Abstract
Lipopolysaccharide (LPS, endotoxin) is a structural component of the Gram negative outer membrane. The lipid A moiety of LPS binds to the LPS receptor complex expressed by leukocytes, endothelial cells and parenchymal cells and is the primary component of Gram negative bacteria that is recognized by the immune system. Activation of the LPS receptor complex by native lipid A induces robust cytokine production, leukocyte activation and inflammation, which is beneficial for clearing bacterial infections at the local level but can cause severe systemic inflammation and shock at higher challenge doses. Interestingly, prior exposure to LPS renders the host resistant to shock caused by subsequent LPS challenge, a phenomenon known as endotoxin tolerance. Treatment with lipid A has also been shown to augment the host response to infection and to serve as a potent vaccine adjuvant. However, the side effects associated with the pronounced inflammatory response limits the use of native lipid A as a clinical immunomodulator. More recently, analogs of lipid A have been developed that possess attenuated pro-inflammatory activity but retain attractive immunomodulatory properties. The lipid A analog monophosphoryl lipid A (MPLA) exhibits approximately 1/1000th of the toxicity of native lipid A but retains potent immunoadjuvant activity. As such, MPLA is currently employed as an adjuvant in several human vaccine preparations. Due to the potency of lipid A analogs as immunoadjuvants, numerous laboratories are actively working to identify and develop new lipid A mimetics and to optimize their efficacy and safety. Based on those characteristics, lipid A analogs represent an attractive family of immunomodulators.
Keywords: endotoxin, lipid A, TLR4, adjuvant, innate immunity, TRIF, MyD88
Lipopolysaccharide recognition and signaling
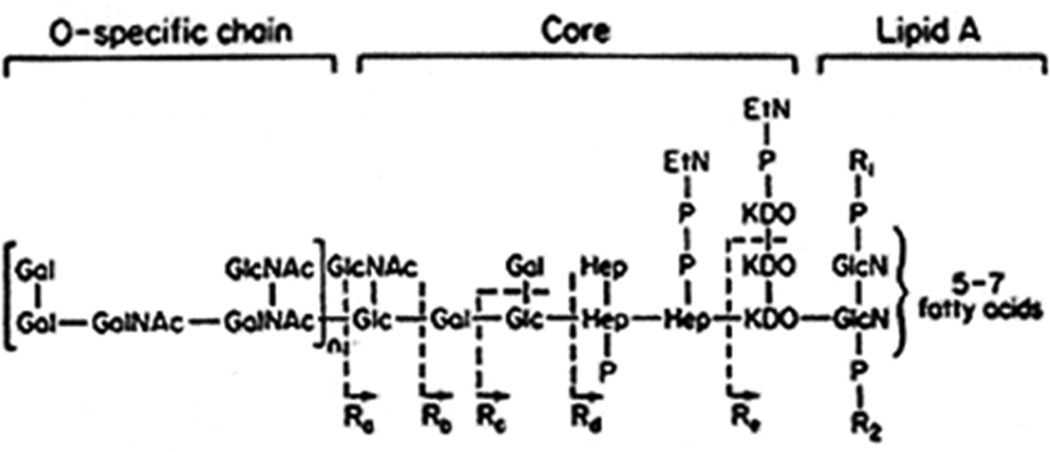
Lipopolysaccharide (LPS, endotoxin) is a glycolipid that is embedded in the outer membrane of Gram negative bacteria and plays a crucial role in maintaining the structural integrity of the organism (1, 2). LPS is composed of 3 major biochemical domains (Figure 1). The O-specific chain is a repetitive glycan polymer that projects outside of the outer membrane onto the surface of bacteria. The presence and structure of the O-specific chain contributes to the antigenicity, morphological appearance and antibiotic sensitivity of Gram negative bacteria (3). The core domain is a phosphorylated oligosaccharide that links the O-specific chain to the lipid A moiety and is important for maintaining structural viability (4). Lipid A is a heat-stable phosphorylated glucosamine disaccharide with multiple fatty acid side chains that anchors the LPS molecule into the lipid bilayer of the bacterial outer membrane (5). Release of lipid A into the local environment requires disruption of the microbial outer membrane, which occurs as a result of microbial proliferation and death.
Figure 1.
Generic structure of lipopolysaccharide. Lipopolysaccharide is comprised of three major structural components, the outward facing O-specific chain, the core segment that links the O-specific chain to lipid A and the lipid A component that embeds in the outer membrane of Gram negative bacteria.
Lipid A is avidly recognized by leukocytes and other cell types and is the major factor that alerts the immune system to the presence of infection with Gram negative organisms. The importance of lipid A recognition is evident by the widespread expression of the endotoxin receptor complex on macrophages, neutrophils, dendritic cells, mast cells, endothelial cells and natural killer cells as well as several populations of parenchymal cells (6, 7). Cells recognize lipid A via a surface receptor complex that is composed of the proteins myeloid differentiation factor 2 (MD2) and toll-like receptor 4 (TLR4) (8, 9) (Figure 2). LPS binding protein (LBP) as well as the membrane-bound and soluble forms of CD14, bind LPS in the systemic and interstitial environments and play important roles in facilitating the presentation of LPS to the endotoxin receptor complex (10). Binding of LPS to MD2 causes conformational changes in TLR4 that facilitate TLR4 dimerization or oligomerization and activation of downstream signaling (9, 11). TLR4 activation triggers two major downstream intracellular signaling pathways, one that depends on the adaptor protein myeloid differentiation factor 88 (MyD88) and the other that requires recruitment of Toll-interleukin-1 (IL-1) receptor (TIR)-domain–containing adaptor inducing interferon-β (TRIF) (12) (Figure 2). Activation of the MyD88-dependent signaling pathway is precipitated by conformational changes in the intracellular TIR domain of TLR4, which facilitates recruitment of the linking proteins TIRAP and MyD88 (13, 14). The clustering of IRAK4 molecules on MyD88 facilitates IRAK1 phosphorylation and activation, recruitment of TRAF6 and engagement of TAK1 (15). TAK1 activates mitogen-activated protein (MAP) kinases and the transcription factor activator protein (AP)-1 as well as I kappa B kinases (IKK) that facilitate activation of the nuclear factor kappa B (NF-κB) pathway. AP-1 and NF-κB translocate to the nucleus to facilitate transcription of pro-inflammatory gene products such as tumor necrosis factor α (TNFα), interleukin-12 (IL-12) and inducible nitric oxide synthase (iNOS), among others (16).
Figure 2.
LPS receptor signaling pathways. Activation of the LPS receptor complex induces TLR4 dimerization/oligomerization with rapid activation of the MyD88-depedent signaling pathways as described in the text. Activated TLR4 is then endocytosed and the TRIF-dependent signaling pathway is induced.
After activation of the MyD88-associated pathway, the LPS/MD-2/TLR4 conglomerate translocates to endosomes and associates with the linking proteins TRIF and TRAF3 (Figure 2). Endosomal compartmentalization is made possible through activation of phosphatidylinositol-3-OH kinase (PI3K), specifically the p110 isoform which leads to the generation of phosphatidylinositol-[3,4,5]-triphosphate (IPI3). The result is dissociation of MAL from the plasma membrane leading to its degradation (17). TRIF facilitates delayed activation of the MAP kinase/AP-1 and NF-κB signaling pathways through engagement of TRAF-6 and RIP-1, respectively (18). The TBK/interferon regulatory factor 3 (IRF3) pathway is activated by TRAF3 and results in the production of type I interferons (IFN) and their associated gene products (19). The endotoxin receptor complex is ultimately sorted to late endosomes/lysosomes for degradation and signal termination (20). TLR4 is unique among toll-like receptors in that its activation induces both MyD88- and TRIF-dependent signaling (21). All other TLRs selectively signal through either the MyD88- or TRIF-dependent signaling pathways.
The family of TLR4 agonists
Lipid A is the bioactive component of the LPS molecule. Native Escherichia coli (E. coli) lipid A is composed of a disaccharide backbone containing 2 phosphate groups and 6 acyl side chains (Figure 3) (22). That structural conformation, which is common to many Gram negative bacteria, strongly activates TLR4 signaling and has potent pro-inflammatory activity (23). Yet, alterations in lipid A structure can markedly affect host responses. Differences in the number, arrangement and length of acyl side chains as well as the location and conformation of charged groups greatly alter the biological properties of lipid A mimetics. Hexaacylated, diphosphoryl lipid A, as described above for native lipid A from E. coli, potently induces pro-inflammatory cytokine production. However, tetraacylated lipid A is a TLR4 antagonist (4). Other manipulations of the lipid A structure result in molecules that preferentially activate MyD88- or TRIF-dependent signaling, respectively. Large scale MyD88 activation causes production of pro-inflammatory mediators, which are important for local clearance of bacteria but can be harmful when overproduced systemically (24). Thus, the inflammatory side effects of most MyD88-biased immunomodulators precludes their use in the clinical setting (25, 26). Activation of the TRIF-dependent signaling pathway induces production of type I interferons (IFNα/β), which are important for generating an effective host response to viral and bacterial infection and facilitating the immunoadjuvant activity of lipid A mimetics (27) (Figure 2). Recent studies indicate that TRIF-biased TLR4 agonists are attractive immunomodulators due to their potency as immunoadjuvants and low inflammatory toxicity (28, 29).
Figure 3.
Molecular structures of native E. coli lipid A and monophosphoryl lipid A. MPLA is produced either de novo or by hydrolysis of native diphosphoryl lipid A, leading to the removal of one phosphate group and altered fatty acid side chains, resulting in a conformation with substantially reduced toxicity.
