Abstract
Liver cancer is the third leading cause of cancer deaths worldwide but no effective treatment toward liver cancer is available so far. Therefore, there is an unmet medical need to identify novel therapies to efficiently treat liver cancer and improve the prognosis of this disease. Here we report that berbamine (BBM) and one of its derivatives, bbd24, potently suppressed liver cancer cell proliferation and induced cancer cell death by targeting Ca2+/calmodulin-dependent protein kinase II (CAMKII). Furthermore, BBM inhibited the in vivo tumorigenicity of liver cancer cells in NOD/SCID mice, and down-regulated the self-renewal abilities of liver cancer initiating cells. Chemical inhibition or short hairpin RNAs-mediated knockdown of CAMKII recapitulated the effects of BBM, while overexpression of CAMKII promoted cancer cell proliferation and increased the resistance of liver cancer cells to BBM treatments. Western blot analyses of human liver cancer specimens showed that CAMKII was hyperphosphorylated in liver tumors compared with the paired peri-tumor tissues, which supports a role of CAMKII in promoting human liver cancer progression and the potential clinical use of BBM for liver cancer therapies. Our data suggests that BBM and its derivatives are promising agents to suppress liver cancer growth by targeting CAMKII.
Keywords: Berbamine, CAMKII, liver cancer, cancer stem cells, CD133
Introduction
Liver cancer is the third leading cause of cancer deaths globally due to its high incidence and the lack of effective treatments. In the United States, it is now among the ten most common cancers, and its prevalence has been increased dramatically in the recent years (1). The major type of liver cancers is hepatocellular carcinoma (HCC), and the risk factors for HCC include infection of hepatitis B or C virus, over-consumption of alcohol, and insults from xenobiotics (2).
Surgical removal of liver tumor is a primary therapy for patients who have relatively small tumors and a still well-functioning liver (3). For patients at late stages or with cirrhosis, other therapies such as arterial chemoembolization, radio frequency ablation, percutaneous ethanol injection, proton beam, chemotherapy, and liver transplantation might be applied (4). However, the outcomes of these therapies are not satisfying. Therefore, novel treatments such as target therapies are in urgent need to improve the prognosis of the liver cancer patients (5). Currently, sorafenib is a widely used target therapy drug that specifically blocks angiogenesis and other growth signaling of liver tumor (6). Nevertheless, this drug only prolongs the patient life by 3 months in average and even shorter in patients under poor conditions potentially due to the resistance of cancer stem cells to this drug (7).
Many compounds in use today for cancer medicine are derived from natural products of plants and marine organisms (8, 9). Berbamine (BBM) is a natural bisbenzylisoquinoline product isolated from traditional Chinese herbal medicine Berberis amurensis and has been used to treat inflammatory and other diseases for centuries (10). Recent studies suggest that BBM and its derivatives also possess anti-tumor activities for chronic myeloid leukemia, breast cancer, and melanoma (11–15).
In this study, we report that BBM and its derivative bbd24 (Figure 1) are potent to suppress the growth of liver cancers, as well as cancer initiating cells. The Ca2+/calmodulin-dependent protein kinase II (CAMKII) is identified as a BBM target in liver cancer. These results implicate that targerting CAMKII by BBM and its derivatives may provide a novel approach to treat liver cancer.
Figure 1.

Structures of BBM and its derivative 2-methylbenzoyl berbamine (bbd24).
Materials and Methods
Cell culture, survival/proliferation assay, and sphere formation assay
HepG2, PLC/PRF/5, SK-Hep-1, and SNU398 cells were ordered from the American Type Culture Collection. MHCC97H cells were from the Cell Resources of Shanghai Institutes for Life Sciences, Chinese Academy of Sciences. Huh7 cells were from Japanese Collection of Research Bioresources Cell Bank. CL48 was a gift from Dr. Yun Yen’s lab at the City of Hope Medical Center. All these cell lines were authenticated by the providers, and were frozen in liquid nitrogen soon after arrival. The experiments with these cells were performed within 6 generations after resuscitation.
CL48, Huh7, PRL/PRF/5, and MHCC97H cells were cultured in DMEM containing 10% FBS. HepG2 cells were maintained in MEM containing 10% FBS. SK-Hep-1 and SNU398 cells were maintained in RPMI-1640 containing 10% FBS. MTS assay measuring amount of survival cells was performed with the CellTiter 96 Aqueous Cell Proliferation Kit (Promega, Madison, WT). The IC50 was defined as the drug concentration that induced 50% viability decrease. The sphere formation assay followed a protocol described elsewhere (16). The maintenance of the cells before the sphere formation assay was performed with digestion and cell passage every 3 days and a subculture ratio for 70–80% confluence before passage.
