Background: Staphylococcus aureus manipulates blood coagulation by secreting von Willebrand factor binding protein (vWbp) and coagulase.
Results: vWbp forms a macromolecular complex with prothrombin, fibrinogen, factor XIII, and fibronectin.
Conclusion: vWbp activates FXIII in a non-proteolytic manner and recruits fibronectin to staphylococcal clots.
Significance: Activation of FXIII by vWbp represents a novel virulence strategy to promote formation of cross-linked fibrin cables in human plasma.
Keywords: Coagulation Factors, Fibrin, Fibrinogen, Fibronectin, Staphylococcus aureus, Coagulase, Factor XIII, vWbp
Abstract
Staphylococcus aureus secretes coagulase (Coa) and von Willebrand factor-binding protein (vWbp) to activate host prothrombin and form fibrin cables, thereby promoting the establishment of infectious lesions. The D1-D2 domains of Coa and vWbp associate with, and non-proteolytically activate prothrombin. Moreover, Coa encompasses C-terminal tandem repeats for binding to fibrinogen, whereas vWbp has been reported to associate with von Willebrand factor and fibrinogen. Here we used affinity chromatography with non-catalytic Coa and vWbp to identify the ligands for these virulence factors in human plasma. vWbp bound to prothrombin, fibrinogen, fibronectin, and factor XIII, whereas Coa co-purified with prothrombin and fibrinogen. vWbp association with fibrinogen and factor XIII, but not fibronectin, required prothrombin and triggered the non-proteolytic activation of FXIII in vitro. Staphylococcus aureus coagulation of human plasma was associated with the recruitment of prothrombin, FXIII, and fibronectin as well as the formation of cross-linked fibrin. FXIII activity in staphylococcal clots could be attributed to thrombin-dependent proteolytic activation as well as vWbp-mediated non-proteolytic activation of FXIII zymogen.
Introduction
Staphylococcus aureus is the microbial agent of soft tissue abscesses and bloodstream infections (1), which are frequent causes of infectious disease mortality in the United States (2–4). Antibiotic therapy has been challenged by the emergence and spread of multidrug-resistant strains, designated methicillin-resistant S. aureus or MRSA (5). Currently, there is an unmet clinical need for the development of new therapeutics to treat S. aureus skin and bloodstream infections.
Under physiological conditions, hemostasis is controlled by a cascade of serine proteases with rapid and highly localized activation in response to vascular damage (6). The hemostatic system is also responsible for preventing the dissemination of microbial invaders (7). For example, the intrinsic blood coagulation cascade is activated by negatively charged surfaces, as occurs with many different bacterial species (8). The relevance of this innate defense mechanism has been demonstrated for Streptococcus pyogenes, the causative agent of pharyngitis and skin infections (9, 10). Furthermore, microbial recognition by pathogen-associated molecular pattern recognition receptors triggers the release of cytokines, which activate the extrinsic blood coagulation cascade, thereby depositing fibrin around bacterial invaders (11). Fibrin deposition around microbes generates peptides with antimicrobial activity that attract phagocytes for opsonophagocytic removal of invading pathogens.
S. aureus has evolved a unique virulence strategy that usurps the hemostatic system for pathogen survival and replication in infected tissues. Exploiting the presence of coagulation factors in their zymogen form in the bloodstream, all clinical isolates of S. aureus secrete two hemostasis factors, coagulase (Coa)2 (12) and von Willebrand factor binding protein (vWbp) (13), that bind to and activate prothrombin in a non-proteolytic manner (14). Two N-terminal residues from Coa or vWbp insert into the activation cleft of the zymogen to form an equimolar complex designated staphylothrombin (Coa-prothrombin and vWbp-prothrombin) (14). Both forms of staphylothrombin display a novel exosite otherwise not found in proteolytically activated thrombin (14, 15). Both complexes cleave fibrinogen and form fibrin cables; however, neither has been reported to cleave or activate the other zymogen substrates of thrombin (15, 16).
Coa and vWbp contribute to the pathogenesis of S. aureus sepsis, endocarditis, and abscess formation in murine infection models (17–19). Furthermore, Coa and vWbp have been implicated as protective antigens (18, 19). McAdow et al. (20) reported that immune sera of mice harbored antibodies that were directed mainly against the N-terminal D1-D2 domains and neutralized their ability to bind and activate prothrombin. Nevertheless, the C-terminal domains of Coa and vWbp, when purified and used as vaccine antigens, also raised protective antibody responses (20). We presume that these antibodies may block the association of Coa and vWbp with other host proteins and disrupt the pathogenesis of staphylococcal infections.
Coa and vWbp share significant structural homology in the D1-D2 domains; however, their C-terminal domains are dissimilar (15, 21). The C-terminal domain of Coa is comprised of tandem repeats of a 27-residue peptide also found in the N-terminal domain of Efb (22). The C-terminal domain of vWbp is found in another uncharacterized gene product of S. aureus. Presumably, this mosaic structure endows coagulases with specific functions. Indeed, vWbp was initially characterized because of its ability to bind von Willebrand factor (vWF) (13). A 26-amino acid peptide sequence within the C-terminal region of vWbp was identified as the minimal binding region; however, the physiological significance of this interaction has not been established (13). Here we sought to identify the ligands of Coa and vWbp in human plasma and to analyze the role of these complexes in staphylococcal interferences with host hemostasis.
EXPERIMENTAL PROCEDURES
Ethics Statement
Blood donations were obtained from anonymous healthy adult donors. Written, informed consent was obtained from participants at the time of collection. The procedure was reviewed and approved by the Institutional Review Board at The University of Chicago.
Reagents
Human fibrinogen (Sigma), prothrombin (Innovative Research), coagulation factor XIII (FXIII; Hematologic Technologies), and von Willebrand factor (Hematologic Technologies) were used for affinity chromatography studies at 9, 1.4, 0.03, and 0.4 μm, respectively. When needed, lepirudin was used at a final concentration of 10 μg ml−1 and CaCl2 was used at 5 mm. FXIII-deficient plasma was obtained from Affinity Biologicals. Commercial antibodies used in the study included sheep anti-human prothrombin, HRP-conjugated (Affinity Biologicals), mouse anti-human factor XIIIa (FXIIIa; Thermo Scientific), rabbit anti-human FXIIIB (Abcam), rabbit anti-fibronectin (Abcam), goat anti-human vWF, HRP-conjugated (Thermo Scientific), anti-mouse IgG, HRP-conjugated (Cell Signaling), and anti-rabbit IgG, HRP-conjugated (Cell Signaling).
Bacterial Strains and Growth
S. aureus Newman (23) was grown in tryptic soy broth at 37 °C. Escherichia coli strains DH5α (24) and BL21 (DE3) (25) were grown in Luria-Bertani (LB) broth with 100 μg ml−1 ampicillin at 37 °C. To examine S. aureus-mediated coagulation, overnight cultures of Newman (wild-type) and isogenic coa, vwb, or coa/vwb mutants (18) were diluted 1:100 in fresh tryptic soy broth and grown at 37 °C until they reached A600 0.4. One ml of culture was centrifuged at 7000 × g, and bacterial sediment was washed and suspended in 1 ml of sterile PBS to generate a suspension of 1 × 108 cfu ml−1.
