Abstract
Background
The purpose of this study was to determine whether interventions including components to improve adherence to antihypertensive medications in patients after stroke/transient ischemic attack (TIA) improve adherence and blood pressure control.
Methods and Results
We searched MEDLINE, EMBASE, CINAHL, BNI, PsycINFO, and article reference lists to October 2012. Search terms included stroke/TIA, adherence/prevention, hypertension, and randomized controlled trial (RCT). Inclusion criteria were participants with stroke/TIA; interventions including a component to improve adherence to antihypertensive medications; and outcomes including blood pressure, antihypertensive adherence, or both. Two reviewers independently assessed studies to determine eligibility, validity, and quality. Seven RCTs were eligible (n=1591). Methodological quality varied. All trials tested multifactorial interventions. None targeted medication adherence alone. Six trials measured blood pressure and 3 adherence. Meta‐analysis of 6 trials showed that multifactorial programs were associated with improved blood pressure control. The difference between intervention versus control in mean improvement in systolic blood pressure was −5.3 mm Hg (95% CI, −10.2 to −0.4 mm Hg, P=0.035; I2=67% [21% to 86%]) and in diastolic blood pressure was −2.5 mm Hg (−5.0 to −0.1 mm Hg, P=0.046; I2=47% [0% to 79%]). There was no effect on medication adherence where measured.
Conclusions
Multifactorial interventions including a component to improve medication adherence can lower blood pressure after stroke/TIA. However, it is not possible to say whether or not this is achieved through better medication adherence. Trials are needed of well‐characterized interventions to improve medication adherence and clinical outcomes with measurement along the hypothesized causal pathway.
Keywords: blood pressure, hypertension, prevention, stroke
Introduction
The number of strokes and their impact on morbidity and mortality continue to increase globally because of population aging, and there is a clear opportunity for better preventive effort.1 Among those who survive a stroke or a transient ischemic attack (TIA), the risk of further stroke is high, ranging from 15% to 42% over 5 years.2–3 Indeed, recurrent stroke accounts for up to 40% of all strokes.4
Recurrent stroke is associated with higher mortality than first stroke, and functional recovery is often poorer,5 so secondary prevention matters. Lowering systolic blood pressure (SBP) by 5 mm Hg or diastolic blood pressure (DBP) by 2.5 mm Hg reduces the incidence of stroke by 15% to 20%, independent of prevalent vascular disease and hypertension.6
However, blood pressure control after stroke is suboptimal, with up to 41% of patients having a SBP >140 mm Hg.7 Blood pressure targets for secondary prevention have been recently lowered to 130/80 mm Hg,8 and some guidelines9 suggest treating all patients with a previous stroke or TIA with antihypertensive medication regardless of blood pressure, unless contraindicated. Patient adherence to antihypertensive therapy is likely to be a major barrier to implementation of these guidelines.10
In primary prevention, a range of interventions to improve adherence have been evaluated. Simplification of dosage regimen improved adherence to antihypertensive drugs although the effect on blood pressure is unclear.11 Where significant effects on blood pressure have been reported, notably in the Hypertension Detection and Follow‐Up study,12 an organized system of regular reviews was linked to medication intensification, and medication adherence was not measured.11,13 Evidence remains uncollated for people with stroke who may be particularly motivated but face special challenges in taking their medicines as prescribed. We performed a systematic review of randomized controlled trials of interventions that included a component to improve adherence to antihypertensive drugs in adults with stroke/TIA to assess the impact of these interventions on blood pressure and adherence.
Methods
Eligible studies included adults with confirmed history of stroke/TIA, randomized to interventions including a component to improve adherence to antihypertensive medications and measuring blood pressure or patients’ adherence to antihypertensive medications.
Search Method and Study Selection
We searched Medline (1966 to October 2012), Embase (1980 to October 2012), CINAHL (1981 to October 2012), PsycINFO (1806 to October 2012), and BNI (1985 to October 2012). Search terms covered adherence, prevention, hypertension, clinical terms for TIA/stroke, and terms for randomized controlled trial (search strategy in Table S1). We adapted the search for each database without language restrictions. Reference lists of all included articles were also searched manually.
One reviewer (A.D.S.) screened all titles and abstracts, and 20% were checked independently by W.H., with differences agreed by consensus. The full text was examined for articles in which a definite decision to reject could not be made based on title and abstract alone. Two reviewers (A.D.S. and W.H.) independently assessed all full‐text articles, and those not meeting the inclusion criteria by both researchers were excluded.
Two translators assessed foreign‐language articles with relevant titles or English abstracts. All translators were familiar with medical literature and terminology. Validation of the data extraction form was performed by A.D.S., W.H., A.L.K., and A.F.
Data Extraction
The data extraction form was created and standardized over 3 meetings between 2 reviewers (A.D.S. and W.H.) until agreement was reached by comparing extractions independently obtained on 3 randomly selected included studies.
Two authors (A.D.S., W.H.) independently extracted data on blood pressure and antihypertensive adherence and resolved disagreements through discussion.