Investigators in academia and industry are working to produce lipid A mimetics that have desirable biological properties using both de novo synthesis and bacterial engineering. The de novo synthesis of homogeneous lipid A mimetics has been complicated by difficulties in producing compounds that possess consistent acyl chain length and conformation. Bioengineering approaches have encountered similar difficulties. Bacteria have been generated that can produce monophophorylated lipid A but challenges have arisen in generating moieties with optimized acyl chain numbers and conformation. Numerous efforts have been made to engineer E. coli and Salmonella species to produce single lipid A species. Those attempts have been complicated by numerous factors, the most important being the requirement of LPS heterogeneity to optimize the stability of the bacterial outer membrane. However, recent studies have reported the use of combinatorial enzyme expression strategies that appear effective in engineering E. coli to produce selective and structurally homogeneous lipid A subtypes and possess potent immunoadjuvant activity (22).
Monophosphoryl lipid A (MPLA) is a heterogeneous mixture of lipid A derivatives created by successive acid and base hydrolysis of lipid A from Salmonella minnesota 595 (30). The predominant species created from that process is 3-O-deacyl-4-monophosphoryl lipid A (Figure 3). MPLA possesses attractive biological characteristics as an immunoadjuvant such as augmentation of T helper 1 (Th1) activity and antigen-induced T cell clonal expansion. Systemic administration of MPLA will induce endotoxin tolerance in humans and experimental animals as indicated by a reduction of circulating pro-inflammatory cytokine concentrations and attenuation of hemodynamic alterations in response to a subsequent LPS challenge (31, 32). Yet, MPLA possesses approximately 1/1000th of the systemic pro-inflammatory activity of native E. coli lipid A in humans (4). That characteristic is likely due to weak MPLA-induced activation of the MyD88-dependent signaling pathway. Compared to native lipid A, MPLA inefficiently induces recruitment of TRAF6 to IRAK-1 resulting in attenuated activation of MAP kinase and NF-κB signaling, an effect that appears to be secondary to inefficient MPLA-induced TLR4/MD-2 heterodimerization (33). However, MPLA potently activates TRIF-dependent signaling as indicated by phosphorylation of IRF3 and induction of TRIF-biased gene products such as CXCL10, MCP-1 and RANTES (22, 28). Due to the described biological properties, alum-absorbed MPLA has gained worldwide acceptance as an adjuvant in vaccine preparations and is a component of commercially available papilloma and hepatitis virus vaccines (34). Thus, MPLA currently serves as the standard for TLR4-based immunoadjuvants.
Other attractive lipid A mimetics are also under development. Of note, Bowen et al described a series of monosaccharide lipid A mimetics termed aminoalkyl glucosamine-4-phosphates (AGPs) and reported the biological properties of two AGPs known as CRX-527 and CRX-547 (Figure 3) (35). The compounds contain three (R)-3-decanoyloxytetradecanoyl residues that are N- or O-linked to an O-glucosaminyl serine backbone. They differ only in the configuration of the D-glycon stereocenter. CRX-527 potently induces production of both MyD88- (e.g. TNF α) and TRIF (e.g. CXCL10, RANTES)-dependent cytokines by cultured human monocytes and dendritic cells whereas CRX-547 retains the ability to induce TRIF-dependent cytokines while production of MyD88-dependent gene products is attenuated. CRX-547-induced RANTES production requires TLR4 endocytosis and activation of TRIF-dependent signaling. NF-κB pathway activation and translocation is minimal. Thus, CRX-547 has characteristics that are biologically similar to MPLA. The efficacy of CRX-547 as an immunomodulator in vivo remains to be further investigated.
The phenomenon of endotoxin tolerance
Exposure to low or moderate doses of LPS or lipid A results in a state of “endotoxin tolerance” that renders the host hyporesponsive to a subsequent LPS or lipid A challenge. Endotoxin tolerance is characterized by attenuated production of pro-inflammatory cytokines such as TNFα, IL-6 and interferon (IFN) γ, and increased production of anti-inflammatory mediators such as IL-10, TGFβ and IL-1 receptor antagonist (IL-1ra) in response to a second endotoxin challenge (36–38). Those alterations allow the endotoxin tolerant host to survive a normally lethal secondary challenge with endotoxin. Thus, endotoxin tolerance is thought to be an adaptive mechanism designed to protect the host from inflammatory injury caused by repeated or excessive exposure to LPS or Gram negative infection (39). Moreover, the phenomenon is not limited to protection from high dose LPS challenge. LPS priming also attenuates pro-inflammatory cytokine production in response to other inflammatory stimuli such as Gram positive infection, ischemia-reperfusion injury or hemorrhagic shock (39–41). The primary cells involved in endotoxin tolerance are monocytes, dendritic cells and macrophages, essentially all cells that express CD14 (42, 43). On initial exposure to LPS, an increased production of IL-1, TNFα, IL-6 and IL-8 is observed. However, in cells that are CD14+, re-exposure to LPS results in reduced cytokine production (43).
Cross tolerance has also been shown to exist between TLR agonists. LPS exposure attenuates pro-inflammatory cytokine production in response to agonists for other toll-like receptors (TLR2, TLR5, etc) whereas non-TLR4 agonists induce cross tolerance to LPS challenge (44–46). Some concerns exist that perceived cross tolerance among TLR agonists may have been caused by contamination of supposedly pure TLR agonist preparations with agonists for other TLR (47). For example, some studies were performed using LPS preparations that may have been contaminated with agonists for TLR2. However, follow-up studies using ultrapure TLR agonist preparations have supported the concept of cross-tolerance between TLR agonists (48). Thus, the phenomenon of LPS tolerance appears to be a broadly applied mechanism to regulate acute pro-inflammatory responses and protect the host from inflammation-induced injury.
A variety of molecular mechanisms have been described that underlie the development of endotoxin tolerance, which might best be characterized as a state of “cellular reprogramming” (41). Defects in TLR4 signaling have been described at the receptor, adaptor protein, signaling molecule and transcription factor levels and likely represent negative feedback at multiple levels (39) (Table 1). At the present time, most alterations in TLR4 signaling have mapped to the MyD88-dependent pathway. However, some studies have implicated a role for the TRIF pathway in the development of LPS tolerance but further research is needed to define specific mechanisms (19, 49).
Initial LPS exposure induces robust TLR4-mediated activation of NF-κB and AP-1 (Figure 2). As noted earlier in this review, NF-κB and AP-1 translocation are the major factors that regulate the expression of pro-inflammatory gene products in response to LPS (50). At the same time, inhibitors of TLR4, NF-κB and AP-1 signaling are induced such as inhibitor of κB (IκB), MAP kinase phosphatase-1, interleukin receptor-associated kinase M (IRAK-M), suppressor of cytokine signaling-1 (SOCS-1) and RelB (36, 51, 52). Those inhibitors attenuate NF-κB and AP-1 activation and translocation and serve to regulate the LPS-induced inflammatory response to prevent uncontrolled expression of pro-inflammatory mediators. IκBα sequesters p65/RelA heterodimers in the cytoplasm, reduces nuclear translocation and decreases production of LPS-induced gene products (53). The function of interleukin receptor associated kinase 1 (IRAK-1), which is essential for mobilization of p65 from the cytoplasm, is disrupted by IRAK-M (54). RelB forms heterodimers with p50 and generates a transcriptional suppressor that decreases LPS-induced pro-inflammatory gene expression (55). Additional studies show that RelB silences gene transcription by binding to histone methyltransferase G9A (56). Similarly, over-expression of NF-κB p50 homodimers has been described during endotoxin tolerance. The transcriptionally inactive p50 homodimers compete for binding with activating p50/p65 on NF-κB consensus sequences on gene promoters and act to inhibit pro-inflammatory gene expression (57). MAPK phosphatase-1 serves to reduce MAP kinase phosphorylation and, ultimately, attenuates AP-1 translocation (58). Evidence indicates that LPS-induced expression of MAP kinase phosphatase-1, RelB and IRAK-M are induced by activation of the phosphoinositide-3-kinase (PI3K) pathway. Thus, PI3K activation may facilitate the development of LPS tolerance (59).
At the nuclear level, changes in histone methylation, acetylation and ubiquitination have been described that cause changes in gene transcription. Foster and colleagues described selective chromatin modifications after LPS exposure that resulted in suppressed expression of many pro-inflammatory gene products but sustained or increased suppression of other gene products that are typically important for anti-microbial immunity (60). Other recent work has implicated inhibitory microRNA (miRNA) expression as a mechanism of post-transcriptional down-regulation of pro-inflammatory gene expression after LPS exposure. LPS treatment augments the expression of miRNAs such as miR146 and miR155 (61). The miR146 attenuates pro-inflammatory gene expression by antagonizing IL-1R and TLR4 signaling through post-transcriptional regulation of IRAK-1 and TRAF-6 (62). IKKε is targeted by miR155, resulting in alterations in TLR4/NF-κB signaling (63). Altered expression of other miRNAs has also been described in models of endotoxin tolerance. Further work is needed to fully define their molecular mechanisms of action. Histone methylation is another documented mechanism of pro-inflammatory gene silencing during endotoxin tolerance. Studies show that chromatin binding of HMGB1 and histone H1, which is facilitated by G9A-facilitated histone H3K9 methylation, is responsible for TNFα gene silencing in endotoxin tolerant THP-1 human monocytes (64, 65).
Suppression of cytokine signaling has also been observed during endotoxin tolerance. SOCS-1 is a potent inhibitor of JAK-STAT signaling. Nakagawa et al (66) reported that SOCS-1 is rapidly induced by LPS and that SOCS-1-deficient mice are highly sensitive to LPS-induced inflammatory injury. The group further reported that SOCS-1 deficient mice do not develop endotoxin tolerance. In a separate study, Liu and colleagues reported that SOCS-1 facilitated the endotoxin tolerant phenotype by inhibiting NF-κB signaling (67).
