Abstract
The primary motor cortex (M1) possesses a functional somatotopic structure -representations of adjacent within-limb joints overlap to facilitate coordination while maintaining discrete centers for individuated movement. We examined whether similar organization exists across other sensorimotor cortices. Twenty-four right-handed healthy subjects underwent functional Magnetic Resonance Imaging (fMRI) while tracking complex targets with flexion/extension at right finger, elbow and ankle separately. Activation related to each joint at false discovery rate of .005 served as its representation across multiple regions. Within each region, we identified the Center of Mass (COM) for each representation, and overlap between representations of within-limb (finger and elbow) and between-limb joints (finger and ankle). Somatosensory (S1) and premotor cortices (PMC) demonstrated greater distinction of COM and minimal overlap for within- and between-limb representations. Contrarily, M1 and supplementary motor area (SMA) showed more integrative somatotopy with higher sharing for within-limb representations. Superior and inferior parietal lobule (SPL and IPL) possessed both types of structure. Some clusters exhibited extensive overlap of within- and between-limb representations, while others showed discrete COMs for within-limb representations. Our results help infer hierarchy in motor control. Areas as S1 may be associated with individuated movements, while M1 may be more integrative for coordinated motion; parietal associative regions may allow switch between both modes of control. Such hierarchy creates redundant opportunities to exploit in stroke rehabilitation. Use of complex rather than traditionally used simple movements was integral to illustrating comprehensive somatotopic structure; complex tasks can potentially help understand cortical representation of skill and learning-related plasticity.
Keywords: Somatotopy, Representation, Motor Map, Motor Control, Movement, Posterior Parietal Cortex, Primary Motor Cortex or M1, Functional MRI or fMRI
1. INTRODUCTION
Traditionally, the primary motor cortex (M1) was known to be organized with a point-to-point layout of cortical representations of joints and muscles, a feature known as discrete somatotopy (Foerster, 1936; Grafton et al., 1993; Kawashima and Fukuda, 1994; Penfield, 1937; Penfield, 1950; Rao SM, 1995; Woolsey et al., 1979). A more recent concept of M1 organization, instead, demonstrates that cortical representations of adjacent joints overlap while maintaining distinction between their centers (Beisteiner et al., 2001; Dechent and Frahm, 2003; Plow et al., 2010). Evidence of organization within M1, thus, suggests a balance between discrete and distributed structure, an organization that is now termed functional somatotopy. We have previously discussed (Plow et al., 2010; Plow and Carey, 2012), along with others (Beisteiner et al., 2001; Hlustik and Mayer, 2006; Kleinschmidt et al., 1997; Molina-Luna et al., 2008; Nudo and Milliken, 1996; Pascual-Leone et al., 1996), that such a structure may afford flexibility to M1, where sharing of cortical substrates may create opportunities for coordinated movements involving within-limb joints (such as index finger and elbow in reaching to grasp), while disparate centers may allow discrete control for individuated movements at the respective joints. It remains unclear, however, whether other cortices that participate in movements, such as higher motor areas, primary sensory and associative areas demonstrate similar somatotopic structure.
To define the somatotopic structure across cortical networks, it is critical to first choose an ideal motor task. Somatotopy within M1 has been routinely studied using simple volitional flexion-extension movements during functional Magnetic Resonance Imaging (fMRI) (Kapreli et al., 2006; Lotze et al., 2000; Luft et al., 2002). However, to define somatotopic structure across comprehensive cortices complex movements may offer a better model because 1) complex movements, unlike simple, are associated with greater task preplanning, error-detection and correction, thereby eliciting widespread fMRI activation (Beisteiner et al., 2001; Carey et al., 2006; Dechent and Frahm, 2003; Hlustik et al., 2001; Kleinschmidt et al., 1997; Luft et al., 2002) and 2) complex movements are more strongly applicable to motor skills, and learning such movements initiates adaptive plasticity across representations in M1 (Kleim et al., 1998; Nudo and Milliken, 1996; Plautz et al., 2000). Thus, by defining the organization of representations based on complex motor tasks, we can more accurately deduce a region’s role in motor skill and motor control.
The purpose of the present study was to explore the somatotopic structure across cortical substrates besides M1 using complex movements with fMRI. We employed joint-tracking involving flexion/extension to precisely follow moving target waveforms (Bhatt et al., 2007; Carey et al., 2002; Carey et al., 2006) because this task maximally elicits activation of higher cortical substrates besides M1 (Bhatt et al., 2007; Carey et al., 2002), more so than simple movements (Carey et al., 2006). Furthermore, training upon joint tracking initiates comparable mechanisms of plasticity of representations in M1 (Plow and Carey, 2012) as described with learning of skill in animal studies (Kleim et al., 1998; Nudo and Milliken, 1996; Plautz et al., 2000).
Subjects performed joint tracking at index finger, elbow and ankle, simultaneous with fMRI. We defined activation related to these individual joints as their movement representations. Within each active cortical region, we identified centers of and calculated overlap between representations of within-limb (finger and elbow) and between-limb (finger and ankle) joints. In doing so, we investigated whether the somatotopic structure for a region resembled that which is now established for M1 (Beisteiner et al., 2001; Carey et al., 2006; Dechent and Frahm, 2003)- ‘functional somatotopy’, containing overlapping yet distinctive within-limb representations. By comparing the extent of functional somatotopy across regions, we intended to gain better understanding of how regions aligned in the hierarchy of motor control. It may be that the role that a region plays in motor control is defined through its somatotopy, whether integrative or distinctive. This carries significance for defining prognosis for patients with cortical damage, and offers unique, parallel substrates that could substitute for damaged areas and serve as alternate targets for brain stimulation in rehabilitation.
2. Results
Joint tracking at right finger, elbow and ankle were performed alternating with rest in a repeating block sequence during fMRI. The order of repeating blocks was randomized across subjects. A schematic of the set-up in the MRI scanner is shown in Fig 1a and an example of sequence of blocks is shown in Fig 1b.
Figure 1.
a and 1b: Experimental description: 1a) Schematic depicting a subject performing a tracking task in the MRI. Task is performed separately at differing joints, right index finger, elbow and ankle, using flexion/extension. Movement at the joint is recorded via special sensors (see section 4.2). Subject uses flexion/extension at the designated joint to follow a moving target waveform presented on a projection screen that is viewed through a rear-projection mirror attached to the MRI head coil. Prompts at the bottom of the screen indicate the block- rest or finger, elbow or ankle tracking. Accuracy of tracking is emphasized as subjects can view their response and its relation to the moving target waveform in real-time. 1b) Repeating sequence of blocks for finger (F), elbow (E), ankle (A) tracking and rest (R).
