Abstract
Developments in digital ultrasonography (US) technology and the use of high-frequency broadband transducers have increased the quality of US imaging, particularly of superficial tissues. Thus, US, particularly color US or power Doppler US, in which high-resolution transducers are used, has become an important imaging modality in the assessment of rheumatic diseases. Furthermore, therapeutic interventions and biopsies can be performed under US guidance during the assessment of lesions. In this era of effective treatments, such as biologics, improvements in synovial inflammation in rheumatoid arthritis as well as changes in enthesitis in spondyloarthropathies, including ankylosing spondylitis and psoriatic arthritis, can be monitored effectively using gray-scale and/or power Doppler US. US is also a good imaging modality for crystal arthropathies, including gout and pseudogout, in which synovitis, erosions, tophi, and crystal deposition within or around the joint can be visualized readily. Vascular and tenosynovial structures, as well as the salivary glands, can be assessed with US in vasculitis and connective tissue disorders, including systemic lupus erythematosus and Sjögren’s syndrome. Current research is focused on improving the sensitivity, specificity, validity, and reproducibility of US findings. In this review, we summarized the role of US, particularly power Doppler US, in rheumatic diseases and inflammation in superficial tissues.
Developments in digital ultrasonography (US) technology and the use of high-frequency broadband transducers have increased the quality of US imaging, particularly of superficial tissues. The increase in spatial resolution has facilitated evaluation of normal and pathological structures using US, and the increased sensitivity of color Doppler US (CDUS) for low-flow signals has enabled the evaluation of vascularity in these structures.
Thus, US, particularly CDUS or power Doppler US (PDUS), in which high-resolution transducers are used, has become the primary imaging modality for superficial tissues. US can be used as a first-step screening tool to assess the presence of superficial soft tissue masses and thus can be used to evaluate the need for other imaging techniques. High-frequency US, in some cases, allows more specific diagnoses by characterizing the anatomy of the superficial tissues and flow within the lesions or neighboring tissues (1–4).
Indeed, US and Doppler US can define the cystic and solid nature of lesions and thus provide a final diagnosis, in some cases without the need for another imaging modality. Furthermore, therapeutic interventions and biopsies can be performed under US guidance during the assessment of lesions. Changes in the size and vascularity of lesions, clinical responses, and local tumor recurrence can also be followed by CDUS/PDUS (1, 3). In this review, we emphasized the role of US, particularly PDUS, in rheumatic diseases and inflammation in superficial tissues.
Clinical and technical requirements
Knowledge of the basic anatomy and pathology of the joints and soft tissues is essential for US evaluations. A PDUS device with an at least 10-MHz linear transducer is used for joint assessment. Knowledge of the indications and limitations of the technique and adequate technical experience in US imaging are also required. During examination, low-wall filters and low pulse repetition frequency should be used for the detection of low-velocity flows. Excessive pressure should not be applied with the probe to avoid decreased flow signals (Fig. 1).
Figure 1. a–c.
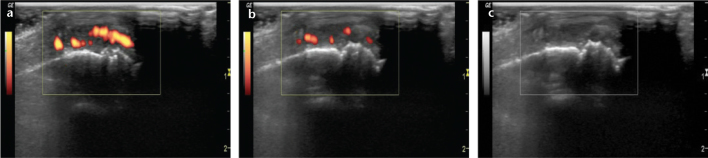
Change in the flow pattern of Doppler US with pressure. Right second metacarpophalangeal joint of a patient with rheumatoid arthritis shows flow signals within the joint (a). The flow signals reduce with mild pressure applied with the probe over the joint (b). The flow disappears with excessive pressure applied with the probe over the joint (c).
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by synovial inflammation, synovial hypertrophy, and pannus formation, causing joint destruction and associated deformities. Pannus formation leads to bony erosions that are specific to RA (1, 2). US can be used in the assessment and monitoring of RA and can facilitate recognition of synovitis and erosions even in the early stages of the disease. It also allows disease progression and treatment responses to be monitored (1).
Gray-scale images often identify synovium in inflammatory arthritis and allow measurement of synovial thickness and the size of effusions (3). PDUS has increased the sensitivity for detecting early disease and can help in differentiating between active and inactive inflammation (4). Doppler US results also correlated well with histopathology in the detection of synovitis (5). US is as effective as magnetic resonance imaging (MRI) in detecting synovitis and tenosynovitis not visible by plain radiographs in early arthritis (6). US can visualize erosions more readily than plain radiographs in RA (6).
