Abstract
Gonadotrophin-releasing hormone (GnRH) neurones of the hypothalamic-pituitary-gonadal (HPG) axis drive reproductive function and undergo age-related decreases in activation during the transition to reproductive senescence. Decreased GnRH secretion from the median eminence (ME) partially arises from attenuated glutamatergic signaling via the NMDA receptor (NMDAR), and may be due to changing NMDAR stoichiometry to favor NR2b over NR2a subunit expression with aging. We have previously shown that the systemic inhibition of NR2b-containing receptors with ifenprodil, an NR2b-specific antagonist, stimulates parameters of luteinising hormone (used as a proxy for GnRH) release in both young and middle-aged females. Here, we chronically administered ifenprodil, an NR2b-specific antagonist, at the site of GnRH terminals in the median eminence (ME) or at GnRH perikarya in the preoptic area, in reproductively senescent middle-aged female rats to determine whether NR2b antagonism could restore aspects of reproductive functionality. Effects on oestrous cyclicity, serum hormones, and protein expression of GnRH, NR2b, and phosphorylated NR2b (Tyr-1472) in the ME were measured. Chronic ifenprodil treatment in the ME, but not the preoptic area, altered oestrous cyclicity by increasing the percentage of days spent in pro-oestrus. This was accompanied by increased GnRH fluorescence intensity in the external ME zone and a greater proportion of GnRH terminals that co-labelled with pNR2b with treatment. We also observed changes in the relationships between protein immunofluorescence, serum hormone levels, and other aspects of reproductive physiology in acyclic females, as revealed by bionetwork analysis. Together, these data support the hypothesis that NMDAR-NR2b expression and phosphorylation state play a role in reproductive senescence and highlight the ME as a major player in reproductive aging.
Keywords: Median Eminence, Menopause, Reproductive senescence, NMDA receptor, GnRH
Introduction
Aging in females of many species, including humans, leads to a loss of reproductive function and may be associated with impaired quality of life (1). However, the complex mechanisms underlying this process remain unclear. Reproductive function is controlled by the hypothalamic-pituitary-gonadal (HPG) axis, governed by the hypothalamic gonadotrophin-releasing hormone (GnRH) neurones. GnRH cells send axonal projections to the median eminence (ME) at the base of the hypothalamus, and secrete their decapeptide into the portal capillary system leading to the anterior pituitary. There, GnRH drives the release of luteinising hormone (LH) and follicle-stimulating hormone (FSH), which in turn stimulate sex steroid hormone secretion from the ovaries. Although all three levels of the HPG axis are involved in reproductive aging, accumulating evidence suggests that GnRH neuronal dysfunction is an initial factor in reproductive senescence (2–6).
During aging, the stimulatory effects of glutamate signaling through the NMDA receptors (NMDARs) on GnRH neurones decrease (7,8). Some of this change may be mediated by the ME, as in vitro incubated arcuate/ME fragments perfused with NMDAR agonists show reduced GnRH release from middle-aged compared to young rats (9). The presence and stoichiometry of NMDARs on GnRH cell bodies and terminals may determine the relative stimulatory effect of glutamate on GnRH release. Previous studies showed increased colocalisation of the NR2b subunit on GnRH somata with aging in ovarian-intact rats (10). NR2b-containing receptors open more slowly and less reliably than NR2a-containing receptors, and the slower channel kinetics of NR2b-containing receptors, relative to NR2a, may attenuate excitatory stimulation of glutamate on GnRH neurons (11). In support of a relative inhibitory influence of the NR2b subunit, intraperitoneal injections of ifenprodil, an NR2b-specific antagonist, increased parameters of pulsatile LH release (used as a proxy for GnRH release) in both young and middle-aged rats (12). Consequently, systemic ifenprodil exposure may affect GnRH release directly from GnRH terminals or affect activation at GnRH cell bodies.
The goal of this study was to determine the effect of chronic NR2b-antagonism at GnRH terminals or cell bodies on reproductive physiology in middle-aged acyclic females. To do this, we infused ifenprodil directly into the ME or the preoptic area for approximately 28 days, and tracked changes in oestrous cyclicity and somatic markers. Then, quantitative double-labelling of NR2b and GnRH in the ME of these rats was conducted to determine the extent of ifenprodil action on the protein expression of GnRH, NR2b or their colocalisation. Immunohistochemistry staining was repeated with tyrosine (Tyr-1472) phosphorylated NR2b (pNR2b) and GnRH, as phosphorylation can affect NMDAR trafficking (13) and functional channel properties (14).
Materials and methods
Animal care and husbandry
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas at Austin, and conformed to all NIH and USDA guidelines. The experiments were conducted in a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
Subjects were 37 middle-aged female Sprague-Dawley rats (Harlan, Houston, TX) aged 9–11 months upon arrival and pair-housed on a 12 h light-dark cycle. Rats received food and water ad libitum. Rats were handled for 5 min/day for 5 days before beginning to monitor cycle status. This was determined by microscopic analysis of cell morphology via a daily vaginal lavage of sterile saline. Surgery was performed after rats entered persistent oestrus (PE), a senescent stage defined as a minimum of 12 continuous days of oestrus.
Surgery
Subjects were anesthetized with 4% isoflurane/95% O2 (E-Z Anesthesia, Euthanex Corp, Palmer, PA) then shaved from the top of the eyes to the thoracic area. Rats were placed on a heating pad with their nose in a breathing cone connected to the anaesthesia machine and placed in the flat skull position in a Kopf stereotaxic frame and maintained on 1.5–2.5% isoflurane. One 30 gauge cannula was lowered into the ME using an 8 degree angle (to avoid hitting the sagittal venous sinus) with the following atlas coordinates (from Bregma, in mm): −3.0 A/P, −1.5 LAT, and −10.1 D/V or the preoptic area: −0.2 A/P, −1.3 LAT, and −8.6 D/V. A prefilled Alzet® osmotic pump was connected to the cannula with tubing (ID = 0.03″, OD = 0.048″, DURECT #PE60) and inserted s.c. between the scapulae, delivering a constant flow of 0.11 μl per minute of either ifenprodil tartartic acid (2.27 nM) dissolved in sterile saline (ME: n = 9, preoptic area: n = 9) or tartaric acid vehicle in sterile saline (ME: n = 9, preoptic area: n = 10). The dosage (2–10 nM) was based on the ability of ifenprodil to inhibit NMDARs in brain membranes in vitro (15,16), which is too low to affect NR2a subunits (17). At the end of surgery, the incision was sutured using 9 mm wound clips. The cannula was secured using dental cement (Instech Laboratories, Plymouth Meeting, PA). If needed, wound clips (7 mm) were applied rostral and caudal to the cement. A single 5 mg/kg injection of carprofen (Rimadyl, Pfizer) was administered s.c. as a post-operative analgesic. Both incisions were treated with a topical antibiotic containing pramoxine hydrochloride (Neosporin). Rats were allowed to awaken ~5–10 min later and then individually housed. Animals were checked and vaginal smears performed daily for up to 29 days post-surgery. One female died after surgery and was excluded from the study.