Change in Blood Oxygen-level Dependent (BOLD) fMRI contrast during tracking versus rest was analyzed. Multi-subject random-effects general linear model (GLM) analysis illustrated regions with significantly greater activation during tracking than rest. Within each region, activation related to tracking at an individual joint was called its ‘representation’. Volume (size) and location (center of mass) of representation was defined for each representation. Overlap between within-limb (finger and elbow) and between-limb (finger and ankle) representations were also calculated for each region. While center of mass and overlap was aimed to understand the integrative versus distinctive nature of representations within a region, volume of activation of representations facilitated comparison across regions.
2.1 Overall Activation Related to Tracking: Contrast of Finger + Elbow + Ankle vs. Rest
Primary motor and somatosensory (M1 and S1), higher-order motor [premotor cortex (PMC) and supplementary motor (SMA)] and posterior parietal cortices [superior parietal lobule (SPL), inferior parietal lobule (IPL) & Precuneus (PrCU)] were active in the left hemisphere with right-sided tracking. M1, S1, SMA, IPL and dorsolateral prefrontal cortex (DLPFC) were active on the right (Fig 2).
Figure 2.
Cortical Substrates of a tracking: Overall statistical parametric maps of complex visuomotor tracking task tested across upper and lower limb joints (F+E+A vs. rest contrast). Activated regions include supplementary motor area (SMA), premotor cortex (PMC), primary motor cortex (M1), somatosensory cortex (S1), superior parietal lobule (SPL), inferior parietal lobule (IPL), dorsolateral prefrontal cortex (DLPFC) and precuneus (PrCU) in the left and SMA, M1, S1, IPL and DLPFC in the right hemisphere. Regions have only been labeled on the right so that their homologues on the left can be viewed clearly. The color scale represents T-scores of intensity of activation threshold at 4.58, FDR = 0.005.
2.2: Activation Related to Tracking at each joint: Representations revealed with individual contrasts of Finger vs. Rest, Elbow vs. Rest and Ankle vs Rest
Tracking at finger, elbow and ankle (Fig 3), separately, showed distributed activation across regions discussed above. Activation in the left hemisphere included M1, S1, PMC, SMA, SPL, IPL and PrCU. Tracking at finger elicited the largest volume of activation in M1 (F2,42=9.0, p = .001), S1 (F1.16, 24.3=52.3, p <.001), PMC (F1.17, 24.7=19.3,p = .001), SPL (F2,42=6.632, p = .003) and IPL (F1.25,26.2=35.5, p < .001), while tracking at ankle generated the largest activation in SMA (F2,42=4.1, p < .05)(Fig. 4). In the right hemisphere, IPL was active across all tracking conditions, while S1 and SMA were active only in finger and ankle tracking; again, tracking with finger was associated with the highest volume of activation in S1 (F1,21=24.3, p < .001), SMA (F1,21=35.0, p = .008) and IPL (F1.34,28.2=15.0 p < .001).
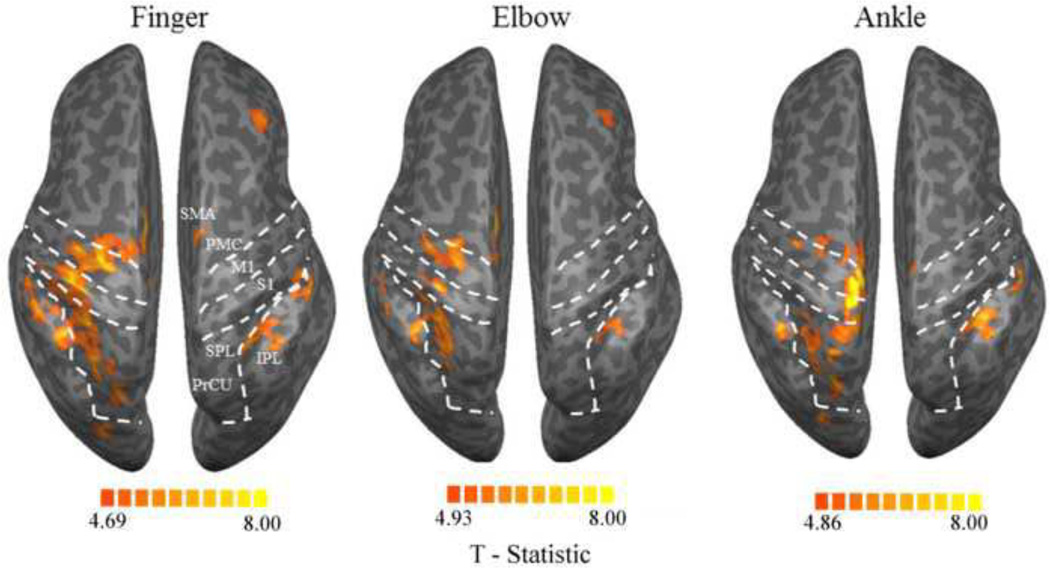
Figure 3.
Activation-based representations of individual joints: Statistical parametric maps (FDR < 0.005) of finger (F), elbow (E) and ankle (A) tracking vs. rest. Activated regions include supplementary motor area (SMA), premotor cortex (PMC), primary motor cortex (M1), somatosensory cortex (S1), superior parietal lobule (SPL) (left hemisphere), inferior parietal lobule (IPL), dorsolateral prefrontal cortex (DLPFC) (right hemisphere) and precuneus (PrCU) (only with finger and ankle tracking in the left hemisphere). The color scale represents T-scores threshold at 4.69 (finger), 4.93 (elbow) and 4.86 (ankle), FDR = 0.005.
Figure 4.
Size of Activation: Volume of activation (number of voxels) related to tracking at each joint plotted for each cortical region within the left hemisphere. Tracking at finger elicited the largest volume of activation in M1, S1, PMC, SPL and IPL, where ankle and elbow generated the largest activation in SMA . (*p <.05 and **p <.001).
Somatotopic Structure across Primary Motor and Sensory Cortices
Left M1 has little COM differentiation, and extensive overlap (67%), between activation of within-limb joints (Fig 5). M1 does, however, show considerable differentiation between COMs for between-limb representations (Fig. 6a, Table 1) with minimal between-limb overlap (Fig 5). S1, contrarily, displayed remarkable COM differentiation between activation associated with within-limb joints (Fig. 6a, Table 1); COM for finger lay inferior, lateral and anterior to that of elbow, with only a limited overlap between the two (14%) (Fig. 5). Activation of ankle, as in M1 (Fig. 6a, Table 1), showed no overlap with finger (Fig. 5).
Figure 5.
Overlap: Degree of overlap between finger and elbow (FE) and finger and ankle (FA) in the right and left hemisphere. Values are a percentage of shared voxels within each given region – SMA, PMC, M1, S1, IPL, SPL, PrCU and DLPFC.
Figure 6.
Center of Mass: Talairach center of mass between finger (F), elbow (E) and ankle (A) tracking across multiple regions of the brain in the left hemisphere and right hemisphere. Regions represented are primary motor cortex (M1), somatosensory cortex (S1), Premotor Cortex (PMC), supplementary motor area (SMA), inferior parietal lobule (IPL) and superior parietal lobule (SPL). The SPL and IPL demonstrated multiple clusters during F and A activation; they are identified as Fc1, Fc2, Ac1 and Ac2. X, Y, Z-axes coordinates are in mm. All coordinates are represented in table 1 and 2 in bold text. The points represent means (±SD).