Compound
Cisplatin and 5′-FU were from Sigma-aldrich (St. Louis, MO) and dissolved in DMSO. For in vitro experiments, BBM and bbd24 were dissolved in DMSO. BBM was dissolved in pure sterile water for animal experiments. 500 ng/ml of the tetracyclin derivative Doxycycline (DOX) (Clontech, Mountain View, CA) was used for induction of CAMKIIγ expression in cell cultures.
Xenograft
5 × 106 Huh7 cells in 50% Matrigel (BD bioscience, San Jose, CA) dissolved in PBS were inoculated in a NOD/SCID mouse. 5 × 106 SK-Hep-1 cells were applied for each xenograft without Matrigel. 100 mg/kg of BBM was orally treated to mice with a regimen of twice a day for 5 consecutive days after the tumors reached a size of 2 mm in diameter. After 2 days withdraw, the regimen was repeated once. All the procedures followed the National Institutes of Health guidelines for the care and use of laboratory animals.
Cell death analysis and flow cytometry
The cell death analysis was performed with the FITC Annexin V Apoptosis Detection Kit I from BD Pharmigen (San Jose, CA) according to the manufacture’s instruction. PE-conjugated anti-human CD133/1 antibody was ordered from Meltenyi Biotec (Auburn, CA) for flow cytometry (FACS) analysis. The purity of the sorted cells was tested with PE-conjugated anti-human CD133/2 antibody. The FITC-conjugated anti-human CD90 antibody for MHCC97H cells was from Biolegend (San Diego, CA).
CAMKIIγ overexpression and knockdown
The human CAMKIIγ coding sequence with a kozak site was cloned into the retroviral vectors pMSCV-puro (Addgene 24828) and pRetroX-Tight-puro (Clontech, Mountain View, CA). A MOI of 3–5 was used for retroviral transduction of the liver cancer cells. The retroviral experiments were performed following the manual of Retro-X™ Tet-On® Advanced Inducible Expression System. A lentiviral vector pLKO.1-TRC (Addgene 10878) was used for the knockdown of CAMK2γ. The following targets in the coding sequences were selected for the design of shRNAs: GGATATGTCGACTTCTGAAAC, GGAGCCTATGATTTCCCATCA, GCCACAAACCACTGTGGTACA, GCATCCATGATGCATCGTCAGGA. A MOI of 3 was applied for the infection of the target cells. Puromycin (Sigma-aldrich, St. Louis, MO) was used to select the cells after lentiviral infection. The stable cells were used for the following animal experiments. Both retroviruses and lentiviruses were packaged in Hek293T cells and titrated with HT1080 cells.
Western blot
Cell/liver lysis and lysate preparation were previously described (17). Antibodies that identified phospho-CAMKII, CAMKIIγ, Bcl-2, and HSP70 were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin and anti-LC3 antibodies were from Sigma-aldrich (St. Louis, MO). All the other antibodies were from Cell Signaling Technology (Danvers, MA).
Human HCC specimen analyses
The frozen samples of the HCC patients were provided by the City of Hope National Medical Center, and the pathological description and analyses were performed by the pathologists in the Department of Pathology. The lysates of tumor and non-tumor adjacent tissues were prepared with the T-PER tissue Protein Extraction Reagent (Pierce, Rockford, IL). The pCAMKII signals were quantified with the software ImageJ.
Statistical analyses
All the data were reported as mean ± SEM. Two-tailed Student’s t test was used to determine the significance of differences between data groups.
Results
BBM inhibits the growth of liver cancer cells in vitro and in vivo
We first determined the effect of BBM (Figure 2A–B) on the growth of a variety of liver cancer cells from different origins or different stages of cancer progression (18). The results show that BBM potently inhibited the growth of epithelial liver cancer cell lines, including Huh7, HepG2, MHCC97H, and PLC/PRF/5, with an IC50 as low as 5.2 μg/ml (Figure 2A). In addition, berbamine also suppressed the growth of mesenchymal-like liver cancer cell lines either from an endothelial origin (SK-Hep-1) or from the cells that has undergone epithelial-to-mesenchymal transition (SNU398) (Figure 2B). In contrast, an embryonic liver cell line, CL48, which is from a normal fetus liver, was much less sensitive to BBM. The high IC50 (55.3 μg/ml) of CL48 indicated that the effects of BBM were more specific to the transformed liver cells in a dose-dependent manner (Table S1).
Figure 2. Berbamine (BBM) suppresses liver cancer cell growth and inhibited CAMKII phosphorylation.
A&B. Cell proliferation was assayed 72 hours after BBM treatment for (A) liver cancer cells with epithelial morphology and (B) two mesenchymal–like cell lines and a normal embryonic liver cell line, CL48. C. Huh7 and SK-Hep-1 cells were used to generate xenografts. On the indicated days after starting BBM treatment, the sizes of Huh7 xenograft were measured. D. On Day 26, the mice were euthanized and the tumors were weighed. E. On the indicated days, the SK-Hep-1 xenograft tumors were measured. F. On Day 30, the tumors were weighed. *, p < 0.05.