Brain processing
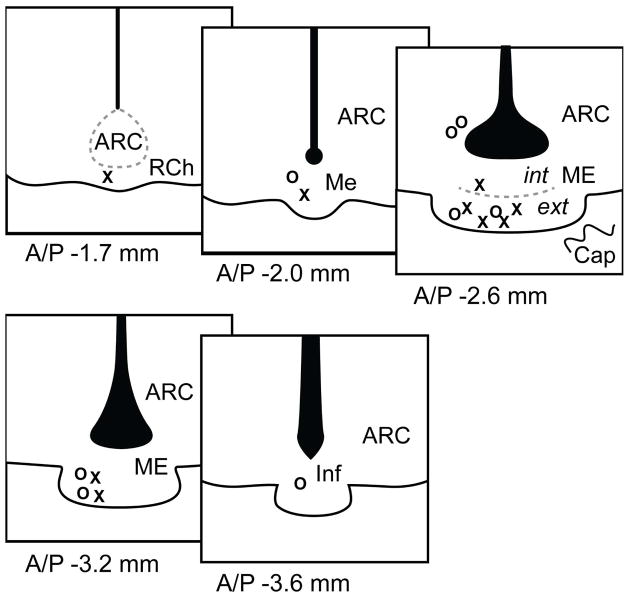
The day of euthanasia was approximately 4 weeks post-surgery and whenever possible on pro-oestrus in those rats exhibiting some cyclicity. Animals with repeated days in oestrus (PE) by the 4 week date were euthanized on oestrus. Regardless of cycle stage, humane euthanasia took place 3 hours before the dark period to measure possible increases in oestradiol and LH in pro-oestrous rats. Rats were deeply anesthetized with 150 mg/kg ketamine and 30 mg/kg xylazine and lack of reflex confirmed. Through the transcardial pathway, rats were perfused by flushing with 1% paraformaldehyde in PBS for 1 min followed by 4% paraformaldehyde in PBS for 10 min (50 ml/min). Brains were postfixed overnight in 4% paraformaldehyde and stored in phosphate buffer with 1% sodium azide at 4°C. Pituitary weight and uterine horn diameter were recorded; no ovarian specimens were taken. Pituitary weight was normalized to body weight and reported as the pituitary somatic index (PSI). Brains were embedded in 4% low melting agarose and sectioned at 50 μm on a vibratome (Leica, Wetzlar, Germany). Sections were stored in PBS+azide until immunohistochemical processing. The cannula track was observed during sectioning, and ME cannula placement spanned from the retrochiasmatic area (A/P −1.7 mm) to caudal ME/infundibulum (A/P −3.6 mm; Figure 1). Placements were all within 0.5 mm of the ME and, due to the high density of white fiber tracks in this region that aid the diffusion of compounds in the brain, we estimate that drug infusion was within the area of GnRH terminals (18). Preoptic area cannula placements were localized to the medial preoptic area (mPOA) or anteroventral periventricular nucleus (AVPV), within the region of GnRH cell bodies (Supplemental Figure 1). One female (Preoptic area-Vehicle) was excluded from analysis due to cannula placement in the bed nucleus of the stria terminalis.
Figure 1. ME Cannula Placement.
Cannula placements are indicated for vehicle (X) and ifenprodil (O) treated animals. For immunohistochemistry and microscopic analysis, we subdivided the ME into the pericapillary region of the external zone and the periventricular region of the internal zone (depicted in the medial ME section at A/P −2.6 mm). Abbreviations: ARC = arcuate nucleus, RCh = retrochiasmatic nucleus, ME = median eminence, int = internal zone, ext = external zone, Cap = capillary region, Inf = Infundibulum, third ventricle shown in black.
Hormone assays
Immediately before perfusion, blood was collected from the anaesthetised rat by cardiac puncture, allowed to clot and then centrifuged at 2300 × g for 5 min. Serum was collected and stored at −80°C until analysis. Serum levels of LH were measured in duplicate 50 μl samples in the laboratory of Dr. Michael J. Woller (University of Wisconsin, Whitewater, WI) using a double antibody competitive binding radioimmunoassay (RIA). The rat LH RP-3 standard was used, kindly provided by Dr. A.F. Parlow through the National Hormone and Pituitary Program of NIDDK. All samples were measured in one assay and the intra-assay variability was 4.81%.
Concentrations of serum oestradiol were detected in duplicate using the oestradiol RIA kit, as recommended by the manufacturer (Cat.No. DSL-4800, Beckman Coulter, Webster, TX). Assay sensitivity was 2.2 pg/ml. All samples were run in a single assay and intra-assay variability was 2.96%. Serum progesterone concentrations were measured in triplicate using the progesterone competitive enzyme immunoassay (Cat.No. 582601, Caymen Chemical, Ann Arbor, MI), according to the manufacturer’s recommended protocol. The assay detection limit was 10 pg/ml. Serum was diluted 1:100, and samples were run on two plates. The inter-plate variability was 8.62% and the intra-assay variability was 4.9%.
Semi-quantitative immunohistochemistry and confocal microscopy
Double-label immunofluorescence histochemistry was performed to quantify NR2b, pNR2b and GnRH expression in axon terminals and varicosities in the ME. For each antibody combination, all tissues were processed in a single run to obviate any inter-run variability. Three sections of the ME were selected in a 1:7 series, corresponding to the rostral, medial and caudal levels. First, antigen retrieval with citrate buffer (pH 8.5) for 30 min at 70°C was used to ensure penetration of the antibodies (19). To prevent non-specific binding, sections were incubated for 1.5 hr in 10% normal goat serum (NGS), 10% normal horse serum (NHS) and 2% bovine serum albumin. Next, sections were incubated with 1) rabbit anti-NR2b [1:1000, NB300-106, Novus Biologicals, Littleton, CO; (20,21)] and the mouse monoclonal GnRH [1:1000, Hu11b, kindly provided by Dr. H.F. Urbanski; (22)]; or 2) rabbit anti-pNR2b-Tyr1472 [1:1000, p1516-1472, PhosphoSolutions, Aurora, CO; (23)] and mouse anti-GnRH for 72 hr at 4°C in 2% NGS/NHS. Texas red-conjugated horse anti-mouse (TXRED; 1:400, Vector Laboratories, Burlingame, CA) and fluorescein-conjugated goat anti-rabbit (FITC; 1:400, Vector Laboratories) were used as secondary antibodies in 5% NGS/NHS for 2 hr at room temperature. Finally, 0.1% Sudan black staining was used to mitigate auto-fluorescence (24). Sections were mounted, and slides were coverslipped with VectaShield (Vector Laboratories). All antibodies have been previously validated using Western blot analysis or preadsorption control (21–23). Primary antibodies were omitted in control samples, and no staining was observed (Supplemental Figure 2). We also performed preadsorption control experiments in which the primary antibodies were incubated in excess blocking peptide (pNR2b and NR2b: Phosphosolutions, GnRH: Sigma) for 30 min at room temperature prior to immunohistochemistry. Preadsorbed controls showed greatly reduced staining (Supplemental Figure 2).
