Abstract
Septoria represents a genus of plant pathogenic fungi with a wide geographic distribution, commonly associated with leaf spots and stem cankers of a broad range of plant hosts. A major aim of this study was to resolve the phylogenetic generic limits of Septoria, Stagonospora, and other related genera such as Sphaerulina, Phaeosphaeria and Phaeoseptoria using sequences of the the partial 28S nuclear ribosomal RNA and RPB2 genes of a large set of isolates. Based on these results Septoria is shown to be a distinct genus in the Mycosphaerellaceae, which has mycosphaerella-like sexual morphs. Several septoria-like species are now accommodated in Sphaerulina, a genus previously linked to this complex. Phaeosphaeria (based on P. oryzae) is shown to be congeneric with Phaeoseptoria (based on P. papayae), which is reduced to synonymy under the former. Depazea nodorum (causal agent of nodorum blotch of cereals) and Septoria avenae (causal agent of avenae blotch of barley and rye) are placed in a new genus, Parastagonospora, which is shown to be distinct from Stagonospora (based on S. paludosa) and Phaeosphaeria. Partial nucleotide sequence data for five gene loci, ITS, LSU, EF-1α, RPB2 and Btub were generated for all of these isolates. A total of 47 clades or genera were resolved, leading to the introduction of 14 new genera, 36 new species, and 19 new combinations.
Taxonomic novelties:
New genera - Acicuseptoria Quaedvlieg, Verkley & Crous, Cylindroseptoria Quaedvlieg, Verkley & Crous, Kirstenboschia Quaedvlieg, Verkley & Crous, Neoseptoria Quaedvlieg, Verkley & Crous, Neostagonospora Quaedvlieg, Verkley & Crous, Parastagonospora Quaedvlieg, Verkley & Crous, Polyphialoseptoria Quaedvlieg, R.W. Barreto, Verkley & Crous, Ruptoseptoria Quaedvlieg, Verkley & Crous, Septorioides Quaedvlieg, Verkley & Crous, Setoseptoria Quaedvlieg, Verkley & Crous, Stromatoseptoria Quaedvlieg, Verkley & Crous, Vrystaatia Quaedvlieg, W.J. Swart, Verkley & Crous, Xenobotryosphaeria Quaedvlieg, Verkley & Crous, Xenoseptoria Quaedvlieg, H.D. Shin, Verkley & Crous. New species - Acicuseptoria rumicis Quaedvlieg, Verkley & Crous, Caryophylloseptoria pseudolychnidis Quaedvlieg, H.D. Shin, Verkley & Crous, Coniothyrium sidae Quaedvlieg, Verkley, R.W. Barreto & Crous, Corynespora leucadendri Quaedvlieg, Verkley & Crous, Cylindroseptoria ceratoniae Quaedvlieg, Verkley & Crous, Cylindroseptoria pistaciae Quaedvlieg, Verkley & Crous, Kirstenboschia diospyri Quaedvlieg, Verkley & Crous, Neoseptoria caricis Quaedvlieg, Verkley & Crous, Neostagonospora caricis Quaedvlieg, Verkley & Crous, Neostagonospora elegiae Quaedvlieg, Verkley & Crous, Paraphoma dioscoreae Quaedvlieg, H.D. Shin, Verkley & Crous, Parastagonospora caricis Quaedvlieg, Verkley & Crous, Parastagonospora poae Quaedvlieg, Verkley & Crous, Phlyctema vincetoxici Quaedvlieg, Verkley & Crous, Polyphialoseptoria tabebuiae-serratifoliae Quaedvlieg, Alfenas & Crous, Polyphialoseptoria terminaliae Quaedvlieg, R.W. Barreto, Verkley & Crous, Pseudoseptoria collariana Quaedvlieg, Verkley & Crous, Pseudoseptoria obscura Quaedvlieg, Verkley & Crous, Sclerostagonospora phragmiticola Quaedvlieg, Verkley & Crous, Septoria cretae Quaedvlieg, Verkley & Crous, Septoria glycinicola Quaedvlieg, H.D. Shin, Verkley & Crous, Septoria oenanthicola Quaedvlieg, H.D. Shin, Verkley & Crous, Septoria pseudonapelli Quaedvlieg, H.D. Shin, Verkley & Crous, Setophoma chromolaenae Quaedvlieg, Verkley, R.W. Barreto & Crous, Setoseptoria phragmitis Quaedvlieg, Verkley & Crous, Sphaerulina amelanchier Quaedvlieg, Verkley & Crous, Sphaerulina pseudovirgaureae Quaedvlieg, Verkley & Crous, Sphaerulina viciae Quaedvlieg, H.D. Shin, Verkley & Crous, Stagonospora duoseptata Quaedvlieg, Verkley & Crous, Stagonospora perfecta Quaedvlieg, Verkley & Crous, Stagonospora pseudocaricis Quaedvlieg, Verkley, Gardiennet & Crous, Stagonospora pseudovitensis Quaedvlieg, Verkley & Crous, Stagonospora uniseptata Quaedvlieg, Verkley & Crous, Vrystaatia aloeicola Quaedvlieg, Verkley, W.J. Swart & Crous, Xenobotryosphaeria calamagrostidis Quaedvlieg, Verkley & Crous, Xenoseptoria neosaccardoi Quaedvlieg, H.D. Shin, Verkley & Crous. New combinations - Parastagonospora avenae (A.B. Frank) Quaedvlieg, Verkley & Crous, Parastagonospora nodorum (Berk.) Quaedvlieg, Verkley & Crous, Phaeosphaeria papayae (Speg.) Quaedvlieg, Verkley & Crous, Pseudocercospora domingensis (Petr. & Cif.) Quaedvlieg, Verkley & Crous, Ruptoseptoria unedonis (Roberge ex Desm.) Quaedvlieg, Verkley & Crous, Septorioides pini-thunbergii (S. Kaneko) Quaedvlieg, Verkley & Crous, Sphaerulina abeliceae (Hiray.) Quaedvlieg, Verkley & Crous, Sphaerulina azaleae (Voglino) Quaedvlieg, Verkley & Crous, Sphaerulina berberidis (Niessl) Quaedvlieg, Verkley & Crous, Sphaerulina betulae (Pass.) Quaedvlieg, Verkley & Crous, Sphaerulina cercidis (Fr.) Quaedvlieg, Verkley & Crous, Sphaerulina menispermi (Thüm.) Quaedvlieg, Verkley & Crous, Sphaerulina musiva (Peck) Quaedvlieg, Verkley & Crous, Sphaerulina oxyacanthae (Kunze & J.C. Schmidt) Quaedvlieg, Verkley & Crous, Sphaerulina patriniae (Miura) Quaedvlieg, Verkley & Crous, Sphaerulina populicola (Peck) Quaedvlieg, Verkley & Crous, Sphaerulina quercicola (Desm.) Quaedvlieg, Verkley & Crous, Sphaerulina rhabdoclinis (Butin) Quaedvlieg, Verkley & Crous, Stromatoseptoria castaneicola (Desm.) Quaedvlieg, Verkley & Crous. Typifications: Epitypifications - Phaeosphaeria oryzae I. Miyake, Phaeoseptoria papayae Speg.; Neotypification - Hendersonia paludosa Sacc. & Speg.
Key words: Capnodiales, Multi-Locus Sequence Typing (MLST), Mycosphaerella, Mycosphaerellaceae, Phaeoseptoria, Phaeosphaeria, Phaeosphaeriaceae, Pleosporales, Septoria, Sphaerulina, Stagonospora, systematics
INTRODUCTION
Fungal species belonging to Septoria are among the most common and widespread leaf-spotting fungi worldwide. Septoria Sacc. (Mycosphaerella, Capnodiales, Dothideomycetes) is based on Septoria cytisi, which was first described by Desmazières (1847) as a pathogen of Cytisus laburnum (= Laburnum anagyroides). The genus Septoria is extremely large, and during the past 150 years more than 2000 taxa have been ascribed to this asexual genus (Verkley & Priest 2000, Verkley et al. 2004). Presently, Septoria s.lat. represents a polyphyletic assembly of genera that cluster mostly in the Mycosphaerellaceae (a family incorporating many plant pathogenic coelomycetes), although fungi with septoria-like morphology have also evolved outside this family (Crous et al. 2009a, c). Although many species of Septoria have mycosphaerella-like sexual states, the name Mycosphaerella does not apply to them, and should not be used in this context.
Following a proposal accepted by the International Code of Nomenclature for algae, fungi and plants (ICN), the generic name Septoria Sacc. was conserved over the older synonym Septaria Fr. (original spelling). The arguments preceding the typification of Septoria and subsequent proposals for name conservation by Wakefield (1940), Rogers (1949) and Donk (1964) between Septoria sensu Saccardo or Septaria Fries were various. In the end the committee for fungi appointed by the ICN followed the recommendation of Donk (1964), and decided on Septoria Sacc. over Septaria Fr., arguing that Septoria Sacc. had already been in prevalent use for many years, and should therefore be accepted as the correct name.
After examining several herbarium specimens of S. cytisi, Sutton (1980) circumscribed Septoria as follows: Mycelium immersed, branched, septate, pale brown. Conidiomata pycnidial, immersed, separate or aggregated (but not confluent), globose, papillate (or not), brown, thin-walled of pale brown textura angularis, often with a smaller-celled inner layer, somewhat darker and more thick-walled around the ostiole. Conidiophores reduced to conidiogenous cells. Conidiogenous cells holoblastic, either determinate or indeterminate, with a limited number of sympodial proliferations. Each locus has a broad, flat, unthickened scar, discrete, hyaline, smooth, ampulliform, doliiform or lageniform to short cylindrical. Conidia hyaline, multiseptate, filiform, smooth and either continuous or constricted at septa. Later work by Constantinescu (1984), Sutton & Pascoe (1987, 1989) and Farr (1991, 1992) augmented Sutton’s previous generic circumscription by also including species with sympodial, enteroblastic and percurrent conidial proliferation. Furthermore, based on similarities in conidiomatal development, von Arx (1983) and Braun (1995) adopted an even wider concept of Septoria that included the acervular forms normally accommodated in Phloeospora.
Morphological traits in Septoria are generally conserved, and specific morphological characters by which to describe and identify Septoria and septoria-like species are limited. This lack of specific morphological characters caused Septoria taxonomy to be largely dependent on associated host data, leading to many of the described species only being identifiable by host plant, and by variation in informative supplementary characters like conidial length, width and septation (Jørstad 1965, 1967, Sutton 1980). Of these supplementary characters, conidial width appears to be the most stable (i.e. it shows the least amount of intraspecific variation) and in most Septoria species, intraspecific conidial width rarely varies more than 1 μm (Priest 2006).
This reliance on host data in Septoria taxonomy is far from perfect, and should be avoided for identification purposes (see Verkley et al. 2013, this volume). Extensive host inoculation experiments by Beach (1919) and Teterevnikova-Babayan (1987) have shown that identification of Septoria spp. by host specificity alone is error prone because many Septoria species are not restricted to a single specific host (i.e. several taxa have broader host ranges). Septoria species like S. lactucicola and S. lycopersici can not only infect multiple plant species within the same genus, but can also infect plants belonging to closely allied families and genera. In contrast to this, morphologically well distinguishable Septoria species can also parasitise the same hosts (e.g. multiple distinct Septoria species can be found on both Chrysanthemum and Rubus hosts) (Demaree & Wilcox 1943, Punithalingam 1976, Shin & Sameva 2004). Because host specificity has been one of the main criteria used for describing new, morphologically indistinguishable Septoria species over the past 150 years, one can expect that a certain number of described taxa are in fact synonyms of species from related hosts.
Septoria and septoria-like genera in the molecular era
Although it had previously been speculated by Sutton (1980) that Septoria was in fact polyphyletic, definitive proof of this hypothesis awaited the introduction of molecular techniques. Cunfer & Ueng (1999) were the first to use rDNA sequence data of the internal transcribed spacer region (ITS) to postulate that Zymoseptoria tritici (then known as Septoria tritici) and several Stagonospora spp. (a morphologically similar genus, previously linked to Septoria) actually belonged to two distinct genera. Verkley et al. (2004) extended this study by employing a combination of 28S nrDNA (LSU) and ITS data to prove that Septoria was in fact both poly- and paraphyletic. Their work showed that septoria-like species such as Z. tritici and Z. passerinii were more closely related to Ramularia than to the majority of the other Septoria species used in their datasets.
Feau et al. (2006) were the first to use a multi-locus polyphasic sequencing approach to reliably identify Septoria spp. Besides ITS and LSU sequence data, they also used β-tubulin (Btub) sequence data to separate closely related species into distinct monophyletic groups that frequently correlated with their respective host families. These results supported the approach of using multi-gene sequence data for studying a large collection of Septoria strains at species level.
Septoria s. str. was finally demarcated when Quaedvlieg et al. (2011) managed to obtain both ITS and LSU sequence data from S. cytisi herbarium specimens. Phylogenetic analysis of the obtained S. cytisi LSU sequence data clearly proved that Z. tritici and Z. passerinii [as previously indicated by Cunfer & Ueng (1999) and Verkley et al. (2004)] did not belong to Septoria s. str., but in fact belonged to a separate genus, closely related to Ramularia. These two species were subsequently split off from Septoria and placed in a new genus, Zymoseptoria (named for the yeast-like state produced in culture). Since the initial Zymoseptoria paper, five additional species from members of Poaceae have been described in this genus (Crous et al. 2012a, Stukenbrock et al. 2012).
Septoria-like asexual genera
Since the description of Septoria by Desmazières (1847), several additional septoria-like genera (pycnidial/acervular/stromatic conidioma with filiform conidia) have been described which could be mistaken for Septoria s. str.
The two economically most important septoria-like genera are probably Zymoseptoria (sexual morph mycosphaerella-like) and Parastagonospora (sexual morph phaeosphaeria-like; see below). Both of these genera are pathogenic on Poaceae (grasses) and are directly or indirectly responsible for significant annual crop losses worldwide on cereals such as barley and wheat (Eyal et al. 1987). Quaedvlieg et al. (2011) determined that Zymoseptoria formed a distinct clade in the Mycosphaerellaceae, while Stagonospora was found to cluster in the Phaeosphaeriaceae within the Pleosporales, near other genera like Phoma and Phaeosphaeria (Cunfer & Ueng 1999, Solomon et al. 2006) which contain important plant pathogens. However, besides Zymoseptoria and Parastagonospora there are many other, lesser-known septoria-like genera awaiting elucidation. The goal of the present study is therefore to conduct an in-depth morphological and molecular analysis of these septoria-like genera, and resolve the affinities of Stagonospora and its purported sexual morph, Phaeosphaeria. To this end a collection of 370 Septoria and septoria-like isolates (Table 1) were subjected to morphological examination and multi-gene DNA analyses.
Table 1.
Collection details and GenBank accession numbers of isolates included in this study.
| Species | Isolate no.1 | Host | Location | Collector |
GenBank accession no.2 |
||||
|---|---|---|---|---|---|---|---|---|---|
| EF-1α | Btub | RPB2 | LSU | ITS | |||||
| Acicuseptoria rumicis | CBS 522.78 | Rumex alpinus | France | H.A. van der Aa | KF253105 | KF252643 | KF252153 | KF251648 | KF251144 |
| Boeremia telephii | CBS 135415; S670 | Lavatera thuringiaca | Germany | U. Damm | – | KF252644 | KF252154 | KF251649 | KF251145 |
| Caryophylloseptoria lychnidis | CBS 109098 | Silene pratensis | Austria | G.J.M. Verkley | KF253234 | KF252768 | KF252292 | KF251790 | KF251286 |
| CBS 109099 | Silene pratensis | Austria | G.J.M. Verkley | KF253235 | KF252769 | KF252293 | KF251791 | KF251287 | |
| CBS 109101 | Silene pratensis | Austria | G.J.M. Verkley | KF253236 | KF252770 | KF252294 | KF251792 | KF251288 | |
| CBS 109102 | Silene pratensis | Austria | G.J.M. Verkley | KF253237 | KF252771 | KF252295 | KF251793 | KF251289 | |
| Car. pseudolychnidis | CBS 128614 | Lychnis cognata | South Korea | H.D. Shin | KF253238 | KF252772 | KF252296 | KF251794 | KF251290 |
| CBS 128630 | Lychnis cognata | South Korea | H.D. Shin | KF253239 | KF252773 | KF252297 | KF251795 | KF251291 | |
| Car. silenes | CBS 109100 | Silene nutans | Austria | G.J.M. Verkley | KF253240 | KF252774 | KF252298 | KF251796 | KF251292 |
| CBS 109103 | Silene pratensis | Austria | G.J.M. Verkley | KF253241 | KF252775 | KF252299 | KF251797 | KF251293 | |
| Car. spergulae | CBS 397.52 | Dianthus caryophyllus | Netherlands | Schouten | KF253243 | KF252777 | KF252301 | KF251799 | KF251295 |
| CBS 109010 | Spergula morisonii | Netherlands | A. Aptroot | KF253242 | KF252776 | KF252300 | KF251798 | KF251294 | |
| Cercospora beticola | CBS 124.31; CPC 5070 | Beta vulgaris | Romania | – | KF253106 | KF252645 | KF252155 | KF251650 | KF251146 |
| Cer. capsici | CBS 118712 | – | Fiji | P. Tyler | KF253244 | KF252778 | KF252302 | KF251800 | KF251296 |
| Cer. zebrina | CBS 137.56 | Hedysarum coronarium | Italy | M. Ribaldi | KF253245 | KF252779 | KF252303 | KF251801 | KF251297 |
| CBS 118790; IMI 262766 | Trifolium subterraneum | Australia | M.J. Barbetti | KF253107 | KF252646 | KF252156 | KF251651 | KF251147 | |
| Chaetosphaeronema hispidulum | CBS 216.75 | Anthyllis vulneraria | Germany | R. Schneider | KF253108 | KF252647 | KF252157 | KF251652 | KF251148 |
| Coniothyrium carteri | CBS 105.91 | Quercus robur | Germany | H. Schill | KF253165 | KF252700 | KF252214 | KF251712 | KF251209 |
| CBS 101633 | Quercus sp. | Netherlands | – | KF253166 | KF252701 | KF252215 | KF251713 | KF251210 | |
| Con. glycinicola | CBS 124141 | Glycine max | Zimbabwe | C. Lavy | KF253167 | KF252702 | KF252216 | KF251714 | KF251211 |
| Con. sidae | CBS 135108; CPC 19602 | Sida sp. | Brazil | R.W. Barreto | KF253109 | KF252648 | KF252158 | KF251653 | KF251149 |
| Corynespora leucadendri | CBS 135133; CPC 19345 | Leucadendron sp. | South Africa | S. Lee | KF253110 | KF252639 | KF252159 | KF251654 | KF251150 |
| Cylindroseptoria ceratoniae | CBS 477.69 | Ceratonia siliqua | Spain | H.A. van der Aa | KF253111 | KF252649 | KF252160 | KF251655 | KF251151 |
| Cyl. pistaciae | CBS 471.69 | Pistacia lentiscus | Spain | H.A. van der Aa | KF253112 | KF252650 | KF252161 | KF251656 | KF251152 |
| Cytostagonospora martiniana | CBS 135102; CPC 17727 | Acacia pycnantha | Australia | P.W. Crous | KF253113 | KF252651 | KF252162 | KF251657 | KF251153 |
| Dissoconium commune | CPC 12397 | Eucalyptus globulus | Australia | I. Smith | KF253190 | KF252724 | KF252242 | KF251740 | KF251237 |
| Dothistroma pini | CBS 116484 | Pinus nigra | USA | G. Adams | JX901622 | JX902193 | JX901948 | JX901824 | JX901736 |
| CBS 116485 | Pinus nigra | USA | G. Adams | JX901625 | JX902196 | JX901951 | JX901827 | JX901739 | |
| CBS 116487 | Pinus nigra | USA | G. Adams | JX901620 | JX902191 | JX901946 | JX901822 | GU214532 | |
| CBS 121005 | Pinus pallasiana | Russia | T. S. Bulgakov | KF253115 | KF252653 | – | KF251659 | KF251155 | |
| CBS 121011 | Pinus pallasiana | Russia | A.C. Usichenko | KF253250 | – | KF252307 | KF251806 | KF251302 | |
| Dot. septosporum | CBS 383.74 | Pinus coulteri | France | M. Morelet | KF253251 | – | KF252308 | KF251807 | KF251303 |
| CPC 16798 | Pinus mugo ‘Rostrata’ | Netherlands | W. Quaedvlieg | JX901627 | JX902198 | JX901953 | JX901829 | JX901741 | |
| CPC 16799 | Pinus mugo | Netherlands | W. Quaedvlieg | JX901628 | JX902199 | JX901954 | JX901830 | JX901742 | |
| Kirstenboschia diospyri | CBS 134911; CPC 19869 | Diospyros whyteana | South Africa | P.W. Crous | KF253116 | KF252640 | KF252164 | KF251660 | KF251156 |
| CPC 19870 | Diospyros whyteana | South Africa | P.W. Crous | KF253117 | KF252641 | KF252165 | KF251661 | KF251157 | |
| Lecanosticta acicola | CBS 322.33 | – | – | P.V. Siggers | JX901639 | JX902213 | JX901968 | JX901844 | JX901755 |
| CBS 133791 | Pinus strobus | USA | B. Ostrofsky | KC013002 | KC013008 | KC013014 | KC013017 | KC012999 | |
| Lec. brevispora | CBS 133601 | Pinus sp. | Mexico | J.Y. Morales | JX901649 | JX902224 | JX901979 | JX901855 | JX901763 |
| Lec. guatamalensis | IMI 281598 | Pinus oocarpa | Guatemala | H.C. Evans | JX901650 | JX902225 | JX901980 | JX901856 | JX901764 |
| Lec. longispora | CBS 133602 | Pinus sp. | Mexico | J.Y. Morales | JX901651 | JX902227 | JX901982 | JX901858 | JX901766 |
| Leptosphaeria albopunctata | CBS 254.64 | Spartina alterniflora | USA | J. Kohlmeyer | KF253118 | KF252654 | KF252166 | KF251662 | KF251158 |
| Mycosphaerella brassicicola | CBS 228.32 | Brassica oleracea | Denmark | C.A. Jörgensen | KF253252 | KF252783 | KF252309 | KF251808 | KF251304 |
| CBS 267.53 | Brassica oleracea | Netherlands | F. Quak | KF253253 | KF252784 | KF252310 | KF251809 | KF251305 | |
| Mycosphaerella sp. | CBS 135464; CPC 11677 | Draba nemorosa var. hebecarpa | South Korea | H.D. Shin | – | KF252786 | KF252312 | KF251811 | KF251307 |
| Neoseptoria caricis | CBS 135097; S653 | Carex acutiformis | Netherlands | W. Quaedvlieg | – | – | KF252167 | KF251663 | KF251159 |
| Neosetophoma samarorum | CBS 138.96 | Phlox paniculata | Netherlands | – | KF253119 | KF252655 | KF252168 | KF251664 | KF251160 |
| CBS 139.96 | Poa sp. | Netherlands | – | KF253120 | KF252656 | KF252169 | KF251665 | KF251161 | |
| CBS 568.94 | Urtica dioica | Netherlands | G.J.M. Verkley | KF253121 | KF252657 | KF252170 | KF251666 | KF251162 | |
| Neostagonospora caricis | CBS 135092; S616 | Carex acutiformis | Netherlands | W. Quaedvlieg | – | KF252658 | KF252171 | KF251667 | KF251163 |
| Neost. elegiae | CBS 135101; CPC 16977 | Elegia cuspidata | South Africa | S. Lee | KF253122 | KF252659 | KF252172 | KF251668 | KF251164 |
| Paraphoma chrysanthemicola | CBS 172.70 | Chrysanthemum morifolium | Netherlands | R. Schneider | KF253123 | KF252660 | KF252173 | KF251669 | KF251165 |
| CBS 522.66 | Chrysanthemum morifolium | UK | – | KF253124 | KF252661 | KF252174 | KF251670 | KF251166 | |
| Parap. dioscoreae | CBS 135100; CPC 11357 | Dioscorea tokoro | South Korea | H.D. Shin | KF253125 | KF252662 | KF252175 | KF251671 | KF251167 |
| CPC 11355 | Dioscorea tokoro | South Korea | H.D. Shin | KF253126 | KF252663 | KF252176 | KF251672 | KF251168 | |
| CPC 11361 | Dioscorea tokoro | South Korea | H.D. Shin | KF253127 | KF252664 | KF252177 | KF251673 | KF251169 | |
| Parap. fimeti | CBS 170.70 | Apium graveolens | Netherlands | M.A. de Waard | KF253128 | KF252665 | KF252178 | KF251674 | KF251170 |
| CBS 368.91 | Juniperus communis | Switzerland | – | KF253129 | KF252666 | KF252179 | KF251675 | KF251171 | |
| Parap. radicina | CBS 111.79 | Malus sylvestris | Netherlands | G.H. Boerema | KF253130 | KF252667 | KF252180 | KF251676 | KF251172 |
| CBS 102875 | Lycopersicon esculentum | Germany | – | KF253131 | KF252668 | KF252181 | KF251677 | KF251173 | |
| Parastagonospora avenae | CBS 289.69 | Lolium perenne | Germany | U.G. Schlösser | KF253132 | KF252669 | KF252182 | KF251678 | KF251174 |
| CBS 290.69 | Lolium perenne | Germany | U.G. Schlösser | KF253133 | KF252670 | KF252183 | KF251679 | KF251175 | |
| Paras. caricis | CBS 135671; S615 | Carex acutiformis | Netherlands | W. Quaedvlieg | KF253134 | KF252671 | KF252184 | KF251680 | KF251176 |
| Paras. nodorum | CBS 110109 | Lolium perenne | Denmark | M.P.S. Câmara | KF253135 | KF252672 | KF252185 | KF251681 | KF251177 |
| Paras. “nodorum” | CBS 259.49 | Triticum sp. | Canada | – | KF253143 | KF252679 | KF252192 | KF251688 | KF251185 |
| Paras. poae | CBS 135089; S606 | Poa sp. | Netherlands | S.I.R. Videira | KF253136 | KF252673 | KF252186 | KF251682 | KF251178 |
| CBS 135091; S613 | Poa sp. | Netherlands | S.I.R. Videira | KF253137 | KF252674 | KF252187 | KF251683 | KF251179 | |
| Passalora depressa | CPC 14915 | Angelica gigas | South Korea | H.D. Shin | KF253256 | KF252788 | KF252314 | KF251813 | KF251309 |
| Pas. dioscoreae | CBS 135460; CPC 10855 | Dioscorea tokoro | South Korea | H.D. Shin | KF253257 | KF252789 | KF252315 | KF251814 | KF251310 |
| CBS 135463; CPC 11513 | Dioscorea tenuipes | South Korea | H.D. Shin | KF253258 | KF252790 | KF252316 | KF251815 | KF251311 | |
| Phaeophleospora eugeniae | CPC 15143 | Eugenia uniflora | Brazil | A.C. Alfenas | KF253138 | KF252642 | – | JX901875 | KF251180 |
| CPC 15159 | Eugenia uniflora | Brazil | A.C. Alfenas | JX901667 | JX902245 | JX901999 | JX901876 | FJ493189 | |
| “Phaeosphaeria” alpina | CBS 456.84 | Phleum alpinum | Switzerland | A. Leuchtmann | KF253139 | KF252675 | KF252188 | KF251684 | KF251181 |
| Phaeos. caricicola | CBS 603.86 | Carex pendula | Switzerland | A. Leuchtmann | KF253140 | KF252676 | KF252189 | KF251685 | KF251182 |
| Phaeos. juncicola | CBS 110108 | Phlox sp. | Netherlands | M.P.S. Câmara | KF253141 | KF252677 | KF252190 | KF251686 | KF251183 |
| Phaeos. nigrans | CBS 307.79 | Zea mays | Switzerland | – | KF253142 | KF252678 | KF252191 | KF251687 | KF251184 |
| Phaeos. oryzae | CBS 110110 | Oryza sativa | South Korea | L. Hausch | – | KF252680 | KF252193 | KF251689 | KF251186 |
| Phaeos. papayae | CBS 135416 | Carica papaya | Brazil | A.C. Alfenas | – | KF252681 | KF252194 | KF251690 | KF251187 |
| “Phaeos.” phragmiticola | CBS 459.84 | Phragmites australis | Switzerland | A. Leuchtmann | KF253144 | KF252682 | KF252195 | KF251691 | KF251188 |
| “Phaeos.” pontiformis | CBS 117487 | – | Netherlands | J. Harrak | KF253145 | KF252683 | KF252196 | KF251692 | KF251189 |
| Phaeosphaeria sp. | CBS 206.87 | Zea mays | Gabon | J.L. Notteghem | KF253146 | KF252684 | KF252197 | KF251693 | KF251190 |
| CBS 135465; CPC 11894 | Zea mays | South Africa | P.W. Crous | KF253147 | KF252685 | KF252198 | KF251694 | KF251191 | |
| “Phaeos.” typharum | CBS 296.54 | Nardus stricta | Switzerland | L.E. Wehmeyer | KF253148 | KF252686 | KF252199 | KF251695 | KF251192 |
| “Phaeos.” vagans | CBS 604.86 | Calamagrostis arundinacea | Sweden | A. Leuchtmann | KF253149 | KF252687 | KF252200 | KF251696 | KF251193 |
| phaeosphaeria-like sp. | CBS 123.76 | Prunus domestica | Serbia | M. Arseijevic | KF253150 | KF252688 | KF252201 | KF251697 | KF251194 |
| CBS 135461; CPC 11231 | Musa sp. | Mauritius | Y. Jaufeerally-Fakim | KF253151 | KF252689 | KF252202 | KF251698 | KF251195 | |
| CBS 135466; CPC 12131 | Acacia crassicarpa | Thailand | W. Himaman | KF253153 | KF252691 | KF252204 | KF251700 | KF251197 | |
| CBS 135469; CPC 12881 | Pinus monticola | USA | G. Newcombe & R.G. Ganley | KF253154 | KF252692 | KF252205 | KF251701 | KF251198 | |
| CPC 12130 | Acacia crassicarpa | Thailand | W. Himaman | KF253152 | KF252690 | KF252203 | KF251699 | KF251196 | |
| Phaeosphaeriopsis glaucopunctata | CBS 653.86 | Ruscus aculeatus | Switzerland | A. Leuchtmann | KF253155 | KF252693 | KF252206 | KF251702 | KF251199 |
| Phloeospora ulmi | CBS 344.97 | Ulmus glabra | Austria | W. Gams | KF253158 | KF252696 | – | KF251705 | KF251202 |
| CBS 613.81 | Ulmus sp. | Austria | H.A. van der Aa | KF253159 | KF252697 | KF252208 | KF251706 | KF251203 | |
| CBS 101564 | Ulmus sp. | Netherlands | H.A. van der Aa | KF253156 | KF252694 | KF252207 | KF251703 | KF251200 | |
| CBS 109835 | Ulmus sp. | Netherlands | G.J.M. Verkley | KF253157 | KF252695 | – | KF251704 | KF251201 | |
| Phlogicylindrium eucalyptorum | CBS 111680 | Eucalyptus nitens | Australia | P.W. Crous | KF253160 | KF252698 | KF252209 | KF251707 | KF251204 |
| CBS 111689 | Eucalyptus nitens | Australia | P.W. Crous | KF253161 | – | KF252210 | KF251708 | KF251205 | |
| Phlyctema vincetoxici | CBS 123726 | Vincetoxicum officinale | Czech Republic | G.J.M. Verkley | KF253162 | KF252699 | KF252211 | KF251709 | KF251206 |
| CBS 123727 | Vincetoxicum officinale | Czech Republic | G.J.M. Verkley | KF253163 | – | KF252212 | KF251710 | KF251207 | |
| CBS 123743 | Vincetoxicum officinale | Czech Republic | G.J.M. Verkley | KF253164 | – | KF252213 | KF251711 | KF251208 | |
| Phoma herbarum | CBS 615.75 | Rosa multiflora | Netherlands | G.H. Boerema | KF253168 | KF252703 | KF252217 | KF251715 | KF251212 |
| Polyphialoseptoria tabebuiae-serratifoliae | CBS 112650 | Tabebuia serratifolia | Brazil | A.C. Alfenas | KF253169 | KF252704 | KF252218 | KF251716 | KF251213 |
| Pol. terminaliae | CBS 135106; CPC 19611 | Terminalia catappa | Brazil | R.W. Barreto | KF253170 | KF252705 | KF252219 | KF251717 | KF251214 |
| CBS 135475; CPC 19487 | Terminalia catappa | Brazil | R.W. Barreto | KF253171 | – | KF252220 | KF251718 | KF251215 | |
| Pseudocercospora chiangmaiensis | CBS 123244 | Eucalyptus camaldurensis | Thailand | R. Cheewangkoon | JX901676 | JX902254 | JX902008 | JX901885 | JX901781 |
| Pse. eucalyptorum | CBS 116303 | Eucalyptus nitens | South Africa | P.W. Crous | KF253172 | KF252706 | KF252221 | KF251719 | KF251216 |
| CPC 13816 | Eucalyptus glaucescens | UK | S. Denman | KF253230 | KF252764 | KF252288 | KF251786 | KF251282 | |
| Pse. madagascariensis | CBS 124155 | Eucalyptus camaldulensis | Madagascar | M.J. Wingfield | KF253265 | – | KF252322 | KF251822 | KF251318 |
| Pse. natalensis | CBS 111069 | Eucalyptus nitens | South Africa | T. Coutinho | KF302389 | KF302384 | KF302393 | KF302405 | KF302399 |
| Pse. norchiensis | CBS 120738 | Eucalyptus sp. | Italy | W. Gams | JX901684 | JX902263 | JX902017 | JX901894 | JX901785 |
| Pse. robusta | CBS 111175 | Eucalyptus robur | Malaysia | M.J. Wingfield | JX901694 | JX902273 | JX902027 | JX901904 | DQ303081 |
| Pse. schizolobii | CBS 120029 | Schizolobium parahybum | Ecuador | M.J. Wingfield | KF253269 | KF252798 | KF252326 | KF251826 | KF251322 |
| Pse. tereticornis | CPC 13299 | Eucalyptus tereticornis | Australia | P.W. Crous | JX901701 | JX902280 | JX902034 | JX901911 | GQ852770 |
| Pseudocercosporella capsellae | CBS 127.29 | – | – | K. Togashi | KF253273 | KF252801 | KF252330 | KF251830 | KF251326 |
| CBS 112032 | Brassica sp. | UK | R. Evans | KF253267 | KF252797 | KF252324 | KF251824 | KF251320 | |
| CBS 112033 | Brassica sp. | UK | R. Evans | KF253254 | KF252785 | KF252311 | KF251810 | KF251306 | |
| CBS 118412 | Brassica sp. | New Zealand | C.F. Hill | KF253272 | KF252800 | KF252329 | KF251829 | KF251325 | |
| “Pella.” magnusiana | CBS 114735 | Geranium silvaticum | Sweden | E. Gunnerbeck | KF253274 | KF252802 | – | KF251831 | KF251327 |
| Pella. pastinacae | CBS 114116 | Laserpitium latifolium | Sweden | L. Holm | KF253275 | KF252803 | KF252331 | KF251832 | KF251328 |
| Pseudoseptoria collariana | CBS 135104; CPC 18119 | Bambusoideae sp. | Iran | A. Mirzadi Gohari | KF253174 | KF252707 | KF252223 | KF251721 | KF251218 |
| Pseudos. obscura | CBS 135103; CPC 18118 | Bambusoideae sp. | Iran | A. Mirzadi Gohari | KF253175 | KF252708 | KF252224 | KF251722 | KF251219 |
| Ramularia endophylla | CBS 113265 | Quercus robur | Netherlands | G.J.M. Verkley | KF253176 | KF252709 | KF252225 | KF251723 | KF251220 |
| Ram. eucalypti | CBS 120726 | Eucalyptus grandis var. grandiflora Maiden | Italy | W. Gams | KF253177 | KF252710 | KF252226 | KF251724 | KF251221 |
| Ram. lamii | CPC 11312 | Leonurus sibiricus | South Korea | H.D. Shin | KF253178 | KF252711 | KF252227 | KF251725 | KF251222 |
| Ram. pratensis | CPC 11294 | Rumex crispus | South Korea | – | KF253179 | KF252712 | KF252228 | KF251726 | KF251223 |
| Ramularia sp. | CBS 115913 | Cerastium semidecandrum | Netherlands | A. Aptroot | KF253180 | – | KF252229 | KF251727 | KF251224 |
| Readeriella angustia | CBS 124998 | Eucalyptus delegatensis | Australia | B.A. Summerel | KF253181 | KF252713 | KF252230 | KF251728 | KF251225 |
| Rea. eucalypti | CPC 13401 | Eucalyptus sp. | Portugal | P.W. Crous | KF253173 | – | KF252222 | KF251720 | KF251217 |
| Rea. readeriellophora | CPC 12920 | Eucalyptus sp. | Australia | A. Carnegie | KF253114 | KF252652 | KF252163 | KF251658 | KF251154 |
| Ruptoseptoria unedonis | CBS 355.86 | Arbutus unedo | France | H.A. van der Aa | – | KF252715 | KF252233 | KF251731 | KF251228 |
| CBS 755.70 | Arbutus unedo | Croatia | J.A. von Arx | – | KF252716 | KF252234 | KF251732 | KF251229 | |
| Sclerostagonospora phragmiticola | CBS 338.86 | Phragmites australis | France | H.A. van der Aa | KF253184 | KF252717 | KF252235 | KF251733 | KF251230 |
| Septoria abei | CBS 128598 | Hibiscus syriacus | South Korea | H.D. Shin | KF253280 | KF252805 | KF252336 | KF251837 | KF251333 |
| Sep. “agropyrina” | CBS 387.64 | – | Japan | – | KF302392 | KF302387 | KF302398 | KF302410 | KF302404 |
| Sep. anthrisci | CBS 109020 | Anthriscus sp. | Austria | G.J.M. Verkley | KF253286 | KF252811 | KF252340 | KF251843 | KF251339 |
| Sep. anthurii | CBS 346.58 | Anthurium scherzerianum | Germany | R. Schneider | KF253288 | KF252813 | KF252342 | KF251845 | KF251341 |
| Sep. apiicola | CBS 400.54 | Apium graveolens | Netherlands | J.A. von Arx | KF253292 | KF252817 | KF252346 | KF251849 | KF251345 |
| “Sep.” arundinacea | CBS 133.68 | Phragmites australis | Netherlands | H.A. van der Aa | KF253185 | KF252718 | KF252236 | KF251734 | KF251231 |
| CBS 281.72 | Phragmites australis | Netherlands | J.W. Veenbaas-Rijks | KF253186 | KF252719 | KF252237 | KF251735 | KF251232 | |
| Sep. astericola | CBS 128593 | Aster yomena | South Korea | H.D. Shin | KF253294 | KF252819 | KF252348 | KF251851 | KF251347 |
| Sep. astragali | CBS 109116 | Astragalus sp. | Austria | G.J.M. Verkley | KF253298 | KF252823 | KF252352 | KF251855 | KF251351 |
| CBS 123878 | Astragalus glycyphyllos | Czech Republic | G.J.M. Verkley | KF253297 | KF252822 | KF252351 | KF251854 | KF251350 | |
| Sep. atropurpurea | CBS 348.58 | Aster canus | Germany | R. Schneider | KF253299 | KF252824 | KF252353 | KF251856 | KF251352 |
| Sep. bothriospermi | CBS 128599 | Bothriospermum tenellum | South Korea | H.D. Shin | KF253301 | KF252826 | KF252355 | KF251858 | KF251354 |
| Sep. bupleuricola | CBS 128603 | Bupleurum falcatum | South Korea | H.D. Shin | KF253303 | KF252828 | KF252357 | KF251860 | KF251356 |
| Sep. calendulae | CBS 349.58 | Calendula arvensis | Italy | R. Schneider | KF253304 | KF252829 | KF252358 | KF251861 | KF251357 |
| Sep. callistephi | CBS 128590 | Callistephus chinensis | South Korea | H.D. Shin | KF253305 | KF252830 | KF252359 | KF251862 | KF251358 |
| Sep. campanulae | CBS 128604 | Campanula takesimana | South Korea | H.D. Shin | KF253308 | KF252833 | KF252362 | KF251865 | KF251361 |
| Sep. cerastii | CBS 128612 | Cerastium holosteoides | South Korea | H.D. Shin | KF253311 | KF252836 | KF252365 | KF251868 | KF251364 |
| Sep. cf. agrimoniicola | CBS 128585 | Agrimonia pilosa | South Korea | H.D. Shin | KF253283 | KF252808 | KF252337 | KF251840 | KF251336 |
| CBS 128602 | Agrimonia pilosa | South Korea | H.D. Shin | KF253284 | KF252809 | KF252338 | KF251841 | KF251337 | |
| Sep. cf. rubi | CBS 128646 | Rubus crataegifolius | South Korea | H.D. Shin | KF253314 | KF252839 | KF252368 | KF251871 | KF251367 |
| Sep. cf. stachydicola | CBS 128668 | Stachys riederi var. japonica | South Korea | H.D. Shin | KF253512 | KF253033 | KF252558 | KF252070 | KF251565 |
| Sep. chelidonii | CBS 128607 | Chelidonium majus | South Korea | H.D. Shin | KF253319 | KF252844 | KF252373 | KF251876 | KF251372 |
| Sep. chromolaenae | CBS 113373 | Chromolaena odorata | Cuba | S. Neser | KF253321 | KF252846 | KF252375 | KF251878 | KF251374 |
| Sep. chrysanthemella | CBS 128622 | Chrysanthemum boreale | South Korea | H.D. Shin | KF253323 | KF252848 | KF252377 | KF251880 | KF251376 |
| CBS 128716 | – | South Africa | E. Oh | KF253325 | KF252850 | KF252379 | KF251882 | KF251378 | |
| Sep. cirsii | CBS 128621 | Cirsium setidens | South Korea | H.D. Shin | KF253328 | KF252853 | KF252382 | KF251885 | KF251381 |
| Sep. citricola | CBS 356.36 | Citrus sinensis | Italy | G. Ruggieri | KF253329 | KF252854 | KF252383 | KF251886 | KF251382 |
| Sep. clematidis | CBS 108983 | Clematis vitalba | Germany | G.J.M. Verkley | KF253330 | KF252855 | KF252384 | KF251887 | KF251383 |
| Sep. codonopsidis | CBS 128620 | Codonopsis lanceolata | South Korea | H.D. Shin | KF253333 | KF252858 | KF252387 | KF251890 | KF251386 |
| Sep. convolvuli | CBS 128627 | Calystegia soldanella | South Korea | H.D. Shin | KF253336 | KF252861 | KF252390 | KF251893 | KF251389 |
| Sep. coprosma | CBS 113391 | Coprosma robusta | New Zealand | G.J.M. Verkley | KF253255 | KF252787 | KF252313 | KF251812 | KF251308 |
| Sep. crepidis | CBS 128608 | Youngia japonica | South Korea | H.D. Shin | KF253337 | KF252862 | KF252391 | KF251894 | KF251390 |
| CBS 128619 | Youngia japonica | South Korea | H.D. Shin | KF253338 | KF252863 | KF252392 | KF251895 | KF251391 | |
| Sep. cretae | CBS 135095; CPC 651 | Nerium oleander | Greece | U. Damm | – | KF252720 | KF252238 | KF251736 | KF251233 |
| Sep. cruciatae | CBS 123747 | Galium odoratum | Czech Republic | G.J.M. Verkley | KF253340 | KF252865 | KF252394 | KF251897 | KF251393 |
| Sep. cucubali | CBS 102386 | Saponaria officinalis | Netherlands | G.J.M. Verkley | KF253344 | KF252869 | KF252398 | KF251901 | KF251397 |
| Sep. cucurbitacearum | CBS 178.77 | Cucurbita maxima | New Zealand | H.J. Boesewinkel | KF253346 | – | KF252400 | KF251903 | KF251399 |
| Sep. dearnessii | CBS 128624 | Angelica dahurica | South Korea | H.D. Shin | KF253347 | KF252871 | KF252401 | KF251904 | KF251400 |
| Sep. digitalis | CBS 391.63 | Digitalis lanata | Czech Republic | V. Holubová | KF253349 | KF252873 | KF252403 | KF251906 | KF251402 |
| Sep. dysentericae | CBS 131892; CPC 12328 | Inula britannica | South Korea | H.D. Shin | KF253353 | KF252877 | KF252406 | KF251910 | KF251406 |
| Sep. epambrosiae | CBS 128629 | Ambrosia trifida | South Korea | H.D. Shin | KF253356 | KF252880 | KF252407 | KF251913 | KF251409 |
| Sep. epilobii | CBS 109084 | Epilobium fleischeri | Austria | G.J.M. Verkley | KF253358 | KF252882 | KF252409 | KF251915 | KF251411 |
| CBS 109085 | Epilobium fleischeri | Austria | G.J.M. Verkley | KF253359 | KF252883 | KF252410 | KF251916 | KF251412 | |
| Sep. erigerontis | CBS 186.93 | Erigeron annuus | Italy | M. Vurro | KF253364 | KF252887 | KF252537 | KF252048 | KF251543 |
| CBS 109094 | Erigeron annuus | Austria | G.J.M. Verkley | KF253360 | KF252884 | KF252411 | KF251917 | KF251413 | |
| CBS 131893; CPC 12340 | Erigeron annuus | South Korea | H.D. Shin | KF253363 | KF252888 | KF252414 | KF251920 | KF251416 | |
| Sep. eucalyptorum | CBS 118505 | Eucalyptus sp. | India | W. Gams | KF253365 | KF252889 | KF252415 | KF251921 | KF251417 |
| Sep. exotica | CBS 163.78 | Hebe speciosa | New Zealand | H.J. Boesewinkel | KF253366 | KF252890 | KF252416 | KF251922 | KF251418 |
| Sep. galeopsidis | CBS 191.26 | Galeopsis sp. | – | C. Killian | KF253370 | KF252894 | KF252420 | KF251926 | KF251422 |
| CBS 102314 | Galeopsis tetrahit | Netherlands | G.J.M. Verkley | KF253371 | KF252895 | KF252421 | KF251927 | KF251423 | |
| CBS 102411 | Galeopsis tetrahit | Netherlands | G.J.M. Verkley | KF253372 | KF252896 | KF252422 | KF251928 | KF251424 | |
| Sep. gentianae | CBS 128633 | Gentiana scabra | South Korea | H.D. Shin | KF253374 | KF252898 | KF252424 | KF251930 | KF251426 |
| “Sep.” gladioli | CBS 121.20 | – | – | W.J. Kaiser | KF253375 | KF252899 | KF252425 | KF251931 | KF251427 |
| CBS 353.29 | – | Netherlands | J.C. Went | KF253376 | KF252900 | KF252426 | KF251932 | KF251428 | |
| Sep. glycinicola | CBS 128618 | Glycine max | South Korea | H.D. Shin | KF253378 | KF252902 | KF252427 | KF251934 | KF251430 |
| Sep. helianthi | CBS 123.81 | Helianthus annuus | – | M. Muntañola | KF253379 | KF252903 | KF252428 | KF251935 | KF251431 |
| Sep. hibiscicola | CBS 128615 | Hibiscus syriacus | South Korea | H.D. Shin | KF253382 | KF252906 | KF252431 | KF251938 | KF251434 |
| Sep. hippocastani | CBS 411.61 | Aesculus hippocastanum | Germany | W. Gerlach | KF253383 | KF252907 | KF252432 | KF251939 | KF251435 |
| CPC 23103; MP11 | Aesculus sp. | Netherlands | S.I.R. Videira | KF253510 | KF253031 | KF252556 | KF252068 | KF251563 | |
| Sep. justiciae | CBS 128625 | Justicia procumbens | South Korea | H.D. Shin | KF253385 | KF252909 | KF252434 | KF251941 | KF251437 |
| Sep. lactucae | CBS 352.58 | Lactuca sativa | Germany | G. Sörgel | KF253388 | KF252912 | KF252437 | KF251944 | KF251440 |
| CBS 108943 | Lactuca sativa | Netherlands | P. Grooteman | KF253387 | KF252911 | KF252436 | KF251943 | KF251439 | |
| Sep. lamiicola | CBS 123884 | Lamium sp. | Czech Republic | G.J.M. Verkley | KF253397 | KF252921 | KF252446 | KF251953 | KF251449 |
| Sep. lepidiicola | CBS 128635 | Lepidium virginicum | South Korea | H.D. Shin | KF253398 | KF252922 | KF252447 | KF251954 | KF251450 |
| Sep. leptostachyae | CBS 128613 | Phryma leptostachya | South Korea | H.D. Shin | KF253399 | KF252923 | KF252448 | KF251955 | KF251451 |
| CBS 128628 | Phryma leptostachya | South Korea | H.D. Shin | KF253400 | KF252924 | KF252449 | KF251956 | KF251452 | |
| Sep. leucanthemi | CBS 109090 | Chrysanthemum leucanthemum | Austria | G.J.M. Verkley | KF253403 | KF252927 | KF252452 | KF251959 | KF251455 |
| Sep. limonum | CBS 419.51 | Citrus limonum | Italy | G. Goidánich | KF253407 | KF252931 | KF252456 | KF251963 | KF251459 |
| Sep. linicola | CBS 316.37 | Linum usitatissimum | – | H.W. Hollenweber | KF253408 | KF252932 | KF252457 | KF251964 | KF251460 |
| Sep. lycoctoni | CBS 109089 | Aconitum vulparia | Austria | G.J.M. Verkley | KF253409 | KF252933 | KF252458 | KF251965 | KF251461 |
| Sep. lycopersici | CBS 128654 | Lycopersicon esculentum | South Korea | H.D. Shin | KF253410 | KF252934 | KF252459 | KF251966 | KF251462 |
| Sep. lycopicola | CBS 128651 | Lycopus ramosissimus | South Korea | H.D. Shin | KF253412 | KF252936 | KF252461 | KF251968 | KF251464 |
| Sep. lysimachiae | CBS 102315 | Lysimachia vulgaris | Netherlands | G.J.M. Verkley | KF253413 | KF252937 | KF252462 | KF251969 | KF251465 |
| CBS 123795 | Lysimachia sp. | Czech Republic | G.J.M. Verkley | KF253417 | KF252941 | KF252466 | KF251973 | KF251469 | |
| Sep. malagutii | CBS 106.80 | Solanum sp. | Peru | G.H. Boerema | KF253418 | – | KF252467 | KF251974 | KF251470 |
| Sep. matricariae | CBS 109001 | Matricaria discoidea | Netherlands | G.J.M. Verkley | KF253420 | KF252943 | KF252469 | KF251976 | KF251472 |
| Sep. mazi | CBS 128755 | Mazus japonicus | South Korea | H.D. Shin | KF253422 | KF252945 | KF252471 | KF251978 | KF251474 |
| Sep. melissae | CBS 109097 | Melissa officinalis | Netherlands | H.A. van der Aa | KF253423 | KF252946 | KF252472 | KF251979 | KF251475 |
| Sep. napelli | CBS 109105 | Aconitum napellus | Austria | G.J.M. Verkley | KF253426 | KF252949 | KF252474 | KF251982 | KF251478 |
| Sep. obesa | CBS 354.58 | Chrysanthemum indicum | Germany | R. Schneider | KF253431 | – | KF252479 | KF251987 | KF251483 |
| CBS 128588 | Artemisia lavandulaefolia | South Korea | H.D. Shin | KF253428 | KF252951 | KF252476 | KF251984 | KF251480 | |
| CBS 128623 | Chrysanthemum indicum | South Korea | H.D. Shin | KF253429 | KF252952 | KF252477 | KF251985 | KF251481 | |
| Sep. oenanthicola | CBS 128649 | Oenanthe javanica | South Korea | H.D. Shin | KF253187 | KF252721 | KF252239 | KF251737 | KF251234 |
| Sep. oenanthis | CBS 128667 | Cicuta virosa | South Korea | H.D. Shin | KF253432 | KF252953 | KF252481 | KF251989 | KF251485 |
| Sep. orchidearum | CBS 457.78 | Listera ovata | France | H.A. van der Aa | KF253435 | KF252956 | KF252483 | KF251991 | KF251487 |
| CBS 128631 | Cyclamen fatrense | South Korea | H.D. Shin | KF253434 | KF252955 | KF252482 | KF251990 | KF251486 | |
| Sep. pachyspora | CBS 128652 | Zyathoxylum schinifolium | South Korea | H.D. Shin | KF253437 | KF252958 | KF252485 | KF251993 | KF251488 |
| Sep. paridis | CBS 109108 | Viola sp. | Austria | G.J.M. Verkley | KF253440 | KF252961 | KF252488 | KF251996 | KF251491 |
| CBS 109111 | Paris quadrifolia | Austria | G.J.M. Verkley | KF253438 | KF252959 | KF252486 | KF251994 | KF251489 | |
| Sep. passiflorae | CBS 102701 | Passiflora edulis | New Zealand | C.F. Hill | KF253442 | KF252963 | KF252490 | KF251998 | KF251493 |
| Sep. perillae | CBS 128655 | Perilla frutescens | South Korea | H.D. Shin | KF253444 | KF252965 | KF252491 | KF252000 | KF251495 |
| Sep. petroselini | CBS 182.44 | Petroselinum sativum | Netherlands | S.D. de Wit | KF253446 | KF252967 | KF252493 | KF252002 | KF251497 |
| Sep. phlogis | CBS 128663 | Phlox paniculata | South Korea | H.D. Shin | KF253448 | KF252969 | KF252495 | KF252004 | KF251499 |
| Sep. polygonorum | CBS 347.67 | Polygonum persicaria | Netherlands | H.A. van der Aa | KF253455 | KF252976 | KF252502 | KF252011 | KF251506 |
| CBS 109834 | Polygonum persicaria | Netherlands | G.J.M. Verkley | KF253453 | KF252974 | KF252500 | KF252009 | KF251504 | |
| Sep. posoniensis | CBS 128645 | Chrysosplenium japonicum | South Korea | H.D. Shin | KF253456 | KF252977 | KF252503 | KF252012 | KF251507 |
| Sep. protearum | CBS 177.77 | Fragaria sp. | New Zealand | H.J. Boesewinkel | KF253463 | KF252984 | KF252509 | KF252019 | KF251514 |
| CBS 390.59 | Ligustrum vulgare | Italy | M. Ribaldi | KF253467 | KF252987 | KF252513 | KF252023 | KF251518 | |
| CBS 566.88 | Hedera helix | France | H.A. van der Aa | KF253470 | KF252990 | KF252515 | KF252026 | KF251521 | |
| CBS 778.97 | Protea cynaroides | South Africa | L. Viljoen | KF253472 | KF252992 | KF252517 | KF252028 | KF251523 | |
| CBS 135477; CPC 19675 | Zantedeschia aethiopica | South Africa | P.W. Crous | KF253473 | KF252993 | KF252518 | KF252029 | KF251524 | |
| Sep. pseudonapelli | CBS 128664 | Aconitum pseudolaeve var. erectum | South Korea | H.D. Shin | KF253475 | KF252995 | KF252520 | KF252031 | KF251526 |
| Sep. putrida | CBS 109088 | Senecio nemorensis | Austria | G.J.M. Verkley | KF253477 | KF252997 | KF252522 | KF252033 | KF251528 |
| Sep. rumicum | CBS 503.76 | Rumex acetosa | France | H.A. van der Aa | KF253478 | KF252998 | KF252523 | KF252034 | KF251529 |
| Sep. saccardoi | CBS 128756 | Lysimachia vulgaris | South Korea | H.D. Shin | KF253479 | KF252999 | KF252524 | KF252035 | KF251530 |
| Sep. scabiosicola | CBS 102334 | Knautia arvensis | Netherlands | G.J.M. Verkley | KF253481 | KF253001 | KF252526 | KF252037 | KF251532 |
| CBS 102336 | Knautia arvensis | Netherlands | G.J.M. Verkley | KF253483 | KF253003 | KF252528 | KF252039 | KF251534 | |
| CBS 108981 | Knautia arvensis | Germany | G.J.M. Verkley | KF253484 | KF253004 | KF252529 | KF252040 | KF251535 | |
| CBS 109093 | Knautia dipsacifolia | Austria | G.J.M. Verkley | KF253487 | KF253007 | KF252532 | KF252043 | KF251538 | |
| Sep. senecionis | CBS 102366 | Senecio fluviatilis | Netherlands | G.J.M. Verkley | KF253492 | KF253012 | KF252538 | KF252049 | KF251544 |
| CBS 102381 | Senecio fluviatilis | Netherlands | G.J.M. Verkley | KF253493 | KF253013 | KF252539 | KF252050 | KF251545 | |
| Sep. siegesbeckiae | CBS 128659 | Siegesbeckia glabrescens | South Korea | H.D. Shin | KF253494 | KF253014 | KF252540 | KF252051 | KF251546 |
| CBS 128661 | Siegesbeckia pubescens | South Korea | H.D. Shin | KF253495 | KF253015 | KF252541 | KF252052 | KF251547 | |
| Sep. sii | CBS 102370 | Berula erecta | Netherlands | G.J.M. Verkley | KF253497 | KF253017 | KF252543 | KF252054 | KF251549 |
| Sep. sisyrinchii | CBS 112096 | Sysirinchium sp. | New Zealand | C.F. Hill | KF253499 | KF253019 | KF252545 | KF252056 | KF251551 |
| Septoria sp. | CBS 128650 | Taraxacum officinale | South Korea | H.D. Shin | KF253504 | KF253024 | KF252550 | KF252061 | KF251556 |
| CBS 128658 | Chrysosplenium japonicum | South Korea | H.D. Shin | KF253505 | KF253025 | KF252551 | KF252062 | KF251557 | |
| CBS 128757 | Sonchus asper | South Korea | H.D. Shin | KF253500 | KF253020 | KF252546 | KF252057 | KF251552 | |
| CBS 135472; CPC 19304 | Vigna unguiculata ssp. sesquipedalis | Austria | P.W. Crous | KF253506 | KF253026 | KF252552 | KF252063 | KF251558 | |
| CBS 135474; CPC 19485 | Conyza canadensis | Brazil | R.W. Barreto | KF253507 | KF253027 | KF252553 | KF252064 | KF251559 | |
| CBS 135478; CPC 19716 | Eucalyptus sp. | India | W. Gams | KF253188 | KF252722 | KF252240 | KF251738 | KF251235 | |
| CBS 135479; CPC 19793 | Syzygium cordatum | South Africa | P.W. Crous | – | KF253029 | KF252555 | KF252066 | KF251561 | |
| CPC 19976 | Feijoa sellowiana | Italy | G. Polizzi | KF253509 | KF253030 | – | KF252067 | KF251562 | |
| CPC 21105 | Cluvia sp. | South Africa | P.W. Crous | – | – | KF302396 | KF302408 | KF302402 | |
| CPC 23104 | – | Italy | E. van Agtmaal | KF253511 | KF253032 | KF252557 | KF252069 | KF251564 | |
| Sep. stachydis | CBS 347.58 | Aster canus | Germany | R. Schneider | KF253295 | KF252820 | KF252349 | KF251852 | KF251348 |
| CBS 102326 | Stachys sylvatica | Netherlands | G.J.M. Verkley | KF253514 | KF253035 | KF252560 | KF252072 | KF251567 | |
| CBS 109115 | Campanula glomerata | Austria | G.J.M. Verkley | KF253502 | KF253022 | KF252548 | KF252059 | KF251554 | |
| CBS 109127 | Stachys sylvatica | Austria | G.J.M. Verkley | KF253517 | KF253038 | KF252563 | KF252075 | KF251570 | |
| Sep. stellariae | CBS 102376 | Stellaria media | Netherlands | G.J.M. Verkley | KF253521 | KF253042 | KF252567 | KF252079 | KF251574 |
| “Sep.” steviae | CBS 120132 | Stevia rebaudiana | Japan | J. Ishiba | KF253191 | – | KF252243 | KF251741 | KF251238 |
| “Sep.” tanaceti | CBS 358.58 | Tanacetum vulgare | Germany | R. Schneider | KF253192 | – | KF252244 | KF251742 | KF251239 |
| Sep. taraxaci | CBS 567.75 | Taraxacum sp. | Armenia | H.A. van der Aa | KF253524 | KF253045 | KF252570 | KF252082 | KF251577 |
| Sep. tinctoriae | CBS 129154 | Serratula coronata | South Korea | H.D. Shin | KF253525 | KF253046 | KF252571 | KF252083 | KF251578 |
| Sep. tormentillae | CBS 128643 | Potentilla fragarioides | South Korea | H.D. Shin | KF253526 | KF253047 | KF252572 | KF252084 | KF251579 |
| CBS 128647 | Potentilla fragarioides | South Korea | H.D. Shin | KF253527 | KF253048 | KF252573 | KF252085 | KF251580 | |
| Sep. urticae | CBS 102316 | Glechoma hederacea | Netherlands | G.J.M. Verkley | KF253528 | KF253049 | KF252574 | KF252086 | KF251581 |
| CBS 102375 | Urtica dioica | Netherlands | G.J.M. Verkley | KF253530 | KF253051 | KF252576 | KF252088 | KF251583 | |
| Sep. verbascicola | CBS 102401 | Verbascum nigrum | Netherlands | G.J.M. Verkley | KF253531 | KF253052 | KF252577 | KF252089 | KF251584 |
| Sep. verbenae | CBS 113438 | Verbena officinalis | New Zealand | G.J.M. Verkley | KF253532 | KF253053 | KF252578 | KF252090 | KF251585 |
| Sep. villarsiae | CBS 514.78 | Nymphoides peltata | Netherlands | H.A. van der Aa | KF253534 | KF253055 | KF252580 | KF252092 | KF251587 |
| Sep. violae-palustris | CBS 128644 | Viola selkirkii | South Korea | H.D. Shin | KF253537 | KF253058 | KF252583 | KF252095 | KF251590 |
| CBS 128660 | Viola yedoensis | South Korea | H.D. Shin | KF253538 | KF253059 | KF252584 | KF252096 | KF251591 | |
| septoria-like sp. | CBS 134910; CPC 19500 | Tibouchina herbacea | Brazil | D.F. Parreira | KF302391 | KF302386 | KF302397 | KF302409 | KF302403 |
| CBS 135471; CPC 19294 | Corymbia gummifera | Australia | P.W. Crous | KF253193 | KF252725 | KF252245 | KF251743 | KF251240 | |
| CBS 135473; CPC 19311 | Phragmites sp. | USA | – | KF253194 | KF252726 | KF252246 | KF251744 | KF251241 | |
| CBS 135481; CPC 22154; S672 | Polygonatum sp. | Netherlands | U. Damm | – | – | KF252247 | KF251745 | KF251242 | |
| Septorioides pini-thunbergii | CBS 473.91 | Pinus thunbergii | Japan | S. Kaneko & Y. Zinno | – | KF252727 | KF252248 | KF251746 | KF251243 |
| Setophoma chromolaenae | CBS 135105; CPC 18553 | Chromolaena odorata | Brazil | R.W. Barreto | KF253195 | KF252728 | KF252249 | KF251747 | KF251244 |
| Setop. sacchari | CBS 333.39 | Saccharum officinarum | Brazil | A.A. Bitancourt | – | – | KF252250 | KF251748 | KF251245 |
| Setop. terrestris | CBS 335.29 | Allium sativum | USA | H.N. Hansen | KF253196 | KF252729 | KF252251 | KF251749 | KF251246 |
| CBS 335.87 | Allium cepa | Senegal | – | KF253197 | KF252730 | KF252252 | KF251750 | KF251247 | |
| CBS 377.52 | Allium cepa | – | R.H. Larson | KF253198 | KF252731 | KF252253 | KF251751 | KF251248 | |
| CBS 135470; CPC 18417 | Zea mays | South Africa | S. Lamprecht | KF253189 | KF252723 | KF252241 | KF251739 | KF251236 | |
| Setoseptoria phragmitis | CBS 114802 | Phragmites australis | Hong Kong | K.D. Hyde | KF253199 | KF252732 | KF252254 | KF251752 | KF251249 |
| CBS 114966 | Phragmites australis | Hong Kong | K.D. Hyde | KF253200 | KF252733 | KF252255 | KF251753 | KF251250 | |
| Sphaerulina abeliceae | CBS 128591 | Zelkova serrata | South Korea | H.D. Shin | KF253539 | – | KF252585 | KF252097 | KF251592 |
| Sph. aceris | CBS 687.94 | Acer pseudoplatanus | Netherlands | G.J.M. Verkley | KF253542 | KF253061 | KF252588 | KF252100 | KF251595 |
| Sph. amelanchier | CBS 102063 | Actinidia deliciosa | New Zealand | C.F. Hill | KF253581 | KF253096 | KF252627 | KF252140 | KF251635 |
| CBS 135110; MP8 | Amelanchier sp. | Netherlands | S.I.R. Videira | KF253543 | KF253062 | KF252589 | KF252101 | KF251596 | |
| CPC 23105; MP22 | Quercus sp. | Netherlands | S.I.R. Videira | KF253544 | KF253063 | KF252590 | KF252102 | KF251597 | |
| CPC 23106; MP7 | Castanea sp. | Netherlands | S.I.R. Videira | KF253545 | KF253064 | KF252591 | KF252103 | KF251598 | |
| CPC 23107; MP9 | Betula sp. | Netherlands | S.I.R. Videira | KF253583 | KF253098 | KF252626 | KF252139 | KF251634 | |
| Sph. azaleae | CBS 352.49 | Rhododendron sp. | Belgium | J. van Holder | KF253547 | KF253066 | KF252593 | KF252105 | KF251600 |
| CBS 128605 | Rhododendron sp. | South Korea | H.D. Shin | KF253546 | KF253065 | KF252592 | KF252104 | KF251599 | |
| Sph. berberidis | CBS 324.52 | Berberis vulgaris | Switzerland | E. Müller | KF253548 | KF253067 | KF252594 | KF252106 | KF251601 |
| Sph. betulae | CBS 116724 | Betula pubescens | Netherlands | S. Green | KF253549 | KF253068 | KF252595 | KF252107 | KF251602 |
| CBS 128600 | Betula platyphylla var. japonica | South Korea | H.D. Shin | KF253552 | KF253071 | KF252598 | KF252110 | KF251605 | |
| Sph. cercidis | CBS 501.50 | Cercis siliquastrum | Netherlands | G. van den Ende | KF253556 | KF253075 | KF252601 | KF252113 | KF251608 |
| CBS 118910 | Eucalyptus sp. | France | P.W. Crous | KF253553 | KF253072 | KF252602 | KF252114 | KF251609 | |
| CBS 128634 | Cercis siliquastrum | Argentina | H.D. Shin | KF253554 | KF253073 | KF252599 | KF252111 | KF251606 | |
| CBS 129151 | Cercis siliquastrum | Argentina | H.D. Shin | KF253555 | KF253074 | KF252600 | KF252112 | KF251607 | |
| Sph. cornicola | CBS 102324 | Cornus sp. | Netherlands | A. van Iperen | KF253557 | KF253076 | KF252603 | KF252115 | KF251610 |
| CBS 102332 | Cornus sp. | Netherlands | A. van Iperen | KF253558 | KF253077 | KF252604 | KF252116 | KF251611 | |
| Sph. frondicola | CBS 391.59 | Populus pyramidalis | Germany | R. Schneider | KF253572 | – | KF252617 | KF252130 | KF251625 |
| Sph. gei | CBS 102318 | Geum urbanum | Netherlands | G.J.M. Verkley | KF253560 | KF253079 | KF252605 | KF252118 | KF251613 |
| CBS 128632 | Geum japonicum | South Korea | H.D. Shin | KF253562 | KF253081 | KF252607 | KF252120 | KF251615 | |
| Sph. hyperici | CBS 102313 | Hypericum sp. | Netherlands | G.J.M. Verkley | KF253563 | KF253082 | KF252608 | KF252121 | KF251616 |
| Sph. menispermi | CBS 128666 | Menispermum dauricum | South Korea | H.D. Shin | KF253564 | KF253083 | KF252609 | KF252122 | KF251617 |
| CBS 128761 | Menispermum dauricum | South Korea | H.D. Shin | KF253565 | KF253084 | KF252610 | KF252123 | KF251618 | |
| Sph. musiva | CBS 130570 | Populus deltoides | Canada | J. LeBoldus | JX901725 | JX902304 | JX902058 | JX901935 | JX901812 |
| Sph. myriadea | CBS 124646 | Quercus dentata | Japan | K. Tanaka | KF253201 | KF252734 | KF252256 | KF251754 | KF251251 |
| Sph. oxyacanthae | CBS 135098; S654 | Crataegus sp. | Netherlands | W. Quaedvlieg | KF253202 | KF252735 | KF252257 | KF251755 | KF251252 |
| Sph. patriniae | CBS 128653 | Patrinia scabiosaefolia | South Korea | H.D. Shin | KF253570 | KF253087 | KF252615 | KF252128 | KF251623 |
| Sph. populicola | CBS 100042 | Populus trichocarpa | USA | G. Newcombe | KF253573 | – | KF252618 | KF252131 | KF251626 |
| Sph. pseudovirgaureae | CBS 135109; S669 | Solidago gigantea | Netherlands | S.I.R. Videira | KF253203 | KF252736 | KF252258 | KF251756 | KF251253 |
| Sph. quercicola | CBS 663.94 | Quercus robur | Netherlands | H.A. van der Aa | KF253577 | KF253092 | KF252622 | KF252135 | KF251630 |
| CBS 109009 | Quercus rubra | Netherlands | G.J.M. Verkley | KF253574 | KF253089 | KF252619 | KF252132 | KF251627 | |
| CBS 115016 | Quercus robur | Netherlands | G.J.M. Verkley | KF253575 | KF253090 | KF252620 | KF252133 | KF251628 | |
| CBS 115136 | Quercus robur | Netherlands | G.J.M. Verkley | KF253576 | KF253091 | KF252621 | KF252134 | KF251629 | |
| CBS 115137 | Quercus robur | Netherlands | G.J.M. Verkley | KF302390 | KF302385 | KF302394 | KF302406 | KF302400 | |
| Sph. socia | CBS 355.58 | Rosa sp. | – | – | KF253579 | KF253094 | KF252624 | KF252137 | KF251632 |
| CBS 357.58 | Chrysanthemum leucanthemum | Germany | R. Schneider | KF253580 | KF253095 | KF252625 | KF252138 | KF251633 | |
| Sph. tirolensis | CBS 109017 | Rubus idaeus | Austria | G.J.M. Verkley | KF253584 | KF253099 | KF252629 | KF252142 | KF251637 |
| CBS 109018 | Rubus idaeus | Austria | G.J.M. Verkley | KF253585 | KF253100 | KF252630 | KF252143 | KF251638 | |
| Sph. viciae | CBS 131898 | Vicia amurense | South Korea | H.D. Shin | KF253586 | KF253101 | KF252631 | KF252144 | KF251639 |
| Sph. westendorpii | CBS 117478 | Rubus fruticosus | Netherlands | G.J.M. Verkley | KF253589 | KF253104 | KF252634 | KF252147 | KF251642 |
| Stagonospora cf. paludosa | CBS 130005 | Carex sp. | Russia | – | KF253204 | KF252737 | KF252259 | KF251757 | KF251254 |
| Sta. duoseptata | CBS 135093; S618 | Carex acutiformis | Netherlands | W. Quaedvlieg | KF253205 | KF252738 | KF252260 | KF251758 | KF251255 |
| “Sta.” foliicola | CBS 110111 | Phalaris arundinacea | USA | N. O’Neil | KF253206 | KF252739 | KF252261 | KF251759 | KF251256 |
| Sta. paludosa | CBS 135088; S601 | Carex acutiformis | Netherlands | W. Quaedvlieg | KF253207 | KF252740 | KF252262 | KF251760 | KF251257 |
| Sta. perfecta | CBS 135099; S656 | Carex acutiformis | Netherlands | W. Quaedvlieg | KF253208 | – | KF252263 | KF251761 | KF251258 |
| Sta. pseudocaricis | CBS 135132; S610 | Carex acutiformis | France | A. Gardiennet | KF253210 | KF252742 | KF252265 | KF251763 | KF251260 |
| CBS 135414; S609 | Carex acutiformis | France | A. Gardiennet | – | KF302383 | KF302395 | KF302407 | KF302401 | |
| Sta. pseudovitensis | CBS 135094; S620 | Carex acutiformis | Netherlands | W. Quaedvlieg | KF253211 | KF252743 | KF252266 | KF251764 | KF251261 |
| S602 | Carex acutiformis | Netherlands | W. Quaedvlieg | KF253212 | KF252744 | KF252267 | KF251765 | KF251262 | |
| Stagonospora sp. | CBS 135096; 652 | Carex acutiformis | France | A. Gardiennet | – | – | KF252268 | KF251766 | KF251263 |
| Sta. uniseptata | CBS 135090; S611 | Carex acutiformis | Netherlands | W. Quaedvlieg | – | KF252745 | KF252269 | KF251767 | KF251264 |
| CPC 22150; S608 | Carex acutiformis | Netherlands | W. Quaedvlieg | KF253214 | KF252747 | KF252271 | KF251769 | KF251266 | |
| CPC 22151; S607 | Carex acutiformis | Netherlands | W. Quaedvlieg | KF253213 | KF252746 | KF252270 | KF251768 | KF251265 | |
| stagonospora-like sp. | CBS 516.74 | Triticum aestivum | Brazil | Y.R. Mehta | KF253215 | KF252748 | KF252272 | KF251770 | KF251267 |
| CBS 135482; CPC 22155; S526 | Poa sp. | Netherlands | W. Quaedvlieg | KF253216 | KF252749 | KF252273 | KF251771 | KF251268 | |
| CBS 135483; CPC 22157; S617 | Carex acutiformis | Netherlands | W. Quaedvlieg | KF253217 | KF252750 | KF252274 | KF251772 | KF251269 | |
| S619 | Carex acutiformis | Netherlands | W. Quaedvlieg | KF253218 | KF252751 | KF252275 | KF251773 | KF251270 | |
| Stromatoseptoria castaneicola | CBS 102322 | Castanea sativa | Netherlands | G.J.M. Verkley | KF253219 | KF252752 | KF252276 | KF251774 | KF251271 |
| CBS 102377 | Castanea sativa | Netherlands | G.J.M. Verkley | KF253220 | KF252753 | KF252277 | KF251775 | KF251272 | |
| Teratosphaeria juvenalis | CBS 111149 | Eucalyptus cladocalyx | South Africa | P.W. Crous | KF253221 | KF252754 | KF252278 | KF251776 | KF251273 |
| Ter. molleriana | CBS 111164 | Eucalyptus globulus | Portugal | M.J. Wingfield | KF253222 | KF252755 | KF252279 | KF251777 | KF251274 |
| Ter. parva | CBS 119901 | Eucalyptus globulus | Ethiopia | A. Gezahgne | KF253223 | KF252756 | KF252280 | KF251778 | KF251275 |
| Ter. pseudoeucalypti | CBS 124577 | Eucalyptus grandis × E. camaldulensis | Australia | V. Andjic | KF253224 | KF252757 | KF252281 | KF251779 | KF251276 |
| Ter. suberosa | CPC 13106 | Eucalyptus dunnii | Australia | A.J. Carnegie | KF253183 | – | KF252232 | KF251730 | KF251227 |
| Ter. toledana | CBS 113313 | Eucalyptus sp. | Spain | P.W. Crous & G. Bills | KF253225 | KF252758 | KF252282 | KF251780 | KF251277 |
| Vrystaatia aloeicola | CBS 135107; CPC 20617 | Aloe maculata | South Africa | P.W. Crous & W.J. Swart | – | KF252759 | KF252283 | KF251781 | KF251278 |
| Xenobotryosphaeria calamagrostidis | CBS 303.71 | Calamagrostis sp. | Italy | G.A. Hedjaroude | KF253226 | KF252760 | KF252284 | KF251782 | KF251279 |
| Xenoseptoria neosaccardoi | CBS 120.43 | Cyclamen persicum | Netherlands | Roodenburg | KF253227 | KF252761 | KF252285 | KF251783 | KF251280 |
| CBS 128665 | Lysimachia vulgaris var. davurica | South Korea | H.D. Shin | KF253228 | KF252762 | KF252286 | KF251784 | KF251281 | |
| Zasmidium anthuriicola | CBS 118742 | Anthurium sp. | Thailand | C.F. Hill | KF253229 | KF252763 | KF252287 | KF251785 | FJ839626 |
| Zas. citri | CPC 13467 | Eucalyptus sp. | Thailand | W. Himaman | KF253182 | KF252714 | KF252231 | KF251729 | KF251226 |
| Zas. lonicericola | CBS 125008 | Lonicera japonica | South Korea | H.D. Shin | KF253231 | KF252765 | KF252289 | KF251787 | KF251283 |
| Zas. nocoxi | CBS 125009 | Twig debris | USA | P.W. Crous | KF253232 | KF252766 | KF252290 | KF251788 | KF251284 |
| Zas. scaevolicola | CBS 127009 | Scaevola taccada | Australia | R.G. Shivas & P.W Crous | KF253233 | KF252767 | KF252291 | KF251789 | KF251285 |
| Zymoseptoria brevis | CBS 128853 | Phalaris minor | Iran | – | JQ739777 | JF700968 | JF700799 | JQ739833 | JF700867 |
| CPC 18109 | Phalaris paradoxa | Iran | – | JQ739779 | JF700970 | JF700801 | JQ739835 | JF700869 | |
| CPC 18112 | Phalaris paradoxa | Iran | – | JQ739782 | JF700973 | JF700804 | JQ739838 | JF700872 | |
| Zym. halophila | CBS 128854; CPC 18105 | Hordeum glaucum | Iran | M. Razavi | KF253592 | – | JF700808 | KF252150 | KF251645 |
| Zym. passerinii | CBS 120384 | Hordeum vulgare | USA | S. Ware | JQ739788 | JF700878 | JF700979 | JQ739844 | JF700810 |
| CBS 120385 | Hordeum vulgare | USA | S. Ware | JQ739789 | JF700980 | JF700811 | JQ739845 | JF700879 | |
| Zym. pseudotritici | CBS 130976 | Dactylis glomerata | Iran | M. Javan-Nikkhah | JQ739772 | JN982484 | JN982482 | JQ739828 | JN982480 |
| Zym. tritici | CPC 18117 | Avena sp. | Iran | – | JQ739801 | JF700986 | JF700817 | JQ739857 | JF700885 |
CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at CBS; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, U.K.; MP: Working collection of Sandra Videira; S: Working collection of William Quaedvlieg.
Btub: β-tubulin; EF-1α: Translation elongation factor 1-alpha; ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: 28S large subunit of the nrRNA gene; RPB2: RNA polymerase II second largest subunit.
MATERIALS AND METHODS
Isolates
Symptomatic leaves were incubated in moist chambers for up to 1 wk to enhance sporulation before single conidial colonies were established on 2 % malt extract agar (MEA) (Crous et al. 2009d). Leaf spots bearing ascomata were soaked in water for approximately 2 h, after which they were attached to the inner surface of Petri dish lids over plates containing MEA. Ascospore germination patterns were examined after 24 h, and single ascospore cultures established as described previously (Crous et al. 1991, Crous 1998). Colonies were sub-cultured onto synthetic nutrient-poor agar (SNA) containing sterile Hordeum vulgare (barley) and Urtica dioica (stinging nettle) stems, potato-dextrose agar (PDA), oatmeal agar (OA), and MEA (Crous et al. 2009d), and incubated at 25 °C under continuous near-ultraviolet light to promote sporulation. Isolates were also obtained from the culture collections of the CBS-KNAW Fungal Biodiversity Centre (CBS) in Utrecht, and the working collection of Pedro Crous (CPC). Reference strains were deposited CBS (Table 1).
DNA extraction, amplification and sequencing
Genomic DNA was extracted from fungal mycelium growing on MEA, using the UltraClean® Microbial DNA Isolation Kit (Mo Bio Laboratories, Inc., Solana Beach, CA, USA). Strains (Table 1) were screened for five loci (β-tubulin (Btub), internal transcribed spacer (ITS), Translation elongation factor 1-alpha (EF-1α) 28S nrDNA (LSU) and RNA polymerase II second largest subunit (RPB2) using the primer sets listed in Table 2. The PCR amplifications were performed in a total volume of 12.5 μL solution containing 10-20 ng of template DNA, 1 × PCR buffer, 0.7 μL DMSO (99.9 %), 2 mM MgCl2, 0.4 μM of each primer, 25 μM of each dNTP and 1.0 U Taq DNA polymerase (GoTaq, Promega). PCR amplification conditions were set as follows: an initial denaturation temperature of 96 °C for 2 min, followed by 40 cycles of denaturation temperature of 96 °C for 45 s, primer annealing at the temperature stipulated in Table 2, primer extension at 72 °C for 90 s and a final extension step at 72 °C for 2 min. The resulting fragments were sequenced using the PCR primers together with a BigDye Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems, Foster City, CA). Sequencing reactions were performed as described by Cheewangkoon et al. (2008). All novel sequences were deposited in NCBI’s GenBank database and alignments and phylogenetic trees in TreeBASE.
Table 2.
Primer combinations used during this study for generic amplification and sequencing.
| locus | Primer | Primer sequence 5’ to 3’ |
Annealing temperature (°C) |
Orientation | Reference |
|---|---|---|---|---|---|
| Translation elongation factor-1α | EF1-728F | CATCGAGAAGTTCGAGAAGG | 52 | Forward | Carbone & Kohn (1999) |
| EF-2 | GGARGTACCAGTSATCATGTT | 52 | Reverse | O’Donnell et al. (1998) | |
| β-tubulin | T1 | AACATGCGTGAGATTGTAAGT | 52 | Forward | O’Donnell & Cigelnik (1997) |
| β-Sandy-R | GCRCGNGGVACRTACTTGTT | 52 | Reverse | Stukenbrock et al. (2012) | |
| RNA polymerase II second largest subunit | fRPB2-5F | GAYGAYMGWGATCAYTTYGG | 49 | Forward | Liu et al. (1999) |
| fRPB2-414R | ACMANNCCCCARTGNGWRTTRTG | 49 | Reverse | Quaedvlieg et al. (2011) | |
| LSU | LSU1Fd | GRATCAGGTAGGRATACCCG | 52 | Forward | Crous et al. (2009a) |
| LR5 | TCCTGAGGGAAACTTCG | 52 | Reverse | Vilgalys & Hester (1990) | |
| ITS | ITS5 | GGAAGTAAAAGTCGTAACAAGG | 52 | Forward | White et al. (1990) |
| ITS4 | TCCTCCGCTTATTGATATGC | 52 | Reverse | White et al. (1990) |
Phylogenetic analyses
A basic alignment of the obtained sequence data was first done using MAFFT v. 7 [(http://mafft.cbrc.jp/alignment/server/index.html) (Katoh et al. 2002)] and if necessary, manually improved in BioEdit v. 7.0.5.2 (Hall 1999). To check the congruency of the RPB2 and LSU dataset, a 70 % neighbour-joining (NJ) reciprocal bootstrap method with maximum likelihood distance was performed (Mason-Gamer & Kellogg 1996, Lombard et al. 2010). Bayesian analyses (critical value for the topological convergence diagnostic set to 0.01) were performed on the concatenated loci using MrBayes v. 3.2.1 (Huelsenbeck & Ronquist 2001) as described by Crous et al. (2006) using nucleotide substitution models that were selected using MrModeltest v.2.3 (Table 3) (Nylander 2004). In order to keep the trees manageable for publication, two separate Bayesian trees were run. The first tree was run with all the Septoria and septoria-like isolates that either belonged to, or where more closely related to the Mycosphaerellaceae (Fig. 1) while the second tree contained all the septoria-like isolates either belonging to, or being more closely related to the Phaeosphaeriaceae (Fig. 2). Parastagonospora nodorum (CBS 259.49) was used as outgroup for the Mycosphaerellaceae dataset, while Dothistroma pini (CBS 121005) was used as outgroup for the Phaeosphaeriaceae dataset. As the novel genera and species described in this study were already clearly distinquishable in the LSU/RPB2 trees, the ITS, EF-1α and Btub sequence data of these isolates were deposited in GenBank without their subsequent trees being published in this paper.
Table 3.
Amplification success, phylogenetic data and the substitution models used in the phylogenetic analysis, per locus.
| Locus | RPB2 | LSU |
|---|---|---|
| Amplification succes (%) | 99.20 % | 100 % |
| Number of characters | 327 | 792 |
| Unique site patterns | 197 | 216 |
| Substitution model used | GTR-I-gamma |
GTR-I-gamma |
| Number of generations (1000×) | 2575 | |
| Total number of trees (n) | 5152 | |
| Sampled trees (n) | 3864 | |
Fig. 1.
A Bayesian 50 % majority rule RPB2/LSU consensus tree containing all Septoria and septoria-like taxa available at the CBS, which cluster in or near the Mycosphaerellaceae. Bayesian posterior probabilities support values for the respective nodes are displayed in the tree. A stop rule (set to 0.01) for the critical value for the topological convergence diagnostic was used for the Bayesian analysis. The tree was rooted to Phaeosphaeria nodorum (CBS 259.49). The scalebar indicates 0.1 expected changes per site.
Fig. 2.
A Bayesian 50 % majority rule RPB2/LSU consensus tree containing all Septoria and septoria-like taxa available at the CBS, which cluster in or near the Phaeosphaeriaceae. Bayesian posterior probabilities support values for the respective nodes are displayed in the tree. A stop rule (set to 0.01) for the critical value for the topological convergence diagnostic was used for the Bayesian analysis. The tree was rooted to Dothistroma pini (CBS 121005). The scalebar indicates 0.01 expected changes per site.
Taxonomy
Taxonomic descriptions were based on isolates sporulating in culture. Diseased leaf tissue was viewed under a Zeiss V20 Discovery stereo-microscope, while a Zeiss Axio Imager 2 light microscope with differential interference contrast (DIC) illumination and an AxioCam MRc5 camera with Zen software was used to capture morphological structures. Adobe Photoshop CS3 was used for the final editing of acquired images and photographic preparations. For measurements, 30-50 replicates of all relevant morphological features were made at ×1000 magnification. Colony characters and pigment production were noted after 2-4 wk of growth on MEA, PDA and OA (Crous et al. 2009d) incubated at 25 °C in the dark. Colony colours (surface and reverse) were rated according to the colour charts of Rayner (1970).
RESULTS
DNA sequencing and phylogenetic analysis
The RPB2 and LSU sequence datasets did not show any conficts in both the Mycosphaerellaceae and Phaeosphaeriaceae tree topologies for the 70 % reciprocal bootstrap trees, allowing us to combine them in the multigene analyses. For the Mycosphaerellaceae tree, the gene boundaries were: 1-327 bp for RPB2 and 332-1120 bp for LSU. For the Phaeosphaeriaceae tree (Fig. 2), the gene boundaries were 1-777 bp for LSU and 782-1108 bp for RPB2. During the generation of the Mycosphaerellaceae tree (Fig. 1), a total of 57 048 trees were sampled out of the generated 76 062 trees (75 %). During the generation of the Phaeosphaeriaceae tree (Fig. 2), a total of 2844 trees were sampled out of the generated 3792 trees (75 %).
Taxonomy
A total of 347 isolates representing 170 species were subjected to DNA analysis and morphological comparison. Phylogenetic analyses based on the LSU and RPB2 genes resolved a total of 47 clades of which 26 contained species belonging to the Septoria (-like) complex. These 47 resolved clades belong to a multitude of different families within the Dothidiomycetes ranging from the Mycosphaerellaceae in the Capnodiales to the Lentitheciaceae in the Pleosporales. It is still unclear within the Dothidiomycetes where the phylogenetic family borders are located, or even how many phylogenetically substainable families there actually are. The family annotation in the phylogenetic trees (Figs 1, 2) is therefore based on the closest LSU neighbour that was available in GenBank, with clades treated as incertae sedis if no closer relationship than 97 % could be found.
Septoria and septoria-like genera
In addition to Septoria s. str., numerous septoria-like genera (pycnidial/acervular/stromatic conidioma with filiform conidia) have since been described. Although the majority of these have no ex-type culture available for DNA analysis, many have type material deposited in herbaria, which were available for morphological examination. A summary of these genera is provided below.
Pycnidial forms
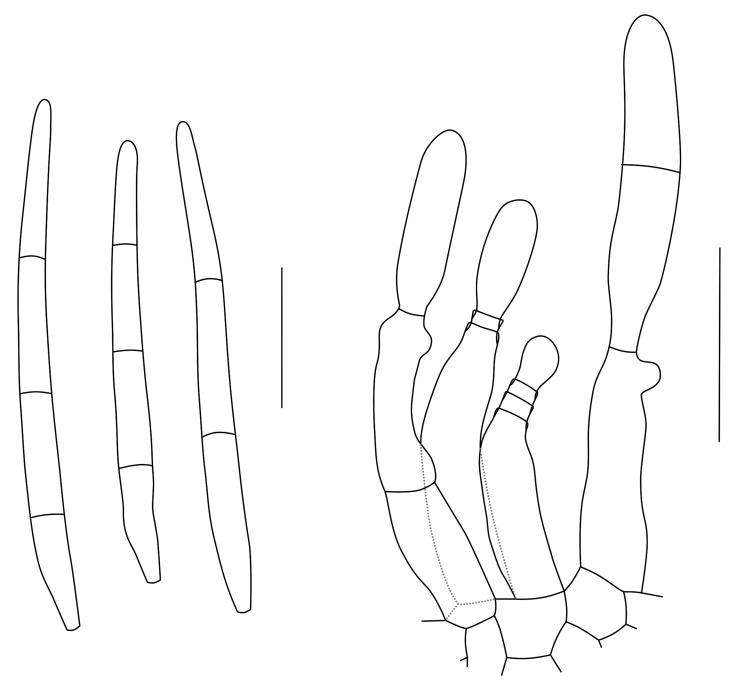
Cytostagonospora Bubák, Ann. Mycol. 14: 150. 1916. Fig. 3.
Fig. 3.
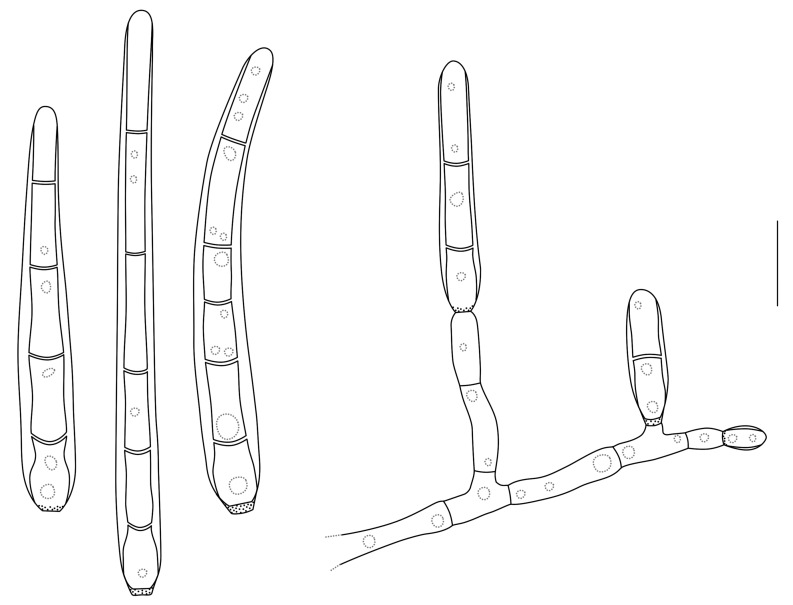
Conidia and conidiogenous cells of Cytostagonospora photiniicola (redrawn from Sutton 1980). Scale bar = 10 μm.
Mycelium immersed, dark brown, branched, septate. Conidiomata pycnidial, amphigenous, separate, globose, dark brown to black, immersed, unilocular, thick-walled, clypeate; walls of dark brown, thick-walled textura angularis to textura globulosa, becoming hyaline towards the conidiogenous region, extending in the upper part to become a circular clypeus of similar thickness to the wall. Ostiole central, circular, papillate to shortly rostrate, depressed, situated immersed within the clypeus. Conidiophores reduced to conidiogenous cells. Conidiogenous cells holoblastic, determinate, discrete, lageniform, hyaline, smooth, formed from the inner cells of the pycnidial wall. Conidia hyaline, 0-2-euseptate, not constricted at septa, base truncate, apex obtuse, thin-walled, eguttulate, smooth, filiform, often curved (Sutton 1980).
Type species: C. photiniicola Bubák, Ann. Mycol. 14(3-4): 150. 1916.
Notes: Von Arx (1983) and Sutton (1980) disagreed about the link of Cytostagonospora to Septoria. Von Arx treated it as a synonym of Septoria, while Sutton retained it as a separate genus.
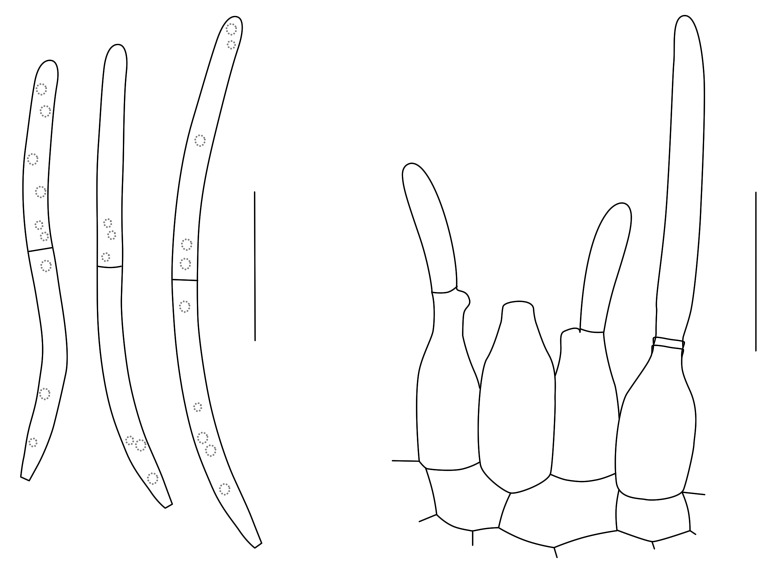
Dearnessia Bubák, Hedwigia 58: 25. 1916.
Mycelium hyaline to brown, branched, septate. Conidiomata pycnidial, amphigenous, separate, globose, immersed, brown; wall of thin-walled textura angularis. Ostiole central, circular, papillate. Setae ostiolar, approximately straight, unbranched, tapered towards apex, dark brown, smooth, thin-walled, septate. Conidiogenous cells holoblastic, determinate, discrete, doliiform to ampulliform, hyaline, smooth and formed from the inner layer of the pycnidial wall. Conidia cylindrical to irregular, hyaline, 1-multi-transversely euseptate, rarely with 1-2 longitudinal eusepta, continuous or constricted, often tapered at the apex, base truncate, thin-walled, smooth, guttulate or not (Sutton 1980).
Type species: D. apocyni Bubák, Hedwigia 58: 25. 1916.
Dearnessia apocyni Bubák, Hedwigia 58: 25. 1916. Figs 4, 5.
Fig. 4.
Conidia and conidiogenous cells of Dearnessia apocyni (F43227). Scale bars = 10 μm.
Fig. 5.