In contrast to LPS tolerance, more recent work has shown that exposure to ultralow doses of LPS (<100 pg/ml) will actually prime the host to mount an exaggerated inflammatory response. Work by Deng et al shows that exposure to very low dose LPS removes transcriptional suppressors from the promoters of pro-inflammatory genes and sensitizes the host to inflammatory challenge (59). In that setting, the production of pro-inflammatory mediators is amplified in response to a secondary inflammatory stimulus, resulting in amplification of inflammatory injury. Evidence indicates that low dose LPS exposure suppresses PI3K signaling and, consequently, RelB expression. The decrease in RelB primes the host for an amplified pro-inflammatory response upon secondary LPS exposure. This is in contrast to RelB induction that occurs in response to treatment with LPS at doses of > 1 ng/ml. Based on their findings, Deng et al postulate that PI3K/RelB signaling serves as a switch to differentiate between LPS priming and tolerance (59). They further hypothesize that chronic exposure to low concentrations of LPS may predispose patients with obesity, aging or low grade infection to morbidity and mortality in response to a normally innocuous inflammatory insult.
Many investigators have postulated that endotoxin tolerance renders the host more susceptible to secondary infections and represents a state of immunosuppression. This presumption is based on the attenuated pro-inflammatory cytokine response present in the endotoxin tolerant host, which has also been observed in patients suffering sepsis, major trauma and thermal injury, all of which are predisposed to secondary infections (41, 68, 69). Monocytes and macrophages harvested from immunocompromised septic patients exhibit suppressed LPS-induced cytokine production, a characteristic that resembles the endotoxin tolerant phenotype (39). However, recent evidence indicates that prior exposure to lipid A mimetics may actually augment the host response to infection. Thus, despite affecting cytokine responses in similar ways, the functional response to secondary infection may be quite different when comparing the LPS tolerant host to those suffering trauma, burns and sepsis.
Biological properties of TLR4-based immunoadjuvants
Due to their ability to stimulate innate and adaptive immune responses, TLR4 agonists have emerged as attractive vaccine adjuvants. The high fidelity antibody responses that are induced by modern vaccines require T cell help to facilitate isotype switching, B cell maturation and more robust immunoglobulin production (Figure 4). TLR4 agonists induce T cell activation, clonal expansion and Th1 polarization indirectly through stimulation and recruitment of antigen-presenting cells (APC) such as dendritic cells, macrophages and monocytes (28, 70). Kwissa and colleagues showed that intradermal injection of MPLA into non-human primates induces recruitment of CD14+CD16− monocytes and myeloid dendritic cells into draining lymph nodes (70). Native lipid A also potently induces chemoattraction of APC at sites of injection (71). The recruited APC exhibit an activated phenotype characterized by increased class II major histocompatibility complex (MHCII) and co-stimulatory (e.g. CD80/86/40) molecule expression, more robust antigen presentation and enhanced cytokine and chemokine production (72, 73).
Figure 4.
Cellular mechanisms by which TLR4 agonists mediate immunoadjuvant functions. TLR4 agonists facilitate T cell clonal expansion and activation by augmenting cytokine production and antigen presentation. The augmentation of Th1 polarity and activation facilitates B cell immunoglobulin production.
Unlike alum, which tends to generate Th2 responses, TLR4 agonists facilitate Th1 polarization characterized by robust production of IFNγ and isotype switching to opsinizing IgG1 and IgG3 immunoglobulins (74–76). MPLA, as well as other TLR4 agonists, also potently induce antigen-specific T cell clonal expansion and primes the expanded T cell populations for long-term survival (28, 77). Although B cells are reported to express TLR4 and play an active role in eliciting T cell help during humoral immune responses, little is known about the direct impact of TLR4 agonists on the antigen presenting and T cell activation properties of B cells. Based on current understanding, it appears that the expansion of antigen specific Th1 cells by APC facilitates B cell-mediated T cell help, which augments B cell activation, isotype switching and immunoglobulin production (Figure 4).
MPLA is currently the only TLR4 agonist employed in commercially available vaccine preparations and is a component of currently available human papilloma virus (HPV) and hepatitis B vaccine preparations (28). Adding MPLA to vaccine preparations boosts serum antibody titers by 10–20 fold compared to vaccine alone and preferentially induces production of IgG2a (78). Current vaccines utilize MPLA absorbed onto aluminum hydroxide or aluminum phosphate, a preparation known as ASO4. Addition of ASO4 to existing hepatitis B vaccine induced higher antibody titers and increased rates of seroprotection in immunocompromised patients (79). ASO4 was also effective in boosting antibody titers when added HPV-16 and HPV-18 vaccines (80). In all cases, MPLA-containing vaccine preparations were well tolerated and generated side effect profiles that were similar to existing vaccines (81). A small increase in the incidence of pain, erythema and swelling at the injection site was noted in ASO4-containing vaccine preparations but compliance with the vaccine schedule was maintained and the incidence of serious events was not increased (82, 83). That safety profile was sufficient to allow for licensure of ASO4-containing vaccines in Europe, the United States and Argentina. Due to the success of MPLA as a vaccine adjuvant, numerous labs have produced and evaluated new lipid A mimetics as potential vaccine adjuvants and immunomodulators (35, 75, 84). However, none of the other preparations are currently available in commercially produced vaccine preparations.
Recent studies show that the immunoadjuvant effects of TLR4 agonists are biased toward activation of the TRIF-dependent signaling pathway. Mata-Haro and colleagues reported that MPLA is a weak inducer of MyD88-dependent cytokine production compared to LPS but is equipotent with LPS as an inducer of TRIF-biased cytokines and T cell clonal expansion (28). They further demonstrated that TLR4-mediated T cell activation and clonal expansion is attenuated in mice lacking TRIF-, but not MyD88-, dependent signaling. In further studies, Gandhapudi et al confirmed the importance of TRIF-dependent signaling for TLR4 agonist-induced APC maturation as well as T cell clonal expansion and survival (85). They showed that type I IFN is the central TRIF-associated gene product driving TLR4-facilitated immunoadjuvant effects including APC maturation and T cell proliferation and survival, which is consistent with reports by other investigators showing the importance of type I IFN for augmentation of antigen presentation and immunoadjuvant activity (86). Thus, current reports indicate that TRIF-biased signaling is important for facilitating the immunoadjuvant effects of TLR4 agonists. However, further research is needed to fully define the immunological mechanisms involved. Nevertheless, investigators in industry and academia are actively pursuing the development of TLR4 agonists that preferentially induce TRIF-dependent signaling.
Augmentation of innate antimicrobial immunity by TLR4 agonists
Although TLR4 agonists have gained acceptance as vaccine adjuvants, less attention has been paid to their effectiveness as agents to augment innate antimicrobial responses. LPS has long been recognized as an agent with potent immunomodulatory properties. However, its significant toxicity has precluded its use as an immunomodulator in humans. In addition, systemic administration of LPS causes development of endotoxin tolerance and there is a persistent impression that the endotoxin tolerant phenotype represents a state of immunosuppression. That impression is based on the observation that pro-inflammatory cytokine production in response to secondary inflammatory stimuli is suppressed in animals that received prior LPS exposure, a characteristic that is also common in conditions that are known to render the host more susceptible to infection such as sepsis, trauma or major burns (87–89). Legitimate concerns have been raised as to whether suppression of inflammatory responses during endotoxin tolerance would interfere with normal anti-microbial immune responses and thus predispose patients to nosocomial infection. Intact cytokine responses have proven to be necessary for elimination of microbial pathogens during infection of naïve animals with small numbers of replicating pathogen. Furthermore, mice deficient in pro-inflammatory cytokines such as IFN-γ and TNF-α are resistant to inflammatory injury but are highly susceptible to otherwise sub-lethal bacterial infections (90–92). Additionally, endotoxin tolerance leads to an increase in the production of the anti-inflammatory cytokine IL-10 in response to secondary infectious challenge. The effects of IL-10 include inhibition of Th1 cytokine production and attenuation of MHC class II and co-stimulatory molecule expression on macrophages (93, 94). Elevated IL-10 levels are also characteristic following severe trauma and may contribute to post-injury immunoparalysis (69). Exogenous administration of IL-10 leads to suppression of endotoxin-induced IFN-γ and IL-12 production in the same way, leading to suppression of immune responses and increased susceptibility to infection (95). Thus, the role of IL-10 in perpetuating susceptibility to infection is well documented (96–98). However, Varma et al. demonstrated that, while endotoxin tolerant mice exhibit depressed IFN-γ and IL-12 and elevated IL-10 production in response to infection, LPS-primed mice were able to clear a Pseudomonas aeruginosa infection more effectively than non-tolerant mice (99). Depletion of IL-10 in endotoxin tolerant mice further augmented bacterial clearance, suggesting IL-10 does have a negative impact on the clearance of bacteria, but not enough to negate the beneficial effects of endotoxin priming on bacterial clearance.
Although a small number of studies reported increased susceptibility to infection after prior LPS exposure (100), an emerging body of literature demonstrates that systemic treatment with low to moderate doses of LPS will augment innate antimicrobial responses in a variety of infection models. Deng et al. demonstrate that higher levels of LPS in mice, whether through exogenous administration or through impaired LPS clearance, lead to enhanced macrophage-mediated clearance of bacteria in a model of polymicrobial sepsis (101). Mice treated with LPS show a drastic reduction in bacterial burden after challenge with either Pseudomonas aeruginosa or Salmonella enterica serovar Typhimurium despite markedly reduced serum and lung IFN-γ, TNF-α, IL-6 and IL-12 concentrations (102, 103). Since bacterial clearance was enhanced in the face of decreased IFN-γ and IL-12 production, this suggests that these pro-inflammatory cytokines are not necessary for a competent innate immune response to infection in mice receiving prophylactic treatment with LPS. However, the reduced cytokine production observed in infected LPS-primed mice may also be due to improved bacterial clearance and reduced systemic inflammation due to a decreased bacterial burden. Further studies are needed to define the mechanisms by LPS priming improves bacterial clearance in the face of reduced systemic cytokine production.