We then further determined the anti-tumor effects of BBM on a xenograft animal model. Two liver cancer cell lines, Huh7 (epithelial) and SK-Hep-1 (mesenchymal-like), were inoculated into NOD/SCID mice by subcutaneous injection. The oral BBM treatment greatly suppressed the growth of Huh7 xenografted tumors over the time (Figure 2C) and led to a tumor reduction by 70% based on the tumor weight (Figure 2D, Figure S1A). Consistent with the in vitro experiments, the growth of SK-Hep-1 cells in NOD/SCID mice was less sensitive to BBM than that of Huh7 (Figure 2E). Nevertheless, there was still a significant suppression of the growth of the SK-Hep-1 xenograft with more than 50% reduction of the tumor weight (Figure 2F, Figure S1B). These results clearly demonstrate that BBM is a very potent natural compound to suppress the growth of liver cancer.
BBM and its derivative, bbd24, induced deaths of liver cancer cells by targeting CAMKII
To determine the mechanisms by which BBM inhibits the growth of liver cancer cells, we examined whether BBM inhibited the liver cancer growth by inducing cell deaths. Huh7 cells were analyzed by flow cytometry with the Propidium Iodide (PI) and Annexin V staining at 72 hours after BBM treatment. 80% cells underwent cell deaths by either apoptosis or necrosis (Figure 3A). A biotinylated BBM was used to study whether BBM bound to plasma membrane receptors or penetrated plasma membrane to affect cytoplasmic molecules. Accompanying with significant cell death morphology of nucleus fragmentation, BBM appeared to be able to enter plasma membrane and stay in the cytoplasm, indicating that the major targets of BBM are probably cytoplasmic and/or membrane receptor-activated signaling molecules (Figure S2).
Figure 3. BBM and its derivative bbd24 induce liver cancer deaths and inhibit the phosphorylation of CAMKII.
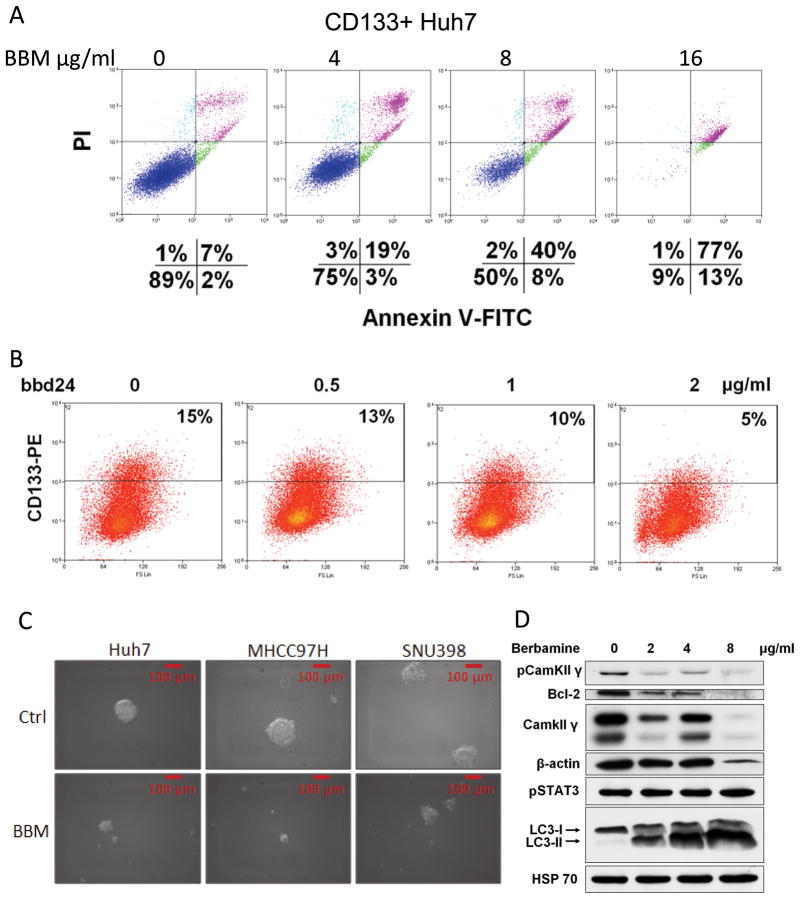
A. Huh7 cells were incubated with 16 μg/ml BBM for 72 hours. The cells were then stained with PI and Annexin V-FITC. B. Huh7 cells, SK-Hep-1, and MHCC97H were treated with BBM for 48 hours, and then their lysates were analyzed by Western blot. HSP70 was used as a loading control. C. A derivate of BBM, bbd24, was applied to Huh7 cells with a concentration of 2 ug/ml. The photos were taken 6 hour after the treatment. D. The same cells were analyzed by flow cytometry at the different time points. E. Huh7 cells were treated with bbd24 for 48 hours, and then their lysates were analyzed by Western blot.