Table I.
Mean values of Talairach x, y, z COM coordinates of the left hemisphere
| Finger | |||||
|---|---|---|---|---|---|
| Talairach | Total# Voxel |
Intensity | |||
| X | Y | Z | |||
| M1 | −29 | −17 | 56 | 3300 | 6.13 |
| S1 | −38 | −26 | 52 | 4577 | 5.75 |
| PMC | −17 | −14 | 59 | 1068 | 5.36 |
| SMA | −4 | −9 | 54 | 1094 | 6.13 |
| SPL | −32 | −47 | 52 | 4776 | 5.68 |
| IPLcluster1 | −53 | −29 | 41 | 1638 | 5.57 |
| IPLcluster2 | −38 | −46 | 47 | 1290 | 5.68 |
| IPLcluster3 | −38 | −34 | 40 | 339 | 5.14 |
| PrCU | −21 | −67 | 52 | 914 | 5.58 |
| Elbow | |||||
| Talairach | Total# Voxel |
Intensity | |||
| X | Y | Z | |||
| M1 | −28 | −17 | 57 | 2276 | 5.93 |
| S1cluster1 | −28 | −25 | 62 | 635 | 6.21 |
| S1cluster2 | −38 | −20 | 56 | 204 | 5.74 |
| PMCcluster1 | −21 | −15 | 51 | 167 | 5.97 |
| SMA | −4 | −11 | 54 | 1291 | 6.14 |
| SPL | −32 | −45 | 53 | 3553 | 5.84 |
| IPLcluster1 | −35 | −45 | 46 | 816 | 5.95 |
| IPLcluster2 | −55 | −28 | 38 | 306 | 5.62 |
| IPLcluster3 | −52 | −35 | 42 | 112 | 5.73 |
| Ankle | |||||
| Talairach | Total# Voxel |
Intensity | |||
| X | Y | Z | |||
| M1cluster1 | −6 | −30 | 65 | 1155 | 7.14 |
| M1cluster2 | −25 | −13 | 58 | 336 | 5.43 |
| M1cluster3 | −38 | −15 | 51 | 243 | 6.05 |
| S1 | −6 | −36 | 64 | 1190 | 6.78 |
| PMCcluster1 | −15 | −18 | 67 | 236 | 7.15 |
| PMCcluster2 | −22 | −14 | 50 | 119 | 5.29 |
| SMA | −5 | −19 | 63 | 1512 | 6.41 |
| SPLcluster1 | −30 | −51 | 52 | 2158 | 5.65 |
| SPLcluster2 | −12 | −47 | 63 | 1521 | 5.84 |
| IPL | −36 | −47 | 49 | 673 | 5.76 |
| PrCU | −22 | −68 | 51 | 845 | 5.93 |
Somatotopic Structure across Higher Motor Cortices
Similar to M1, left SMA demonstrated little COM differentiation between activation associated with finger and elbow tracking (Fig. 6a, Table 1), further reinforced by findings of considerable (50%) sharing (Fig. 5). Activation related to ankle task, however, remained distinct from finger and elbow and showed only a minimal between-limb (finger-ankle) overlap. PMC, on the other hand, demonstrated greater distinction between COMs for activation related to within-limb joints, similar to S1. As in SMA, PMC showed that activation related to ankle task appeared superior and medial to that for finger and elbow, with only a modest degree of between-limb sharing amongst them.
Somatotopic Structure across Parietal Associative Areas
Unlike the higher motor cortices, the parietal associative areas showed more than one major cluster of activation for each tracking condition, yielding multiple activation locations. SPL demonstrated little COM differentiation between finger and elbow activation with 49% overlap between their representations. The main cluster of activation related to ankle task (2158 voxels) showed little differentiation from finger or elbow. However, a second activation cluster for ankle task (1521 voxels) was superior and medial to that of finger and elbow tasks and showed no voxel overlap with either (Fig. 5), suggesting strongly-overlapping clusters as well as highly distinctive ones for between-limb joints.
IPL shows two major clusters of activation during finger task, one located near the supramarginal gyrus that is inferior, lateral and anterior to the other identified near the intra-parietal sulcus (Fig. 6a, Table 1). The cluster near the supramarginal gyrus shows very distinctive COMs with limited within-limb overlap and no between-limb overlap (Fig. 5). The cluster near the intra-parietal sulcus, however, has a non-distinct COM with considerable within- and between-limb overlap (54% and 49%) of activation. PrCU activation was only present in the finger and ankle task. There is very little COM differentiation between these two tasks, further demonstrated by 59% overlap.
Somatotopic Structure across Cortices in the Right Hemisphere
The right hemisphere only showed activation in S1, SMA and IPL. S1 activation was limited to finger and ankle tasks where little differentiation was observed between their respective COMs (Fig 6b, Table 2). SMA activation on the right shows similarities with those on the left with great differentiation between COMs associated with finger and ankle tasks. Right IPL, unlike its homologue on the left, shows little COM differentiation between activation for finger, elbow and ankle tasks and in fact shows an even greater between-limb overlap (48% in main cluster & 53% in secondary cluster) compared to within-limb (34%) (Fig 5).
Table II.
Mean values of Talairach x, y, z COM coordinates of the right hemisphere
| Finger | |||||
|---|---|---|---|---|---|
| Talairach | Total# Voxel |
Intensity | |||
| X | Y | Z | |||
| S1 | 52 | −23 | 39 | 597 | 5.96 |
| SMA | 5 | −8 | 59 | 433 | 5.54 |
| IPLcluster 1 | 34 | −44 | 50 | 2087 | 5.94 |
| IPLcluster2 | 52 | 26 | 40 | 763 | 5.79 |
| DLPFC | 34 | 32 | 31 | 516 | 5.39 |
| Elbow | |||||
| Talairach | Total# Voxel |
Intensity | |||
| X | Y | Z | |||
| IPL | 34 | −42 | 48 | 696 | 5.74 |
| DLPFC | 33 | 31 | 32 | 322 | 5.52 |
| Ankle | |||||
| Talairach | Total # Voxel |
Intensity | |||
| X | Y | Z | |||
| S1 | 53 | −21 | 37 | 165 | 6 |
| SMA | 3 | −21 | 66 | 240 | 5.66 |
| IPLcluster 1 | 36 | −41 | 47 | 1409 | 5.79 |
| IPLcluster2 | 53 | −25 | 39 | 448 | 5.6 |
3. Discussion
Our aim was to investigate the somatotopic structure across cortical substrates involved in complex movement. We observed that regions vary along the continuum of discrete versus functional somatotopy. Complex movement tracking elicited activation in M1, S1, SMA, PMC, SPL, IPL and PrCU as demonstrated previously using complex tasks (Carey et al., 2006; Meister et al., 2005; Shibasaki et al., 1993). While S1 and PMC demonstrate greater distinction between within- and between-limb representations, M1 and SMA show a more integrative somatotopy with higher sharing for within-limb representations of finger and elbow. Parietal associative areas (SPL and IPL) show a more intriguing structure, where some activation clusters exhibit extensive overlap of all within- and between-limb representations while other clusters show discrete arrangement of within-limb representations.