Pre-treatment with LPS also elicits protection against infection with organisms other than gram-negative bacteria. In a mouse model of Cryptococcus neoformans fungal infection, LPS pre-treatment (2.5 ug) for two days resulted in decreased fungal burden and improved survival but lower levels of IL-1β, TNF-α, IFN-γ, and IL-6 in the spleen, blood and lungs in response to fungal infection (104). Additionally, LPS pre-treatment facilitated enhanced bacterial clearance and improved survival in a model of mouse Staphylococcus aureus infection (105). In further studies, Wheeler et al. demonstrated improved survival and enhanced bacterial clearance following LPS-pre-treatment in a model of polymicrobial sepsis induced by cecal ligation and puncture (CLP) (106). Thus, it appears that endotoxin-induced augmentation of innate antimicrobial immune responses is not limited to LPS-containing Gram negative organisms, but extends to a variety of bacterial and fungal infection models. Furthermore, LPS from E. coli-, Pseudomonas and Salmonella are equally effective at improving bacterial clearance and decreasing mortality after challenge with a variety of organisms (99, 102, 103, 105). Thus, the origin of the LPS used for priming is irrelevant to its function and LPS appears to induce nonspecific enhancement of innate antimicrobial immunity against a wide spectrum of pathogens.
Monophosphoryl lipid A
As mentioned previously in this review, the heightened toxicity of LPS in humans has precluded its use as an immunomodulator in clinical studies. The identification of less toxic derivatives of LPS, has allowed for more clinically relevant investigations of TLR4 agonists. Numerous reports indicate that a variety of TLR4 agonists possess the ability to endotoxin tolerance and enhance host responses to microbial pathogens. MPLA preferentially induces TLR4 signaling through the TRIF-dependent signaling pathway, resulting in the stimulation of beneficial immune responses without the excessive production of pro-inflammatory cytokines (28). MPLA can be infused into humans at 5 ug/kg without significant toxicity, a dose that is at least 1000-fold higher than the tolerable dose of native lipid A (31). MPLA will also augment innate host resistance to infection in experimental models. Roquilly et al reported that treatment of mice exposed to non-lethal hemorrhagic shock protects from developing post-injury pneumonia (107). Other investigators have reported enhanced host resistance to infection with H. influenza, M. catarhallis, E. coli and S. epidermidis after MPLA treatment (108, 109). Romero et al reported the protection of mice from clinically relevant models of systemic bacterial infection following prophylactic treatment with MPLA (110). Both intravenous and intraperitoneal pre-treatment with MPLA improved survival, enhanced bacterial clearance and attenuated pro-inflammatory cytokine production during polymicrobial infection induced by CLP. In further studies, that group showed that treatment of burned mice with MPLA enhanced their resistance to Pseudomonas burn wound infection. This protection was mediated, in part, by enhanced recruitment of myeloid cells to the site of infection, most notably neutrophils.
Aminoalkyl glucosaminide 4-phosphates (AGPs)
In addition to MPLA, a family of synthetic lipid A mimetics termed aminoalkyl glucosaminide phosphates (AGPs) can also facilitate innate resistance to infectious challenge. Prophylactic treatment of mice with AGPs has been shown to improve survival following lethal challenge with Listeria monocytogenes or influenza virus. Protection was mediated by a reduction in systemic viral or bacterial load in inoculated animals, and was found to be TLR4-dependent (111). Another study found the prophylactic treatment of mice with AGPs led to protection from Yersinia pestis infection, an effect that was also found to be dependent upon TLR4 signaling, as TLR4-deficient mice were not protected by MPLA treatment. Protection was associated with a reduction of bacterial load in lung tissue and an enhanced mobilization of neutrophils to the lung (112). In further studies, AGP treatment was shown to decrease bacterial burden and improve survival in an experimental model of pneumonic tularemia (113).
The prospect of developing prophylactic therapies that enhance innate microbial immunity while controlling excessive inflammation may be particularly beneficial for atrisk or immune compromised individuals who are at increased risk of developing severe secondary and nosocomial infections. This approach has significant clinical relevance, as TLR4 agonists could be administered to patients prior to undergoing high risk surgical procedures to lessen the incidence or severity of postoperative infection and inflammation-induced morbidity. Patients that have suffered major trauma or burns or that have survived the acute phase of sepsis are also at increased risk of developing nosocomial infections and might benefit from immunoprophylaxis facilitated by administration of lipid A mimetics.
Cellular mechanisms by which TLR4 agonists enhance innate anti-microbial responses
The mechanisms by which pre-treatment with TLR4 agonists leads to enhanced clearance of infection to subsequent pathogen exposure remain to be fully elucidated. Several studies have focused on the enhancement of innate effector cell responses induced by prior exposure to TLR4 agonists. Enhancements in the recruitment and phagocytic functions of macrophages and neutrophils have been observed. Improved recruitment, expansion and/or functional modifications of these innate effector cells responsible for pathogen clearance could explain enhanced clearance of infection.
Wheeler and colleagues demonstrated that LPS pre-treatment improved bacterial clearance and survival in a model of CLP-induced sepsis. They further showed that LPS treatment increased phagocytosis by macrophages after in vivo infection with fluorochrome-tagged E. coli or S. aureus (106). These findings confirmed earlier reports which showed that preconditioning macrophages and mononuclear cells with small doses of LPS resulted in both decreased pro-inflammatory cytokine production in response to a subsequent dose of LPS in vitro or in vivo and increased bacterial phagocytosis (114). Other studies have demonstrated a potential role of hepatic macrophages (Kupffer cells) in LPS-augmented bacterial clearance (103, 115, 116).
Increased recruitment of innate effector cells to the site of infection may also underlie enhanced bacterial clearance in mice receiving prophylactic treatment with TLR4 agonists. Some studies have shown that both LPS and MPLA facilitate local recruitment of neutrophils to sites of infection but do not change the phagocytic activity of macrophages and neutrophils on a per cell basis (104, 106, 110, 117). The accumulation of neutrophils at the site of infection appears to be critical for augmented bacterial clearance since neutrophil depletion ablated the beneficial antimicrobial effects of lipid A mimetics. Yet, the mechanisms by which TLR4 agonists facilitate myeloid cell recruitment to sites of infection remain to be determined. However, several potential mechanisms warrant investigation (Table 2). Perhaps TLR4 agonist pre-treatment is able to enhance the directionality and/or responsiveness of myeloid cells in response to pathogen detection, allowing effector cells to be more rapidly and efficiently recruited to site of infection, where they can clear the pathogen. Those changes could be mediated by increased expression of chemokines and/or chemoattractant receptors by leukocytes from subjects treated with lipid A mimetics. Lipid A mimetics may also increase expression of adhesion molecules on leukocytes and/or endothelial cells resulting in improved leukocyte binding and chemotaxis. As noted earlier, the ability of lipid A mimetics to improve the phagocytic and killing functions of neutrophils is somewhat controversial and requires further investigation.
Conclusions
Lipid A is a potent activator of innate immune responses. Activation of the LPS receptor complex by native lipid A induces robust cytokine production, leukocyte activation and inflammation, which provides attractive immunological characteristics but is associated with the pronounced inflammatory responses and limits its use as a clinical immunomodulator. Analogs of lipid A have been generated that possess greatly attenuated pro-inflammatory activity but retain attractive properties as immunoadjuvants. For example, the lipid A analog monophosphoryl lipid A (MPLA) exhibits approximately 1/1000th of the toxicity of native lipid A but retains potent immunoadjuvant activity and is currently employed as an adjuvant in several human vaccine preparations. Due to the potency of lipid A analogs as immunoadjuvants, numerous laboratories are actively working to identify and develop new lipid A mimetics and to optimize their efficacy and safety. In addition to their value as vaccine immunoadjuvants, lipid A analogs potently facilitate innate immune responses to bacterial, viral and fungal infections when given prophylactically. Treatment with lipid A mimetics augments the recruitment of myeloid cells, particularly neutrophils, to sites of infection and may enhance neutrophil phagocytic and killing functions. Based on those characteristics, lipid A analogs represent an attractive family of immunomodulators that could have clinical application in patients at risk for developing opportunistic and/or nosocomial infections.