Several research groups have evaluated the role of US in monitoring the response to treatment in patients with RA and showed significant decreases in joint cavity widening, synovial perfusion, and vascularization after intra-articular steroid injections and treatments with biologics agents (7, 8). US findings have been shown to correlate positively with radiographic progression (9).
Semiquantitative scoring systems to grade vascularization, amount of synovial fluid, and synovial hypertrophy have also been developed and used in clinical trials or practice. The most commonly used grading system for vascularization consists of three grades, ranging from 0 to 3 according to the PDUS signal intensity, as follows: grade 0, no Doppler signal or no flow; grade 1, single vessel signal or mild flow; grade 2, confluent signals or moderate flow; and grade 3, more than 50% of the area of the synovial membrane with signal or severe flow (5, 10).
Although there is not yet enough evidence showing which joints and synovial recesses are the best predictors of disease outcome or response to treatment, Scheel et al. (11) suggested that US examination can be simplified by focusing on the palmar side of the finger joints with a semiquantitative grading of the II–IV metacarpophalangeal and proximal interphalangeal joints. Evaluation of the metacarpophalangeal, proximal interphalangeal, and metatarsophalangeal joints can be particularly useful in patients with early and established disease (12).
Doppler US is a reliable method of assessing the vascularization of the hand and wrist joints in RA (Fig. 2). Joint destruction is closely associated with increased vascularization and flow pattern (13, 14). Demonstration of this vascularization during the early period of the disease can lead to an early diagnosis of RA and may lead to effective treatment before erosions and bony destruction occur.
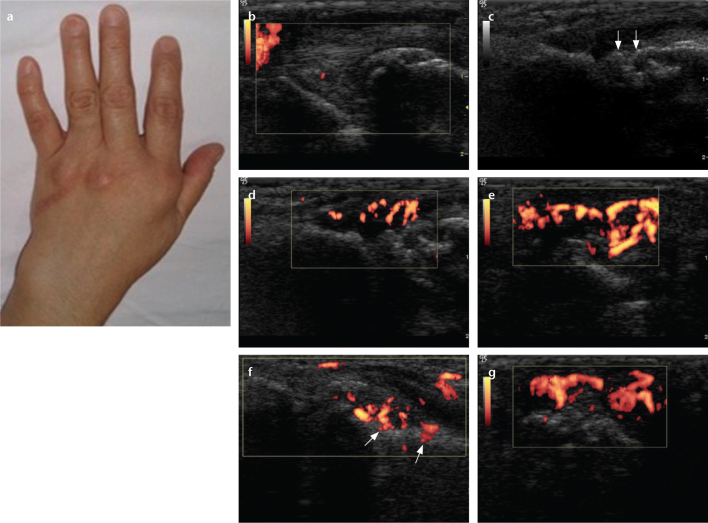
Figure 2. a–g.
The left hand of a 48-year-old female with rheumatoid arthritis. Photograph of the left hand (a) shows swelling in the wrist and second finger. Longitudinal power Doppler image (b) shows minimally increased vascularity in the left ulnocarpal joint. Longitudinal gray-scale US (c) shows cortical irregularity (arrows) in the second left metacarpophalangeal joint. Longitudinal power Doppler (d), transverse power Doppler (e), angled longitudinal power Doppler (f), volar aspect (g), and longitudinal power Doppler (f) images show severely increased vascularity with cortical penetration (f, arrows) in the second left metacarpophalangeal joint.
In one study, the sensitivity of PDUS in detecting synovitis in RA was 88.8%, and the specificity was 97.1%, with reference to dynamic contrast-enhanced MRI (15). In the same study, the authors noted that contrast enhancement did not correlate with PDUS scores.
Ankylosing spondylitis
Ankylosing spondylitis (AS) is an inflammatory disorder and the largest subgroup of the spondyloarthropathies (SpA). Although AS affects primarily the axial skeleton, peripheral joints and extra-articular structures may also be involved. The main use of US in AS and SpA is the detection of peripheral joint pathologies, including synovial effusion, hypertrophy, enthesopathy, and increased Doppler signals at sites of entheses (16, 17).