Stacks of fluorescent images spaced 0.5 μm apart (for a total of 5 μm) were obtained with a 1.25 NA 40X oil immersion lens, optical zoom 2 on a confocal laser scanning microscope (TCS SP2; Leica Microsystems, Mannheim, Germany). Sections were scanned sequentially between frames at excitation wavelengths of 488 nm and 568 nm using line averaging of 4. High resolution images of GnRH/pNR2b colocalisation in the external zone of the ME were obtained with the same confocal settings, except using a 1.4 NA 63X oil immersion lens. Colocalisation studies were conducted with Imaris 7.6 software (BitPlate, Zurich, Switzerland) using the automatic thresholding technique (25) to measure colocalised voxels of approximately 1×1×1 μm dimension. The number and mean signal intensity of punctate staining for FITC, TXRED and colocalised fluorescence in the ME were quantified using the Imaris spot function after background correction and using the same mask threshold settings across all subjects (26). Number of puncta was normalized to the volume sampled to give density values. The internal and external zones of the ME were separately analysed from each section using the Imaris contour function (Figure 1). Fluorescence intensity was used as a relative, semi-quantitative measure of protein expression in axons and terminals while preserving the anatomical distribution of the protein (27–29). All image acquisition and Imaris analyses were performed blind to treatment.
Statistical analysis
The SPSS 18.0 software package (SPSS, Inc., Chicago, IL) was used to analyse all data and group differences were considered statistically significant at the two-tailed p < 0.05 level. The effects of NR2b antagonism on cycle status, somatic markers, and hormone data were analysed using One-Way ANOVA. If data did not meet the assumptions of homogeneity of variance (Levene’s Test p > 0.1) or normal distribution of residuals (Shapiro-Wilk Test p > 0.1), a nonparametric Kruskal-Wallis test was used. No main or interaction effects of oestrous cycle at euthanasia (oestrus versus pro-oestrus) were found.
The fluorescence signal intensity and density data obtained from Imaris were analysed using linear mixed modelling (LMM) with the diagonal repeated covariance type to determine the effects of NR2b antagonism after removing outliers with the Grubb’s Test. Only one sample was removed from any group, with 2 outliers removed per dataset on average. LMM is the preferred model for handling data with missing values, correlated errors, and nonconstant variability (30). Animal ID was included as a random factor in the model to adjust for individual variability. Treatment (ifenprodil or vehicle) and ME Zone (internal or external) were included as fixed factors, and ME Level (rostral, medial and caudal), ME Zone and IHC Run (for GnRH only) were included as repeated variables. When a Region x Treatment interaction was found, paired t-tests were performed to compare treatment groups. Main effects of ME Level, or Level x Treatment interactions were examined, but no effects were found except for GnRH expression.
To examine relationships among protein expression, hormones and somatic markers within each treatment group, a correlation network was created based on a bootstrapping technique (31,32). Briefly, original paired data were resampled with replacement for 1000 repetitions to build a distribution of Pearson’s correlation coefficients using Matlab software (The Mathworks, Natick, MA). Significance of the Pearson’s correlations coefficients for each interaction was determined based on the bootstrapped distribution after correcting for multiple comparisons with the Benjamini-Hochberg False Discovery Rate at p < 0.05. Significant Pearson coefficients were uploaded into Cytoscape v2.8.3 (33) to create a correlation network.
Results
Reproductive physiology
Surgical implantation of the cannula into the ME and preoptic area of persistent oestrous middle-aged rats caused some transitory changes to oestrous cyclicity in both groups, probably due to general stimulation of these regions. However, the nature and persistence of these cycles differed significantly, with infusion of ifenprodil into the ME significantly increasing the percentage of days in pro-oestrus, as determined by the presence of nucleated cells in the vaginal smear, relative to the vehicle group (H(1) = 7.430, p = 0.006; Figure 2). Discriminate analysis of the treated rats validated that the percentage of days in pro-oestrus after surgery was due to ifenprodil and not to other variables, such as age, body weight, or days in persistent oestrus before surgery. By contrast, ifenprodil infusion into the preoptic region did not alter oestrous cyclicity, compared to vehicle (F(1,16) = 0.516, p = 0.483; Supplemental Table 1).
Figure 2. Effect of Ifenprodil Treatment in the ME on Vaginal Cytology.
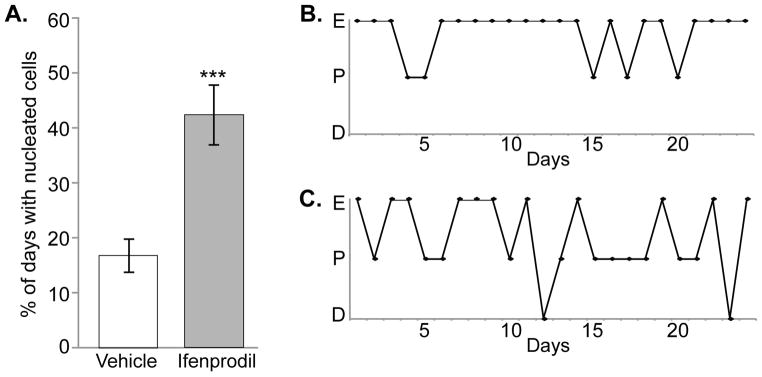
Daily vaginal cytology was used to monitor changes in oestrous cyclicity during chronic infusion with either Ifenprodil or Vehicle for ~28 days. A. Percentage of days spent in pro-oestrus during treatment, as determined by the presence of nucleated cells in the vaginal smear. B, C. Representative estrous cycles of a Vehicle (B) and an Ifenprodil (C) rat are shown. Abbreviations: E = oestrus, P = pro-oestrus, D = dioestrus. *** p ≤ 0.001.
One-Way ANOVA did not reveal group differences in the following measures: number of days in PE before surgery, age at death, changes in body weight before surgery and at the time of death, pituitary somatic index, uterine diameter, or serum hormone levels for either ME-cannula (Table 1) or preoptic-cannula females (Supplemental Table 1). The subsequent experiments focused on GnRH and NR2b or pNR2b protein expression of the ME-cannula brains to elucidate neuroendocrine mechanisms underlying the ifenprodil-induced change in cyclicity.
Table 1.
Somatic markers and circulating hormone levels after chronic ifenprodil or vehicle treatment into the ME
| ME | |||
|---|---|---|---|
| Measure | Vehicle | Ifenprodil | p value |
| Days in persistent oestrus pre-surgery | 28.6 (3.62) | 28.0 (5.11) | 0.930 |
| Age (mo) | 14.0 (0.24) | 13.9 (0.24) | 0.790 |
| Pre-surgery body weight (g) | 314.2 (2.75) | 299.6 (12.55) | 0.564 |
| Post-surgery body weight (g) | 301.0 (4.10) | 295.2 (8.98) | 0.723 |
| Pituitary somatic index | 5.5E-05 (2.30E-06) | 5.4E-05 (1.21E-06) | 1.000 |
| Uterine diameter (mm) | 3.6 (0.15) | 3.2 (0.14) | 0.106 |
| Serum LH (ng/μl) | 3.5 (0.99) | 2.5 (0.80) | 0.269 |
| Serum P4 (ng/μl) | 11.3 (2.94) | 6.0 (0.77) | 0.178 |
| Serum E2 (pg/μl) | 10.3 (3.70) | 6.7 (1.20) | 0.757 |
Data are shown as Mean (SEM). Terminal measures (pituitary, uterus, serum hormones) were collected ~28 days post-surgery.
Abbreviations: LH = luteinising hormone, P4 = progesterone, E2 = oestradiol. P-values are indicated for effects of treatment on each parameter. N=9 rats per group.