Our previous study in chronic myeloid leukemia showed that BBM targeted CAMKII by blocking its ATP binding pocket (15). Indeed, among many signal pathways that were examined in different BBM-treated liver cancer cell lines, including Huh7, SK-Hep-1, and MHCC97H cells, phosphorylation of CAMKII was universally inhibited by BBM (Figure 3B). The strongest inhibition of CAMKII phosphorylation was observed in Huh7 cells, which is the cell line that was the most sensitive to BBM. BBM also suppressed CAMKII downstream signaling pathways in these cancer cells. In Huh7 cells, activities of the CAMKII downstream/target genes Bcl-2 and STAT3 were reduced by BBM treatment. While in SK-Hep-1 and MHCC97H cells, activities of AKT, ERK, and STAT3 were suppressed to different extents. Of note, the ablation of β-actin expression implied that BBM might directly affect cytoskeleton and thereby impact cell deaths. In addition, BBM induced cleavage of LC3, Caspase 3, and PARP, which implied that BBM caused autophagy and apoptosis in all the 3 liver cancer cell lines.
A derivative of BBM as well as a more potent CAMKII inhibitor (15), bbd24, has an low IC50 of 1.69 μg/ml for Huh7 cells and can induce cell detachment as soon as 6 hours after the treatment (Figure 3C). This derivative quickly induced Huh7 cell deaths, such as apoptosis, at a very low concentration of 2 μg/ml (Figure 3D). A significant reduction of phosphorylation of CAMKII and its downstream target ERK was observed with 1 μg/ml bbd24, a dose that also strongly reduced β-actin levels (Figure 3E).
BBM and bbd24 inhibit the growth of the liver cancer initiating cells
Recent research implies that cancer stem cells or cancer initiating cells are responsible for cancer initiation, chemo-resistance and re-initiation of cancer after therapy (19). Therefore, effects of BBM on this population of liver cancer cells were evaluated. CD133 (AC133, prominin-1), a membrane-associated glycoprotein first identified in mouse neuroepithelium, has been widely used as a marker to enrich liver cancer initiating cells (20, 21). Therefore, this marker was chosen for sorting Huh7 stem cells. Consistent with previous studies, the CD133+ Huh7 cells expressed high levels of β-catenin and Bcl-2 (Figure S3A), the key genes for stem cell survival and self-renewal. However, the CD133+ Huh7 cells did not show increased survival capabilities under BBM treatment than the CD133- population (Figure S3B). More interestingly, this population appeared to be equally, if not more, sensitive to BBM treatment than the parental Huh7 cells did, as 4–8 μg/ml BBM was sufficient to induce cell deaths of them (Figure 4A, Figure S3C).
Figure 4. BBM preferentially targets liver cancer initiating cells and suppresses the phosphorylation of CAMKII.
A. A series of doses of BBM were applied to the sorted CD133+ Huh7 cells, the death of which was analyzed by flow cytometry. B. Huh7 cells were treated with bbd24 for 24 hours, and then labeled with CD133-PE antibody and analyzed. C. Huh7, MHCC97H, and SNU398 cells were treated with BBM for 48 hours, and then trysinized and re-suspended with sphere formation conditioning medium, and seeded in low attachment plates. The spheres were photographed 1 week after seeding cells. D. The sorted CD133+ Huh7 cells were treated with BBM for 48 hours and then analyzed with Western blot.
The regular chemotherapy drugs can enrich the CD133+ population, which is regarded as a reason of liver cancer recurrence after chemotherapy. In contrast, the BBM derivative bbd24 decreased the percentage of the CD133+ cells in a dose-dependent manner (Figure 4B). Because cancer stem cells are able to survive under anchorage-independent conditions (16, 21), the sphere formation assay is used to evaluate the characteristics and behaviors of stem cells, which excludes the potential bias and controversy caused by the application of certain stem cell markers, such as CD133. BBM was indeed also an agent that strongly inhibited hepatosphere formation of different liver cancer cell lines (Figure 4C, Table S2), with the doses (Huh7: 1.5 μg/ml; MHCC97H: 1.5 μg/ml; SNU398: 2.5 μg/ml) that were much lower than its IC50s on these cell lines (Huh7: 5.2 μg/ml; MHCC97H: 13.7 μg/ml; SNU398:14.2 μg/ml). These results suggest that BBM preferentially targets liver cancer stem cells. Furthermore, Western blot analysis showed that BBM inhibited CAMKII signaling in the CD133+ Huh7 cells (Figure 4D), indicating that BBM also targets CAMKII in liver cancer stem cells.
