolic syndrome (42–45). A second pathway involves regulation of cellular metabolism and programming by insulin and insulin-like growth factors (IGF). Insulin induces airway smooth muscle hypercontractility (46). Furthermore, IGF-1 levels are increased in the airways of the mouse model of asthma, with IGF-1 neutralization resulting in improvement in airway resistance, inflammation, and wall thickening (47). High systemic levels of insulin, a marker of insulin resistance, are seen in subjects with the metabolic syndrome. Insulin resistance has also been hypothesized to explain the associations of both obesity and the metabolic syndrome with asthma. In fact, insulin resistance was shown to predict development of wheeze and asthma-like symptoms, after adjustment for BMI or waist circumference, in one longitudinal study (29). However, our longitudinal data show that insulin resistance does not adequately explain the association between either BMI or the metabolic syndrome with incident asthma.
Other components of the metabolic syndrome, such as hypertriglyceridemia, were also independently associated with asthma in one large pediatric study (12). The metabolic syndrome itself was a significant predictor of asthma-like symptoms in a large cross-sectional study of Korean adults, with stronger associations seen for abdominal adiposity and hypertension components of the syndrome (30). However, this study made no adjustments for BMI. Other cross-sectional and case-control studies also found the metabolic syndrome to be a predictor for prevalent asthma in both adults (7, 29, 48) and children (11, 12, 49). Our data, however, suggest that the metabolic syndrome does not predict incident asthma in women independent of BMI. Our study is different from the previously mentioned studies in the following respects: sex-stratified analyses, adjustment for BMI and insulin resistance, longitudinal analysis with incident asthma as the outcome, stepwise analyses to compare the relative strengths of associations, and use of time-varying predictors over a 25-year period. These differences in statistical and analytical approach may explain the differences in results between our study and the previously mentioned studies. Furthermore, there is a controversy regarding the usefulness of the metabolic syndrome, and some investigators are moving away from using this cluster of variables and instead focusing on its individual components. We also show that some components of the metabolic syndrome may be more important than others vis-à-vis asthma but do not find their association with incident asthma to be independent of BMI. On the other hand, the association between BMI and incident asthma is inadequately explained by the metabolic syndrome, and therefore other factors play an important role in this association. These factors include biomechanical, inflammatory, and metabolic abnormalities associated with obesity that may not be easily measured by the otherwise standard metabolic syndrome definition that we used.
Our study has several limitations. Incident asthma definition was based on self-reported provider-diagnosed asthma, which may have misclassification bias as compared with objective tests. However, obesity has not been shown to be associated with misclassification of asthma diagnosis in a carefully performed study by Aaron and colleagues (50). Our findings may be explained by a greater erroneous overdiagnosis in incident asthma among women. However, this is unlikely, as others have shown that men are more likely to be erroneously overdiagnosed with asthma than women (50). Asthma self-report may include early emphysema or chronic bronchitis, particularly among women smokers. Emphysema is, however, an unlikely explanation, because most subjects in our study had normal spirometric data. Another potential explanation for the absence of association in men may be their relatively fewer incident asthma cases as compared with women in our cohort. However, we do not think that this explains our findings, because the hazard ratios are nearly 1.0 in men and stronger in women in Table 2, where the metabolic syndrome is the predictor, and sex interactions are largely significant in Table 3, where BMI is the predictor. Furthermore, recall bias may misclassify age of onset of disease and erroneously classify pediatric disease on recrudescence as incident adult-onset disease. However, 95.2% of all subjects with asthma consistently classified their disease onset status as pediatric or adult at subsequent examination visits. Recall bias related to medication component of the asthma definitions was minimized by trained research assistants verifying pill containers and inhalers. The CARDIA cohort may not be representative of the entire U.S. population but is still a very relevant cohort, because it is representative of the urban and suburban populations in the United States. Finally, the attenuation of the metabolic syndrome association when adjusted for BMI may be a case of overadjustment, because the variables may be collinear. However, if that were the case, the BMI associations should also lose statistical significance when additionally adjusted for the metabolic syndrome, which was not the case.
In summary, using a longitudinal national cohort with 25 years of follow-up data, our study found that the metabolic syndrome does not adequately explain the association of BMI and incident asthma among women. This supports the notion that asthma in the obese subject has complex origins, with multiple mechanisms contributing to its pathophysiology (51). These mechanisms involve biomechanical, inflammatory, and metabolic abnormalities associated with obesity that may not be easily measured by the metabolic syndrome definition.