Enthesitis is the major finding in peripheral pathology in SpA. US findings include increased fascia and tendon thickness, fibrillar separation, caused by intratendinous edema, bursitis, and different patterns of PDUS signals. In the later stages, enthesal calcifications, enthesophytes, bone erosions, and cortical changes at the entheses may be seen (18–20). US has been suggested to be superior to MRI for detecting enthesitis (21). Increased vascularity within the entheses, suggestive of inflammation, has been demonstrated in various studies assessing patients with AS. These entheses are usually superficial structures and use of PDUS enables assessment of the involvement, extent of inflammation, and response to therapy. Our group reported previously that pain and tenderness at these enthesitis sites correlated with increased vascularization (22). We also underscored that PDUS examination of entheses may be useful and complementary to a clinical examination in patients with SpA. Entheses showing high vascularity on PDUS were more likely to cause pain. In all patients, abnormal vascularization was detected at least one enthesis, and abnormal vascularization was frequently seen at points where the enthesis adhered to cortical bone but were rare in bursae. The most frequent sites of increased vascularization were the Achilles tendon insertion (Fig. 3), the first costochondral joint, and the anterior superior iliac spine (22).
Figure 3. a, b.
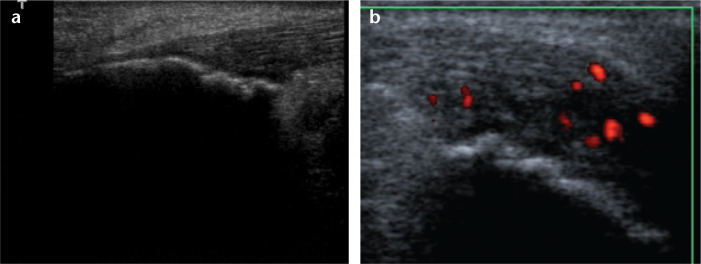
Longitudinal US image (a) shows cortical irregularity at the Achilles tendon insertion, and transverse power Doppler image (b) shows grade 2 vascularization at the Achilles tendon insertion.
The first and still commonly used US scoring system for enthesitis is the Glasgow Enthesitis Scoring System (GUESS), developed by Balint et al. (23) in 2002. GUESS was designed to assess five enthesal sites in the lower limbs: the superior pole of the patella (quadriceps tendon insertion), the inferior pole of the patella (patellar ligament origin), the patellar ligament insertion at the tibial tuberosity, the Achilles tendon, and the plantar aponeurosis. In this scoring system, only gray-scale findings are included. The possible maximum total score in GUESS is 36. The US score is also calculated separately as a soft tissue score (enthesal thickness and bursitis) and a bone score (erosions and enthesophytes). However, GUESS may not be sufficiently sensitive to detect responses to treatment (24). Another scoring system was proposed by D’Agostino et al., (20) which classified five distinct patterns according to the different combinations of abnormal gray-scale and/or PDUS features. Gray-scale US has also been used to view sacroiliac joints and CDUS to estimate arterial flow around sacroiliac joints (25).
Psoriatic arthritis
Several studies of the diagnostic potential of US in the assessment of patients with psoriatic arthritis (PsA) have been reported. US findings in PsA are similar to those in patients with other inflammatory conditions, because the disease may involve synovial joints, the spine, and entheses. Disease activity can be assessed by evaluating synovial proliferation, joint effusion, synovial hyperemia, and changes on bone surfaces (cortical changes including periost reactions). Entheses and tendon involvement are characteristic features of the spondyloarthritides, probably most prominent in PsA, accessible mostly on the plantar fascia and Achilles tendon insertion (26). A consensus definition of enthesopathy has been suggested by Outcomes Measures in Rheumatology (OMERACT) group, which defined enthesopathy as “abnormally hypoechoic (loss of normal fibrillar architecture) and/or thickened tendon or ligament at its bony attachment, seen in two perpendicular planes that may exhibit Doppler signal and/or bony changes including enthesophytes, erosions, or irregularity” (3). US has higher sensitivity in the detection of joint inflammation with respect to clinical assessment in PsA (27).
Dactylitis or “sausage digit” is a common manifestation of PsA that can be evaluated by US. In dactylitis, abnormalities include tenosynovitis of the finger or toe flexor tendons, synovitis, and diffuse soft-tissue edema, and increased PDUS signals can be observed (19, 20). In active and acute dactylitis, swelling and increased vascularization may be seen readily with Doppler US. After juxta-articular or periosteal reactions, capsular enthesophytes, and enthesopathy at the attachment of the deep flexor tendons can also be seen on US (28).
PsA is an inflammatory form of arthritis that affects ∼1% of the population and may be as severe as RA. Hand and wrist joints are typically involved. Inflammatory synovitis is the earliest change in arthritis and its detection is important in terms of diagnosis and early treatment. US findings are usually seen in the metacarpophalangeal, carpal, proximal interphalangeal, and distal interphalangeal joints (Fig. 4).