A.D.S. and W.H. classified intervention and control strategies independently. They initially used behavior change techniques (BCTs) Taxonomy V114 to identify intervention components, but could not extract meaningful data on BCTs used because of poor reporting, particularly in relation to patient‐directed interventions like “education” and “lifestyle.” Therefore, intervention strategies were described more broadly, faithful to the intervention descriptions by the authors. Strategies were grouped into verbal information/advice on disease and secondary‐prevention drug treatment, goal setting, supply of printed information/advice material, screening for depression, personalized instructions, and integrated care (see data extraction elements in Table S4).
Quality Assessment
A.D.S. and W.H. appraised each study independently for risk of bias, using accepted guidance.15 We considered sequence generation, allocation concealment, blinding of study personnel and participants, incomplete outcome data, selective outcome reporting, adequacy of the power calculation, and use of intention‐to‐treat analysis (Table S3).
Statistical Analysis
We calculated pooled effect estimates for systolic blood pressure (SBP) and diastolic blood pressure (DBP) for 6 trials in which these outcomes were reported. We fitted random effects meta‐analyses models to allow for heterogeneity between studies in RevMan. We used pooled difference in mean improvement of blood pressure from the intervention together with the pooled difference in mean improvement of blood pressure from the control arms of the trials to estimate the effect of the intervention on blood pressure control. For each analysis, we calculated the I2 statistic to estimate the proportion of the observed variance in effects across studies that indicates real differences rather than random error, with 95% confidence intervals using Stata. We used values of 25%, 50%, and 75% as boundary limits for low, moderate, and high heterogeneity.16 Significance was set at P<0.05, and 95% confidence intervals are quoted throughout.
Not all the trials reported the necessary data directly, so we transformed and estimated these as necessary (see Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0).15 If standard deviations of blood pressure measures at follow‐up were not supplied,17 we carried forward the baseline values. If the studies reported 95% confidence intervals only,18–19 we calculated standard deviations using the following formula: standard deviation=(confidence interval [CI]×square root [n])/1.96.
For the blood pressure measurements in the meta‐analysis, we used data collected at follow‐up times. In all studies blood pressure measurements were recorded at a single follow‐up time after the end of the intervention. Follow‐ups were carried out straight after the last intervention session in 2 studies,20–21 2 months later in 3 trials,18–19,22 and 3 months later in 1.17
We performed sensitivity analyses to explore the impact of excluding:
Relatively small studies (with <50 participants per randomization group).
Studies considered at high risk of bias.
Studies that did not measure adherence.
Studies that did not properly describe the adherence component of the intervention.
Three trials included a measure of adherence. The range of outcome measures and the diversity of metrics used to ascertain adherence prevented pooling for meta‐analysis.
Results
Study Selection
We included 8 articles referring to 7 separate randomized controlled trials after screening 7518 titles and abstracts and reviewing 48 full texts (Figure 1). Eight trials required further consideration after full‐text reading. We excluded 3 studies that made no distinction between adherence to antihypertensive and other medications.23–25 We also excluded 1 study because the primary aim was to improve health professionals’ adherence to prescribing antihypertensives rather than patients’ adherence26 and 2 further studies in which the outcomes were measured in a population of patients with cardiovascular events that included only a minority of patients with stroke.27–28 These trials were excluded after contacting the authors and finding that separate outcome measures were not available. We also assessed 2 foreign‐language papers, 1 Chinese29 and 1 German.30 One was excluded as adherence to antihypertensive medication was not measured, and the other study because the intervention was aimed at improving practitioners’ management of blood pressure rather than patients’ adherence.
Figure 1.

Study flow. TIA indicates transient ischemic attack.
Eight trials that were identified as potentially eligible were excluded as the results were not available at time of submission (see study characteristics in Table S5).31–38
Participants’ Characteristics
In 7 trials, 1591 patients living in the community with an average age between 63 and 74 years were randomized. All trials except 139 excluded patients with significant cognitive impairment or with serious comorbidities (see inclusion criteria from Table S2).
Two studies included only patients with a history of stroke, whereas the other 5 had a different proportion of patients with stroke and TIA (Figure 2, Table). The proportion of people with prior diagnosis of hypertension varied from 43% to 100%.
Figure 2.
Representation of interventions from the 7 trials included in the review. See label on top right for explanation. Sizes correlated with percentge of involvement. *Significant blood pressure improvement in the intervention group. m Indicates months; TIA, transient ischemic attack; HTN, hypertension; f/u, follow‐up; GP, general practitioner.
Table.