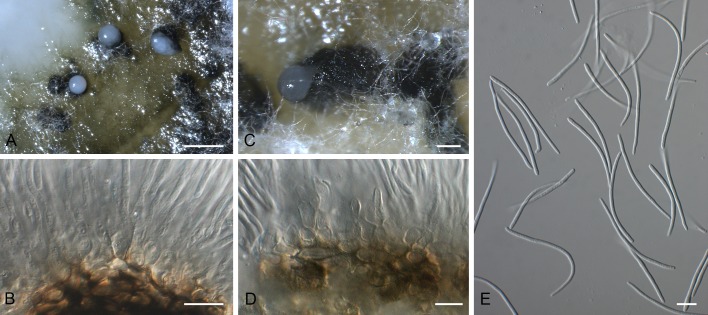
Dearnessia apocyni (F43227). A. Leaf spot. B, C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Leaf spots amphigenous, irregular, feathery to angular, dark brown, 3-6 mm diam, surrounded by a wide chlorotic zone up to 3 mm diam. Conidiomata epiphyllous, pycnidial, erumpent, up to 150 μm diam, with central ostiole; wall of 3-6 layers of brown textura angularis. Conidiogenous cells doliiform, globose to subcylindrical, hyaline, smooth, thin-walled, mode of proliferation obscure, 5-10 × 4-6 μm. Conidia hyaline, smooth, subcylindrical to obclavate, apex obtuse, base truncate to subobtuse, straight to irregular (lateral swellings?), 1-4-septate, 16-33 × 5-8 μm.
Specimen examined: Canada, Ontario, London, on leaves of Apocynum androsaemifolium (Apocynaceae), 11 Aug. 1910, J. Dearness, holotype F43227.
Notes: Because the specimen is in poor condition, no definite conclusion could be reached about its potential relationships. However, D. apocyni does appear septoria-like in general morphology.
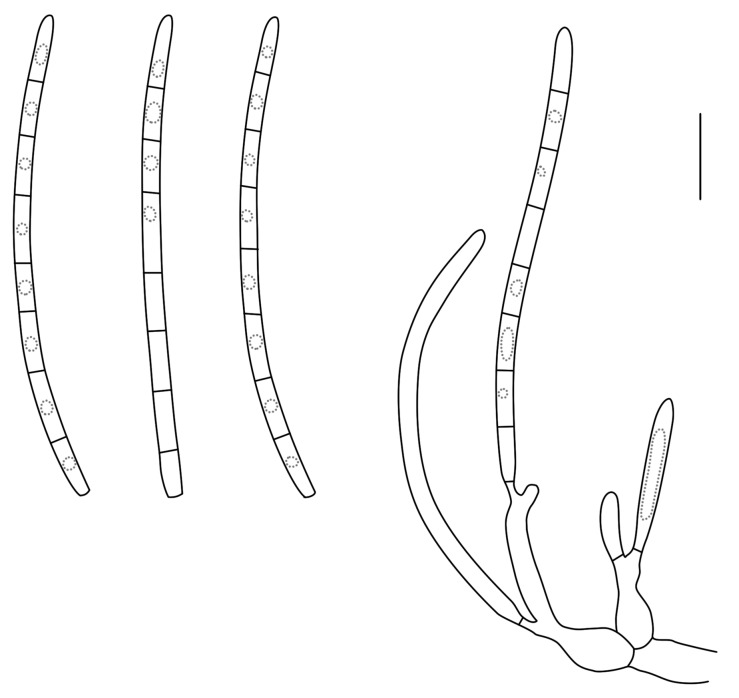
Jahniella Petr., Ann. Mycol. 18: 123. 1921. [1920]. Figs 6, 7.
Fig. 6.
Conidia and conidiogenous cells of Jahniella bohemica (redrawn from Sutton 1980). Scale bar = 10 μm.
Fig. 7.

