Abstract
Current data support a role for gut colonization in maintaining balanced mucosal and systemic immune responses and have suggested aberrant innate immune recognition of enteric bacteria as an initiator of the adaptive immune damage associated with inflammatory bowel disease (Crohn's disease and ulcerative colitis). In fact, data from human studies and experimental mouse models have implicated transformation of the gut microbiota from a beneficial symbiotic state to one of imbalance or “dysbiosis” in the pathogenesis of several autoinflammatory diseases, including allergic skin and respiratory disorders, rheumatoid arthritis, type I diabetes, and colorectal cancer. The host has evolved to co-exist and maintain a mutualistic relationship with the commensal microbes of the gut, and it is the function of the host innate immune system to initiate and maintain this homeostasis, while retaining the ability to respond appropriately to pathogenic organisms. In this review, we discuss the molecular and cellular interactions of the mucosal immune system that decide this delicate balance of mutualism. Furthermore, we will highlight the role of dendritic cells in preserving this precarious balance and how gene products of commensal microbes may play an integral role in re-establishing this balance once it has gone awry.
Introduction
The human colon is estimated to contain more than 70% of all the microbes in the body with a dense and diverse bacterial consortium of approximately 1012 microorganisms per gram of luminal contents, of which more than 99% are anaerobes and include the phyla, Firmicutes (64%), Bacteroidetes (23%), Proteobacteria (8%), and Actinobacteria (3%) (Fig. 1) (Whitman and others 1998; Hakansson and Molin 2011; Barengolts 2013). The gastrointestinal (GI) tract, as a whole, is a nutrient-rich environment with abundant niches and habitats, and surface areas that approximate the size of a tennis court, making it an optimal site for bacterial colonization (Ley and others 2006; Sekirov and others 2010). The relationship between this microbial community that colonizes the GI tract and the host is one of reciprocity, as these microorganisms help shape the host immune system. Case in point, germ-free (GF) animals have poorly developed gut-associated lymphoid tissues (GALT), including fewer T cells, dendritic cells (DCs), weaker antibody responses, and impaired oral tolerance (Dongarra and others 2013). Furthermore, disruption of the normal balance of this commensal bacterial community or “dysbiosis” is associated with an increased risk of infection and the development of inflammatory diseases, including allergic skin and respiratory disorders, rheumatoid arthritis, type I diabetes, and colorectal cancer (CRC) (Sobhani and others 2011; Smelt and others 2012).
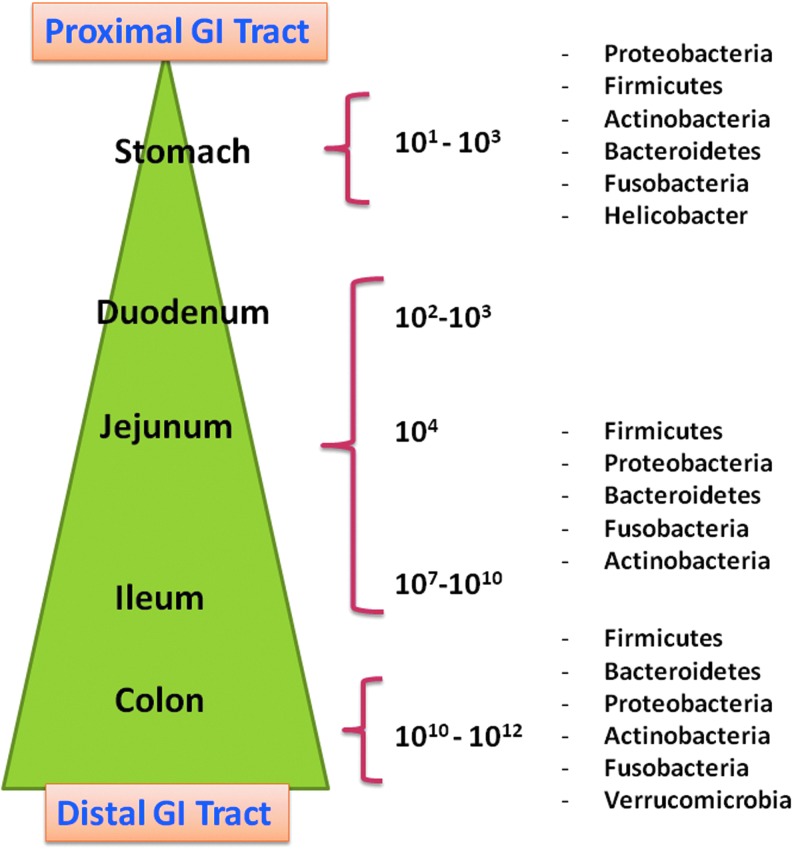
FIG. 1.
Composition of intestinal microbiome in the gastrointestinal tract. The estimated numbers of colony-forming units of bacteria/gram of luminal contents and most prevalent bacterial phyla in that location are shown. The numbers are low in the gastric juices of the stomach and then steadily increase abroad, with the highest numbers found in the colon (Hakansson and Molin 2011).
For example, colonization with the gut commensal, Escherichia coli NC101, promoted invasive colorectal carcinoma in GF, colitis-susceptible Il-10−/− mice treated with the colon-specific carcinogen, azoxymethane (Arthur and others 2012). This promotion of tumorigenesis was related to the DNA-damaging, polyketide synthase (pks) pathogenicity island of the bacteria, and mucosal pks+ E. coli were found in a high percentage of inflammatory bowel diseases (IBD) and CRC patients. These data suggest that colitis could promote tumorigenesis by altering microbial composition and inducing the expansion of microorganisms with the ability to damage DNA (Arthur and others 2012).
The state of dysbiosis can be induced by infection, inflammation, host genetics, drugs, and diet, and can result in increased numbers of Gram-negative bacteria and translocation of their gene products such as lipopolysaccharide (LPS) across inflamed intestinal epithelia, leading to systemic inflammation (Chassaing and Darfeuille-Michaud 2011; Honda and Littman 2012). Moreover, investigators have found an association between the gut microbiota and metabolic homeostasis, with reduced bacterial diversity, differential gene expression, and metabolic pathways in the microbiome of obese individuals versus their lean counterparts (Turnbaugh and others 2009).
Understanding the gut microbiome is critical in order to appreciate its influence on mucosal immunity and the pathology of nutritional and GI diseases; and many factors, including age, genetics, and diet, can influence its composition (Gill and others 2006). Some investigators have suggested that the microbiome of most people can be categorized into 3 “enterotypes” based on the dominant genera, Bacteroides, Prevotella, or Ruminococcus (Arumugam and others 2011), and that these enterotypes or patterns are influenced primarily by the diet, with long-term consumption of protein and animal fat associated with Bacteroides and carbohydrate consumption linked with the Prevotella enterotype (Wu and others 2011). Indeed, consumption of a diet which is high in saturated (milk) fat altered bile composition and promoted the expansion of a pathogenic intestinal bacterium and a pro-inflammatory Th1 response with increased colitis in genetically susceptible mice that lack the anti-inflammatory cytokine, IL-10 (Devkota and others 2012). Recently, the notion of different and distinct enterotypes has been debated, with some investigators favoring the idea of a “continuum or gradient” of genera and species rather than discontinuous groups or enterotypes (Jeffery and others 2012; Shanahan 2012).
Increased awareness that an altered microbiome plays a role in the pathogeneses of many disease processes has led to the hypothesis that improving the health of the microflora can result in decreased disease activity (Sanders 2011). Here, we focus on intestinal microbes and their effects on immune modulation, and the key role that DCs play in the delicate balance between regulation and inflammation to shape the immune response at mucosal surfaces.
Intestinal Microbiota
The general mechanisms by which commensal microbes are thought to benefit the host include maintaining the integrity of the intestinal epithelial barrier, promoting gut microbial diversity, inhibiting pathogen attachment and proliferation, modulating pain perception via differential expression of opioid receptors, and regulating the innate and adaptive immune responses by suppressing pro-inflammatory cytokine production (Reiff and Kelly 2010; Andrews and Tan 2012). A number of in vitro systems and animal models are currently under study to evaluate orally administered microbes and to better predict their effects on immune modulation for potential prophylactic or therapeutic use in patients suffering from IBD, atopic dermatitis, or rheumatoid arthritis (Vanderpool and others 2008; Gad and others 2011). Understanding the complicated nexus between the microbiota, the host immune system, and therapeutically administered gut microbes is critical to the development of innovative, effective strategies that target the intestinal microbiome. However, as investigators develop therapeutic inventions in the manipulation of the microbiota, it should be considered that typically, large numbers of viable microbes (108 to 109 viable bacteria per daily dose) are necessary to induce a measurable response in the host, and that administration of these supplements should closely resemble that of normal food consumption (van Baarlen and others 2011).
The commensal microbes in the gut far outnumber the host epithelial and immune cells; thus, a dietary microorganism could achieve transient dominance in the intestines with the appropriate dose (Booijink and others 2010; Bron and others 2012). Conventional treatments for IBD have traditionally focused on suppressing the immune system with the administration of steroids and/or biologics such as anti-tumor necrosis factor (TNF) antibodies; however, the concept of restoring the balance of the commensal microbiota and eliminating pro-inflammatory bacterial antigens in the gut has raised the interest of clinicians and patients in this line of therapy (Veerappan and others 2012). The beneficial effects of commensal microbes as adjunct treatment modalities for patients who do not respond to standard therapy with intestinal inflammatory diseases are important to explore, especially with children, in whom the use of steroids could lead to further growth retardation (Meijer and Dieleman 2011).