Recombinant Proteins
For binding studies, coding sequence of full-length mature vWbp or its truncations was extended to encode an N-terminal six-histidyl tag (H6) and a C-terminal strep tag (strep) and cloned into pET22b using the primers listed in Table 1. Recombinant plasmids were transformed into E. coli BL21 (DE3). Overnight cultures of E. coli BL21 (DE3) were diluted 1:100 into fresh media, grown at 37 °C to A600 0.6, induced with 1 mm isopropyl β-d-1-thiogalatopyranoside, and grown for an additional 3 h. Bacterial cells were sedimented by centrifugation, suspended in column buffer (100 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1 mm EDTA)), and disrupted with a French pressure cell at 10,000 p.s.i. Lysates were cleared of membrane and insoluble components by ultracentrifugation at 40,000 × g for 30 min. Proteins in the soluble lysate were subjected to Strep-Tactin affinity chromatography. Proteins were eluted in column buffer containing 2.5 mm d-desthiobiotin and subsequently dialyzed against PBS. Immediately before use, protein concentrations were determined with the BCA protein assay (Thermo Scientific). Activity was monitored by the ability to cleave chromogenic thrombin substrate S-2238 (Chromogenix). Coastrep was cloned into pET15b using the primers listed in Table 1 and purified as described above. To assess FXIII activity, the coding sequence of full-length mature vWbpstrep with a C-terminal strep-tag was cloned into pET22b using the primers listed in Table 1 and purified as described above.
TABLE 1.
Sequences of oligonucleotides used in this study
| Primer | Sequence |
|---|---|
| vWbp_fwd_NcoI_His | GGAATTCCCATGGGCAGCAGCCATCATCATCATCATCATGTGGTTTCTGGGGAGAAG |
| vWbp_rev_XhoI_strep | CATGCTCGAGTTATTTTTCGAACTGCGGGTGGCTCCATTTGCCATTATATACTTTATTGATTT |
| N1_fwd_NcoI_His | GGAATTCCCATGGGCAGCAGCCATCATCATCATCATCATAATGAAGAGGAGCAATTAAAG |
| N2_fwd_NcoI_His | GGAATTCCCATGGGCAGCAGCCATCATCATCATCATCATGCAGCAAAAAGTGATGAATC |
| N3_fwd_NcoI_His | GGAATTCCCATGGGCAGCAGCCATCATCATCATCATCATACATCACCGACTACATATACT |
| N4_fwd_NcoI_His | GGAATTCCCATGGGCAGCAGCCATCATCATCATCATCATATTTATAATGCACCAAAACAATTG |
| C1_rev_XhoI_strep | CATGCTCGAGTTATTTTTCGAACTGCGGGTGGCTCCAAATTTGTTGCTGAGTTTGACG |
| C2_rev_XhoI_strep | CATGCTCGAGTTATTTTTCGAACTGCGGGTGGCTCCATGGTTTCTTTGTTGGTGCA |
| C3_rev_XhoI_strep | CATGCTCGAGTTATTTTTCGAACTGCGGGTGGCTCCATTCAGTGTCAGATTTTAATTGAGCC |
| C4_rev_XhoI_strep | CATGCTCGAGTTATTTTTCGAACTGCGGGTGGCTCCAAAAGTCTTTCAATTCAGGGTTATC |
| vWbp_fwd_NcoI | AAAACCATGGTGGTTTCTGGGGAGAAGAAT |
| Coa_fwd_NdeI | AAAACATATGATAGTAACAAAGGATTATAGTGGGA |
| Coa_rev_XhoI_strep | AAAACTCGAGTTATTTTTCGAACTGCGGGTGGCTCCATTTTGTTACTCTAGGCCCATATGT |
Affinity Chromatography of Human Plasma Proteins
Strep-Tactin-Sepharose (IBA) was equilibrated in PBS buffer and charged with 100 nmol of H6vWbpstrep, N1–4, C1–4, or Coastrep. Citrate-plasma from healthy human volunteers (500 μl) was diluted 1:1 in PBS (1.5 mm NaH2PO4, 8.5 mm Na2HPO4, 67 mm NaCl, pH 7.2, containing only traces of calcium (<0.002%), and magnesium (<0.001%) was applied by gravity flow over the resin followed by extensive washing in PBS. Bound proteins were recovered by boiling the resin in sample buffer. Where indicated, human plasma was substituted with 500 μl of purified human plasma proteins in PBS.
SDS-PAGE and Immunoblot
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) was performed for extracts prepared in sample buffer (62.5 mm Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 5% 2-mercaptoethanol, 0.01% bromphenol blue). Gels were cast at 12% polyacrylamide, and proteins in extracts were separated by electrophoresis at 25 mA for 50 min. Proteins were either stained with 0.2% Coomassie Brilliant Blue (Sigma) or electrotransferred to polyvinylidene difluoride (PVDF) membrane (Millipore) for immunoblot analysis. Blots were developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific).
Assessing FXIII Activity
1.5 μm fibrinogen was incubated with 100 nm thrombin, 200 nm prothrombin, 200 nm vWbpstrep, and/or 20 nm FXIII. Lepirudin or CaCl2 was added to the reaction volume as needed. Insoluble reaction products were isolated by sedimentation at 20,000 × g for 10 min, and the sediments were washed 3 times in PBS and boiled in sample buffer supplemented with 4 m urea. To assess FXIII activity in human plasma, fresh blood was obtained from consenting volunteers and anticoagulated with 10 mm sodium citrate. Bacterial suspension (50 μl) was added to 50 μl of plasma in the presence of 5 mm CaCl2 and incubated at 37 °C for 2 or 24 h. Insoluble reaction products were isolated as described above.
Mass Spectrometry
To identify Coomassie-stained proteins in SDS-PAGE gels, bands were excised, placed into microcentrifuge tubes with double distilled H2O, and submitted to the Taplin Mass Spectrometry Facility at Harvard Medical School, Cambridge, MA, for microcapillary LC/MS/MS mass spectrometry. Any protein with three or more unique peptide matches was considered as a hit. The complete list of identified proteins is provided in Table 2.