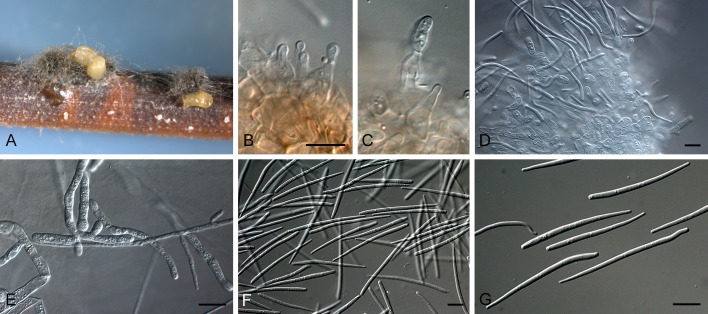
Jahniella bohemica [K(M) 180917]. A. Vertical section through conidioma. B. Ostiolar region with loose cells. C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Mycelium branched, immersed, septate, brown. Conidiomata pycnidial, superficial on epidermis, immersed, separate, globose, papillate, dark brown, thick-walled, sclerenchymatic; wall consisting of an outer layer of dark brown, thick-walled textura angularis, a middle layer of 8 cells thick, of hyaline to pale brown, thick-walled cells, and an inner layer of thin-walled, hyaline, irregular cells. Ostiole single, circular, with a distinct channel and hyaline periphysoid cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells holoblastic, determinate, discrete, hyaline, ampulliform, lining the wall of the pycnidium. Conidia straight or slightly curved, hyaline, thin-walled, smooth, 3-4-euseptate, eguttulate, truncate at the base, slightly tapered to the apex (Sutton 1980).
Type species: J. bohemica Petr., Ann. Mycol. 18(4-6): 123. 1921. [1920]
Specimen examined: Czech Republic, Bohemia, on stems of Scrophularia nodosa (Scrophulariaceae), 18 Mar. 1916, J. Jahn, holotype K(M) 180917, slides ex BPI.
Note: The specimen correlates closely with the description provided by Sutton (1980), except that the conidiomata are superficial, not immersed in the epidermis.
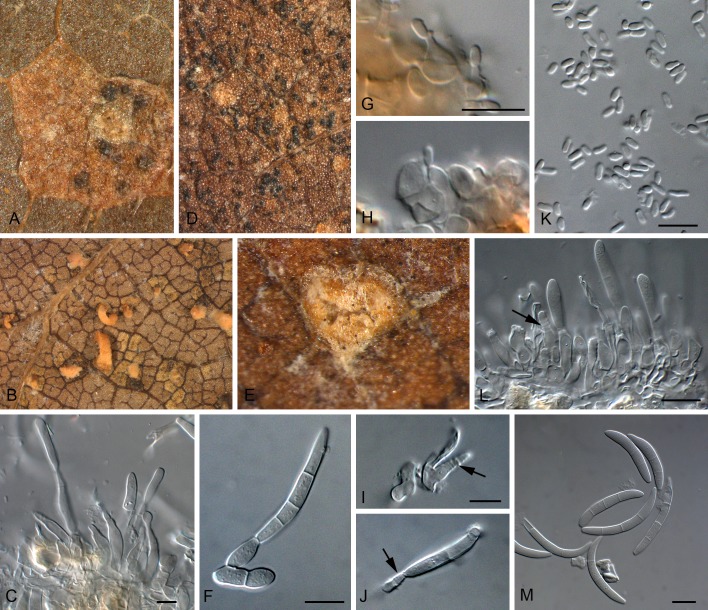
Megaloseptoria Naumov, Morbi Plantarum 14: 144. 1925. Figs 8, 9.
Fig. 8.
Conidia and conidiogenous cells of Megaloseptoria mirabilis (MA-Fungi 6978-1). Scale bars = 10 μm.
Fig. 9.

