Abstract
Neonatal exposure to a selective serotonin reuptake inhibitor (SSRI) leads to decreased left ventricular volumes and sympathetic activation in adult mice. We hypothesized this neonatal SSRI exposure-induced small left heart syndrome would increase post-myocardial infarction morbidity and mortality. C57BL/6 mice received saline or sertraline (5 mg/kg IP) on post-natal days 1–14. At 5 months, male mice underwent coronary artery ligation and were monitored by radiotelemetry until death or 4 weeks post-ligation. Following ligation, SSRI exposed mice had increased heart rates (SSRI 516 ± 13 bpm, control 470 ± 15 bpm, p<0.05). SSRI-exposed mice had significant reductions in left ventricular systolic volumes both before and after coronary ligation (SSRI: baseline 20 ± 3 µL, post-MI 37 ± 10 µL; control: baseline 30 ± 3 µL, post-MI 65 ± 23 µL). Post-MI echocardiography showed significantly decreased ejection fraction in control mice (baseline 60 ± 4%, post-MI 41 ± 2%, p <0.01) but not SSRI-exposed mice (baseline 65 ± 3%, post-MI 53 ± 7%). Neonatal SSRI exposure did not significantly alter post-MI survival. We conclude that the preexisting SSRI-induced small left heart syndrome may provide protection from post-MI ventricular dilation.
Keywords: exposure, selective serotonin reuptake inhibitors, myocardial infarction, echocardiography, telemetry
INTRODUCTION
Over the past 4 years, selective serotonin reuptake inhibitors (SSRIs) have been the most commonly prescribed antidepressants in America.1 Annual prescriptions for sertraline (Zoloft) have increased dramatically from 10.8 million in 2006 to 35.7 million in 2010.1 With this widespread use, there have been some intriguing observations. Observational studies have suggested a potential protective effect of SSRI administration in patients with a history of myocardial infarction (MI).2,3
Acute MI is a leading cause of death in adults. Impaired ventricular function and heart failure post-MI are influenced by left ventricular remodeling. In the early phase of healing, thinning and dilation of the infarcted myocardial wall occurs.4 The later phase of healing is marked by myocyte hypertrophy, interstitial fibrosis, and left ventricular dilation in the surviving myocardium.4 In addition to structural changes, acute MI is accompanied by an imbalance in the autonomic nervous system activity, with increased sympathetic nervous system activation.5 Notably, increased heart rate and decreased heart rate variability, non-specific markers of sympathetic activation, are independent risk factors for post-MI morbidity and mortality.6–10 Given the established links between sympathetic activation and post-MI mortality,9,11 SSRI-induced sympatho-inhibition might provide some cardioprotection.2,3,12,13 In the randomized, placebo-controlled Sertraline Antidepressant Heart Attack Randomized Trial (SADHART), a 4-month course of sertraline reduced post-MI mortality.3
Coincident with the increased use of SSRIs in the general population, SSRI use during pregnancy has been steadily increasing and is now estimated to affect 6.2% of pregnancies.14–17 Pharmacokinetic data in humans and epidemiologic studies have shown significant fetal exposure occurs during maternal SSRI therapy.18–20 Intrauterine exposure is associated with decreased fetal growth, impaired neonatal adaptation, and increased risk of cardiac malformations.19–21 Although multiple population based studies have been completed to evaluate for structural heart disease secondary to intrauterine SSRI exposure,22–24 no information is known regarding any histopathologic changes from SSRI exposure. While the neonatal effects from intrauterine SSRI exposure are well established, the long-term effects of exposure remain unclear.
According to the concept of developmental plasticity, when environmental exposures occur during critical windows of development, adaptive or homeostatic mechanisms may evolve to optimize the potential for short-term survival, but these changes may be maladaptive once the exposure ceases. Consistent with this theory, animal studies have demonstrated a persistent suppression in endogenous serotonin production in response to neonatal SSRI exposure.25–27 In addition to behavioral phenotypes consistent with rebound depression, we have shown SSRI-exposed mice have reduced left ventricular volumes and a programmed hypermetabolic state.27,28 We further demonstrated that mice exposed to neonatal sertraline have increased adult heart rates, increased very low frequency heart rate variability, and increased urinary excretion of noradrenaline, together suggesting increased sympathetic tone.27 Because impaired cardiac remodeling and increased sympathetic tone correlates with increased post-MI morbidity and mortality, we hypothesized that neonatal SSRI exposed mice will have increased mortality following myocardial infarction in adulthood.