TABLE 2.
vWbp ligands identified during Strep-Tactin affinity chromatography of human citrate-plasma
| Sample number | Electrophoretic mobility | Proteins identified (number of unique peptide matches) |
|---|---|---|
| #1 | 200 kDa | Isoform 1 of fibronectin (60) |
| Isoform 1 of Fibrinogen α-chain (14) | ||
| Putative uncharacterized protein ALB (12) | ||
| Fibrinogen β-chain (6) | ||
| α-2-Macroglobulin (4) | ||
| #2 | 170 kDa | Isoform 1 of fibrinogen α-chain (30) |
| Isoform 1 of fibronectin (19) | ||
| Fibrinogen β-chain (11) | ||
| Putative uncharacterized protein ALB (11) | ||
| Isoform γ-B of fibrinogen γ-chain (6) | ||
| 55-kDa protein (4) | ||
| Elongation factor 1-a2 (3) | ||
| #3 | 130 kDa | Isoform 1 of fibrinogen α-chain (37) |
| Isoform γ-B of fibrinogen γ-chain (25) | ||
| Putative uncharacterized protein ALB (7) | ||
| Isoform 1 of fibronectin (5) | ||
| Fibrinogen β-chain (4) | ||
| SNC66 protein (3) | ||
| #4 | 100 kDa | Isoform 1 of fibrinogen α-chain (23) |
| F2 prothrombin (fragment) (14) | ||
| Coagulation factor XIII A chain (13) | ||
| Isoform γ-B of fibrinogen γ-chain (7) | ||
| Glyceraldehyde-3-phosphate dehydrogenase (4) | ||
| Putative uncharacterized protein ALB (4) | ||
| Peroxiredoxin-2 (4) | ||
| Peroxiredoxin-1 (4) | ||
| Coagulation factor XIII B chain (3) | ||
| #5 | 70 kDa | Isoform 1 of fibrinogen α-chain (60) |
| Fibrinogen β-chain (19) | ||
| Putative uncharacterized protein ALB (16) | ||
| Isoform γ-B of fibrinogen γ-chain (6) | ||
| SNC66 protein (6) | ||
| #6 | 60 kDa | Fibrinogen β-chain (60) |
| Isoform 1 of fibrinogen α-chain (32) | ||
| Isoform γ-B of fibrinogen γ-chain (21) | ||
| FLJ00385 protein (fragment) (5) | ||
| PRO2275 (4) | ||
| Putative uncharacterized protein DKFZp686N02209 (3) | ||
| Isoform 1 of α-1-antitrypsin (3) | ||
| #7 | 55 kDa | Isoform γ-B of fibrinogen γ-chain (52) |
| Fibrinogen β-chain (39) | ||
| Isoform 1 of fibrinogen α-chain (24) | ||
| #8 | 45 kDa | Isoform 1 of fibrinogen α-chain (29) |
| Fibrinogen β-chain (14) | ||
| Isoform γ-B of fibrinogen γ-chain (13) | ||
| #9 | 35 kDa | Isoform 1 of fibrinogen α-chain (36) |
| Fibrinogen β-chain (16) | ||
| Isoform γ-B of Fibrinogen γ-chain (11) |
RESULTS
Identification of vWbp Ligands in Human Plasma
To identify interacting ligands of vWbp, we generated a non-catalytic variant with an N-terminal six histidyl tag and a C-terminal strep tag: H6vWbpstrep. Human plasma represents a heterogeneous mixture of >300 distinct proteins with a wide range of concentration (26). Chromatography of human plasma proteins on Strep-Tactin resin charged with H6vWbpstrep identified several proteins as vWbp ligands (Fig. 1A). Binding of these ligands did not occur on Strep-Tactin beads alone (Fig. 1A). Prominent species were subjected to microcapillary LC-MS/MS for identification (Table 2). H6vWbpstrep retained human prothrombin as well as the α-, β- and γ-chains of fibrinogen in addition to fibronectin and factor XIII (Fig. 1A). Of note, vWF was not identified.
FIGURE 1.

Identification of vWbp ligands isolated from human plasma and comparative analysis with Coa. A, human plasma (500 μl) was flowed over Strep-Tactin resin uncharged (control) or charged with H6vWbpstrep (100 nmol). Bound proteins were eluted by boiling the resin in sample buffer, separated by SDS-PAGE, and visualized after Coomassie staining. The identity of proteins labeled 1–9 was performed by microcapillary LC/MS/MS techniques. A complete list of protein hits can be found in Table 2. PT, prothrombin; FG, fibrinogen. B and C, for the comparative analysis of Coa and vWbp ligands, human plasma (500 μl) was flowed over Strep-Tactin resin uncharged (control) or charged with either H6vWbpstrep (100 nmol) or Coastrep (100 nmol). Bound proteins were eluted by boiling the resin in sample buffer and separated by SDS-PAGE. Proteins in gels were visualized by Coomassie staining (B) or after transfer to PVDF membranes for immunoblotting using specific antibodies against prothrombin, FXIII subunit A (FXIII A), and fibronectin (C).
Fibronectin is a high molecular weight glycoprotein found in blood and in the extracellular matrix (27). Fibronectin interacts with a wide array of macromolecules, most notably with fibrinogen and fibrin, highlighting its importance in blood coagulation and other physiological processes (27). FXIII is the zymogen precursor to the active transglutaminase factor XIIIa, also known as fibrin cross-linking factor (28). Plasma FXIII is a tetramer of two copies each of the A and B subunits. During the final stages of hemostasis, thrombin activates FXIII by cleaving a 37-residue activation peptide from the N terminus of the A subunit. In the presence of calcium, factor XIIIa catalyzes the formation of ϵ-(γ-glutamyl)-lysine isopeptide bonds in polymerized fibrin strands (28). The product of this reaction, cross-linked fibrin, is endowed with increased stability and resistance to fibrinolysis (29).
FXIII Binds vWbp but Not Coa
To investigate whether or not fibronectin and FXIII are unique ligands of vWbp, we subjected human plasma to affinity chromatography on Strep-Tactin resin that had been charged with either H6vWbpstrep or Coastrep (Fig. 1B). Because its N-terminal formyl-methionine is not removed from the translation product, Coastrep is catalytically inactive (Ref. 21 and data not shown). In accordance with previous reports (15, 21), Coastrep and H6vWbpstrep both retained prothrombin and fibrinogen after affinity chromatography experiments with human plasma (Fig. 1, B and C). Coastrep also retained fibronectin (Fig. 1B). FXIII, on the other hand, was only retained on Strep-Tactin beads charged with H6vWbpstrep but not with Coastrep (Fig. 1C). Thus, FXIII appears to represent a unique ligand of vWbp.
vWbp Binding Sites for Prothrombin, Fibrinogen, Fibronectin, and FXIII
A panel of recombinant H6vWbpstrep variants with serial deletions of N- and C-terminal amino acid sequences was generated, and protein products were purified (Fig. 2A). These variants were subjected to affinity chromatography experiments with human plasma and ligand binding monitored via Coomassie-stained SDS-PAGE and immunoblotting (Fig. 2, B and C). Prothrombin and fibrinogen did not bind to vWbp variants with N-terminal truncations (N1-N4), suggesting that the D1 domain of vWbp is necessary for its association with both plasma proteins (Fig. 2B). On the other hand, vWbp variants C1–3, with C-terminal truncations, were able to retain prothrombin and fibrinogen during affinity chromatography; this attribute was abolished after further truncation of the D2 domain (Fig. 2, B and C). Thus, vWbp D1-D2 domains are necessary and sufficient for prothrombin and fibrinogen binding. These data are in accordance with previous studies (15). The A and B chains of FXIII were not retained during affinity chromatography of human plasma with the vWbp N1-N4 variants (Fig. 2, B and C). FXIII bound to the C1 and C2 truncations but not to the C3 no the C4 variants (Fig. 2, B and C). These results suggest that FXIII requires the D1 and D2 domains as well as the central domain of vWbp for binding. In contrast to prothrombin, fibrinogen, and FXIII, fibronectin bound to the vWbp N1-N4 variants but not to C1 or any further truncation at the C terminus of vWbp. Thus, fibronectin binds to the C-terminal domain of vWbp in a manner that does not require prothrombin, fibrinogen, or FXIII (Fig. 2, B and C).