Direct Inhibition of CAMKII recapitulates BBM’s effects on liver cancer cells and cancer initiating cells
To investigate whether the effects of BBM can be attributed to its effect on down-regulation of CAMKII activity, we first examined whether direct inhibition of CAMKII by a chemical inhibitor KN93 could mimic BBM’s cytotoxicities on liver cancer cell lines. Compared with its structural analog control KN92, KN93 exerted the survival inhibition to a series of liver cell lines in a dose-dependent manner (Figure 5A), which further confirmed the critical role of CAMKII in liver cancer progression. In addition, the effects of the CAMKII inhibitor were tested on the liver cancer initiating cells. KN93 significantly minimized the stem cell populations, the CD133+ Huh7 population and the CD90+ MHCC97H population, in their parental cells (Figure 5B, Figure S4–5). Moreover, KN93, but not KN92, also exhibited strong capabilities to inhibit the hepatosphere formations of liver cancer cells at 5 μM, a concentration that did not significantly inhibit the proliferation of these liver cancer cells (Figure 5C, Table S2).
Figure 5. Responses of liver cancer cell lines to a chemical inhibitor of CAMKII, KN93.
A. A series of doses of KN93 and its non-targeting analogue KN92 were applied to the liver cancer cell lines. Cell proliferation assay with MTS was performed 72 hours after the treatment. *, p < 0.05. B. The Huh7 and MHCC97H cells under 5 μM KN93 were labeled with CD133-PE and CD90-FITC antibodies, respectively, and then analyzed by flow cytometry. C. Huh7, MHCC97H, and SNU398 cells were treated with KN93 or KN92 for 48 hours, and then trysinized and resuspended with sphere formation conditioning medium, and seeded in low attachment plates.
Our previous studies showed that BBM interacted with the gamma isoform of CAMKII (CAMKIIγ), and another study showed that the most abundant CAMKII in liver was also the gamma subtype (22). However, there has been no report about the role of CAMKIIγ in hepatocarcinogenesis. Four short hairpin RNAs (shRNAs) targeting CAMKIIγ were designed and verified for their knockdown efficiency individually in Huh7 cells by transdution of lentiviral vectors (Figure 6A). Then the shRNAs were pooled and used to transduce HepG2, Huh7, and MHCC97H cells. The knockdown of CAMKIIγ greatly inhibited the cell proliferation in vitro (Figure 6B) and led to slight morphological changes compared with the cells transduced with the scramble control shRNA. The transduced Huh7 cells were further selected by puromycin and used to generate stable cells, which thereafter were inoculated into NOD/SCID mice. Although no significant difference was observed in the initial phases of xenograft growth, the knockdown of CAMKIIγ in Huh7 cells showed a considerable reduction of tumor volume and weight in the later stages (Figure 6C, Figure S6).
Figure 6. Knockdown of CAMKIIγ suppresses liver cancer cell growth, and overexpression of CAMKIIγ antagonizes the effect of BBM.
A. shRNAs were expressed by a lentiviral vector pLKO.1-puro. An MOI of 3 was applied for infection of Huh7 cells. Each of the 4 pairs of shRNAs targeting CAMKIIγ and a scramble control was tested in Huh7 cells by Western blot. B. Cell proliferation assay with MTS was performed 96 hours after infection of Huh7, SK-hep-1, and MHCC97H by the pool of the 4 lentiviral vectors. C. The transduced Huh7 cells were selected by puromycin. The stable cells were used for the xenograft on NOD/SCID mice. The tumor sizes were measured on Day 7, Day 15, and Day 28 after the s.c. injection. The tumors were weighed on Day 28. *, p < 0.05. D. Overexpression of CAMKIIγ by infecting HepG2 and Huh7 cells with a retroviral vector pMSCV. E. The enhanced growth of the CAMKIIγ overexpression HepG2 and Huh7 cells. F. Reduced cytotoxicity of 5-FU by the CAMKIIγ overexpression in HepG2 cells. G. Tetracycline-derivative doxycycline (DOX) induction of CAMKIIγ by infecting HepG2 and Huh7 cells with a retroviral vector pRetroX-Tight-puro. H. DOX-induced CAMKIIγ expression antagonized the cytotoxicity of 72 hours BBM treatment. *, p < 0.05. I. The stable HepG2 and Huh7 cells with CAMKIIγ knockdown were treated with BBM at indicated concentrations. The cell survival was measured by MTS assay.
Overexpression of CAMKIIγ promotes liver cancer regrowth, and resistance to 5′-FU and BBM
To verify that CAMKIIγ is the major target of BBM, we analyzed the function of CAMKIIγ in liver cancer cells by overexpressing CAMKIIγ with retroviral vectors. An MSCV retroviral vector increased CAMKIIγ expression by 3–10 times in HepG2 and Huh7 cells (Figure 6D). This overexpression slightly enhanced the cancer cell proliferation in vitro (Figure 6E), and promoted the chemoresistance to 5′-FU, a conventional chemotherapy drug (Figure 6F). To exclude the interference from antibiotic/antiseptic selection during generation of stable cell lines, especially on the phosphorylation of CAMKIIγ, two tetracycline-inducible CAMKIIγ expression cell lines were further generated with Huh7 and HepG2 cells in order to evaluate whether CAMKIIγ expression diminished the BBM’s effects (Figure 6G). As expected, DOX-induced CAMKIIγ expression successfully reduced the suppression of liver cancer growth by BBM, which was more prominent in HepG2 cells probably due to the more robust induction of CAMKIIγ expression in this cell line (Figure 6H). In addition, knockdown of CAMKIIγ decreased the liver cancer cells’ sensitivity to BBM (Figure 6I). These results altogether demonstrate that CAMKIIγ is a target of BBM effect in liver cancer cells.