Innate Intestinal Immunity
The innate immune system is the first line of the host defense and is critical to the development of the adaptive immune response through the activation and expansion of T lymphocytes. The mucosae of the GI, respiratory, and genitourinary tracts are the main entry points for pathogens; thus, effective immune responses at these surfaces are crucial to prevent infection. However, regulatory mechanisms should also exist to avert damaging inflammatory reactions against benign antigens (Ruane and Lavelle 2011). Consequently, mammals have evolved to efficiently respond to invading pathogens while preventing destructive inflammatory responses against constituents of the diet and the commensal microbiome of the gut. Usually, the host immune system is tolerant of the nonself, dietary, and environmental antigens in the intestine and the large and diverse microflora that represent the commensal gut microbiota. However, unbalanced or hyperactive immune responses against luminal antigens, including commensal microbes, are thought to underlie the pathogenesis of IBD (Crohn's disease and ulcerative colitis) and lead to severe inflammation and tissue damage (Guarner 2005). In fact, a recent investigation identified pathogen-driven selection as the major driving force for human genetic adaptation to local environments (Fumagalli and others 2011; Mayer and others 2012). Among the genes targeted by pathogen-driven selection, there was an overrepresentation of loci associated with autoimmune diseases, such as type I diabetes, ulcerative colitis, celiac disease, and multiple sclerosis, loci that are likely related to pathogen resistance/susceptibility (Fumagalli and others 2011).
Innate immune receptors that are important for the recognition of conserved motifs derived from intestinal microbes, including commensal bacteria or potential pathogens, comprise Toll-like receptors (TLRs), nucleotide oligomerization domain (NOD)-like receptors, retinoic acid-inducible gene I (RIG)-like receptors, and C-type lectin receptors (CLRs) (Lavelle and others 2010). TLRs play particularly critical roles in microbial recognition, homeostasis, and immune defense in the gut, and are found on epithelial cells, stromal cells, and immune cells such as DCs, monocytes, macrophages, and B lymphocytes (Capelluto 2012). These receptors are expressed on the surface of the cell membrane and within the cytosol, on the endoplasmic reticulum, endosomes, and lysosomes (Blasius and Beutler 2010). Each TLR is able to recognize a discrete set of microbial ligands; TLR2/TLR6 recognizes lipopeptides such as lipoteichoic acid (LTA); TLR3 recognizes viral-derived dsRNA; TLR4 recognizes LPS; TLR5 recognizes flagellin; and TLR9 recognizes CpG DNA. (Cario 2010). In the healthy gut, TLR3 and TLR5 seem to be constitutively expressed, whereas TLR2 and TLR4 are expressed at very low levels, suggesting that the expression of these 2 receptors is carefully regulated to avoid autoinflammatory immune activation in response to commensal microbes (Kelly and Mulder 2012).
TLR expression is also spatially regulated within the gut to maintain colonic homeostasis. Thus, while TLR9 is expressed on the apical surface of epithelial cells that face the intestinal lumen, inflammatory signaling, as evidenced by NF-κB activation, only occurs when the cognate ligand is bound by TLR9 that is expressed on the basolateral surface of the epithelial cells which face adjacent epithelium and the underlying basement membrane. (Lee and others 2006). In support of the importance of TLR signaling in maintaining tolerance, multiple studies of dextran sodium sulfate (DSS)-induced experimental models of colitis have shown that mice lacking TLR2, TLR4, TLR9, or the TLR adaptor molecule, myeloid differentiation primary response gene 88 (MyD88), are very susceptible to induced colitis (Nishio and Honda 2012). MyD88 is an adaptor molecule employed by all TLRs except TLR3 that transmits signals, resulting in the induction of inflammatory cytokines via the activation of NF-κB and MAP kinase (Kawai and Akira 2011). Negative modulators of TLR signaling such as Toll-interacting protein are also preferentially expressed in intestinal epithelial cells and are another mechanism utilized in the gut to promote a tolerant environment to enteric bacteria (Capelluto 2012; Kubinak and Round 2012). Another important class of receptors, CLRs, binds to Ca2+ or carbohydrate ligands of self or nonself origin. Similar to TLRs, CLR expression varies among distinct DC subsets (Hammer and Ma 2013). In human DCs, certain species of lactobacilli, Lactobacillus reuteri and Lactobacillus casei, induce DC maturation and the induction of regulatory T lymphocytes (Tregs) and IL-10 production via the CLR, DC-specific ICAM3-grabbing nonintegrin (DC-SIGN) (Smits and others 2005; Bron and others 2012).
Intestinal DCs
The exact manner in which the intestinal immune system discriminates between pathogenic and commensal microorganisms is still unclear; however, we know that Foxp3+ Tregs and the professional antigen-presenting cells, DCs, play critical roles in maintaining tolerance and immune homeostasis in the gut. Intestinal DCs appear to generally favor the induction of Th2 or Treg responses (Coombes and Maloy 2007). DCs are at the interface between the innate and adaptive immune systems and are strategically positioned within the intestine, residing in the GALT, including mesenteric lymph nodes (MLNs) and Peyer's patches, as well as being dispersed throughout the subepithelial lamina propria (Varol and others 2010). DCs interact with bacteria that have gained access to the GALT via transcytosis across specialized enterocytes called microfold or M cells, and sense these microbes through pattern recognition receptors such as TLRs.
DCs as a family express the integrin, CD11c (Hammer and Ma 2013). However, CD11c expression alone cannot definitively identify a cell as a DC, as there are CD11c+ intestinal eosinophils and a “controversial” population of gut resident CX3CR1+CD103− “DCs” (Pabst and Bernhardt 2010). Lamina propria DCs are further classified by virtue of their expression of the α-integrin, CD103. CX3CR1−CD103+ DCs differentiate from conventional myeloid DC precursors in an Flt3L-dependent manner, while CX3CR1+CD103− DCs express the fractalkine receptor, CX3CR1, and are derived from monocytes in a GM-CSF-dependent process (Varol and others 2009; Rescigno 2011). The primary role of CX3CR1+CD103− DCs is to sample intestinal luminal antigens across the epithelial cell layer; it is worth noting that GF mice have a decreased number of these CX3CR1+CD103− DCs, which also exhibit a lower number of intraepithelial protrusions when compared with conventionally housed animals (Niess and Adler 2010; Swiatczak and Rescigno 2012). Unlike their CD103+ counterparts, CX3CR1+CD103− DCs have been reported to not typically migrate and have limited ability to activate T cells; they are, however, involved in the re-stimulation of T cells after challenge (Swiatczak and Rescigno 2012). Interestingly, it has recently been shown that CX3CR1hi cells are able to traffic noninvasive Salmonella to MLNs in a CCR-7-dependent manner, and to induce T-cell responses and IgA production in the absence of Myd88 or under conditions of antibiotic-induced dysbiosis (Diehl and others 2013). The authors of this study concluded that the Myd88-dependent detection of commensal gut bacteria functions to reduce bacteria trafficking to the MLNs by the CX3CR1hi population in order to down-regulate intestinal immune responses and autoinflammation (Diehl and others 2013).
CX3CR1+ DCs are exposed not only to luminal antigens, but also to circulating antigens because of their proximity to the fenestrated capillaries that run through the lamina propria before entering the draining lymphatics. In several elegant experiments, Reinecker and colleagues recently demonstrated that resident CX3CR1+ DCs in the lamina propria could process blood-derived antigens and cross-present them to naïve CD8+ T cells, initiating their differentiation into intraepithelial lymphocytes that express IL-9, IL-10, and IL-13, and regulate CD4+ T-cell activation in the small intestine (Chang and others 2013).
Conversely, it is the primary role of CX3CR1−CD103+ DCs to migrate and transport microbial antigens from the lamina propria to the MLNs in a CCR7-dependent manner. Expression of CCR7 is required for the CD103+ DCs to reach the interfollicular T-cell areas of the draining lymph nodes, where they can interact with T cells (Jang and others 2006), and induce the expression of the gut homing receptors, CCR9 and the α4β7 integrin on naïve T cells during priming in the steady state and during inflammation (Bar-On and others 2011; Swiatczak and Rescigno 2012). CCR9 and α4β7 are essential for T-cell migration from the lymph node to the small intestine (Ruane and Lavelle 2011). Due to the many phenotypic and functional differences between CX3CR1+CD103− DCs and CX3CR1−CD103+ DCs, it is not surprising that some investigators have proposed that CX3CR1+CD103− DCs should not be classified as “genuine” DCs, but rather be considered a group of phagocytes in between a macrophage and a DC (Hume 2008; Pabst and Bernhardt 2010; Swiatczak and Rescigno 2012). Further studies are required that focus on intestinal DC subsets and their function in co-stimulation/co-regulation, luminal antigen sampling, and the activation of T-cell subsets.
DC subsets also express distinct and diverse sets of TLRs (Kinnebrew and Pamer 2012), especially in the lamina propria of the small intestine (Fujimoto and others 2011). For example, bone marrow-derived CD11c+ DCs express high levels of TLR4 in order to efficiently recognize Gram-negative pathogens; conversely, lamina propria CD11c+ DCs express significantly less TLR4 so as not to respond to the LPS that is a constant presence in the gut lumen (Cerovic and others 2009). However, not all TLRs are down-regulated in intestinal DCs. Murine CD11b+ CD11c+ small intestinal lamina propria DCs express TLR5 and respond to the cognate ligand, flagellin, by inducing IgA+ plasma cells and antigen-specific Th17 and Th1 subsets to generate protective immunity against pathogens, not tolerance (Uematsu and others 2008). A minor population of plasmacytoid DCs (pDCs) is also found within the intestinal lamina propria; pDCs express the nucleic acid sensors, TLR7 and TLR9, and are capable of high levels of type I interferon (IFN) (Belz and Nutt 2012). IFNα/β secretion by pDCs can promote the maturation and differentiation of myeloid DCs, increase the cytotoxicity of CD8+ T cells and natural killer cells, and enhance the production and class-switching of antibodies (Ronnblom and Eloranta 2013). TLR2, 3, 4, 5, 7, 8, and 9 are the main TLRs that are able to recognize microbial products, and all of these receptors can play a role in the induction of IFNα/β, except for TLR5 (Monroe and others 2010). TLR9 recognizes unmethylated CpG motifs, which are common in bacterial and viral DNA (Walsh and others 2012), and mediates protection against the development of experimental colitis via the induction of anti-inflammatory IFNα/β by bone marrow-derived DCs (Katakura and others 2005). Interestingly, a polymorphism in the promoter of TLR9 has also been associated with Crohn's disease (Torok and others 2004).