NR2b and pNR2b protein expression
Punctate staining for processes and terminals was observed in the ME for both NR2b and pNR2b (Figure 3). LMM statistical analysis revealed significant effects of ME Zone for NR2b density (p = 0.017) and NR2b intensity (p = 0.011; Figure 4a, b). NR2b density was higher in the external compared to the internal zone, whereas NR2b fluorescence intensity was higher in the internal compared to the external zone. Phosphorylated NR2b density was also significantly different in the two ME Zones (p = 2.9E-5), with a higher density in the external versus internal zone (Figure 4c). No treatment effects were found for either NR2b or pNR2b.
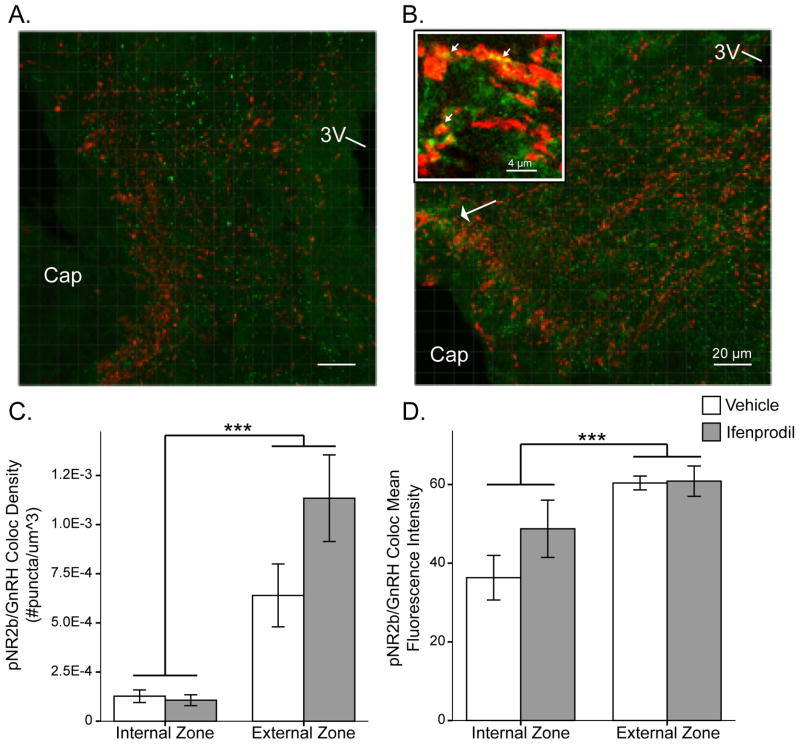
Figure 3. Colocalisation of NR2b/GnRH and pNR2b/GnRH.
Representative confocal micrographs in the ME of double-label immunofluorescence of A) NR2b (green) with GnRH (red); and B) pNR2b (green) with GnRH (red). No colocalisation of GnRH was seen with NR2b but a small amount was observed with pNR2b in the external zone of the ME (white arrowhead). A single confocal slice is shown in the inset of B demonstrating a higher resolution image of GnRH/pNR2b colocalisation in the external zone of the ME (small white arrowheads). Linear mixed modelling revealed GnRH/pNR2b colocalisation density and mean intensity levels were higher in the external zone. Raw data are graphed in C and D (not estimated means from model). Abbreviations: 3V = third ventricle, Cap = capillary region, *** p value ≤0.001. Scale bar = 20 μm for A and B, 4 μm for inset.
Figure 4. Effect of ME Zone and Ifenprodil Treatment in the ME on NR2b and pNR2b Expression.
Imaris software was used to analyse punctate labeling of NR2b and pNR2b subunit expression in the ME. A, C) Density of NR2b and pNR2b punctate staining, respectively. B, D) Mean fluorescence intensity of NR2b and pNR2b, respectively. No effects of treatment were found. Linear mixed modelling revealed that NR2b and pNR2b densities were higher in the external zone of the ME, whereas NR2b fluorescence intensity was higher in the internal zone of the ME. Raw data are graphed here (not estimated means from model). * p ≤ 0.05, *** p ≤ 0.001.
GnRH protein expression
Punctate staining of GnRH fibers and terminals was observed in the ME (Figure 3). GnRH density (p = 2.1E-11) and average intensity (p = 0.001) were both greater in the external versus internal zone (Figure 5). Furthermore, a Treatment x Zone interaction was revealed for GnRH intensity levels (p = 0.021). A paired t-test revealed that GnRH fluorescence intensity was significantly increased by ifenprodil treatment in the external zone (p=0.003; Figure 5b). GnRH density was higher in the medial and caudal sections compared to rostral (data not shown), as found previously (28). This contrasts the results for NR2b and pNR2b, and colocalised protein expression, none of which showed differences from rostral to caudal.
Figure 5. Effect of ME Zone and Ifenprodil Treatment in the ME on GnRH Expression.
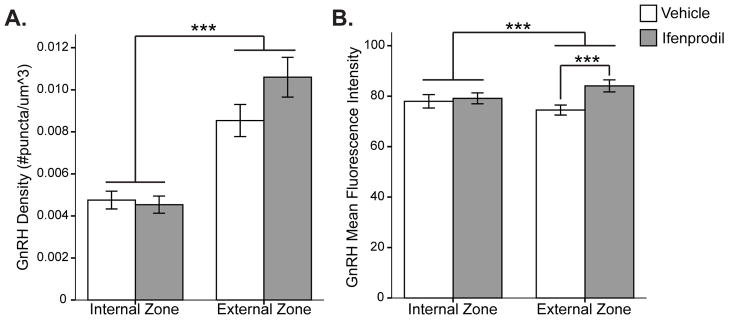
Imaris software was used to analyse the A) density and B) mean fluorescence intensity of GnRH punctate staining in the ME. Linear mixed modelling revealed that GnRH density and fluorescence intensity were higher in the external zone of the ME. A Treatment x Zone interaction was found for GnRH intensity levels (p = 0.021), and a paired T-test revealed that GnRH fluorescence intensity was significantly increased by ifenprodil treatment in the external zone. Raw data are graphed here (not estimated means from model). *** p ≤ 0.001.
Colocalisation of GnRH with NR2b or pNR2b
No quantifiable colocalisation was observed between NR2b and GnRH in the ME due to the double-label signal being at the level of detection (Figure 3a). However, a small amount of colocalisation was seen between pNR2b and GnRH (Figure 3b). GnRH and pNR2b colocalisation density (p = 7.1E-7) and mean intensity (p = 8.5E-5) were higher in the external compared to internal zone (Figure 3c, d). LMM analysis also revealed a trend for Treatment (p = 0.069) and Treatment x Zone interaction (p = 0.069) for GnRH/pNR2b colocalisation density. Further analysis of colocalisation parameters revealed other treatment effects (Table 2). A lower percentage of pNR2b voxel volume (p = 0.035) and pNR2b voxel fluorescence intensity (p = 0.034) were colocalised with GnRH with ifenprodil treatment. Thus, a lesser proportion of pNR2b colabelled with GnRH in the ifenprodil (14% volume/13% intensity) than in the vehicle group (20% volume/20% intensity). Conversely, a greater percentage of GnRH voxel volume (trend; p = 0.062) and GnRH voxel intensity (p = 0.016) were colocalised with pNR2b with ifenprodil; i.e. a greater proportion of GnRH was colabelled with pNR2b with treatment (ifenprodil: 13% volume/14% intensity vs. vehicle: 7% volume/6% intensity).