The varying nature of somatotopic structure revealed across areas may align with their roles in hierarchical processing of complex motor control. Discrete somatotopy in S1 may serve to focus or refine the perception of individual joints in movement, while integrative structure in IPL and SPL that contains discrete as well as overlapping and functionally somatotopic sub-regions may offer flexibility in switching between individuated versus coordinated movement. Thus, use of a complex motor task helped illustrate comprehensive cortical substrates participating in motor control. Since the task could be uniformly applied to different joints, we were able to define their respective representations to illustrate somatotopic structure.
Somatotopic Structure across Cortical Substrates of Motor task
Primary motor and sensorimotor cortices
Somatotopic structure varied between M1 and S1. We witnessed considerable overlap of within-limb representations in M1 as shown in our previous study (Plow et al., 2010) and those of others (Beisteiner et al., 2001; Dechent and Frahm, 2003; Hlustik et al., 2001; Kleinschmidt et al., 1997; Luft et al., 2002) and only minimal between-limb sharing. But, in S1, there was considerable separation of COMs of representations of finger and elbow with only minimal overlap, while between-limb overlap was lacking. These findings resonate with classical evidence that M1 possesses a more integrated somatotopy compared to S1 (Hlustik et al., 2001). It is believed S1 somatotopy function is a true representation of the body surface where M1 functions to bring together functionality across limbs and joints (Sanes and Schieber, 2001). This discrete somatotopic representation allows for greater proprioceptive processing across different body parts (Hlustik et al., 2001). The cohesive structure in M1 is ascribed to its extensive horizontal connections spanning across adjacent within-limb representations (Donoghue et al., 1992; Huntley and Jones, 1991; Wu et al., 2000) and may explain its flexibility for change and exchange of motor skill in learning (Plow and Carey, 2012). Further, besides horizontal connections within M1, another substrate for integrative control of within-limb joints has recently been revealed with retrograde viral transneuronal transport. In rhesus monkeys, Rathelot and Strick (2009), demonstrate that a caudal region of M1 offers direct connections to motor neurons controlling proximal and distal upper limb joints. They suggest that such a region in M1 may be present in humans, besides some specific species of non-human primates. Therefore, integrative control of finger and elbow joints in M1 may be served by evidence of representation-to-representation horizontal connections as well as direct corticomotorneuronal control from caudal M1.
Premotor and Supplementary motor cortices
The higher motor areas, important for their contribution to motor-planning and organization of sequence of movement in tracking (Crammond and Kalaska, 2000; Gerloff et al., 1997), differ in the extent of their functional somatotopy, similar to M1 and S1. Sharing of within-limb representations was prominent in the SMA. PMC, however, seemingly possesses a more distinctive somatotopic structure compared to the M1 or SMA although limited activation in PMC may have skewed results. There appears to be a bias towards greater activation with finger in PMC, whereas significant activation in the SMA was devoted to elbow and ankle rather than finger. These results coincide with findings in animals (Wu et al. 2000) that distal forelimb is disproportionately represented in PMC, while hindlimb representations are strongly evident in the SMA. These disparities in representations between PMC and SMA align with their roles in movement control. PMC makes substantial contributions towards the corticospinal pool devoted to fingers and dexterity (Dum and Strick 2002), independently of M1; thus, in recovery from focal strokes affecting M1, PMC can serves as an alternate locus for controlling prehension (Schulz et al., 2012; Zeiler et al., 2013). On the other hand, the disproportionately large representation of ankle in the SMA, as also noted in our previous work (LaPointe et al., 2009), potentially relates to its prominent role in postural control and control of proximal and axial movements (Massion, 1992).
Parietal Associative Areas
The parietal associative areas demonstrate an even more intriguing structure. Both IPL and SPL show several activation clusters, which coincide with their role in sustained visuo-spatial attention (Culham et al., 1998; Imaruoka et al., 2005; Shomstein and Behrmann, 2006; Yantis et al., 2002); yet, each cluster reflects differing somatotopic structure. For instance, in case of IPL, a major cluster located proximate to the intraparietal sulcus was commonly active across finger, elbow and ankle tracking, suggesting complete within- and between-limb overlap. However, in another cluster located closer to the supramarginal gyrus, we noted more distinctive within-limb representations, aligning with its role in precise manipulation and grasping (Grefkes and Fink, 2005). Evidence of organization of the posterior parietal cortex into sub-regions is also noted in animal studies where different portions have distinct arrangement for individual body parts (Hyvarinen, 1981). The SPL and IPL cortices possess, what we can call ‘distributed functional’ somatotopy, such that certain sub-regions provide extensive within- and across-limb overlap while others display a discrete somatotopic frame. These associative areas may thus be able to switch promptly between differing modes of movement control- coordinated versus individuated, signifying their parallel, yet advanced contribution to motor control, compared to primary and higher-order motor cortices (Imamizu and Shimojo, 1995).
Right Hemisphere
Observations of the somatotopic arrangement in the ipsilateral hemisphere allude to its executive role in complex movements. The significant activation observed in the right IPL supports its dominant role in sustained attention to bilateral visual fields (Culham et al., 1998; Imaruoka et al., 2005; Shomstein and Behrmann, 2006; Yantis et al., 2002) that is continually required to modify movement (Callaert et al., 2011), as in the case of our tracking task. Activation of the right DLPFC complements that of IPL through its role in working memory (Robertson et al., 2001) which is important for online visual feedback to maintain tracking accuracy. Activation of the ipsilateral SMA has been previously shown during complex movements, where it is believed bilateral SMA activation is important for the generation of a complex task (Fried et al., 1991; Shibasaki et al., 1993). However, we acknowledge that role of activation in areas may be more speculative; for instance, activation of DLPFC may be related to feedback monitoring than complex movement per se. To delineate whether somatotopic structure revealed across areas besides M1, indeed relates to their role in movement or the task preplanning, error-detection and correction that is inherent in complex tasks, future experiments can build control tasks that seek to highlight activation related to such resources or virtual inactivation of key regions with inhibitory repetitive transcranial magnetic stimulation may suggest causality.