Soluble Mediators of Tolerance in the Gut
The intestinal cytokine milieu is characterized by high levels of anti-inflammatory IL-10, transforming growth factor (TGF-β), and the vitamin A metabolite, retinoic acid (RA), which is produced by epithelial, stromal, and immune cells, especially CD103+ DCs; in fact, mice deficient in IL-10 and TGF-β develop spontaneous colitis, (Abraham and Medzhitov 2011). RA production is considered a specialized function of lamina propria CD103+ DCs, although stromal cells in the MLNs can also produce this metabolite (Bar-On and others 2011). RA plays a critical role in mucosal immunity and is involved in the induction of gut-homing specificity in activated T cells, inducible Tregs (iTregs), and IgA-secreting plasma cells, in addition to suppressing the differentiation of Th17 cells (Molenaar and others 2011). In fact, the unique ability of CD103+ DCs to generate iTregs and induce gut-homing receptor expression is dependent on their production of RA (Stock and others 2013). CD103+ DCs from the lamina propria and the MLNs express much higher levels of the RA producing enzyme, retinaldehyde dehydrogenase (RALDH), than do CD103− DCs (Coombes and others 2007). It has also been shown that murine intestinal lamina propria DCs secrete the aforementioned anti-inflammatory mediators, IL-10, TGF-β, and RA, in a β-catenin dependent manner, unlike splenic DCs. Thus, intestinal and peripheral DCs utilize distinct tolerogenic molecular pathways, further emphasizing the unique role of intestinal DCs in mediating oral tolerance toward dietary antigens and gut commensal microorganisms (Manicassamy and others 2010).
In vitro, the cytokine, TGF-β, has been implicated in the maintenance of human monocyte-derived DCs and mouse bone marrow-derived DCs in an immature and tolerogenic state by means of low expression levels of MHC II and co-stimulatory molecules, and decreased IL-12 production (Ramalingam and others 2012). In vivo, loss of TGF-β signaling in DCs renders these cells more pro-inflammatory and leads to the development of autoimmune disease in mice (Ramalingam and others 2012). Autocrine TGF-β has also been shown to maintain long-term tolerance in murine pDCs by sustaining the activation of indoleamine 2,3-dioxygenase (IDO), the immunomodulatory enzyme that catalyzes the degradation of tryptophan and generates toxic kynurenine metabolites which limit activated T-cell responses (Pallotta and others 2011).
Cellular Mediators of Tolerance in the Gut
Natural Tregs (nTregs) are Foxp3+CD4+CD25+ T cells arising from the thymus, while inducible Tregs (iTregs) are CD4+ T cells generated in the periphery through induction of Foxp3 after antigen stimulation or co-stimulation from antigen presenting cells; both subsets play important roles in suppressing self-reactive T cells and the onset of autoimmunity (Yadav and others 2012). Natural Tregs are thought to prevent autoimmunity by increasing the threshold for response to self antigens, while iTregs suppress the immune response in chronically inflamed and/or transplanted tissues, tumors, and in the lamina propria of the gut and in the MLNs during induction of oral tolerance to food allergens and commensal microbes (Curotto de Lafaille and Lafaille 2009). The development of iTregs is dependent on the secretion of TGF-β, IL-2, and RA in the intestinal environment, and RA is necessary for sustained expansion of iTregs in the gut (Hadis and others 2011). Indeed, many of the immunomodulatory effects of commensal microbes are attributed to the induction and expansion of Tregs (Molloy and others 2012).
Murine and human Tregs express the transcription factor, Foxp3, which regulates the expression of approximately 1100 genes, many of which are responsible for these cells' regulatory characteristics, such as CD25, cytotoxic T lymphocyte antigen-4 (CTLA-4), and the absence of IL-2 production. Tregs suppress pro-inflammatory immune responses through the production of anti-inflammatory cytokines, including IL-10 and TGF-β, and the surface expression of inhibitory molecules, such as CTLA-4 and lymphocyte activation gene-3 (LAG-3) (Wing and Sakaguchi 2012). The transfer of CD4+CD25+ Tregs efficiently attenuated established colitis in an experimental model of the disease (Mottet and others 2003), and a deficiency of this cell population has been found in patients with ulcerative colitis (Takahashi and others 2006). Although these studies highlight the role of thymic-derived or natural Tregs, subsequent studies have emphasized the importance of iTregs for disease resolution (Haribhai and others 2009). The induction of Tregs by regulatory DCs in the gut seems to be particularly important for microbial and colonic health. In support of this idea, colonic Tregs were found to express T-cell receptor repertoires that were distinct from those found on Tregs from other organs and were also specific for antigens encoded by commensal microbes (Lathrop and others 2011). A number of groups have also reported that Tregs may become pro-inflammatory in certain microenvironments, simultaneously expressing Foxp3 and the Th17 defining transcription factor, RORγt, and secreting IL-17 (Hovhannisyan and others 2011; Wing and Sakaguchi 2012).
Gut Microbes and Immune Modulation
Gut microbes interact with the intestinal environment via cellular components such as LTAs, polysaccharides, and DNA, and can have direct antibacterial effects on intestinal pathogens via the production of bacteriocins and conjugated linoleic acid (O'Shea and others 2012). These microorganisms regulate immunomodulatory functions through their interactions with TLRs expressed on the surfaces of epithelial cells and DCs (Abreu 2010), and different bacteria stimulate different and distinct TLRs on host cells (Vanderpool and others 2008). For example, “VSL#3,” which is a proprietary preparation comprising of 8 different Gram-positive species (Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, L. casei, Lactobacillus plantarum, Bifidobacterium longum, Bifidobacterium infantis, Bifidobacterium breve, and Streptococcus salivarius subsp. thermophilus), reduced DSS-induced colitis in wild-type, but not TLR9-deficient mice, indicating an important role for this intracellular TLR in VSL#3's immune modulation (Rachmilewitz and others 2004). Moreover, 6 weeks of VSL#3 treatment in 32 human patients with mild to moderate ulcerative colitis, which was not responding to conventional therapy, resulted in a combined induction of remission/response rate of 77% and no adverse effects reported (Bibiloni and others 2005).
The mechanisms that underlie this protective effect were recently studied in a rat experimental model of DSS-induced colitis. Administration of VSL#3 for 7 days resulted in a decrease in TNF-α and IL-6 and increased IL-10 expression in excised colonic tissue. This decrease in pro-inflammatory cytokines and increase in the anti-inflammatory cytokine, IL-10, was also seen in the sera of these animals and was found to result from inhibition of the PI3K/Akt and NF-κB signaling pathways (Dai and others 2013). In addition to the effects of administration of whole cell preparations of VSL#3, DNA alone isolated from this Gram-positive mixture decreased TNF and IFN-γ production and LPS-induced IL-8 secretion in murine splenocytes and colonic epithelial cells in vitro and in vivo (Jijon and others 2004). Increased expression levels of the chemotactic cytokine, IL-8, have been shown to correlate with disease activity in inflamed mucosal biopsies from ulcerative colitis patients (Gologan and others 2012).
Decreased exposure to enteric microbes due to better sanitation and vaccination, the increased use of antibiotics, and adoption of a high-fat, Western diet has also been postulated to contribute to the increasing incidence of food allergies in developed countries (Feehley and others 2012). Accordingly, VSL#3 has also been evaluated in several murine models of allergy and anaphylaxis. Oral VSL#3 treatment of mice sensitized to the major food allergen, shrimp tropomyocin, resulted in the shifting of a polarized Th2 response to a Th1/Treg response, with decreased IL-4, IL-5, and IL-13 production and increased Foxp3, IL-10, IL-27, IFNγ, and TGF-β in the jejunum of the mice after intragastric challenge with the antigen. (Schiavi and others 2011).
Administration of another microbial mixture, “IRT5,” consisting of L. acidophilus, L. casei, L. reuteri, Bifidobacterium bifidium, and Streptococcus thermophilus, induced high levels of IL-10, TGF-β, and IDO in MLN CD11c+ DCs, which promoted the generation of Foxp3 Tregs in inflamed areas using experimental murine models of IBD, atopic dermatitis, and rheumatoid arthritis (Kwon and others 2010). Furthermore, oral administration of IRT5 significantly reduced clinical symptoms such as weight loss, body trembling, and grip strength in a mouse model of myasthenia gravis, which is a T-cell-dependent antibody-mediated autoimmune disease against the acetylcholine receptor (AChR) at the neuromuscular junction (Chae and others 2012). This effect was mediated by down-regulation of the effector functions of AChR-reactive T and B lymphocytes. Administration of IRT5 decreased AChR-reactive lymphocyte proliferation, anti-AChR reactive IgG levels, and pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-6, and IL-17. Down-regulation of inflammatory molecules in AChR-reactive lymphocytes after IRT5 administration was modulated by regulatory DCs that expressed high levels of TGF-β, IL-10, arginase 1, and the RA-producing gene, aldh1a2. Moreover, DCs isolated from the IRT5-fed mice effectively induced the differentiation of CD4+ T cells into CD4+Foxp3+ iTregs compared with DCs from control animals. These data suggest that oral administration of IRT5 may modulate systemic antibody-mediated autoimmune diseases such as myasthenia gravis (Chae and others 2012).
