Background: Endothelial cells respond to proangiogenic cues during angiogenesis.
Results: Receptor for activated C kinase 1 (RACK1) and vimentin are up-regulated and complex with focal adhesion kinase (FAK). RACK1 and vimentin also regulate FAK expression and activation during sprouting.
Conclusion: RACK1 and vimentin complex with and regulate FAK.
Significance: These data uncover a new intermolecular complex that regulates sprouting angiogenesis by modulating focal adhesions.
Keywords: Angiogenesis, Collagen, Endothelial Cell, Focal Adhesion Kinase, Morphogenesis, RACK1, Vimentin
Abstract
Angiogenesis is critical for many physiological and pathological processes. To identify molecules relevant to angiogenesis, we performed a proteomic screen comparing invading versus non-invading endothelial cells in three-dimensional collagen matrices. We found up-regulated levels of receptor for activated C kinase 1 (RACK1) and the intermediate filament protein vimentin that correlated with increased endothelial cell invasion. Because both RACK1 and vimentin have been linked to focal adhesion kinase (FAK), we investigated whether this pathway regulated invasion. RACK1 depletion reduced invasion responses, and this was associated with attenuated activation of FAK. Knockdown of vimentin significantly decreased levels of phosphorylated and total FAK. Treatment with a pharmacological inhibitor of FAK dose-dependently reduced invasion, indicating a crucial role for FAK activity during invasion. Because RACK1 and vimentin were both up-regulated with sphingosine 1-phosphate treatment, required for invasion, and regulated FAK, we tested whether they complexed together. RACK1 complexed with vimentin, and growth factors enhanced this interaction. In addition, RACK1, vimentin, and FAK formed an intermolecular complex in invading endothelial cultures in three dimensions in response to stimulation by sphingosine 1-phosphate and growth factors. Moreover, depletion of RACK1 decreased the association of vimentin and FAK, suggesting that RACK1 was required for stabilizing vimentin-FAK interactions during sprouting. Silencing of vimentin and RACK1 decreased cell adhesion and focal contact formation. Taken together, these results demonstrate that proangiogenic signals converge to enhance expression and association of RACK1 and vimentin, which regulated FAK, resulting in successful endothelial sprout formation in three-dimensional collagen matrices.
Introduction
Angiogenesis, which is defined as the development of new blood vessels from pre-existing ones, is a fundamental step in the growth and development of new vasculature. It plays vital roles in several physiological processes such as pregnancy, ovulation, and wound healing and pathological conditions such as cancer (1, 2). During angiogenesis, normally quiescent endothelial cells (ECs)2 that line the vasculature are stimulated by factors that promote basement membrane degradation, sprout initiation, migration, lumen formation, and stabilization (3–5). Because standard two-dimensional cell culture techniques cannot completely replicate this dynamic process, in vitro three-dimensional models that mimic natural angiogenesis can be used (6–10). In this study, we utilized a model in which collagen matrices containing sphingosine 1-phosphate (S1P) are combined with vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) to trigger sprouting and robust EC invasion (11, 12). S1P, a platelet-derived bioactive lipid, plays a crucial role in several cellular processes in ECs such as adherens junction assembly, chemotaxis, and morphogenesis into capillary-like networks (13–16). S1P acts through G protein-coupled endothelial differentiation gene (EDG) receptors, EDG-1 (S1P1) and EDG-3 (S1P3) in ECs (17). To identify regulators of S1P-induced EC invasion, we designed a proteomic screen comparing invading versus non-invading ECs.
We report here that receptor for activated C kinase 1 (RACK1) was differentially expressed in two independent proteomic screens designed to dissect the downstream targets of S1P-induced endothelial invasion. RACK1 is a member of the tryptophan-aspartate (WD) repeat family of proteins and was originally named for associating with protein kinase C β type III. However, it is now well known that RACK1 binds several proteins and can shuttle or anchor them to specific subcellular locations and stabilize their activity (18, 19). RACK1 is critical for development (20–22), cell proliferation, migration (23–26), and circadian rhythms (27). RACK1 mediates effects on cell migration mostly through regulation of focal adhesion (FA) assembly by promoting focal adhesion kinase (FAK) activation downstream of integrin clustering and adhesion (24, 25, 28, 29). Although studies in RACK1-null mice have not been reported, Berns et al. (30) reported that RACK1 was up-regulated during cord formation in bovine aortic endothelial cells in vitro and in angiogenically active tissues such as corpora lutea, ovarian follicles, and human carcinomas in vivo. Although these reports suggest a relevant role for RACK1 during angiogenesis, the molecular events directed by RACK1 remain unclear.
The major intermediate filament protein, vimentin, was also identified in a proteomic screen as a downstream target of S1P-induced EC invasion. Our laboratory has shown that vimentin depletion in ECs resulted in poor invasion responses (31). Recent studies in mouse fibroblasts suggest that vimentin may stabilize FA assembly (32) and regulate FA contact size in response to shear stress (33). FAs are dynamic macromolecular assemblies that link cells to the extracellular matrix (34–38). FAK is a major tyrosine kinase protein concentrated at FAs. Extracellular matrix engagement and integrin assembly promote FAK phosphorylation, which further recruits and phosphorylates other proteins at FAs to promote cytoskeletal rearrangement and cell migration (39–41). FAK has been extensively studied in cell migration, angiogenesis, and cardiac morphogenesis (42–45).
In this study, we show for the first time that a trimolecular complex between RACK1, vimentin, and FAK is stimulated by proangiogenic factors in three-dimensional invading cultures. Knockdown of RACK1 resulted in poor invasion responses and attenuated FAK activation, whereas vimentin depletion resulted in decreased FAK expression in ECs. Furthermore, RACK1 was needed to stabilize the complex between vimentin and FAK.
EXPERIMENTAL PROCEDURES
Endothelial Cell Culture and Invasion
Human umbilical vein ECs (HUVECs; Lonza, Allendale, NJ), passages 3–6, were cultured as described previously (11). Three-dimensional invasion experiments were established using 40 ng/ml VEGF and bFGF alone (GF), S1P and GF (S1P + GF), or S1P and GF plus 0.1 μg/ml pertussis toxin (S1P + GF + PTX). Collagen matrices (2.5 mg/ml) containing 0 or 1 μm S1P (Avanti Polar Lipids, Alabaster, AL) were prepared as described previously, and invasion responses where indicated were quantified for invasion density as described previously in detail (11).
Proteomic Screen 1: Differential In-gel Electrophoresis (DIGE)
Collagen gels from invading cultures (S1P + GF or S1P + GF + PTX) were collected (12.5 h) and incubated in M199 containing 10 μg/ml collagenase (Sigma) and HALT phosphatase inhibitor mixture (Thermo Scientific, Ashville, NC) at 37 °C for 10 min. For each condition, 240 gels were prepared. Cells were pelleted at 500 × g for 5 min and washed once in ice-cold PBS. Cell pellets were flash frozen in liquid nitrogen and stored at −80 °C. Frozen cell pellets were lysed by thawing and pipetting on ice in 1 ml of lysis solution I (0.3% SDS, 200 mm dithiothreitol, 50 mm Tris, pH 7.5, broad range protease inhibitors (GE Healthcare), HALT phosphatase inhibitor mixture (Thermo Scientific). Proteins were further solubilized by heating at 100 °C for 10 min followed by incubating on ice for 5 min. Lysates were sonicated with a 15-s burst (amplitude setting, 60) on ice using a Sonics Vibracell sonicator to further fragment DNA and cytoskeletal structures. Nucleic acids were digested by adding DNase/RNase mixture (GE Healthcare) and rotating the lysates at 4 °C for 45 min. Lysates were delipidated in chloroform-methanol by adding 4 ml of methanol and vortexing for 30 s followed by adding 1 ml of chloroform and vortexing for 30 s and finally by adding 3 ml of Millipore-purified water and vortexing for 60 s. Samples were rotated at room temperature for 15 min, transferred to Corex glass tubes, and centrifuged at 5500 × g in a Sorvall JA20 rotor for 20 min at room temperature. The interphase containing the proteins was transferred to a microcentrifuge tube and mixed with 0.5 ml of methanol. After spinning for 10 min in a microcentrifuge, the pellets were resuspended in 0.6 ml of water, and proteins were reprecipitated by adding a 0.8 volume of ice-cold acetone and incubating on ice for 15 min. Proteins were pelleted out of the acetone solution by spinning in a microcentrifuge for 15 min and resolubilized in 100 ml of a two-dimensional gel proteomic solubilization buffer (9.9 m urea, 4% CHAPS, 14% thiourea, 4.8% SDS, 10 mm Tris, pH 7.5, 40 mm DTT). Proteins were allowed to solubilize for 1 h by rotating at room temperature and then desalted through microspin desalting columns (Pierce) into desalting solution (9.9 m urea, 4% CHAPS). Protein concentrations were determined by the BCA protein assay after pretreating and reprecipitating an aliquot of each sample with BCA compatibility reagent and Compat-Able protein assay preparation set (Pierce catalog numbers 23250 and 23215, respectively) to eliminate interference from BCA protein reagent with thiourea. After determining protein concentration, the samples were adjusted to 50 mg of protein/10 ml of 9.9 m urea, 4% CHAPS and subjected to a second spin through a desalting microspin column into DIGE labeling buffer (9.9 m urea, 4% CHAPS, 30 mm Tris-HCl, pH 8).