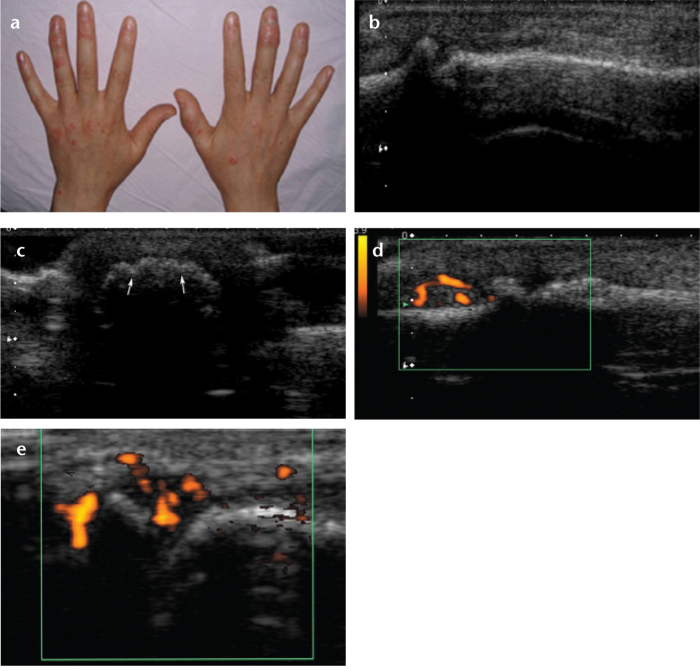
Figure 4. a–e.
A 23-year-old female with psoriatic arthritis. Photograph of the hands (a) shows psoriatic skin changes and swelling, especially in the right third distal interphalangeal joint and left fourth distal interphalangeal joint. Longitudinal (b) and transverse gray-scale (c) US show soft-tissue swelling and erosions (c, arrows) in the right third distal interphalangeal joint. Longitudinal power Doppler image (d) shows increased vascularity in the right third distal interphalangeal joint. Another longitudinal power Doppler image (e) shows increased vascularity in the right first distal interphalangeal joint.
Gout
US has become a useful diagnostic tool in crystal arthropathies in which crystals of various types may be deposited within articular and periarticular tissues, leading to inflammation and damage. Bone erosions, tendinopathy, cartilage involvement, and bursitis are frequently imaged in crystal arthropathies.
Gouty arthritis is a form of inflammatory arthritis characterized by tissue deposition of monosodium urate (MSU) crystals. Deposition of MSU crystals on the hyaline cartilage surface leads to hyperechoic enhancement of the superficial margin of the cartilage layer (29). Tophi appear as nodular deposits; the most frequent locations are the fingers, toes, and olecranon bursae. After sufficient deposition, tophi appear as hyperechoic bands with a posterior acoustic shadow (30). The prevalence of US signs from MSU crystal aggregates is highest at the first metatarsophalangeal and the talo-navicular joints (Figs. 5, 6) (31).
Figure 5. a, b.
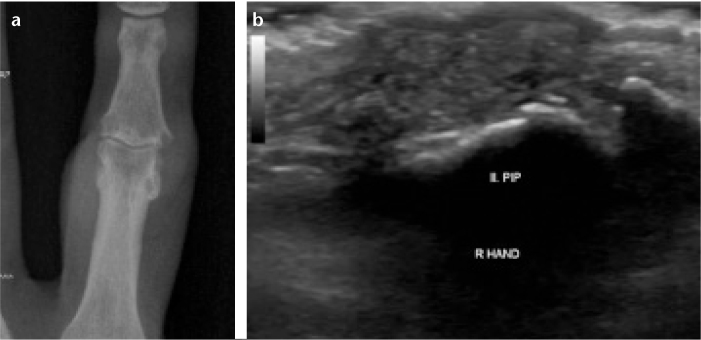
Gouty arthritis. The right hand second proximal interphalangeal (PIP) joint posteroanterior X-ray (a) and longitudinal B mode US (b) show erosion and tophi.
Figure 6. a, b.
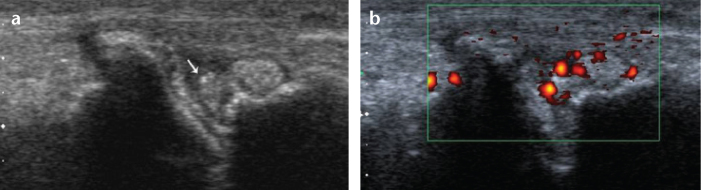
Gouty arthritis. Longitudinal B-mode (a) and longitudinal power Doppler US (b) show tophi (a, arrow) and synovial vascularity and inflammation in the right third metatarsophalangeal joint.