Information Used for the Analysis of Interventions to Improve Blood Pressure (BP)
| Trial Year Country Number of Patients | Intervention Components as Described in Paper | BP Improvement Intervention (SBP and DBP) Control (SBP and DBP) Mean (SD) | Mean Age of Participants (years) | Average Duration of Intervention/Last Follow‐Up (months) | Baseline BP Intervention (SBP and DBP) Control (SBP and DBP) Mean (SD) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention Content | Delivery | Provider and Settings | Intensity | Population: Stroke/TIA% Previous Episodes% Time Since First TIA/Stroke Hypertensive% | |||||
| Joubert20 2009 Australia n=233 |
Verbal information/advice on disease/treatment Personalised instructions Targeting multiple behaviours (adherence‐lifestyle) Screening for depression. |
Face to face Telephone |
Nurses Primary care physicians Hospital Home GP surgeries |
Regular reviews (Pre‐discharge education, 6 sessions over 12 months with GP, 6 telephone calls to patients, 6 follow‐up telephone calls to GPs) |
Stroke 85% TIA 15% Primary care physicians Not reported <1 month Not reported |
−6.0 (20.1) 1.0 (12.3) 1.8 (24.2) 2.8 (13) |
68 C 63 I |
12/12 | 134.2 (17) 76.1 (11.7) 131.2 (19.2) 75.6 (12.0) |
| Adie22 2010 UK n=56 |
Verbal information/advice on disease/treatment Motivational interviewing Goal setting Targeting multiple behaviours (adherence‐lifestyle) Educative printed material |
Phone + written material |
Researcher Home |
Regular reviews (4 sessions of 20 minutes over 4 months) |
Minor stroke 57% TIA 43% 12.5% recurrent stroke/TIA <1 month 100% hypertensive |
−2.6 (25) −10.0 (12.0) −4.6 (22.4) −8.4 (14) |
72 | 4/6 | 163.7 (19.3) 87.6 (11.4) 167.0 (15.5) 82.8 (14.7) |
| Chiu21 2008 Taiwan n=160 |
Verbal information/advice on disease/treatment Goal setting |
Face to face | Pharmacists Hospital outpatient |
Regular reviews (6 sessions of 1hour over 6 months) |
Stroke 48% recurrent stroke >12 months 96% hypertensive |
−11.6 (15.1) −7.4 (9.2) 1.2 (12.6) −0.7 (10) |
65 | 6/6 | 143.5 (19.9) 83.4 (11.8) 142.6 (17.2) 81.7 (11.4) |
| Ellis18 2005 Scotland n=205 |
Verbal information/advice on disease/treatment Personalised instructions and patient health records Targeting multiple behaviours (adherence‐lifestyle) Educative printed material |
Face to face + written material |
Nurses (stroke nurse specialists) Hospital outpatient |
Regular reviews (3 sessions of 30 minutes over 3 months) |
Stroke 68% TIA 32% 31% recurrent stroke/TIA <3 months 70% hypertensive |
−7.8 (27) −2.2 (17.8) −2.2 (25.6) −1.2 (22.7) |
66 C 64 I |
3/5 | 156.2 (27.2) 83.4 (18.3) 151.1 (28.7) 80.0 (16.7) |
| Hornnes19 2011 Denmark n=349 |
Verbal information/advice on disease/treatment Personalised instructions Targeting multiple behaviours (adherence‐lifestyle) |
Face to face | Nurses Home |
Regular reviews (4 sessions of 1hour over 10 months) |
Stroke 72% TIA 24% first episode or recurrence 71% hypertensive |
0.1 (25.8) −0.1 (19.5) 0.7 (25.6) 2.4 (19.5) |
68 C 70 I |
12/14 | 139.3 (24.8) 82.1 (14.3) 141.7 (23.0) 83.6 (12.0) |
| Maasland17 2007 Netherlands n=65 |
Information/advice on disease/treatment Personalised instructions Targeting multiple behaviours (adherence‐lifestyle) |
Computer | Computer | Single session Hospital outpatient |
Stroke 54% TIA 46% 17.5% recurrent stroke/TIA <3 months 43% hypertensive |
−8.4 (23) −5.4 (10) −6.9 (10.0) −6.2 (8.0) |
63 C 65 I |
0/3 | 144 (23) 84 (10) 140 (16) 86 (8) |
Caregivers received the intervention together with patients in 2 studies.20,39 Their degree of involvement, though, was not reported.
Intervention Characteristics
A wide range of interventions were evaluated (Table, Figure 2, and Table S2). All interventions were complex with multiple aims and components that were generally poorly described.
There was considerable variation in terms of size, setting, duration of intervention, and study population (Table and Table S2). Most interventions included ≥4 face‐to‐face sessions. Primary care doctors and nurses delivered the intervention in 3 studies,18–20 pharmacists in 1,21 and a researcher in 1.22 The intervention was delivered through a computer in 1 study17 and by a written hard‐copy “keeping well plan” for patients and evidence‐based secondary prevention plan tailored to patients for general practitioners (GPs) in another study.39 The interventions were delivered in a variety of settings, including GP surgery, home, and the hospital (Figure 2). Final follow‐up was carried out between 0 and 3 months after the last intervention session.