Protein samples were prepared for DIGE, which is based on prelabeling of different protein fractions with cyanine-based fluors (CyDye DIGE Fluor minimal dyes) and their subsequent co-electrophoresis in a single two-dimensional gel. The DIGE technology has the advantages of allowing elimination of inconsistencies based on gel to gel variations and exact quantification of proteins spots separated by gel electrophoresis. Proteins in DIGE labeling buffer (150 mg in a 30-ml volume) were covalently attached to CyDyes (Cy5, Cy3, and Cy2) using the manufacturer's protocol (GE Healthcare). A 500-mg aliquot (75-ml volume) of a pooled total protein preparation was then added to each CyDye-labeled protein sample. This allowed each gel to be a preparative gel for picking spots for mass spectrometry. The CyDye-labeled preparative protein samples were then passed through a desalting microspin column equilibrated with desalting solution to remove free CyDye reagents and buffer. Desalted CyDye-labeled protein lysates (∼100 ml) were mixed with 400 ml of Destreak Rehydration sample buffer (GE Healthcare) containing 0.077 mg of DTT. Protein samples were solubilized by rotating for 1 h at room temperature. After adding another 0.077 mg of DTT and ampholytes (100× Bio-Lyte pI 3–10 ampholytes, Bio-Rad), samples were rotated for 30 min at room temperature and then allowed to rehydrate pH 3–10 Immobiline dry strip gel strips (GE Healthcare) for 18 h. Rehydrated Immobiline dry strips were subjected to stepwise isoelectric focusing at 150 V for 6 h, 500 V for 1 h, 1000 V for 1 h, and 8000 V for 6 h in an Ettan IPGphorII unit. Proteins were then separated in the second dimension on large format (27 × 21-cm) 8–16% gradient SDS-polyacrylamide gels at 5 watts/gel for 9.5 h. CyDye-labeled proteins in the two-dimensional gels were imaged with a Typhoon 9200 laser scanner. A gel selected for spot picking was counterstained with SYPRO Red for 6 h in 7.5% acetic acid, destained for 1 h in 7.5% acetic acid, and reimaged in a Typhoon 9200 laser scanner. Image analysis was performed using the Biological Variation Analysis module of DeCyder software version 5.0 (GE Healthcare), which first normalizes each sample to its respective in-gel Cy2 internal standard and then matches all controls and samples between different gels. Comparing each group in the Biological Variation Analysis module generated average expression ratios and Student's t tests of individual protein spots.
Proteomic Screen 2
Two-dimensional gel electrophoresis was performed as described previously (12).
In-gel Digestion
Spot picking and in-gel digestion were carried out robotically at the Protein Analysis Laboratory, Texas A&M University, on selected SYPRO Red-stained preparative gels using the Ettan Spot Picker and the Ettan Digester (GE Healthcare). For in-gel digestion, protein spots of interest (1.4 expression ratio or greater) were excised, and the gel plugs were washed twice for 15 min in 50% acetonitrile, 50 mm ammonium bicarbonate. The plugs were washed once more with 100% acetonitrile for 15 min and then dried by centrifugal lyophilization for 30 min. In-gel digestion was conducted by adding 20 ml of trypsin (20 ng/ml) and incubating for 15 min. After adding 100 μl of 25 mm ammonium bicarbonate, the gel plugs were incubated at 37 °C overnight. The supernatants were removed and saved. A solution of 80% acetonitrile, 0.1% trifluoroacetic acid was added to the gel plug for 30 min. The supernatant was then removed and pooled with the first supernatant. The peptide volume was reduced down to 10 μl by centrifugal lyophilization and then subjected to LCMS.
Protein Identification
LCMS was carried out on a Thermo Finnigan (Thermo Electron, Asheville, NC) LCQDecaXP (ESI-TRAP model). Samples were run using an in-house packed C18 reverse phase nanospray needle. Mass spectra were used to interrogate human sequences in the NCBInr database (May 2009; 478,579 entries; human database). The automatic data analysis and database searching were fulfilled by the TurboSEQUEST software in the Bioworks Browser (version 3.3.1 SP1). Searches with TurboSEQUEST were performed to allow for a maximum of two missed trypsin cleavages. Additional TurboSEQUEST search parameters were as follows: mass typesetting was monoisotopic precursor and fragments; threshold tolerance was 50,000; peptide tolerance, precursor ion tolerance, and fragment ion tolerance were 2.5000, 1.4000, and 0.0001 atomic mass units, respectively. Ions and ion series calculated were B and Y ions. Searches were also conducted using the Mascot search engine (Matrix Science). The database searched by Mascot was NCBInr 20070216 (4,626,804 sequences; 1,596,079,197 residues), and the taxonomy was Homo sapiens (human) (191,437 sequences). Mascot search parameters were as follows: fixed modifications, carboxymethyl; variable modifications, oxidation (Met); mass values, monoisotopic; protein mass, unrestricted; peptide mass tolerance, ±1.8 Da; fragment mass tolerance, ±0.8 Da. The search identification had a statistically significant p < 0.05 (based on mass/mass spectra). Redundancy of proteins that appeared in the database under different names and accession numbers was eliminated. If more than one protein was identified in one spot, the single protein member with the highest protein score (top rank) was singled out from the multiprotein family.
Immunoblotting
Total cell lysates were prepared by solubilizing collagen matrices in boiling 1.5× Laemmli sample buffer at 95 °C for 10 min. Protein samples were resolved by 8.5–14% SDS-PAGE, transferred onto Immobilon PVDF membranes (Millipore), blocked with 5% nonfat dry milk or 5% BSA, washed, and probed with primary antibodies overnight at 4 °C. Membranes were incubated with HRP-conjugated secondary antibodies, washed, and developed with Immobilon Western Chemiluminescent HRP Substrate (Millipore) and HyBlot CL autoradiography film (Denville Scientific, South Plainfield, NJ). The antibodies used were directed to vimentin (sc-5565 and sc-6260, Santa Cruz Biotechnology), annexin A2 (ANXA2) (AF3928, BD Transduction Laboratories), RACK1 (sc-17754, Santa Cruz Biotechnology; ab119442, Abcam, Cambridge, MA), α-tubulin (T6199, Sigma-Aldrich), FAK (3285, Cell Signaling Technology, Danvers, MA; 05-537, Millipore), phosphorylated FAK (pFAK) (Tyr-397; 8556, Cell Signaling Technology), β2-microglobulin (β2M) (M8523, Sigma), GAPDH (ab8245, Abcam), VE-cadherin (sc-52751, Santa Cruz Biotechnology), and filamin A (MCA464ST, Bio-Rad) and HRP-conjugated secondary antibodies (Dako, Carpinteria, CA).
Generation of Stable Cell Lines Using shRNA
Lentiviral vectors specific for RACK1 (catalog number SHCLNG-NM006098), vimentin (catalog number SHCLNG-NM011701), and β2M (catalog number SHCLNG-NM004048) were purchased from Sigma-Aldrich. Lentiviral particles were generated by transfecting 1.25 μg of backbone shRNA lentiviral plasmid with 3.75 μg of VIRAPOWER packaging mixture (Invitrogen) into confluent 293FT cells (Invitrogen) using Lipofectamine 2000 (Invitrogen) in T25 flasks. Viral supernatants were harvested at 60 h, centrifuged at 1000 × g for 10 min, and filtered through a 0.45-μm filter (Millipore, Rockland, MA). 25–30% confluent T25 flasks of HUVECs were infected with 2 ml of viral supernatant, 3 ml of endothelial growth medium, and Polybrene (12 μg/ml; Sigma) for 4 h. HUVECs were allowed to grow for 4 days prior to use in experiments.
mRNA Extraction and PCR Analysis
HUVECs were treated with shRNAs to RACK1 (shRACK1-1, TRCN 0000273167; shRACK1-2, TRCN 0000006472; shRACK1-3, TRCN 0000006471) and β2M (shβ2M-1, TRCN 0000057254; shβ2M-2, TRCN 0000057255). RNA was extracted from HUVECs expressing shRNA as well as an untreated control using an RNeasy minikit (Qiagen). Eluted RNA was treated with RNase-free DNase (Qiagen) for 10 min at room temperature and inactivated at 65 °C for 15 min. RNA quality was assessed with an agarose gel. cDNA was generated using the SuperScript III First-Strand Synthesis System (Invitrogen) using 1 μg of RNA and oligo(dT)20. Amplicons were run on 2% agarose gels. The primers used for this study were: ANXA2 (NCBI Reference Sequence NM_001002858.2; 262 bp; 5′-CAGAGGATGCTCTGTCATTG-3′ and 5′-GGCTTGTTCTGAATGCACTG-3′); FAK (NM_153831.3; 217 bp; 5′-CTGGCTACCCTGGTTCACAT-3′ and 5′-TGTTGCTGTCGGATTAGACG-3′); vimentin (NM_003380; 177 bp; 5′-GGGACCTCTACGAGGAGGAG-3′ and 5′-AAGATTGCAGGGTGTTTTCG-3′); RACK1 (NM_006098.4, 1000 bp; 5′-CCACCATGACTGAGC-3′ and 5′-GCGTGTGCCAATGGT-3′); VE-cadherin (NM_001795.3, 182 bp; 5′-CCAGGTATGAGATCGTGGTG-3′ and 5′-AAACAGAGAGCCCACAGAGG-3′); β2M (NM_004048.2, 158 bp; 5′-TTTCATCCATCCGACATTGAAG-3′ and 5′-ACACGGCAGGCATACTCATC-3′); filamin-A (NM_001456.3, 243 bp; 5′-TCCAGCAGAACACTTTCACG-3′ and 5′-CGATGGACACCAGTTTGATG-3′), and GAPDH (NM_002046.4, 228 bp; 5′-CGACCACTTTGTCAAGCTCA-3′ and 5′-AGGGGTCTACATGGCAACTG-3′).