Acknowledgments
Acknowledgment
The authors thank William S. Beckett, M.D., M.P.H., Primary Care Center, Mount Auburn Hospital, Cambridge, MA, for his careful critique of the manuscript.
Footnotes
Supported by National Institutes of Health, CARDIA contracts N01-HC-48047-50 and N01-HC-95095 and an ancillary study, R01 HL 53560. A.S. is additionally supported by National Institutes of Health grants 1K23HL094531-01, 5M01RR00997, and 8 UL1TR000041.
Author Contributions: N.A. was responsible for hypothesis generation, study design, data analysis, data interpretation, and manuscript preparation. C.Q. was responsible for data analysis, data interpretation, and manuscript preparation. L.J.S. was responsible for data interpretation and manuscript preparation. A.A. was responsible for data administration and manuscript preparation. B.T. was responsible for data interpretation and manuscript preparation. M.S. was responsible for data interpretation and manuscript preparation. D.R.J. was responsible for data analysis, data interpretation, and manuscript preparation. A.S. was responsible for hypothesis generation, study design, data collection, data analysis, and manuscript preparation.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201303-0457OC on June 19, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization. 2011 obesity and overweight, fact sheet no 311 [accessed 2012 May 12]. Available from: www.who.int/mediacentre
- 2.World Health Organization. 2011. asthma, fact sheet no 307 [accessed May 2011]. Available from: www.who.int/mediacentre
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 4.National surveillance of asthma: United states, 2001–2010. U.S. Department of Health and Human Services Centers for Disease Control and Prevention National Center for Health Statistics. November 2012. Vital and Health Statistics Series 3, Number 35. [PubMed]
- 5.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment Of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 7.Appleton SL, Adams RJ, Wilson DH, Taylor AW, Ruffin RE North West Adelaide Health Study Team. Central obesity is associated with nonatopic but not atopic asthma in a representative population sample. J Allergy Clin Immunol. 2006;118:1284–1291. doi: 10.1016/j.jaci.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Von Behren J, Lipsett M, Horn-Ross PL, Delfino RJ, Gilliland F, McConnell R, Bernstein L, Clarke CA, Reynolds P. Obesity, waist size and prevalence of current asthma in the California Teachers Study cohort. Thorax. 2009;64:889–893. doi: 10.1136/thx.2009.114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agrawal A, Mabalirajan U, Ahmad T, Ghosh B. Emerging interface between metabolic syndrome and asthma. Am J Respir Cell Mol Biol. 2011;44:270–275. doi: 10.1165/rcmb.2010-0141TR. [DOI] [PubMed] [Google Scholar]
- 10.Al-Shawwa B, Al-Huniti N, Titus G, Abu-Hasan M. Hypercholesterolemia is a potential risk factor for asthma. J Asthma. 2006;43:231–233. doi: 10.1080/02770900600567056. [DOI] [PubMed] [Google Scholar]
- 11.Al-Shawwa BA, Al-Huniti NH, DeMattia L, Gershan W. Asthma and insulin resistance in morbidly obese children and adolescents. J Asthma. 2007;44:469–473. doi: 10.1080/02770900701423597. [DOI] [PubMed] [Google Scholar]
- 12.Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G. Metabolic abnormalities in children with asthma. Am J Respir Crit Care Med. 2011;183:441–448. doi: 10.1164/rccm.201004-0603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 14.Cutter GR, Burke GL, Dyer AR, Friedman GD, Hilner JE, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Manolio TA, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12(1, suppl):1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 15.Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, Jacobs DR, Jr, Liu K, Orden S, Pirie P, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) study. Control Clin Trials. 1987;8(4, suppl):68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 16.Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol. 1960;15:377–382. doi: 10.1152/jappl.1960.15.3.377. [DOI] [PubMed] [Google Scholar]