Several US features facilitate the differential diagnosis of crystal arthropathies; for example, in chronic gout, MSU deposition can be visualized as a “double contour sign,” a hyperechoic irregular band on the surface of anechoic articular cartilage (30). US and MRI findings are consistent in the detection of tophi (32). US is also sensitive to determine resolution of the double contour sign after urate-lowering therapy (33). A decrease in PDUS signals has also been shown after colchicine therapy (34).
Pseudogout
US findings of calcium pyrophosphate deposition (CPPD) differ from those of gouty arthritis. US is more sensitive than conventional radiography in detecting pyrophosphate crystals (35). In CPPD, hyperechoic crystals form a thin linear band within the anechoic articular cartilage, especially in the Achilles tendons or plantar fascia (29, 36), and hyperechoic rounded areas in fibrocartilage, typically in the triangular ligament of the wrist and in the menisci of the knee (29, 35). Acoustic shadowing is absent behind the calcifications in CPPD (37). Intracartilaginous hyperechoic enhancement correlates with the existence of CPPD crystals in the synovial fluid, with a specificity of 98% (35).
Other rheumatic disorders
Relatively few studies have characterized US findings in systemic lupus erythematosus (SLE). Synovial hypertrophy and effusions have been described in addition to erosions and tenosynovitis in the hand joints of patients with SLE (38). Synovitis and tenosynovitis in the upper and lower extremities have been defined in juvenile patients with SLE (39). US has been also used to define improvement in synovitis in patients undergoing treatment (40).
The utility of US is not limited to articular or periarticular imaging in rheumatic diseases. Submandibular gland US showed similar diagnostic value to invasive sialography in Sjögren’s syndrome (41).
Several clinical features of systemic sclerosis can be evaluated using US, including joint and tendon involvement, the presence of subcutaneous calcification, and skin changes. The most common findings are soft tissue calcifications and lessening of the distance between the phalangeal apex and the skin surface. US may also show thickening and decreased echogenicity of the dermis during the early phases of the disease.
US also has the ability to demonstrate carpal tunnel syndrome (CTS) by the increased cross-sectional area of the median nerve at the level of the carpal inlet, and can diagnose CTS with sensitivity comparable to other techniques (42). It is a practical first-line screening test and allows differentiation of the primary and secondary forms of CTS (43). Furthermore, US can be used to assess local anatomy and alternative pathologies that might be causing the CTS symptoms, such as a space-occupying lesions, amyloid deposition, erosions, cysts, or synovitis.
US is also useful for the diagnosis of proximal deep vein thrombosis in Behçet’s disease or anti-phospholipid syndrome (44). In large-vessel arteritis, US can detect homogenous wall thickening, stenosis, or occlusion (45).
Power Doppler US for evaluating treatment response in rheumatic diseases
PDUS is an ideal method for assessing the response to treatments in rheumatic diseases. Ultrasonographic evaluation of the existence of synovitis may also predict future structural damage in patients with RA (46). In a recent study, gray-scale, power Doppler, and contrast-enhanced US were suggested to be accurate tools for the detection and follow-up of synovitis in rheumatic wrist and finger joints, and contrast-enhanced US has been reported to be more sensitive than MRI in these joints (47). In this era of effective treatments for rheumatic diseases, such as biologics, attention is focused on feasible therapy monitoring techniques. US and PDUS seem to be among the most promising modalities for monitoring the efficacy of treatments for rheumatic diseases (48–50).