Lactobacilli
In particular, microbes belonging to the genus, Lactobacillus, have been clinically tested with favorable outcomes for the relief of symptoms of immunopathologies, including IBD and atopy (Dongarra and others 2013). This is due, in part, to induced changes in the immune system, as certain Lactobacillus species are known to stimulate DCs to produce pro-inflammatory and anti-inflammatory cytokines that direct T-cell responses (Christensen and others 2002; Mohamadzadeh and others 2005; Konstantinov and others 2008). Given the species-specific differential signaling of lactobacilli cell surface components, including LTA and surface layer proteins (Slps), a thorough evaluation of these surface molecules is critical to achieve appropriate immune responses. Dissecting the consequences of host immune cell-microbial interactions is of particular importance in cases in which pre-existing inflammation or the propensity for inflammation might be exacerbated or promoted.
Modification of the bacterial genome is one approach that is used to enhance the regulatory effects of the gut microbiota. For example, studies in our laboratory have demonstrated that oral treatment with a novel strain of L. acidophilus deficient in LTA is sufficient to alter the immune balance from pro-inflammatory to anti-inflammatory or regulatory. This shift in immune regulation effectively ameliorated inflammation-induced colitis and colonic polyposis in experimental murine models by down-regulating IL-12 and TNF-α, and enhancing IL-10 production by DCs (Fig. 2) (Mohamadzadeh and others 2011; Khazaie and others 2012). LTA is regarded as the Gram-positive counterpart of the potent pro-inflammatory Gram-negative stimulus, LPS, as both can trigger the secretion of pro-inflammatory cytokines and the activation of NF-κB and other pro-inflammatory mediators in multiple cell types (Lebeer and others 2012). LTA is a glycolipid found in the cell wall of many Gram-positive bacterial strains, including L. acidophilus, which is believed to facilitate the adhesion, colonization, and invasion of host cells (Reichmann and Gründling 2011). In addition to the likely role of LTA in Lactobacillus adhesion to mucosal surfaces, this molecule also promotes immune cellular activation via TLR2/TLR6 with CD14 and CD36 as co-receptors, which then activate downstream pro-inflammatory cytokine signaling cascades (Nilsen and others 2008; Chang and others 2010; Saber and others 2011). Notwithstanding, conflicting reports have suggested that LTA from certain Lactobacillus species induces anti-inflammatory cytokine production (IL-10), and results in the generation of pro-inflammatory mediators only in the context of pre-existing inflammatory conditions (e.g., co-culture with IFNγ) (Kaji and others 2010; Kang and others 2011). Taken together, these data suggest that the functions of LTA might differ between bacterial species (beneficial lactobacilli versus pathogenic), as well as depend on the status of the local cytokine microenvironment (steady state versus pro-inflammatory).
FIG. 2.
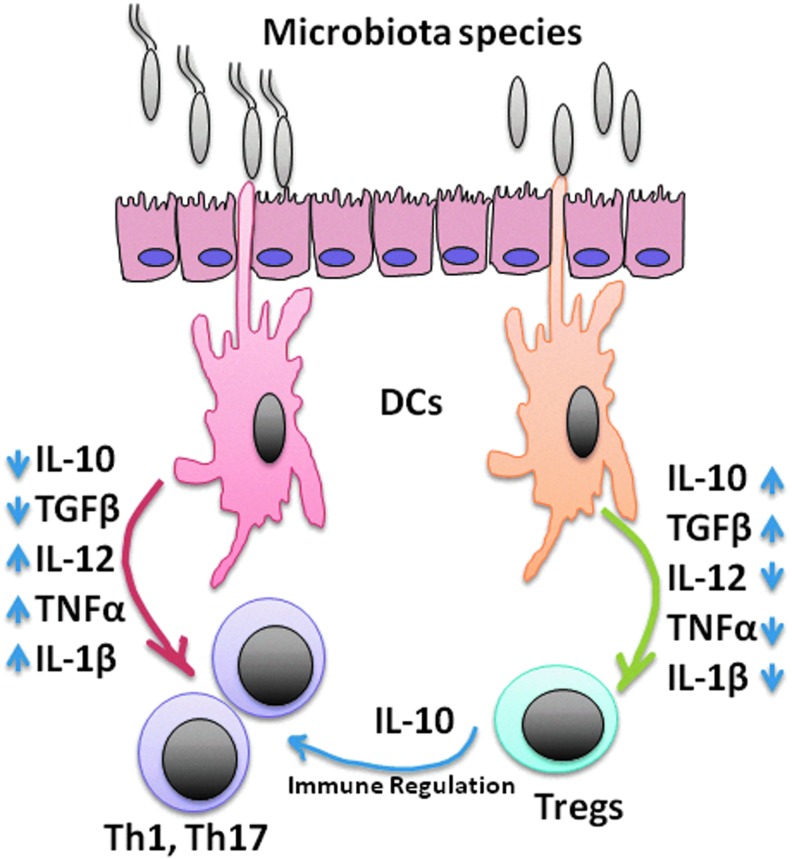
Differential activation of dendritic cells (DCs) by microbial communities. DCs can be activated by pathogenic bacteria to induce protective immunity via Th1/Th17 polarization; conversely, beneficial bacteria or their gene products can activate DCs to induce the development of Tregs, which will control the functions of protective Th1 and Th17 immune responses.
To clarify the in vivo effects of L. acidophilus-LTA, an L. acidophilus strain with a specific deletion of the gene encoding phosphoglycerol transferase, an enzyme required for the biosynthesis of LTA, was developed. As opposed to treatment with the wild-type strain, oral inoculation with LTA-deficient L. acidophilus not only prevented DSS and pathogenic T cell-induced colitis, but also significantly resolved established colitis, as measured by decreased percent weight loss, lower diarrhea and fecal occult blood scores, and reduced disease activity indices (Mohamadzadeh and others 2011). In addition, LTA-deficient L. acidophilus dramatically decreased colonic polyposis in genetically predisposed animals (Khazaie and others 2012). While protection from colitis in our studies correlated with an increase in IL-10-producing DCs and the number of iTregs (Mohamadzadeh and others 2011; Khan and others 2012), polyposis reversal coincided with an overall dampening of local and systemic immunity that was linked with restoration of Treg function and stability (Khazaie and others 2012). Importantly, pro-inflammatory iTregs have also been identified in CRC patients (Blatner and others 2012), further supporting the clinical applicability of LTA-deficient L. acidophilus in the treatment of intestinal disease given its potential ability to prevent the formation of pro-inflammatory Foxp3+RORγt+ Tregs.
Moreover, in vitro co-culture of DCs with LTA-deficient L. acidophilus led to a regulatory DC phenotype, as demonstrated by enhanced IL-10 secretion, decreased expression of co-stimulatory molecules, and decreased IL-12 and TNF-α production. Conversely, no beneficial effects were induced in IL-10−/− mice in vivo, delineating the important role of IL-10 in the control of pathogenic intestinal inflammation in our model, similar to the previous findings of others (Asseman and others 1999; Grangette and others 2005; Rubtsov and others 2008).
Activation of MAPK signaling pathways differentially controls features of both innate and adaptive immune responses (Zassadowski and others 2012). Increased IL-10 production by regulatory DCs has previously been found to be dependent on extracellular signal-regulated kinase (ERK)1/2 activation, while suppressed IL-12 secretion resulted from impaired p38 activation (Qian and others 2006). Indeed, significant and sustained ERK1/2 activation was measured in colonic tissues from mice orally treated with LTA-deficient L. acidophilus, whereas the wild-type strain promoted p38 phosphorylation (Saber and others 2011). Furthermore, DC stimulation with LTA-deficient L. acidophilus only weakly resulted in TLR2-dependent cytokine production and did not enhance the expression of this TLR; these data indicate that LTA is, in fact, the pro-inflammatory molecule which is most strongly associated with TLR2 signaling by L. acidophilus in DCs, and that the in vivo regulatory response noted after LTA-deficient L. acidophilus treatment is a direct consequence of its absence.
Another example of immunomodulation in the gut and its absolute specificity for a particular strain of bacteria or the differential expression of a surface molecule was recently published by Macho Fernandez and colleagues (2011). This laboratory found that the peptidoglycan (PGN) of Lactobacillus salivarius Ls33 mediated this strain's ability to protect against 2,4,6-trinitrobenzene sulfonic acid colitis in an NOD-2 dependent mechanism, while L. acidophilus NCFM could not confer protection. This was explained by the increased turnover of biologically active muropeptides within the PGN of L. salivarius, including M-tri-Lys, an NOD2 ligand that exhibits anti-inflammatory properties. The NOD2 receptor is primarily expressed by myeloid cells, including DCs, and binds the muramyl dipeptides of PGN, which are continuously released during cell wall synthesis throughout bacterial division and growth (Kinnebrew and Pamer 2012). The protection against colitis was accompanied in vivo by increased expression of the immunosuppressive molecule, IDO, in the colon, and in CD11c+ DCs within the MLNs. When comparing the ability to activate bone marrow-derived DCs in vitro, the PGN of both strains were poor inducers of the pro-inflammatory mediators, IL-12p70 or CCL3. In addition, only the PGN of L. salivarius induced high amounts of IL-10 by the DCs (Macho Fernandez and others 2011).
Modification of microbial dose is another approach by which immunomodulation is affected. Human monocyte-derived DCs cultured with a wide range of doses of the well characterized strain, Lactobacillus rhamnosus Lcr35, exhibited a strong dose-dependent increase in Th1/Th17 skewing cytokines (TNF-α, IL-1β, IL-12p40, IL-12p70, and IL-23), but only a modest increase in IL-10 production in DCs treated with the highest dose. At lower MOI, no changes in the expression of pro- or anti-inflammatory genes were detected. There was also a dose-dependent maturation of the DC membrane phenotype, with increased expression of CD86, CD83, HLA-DR, and TLR4, and decreased DC-SIGN, mannose receptor (MR), and CD14 in DCs treated with the highest dose (Evrard and others 2011). The MR is a marker of immature DCs in human monocyte-derived DCs and mouse bone marrow-derived DCs (Martinez-Pomares 2012). These data suggest that orally administered commensal microbes can result in very different effects on the immune system depending on the dose and frequency of administration. Manipulation of the dose may allow a certain microbe to be used for the treatment of different diseases that require enhancement of either pro- or anti-inflammatory responses (Evrard and others 2011).