CAMKIIγ is hyperphosphorylated in human liver tumors
Since CAMKII has not been characterized before in liver cancer, it is potentially an unidentified proto-oncogene for human liver cancer. Therefore, we examined whether CAMKII is hyperphosphorylated in liver tumors compared with non-tumor adjacent tissues. The phosphorylation of CAMKII (pCAMKII) in liver tumors was up-regulated in 8 of 14 pairs of liver cancer specimens (Figure 7A, Table S3). More interestingly, the up-regulation of pCAMKII in tumors is more frequent in the liver cancer patients with higher stages of hepatocarcinomas (Figure 7B), classified by the TNM Classification of Malignant Tumours staging system (the American Joint Committee on Cancer 2009). In average, the levels of pCAMKII in high stage tumors (T=3) were at least 2 times higher than that in low stage tumors (T=1 or 2) (Figure 7C). The phophorylation of the potential CAMKII substrates and oncogenic proteins, ERK1/2, AKT1/2, and STAT3 (Y705), were mostly correlated with the pCAMKII levels between tumors and non-tumor tissues (Figure 7A). In contrast, mTOR phosphorylation and β-catenin overexpression in these HCC specimens were not greatly changed in the tumors compared with the paired peri-tumor tissues.
Figure 7. CAMKII is hyperphosphorylated in human HCC compared with the paired peri-tumor tissues.
A. Western blot analyses of oncogenic signaling in liver tumors from patients with different tumor stages. HSP70 was used as a loading control. B. Tumor stages in patients with different pCAMKII between tumors and non-tumor tissues. ‘Down’ means the patients have down-regulated CAMKII phosphorylation in tumors compared with that in non-tumor liver tissues, while ‘Up’ means the patients have higher CAMKII phosphorylation in tumors than that in non-tumor tissues. *, p < 0.05. C. The quantification of CAMKII phosphorylation in patients of different tumor stages.
Discussion
Natural compounds and their derivatives are emerging attractive new generation of anti-cancer drug candidates. BBM is a traditional natural product that has been used for centuries to treat inflammatory diseases. However, the anti-cancer activities of BBM are just recently being elucidated. The most promising results are from the studies of leukemia, where the IC50 of the natural form of BBM after 72 hours treatment was from 4.80 to 7.50 μg/ml (14). More importantly, a clinical study has demonstrated the efficacy of BBM (12). In contrast, for other cancer cells such as melanoma and breast cancer cells, the IC50 of BBM is usually higher than 15.0 μg/ml (11, 13). In our studies, the lower IC50s of the natural form of BBM were observed for liver cancer cell lines, and the BBM derivative bbd24 has an IC50 as low as 1.67 μg/ml. Moreover, the xenograft studies showed the BBM reduced the liver tumor growth by 70% at a very tolerable dose for animals (15). Therefore, BBM and its derivatives have great potentials to target liver cancer.
CaMKIIs are serine/threonine-specific protein kinases that respond to the fluctuation of Ca2+. The previous studies have shown that CaMKIIs play key roles in three aspects. In the neural system, CaMKIIs (alpha and beta isoforms) are important mediators of memory and learning (23). In the circulation system, CaMKIIs (delta isoforms) are necessary for Ca2+ homeostasis in hearts and regulate function of cardiomyocytes (24). In the immune system, CaMKIIs (gamma isoforms) are required for T cell memory, positive T-cell selection, and CD8+ T cell activation (25, 26). The roles of CAMKIIs in cancer are unclear, although there have been a few reports indicating its potential roles in prostate cancer, basal cell carcinoma, and leukemia (27–29). Here, we report for the first time that CAMKII is a target of BBM in suppressing liver tumor growth.
Furthermore, through targeting CAMKII, BBM’s inhibition on cancer cells might have broader effects beyond affecting JAK/STAT3 and p210bcr-abl as previously reported (11, 14, 15), because CAMKIIγ directly impacts many other signal pathways including STAT1, NF-κB, JNK, ERK1/2, FOXO1, and Wnt/β-catenin (22, 30–32). These genes, together with STAT3, form a signal network that can plays critical roles in hepatocarcinogenesis, which is demonstrated by many experimental animal studies. CAMKIIγ, as an upstream molecule that directly responds to extracellular stimuli and modulates many receptor/adaptor/kinase/transcription factor interactions, could be also involved in liver cancer initiation and progression. Particularly, ERK, AKT, and STAT3, which play central roles in hepatocarcinogenesis (33), seem to be the major downstream of CAMKII in human HCC specimens based on this study. However, this point needs to be verified with systemic statistical analyses of a bigger cohort of HCC patients. Nevertheless, hyperphosphorylation of CAMKII is significantly correlated with human HCC stages, which indicates that CAMKII may enhance HCC progression by promoting cell cycling and preventing cell deaths. These results, together with our in vivo xenograft studies and in vitro cell culture experiments, implicate an oncogenic role of CAMKII in liver cancer.