Megaloseptoria mirabilis (MA-Fungi 6978-1). A. Conidiomata on host tissue. B. Conidiogenous cells. C. Conidia. Scale bars = 10 μm.
Mycelium immersed, branched, septate, brown. Conidiomata pycnidial, separate, globose, slightly papillate, dark brown to black, superficial, sessile, often aggregated in groups, unilocular, thick-walled; wall of several cell layers of brown textura angularis, more darkly pigmented on the outside. Ostiole single, circular. Conidiophores hyaline, branched, septate (mainly at the base), smooth, straight or irregular, formed from the inner cells of the pycnidial wall. Conidiogenous cells enteroblastic, determinate, discrete or integrated, doliiform, ampulliform or irregularly cylindrical, hyaline, smooth, collarette evident, channel wide, periclinal thickening present. Conidia hyaline to pale brown with several transverse eusepta, continuous, tapered near the obtuse apex and truncate base, thin-walled, smooth, cylindrical, straight or slightly curved, often with 2 guttules in each cell (Sutton 1980).
Type species: M. mirabilis Naumov, Morbi Plant. Script. Sect. Phytopath. Hort. Bot. Prince. USSR 14: 144. 1925.
Megaloseptoria mirabilis Naumov, Morbi Plantarum 14: 144. 1925.
Conidiomata aggregated in a black stroma at the ends of branchlets, globose, black, smooth, with central ostiole, up to 600 μm diam, papillate; wall of 3-8 layers of dark brown textura angularis. Conidiogenous cells lining the cavity, subcylindrical to ampulliform, hyaline, smooth, 7-15 × 4-8 μm; proliferating percurrently near apex. Conidia solitary, scolecosporous, variously curved, subcylindrical, tapering in upper third to obtuse apex, base truncate, 3-4 μm diam, transversely 30-40-septate, (170-)200-250 × (5-)6(-7) μm.
Specimen examined: Switzerland, Zürich, St. Schnach., on branchlets of Pinus pungens var. glauba (Pinaceae), 10 July 1951, E. Müller, holotype MA-Fungi 6978-1.
Note: Megaloseptoria differs from Septoria in that the conidiomata are aggregated in a black stroma, which is not the case in Septoria s. str.
Phaeoseptoria Speg., Revista Mus. La Plata 15(2): 39. 1908.
Leaf spots angular-subcircular, 0.5-3 mm diam, becoming confluent. Conidiomata pycnidial, epiphyllous, subepidermal, black, 60-90 μm diam. Conidiogenesis cells hyaline, smooth, holoblastic (?). Conidia filiform, obclavate, smooth, 1-3-euseptate, medium brown, 30 × 3 μm (Saccardo & Trotter 1913, Walker et al. 1992, Crous et al. 1997).
Type species: P. papayae Speg., Revista Mus. La Plata 15(2): 39. 1908.
Notes: Phaeoseptoria papayae was originally described from leaf spots on Carica papaya collected in the São Paulo Botanical Garden, Brazil. Presently there are numerous clades that contain isolates conforming to this morphology, and this matter can only be resolved once fresh material of P. papayae has been recollected to clarify its phylogeny (see below).
Pseudoseptoria Speg., Ann. Mus. Nac. B. Aires, Ser. 3 13: 388. 1910.
Mycelium immersed, branched, septate, pale brown. Conidiomata pycnidial, solitary or linearly aggregated, immersed, brown, globose, unilocular; walls thin, of pale brown textura angularis. Ostiole distinct, central, circular. Conidiophores reduced to conidiogenous cells. Conidiogenous cells discrete, determinate or indeterminate, hyaline, smooth, ampulliform with a prominent cylindrical papilla with several percurrent proliferations at the apex. Conidia falcate, fusoid, acutely rounded at each end, hyaline, aseptate, guttulate, smooth, thin-walled (Sutton 1980).
Type species: P. donacicola Speg., Ann. Mus. Nac. B. Aires, Ser. 3 13: 388. 1910.
Note: Species of Pseudoseptoria are plant pathogenic to members of Poaceae.
Rhabdospora (Durieu & Mont. ex Sacc.) Sacc., Syll. Fung. (Abellini) 3: 578. 1884. nom. cons.
Basionym: Septoria sect. Rhabdospora Durieu & Mont., in Durieu, Expl. Sci. Alg. 1 (livr. 15): 592. 1849. [1846-1849].
Type species: R. oleandri Durieu & Mont., in Durieu, Expl. Sci. Alg. 1 (livr. 15): 593. 1849 [1846-1849].
Notes: Rhabdospora is a poorly defined genus, originally established to accommodate septoria-like species occurring on stems (Priest 2006). Of the 11 species treated by Sutton (1980), most are currently placed in Septoria. This genus is in need of revision pending the recollection of fresh material (on Nerium oleander from Algeria).
Sclerostagonospora Höhn., Hedwigia 59: 252. 1917.
Conidiomata pycnidial, immersed, separate, dark brown to black, globose, unilocular; walls thin, composed of thick-walled, dark brown textura angularis; ostiole single, circular, central, papillate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells holoblastic, determinate, discrete, hyaline, smooth, ampulliform to irregular, formed from the inner cells of the pycnidial wall. Conidia subcylindrical, pale brown, thin-walled, minutely verruculose, 3-euseptate, sometimes slightly constricted at the septa (from Sutton 1980).
Type species: S. heraclei (Sacc.) Höhn., Hedwigia 59: 252. 1917.
Note: Sclerostagonospora differs from Stagonospora in having pigmented conidia.
Septoria (Sacc.) Sacc., Syll. Fung 3: 474. 1884. nom. cons. Figs 10, 11.
Fig. 10.
Conidia and conidiogenous cells of Septoria cytisi (BPI USO 378994). Scale bars = 10 μm.
Fig. 11.

Septoria cytisi (BPI USO 378994). A. Leaf with symptoms. B. Close-up of leaf spot with conidiomata. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
= Septaria Fr., Novit. Fl. Svec. 5: 78. 1819. nom. rejic.
Mycelium slow-growing, pale brown, septate, immersed. Conidiomata pycnidial, immersed, separate or aggregated (but not confluent), globose, papillate (or not), brown, wall of thin, pale brown textura angularis, inner layer of flattened, hyaline textura angularis, frequently somewhat darker and more thick-walled around the ostiole. Ostiole single, circular, central. Conidiophores reduced to conidiogenous cells. Conidiogenous cells holoblastic, either determinate or indeterminate, proliferating sympodially and/or percurrently, hyaline, smooth, ampulliform, dolliform or lageniform to short cylindrical; scars unthickened. Conidia hyaline, multiseptate, filiform, smooth, continuous or constricted at septa. Sexual states are mycosphaerella-like.
Type species: S. cytisi Desm., Ann. Sci. Nat. Bot. 8: 24. 1847.
Specimen examined: Slovakia, Muehlthal near Bratislava (Pressburg), on leaves of Laburnum anagyroides (Leguminosae), 1884, J.A. Baeumler, BPI USO 378994.
Note: The ITS and LSU sequences of this specimen were published respectively under GenBank accession numbers JF700932 and JF700954.
Stagonospora (Sacc.) Sacc., Syll. Fung. (Abellini) 3: 445. 1884. nom. cons.
Basionym: Hendersonia subgen. Stagonospora Sacc., Michelia 2 (no. 6): 8. 1880.
Conidiomata pycnidial, immersed, unilocular, globose, separate, ostiolate; walls of dark brown, thick-walled textura angularis, and on the inside of hyaline, thin-walled, flattened cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells doliiform, hyaline, with several percurrent proliferations at the apex, formed from the inner cells of the pycnidial wall. Conidia hyaline, smooth to finely verruculose, 1-multiseptate, cylindrical or fusoid-ellipsoidal, straight or slightly curved, often guttulate, constricted or not at septa.
Type species: S. paludosa (Sacc. & Speg.) Sacc., Syll. Fung. (Abellini) 3: 453. 1884.
Stenocarpella Syd. & P. Syd., Ann. Mycol. 15(3-4): 258. 1917. Fig. 12.
Fig. 12.
Stenocarpella maydis (top) and S. macrospora (bottom) (redrawn from Sutton 1980). Scale bars = 10 μm.
Mycelium immersed, brown, branched, septate. Conidiomata pycnidial, separate or sometimes confluent, globose or elongated, dark brown, subepidermal, unilocular, thick-walled; walls composed of dark brown, thick-walled textura angularis. Ostiole single, circular, papillate, protruding. Conidiophores usually absent. Conidiogenous cells cylindrical, hyaline, determinate, discrete, phialidic, with collarette and minute periclinal thickening, lining the inner layer of the pycnidial wall. Conidia subcylindrical, straight or curved, fusiform, apex obtuse, base tapered, truncate, thick-walled, smooth-walled, granular, pale to medium brown, 0-3-euseptate. Beta conidia hyaline, scolecosporous, curved (Crous et al. 2006, Lamprecht et al. 2011).
Type species: S. zeae Syd. & P. Syd., Ann. Mycol. 15(3-4): 258. 1917. [= S. macrospora (Earle) B. Sutton]
Specimens examined: South Africa, KwaZulu-Natal, Hlabisa, rain-damaged Bt Zea mays hybrid (Poaceae), 2003-04 season, J. Rheeder (ex-epitype, CBS 117560 =MRC 8615, designated in Crous et al. 2006); KwaZulu-Natal, Zea mays kernels, 2005, P. Caldwell, CPC 11863 = CBS 128560.
Notes: Stenocarpella presently contains two species, S. macrospora and S. maydis, both causing “Diplodia ear rot of maize”. These two taxa were previously assigned to Diplodia and Macrodiplodia, respectively (Petrak & Sydow 1927, Sutton 1964). Several years later, Sutton re-examining these taxa and placed them in their own genus, Stenocarpella (Sutton 1977, 1980). Recent phylogenetic studies confirmed that these taxa indeed cluster by themselves within the Diaporthales (Crous et al. 2006, Lamprecht et al. 2011), supporting the decision of Sutton (1980).
Trichoseptoria Cavara, Atti Ist. Bot. Univ. Lab. Crittog. Pavia 2: 40. 1892.
Type species: T. alpei Cavara, Atti Ist. Bot. Univ. Lab. Crittog. Pavia 2: 40. 1892.
Notes: Not much is known about this septoria-like genus, except that it is distinguished from Septoria by having setae on its pycnidia with 1-2-septate, hyaline conidia. This genus is in further need of revision once fresh material has been recollected (Citrus vulgaris, Belgiojoso, Alps).
Zymoseptoria Quaedvlieg & Crous, Persoonia 26: 64. 2011.
Conidiomata pycnidial, semi-immersed to erumpent, dark brown to black, subglobose, with central ostiole; wall of 3-4 layers of brown textura angularis. Conidiophores hyaline and smooth, 1-2-septate, or reduced to conidiogenous cells, lining the inner cavity. Conidiogenous cells are tightly aggregated and ampulliform to doliiform or subcylindrical, phialidic with periclinal thickening, or with 2-3 inconspicuous, percurrent proliferations at the apex. Conidia (Type I) solitary, hyaline, smooth, guttulate, narrowly cylindrical to subulate, tapering towards acutely rounded apex, with bluntly rounded to truncate base, transversely euseptate; hila not thickened nor darkened. On OA and PDA media plates the aerial hyphae disarticulate into phragmospores (Type II conidia) that again give rise to Type I conidia via microcyclic conidiation; yeast-like growth and microcyclic conidiation (Type III conidia) common on agar media (Quaedvlieg et al. 2011).
Type species: Z. tritici (Desm.) Quaedvlieg & Crous, Persoonia 26: 67. 2011.
Notes: Zymoseptoria was split off from Septoria s. str. and redescribed by Quaedvlieg et al. (2011) based on LSU sequence data when said authors delimitated Septoria s. str. by sequencing the ITS and LSU sequences out of S. cytisi herbarium material. Phylogenetic analysis showed that Zymoseptoria species cluster within a distinct clade inside the Mycosphaerellaceae that is closely related to Ramularia, but located distant from Septoria s. str.
Acervular forms
Asteromidium Speg., Ann. Soc. cient. argent. 26(1): 66. 1888. Figs 13, 14.
Fig. 13.
Conidia and conidiogenous cells of Asteromidium imperspicuum (redrawn from Sutton 1980). Scale bar = 10 μm.
Fig. 14.
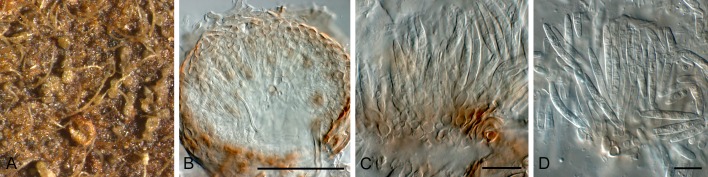
Asteromidium imperspicuum [K(M) 180228]. A. Conidiomata on host surface. B. Section through conidioma. C, D. Conidiogenous cells and conidia. Scale bars: B = 75 μm, all others = 10 μm.
Mycelium immersed, branched, septate, hyaline. Conidiomata acervular, subcuticular, separate or confluent, pulvinate to doliiform, at the base, composed of hyaline to pale brown, thin-walled textura angularis which extends laterally, finally with separate cells dispersed in a mucilaginous matrix to form the overlying wall; cuticle discoloured and occasionally pseudoparenchymatous, walls adjacent to the upper epidermal wall also discoloured; dehiscence irregular. Conidiogenous cells holoblastic, discrete, indeterminate, ± cylindrical, hyaline, smooth, with 1-2 sympodial proliferations, scars unthickened, flat, formed from the basal and lateral walls. Conidia cylindrical to fusoid, gently tapered at each end, apex obtuse, base truncate, thin-walled, guttulate to granular, hyaline, 3-septate (Sutton 1980).
Type species: A. imperspicuum Speg., Ann. Soc. cient. argent. 26(1): 66. 1888.
Specimen examined: Paraguay, on leaves of Sapindaceae, 1883, isotype K(M) 180228, ex B. Balansa Pl. du Paraguay No. 4085.
Notes: This genus has to be recollected (Sapindaceae, Paraguay) to allow for a molecular comparison to other existing genera in this complex. The morphology of the specimen examined correlates well with the description provided by Sutton (1980).
Ciferriella Petr., Ann. Mycol. 28(5/6): 409. 1930.
Type species: C. domingensis Petr. & Cif., Ann. Mycol. 28(5/6): 409. 1930.
= Pseudocercospora Speg., Anales Mus. Nac. Hist. Nat. B. Aires, Ser. 3, 20: 437. 1910.
Pseudocercospora domingensis (Petr. & Cif.) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804401. Figs 15, 16.
Fig. 15.
Conidia and conidiogenous cells of Pseudocercospora domingensis (NY No 01048475). Scale bars = 10 μm.
Fig. 16.

Pseudocercospora domingensis (NY No 01048475). A. Leaf spot. B, C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Basionym: Ciferriella domingensis Petr. & Cif., Ann. Mycol. 28(5/6): 409. 1930.
Leaf spots amphigenous, subcircular, medium brown with dark purple margin, 1.5-6 mm diam. Sporulation hypophyllous, fasciculate to sporodochial, brown, arising from a brown stroma, up to 50 μm diam. Conidiophores medium brown, smooth, subcylindrical, 0-2-septate, straight to once geniculate, 15-20 × 3-5 μm. Conidiogenous cells terminal, brown, smooth to finely verruculose, ampulliform to subcylindrical, proliferating sympodially or percurrently, tapering to a truncate apex, 2 μm diam, 10-15 × 3-4 μm. Conidia brown, smooth, straight to slightly curved, obclavate, apex subobtuse, base obconically truncate, 0-3-septate, 35-60 × 3-4 μm.
Specimen examined: Dominican Republic, on Vitex umbrosa (Lamiaceae), 26 May 1929, coll. R. Ciferri, det. F. Petrak, holotype ex N.Y. Bot. Gard. No 01048475.
Notes: The dimensions of the conidia and conidiophores correlate with the observations of Sutton (1980). However, the conidiomata are sporodochial to fasciculate, and not acervular. Ciferriella domingensis is a typical Pseudocercospora sensu Crous et al. (2013). Based on the species presently known from Vitex (Crous & Braun 2003), it appears to represent a distinct taxon, for which a new combination in Pseudocercospora is proposed.
Colletogloeum Petr., Sydowia 7: 368. 1953.
Mycelium immersed, branched, septate, hyaline to pale brown. Conidiomata acervular, epidermal to subepidermal, separate, occasionally confluent, composed of hyaline to pale brown, thin-walled textura angularis. Conidiophores hyaline to pale brown, sparsely branched, septate, smooth, cylindrical or slightly irregular, formed from the upper cells of the acervulus. Conidiogenous cells integrated or discrete, indeterminate, cylindrical or doliiform, with several percurrent proliferations at apex. Conidia hyaline to pale brown, 0-multiseptate, straight, curved or irregular, truncate at the base, obtuse at the apex, usually thin-walled, smooth, guttulate or not.
Type species: C. dalbergiae (S. Ahmad) Petr., Sydowia 7: 369. 1953. [= C. sissoo (Syd.) B. Sutton, Mycol. Pap. 97: 14. 1964.]
Notes: The exact taxonomic position of Colletogloeum was unclear for a long time as it included many species that appear to represent asexual morphs of Teratosphaeria. Crous et al. (2009a, b, c) used ITS sequence data from a specimen representative of C. sissoo (IMI 119162) to demonstate that the type of Colletogloeum clustered near the Pseudocercospora complex within the Mycosphaerellaceae.
Cylindrosporium Grev., Scott. crypt. fl. (Edinburgh) 1: pl. 27. 1822.
= Cylindrodochium Bonord., Handb. Allgem. mykol. (Stuttgart): 132. 1851.
Mycelium immersed, branched, septate, hyaline. Conidiomata acervular, white, slimy, subcuticular, separate or confluent, formed of pale brown to hyaline, thin-walled textura angularis; dehiscence irregular. Conidiophores hyaline, parallel, branched only at the base, 1-2-septate, smooth, formed from the upper pseudoparenchyma. Conidiogenous cells enteroblastic, phialidic, integrated, cylindrical, hyaline, smooth. Conidia straight or slightly curved, aseptate, cylindrical, thin-walled, smooth, hyaline, eguttulate (Sutton 1980).
Type species: C. concentricum Unger, Exanth. Pflanzen (Wien) 2: 9. 1833.
Notes: Sutton (1980), Von Arx (1983), Deighton (1987) and Braun (1990) could not agree on the taxonomic status of this genus, which is associated with light leaf spot of oil seed rape (sexual morph Pyrenopeziza brassicae). This genus is in need of revision, awaiting the recollection of fresh material of C. concentricum (on Pulmonaria officinalis, Germany).
Phloeospora Wallr., Fl. Crypt. Germ. (Norimbergae) 2: 176. 1833.
Mycelium immersed, septate, hyaline. Conidiomata acervular, subepidermal, circular, discrete or confluent, composed of hyaline to pale brown, thin-walled textura angularis; dehiscence irregular. Conidiophores reduced to conidiogenous cells or with one or two supporting cells, branched at base or not. Conidiogenous cells holoblastic, annellidic, occasionally also sympodial, discrete, indeterminate hyaline, smooth, cylindrical, with several apical inconspicuous annellations, formed from the upper cells of the acervuli. Conidia hyaline, septate, smooth, guttulate or not, cylindrical, curved, attenuated towards the apices, apex obtuse to subobtuse, base truncate, with minute marginal frill.
Type species: P. ulmi (Fr.) Wallr., Fl. Crypt. Germ. (Norimbergae) 2: 177. 1833.
Notes: Sexual morphs of Phloeospora have been linked to genera that resemble the concepts of Mycosphaerella, Didymella and Sphaerulina. Verkley & Priest (2000) already noted that this genus is heterogeneous and in need of revision. The phylogenetic analysis performed in this study confirmed that Phloeospora (based on P. ulmi) clusters close to, but separate from Septoria s. str. (Fig. 1).
Phloeosporella Höhn., Ann. Mycol. 22: 201. 1924. Fig. 17.
Fig. 17.
Conidia and conidiogenous cells of Phloeosporella ceanothi (redrawn from Sutton 1980). Scale bar = 10 μm.
Mycelium immersed, branched, septate, hyaline. Conidiomata acervular, subepidermal, ± circular, discrete, composed of hyaline to pale brown, thin-walled textura angularis. Conidiogenous cells holoblastic, sympodial, discrete, indeterminate, hyaline, smooth, lageniform to cylindrical, with 1-2 broad, flat unthickened apical scars, formed from the upper pseudoparenchyma. Conidia hyaline, 2-euseptate, thin-walled, smooth, guttulate, straight, curved or irregular, tapered gradually to an obtuse apex and abruptly to a truncate base (Sutton 1980).
Type species: P. ceanothi (Ellis & Everh.) Höhn., Ann. Mycol. 22(1-2): 201. 1924.
Notes: Not much is known of the sexual state of this genus, but P. padi has been linked to Blumeriella jaapii (Sutton 1980). A phylogenetic analysis performed on available isolates (unpubl. data) indicated that Phloeosporella is polyphyletic. However, as the type is not known from culture (on Ceanothus, California, USA), this matter could not be resolved.
Septogloeum Sacc., Michelia 2(6): 11. 1880.
Mycelium immersed, branched, septate, hyaline. Conidiomata acervular, epidermal to subepidermal, separate or confluent, formed of pale brown thin-walled pseudoparenchyma. Conidiophores short, stout, 1-2-septate, hyaline, smooth, branched at the base, formed from the upper pseudoparenchyma. Conidiogenous cells phialidic, discrete or integrated, determinate, cylindrical, doliiform to obpyriform, hyaline, smooth, with minute collarette and prominent periclinal thickening. Conidia hyaline, 1-3-euseptate, thin-walled, smooth, eguttulate, base truncate, apex obtuse, straight or curved, constricted, obovoid (Sutton 1980).
Type species: S. carthusianum (Sacc.) Sacc., Michelia 2(6): 11. 1880.
Notes: Although more than 120 species of Septogloeum have been described, the genus was reduced to just two species by Sutton & Pollack (1974). Sexual morphs have been linked to Pleuroceras in the Diaporthales (Monod 1983). The genus is in need of revision pending fresh collections.
Xenocylindrosporium Crous & Verkley, Fungal Planet 44. 2009.
Conidiomata immersed, black, opening by irregular rupture, acervuloid, up to 300 μm diam; wall consisting of 3-4 layers of pale brown textura angularis. Conidiophores hyaline, smooth, subcylindrical, branched, septate, or reduced to ampulliform conidiogenous cells. Conidiogenous cells hyaline, smooth, ampulliform to subcylindrical, terminal or lateral on septate conidiophores, monophialidic with minute periclinal thickening. Conidia solitary, hyaline, smooth, curved, widest in middle, tapering to acutely rounded apex and truncate base, 0 -1-septate.
Type species: X. kirstenboschense Crous & Verkley, Fungal Planet 44. 2009.
Stromatic forms
Dothistroma Hulbary, Bull. Ill. Nat. Hist. Surv. 21: 235. 1941.
Mycelium immersed, branched, septate, pale brown to hyaline. Conidiomata sometimes acervular, initially subepidermal later erumpent, composed of pale brown, thin-walled textura angularis, sometimes eustromatic, multilocular and of darker brown, thick-walled tissue. Stromata are strongly erumpent and finally pulvinate. Conidiogenous cells holoblastic, discrete, determinate, ampulliform, hyaline, smooth, non-proliferating, formed from the upper cells of stroma or from inner cells of the locular walls. Conidia hyaline, straight or curved, filiform, 1-5-euseptate, continuous, thin-walled and smooth (Barnes et al. 2004).
Type species: D. pini Hulbary, Bull. Ill. Nat. Hist. Surv. 21: 235. 1941.
Notes: Dothistroma sexual morphs are mycosphaerella-like (Evans 1984), and the two species of Dothistroma that have been subjected to DNA sequencing (D. septosporum and D. pini) cluster together in the “Dothistroma clade” as described by Crous et al. (2009a, c). Because of a lack of recognisable morphological characteristics, it is virtually impossible to discriminate between D. septosporum and D. pini without molecular tools (Barnes et al. 2004). Multiple morphological varieties of both D. septosporum and D. pini have been described based on differences in conidia length alone (e.g. D. septosporum var. keniense). However, controversy exists as to whether spore size represents an adequate characteristic to distinguish among these Dothistroma varieties, as since the introduction of molecular tools only D. septosporum and D. pini have been confirmed as distinct species.
Phlyctaeniella Petr., Ann. Mycol. 20: 323. 1922. Fig. 18.
Fig. 18.
Conidia and conidiogenous cells of Phlyctaeniella humuli (IMI 202260) (redrawn from Sutton 1980). Scale bar = 10 μm.
Mycelium immersed, branched, septate, hyaline. Conidiomata eustromatic, separate, immersed, pale brown, globose, unilocular, scarcely erumpent; side wall and base of several cell layers of hyaline, thin-walled textura angularis, above of larger pale brown tissue. Ostiole indistinct, and dehiscence by rupture of the upper wall. Conidiophores hyaline, smooth, septate, irregularly branched, especially at the base, formed from the inner cells of the stroma wall. Conidiogenous cells phialidic, integrated or discrete, determinate, hyaline, markedly tapered at the apices, smooth, with apical or lateral apertures, collarette minute, with periclinal thickening; only rarely becoming percurrent. Conidia hyaline, smooth, thin-walled, irregularly guttulate, filiform, straight, curved or irregular, multiseptate (Sutton 1980).
Type species: P. polonica Petr., Ann. Mycol. 20: 323. 1922.
Note: Fresh material needs to be collected of this taxon (on Aruncus silvestris, Austria), before its taxonomy can be resolved.
Septocyta Petr., Ann. Mycol. 25: 330. 1927. Figs 19, 20.
Fig. 19.
Conidia and conidiogenous cells of Septocyta ramealis (PDD 51271). Scale bars = 10 μm.
Fig. 20.