Significance and Future Directions
Our findings carry significance for the understanding of skill and its representation. By studying organization of activation-based representations across cortical regions, we have been able to create a template that can be utilized in the future for understanding representation of skill and cortical plasticity associated with learning of skill. Motor skill may be represented in effector-specific manner, where skill learned is specific to the kinematic properties of that joint, or an effector-independent manner, where skill learned is general and is easily transferable to multiple joints. We believe that discrete somatotopy with an organization of point-to-point representations, aligns with effector-specificity (Brashers-Krug et al., 1996; Vangheluwe et al., 2006), while functional somatotopic organization may fit well with effector-independence since overlap could allow skill to be uniformly applied across different joints (van Mier and Petersen, 2006). Further, following brain damage, such as stroke, knowing whether substrates that remain represent skill in effector-specific or effector-independent coordinates would help define prognosis of recovery and develop individualized therapeutic protocols. For example, re-learning of skill in stroke is associated with activation of higher motor areas (Bhatt et al., 2007; Carey et al., 2002; Hamzei et al., 2012; Marshall et al., 2000; Ward et al., 2003), and is evidenced to be functionally-adaptive (Abela et al., 2012; Jang et al., 2005; Page et al., 2009). Novel interventions can be created that help reorganize the nature of somatotopic structure. Noninvasive brain stimulation, such as repetitive transcranial magnetic stimulation (Carey et al., 2010; Corti et al., 2012)or transcranial direct current stimulation (Bolognini et al., 2009; Zimerman et al., 2012), may assist in transient re-mapping of cortical topography once patient-specific sites, guided by effector-specific or –generalizable treatment goals.
Limitations
Our aim was to decipher the somatotopic structure across cortical networks involved in complex movements. Even though we chose a complex task to elicit more widespread activation, the use of a complex task introduces a number of confounds (e.g. attention, prediction and learning). These confounds do make it difficult to analyze functional somatotopy with specificity as it relates to individual motor movements. However, we believe the possible confounds are important to examine somatotopic activation with true representations of a complex motor task. In the future, it will be beneficial to conduct an experiment which can identify the varying contributions of these multiple confounds by including a comparison between simple repetitive, passive and a complex task in order to identify regional contribution of the confounds to the complex task. Further, investigating somatotopy from a direct perspective by including portions of the scan where subject move joints simultaneously will prove beneficial. A second limitation is that we did not control for differing levels of difficulty in accomplishing tracking at finger versus elbow or ankle. However, because our aim was to investigate functional vs. discrete somatotopic structure, if we controlled for task difficulty, we would be unable to accurately reflect the functional representation of movement at each joint, influencing interpretation of somatotopic structure. A third limitation is that individual experience, motor-skill and motor learning does play an impact on BOLD-signal changes (Floyer-Lea and Matthews, 2005), which could have confounded our results. Future studies will build in sufficient time for learning of skill, so that all joints reach an asymptote level of performance; comparison of BOLD activation then would primarily relate to movement at the joint, than the confound of its level of skill. Lastly, we recognize that neural networks don’t always rely on spatial proximity for integration (Olshausen and Field, 2004). Future studies can look at time-series based correlation with resting state fMRI in order to investigate whether ‘distinct’ representative foci are indeed functionally distinct or still integrative
Conclusions
In conclusion we have found differences in the degree of discrete versus functional somatotopy across motor and associative regions. Primary sensory and premotor areas show highly discrete organization of movement representations, where primary motor and supplementary motor areas show overlap of within-limb representations. Parietal associative areas show a distributed structure, with highly overlapping within- and between-limb representations and as well as discrete somatotopy. Based on their differing somatotopic structure, we can project their specific roles in movement control. While primary areas may play a role in control across individual or some multi-joint movements, associative areas may exert hierarchically advanced control allowing them to switch from individuated to multi-joint, multi-limb control. The structure revealed here for each region may serve as a template for future to understand representation of skill because we not only signify gradients of somatotopy across regions but also show hierarchical functional specialization across networks.
4. Experimental Procedure
4.1 Participants
Subjects were recruited through convenience sampling from the campus of the University of Minnesota, Minneapolis. Twenty-four right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971), healthy college students [20 females and 4 males, mean (±SD) age (25.72 ± 2.9 years)] were enrolled. Exclusion criteria were claustrophobia, pregnancy and indwelling metallic implants or medical devices incompatible with MRI and any neuromuscular or musculoskeletal conditions affecting function of upper or lower limbs. The study was approved by the Institutional Review Board of the University of Minnesota.
4.2 Complex Motor Task with fMRI
Subjects performed joint tracking of finger, ankle and elbow simultaneous with fMRI scanning (Fig 1a). This study is based on the same dataset as used in our previous study (Plow et al., 2010); however, the data presented is analyzed differently to address novel perspectivesto explore somatotopic profile across sensory, motor and higher motor associative regions. Extensive detail of methodology can be found in Plow et al 2010.
The three different joints were tested separately on the right side: the metacarpophalangeal (MCP) joint of the index finger, the elbow and the ankle. Movement at these joints was recorded using customized electrogoniometers attached to potentiometers (ETI Systems, Carlsbad, CA). The potentiometer recording finger motion was centered lateral to the MCP, the potentiometer measuring elbow movement was positioned at the lateral epicondyle, while the potentiometer noting ankle movements was placed one inch below the medial malleolus. While tracking with finger and ankle was performed in the sagittal plane, elbow tracking was performed with a pronated forearm in the transverse plane so as not to interfere with magnetic field lines. Subjects performed joint tracking in a 3T scanner as a tracking test, composed of 25 blocks of 4 repeating and alternating conditions (rest, finger tracking, elbow tracking, and ankle tracking) (Fig 1b) as described in Plow et al. (2010). Cues appeared at the bottom of the screen, without obstructing view of the target waveform, and were denoted by identifiers as, “Rest”, “Finger” “Elbow” or “Ankle”. In order to maintain similar visual stimulus, subjects could still view the target waveform at rest, but they were asked to not move any joint. Prior to entering the 3T, subjects were allotted practice time in a mock scanner in order to mitigate extraneous muscle movement in unwanted limbs. Subjects were allotted 3 trials of 10 seconds where the target waveform was at a sine wave at a frequency of .4 Hz and amplitude of 15%–85%. Subjects were able to demonstrate no extraneous EMG activity beyond that of the joint of interest. Once in the fMRI subjects viewed a different random sine wave at the same frequency and range in amplitude within the mock scanner.
4.3 Data Acquisition
Simultaneous with the tracking test, Blood Oxygenation Level-Dependent (BOLD) fMRI data were collected. The first three fMRI volumes were dismissed due to T1 saturation effects. The tracking test was initiated at the 4th volume, simultaneous with which T2* - weighted images were acquired. Images were collected in the transverse plane using gradient Echo Planar Imaging (EPI) sequence (TE = 36 msec, TR = 3000 msec, FA = 80°, FOV = 256 mm × 256 mm). The in-plane resolution was 1.5 × 1.5 mm2 while the slice thickness was 1.5mm, as this resolution is considered optimal to capture task-based activation in M1 (Hyde et al., 2001; Hyde, 2001). At such high resolution, we acquired images up to a depth of 63 mm, extending from the superior pole, in 42 interleaved slices. Throughout the fMRI scan, approximately 300 imaged volumes were collected. In order to acquire a template for fMRI coregistration, landmarks were identified utilizing a high resolution (1 mm3), T1-weighted, 3D anatomical image (3D FLASH, TE= TR = 20 msec, FA = 30°, acquisition time = 10:44 min).