Bifidobacteria
Differences in the composition of the intestinal microbiome likely underlie the symptoms of irritable bowel syndrome (IBS) by promoting abnormal bacterial fermentation in the colon (King and others 1998). Excessive amounts of H2S production from sulfate-reducing bacteria (SRB) in the lumen of the colon have been linked to increased GI motility, which is characteristic of IBS, as well as to the pathogenesis of UC and potentially CRC (Medani and others 2011). Butyrate is one of the primary products of colonic bacterial fermentation, and the respiration of human colonic epithelial cells is dependent on butyrate oxidation. H2S impairs this oxidation of butyrate and other short chain fatty acids, leading to pathologic changes in the colonocytes, including increased permeability (Fig. 3). The impairment of butyrate oxidation by H2S has been identified in both active and quiescent UC; however, evaluation of the pathophysiologic role of H2S is ongoing, as accurate and representative measurements of this water-soluble gas are difficult to ascertain, and interspecies differences in its biologic effects have complicated the investigations (Medani and others 2011).
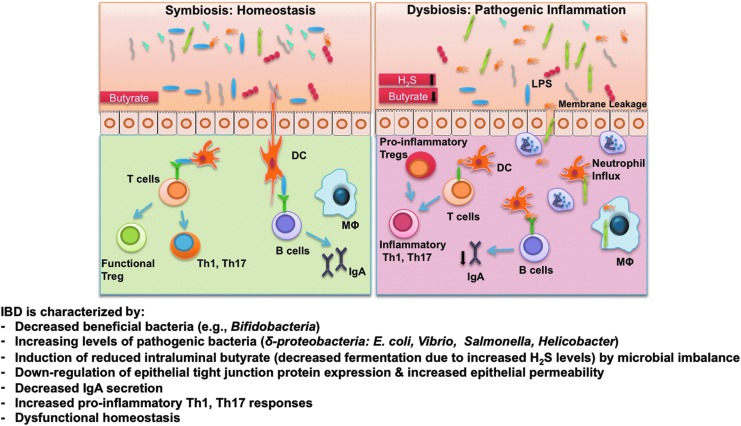
FIG. 3.
Overview of dysfunctional homeostasis that occurs during inflammatory bowel diseases (IBD). Microbial dysbiosis in IBD can be elicited by a reduced prevalence of putative beneficial bifidobacteria with concomitant increase in sulfate-reducing bacteria. The respiration of human colonocytes is dependent on butyrate oxidation. H2S impairs this oxidation of butyrate, leading to pathologic changes, including increased epithelial permeability and leakage, by which increased numbers of bacteria and their gene products (e.g., lipopolysaccharide [LPS]) enter the lamina propria, which demonstrates immune dysfunction due, in part, to low levels of IgA. Insufficient bacterial clearance may result in pattern recognition receptor (e.g., TLR, C-type lectins, NOD) stimulation, and increased levels of pro-inflammatory cytokines and stimulatory molecules in innate immune cells, which then activate pathogenic T-cell responses. In addition, dysfunctional regulatory responses that are dictated by regulatory DCs and Tregs lead to breakdown of immune tolerance, amplifying the recruitment of neutrophils and pro-inflammatory T-cell subsets (Th1, Th17, pro-inflammatory Tregs), all of which result in devastating disease progression.
Researchers have determined that patients with IBS also have significantly lower levels of beneficial bifidobacteria in duodenal brush and stool samples compared with healthy individuals (Kerckhoffs and others 2009). Recently, Weiss and others recently analyzed the cytokine profile of bone marrow-derived DCs stimulated by 27 different lactobacilli and 16 bifidobacteria. As previously mentioned, there are distinct species and strain specificities with microbe-induced immunoregulation; accordingly, these investigators found that different groups of lactobacilli have distinct and opposite effects on IL-12, TNF-α, and IFN-β secretion by DCs, while all bifidobacteria tested resulted in decreased production of those pro-inflammatory cytokines (Weiss and others 2011). Furthermore, B. bifidum Z9 down-regulated the pro-inflammatory and Th1 skewing cytokine profile induced by L. acidophilus NCFM in DCs; however, L. acidophilus NCFM could not modulate the B. bifidum Z9-induced cytokine response (Weiss and others 2010).
Different strains of intestinal bacteria have been found to promote the development of intestinal T lymphocytes by distinct mechanisms; therefore, B. breve and Lactobacillus casei were recently analyzed for their ability to induce IL-10 producing Tregs in the colon using several murine models (Jeon and others 2012). Increased IL-10+ CD4+ Tregs were induced with oral administration of B. breve, but not L. casei; this induction was mediated by intestinal CD103+ DCs via the TLR2/MyD88-dependent production of IL-10 and IL-27. Furthermore, B. breve supplementation significantly decreased intestinal inflammation in the mice. Since previous studies have shown that oral administration of B. breve failed to induce colonic Tregs in GF mice, the authors of this study postulated that B. breve may require the presence of other commensal bacteria to be recognized by intestinal DCs (Jeon and others 2012).
Probiotics
An altered microbiome can have a profound impact on both the local and systemic immune responses in the host. This realization has opened up new approaches to improve the health of the microflora to decrease disease activity; administration of gut commensal microbes may be such an intervention (Sanders 2011). Probiotics are defined as “live microorganisms which, when consumed in adequate amounts as part of food, confer a health benefit on the host” (Lilly and Stillwell 1965; Guarner and Schaafsma 1998). The administration of gut commensal microorganisms does not always involve the goal of dampening inflammation. In fact, certain gut microbes can also stimulate the immune system and host defense mechanisms. Thus, exogenous administration of microorganisms is also a promising therapeutic strategy to supplement antibiotics in the treatment of GI pathogens that colonize the gut, especially in immunocompromised patients (Kinnebrew and Pamer 2012). A meta-analysis of several clinical trials has shown an association between probiotic supplementation and reduced incidence of necrotizing enterocolitis, sepsis, and lower mortality in preterm infants (Deshpande and others 2010). An experimental model of necrotizing enterocolitis confirmed these results, as the pro-inflammatory cytokines, IFNα/β, IL-1β, IL-6, and IFNγ, were down-regulated in the ileum of neonatal rats supplemented with the probiotic, Saccharomyces boulardii lyo. Administration of prebiotics, which are nondigestible, but fermentable carbohydrates such as fructo- and galacto-oligosaccharides that stimulate the growth and activity of probiotic bacteria, also resulted in anti-inflammatory effects in this model (D'Souza and others 2012).
The Gram-positive genera, Lactobacillus, Bifidobacterium, and Streptococcus, as well the nonpathogenic Gram-negative, Escherichia coli Nissle 1917, and yeasts such as Saccharomyces are among the most commonly studied and commercially available probiotics (Andrews and Tan 2012; Tsai and others 2012). Probiotic bacteria have been shown to attenuate some of the clinical signs associated with inflammatory and autoimmune diseases, however, the modes of action are very species and strain specific (Yan and Polk 2011). Thus, selection of the appropriate microbe is critical for the success of these bacteria in mitigating disease, and many questions remain involving the nature of the cellular interactions and immunomodulation in the host with probiotic administration. Compounding this situation is the lack of any true regulatory body to ensure microbial viability in commercial preparations, the absence of a gold standard for bacterial cultivation methods, and little experimental proof that the probiotic strains in question reach the intestines in sufficient number (van Belkum and Nieuwenhuis 2007). Nonetheless, more rigorous mechanistic studies are required while focusing on probiotic bacteria and their effects on health in order to elucidate why clinical trials using probiotics do not demonstrate similar immune regulation.
Concluding Remarks
It is clear that intestinal DCs shape mucosal immunity by directing the induced T cell response. It is also clear that the gut microbiota and their gene products can impact intestinal DC function. Thus, accumulating evidence suggests that alteration of the composition of the microbiota can affect microbial-host interactions and immune homeostasis. Collectively, this review suggests the mechanisms by which beneficial gut commensal microbes can drive healthy immune function in an experimental setting. However, species and strain specificity, the health and immune status of the patient, the local cytokine microenvironment (steady state versus pro-inflammatory), and the composition of the microbiome are critical to be considered when selecting a microbe with which to develop a nutritional therapeutic intervention for disease. Furthermore, many of the health-promoting effects of gut microbes have been proposed for the prevention or treatment of certain immunopathologies, raising questions about the immunomodulatory consequences or rationale for the administration of gut commensal bacteria to healthy individuals.
Acknowledgments
This work was supported in part by NIH Grant 1R01AI098833-01, DoD Grant CA111002, and NIH/NCRR Clinical & Translational Science Award to the University of Florida (UL1 RR029890).
Author Disclosure Statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this article apart from those disclosed. No competing financial interests exist.