Table 2.
Colocalisation of pNR2b and GnRH after chronic ifenprodil or vehicle treatment in the ME
| Measure | Vehicle | Ifenprodil | p value |
|---|---|---|---|
| Total number of colocalised voxels | 5593 (1014) | 4617 (1037) | 0.511 |
| % of total region of interest colocalised | 0.669 (0.111) | 0.699 (0.116) | 0.850 |
| % of volume of pNR2b voxels colocalised | 20.7 (2.2) | 14.0 (2.2) | 0.035* |
| % of volume of GnRH voxels colocalised | 7.1 (2.2) | 13.4 (2.3) | 0.062+ |
| % of intensity of pNR2b voxels colocalised | 20.5 (2.1) | 13.9 (2.1) | 0.034* |
| % of intensity of GnRH voxels colocalised | 6.5 (1.9) | 14.1 (2.0) | 0.016* |
Data are shown as estimated means (SEM).
+p value ≤ 0.1 (trend),
p value ≤ 0.05. N=9 rats per group.
Correlation network analysis
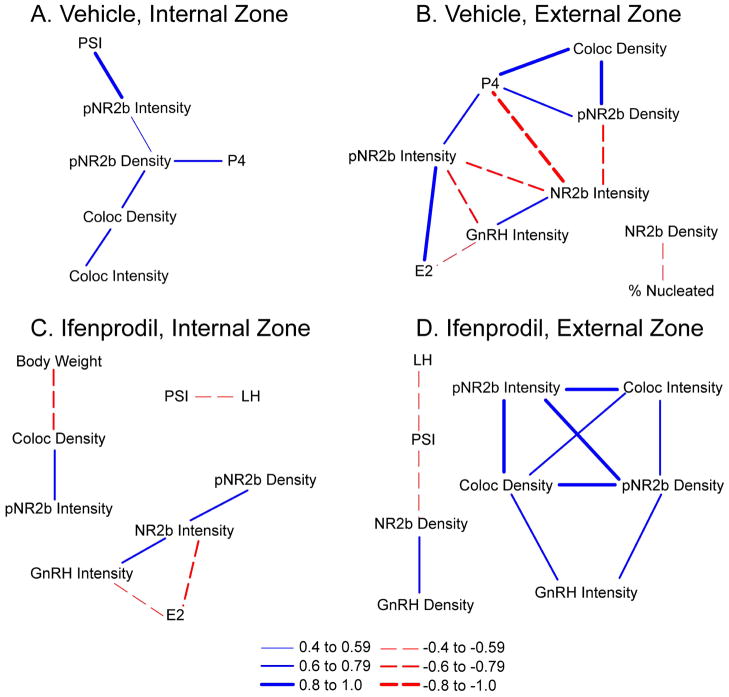
In order to determine the relationship between protein expression and physiological endpoints and how these relationships may change with treatment, we produced a correlation network for ifenprodil and vehicle groups. Although correlation does not imply direct causation, bionetwork analysis is a valuable tool for revealing how cellular and physiological endpoints interact within a system. Because many parameters for protein expression displayed an effect of ME Zone, networks were created separately for the external and internal zones (Figure 6). All four networks showed positive correlations between expression of pNR2b/GnRH colocalisation and pNR2b alone. Key differences between the ifenprodil- and vehicle-treated groups were primarily observed in the external zone networks. For example, only the Ifenprodil-External Zone network showed positive correlations among GnRH intensity, pNR2b and colocalised pNR2b/GnRH densities. Also, only the Vehicle-External Zone network demonstrated a negative correlation between NR2b density and the percentage of days in pro-oestrus. Networks of the vehicle treatment were also the only ones to exhibit correlations with circulating progesterone concentrations.
Figure 6. Network Analysis.
A correlation network of the ME-cannula data was made for the ifenprodil and vehicle groups to examine the relationships among protein expression, hormone levels and other physiological endpoints. Networks were created separately for the external and internal zones of the ME and only significant correlations are shown (p < 0.05 after Benjamini-Hochberg False Discovery Rate). Positive Pearson’s r correlation coefficients are indicated with solid blue lines and negative Pearson’s r coefficients are indicated with dashed red lines. Thicker lines correspond to higher Pearson’s r coefficients. PSI = pituitary somatic index, E2 = oestradiol, P4 = progesterone
Discussion
During the transition to reproductive senescence in rats, there is a marked reduction in the glutamatergic excitatory stimulation of GnRH neurones. Decreased glutamate release from afferent projections (8,34) together with changes in glutamate receptor expression and function (9) contribute to the loss of excitatory tone in the anterior hypothalamus with aging. We tested our hypothesis that NMDA-NR2b receptors attenuate excitatory stimulation of glutamate during aging using the selective antagonist ifenprodil, a phenylethanolamine derivative that stabilizes the closed-channel configuration (17,35). In this study we found that chronic ifenprodil infusion into the ME, but not the preoptic area, caused female acyclic rats to significantly increase the percentage of days spent in pro-oestrus. Ifenprodil treatment into the ME also increased GnRH immunofluorescence and colocalisation with pNR2b in the ME and altered correlation networks of protein immunofluorescence and measures of reproductive physiology.
Chronic antagonism of NMDA-NR2b receptors in the median eminence partially stimulates the HPG axis in acyclic females
Chronic inhibition of NMDA-NR2b receptors at the site of GnRH terminals partially activated the HPG axis in acyclic females by increasing the percentage of days in pro-estrus (nucleated vaginal smears). Nucleated cells are associated with the rise in oestradiol, GnRH and LH levels that trigger ovulation, whereas disruptions of oestrous cyclicity manifest as increases in oestrus or dioestrus days with concomitant decreases in the proportion of nucleated (pro-oestrus) days (36). The percentage of nucleated days is a pertinent measure of reproductive function and can help sort females as they progress through reproductive aging (32). Despite the increase in pro-oestrus days with ifenprodil infusion into the ME, we emphasize that the drug did not reinstate normal cycles or ovulation. Reproductive aging results from a combination of both decreased excitatory stimulation and increased inhibitory inputs (37). In order to fully restore reproductive function, a combination of manipulations is likely necessary.
Interestingly, even vehicle administration, both in the ME and preoptic area, initially modulated oestrous cyclicity. Trophic factors released from glial cells upon the insertion of the cannula are likely responsible, as they could temporarily shift the overall balance of inputs to a stimulatory state. Consistent with this idea, hypothalamic lesions accelerate the pubertal transition in females due to TGFα release from reactive astrocytes (38). Surprisingly, ifenprodil infusion into the preoptic area region did not further modulate oestrous cyclicity over vehicle. The diffuse distribution of the GnRH cell bodies may preclude sufficient ifenprodil exposure. Alternatively, age-related decreases in glutamate and increases in GABA release in the anterior hypothalamus (34) may prevent ifenprodil from having a strong effect in this region. However, ifenprodil’s effects in the ME suggest that the NR2b subunit plays a role in reproductive aging, at least in that region. Supporting this hypothesis, a previous study found that NR2b subunit gene expression in the ME was significantly affected by the transition from cyclicity to acyclicity in females (32).