In all studies interventions included information and advice about stroke and the role of preventive drugs. In 5 studies this information and advice were tailored to individual patient characteristics according to their risk factors profile for stroke recurrence. A goal‐setting technique was used in 3 studies, with blood pressure targets assigned to patients.20–22 Three studies supplied written information.18,22,39 Only 1 intervention was explicitly theory based, using social‐cognitive theory. This intervention aimed to translate knowledge (of hypertension and its treatment) into effective patient behavior change (improved adherence and BP control), using motivational interviewing.22
Six trials included additional information/advice on treatments other than antihypertensive drugs (cholesterol‐ and glucose‐lowering medications, anticoagulants),17–18,17–22,39 and 5 gave information on lifestyle risk factors (eg, smoking cessation, weight reduction).17–18,20,22,39
Control Interventions
Control groups were described as receiving “usual care” in 4 of 7 studies. In the other studies, control care included generic risk factor advice once from a stroke nurse specialist,18 health education from a neurologist,17 and advice on healthy lifestyle choices from the multidisciplinary stroke team.19
Study Quality
Study quality was variable (Table S3). All studies were judged at risk of bias in at least 2 domains, but only 1 study21 was judged to be at high risk of bias. Blinding of participants was not possible with these types of intervention. Outcome assessors were clearly blinded to treatment allocation in 3 studies.
Intervention Effects on Blood Pressure
Six studies17–22 examined the effect of interventions on systolic and diastolic blood pressure (Table). Pooled analysis showed that interventions were associated with a significant (P=0.03) reduction in SBP of −5.3 mm Hg (95% CI, −10.2 to −0.4 mm Hg), I2=67% (21% to 86%). Pooled data on difference in mean DBP showed that interventions were associated with a reduction of −2.5 mm Hg (95% CI, −5 to −0.1 mm Hg), I2=47% (0% to 79%); P=0.05 (Figure 3).
Figure 3.
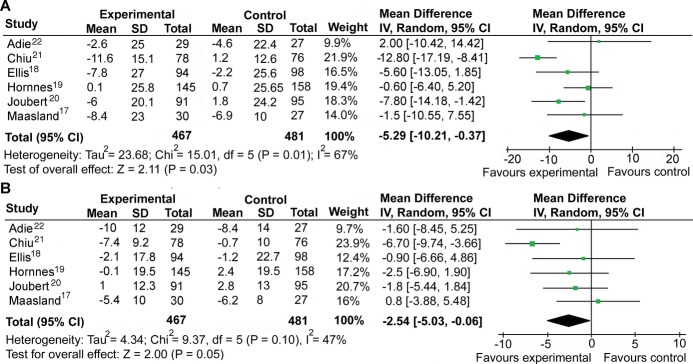
Meta‐analysis of effect of interventions on systolic blood pressure (A) and diastolic blood pressure (B). SD indicates standard deviation; CI, confidence interval.
Intervention Effect on Adherence to Antihypertensive Medications
The effect of the intervention on patients’ adherence to antihypertensive medications was small and not significant in any of the studies. Adherence was self‐reported in 2 studies,19,39 undefined in 1,17 and assessed from refilling prescription data (persistence of use of antihypertensives) in a further study40 (see Table S3). Three trials17,19,40 reported on both adherence and blood pressure changes and found no effect on either outcome.
In 1 trial19adherence, defined as missing no fewer than 2 doses in the previous 2 weeks, was the same (98% versus 99%) in both arms; the second trial39 measured self‐reported adherence, and treatment with antihypertensives was 63% and 66% in the intervention and control arms, respectively; 92% of patients in both control and intervention groups were adherent to blood‐pressure‐lowering medications in the third trial,17 although the method for measuring this outcome was not reported. Another trial40 reported persistence with antihypertensive therapy evaluated by comparing medication details at 3‐year follow‐up, which was 95% and 97% for the intervention and control groups, respectively.
The trials did not use objective measures of adherence (eg, rate of prescription refills, electronic medication monitors) or assess adherence among different classes (eg, calcium antagonists, diuretics, beta‐blockers, angiotensin inhibitors).
Sensitivity Analysis of Blood Pressure Outcomes
After exclusion of relatively small studies17,22 from the meta‐analysis, significant reductions in BP for the intervention care compared with the usual care group were observed (Figure 4A). Pooling data from the studies excluding the study at highest risk of bias showed smaller but still significant improvement in SBP, whereas the effect on DBP was reduced (Figure 4B). A further analysis was performed to check sensitivity to outcome, including only those studies that measured adherence as outcome,17–18,40 failing to detect a difference in either adherence or BP (Figure 4C). A final analysis on studies that fully described the adherence intervention19,22 (Figure 4D) showed no effect on SBP/DBP.
Figure 4.
Sensitivity analysis to explore the impact of excluding: A, relatively small studies (with <50 participants per randomization group); B, the study considered at high risk of bias; C, studies that did not measure adherence; D, studies that did not properly describe the adherence component of the intervention. SBP indicates systolic blood pressure; DBP, diastolic blood pressure.
Discussion
There is little evidence to inform approaches to improve blood pressure control through adherence to antihypertensive drugs among patients with stroke or TIA. A rigorous search discovered only 6 randomized controlled trials of relevant interventions. All included multiple components and together demonstrated clinically important effects on both systolic and diastolic blood pressure. There was no evidence that this effect was a result of improved adherence; few studies measured adherence, and none found an intervention effect.