METHODS
Animal Model
All procedures were approved by the University of Iowa Animal Care and Use Committee. Pregnant C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were allowed natural delivery. Pups were culled into litters of 6. Within each litter, pups were randomized to receive intraperitoneal saline (10 ml/kg/d) or sertraline (5 mg/kg/d). Injections were administered from postnatal day 1 to 14 to encompass a developmental window similar to the third trimester of human pregnancy. The sertraline dose was chosen to reflect exposure under the assumption that maternal therapy is prescribed at the lowest effective dose (50 to 100 mg/day).28 This dose conversion accounted for differences in body surface area, 70% oral bioavailability, and 29% placental transfer efficiency and the approximation was validated by serum sampling, as detailed in our initial publication.28 Pups were observed daily during injections. Sertraline-exposed and control pups were nursed by the same mother to minimize environmental influences on growth and development.
Hematoxylin-eosin & Masson’s Trichrome Staining
At 14 days and 180 days, hearts were harvested under isoflurane anesthesia and atria were removed. Ventricles were washed with cold PBS then fixed in formalin overnight at 4° C. Paraffin-embedded samples were sectioned (5µm thick) and hematoxylin-eosin stained following standard protocols. Masson’s trichrome stain was also performed to assess for fibrosis.
Baseline Measurements
Echocardiograms were obtained as previously described on control and sertraline-exposed male mice.27 Anesthesia was titrated to minimize movement yet maintain heart rate between 450 and 600 beats per minute. Measurements and calculations were made in accordance with the American Society for Echocardiography Guidelines.29 Volumes were calculated based on Teicholz et al formulas.30 Beginning at 5 months of age, electrocardiogram telemeters (TA-F10; Data Sciences International, St. Paul, MN) were implanted as previously described.27 Following recovery, baseline recordings were sampled for 10 seconds every 5 minutes over a period of 60 hours. Locomotor activity was digitally captured based on the fluctuation in radiotransmitter signal strength captured by the receiver’s array of antennae.
Coronary Ligations
Following baseline recordings, adult mice were anesthetized. After tracheal intubation and establishment of mechanical ventilation, a small thoracotomy was made. The proximal left coronary artery was ligated as previously described.31 Mice were monitored by telemeters following coronary ligations until death or 4 weeks post-ligation. Echocardiograms were repeated at 4 weeks.
Infarct area
Infarct area was measured on immersion-fixed ventricular sections. Ventricles were cut into 1 mm-thick sections and stained with 1% triphenyltetrazolium chloride (TTC) to delineate metabolically active versus necrotic myocardium.32 Images of each section were acquired using a stereoscope, and the percentage of the left ventricular free wall that was infarcted was measured using ImageJ software as previously described.31
Data Analysis
All values are presented as means ± SEM. Statistical comparisons were performed by two-tailed t-tests. P<0.05 was considered significant.
RESULTS
Exposure Model
Pup weights on the final day of exposure demonstrated a mild neonatal growth-restriction in sertraline exposed mice (control 7.35 ± 0.15 g, sertraline 6.8 ± 0.12 g, p=0.011) which resolved by 5 months of age (control 31.2 ± 1.0 g, sertraline 29.9 ± 0.9 g, NS).
Hearts were harvested from 4 SSRI-exposed and 4 control mice on both P14 and P180. As shown in Figure 1, there appeared to be increased fibrosis in the hearts from sertraline-exposed mice, despite a reduction in the density of nuclear staining. Given that myocytes only contribute to 20–30% of the total nuclei in the adult mouse heart33,34 and the cell types are interspersed within the myocardium, it is not possible to define the etiology of this morphologic difference in full tissue sections.
Figure 1.