- 17.Ding DJ, Martin JG, Macklem PT. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol. 1987;62:1324–1330. doi: 10.1152/jappl.1987.62.3.1324. [DOI] [PubMed] [Google Scholar]
- 18.Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol. 2004;114:254–259. doi: 10.1016/j.jaci.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 19.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, Kercsmar CM, Visness CM, Matsui EC, Steinbach SF, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125:584–592. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sood A, Ford ES, Camargo CA., Jr Association between leptin and asthma in adults. Thorax. 2006;61:300–305. doi: 10.1136/thx.2004.031468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sood A, Cui X, Qualls C, Beckett WS, Gross MD, Steffes MW, Smith LJ, Jacobs DR., Jr Association between asthma and serum adiponectin concentration in women. Thorax. 2008;63:877–882. doi: 10.1136/thx.2007.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sood A, Qualls C, Schuyler M, Thyagarajan B, Steffes MW, Smith LJ, Jacobs DR., Jr Low serum adiponectin predicts future risk for asthma in women. Am J Respir Crit Care Med. 2012;186:41–47. doi: 10.1164/rccm.201110-1767OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunnbjornsdottir MI, Omenaas E, Gislason T, Norrman E, Olin AC, Jogi R, Jensen EJ, Lindberg E, Bjornsson E, Franklin K, et al. Obesity and nocturnal gastro-oesophageal reflux are related to onset of asthma and respiratory symptoms. Eur Respir J. 2004;24:116–121. doi: 10.1183/09031936.04.00042603. [DOI] [PubMed] [Google Scholar]
- 24.Dixon AE, Clerisme-Beaty EM, Sugar EA, Cohen RI, Lang JE, Brown ED, Richter JE, Irvin CG, Mastronarde JG. Effects of obstructive sleep apnea and gastroesophageal reflux disease on asthma control in obesity. J Asthma. 2011;48:707–713. doi: 10.3109/02770903.2011.601778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teodorescu M, Consens FB, Bria WF, Coffey MJ, McMorris MS, Weatherwax KJ, Palmisano J, Senger CM, Ye Y, Kalbfleisch JD, et al. Predictors of habitual snoring and obstructive sleep apnea risk in patients with asthma. Chest. 2009;135:1125–1132. doi: 10.1378/chest.08-1273. [DOI] [PubMed] [Google Scholar]
- 26.Bahreinian S, Ball GD, Colman I, Becker AB, Kozyrskyj AL. Depression is more common in girls with nonatopic asthma. Chest. 2011;140:1138–1145. doi: 10.1378/chest.11-0219. [DOI] [PubMed] [Google Scholar]
- 27.de Miguel Díez J, Hernández Barrera V, Puente Maestu L, Carrasco Garrido P, Gómez García T, Jiménez García R.Psychiatric comorbidity in asthma patients. Associated factors J Asthma 2011;48253–258. [DOI] [PubMed] [Google Scholar]
- 28.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 29.Thuesen BH, Husemoen LL, Hersoug LG, Pisinger C, Linneberg A. Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clin Exp Allergy. 2009;39:700–707. doi: 10.1111/j.1365-2222.2008.03197.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee EJ, In KH, Ha ES, Lee KJ, Hur GY, Kang EH, Jung KH, Lee SY, Kim JH, Shin C, et al. Asthma-like symptoms are increased in the metabolic syndrome. J Asthma. 2009;46:339–342. doi: 10.1080/02770900802660931. [DOI] [PubMed] [Google Scholar]
- 31.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 32.Beckett WS, Jacobs DR, Jr, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med. 2001;164:2045–2050. doi: 10.1164/ajrccm.164.11.2004235. [DOI] [PubMed] [Google Scholar]
- 33.McLachlan CR, Poulton R, Car G, Cowan J, Filsell S, Greene JM, Taylor DR, Welch D, Williamson A, Sears MR, et al. Adiposity, asthma, and airway inflammation. J Allergy Clin Immunol. 2007;119:634–639. doi: 10.1016/j.jaci.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 34.Herrera-Trujillo M, Barraza-Villarreal A, Lazcano-Ponce E, Hernandez B, Sanin LH, Romieu I. Current wheezing, puberty, and obesity among Mexican adolescent females and young women. J Asthma. 2005;42:705–709. doi: 10.1080/02770900500265306. [DOI] [PubMed] [Google Scholar]