Populations
Populations were highly selected. A third of patients with stroke have difficulty with communication,41 one fifth of whom suffer from aphasia.42 Stroke survivors may have shortened attention spans or may experience deficits in short‐term memory, comprehension, or engagement in complex mental activities, requiring behavioral interventions tailored to these impairments.43–47 Yet only 1 study39 included patients with cognitive deficits and multiple morbidities. Patients with significant communication difficulties were largely excluded. Perhaps not surprisingly, therefore, results from this selected population with stroke are consistent with those from similar trials in primary prevention of cardiovascular disease.11–13,48
Only in 1 study39 were patients’ ethnicity and social class recorded and primary outcomes adjusted for, with no significant effect on adherence. Patients’ “education” was accounted for in 2 studies.17,21 The impact of culture, socioeconomic status, health care coverage systems, and availability of free care and medications was not studied, although likely to have influenced adherence.
Interventions and Their Delivery
Interventions and their delivery were poorly described. Interventions were commonly adapted from those in studies of primary prevention of cardiovascular disease, including components such as an organized system of regular review, giving patients information or advice on disease and treatment tailored to individual risk factor profiles, goal setting, and motivational interviewing. Although educational interventions were not promising in primary prevention,13 they were incorporated into all secondary prevention trials as information and advice on stroke and preventive drug treatments. Yet simplification of the overall drug regimen was not used despite being the most promising strategy to improve adherence to antihypertensive medications in primary prevention trials,11 with a study suggesting feasibility in patients with stroke.49 Future interventions may use the BCT taxonomy14 to aid precise specification of the behavior change techniques used and the criteria defined by the CONSORT statement and Davidson et al50 to describe other important intervention components (eg, mode of delivery, fidelity).
There was a surprising lack of attention to epidemiological or qualitative data available to inform interventions that might be more effective with this patient group, perhaps because of the selected study population as detailed above. One qualitative study among patients with stroke identified priorities of longer time for communication, simple language, short sentences and large text, and uncluttered design for written materials.51
Family members or caregivers were only included as recipients of the intervention in 2 trials despite evidence that their involvement improves adherence52–53 and that they can find giving medicines difficult.54
Greater attention to physician training in intensification of antihypertensive medication prescribing and simplification of overall drug regimens might also be fruitful. In the effective Hypertension Detection and Follow‐Up study, medication intensification rather than adherence was the main target.12 It is seldom possible to untangle the effects of intervening on medication adherence from regimen intensification because in most studies patients were advised to see their doctors for medication review if their blood pressure was not at target, and regimen intensification was rarely measured.
Similarly to primary prevention studies, nurse and pharmacist involvement in a whole‐systems approach to prescribing might be fruitful. Systems of regular reviews, linked to medication intensification and medication adherence counseling by pharmacists or nurses lead to higher achievement of blood pressure goals (conference abstracts).33,37
Measurement
Measurement of blood pressure and adherence was inconsistent across studies. Where adherence was measured, self‐report was used, and objective measures such as pill count devices or electronic monitoring were absent.17,19,39
Interventions showed considerable heterogeneity in terms of design and settings. Despite using appropriate meta‐analytic techniques with random‐effect models, we were unable to control fully for these differences, and the small number of studies meant that the degree of heterogeneity was uncertain (the confidence interval of I2 ranged from 0% to 86%).
During the period covered by the trials (2002–2011) optimal goals for blood pressure after stroke/TIA changed internationally as well as policies to reinforce them. For example in the United Kingdom the introduction of the quality and outcome frameworks payment to GPs in improving usual care management of risk factors may partially explain the failure to provide evidence of intervention effectiveness in some studies.18,22,39 This could be attributed to improved standards of care received by participants from both arms of the trials.
Given the relatively small number of trials that we identified and their small size (the largest only had 349 participants), publication bias is a concern. It is likely to be negative studies that are not published; therefore, this will not affect our finding that no studies have demonstrated an improvement in adherence, but may mean that we have overestimated the value of multifactorial interventions on blood pressure lowering in this population.
Future Work
Future work should improve on the weaknesses of current evidence, yet review of the designs of 8 additional randomized controlled trial protocols identified by the search strategy31–38 showed little sign of doing so. Trials still excluded participants with significant cognitive and communication impairments; only 2 trial protocols took into account stroke disabilities in the form of adding brief one‐on‐one sessions34 or by providing practical problem solving.35
Although most protocols measured both blood pressure and adherence, only 1 used the gold standard objective measure of adherence with electronic pill containers.31 Caregivers were additional recipients of the intervention in only 1 trial protocol35 for participants with stroke and moderate to severe disabilities. Only 1 trial protocol specified measurement along a hypothesized causal pathway,31 with other studies continuing to test multifactorial interventions with poorly specified multiple components, with no details of how to isolate their effects on the outcomes measured.
Conclusions
On the basis of the limited data available, there is evidence that multifactorial interventions can be effective in lowering blood pressure in a selected population of patients with stroke or TIA living in the community, although it is not possible to isolate which component(s) of the interventions account for this effect. The effects size is compatible with a 15% to 20% reduction in stroke recurrences.6
There is a paucity of studies of interventions to improve medication adherence and blood pressure control after stroke/TIA, when disabilities and cognitive impairment might make adherence particularly difficult.