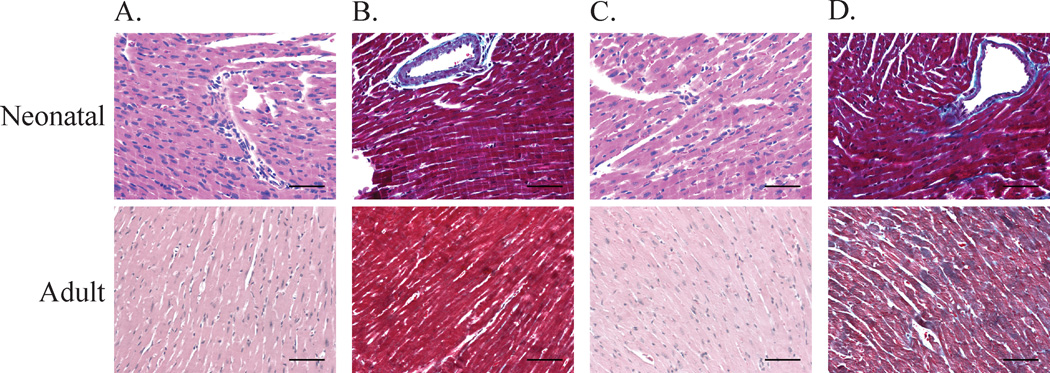
Hematoxylin-eosin (A & C) and Masson’s Trichrome (B & D) Staining in neonatal (top row) and adult hearts (bottom row). Stains from control mice are shown in panel A and B and SSRI-exposed mice are shown in panel C and D. Bar = 100 µm.
Increased Heart Rates Post-Myocardial Infarction
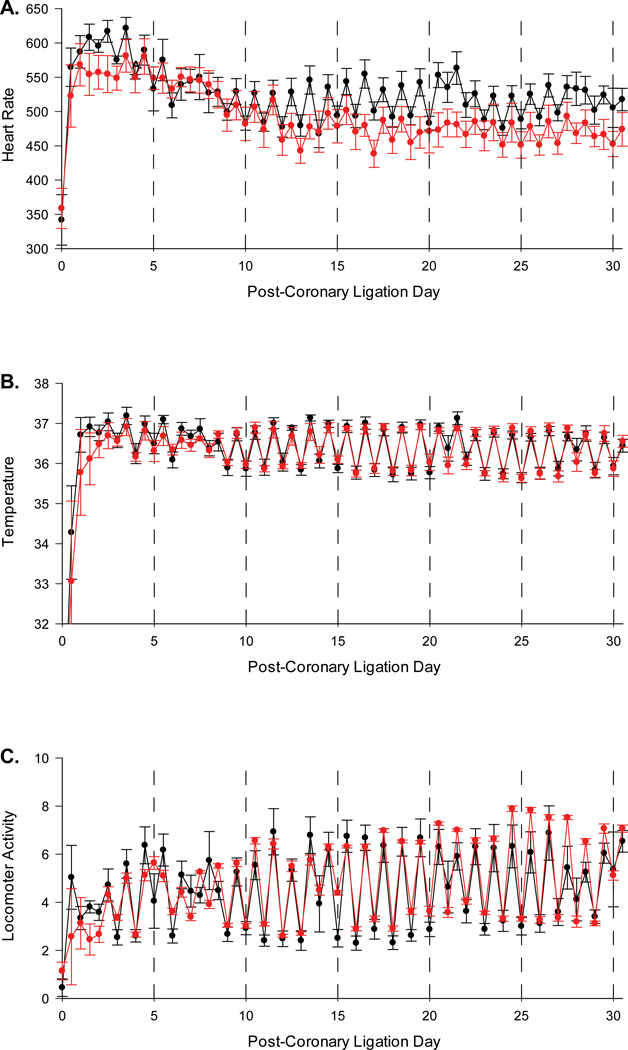
Following coronary artery ligation, sertraline exposed mice had increased heart rates compared to control mice (control 470 ± 15 bpm, sertraline 516 ± 13 bpm, p<0.05, Figure 2A). No significant differences were noted in temperature or locomotor activity levels between the groups (Figure 2B and 2C, respectively).
Figure 2.
A–C. Heart rate (A), temperature (B), and activity level (C) recordings over a 30 day period in sertraline exposed mice (black line) and control mice (red line).
Preserved Cardiac Function in SSRI Exposed Mice despite Increased LV Volumes
Baseline echocardiograms in sertraline exposed mice revealed decreased left ventricular (LV) internal diameters in diastole, decreased LV volumes in systole and diastole, and decreased stroke volumes (Table 1). Coronary ligation significantly increased systolic LV volumes of SSRI mice, but only to a level slightly greater than the baseline LV volumes of control mice (Table 1). Coronary ligations significantly decreased the ejection fractions of control but not SSRI exposed mice (Table 1). No significant differences were observed in stroke volume post-ligation. No significant differences were observed in heart weights or heart weight: body weight ratio four weeks post-myocardial infarction (Table 2).