shRACK1 Rescue Experiments
HUVEC cDNA was used to amplify RACK1 using primers 5′-GCAAGCTTGCCACCATGACTGAGCAC-3′ and 5′-GCGGTACCGCGTGTGCCAATGGTCAG-3′. RACK1 amplicons were ligated into the pEGFP-C2 vector in the HindIII and KpnI sites. The resulting EGFP-RACK1 fusion construct was amplified using 5′-AGGATATCCTAGCGTGTGCCAATGGT-3′ and 5′-AGGTCGACGCCACCATGGTGAGCAA-3′ primers. EGFP-RACK1 fusion was inserted into pENTR4 vector using SalI and EcoRV restriction sites. Inserts were validated by sequencing and recombined with pLenti6/V5-DEST (Invitrogen) in Stbl3 cells (Invitrogen). After confirming expression, EGFP-RACK1 in pLenti6/V5 was mutated at shRACK1-1 target site using the QuikChange Lightning site-directed mutagenesis kit (Stratagene, Invitrogen) according to the manufacturer's instructions. The rescue construct was designated as R3 because primers incorporated three base pair changes (5′-GGAAACTGACCCGGGACGAGACCAACTACGGAATTCCACAG-3′ and 5′-CCTTTGACTGGGCCCTGCTCTGGTTGATGCCTTAAGGTGTC-3′). In rescue experiments, HUVECs infected with shRNA directed against β2M (shβ2M-1) or RACK1 (shRACK1-1) were transduced for 6 h, fed, and cultured for 26 h before transducing with lentiviruses delivering R3 (or green fluorescent protein (GFP) control (12)) rescue constructs. Lentiviruses (5 ml) were generated by transfecting 2.5 μg of backbone plasmids into confluent 293FT cells (Invitrogen) with 7.5 μg of VIRAPOWER packaging vectors (Invitrogen) using Lipofectamine 2000 (Invitrogen). Viral particles were harvested at 60 h, centrifuged at 1000 × g for 10 min, and filtered through a 0.45-μm filter (Millipore). T25 flasks transduced with shβ2M-1 or shRACK1-1 (40% confluent) were infected with 4.5 ml of viral supernatant and Polybrene (12 μg/ml; Sigma) overnight. Another 4.5 ml of viral supernatant and Polybrene were added to ECs and transduced for 48 h. HUVECs were cultured for 4 days before testing in invasion experiments.
Immunoprecipitation
In two-dimensional experiments, ECs (3 × 106) cultured in 75-cm2 flasks were treated for 1 h with or without 1 μm S1P or 40 ng/ml VEGF and bFGF. Cells were placed on ice, washed twice with 10 ml of cold PBS, lysed in 1 ml of cold lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 1× protease inhibitor mixture (Roche Diagnostics), 1 mm PMSF), and incubated for 10 min on ice with occasional mixing. Lysates were centrifuged at 16,000 × g for 15 min at 4 °C. Supernatants were collected and incubated with protein G magnetic beads (5 μl) for 1 h at 4 °C with agitation for preclearing. Supernatants (1 ml) were incubated with 2 μg of antiserum directed to vimentin (sc-5565), RACK1 (sc-17754), or FAK (05-537) for 18 h at 4 °C with agitation. Protein G magnetic beads (10 μl) were added for 2 h at 4 °C. Beads were washed six times with 1 ml of lysis buffer without protease inhibitors, eluted in 1% SDS, and analyzed by Western blotting. For three-dimensional experiments, 30 gels with 30,000 ECs each were collected in 600 μl of cold lysis buffer. Mixtures were incubated for 30 min at 4 °C with gentle agitation. Cell lysates were centrifuged at 16,000 × g for 20 min at 4 °C. The remaining steps were performed as above.
Immunofluorescence and Co-localization Studies
Cells were seeded on collagen-coated coverslips (20 μg/ml) and allowed to grow overnight. In wound assays, cells were wounded with a pipette tip and washed twice with M199. Cells were treated for the times indicated with 1 μm S1P or 40 ng/ml growth factors (or both), fixed in 4% paraformaldehyde, rinsed three times in Tris-glycine buffer (0.3% Tris, 1.5% glycine), and permeabilized with 0.5% Triton X-100 for 20 min with gentle agitation before blocking in buffer containing 0.5% Triton X-100, 1% BSA, and 1% serum overnight at 4 °C. Primary antibodies to vimentin (sc-5565), RACK1 (sc-17754), pFAK (8556), and vinculin (V9131, Sigma) were added in blocking buffer (1:50) for 3 h at room temperature, washed, and incubated with Alexa Fluor 488- or 594-conjugated secondary antibodies (Molecular Probes) (1:300) in blocking buffer for 1 h. After washing, coverslips were mounted and imaged using a Nikon TI A1R inverted confocal microscope. For quantification of co-localization, intensity profiles of RACK1 (red) and vimentin (green) along randomly selected lines in the image were plotted using NIS-Elements software. The average Pearson's correlation coefficient from n number of images for each treatment group was statistically analyzed.
Imaging and Quantification of Invasion Density and Distance
Top and side views of invading ECs were captured using an Olympus CKX41 microscope equipped with a Q-Color3 camera. Invasion densities were quantified by counting fixed cultures under transmitted light using an eyepiece equipped with a reticle displaying a 10 × 10 ocular grid. For each condition, at least three random fields were selected, and the number of invading cells per field was counted manually. Data are reported as mean numbers of invading cells per field (±S.D.). Area is indicated in each figure. Invasion length was measured using digital images taken from a side view of cultures at 10× magnification using Image-Pro Analyzer 7.0. Data are reported as average invasion distance in micrometers (±S.E.). Approximately 100 cells per treatment group were included in analyses.
Cell Adhesion Assays
High binding EIA/RIA 96-well plates (Corning-Costar) were coated overnight with various doses of fibronectin and blocked with 10 mg/ml BSA for 45 min. ECs expressing shRNAs directed to β2M, RACK1, and vimentin (30,000 cells/well) were incubated at 37 °C for 15 min before removing unbound cells. Plates were fixed in 3% formalin for 3 h before staining with 0.1% Amido Black in 10% acetic acid and 30% methanol for 15 min. Plates were washed and dried before adding 50 μl of 2 n NaOH/well. Raw absorbances (595 nm) were measured using a Victor X3 plate reader (PerkinElmer Life Sciences) for triplicate wells. Data are reported as average absorbances (595 nm) (±S.D.).
RESULTS
Identification of RACK1 as a Regulator of S1P-induced Invasion in ECs
The assay used in this study is a defined three-dimensional model of EC invasion in which robust and rapid invasion responses are triggered by S1P, bFGF, and VEGF (11). To identify intracellular targets of S1P that regulate invasion, ECs were treated with GF, S1P + GF, or S1P + GF + PTX to inhibit sprouting responses. PTX blocks S1P receptor activity by catalyzing ADP-ribosylation of α subunits of heteromeric G proteins (Go, Gi, and Gt), thus preventing G proteins from interacting with their receptors and interfering with intracellular communication (46). Dose-response experiments determined that 0.1 ng/ml PTX blocked S1P- and GF-induced invasion (data not shown). Treatment with PTX effectively blocked invasion in ECs to control levels with GF alone (Fig. 1A). Quantification of invasion density showed significantly more sprouting with S1P + GF treatment compared with GF or S1P + GF + PTX treatment (Fig. 1B).
FIGURE 1.

Effect of PTX on S1P- and GF-induced EC invasion. A, photographs illustrating invasion responses (upper panels, top view; lower panels, side view). ECs were treated with GF, S1P + GF, or S1P + GF + PTX. Cultures were fixed in 3% glutaraldehyde at 12.5 h, stained with toluidine blue, and photographed. Black arrowheads indicate invading cells. Scale bars, 50 μm. B, quantification of EC invasion density in response to various treatments shown in panel A. Data represent average numbers of invading cells per 1-mm2 field. Error bars represent S.D. (n = 5 fields; Student's t test; ***, p < 0.001 compared with other treatments).
Protein lysates from invading EC cultures (12.5 h) shown in Fig. 1A were analyzed in two independent proteomic screens designed to dissect downstream regulators of S1P signaling in ECs. Protein identification was carried out using two search engines, TurboSEQUEST and Mascot, and the identified target proteins were ranked according to their XCorr values where a score above 2.0 is indicative of a good correlation. Differential in-gel electrophoresis (proteomic screen 1) revealed at ANXA2, RACK1, and vimentin were up-regulated with S1P + GF treatment compared with S1P + GF + PTX (Table 1). In addition, several proteins were found to be down-regulated in S1P-induced invasion compared with non-invading ECs (Table 2). An independent screen using a distinct approach (proteomic screen 2) confirmed that ANXA2 and RACK1 were up-regulated with S1P and GF treatment compared with S1P + GF + PTX (Table 3).
TABLE 1.
Up-regulated proteins identified in proteomic screen 1
TQ, TurboSEQUEST; M, Mascot; Score XC, XCorr value.

TABLE 2.
Down-regulated proteins identified in proteomic screen 1
TQ, TurboSEQUEST; M, Mascot; Score XC, XCorr value.

TABLE 3.
Up-regulated proteins identified in proteomic screen 2
Score XC, XCorr value.
| Predicted protein match | Score XC | Sequence coverage | NCBI Protein database accession number |
|---|---|---|---|
| % | |||
| Annexin A2 | 100.24 | 27.14 | GI:119574085.0 |
| RACK1 | 50.23 | 12 | GI:83641897 |
| Lamin B2 | 60.19 | 11.33 | GI:119589784.0 |
| Peroxiredoxin 1 | 40.19 | 19.59 | GI:55959887.0 |
Up-regulation of ANXA2, RACK1, and vimentin were confirmed in separate experiments using Western blotting (Fig. 2A). Although we observed up-regulation of ARPC2 (Table 1), a requirement for ARPC2 in S1P- and GF-induced EC invasion was not confirmed (data not shown); thus, ARPC2 was not investigated further. ANXA2, RACK1, and vimentin protein expression levels observed with PTX treatment were consistent with GF alone (Fig. 2). Quantification of the data revealed significant up-regulation of ANXA2, RACK1, and vimentin with S1P + GF treatment compared with GF alone or S1P + GF + PTX treatment (Fig. 2B). No changes in the loading control, β2M, were observed between treatment groups (Fig. 2A).