Supplementary Material
Acknowledgments
Supported by NIH Grants R01 GM66885 and R01 GM104306
Footnotes
There is no conflict of interest
References
- 1.Schweizer HP. Understanding efflux in Gram-negative bacteria: opportunities for drug discovery. Expert Opin Drug Discov. 2012;7(7):633–642. doi: 10.1517/17460441.2012.688949. Epub 2012/05/23. doi: 10.1517/17460441.2012.688949. PubMed PMID: 22607346. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt H, Hansen G, Singh S, Hanuszkiewicz A, Lindner B, Fukase K, et al. Structural and mechanistic analysis of the membrane-embedded glycosyltransferase WaaA required for lipopolysaccharide synthesis. Proc Natl Acad Sci U S A. 2012;109(16):6253–6258. doi: 10.1073/pnas.1119894109. Epub 2012/04/05. doi: 10.1073/pnas.1119894109. PubMed PMID: 22474366; PubMed Central PMCID: PMC3341020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalis C, Kanzler B, Lembo A, Poltorak A, Galanos C, Freudenberg MA. Toll-like receptor 4 expression levels determine the degree of LPS-susceptibility in mice. Eur J Immunol. 2003;33(3):798–805. doi: 10.1002/eji.200323431. Epub 2003/03/05. doi: 10.1002/eji.200323431. PubMed PMID: 12616500. [DOI] [PubMed] [Google Scholar]
- 4.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458(7242):1191–1195. doi: 10.1038/nature07830. Epub 2009/03/03. doi: 10.1038/nature07830. PubMed PMID: 19252480. [DOI] [PubMed] [Google Scholar]
- 5.Asai Y, Hashimoto M, Fletcher HM, Miyake K, Akira S, Ogawa T. Lipopolysaccharide preparation extracted from Porphyromonas gingivalis lipoprotein-deficient mutant shows a marked decrease in toll-like receptor 2-mediated signaling. Infect Immun. 2005;73(4):2157–2163. doi: 10.1128/IAI.73.4.2157-2163.2005. Epub 2005/03/24. doi: 10.1128/IAI.73.4.2157-2163.2005. PubMed PMID: 15784558; PubMed Central PMCID: PMC1087447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Namas R, Zamora R, An G, Doyle J, Dick TE, Jacono FJ, et al. Sepsis: Something old, something new, and a systems view. J Crit Care. 2012;27(3):e1–e11. doi: 10.1016/j.jcrc.2011.05.025. 314. Epub 2011/07/30. doi: 10.1016/j.jcrc.2011.05.025. PubMed PMID: 21798705; PubMed Central PMCID: PMC3206132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandenbon A, Teraguchi S, Akira S, Takeda K, Standley DM. Systems biology approaches to toll-like receptor signaling. Wiley Interdiscip Rev Syst Biol Med. 2012;4(5):497–507. doi: 10.1002/wsbm.1178. Epub 2012/06/21. doi: 10.1002/wsbm.1178. PubMed PMID: 22714995; PubMed Central PMCID: PMC3465798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86(1):9–22. doi: 10.1038/labinvest.3700366. Epub 2005/12/17. doi: 10.1038/labinvest.3700366. PubMed PMID: 16357866. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter S, O'Neill LA. Recent insights into the structure of Toll-like receptors and post-translational modifications of their associated signalling proteins. Biochem J. 2009;422(1):1–10. doi: 10.1042/BJ20090616. Epub 2009/07/25. doi: 10.1042/BJ20090616. PubMed PMID: 19627256. [DOI] [PubMed] [Google Scholar]
- 10.Ebong SJ, Goyert SM, Nemzek JA, Kim J, Bolgos GL, Remick DG. Critical role of CD14 for production of proinflammatory cytokines and cytokine inhibitors during sepsis with failure to alter morbidity or mortality. Infect Immun. 2001;69(4):2099–2106. doi: 10.1128/IAI.69.4.2099-2106.2001. Epub 2001/03/20. doi: 10.1128/IAI.69.4.2099-2106.2001. PubMed PMID: 11254563; PubMed Central PMCID: PMC98135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65(20):3231–3240. doi: 10.1007/s00018-008-8228-6. Epub 2008/08/01. doi: 10.1007/s00018-008-8228-6. PubMed PMID: 18668203; PubMed Central PMCID: PMC2647720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. Toll-like receptor downstream signaling. Arthritis Res Ther. 2005;7(1):12–19. doi: 10.1186/ar1469. Epub 2005/01/12. doi: 10.1186/ar1469. PubMed PMID: 15642149; PubMed Central PMCID: PMC1064894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. Epub 2006/10/20. PubMed PMID: 17048703. [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2(2):253–258. doi: 10.1016/s1097-2765(00)80136-7. Epub 1998/09/12. PubMed PMID: 9734363. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Commane M, Jiang Z, Stark GR. IL-1-induced NFkappa B and c-Jun N-terminal kinase (JNK) activation diverge at IL-1 receptor-associated kinase (IRAK) Proc Natl Acad Sci U S A. 2001;98(8):4461–4465. doi: 10.1073/pnas.071054198. Epub 2001/04/05. doi: 10.1073/pnas.071054198. PubMed PMID: 11287640; PubMed Central PMCID: PMC31857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis. 2003;35(9):555–562. doi: 10.1080/00365540310015683. Epub 2003/11/19. PubMed PMID: 14620134. [DOI] [PubMed] [Google Scholar]
- 17.Aksoy E, Taboubi S, Torres D, Delbauve S, Hachani A, Whitehead MA, et al. The p110delta isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nature immunology. 2012;13(11):1045–1054. doi: 10.1038/ni.2426. Epub 2012/10/02. doi: 10.1038/ni.2426. PubMed PMID: 23023391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9(4):361–368. doi: 10.1038/ni1569. Epub 2008/02/26. doi: 10.1038/ni1569. PubMed PMID: 18297073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Z, Mak TW, Sen G, Li X. Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci U S A. 2004;101(10):3533–3538. doi: 10.1073/pnas.0308496101. Epub 2004/02/26. doi: 10.1073/pnas.0308496101. PubMed PMID: 14982987; PubMed Central PMCID: PMC373497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husebye H, Halaas O, Stenmark H, Tunheim G, Sandanger O, Bogen B, et al. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. Embo J. 2006;25(4):683–692. doi: 10.1038/sj.emboj.7600991. Epub 2006/02/10. doi: 10.1038/sj.emboj.7600991. PubMed PMID: 16467847; PubMed Central PMCID: PMC1383569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–643. doi: 10.1126/science.1087262. Epub 2003/07/12. doi: 10.1126/science.1087262. PubMed PMID: 12855817. [DOI] [PubMed] [Google Scholar]
- 22.Needham BD, Carroll SM, Giles DK, Georgiou G, Whiteley M, Trent MS. Modulating the innate immune response by combinatorial engineering of endotoxin. Proc Natl Acad Sci U S A. 2013;110(4):1464–1469. doi: 10.1073/pnas.1218080110. Epub 2013/01/09. doi: 10.1073/pnas.1218080110. PubMed PMID: 23297218; PubMed Central PMCID: PMC3557076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raetz CR, Guan Z, Ingram BO, Six DA, Song F, Wang X, et al. Discovery of new biosynthetic pathways: the lipid A story. J Lipid Res. 2009;50(Suppl):S103–S108. doi: 10.1194/jlr.R800060-JLR200. Epub 2008/11/01. doi: 10.1194/jlr.R800060-JLR200. PubMed PMID: 18974037; PubMed Central PMCID: PMC2674688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akira S. Toll-like receptors: lessons from knockout mice. Biochem Soc Trans. 2000;28(5):551–556. doi: 10.1042/bst0280551. Epub 2000/10/25. PubMed PMID: 11044373. [DOI] [PubMed] [Google Scholar]
- 25.Bolz DD, Sundsbak RS, Ma Y, Akira S, Weis JH, Schwan TG, et al. Dual role of MyD88 in rapid clearance of relapsing fever Borrelia spp. Infect Immun. 2006;74(12):6750–6760. doi: 10.1128/IAI.01160-06. Epub 2006/10/13. doi: 10.1128/IAI.01160-06. PubMed PMID: 17030581; PubMed Central PMCID: PMC1698049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, et al. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A. 2009;106(7):2348–2352. doi: 10.1073/pnas.0808146106. Epub 2009/02/03. doi: 10.1073/pnas.0808146106. PubMed PMID: 19181857; PubMed Central PMCID: PMC2650125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16(1):3–9. doi: 10.1016/j.smim.2003.10.003. Epub 2004/01/31. PubMed PMID: 14751757. [DOI] [PubMed] [Google Scholar]
- 28.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–1632. doi: 10.1126/science.1138963. Epub 2007/06/16. doi: 10.1126/science.1138963. PubMed PMID: 17569868. [DOI] [PubMed] [Google Scholar]
- 29.Cekic C, Casella CR, Eaves CA, Matsuzawa A, Ichijo H, Mitchell TC. Selective activation of the p38 MAPK pathway by synthetic monophosphoryl lipid A. J Biol Chem. 2009;284(46):31982–31991. doi: 10.1074/jbc.M109.046383. Epub 2009/09/18. doi: 10.1074/jbc.M109.046383. PubMed PMID: 19759006; PubMed Central PMCID: PMC2797270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaar O, Cazan D, Klimek L, Larenas-Linnemann D, Calderon MA. Adjuvants for immunotherapy. Curr Opin Allergy Clin Immunol. 2012;12(6):648–657. doi: 10.1097/ACI.0b013e32835a11d6. Epub 2012/10/24. doi: 10.1097/ACI.0b013e32835a11d6. PubMed PMID: 23090384. [DOI] [PubMed] [Google Scholar]
- 31.Astiz ME, Rackow EC, Still JG, Howell ST, Cato A, Von Eschen KB, et al. Pretreatment of normal humans with monophosphoryl lipid A induces tolerance to endotoxin: a prospective, double-blind, randomized, controlled trial. Crit Care Med. 1995;23(1):9–17. doi: 10.1097/00003246-199501000-00006. Epub 1995/01/01. PubMed PMID: 8001393. [DOI] [PubMed] [Google Scholar]