4.3 Data Analysis: fMRI
Pre-processing, first-and second-level fMRI analysis
Brain Voyager QX (Brain Innovation B.V., Maastricht, Netherlands) software version 1.8 was used for analysis of fMRI. Pre-processing of fMRI data corrected for differences in slice scan time acquisition, head motion artifacts and temporal linear trends as described in Plow et al. 2010. A General Linear Model (GLM) analysis was performed on individual subjects. The GLM was used in order to explore task specific activation volume and intensity across regions (described below). Once fMRI data was registered with the individual’s high-resolution anatomical images, it was transformed to standard Talairach space (Tournoux, 1988). Following the individual analysis, a group random-effects analysis was performed across all subjects. Three predictors, one for each effector: finger, elbow and ankle were included in the multi-subject GLM. Finger, elbow and ankle predictors were derived by convolving a box-car waveform with a double-gamma hemodynamic response function to model the hemodynamic response of BOLD signal (Friston et al., 1998; Goebel et al., 2006).
Hypothesis-driven Analyses
After fitting the random-effects GLM, and accounting for effects of temporal serial correlations in data (Goebel et al., 2006), multi-subject t-maps of activation related to finger, elbow and/or ankle predictors were generated. Maps were thresholded using a false discovery rate (Genovese et al., 2002) of .005 at a minimum cluster size set to 100 active voxels. An overall statistical parametric map was generated for the following statistical contrast: Finger+Elbow+Ankle vs. rest, which illustrated overall activation associated with tracking, regardless of the joint. Activated cortical substrates were identified based on landmarks from Talairach Daemon (Lancaster et al., 2000) and from previously-defined criteria (Critchley, 1953; Dassonville et al., 2001). These regions included primary motor (M1), primary sensory (S1), premotor cortex (PMC), supplementary motor area (SMA), superior parietal lobule (SPL), inferior parietal lobule (IPL), precuneus (PrCU) and dorsolateral prefrontal cortex (DLPFC).
To address the primary purpose of study, i.e. the nature of somatotopy within each region identified above, we examined activation patterns associated with tracking at index finger, elbow and ankle; individual statistical parametric maps were created with contrasts: Finger vs. rest, Elbow vs. rest and Ankle vs. rest. We computed the following variables to comprehensively describe the somatotopic structure for each previously defined region.
Center of Mass (COM): COM is the most commonly used metric to define pattern of somatotopy within a region (Dechent and Frahm, 2003; Lotze et al., 2000; Plow et al., 2010). It denotes the average location of all constituent active voxels (Tournoux, 1988). COM has demonstrated high intra-session reliability (Lotze et al., 2000; Loubinoux et al., 2001; Waldvogel et al., 1999). COM was defined in terms of Talairach x, y and z coordinates, with x referring to medio-lateral plane, y to the antero-posterior plane and z indicating the supero-inferior plane.
- Overlap: Overlap symbolizes sharing between activation/representations related to movement of different joints/effectors and has helped visualize functional somatotopy. (Dechent and Frahm, 2003; Hlustik et al., 2001; Kapreli et al., 2007; Plow et al., 2010; Weiss et al., 2012). Overlap was computed by overlaying individual statistical parametric maps for Finger vs. rest, Elbow vs. rest and Ankle vs. rest contrasts. For each region, we computed overlapping or common voxels for pairs of within-limb activation (finger and elbow) and between-limb activation (finger and ankle) using the formula below (Kapreli et al., 2007; Plow et al., 2010).
We also looked at between-limb activation of elbow and ankle; however, overlap results were similar to those of finger and ankle thus corroborating the between-limb specificity findings (data not shown). In our illustrations, we show overlap using color-coded gradient maps; if overlap between two statistical parametric maps is absent, then monochromatic colors are used, whereas if they overlap, the color of the overlapping region appears as a mix of constituent colors of individual maps. Activation Volume: Volume has generally not been used to define somatotopy of a region. But, we studied this to understand whether a region showed greater volume during tracking with one joint vs. another to realize where its structure lay along the continuum of discrete- versus functional-somatotopy. Activation volume was derived through individual statistical parametric maps for Finger vs. rest, Elbow vs. rest and Ankle vs. rest and examined on an individual subject-by-subject level. We determined the total number of activated voxels for each region and compared across task.
4.4 Statistical Analysis
We compared all metrics, for each region. The dependent variables were compared across regions for the F+E+A vs. rest contrast and across tasks within-regions for the individual joint contrasts using a repeated measures ANOVA at α = .05 using Bonferroni correction. SPSS 18.0 (IBM, Chicago) was used for statistical analysis.
Highlights.
We studied somatotopy across multiple cortical networks involved in complex movement.
Somatosensory and premotor cortices exhibited discrete somatotopy with greater distinction between adjacent representations.
Primary motor cortex and supplementary motor cortex exhibited functional somatotopy with overlapping representations.
Posterior parietal areas showed discrete and functional somatotopic representations.
Differences in degree of somatotopy allude to their role with complex movement.