Septocyta ramealis (PDD 51271). A. Conidiomata on host tissue. B, C. Conidiogenous cells. D. Conidia. Scale bar = 10 μm.
Mycelium immersed, branched, septate, hyaline to pale brown. Conidiomata eustromatic, immersed, separate, erumpent, dark brown to black, finally opening widely, unilocular, multilocular or convoluted, thick-walled; wall of pale brown, thin-walled textura angularis except in the dehiscent region which is darker brown and more thick-walled. Ostiole absent, dehiscence by breakdown of the upper wall. Conidiogenous cells are holoblastic, sympodial with 1-3 apical, scarcely protruding, unthickened denticles, indeterminate, discrete, ampulliform to lageniform, hyaline, smooth, formed from the inner cells of the locular walls. Conidia hyaline, 1-3 euseptate, smooth, straight or slightly curved, acicular, apex obtuse, base truncate, often with minute guttules associated with septa (Sutton 1980).
Type species: S. ramealis (Roberge ex Desm.) Petr., Ann. Mycol. 25: 330. 1927.
Septocyta ramealis (Roberge ex Desm.) Petr., Ann. Mycol. 25: 330. 1927.
Conidiomata eustromatic to pycnidial, black, up to 160 μm diam, aggregated in clusters, erumpent through ruptures in epidermis, convulated; wall of 3-8 layers of brown textura angularis. Conidiophores lining the inner cavity, reduced to conidiogenous cells, or one or two supporting cells. Conidiogenous cells hyaline, smooth, ampulliform, proliferating sympodially and percurrently near apex, also with lateral denticle-like protrusions, 6-12 × 2.5-4 μm. Conidia hyaline, smooth, guttulate, (9-)20-30(-35) × 1.5(-2) μm, 1(-3)-septate, irregularly curved, subcylindrical, apex obtuse, base tapering slightly to truncate hilum, 0.5 μm diam.
Specimen examined: Germany, Brandenberg, on Rubus fructicosus (Rosaceae), 7 June 1923, coll. P. Sydow, det. H. Sydow, Sydow Mycoth. Germ. PDD 51271.
Notes: Septocyta ramealis, the type of Septocyta, has a long list of synonyms. The specimen examined here (PDD 51271), differs somewhat from the description provided by Sutton (1980), and appears to represent a species of Septoria s. str., as the mode of conidiogenesis is not that different. Presently there is a single ITS sequence labelled as S. ruborum available on GenBank (JN133277.1), placing it in the middle of Septoria s. str. As no type material of S. ramealis could be located, this matter remains unresolved.
Septopatella Petr., Ann. Mycol. 23: 128. 1925.
Mycelium immersed, branched, septate, hyaline to subhyaline. Conidiomata superficial, often subtended by a superficial, pale brown, septate, branched mycelium, pulvinate, separate to occasionally aggregated, dark brown to black, finally opening widely, cupulate; basal wall of small-celled, brown, thin-walled textura angularis, becoming textura porrecta as it merges into the periclinal walls; a hypostroma attaches the conidioma to the substrate; Ostiole absent. Conidiophores hyaline, septate, branched at the base, thin-walled, cylindrical, formed from the gelatinized basal wall of the conidioma. Conidiogenous cells holoblastic, sympodial, integrated, indeterminate, cylindrical, hyaline, smooth, produced as 2-3 branches from the apex of the conidiophores. Conidia hyaline, 3-4-euseptate, thin-walled, smooth, minutely guttulate, straight or curved, occasionally irregularly filiform (Dyko & Sutton 1979, Sutton 1980).
Type species: S. septata (Jaap) Petr., Ann. Mycol. 23: 129. 1925.
Note: Not much is known about this genus, and as no cultures of S. septata are presently available (on Pinus montana, Czech Republic) this matter cannot be resolved.
Stictosepta Petr., Sydowia 17: 230. 1964. [1963]. Fig. 21.
Fig. 21.
Conidia and conidiogenous cells of Stictosepta cupularis (redrawn from Sutton 1980). Scale bar = 10 μm.
Mycelium immersed, branched, septate, hyaline. Conidiomata eustromatic, immersed, globose to collabent, papillate, unilocular, often convoluted, hyaline; walls thick, of hyaline, thin-walled textura intricata. Ostiole central and circular, single, furfuraceous. Conidiophores hyaline, septate, branched, anastomosing, formed from the inner cells of the locular wall. Conidiogenous cells sympodial or synchronous, integrated, indeterminate, hyaline, thin-walled, with usually two small, unthickened, apical, slightly protuberant conidiogenous loci. Conidia hyaline, thin-walled, smooth, multiseptate, slightly constricted at the septa, each cell medianly guttulate, straight or curved, base truncate, apex obtuse (Sutton 1980).
Type species: S. cupularis Petr., Sydowia 17: 230. 1964. [1963].
Note: Not much is known about this genus, but as no isolate of S. cupularis is presently available (on stems of Fraxinus, Czech Republic), it will not be treated here.
Sexual morphs linked to Septoria
Several sexual genera have been linked to Septoria and allied genera in literature, but very few have been confirmed in culture. Most sexual states cluster in the Mycosphaerella complex.
Mycosphaerella Johanson, Öfvers. K. Svensk. Vetensk.-Akad. Förhandl. 41(no. 9): 163. 1884.
= Ramularia Unger, Exanth. Pflanzen (Wien): 119. 1833.
Mycelium immersed to superficial, septate, hyaline, branched. Caespituli usually whitish to greyish on host tissue. Conidiophores fasciculate to synnematal, rarely solitary, or forming small sporodochia, emerging through stomata, from inner hyphae or stromata; conidiophores straight, subcylindric to geniculate-sinuous, aseptate or septate, hyaline, occasionally branched, smooth, rarely rough. Conidiogenous cells integrated, terminal, polyblastic, elongating sympodially, apex more or less straight to geniculate-sinuous or strongly curved, cicatrized, conidial scars hardly to conspicuously thickened, but always darkened, refractive. Conidia solitary to catenate, sometimes in branched chains, 0-4(-multi)-septate, hyaline, ellipsoid-ovoid to cylindrical-fusoid, rarely filiform, occasionally constricted at the septa, smooth to verruculose-echinulate; hila distinct, slightly to conspicuously thickened, darkened, refractive; conidial secession schizolytic. Ascomata immersed to superficial, uniloculate, globose to subglobose with papillate, central, periphysate ostiole, dark brown to black, scattered or gregarious. Peridium of 3-6 layers of thin- to thick-walled textura angularis, dark brown to black. Hamathecium dissolves at maturity, and no stromatic tissue remains between the asci. Asci bitunicate, fissitunicate, 8-spored, cylindrical to cylindric-clavate, ovoid to ampulliform or saccate, sessile to subsessile, apex rounded with distinct or indistinct ocular chamber. Ascospores bi- to tri- or multiseriate, ellipsoid-fusoid to obclavate or subcylindrical, hyaline, medianly 1-septate, often constricted at the septum, smooth-walled, granular to guttulate, mostly lacking any sheath.
Type species: Ramularia pusilla Unger, Exanth. Pflanzen (Wien): 169. 1833.
Notes: Species of Ramularia (including the Mycosphaerella sexual morph) have evolved over a broad developmental and physiological adaptation range that includes endophytes, saprophytes and symbionts. However, for a major part Ramularia consists of a wide range of narrow host range, foliicolous plant pathogens which are the cause of significant economical losses in both temperate and tropical crops worldwide (Crous et al. 2001). Verkley et al. (2004) showed that Mycosphaerella s. str. (linked to M. punctiformis) was in fact restricted to species with Ramularia anamorphs, leaving many “Mycosphaerella” species to be disposed to other genera. In employing the one fungus = one name concept (Hawksworth et al. 2011, Wingfield et al. 2012), the choice is to use Ramularia over Mycosphaerella, as the former is monophyletic and recently monographed (Braun 1995, 1998), while Mycosphaerella is poly- and paraphyletic, and consists of more than 40 genera, many as yet untreated (Crous et al. 2009c)
Sphaerulina Sacc., Michelia 1(no. 4): 399. 1878.
Ascomata pseudothecial, immersed, subepidermal, erumpent at the top, single to clustered, globose, papillate. Ostiole central, with hyaline periphyses; wall of textura angularis, composed of 2-4 layers of brown cells. Hamathecium dissolving at maturity. Asci bitunicate, fissitunicate, clustered, cylindrical to obclavate, rounded at apex, with or without a shallow apical chamber, short-stipitate or sessile, with 8 biseriate to triseriate ascospores. Ascospores subcylindrical to fusiform, rounded at ends, slightly tapered, straight or slightly curved, 1-3-septate, with a primary septum nearly median, hyaline, smooth, without sheath or appendages.
Type species: Sphaerulina myriadea (DC.) Sacc., Michelia 1(no. 4): 399. 1878.
Notes: The genus Sphaerulina was chiefly separated from Mycosphaerella on the basis of ascospore septation (Crous et al. 2011). Sphaerulina myriadea, which occurs on hosts in the Fagaceae, appears to be a species complex. Results in this paper show that Sphaerulina myriadea clusters together with many septoria-like species in a clade that is distinct, but very closely related to Septoria s. str. The septoria-like species in this Sphaerulina clade were subsequently rediscribed in Sphaerulina. Species including ones with 1-septate ascospores and septoria-like asexual morphs are treated below and by Verkley et al. (2013).
Treatment of phylogenetic clades
Based on the phylogenetic data generated in this study, we were able to delineate several clades in the Septoria complex. Recognised clades, as well as novel species and genera, are described and discussed below. Taxa with descriptions that are freely available online in MycoBank or open access journals, are not repeated here.
Clade 1: Septoria
Description: See above.
Type species: S. cytisi Desm., Ann. Sci. Nat. Bot. 8: 24. 1847.
Septoria cf. agrimoniicola Bondartsev, Mater. mikol. obslêed. Ross. 2: 6. 1921.
Leaf spots on the upper leaf surface, distinct, scattered, brown with purplish margin, circular to angular, sometimes vein-limited, discrete lesions 2-4 mm diam, reaching 10 mm wide when confluent, finally the center becoming pale colored to nearly whitish; on the lower leaf surface similar but discoloured (Shin & Sameva 2004). On sterile Carex leaves on WA. Conidiomata pycnidial, separate but frequently aggregated and linked by brown stromatic tissue in a stroma; globose, black, exuding a creamy conidial mass via a central ostiole; conidiomata up to 350 μm diam; wall of 6-12 layers of dark brown, thick-walled textura angularis. Conidiophores reduced to conidiogenous cells or 1-2 supporting cells, hyaline, subcylindrical, lining the inner layer of conidioma. Conidiogenous cells hyaline, smooth, subcylindrical to ampulliform, 10-17 × 3-4 μm; proliferating sympodially but also percurrently near apex. Conidia hyaline, smooth, guttulate, filiform, apex subobtuse, base long obconically truncate, 1-4-septate, (20-)25-35(-40) × 1.5-2(-2.5) μm; microcyclic conidiation observed.
Culture characteristics: Colonies on PDA flat, undulate with sparse, white aerial mycelium, surface olivaceous-black, reverse olivaceous-black, after 14 d, 3.5 cm diam; on MEA with sparse white aerial mycelium, surface olivaceous-black, reverse olivaceous-black, after 14 d, 5 cm diam; on OA with sparse white aerial mycelium, surface olivaceous, reverse olivaceous, after 14 d, 3 cm diam.
Specimen examined: South Korea, Guri, on leaves of Agrimonia pilosa (Rosaceae), 11 Jul. 2009, H.D. Shin (CBS H-21279, culture CBS 128602 =KACC 44644 = SMKC 24292).
Notes: This fungus was first reported from Korea by Shin & Sameva (2002) as S. agrimoniicola, and fits well with the original description of this European taxon. However, fresh European collections and cultures are required for comparison, as S. agrimoniicola may well be restricted to Europe.
Septoria cf. stachydicola Hollós, Mathem. Természettud. Közlem. Magg. Tudom. Akad. 35(1): 60. 1926.
Leaf spots on the upper leaf surface distinct, scattered, brown with purplish margin, circular to angular, sometimes vein-limited, discrete lesions 2-4 mm diam, reaching 10 mm wide when confluent, finally the center becoming paler or nearly whitish; on the lower leaf surface similar but discoloured (Shin & Sameva 2004). On OA. Conidiomata solitary to aggregated, black, globose, becoming somewhat papillate, up to 250 μm diam, opening by means of central ostiole, up to 40 μm diam; wall of 6-8 layers of thick-walled, brown textura angularis; exuding a creamy conidial mass. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner wall layer, hyaline, discrete, ampulliform to lageniform, 4-10 × 3-5 μm, proliferating sympodially or percurrently with inconspicuous proliferations. Conidia filiform, curved or flexuous, rarely straight, (60-)65-75(-90) × 1.5-2(-3) μm, hyaline, guttulate, 4-7(-11)-septate, apex subobtuse, slightly tapering from basal septum to truncate base, 1.5-2 μm.
Culture characteristics: Colonies on PDA erumpent, with feathery margin, with sparse white aerial mycelium, surface greenish-black, reverse olivaceous-black, after 14 d, 2.5 cm diam; on MEA with sparse white aerial mycelium, surface cinnamon to olivaceous-black in the younger patches, reverse cinnamon to olivaceous-black in patches, after 14 d, 4 cm diam; on OA with sparse white aerial mycelium, surface greenish-black, reverse fuscous-black, after 14 d, 3 cm diam.
Specimen examined: South Korea, Incheon, leaf of Stachys riederi var. japonica (Lamiaceae), 14 Aug. 2008, H.D. Shin (CBS H-21278, culture CBS 128668 =KACC 44796 = SMKC 24663).
Note: The Korean collection was originally identified as Septoria stachydicola, which fits the original description provided for this taxon (Shin & Sameva 2004). However, authentic European material is required for a comparison to confirm this identification, as we suspect S. stachydicola may be restricted to Europe.
Septoria cretae Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804402. Figs 22, 23.
Fig. 22.
Conidia and conidiogenous cells of Septoria cretae (CBS 135095). Scale bar = 10 μm.
Fig. 23.
Septoria cretae (CBS 135095). A. Colony sporulating in culture. B-F. Conidiophores and conidiogenous cells giving rise to conidia. G. Conidia. Scale bars = 10 μm.
Etymology: Named after Crete, the island from where it was collected.
On sterile Carex leaves on WA. Conidiomata up to 250 μm diam, brown, immersed, subepidermal, pycnidial, subglobose with central ostiole, exuding creamy conidial mass; wall of 2-3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells, or with a supporting cell that gives rise to several conidiogenous cells. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, ampulliform to subcylindrical, straight to curved, proliferating sympodially near apex, 10-20 × 2-3.5 μm. Conidia hyaline, smooth, thin-walled, subcylindrical to narrowly obclavate, granular, with subobtuse apex and obconically truncate to truncate base, 1-3-septate, (8-)15-22(-27) × 2(-3) μm.
Culture characteristics: Colonies on PDA erumpent, with feathery margin, lacking aerial mycelium, surface fuscous-black, reverse olivaceous-black, after 14 d, 3.5 cm diam; on MEA surface fuscous-black, reverse olivaceous-black, after 14 d, 4 cm diam; on OA surface fuscous-black, reverse fuscous-black, after 14 d, 3.5 cm diam.
Specimen examined: Greece, Crete, on leaves of Nerium oleander (Apocynaceae), 7 Jul. 2012, U. Damm, (holotype CBS H-21277, culture ex-type CBS 135095).
Notes: Several species of Septoria are known on Nerium oleander, namely S. juliae [conidia 1-6(-7)-septate, 26-54 × 2.5-5.5 μm], S. neriicola (conidia 1-septate, 30-40 × 0.7-1 μm), S. oleandriicola [conidia 1-3-septate, 12.5-22.5-37.5(-40) × 2.5-3(-4.5) μm], S. oleandrina (conidia 0-1-septate, 9-19 × 1-1.5 μm), and S. roll-hansenii (conidia 0-4-septate, 25-39 × 3-4 μm) (Bedlan 2011), which differ from S. cretae based on conidial dimensions and septation.
Septoria glycinicola Quaedvlieg, H.D. Shin, Verkley & Crous, sp. nov. MycoBank MB804403. Fig. 24.
Fig. 24.

Septoria glycinicola (CBS 128618). A, B. Colonies sporulating on PDA. C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus on which it was collected, Glycine.
On OA. Conidiomata forming in concentric circles, pycnidial, separate, black, globose, up to 150 μm diam, opening by a central ostiole, up to 30 μm diam, exuding a creamy conidial mass; wall consisting of 3-6 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity, hyaline, smooth, ampulliform, 10-16 × 2.5-3.5 μm, proliferating sympodially near apex, holoblastic. Conidia hyaline, smooth, guttulate to granular, subcylindrical to narrowly obclavate, irregularly to gently curved, apex subobtuse, base long obconically truncate, 3-6-septate, (33-)45-55(-65) × (1.5-)2 μm.
Culture characteristics: Colonies on PDA flat, circular, with sparse black aerial mycelium with black tufts, surface patches of olivaceous-black to fawn in the younger parts, reverse with patches of olivaceous-black in the older parts to mouse-grey and pale purplish grey in the younger mycelium, after 14 d, 6.5 cm diam, pinkish exudate; on OA lobate, with sparse white aerial mycelium, surface patches of vinaceous to olivaceous-black, reverse fuscous-black to vinaceous-buff; after 14 d, 8.5 cm diam, pinkish exudate; on MEA with radial lobes, very short white aerial mycelium, surface fuscous-black, reverse olivaceous-black; after 14 d, 4.5 cm diam.
Specimen examined: South Korea, Namyangju, on leaves of Glycine max (Fabaceae), 22 Sep. 2008, H.D. Shin (holotype CBS H-21270, culture ex-type CBS 128618 =KACC 43091 = SMKC 22879).
Notes: Septoria glycines is the common Septoria species associated with brown spot of soybeans. Septoria glycinicola is distinct from S. glycines (conidia 1-4 septate, 21-45 × 1.5-2 μm) in that it has larger conidia.
Septoria oenanthicola Quaedvlieg, H.D. Shin, Verkley & Crous, sp. nov. MycoBank MB804405. Fig. 25.
Fig. 25.
Septoria oenanthicola (CBS 128649). A. Colony sporulating on MEA. B. Section through conidiomata. C-G. Conidiogenous cells. H. Conidia. Scale bars: B = 200 μm, all others = 10 μm.
Etymology: Named after the host genus from which it was collected, Oenanthe.
On sterile Carex leaves on WA. Conidiomata pycnidial, separate but aggregated, black, globose, up to 200 μm diam, opening by central ostiole, up to 20 μm diam, exuding a creamy conidial mass; wall consisting of dark brown, thickened, 6-10 layers of textura angularis. Conidiophores reduced to conidiogenous cells or to one supporting cell. Conidiogenous cells hyaline, smooth, 3-5 × 3-7 μm, ampulliform, proliferating sympodially near apex, holoblastic. Conidia hyaline, smooth, guttulate, subcylindrical to narrowly obclavate, apex subobtuse, base long obconically truncate, 1-6-septate, (17-)25-45(-55) × (2-)2.5(-3) μm.
Culture characteristics: Colonies on PDA flat, undulate with sparse, white aerial mycelium, surface olivaceous-grey, reverse olivaceous, after 14 d, 2.5 cm diam; on MEA with sparse, white aerial mycelium, surface olivaceous-grey, reverse olivaceous-black, after 14 d, 5 cm diam; on OA with sparse white aerial mycelium, surface olivaceous-grey, reverse olivaceous, after 14 d, 3 cm diam.
Specimen examined: South Korea, Yangpyeong, on leaves of Oenanthe javanica (Apiaceae), 25 May 2006, H.D. Shin (holotype CBS H-21281, culture ex-type CBS 128649 =KACC 42394 = SMKC 21807).
Notes: This fungus was originally recorded from Korea by Shin (1998) as Septoria oenanthis. However, conidia of Korean specimens (30-60 ×1.5-2.5 μm; Shin & Sameva 2004) are much larger than that of the American type collection (20-35 × 1.5-2 μm; Saccardo 1895), and therefore better treated as a separate taxon.
Septoria pseudonapelli Quaedvlieg, H.D. Shin, Verkley & Crous, sp. nov. MycoBank MB804404. Fig. 26.
Fig. 26.
Septoria pseudonapelli (CBS 128664). A. Colony sporulating on PDA. B. Section through conidioma. C-E. Conidiogenous cells. F. Conidia. Scale bars: B = 125 μm, all others = 10 μm.
Etymology: Named after its morphological similarity to Septoria napelli.
Leaf spots on the upper leaf surface, scattered to confluent, distinct, angular to irregular, usually vein-limited, small to large, up to 30 mm when confluent, at first appearing small angular brown discoloration, later turning blackish brown with or without distinct border line, finally central area becoming blackish and surrounded by pale greenish margin; on the lower leaf surface similar but discoloured (Shin & Sameva 2004). On sterile Carex leaves on WA. Conidiomata pycnidial, separate, black, globose, papillate with short neck (at times 1-2 necks develop), up to 250 μm wide, 500 μm high with central ostiole; wall of 5-7 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells ampulliform, lining the inner cavity, hyaline, smooth, with sympodial or apical percurrent proliferation, 10-13 × 5-7 μm. Conidia filiform, curved to flexuous, (50-)75-90(-100) × (2.5-)3(-3.5) μm, hyaline, guttulate, 4-10-septate, apex subobtuse, base obconically truncate, 2 μm diam.
Culture characteristics: Colonies on PDA flat, undulate with sparse, white aerial mycelium, surface olivaceous-black, reverse olivaceous-black, after 14 d, 2 cm diam; on MEA with sparse white aerial mycelium, surface olivaceous-black, reverse olivaceous-black, after 14 d, 4 cm diam; on OA with sparse white aerial mycelium, surface olivaceous, reverse olivaceous, after 14 d, 2 cm diam.
Specimen examined: South Korea, Chuncheon, on leaves of Aconitum pseudolaeve var. erectum (Ranunculaceae), 4 Sep. 2008, H.D. Shin (holotype CBS H-21280, culture ex-type CBS 128664 =KACC 43952 = SMKC 23638).
Notes: This taxon was originally reported as Septoria napelli from Korea by Shin & Sameva (2004), and broadly corresponds with the original description provided for this taxon (Petrak 1957). However, we have examined European material authentic for the name (see Verkley et al. 2013, this issue), from which the Korean fungus is genetically different. Based on these observations we describe the Korean collection as new.
Clade 2: Sphaerulina
Sphaerulina Sacc., Michelia 1(no. 4): 399. 1878.
Description: See above.
Type species: Sphaerulina myriadea (DC.) Sacc., Michelia 1(no. 4): 399. 1878.
Specimens examined: Germany, Driesen, Lasch [Rabenhorst, Fungi Eur. no. 149] (L). Japan, Aomori, Tsugaru, Kidukuri, Bense-marsh (40°51’53” N, 140°17’42”E), on leaves of Q. dentata, 21 Apr. 2007, K. Tanaka 2243 (HHUF 29940; single ascospore culture CBS 124646 =JCM 15565). UK, on leaves of Quercus robur (Fagaceae), J.E. Vize [Microfungi Brit. Ex. No. 195] (ex IMI 57186, K(M) 167735). USA, California: Sequoia National Park. alt. 2590 m, on leaves of Castanopsis sempervirens, 18 Jun. 1931, H.E. Parks (BPI 623686); Lake Co., Hoberg’s Resort, on leaves of Q. kelloggii, 15 May 1943, V. Miller (BPI 623707); Maryland, Marlboro, on leaves of Q. alba, 26 Apr. 1929, C.L. Shear (BPI 623705); Texas, Houston, on leaves of Q. alba, 8 Apr. 1869, H.W. Ravenel (BPI 623704).
Notes: Sivanesan (1984) linked Sphaerulina to Septoria, Cercospora and Cercosporella asexual morphs, though these were never confirmed based on DNA data. The latter two genera have since been shown to be distinct (Crous et al. 2013, Groenewald et al. 2013; this volume), which leaves septoria-like asexual morphs such as Sphaerulina rubi Demaree & Wilcox (linked to Cylindrosporium rubi Ellis & Morgan), and S. rehmiana (linked to Septoria rosae), which confirms the results obtained here (Fig. 1).
Sphaerulina abeliceae (Hiray.) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804406.
Basionym: Septoria abeliceae Hiray., Mem. Col. Agr. Kyoto. Imp. Univ. 13(3): 33. 1931.
Specimen examined: South Korea, Jeonju, on leaves of Zelkova serrata (Ulmaceae), 29 Oct. 2006, H.D. Shin, CBS 128591 =KACC 42626.
Sphaerulina amelanchier Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804407. Figs 27, 28.
Fig. 27.
Conidia, conidiogenous cells, ascospore and ascus of Sphaerulina amelanchier (CBS 135110). Scale bars = 10 μm.
Fig. 28.
Sphaerulina amelanchier (CBS 135110). A. Colony on PDA. B. Conidiogenous cells. C. Ascomata on host tissue. D. Germinating ascospore. E, F. Asci. G. Ascospores. H. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Amelanchier.
On sterile Carex leaves on WA. Conidiomata pycnidial, brown, separate, immersed, globose, up to 150 μm diam, exuding a creamy conidial mass via central ostiole; wall of 3-6 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, smooth, subcylindrical, irregularly curved, branched to once geniculate-sinuous, 5-20 × 3-4 μm; proliferating sympodially. Conidia hyaline, smooth, guttulate, filiform, narrowly obclavate, apex subacutely rounded, base long obconically truncate, 1-8-septate, (25-)40-55(-60) × (1.5-)2(-2.5) μm; microcyclic conidiation common. Ascomata globose, brown, separate, immersed to erumpent, up to 150 μm diam. Asci broadly ellipsoid to obclavate, 22-35 × 7-9 μm; apical chamber visible, 1-1.5 μm diam. Ascospores fusoid-ellipsoid, hyaline, smooth, granular, not to slightly constricted at median septum, widest just above septum, prominently curved, (13-)17-20(-25) × (2.5-)3(-3.5) μm. Ascospores germinating from both ends, with germ tubes parallel to the long axis, developing lateral branches and becoming constricted at septum, 3-4 μm diam.
Culture characteristics: Colonies on PDA radially striate with lobate edge, sparse white aerial mycelium, surface fuscous-black to buff for the younger tissue, reverse cinnamon to olivaceous-black, after 14 d, 3 cm diam; on MEA surface patches of hazel to fawm to fuscous-black, reverse sepia to olivaceous-black, after 14 d, 4.5 cm diam; on OA surface pale-vinaceous to fuscous-black, reverse cinnamon to fuscous-black, after 14 d, 3 cm diam.
Specimen examined: Netherlands, Houten, on leaf litter of Amelanchier sp. (Rosaceae), 28 Mar. 2012, S. Videira (holotype CBS H-21282, culture ex-type CBS 135110 =MP8 = S544).
Note: Presently there are no known species of septoria-like fungi known from Amelanchier. Phylogenetically, it is similar to Sphaerulina rhabdoclinis (conidia 8-30 × 1.5-2 μm), which infects needles of Pseudotsuga menziesii. Phylogenetically similar isolates occur on Betula, Castanea and Quercus. More isolates and molecular data are required to resolve this complex.
Sphaerulina azaleae (Voglino) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804408.
Basionym: Septoria azaleae Voglino, Syll. Fung. (Abellini) 14(2): 976. 1899.
≡ Phloeospora azaleae (Voglino) Priest, Fungi of Australia: 224. 2006.
Specimens examined: Belgium, on leaves of Rhododendron sp. (Ericaceae), J. van Holder, CBS 352.49. South Korea, Hongcheon, on leaves of Rhododendron sp., 18 Oct. 2009, H.D. Shin, KACC 44865 = CBS 128605.
Sphaerulina berberidis (Niessl) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804409.
Basionym: Septoria berberidis Niessl, in Rabenhorst, Bot. Ztg. 24: 411. 1866.
-
= Sphaerella berberidis Auersw., in Gonnermann & Rabenhorst, Mycol. eur. Abbild. Sämmtl. Pilze Eur. 5-6: 3. 1869 (nom. nov. for Sphaeria berberis Nitschke ex Fuckel).
≡ Mycosphaerella berberidis (Auersw.) Lindau, in Engler & Prantl, Nat. Pflanzenfam., Teil. I (Leipzig) 1(1): 424. 1897.
Description in vitro (CBS 116724): Colonies on OA 16-20 mm diam after 14 d, with an even, colourless margin; colonies spreading to restricted, somewhat elevated in the centre, the surface covered by a dense mat of pure white, woolly aerial mycelium; reverse in the centre dark brick to brown vinaceous, surrounded by cinnamon tinges; small amounts of a yellow to greenish pigment diffusies into the surrounding medium. Colonies on MEA 8-10 mm diam after 14 d, with an even to slighlty ruffled vinaceous buff margin; colonies restricted, pustulate, the surface ochraceous or darker, with diffuse to locally more dense finely felted grey aerial mycelium; reverse brown vinaceous to vinaceous buff. Culture remained sterile.
Specimen examined: Switzerland, Kt. Graubünden, Rodels-Realta, on Berberis vulgaris (Berberidaceae), 2 Jun. 1951, E. Müller, specimen CBS-H4984, culture CBS 324.52.
Sphaerulina betulae (Pass.) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804410.
Basionym: Septoria betulae Pass., Primo Elenc. Funghi Parm.: no. 52. 1867.
Specimens examined: Netherlands, Olst, leaves of Betula pubescens (Betulaceae), Sep. 2004, S. Green, CBS 116724. South Korea, Hongcheon, leaves of B. platyphylla var. japonica, 27 May 2008, H.D. Shin, CBS 128600 =KACC 43769.
Sphaerulina cercidis (Fr.) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804411.
Basionym: Septoria cercidis Fr., in Léveillé, Ann. Sci. Nat., Bot., Sér. 3 9: 251. 1848.
= Septoria provencialis Crous, Stud. Mycol. 55: 127. 2006.
Specimens examined: Argentina, La Plata, on Cercis siliquastrum (Caesalpiniaceae), 12 Feb. 2008, H.D. Shin, KACC 43596 = CBS 129151; on C. siliquastrum, 1 Sep. 2007, H.D. Shin, KACC 44497 = CBS 128634. France, Provence, Cheval Blanc camping site, on leaves of Eucalyptus sp., 29 Jul. 2005, P.W. Crous, holotype of S. provincialis, CBS H-19701, culture ex-type CBS 118910. Netherlands, on C. siliquastrum, Sep. 1950, G. van den Ende, CBS 501.50.
Sphaerulina menispermi (Thüm.) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804412.
Basionym: Septoria menispermi Thüm., Pilzflora Siber.: no. 818. 1880.
Specimens examined: South Korea, Chuncheon, on leaves of Menispermum dauricum (Menispermaceae), 16 Jun. 2008, H.D. Shin, KACC 43848 = CBS 128761; Pyeongchang, on leaves of M. dauricum, 23 Sep. 2008, H.D. Shin, KACC 43968 = CBS 128666.
Sphaerulina musiva (Peck) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804413.
Basionym: Septoria musiva Peck, Ann. Rep. N.Y. St. Mus. Nat. Hist. 35: 138. 1883 [1881]
-
= Mycosphaerella populorum G.E. Thomps., Phytopathology 31: 246. 1941.
≡ Davidiella populorum (G.E. Thomps.) Aptroot, CBS Diversity Ser. (Utrecht) 5: 164. 2006.
= Cylindrosporium oculatum Ellis & Everh., J. Mycol. 5(3): 155. 1889.
Specimen examined: Canada, Quebec, leaf spot of Populus deltoids (Salicaceae), J. LeBoldus, CBS 130570.
Sphaerulina oxyacanthae (Kunze & J.C. Schmidt) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804414. Figs 29, 30.
Fig. 29.
Conidia and conidiogenous cells of Sphaerulina oxyacanthae (CBS 135098). Scale bars = 10 μm.
Fig. 30.
Sphaerulina oxyacanthae (CBS 135098). A. Leaves with leaf spots. B. Close-up of conidiomata. C. Section though conidioma. D-F. Conidiogenous cells. G. Conidia (note appendages). Scale bars: C = 150 μm, all others = 10 μm.
Basionym: Septoria oxyacanthae Kunze & J.C. Schmidt, Myk. Hefte (Leipzig) 2: 108. 1823.
≡ Phloeospora oxyacanthae (Kunze & J.C. Schmidt) Wallr., Fl. Crypt. Germ. (Norimbergae) 2: 117. 1833.
Leaf spots amphigenous, medium to dark brown, subcircular to angular, 1-6 mm diam, with dark brown border. Conidiomata epiphyllous, up to 150 μm diam, brown, immersed, subepidermal, opening by irregular rupture of upper layer, with 3-4 apical flaps, exuding a long crystalline flame-like cirrhus of conidia; wall 3-8 layers of brown textura angularis. On sterile Carex leaves on WA. Conidiophores reduced to conidiogenous cells, or with one supporting cell that can become fertile, forming a lateral conidiogenous locus just below the septum, 10-20 × 2.5-4 μm. Conidiogenous cells hyaline, smooth, aggregated, lining the inner cavity, terminal and lateral, ampulliform, 5-10 × 2.5-3.5 μm; proliferating several times percurrently near apex. Conidia hyaline, smooth, guttulate, 6-12-septate, falcate, widest in lower third of conidium, flexuous, apical cell tapering to subacute apex, forming a curved apical appendage-like elongation, 10-17 μm long, median cells are 5-10 μm long, basal cell forming an eccentric appendage that tapers to a subacutely rounded base, scar approximately 2-4 μm below basal septum; basal cell (incl. appendage) 11-20 μm long, conidia (60-)75-90(-100) × 2(-2.5) μm.
Culture characteristics: Colonies on PDA umbonate with undulate edge and sparse, white aerial mycelium, surface isabelline, reverse greyish sepia, after 14 d, 3 cm diam; similar on MEA and PDA.
Specimen examined: Netherlands, Wageningen, 51°57’50.43”N 5°41’0.41”E, on leaves of Crataegus sp. (Rosaceae), Sep. 2012, W. Quaedvlieg (CBS H-21291, culture CBS 135098 =S654).
Notes: Several septoria-like species have been described from leaves of Crataegus (Farr & Rossman 2013). The present collection matches the description of Septoria oxyacanthae (leaf spots on Crataegus oxyacantha in Germany, conidia 8-12-septate; conidial dimensions not given). Unfortunately we have been unable to locate type material of this species.
Sphaerulina patriniae (Miura) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804415.
Basionym: Septoria patriniae Miura, Flora of Manchuria and East Mongolia, III Cryptogams, Fungi (Industr. Contr. S. Manch. Rly 27) 3: 465. 1928.
Specimen examined: South Korea, Pocheon, on leaves of Patrinia scabiosaefolia (Valerianaceae), 20 Aug. 2006, H.D. Shin, KACC 42518 = CBS 128653.
Sphaerulina populicola (Peck) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804416.
Basionym: Septoria populicola Peck, Ann. Rep. N.Y. St. Mus. 40: 59. 1887.
= Septoria populicola House, Bull. N.Y. St. Mus.: 59. 1920. (nom. illegit.)
= Mycosphaerella populicola C.H. Thomps., Phytopathology 31: 251. 1941.
Specimen examined: USA, Washington, Puyallup, on Populus trichocarpa (Salicaceae), 2 May 1997, G. Newcombe, CBS 100042.
Sphaerulina pseudovirgaureae Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804417. Figs 31, 32.
Fig. 31.
Conidia, conidiogenous loci on a hypha, and conidiogenous cells of Sphaerulina pseudovirgaureae (CBS 135109). Scale bars = 10 μm.
Fig. 32.
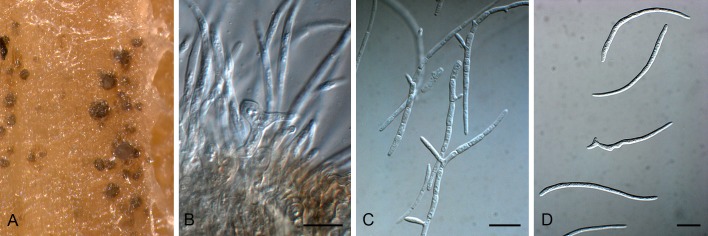
Sphaerulina pseudovirgaureae (CBS 135109). A. Conidiomata forming in culture. B. Conidiogenous cells. C. Microcyclic conidiation. D. Conidia. Scale bars = 10 μm.
Etymology: Named after its similarity to Septoria virgaureae.
Conidiomata pycnidial, separate, erumpent, globose, up to 120 μm diam, dark brown, exusing a creamy conidial cirrhus through central ostiole, somewhat papillate; wall of 2-3 laters of brown textura angularis. Conidiophores reduced to conidiogenous cells or with one supporting cell, subcylindrical, 0-1-septate, branched below or not, pale brown at base, 10-20 × 3-5 μm. Conidiogenous cells integrated, hyaline, but pale brown at base, smooth, proliferating sympodially near apex, 7-17 × 2-3 μm. Conidia solitary, hyaline, smooth, guttulate, subcylindrical to narrowly obclavate, scolecosporous, irregularly curved, apex subobtuse, base truncate or narrowly obconically truncate, 3-10-septate, (30-)40-60(-80) × 2.5(-3) μm.
Culture characteristics: Colonies spreading, erumpent with sparse aerial mycelium and smooth, lobate margin and folded surface; reaching 13 mm diam after 2 wk. On MEA surface saffron with patches of dirty white, reverse saffron to orange; on PDA surface and reverse saffron; on OA surface saffron.
Specimen examined: Netherlands, Nijmegen, de Duffelt, on leaves of Solidago gigantea (Asteraceae), Aug. 2012, S. Videira (holotype CBS H-21327, culture ex-type CBS 135109 =S669).
Notes: Several septoria-like species have been recorded on Solidago (Farr & Rossman 2013). Of these taxa Sphaerulina pseudovirgaureae is most similar to Septoria virguareae (conidia 80-100 × 1.5 μm) except that its conidia are shorter and wider.
Sphaerulina quercicola (Desm.) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804419. Figs 33, 34.
Fig. 33.
Ascospores and asci of Sphaerulina quercicola (CBS 113266). Scale bar = 10 μm.
Fig. 34.