- 35.Wasir JS, Misra A, Vikram NK, Pandey RM, Gupta R. Comparison of definitions of the metabolic syndrome in adult Asian Indians. J Assoc Physicians India. 2008;56:158–164. [PubMed] [Google Scholar]
- 36.Weiss ST. Obesity: insight into the origins of asthma. Nat Immunol. 2005;6:537–539. doi: 10.1038/ni0605-537. [DOI] [PubMed] [Google Scholar]
- 37.Musaad SM, Patterson T, Ericksen M, Lindsey M, Dietrich K, Succop P, Khurana Hershey GK.Comparison of anthropometric measures of obesity in childhood allergic asthma: central obesity is most relevant J Allergy Clin Immunol 20091231321–1327.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romieu I, Avenel V, Leynaert B, Kauffmann F, Clavel-Chapelon F. Body mass index, change in body silhouette, and risk of asthma in the E3N cohort study. Am J Epidemiol. 2003;158:165–174. doi: 10.1093/aje/kwg131. [DOI] [PubMed] [Google Scholar]
- 39.Leone N, Courbon D, Berr C, Barberger-Gateau P, Tzourio C, Alpérovitch A, Zureik M. Abdominal obesity and late-onset asthma: cross-sectional and longitudinal results: the 3C study. Obesity (Silver Spring) 2012;20:628–635. doi: 10.1038/oby.2011.308. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad T, Mabalirajan U, Ghosh B, Agrawal A. Altered asymmetric dimethyl arginine metabolism in allergically inflamed mouse lungs. Am J Respir Cell Mol Biol. 2010;42:3–8. doi: 10.1165/rcmb.2009-0137RC. [DOI] [PubMed] [Google Scholar]
- 41.Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM., Jr Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170:148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- 42.Mabalirajan U, Ahmad T, Leishangthem GD, Joseph DA, Dinda AK, Agrawal A, Ghosh B. Beneficial effects of high dose of L-arginine on airway hyperresponsiveness and airway inflammation in a murine model of asthma. J Allergy Clin Immunol. 2010;125:626–635. doi: 10.1016/j.jaci.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad T, Mabalirajan U, Sharma A, Aich J, Makhija L, Ghosh B, Agrawal A. Simvastatin improves epithelial dysfunction and airway hyperresponsiveness: from asymmetric dimethyl-arginine to asthma. Am J Respir Cell Mol Biol. 2011;44:531–539. doi: 10.1165/rcmb.2010-0041OC. [DOI] [PubMed] [Google Scholar]
- 44.Wu G, Collins JK, Perkins-Veazie P, Siddiq M, Dolan KD, Kelly KA, Heaps CL, Meininger CJ. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr. 2007;137:2680–2685. doi: 10.1093/jn/137.12.2680. [DOI] [PubMed] [Google Scholar]
- 45.Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin inhibits airway hyperreactivity: implications for the mevalonate pathway and beyond. Am J Respir Crit Care Med. 2009;180:731–740. doi: 10.1164/rccm.200901-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gosens R, Nelemans SA, Hiemstra M, Grootte Bromhaar MM, Meurs H, Zaagsma J. Insulin induces a hypercontractile airway smooth muscle phenotype. Eur J Pharmacol. 2003;481:125–131. doi: 10.1016/j.ejphar.2003.08.081. [DOI] [PubMed] [Google Scholar]
- 47.Yamashita N, Tashimo H, Ishida H, Matsuo Y, Arai H, Nagase H, Adachi T, Ohta K. Role of insulin-like growth factor-I in allergen-induced airway inflammation and remodeling. Cell Immunol. 2005;235:85–91. doi: 10.1016/j.cellimm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Terzano C, Morano S, Ceccarelli D, Conti V, Paone G, Petroianni A, Graziani E, Carnovale A, Fallarino M, Gatti A, et al. Effect of insulin on airway responsiveness in patients with type 2 diabetes mellitus: a cohort study. J Asthma. 2009;46:703–707. doi: 10.1080/02770900903056203. [DOI] [PubMed] [Google Scholar]
- 49.Arshi M, Cardinal J, Hill RJ, Davies PS, Wainwright C. Asthma and insulin resistance in children. Respirology. 2010;15:779–784. doi: 10.1111/j.1440-1843.2010.01767.x. [DOI] [PubMed] [Google Scholar]
- 50.Aaron SD, Vandemheen KL, Boulet LP, McIvor RA, Fitzgerald JM, Hernandez P, Lemiere C, Sharma S, Field SK, Alvarez GG, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121–1131. doi: 10.1503/cmaj.081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation J Allergy Clin Immunol 2011128508–515.e1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]