FIGURE 2.
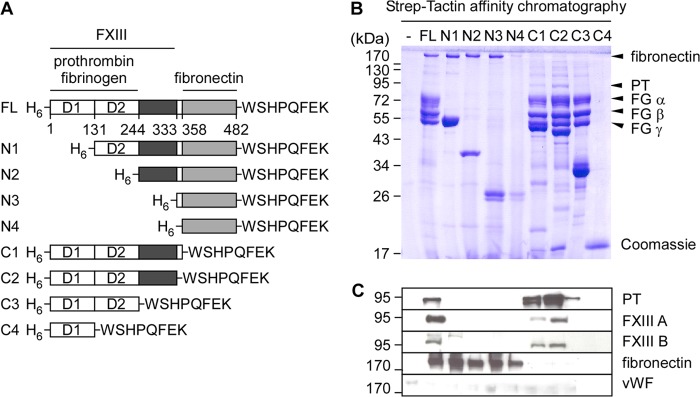
Mapping the functional domains of vWbp. A, diagram illustrating the primary translational products of mature full-length vWbp and truncated variants. Each variant includes an N-terminal six histidyl tag (H6) and a C-terminal strep tag (WSHPQFEK). The binding sites for prothrombin, fibrinogen, FXIII, and fibronectin are delineated. Numbers indicate amino acid residues within the mature protein. B and C, human plasma (500 μl) was flowed over Strep-Tactin resin uncharged (control) or charged with 100 nmol of proteins shown in A. Samples were eluted by boiling the resin in sample buffer and examined as described in Fig. 1. Immunoblotting was performed for prothrombin (PT), FXIII subunit A (FXIII A) and B (FXIIIB), fibronectin, and vWF.
H6vWbpstrep Does Not Retain Plasma-borne vWF
vWbp was initially identified by shotgun phage display of S. aureus genome-encoded peptides panned against recombinant vWF (13). In addition to measuring the binding of recombinant vWbp to both immobilized and soluble forms of vWF, Bjerketorp et al. (13) purified vWF from human serum by chromatography on resin charged with rvWbp-part (residues 124–392). These data were interpreted as a demonstration of the specific interaction between vWbp and vWF in human serum (13). Of note, the variant rvWbp-part lacks the D1 domain and the C-terminal domain of vWbp and, therefore, cannot bind prothrombin, fibrinogen, FXIII, or fibronectin. Using full-length, catalytically inactive H6vWbpstrep for affinity chromatography of human plasma, we failed to detect an interaction between vWbp and vWF (Fig. 2C), although vWF was clearly present in the plasma sample (Fig. 3A). To test whether full-length vWbp can bind vWF, purified human vWF was subjected to affinity chromatography on H6vWbpstrep-charged resin. vWF was indeed retained on H6vWbpstrep resin but not on Coastrep charged control beads (Fig. 3B).
FIGURE 3.

vWbp binds purified human vWF. A, the presence of vWF in human plasma was examined by immunoblot using aliquots of plasma diluted 10, 50, and 100 times and compared with purified vWF. Aliquots were boiled in sample buffer, and proteins were separated by SDS-PAGE before transfer to the PVDF membrane. Immunoblotting with human vWF-specific antibodies reveals immunoreactive species with the expected electrophoretic mobility (225 kDa). B, H6vWbpstrep or Coastrep (100 nmol) were immobilized on Strep-Tactin resin, and 500 μl of human vWF (0.4 μm) was flowed over the resin. Uncharged Strep-Tactin resin was used as a control. Bound proteins were eluted by boiling the resin in sample buffer and separated by SDS-PAGE. Proteins in gels were visualized by Coomassie staining. The density of bands corresponding to vWF was determined using the Gel Analysis function of ImageJ. The percentage of bound vWF was calculated as the density of vWF retained over input. Data are representative of three independent experiments. Statistical significance was analyzed with the two-tailed t test.
Requirements for Prothrombin, Fibrinogen, and FXIII Binding to vWbp
As part of their investigation of vWbp-prothrombin, Kroh et al. reported that neither of the two proteins by themselves associated with fibrinogen (15). However, assembly of the novel exosite on the vWbp staphylothrombin complex allowed for fibrinogen binding, as previously reported for the Coa(1–325)-prethrombin 2 complex (14). To test whether vWbp-prothrombin assembly is required for the fibrinogen binding activity of vWbp in human plasma, we immobilized H6vWbpstrep on Strep-Tactin resin and added fibrinogen in the presence or absence of prothrombin. Samples were analyzed by Coomassie-stained SDS-PAGE, which revealed that fibrinogen occurred with H6vWbpstrep-prothrombin complexes but not with H6vWbpstrep alone (Fig. 4, A and B).
FIGURE 4.

Analysis of ligand binding to vWbp. A, human fibrinogen (FG; 9 μm in 500 μl) was flowed alone or in the presence of human prothrombin (PT; 1.4 μm) over uncharged Strep-Tactin resin or resin charged with H6vWbpstrep (100 nmol). Samples were eluted by boiling the resin in sample buffer and examined as in Fig. 1 after Coomassie staining of SDS-PAGE (A) and immunoblot against prothrombin (B). C and D, to decipher the biochemical basis for the interaction between FXIII and vWbp, human FXIII (0.03 μm in 500 μl) was flowed alone or in the presence of human fibrinogen (9 μm) and/or human prothrombin (1.4 μm) over uncharged Strep-Tactin resin (−) resin charged with H6vWbpstrep (100 nmol), or Coastrep (100 nmol). Samples were eluted by boiling the resin in sample buffer and examined as in Fig. 1 after Coomassie staining of SDS-PAGE (C) and immunoblot against prothrombin and FXIII subunit A (D).
The minimal binding site of vWbp for association with FXIII in human plasma encompasses its D1-D2 domains. We wondered whether prothrombin and fibrinogen are required for the association of vWbp with FXIII. To test this, purified FXIII was chromatographed on H6vWbpstrep immobilized on Strep-Tactin beads. When the eluate was analyzed by immunoblotting with antibodies against the A subunit, FXIII binding to vWbp could not be detected (Fig. 4, C and D). The addition of prothrombin to FXIII did not promote vWbp association; however, when subjecting prothrombin, fibrinogen, and FXIII to affinity chromatography, all three plasma proteins were retained on H6vWbpstrep charged Strep-Tactin beads (Fig. 4, C and D). As a control, chromatography of prothrombin, fibrinogen, and FXIII on Coastrep-charged resin led to the formation of Coastrep-prothrombin-fibrinogen complexes but not to the retention of FXIII (Fig. 4, C and D). Thus, FXIII associates specifically with vWbp-prothrombin-fibrinogen complexes but not with Coa-prothrombin-fibrinogen.