Table 1.
Echocardiograms at baseline and four weeks post-coronary artery ligations.
| Control | |||||||
| Day |
LVIDd (mm) |
LVIDs (mm) |
LVVd (µl) |
LVVs (µl) |
SV (µl) |
SF (%) |
EF (%) |
| 0 | 4.1 + 0.0 | 2.8 + 0.1 | 74 + 2 | 30 + 3 | 44 + 3 | 31 + 3 | 60 + 4 |
| 28 | 4.7 + 0.7 | 3.8 + 0.6 | 109 + 37 | 65 + 23 | 44 + 14 | 20 + 1** | 41 + 2** |
| Sertraline | |||||||
| Day |
LVIDd (mm) |
LVIDs (mm) |
LVVd (µl) |
LVVs (µl) |
SV (µl) |
SF (%) |
EF (%) |
| 0 | 3.6 +0.1* | 2.4 + 0.1 | 56 + 5* | 20 + 3* | 36 + 3* | 35 + 2 | 65 + 3 |
| 28 | 3.9 + 0.4 | 3.1 + 0.5 | 75 + 14 | 37 + 10** | 38 + 6 | 28 + 2 | 53 + 7 |
P<0.05 compared to controls,
P< 0.05 compared to baseline.
LVIDd = left ventricular internal diameter in diastole, LVIDs = left ventricular internal diameter in systole, LVVd = left ventricular volume in diastole, LVVs = left ventricular volume in systole, SV = stroke volume, SF = shortening fraction, and EF = ejection fraction.
Table 2.
Body weights and heart weights at time of sacrifice following myocardial infarctions.
| Control (N=5) | SSRI (N=9) | |
|---|---|---|
| Heart wts (mg) | 196 ± 14 | 193 ± 13 |
| Body wts (g) | 34.9 ± 2.3 | 33.4 ± 0.8 |
| Heart wt/Body wt (mg/g) | 5.6 ± 0 | 5.8 ± 0.2 |
Mortality Post-Myocardial Infarction
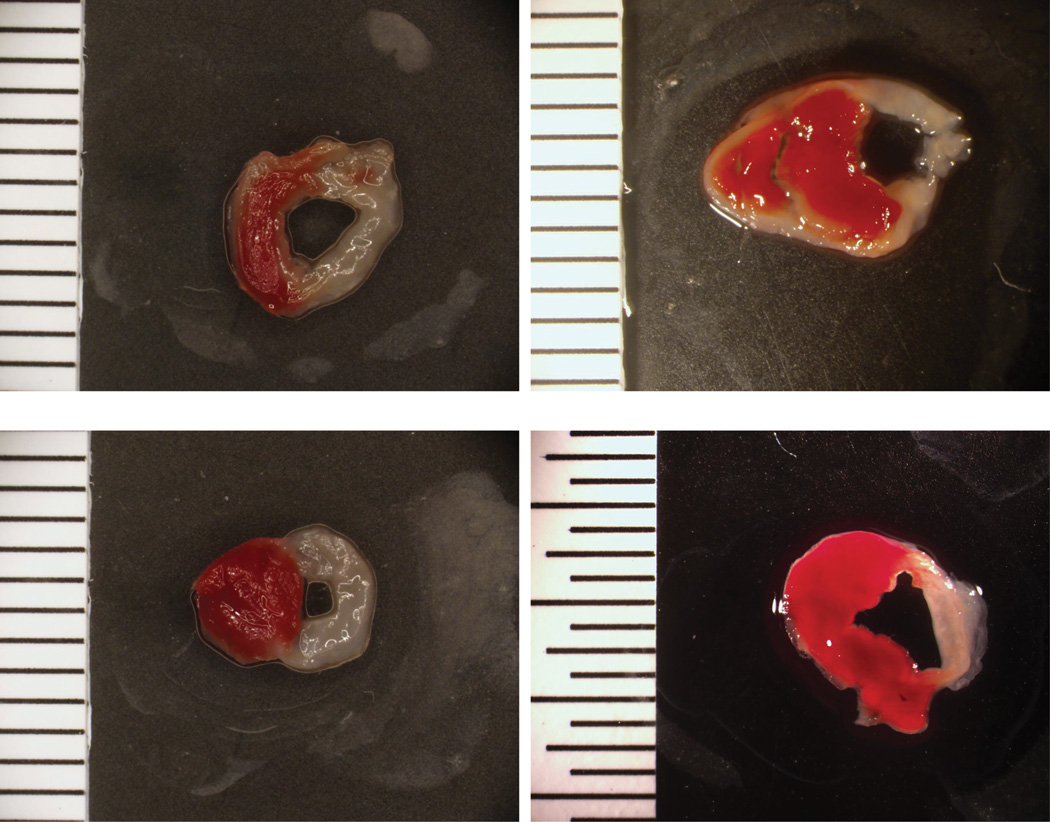
Nine control and 11 SSRI-exposed mice underwent coronary artery ligations. Twenty five percent of mice (3 controls, 2 SSRI) died within 24 hours following coronary artery ligation, but the remaining mice survived 4 weeks. There were no significant differences in infarct size between the groups (control 42.8 ± 10.0%, sertraline 37.9 ± 6.5%, p=0.68). Figure 3 demonstrates a representative sample of infarcts in sertraline and control mice.