References
- Abraham C. Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140(6):1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10(2):131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- Andrews JM. Tan M. Probiotics in luminal gastroenterology: the current state of play. Int Med J. 2012;42(12):1287–1291. doi: 10.1111/imj.12015. [DOI] [PubMed] [Google Scholar]
- Arthur JC. Perez-Chanona E. Muhlbauer M. Tomkovich S. Uronis JM. Fan TJ. Campbell BJ. Abujamel T. Dogan B. Rogers AB. Rhodes JM. Stintzi A. Simpson KW. Hansen JJ. Keku TO. Fodor AA. Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338(6103):120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M. Raes J. Pelletier E. Le Paslier D. Yamada T. Mende DR. Fernandes GR. Tap J. Bruls T. Batto JM. Bertalan M. Borruel N. Casellas F. Fernandez L. Gautier L. Hansen T. Hattori M. Hayashi T. Kleerebezem M. Kurokawa K. Leclerc M. Levenez F. Manichanh C. Nielsen HB. Nielsen T. Pons N. Poulain J. Qin J. Sicheritz-Ponten T. Tims S. Torrents D. Ugarte E. Zoetendal EG. Wang J. Guarner F. Pedersen O. de Vos WM. Brunak S. Dore J. Antolin M. Artiguenave F. Blottiere HM. Almeida M. Brechot C. Cara C. Chervaux C. Cultrone A. Delorme C. Denariaz G. Dervyn R. Foerstner KU. Friss C. van de Guchte M. Guedon E. Haimet F. Huber W. van Hylckama-Vlieg J. Jamet A. Juste C. Kaci G. Knol J. Lakhdari O. Layec S. Le Roux K. Maguin E. Merieux A. Melo Minardi R. M'Rini C. Muller J. Oozeer R. Parkhill J. Renault P. Rescigno M. Sanchez N. Sunagawa S. Torrejon A. Turner K. Vandemeulebrouck G. Varela E. Winogradsky Y. Zeller G. Weissenbach J. Ehrlich SD. Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseman C. Mauze S. Leach MW. Coffman RL. Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190(7):995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On L. Zigmond E. Jung S. Management of gut inflammation through the manipulation of intestinal dendritic cells and macrophages? Semin Immunol. 2011;23(1):58–64. doi: 10.1016/j.smim.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Barengolts E. Vitamin D and prebiotics may benefit the intestinal microbacteria and improve glucose homeostasis in prediabetes and type 2 diabetes. Endocr Pract. 2013;19(3):497–510. doi: 10.4158/EP12263.RA. [DOI] [PubMed] [Google Scholar]
- Belz GT. Nutt SL. Transcriptional programming of the dendritic cell network. Nat Rev Immunol. 2012;12(2):101–113. doi: 10.1038/nri3149. [DOI] [PubMed] [Google Scholar]
- Bibiloni R. Fedorak RN. Tannock GW. Madsen KL. Gionchetti P. Campieri M. De Simone C. Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100(7):1539–1546. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- Blasius AL. Beutler B. Intracellular toll-like receptors. Immunity. 2010;32(3):305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Blatner NR. Mulcahy MF. Dennis KL. Scholtens D. Bentrem DJ. Phillips JD. Ham S. Sandall BP. Khan MW. Mahvi DM. Halverson AL. Stryker SJ. Boller A-M. Singal A. Sneed RK. Sarraj B. Ansari MJ. Oft M. Iwakura Y. Zhou L. Bonertz A. Beckhove P. Gounari F. Khazaie K. Expression of RORγt Marks a Pathogenic Regulatory T Cell Subset in Human Colon Cancer. Sci Trans Med. 2012;4(164):164ra159. doi: 10.1126/scitranslmed.3004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booijink CC. El-Aidy S. Rajilic-Stojanovic M. Heilig HG. Troost FJ. Smidt H. Kleerebezem M. De Vos WM. Zoetendal EG. High temporal and inter-individual variation detected in the human ileal microbiota. Environ Microbiol. 2010;12(12):3213–3227. doi: 10.1111/j.1462-2920.2010.02294.x. [DOI] [PubMed] [Google Scholar]
- Bron PA. van Baarlen P. Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol. 2012;10(1):66–78. doi: 10.1038/nrmicro2690. [DOI] [PubMed] [Google Scholar]
- Capelluto DG. Tollip: a multitasking protein in innate immunity and protein trafficking. Microbes Infect. 2012;14(2):140–147. doi: 10.1016/j.micinf.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Cario E. Toll-like receptors in inflammatory bowel diseases: a decade later. Inflamm Bowel Dis. 2010;16(9):1583–1597. doi: 10.1002/ibd.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerovic V. Jenkins CD. Barnes AG. Milling SW. MacPherson GG. Klavinskis LS. Hyporesponsiveness of intestinal dendritic cells to TLR stimulation is limited to TLR4. J Immunol. 2009;182(4):2405–2415. doi: 10.4049/jimmunol.0802318. [DOI] [PubMed] [Google Scholar]
- Chae CS. Kwon HK. Hwang JS. Kim JE. Im SH. Prophylactic effect of probiotics on the development of experimental autoimmune myasthenia gravis. PloS One. 2012;7(12):e52119. doi: 10.1371/journal.pone.0052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC. Lin KH. Tai YT. Chen JT. Chen RM. Lipoteichoic acid-induced TNF-α and IL-6 gene expressions and oxidative stress production in macrophages are suppressed by ketamine through downregulating Toll-like receptor 2-mediated activation oF ERK1/2 and NFκB. Shock. 2010;33(5):485–492. doi: 10.1097/SHK.0b013e3181c3cea5. [DOI] [PubMed] [Google Scholar]
- Chang SY. Song JH. Guleng B. Cotoner CA. Arihiro S. Zhao Y. Chiang HS. O'Keeffe M. Liao G. Karp CL. Kweon MN. Sharpe AH. Bhan A. Terhorst C. Reinecker HC. Circulatory antigen processing by mucosal dendritic cells controls CD8(+) T cell activation. Immunity. 2013;38(1):153–165. doi: 10.1016/j.immuni.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B. Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1720–1728. doi: 10.1053/j.gastro.2011.01.054. [DOI] [PubMed] [Google Scholar]
- Christensen HR. Frokiaer H. Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168(1):171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- Coombes JL. Maloy KJ. Control of intestinal homeostasis by regulatory T cells and dendritic cells. Semin Immunol. 2007;19(2):116–126. doi: 10.1016/j.smim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Coombes JL. Siddiqui KR. Arancibia-Carcamo CV. Hall J. Sun CM. Belkaid Y. Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille MA. Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30(5):626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- D'Souza A. Cai CL. Kumar D. Cai F. Fordjour L. Ahmad A. Valencia G. Aranda JV. Beharry KD. Cytokines and Toll-like receptor signaling pathways in the terminal ileum of hypoxic/hyperoxic neonatal rats: benefits of probiotics supplementation. Am J Trans Res. 2012;4(2):187–197. [PMC free article] [PubMed] [Google Scholar]
- Dai C. Zheng CQ. Meng FJ. Zhou Z. Sang LX. Jiang M. VSL#3 probiotics exerts the anti-inflammatory activity via PI3k/Akt and NF-kappaB pathway in rat model of DSS-induced colitis. Mol Cell Biochem. 2013;374(1–2):1–11. doi: 10.1007/s11010-012-1488-3. [DOI] [PubMed] [Google Scholar]
- Deshpande G. Rao S. Patole S. Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010;125(5):921–930. doi: 10.1542/peds.2009-1301. [DOI] [PubMed] [Google Scholar]
- Devkota S. Wang Y. Musch MW. Leone V. Fehlner-Peach H. Nadimpalli A. Antonopoulos DA. Jabri B. Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature. 2012;487(7405):104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl GE. Longman RS. Zhang JX. Breart B. Galan C. Cuesta A. Schwab SR. Littman DR. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature. 2013;494(7435):116–120. doi: 10.1038/nature11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongarra ML. Rizzello V. Muccio L. Fries W. Cascio A. Bonaccorsi I. Ferlazzo G. Mucosal immunology and probiotics. Curr Allergy Asthma Rep. 2013;13(1):19–26. doi: 10.1007/s11882-012-0313-0. [DOI] [PubMed] [Google Scholar]
- Evrard B. Coudeyras S. Dosgilbert A. Charbonnel N. Alame J. Tridon A. Forestier C. Dose-dependent immunomodulation of human dendritic cells by the probiotic Lactobacillus rhamnosus Lcr35. PloS One. 2011;6(4):e18735. doi: 10.1371/journal.pone.0018735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feehley T. Stefka AT. Cao S. Nagler CR. Microbial regulation of allergic responses to food. Semin Immunopathol. 2012;34(5):671–688. doi: 10.1007/s00281-012-0337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K. Karuppuchamy T. Takemura N. Shimohigoshi M. Machida T. Haseda Y. Aoshi T. Ishii KJ. Akira S. Uematsu S. A new subset of CD103+CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity. J Immunol. 2011;186(11):6287–6295. doi: 10.4049/jimmunol.1004036. [DOI] [PubMed] [Google Scholar]
- Fumagalli M. Sironi M. Pozzoli U. Ferrer-Admetlla A. Pattini L. Nielsen R. Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet. 2011;7(11):e1002355. doi: 10.1371/journal.pgen.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad M. Ravn P. Soborg DA. Lund-Jensen K. Ouwehand AC. Jensen SS. Regulation of the IL-10/IL-12 axis in human dendritic cells with probiotic bacteria. FEMS Immunol Med Microbiol. 2011;63(1):93–107. doi: 10.1111/j.1574-695X.2011.00835.x. [DOI] [PubMed] [Google Scholar]
- Gill SR. Pop M. Deboy RT. Eckburg PB. Turnbaugh PJ. Samuel BS. Gordon JI. Relman DA. Fraser-Liggett CM. Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gologan S. Iacob R. Iancu D. Iacob S. Cotruta B. Vadan R. Catuneanu AM. Constantinescu I. Barbarii L. Gheorghe C. Diculescu M. Inflammatory gene expression profiles in Crohn's disease and ulcerative colitis: a comparative analysis using a reverse transcriptase multiplex ligation-dependent probe amplification protocol. J Crohn's Colitis. 2012 doi: 10.1016/j.crohns.2012.08.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Grangette C. Nutten S. Palumbo E. Morath S. Hermann C. Dewulf J. Pot B. Hartung T. Hols P. Mercenier A. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci U S A. 2005;102(29):10321–10326. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner F. The intestinal flora in inflammatory bowel disease: normal or abnormal? Curr Opin Gastroenterol. 2005;21(4):414–418. [PubMed] [Google Scholar]