- 32.Astiz ME, Rackow EC, Kim YB, Weil MH. Monophosphoryl lipid A induces tolerance to the lethal hemodynamic effects of endotoxemia. Circ Shock. 1991;33(2):92–97. Epub 1991/02/01. PubMed PMID: 2049817. [PubMed] [Google Scholar]
- 33.Casella CR, Mitchell TC. Inefficient TLR4/MD-2 Heterotetramerization by Monophosphoryl Lipid A. PLoS One. 2013;8(4):e62622. doi: 10.1371/journal.pone.0062622. Epub 2013/05/03. doi: 10.1371/journal.pone.0062622. PubMed PMID: 23638128; PubMed Central PMCID: PMC3637451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alving CR, Peachman KK, Rao M, Reed SG. Adjuvants for human vaccines. Curr Opin Immunol. 2012;24(3):310–315. doi: 10.1016/j.coi.2012.03.008. Epub 2012/04/24. doi: 10.1016/j.coi.2012.03.008. PubMed PMID: 22521140; PubMed Central PMCID: PMC3383374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowen WS, Minns LA, Johnson DA, Mitchell TC, Hutton MM, Evans JT. Selective TRIF-dependent signaling by a synthetic toll-like receptor 4 agonist. Sci Signal. 2012;5(211) doi: 10.1126/scisignal.2001963. ra13. Epub 2012/02/18. doi: 10.1126/scisignal.2001963. PubMed PMID: 22337809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong Y, Medvedev AE. Induction of endotoxin tolerance in vivo inhibits activation of IRAK4 and increases negative regulators IRAK-M, SHIP-1, and A20. J Leukoc Biol. 2011;90(6):1141–1148. doi: 10.1189/jlb.0611273. Epub 2011/09/22. doi: 10.1189/jlb.0611273. PubMed PMID: 21934070; PubMed Central PMCID: PMC3236548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varma TK, Toliver-Kinsky TE, Lin CY, Koutrouvelis AP, Nichols JE, Sherwood ER. Cellular mechanisms that cause suppressed gamma interferon secretion in endotoxin-tolerant mice. Infect Immun. 2001;69(9):5249–5263. doi: 10.1128/IAI.69.9.5249-5263.2001. Epub 2001/08/14. PubMed PMID: 11500393; PubMed Central PMCID: PMC98633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salkowski CA, Detore G, Franks A, Falk MC, Vogel SN. Pulmonary and hepatic gene expression following cecal ligation and puncture: monophosphoryl lipid A prophylaxis attenuates sepsis-induced cytokine and chemokine expression and neutrophil infiltration. Infect Immun. 1998;66(8):3569–3578. doi: 10.1128/iai.66.8.3569-3578.1998. Epub 1998/07/23. PubMed PMID: 9673235; PubMed Central PMCID: PMC108388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30(10):475–487. doi: 10.1016/j.it.2009.07.009. Epub 2009/09/29. doi: 10.1016/j.it.2009.07.009. PubMed PMID: 19781994. [DOI] [PubMed] [Google Scholar]
- 40.Flohe S, Lendemans S, Schade FU, Kreuzfelder E, Waydhas C. Influence of surgical intervention in the immune response of severely injured patients. Intensive Care Med. 2004;30(1):96–102. doi: 10.1007/s00134-003-2041-3. Epub 2003/11/08. doi: 10.1007/s00134-003-2041-3. PubMed PMID: 14605804. [DOI] [PubMed] [Google Scholar]
- 41.Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10(5):233. doi: 10.1186/cc5055. Epub 2006/10/19. doi: 10.1186/cc5055. PubMed PMID: 17044947; PubMed Central PMCID: PMC1751079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gahring LC, Daynes RA. Desensitization of animals to the inflammatory effects of ultraviolet radiation is mediated through mechanisms which are distinct from those responsible for endotoxin tolerance. J Immunol. 1986;136(8):2868–2874. Epub 1986/04/15. PubMed PMID: 2420872. [PubMed] [Google Scholar]
- 43.Granowitz EV, Porat R, Mier JW, Orencole SF, Kaplanski G, Lynch EA, et al. Intravenous endotoxin suppresses the cytokine response of peripheral blood mononuclear cells of healthy humans. J Immunol. 1993;151(3):1637–1645. Epub 1993/08/01. PubMed PMID: 7687636. [PubMed] [Google Scholar]
- 44.Dobrovolskaia MA, Medvedev AE, Thomas KE, Cuesta N, Toshchakov V, Ren T, et al. Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: effects of TLR "homotolerance" versus "heterotolerance" on NF-kappa B signaling pathway components. J Immunol. 2003;170(1):508–519. doi: 10.4049/jimmunol.170.1.508. Epub 2002/12/24. PubMed PMID: 12496438. [DOI] [PubMed] [Google Scholar]
- 45.Jacinto R, Hartung T, McCall C, Li L. Lipopolysaccharide- and lipoteichoic acid-induced tolerance and cross-tolerance: distinct alterations in IL-1 receptor-associated kinase. J Immunol. 2002;168(12):6136–6141. doi: 10.4049/jimmunol.168.12.6136. Epub 2002/06/11. PubMed PMID: 12055225. [DOI] [PubMed] [Google Scholar]
- 46.Li CH, Wang JH, Redmond HP. Bacterial lipoprotein-induced self-tolerance and cross-tolerance to LPS are associated with reduced IRAK-1 expression and MyD88-IRAK complex formation. J Leukoc Biol. 2006;79(4):867–875. doi: 10.1189/jlb.0905505. Epub 2006/02/08. doi: 10.1189/jlb.0905505. PubMed PMID: 16461741. [DOI] [PubMed] [Google Scholar]
- 47.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165(2):618–622. doi: 10.4049/jimmunol.165.2.618. Epub 2000/07/06. PubMed PMID: 10878331. [DOI] [PubMed] [Google Scholar]
- 48.Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, et al. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J Immunol. 2007;178(2):1164–1171. doi: 10.4049/jimmunol.178.2.1164. Epub 2007/01/05. PubMed PMID: 17202381. [DOI] [PubMed] [Google Scholar]
- 49.Sato S, Takeuchi O, Fujita T, Tomizawa H, Takeda K, Akira S. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int Immunol. 2002;14(7):783–791. doi: 10.1093/intimm/dxf046. Epub 2002/07/04. PubMed PMID: 12096038. [DOI] [PubMed] [Google Scholar]
- 50.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13(2):85–94. doi: 10.1016/s0898-6568(00)00149-2. Epub 2001/03/21. PubMed PMID: 11257452. [DOI] [PubMed] [Google Scholar]
- 51.Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12(3):133–150. doi: 10.1179/096805106X102255. Epub 2006/05/25. doi: 10.1179/096805106X102255. PubMed PMID: 16719986. [DOI] [PubMed] [Google Scholar]
- 52.Piao W, Song C, Chen H, Diaz MA, Wahl LM, Fitzgerald KA, et al. Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling. J Leukoc Biol. 2009;86(4):863–875. doi: 10.1189/jlb.0309189. Epub 2009/08/07. doi: 10.1189/jlb.0309189. PubMed PMID: 19656901; PubMed Central PMCID: PMC2796624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. Epub 2010/05/12. doi: 10.1101/cshperspect.a001651. PubMed PMID: 20457564; PubMed Central PMCID: PMC2882124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110(2):191–202. doi: 10.1016/s0092-8674(02)00827-9. Epub 2002/08/02. PubMed PMID: 12150927. [DOI] [PubMed] [Google Scholar]
- 55.Lawrence T, Fong C. The resolution of inflammation: anti-inflammatory roles for NF-kappaB. Int J Biochem Cell Biol. 2010;42(4):519–523. doi: 10.1016/j.biocel.2009.12.016. Epub 2009/12/23. doi: 10.1016/j.biocel.2009.12.016. PubMed PMID: 20026420. [DOI] [PubMed] [Google Scholar]
- 56.Chen X, El Gazzar M, Yoza BK, McCall CE. The NF-kappaB factor RelB and histone H3 lysine methyltransferase G9a directly interact to generate epigenetic silencing in endotoxin tolerance. J Biol Chem. 2009;284(41):27857–27865. doi: 10.1074/jbc.M109.000950. Epub 2009/08/20. doi: 10.1074/jbc.M109.000950. PubMed PMID: 19690169; PubMed Central PMCID: PMC2788836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7(12):1291–1297. doi: 10.1038/nm1201-1291. Epub 2001/12/01. doi: 10.1038/nm1201-1291. PubMed PMID: 11726968. [DOI] [PubMed] [Google Scholar]
- 58.Su J, Xie Q, Wilson I, Li L. Differential regulation and role of interleukin-1 receptor associated kinase-M in innate immunity signaling. Cell Signal. 2007;19(7):1596–1601. doi: 10.1016/j.cellsig.2007.02.009. Epub 2007/03/24. doi: 10.1016/j.cellsig.2007.02.009. PubMed PMID: 17379480; PubMed Central PMCID: PMC1978187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deng H, Maitra U, Morris M, Li L. Molecular mechanism responsible for the priming of macrophage activation. J Biol Chem. 2013;288(6):3897–3906. doi: 10.1074/jbc.M112.424390. Epub 2012/12/25. doi: 10.1074/jbc.M112.424390. PubMed PMID: 23264622; PubMed Central PMCID: PMC3567643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7147):972–978. doi: 10.1038/nature05836. Epub 2007/06/01. doi: 10.1038/nature05836. PubMed PMID: 17538624. [DOI] [PubMed] [Google Scholar]
- 61.El Gazzar M, McCall CE. MicroRNAs distinguish translational from transcriptional silencing during endotoxin tolerance. J Biol Chem. 2010;285(27):20940–20951. doi: 10.1074/jbc.M110.115063. Epub 2010/05/04. doi: 10.1074/jbc.M110.115063. PubMed PMID: 20435889; PubMed Central PMCID: PMC2898346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. Epub 2006/08/04. doi: 10.1073/pnas.0605298103. PubMed PMID: 16885212; PubMed Central PMCID: PMC1567904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179(8):5082–5089. doi: 10.4049/jimmunol.179.8.5082. Epub 2007/10/04. PubMed PMID: 17911593. [DOI] [PubMed] [Google Scholar]