More importantly, we also observed that liver cancer initiating cells were more sensitive to BBM than non-cancer initiating cells based on the CD133 expression and the hepatosphere formation assays. Recurrence after surgical liver tumor removal is a long-lasting clinical issue. Chemoresistance of liver cancer cells is also an important reason for the high-death rates of the patients. Recent studies have shown that liver cancer stem cells may be responsible for cancer recurrence and chemoresistance. Therefore, BBM and its derivatives could be of great value to treat the patients with advanced progression. Application of BBM alone or in combination with other drugs may provide new approaches for liver cancer therapies. In addition, a variety of CAMKII inhibitors have been developed recently (34, 35), which also possess potential clinical values.
In conclusion, we have shown that BBM and its derivative bbd24 strongly suppress the growth of liver cancer cells by targeting CAMKII. Therefore, targeting CAMKII by natural products may hold great promise to develop new strategies for liver cancer therapy.
Supplementary Material
Acknowledgments
We thank the Research Pathology Core and Department of Pathology at City of Hope Helford Clinical Research Hospital for pathological analyses, and Dr. Richard Ermel and the Animal Resource center for the technique support in animal experiments.
Footnotes
Competing interests
The authors have no conflicts of interest to disclose.
Financial information:
W. Huang is supported by NCI R01-CA139158. This work was also supported in part by the National Natural Science Foundation of China (81070420 and 81270601) to R. Xu, Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents.
Author contribution:
ZM designed and performed most of the experiments, analyzed the results, and wrote the paper. TL performed flow cytometry and some Western blot experiments. XM and XW assisted in the real-time PCR and the immunostaining. XW also participated in the pathological analysis of human HCC specimens. CVN, YG, HZ, JT, GL, and JW provided technical assistance. YW and YY provided the human HCC specimens and made intelligent contribution. RX and WH initiated and supervised the project, obtained the funding, and revised the manuscript.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892–9. doi: 10.1200/JCO.2005.03.196. [DOI] [PubMed] [Google Scholar]
- 3.Kassahun WT, Fangmann J, Harms J, Hauss J, Bartels M. Liver resection and transplantation in the management of hepatocellular carcinoma: a review. Experimental and clinical transplantation: official journal of the Middle East Society for Organ Transplantation. 2006;4:549–58. [PubMed] [Google Scholar]
- 4.Carr BI. Some new approaches to the management of hepatocellular carcinoma. Seminars in oncology. 2012;39:369–73. doi: 10.1053/j.seminoncol.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka S, Arii S. Molecular targeted therapies in hepatocellular carcinoma. Seminars in oncology. 2012;39:486–92. doi: 10.1053/j.seminoncol.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. The New England journal of medicine. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Xin HW, Ambe CM, Hari DM, Wiegand GW, Miller TC, Chen JQ, et al. Label-retaining liver cancer cells are relatively resistant to sorafenib. Gut. 2013 doi: 10.1136/gutjnl-2012-303261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra BB, Tiwari VK. Natural products: an evolving role in future drug discovery. European journal of medicinal chemistry. 2011;46:4769–807. doi: 10.1016/j.ejmech.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 9.Schumacher M, Kelkel M, Dicato M, Diederich M. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2010. Molecules. 2011;16:5629–46. doi: 10.3390/molecules16075629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kupeli E, Kosar M, Yesilada E, Husnu K, Baser C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life sciences. 2002;72:645–57. doi: 10.1016/s0024-3205(02)02200-2. [DOI] [PubMed] [Google Scholar]
- 11.Nam S, Xie J, Perkins A, Ma Y, Yang F, Wu J, et al. Novel synthetic derivatives of the natural product berbamine inhibit Jak2/Stat3 signaling and induce apoptosis of human melanoma cells. Molecular oncology. 2012;6:484–93. doi: 10.1016/j.molonc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Tan Y, Wu G, Liu L, Wang Y, Luo Y, et al. Berbamine overcomes imatinib-induced neutropenia and permits cytogenetic responses in Chinese patients with chronic-phase chronic myeloid leukemia. International journal of hematology. 2011;94:156–62. doi: 10.1007/s12185-011-0887-7. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Liu Q, Zhang Y, Liu K, Yu P, Liu K, et al. Suppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of action. Molecular cancer. 