- Guarner F. Schaafsma GJ. Probiotics. Int J Food Microbiol. 1998;39(3):237–238. doi: 10.1016/s0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- Hadis U. Wahl B. Schulz O. Hardtke-Wolenski M. Schippers A. Wagner N. Muller W. Sparwasser T. Forster R. Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34(2):237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Hakansson A. Molin G. Gut microbiota and inflammation. Nutrients. 2011;3(6):637–682. doi: 10.3390/nu3060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer GE. Ma A. Molecular control of steady-state dendritic cell maturation and immune homeostasis. Ann Rev Immunol. 2013;31:743–791. doi: 10.1146/annurev-immunol-020711-074929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haribhai D. Lin W. Edwards B. Ziegelbauer J. Salzman NH. Carlson MR. Li SH. Simpson PM. Chatila TA. Williams CB. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J Immunol. 2009;182(6):3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K. Littman DR. The microbiome in infectious disease and inflammation. Ann Rev Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovhannisyan Z. Treatman J. Littman DR. Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140(3):957–965. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume DA. Differentiation and heterogeneity in the mononuclear phagocyte system. Mucosal Immunol. 2008;1(6):432–441. doi: 10.1038/mi.2008.36. [DOI] [PubMed] [Google Scholar]
- Jang MH. Sougawa N. Tanaka T. Hirata T. Hiroi T. Tohya K. Guo Z. Umemoto E. Ebisuno Y. Yang BG. Seoh JY. Lipp M. Kiyono H. Miyasaka M. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol. 2006;176(2):803–810. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- Jeffery IB. Claesson MJ. O'Toole PW. Shanahan F. Categorization of the gut microbiota: enterotypes or gradients? Nat Rev Microbiol. 2012;10(9):591–592. doi: 10.1038/nrmicro2859. [DOI] [PubMed] [Google Scholar]
- Jeon SG. Kayama H. Ueda Y. Takahashi T. Asahara T. Tsuji H. Tsuji NM. Kiyono H. Ma JS. Kusu T. Okumura R. Hara H. Yoshida H. Yamamoto M. Nomoto K. Takeda K. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathogens. 2012;8(5):e1002714. doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jijon H. Backer J. Diaz H. Yeung H. Thiel D. McKaigney C. De Simone C. Madsen K. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126(5):1358–1373. doi: 10.1053/j.gastro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Kaji R. Kiyoshima-Shibata J. Nagaoka M. Nanno M. Shida K. Bacterial teichoic acids reverse predominant IL-12 production induced by certain lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages. J Immunol. 2010;184(7):3505–3513. doi: 10.4049/jimmunol.0901569. [DOI] [PubMed] [Google Scholar]
- Kang SS. Ryu YH. Baik JE. Yun CH. Lee K. Chung DK. Han SH. Lipoteichoic acid from Lactobacillus plantarum induces nitric oxide production in the presence of interferon-γ in murine macrophages. Mol Immunol. 2011;48(15–16):2170–2177. doi: 10.1016/j.molimm.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Katakura K. Lee J. Rachmilewitz D. Li G. Eckmann L. Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115(3):695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T. Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kelly D. Mulder IE. Microbiome and immunological interactions. Nutr Rev. 2012;70(Suppl 1):S18–S30. doi: 10.1111/j.1753-4887.2012.00498.x. [DOI] [PubMed] [Google Scholar]
- Kerckhoffs AP. Samsom M. van der Rest ME. de Vogel J. Knol J. Ben-Amor K. Akkermans LM. Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J Gastroenterol. 2009;15(23):2887–2892. doi: 10.3748/wjg.15.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MW. Zadeh M. Bere P. Gounaris E. Owen J. Klaenhammer T. Mohamadzadeh M. Modulating intestinal immune responses by lipoteichoic acid-deficient Lactobacillus acidophilus. Immunotherapy. 2012;4(2):151–161. doi: 10.2217/imt.11.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaie K. Zadeh M. Khan MW. Bere P. Gounari F. Dennis K. Blatner NR. Owen JL. Klaenhammer TR. Mohamadzadeh M. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci U S A. 2012;109(26):10462–10467. doi: 10.1073/pnas.1207230109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TS. Elia M. Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352(9135):1187–1189. doi: 10.1016/s0140-6736(98)02146-1. [DOI] [PubMed] [Google Scholar]
- Kinnebrew MA. Pamer EG. Innate immune signaling in defense against intestinal microbes. Immunol Rev. 2012;245(1):113–131. doi: 10.1111/j.1600-065X.2011.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinov SR. Smidt H. de Vos WM. Bruijns SC. Singh SK. Valence F. Molle D. Lortal S. Altermann E. Klaenhammer TR. van Kooyk Y. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci U S A. 2008;105(49):19474–19479. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubinak JL. Round JL. Toll-like receptors promote mutually beneficial commensal-host interactions. PLoS Pathogens. 2012;8(7):e1002785. doi: 10.1371/journal.ppat.1002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HK. Lee CG. So JS. Chae CS. Hwang JS. Sahoo A. Nam JH. Rhee JH. Hwang KC. Im SH. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A. 2010;107(5):2159–2164. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop SK. Bloom SM. Rao SM. Nutsch K. Lio CW. Santacruz N. Peterson DA. Stappenbeck TS. Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle EC. Murphy C. O'Neill LA. Creagh EM. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol. 2010;3(1):17–28. doi: 10.1038/mi.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeer S. Claes IJ. Vanderleyden J. Anti-inflammatory potential of probiotics: lipoteichoic acid makes a difference. Trends Microbiol. 2012;20(1):5–10. doi: 10.1016/j.tim.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Lee J. Mo JH. Katakura K. Alkalay I. Rucker AN. Liu YT. Lee HK. Shen C. Cojocaru G. Shenouda S. Kagnoff M. Eckmann L. Ben-Neriah Y. Raz E. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8(12):1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- Ley RE. Peterson DA. Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Lilly DM. Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147(3659):747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- Macho Fernandez E. Valenti V. Rockel C. Hermann C. Pot B. Boneca IG. Grangette C. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut. 2011;60(8):1050–1059. doi: 10.1136/gut.2010.232918. [DOI] [PubMed] [Google Scholar]
- Manicassamy S. Reizis B. Ravindran R. Nakaya H. Salazar-Gonzalez RM. Wang YC. Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329(5993):849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92(6):1177–1186. doi: 10.1189/jlb.0512231. [DOI] [PubMed] [Google Scholar]
- Mayer CT. Berod L. Sparwasser T. Layers of dendritic cell-mediated T cell tolerance, their regulation and the prevention of autoimmunity. Front Immunol. 2012;3:183. doi: 10.3389/fimmu.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medani M. Collins D. Docherty NG. Baird AW. O'Connell PR. Winter DC. Emerging role of hydrogen sulfide in colonic physiology and pathophysiology. Inflamm Bowel Dis. 2011;17(7):1620–1625. doi: 10.1002/ibd.21528. [DOI] [PubMed] [Google Scholar]
- Meijer BJ. Dieleman LA. Probiotics in the treatment of human inflammatory bowel diseases: update 2011. J Clin Gastroenterol. 2011;45(Suppl):S139–S144. doi: 10.1097/MCG.0b013e31822103f7. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M. Olson S. Kalina WV. Ruthel G. Demmin GL. Warfield KL. Bavari S. Klaenhammer TR. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci U S A. 2005;102(8):2880–2885. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamadzadeh M. Pfeiler EA. Brown JB. Zadeh M. Gramarossa M. Managlia E. Bere P. Sarraj B. Khan MW. Pakanati KC. Ansari MJ. O'Flaherty S. Barrett T. Klaenhammer TR. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4623–4630. doi: 10.1073/pnas.1005066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar R. Knippenberg M. Goverse G. Olivier BJ. de Vos AF. O'Toole T. Mebius RE. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J Immunol. 2011;186(4):1934–1942. doi: 10.4049/jimmunol.1001672. [DOI] [PubMed] [Google Scholar]
- Molloy MJ. Bouladoux N. Belkaid Y. Intestinal microbiota: shaping local and systemic immune responses. Semin Immunol. 2012;24(1):58–66. doi: 10.1016/j.smim.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe KM. McWhirter SM. Vance RE. Induction of type I interferons by bacteria. Cell Microbiol. 2010;12(7):881–890. doi: 10.1111/j.1462-5822.2010.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottet C. Uhlig HH. Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170(8):3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- Niess JH. Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol. 2010;184(4):2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- Nilsen NJ. Deininger S. Nonstad U. Skjeldal F. Husebye H. Rodionov D. von Aulock S. Hartung T. Lien E. Bakke O. Espevik T. Cellular trafficking of lipoteichoic acid and Toll-like receptor 2 in relation to signaling: role of CD14 and CD36. J Leukoc Biol. 2008;84(1):280–291. doi: 10.1189/jlb.0907656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio J. Honda K. Immunoregulation by the gut microbiota. Cell Mol Life Sci. 2012;69(21):3635–3650. doi: 10.1007/s00018-012-0993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea EF. Cotter PD. Stanton C. Ross RP. Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189–205. doi: 10.1016/j.ijfoodmicro.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Pabst O. Bernhardt G. The puzzle of intestinal lamina propria dendritic cells and macrophages. Eur J Immunol. 2010;40(8):2107–2111. doi: 10.1002/eji.201040557. [DOI] [PubMed] [Google Scholar]