Acknowledgments
This work was supported by the University of Minnesota’s Doctoral Dissertation Fellowship to [E. B. P.]; National Center for Research Resources at the National Institutes of Health (grant numbers P41 RR008079, M01-RR00400 to Center for Magnetic Resonance and Research, Minneapolis, MN); National Institutes of Health supporting investigator roles-1K01HD069504 (E. B. P.) and 1 R01 HD 053153-01A2 and 1 RC1 HD063838-01 (J. R. C) and the Program in Physical Therapy at the University of Minnesota. The authors would like to declare that Machado A. has ownership interest with IntElect Medical, ATI, Cardionomics, Boston Scientific and is on the consultant/advisory Board with IntElect Medical, Monteris. The authors would like to thank Ms. Pooja Arora, Ms. Megan Pline, Ms. Meagan Binenstock, and Dr. Kathleen Anderson for their assistance in data collection and analysis. Also, the authors would like to thank Drs. Theresa Kimberley, James Ashe and Carl Kukulka for valuable feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abela E, Missimer J, Wiest R, Federspiel A, Hess C, Sturzenegger M, Weder B. Lesions to primary sensory and posterior parietal cortices impair recovery from hand paresis after stroke. PLoS One. 2012;7:e31275. doi: 10.1371/journal.pone.0031275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisteiner R, Windischberger C, Lanzenberger R, Edward V, Cunnington R, Erdler M, Gartus A, Streibl B, Moser E, Deecke L. Finger somatotopy in human motor cortex. Neuroimage. 2001;13:1016–1026. doi: 10.1006/nimg.2000.0737. [DOI] [PubMed] [Google Scholar]
- Bhatt E, Nagpal A, Greer KH, Grunewald TK, Steele JL, Wiemiller JW, Lewis SM, Carey JR. Effect of finger tracking combined with electrical stimulation on brain reorganization and hand function in subjects with stroke. Exp Brain Res. 2007;182:435–447. doi: 10.1007/s00221-007-1001-5. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- Callaert DV, Vercauteren K, Peeters R, Tam F, Graham S, Swinnen SP, Sunaert S, Wenderoth N. Hemispheric asymmetries of motor versus nonmotor processes during (visuo)motor control. Hum Brain Mapp. 2011;32:1311–1329. doi: 10.1002/hbm.21110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, Ugurbil K. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- Carey JR, Greer KR, Grunewald TK, Steele JL, Wiemiller JW, Bhatt E, Nagpal A, Lungu O, Auerbach EJ. Primary motor area activation during precision-demanding versus simple finger movement. Neurorehabil Neural Repair. 2006;20:361–370. doi: 10.1177/1545968306289289. [DOI] [PubMed] [Google Scholar]
- Carey JR, Anderson DC, Gillick BT, Whitford M, Pascual-Leone A. 6-Hz primed low-frequency rTMS to contralesional M1 in two cases with middle cerebral artery stroke. Neurosci Lett. 2010;469:338–342. doi: 10.1016/j.neulet.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti M, Patten C, Triggs W. Repetitive transcranial magnetic stimulation of motor cortex after stroke: a focused review. Am J Phys Med Rehabil. 2012;91:254–270. doi: 10.1097/PHM.0b013e318228bf0c. [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Prior information in motor and premotor cortex: activity during the delay period and effect on pre-movement activity. J Neurophysiol. 2000;84:986–1005. doi: 10.1152/jn.2000.84.2.986. [DOI] [PubMed] [Google Scholar]
- Critchley. The Parietal Lobes. London: Arnold; 1953. Vol., ed.^eds. [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RB. Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol. 1998;80:2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J. The effect of stimulus-response compatibility on cortical motor activation. Neuroimage. 2001;13:1–14. doi: 10.1006/nimg.2000.0671. [DOI] [PubMed] [Google Scholar]
- Dechent P, Frahm J. Functional somatotopy of finger representations in human primary motor cortex. Hum Brain Mapp. 2003;18:272–283. doi: 10.1002/hbm.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Leibovic S, Sanes JN. Organization of the forelimb area in squirrel monkey motor cortex: representation of digit, wrist, and elbow muscles. Exp Brain Res. 1992;89:1–19. doi: 10.1007/BF00228996. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews PM. Distinguishable brain activation networks for short- and long-term motor skill learning. Journal of neurophysiology. 2005;94:512–518. doi: 10.1152/jn.00717.2004. [DOI] [PubMed] [Google Scholar]
- Foerster O. The motor cortex in man in the light of Hughlings Jackson's doctrines. Brain. 1936;59:135–159. [Google Scholar]
- Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, Spencer DD. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 1991;11:3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain. 1997;120((Pt 9)):1587–1602. doi: 10.1093/brain/120.9.1587. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Woods RP, Mazziotta JC. Within-arm somatotopy in human motor areas determined by positron emission tomography imaging of cerebral blood flow. Exp Brain Res. 1993;95:172–176. doi: 10.1007/BF00229666. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei F, Lappchen CH, Glauche V, Mader I, Rijntjes M, Weiller C. Functional plasticity induced by mirror training: the mirror as the element connecting both hands to one hemisphere. Neurorehabil Neural Repair. 2012;26:484–496. doi: 10.1177/1545968311427917. [DOI] [PubMed] [Google Scholar]
- Hlustik P, Solodkin A, Gullapalli RP, Noll DC, Small SL. Somatotopy in human primary motor and somatosensory hand representations revisited. Cereb Cortex. 2001;11:312–321. doi: 10.1093/cercor/11.4.312. [DOI] [PubMed] [Google Scholar]
- Hlustik P, Mayer M. Paretic hand in stroke: from motor cortical plasticity research to rehabilitation. Cognitive & Behavioral Neurology. 2006;19:34–40. [PubMed] [Google Scholar]
- Huntley GW, Jones EG. Relationship of intrinsic connections to forelimb movement representations in monkey motor cortex: a correlative anatomic and physiological study. Journal of neurophysiology. 1991;66:390–413. doi: 10.1152/jn.1991.66.2.390. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Biswal BB, Jesmanowicz A. High-resolution fMRI using multislice partial k-space GR-EPI with cubic voxels. Magn Reson Med. 2001;46:114–125. doi: 10.1002/mrm.1166. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Hyde Biswal BB, Jesmanowicz A. Optimum voxel size in fMRU. International society for magnetic resonance in medicine. 2001:240. [Google Scholar]
- Hyvarinen J. Regional distribution of functions in parietal association area 7 of the monkey. Brain Res. 1981;206:287–303. doi: 10.1016/0006-8993(81)90533-3. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Shimojo S. The locus of visual-motor learning at the task or manipulator level: implications from intermanual transfer. Journal of Experimental Psychology: Human Perception & Performance. 1995;21:719–733. doi: 10.1037//0096-1523.21.4.719. [DOI] [PubMed] [Google Scholar]