Sphaerulina quercicola (CBS 663.94). A. Leaves with leaf spots. B. Close-up of lesion. C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Basionym: Septoria incondita var. quercicola Desm., Ann. Sci. nat., Sér. 3, Bot. 20: 95. 1853.
≡ Septoria quercicola (Desm.) Sacc., Michelia 1: 174. 1879.
≡ Phleospora quercicola (Desm.) Sacc., in P. A. Saccardo & D. Saccardo, 1906. Syll. Fung. 18: 490. 1906.
= Septoria quercina Fautr., in Fautrey & Lambotte, Revue Mycol. 17: 170. 1895 (nom. illeg., art. 53; non Desmazières, 1847). Nom. nov. pro Septoria quercicola f. macrospora Roum., Revue Mycol. 13: 80. 1891.
Description in vivo. Symptoms definite, small hologenous leaf spots, scattered or in clusters, in the centre orange brown, pale yellowish brown to white, usually delimited by a blackened, somewhat elevated zone, the surrounding leaf tissues becoming red or yellow. Conidiomata pycnidial or acervuloid, one to a few in each leafspot, scattered, semi-immersed, predominantly hypophyllous, pale to dark brown, lenticular to globose, 100-200 μm diam; ostiolum often not well-developed, initially circular, central, soon opening widely, lacking distinctly differentiated cells; conidiomatal wall composed of textura angularis without distinctly differentiated layers and sometimes only well-developed in the lower part of the conidioma, mostly 10-15 μm thick, the outer cells with brown, somewhat thickened walls and 4.5-8 μm diam, the inner cells hyaline, thin-walled, 3-8 μm diam. Conidiogenous cells hyaline, discrete or integrated in simple, short, (1-)3-5-septate conidiophores which may be branched at the base, doliiform, cylindrical, or ampuliform, hyaline, holoblastic, proliferating percurrently with one to several, more or less distinct annellations, or sympodially, sometimes both types of proliferation occurring in a single conidiogenous cell, 4.5-16(-22.5) × 3-4.5 μm. Conidia cylindrical, curved or flexuous, broadly rounded at the apex which is provided with a cap of mucilaginous material, attenuated gradually to a broadly or more narrowly truncate base which often is also provided with an amorphous mass of mucilaginous material, hyaline, (0-)1-3-septate, constricted around the septa, sometimes at one or more septa also some amorphous mucilaginous material may be present, contents with numerous small oil droplets, (32.5-)38-50(-65) × 3-4 μm. Ascomata not clearly associated with leaf spots, pseudothecial, predominantly hypophyllous, black, subepidermal, erumpent to superficial, globose, 100-150 μm diam; apical ostiole 5-10 μm wide; wall consisting of 2-3 layers of medium brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, broadly ellipsoidal to subcylindrical, straight to slightly curved, 8-spored, 35-50 × 9-12 μm. Ascospores tri- to multiseriate, overlapping, hyaline, guttulate, thin-walled, curved, rarely straight, fusoid-ellipsoidal with obtuse ends, widest at septum or just above, medianly 1-septate, not constricted at the septum, tapering towards both ends, (13-)15-18(-20) × (3.5-)4-4.5(-5) μm (av. 17 × 4.5 μm).
Culture characteristics: Colonies on OA reaching 5-7 mm diam in 21 d, with an even to undulating, colourless margin; colonies restricted, irregularly pustulate, immersed mycelium appearing dark greyish to olivaceous black, rosy buff near the margin, covered mostly with a dense mat of woolly, pure white or greyish aerial mycelium; reverse in the centre brown vinaceous or more greyish black, surrounded by brick to rosy buff. Pycnidia developing on the agar surface in the centre, releasing droplets of rosy-buff conidial slime. Colonies on MEA reaching 4-6(-8) mm diam in 21 d, with an even, to irregularly undulating margin which is mostly hidden under the aerial mycelium; colonies restricted, irregularly pustulate, the surface mostly blackish or very dark grey, covered by dense to diffuse, finely felted, white aerial mycelium; reverse mostly olivaceous black, near the margin cinnamon to buff. Numerous single and aggregated pycnidia developing on the colony surface in the centre, releasing milky white to rosy buff conidial slime. Conidia as in planta (CBS 663.94) though on average considerably longer, 51.5-74.5 × 3-4(-4.5) μm (OA), the apex, base and area around septa normally both provided with mucilaginous material as described above, (0-)1-3(-5)-septate.
Specimens examined: Austria, endophyte culture ex twig of Quercus petraea (Fagaceae), Aug. 1991, E. Halmschlager 212 (H. A. van der Aa 10986), CBS 456.91. France, loc. unknown, on leaves of Quercus sp. (“divers Chênes”), distributed in Desmazières, Pl. crypt. Fr., Fasc. 43, no. 2193 (PC, type of Septoria incondita var. quercicola Desm.). Netherlands, Utrecht, Baarn, on living leaves of Q. robur, 11 Aug. 1994, G. Verkley 225 (CBS H-21188), living culture CBS 663.94; prov. Utrecht, Soest, De Stompert, on living leaves of Q. rubra, 15 Aug. 1995, G. Verkley 310 (CBS H-21189), CBS 791.95; Same loc., dead fallen leaves of Q. robur, Apr. 2003, G. Verkley s.n., single ascospore-isolate CBS 113266 (’Crous 3’); Same loc., G. Verkley & I. van Kempen, endophyte isolates ex green leaves of Q. robur CBS 115016, 115136, 115137; Prov. Gelderland, Amerongen, Park Kasteel Amerongen, leaf spot of Q. rubra, 11 Jul. 2000, G. Verkley 973 (CBS H-21231), living culture CBS 109009; Prov. Utrecht, Amelisweerd, on dead leaves of Q. robur, 25 Apr. 2005, G. Verkley 3108A, culture CBS 117803, CPC 12097.
Sphaerulina rhabdoclinis (Butin) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804420. Fig. 35.
Fig. 35.
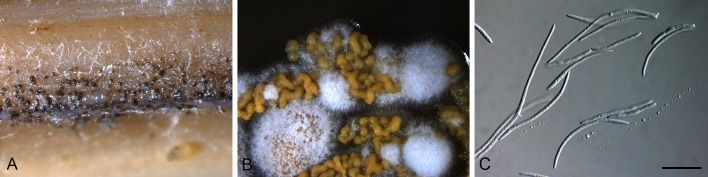
Sphaerulina rhabdoclinis (CBS 102195). A. Conidiomata forming in culture. B. Sporulation on PDA. C. Conidia. Scale bar = 10 μm.
Basionym: Dothistroma rhabdoclinis Butin, For. Path. 30: 196. 2000.
Specimen examined: Germany, Wolfenbüttel, on needles of Pseudotsuga menziesii (Pinaceae), 24 May 1998, H. Butin, culture ex-type CBS 102195.
Note: Sphaerulina rhabdoclinis is phylogenetically closely related to S. amelanchier, which appears to be a species complex occurring on unrelated hosts (see Verkley et al. 2013).
Sphaerulina viciae Quaedvlieg, H.D. Shin, Verkley & Crous, sp. nov. MycoBank MB804418. Figs 36, 37.
Fig. 36.
Conidia and conidiogenous cells of Sphaerulina viciae (CBS 131898). Scale bars = 10 μm.
Fig. 37.
Sphaerulina viciae (CBS 131898). A. Conidiomata forming in culture. B, C, E. Conidiogenous cells. D, F. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Vicia.
On Anthriscus stem. Conidiomata pycnidial, solitary, erumpent, brown, globose, up to 150 μm diam, with central ostiole; wall of 3-6 layers of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity, hyaline, smooth, subcylindrical, tapering and proliferating sympodially at apex, 5-10 × 3-4 μm. Conidia hyaline, smooth, guttulate, subcylindrical, irregularly curved, apex obtuse, base truncate, (3-)6-multiseptate, not or slightly constricted at septa (especially constricted on SNA, OA and MEA), (45-)55-75(-110) × (2.5-)3(-3.5) μm.
Culture characteristics: Colonies erumpent, spreading with folded surface and sparse aerial mycelium, and smooth, lobate margin; reaching 12 mm diam after 2 wk. On MEA and PDA surface and reverse olivaceous-grey. On OA surface pale olivaceous-grey.
Specimen examined: South Korea, on leaves of Vicia amurense (Fabaceae), 12 Aug. 2004, H.D. Shin (holotype CBS H-21283, culture ex-type CPC 11414, 11416, 11415 = CBS 131898).
Notes: Several septoria-like species are known from Vicia (Farr & Rossman 2013). Of these, Sphaerulina viciae is most similar to Septoria viceae (conidia 30-60 × 2.5 μm), but distinct in having longer and wider conidia.
Clade 3: Caryophylloseptoria
Description: See Verkley et al. (2013)
Type species: Caryophylloseptoria lychnidis (Desm.) Verkley, Quaedvlieg & Crous.
Caryophylloseptoria pseudolychnidis Quaedvlieg, H.D. Shin, Verkley & Crous, sp. nov. MycoBank MB804481. Fig. 38.
Fig. 38.
Caryophylloseptoria pseudolychnidis (CBS 128630). A. Colony sporulating on MEA. B. Vertical section through conidiomata. C, D. Conidiogenous cells. E. Conidia. Scale bar: B = 250 μm, others = 10 μm.
Etymology: Named after its morphological similarity to Septoria lychnidis.
Leaf spots on the upper leaf surface, scattered to confluent, distinct, circular, angular to irregular, usually very large, reaching up to 20 mm diam, often surrounded with yellow halo, lacking concentric rings, initially dark brown with pale green border, becoming brown to dark brown, finally turning greyish brown to pallid in the centre; on the lower leaf surface greyish brown to brown with yellowish margin (Shin & Sameva 2004). On sterile Carex leaves on WA. Conidiomata pycnidial, globose, up to 250 μm diam, black with central ostiole, but frequently splitting open at maturity, appearing acervular; wall of 6-8 layers of dark brown textura angularis. Conidiophores subcylindrical, lining the inner cavity, hyaline, smooth, reduced to conidiogenous cells, or with 1-2 supporting cells, frequently branched at base, 10-25 × 3-5 μm. Conidiogenous cells subcylindrical to ampulliform, 7-15 × 3-5 μm; proliferating sympodially or percurrently near apex. Conidia hyaline, smooth, guttulate, cylindrical, apex obtuse to subobtuse, base truncate, 3-3.5 μm; 1-3(-5)-septate, (25-)32-45(-50) × (2-)2.5-3(-3.5) μm.
Culture characteristics: Colonies on PDA flat, undulate, very sparse, mixed grey and white aerial mycelium, surface isabelline to fuscous-black, reverse olivaceous-black to isabelline for the younger tissue, after 14 d, 3 cm diam; on MEA umbonate, striate, undulate, surface fuscous-black to honey for the younger tissue after 14 d 3.5 cm diam; on OA surface dark-mouse-grey, reverse iron-grey to mouse-grey.
Specimen examined: South Korea, Yangpyeong, Jungmi mountain, on leaves of Lychnis cognata (Caryophyllaceae), 27 May 2007, H.D. Shin (holotype CBS H-21292, culture ex-type CBS 128630 =KACC 43866 = SMKC 23519).
Notes: Shin (1995) recorded this species for the first time in Korea, while Shin & Sameva (1999) provided a full morphologial description. Although it compared well with the original description of this European taxon, its conidia tend to be smaller than those of S. lychnidis (50-70 × 2.5-3 μm), of which we have also examined European material (see Verkley et al. 2013, this issue).
Clade 4: pseudocercosporella-like
Note: See Frank et al. (2010).
Clade 5: Cercospora
Note: See Groenewald et al. (2013).
Clade 6: Phloeospora
Description: See above.
Type species: P. ulmi (Fr.) Wallr., Fl. Crypt. Germ. (Norimbergae) 2: 177. 1833.
Phloeospora ulmi (Fr.) Wallr., Fl. Crypt. Germ. (Norimbergae) 2: 177. 1833. Figs 39, 40.
Fig. 39.
Conidia and conidiogenous cells of Phloeospora ulmi (CBS 613.81). Scale bars = 10 μm.
Fig. 40.
Phloeospora ulmi (CBS 613.81). A, B, D, E. Conidiomata bursting through host tissue. G, H. Microconidiogenous cells. K. Spermatia. C, F, I, J, L. Macroconidiogenous cells (arrows denote percurrent proliferation). M. Conidia. Scale bars = 10 μm.
≡ Septoria ulmi Fr. [as ‘Septaria’], Novit. Fl. Svec. 5(cont.): 78. 1819.
≡ Septogloeum ulmi (Fr. & Kunze) Died., Krypt. Fl. Brandenburg (Leipzig) 9: 836. 1915.
≡ Cylindrosporium ulmi (Fr.) Vassiljevsky, Fungi Imperfecti Parasitici 2: 580. 1950.
= Mycosphaerella ulmi Kleb., Z. PflKrankh. 12: 257. 1902.
= Sphaerella ulmi (Kleb.) Sacc. & D. Sacc., Syll. Fung. (Abellini) 17: 642. 1905.
Leaf spots angular, vein limited, separate, becoming somewhat confluent, initially small yellow-green spots that finally turn brown. Conidiomata acervular, hypophyllous, separate, subepidermal, composed of thin-walled, medium brown textura angularis, up to 200 μm diam, opening by irregular rupture, and exuding a prominent cirrhus of orange to yellow-orange conidia. Conidiophores reduced to conidiogenous cells, or with 1-2 supporting cells, branched below or not, subcylindrical, 10-30 × 4-5 μm. Conidiogenous cells hyaline, smooth, subcylindrical, straight to once geniculate, with numerous prominent percurrent proliferations at apex, 10-15 × 4-5 μm. Conidia solitary, hyaline, smooth, straight to curved, guttulate or not, fusiform, tapering towards an obtuse or subobtuse apex, and truncate base, 2-3 μm diam, with minute marginal frill, 3-5-septate, (20-)30-50(-60) × (3.5-)4-5(-6) μm. Leaf spots also contain black spermatogonia and ascomata.
Specimens examined: Austria, Innsbruck, near Hungerburg, on leaves of Ulmus sp. (Ulmaceae), 21 Sep. 1981, H.A. van der Aa, CBS H-14740, H-14861, culture CBS 613.81; Innsbruck, road to Hungerburg, on leaves of Ulmus glabra, 20 Oct. 1996, W. Gams, CBS 344.97. Netherlands, Baarn, garden of CBS, Oosterstraat 1, on leaves of Ulmus sp., 26 Aug. 1998, H.A. van der Aa, CBS H-14739, culture CBS 101564. Unknown, on leaves of Ulmus pedunculata, 15 Jul. 1901, A. van Luijk, CBS H-920.
Note: Distinct from Septoria s. str. by having acervuli, and conidiogenous cells with prominent percurrent proliferation.
Clade 7: septoria-like
Septoria gladioli Pass., in Rabenhorst, Fungi europ. exsicc.: no. 1956. 1875. Passerini, Atti Soc. crittog. ital. 2: 41. 1879.
Descripton in vitro (18 °C, NUV). CBS 121.20: Colonies on OA 15-18 mm diam after 21 d, with an even to slightly ruffled, colourless margin; colonies plane, immersed mycelium olivaceous black, fading over amber towards the margin, aerial mycelium absent; reverse concolorous. No sporulation observed. Colonies on MEA 10-15 mm diam after 21 d, with an even, pale luteous to amber margin; colonies restricted, irregularly pustulate to cerebriform, immersed mycelium ochreous to salmon, covered by diffuse, finely felted, white aerial mycelium; reverse in the centre rust, fading towards the margin over apricot to pale luteous. No sporulation observed. CBS 353.29: Colonies on OA 16-20 mm diam after 21 d, with an even to slightly ruffled, colourless margin; colonies plane, immersed mycelium rosy buff mixed with some olivaceous grey, aerial mycelium absent; reverse mainly pale purplish grey to pale mouse grey. No sporulation observed. Colonies on MEA 14-22(-26) mm diam after 21 d, with an even to lobed, buff margin; colonies restricted, elevated towards the centre, radially striate, immersed mycelium greenish olivaceous fading to ochreous or buff salmon, the central part mostly covered by diffuse, finely felted, white aerial mycelium; reverse in the centre dark brick to isabelline or hazel, fading towards the margin over pale cinnamon to buff. No sporulation observed.
Specimen examined: Netherlands, Mar. 1929, J.C. Went, CBS 353.29. Unknown location and host, 1920, W.J. Kaiser, CBS 121.20.
Notes: Priest (2006) provided a complete description of S. gladioli on host material, based on observations of an isotype in MEL, and several specimens on Gladiolus cultivars collected in Australia. The two strains available from the CBS are old and sterile, and show some differences that also seem to be reflected in the DNA data obtained. Septoria gladioli is the only species of septorioid fungi described from the genus Gladiolus. An unusual feature of the species is that it overwinters as “sclerotia”, that cause leaf infections in the next season (Priest 2006). The conidiogenous cells are holoblastic and very distinctly proliferate percurrently to form subsequent conidia, but no sympodial proliferation has been reported. Based on the multilocus phylogeny, the aforementioned isolates should be placed in their own genus, with the genus Phloeospora as its closest relative. Recollecting material will be required to determine the generic disposition, the delimitation of the taxa (as there seem to be at least two) and to which of these taxa the name Septoria gladioli should be applied.
Clade 8: passalora-like
Passalora dioscoreae (Ellis & G. Martin) U. Braun & Crous, in Crous & Braun, CBS Biodiversity Ser. (Utrecht) 1: 162. 2003.
Specimen examined: South Korea, on leaves of Dioscorea tokoro (Dioscoreaceae), 24 Oct. 2003, H.D. Shin (CPC 10855); ibid., on leaves of Dioscorea tenuipes, 1 Jan. 2004, H.D. Shin (CPC 11513).
Notes: Passalora dioscoreae is not congeneric with the type species of the genus, P. bacilligera. The taxonomy of Passalora and its relatives will be treated in a future publication (Videira et al., in prep.).
Clade 9: Neoseptoria
Neoseptoria Quaedvlieg, Verkley & Crous, gen. nov. MycoBank MB804421.
Etymology: Resembling the genus Septoria.
Foliicolous. Conidiomata black, immersed, subepidermal, pycnidial, subglobose with central ostiole, exuding creamy conidial mass; wall of 2-3 layers of brown textura angularis. Conidiophores 0-2-septate, subcylindrical, hyaline to pale brown at base, smooth, straight to geniculate-sinuous. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, subcylindrical to ampulliform, straight to geniculate-sinuous; proliferating several times percurrently near apex, rarely sympodially. Conidia scolecosporous, hyaline, smooth, flexuous, rarely straight, granular, thin-walled, narrowly obclavate, apex subobtuse, base long obconically truncate, tapering to a truncate hilum, 3-multiseptate.
Type species: Neoseptoria caricis Quaedvlieg, Verkley & Crous.
Note: The genus Neoseptoria is morphologically similar to Septoria, but distinct in having mono- to polyphialidic conidiogenous cells that proliferate percurrently at the apex.
Neoseptoria caricis Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804422. Figs 41, 42.
Fig. 41.
Conidia and conidiogenous cells of Neoseptoria caricis (CBS 135097). Scale bars = 10 μm.
Fig. 42.
Neoseptoria caricis (CBS 135097). A, B. Conidiomata developing in culture. C, D. Conidiogenous cells. E, F. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus on which it occurs, Carex.
On sterile Carex leaves on WA. Conidiomata up to 150 μm diam, black, immersed, subepidermal, pycnidial, subglobose with central ostiole, exuding creamy conidial mass; wall of 2-3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells, or 0-2-septate, subcylindrical, hyaline to pale brown at base, smooth, straight to geniculate-sinuous, 10-30 × 2.5-3.5 μm. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, subcylindrical to ampulliform, straight to geniculate-sinuous, 8-15 × 2.5-3 μm; proliferating several times percurrently near apex, rarely sympodially. Conidia scolecosporous, hyaline, smooth, flexuous, rarely straight, granular, thin-walled, narrowly obclavate, apex subobtuse, base long obconically truncate, tapering to a truncate hilum, 1.5-2 μm diam, 3(-5)-septate, (40-)55-68(-80) × (2.5-)3(-3.5) μm.
Culture characteristics: Colonies on PDA erumpent, undulate, lacking aerial mycelium, reverse iron-grey, after 14 d, 3 cm diam; on MEA reverse greyish sepia, after 14 d, 3 cm diam, with fine, pale pink to orange aerial mycelium; on OA similar to MEA, but with pinkish tufts of aerial mycelium.
Specimen examined: Netherlands, Wageningen, on leaves of Carex acutiformis (Cyperaceae), Aug. 2012, W. Quaedvlieg (holotype CBS H-21293, culture ex-type CBS 135097 =S653).
Notes: Several septoria-like species have been described from Carex (Farr & Rossman 2013). Of these, N. caricis is most similar to S. caricicola (conidia 25-55 × 4 μm; (6-)7(-8)-septate), but distinct in having longer and narrower conidia with less septa.
Clade 10: Pseudocercospora
Note: See Crous et al. (2013)
Clade 11: Zymoseptoria
Note: See Quaedvlieg et al. (2011).
Clade 12: Ramularia
Clade 13: Dothistroma
Note: See Barnes et al. (2004).
Clade 14: Stromatoseptoria
Stromatoseptoria Quaedvlieg, Verkley & Crous, gen. nov. MycoBank MB804423.
Etymology: Stroma = referring to central stoma in pycnidium that gives rise to conidiophores; Septoria = septoria-like morphology.
Foliicolous, plant pathogenic. Conidiomata pycnidial, hypophyllous, subglobose to lenticular, very pale brown to dark brown, immersed to erumpent, exuding conidia in white cirrhus; ostiolum central, circular, surrounding cells concolorous; conidiomatal wall composed of a homogenous tissue of hyaline to very pale brown, angular to irregular cells. Conidiophores subcylindrical, branched, hyaline, septate. Conidiogenous cells hyaline, discrete or integrated, cylindrical or narrowly ampulliform, holoblastic, often also proliferating percurrently. Conidia cylindrical, slightly to distinctly curved, broadly rounded apex, attenuated towards a truncate base, transversely euseptate, mostly constricted at septa.
Type species: Stromatoseptoria castaneicola (Desm.) Quaedvlieg, Verkley & Crous.
Notes: Stromatoseptoria is distinguished from Septoria based on the central cushion or stroma that gives rise to its conidiophores (sensu Coniella and Pilidiella; van Niekerk et al. 2004), and conidia that tend to be olivaceous-brown in mass, and also turn olivaceous and verruculose with age.
Stromatoseptoria castaneicola (Desm.) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804424. Fig. 43.
Fig. 43.