- Pallotta MT. Orabona C. Volpi C. Vacca C. Belladonna ML. Bianchi R. Servillo G. Brunacci C. Calvitti M. Bicciato S. Mazza EM. Boon L. Grassi F. Fioretti MC. Fallarino F. Puccetti P. Grohmann U. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12(9):870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- Qian C. Jiang X. An H. Yu Y. Guo Z. Liu S. Xu H. Cao X. TLR agonists promote ERK-mediated preferential IL-10 production of regulatory dendritic cells (diffDCs), leading to NK-cell activation. Blood. 2006;108(7):2307–2315. doi: 10.1182/blood-2006-03-005595. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D. Katakura K. Karmeli F. Hayashi T. Reinus C. Rudensky B. Akira S. Takeda K. Lee J. Takabayashi K. Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126(2):520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Ramalingam R. Larmonier CB. Thurston RD. Midura-Kiela MT. Zheng SG. Ghishan FK. Kiela PR. Dendritic cell-specific disruption of TGF-beta receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J Immunol. 2012;189(8):3878–3893. doi: 10.4049/jimmunol.1201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann NT. Gründling A. Location, synthesis and function of glycolipids and polyglycerolphosphate lipoteichoic acid in Gram-positive bacteria of the phylum Firmicutes. FEMS Microbiol Lett. 2011;319(2):97–105. doi: 10.1111/j.1574-6968.2011.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff C. Kelly D. Inflammatory bowel disease, gut bacteria and probiotic therapy. International journal of medical microbiology: IJMM. 2010;300(1):25–33. doi: 10.1016/j.ijmm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Rescigno M. Dendritic cells in bacteria handling in the gut. J Leukoc Biol. 2011;90(4):669–672. doi: 10.1189/jlb.0311141. [DOI] [PubMed] [Google Scholar]
- Ronnblom L. Eloranta ML. The interferon signature in autoimmune diseases. Curr Opin Rheumatol. 2013;25(2):248–253. doi: 10.1097/BOR.0b013e32835c7e32. [DOI] [PubMed] [Google Scholar]
- Ruane DT. Lavelle EC. The role of CD103(+) dendritic cells in the intestinal mucosal immune system. Front Immunol. 2011;2:25. doi: 10.3389/fimmu.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP. Rasmussen JP. Chi EY. Fontenot J. Castelli L. Ye X. Treuting P. Siewe L. Roers A. Henderson WR. Muller W. Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Saber R. Zadeh M. Pakanati KC. Bere P. Klaenhammer T. Mohamadzadeh M. Lipoteichoic acid-deficient Lactobacillus acidophilus regulates downstream signals. Immunotherapy. 2011;3(3):337–347. doi: 10.2217/imt.10.119. [DOI] [PubMed] [Google Scholar]
- Sanders ME. Impact of probiotics on colonizing microbiota of the gut. J Clin Gastroenterol. 2011;45(Suppl):S115–S119. doi: 10.1097/MCG.0b013e318227414a. [DOI] [PubMed] [Google Scholar]
- Schiavi E. Barletta B. Butteroni C. Corinti S. Boirivant M. Di Felice G. Oral therapeutic administration of a probiotic mixture suppresses established Th2 responses and systemic anaphylaxis in a murine model of food allergy. Allergy. 2011;66(4):499–508. doi: 10.1111/j.1398-9995.2010.02501.x. [DOI] [PubMed] [Google Scholar]
- Sekirov I. Russell SL. Antunes LC. Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Shanahan F. The colonic microbiota and colonic disease. Curr Gastroenterol Rep. 2012;14(5):446–452. doi: 10.1007/s11894-012-0281-5. [DOI] [PubMed] [Google Scholar]
- Smelt MJ. de Haan BJ. Bron PA. van Swam I. Meijerink M. Wells JM. Faas MM. de Vos P. L. plantarum, L. salivarius, and L. lactis attenuate Th2 responses and increase Treg frequencies in healthy mice in a strain dependent manner. PloS One. 2012;7(10):e47244. doi: 10.1371/journal.pone.0047244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits HH. Engering A. van der Kleij D. de Jong EC. Schipper K. van Capel TM. Zaat BA. Yazdanbakhsh M. Wierenga EA. van Kooyk Y. Kapsenberg ML. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115(6):1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Sobhani I. Tap J. Roudot-Thoraval F. Roperch JP. Letulle S. Langella P. Corthier G. Tran Van Nhieu J. Furet JP. Microbial dysbiosis in colorectal cancer (CRC) patients. PloS One. 2011;6(1):e16393. doi: 10.1371/journal.pone.0016393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock A. Napolitani G. Cerundolo V. Intestinal DC in migrational imprinting of immune cells. Immunol Cell Biol. 2013;91(3):240–249. doi: 10.1038/icb.2012.73. [DOI] [PubMed] [Google Scholar]
- Swiatczak B. Rescigno M. How the interplay between antigen presenting cells and microbiota tunes host immune responses in the gut. Semin Immunol. 2012;24(1):43–49. doi: 10.1016/j.smim.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Takahashi M. Nakamura K. Honda K. Kitamura Y. Mizutani T. Araki Y. Kabemura T. Chijiiwa Y. Harada N. Nawata H. An inverse correlation of human peripheral blood regulatory T cell frequency with the disease activity of ulcerative colitis. Dig Dis Sci. 2006;51(4):677–686. doi: 10.1007/s10620-006-3191-2. [DOI] [PubMed] [Google Scholar]
- Torok HP. Glas J. Tonenchi L. Bruennler G. Folwaczny M. Folwaczny C. Crohn's disease is associated with a toll-like receptor-9 polymorphism. Gastroenterology. 2004;127(1):365–366. doi: 10.1053/j.gastro.2004.05.051. [DOI] [PubMed] [Google Scholar]
- Tsai YT. Cheng PC. Pan TM. The immunomodulatory effects of lactic acid bacteria for improving immune functions and benefits. Appl Microbiol Biotechnol. 2012;96(4):853–862. doi: 10.1007/s00253-012-4407-3. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ. Hamady M. Yatsunenko T. Cantarel BL. Duncan A. Ley RE. Sogin ML. Jones WJ. Roe BA. Affourtit JP. Egholm M. Henrissat B. Heath AC. Knight R. Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S. Fujimoto K. Jang MH. Yang BG. Jung YJ. Nishiyama M. Sato S. Tsujimura T. Yamamoto M. Yokota Y. Kiyono H. Miyasaka M. Ishii KJ. Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9(7):769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- van Baarlen P. Troost F. van der Meer C. Hooiveld G. Boekschoten M. Brummer RJ. Kleerebezem M. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4562–4569. doi: 10.1073/pnas.1000079107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum A. Nieuwenhuis EE. Life in commercial probiotics. FEMS Immunol Med Microbiol. 2007;50(3):281–283. doi: 10.1111/j.1574-695X.2007.00290.x. [DOI] [PubMed] [Google Scholar]
- Vanderpool C. Yan F. Polk DB. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm Bowel Dis. 2008;14(11):1585–1596. doi: 10.1002/ibd.20525. [DOI] [PubMed] [Google Scholar]
- Varol C. Vallon-Eberhard A. Elinav E. Aychek T. Shapira Y. Luche H. Fehling HJ. Hardt WD. Shakhar G. Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31(3):502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Varol C. Zigmond E. Jung S. Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat Rev Immunol. 2010;10(6):415–426. doi: 10.1038/nri2778. [DOI] [PubMed] [Google Scholar]
- Veerappan GR. Betteridge J. Young PE. Probiotics for the treatment of inflammatory bowel disease. Curr Gastroenterol Rep. 2012;14(4):324–333. doi: 10.1007/s11894-012-0265-5. [DOI] [PubMed] [Google Scholar]
- Walsh D. McCarthy J. O'Driscoll C. Melgar S. Pattern recognition receptors-molecular orchestrators of inflammation in inflammatory bowel disease. Cytokine Growth Factor Rev. 2013;24(2):91–104. doi: 10.1016/j.cytogfr.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Weiss G. Christensen HR. Zeuthen LH. Vogensen FK. Jakobsen M. Frokiaer H. Lactobacilli and bifidobacteria induce differential interferon-beta profiles in dendritic cells. Cytokine. 2011;56(2):520–530. doi: 10.1016/j.cyto.2011.07.024. [DOI] [PubMed] [Google Scholar]
- Weiss G. Rasmussen S. Nielsen Fink L. Jarmer H. Nohr Nielsen B. Frokiaer H. Bifidobacterium bifidum actively changes the gene expression profile induced by Lactobacillus acidophilus in murine dendritic cells. PloS One. 2010;5(6):e11065. doi: 10.1371/journal.pone.0011065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman WB. Coleman DC. Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A. 1998;95(12):6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing JB. Sakaguchi S. Multiple treg suppressive modules and their adaptability. Front Immunol. 2012;3:178. doi: 10.3389/fimmu.2012.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD. Chen J. Hoffmann C. Bittinger K. Chen YY. Keilbaugh SA. Bewtra M. Knights D. Walters WA. Knight R. Sinha R. Gilroy E. Gupta K. Baldassano R. Nessel L. Li H. Bushman FD. Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M. Louvet C. Davini D. Gardner JM. Martinez-Llordella M. Bailey-Bucktrout S. Anthony BA. Sverdrup FM. Head R. Kuster DJ. Ruminski P. Weiss D. Von Schack D. Bluestone JA. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209(10):1713–1722. doi: 10.1084/jem.20120822. S1–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F. Polk DB. Probiotics and immune health. Curr Opin Gastroenterol. 2011;27(6):496–501. doi: 10.1097/MOG.0b013e32834baa4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zassadowski F. Rochette-Egly C. Chomienne C. Cassinat B. Regulation of the transcriptional activity of nuclear receptors by the MEK/ERK1/2 pathway. Cell Signal. 2012;24(12):2369–2377. doi: 10.1016/j.cellsig.2012.08.003. [DOI] [PubMed] [Google Scholar]