Conclusion
In conclusion, US can be considered to be a stethoscope for physicians in musculoskeletal medicine. US can assist the rheumatologist in making therapeutic decisions and in monitoring responses to therapy. Much current research in US is focused on continued improvements in the sensitivity, specificity, validity, and reproducibility of US findings.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Ribbens C, Andre B, Marcelis S, et al. Rheumatoid hand joint synovitis: gray-scale and power Doppler US quantifications following anti-tumor necrosis factor-alpha treatment: pilot study. Radiology. 2003;229:562–569. doi: 10.1148/radiol.2292020206. [DOI] [PubMed] [Google Scholar]
- 2.Wakefield RJ, Gibbon WW, Conaghan PG, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum. 2000;43:2762–2770. doi: 10.1002/1529-0131(200012)43:12<2762::AID-ANR16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Wakefield RJ, Balint PV, Szkudlarek M, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–2487. [PubMed] [Google Scholar]
- 4.Brown AK. Using ultrasonography to facilitate best practice in diagnosis and management of RA. Nat Rev Rheumatol. 2009;5:698–706. doi: 10.1038/nrrheum.2009.227. [DOI] [PubMed] [Google Scholar]
- 5.Walther M, Harms H, Krenn V, Radke S, Faehndrich TP, Gohlke F. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:331–338. doi: 10.1002/1529-0131(200102)44:2<331::AID-ANR50>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Wakefield RJ, O’Connor PJ, Conaghan PG, et al. Finger tendon disease in untreated early rheumatoid arthritis: a comparison of ultrasound and magnetic resonance imaging. Arthritis Rheum. 2007;57:1158–1164. doi: 10.1002/art.23016. [DOI] [PubMed] [Google Scholar]
- 7.Hau M, Kneitz C, Tony HP, Keberle M, Jahns R, Jenett M. High resolution ultrasound detects a decrease in pannus vascularisation of small finger joints in patients with rheumatoid arthritis receiving treatment with soluble tumour necrosis factor alpha receptor (etanercept) Ann Rheum Dis. 2002;61:55–58. doi: 10.1136/ard.61.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terslev L, Torp-Pedersen S, Qvistgaard E, Danneskiold-Samsoe B, Bliddal H. Estimation of inflammation by Doppler ultrasound: quantitative changes after intra-articular treatment in rheumatoid arthritis. Ann Rheum Dis. 2003;62:1049–1053. doi: 10.1136/ard.62.11.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naredo E, Collado P, Cruz A, et al. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum. 2007;57:116–124. doi: 10.1002/art.22461. [DOI] [PubMed] [Google Scholar]
- 10.Iagnocco A, Epis O, Delle Sedie A, et al. Ultrasound imaging for the rheumatologist. XVII. Role of colour Doppler and power Doppler. Clin Exp Rheumatol. 2008;26:759–762. [PubMed] [Google Scholar]
- 11.Scheel AK, Hermann KG, Kahler E, et al. A novel ultrasonographic synovitis scoring system suitable for analyzing finger joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2005;52:733–743. doi: 10.1002/art.20939. [DOI] [PubMed] [Google Scholar]
- 12.Szkudlarek M, Klarlund M, Narvestad E, et al. Ultrasonography of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis: a comparison with magnetic resonance imaging, conventional radiography and clinical examination. Arthritis Res Ther. 2006;8:R52. doi: 10.1186/ar1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozgocmen S, Kiris A, Kocakoc E, Ardicoglu O, Kamanli A. Evaluation of metacarpophalangeal joint synovitis in rheumatoid arthritis by power Doppler technique: relationship between synovial vascularization and periarticular bone mineral density. Joint Bone Spine. 2004;71:384–388. doi: 10.1016/j.jbspin.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Kiris A, Ozgocmen S, Kocakoc E, Ardicoglu O. Power Doppler assessment of overall disease activity in patients with rheumatoid arthritis. J Clin Ultrasound. 2006;34:5–11. doi: 10.1002/jcu.20175. [DOI] [PubMed] [Google Scholar]