Stromatoseptoria castaneicola (CBS 102320). A. Colony sporulating on MEA. B. Stroma giving rise to conidiogenous cells. C, D. Conidiogenous cells. E. Conidia. Scale bars: B = 200 μm, all others = 10 μm.
Basionym: Septoria castaneicola Desm., Ann. Sci. Nat., Sér. 3, Bot. 8: 26. 1847.
≡ (?) Phleospora castanicola (Desm.) D. Sacc., Mycoth. Ital., Cent. 1-2, no. 173.
= Septoria gilletiana Sacc., Michelia 1: 359. 1878.
-
?= Septoria castaneae Lév., Ann. Sci. Nat., Sér. 3, Bot. 5: 278. 1846.
≡ Cylindrosporium castaneae Krenner, Bot. Közl. 41(3-4): 126. 1944.
Description in vivo. Leaf spots numerous, small, angular, and often merging to irregular patterns, visible on both sides of the leaf, initially pale yellowish brown, later reddish brown with a narrow, darker border; Conidiomata pycnidial, hypophyllous, several in each leaf spot, subglobose to lenticular, very pale brown to dark brown, usually fully immersed, 80-150(-200) μm diam, releasing conidia in white cirrhi; ostiolum not well-differentiated, central, circular, 18-50 μm wide, surrounding cells concolorous; conidiomatal wall about 10-17 μm thick, composed of a homogenous tissue of hyaline to very pale brown, angular to irregular cells 4-10 μm diam; Conidiophores subcylindrical, branched at base, hyaline, smooth, 1-2-septate; base frequently brown, verruculose. Conidiogenous cells hyaline, discrete or integrated in conidiophores cylindrical or narrowly ampulliform, holoblastic, often also proliferating percurrently with up to 3 closely positioned annellations, 7-17(-20) × 3-4(-5) μm. Conidia cylindrical, slightly to distinctly curved, irregularly bent or flexuous, with a relatively broadly rounded apex, attenuated towards a truncate base, basal and apical cell often both wider than intermediate cells, (0-)2-3(-4)-septate, mostly constricted around the septa in the living state, hyaline, contents with several oil-droplets and granular material in each cell in the living state, with granular contents in the rehydrated state, 30-46 × 3-4 μm (“T”; rehydrated, “NT” 2-3 μm wide). Conidia are olivaceous-brown in mass, and older conidia also turn olivaceous and verruculose, and at times anastomose in culture.
Culture characteristics: Colonies (CBS 102322) on OA reaching 4-8 mm diam in 25 d (9-12 mm in 33 d), with an even, glabrous, buff margin; colonies restricted, up to 1 mm high, immersed mycelium homogeneously buff, where conidiomatal complexes develop dark brick to black, in part covered by pure white, dense, appressed and woolly aerial mycelium, later a salmon haze occurs in the immersed mycelium; reverse buff, locally cinnamon to sepia. Colonies on CMA reaching (4-)7-11 mm diam in 25 d (8-12 mm in 33 d), as on OA, but with a halo of reddish to salmon, diffusing pigment, which becomes more intense after 33 d, and immersed mycelium in the centre darker, and aerial mycelium more strongly developed, later becoming locally salmon or citrine; reverse brick and dark brick, surrounded by a reddish to salmon zone. Colonies on MEA reaching 6.5-9 mm diam in 25 d (9-11.5 mm in 33 d), with an even, buff to cinnamon margin, entirely hidden under the aerial mycelium, with a very faint halo of diffusing pigment; colonies restricted, up to 4 mm high, hemispherical to irregularly pustulate, entirely covered by a dense mat of felted aerial mycelium, which, especialy in the centre, attains a rosy buff or primrose to citrine haze; reverse cinnamon to hazel, around a brick to dark brick centre. Colonies on CHA reaching 7-9 mm diam in 25 d (9-11 mm in 33 d), as on MEA, but no diffusing pigment observed around the colonies. Conidiomata on OA developing after 10-15 d, black, globose, single or merged to complexes up to 250 μm diam, releasing milky white conidial slime. Conidiogenous cells as in planta. Conidia as in planta, mostly 3-septate, 30-45 × 3.5-4.5 μm (CBS 102320, OA, “T”; “NT” 3 μm wide).
Specimens examined: Austria, Tirol, Klausen, on leaves of Castanea vesca (Fagaceae), Aug., distributed in F. von Höhnel, Krypt. exsicc. no. 415, (PC0084576, PC0084583). France, Lébisey, Aug. and Sep. 1843, M. Roberge, ‘Coll. Desmazières 1863, no. 8’, on leaves of Castanea sativa (PC0084574, type of Septoria castanicola Desm.); same substr., Meudon, 1 Aug. 1849 (PC0084571, PC0084589, PC0084590, PC0084591) and Jul. 1852 (PC0084572); same substr., loc. and date unknown, ‘Coll. Desmazières 1863, no. 8’ (PC0084570); Seine-et-Marne, Fontainebleau, Sep 1881, distributed in Roumeguère, Fungi Gallici exsicc. no. 2029 (PC0084575). Netherlands, prov. Utrecht, Baarn, Lage Vuursche, on living leaves of Castanea sativa, 29 Aug. 1999, G. Verkley 912 (CBS H-21200), cultures CBS 102320-102322; same substr., prov. Limburg, St. Jansberg, 9 Sep. 1999, G. Verkley 932 (CBS H-21214), culture CBS 102377; same substr., prov. Limburg, Molenhoek, Heumense Schans (46-12-55), 23 Aug. 2004, G. Verkley & M. Starink 3040, culture CBS 116464.
Notes: According to the original diagnosis that Desmazières published in 1847 based on material on Castanea collected in autumn, the conidia are elongated, thin and curved, and about 40 μm in length. No further details like conidial septa were given. The material PC0084574 is the only collection received from PC that antidates the publication and assumedly is the type. It consists of several leaves with numerous pycnidia in leaf spots, some of which belong to Septoria castaneicola with the characteristic conidia, but most are a spermatial state of most likely the Mycosphaerella punctiformis complex (= Ramularia, Verkley et al. 2004).
Teterevnikova-Babayan (1987) treated S. castaneicola Desm. as a synonym of S. castaneae Lév., and both originally were described from the same host, Castanea sativa (syn. C. vesca). Teterevnikova-Babayan (1987) described the conidia as 3-septate, 25-40 × 2.5-4.5 μm, which is in fairly good agreement with present observations. The type of S. castanaea Lév. could not be studied and the name remains doubtful. Even though Léveillé described symptoms that match those of S. castaneicola fairly well, he described the conidia as aseptate, and failed to give information about their size.
Clade 15: Lecanosticta
Note: See Quaedvlieg et al. (2012).
Clade 16: Phaeophleospora
Clade 17: Cytostagonospora
Cytostagonospora Bubák, Ann. Mycol. 14: 150. 1916.
Description: See above.
Type species: Cytostagonospora photiniicola Bubák [as “photinicola”], Ann. Mycol. 14: 150. 1916.
Cytostagonospora martiniana (Sacc.) B. Sutton & H.J. Swart, Trans. Br. mycol. Soc. 87: 99. 1986. Figs 44, 45.
Fig. 44.
Conidia and conidiogenous cells of Cytostagonospora martiniana (CBS 135102). Scale bars = 10 μm.
Fig. 45.
Cytostagonospora martiniana (CBS 135102). A. Leaf spot. B. Conidiomata forming in culture. C-F. Conidiogenous cells. G. Conidia. Scale bars = 10 μm.
Basionym: Septoria martiniana Sacc., Syll. Fung. (Abellini) 10: 351. 1892.
= Septoria phyllodiorum Cooke & Massee, Grevillea 19(90): 47. 1890, non S. phyllodiorum Sacc., Hedwigia 29: 156. 1890.
On sterile Carex leaves on WA. Leaf spots amphigenous, circular, grey to brown with raised dark brown border, 1-3 mm diam. Conidiomata immersed, subepidermal, epiphyllous, solitary to aggregated with stromatic tissue, with central ostiolar opening exuding a creamy to white conidial mass, rupturing at maturity (pycnidial to acervular), brown, globose, up to 400 μm diam; wall of 3-6 layers of brown textura angularis. Conidiophores hyaline, smooth, subcylindrical, 0-5-septate, branched or not, 10-15(-50) × 3-4 μm, giving rise to terminal and lateral conidiogenous cells. Conidiogenous cells hyaline, smooth, subcylindrical or ampulliform, 4-8 × 3-4 μm, polyphialidic, with apical and lateral loci, with visible periclinal thickening, at times also proliferating percurrently (both modes can also be present on the same conidiogenous cell). Conidia hyaline, smooth, granular, irregularly curved, subcylindrical to narrowly obclavate, apex subobtuse, base long, obconically truncate, (1-)3-septate, (18-)32-45(-50) × (1.5-)2(-3) μm; base not thickened, 0.5-1 μm diam.
Culture characteristics: Colonies on PDA convex, erumpent with feathery margin, lacking aerial mycelium, surface fuscous-black, reverse olivaceous-black, after 14 d, 4 cm diam, with a beautifull purple exudate at the outer edges; on MEA, after 14 d, 3.5 cm diam, lacking any exudate; on OA surface fuscous-black, reverse olivaceous-grey, after 14 d, 4 cm diam, purplish-red coloured exudate.
Specimen examined: Australia, Warneet close to Melbourne, S38°13’37.8” E145°18’25.4”, on leaves of Acacia pycnantha (Mimosaceae), 21 Oct. 2009, P.W. Crous (specimen CBS H-21297, culture CBS 135102 =CPC 17727).
Notes: The present collection matches the description of Cytostagonospora martiniana provided by Sutton & Swart (1986). As discussed by the authors, this genus is distinct from Septoria s. str. based on its conidiomata aggregated in stromatic tissue, and unique mode of conidiogenesis. In culture conidiogenous cells exhibited a mixture of sympodial proliferation, or were polyphialidic with periclinal thickening, but also proliferated percurrently. Species of Septoria occurring on Acacia were treated by Sutton & Pascoe (1987).
Clade 18: Zasmidium
Clade 19: Polyphialoseptoria
Polyphialoseptoria Quaedvlieg, R.W. Barreto, Verkley & Crous, gen. nov. MycoBank MB804425.
Etymology: Polyphialo = polyphialides; Septoria = septoria-like.
Foliicolous, plant pathogenic. Conidiomata brown, erumpent, pycnidial (acervular in culture), globose, brown; wall of 3-6 layers of pale brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, smooth, subcylindrical to ampulliform; proliferating sympodially at apex, forming polyphialides with minute periclinal thickening, or as solitary loci on superficial mycelium in culture. Conidia hyaline, smooth, granular to guttulate, scolecosporous, irregularly curved, apex subobtuse, base long obconically truncate, transversely multi-euseptate, in older cultures disarticulating at septa; microcyclic conidiation also common in older cultures.
Type species: Polyphialoseptoria terminaliae Quaedvlieg, R.W. Barreto, Verkley & Crous.
Polyphialoseptoria tabebuiae-serratifoliae Quaedvlieg, Alfenas & Crous, sp. nov. MycoBank MB804427. Figs 46, 47.
Fig. 46.
Conidia and conidiogenous loci on hypha of Polyphialoseptoria tabebuiae-serratifoliae (CBS 112650). Scale bars = 10 μm.
Fig. 47.

Polyphialoseptoria tabebuiae-serratifoliae (CBS 112650). A. Conidiomata forming in culture. B. Conidiogenous cells. C. Conidia. Scale bars = 10 μm.
Etymology: Named after its host, Tabebuia serratifolia.
Leaf spots variable in number on mature leaves; initially as small spots or purple-brown areas, with the inner part becoming grey-white with age, surrounded by a purple-brown halo. Conidiomata developing on sterile barley leaves on WA, pale cream in colour, erumpent, globose, up to 180 μm diam; wall of 2-3 layers of pale brown textura angularis. Conidiophores hyaline, smooth, cylindrical, septate, branched, 10-35 × 1.5 μm. Conidiogenous cells terminal and lateral, cylindrical, hyaline, smooth, proliferating sympodially, 10-15 × 1.5 μm. Conidia solitary, hyaline, smooth, granular, irregularly curved, subcylindrical, apex subobtuse, base truncate, (0-)1-3(-4)-septate, (15-)25-35(-55) × 1.5(-2) μm.
Culture characteristics: Colonies flat, spreading, with sparse aerial mycelium and smooth, even margins, reaching 40 mm diam after 2 wk. On OA surface dirty pink; on PDA surface and reverse dirty white. On MEA surface folded, dirty white, reverse cinnamon.
Specimen examined: Brazil, Minas Gerais, Viçosa, on leaves of Tabebuia serratifolia (Bignoniaceae), 1999, A.C. Alfenas (holotype CBS H-21299, culture ex-type CBS 112650).
Notes: Inácio & Dianese (1998) described Septoria tabebuiae-impetiginosae on T. impetiginosa (conidia 25-67 × 2-4 μm, 2-6-septate), and also compared this species to S. tabebuiae (18-40 × 1.7-2.5 μm, aseptate conidia) on T. berteroi, and S. cucutana (34-40 × 0.8-1 μm) on T. pentaphylla and T. spectabilis. Furthermore, they also referred to an undescribed species Ferreira (1989) mentioned on T. serratifolia in Viçosa, Minas Gerais, which is named as S. tabebuiae-serratifoliae in the present study. Polyphialoseptoria tabebuiae-serratifoliae is distinct from species of Septoria known from Tabebuia based on its conidial morphology.
Polyphialoseptoria terminaliae Quaedvlieg, R.W. Barreto, Verkley & Crous, sp. nov. MycoBank MB804426. Fig. 48.
Fig. 48.
Polyphialoseptoria terminaliae (CBS 135106). A. Leaves with leaf spots. B, C. Conidiomata sporulating in culture. D-F. Conidiogenous cells and loci. G. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Terminalia.
Leaf spots irregular to subcircular, amphigenous, mostly aggregated along leaf veins, pale brown, 3-8 mm diam, surrounded by a prominent, wide, red-purple border. On sterile Carex leaves on WA. Conidiomata brown, erumpent, pycnidial (acervular in culture), up to 600 μm diam, globose, brown, exuding a crystalline cirrhus of conidia; wall of 3-6 layers of pale brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, smooth, subcylindrical to ampulliform, 5-10 × 3-4 μm; proliferating sympodially at apex, forming polyphialides with minute periclinal thickening, or as solitary loci on superficial mycelium in culture. Conidia hyaline, smooth, granular to guttulate, scolecosporous, irregularly curved, apex subobtuse, base long obconically truncate (1-1.5 μm diam), multiseptate (-16), in older cultures disarticulating at septa; microcyclic conidiation also common in older cultures, (40-)75-120(-140) × 2-3(-3.5) μm.
Culture characteristics: Colonies on PDA erumpent with feathery margin, lacking aerial mycelium, surface fuscous-black, reverse olivaceous-black to buff in the younger tissue, after 14 d, 1 cm diam; on MEA surface and reverse isabelline to greyish-sepia; on OA surface pale-vinaceous, reverse rosy-buff to buff.
Specimen examined: Brazil, Minas Gerais, Viçosa, on leaves of Terminalia catappa (Combretaceae), 18 May 2010, R.W. Barreto (holotype CBS H-21298, culture ex-type CBS 135106 =CPC 19611); ibed., (CBS 135475 =CPC 19487)
Notes: As far as we could establish there are presently no species of Septoria described from Terminalia, and as this taxon is distinct from all taxa in GenBank, we herewith describe it as a novel species. A Septoria sp. has been reported on leaves of Terminalia sp. in Florida and Venezuela (Farr & Rossman 2013). Polyphialoseptoria is distinct from Septoria based on the presence of polyphialides. Neoseptoria also has phialides as observed in Polyphialoseptoria, but these tend to chiefly be monophialides.
Clade 20: Ruptoseptoria
Ruptoseptoria Quaedvlieg, Verkley & Crous, gen. nov. MycoBank MB804428.
Etymology: Rupto = irregular rupture of conidiomata; Septoria = septoria-like.
Foliicolous, plant pathogenic. Conidiomata black, appressed, elongated, pycnidial, but opening via irregular rupture, convulated; exuding a creamy white conidial mass; outer wall dark brown, crusty, consisting of 6-8 layers of dark brown textura angularis; giving rise to 2-3 inner layers of pale brown to hyaline textura angularis. Conidiophores lining the inner cavity, hyaline, smooth or pale brown, verruculose at base, branched below, septate, subcylindrical. Conidiogenous cells integrated, terminal, subcylindrical, smooth; proliferating sympodially at apex, or apex phialidic with minute periclinal thickening. Conidia solitary, hyaline, smooth, guttulate, subcylindrical to narrowly obclavate, gently to irregularly curved, apex subobtuse, base truncate to narrowly obovoid, transversely septate.
Type species: Ruptoseptoria unedonis (Roberge ex Desm.) Quaedvlieg, Verkley & Crous.
Ruptoseptoria unedonis (Roberge ex Desm.) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804429. Figs 49, 50.
Fig. 49.
Conidia and conidiogenous cells of Ruptoseptoria unedonis (CBS 355.86). Scale bars = 10 μm.
Fig. 50.
Ruptoseptoria unedonis (CBS 355.86). A, C. Conidiomata forming in culture. B, D. Conidiogenous cells. E. Conidia. Scale bars: A = 450 μm, C = 110 μm, all others = 10 μm.
Basionym: Septoria unedonis Roberge ex Desm., Ann. Sci. Nat., Bot., Sér. 3(8): 20. 1847.
-
= Sphaerella arbuticola Peck, Bull. Torrey Bot. Club 10(7): 75. 1883.
≡ Mycosphaerella arbuticola (Peck) Jaap, Ann. Mycol. 14(1/2): 13. 1916.
≡ Mycosphaerella arbuticola (Peck) House, Contr. Univ. Mich. Herb. 9(8): 587. 1972.
Leaf spots numerous, small, amphigenous, irregular to subcircular, whitish in the middle, with very broad, purple borders. Conidiomata black, appressed, elongated, pycnidial, but opening via irregular rupture, convulated, up to 450 μm diam, exuding a creamy white conidial mass; outer wall dark brown, crusty, consisting of 6-8 layers of dark brown textura angularis; giving rise to 2-3 inner layers of pale brown to hyaline textura angularis. Conidiophores lining the inner cavity, hyaline, smooth or pale brown, verruculose at base, branched below, 1-2-septate, subcylindrical, 10-15 × 2-4 μm. Conidiogenous cells integrated, terminal, subcylindrical, smooth, 6-12 × 2.5-3.5 μm; proliferating sympodially at apex, or apex phialidic with minute periclinal thickening. Conidia solitary, hyaline, smooth, guttulate, subcylindrical to narrowly obclavate, gently to irregularly curved, apex subobtuse, base truncate to narrowly obovoid, 1-3(-6)-septate, (25-)30-47(-56) × 2(-3) um.
Culture characteristics: Colonies on OA spreading with moderate aerial mycelium and smooth, even margins; surface olivaceous-grey in outer region, centre dirty white to pale pink, reverse iron grey; on MEA surface dark-mouse-grey to mouse-grey, reverse greenish-black; on PDA surface mouse-grey to dark-mouse-grey, reverse greenish-black.
Specimen examined: France, Seignosse le Penon, Lamdes, Forest communale de Seignosse, on leaves of Arbutus unedo (Ericaceae), Aug. 1986, H.A. van der Aa (CBS H-14645, culture CBS 355.86).
Notes: Mycosphaerella arbuticola (CBS 355.86) is a species pathogenic to Arbutus menziesii in California (Aptroot 2006), clusters with “Septoria” unedonis (CBS 755.70, CBS H-18192), which is associated with leaf spots on Arbutus unedo in Croatia, and elsewhere in Europe. Based on these results, the sexual-asexual link between these two names is confirmed. Morphologically, however, Ruptoseptoria is similar to Septoria, and can only be distinguished based on its conidiomata that are convulated, opening by irregular rupture, and conidiogenous cells that are frequently phialidic.
Clade 21: Dissoconium (Dissoconiaceae)
Note: See Li et al. (2012).
Clade 22: Readeriella (Teratosphaeriaceae)
Clade 23: Teratosphaeria
Note: See Crous et al. (2007, 2009c).
Clade 24: septoria-like
Specimen examined: Brazil, Nova Friburgo, on leaves of Tibouchina herbacea (Melastomataceae), 15 Dec. 2007, D.F. Parreira (CBS 134910 =CPC 19500).
Note: The taxonomy of this species could not be resolved, as isolate CPC 19500 proved to be sterile.
Clade 25: Cylindroseptoria
Cylindroseptoria Quaedvlieg, Verkley & Crous, gen. nov. MycoBank MB804430.
Etymology: Cylindro = cylindrical conidia; Septoria = septoria-like.
Conidiomata pycnidial with central ostiole, or cupulate, separate, brown, short-stipitate, tapering towards base; rim with elongated brown, thick-walled cells with obtuse ends; rim covered with mucoid layer that flows over from conidiomatal cavity, filled with conidial mass; wall of 3-4 layers of medium brown textura angularis, becoming hyaline towards inner region. Conidiogenous cells hyaline, smooth, ampulliform, lining inner cavity, with prominent periclinal thickening at apex. Conidia solitary, hyaline, smooth, granular or not, cylindrical with obtuse apex, tapering at base to truncate scar, aseptate.
Type species: Cylindroseptoria ceratoniae Quaedvlieg, Verkley & Crous.
Cylindroseptoria ceratoniae Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804431. Figs 51, 52.
Fig. 51.
Conidia and conidiogenous cells of Cylindroseptoria ceratoniae (CBS 477.69). Scale bar = 10 μm.
Fig. 52.

Cylindroseptoria ceratoniae (CBS 477.69). A, B. Conidiomata forming in culture. C, D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars: B = 45 μm, all others = 10 μm.
Etymology: Named after the host genus on which it occurs, Ceratonia.
Conidiomata separate, brown, cupulate, short-stipitate, rim up to 300 μm diam, 100-180 μm tall, tapering towards base, 20-50 μm diam (on Anthriscus sylvestris stems, not on OA or PDA, where they appear more flattened with agar surface); rim with elongated brown, thick-walled cells with obtuse ends, 5-12 × 4-5 μm; rim covered with mucoid layer that flows over from conidiomatal cavity, filled with conidial mass; wall of 3-4 layers of medium brown textura angularis, becoming hyaline towards inner region. Conidiogenous cells hyaline, smooth, ampulliform, lining inner cavity, 7-12 × 4-6 μm; apex 2 μm diam, with prominent periclinal thickening. Conidia solitary, hyaline, smooth, granular or not, cylindrical with obtuse apex, tapering at base to truncate scar 1 μm diam, aseptate, (10-) 12-14(-16) × 3(-3.5) μm.
Culture characteristics: Colonies spreading, reaching 28 mm diam after 2 wk, with sparse aerial mycelium and even, lobate margins. On MEA surface iron-grey, reverse olivaceous-grey. On OA surface olivaceous-grey. On PDA surface and reverse iron-grey.
Specimen examined: Spain, Mallorca, Can Pastilla, on leaves of Ceratonia siliqua (Caesalpinaceae), 24 May 1969, H.A. van der Aa (holotype CBS H-21300, culture ex-type CBS 477.69).
Notes: Cylindroseptoria ceratoniae is quite distinct in that it has cup-shaped acervuli, ampilliform conidiogenous cells with periclinal thickening, and hyaline, aseptate, cylindrical conidia. Cylindroseptoria needs to be compared with Satchmopsis (infundibular conidiomata), Cornucopiella (tubular conidiomata) and Thaptospora (cylindrical / lageniform / campanulate conidiomata), but the combination of cupulate conidiomata and cylindrical, and aseptate conidia is distinct.
Cylindroseptoria pistaciae Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804432. Figs 53, 54.
Fig. 53.
Conidia and conidiogenous cells of Cylindroseptoria pistaciae (CBS 471.69). Scale bars = 10 μm.
Fig. 54.

Cylindroseptoria pistaciae (CBS 471.69). A, B. Conidiomata sporulating in culture. C, D. Intercalary chains of chlamydospore-like cells. E, F. Conidiogenous cells. G, H. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus on which it occurs, Pistacia.
Conidiomata pycnidial, erumpent, globose, black, separate, with black crusty outer layer of cells, up to 200 μm diam, with central ostiole; wall of 3-6 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic (mostly monophialidic, but a few observed to also be polyphialidic), lining the inner cavity, hyaline, smooth, ampulliform, 5-8 × 3-4 μm, proliferating percurrently (inconspicuous) or with periclinal thickening at apex (also occurring as solitary loci on superficial hyphae surrounding pycnidia). Conidia hyaline, smooth, cylindrical, mostly straight, rarely slightly curved, apex subobtuse, base truncate, guttulate, aseptate, (9-)11-13(-18) × 2.5-3(-3.5) μm.
Culture characteristics: Colonies on PDA flat, circular, lacking aerial mycelium, surface fuscous-black, reverse olivaceous-black, after 14 d, 3.5 cm diam; on MEA surface fuscous-black, reverse olivaceous-black, after 14 d, 4.5 cm diam; on OA similar to PDA.
Specimen examined: Spain, Mallorca, El Arenal, on leaves of Pistacia lentiscus (Anacardiaceae), 25 May 1969, H.A. van der Aa (holotype CBS H-21301, culture CBS 471.69).
Notes: Cylindroseptoria pistaciae is tentatively placed in Cylindroseptoria, as it has pycnidial rather than cupulate conidiomata. However, synapomorphies with Cylindroseptoria include phialides with periclinal thickening, and cylindrical, aseptate conidia. Further collections are required to determine if conidiomatal anatomy is more important than conidiogenesis and conidial morphology. For the present, however, the generic circumscription of Cylindroseptoria has been widened to include taxa with pycnidial conidiomata. Cylindroseptoria pistaciae could be confused with Septoria pistaciae, though conidia of the latter are 20-30 × 1.6 μm, and are 1(-3)-septate (Chitzanidis & Michaelides 2002).
Clade 26: Pseudoseptoria
Pseudoseptoria Speg., Ann. Mus. Nac. B. Aires, Ser. 3 13: 388. 1910.
= Aphanofalx B. Sutton, Trans. Brit. Mycol. Soc. 86: 21. 1986.
Caulicolous and foliicolous, plant pathogenic or saprobic. Conidiomata stromatic, pycnidioid, unilocular, glabrous, black, ostiolate; wall of textura angularis, in some cases cells in the upper wall larger and darker than cells in the lower wall. Conidiophores reduced to conidiogenous cells lining the cavity of the conidioma. Conidiogenous cells discrete or integrated, cylindrical or lageniform, colourless, smooth-walled, invested in mucus, with a prominent cylindrical papilla with several percurrent proliferations at the apex; collarette prominent and extanding past conidia, or reduced and inconspicuous. Conidia fusiform, lunate or irregular, curved, unicellular, colourless, smooth-walled with or without an excentric basal appendage, continuous with conidium body, plectronoid to podiform, or with a blunt or spathulate distal end.
Type species: P. donacicola Speg., Ann. Mus. Nac. B. Aires, Ser. 3 13: 388. 1910. [= P. donacis (Pass.) B. Sutton].
Pseudoseptoria collariana Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804433. Fig. 55.
Fig. 55.
Pseudoseptoria collariana (CBS 135104). A, B. Colonies sporulating in culture. C-F. Conidiogenous cells with prominent collarettes. G, H. Conidia. Scale bars: A = 400 μm, all others = 10 μm.
Etymology: Named after its prominently flared collarettes, forming a sleeve.
On sterile Carex leaves on WA. Conidiomata immersed to erumpent, globose, dark brown, up to 400 μm diam, unilocular, opening via central ostiole; wall of 6-10 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells, or branched at the base with one supporting cell that is dark brown, encased in a mucilaginous matrix. Conidiogenous cells subcylindrical to ampulliform, hyaline, smooth to pale brown, finely verruculose, 18-35 × 3.5-8 μm; apical region with numerous conspicuous percurrent proliferations, with long, prominent collarettes that completely enclose and extend above young, developing conidia, but disintegrating into a mucoid mass with age. Conidia fusiform, lunate, curved, aseptate, hyaline, smooth, tapering to an subobtuse to spathulate apex, base truncate (1 μm diam), with a single, unbranched, eccentric basal appendage, 2-4 μm long; conidia (from apex to hilum) (24-)26-28(-30) × (2.5-)3 μm.
Culture characteristics: Colonies on PDA flat, round with feathery margins, lacking aerial mycelium, surface olivaceous-black to rosy-buff for younger tissue, reverse olivaceous-black, to rosy-buff for younger tissue, after 14 d 1.5 cm diam; on MEA surface olivaceous-black to buff for younger tissue, reverse olivaceous-black to brick for younger tissue, after 14 d, 2 cm diam; on OA similar to MEA.
Specimen examined: Iran, Golestan Province, on leaves of Bamboo (Poaceae), 12 May 2009, A. Mirzadi Gohari (holotype CBS H-21302, culture ex-type CBS 135104 =CPC 18119).
Pseudoseptoria obscura Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804434. Fig. 56.
Fig. 56.
Pseudoseptoria obscura (CBS 135103). A, B. Colony sporulating in culture. C. Chlamydospore-like cells developing. D, E. Conidiogenous cells. F-H. Conidia. Scale bars: B = 250 μm, all others = 10 μm.
Etymology: Named after the obscure basal appendage that occurs on some conidia.
On sterile Carex leaves on WA. Conidiomata immersed to erumpent, globose, dark brown, up to 250 μm diam (smaller than in 18119), unilocular, opening via central ostiole; wall of 3-6 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells subcylindrical to doliiform, hyaline, smooth to pale brown, finely verruculose, 6-12 × 2-5 μm; apical region with numerous inconspicuous to conspicuous percurrent proliferations; collarettes absent to prominent. Conidia fusiform, lunate, curved, aseptate, hyaline, smooth, tapering to an subobtuse apex; base truncate, rarely with a single, unbranched, eccentric basal appendage, 1-2 μm long; conidia (from apex to hilum) (8-) 12-14(-15) × (2-)2.5(-3) μm.
Culture characteristics: Colonies on PDA flat, undulate with feathery margins, lacking aerial mycelium, surface concentric rings of fuscous-black to pale purplish grey to fuscous-black, reverse concentric rings of greyish-sepia to fawn to fuscous-black, after 14 d, 2 cm diam; on MEA similar to PDA; OA flat, undulate, lacking aerial mycelium, surface fuscous-black to purplish grey for the younger tissue, reverse greyish-sepia to vinaceous-buff for the younger tissue.
Specimen examined: Iran, Golestan Province, on leaves of Bamboo (Poaceae), 12 May 2009, A. Mirzadi Gohari (holotype CBS H-21303, culture ex-type CBS 135103 =CPC 18118).
Notes: Species of the genus Aphanofalx occur on members of Poaceae, presumably as saprobes. The genus is characterised by having taxa with pycnidial conidiomata, and percurrently proliferating conidiogenous cells, and hyaline, aseptate conidia with a basal, excentric appendage. In contrast, species of Pseudoseptoria are known to occur on members of Poaceae as plant pathogens. The genus is also characterised by having taxa with pycnidial conidiomata, and percurrently proliferating conidiogenous cells, and hyaline, aseptate conidia that lack basal appendages. During this study we also investigated three strains identified as P. donasis (CBS 291.69, 313.68 and 417.51), the type species of Pseudoseptoria. Much to our surprise they formed a monophyletic lineage (results not shown) with the two strains described here (which have basal appendages), suggesting that Pseudoseptoria represents an older name for Aphanofalx, and that the basal appendage is a species-specific character, as also found in other groups of coelomycetes (Crous et al. 2012b).
Aphanofalx is presently known from two species, A. mali (conidia 26-33 × 2-2.5 μm), and A. irregularis (conidia 12-28(-31) × (2-)2.5-3(-3.5) μm (Nag Raj 1993). Pseudoseptoria collariana [conidia (24-) 26-28(-30) × (2.5-)3 μm] and P. obscura [conidia (8-)12-14(-15) × (2-)2.5(-3) μm] are easily distinguished from these taxa based on their conidial dimensions. The three species of Pseudoseptoria treated by Sutton (1980), namely P. donacis (conidia 20-23 × 2-2.5 μm), P. stromaticola (conidia 16-18.5 × 2 μm) and P. bromigena (conidia 20-23 × 2-2.5 μm) can be distinguished from P. collorata and P. obscura by conidial dimensions, and lacking basal conidial appendages.
Clade 27: Parastagonospora
Parastagonospora Quaedvlieg, Verkley & Crous, gen. nov. MycoBank MB804435.
Etymology: Resembling the genus Stagonospora.
Foliicolous, plant pathogenic. Ascocarps immersed, globose, becoming depressed, medium brown to black; wall of 3-6 layers of thick-walled, brown textura angularis; ostiole slightly papillate. Asci clavate, cylindrical or curved, shortly stipitate, 8-spored; ascus wall thick, bitunicate. Ascospores fusoid, subhyaline to pale brown, transversely euseptate (-3), constricted at the septa, penultimate cell swollen. Pseudoparaphyses filiform, hyaline, septate. Conidiomata black, immersed, subepidermal, pycnidial, subglobose with central ostiole, exuding creamy conidial mass; wall of 2-3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, ampulliform to subcylindrical, with percurrent proliferation near apex. Conidia hyaline, smooth, thin-walled, cylindrical, granular to multi-guttulate, with obtuse apex and truncate base, transversely euseptate.
Type species: Parastagonospora nodorum (Berk.) Quaedvlieg, Verkley & Crous.
Notes: The genus Parastagonospora is introduced to accommodate several serious cereal pathogens that were formerly accommodated in either Septoria/Stagonospora, or Leptosphaeria/Phaeosphaeria. As shown previously, Septoria is not available for these fungi (Quaedvlieg et al. 2011), and neither is Leptosphaeria (de Gruyter et al. 2013). Furthermore, in the present study we also clarify the phylogenetic positions of Stagonospora and Phaeosphaeria, which cluster apart from this group of cereal pathogens, which are best accommodated in their own genus, Parastagonospora.
Parastagonospora is distinguished from Stagonospora in that Stagonospora has conidiogenous cells that proliferate percurrently, or via phialides with periclinal thickening, and conidia that are subcylindrical to fusoid-ellipsoidal. Sexual morphs known for species of Parastagonospora are phaeosphaeria-like, whereas those observed for Stagonospora s. str. are didymella-like.
Parastagonospora avenae (A.B. Frank) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804436.
Basionym: Septoria avenae A.B. Frank, Ber. Dt. Bot. Ges. 13: 64. 1895.
≡ Stagonospora avenae (A.B. Frank) Bissett [as ‘avena’], Fungi Canadenses, Ottawa 239: 1. 1982
-
= Leptosphaeria avenaria G.F. Weber, Phytopath. 12: 449. 1922.
≡ Phaeosphaeria avenaria (G.F. Weber) O.E. Erikss., Ark. Bot., Ser. 2 6: 408. 1967.
= Pleospora tritici Garov., Arch. Triennale Lab. Bot. Crittog. 1: 123. 1874.
Specimens examined: Germany, Kiel-Kitzeberg, on Lolium multiflorum, 1968, U.G. Schlösser, CBS 290.69, CBS 289.69.
Notes: Although the oldest epithet for this taxon is Pleospora tritici (1874), “avenae” has been well established in literature, and accepted by the community. We thus recommend that this epithet be retained for this pathogen. Parastagonospora avenae leaf blotch of barley and rye (f.sp. tritici), appears distinct from the pathogen on oats (f.sp. avenaria) (Cunfer 2000), and further research is required to resolve this issue.
Parastagonospora caricis Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804437. Figs 57, 58.
Fig. 57.
Conidia and conidiogenous cells of Parastagonospora caricis (CBS H-21304). Scale bars = 10 μm.
Fig. 58.