The ME contains projections of diverse neurosecretory cells and interneurones, and so the stimulatory effect of ifenprodil treatment on GnRH release may be exerted directly onto GnRH terminals or indirectly via regulatory inputs to the GnRH system, such as through oestrogen receptor-positive cells (39) or GABAergic interneurones (40). Although this has not been studied in the ME, NMDARs may also be expressed in glial cells, which are abundant within the ME (41). However, in that case we would expect to see cell cytosolic labeling characteristic of glia, which we did not. Also, the ME and anterior pituitary tightly interact, and while most actions of glutamate are believed to be exerted at the hypothalamis, ifenprodil could potentially act on pituicytes via diffusion through the portal capillary system to affect the HPG axis (42).
Protein expression of NR2b, pNR2b and their colocalisation with GnRH in the ME
While the density of GnRH puncta did not change in ME-cannula, ifenprodil-treated females, many studies do not find changes in GnRH distribution with aging (4,43). However, the GnRH fluorescence intensity results suggest an increase in the amount of GnRH decapeptide within each punctum. Fluorescence intensity levels indicate relative expression between groups (27–29), but we believe our results are biologically relevant because immunohistochemical analyses for each pair of antibodies were performed in a single run. Notably, GnRH intensity was only increased in the external zone of the ME, where GnRH terminals lie adjacent to the portal capillaries. Though small, the specificity of this increase to the external ME may reflect increased pools of GnRH poised to be released, thereby signaling to the pituitary, and subsequently the ovary, to affect vaginal cytology. An increase in the amount of GnRH in the terminals could be interpreted as an increase in the synthesis of GnRH with treatment or as an accumulation of GnRH in the terminals due to decreased release, something that could not be differentiated by the present in vivo study. Future experiments to identify changes in GnRH gene expression, or microdialysis of GnRH release in the portal capillary system, will help clarify how ifenprodil treatment in the ME affects GnRH expression and release.
To our knowledge, the distribution of the NR2b subunit in the ME has not been described. Punctate labeling of NR2b and pNR2b suggest they are expressed on processes and terminals extending into the ME. There are few synapses in the ME so NMDAR expression on nerve processes is likely extrasynaptic. Both NR2b and pNR2b showed greater puncta density in the external zone of the ME, in close proximity to modulate GnRH release from their terminals. Because the NR1 subunit has been found within GnRH terminals (44), we measured colocalisation of NR2b, and of pNR2b, with GnRH in the ME. Unfortunately, we were unable to quantify the amount of colocalisation between NR2b and GnRH, because the levels were at the limit of detection. However, a small amount of colocalisation was observed between pNR2b and GnRH, particularly within the external zone where NR1 and GnRH colocalisation was also primarily found (44). However, based on limited resolution (1 × 1 × 1 μm, approximately the size of one terminal) we were unable to confidently determine whether pNR2b was expressed within the GnRH terminal itself or within a separate cell axon closely abutting the GnRH terminal. Future work will require a higher resolution approach such as electron microscopy. Despite these limitations, this technique provides structural evidence that NMDAR-NR2b-mediated regulation of GnRH may take place at the GnRH terminals. Intriguingly, the vehicle group showed a negative correlation between NR2b expression in the external zone and percentage of nucleated days, substantiating an inhibitory effect of NR2b expression on reproductive function in acyclic female rats.
The pNR2b primary antibody used in this study recognises NR2b that has been phosphorylated by tyrosine kinase at residue Tyr-1472, which enhances surface expression of the NMDAR through associations with scaffolding proteins (13) and potentiates NMDAR-mediated currents (45). Compared to vehicle, the ME-cannula, ifenprodil-treated rats showed a trend for increased pNR2b/GnRH colocalisation in the external zone, and a greater proportion of GnRH voxels that were colocalised with pNR2b. Although it is unclear how chronic ifenprodil treatment may upregulate pNR2b on GnRH terminals, studies in cultured cortical neurons demonstrated that NR2b protein, but not NR2a, was upregulated after 1-day of blockade of NR2b-containing receptors with ifenprodil (14) as well as after 2-day blockade of synaptic activity with the Na+ channel blocker tetrodotoxin (46). Future studies are needed to clarify the effects of chronic ifenprodil exposure on the expression and phosphorylation state of NR2b. This would be informative not only for studies on the neurobiology of reproduction but also for clinical applications of ifenprodil (47).
Role of the NMDAR in the median eminence during reproductive aging
No group differences in serum hormone concentrations or somatic markers were found on individual endpoints. However, network analysis revealed that their relationships with other parameters were affected by treatment. Network analysis is a useful tool that integrates hypothalamic and peripheral data to give a more holistic perspective on changes with treatment. Here, we used this method to identify common relationships among protein immunofluorescence, hormone and somatic data within treatment groups to better understand hypothalamic NR2b antagonism on reproductive neurophysiology. Although we expected to see increased circulating LH levels with NR2b-antagonism, the LH surge is difficult to “catch” using a single timepoint (approximately 3 hours prior to lights out), especially in view of the delayed surge in middle-aged females (2,5). On the other hand, the network analysis revealed negative associations between oestradiol levels and GnRH intensity in both vehicle and ifenprodil-treated females. This finding may indicate that negative hormonal feedback onto the GnRH system is sustained with aging, as has been found in rodents and humans (48,49). Alternatively, this relationship may indicate positive feedback of estradiol depleting the pool of readily releasable GnRH. However, as many of these subjects were euthanized on persistent oestrus, positive feedback would not be expected. Correlations between circulating progesterone and protein expression were lost in the ifenprodil group, compared to control. Because ifenprodil exposure was directly targeted to the brain, treatment may uncouple some effects of ovarian hormone signaling, but further experiments are needed to understand the effect of the hormonal milieu on the processes described in this study.
Although LMM statistical analysis did not reveal treatment effects on pNR2b or NR2b expression levels, their relationship with other variables as determined by network analysis did show interesting treatment differences, particularly in the external zone closest to the portal capillary vasculature. While vehicle-treated females displayed a negative correlation between pNR2b and GnRH expression, ifenprodil-treated females showed a positive relationship between pNR2b and GnRH expression. In other words, the presence of pNR2b is inhibitory to GnRH expression in the external zone until the NMDA-NR2b receptors are antagonised. In the ifenprodil-treated females, those expressing more pNR2b benefit the most from ifenprodil antagonism, resulting in higher levels of GnRH expression. Furthermore, GnRH intensity was very highly correlated with pNR2b/GnRH colocalisation expression in the ifenprodil group, compared to vehicle, suggesting this effect may be occurring directly on GnRH terminals. However, intensity levels are only an indirect measurement of GnRH and must be interpreted with caution. Together, these studies suggest that the NR2b subunit in particular, and NMDARs in general, are a factor in acyclicity, possibly due to age-related changes in the channel kinetics of NMDARs such that the receptors are inhibitory to GnRH neurons or to the circuitry regulating them (50). With NMDA-NR2b blockage, other NMDAR heteromers, such as NR1/NR2a receptors, may gain a larger influence in the mediating effects of glutamate on GnRH neurones.