- 15.Szkudlarek M, Court-Payen M, Strandberg C, Klarlund M, Klausen T, Ostergaard M. Power Doppler ultrasonography for assessment of synovitis in the meta-carpophalangeal joints of patients with rheumatoid arthritis: a comparison with dynamic magnetic resonance imaging. Arthritis Rheum. 2001;44:2018–2023. doi: 10.1002/1529-0131(200109)44:9<2018::AID-ART350>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 16.Backhaus M, Burmester GR, Sandrock D, et al. Prospective two year follow up study comparing novel and conventional imaging procedures in patients with arthritic finger joints. Ann Rheum Dis. 2002;61:895–904. doi: 10.1136/ard.61.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierrez M, Filippucci E, De Angelis R, Filosa G, Kane D, Grassi W. A sonographic spectrum of psoriatic arthritis: “the five targets”. Clin Rheumatol. 2010;29:133–142. doi: 10.1007/s10067-009-1292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Agostino MA, Aegerter P, Jousse-Joulin S, et al. How to evaluate and improve the reliability of power Doppler ultrasonography for assessing enthesitis in spondylarthritis. Arthritis Rheum. 2009;61:61–69. doi: 10.1002/art.24369. [DOI] [PubMed] [Google Scholar]
- 19.de Miguel E, Cobo T, Munoz-Fernandez S, et al. Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann Rheum Dis. 2009;68:169–174. doi: 10.1136/ard.2007.084251. [DOI] [PubMed] [Google Scholar]
- 20.D’Agostino MA, Said-Nahal R, Hacquard-Bouder C, Brasseur JL, Dougados M, Breban M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: a cross-sectional study. Arthritis Rheum. 2003;48:523–533. doi: 10.1002/art.10812. [DOI] [PubMed] [Google Scholar]
- 21.Kamel M, Eid H, Mansour R. Ultrasound detection of heel enthesitis: a comparison with magnetic resonance imaging. J Rheumatol. 2003;30:774–778. [PubMed] [Google Scholar]
- 22.Kiris A, Kaya A, Ozgocmen S, Kocakoc E. Assessment of enthesitis in ankylosing spondylitis by power Doppler ultrasonography. Skeletal Radiol. 2006;35:522–528. doi: 10.1007/s00256-005-0071-3. [DOI] [PubMed] [Google Scholar]
- 23.Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis. 2002;61:905–910. doi: 10.1136/ard.61.10.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genc H, Duyur Cakit B, Nacir B, Saracoglu M, Kacar M, Erdem HR. The effects of sulfasalazine treatment on enthesal abnormalities of inflammatory rheumatic diseases. Clin Rheumatol. 2007;26:1104–1010. doi: 10.1007/s10067-006-0460-6. [DOI] [PubMed] [Google Scholar]
- 25.Spadaro A, Iagnocco A, Baccano G, Ceccarelli F, Sabatini E, Valesini G. Sonographic-detected joint effusion compared with physical examination in the assessment of sacroiliac joints in spondyloarthritis. Ann Rheum Dis. 2009;68:1559–1563. doi: 10.1136/ard.2008.093351. [DOI] [PubMed] [Google Scholar]
- 26.Hyslop E, McInnes IB, Woodburn J, Turner DE. Foot problems in psoriatic arthritis: high burden and low care provision. Ann Rheum Dis. 2010;69:928. doi: 10.1136/ard.2009.111971. [DOI] [PubMed] [Google Scholar]
- 27.Delle Sedie A, Riente L, Filippucci E, et al. Ultrasound imaging for the rheumatologist. XXXII. Sonographic assessment of the foot in patients with psoriatic arthritis. Clin Exp Rheumatol. 2011;29:217–222. [PubMed] [Google Scholar]
- 28.Fournie B, Margarit-Coll N, Champetier de Ribes TL, et al. Extrasynovial ultrasound abnormalities in the psoriatic finger. Prospective comparative power-doppler study versus rheumatoid arthritis. Joint Bone Spine. 2006;73:527–531. doi: 10.1016/j.jbspin.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Grassi W, Meenagh G, Pascual E, Filippucci E. “Crystal clear”-sonographic assessment of gout and calcium pyrophosphate deposition disease. Semin Arthritis Rheum. 2006;36:197–202. doi: 10.1016/j.semarthrit.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Wright SA, Filippucci E, McVeigh C, et al. High-resolution ultrasonography of the first metatarsal phalangeal joint in gout: a controlled study. Ann Rheum Dis. 2007;66:859–864. doi: 10.1136/ard.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filippucci E, Meenagh G, Delle Sedie A, et al. Ultrasound imaging for the rheumatologist XXXVI. Sonographic assessment of the foot in gout patients. Clin Exp Rheumatol. 2011;29:901–905. [PubMed] [Google Scholar]
- 32.Perez-Ruiz F, Martin I, Canteli B. Ultrasonographic measurement of tophi as an outcome measure for chronic gout. J Rheumatol. 2007;34:1888–1893. [PubMed] [Google Scholar]
- 33.Thiele RG, Schlesinger N. Ultrasonography shows disappearance of monosodium urate crystal deposition on hyaline cartilage after sustained normouricemia is achieved. Rheumatol Int. 2010;30:495–503. doi: 10.1007/s00296-009-1002-8. [DOI] [PubMed] [Google Scholar]