Non-proteolytic Activation of FXIII by vWbp-Prothrombin
Plasma FXIII represents a heterotetramer assembled from two A and two B subunits (28). The A subunit harbors the active site (30), whereas the B subunit fulfills a regulatory function (31). Under physiological conditions, activation of FXIII involves thrombin-mediated cleavage of the activation peptides from both A subunits to generate A′ products (30). Calcium and fibrin then induce dissociation of the B subunits from the A′ dimer, thereby exposing the active site of the now fully active A* dimer (for review, see Ref. 29). FXIII-mediated cross-linking initially occurs between the properly aligned γ-chains of fibrin cables, resulting in the formation of γ dimers (32). Over time, intermolecular cross-linking between α-chains creates oligomers and α-chain multimers as well as complexes between α- and γ-chains that can be visualized as higher molecular weight species on SDS-PAGE (32).
To examine whether vWbp-prothrombin activates FXIII in vitro, we purified vWbpstrep, which once complexed with prothrombin, cleaves fibrinogen to form fibrin cables (18). Purified human FXIII was incubated with vWbpstrep in the presence or absence of human prothrombin and fibrinogen. Purified proteins and their reaction products were centrifuged, and fibrin cables were solubilized with urea and analyzed by Coomassie-stained SDS-PAGE (Fig. 5A). As controls, incubation of FXIII with fibrinogen did not generate significant amounts of γ-chain dimers or α-chain multimers and did not lead to FXIII A cleavage. Incubation with thrombin converted FXIII A to FXIII A′ and A*, which catalyzed the formation of γ-chain dimers and α-chain multimers in polymerized fibrin cables (Fig. 5A). This sequence of fibrin cross-linking reactions was blocked in the absence of calcium ions or in the presence of lepirudin, a recombinant form of hirudin that functions as a direct inhibitor of thrombin (33) (Fig. 5A). The addition of vWbpstrep-prothrombin to fibrinogen and FXIII also led to the sedimentation of fibrin cables and to the formation of γ-chain dimers or α-chain multimers (32). Unlike thrombin, vWbpstrep-prothrombin did not cleave FXIII A and its activation of FXIII was not inhibited by lepirudin (Fig. 5, A and B). vWbpstrep-prothrombin-mediated activation of FXIII required calcium ions, as the formation of γ-chain dimers and α-chain multimers did not occur in samples where the divalent cation had been omitted (34) (Fig. 5, A and B).
FIGURE 5.

vWbpstrep-prothrombin-fibrinogen complex activates FXIII in a non-proteolytic manner. Formation of cross-linked fibrin products was monitored after incubation at 37 °C for 1 h with the following factors in various combinations: human FXIII (20 nm), human thrombin (100 nm), human prothrombin (200 nm), vWbpstrep (200 nm), fibrinogen (1.5 μm), and CaCl2 (5 mm). Where indicated, lepirudin was added to the reaction. Reaction products were subjected to centrifugation, and sediments were washed three times with PBS and boiled in sample buffer supplemented with urea (4 m). Solubilized protein samples were examined with Coomassie-stained SDS-PAGE (A) and immunoblot against FXIII subunits A and B (B).
The canonical view of FXIII activation centers on the proteolytic removal of the 37-residue activation peptide from the N terminus of the A subunit of FXIII by thrombin (29). Immunoblotting of solubilized fibrin clots revealed the cleavage of the A subunit in samples treated with thrombin as indicated by the appearance of A′ species (Fig. 5B). No cleavage of FXIII A was detectable in the presence of vWbpstrep-prothrombin despite the presence of cross-linked-fibrin (Fig. 5, A and B). We, therefore, conclude that vWbpstrep-prothrombin-fibrinogen complex can activate FXIII in a non-proteolytic manner as has been reported for coagulase-mediated activation of prothrombin (14) or staphylokinase-mediated activation of plasminogen (35). The B subunit of FXIII was present in all samples in which a fibrin clot had been formed (Fig. 5, A and B).
FXIII and Fibronectin Are Incorporated into Staphylococcal Coagulation Products
We wondered whether the vWbp-mediated clotting reactions observed in vitro occur during infection. To test this, wild-type S. aureus Newman (23) and its variants lacking either coagulase (coa), vWbp (vwb), or both (coa/vwb) (18) were incubated with calcium-supplemented human plasma. In accordance with previously published reports (18), S. aureus as well as the coa and vwb mutants promoted the formation of fibrin cables that sedimented with the bacteria and, after solubilization with urea, were detected via Coomassie-stained SDS-PAGE (Fig. 6A). This was not observed for the coa/vwb mutant (Fig. 6A).
FIGURE 6.

FXIII and fibronectin are incorporated into staphylococcal fibrin clots. S. aureus Newman or isogenic mutants coa, vwb, or coa/vwb were grown to mid-log phase. Cells were washed, taken up in PBS, and incubated in the presence of 5 mm calcium and human plasma untreated (normal) or treated with lepirudin. A PBS control with no bacterial cell (−) was included. After incubation at 37 °C, the samples were subjected to centrifugation after 2 and 24 h. Pellets were washed three times in PBS and boiled in sample buffer supplemented with urea (4 m) to solubilize clots. Samples were examined after Coomassie staining of SDS-PAGE (A) and immunoblot against prothrombin (PT), FXIII subunit A and B, and fibronectin (B).
S. aureus-mediated fibrin clots catalyzed the formation of γ-chain dimers and α-chain multimers within polymerized fibrin cables; this did not occur in human plasma inoculated with the coa/vwb mutant strain (Fig. 6A). Immunoblotting experiments revealed that FXIII (A and B) and fibronectin were incorporated in these clots (Fig. 6B). Fibrin clot formation, FXIII and fibronectin recruitment, and formation of γ-chain dimers and α-chain multimers occurred after incubation with coa or vwb mutants but not with the coa/vwb strain (Fig. 6, A and B). The formation of fibrin γ-chain dimers and α-chain multimers in clots formed by the vwb mutant, but not in clots generated by the wild-type or coa mutant strains, was reduced when plasma samples were treated with lepirudin (Fig. 6, A and B). Taken together these data suggest that S. aureus promotes fibrin clotting via the secretion of Coa and vWbp. Fibrin cross-linking is promoted by two mechanisms, the thrombin-dependent pathway that can be blocked with lepirudin and the vWbp-mediated recruitment and FXIII activation pathway that cannot be inhibited with lepirudin as it involves the non-proteolytic activation of FXIII A via vWbp-prothrombin-fibrinogen. S. aureus failed to induce fibrin γ-chain dimers and α-chain multimers in FXIII-depleted plasma (Fig. 7). After incubation of staphylococci in plasma for 24 h, cleavage of FXIII A to A′ was readily detected, and this was blocked by treatment with lepirudin (Fig. 6B). Thus, thrombin-mediated activation of FXIII indeed occurs in plasma harboring staphylococci. The conversion of prothrombin to activated thrombin occurs presumably via the contact system (36) and must account for the observed fibrin-cross-linking in plasma inoculated with the vwb mutant strain.