Figure 3.
Myocardial infarctions following coronary artery ligations in sertraline exposed (top) and control mice (bottom).
DISCUSSION
Given the increase in fetal SSRI exposure in the last decade, it is crucial to determine if this population will be at greater risk for cardiovascular disease. We are the first to investigate long term cardiovascular effects from sertraline exposure. The key finding in this model was that sertraline exposed mice had significantly higher heart rates but no differences were seen in infarct size or mortality compared to control mice. Diaz et al. demonstrated in humans with suspected or known coronary artery disease that a resting heart rate ≥ 83 beats per minute at baseline had a significantly higher risk for total mortality and cardiovascular mortality.7 The sertraline exposed mice had a comparable increase in heart rate of approximately 45 bpm, yet they were resistant to the development of post-MI mortality.
One potential explanation for our findings is that sertraline-exposed mice had smaller LV dimensions at baseline. The relatively preserved cardiac function in the SSRI exposed mice post-myocardial infarction could be a result of less LV dilation following myocardial infarction. This is consistent with histology suggesting increased fibrosis in SSRI-exposed mice. The same factor that led to small left heart syndrome at baseline may thus provide protection from post-MI dilated cardiomyopathy. Cardiac remodeling post-myocardial infarction is a dynamic process, involving both infarcted and non-infarcted areas.35 Mouse models of myocardial infarction show acute and chronic changes.36–38 In our model, characteristic findings of decreased shortening fraction and increased LV volumes were observed post-myocardial infarctions.36,39 Function was significantly diminished in control mice, but not sertraline exposed mice post-myocardial infarction. Following myocardial infarction, LV volumes were larger in the control group with subsequently increased dilatation even though infarct sizes were comparable. There were no significant differences in heart weights or heart weight/body weight ratio, but our monitoring time was only four weeks and there were no sham surgeries for comparison. Another consideration for our findings is that in our previous studies sympathetic hyperactivity may have been overestimated. SSRI exposed mice had increased urinary noradrenaline excretion and increased very low frequency heart rate variability, but no measurements of renal nerve sympathetic activity were obtained.
In our exposure model, we injected mice during the first 14 days with a clinically relevant dose of sertraline.40,41 Mild neonatal growth restriction was observed which is consistent with other animal exposure models and SSRI exposed infants.16,42 We have previously reported plasma levels in mice 2 hours after injection of 71.8 + 1.3 ng/mL and plasma levels 12 hours after injection of 13.1 + 0.6 ng/mL.28 Based on these levels, there was an estimated peak concentration of 101 ng/mL, and estimated trough concentration of 1.7 ng/mL. Our projected peak concentration approximates that seen in human pregnancy, and our projected trough approximates umbilical cord levels.28 Although our drug levels were comparable to maternal levels during pregnancy, the placenta provides a partial barrier to fetal exposure. It is therefore possible that the dose we utilized exceeds clinical exposure and the mild neonatal growth restriction it elicited may have been the proximal cause of the decreased adult heart dimensions. Future studies using an intrauterine exposure model will be beneficial to better represent exposure throughout human pregnancy and minimize the error in estimating maternal absorption, metabolism, and placental transport.