FIGURE 2.

Western blot analysis to verify up-regulation of identified proteins. A, verification of ANXA2, VIM, and RACK1 protein up-regulation. ECs treated with GF, S1P + GF, or S1P + GF + PTX were allowed to invade for 12.5 h prior to preparing cell extracts. Antisera specific to ANXA2, VIM, RACK1, and β2M were used for Western blot analyses. B, quantification of protein expression from Western blots using ImageJ software with normalization to β2M. The results represent average values normalized to β2M in arbitrary units (a.u.) from three independent experiments (Student's t test; *, p < 0.05 compared with all other treatments). Error bars represent S.D.
Our laboratory has investigated the role of ANXA2 in separate studies and found that ANXA2 complexed with VE-cadherin upstream of Akt activation to regulate EC sprouting (12), and thus ANXA2 was not investigated in this study. Because RACK1 has been implicated in cell migration and blood vessel formation (30) and we have previously identified a requirement for vimentin (31), we focused our investigation on better understanding the interplay between RACK1 and vimentin in EC invasion responses.
RACK1 Was Required for EC Invasion
Recombinant lentiviruses from three individual shRNA sequences targeted against RACK1 (shRACK1-1, -2, and -3) and two shRNA sequences specific to β2M (shβ2M-1 and -2) were utilized to transduce ECs. To test knockdown efficiency at mRNA and protein levels, PCR analysis using primers specific to RACK1, β2M,and GAPDH was carried out. Efficient silencing of RACK1 mRNA with shRACK1-1 and shRACK1-2 treatment was revealed (Fig. 3A). Western blot analyses of cell lysates revealed knockdown of RACK1 protein that was consistent with mRNA levels (Fig. 3, A and B). β2M control also showed efficient knockdown with shβ2M-1 and -2 treatment of β2M mRNA (Fig. 3A) and protein (Fig. 3B). To test the specificity of shRNA-mediated knockdown of RACK1 and β2M, expression levels of other molecules known to regulate EC sprouting such as vimentin, VE-cadherin, filamin A, and FAK were analyzed (Fig. 3, A and B). No significant changes in mRNA or protein levels of GAPDH, ANXA2, FAK, VE-cadherin, filamin A, or vimentin were seen with shβ2M and shRACK1 treatment (Fig. 3, A and B). To test the effect of RACK1 silencing on S1P- and GF-mediated invasion, ECs were not transduced (WT) or transduced separately with packaging vectors only (mock) or recombinant lentiviruses directed against β2M (shβ2M-1) or RACK1 (shRACK1-1 and -2). Photographs of invading cultures illustrated that knockdown of RACK1 interfered with EC invasion responses stimulated by S1P + GF (Fig. 3C). Western blot analyses of extracts collected from invading cultures showed selective knockdown of RACK1 and β2M proteins by their respective shRNAs (Fig. 3D). Selective knockdown was also confirmed using immunofluorescence staining against RACK1 (data not shown). Quantification of invasion density revealed a significantly decreased number of invading cells with RACK1 silencing compared with shβ2M controls (Fig. 3E). In addition, the length of invading structures was significantly decreased with RACK1 silencing compared with shβ2M controls (Fig. 3F). These results demonstrated that RACK1 was required for S1P- and GF-stimulated EC invasion in three-dimensional collagen matrices.
FIGURE 3.
RACK1 knockdown decreased invasion responses in ECs. A, non-transduced ECs (WT) or ECs transduced with lentiviruses delivering shRNA directed to β2M (shβ2M-1 and -2) or RACK1 (shRACK1-1, -2, and -3) were utilized to generate RNA, cDNA, and RT-PCRs using RACK1-, β2M-, GAPDH-, ANXA2-, FAK-, VE-cadherin-, VIM-, and filamin A-specific primer sets. B, cell lysates from treatment groups as in A were analyzed by Western blotting using RACK1-, β2M-, GAPDH-, ANXA2-, FAK-, VE-cadherin-, VIM-, filamin A-, and tubulin-specific antisera. C, non-transduced ECs (WT) or ECs transduced with lentiviruses delivering shRNA directed to β2M (shβ2M-1) or RACK1 (shRACK1-1 and -2) were tested in three-dimensional invasion assays using S1P + GF. Photographs illustrate the invasion responses (upper panel, top view; lower panel, side view). Scale bars, 50 μm. D, Western blot analyses of whole cell lysates to verify β2M and RACK1 protein suppression using RACK1-, β2M-, and tubulin-specific antisera. Quantification of invasion density (E) and invasion distance (F) at 20 h of invasion is shown. Data in E represent average numbers of invading cells per 1-mm2 field (n = 3 fields; Student's t test; **, p < 0.01 compared with shβ2M). Data in F represent average lengths of structures (n > 80 cells; Student's t test; **, p < 0.01; ***, p < 0.001 compared with shβ2M). A representative experiment is shown (n = 4). Error bars represent S.D. in E and S.E. in F.
To confirm a requirement for RACK1 and test whether RACK1 rescue would restore invasion responses decreased by RACK1 silencing, GFP-RACK1 constructs were mutated using site-directed mutagenesis to alter the shRACK1-1 target site (R3). R3 and GFP control constructs were overexpressed in ECs treated with lentiviruses delivering shRNA targeted to β2M and RACK1 (Fig. 4A). Western blot analyses of cell lysates showed successful knockdown of RACK1 and β2M (Fig. 4B). Also, expression of the R3 rescue construct (∼65 kDa) was successfully detected in shβ2M + R3 and shRACK1 + R3 treatment groups with GFP and RACK1 antisera (Fig. 4B). GFP control was detected with GFP antiserum (30 kDa). Quantification of invasion density (Fig. 4C) and invasion distance (Fig. 4D) revealed similar invasion responses in ECs expressing shβ2M + GFP and shβ2M + R3. Significantly less sprouting was seen with shRACK1 + GFP compared with shβ2M + GFP and shβ2M + R3 (Fig. 4C), consistent with effects of RACK1 knockdown (Fig. 3). Expression of the R3 rescue construct with RACK1-specific shRNA (shRACK1 + R3) resulted in a slight but significant increase of invasion density (Fig. 4C) and invasion distance (Fig. 4D) compared with shRACK1 + GFP. Thus, rescuing with R3 GFP-RACK1 in shRACK1-1-expressing ECs increased the number and length of sprouting ECs compared with GFP control, supporting a requirement for RACK1 in mediating endothelial sprouting responses.
FIGURE 4.
RACK1 rescue partially restored sprouting responses and invasion distance reduced by RACK1 silencing. ECs were transduced with lentiviruses delivering shRNA directed to β2M (shβ2M) or RACK1 (shRACK1) before being transduced with lentiviruses delivering enhanced GFP control or R3 (GFP-RACK1 rescue construct). A, photographs of a side view illustrating invasion responses. Scale bars, 50 μm. B, Western blot analyses of whole cell lysates probed with RACK1-, GFP-, β2M-, and tubulin-specific antisera. Arrows indicate expression of the R3 (65-kDa) construct. C, quantification of invasion density at 22 h. Data represent average numbers of invading cells per 1-mm2 field (n = 4 fields; one-way analysis of variance). Error bars represent S.D. D, quantification of invasion distance from EC monolayer to the tip of invading structures. The results represent average values (n > 100 sprouts; one-way analysis of variance). Error bars represent S.E. C and D, means with the same letter are not significantly different.
RACK1 Depletion Decreased FAK Activation
RACK1 is proposed to act as a scaffold protein and plays a role in various cellular processes including cell migration (23, 25, 47). Most functions of RACK1 associated with cell adhesion, spreading, and migration are linked to its ability to associate with FAK and regulate its activity (25, 28, 48, 49). These studies guided us to investigate whether RACK1 affected FAK activity during EC sprouting in collagen matrices. ECs in all subsequent experiments were transduced with recombinant lentiviruses expressing shRACK1-1 (shRACK1) or shβ2M-1 (shβ2M) and tested in invasion assays. Whole cell extracts were collected from invading cultures at 0, 2, and 3 h and analyzed by Western blotting. We observed that RACK1 and β2M were successfully silenced (Fig. 5A). In addition, FAK activation, indicated by an autophosphorylation event at Tyr-397, was decreased at 2 h in RACK1-depleted ECs compared with shβ2M controls. However, expression of total FAK was not affected. Tubulin and GAPDH loading controls remained constant (Fig. 5A). FAK phosphorylation significantly decreased in ECs expressing shRACK1 compared with shβ2M at 2 h of invasion (Fig. 5B). These results suggested that RACK1 was required for FAK activation in ECs.
FIGURE 5.

Depletion of RACK1 reduced FAK activation. A, ECs expressing shRNA directed to β2M (shβ2M) or RACK1 (shRACK1) were allowed to invade for the time points indicated. Whole cell lysates were prepared and analyzed by Western blot analysis using polyclonal antisera directed to pFAK (Tyr-397) and β2M and monoclonal antibodies directed to total FAK, RACK1, tubulin, and GAPDH. B, intensity levels for pFAK (Tyr-397) and total FAK were normalized to tubulin using ImageJ software. Ratios of normalized pFAK and FAK were plotted in arbitrary units (a.u.) from three independent experiments (Student's t test; *, p < 0.05 compared with shβ2M control). Error bars represent S.D.