FIGURE 7.

FXIII depletion abolishes fibrin cross-linking in staphylococcal clots. S. aureus Newman or isogenic mutants coa, vwb, or coa/vwb were grown to mid-log phase. Cells were washed, taken up in PBS, and incubated in the presence of 5 mm calcium and human plasma that had been depleted of FXIII. A PBS control with no bacterial cell (−) was included. After incubation at 37 °C, the samples were subjected to centrifugation after 24 h. Pellets were washed 3 times in PBS and boiled in sample buffer supplemented with urea (4 m) to solubilize clots. Samples were examined after Coomassie staining of SDS-PAGE (A) and immunoblot against FXIII subunit A and B and fibronectin (B).
DISCUSSION
Unlike non-pathogenic or opportunistic staphylococcal species, the ability to coagulate human blood represents a hallmark of clinical S. aureus isolates (37). Why does S. aureus promote coagulation, a pathway generally appreciated as an innate defense mechanism to limit the dissemination of bacterial invaders (36)? Although the exuberant activation of hemostasis by S. aureus may appear enigmatic, recent results suggest that fibrin clot formation may be a prerequisite for the replication and persistence of staphylococci in host tissues (18). Histopathology of S. aureus abscess lesions revealed the formation of bacterial abscess communities that are enclosed by a pseudocapsule formed via fibrin deposits to form a shield against host immune cells (38). A second layer of fibrin delineates healthy and infected tissues. Purulent lesions with increasing size develop within this second layer and are eventually drained onto the surface of infected organs (18). S. aureus mutants lacking coa and vwb cannot form abscess lesions or persist in organ tissues of intravenously infected mice (18). When analyzed by immunohistochemistry, Coa is found within the pseudocapsule of S. aureus abscess communities, whereas vWbp is distributed throughout the lesion with pronounced accumulation in its periphery (18). S. aureus coagulation has been reconstituted within a three-dimensional collagen matrix supplemented with plasma proteins (39). Under these conditions, staphylococci form two concentric structures, an inner pseudocapsule and an extended, outer microcolony-associated meshwork containing fibrin that shield staphylococci against neutrophils (39). Use of isogenic mutants could attribute the formation of the inner pseudocapsule to Coa, whereas establishment of the outer meshwork required vWbp (39). Together, these observations suggest that Coa and vWbp fulfill partially overlapping yet non-redundant functions during infection (18).
To explore the molecular attributes of vWbp, we employed affinity chromatography of human plasma with vWbp and Coa variants unable to promote staphylothrombin-mediated cleavage of fibrin while retaining the ability to bind both prothrombin and fibrinogen. This approach uncovered prothrombin, fibrinogen, and fibronectin as ligands of both Coa and vWbp and FXIII as a specific ligand of vWbp. Fibrinogen and prothrombin binding involves the D1-D2 domains of vWbp, whereas FXIII binding involved the D1-D2 domains and its central domain. Fibronectin binding occurred at the C-terminal domain of vWbp. These data support a model for the assembly of a supramolecular complex initiated via vWbp-prothrombin that first associates with fibrinogen and then with FXIII. Fibronectin association with vWbp-prothrombin-fibrinogen-FXIII occurs independently of other plasma proteins. vWF does not function as a ligand of vWbp-prothrombin in plasma; presumably, fibrinogen and FXIII occupy the vWF binding site (residues 333–358; Ref, 13). Perhaps vWbp binds vWF in specific host tissues where fibrinogen/FXIII is not available. In contrast to vWbp, Coa has evolved designated fibrinogen binding sites (C-terminal tandem repeats) (17). The binding site of Coa for fibronectin is not yet known; however, it must be distinct from that of vWbp, as amino acids 359–482 lack sequence similarity with Coa. Structure predictions of the C-terminal domain of vWbp suggest a helical segment of ∼50 residues (40) that may be involved in the recruitment of fibronectin.
Recent work proposed that the vWbp staphylothrombin complex displays an exosite with unique substrate specificity as compared with thrombin (14, 15). Indeed, vWbp staphylothrombin cleaves fibrinogen but not the other thrombin substrates: protein C, coagulation factors FV, FIX, FX, FXI, antithrombin III, or heparin cofactor II (15). We observed that association of vWbp-prothrombin did not trigger cleavage of the FXIII A zymogen. Nevertheless, the vWbp-prothrombin-fibrinogen-FXIII complex activated the FXIII A transglutaminase activity, as judged by the formation of fibrin γ-chain dimers and α-chain multimers. Whereas thrombin cleaves fibrinogen and FXIII A at similar rates (41), vWbp-prothrombin cleaved fibrinogen at a much faster rate than it activated FXIII.
When fibrin clots were formed by live bacteria, FXIII was found associated with the products of staphylococcal coagulation (Fig. 6). Surprisingly, even though Coa did not interact with FXIII in vitro, FXIII was recruited to staphylococcal clots formed by vwb mutants. This observation hints at either the presence of another, yet unidentified staphylococcal factor capable of binding FXIII, or at the recruitment of FXIII to the products of Coa- and vWbp-mediated coagulation. Under physiological conditions, zymogen FXIII interacts with fibrinogen γ′ through its B subunits (42). Fibrinogen γ′ is a product of alternative splicing of the γ-chain transcript, causing read-through at the exon IX/intron I junction (43). The product of alternative splicing and translation is the replacement of the final four amino acids at the C-terminal end of the γ-chain with 20 distinct residues (43, 44). The possibility that Coa or any other fibrinogen binding factor of S. aureus may selectively recruit γ′ fibrinogen, which accounts for 10% of fibrinogen in human plasma, into the staphylococcal clot together with FXIII has not yet been investigated. Although entirely speculative, such a scenario could explain the recruitment of FXIII to the staphylococcal clot.
Studies with S. pyogenes reported the proteolytic activation of FXIII via the contact system and the conversion of prothrombin to thrombin (36). Because FXIII is activated by vWbp-prothrombin even in the absence of thrombin, we chose to investigate the possibility of non-proteolytic activation. Although slower in nature than thrombin-mediated cleavage, activation of FXIII by staphylococcal factors did occur within S. aureus clots. Previous studies have already reported the non-proteolytic activation of FXIII. For example, tetrameric A2B2 zymogen can be dissociated at high calcium ion concentrations, resulting in non-proteolytic activation of FXIII A (45–47). Calcium concentrations required for FXIII A activation can be reduced to a physiological range in the presence of fibrin(ogen) (47). Siebenlist et al. (48) suggested that during the lag phase of the cross-linking reaction, fibrin may bind at or near the active site of FXIII, causing a conformational change that renders the zymogen catalytically active and promotes dissociation of A and B subunits. The recruitment of FXIII into staphylococcal clots via vWbp represents yet another mechanism for non-proteolytic activation of the zymogen. Because it has been shown that fibrin, but not fibrinogen, is a substrate for FXIII cross-linking (48), the incorporation of FXIII into the staphylococcal clot may be sufficient for the non-proteolytic activation of the zymogen.