Despite improvements in survival following myocardial infarctions, the incidence of post-MI heart failure is increasing.43–45 Post-MI left ventricular remodeling, consisting of hypertrophy and dilation, attempts to preserve cardiac function, but may become an independent risk factor for development of heart failure.46
Neonatal sertraline exposure impacts adult cardiac morphology and physiology, but no differences were noted in post-MI mortality between SSRI exposed mice and control mice. Instead, sertraline exposed mice had relatively preserved cardiac function and less LV dilatation. Further studies on cellular differences in SSRI exposed mice are needed to determine the impact SSRI exposure may have on adult cardiovascular disease.
Acknowledgments
FINANCIAL SUPPORT
This work was supported by the National Institutes of Health (HL07121, HL102659) and Children’s Miracle Network.
References
- 1.IMS Health. National Prescription Aduit. Parsippany, NJ: IMS; 2010. [Google Scholar]
- 2.Barton DA, Dawood T, Lambert EA, et al. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. 2007;25:2117–2124. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 3.Glassman AH, O'Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 4.Fraccarollo D, Galuppo P, Bauersachs J. Novel therapeutic approaches to post-infarction remodelling. Cardiovasc Res. 2012;94:293–303. doi: 10.1093/cvr/cvs109. [DOI] [PubMed] [Google Scholar]
- 5.Honda T, Kanazawa H, Koga H, Miyao Y, Fujimoto K. Heart rate on admission is an independent risk factor for poor cardiac function and in-hospital death after acute myocardial infarction. J Cardiol. 2010;56:197–203. doi: 10.1016/j.jjcc.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 7.Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26:967–974. doi: 10.1093/eurheartj/ehi190. [DOI] [PubMed] [Google Scholar]
- 8.Kovar D, Cannon CP, Bentley JH, Charlesworth A, Rogers WJ. Does initial and delayed heart rate predict mortality in patients with acute coronary syndromes? Clin Cardiol. 2004;27:80–86. doi: 10.1002/clc.4960270207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji H, Venditti FJ, Jr, Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 11.Soliman EZ, Elsalam MA, Li Y. The relationship between high resting heart rate and ventricular arrhythmogenesis in patients referred to ambulatory 24 h electrocardiographic recording. Europace. 2010;12:261–265. doi: 10.1093/europace/eup344. [DOI] [PubMed] [Google Scholar]
- 12.Shores MM, Pascualy M, Lewis NL, Flatness D, Veith RC. Short-term sertraline treatment suppresses sympathetic nervous system activity in healthy human subjects. Psychoneuroendocrinology. 2001;26:433–439. doi: 10.1016/s0306-4530(01)00002-6. [DOI] [PubMed] [Google Scholar]
- 13.Siepmann M, Grossmann J, Muck-Weymann M, Kirch W. Effects of sertraline on autonomic and cognitive functions in healthy volunteers. Psychopharmacology (Berl) 2003;168:293–298. doi: 10.1007/s00213-003-1448-4. [DOI] [PubMed] [Google Scholar]
- 14.Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198:194 e1–194 e5. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 15.Bakker MK, Kolling P, van den Berg PB, de Walle HE, de Jong van den Berg LT. Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol. 2008;65:600–606. doi: 10.1111/j.1365-2125.2007.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63:898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- 17.Wichman CL, Fothergill A, Moore KM, Lang TR, Heise RH, Jr, Watson WJ. Recent trends in selective serotonin reuptake inhibitor use in pregnancy. J Clin Psychopharmacol. 2008;28:714–716. doi: 10.1097/JCP.0b013e31818b53fd. [DOI] [PubMed] [Google Scholar]
- 18.Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D. Placental passage of antidepressant medications. Am J Psychiatry. 2003;160:993–996. doi: 10.1176/appi.ajp.160.5.993. [DOI] [PubMed] [Google Scholar]
- 19.Levinson-Castiel R, Merlob P, Linder N, Sirota L, Klinger G. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med. 2006;160:173–176. doi: 10.1001/archpedi.160.2.173. [DOI] [PubMed] [Google Scholar]
- 20.Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293:2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- 21.Greene MF. Teratogenicity of SSRIs--serious concern or much ado about little? N Engl J Med. 2007;356:2732–2733. doi: 10.1056/NEJMe078079. [DOI] [PubMed] [Google Scholar]