FAK Activation Was Required for EC Invasion
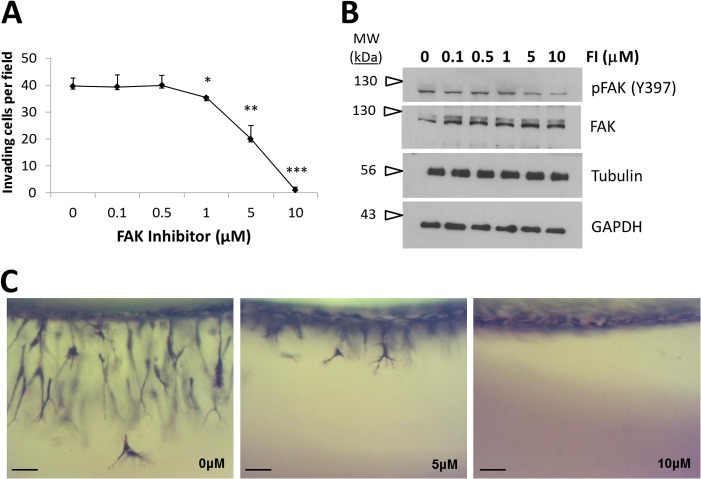
Because RACK1 knockdown decreased FAK activation, we investigated whether FAK activity was required for endothelial sprouting. ECs treated with FAK inhibitor (FI), which selectively blocks FAK phosphorylation at Tyr-397, were allowed to invade collagen matrices overnight. Quantification of invasion responses indicated that FI treatment dose-dependently inhibited invasion, indicating that FAK activation was required for EC invasion (Fig. 6A). No adverse effects on cell monolayers or cell viability were observed with blocking FAK activity (data not shown). Western blot analyses confirmed a dose-dependent decrease in Tyr-397 phosphorylation (Fig. 6B) with FI treatment that correlated with a decrease in EC sprouting (Fig. 6A). Representative side view images of sprouting EC cultures treated with 0, 5, and 10 μm FI illustrated partial and complete inhibition of sprouting at the 5 and 10 μm doses of FI, respectively (Fig. 6C). These results demonstrate a requirement for FAK activation during S1P- and GF-stimulated endothelial sprouting.
FIGURE 6.
EC invasion required FAK activation. A, ECs were preincubated with FI at the concentrations indicated for 20 min at 37 °C prior to seeding on collagen matrices. ECs were allowed to invade for 20 h with S1P + GF. Samples were fixed in 3% glutaraldehyde and stained with toluidine blue. Data represent average numbers of invading cells per 0.25-mm2 field (n = 3; Student's t test; *, p < 0.05; **, p < 0.01; ***, p < 0.001 as compared with 0 μm FI treatment). Error bars represent S.D. B, whole cell extracts from invading cultures from A at 2 h with the indicated FI concentration were immunoblotted with antisera directed against pFAK (Tyr-397), tubulin, and GAPDH. Blots were stripped and reprobed with FAK-specific antiserum. C, representative photographs of a side view of invasion (24 h) with 0, 5, and 10 μm FI treatment. Scale bars, 50 μm.
Vimentin Regulated FAK Expression in ECs
Proteomics studies demonstrated that full-length 60-kDa vimentin was up-regulated in invading versus non-invading ECs (Table 1), and Burgstaller et al. (32) have suggested that networking and anchorage of vimentin intermediate filaments at FAs resulted in cell shape changes, polarization, and prolonged lifespan of FAs in mouse fibroblasts. We have shown previously that vimentin was required for endothelial sprouting (31). Because of the link between vimentin and FA regulation (32) and a known requirement for vimentin in EC sprouting (31), we tested whether vimentin regulated FAK in S1P- and GF-stimulated invasion. ECs transduced with recombinant lentiviruses expressing short hairpin RNAs directed against vimentin (shVIM) or β2M (shβ2M) were allowed to invade collagen matrices. Whole cell extracts collected from invading cultures at 0, 2, and 3 h were analyzed by Western blotting. We observed a decrease in total and phosphorylated FAK levels with vimentin knockdown compared with shβ2M controls (Fig. 7A). Quantification of Western blots revealed a significant difference in the expression of total FAK at all time points between shVIM- and shβ2M-expressing ECs (Fig. 7B). These data suggested that vimentin regulated FAK expression. We reported previously that calpain-dependent vimentin cleavage increased vimentin solubility during EC invasion (31). To determine whether vimentin solubility affected FAK expression, we analyzed invading cultures treated with a calpain inhibitor (calpain inhibitor-III) to block calpain-dependent vimentin cleavage. No change in FAK expression was seen when ECs were treated with a calpain inhibitor (data not shown), suggesting that the solubility state of vimentin did not affect FAK expression during endothelial sprouting.
FIGURE 7.

Vimentin regulated FAK expression in ECs. A, ECs were transduced with shRNA directed to β2M (shβ2M) or vimentin (shVIM) and placed in invasion assays in the presence of S1P + GF. Whole cell lysates were prepared at the indicated time points. Western blot analyses were conducted using polyclonal antisera directed to phospho-FAK (Tyr-397) and β2M as well as monoclonal antisera directed to total FAK, tubulin, GAPDH, and vimentin. B, quantification of FAK protein expression using ImageJ software with normalization to GAPDH. The results represent average values in arbitrary units (a.u.) from three independent experiments (Student's t test; *, p < 0.05 compared with shβ2M expression). Error bars represent S.D.
RACK1 Complexed with Vimentin, and GFs Enhanced This Interaction
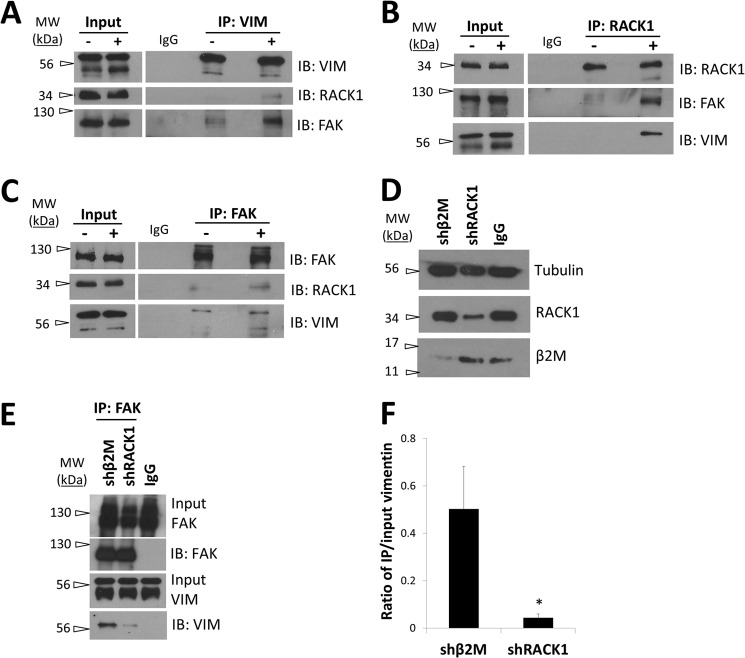
The above data show that RACK1 and vimentin regulate FAK activation and expression. Immunoprecipitation experiments revealed vimentin complexed with RACK1 (Fig. 8A). RACK1 also immunoprecipitated with vimentin antiserum (Fig. 8B), indicating that a complex containing RACK1 and vimentin was formed in EC monolayers stimulated with S1P and GFs. To test whether this association was stimulated by individual proangiogenic stimuli, EC monolayers were untreated or treated with S1P or GF for 1 h. Immunoprecipitations were performed by incubating lysates with RACK1-specific antiserum, and eluates were probed for vimentin and RACK1. Interestingly, we observed an increase in RACK1-vimentin association with GF (but not S1P) stimulation, whereas equivalent amounts of RACK1 were immunoprecipitated in all conditions (Fig. 8C). Data quantified from three independent experiments indicated a significant increase in vimentin that associated with RACK1 following GF treatment compared with no treatment and S1P alone (Fig. 8D).
FIGURE 8.
Growth factor stimulation enhanced RACK1-vimentin complex formation. A, ECs were cultured in T75 flasks for 3 days without replenishing growth medium and stimulated with S1P + GF for 1 h. Immunoprecipitations (IP) were performed using monoclonal RACK1 antibody or isotype control (IgG) and probed for vimentin and RACK1 using Western blot analyses. B, reverse immunoprecipitations were performed using a polyclonal vimentin antibody or isotype control (IgG) and probed for RACK1 and vimentin using Western blot analyses. C, ECs were cultured in T75 flasks for 3 days and stimulated with nothing (control), 1 μm S1P, or GF for 1 h. Immunoprecipitations were performed using monoclonal RACK1 antibody or isotype control (IgG). Eluates were probed with vimentin- and RACK1-specific antisera using Western blotting. D, quantification of co-precipitated vimentin normalized to starting material from Western blots using ImageJ software. The results represent average values in arbitrary units (a.u.) from three independent experiments (Student's t test; **, p < 0.01 compared with control). Error bars represent S.D. E, ECs seeded on glass coverslips were serum-starved for 1 h and treated without (control), with 1 μm S1P, or with 40 ng/ml GFs for 1 h. Following paraformaldehyde fixation, cells were stained with monoclonal anti-RACK1 or polyclonal anti-vimentin antibody and detected with species-specific secondary antibodies conjugated to Alexa Fluor 594 and Alexa Fluor 488. Images were collected using a Nikon A1 confocal laser microscope. Scale bars, 5 μm. F, quantification of RACK1 (red) and vimentin (green) co-localization using Pearson's correlation coefficient. The results represent average values from each treatment group. Error bars represent S.D. n = number of cells per treatment group (one-way analysis of variance with Bonferroni's multiple comparison post hoc test; *, p < 0.05 compared with control). IB, immunoblot.