In summary, S. aureus appears to recruit and activate FXIII within vWbp- and Coa-mediated fibrin clots. FXIII products, fibrin cables with increased stability and resistance to degradation, may fortify the barriers that are formed by staphylococci in abscess communities. Fibronectin, a known substrate of factor XIIIa that is preferentially cross-linked to the α-chain of fibrin (47), was also found associated with staphylococcal clots (Fig. 6). Presumably, fibronectin cross-linking modulates the mechanical and structural properties of the clot (49, 50). If so, vWbp-mediated recruitment of FXIII and fibronectin may favor bacterial escape from innate immune responses and bacterial replication in host tissues.
Finally, fibronectin-binding protein A (FnbpA), a surface protein expressed by most S. aureus strains, also serves as a substrate for factor XIIIa (51). At least in vitro, FnbpA is covalently cross-linked to both fibronectin and fibrin/fibrinogen (51). Thus, in addition to stabilizing the fibrin barriers against phagocytes, S. aureus may also utilize the transglutaminase activity of FXIII to deposit its own proteins in the fibrin scaffold, thereby modulating host immune responses to infection.
Acknowledgments
We thank members of our laboratory for suggestions and discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants AI092711, AI052747 (to O. S.), and AI075258 (to D. M.) (NIAID, Infectious Diseases Branch) and 1-U54-AI-057153 (Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium).
- Coa
- coagulase
- FXIII
- FXIII A and FXIIIB, factor XIII and factor subunits A or B
- vWF
- von Willebrand factor
- vWbp
- von Willebrand factor binding protein
- H6
- His6 tag.
REFERENCES
- 1. Lowy F. D. (1998) Staphylococcus aureus infections. New Engl. J. Med. 339, 520–532 [DOI] [PubMed] [Google Scholar]
- 2. Klevens R. M., Morrison M. A., Nadle J., Petit S., Gershman K., Ray S., Harrison L. H., Lynfield R., Dumyati G., Townes J. M., Craig A. S., Zell E. R., Fosheim G. E., McDougal L. K., Carey R. B., Fridkin S. K. (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298, 1763–1771 [DOI] [PubMed] [Google Scholar]
- 3. Bamberger D. M., Boyd S. E. (2005) Management of Staphylococcus aureus infections. Am. Fam. Physician 72, 2474–2481 [PubMed] [Google Scholar]
- 4. Weems J. J., Jr. (2001) The many faces of Staphylococcus aureus infection. Recognizing and managing its life-threatening manifestations. Postgrad. Med. 110, 24–26 [DOI] [PubMed] [Google Scholar]
- 5. Grundmann H., Aires-de-Sousa M., Boyce J., Tiemersma E. (2006) Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368, 874–885 [DOI] [PubMed] [Google Scholar]
- 6. Adams R. L., Bird R. J. (2009) Coagulation cascade and therapeutics update. Relevance to nephrology. Overview of coagulation, thrombophilias, and history of anticoagulants. Nephrology 14, 462–470 [DOI] [PubMed] [Google Scholar]
- 7. Levi M., Keller T. T., van Gorp E., ten Cate H. (2003) Infection and inflammation and the coagulation system. Cardiovasc. Res. 60, 26–39 [DOI] [PubMed] [Google Scholar]
- 8. Frick I. M., Björck L., Herwald H. (2007) The dual role of the contact system in bacterial infectious disease. Thromb. Haemost. 98, 497–502 [PubMed] [Google Scholar]
- 9. Herwald H., Mörgelin M., Dahlbäck B., Björck L. (2003) Interactions between surface proteins of Streptococcus pyogenes and coagulation factors modulate clotting of human plasma, J. Thromb. Haemost. 1, 284–291 [DOI] [PubMed] [Google Scholar]
- 10. Loof T. G., Morgelin M., Johansson L., Oehmcke S., Olin A. I., Dickneite G., Norrby-Teglund A., Theopold U., Herwald H. (2011) Coagulation, an ancestral serine protease cascade, exerts a novel function in early immune defense. Blood 11, 2589–2598 [DOI] [PubMed] [Google Scholar]
- 11. Levi M., van der Poll T., ten Cate H., van Deventer S. J. (1997) The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur. J. Clin. Invest. 27, 3–9 [DOI] [PubMed] [Google Scholar]
- 12. Loeb L. (1903) The influence of certain bacteria on the coagulation of blood. J. Med. Res. 10, 407–419 [PMC free article] [PubMed] [Google Scholar]
- 13. Bjerketorp J., Nilsson M., Ljungh A., Flock J. I., Jacobsson K., Frykberg L. (2002) A novel von Willebrand factor binding protein expressed by Staphylococcus aureus. Microbiology 148, 2037–2044 [DOI] [PubMed] [Google Scholar]
- 14. Friedrich R., Panizzi P., Fuentes-Prior P., Richter K., Verhamme I., Anderson P. J., Kawabata S., Huber R., Bode W., Bock P. E. (2003) Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature 425, 535–539 [DOI] [PubMed] [Google Scholar]
- 15. Kroh H. K., Panizzi P., Bock P. E. (2009) von Willebrand factor-binding protein is a hysteretic conformational activator of prothrombin. Proc. Natl. Acad. Sci. U.S.A. 106, 7786–7791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hendrix H., Lindhout T., Mertens K., Engels W., Hemker H. C. (1983) Activation of human prothrombin by stoichiometric levels of staphylocoagulase. J. Biol. Chem. 258, 3637–3644 [PubMed] [Google Scholar]
- 17. Panizzi P., Nahrendorf M., Figueiredo J. L., Panizzi J., Marinelli B., Iwamoto Y., Keliher E., Maddur A. A., Waterman P., Kroh H. K., Leuschner F., Aikawa E., Swirski F. K., Pittet M. J., Hackeng T. M., Fuentes-Prior P., Schneewind O., Bock P. E., Weissleder R. (2011) In vivo detection of Staphylococcus aureus endocarditis by targeting pathogen-specific prothrombin activation. Nat. Med. 17, 1142–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng A. G., McAdow M., Kim H. K., Bae T., Missiakas D. M., Schneewind O. (2010) Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog. 6, e1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McAdow M., Kim H. K., Dedent A. C., Hendrickx A. P., Schneewind O., Missiakas D. M. (2011) Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 7, e1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McAdow M., DeDent A. C., Emolo C., Cheng A. G., Kreiswirth B. N., Missiakas D. M., Schneewind O. (2012) Coagulases as determinants of protective immune responses against Staphylococcus aureus. Infect. Immun. 80, 3389–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Panizzi P., Friedrich R., Fuentes-Prior P., Bode W., Bock P. E. (2004) The staphylocoagulase family of zymogen activator and adhesion proteins. Cell. Mol. Life Sci. 61, 2793–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palma M., Shannon O., Quezada H. C., Berg A., Flock J. I. (2001) Extracellular fibrinogen-binding protein, Efb, from Staphylococcus aureus blocks platelet aggregation due to its binding to the α-chain. J. Biol. Chem. 276, 31691–31697 [DOI] [PubMed] [Google Scholar]