Conclusion
The ME is a dynamic region for neurosecretion, and inputs to this area can modulate neuropeptide release independently of the cell body. By specifically inhibiting NMDA-NR2b receptors at the level of the GnRH terminals, but not at the level of the GnRH perikarya in the preoptic region, we were able to induce changes in oestrous cyclicity in previously acyclic females. Chronic ifenprodil infusion into the ME also altered the relationship between NR2b, pNR2b and GnRH protein expression, serum hormone levels, and other aspects of reproductive physiology in acyclic females. Overall, these data suggest the presence and phosphorylation state of the NR2b subunit in the ME is part of the neural network involved in acyclicity at middle age, and may additionally contribute to age-related changes in other neurosecretory cells projecting to this region.
Supplementary Material
Cannula placements are indicated for vehicle (X) and ifenprodil (O) treated animals (+ = excluded female). Abbreviations: AVPV = anteroventral periventricular nucleus, mPOA = medial preoptic area, BnST = bed nucleus of the stria terminalis, ac = anterior commissure, och = optic chiasm, third ventricle shown in black.
Confocal micrographs in the ME of the different controls for A) GnRH (red), B) NR2b (green), and C) pNR2b (green). Standard primary antibody incubation showed robust staining of each antibody. Omission or preadsorption of the primary antibodies greatly reduced or completely eliminated staining. Scale bar = 20 μm.
Acknowledgments
Dr. Michael Woller generously performed the LH RIA, and Dr. A.M. Parlow (NIDDK) provided reagents for the LH RIA. We thank Ashley Liou for help with tissue processing, Dean Kirson and Dr. M. Shel Swenson for the matlab and python scripts for the network analysis, respectively, Luke Magalsky for help creating the figures and Ross Gillette for assistance in troubleshooting cannula placement. This research was funded by NIH grant AG028051 to ACG.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- 1.Nelson HD. Menopause. Lancet. 2008;371:760–770. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 2.Wise PM. Alterations in the proestrous pattern of median eminence LHRH, serum LH, FSH, estradiol and progesterone concentrations in middle-aged rats. Life Sci. 1982;31:165–173. doi: 10.1016/0024-3205(82)90429-5. [DOI] [PubMed] [Google Scholar]
- 3.Rubin BS, Lee CE, King JC. A reduced proportion of luteinizing hormone (LH)-releasing hormone neurons express Fos protein during the preovulatory or steroid-induced LH surge in middle-aged rats. Biol Reprod. 1994;51:1264–1272. doi: 10.1095/biolreprod51.6.1264. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd JM, Hoffman GE, Wise PM. Decline in immediate early gene expression in gonadotropin-releasing hormone neurons during proestrus in regularly cycling, middle-aged rats. Endocrinology. 1994;134:1800–1805. doi: 10.1210/endo.134.4.8137745. [DOI] [PubMed] [Google Scholar]
- 5.Neal-Perry GS, Zeevalk GD, Shu J, Etgen AM. Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area. Biol Reprod. 2008;79:878–888. doi: 10.1095/biolreprod.108.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gore AC, Yeung G, Morrison JH, Oung T. Neuroendocrine aging in the female rat: the changing relationship of hypothalamic gonadotropin-releasing hormone neurons and N-methyl-D-aspartate receptors. Endocrinology. 2000;141:4757–4767. doi: 10.1210/endo.141.12.7841. [DOI] [PubMed] [Google Scholar]
- 7.Gore AC, Oung T, Yung S, Flagg RA, Woller MJ. Neuroendocrine mechanisms for reproductive senescence in the female rat: gonadotropin-releasing hormone neurons. Endocrine. 2000;13:315–323. doi: 10.1385/ENDO:13:3:315. [DOI] [PubMed] [Google Scholar]
- 8.Bonavera JJ, Swerdloff RS, Sinha Hakim AP, Lue YH, Wang C. Aging results in attenuated gonadotropin releasing hormone-luteinizing hormone axis responsiveness to glutamate receptor agonist N-methyl-D-aspartate. J Neuroendocrinol. 1998;10:93–99. doi: 10.1046/j.1365-2826.1998.00177.x. [DOI] [PubMed] [Google Scholar]
- 9.Zuo Z, Mahesh VB, Zamorano PL, Brann DW. Decreased gonadotropin-releasing hormone neurosecretory response to glutamate agonists in middle-aged female rats on proestrus afternoon: a possible role in reproductive aging? Endocrinology. 1996;137:2334–2338. doi: 10.1210/endo.137.6.8641183. [DOI] [PubMed] [Google Scholar]
- 10.Miller B, Gore AC. N-Methyl-D-aspartate receptor subunit expression in GnRH neurons changes during reproductive senescence in the female rat. Endocrinology. 2002;143:3568–3574. doi: 10.1210/en.2002-220346. [DOI] [PubMed] [Google Scholar]
- 11.Santucci DM, Raghavachari S. The effects of NR2 subunit-dependent NMDA receptor kinetics on synaptic transmission and CaMKII activation. PLoS Comput Biol. 2008;4:e1000208. doi: 10.1371/journal.pcbi.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maffucci JA, Walker DM, Ikegami A, Woller MJ, Gore AC. NMDA receptor subunit NR2b: effects on LH release and GnRH gene expression in young and middle-aged female rats, with modulation by estradiol. Neuroendocrinol. 2008;87:129–141. doi: 10.1159/000111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goebel-Goody S, Davies K, Alvestad Linger R, Freund R, Browning M. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Chen WS, Bear MF. Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology. 2007;52:200–214. doi: 10.1016/j.neuropharm.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Burban A, Faucard R, Armand V, Bayard C, Vorobjev V, Arrang JM. Histamine potentiates N-methyl-D-aspartate receptors by interacting with an allosteric site distinct from the polyamine binding site. J Pharmacol Exp Ther. 2010;332:912–921. doi: 10.1124/jpet.109.158543. [DOI] [PubMed] [Google Scholar]
- 16.Grimwood S, Richards P, Murray F, Harrison N, Wingrove PB, Hutson PH. Characterisation of N-methyl-D-aspartate receptor-specific [(3)H]Ifenprodil binding to recombinant human NR1a/NR2B receptors compared with native receptors in rodent brain membranes. J Neurochem. 2000;75:2455–2463. doi: 10.1046/j.1471-4159.2000.0752455.x. [DOI] [PubMed] [Google Scholar]
- 17.Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- 18.Emborg ME, Kordower JH. Delivery of therapeutic molecules into the CNS. Prog Brain Res. 2000;128:323–332. doi: 10.1016/S0079-6123(00)28029-1. [DOI] [PubMed] [Google Scholar]
- 19.Jiao Y, Sun Z, Lee T, Fusco FR, Kimble TD, Meade CA, Cuthbertson S, Reiner A. A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J Neurosci Methods. 1999;93:149–162. doi: 10.1016/s0165-0270(99)00142-9. [DOI] [PubMed] [Google Scholar]
- 20.Maffucci J, Noel ML, Gillette R, Wu D, Gore AC. Age-and hormone-regulation of NMDA receptor subunit NR2b in the anteroventral periventricular nucleus (AVPV) of the female rat: Implications for reproductive senescence. J Neuroendocrinol. 2009;21:506–517. doi: 10.1111/j.1365-2826.2009.01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams MM, Fink SE, Janssen WGM, Shah RA, Morrison JH. Estrogen modulates synaptic N-methyl-D-aspartate receptor subunit distribution in the aged hippocampus. J Comp Neurol. 2004;474:419–426. doi: 10.1002/cne.20148. [DOI] [PubMed] [Google Scholar]