To confirm that GF treatment increased vimentin and RACK1 co-localization, ECs seeded on collagen-coated glass coverslips were serum-starved for 1 h and not treated (control) or treated with S1P or GF and stained with RACK1- and vimentin-specific antisera (Fig. 8E). Quantification of co-localization in each treatment group confirmed that GF treatment significantly increased overlap compared with control or S1P treatment (Fig. 8F). These results support that GFs enhanced complex formation between RACK1 and vimentin.
Vimentin Association with FAK Required RACK1
Thus far, these data show that downstream of proangiogenic signals RACK1 and vimentin complexed to regulate FAK activation. To test whether FAK was a part of this complex as well as whether S1P and GF stimulation enhanced formation of the complex, lysates from ECs invading into three-dimensional collagen matrices treated without or with S1P + GF were collected for immunoprecipitation experiments. We observed that S1P + GF stimulation increased RACK1 and FAK co-immunoprecipitation with vimentin compared with no treatment (Fig. 9A). Reverse immunoprecipitations using RACK1- (Fig. 9B) and FAK-specific antisera (Fig. 9C) were performed to confirm that these interactions were enhanced by S1P + GF treatment. Thus, conditions that stimulate EC invasion enhanced the formation of a RACK1, vimentin, and FAK complex in three-dimensional cultures.
FIGURE 9.
RACK1, vimentin, and FAK complex formation was enhanced during EC invasion. Confluent flasks (75 cm2) of ECs were serum-starved for 2 h, trypsinized, and placed in invasion assays in the absence (−) or presence of S1P + GF (+) for 3 h prior to lysate preparation. Immunoprecipitations (IP) were performed using polyclonal antisera directed to vimentin (A), RACK1 (B), and FAK (C) along with matched isotype controls (IgG). Eluates (A–C) were probed for VIM, FAK, and RACK1 using Western blot analyses and are from one representative experiment (n = 3). D–F, ECs were transduced with lentiviruses delivering shRNAs directed to RACK1 (shRACK1) or β2M (shβ2M) and allowed to invade collagen matrices for 3 h prior to cell lysis and immunoprecipitation. D, Western blot analyses of starting material to verify successful silencing of β2M and RACK1 proteins. E, immunoprecipitations were performed using a monoclonal FAK antibody or isotype control (IgG) and probed for FAK and VIM using Western blot analyses. F, quantification of co-precipitated vimentin normalized to input material from Western blots using ImageJ software. The results represent average values in arbitrary units (a.u.) from three independent experiments (Student's t test; *, p < 0.05 compared with shβ2M control). Error bars represent S.D. IB, immunoblot.
Based on these data, we next tested whether FAK activation was needed to stabilize the complex. Interestingly, FI did not alter complex formation between vimentin, RACK1, and FAK (data not shown). Vimentin binding to FAK remained unaltered with FI, and FAK and vimentin similarly bound RACK1 (data not shown), suggesting that association of RACK1 and vimentin with FAK was independent of FAK activation.
To further investigate the role of RACK1, we tested whether RACK1 silencing altered complex formation. Knockdown of RACK1 and β2M protein levels were confirmed using Western blot analyses (Fig. 9D). RACK1 knockdown resulted in decreased complex formation between full-length vimentin (60 kDa) and FAK compared with β2M knockdown (Fig. 9E). Quantification of data from three independent experiments indicated a significant decrease in vimentin and FAK complex formation with RACK1 knockdown (Fig. 9F). Taken together, these results indicated that the scaffold protein RACK1 was needed to maintain complex formation between vimentin and FAK.
Knockdown of Vimentin and RACK1 Decreased Cell Attachment and Focal Adhesion Formation
We next tested the consequences of vimentin knockdown on cell adhesion, which has been linked to focal adhesion formation (50). ECs expressing shRNA directed to β2M or vimentin were allowed to attach to wells coated with increasing doses of fibronectin. These experiments were conducted rapidly (15 min) using an established method to determine whether vimentin silencing altered cell attachment (51). Cells expressing shRNA directed to vimentin (shVIM) exhibited decreased attachment compared with shβ2M controls (Fig. 10A). Western blot analyses confirmed successful knockdown of both vimentin and β2M (Fig. 10B). These data demonstrate that decreasing vimentin levels in ECs reduced cell attachment (Fig. 10A), which coincided with decreased FAK expression (Fig. 7A). Similar experiments conducted with ECs expressing shRNA directed to RACK1 showed decreased attachment to fibronectin compared with shβ2M controls (Fig. 10C). Western blot analyses confirmed successful knockdown of both RACK1 and β2M (Fig. 10D). In addition to cell attachment, focal adhesion formation (visualized by pFAK Tyr-397 and vinculin staining) was also decreased in ECs expressing shVIM and shRACK1 compared with shβ2M controls. Although large, organized focal adhesions were observed in shβ2M controls as indicated by vinculin and pFAK co-staining (Fig. 10E), smaller and less distinct structures were seen with shVIM and shRACK1 expression.
FIGURE 10.

Vimentin silencing reduced EC adhesion responses. ECs were transduced with shRNA directed to β2M (shβ2M) and vimentin (shVIM) (A) or shβ2M and shRACK1 (C) for 4 days before being plated on wells coated with increasing amounts of fibronectin at the doses indicated for 15 min. Absorbance values (595 nm) represent relative levels of cell attachment, and error bars represent S.D. Results shown are representative of four independent experiments. B, Western blot of cell extracts probed with antisera directed to β2M, α-tubulin, and vimentin from the experiment in A. D, Western blot of cell extracts probed with antisera directed to β2M, α-tubulin, and RACK1 from the experiment in C. E, indirect immunofluorescence staining of ECs expressing shβ2M, shVIM, and shRACK1 using monoclonal antibodies directed against pFAK (Tyr-397) and vinculin. Scale bars, 10 μm.
DISCUSSION
In this study, we performed proteomic analyses to determine changes in protein expression associated with sprouting angiogenesis. The three-dimensional model used in this study incorporated S1P with angiogenic growth factors VEGF and bFGF to stimulate robust EC sprouting (11). The assay mimics a wound environment where S1P combined with VEGF and bFGF to stimulate new blood vessel formation (52). Proteomics analyses from two independent screens showed up-regulation of RACK1 and ANXA2 in invading ECs, suggesting a crucial role for RACK1 downstream of S1P and GF signaling. Garcia and co-workers (53) have previously reported an increase in RACK1, vimentin, and ANXA2 in response to S1P treatment. Our findings suggest that RACK1 regulated sprouting through association with the major intermediate filament protein, vimentin, and consequent regulation of FAK.
Our results are the first to demonstrate a complex formation between RACK1 and vimentin during endothelial sprouting. The WD repeat protein RACK1 is a highly conserved cellular adaptor protein with a seven-bladed β-propeller structure. This unique structure facilitates binding with a variety of proteins either directly or as a part of a larger complex (54–61). RACK1 also shuttles proteins to specific cellular sites, modulating enzymatic activity of its binding partners and stabilizing protein interactions. We found here that GF stimulation increased RACK1 complex formation with full-length vimentin during EC sprouting. The intermediate filament protein vimentin is involved in many important physiological functions such as distribution of organelles, signal transduction, cell polarity, and gene regulation (62–64). Previous work from our laboratory demonstrated a direct requirement of vimentin during angiogenic sprouting. Soluble vimentin was needed for successful membrane type 1 matrix metalloproteinase membrane translocation and endothelial sprout formation (31). The data here support an additional role for vimentin in regulating EC invasion through a unique pathway involving RACK1 and FAK.
FAK, the major focal kinase protein, has been established as a key mediator in cell migration and endothelial morphogenesis. FAK conditional knock-out mice exhibit severe cardiac defects, ventricular hypertrophy, and significantly attenuated chemotaxis in isolated cardiomyocytes resulting in embryonic lethality (43, 44). Deletion of FAK specifically in ECs resulted in defective angiogenesis in the embryo, yolk sac, and placenta as well as hemorrhage, edema, and developmental delay (45). Similar studies by Braren et al. (42) revealed that FAK depletion in ECs resulted in distinctive and irregular embryonic vasculature with reduced vessel growth and vessel regression resulting in lethality between days E10.5 and E11.5. ECs derived from FAK mutants exhibited aberrant lamellipodial extensions, altered actin cytoskeleton, and non-polarized cell movement. These data supported a crucial role for FAK in cardiac and vascular morphogenesis and in regulation of EC survival. In agreement with these data, we observed that blocking FAK activation completely inhibited sprouting in ECs.
Our data also further investigated a connection between vimentin filaments and FAK and to the best of our knowledge are the first to show that vimentin complexed with FAK. Numerous studies have established that vimentin intermediate filaments terminate at focal contacts (65, 66). In fibroblasts, tethering of vimentin filaments to FAs decreased turnover rate and increased stabilization (32). Furthermore, vimentin regulated focal contact assembly and helped stabilize cell-matrix adhesions in ECs subjected to shear stress (33). FAK is an integral component of FAs and functions to assemble focal contacts in response to integrin clustering by extracellular matrix engagement (39, 40) as well as regulate focal adhesion turnover (67). Our data showed that vimentin was required to maintain FAK expression, which is consistent with data showing that loss of vimentin correlated with decreased focal contact size and stability (32, 33). Fitting with these data, loss of vimentin in ECs attenuated cell adhesion responses and decreased phosphorylated and total FAK. Interestingly, we did not observe attenuated levels of FAK when blocking vimentin cleavage with a calpain inhibitor, suggesting that the cleavage state of vimentin had no bearing on FAK expression. The ability of vimentin to regulate FAK expression in ECs has important implications regarding control of cell-matrix interactions during EC sprouting, and these findings help explain the ability of vimentin to control FA size and stability reported by others (65, 66).