- 23. Baba T., Bae T., Schneewind O., Takeuchi F., Hiramatsu K. (2008) Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes, J. Bacteriol. 190, 300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanahan D. (1983) Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166, 557–580 [DOI] [PubMed] [Google Scholar]
- 25. Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. (1990) Use of T7 polymerase to direct expression of cloned genes. Methods Enzymol. 185, 60–89 [DOI] [PubMed] [Google Scholar]
- 26. Anderson N. L., Anderson N. G. (2002) The human plasma proteome. History, character, and diagnostic prospects. Mol. Cell Proteomics 1, 845–867 [DOI] [PubMed] [Google Scholar]
- 27. Mosher D. F., Furcht L. T. (1981) Fibronectin. Review of its structure and possible functions. J. Invest. Dermatol. 77, 175–180 [DOI] [PubMed] [Google Scholar]
- 28. Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. (1973) Human factor XIII from plasma and platelets. Molecular weights, subunit structures, proteolytic activation, and cross-linking of fibrinogen and fibrin. J. Biol. Chem. 248, 1395–1407 [PubMed] [Google Scholar]
- 29. Ariëns R. A., Lai T. S., Weisel J. W., Greenberg C. S., Grant P. J. (2002) Role of factor XIII in fibrin clot formation and effects of genetic polymorphisms. Blood 100, 743–754 [DOI] [PubMed] [Google Scholar]
- 30. Skrzynia C., Reisner H. M., McDonagh J. (1982) Characterization of the catalytic subunit of factor XIII by radioimmunoassay. Blood 60, 1089–1095 [PubMed] [Google Scholar]
- 31. Nagy J. A., Kradin R. L., McDonagh J. (1988) Biosynthesis of factor XIII A and B subunits. Adv. Exp. Med. Biol. 231, 29–49 [DOI] [PubMed] [Google Scholar]
- 32. Siebenlist K. R., Mosesson M. W. (1994) Progressive cross-linking of fibrin γ chains increases resistance to fibrinolysis, J. Biol. Chem. 269, 28414–28419 [PubMed] [Google Scholar]
- 33. Greinacher A., Warkentin T. E. (2008) The direct thrombin inhibitor hirudin. Thromb. Haemost. 99, 819–829 [DOI] [PubMed] [Google Scholar]
- 34. Curtis C. G., Brown K. L., Credo R. B., Domanik R. A., Gray A., Stenberg P., Lorand L. (1974) Calcium-dependent unmasking of active center cysteine during activation of fibrin stabilizing factor. Biochemistry 13, 3774–3780 [DOI] [PubMed] [Google Scholar]
- 35. Parry M. A., Fernandez-Catalan C., Bergner A., Huber R., Hopfner K. P., Schlott B., Gührs K. H., Bode W. (1998) The ternary microplasmin-staphylokinase-microplasmin complex is a proteinase-cofactor-substrate complex in action. Nat. Struct. Biol. 5, 917–923 [DOI] [PubMed] [Google Scholar]
- 36. Loof T. G., Schmidt O., Herwald H., Theopold U. (2011) Coagulation systems of invertebrates and vertebrates and their roles in innate immunity. The same side of two coins? J. Innate Immun. 3, 34–40 [DOI] [PubMed] [Google Scholar]
- 37. Cheng A. G., DeDent A. C., Schneewind O., Missiakas D. (2011) A play in four acts. Staphylococcus aureus abscess formation. Trends Microbiol. 19, 225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng A. G., Kim H. K., Burts M. L., Krausz T., Schneewind O., Missiakas D. M. (2009) Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 23, 3393–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guggenberger C., Wolz C., Morrissey J. A., Heesemann J. (2012) Two distinct coagulase-dependent barriers protect Staphylococcus aureus from neutrophils in a three dimensional in vitro infection model. PLoS Pathog. 8, e1002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kroh H. K., Bock P. E. (2012) Effect of zymogen domains and active site occupation on activation of prothrombin by von Willebrand Factor-binding protein. J. Biol. Chem. 287, 39149–39157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Greenberg C. S., Miraglia C. C., Rickles F. R., Shuman M. A. (1985) Cleavage of blood coagulation factor XIII and fibrinogen by thrombin during in vitro clotting. J. Clin. Invest. 75, 1463–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Siebenlist K. R., Meh D. A., Mosesson M. W. (1996) Plasma factor XIII binds specifically to fibrinogen molecules containing γ chains. Biochemistry 35, 10448–10453 [DOI] [PubMed] [Google Scholar]
- 43. Fornace A. J., Jr., Cummings D. E., Comeau C. M., Kant J. A., Crabtree G. R. (1984) Structure of the human γ-fibrinogen gene. Alternate mRNA splicing near the 3′ end of the gene produces γ A and γ B forms of γ-fibrinogen. J. Biol. Chem. 259, 12826–12830 [PubMed] [Google Scholar]
- 44. Wolfenstein-Todel C., Mosesson M. W. (1981) Carboxyl-terminal amino acid sequence of a human fibrinogen γ-chain variant (γ′). Biochemistry 20, 6146–6149 [DOI] [PubMed] [Google Scholar]
- 45. Muszbek L., Yee V. C., Hevessy Z. (1999) Blood coagulation factor XIII. Structure and function. Thromb. Res. 94, 271–305 [DOI] [PubMed] [Google Scholar]
- 46. Credo R. B., Curtis C. G., Lorand L. (1978) Ca2+-related regulatory function of fibrinogen. Proc. Natl. Acad. Sci. U.S.A. 75, 4234–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blombäck B., Procyk R., Adamson L., Hessel B. (1985) FXIII induced gelation of human fibrinogen. An alternative thiol-enhanced, thrombin-independent pathway. Thromb. Res. 37, 613–627 [DOI] [PubMed] [Google Scholar]
- 48. Siebenlist K. R., Meh D. A., Mosesson M. W. (2001) Protransglutaminase (factor XIII) mediated cross-linking of fibrinogen and fibrin. Thromb. Haemost. 86, 1221–1228 [PubMed] [Google Scholar]
- 49. Okada M., Blombäck B., Chang M. D., Horowitz B. (1985) Fibronectin and fibrin gel structure. J. Biol. Chem. 260, 1811–1820 [PubMed] [Google Scholar]
- 50. Chow T. W., McIntire L. V., Peterson D. M. (1983) Importance of plasma fibronectin in determining PFP and PRP clot mechanical properties. Thromb. Res. 29, 243–248 [DOI] [PubMed] [Google Scholar]
- 51. Matsuka Y. V., Anderson E. T., Milner-Fish T., Ooi P., Baker S. (2003) Staphylococcus aureus fibronectin-binding protein serves as a substrate for coagulation factor XIIIa. Evidence for factor XIIIa-catalyzed covalent cross-linking to fibronectin and fibrin. Biochemistry 42, 14643–14652 [DOI] [PubMed] [Google Scholar]