Future studies should focus on characterizing the target groups that might benefit most from novel or better‐applied interventions to improve adherence and include carers as well as patients and a whole healthcare system approach to prescribing and taking medicines. Attention to the reliability and objectivity of adherence and blood pressure measurement is needed. Multifactorial intervention design should enable measurement of intermediate outcomes along a hypothesized causal pathway to allow isolation of active ingredients and cost‐effectiveness evaluation of interventions.
Sources of Funding
Dr De Simoni was funded by an academic clinical fellowship from the National Institute for Health Research (NIHR) and FSF (Flexibility and Sustainability Funding) from NHS Cambridgeshire at the UK Department of Health. Drs Kinmonth and Farmer are NIHR Senior Investigators. Dr Hardeman's contribution to the study was funded by NIHR Flexibility and Sustainability Funding from NHS Cambridgeshire. Dr Farmer's contribution was funded by NIHR School for Primary Care Research and NIHR Oxford Biomedical Research Consortium.
Disclosures
None.
Acknowledgments
We thank Isla Kuhn, Reader Services Librarian, University of Cambridge Medical Library, for help developing the search strategy; Professor Stephen Sutton, the Primary Care Unit, University of Cambridge, for helpful advice on interpretation of study heterogeneity and calculation of the CI of I2; Richard Parker, the Primary Care Unit, University of Cambridge, for statistical advice on meta‐analysis; and the authors of primary studies included in our review, who responded to requests for clarification or further information about study data.
References
- 1.World Health Organization Preventing Chronic Disease: A Vital Investment. 2005Geneva: World Health Organization [Google Scholar]
- 2.Arima H, Tzourio C, Butcher K, Anderson C, Bousser MG, Lees KR, Reid JL, Omae T, Woodward M, MacMahon S, Chalmers JPROGRESS Collaborative Group Prior events predict cerebrovascular and coronary outcomes in the PROGRESS trial. Stroke. 2006; 37:1497-1502 [DOI] [PubMed] [Google Scholar]
- 3.Hankey G, Jamrozik K, Broadhurst R, Forbes S, Burvill PW, Anderson CS, Stewart‐Wynne EG. Long‐term risk of first recurrent stroke in the Perth Community Stroke Study. Stroke. 1998; 29:2491-2500 [DOI] [PubMed] [Google Scholar]
- 4.Mant J, Wade DT, Winner S. In: Stevens A, Raftery J, Mant J, Simpson S. (eds.). Health care needs assessment: stroke. Health Care Needs Assessment: The Epidemiologically Based Needs Assessment Reviews, First Series. 20042nd edOxford: Radcliffe Medical Press; 141-244 [Google Scholar]
- 5.Jørgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS. Stroke recurrence: predictors, severity, and prognosis. The Copenhagen Stroke Study. Neurology. 1997; 48:891-895 [DOI] [PubMed] [Google Scholar]
- 6.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009; 338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ovbiagele B, Diener HC, Yusuf S, Martin RH, Cotton D, Vinisko R, Donnan GA, Bath PMPROFESS Investigators Level of systolic blood pressure within the normal range and risk of recurrent stroke. JAMA. 2011; 306:2137-2144 [DOI] [PubMed] [Google Scholar]
- 8.Intercollegiate Stroke Working Party National Clinical Guidelines for Stroke. 20083rd edLondon: Royal College of Physicians [Google Scholar]
- 9. Management of patients with stroke or TIA: assessment, investigation, immediate management and secondary prevention. Scottish Intercollegiate Guidelines Network. 2008. Available at http://www.sign.ac.uk/pdf/sign108.pdf Accessed 2012.