- 64.El Gazzar M, Yoza BK, Chen X, Garcia BA, Young NL, McCall CE. Chromatin-specific remodeling by HMGB1 and linker histone H1 silences proinflammatory genes during endotoxin tolerance. Mol Cell Biol. 2009;29(7):1959–1971. doi: 10.1128/MCB.01862-08. Epub 2009/01/23. doi: 10.1128/MCB.01862-08. PubMed PMID: 19158276; PubMed Central PMCID: PMC2655606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El Gazzar M, Yoza BK, Chen X, Hu J, Hawkins GA, McCall CE. G9a and HP1 couple histone and DNA methylation to TNFalpha transcription silencing during endotoxin tolerance. J Biol Chem. 2008;283(47):32198–32208. doi: 10.1074/jbc.M803446200. Epub 2008/09/24. doi: 10.1074/jbc.M803446200. PubMed PMID: 18809684; PubMed Central PMCID: PMC2583293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, et al. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17(5):677–687. doi: 10.1016/s1074-7613(02)00449-1. Epub 2002/11/16. PubMed PMID: 12433373. [DOI] [PubMed] [Google Scholar]
- 67.Liu ZJ, Liu XL, Zhao J, Shi YJ, Yan LN, Chen XF, et al. The effects of SOCS-1 on liver endotoxin tolerance development induced by a low dose of lipopolysaccharide are related to dampen NF-kappaB-mediated pathway. Dig Liver Dis. 2008;40(7):568–577. doi: 10.1016/j.dld.2007.12.019. Epub 2008/04/02. doi: 10.1016/j.dld.2007.12.019. PubMed PMID: 18378198. [DOI] [PubMed] [Google Scholar]
- 68.Adib-Conquy M, Adrie C, Moine P, Asehnoune K, Fitting C, Pinsky MR, et al. NF-kappaB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am J Respir Crit Care Med. 2000;162(5):1877–1883. doi: 10.1164/ajrccm.162.5.2003058. Epub 2000/11/09. PubMed PMID: 11069829. [DOI] [PubMed] [Google Scholar]
- 69.Wolk K, Docke W, von Baehr V, Volk H, Sabat R. Comparison of monocyte functions after LPS- or IL-10-induced reorientation: importance in clinical immunoparalysis. Pathobiology. 1999;67(5–6):253–256. doi: 10.1159/000028104. Epub 2000/03/22. doi: 28104. PubMed PMID: 10725796. [DOI] [PubMed] [Google Scholar]
- 70.Kwissa M, Nakaya HI, Oluoch H, Pulendran B. Distinct TLR adjuvants differentially stimulate systemic and local innate immune responses in nonhuman primates. Blood. 2012;119(9):2044–2055. doi: 10.1182/blood-2011-10-388579. Epub 2012/01/17. doi: 10.1182/blood-2011-10-388579. PubMed PMID: 22246032; PubMed Central PMCID: PMC3311246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alving CR. Lipopolysaccharide, lipid A, and liposomes containing lipid A as immunologic adjuvants. Immunobiology. 1993;187(3–5):430–446. doi: 10.1016/S0171-2985(11)80355-4. Epub 1993/04/01. doi: 10.1016/S0171-2985(11)80355-4. PubMed PMID: 8330907. [DOI] [PubMed] [Google Scholar]
- 72.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta. 2002;1589(1):1–13. doi: 10.1016/s0167-4889(01)00182-3. Epub 2002/03/23. PubMed PMID: 11909637. [DOI] [PubMed] [Google Scholar]
- 73.Kaisho T, Akira S. Regulation of dendritic cell function through Toll-like receptors. Curr Mol Med. 2003;3(4):373–385. doi: 10.2174/1566524033479726. Epub 2003/06/05. PubMed PMID: 12776992. [DOI] [PubMed] [Google Scholar]
- 74.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171(10):4984–4989. doi: 10.4049/jimmunol.171.10.4984. Epub 2003/11/11. PubMed PMID: 14607893. [DOI] [PubMed] [Google Scholar]
- 75.Pantel A, Cheong C, Dandamudi D, Shrestha E, Mehandru S, Brane L, et al. A new synthetic TLR4 agonist, GLA, allows dendritic cells targeted with antigen to elicit Th1 T-cell immunity in vivo. Eur J Immunol. 2012;42(1):101–109. doi: 10.1002/eji.201141855. Epub 2011/10/18. doi: 10.1002/eji.201141855. PubMed PMID: 22002164; PubMed Central PMCID: PMC3517108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watanabe S, Inoue J. Intracellular Delivery of Lipopolysaccharide Induces Effective Th1-Immune Responses Independent of IL-12. PLoS One. 2013;8(7):e68671. doi: 10.1371/journal.pone.0068671. Epub 2013/07/23. doi: 10.1371/journal.pone.0068671. PubMed PMID: 23874715; PubMed Central PMCID: PMC3714268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McAleer JP, Vella AT. Understanding how lipopolysaccharide impacts CD4 T-cell immunity. Crit Rev Immunol. 2008;28(4):281–299. doi: 10.1615/critrevimmunol.v28.i4.20. Epub 2009/01/27. PubMed PMID: 19166381; PubMed Central PMCID: PMC3549535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, et al. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther. 2004;4(7):1129–1138. doi: 10.1517/14712598.4.7.1129. Epub 2004/07/23. doi: 10.1517/14712598.4.7.1129. PubMed PMID: 15268679. [DOI] [PubMed] [Google Scholar]
- 79.Kundi M. New hepatitis B vaccine formulated with an improved adjuvant system. Expert Rev Vaccines. 2007;6(2):133–140. doi: 10.1586/14760584.6.2.133. Epub 2007/04/06. doi: 10.1586/14760584.6.2.133. PubMed PMID: 17408363. [DOI] [PubMed] [Google Scholar]
- 80.Garcon N, Morel S, Didierlaurent A, Descamps D, Wettendorff M, Van Mechelen M. Development of an AS04-adjuvanted HPV vaccine with the adjuvant system approach. BioDrugs. 2011;25(4):217–226. doi: 10.2165/11591760-000000000-00000. Epub 2011/08/06. doi: 10.2165/11591760-000000000-00000. PubMed PMID: 21815697. [DOI] [PubMed] [Google Scholar]
- 81.Descamps D, Hardt K, Spiessens B, Izurieta P, Verstraeten T, Breuer T, et al. Safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for cervical cancer prevention: a pooled analysis of 11 clinical trials. Hum Vaccin. 2009;5(5):332–340. doi: 10.4161/hv.5.5.7211. Epub 2009/02/18. PubMed PMID: 19221517. [DOI] [PubMed] [Google Scholar]
- 82.Sow PS, Watson-Jones D, Kiviat N, Changalucha J, Mbaye KD, Brown J, et al. Safety and immunogenicity of human papillomavirus-16/18 AS04-adjuvanted vaccine: a randomized trial in 10–25-year-old HIV-Seronegative African girls and young women. J Infect Dis. 2013;207(11):1753–1763. doi: 10.1093/infdis/jis619. Epub 2012/12/18. doi: 10.1093/infdis/jis619. PubMed PMID: 23242542; PubMed Central PMCID: PMC3636781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pedersen C, Breindahl M, Aggarwal N, Berglund J, Oroszlan G, Silfverdal SA, et al. Randomized trial: immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-adjuvanted vaccine and combined hepatitis A and B vaccine in girls. J Adolesc Health. 2012;50(1):38–46. doi: 10.1016/j.jadohealth.2011.10.009. Epub 2011/12/23. doi: 10.1016/j.jadohealth.2011.10.009. PubMed PMID: 22188832. [DOI] [PubMed] [Google Scholar]
- 84.Persing DH, Coler RN, Lacy MJ, Johnson DA, Baldridge JR, Hershberg RM, et al. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 2002;10(10 Suppl):S32–S37. doi: 10.1016/s0966-842x(02)02426-5. Epub 2002/10/16. PubMed PMID: 12377566. [DOI] [PubMed] [Google Scholar]
- 85.Gandhapudi SK, Chilton PM, Mitchell TC. TRIF is required for TLR4 mediated adjuvant effects on T cell clonal expansion. PLoS One. 2013;8(2):e56855. doi: 10.1371/journal.pone.0056855. Epub 2013/03/05. doi: 10.1371/journal.pone.0056855. PubMed PMID: 23457630; PubMed Central PMCID: PMC3574014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oh JZ, Kurche JS, Burchill MA, Kedl RM. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood. 2011;118(11):3028–3038. doi: 10.1182/blood-2011-04-348839. Epub 2011/08/05. doi: 10.1182/blood-2011-04-348839. PubMed PMID: 21813451; PubMed Central PMCID: PMC3175780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murphey ED, Lin CY, McGuire RW, Toliver-Kinsky T, Herndon DN, Sherwood ER. Diminished bacterial clearance is associated with decreased IL-12 and interferon-gamma production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock. 2004;21(5):415–425. doi: 10.1097/00024382-200405000-00004. Epub 2004/04/17. PubMed PMID: 15087817. [DOI] [PubMed] [Google Scholar]
- 88.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–1501. doi: 10.1097/TA.0b013e318256e000. Epub 2012/06/15. doi: 10.1097/TA.0b013e318256e000. PubMed PMID: 22695412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wysocka M, Montaner LJ, Karp CL. Flt3 ligand treatment reverses endotoxin tolerance-related immunoparalysis. J Immunol. 2005;174(11):7398–7402. doi: 10.4049/jimmunol.174.11.7398. Epub 2005/05/21. PubMed PMID: 15905588. [DOI] [PubMed] [Google Scholar]
- 90.Dai WJ, Bartens W, Kohler G, Hufnagel M, Kopf M, Brombacher F. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J Immunol. 1997;158(11):5297–5304. Epub 1997/06/01. PubMed PMID: 9164949. [PubMed] [Google Scholar]
- 91.O'Brien DP, Briles DE, Szalai AJ, Tu AH, Sanz I, Nahm MH. Tumor necrosis factor alpha receptor I is important for survival from Streptococcus pneumoniae infections. Infection and immunity. 1999;67(2):595–601. doi: 10.1128/iai.67.2.595-601.1999. Epub 1999/01/23. PubMed PMID: 9916064; PubMed Central PMCID: PMC96360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364(6440):798–802. doi: 10.1038/364798a0. Epub 1993/08/26. doi: 10.1038/364798a0. PubMed PMID: 8395024. [DOI] [PubMed] [Google Scholar]