We show here that RACK1 and vimentin complexed with FAK. Furthermore, we found that FAK inhibition did not change the dynamics of this complex, indicating that complex formation did not require FAK activation. S1P and GF stimulation enhanced the formation of a RACK1, vimentin, and FAK complex in three-dimensional invading cultures. Our results indicated that vimentin and RACK1 were required to maintain expression and activation of FAK, respectively, which were crucial for successful sprouting. Because RACK1 is proposed as a scaffold protein, its functions are commonly ascribed to its ability to associate and stabilize multiple protein interactions. We therefore tested the effect of RACK1 depletion on vimentin-FAK interaction. We showed here for the first time that binding between vimentin and FAK was RACK1-dependent. These findings support a novel scaffold function of RACK1 in stabilizing complex formation between vimentin and FAK and suggest that RACK1 may assist in tethering vimentin filaments to FA complexes (32, 66). Altogether, these data suggest that a RACK1-vimentin-FAK complex is important, although the specific binding domains that mediate the interactions have not been investigated here.
In conclusion, we have demonstrated that RACK1 and vimentin are up-regulated in sprouting versus quiescent ECs. RACK1, vimentin, and FAK form a complex in response to proangiogenic factors during endothelial sprout formation in three-dimensional collagen matrices; FAK (68, 69), RACK1 (30, 70, 71), and vimentin (72, 73) are up-regulated during tumor formation and progression. Because expression of all these molecules is altered during malignant disease, this pathway may regulate invasive, metastatic cell behavior as well. Altogether, our data provide novel insights into the role of RACK1 during endothelial invasion in three-dimensional collagen matrices downstream of S1P signaling.
Acknowledgments
We thank Katherine Beifuss and Dr. Tina Gumienny for assistance with fluorescence microscopy and imaging, Scott Ballard for assistance with two-dimensional electrophoresis and protein identification, and Dr. Shih-Chi Su for cloning EGFP-RACK1 into the pLenti6 vector.
This work was supported, in whole or in part, by National Institutes of Health Grant HL-095786, a Public Health Service grant from the NHLBI (to K. J. B.).
- EC
- endothelial cell
- RACK1
- receptor for activated C kinase 1
- FAK
- focal adhesion kinase
- VIM
- vimentin
- bFGF
- basic fibroblast growth factor
- EDG
- endothelial differentiation gene
- FA
- focal adhesion
- HUVEC
- human umbilical vein EC
- S1P
- sphingosine 1-phosphate
- GF
- growth factor
- PTX
- pertussis toxin
- DIGE
- differential in-gel electrophoresis
- β2M
- β2-microglobulin
- EGFP
- enhanced GFP
- pFAK
- phosphorylated FAK
- ANXA2
- annexin A2
- FI
- FAK inhibitor
- XCorr
- cross correlation value.
REFERENCES
- 1. Carmeliet P. (2003) Angiogenesis in health and disease. Nat. Med. 9, 653–660 [DOI] [PubMed] [Google Scholar]
- 2. Folkman J., D'Amore P. A. (1996) Blood vessel formation: what is its molecular basis? Cell 87, 1153–1155 [DOI] [PubMed] [Google Scholar]
- 3. Adams R. H., Alitalo K. (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8, 464–478 [DOI] [PubMed] [Google Scholar]
- 4. Kalluri R. (2003) Basement membranes: structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer, 3, 422–433 [DOI] [PubMed] [Google Scholar]
- 5. Iruela-Arispe M. L., Davis G. E. (2009) Cellular and molecular mechanisms of vascular lumen formation. Dev. Cell 16, 222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis G. E., Camarillo C. W. (1996) An α2β1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp. Cell Res. 224, 39–51 [DOI] [PubMed] [Google Scholar]
- 7. Egginton S., Gerritsen M. (2003) Lumen formation: in vivo versus in vitro observations. Microcirculation 10, 45–61 [DOI] [PubMed] [Google Scholar]
- 8. Montesano R., Pepper M. S., Vassalli J. D., Orci L. (1992) Modulation of angiogenesis in vitro. EXS 61, 129–136 [DOI] [PubMed] [Google Scholar]
- 9. Nicosia R. F., Villaschi S. (1999) Autoregulation of angiogenesis by cells of the vessel wall. Int. Rev. Cytol. 185, 1–43 [DOI] [PubMed] [Google Scholar]
- 10. Vernon R. B., Sage E. H. (1995) Between molecules and morphology. Extracellular matrix and creation of vascular form. Am. J. Pathol. 147, 873–883 [PMC free article] [PubMed] [Google Scholar]
- 11. Bayless K. J., Kwak H. I., Su S. C. (2009) Investigating endothelial invasion and sprouting behavior in three-dimensional collagen matrices. Nat. Protoc. 4, 1888–1898 [DOI] [PubMed] [Google Scholar]
- 12. Su S. C., Maxwell S. A., Bayless K. J. (2010) Annexin 2 regulates endothelial morphogenesis by controlling AKT activation and junctional integrity. J. Biol. Chem. 285, 40624–40634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peters S. L., Alewijnse A. E. (2007) Sphingosine-1-phosphate signaling in the cardiovascular system. Curr. Opin. Pharmacol. 7, 186–192 [DOI] [PubMed] [Google Scholar]
- 14. Rivera J., Proia R. L., Olivera A. (2008) The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 8, 753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosen H., Goetzl E. J. (2005) Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 5, 560–570 [DOI] [PubMed] [Google Scholar]
- 16. Taha T. A., Mullen T. D., Obeid L. M. (2006) A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim. Biophys. Acta 1758, 2027–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spiegel S., Milstien S. (2003) Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4, 397–407 [DOI] [PubMed] [Google Scholar]
- 18. McCahill A., Warwicker J., Bolger G. B., Houslay M. D., Yarwood S. J. (2002) The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol. Pharmacol. 62, 1261–1273 [DOI] [PubMed] [Google Scholar]
- 19. Sklan E. H., Podoly E., Soreq H. (2006) RACK1 has the nerve to act: structure meets function in the nervous system. Prog. Neurobiol. 78, 117–134 [DOI] [PubMed] [Google Scholar]
- 20. Chen J. G., Ullah H., Temple B., Liang J., Guo J., Alonso J. M., Ecker J. R., Jones A. M. (2006) RACK1 mediates multiple hormone responsiveness and developmental processes in Arabidopsis. J. Exp. Bot. 57, 2697–2708 [DOI] [PubMed] [Google Scholar]
- 21. Guo J., Chen J. G. (2008) RACK1 genes regulate plant development with unequal genetic redundancy in Arabidopsis. BMC Plant Biol. 8, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wehner P., Shnitsar I., Urlaub H., Borchers A. (2011) RACK1 is a novel interaction partner of PTK7 that is required for neural tube closure. Development 138, 1321–1327 [DOI] [PubMed] [Google Scholar]
- 23. Hermanto U., Zong C. S., Li W., Wang L. H. (2002) RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting protein, modulates IGF-I-dependent integrin signaling and promotes cell spreading and contact with extracellular matrix. Mol. Cell. Biol. 22, 2345–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kiely P. A., Baillie G. S., Barrett R., Buckley D. A., Adams D. R., Houslay M. D., O'Connor R. (2009) Phosphorylation of RACK1 on tyrosine 52 by c-Abl is required for insulin-like growth factor I-mediated regulation of focal adhesion kinase. J. Biol. Chem. 284, 20263–20274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kiely P. A., Leahy M., O'Gorman D., O'Connor R. (2005) RACK1-mediated integration of adhesion and insulin-like growth factor I (IGF-I) signaling and cell migration are defective in cells expressing an IGF-I receptor mutated at tyrosines 1250 and 1251. J. Biol. Chem. 280, 7624–7633 [DOI] [PubMed] [Google Scholar]
- 26. Kiely P. A., Sant A., O'Connor R. (2002) RACK1 is an insulin-like growth factor 1 (IGF-1) receptor-interacting protein that can regulate IGF-1-mediated Akt activation and protection from cell death. J. Biol. Chem. 277, 22581–22589 [DOI] [PubMed] [Google Scholar]
- 27. Neer E. J., Schmidt C. J., Nambudripad R., Smith T. F. (1994) The ancient regulatory-protein family of WD-repeat proteins. Nature 371, 297–300 [DOI] [PubMed] [Google Scholar]
- 28. Onishi I., Lin P. J., Diering G. H., Williams W. P., Numata M. (2007) RACK1 associates with NHE5 in focal adhesions and positively regulates the transporter activity. Cell. Signal. 19, 194–203 [DOI] [PubMed] [Google Scholar]
- 29. Adams D. R., Ron D., Kiely P. A. (2011) RACK1, a multifaceted scaffolding protein: structure and function. Cell Commun. Signal. 9, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berns H., Humar R., Hengerer B., Kiefer F. N., Battegay E. J. (2000) RACK1 is up-regulated in angiogenesis and human carcinomas. FASEB J. 14, 2549–2558 [DOI] [PubMed] [Google Scholar]