- 10.Glader EL, Sjolander M, Eriksson M, Lundberg M. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke. 2010; 41:397-401 [DOI] [PubMed] [Google Scholar]
- 11.Schroeder K, Fahey T, Ebrahim S. Interventions for improving adherence to treatment in patients with high blood pressure in ambulatory settings. Cochrane Database Syst Rev. 2004; 2:CD004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hypertension Detection & Follow‐up Program Therapeutic control of blood pressure in the Hypertension Detection and Follow‐up Program. Hypertension Detection and Follow‐up Program Cooperative Group. Prev Med. 1979; 8:2-13 [DOI] [PubMed] [Google Scholar]
- 13.Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010; 3:CD005182. [DOI] [PubMed] [Google Scholar]
- 14.Michie S, Abraham C, Eccles M, Francis J, Hardeman W, Johnston M. Strengthening evaluation and implementation by specifying components of behaviour change interventions: a study protocol. Implement Sci. 2011; 6:1010.1186/1748‐5908‐6‐10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (updated September 2009). 2009West Sussex, UK: Wiley‐Blackwell [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003; 327:557-560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maasland E, Koudstaal PJ, Habbema JD, Dippel DW. Effects of an individualized multimedia computer program for health education in patients with a recent minor stroke or transient ischemic attack—a randomized controlled trial. Acta Neurol Scand. 2007; 115:41-48 [DOI] [PubMed] [Google Scholar]
- 18.Ellis G, Rodger J, McAlpine C, Langhorne P. The impact of stroke nurse specialist input on risk factor modification: a randomised controlled trial. Age Ageing. 2005; 34:389-392 [DOI] [PubMed] [Google Scholar]
- 19.Hornnes N, Larsen K, Boysen G. Blood pressure 1 year after stroke: the need to optimize secondary prevention. J Stroke Cerebrovasc Dis. 2011; 20:16-23 [DOI] [PubMed] [Google Scholar]
- 20.Joubert J, Reid C, Barton D, Cumming T, McLean A, Joubert L, Barlow J, Ames D, Davis S. Integrated care improves risk‐factor modification after stroke: initial results of the Integrated Care for the Reduction of Secondary Stroke model. J Neurol Neurosurg Psychiatry. 2009; 80:279-284 [DOI] [PubMed] [Google Scholar]
- 21.Chiu CC, Wu SS, Lee PY, Huang YC, Tan TY, Chang KC. Control of modifiable risk factors in ischemic stroke outpatients by pharmacist intervention: an equal allocation stratified randomized study. J Clin Pharm Ther. 2008; 33:529-535 [DOI] [PubMed] [Google Scholar]
- 22.Adie K, James MA. Does telephone follow‐up improve blood pressure after minor stroke or TIA? Age Ageing. 2010; 39:598-603 [DOI] [PubMed] [Google Scholar]
- 23.Sit JW, Yip VY, Ko SK, Gun AP, Lee JS. A quasi‐experimental study on a community‐based stroke prevention programme for clients with minor stroke. J Clin Nurs. 2007; 16:272-281 [DOI] [PubMed] [Google Scholar]
- 24.Nir Z, Weisel‐Eichler A. Improving knowledge and skills for use of medication by patients after stroke: evaluation of a nursing intervention. Am J Phys Med Rehabil. 2006; 85:582-592 [DOI] [PubMed] [Google Scholar]
- 25.Banet GA, Felchlia MA. The potential utility of a shared medical record in a “first‐time” stroke population. J Vasc Nurs. 1997; 15:29-33 [DOI] [PubMed] [Google Scholar]
- 26.Johnston SC, Sidney S, Hills NK, Grosvenor D, Klingman JG, Bernstein A, Levin E. Standardized discharge orders after stroke: results of the quality improvement in stroke prevention (QUISP) cluster randomized trial. Ann Neurol. 2010; 67:579-589 [DOI] [PubMed] [Google Scholar]
- 27.Sol BG, van der Graaf Y, van der Bijl JJ, Goessens BM, Visseren FL. The role of self‐efficacy in vascular risk factor management: a randomized controlled trial. Patient Educ Couns. 2008; 71:191-197 [DOI] [PubMed] [Google Scholar]
- 28.Fu D, Fu H, McGowan P, Shen YE, Zhu L, Yang H, Mao J, Zhu S, Ding Y, Wei Z. Implementation and quantitative evaluation of chronic disease self‐management programme in Shanghai, China: randomized controlled trial. Bull World Health Organ. 2003; 81:174-182 [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Y, Li YS, Xu Q, Shi GW, Li HW, Geng JL. The impact of stroke clinic on improving the compliance with the guidelines for secondary prevention of ischemic stroke. Zhonghua Nei Ke Za Zhi. 2007; 46:736-739 [PubMed] [Google Scholar]
- 30.Mols V, Jahn H, Hetzel A, Luckner A, Kampmann M, Niebling W. Quality circles in the secondary prevention of stroke — a controlled interventional study [German] qualitatszirkel in der sekundarpravention nach schlaganfall ‐ eine kontrollierte interventionsstudie. Zeitschrift für Allgemeinmedizin (ZFA). 2005; 81:435-441 [Google Scholar]
- 31.O'Carroll R, Dennis M, Johnston M, Sudlow C. Improving adherence to medication in stroke survivors (IAMSS): a randomised controlled trial: study protocol. BMC Neurol. 2010; 10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacKay‐Lyons M, Gubitz G, Giacomantonio N, Wightman H, Marsters D, Thompson K, Blanchard C, Eskes G, Thornton M. Program of rehabilitative exercise and education to avert vascular events after non‐disabling stroke or transient ischemic attack (PREVENT Trial): a multi‐centred, randomised controlled trial. BMC Neurol. 2010; 10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen V‐HV, Poon J, Tokuda L, Sayers J, Wallis R‐A, Dergalust S. Pharmacist telephone interventions improve adherence to stroke preventive medications and reduce stroke risk factors: a randomized controlled trial. Stroke. 2011; 42/3e244:0039‐-2499 [Google Scholar]