2009;8:81. doi: 10.1186/1476-4598-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu R, Dong Q, Yu Y, Zhao X, Gan X, Wu D, et al. Berbamine: a novel inhibitor of bcr/abl fusion gene with potent anti-leukemia activity. Leukemia research. 2006;30:17–23. doi: 10.1016/j.leukres.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Gu Y, Chen T, Meng Z, Gan Y, Xu X, Lou G, et al. CaMKII gamma, a critical regulator of CML stem/progenitor cells, is a target of the natural product berbamine. Blood. 2012;120:4829–39. doi: 10.1182/blood-2012-06-434894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, et al. CD13 is a therapeutic target in human liver cancer stem cells. The Journal of clinical investigation. 2010;120:3326–39. doi: 10.1172/JCI42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng Z, Wang Y, Wang L, Jin W, Liu N, Pan H, et al. FXR regulates liver repair after CCl4-induced toxic injury. Mol Endocrinol. 2010;24:886–97. doi: 10.1210/me.2009-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng Z, Fu X, Chen X, Zeng S, Tian Y, Jove R, et al. miR-194 is a marker of hepatic epithelial cells and suppresses metastasis of liver cancer cells in mice. Hepatology. 2010;52:2148–57. doi: 10.1002/hep.23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature reviews Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 20.Ma S, Tang KH, Chan YP, Lee TK, Kwan PS, Castilho A, et al. miR-130b Promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell. 2010;7:694–707. doi: 10.1016/j.stem.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. Lupeol targets liver tumor-initiating cells through phosphatase and tensin homolog modulation. Hepatology. 2011;53:160–70. doi: 10.1002/hep.24000. [DOI] [PubMed] [Google Scholar]
- 22.Ozcan L, Wong CC, Li G, Xu T, Pajvani U, Park SK, et al. Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity. Cell metabolism. 2012;15:739–51. doi: 10.1016/j.cmet.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nature reviews Neuroscience. 2012;13:169–82. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bers DM. CaMKII inhibition in heart failure makes jump to human. Circulation research. 2010;107:1044–6. doi: 10.1161/CIRCRESAHA.110.231902. [DOI] [PubMed] [Google Scholar]
- 25.Bui JD, Calbo S, Hayden-Martinez K, Kane LP, Gardner P, Hedrick SM. A Role for CaMKII in T Cell Memory. Cell. 2000;100:457–67. doi: 10.1016/s0092-8674(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 26.McGargill MA, Sharp LL, Bui JD, Hedrick SM, Calbo S. Active Ca2+/calmodulin-dependent protein kinase II gamma B impairs positive selection of T cells by modulating TCR signaling. Journal of immunology. 2005;175:656–64. doi: 10.4049/jimmunol.175.2.656. [DOI] [PubMed] [Google Scholar]
- 27.Nitzki F, Zibat A, Konig S, Wijgerde M, Rosenberger A, Brembeck FH, et al. Tumor stroma-derived Wnt5a induces differentiation of basal cell carcinoma of Ptch-mutant mice via CaMKII. Cancer Res. 2010;70:2739–48. doi: 10.1158/0008-5472.CAN-09-3743. [DOI] [PubMed] [Google Scholar]
- 28.Si J, Mueller L, Collins SJ. CaMKII regulates retinoic acid receptor transcriptional activity and the differentiation of myeloid leukemia cells. J Clin Invest. 2007;117:1412–21. doi: 10.1172/JCI30779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamaeva OA, Kim J, Feng G, McDonald JM. Calcium/calmodulin-dependent kinase II regulates notch-1 signaling in prostate cancer cells. Journal of cellular biochemistry. 2009;106:25–32. doi: 10.1002/jcb.21973. [DOI] [PubMed] [Google Scholar]
- 30.Si J, Collins SJ. Activated Ca2+/calmodulin-dependent protein kinase IIgamma is a critical regulator of myeloid leukemia cell proliferation. Cancer research. 2008;68:3733–42. doi: 10.1158/0008-5472.CAN-07-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Yao M, Li N, Wang C, Zheng Y, Cao X. CaMKII promotes TLR-triggered proinflammatory cytokine and type I interferon production by directly binding and activating TAK1 and IRF3 in macrophages. Blood. 2008;112:4961–70. doi: 10.1182/blood-2008-03-144022. [DOI] [PubMed] [Google Scholar]
- 32.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. The Journal of clinical investigation. 2009;119:2925–41. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He G, Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell research. 2011;21:159–68. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez-Monterrey I, Sala M, Rusciano MR, Monaco S, Maione AS, Iaccarino G, et al. Characterization of a selective CaMKII peptide inhibitor. Eur J Med Chem. 2013;62:425–34. doi: 10.1016/j.ejmech.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 35.Komiya M, Asano S, Koike N, Koga E, Igarashi J, Nakatani S, et al. Synthesis and structure based optimization of 2-(4-phenoxybenzoyl)-5-hydroxyindole as a novel CaMKII inhibitor. Bioorg Med Chem. 2012;20:6840–7. doi: 10.1016/j.bmc.2012.09.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.