- 93.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annual review of immunology. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. Epub 2004/03/23. doi: 10.1146/annurev.immunol.22.012703.104622. PubMed PMID: 15032600. [DOI] [PubMed] [Google Scholar]
- 94.Rossato M, Curtale G, Tamassia N, Castellucci M, Mori L, Gasperini S, et al. IL-10-induced microRNA-187 negatively regulates TNF-alpha, IL-6, and IL-12p40 production in TLR4-stimulated monocytes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(45):E3101–E110. doi: 10.1073/pnas.1209100109. Epub 2012/10/17. doi: 10.1073/pnas.1209100109. PubMed PMID: 23071313; PubMed Central PMCID: PMC3494907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grutz G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. Journal of leukocyte biology. 2005;77(1):3–15. doi: 10.1189/jlb.0904484. Epub 2004/11/04. doi: 10.1189/jlb.0904484. PubMed PMID: 15522916. [DOI] [PubMed] [Google Scholar]
- 96.van der Sluijs KF, van Elden LJ, Nijhuis M, Schuurman R, Pater JM, Florquin S, et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004;172(12):7603–7609. doi: 10.4049/jimmunol.172.12.7603. Epub 2004/06/10. PubMed PMID: 15187140. [DOI] [PubMed] [Google Scholar]
- 97.Metzger DW, Salmon SL, Kirimanjeswara G. Differing Effects of IL-10 in Cutaneous and Pulmonary Francisella tularensis LVS Infection. Infection and immunity. 2013 doi: 10.1128/IAI.00024-13. Epub 2013/03/27. doi: 10.1128/IAI.00024-13. PubMed PMID: 23529615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murphy ML, Wille U, Villegas EN, Hunter CA, Farrell JP. IL-10 mediates susceptibility to Leishmania donovani infection. European journal of immunology. 2001;31(10):2848–2856. doi: 10.1002/1521-4141(2001010)31:10<2848::aid-immu2848>3.0.co;2-t. Epub 2001/10/10. doi: 10.1002/1521-4141(2001010)31:10<2848::AID-IMMU2848>3.0.CO;2-T. PubMed PMID: 11592059. [DOI] [PubMed] [Google Scholar]
- 99.Varma TK, Durham M, Murphey ED, Cui W, Huang Z, Lin CY, et al. Endotoxin priming improves clearance of Pseudomonas aeruginosa in wild-type and interleukin-10 knockout mice. Infection and immunity. 2005;73(11):7340–7347. doi: 10.1128/IAI.73.11.7340-7347.2005. Epub 2005/10/22. doi: 10.1128/IAI.73.11.7340-7347.2005. PubMed PMID: 16239532; PubMed Central PMCID: PMC1273831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mason CM, Dobard E, Summer WR, Nelson S. Intraportal lipopolysaccharide suppresses pulmonary antibacterial defense mechanisms. J Infect Dis. 1997;176(5):1293–1302. doi: 10.1086/514125. Epub 1997/11/14. PubMed PMID: 9359731. [DOI] [PubMed] [Google Scholar]
- 101.Deng M, Scott MJ, Loughran P, Gibson G, Sodhi C, Watkins S, et al. Lipopolysaccharide clearance, bacterial clearance, and systemic inflammatory responses are regulated by cell type-specific functions of TLR4 during sepsis. J Immunol. 2013;190(10):5152–5160. doi: 10.4049/jimmunol.1300496. Epub 2013/04/09. doi: 10.4049/jimmunol.1300496. PubMed PMID: 23562812; PubMed Central PMCID: PMC3644895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murphey ED, Fang G, Varma TK, Sherwood ER. Improved bacterial clearance and decreased mortality can be induced by LPS tolerance and is not dependent upon IFN-gamma. Shock. 2007;27(3):289–295. doi: 10.1097/01.shk.0000245024.93740.28. Epub 2007/02/17. doi: 10.1097/01.shk.0000245024.93740.28. PubMed PMID: 17304110. [DOI] [PubMed] [Google Scholar]
- 103.Lehner MD, Ittner J, Bundschuh DS, van Rooijen N, Wendel A, Hartung T. Improved innate immunity of endotoxin-tolerant mice increases resistance to Salmonella enterica serovar typhimurium infection despite attenuated cytokine response. Infection and immunity. 2001;69(1):463–471. doi: 10.1128/IAI.69.1.463-471.2001. Epub 2000/12/19. doi: 10.1128/IAI.69.1.463-471.2001. PubMed PMID: 11119538; PubMed Central PMCID: PMC97904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rayhane N, Fitting C, Lortholary O, Dromer F, Cavaillon JM. Administration of endotoxin associated with lipopolysaccharide tolerance protects mice against fungal infection. Infection and immunity. 2000;68(6):3748–3753. doi: 10.1128/iai.68.6.3748-3753.2000. Epub 2000/05/19. PubMed PMID: 10816541; PubMed Central PMCID: PMC97672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murphey ED, Fang G, Sherwood ER. Endotoxin pretreatment improves bacterial clearance and decreases mortality in mice challenged with Staphylococcus aureus. Shock. 2008;29(4):512–518. doi: 10.1097/shk.0b013e318150776f. Epub 2007/08/29. doi: 10.1097/shk.0b013e318150776f. PubMed PMID: 17724430. [DOI] [PubMed] [Google Scholar]
- 106.Wheeler DS, Lahni PM, Denenberg AG, Poynter SE, Wong HR, Cook JA, et al. Induction of endotoxin tolerance enhances bacterial clearance and survival in murine polymicrobial sepsis. Shock. 2008;30(3):267–273. doi: 10.1097/shk.0b013e318162c190. Epub 2008/01/17. doi: 10.1097/shk.0b013e318162c190. PubMed PMID: 18197145; PubMed Central PMCID: PMC2754132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roquilly A, Broquet A, Jacqueline C, Gautreau L, Segain JP, de Coppet P, et al. TLR-4 agonist in post-haemorrhage pneumonia: role of dendritic and natural killer cells. Eur Respir J. 2013 doi: 10.1183/09031936.00152612. Epub 2013/01/15. doi: 10.1183/09031936.00152612. PubMed PMID: 23314895. [DOI] [PubMed] [Google Scholar]
- 108.Hirano T, Kodama S, Kawano T, Maeda K, Suzuki M. Monophosphoryl lipid A induced innate immune responses via TLR4 to enhance clearance of nontypeable Haemophilus influenzae and Moraxella catarrhalis from the nasopharynx in mice. FEMS Immunol Med Microbiol. 2011;63(3):407–417. doi: 10.1111/j.1574-695X.2011.00866.x. Epub 2011/11/19. doi: 10.1111/j.1574-695X.2011.00866.x. PubMed PMID: 22092567. [DOI] [PubMed] [Google Scholar]
- 109.Chase JJ, Kubey W, Dulek MH, Holmes CJ, Salit MG, Pearson FC, 3rd, et al. Effect of monophosphoryl lipid A on host resistance to bacterial infection. Infect Immun. 1986;53(3):711–712. doi: 10.1128/iai.53.3.711-712.1986. Epub 1986/09/01. PubMed PMID: 3744562; PubMed Central PMCID: PMC260854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Romero CD, Varma TK, Hobbs JB, Reyes A, Driver B, Sherwood ER. The Toll-like receptor 4 agonist monophosphoryl lipid A augments innate host resistance to systemic bacterial infection. Infect Immun. 2011;79(9):3576–3587. doi: 10.1128/IAI.00022-11. Epub 2011/06/08. doi: 10.1128/IAI.00022-11. PubMed PMID: 21646453; PubMed Central PMCID: PMC3165493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baldridge JR, Cluff CW, Evans JT, Lacy MJ, Stephens JR, Brookshire VG, et al. Immunostimulatory activity of aminoalkyl glucosaminide 4-phosphates (AGPs): induction of protective innate immune responses by RC-524 and RC-529. Journal of endotoxin research. 2002;8(6):453–458. doi: 10.1179/096805102125001064. Epub 2003/04/17. doi: 10.1179/096805102125001064. PubMed PMID: 12697089. [DOI] [PubMed] [Google Scholar]
- 112.Airhart CL, Rohde HN, Bohach GA, Hovde CJ, Deobald CF, Lee SS, et al. Induction of innate immunity by lipid A mimetics increases survival from pneumonic plague. Microbiology. 2008;154(Pt 7):2131–2138. doi: 10.1099/mic.0.2008/017566-0. Epub 2008/07/05. doi: 10.1099/mic.0.2008/017566-0. PubMed PMID: 18599840. [DOI] [PubMed] [Google Scholar]
- 113.Lembo A, Pelletier M, Iyer R, Timko M, Dudda JC, West TE, et al. Administration of a synthetic TLR4 agonist protects mice from pneumonic tularemia. J Immunol. 2008;180(11):7574–7581. doi: 10.4049/jimmunol.180.11.7574. Epub 2008/05/21. PubMed PMID: 18490759; PubMed Central PMCID: PMC3063511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lehner MD, Hartung T. Endotoxin tolerance-mechanisms and beneficial effects in bacterial infection. Reviews of physiology, biochemistry and pharmacology. 2002;144:95–141. doi: 10.1007/BFb0116586. Epub 2002/05/04. PubMed PMID: 11987826. [DOI] [PubMed] [Google Scholar]
- 115.Hafenrichter DG, Roland CR, Mangino MJ, Flye MW. The Kupffer cell in endotoxin tolerance: mechanisms of protection against lethal endotoxemia. Shock. 1994;2(4):251–256. Epub 1994/10/01. PubMed PMID: 7757516. [PubMed] [Google Scholar]
- 116.Ruggiero G, Andreana A, Utili R, Galante D. Enhanced phagocytosis and bactericidal activity of hepatic reticuloendothelial system during endotoxin tolerance. Infection and immunity. 1980;27(3):798–803. doi: 10.1128/iai.27.3.798-803.1980. Epub 1980/03/01. PubMed PMID: 6991430; PubMed Central PMCID: PMC550842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Astiz ME, Saha DC, Carpati CM, Rackow EC. Induction of endotoxin tolerance with monophosphoryl lipid A in peritonitis: importance of localized therapy. J Lab Clin Med. 1994;123(1):89–93. Epub 1994/01/01. PubMed PMID: 8288966. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.