- Imaruoka T, Saiki J, Miyauchi S. Maintaining coherence of dynamic objects requires coordination of neural systems extended from anterior frontal to posterior parietal brain cortices. Neuroimage. 2005;26:277–284. doi: 10.1016/j.neuroimage.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Jang SH, You SH, Hallett M, Cho YW, Park CM, Cho SH, Lee HY, Kim TH. Cortical reorganization and associated functional motor recovery after virtual reality in patients with chronic stroke: an experimenter-blind preliminary study. Arch Phys Med Rehabil. 2005;86:2218–2223. doi: 10.1016/j.apmr.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Kapreli E, Athanasopoulos S, Papathanasiou M, Van Hecke P, Strimpakos N, Gouliamos A, Peeters R, Sunaert S. Lateralization of brain activity during lower limb joints movement. An fMRI study. Neuroimage. 2006;32:1709–1721. doi: 10.1016/j.neuroimage.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Kapreli E, Athanasopoulos S, Papathanasiou M, Van Hecke P, Keleki D, Peeters R, Strimpakos N, Sunaert S. Lower limb sensorimotor network: issues of somatotopy and overlap. Cortex. 2007;43:219–232. doi: 10.1016/s0010-9452(08)70477-5. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Fukuda H. Functional organization of the human primary motor area: an update on current concepts. Rev Neurosci. 1994;5:347–354. doi: 10.1515/revneuro.1994.5.4.347. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. Journal of neurophysiology. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Nitschke MF, Frahm J. Somatotopy in the human motor cortex hand area. A high-resolution functional MRI study. Eur J Neurosci. 1997;9:2178–2186. doi: 10.1111/j.1460-9568.1997.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe KE, Klein JA, Konkol ML, Kveno SM, Bhatt E, DiFabio RP, Carey JR. Cortical activation during finger tracking vs. ankle tracking in healthy subjects. Restor Neurol Neurosci. 2009;27:253–264. [PubMed] [Google Scholar]
- Lotze M, Erb M, Flor H, Huelsmann E, Godde B, Grodd W. fMRI evaluation of somatotopic representation in human primary motor cortex. Neuroimage. 2000;11:473–481. doi: 10.1006/nimg.2000.0556. [DOI] [PubMed] [Google Scholar]
- Loubinoux I, Carel C, Alary F, Boulanouar K, Viallard G, Manelfe C, Rascol O, Celsis P, Chollet F. Within-session and between-session reproducibility of cerebral sensorimotor activation: a test--retest effect evidenced with functional magnetic resonance imaging. J Cereb Blood Flow Metab. 2001;21:592–607. doi: 10.1097/00004647-200105000-00014. [DOI] [PubMed] [Google Scholar]
- Luft AR, Smith GV, Forrester L, Whitall J, Macko RF, Hauser TK, Goldberg AP, Hanley DF. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Hum Brain Mapp. 2002;17:131–140. doi: 10.1002/hbm.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–661. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Meister I, Krings T, Foltys H, Boroojerdi B, Muller M, Topper R, Thron A. Effects of long-term practice and task complexity in musicians and nonmusicians performing simple and complex motor tasks: implications for cortical motor organization. Hum Brain Mapp. 2005;25:345–352. doi: 10.1002/hbm.20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Luna K, Hertler B, Buitrago MM, Luft AR. Motor learning transiently changes cortical somatotopy. Neuroimage. 2008;40:1748–1754. doi: 10.1016/j.neuroimage.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. Journal of neurophysiology. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Sparse coding of sensory inputs. Curr Opin Neurobiol. 2004;14:481–487. doi: 10.1016/j.conb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Page SJ, Szaflarski JP, Eliassen JC, Pan H, Cramer SC. Cortical plasticity following motor skill learning during mental practice in stroke. Neurorehabil Neural Repair. 2009;23:382–388. doi: 10.1177/1545968308326427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Peris M, Tormos JM, Pascual AP, Catala MD. Reorganization of human cortical motor output maps following traumatic forearm amputation. Neuroreport. 1996;7:2068–2070. doi: 10.1097/00001756-199609020-00002. [DOI] [PubMed] [Google Scholar]
- Penfield Ba. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Penfield Ra. A clinical study of localization of function. New York: Hafner Pub. Co; 1950. The cerebral cortex of man. Vol. [Google Scholar]
- Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiology of Learning & Memory. 2000;74:27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- Plow EB, Arora P, Pline MA, Binenstock MT, Carey JR. Within-limb somatotopy in primary motor cortex--revealed using fMRI. Cortex. 2010;46:310–321. doi: 10.1016/j.cortex.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Plow EB, Carey JR. Pilot fMRI investigation of representational plasticity associated with motor skill learning and its functional consequences. Brain Imaging Behav. 2012 doi: 10.1007/s11682-012-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM BJ, Hammeke TA Bandettini PA, Bobholz JA Frost JA, Myklebust BM Jacobson RD, Hyde JS. Somatotopic mapping of the human primary motor cortex with functional magnetic resonance imaging. Neurology. 1995;45:919–924. doi: 10.1212/wnl.45.5.919. [DOI] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci U S A. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Tormos JM, Maeda F, Pascual-Leone A. The role of the dorsolateral prefrontal cortex during sequence learning is specific for spatial information. Cereb Cortex. 2001;11:628–635. doi: 10.1093/cercor/11.7.628. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Schieber MH. Orderly somatotopy in primary motor cortex: does it exist? Neuroimage. 2001;13:968–974. doi: 10.1006/nimg.2000.0733. [DOI] [PubMed] [Google Scholar]
- Schulz R, Park CH, Boudrias MH, Gerloff C, Hummel FC, Ward NS. Assessing the integrity of corticospinal pathways from primary and secondary cortical motor areas after stroke. Stroke. 2012;43:2248–2251. doi: 10.1161/STROKEAHA.112.662619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki H, Sadato N, Lyshkow H, Yonekura Y, Honda M, Nagamine T, Suwazono S, Magata Y, Ikeda A, Miyazaki M, et al. Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain. 1993;116(Pt 6):1387–1398. doi: 10.1093/brain/116.6.1387. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Behrmann M. Cortical systems mediating visual attention to both objects and spatial locations. Proc Natl Acad Sci U S A. 2006;103:11387–11392. doi: 10.1073/pnas.0601813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournoux Ta. Co-planar stereotaxic atlas of the human brain. 1988 [Google Scholar]
- van Mier HI, Petersen SE. Intermanual transfer effects in sequential tactuomotor learning: evidence for effector independent coding. Neuropsychologia. 2006;44:939–949. doi: 10.1016/j.neuropsychologia.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Vangheluwe S, Suy E, Wenderoth N, Swinnen SP. Learning and transfer of bimanual multifrequency patterns: effector-independent and effector-specific levels of movement representation. Experimental Brain Research. 2006;170:543–554. doi: 10.1007/s00221-005-0238-0. [DOI] [PubMed] [Google Scholar]
- Waldvogel D, van Gelderen P, Ishii K, Hallett M. The effect of movement amplitude on activation in functional magnetic resonance imaging studies. J Cereb Blood Flow Metab. 1999;19:1209–1212. doi: 10.1097/00004647-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C, Nettekoven C, Rehme AK, Neuschmelting V, Eisenbeis A, Goldbrunner R, Grefkes C. Mapping the hand, foot and face representations in the primary motor cortex - Retest reliability of neuronavigated TMS versus functional MRI. Neuroimage. 2012;66C:531–542. doi: 10.1016/j.neuroimage.2012.10.046. [DOI] [PubMed] [Google Scholar]
- Woolsey CN, Erickson TC, Gilson WE. Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation. J Neurosurg. 1979;51:476–506. doi: 10.3171/jns.1979.51.4.0476. [DOI] [PubMed] [Google Scholar]
- Wu CW, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 2000;423:140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- Zeiler SR, Gibson EM, Hoesch RE, Li MY, Worley PF, O’Brien RJ, Krakauer JW. Medial premotor cortex shows a reduction in inhibitory markers and mediates recovery in a mouse model of focal stroke. Stroke. 2013;44:483–489. doi: 10.1161/STROKEAHA.112.676940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC. Modulation of Training by Single-Session Transcranial Direct Current Stimulation to the Intact Motor Cortex Enhances Motor Skill Acquisition of the Paretic Hand. Stroke. 2012 doi: 10.1161/STROKEAHA.111.645382. [DOI] [PMC free article] [PubMed] [Google Scholar]