- 22.Kornum JB, Nielsen RB, Pedersen L, Mortensen PB, Norgaard M. Use of selective serotonin-reuptake inhibitors during early pregnancy and risk of congenital malformations: updated analysis. Clin Epidemiol. 2010;2:29–36. doi: 10.2147/clep.s9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356:2675–2683. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ. 2009;339:b3569. doi: 10.1136/bmj.b3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maciag D, Simpson KL, Coppinger D, et al. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 27.Haskell SE, Hermann GM, Reinking BE, et al. Sertraline exposure leads to small left heart syndrome in adult mice. Pediatr Res. 2013;73:286–293. doi: 10.1038/pr.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kummet GJ, Haskell SE, Hermann GM, et al. Neonatal SSRI Exposure Programs a Hypermetabolic State in Adult Mice. J Nutr Metab. 2012;2012:431–574. doi: 10.1155/2012/431574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Kronik G, Slany J, Mosslacher H. Comparative value of eight M-mode echocardiographic formulas for determining left ventricular stroke volume. A correlative study with thermodilution and left ventricular single-plane cineangiography. Circulation. 1979;60:1308–1316. doi: 10.1161/01.cir.60.6.1308. [DOI] [PubMed] [Google Scholar]
- 31.Miller JD, Peotta VA, Chu Y, et al. MnSOD protects against COX1-mediated endothelial dysfunction in chronic heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1600–H1607. doi: 10.1152/ajpheart.01108.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherrer-Crosbie M, Rodrigues AC, Hataishi R, Picard MH. Infarct size assessment in mice. Echocardiography. 2007;24:90–96. doi: 10.1111/j.1540-8175.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 34.Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annu Rev Physiol. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- 35.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 36.Yang F, Liu YH, Yang XP, Xu J, Kapke A, Carretero OA. Myocardial infarction and cardiac remodelling in mice. Exp Physiol. 2002;87:547–555. doi: 10.1113/eph8702385. [DOI] [PubMed] [Google Scholar]
- 37.Yang Z, Berr SS, Gilson WD, Toufektsian MC, French BA. Simultaneous evaluation of infarct size and cardiac function in intact mice by contrast-enhanced cardiac magnetic resonance imaging reveals contractile dysfunction in noninfarcted regions early after myocardial infarction. Circulation. 2004;109:1161–1167. doi: 10.1161/01.CIR.0000118495.88442.32. [DOI] [PubMed] [Google Scholar]
- 38.Benavides-Vallve C, Corbacho D, Iglesias-Garcia O, et al. New strategies for echocardiographic evaluation of left ventricular function in a mouse model of long-term myocardial infarction. PLoS One. 2012;7:e41691. doi: 10.1371/journal.pone.0041691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patten RD, Aronovitz MJ, Deras-Mejia L, et al. Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol. 1998;274:H1812–H1820. doi: 10.1152/ajpheart.1998.274.5.H1812. [DOI] [PubMed] [Google Scholar]
- 40.Johnson RD, Lewis RJ, Angier MK. The distribution of fluoxetine in human fluids and tissues. J Anal Toxicol. 2007;31:409–414. doi: 10.1093/jat/31.7.409. [DOI] [PubMed] [Google Scholar]
- 41.Reis M, Aamo T, Spigset O, Ahlner J. Serum concentrations of antidepressant drugs in a naturalistic setting: compilation based on a large therapeutic drug monitoring database. Ther Drug Monit. 2009;31:42–56. doi: 10.1097/FTD.0b013e31819114ea. [DOI] [PubMed] [Google Scholar]
- 42.Deiro TC, Manhaes-de-Castro R, Cabral-Filho JE, et al. Sertraline delays the somatic growth and reflex ontogeny in neonate rats. Physiol Behav. 2006;87:338–344. doi: 10.1016/j.physbeh.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Velagaleti RS, Pencina MJ, Murabito JM, et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53:13–20. doi: 10.1016/j.jacc.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 45.Chung ES, Dan D, Solomon SD, et al. Effect of peri-infarct pacing early after myocardial infarction: results of the prevention of myocardial enlargement and dilatation post myocardial infarction study. Circ Heart Fail. 2010;3:650–658. doi: 10.1161/CIRCHEARTFAILURE.110.945881. [DOI] [PubMed] [Google Scholar]
- 46.Kuster DW, Merkus D, Kremer A, et al. Left ventricular remodeling in swine after myocardial infarction: a transcriptional genomics approach. Basic Res Cardiol. 2011;106:1269–1281. doi: 10.1007/s00395-011-0229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]