- 31. Kwak H. I., Kang H., Dave J. M., Mendoza E. A., Su S. C., Maxwell S. A., Bayless K. J. (2012) Calpain-mediated vimentin cleavage occurs upstream of MT1-MMP membrane translocation to facilitate endothelial sprout initiation. Angiogenesis 15, 287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burgstaller G., Gregor M., Winter L., Wiche G. (2010) Keeping the vimentin network under control: cell-matrix adhesion-associated plectin 1f affects cell shape and polarity of fibroblasts. Mol. Biol. Cell 21, 3362–3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsuruta D., Jones J. C. (2003) The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J. Cell Sci. 116, 4977–4984 [DOI] [PubMed] [Google Scholar]
- 34. Romer L. H., Birukov K. G., Garcia J. G. (2006) Focal adhesions: paradigm for a signaling nexus. Circ. Res. 98, 606–616 [DOI] [PubMed] [Google Scholar]
- 35. Petit V., Thiery J. P. (2000) Focal adhesions: structure and dynamics. Biol. Cell 92, 477–494 [DOI] [PubMed] [Google Scholar]
- 36. Martin G. M. (1996) Fak and focal adhesions. Jpn. J. Cancer Res. 87, inside front cover [PubMed] [Google Scholar]
- 37. Burridge K., Chrzanowska-Wodnicka M., Zhong C. (1997) Focal adhesion assembly. Trends Cell Biol. 7, 342–347 [DOI] [PubMed] [Google Scholar]
- 38. Burridge K., Chrzanowska-Wodnicka M. (1996) Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12, 463–518 [DOI] [PubMed] [Google Scholar]
- 39. Mitra S. K., Schlaepfer D. D. (2006) Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18, 516–523 [DOI] [PubMed] [Google Scholar]
- 40. Guan J. L. (2010) Integrin signaling through FAK in the regulation of mammary stem cells and breast cancer. IUBMB Life. 62, 268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arold S. T. (2011) How focal adhesion kinase achieves regulation by linking ligand binding, localization and action. Curr. Opin. Struct. Biol. 21, 808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Braren R., Hu H., Kim Y. H., Beggs H. E., Reichardt L. F., Wang R. (2006) Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J. Cell Biol. 172, 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hakim Z. S., DiMichele L. A., Doherty J. T., Homeister J. W., Beggs H. E., Reichardt L. F., Schwartz R. J., Brackhan J., Smithies O., Mack C. P., Taylor J. M. (2007) Conditional deletion of focal adhesion kinase leads to defects in ventricular septation and outflow tract alignment. Mol. Cell. Biol. 27, 5352–5364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peng X., Wu X., Druso J. E., Wei H., Park A. Y., Kraus M. S., Alcaraz A., Chen J., Chien S., Cerione R. A., Guan J. L. (2008) Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc. Natl. Acad. Sci. U.S.A. 105, 6638–6643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shen T. L., Park A. Y., Alcaraz A., Peng X., Jang I., Koni P., Flavell R. A., Gu H., Guan J. L. (2005) Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J. Cell Biol. 169, 941–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaslow H. R., Burns D. L. (1992) Pertussis toxin and target eukaryotic cells: binding, entry, and activation. FASEB J. 6, 2684–2690 [DOI] [PubMed] [Google Scholar]
- 47. Kiely P. A., O'Gorman D., Luong K., Ron D., O'Connor R. (2006) Insulin-like growth factor I controls a mutually exclusive association of RACK1 with protein phosphatase 2A and β1 integrin to promote cell migration. Mol. Cell. Biol. 26, 4041–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Serrels A., Canel M., Brunton V. G., Frame M. C. (2011) Src/FAK-mediated regulation of E-cadherin as a mechanism for controlling collective cell movement: insights from in vivo imaging. Cell. Adh. Migr. 5, 360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kiely P. A., Baillie G. S., Lynch M. J., Houslay M. D., O'Connor R. (2008) Tyrosine 302 in RACK1 is essential for insulin-like growth factor-I-mediated competitive binding of PP2A and β1 integrin and for tumor cell proliferation and migration. J. Biol. Chem. 283, 22952–22961 [DOI] [PubMed] [Google Scholar]
- 50. Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. (1988) Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu. Rev. Cell Biol. 4, 487–525 [DOI] [PubMed] [Google Scholar]
- 51. Bayless K. J., Davis G. E., Meininger G. A. (1997) Isolation and biological properties of osteopontin from bovine milk. Protein Expr. Purif. 9, 309–314 [DOI] [PubMed] [Google Scholar]
- 52. Tonnesen M. G., Feng X., Clark R. A. (2000) Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 5, 40–46 [DOI] [PubMed] [Google Scholar]
- 53. Zhao J., Singleton P. A., Brown M. E., Dudek S. M., Garcia J. G. (2009) Phosphotyrosine protein dynamics in cell membrane rafts of sphingosine-1-phosphate-stimulated human endothelium: role in barrier enhancement. Cell. Signal. 21, 1945–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chang B. Y., Harte R. A., Cartwright C. A. (2002) RACK1: a novel substrate for the Src protein-tyrosine kinase. Oncogene 21, 7619–7629 [DOI] [PubMed] [Google Scholar]
- 55. Yaka R., Thornton C., Vagts A. J., Phamluong K., Bonci A., Ron D. (2002) NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc. Natl. Acad. Sci. U.S.A. 99, 5710–5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Koehler J. A., Moran M. F. (2001) RACK1, a protein kinase C scaffolding protein, interacts with the PH domain of p120GAP. Biochem. Biophys. Res. Commun. 283, 888–895 [DOI] [PubMed] [Google Scholar]
- 57. Rodriguez M. M., Ron D., Touhara K., Chen C. H., Mochly-Rosen D. (1999) RACK1, a protein kinase C anchoring protein, coordinates the binding of activated protein kinase C and select pleckstrin homology domains in vitro. Biochemistry 38, 13787–13794 [DOI] [PubMed] [Google Scholar]
- 58. Usacheva A., Tian X., Sandoval R., Salvi D., Levy D., Colamonici O. R. (2003) The WD motif-containing protein RACK-1 functions as a scaffold protein within the type I IFN receptor-signaling complex. J. Immunol. 171, 2989–2994 [DOI] [PubMed] [Google Scholar]
- 59. Liliental J., Chang D. D. (1998) Rack1, a receptor for activated protein kinase C, interacts with integrin β subunit. J. Biol. Chem. 273, 2379–2383 [DOI] [PubMed] [Google Scholar]
- 60. Lee H. S., Millward-Sadler S. J., Wright M. O., Nuki G., Al-Jamal R., Salter D. M. (2002) Activation of integrin-RACK1/PKCα signalling in human articular chondrocyte mechanotransduction. Osteoarthritis Cartilage 10, 890–897 [DOI] [PubMed] [Google Scholar]
- 61. Rigas A. C., Ozanne D. M., Neal D. E., Robson C. N. (2003) The scaffolding protein RACK1 interacts with androgen receptor and promotes cross-talk through a protein kinase C signaling pathway. J. Biol. Chem. 278, 46087–46093 [DOI] [PubMed] [Google Scholar]
- 62. Helfand B. T., Chou Y. H., Shumaker D. K., Goldman R. D. (2005) Intermediate filament proteins participate in signal transduction. Trends Cell Biol. 15, 568–570 [DOI] [PubMed] [Google Scholar]
- 63. Ivaska J., Pallari H. M., Nevo J., Eriksson J. E. (2007) Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 313, 2050–2062 [DOI] [PubMed] [Google Scholar]
- 64. Oriolo A. S., Wald F. A., Ramsauer V. P., Salas P. J. (2007) Intermediate filaments: a role in epithelial polarity. Exp. Cell Res. 313, 2255–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bershadsky A. D., Tint I. S., Svitkina T. M. (1987) Association of intermediate filaments with vinculin-containing adhesion plaques of fibroblasts. Cell Motil. Cytoskeleton 8, 274–283 [DOI] [PubMed] [Google Scholar]
- 66. Gonzales M., Weksler B., Tsuruta D., Goldman R. D., Yoon K. J., Hopkinson S. B., Flitney F. W., Jones J. C. (2001) Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol. Biol. Cell 12, 85–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ilić D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T. (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539–544 [DOI] [PubMed] [Google Scholar]
- 68. Owens L. V., Xu L., Dent G. A., Yang X., Sturge G. C., Craven R. J., Cance W. G. (1996) Focal adhesion kinase as a marker of invasive potential in differentiated human thyroid cancer. Ann. Surg. Oncol. 3, 100–105 [DOI] [PubMed] [Google Scholar]
- 69. Tremblay L., Hauck W., Aprikian A. G., Begin L. R., Chapdelaine A., Chevalier S. (1996) Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int. J. Cancer 68, 164–171 [DOI] [PubMed] [Google Scholar]
- 70. Al-Reefy S., Mokbel K. (2010) The role of RACK1 as an independent prognostic indicator in human breast cancer. Breast Cancer Res. Treat. 123, 911. [DOI] [PubMed] [Google Scholar]
- 71. Cao X. X., Xu J. D., Xu J. W., Liu X. L., Cheng Y. Y., Wang W. J., Li Q. Q., Chen Q., Xu Z. D., Liu X. P. (2010) RACK1 promotes breast carcinoma proliferation and invasion/metastasis in vitro and in vivo. Breast Cancer Res. Treat. 123, 375–386 [DOI] [PubMed] [Google Scholar]
- 72. Singh S., Sadacharan S., Su S., Belldegrun A., Persad S., Singh G. (2003) Overexpression of vimentin: role in the invasive phenotype in an androgen-independent model of prostate cancer. Cancer Res. 63, 2306–2311 [PubMed] [Google Scholar]
- 73. Alfonso P., Núñez A., Madoz-Gurpide J., Lombardia L., Sánchez L., Casal J. I. (2005) Proteomic expression analysis of colorectal cancer by two-dimensional differential gel electrophoresis. Proteomics 5, 2602–2611 [DOI] [PubMed] [Google Scholar]