- 34.Cheng EM, Cunningham WE, Towfighi A, Sanossian N, Bryg RJ, Anderson TL, Guterman JJ, Gross‐Schulman SG, Beanes S, Jones AS, Liu H, Ettner SL, Saver JL, Vickrey BG. Randomized, controlled trial of an intervention to enable stroke survivors throughout the Los Angeles County safety net to “stay with the guidelines”. Circ Cardiovasc Qual Outcomes. 2011; 4:229-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dromerick AW, Gibbons MC, Edwards DF, Farr DE, Giannetti ML, Sanchez B, Shara NM, Fokar A, Jayam‐Trouth A, Ovbiagele B, Kidwell CS. Preventing recurrence of thromboembolic events through coordinated treatment in the District of Columbia. Int J Stroke. 2011; 6:454-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldfinger JZ, Kronish IM, Fei K, Graciani A, Rosenfeld P, Lorig K, Horowitz CR. Peer education for secondary stroke prevention in inner‐city minorities: design and methods of the prevent recurrence of all inner‐city strokes through education randomized controlled trial. Contemp Clin Trials. 2012; 33:1065-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacKenzie GL. Tailored interventions to improve hypertension management after stroke or TIA — Phase II (Tims II). Stroke. 2011; 42/11e599:0039‐-2499 [PubMed] [Google Scholar]
- 38.Flemming K, Brown R. Utility of a physician directed, nurse based stroke prevention program. Neurology. 2012; 78 [Google Scholar]
- 39.Wolfe CD, Redfern J, Rudd AG, Grieve AP, Heuschmann PU, McKevitt C. Cluster randomized controlled trial of a patient and general practitioner intervention to improve the management of multiple risk factors after stroke: stop stroke. Stroke. 2010; 41:2470-2476 [DOI] [PubMed] [Google Scholar]
- 40.McManus JA, Craig A, McAlpine C, Langhorne P, Ellis G. Does behaviour modification affect post‐stroke risk factor control? Three‐year follow‐up of a randomized controlled trial. Clin Rehabil. 2009; 23:99-105 [DOI] [PubMed] [Google Scholar]
- 41.Warlow C. Stroke: Practical Management. 20083rd edOxford, UKBlackwell Publishing: 598 [Google Scholar]
- 42.Kelly‐Hayes M, Beiser A, Kase CS, Scaramucci A, D'Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003; 12:119-126 [DOI] [PubMed] [Google Scholar]
- 43.Jokinen H, Kalska H, Mantyla R, Pohjasvaara T, Ylikoski R, Hietanen M, Salonen O, Kaste M, Erkinjuntti T. Cognitive profile of subcortical ischaemic vascular disease. J Neurol Neurosurg Psychiatry. 2006; 77:28-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nyenhuis DL, Gorelick PB, Geenen EJ, Smith CA, Gencheva E, Freels S, deToledo‐Morrell L. The pattern of neuropsychological deficits in vascular cognitive impairment‐no dementia (Vascular CIND). Clin Neuropsychol. 2004; 18:41-49 [DOI] [PubMed] [Google Scholar]
- 45.Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz L, Looi JC, Wen W, Zagami AS. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004; 62:912-919 [DOI] [PubMed] [Google Scholar]
- 46.Leys D, Hénon H, Mackowiak‐Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005; 4:752-759 [DOI] [PubMed] [Google Scholar]
- 47.Young J, Forster A. Review of stroke rehabilitation. BMJ. 2007; 334:86-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vermeire E, Wens J, Van Royen P, Biot Y, Hearnshaw H, Lindenmeyer A. Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005; 2:CD003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ireland S, MacKenzie G, Gould L, Dassinger D, Koper A, LeBlanc K. Nurse case management to improve risk reduction outcomes in a stroke prevention clinic. Can J Neurosci Nurs. 2010; 32:7-13 [PubMed] [Google Scholar]
- 50.Davidson KW, Goldstein MG, Kaplan RM, Kaufmann PG, Knatterud GL, Orleans CT, Spring B, Trudeau KJ, Whitlock EP. Evidence‐based behavioral medicine: what is it and how do we achieve it? Ann Behav Med. 2003; 26:161-171 [DOI] [PubMed] [Google Scholar]
- 51.De Simoni A, Kellar I, Sutton S, Farmer A, Blenkinsopp A, Kinmonth AL, Mant J. Developing a Pharmacist Intervention to Improve Adherence to Hypertensive Medications in TIA/Stroke Patients. 2010Norwich: SAPC Annual Conference Abstracts [Google Scholar]
- 52.Chambers JA, O'Carroll RE, Hamilton B, Whittaker J, Johnston M, Sudlow C, Dennis M. Adherence to medication in stroke survivors: a qualitative comparison of low and high adherers. Br J Health Psychol. 2011; 16:592-609 [DOI] [PubMed] [Google Scholar]
- 53.O'Carroll R, Whittaker J, Hamilton B, Johnston M, Sudlow C, Dennis M. Predictors of adherence to secondary preventive medication in stroke patients. Ann Behav Med. 2011; 41:383-390 [DOI] [PubMed] [Google Scholar]
- 54.Travis SS, Bethea LS, Winn P. Medication administration hassles reported by caregivers of dependent elderly persons. J Gerontol. 2000; 55:M412-M417 [DOI] [PubMed] [Google Scholar]