- 34.Filippucci E, Ciapetti A, Grassi W. Sonographic monitoring of gout. Reumatismo. 2003;55:184–186. doi: 10.4081/reumatismo.2003.184. [DOI] [PubMed] [Google Scholar]
- 35.Frediani B, Filippou G, Falsetti P, et al. Diagnosis of calcium pyrophosphate dihydrate crystal deposition disease: ultrasonographic criteria proposed. Ann Rheum Dis. 2005;64:638–640. doi: 10.1136/ard.2004.024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falsetti P, Frediani B, Acciai C, et al. Ultrasonographic study of Achilles tendon and plantar fascia in chondrocalcinosis. J Rheumatol. 2004;31:2242–2250. [PubMed] [Google Scholar]
- 37.Guermazi A, Burstein D, Conaghan P, et al. Imaging in osteoarthritis. Rheum Dis Clin North Am. 2008;34:645–687. doi: 10.1016/j.rdc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Wright S, Filippucci E, Grassi W, Grey A, Bell A. Hand arthritis in systemic lupus erythematosus: an ultrasound pictorial essay. Lupus. 2006;15:501–506. doi: 10.1191/0961203306lu2340oa. [DOI] [PubMed] [Google Scholar]
- 39.Demirkaya E, Ozcakar L, Turker T, et al. Musculoskeletal sonography in juvenile systemic lupus erythematosus. Arthritis Rheum. 2009;61:58–60. doi: 10.1002/art.24090. [DOI] [PubMed] [Google Scholar]
- 40.Torrente-Segarra V, Lisbona-Perez M, Rotes-Sala D, Castro-Oreiro S, Carbonell-Abello J. Clinical, biological and ultrasonographic remission in a patient with musculoskeletal systemic lupus erythematosus with rituximab. Lupus. 2009;18:270–272. doi: 10.1177/0961203308095001. [DOI] [PubMed] [Google Scholar]
- 41.Takagi Y, Kimura Y, Nakamura H, Sasaki M, Eguchi K, Nakamura T. Salivary gland ultrasonography: can it be an alternative to sialography as an imaging modality for Sjogren’s syndrome? Ann Rheum Dis. 2010;69:1321–1324. doi: 10.1136/ard.2009.123836. [DOI] [PubMed] [Google Scholar]
- 42.Mondelli M, Filippou G, Gallo A, Frediani B. Diagnostic utility of ultrasonography versus nerve conduction studies in mild carpal tunnel syndrome. Arthritis Rheum. 2008;59:357–366. doi: 10.1002/art.23317. [DOI] [PubMed] [Google Scholar]
- 43.Fowler JR, Gaughan JP, Ilyas AM. The sensitivity and specificity of ultrasound for the diagnosis of carpal tunnel syndrome: a meta-analysis. Clin Orthop Relat Res. 2011;469:1089–1094. doi: 10.1007/s11999-010-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaitini D. Current approaches and controversial issues in the diagnosis of deep vein thrombosis via duplex Doppler ultrasound. J Clin Ultrasound. 2006;34:289–297. doi: 10.1002/jcu.20236. [DOI] [PubMed] [Google Scholar]
- 45.Arida A, Kyprianou M, Kanakis M, Sfikakis PP. The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Disord. 2010;11:44. doi: 10.1186/1471-2474-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dougados M, Devauchelle-Pensec V, Ferlet JF, et al. The ability of synovitis to predict structural damage in rheumatoid arthritis: a comparative study between clinical examination and ultrasound. Ann Rheum Dis. 2013;72:665–671. doi: 10.1136/annrheumdis-2012-201469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohrndorf S, Hensch A, Naumann L, et al. Contrast-enhanced ultrasonography is more sensitive than grayscale and power Doppler ultrasonography compared to MRI in therapy monitoring of rheumatoid arthritis patients. Ultraschall Med. 2011;32(Suppl 2):E38–44. doi: 10.1055/s-0031-1281770. [DOI] [PubMed] [Google Scholar]
- 48.Capkin E, Kiris A, Karkucak M, et al. Investigation of effects of different treatment modalities on structural and functional vessel wall properties in patients with ankylosing spondylitis. Joint Bone Spine. 2011;78:378–382. doi: 10.1016/j.jbspin.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 49.Gutierrez M, De Angelis R, Bernardini ML, et al. Clinical, power Doppler sonography and histological assessment of the psoriatic plaque: short-term monitoring in patients treated with etanercept. Br J Dermatol. 2011;164:33–37. doi: 10.1111/j.1365-2133.2010.10026.x. [DOI] [PubMed] [Google Scholar]
- 50.Naredo E, Batlle-Gualda E, Garcia-Vivar ML, et al. Power Doppler ultrasonography assessment of entheses in spondyloarthropathies: response to therapy of entheseal abnormalities. J Rheumatol. 2010;37:2110–2117. doi: 10.3899/jrheum.100136. [DOI] [PubMed] [Google Scholar]