- 22.Urbanski HF. Monoclonal antibodies to luteinizing hormone-releasing hormone: production, characterization, and immunocytochemical application. Biol Reprod. 1991;44:681–686. doi: 10.1095/biolreprod44.4.681. [DOI] [PubMed] [Google Scholar]
- 23.Carreño FR, Walch JD, Dutta M, Nedungadi TP, Cunningham JT. Brain-derived neurotrophic factor-tyrosine kinase B pathway mediates NMDA receptor NR2B subunit phosphorylation in the supraoptic nuclei following progressive dehydration. J Neuroendocrinol. 2011;23:894–905. doi: 10.1111/j.1365-2826.2011.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baschong W, Suetterlin R, Laeng RH. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM) J Histochem Cytochem. 2001;49:1565–1572. doi: 10.1177/002215540104901210. [DOI] [PubMed] [Google Scholar]
- 25.Costes SV, Daelemans D, Cho EH, Dobbin A, Pavlakis F, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86:3993–4003. doi: 10.1529/biophysj.103.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banovic D, Khorramshahi O, Owald D, Wichmann C, Riedt T, Fouquet W, Tian R, Sigrist SJ, Aberle H. Drosophila neuroligin 1 promotes growth and postsynaptic differentiation at glutamatergic neuromuscular junctions. Neuron. 2010;66:724–738. doi: 10.1016/j.neuron.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Taylor LE, Kaminoh YJ, Rodesch CK, Flanigan KM. Quantification of dystrophin immunofluorescence in dystrophinopathy muscle specimens. Neuropathol Appl Neurobiol. 2012;38(6):591–601. doi: 10.1111/j.1365-2990.2012.01250.x. [DOI] [PubMed] [Google Scholar]
- 28.Yin W, Wu D, Noel ML, Gore AC. Gonadotropin-releasing hormone neuroterminals and their microenvironment in the median eminence: effects of aging and estradiol treatment. Endocrinol. 2009;150:5498–5508. doi: 10.1210/en.2009-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caviglia S, Luschnig S. The ETS domain transcriptional repressor Anterior open inhibits MAP kinase and Wingless signaling to couple tracheal cell fate with branch identity. Development. 2013;140:1240–1249. doi: 10.1242/dev.087874. [DOI] [PubMed] [Google Scholar]
- 30.Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. J Amer Statist Assoc. 1993;88:9–25. [Google Scholar]
- 31.Walker DM, Kirson D, Perez LF, Gore AC. Molecular profiling of postnatal development of the hypothalamus in female and male rats. Biol Reprod. 2012;87:1–12. doi: 10.1095/biolreprod.112.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker DM, Kermath BA, Woller MJ, Gore AC. Disruption of reproductive aging in female and male rats by gestational exposure to estrogenic endocrine disruptors. Endocrinol. 2013 doi: 10.1210/en.2012-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smoot ME, Ono K, Ruscheinski J, Wang PL, Ideker T. Cytoscape 2. 8: new features for data integration and network visualization. Bioinformatics. 2011;27:431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neal-Perry GS, Zeevalk GD, Shu J, Etgen AM. Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area. Endocrinology. 2005;146:4331–4339. doi: 10.1095/biolreprod.108.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mony L, Krzaczkowski L, Leonetti M, Le Goff A, Alarcon K, Neyton J, Bertand HO, Acher F, Paoletti P. Structural basis of NR2B-selective antagonist recognition by N-methyl-D- aspartate receptors. Mol Pharmacol. 2009;75:60–74. doi: 10.1124/mol.108.050971. [DOI] [PubMed] [Google Scholar]
- 36.Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 37.Kermath BA, Gore AC. Neuroendocrine control of the transition to reproductive senescence: Lessons learned from the female rodent model. Neuroendocrinology. 2012;96:1–12. doi: 10.1159/000335994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Junier MP, Ma YJ, Costa ME, Hoffman G, Hill DF, Ojeda SR. Transforming growth factor alpha contributes to the mechanism by which hypothalamic injury induces precocious puberty. Proc Nat Acad Sci. 1991;88:9743–9747. doi: 10.1073/pnas.88.21.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakraborty TR, Ng L, Gore AC. Colocalization and hormone regulation of estrogen receptor alpha and N-methyl-D-aspartate receptor in the hypothalamus of female rats. Endocrinology. 2003;144:299–305. doi: 10.1210/en.2002-220749. [DOI] [PubMed] [Google Scholar]
- 40.Cserép C, Szabadits E, Szonyi A, Watanabe M, Freund TF, Nyiri G. NMDA Receptors in GABAergic Synapses during Postnatal Development. PLoS ONE. 2012;7:e37753. doi: 10.1371/journal.pone.0037753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verkhratsky A, Kirchhoff F. NMDA Receptors in glia. The Neuroscientist. 2007;13:28–37. doi: 10.1177/1073858406294270. [DOI] [PubMed] [Google Scholar]
- 42.Bhat GK, Mahesh VB, Chu ZW, Chorich LP, Zamorano PL, Brann DW. Localization of the N-methyl-D-aspartate R1 receptor subunit in specific anterior pituitary hormone cell types of the female rat. Neuroendocrinol. 1995;62(2):178–186. doi: 10.1159/000127003. [DOI] [PubMed] [Google Scholar]
- 43.Gore AC. Gonadotropin-releasing hormone neurons, NMDA receptors, and their regulation by steroid hormones across the reproductive life cycle. Brain Res Rev. 2001;37:235–248. doi: 10.1016/s0165-0173(01)00121-7. [DOI] [PubMed] [Google Scholar]
- 44.Yin W, Mendenhall JM, Bratton SB, Oung T, Janssen WGM, Morrison JH, Gore AC. Novel localization of NMDA receptors within neuroendocrine gonadotropin-releasing hormone terminals. Exp Biol Med. 2007;232:662–673. [PubMed] [Google Scholar]
- 45.Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 46.Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- 47.Gogas K. Glutamate-based therapeutic approaches: NR2B receptor antagonists. Curr Opin Pharmacol. 2006;6:68–74. doi: 10.1016/j.coph.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM. Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinol. 2005;146(10):4331–4339. doi: 10.1210/en.2005-0575. [DOI] [PubMed] [Google Scholar]
- 49.Hall JE, Gill S. Neuroendocrine aspects of aging in women. Endocrinol Metab Clin North Am. 2001;30(3):631–646. doi: 10.1016/s0889-8529(05)70205-x. [DOI] [PubMed] [Google Scholar]
- 50.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;500:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cannula placements are indicated for vehicle (X) and ifenprodil (O) treated animals (+ = excluded female). Abbreviations: AVPV = anteroventral periventricular nucleus, mPOA = medial preoptic area, BnST = bed nucleus of the stria terminalis, ac = anterior commissure, och = optic chiasm, third ventricle shown in black.
Confocal micrographs in the ME of the different controls for A) GnRH (red), B) NR2b (green), and C) pNR2b (green). Standard primary antibody incubation showed robust staining of each antibody. Omission or preadsorption of the primary antibodies greatly reduced or completely eliminated staining. Scale bar = 20 μm.