Parastagonospora caricis (CBS H-21304). A. Colony sporulating in culture. B, C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Carex.
On sterile Carex leaves on WA. Conidiomata up to 250 μm diam, black, immersed, subepidermal, pycnidial, subglobose with central ostiole, exuding pale pink conidial cirrhus; wall of 2-3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, ampulliform, 8-15 × 4-6 μm, with percurrent proliferation at apex. Conidia hyaline, smooth, thin-walled, scolecosporous, subcylindrical, with subobtuse apex and truncate base, 7-15-septate, (50-)60-70(-75) × (5-)6 μm.
Culture characteristics: Colonies on PDA flat, undulate, with short, white aerial mycelium, surface olivaceous-black in the older parts, vinaceous-buff in the younger mycelium, reverse olivaceous-black in the older parts, brick in the younger mycelium, after 14 d, 4 cm diam; on MEA convex, fimbriate, surface fawn to hazel, reverse fusceous-black to cinnamon, after 14 d, 3 cm diam; on OA similar to MEA.
Specimen examined: Netherlands, Veenendaal, de Blauwe Hel, on leaves of Carex acutiformis (Cyperaceae), 25 Jul. 2012, W. Quaedvlieg (holotype CBS H-21304, culture ex-type CBS 135671 =S615).
Note: Conidia of P. caricis are larger than those of P. avenae, which are (1-)3(-7)-septate, 17-46 × 2.5-4.5 μm (Bissett 1982), and narrower than those of Stagonospora gigaspora, which are 58-84 × 10-14 μm (Ellis & Ellis 1997).
Parastagonospora nodorum (Berk.) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804438. Fig. 59.
Fig. 59.
Parastagonospora nodorum (CBS H-13909). A, C. Ascomata and conidiomata forming in culture. B, D, F. Asci with ascospores. E, G. Conidia. Scale bars = 10 μm.
Basionym: Depazea nodorum Berk., Gard. Chron., London: 601. 1845.
≡ Septoria nodorum (Berk.) Berk., Gard. Chron., London: 601. 1845.
≡ Stagonospora nodorum (Berk.) E. Castell. & Germano, Annali Fac. Sci. Agr. Univ. Torino 10: 71. 1977. [1975-76]
-
= Leptosphaeria nodorum E. Müll., Phytopath. J. 19: 409. 1952.
≡ Phaeosphaeria nodorum (E. Müll.) Hedjar., Sydowia 22: 79. 1969. [1968]
Specimen examined: Denmark, on Lolium perenne, Feb. 2002, M.P.S. Câmara, CBS 110109.
Notes: Parastagonospora nodorum blotch is an important disease of cereals, having been reported from barley and wheat in most countries where these crops are cultivated (Cunfer 2000). Recent studies have also indicated that P. nodorum probably resembles a species complex, awaiting further morphological characterisation (McDonald et al. 2013).
Parastagonospora poae Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804439. Figs 60, 61.
Fig. 60.
Conidia and conidiogenous cells of Parastagonospora poae (CBS 135091). Scale bars = 10 μm.
Fig. 61.

Parastagonospora poae (CBS 135091). A, B. Conidiomata forming in culture. C-E. Conidiogenous cells. F, G. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Poa.
On sterile Carex leaves on WA. Conidiomata up to 250 μm diam, black, immersed, subepidermal, pycnidial, subglobose with central ostiole, exuding creamy conidial mass; wall of 2-3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, ampulliform to subcylindrical, with percurrent proliferation near apex, 6-10 × 3-4(-5) μm. Conidia hyaline, smooth, thin-walled, cylindrical, granular, with obtuse apex and truncate base, medianly 1-septate, (20-)25-27(-32) × (2-)2.5(-2.5) μm; ends becoming swollen and guttulate with age.
Culture characteristics: Colonies on PDA flat, circular, with sparse, white aerial mycelium, surface dark-mouse-grey, reverse black, after 14 d, 8.5 cm diam; on MEA surface hazel, reverse dark-brick to sepia; OA similar to MEA.
Specimens examined: Netherlands, Wageningen, on leaves of Poa sp. (Poaceae), 2 Aug. 2012, S. Videira J (holotype CBS H-21305, culture ex-type CBS 135089 =S606); Wageningen, on leaves of Poa sp., 2 Aug. 2012, S. Videira CBS 135091 =S613).
Note: Conidia of P. poae are narrower than those of P. nodorum, which are (0-)1-3-septate, 13-28 × 2.8-4.6 μm (Bissett 1982).
Clade 28: Neostagonospora
Neostagonospora Quaedvlieg, Verkley & Crous, gen. nov. MycoBank MB804440.
Etymology: Resembling the genus Stagonospora.
Foliicolous. Conidiomata immersed, pycnidial, globose, exuding a pale luteous to creamy conidial mass; wall of 2-3 layers of pale brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, ampulliform to doliiform, tapering at apex with prominent periclinal thickening. Conidia hyaline, smooth, granular, thin-walled, narrowly fusoid-ellipsoidal to subcylindrical, apex subobtusely rounded, base truncate, widest in middle, transversely euseptate, becoming constricted with age.
Type species: Neostagonospora caricis Quaedvlieg, Verkley & Crous.
Note: Neostagonospora is similar to Stagonospora by having pycnidial conidiomata with euseptate, hyaline, fusoid-ellipsoidal to subcylindrical conidia, but distinct in having conidiogenous cells that are phialidic, with prominent periclinal thickening.
Neostagonospora caricis Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804441. Figs 62, 63.
Fig. 62.
Conidia and conidiogenous cells of Neostagonospora caricis (CBS 135092). Scale bars = 10 μm.
Fig. 63.

Neostagonospora caricis (CBS 135092). A. Conidioma forming in culture. B, C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus on which it occurs, Carex.
On sterile Carex leaves on WA. Conidiomata immersed, pycnidial, globose, up to 200 μm diam, exuding a pale luteous to creamy conidial mass; wall of 2-3 layers of pale brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, ampulliform to doliiform, 5-7 × 5-7 μm; tapering at apex with prominent periclinal thickening. Conidia hyaline, smooth, granular, thin-walled, narrowly fusoid-ellipsoidal, apex subobtusely rounded, base truncate, widest in middle, 1-septate, becoming constricted with age, (10-)13-16(-19) × (3-)3.5(-4) μm.
Culture characteristics: Colonies on PDA flat, undulate, with sparse, powdery white aerial mycelium, surface greyish-sepia to isabelline, reverse olivaceous-grey to pale olivaceous-grey, after 14 d, 8.5 cm diam; on MEA erumpent, circular, with fine white aerial mycelium, surface honey, reverse cinnamon, after 14 d, 6 cm diam; on OA similar to PDA but surface honey, reverse cinnamon.
Specimen examined: Netherlands, Veenendaal, de Blauwe Hel, on leaves of Carex acutiformis (Cyperaceae), Aug. 2012, W. Quaedvlieg (holotype CBS H-21306, culture ex-type CBS 135092 =S616).
Note: Neostagonospora caricis is similar to Septoria caricis (conidia 1-septate, 20-35 × 2.5-3 μm; Ellis & Ellis 1997), although its conidia are shorter.
Neostagonospora elegiae Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804442. Figs 64, 65.
Fig. 64.
Conidia and conidiogenous cells of Neostagonospora elegiae (CBS 135101). Scale bars = 10 μm.
Fig. 65.
Neostagonospora elegiae (CBS 135101). A. Conidioma forming in culture. B-D. Conidiogenous cells. E. Conidia. Scale bars: A = 150 μm, all others = 10 μm.
Etymology: Named after the host genus from which it was collected, Elegia.
On Anthriscus stem. Conidiomata pycnidial, up to 150 μm diam, erumpent, globose, brown, opening by a central ostiole, exuding a crystalline conidial mass; wall consisting of 3-6 layers of pale brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, lining the inner cavity, hyaline, smooth, ampulliform, 4-7 × 4-6 μm; apex with prominent periclinal thickening. Conidia hyaline, smooth, guttulate to granular, scolecosporous, irregularly curved, subcylindrical, apex subobtuse, base truncate (slight taper from apical septum to apex and basal septum to hilum visible in some conidia), (0-)3-septate, (20-)50-65(-70) × (2.5-)3 μm.
Culture characteristics: Colonies spreading, erumpent with moderate aerial mycelium and smooth, even margins; reaching 35 mm diam after 2 wk. On OA pale luteous. On MEA dirty white on surface, luteous in reverse. On PDA dirty white on surface, pale luteous in reverse.
Specimen examined: South Africa, Western Cape Province, Harold Porter Botanical Garden, on leaves of Elegia cuspidata (Restionaceae), 30 Nov. 2001, S. Lee (holotype CBS H-21307, culture ex-type CBS 135101 =CPC 16977).
Notes: No septoria-like fungi are presently known from Elegia (Lee et al. 2004). Neostagonospora elegiae is distinguished from N. caricis based on its conidial morphology.
Clade 29: Phaeosphaeriopsis
Phaeosphaeriopsis M.P.S. Câmara, M.E. Palm & A.W. Ramaley, Mycol. Res. 107: 519. 2003.
Saprobic or plant pathogenic. Ascomata solitary or aggregated, immersed, subepidermal to erumpent, pushing up flaps of the epidermis, globose to pyriform, often papillate, solitary or gregarious in a stroma of scleroplectenchyma or dark brown textura angularis, often surrounded by septate, brown hyphae extending into the host tissues. Asci 8-spored, bitunicate, cylindrical to broadly fusoid, short stipitate, with visible apical chamber. Ascospores uni- to triseriate, cylindrical, broadly rounded at apex, tapering to narrowly rounded base, 4-5-septate, first septum submedian, often constricted, medium brown, echinulate, punctate or verrucose. Asexual morph coniothyrium-like or phaeostagonospora-like. Conidiomata pseudoparenchymatous, sometimes of scleroplectenchyma. Conidiogenous cells lining locule, ampulliform, hyaline, proliferating percurrently, resulting in inconspicuous annellations. Conidia cylindrical, with bluntly rounded ends, 0-3-septate, yellowish brown, punctate (Câmara et al. 2003, Zhang et al. 2012).
Type species: Phaeosphaeriopsis glaucopunctata (Grev.) M.P.S. Câmara, M.E. Palm & A.W. Ramaley, Mycol. Res., 107: 519. 2003.
Phaeosphaeriopsis glaucopunctata (Grev.) M.P.S. Câmara, M.E. Palm & A.W. Ramaley, Mycol. Res. 107: 519. 2003. Figs 66, 67.
Fig. 66.
Conidia and conidiogenous cells of Phaeosphaeriopsis glaucopunctata (CBS 653.86). Scale bar = 10 μm.
Fig. 67.

Phaeosphaeriopsis glaucopunctata (CBS 653.86). A. Colony on MEA. B. Colony on OA. C-F. Conidiogenous cells giving rise to conidia. G. Conidia. Scale bars = 10 μm
Basionym: Cryptosphaeria glaucopunctata Grev., Fl. Edin.: 362. 1824.
≡ Paraphaeosphaeria glaucopunctata (Grev.) Shoemaker & C. E. Babc., Can. J. Bot. 63: 1286. 1985.
-
= Sphaeria rusci Wallr., Fl. Crypt. Germ. 2: 776. 1833.
≡ Leptosphaeria rusci (Wallr.) Sacc., Syll. Fung. 2: 74. 1883.
≡ Paraphaeosphaeria rusci (Wallr.) O. E. Erikss., Ark. Bot., Ser. 2 6: 406. 1967.
Ascomata scattered or aggregated, immersed, globose to subglobose, up to 250 μm diam; peridium up to 25 μm wide, of thick-walled textura angularis; hamathecium of dense, wide, cellular pseudoparaphyses, 3-5 μm diam. Asci 8-spored, bitunicate, cylindrical to broadly fusoid, with a short pedicel and small apical chamber, 50-110 × 10-16 μm. Ascospores uni- to triseriate, cylindrical, medium brown, 4(-5)-septate, without constriction or slightly constricted at the basal septum, the forth cell from the apex usually slightly inflated, the basal cell often longer, 14-28 × (3.5-)5-7.5 μm. Conidiomata pycnidial, immersed, scattered or aggregated, dark brown, subglobose, ostiolate, up to 200 μm diam. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity, ampulliform, hyaline, smooth, 5-10 × 3-6 μm; proliferating percurrently at apex. Conidia aseptate, smooth to finely verruculose, medium brown, subcylindrical, straight to reniform with obtuse ends, (5-)7-9(-10) × (2.5-)3(-5) μm.
Culture characteristics: On PDA colonies flat, spreading, with sparse aerial mycelium and smooth, lobate, even margins, surface primrose, reverse olivaceous-buff, On OA buff with patches of isabelline due to sporulating conidiomata. On MEA dirty white on surface, isabelline in reverse (centre), cinnamon in outer region.
Specimen examined: Switzerland, Kt. Basel-Stadt, Park Basel, on Ruscus aculeatus (Ruscaceae), 25 Sep. 1980, A. Leuchtmann (CBS H-21308, culture CBS 653.86).
Notes: The genus Phaeosphaeriopsis is characterised by having uni- or multiloculate stromata and 4-5-septate ascospores. It presently contains species with coniothyrium-like, and phaeostagonospora-like asexual morphs (e.g. P. musae; Arzanlou & Crous 2006). The type species, Phaeosphaeriopsis glaucopunctata, is associated with leaf spot and necrosis on Ruscus aculeatus (Câmara et al. 2003, Golzar & Wang 2012). The fact that an isolate identified as Chaetosphaeronema hispidulum (lectotype of Chaetosphaeronema) clusters in this clade is puzzling. The genus Chaetosphaeronema is characterised by setose, dark brown pycnidia with thick-walled outer cell layers, producing hyaline, 1-septate conidia (Sutton 1980). Isolate CBS 216.75 proved to be sterile, however, so this matter could unfortunately not be resolved.
Clade 30: Sclerostagonospora
Description: See above.
Type species: S. heraclei (Sacc.) Höhn., Hedwigia 59: 252. 1917.
Sclerostagonospora phragmiticola Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804443. Fig. 68.
Fig. 68.

Sclerostagonospora phragmiticola (CBS 338.86). A. Colony sporulating in culture. B, C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Phragmites.
On sterile Carex leaves on WA. Conidiomata pycnidial, brown, globose, immersed to erumpent, up to 400 μm diam with central ostiole; wall of 6-8 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity of conidioma, hyaline to pale olivaceous, smooth, subcylindrical to doliiform, 6-15 × 3-4 μm, proliferating several times percurrently at apex. Conidia brown, smooth, subcylindrical, apex obtuse, base truncate, straight to gently curved, (1-)3(-5)-euseptate, older conidia swelling, becoming widest in second or third cell from base, (15-)20-25(-27) × (3-)3.5(-4) μm.
Specimen examined: France, Landes, Seignosse, Étang d’Hardy, on leaves of Phragmites australis (Poaceae), 11 June 1986, H.A. van der Aa (holotype CBS H-21309, culture ex-type CBS 338.86).
Notes: Sclerostagonospora caricicola fits the concept of Sclerostagonospora by having pycnidial conidiomata that give rise to hyaline conidiogenous cells that proliferate percurrently, and subcylindrical, pigmented conidia. Until fresh material of the type species, S. heraclei has been recollected and subjected to DNA analysis, the application of this generic name will remain tentative. Several other species cluster in this clade, suggesting that the sexual morph is phaeosphaeria-like.
Clade 31: Phaeosphaeria
Phaeosphaeria I. Miyake, Bot. Mag., Tokyo 23: 93. 1909.
= Phaeoseptoria Speg., Revta Mus. La Plata 15: 39. 1908.
Foliicolous. Ascomata immersed, subepidermal, ellipsoidal to globose, glabrous; ostiole central, devoid of periphyses; wall of 2-3 layers of brown textura angularis. Pseudoparaphyses transversely septate, guttulate, encased in mucous. Asci stipitate, clavate to cylindrical, stalked, biseriate. Ascospores brown, narrowly fusiform, straight or slightly curved, transversely septate, smooth to verruculose, enclosed in a mucoid sheath or not. Conidiomata pycnidial, immersed, becoming erumpent, brown, with central ostiole; wall of 2-3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, ampulliform to subcylindrical or doliiform; proliferating inconspicuously percurrently near apex. Conidia solitary, pale brown, smooth, guttulate, subcylindrical to narrowly obclavate, apex obtuse, base truncate, straight to curved, transversely euseptate, at times slightly constricted at septa; hilum not darkened nor thickened.
Type species: P. oryzae I. Miyake, Bot. Mag. Tokyo, 23(266): 93. 1909.
Notes: Phaeosphaeria (1909; based on P. oryzae) is congeneric with Phaeoseptoria (1908; based on P. papayae). We choose to use the sexual name Phaeosphaeria, as it is well established, and less confused than Phaeoseptoria, which has become a confused concept applied to numerous septoria-like taxa with pigmented conidia (see Walker et al. 1992).
Phaeosphaeria oryzae I. Miyake, Bot. Mag. Tokyo, 23(266): 93. 1909. Figs 69, 70.
Fig. 69.
Asci and ascospores of Phaeosphaeria oryzae (BPI 744438). Scale bars = 10 μm.
Fig. 70.
Phaeosphaeria oryzae (BPI 744438). A. Ascomata on host tissue. B-G. Asci. H. Ascospores. Scale bars = 10 μm.
≡ Pleospora oryzae (I. Miyake) Hara, J. Agric. Soc. Japan 31(361): 17. 1927.
≡ Trematosphaerella oryzae (I. Miyake) Padwick, A manual of rice diseases: 153. 1950.
≡ Leptosphaerella oryzae (I. Miyake) Hara, A monograph of rice diseases: 53. 1959.
≡ Leptosphaerulina oryzae (I. Miyake) Karan, Mycopath. Mycol. Appl. 24: 88. 1964.
= Phaeoseptoria oryzae I. Miyake, J. Coll. Agric. Imp. Univ. Tokyo 2(4): 260. 1910.
Ascomata immersed, subepidermal, ellipsoidal to globose, glabrous, up to 150 μm diam, ostiole central, up to 20 μm diam, devoid of periphyses; wall of 2-3 layers of brown textura angularis. Pseudoparaphyses 2-3 μm diam, transversely septate, guttulate, encased in mucous. Asci stipitate, cylindrical, 30-55 × 7-9 μm, stalked, biseriate. Ascospores brown, narrowly fusiform, straight or slightly curved, (15-)17-20(-23) × 4(-5) μm, 3-septate, uniformly verruculose, enclosed in a mucoid sheath; after discharge, ascospores become prominently swollen, up to 33 μm long and 8 μm wide.
Specimens examined: Japan, No. 196178, on 2, Prov. Susuya Shizuoka, Sep. 1907, ex Herb. Sydow, ex S., as Leptosphaeria oryzae Hori = Phaeosphaeria oryzae I. Miyake, slides prepared by O. Eriksson, lectotype (UPS). Korea, on leaf of Oryza sativa (Poaceae), intercepted at Port San Francisco, CA, 29 Dec. 1997, coll. L. Hausch, det. M.E. Palm, epitype designated here as BPI 744438, culture ex-epitype CBS 110110 (MBT175330).
Notes: Several detailed accounts of this species are available (Eriksson 1967, Shoemaker & Babcock 1989, Fukuhara 2002). The epitype chosen here closely matches the lectotype in morphology.
Phaeosphaeria papayae (Speg.) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804444. Figs 71, 72.
Fig. 71.
Conidia, ascospores and ascus of Phaeosphaeria papayae (CBS H-21310). Scale bars = 10 μm.
Fig. 72.
Phaeosphaeria papayae (CBS H-21310). A. Leaf spot. B. Conidioma with ostiole (arrow). C-E. Conidiogenous cells. F. Conidia. G-K. Asci and ascospores. Scale bars = 10 μm.
Basionym: Phaeoseptoria papayae Speg., Revta Mus. La Plata: 39. 1908.
Leaf spots associated with infections of Asperisporium caricae, amphigenous, pale brown to grey-white, subcircular to angular, 1-5 mm diam, with red-purple margin; conidiomata developing and sporulating on leaves when incubated in moist chambers, with white, fluffy mycelium erumpting from lesions. Conidiomataamphigenous, pycnidial, brown, globose, up to 120 μm diam, with central ostiole, exuding a brown conidial cirrhus; wall of 3-4 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity, hyaline, smooth, ampulliform to subcylindrical or doliiform, 5-12 × 4-6 μm; proliferating inconspicuously percurrently near apex (conidiogenous cells disintegrating at maturity). Conidia solitary, pale brown, smooth, guttulate, subcylindrical to narrowly obclavate, apex obtuse, base truncate, (1-)3(-4)-septate, at times slightly constricted at septa, straight to slightly curved, (15-)26-32(-35) × (2.5-)3 μm; hilum not darkened nor thickened, 2 μm diam. Ascomata developed after 4 wk in culture on sterile nettle stems: aggregated in black clusters, globose, up to 150 μm diam, with central ostiole; wall of 2-3 layers of brown textura angularis. Asci bitunicate, curved to straight, fasciculate, short stipitate with ocular chamber, 40-60 × 8-11 μm. Pseudoparaphyses hyaline, smooth, 2-3 μm, septate, constricted at septa, not anastomosing, hypha-like with obtuse ends, distributed among asci. Ascospores tri to multiseriate, fusoid, curved to straight, brown, verruculose throughout, somewhat constricted at septa with age, second cell from apex swollen, (18-)24-26(-29) × (3-)4(-5) μm.
Culture characteristics: Colonies with abundant aerial mycelium, covering dish within 2 wk at 24 °C, fast growing, olivaceous-grey on MEA (surface and reverse); margins smooth, even, sterile on MEA, PDA and OA, as well as on SNA with sterile barley leaves.
Specimens examined: Brazil, São Paulo, Botanical Garden, on leaves of Carica papaya (Caricaceae), Sep. 1908, IMI 246301, slide ex-holotype; Minas Gerais, Viçosa, UFV campus, on leaves of Carica papaya, Mar. 2013, A.C. Alfenas, epitype designated here as CBS H-21310, culture ex-epitype CBS 135416 (MBT175331).
Notes: It is interesting to note that Walker et al. (1992) also observed Phaeoseptoria papayae to co-occur with Asperisporium caricae on the holotype specimen (noted by Spegazzini as Cercospora caricae), suggesting that the co-occurrence of these two pathogens is quite common. The fresh collection obtained in this study enabled us to elucide the conidiogenesis of the fungus (not observed by Walker et al. 1992), and also designate an epitype specimen. Phylogenetically it is closely related to Phaeosphaeria oryzae, which has Phaeoseptoria oryzae as asexual morph.
Clade 32: Neosetophoma
Neosetophoma Gruyter, Aveskamp & Verkley, Mycologia 102(5): 1075. 2010.
Foliicolous, plant pathogenic. Conidiomata pycnidial, solitary to confluent, on upper surface of agar, globose to irregular, with mycelial outgrowths, or confluent, with papillate ostioles, sometimes developing long necks, honey to olivaceous or olivaceous-black, with up to 10 layers of pseudoparenchymatal cells. Conidiogenous cells hyaline, monophyalidic. Conidia slightly yellowish, 0-1(-3)-septate, ellipsoidal to cylindrical, usally attenuate at one end, often guttulate.
Type species: N. samarorum Gruyter, Aveskamp & Verkley, Mycologia 102(5): 1075. 2010.
Note: The fact that several strains with a phaeosphaeria-like morphology cluster in this clade, suggests that sexual states do exist for species of Neosetophoma.
Clade 33: Paraphoma
Paraphoma Morgan-Jones & J.F. White, Mycotaxon 18: 58. 1983.
Mycelium consisting of branched, septate, subhyaline to pale brown, smooth hyphae. Conidiomata pycnidial, solitary to aggregated, superficial to immersed, dark brown, globose to subglobose, papillate, uniloculate, setose; ostiole circular, single; wall of 3-6 layers of brown textura angularis. Setae copious, straight to flexuous, smooth to verruculose, thick-walled, septate, pale brown to brown. Conidiogenous cells lageniform, monophalidic, formed from inner layer of conidiomatal wall, hyaline to subhyaline, discrete. Conidia ellipsoid, aseptate, hyaline, smooth, guttulate. Chlamydospores if present unicellular.
Type species: P. radicina (McAlpine) Morgan-Jones & J.F. White, Mycotaxon 18: 60. 1983.
Paraphoma dioscoreae Quaedvlieg, H.D. Shin, Verkley & Crous, sp. nov. MycoBank MB804445. Figs 73, 74.
Fig. 73.
Conidia and conidiogenous cells of Paraphoma dioscoreae (CBS 135100). Scale bar = 10 μm.
Fig. 74.

Paraphoma dioscoreae (CBS 135100). A. Conidioma forming in culture. B-E. Conidiogenous cells. F. Conidia. Scale bars: B = 350 μm, all others = 10 μm.
Etymology: Named after the host genus from which it was collected, Dioscorea.
On Anthriscus stem. Conidiomata pycnidial, separate, immersed becoming erumpent, globose, with papillate neck and central ostiole exuding a crystalline conidial mass; conidiomata up to 350 μm diam, neck up to 150 μm diam, of darker brown cells than body, which is pale brown; wall of 3-6 layers of pale brown textura angularis. Conidiophores hyaline, smooth, subcylindrical, reduced to conidiogenous cells, 1-5-septate, irregularly branched, 5-20 × 3-5 μm. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to subcylindrical (long, elongated neck on Anthriscus stem, but not on MEA), 5-15 × 2-3 μm; apex with prominent periclinal thickening, or with several percurrent prolferations (especially on conidiogenous cells with elongated necks). Conidia solitary, straight to slightly curved, hyaline, smooth, aseptate, cylindrical with obtuse ends and a guttule at each end, (5-)6(-7) × 2(-2.5) μm.
Culture characteristics: Colonies flat, spreading with sparse aerial mycelium and even, smooth margins; after 2 wk reaching 30 mm diam on MEA, 40 mm on PDA and 50 mm on OA. On PDA dark brick, reverse fuscous-black. On OA dark brick with patches of sienna and ochreous. On MEA surface dirty white (due to aerial mycelium), also somewhat sectored, reverse umber.
Specimen examined: South Korea, on leaves of Dioscorea tokoro (Dioscoreaceae), 24 Oct. 2003, H.D. Shin (holotype CBS H-21311, culture ex-type CPC 11357 = CBS 135100).
Note: Paraphoma dioscoreae is phylogenetically distinct from the three other species presently known in the genus (de Gruyter et al. 2010).
Clade 34: Xenoseptoria
Xenoseptoria Quaedvlieg, H.D. Shin, Verkley & Crous, gen. nov. MycoBank MB804446.
Etymology: Similar to the genus Septoria s. str., but distinct.
Foliicolous, plant pathogenic. Conidiomata separate, pycnidial, immersed becoming erumpent, globose, brown, developing 1-3 papillate necks, exuding a pink to orange conidial mass; wall of 4-8 layers of brown textura angularis. Conidiophores hyaline, smooth, reduced to conidiogenous cells or septate, branched below. Conidiogenous cells lining the inner cavity, hyaline, smooth, ampulliform to doliiform or subcylindrical, mono- to polyphialidic, with prominent periclinal thickening, but also with percurrent proliferation. Conidia hyaline, smooth, guttulate, scolecosporous, straight to irregularly curved, cylindrical to obclavate, transversely euseptate, tapering to subobtuse apex, base obtuse.
Type species: Xenoseptoria neosaccardoi Quaedvlieg, Verkley & Crous.
Xenoseptoria neosaccardoi Quaedvlieg, H.D. Shin, Verkley & Crous, sp. nov. MycoBank MB804447. Figs 75, 76.
Fig. 75.
Conidia and conidiogenous cells of Xenoseptoria neosaccardoi (CBS 128665). Scale bars = 10 μm.
Fig. 76.
Xenoseptoria neosaccardoi (CBS 128665). A, B. Pycnidia forming in culture. C-E. Conidiogenous cells. F, G. Conidia. Scale bars: B = 170 μm, all others = 10 μm.
Etymology: Resembling Septoria saccardoi, but morphologically distinct.
Leaf spots on the upper leaf surface, scattered, distinct, circular, 2-4 mm diam, initially appearing as reddish brown discolouration, later turning brown to reddish brown without a distinct border line, finally central area becoming greyish brown to dull grey and surrounded by reddish to dark brown margin, reddish pigments may diffuse outward to form a halo; on the lower leaf surface initially showing reddish discolouration, later becoming brown with distinct border line, center greyish brown to grey with indistinct border (Shin & Sameva 2004). On sterile Carex leaves on WA. Conidiomata separate, pycnidial, immersed becoming erumpent, globose, up to 350 μm diam, brown, becoming ostiolate, developing 1-3 papillate necks, exuding a pink to orange conidial mass; wall of 4-8 layers of brown textura angularis. Conidiophores hyaline, smooth, reduced to conidiogenous cells or 1-2-septate, branched below, 10-20 × 4-6 μm. Conidiogenous cells lining the inner cavity, hyaline, smooth, ampulliform to doliiform or subcylindrical, mono- to polyphialidic, with prominent periclinal thickening, but also with percurrent proliferation, 5-15 × 3-5 μm. Conidia hyaline, smooth, guttulate, scolecosporous, straight to irregularly curved, cylindrical to obclavate, (1-)3-septate, (23-)33-45(-48) × (2.5-)3(-4) μm, tapering to subobtuse apex, base obtuse, 2-2.5 μm diam.
Culture characteristics: Colonies flat, spreading, with sparse aerial mycelium and lobate, feathery mergins, reaching 30 mm after 2 wk. On PDA surface iron-grey, reverse olivaceous-grey; on OA surface olivaceous-grey; on MEA surface folded, bay, reverse umber.
Specimen examined: South Korea, Pyeongchang, on leaves of Lysimachia vulgaris var. davurica (Primulaceae), 30 May 2007, H.D. Shin (holotype CBS H-21312, culture ex-type CBS 128665 =KACC 43962 = SMKC 23666).
Notes: An isolate of Septoria saccardoi (CBS 128756) clusters in Septoria s. str., thus well apart from this taxon, which was collected in Korea. The Korean collection closely matches that of the original description of Septoria saccardoi (on Lysimachia vulgaris in Italy), having 3-septate, curved, cylindrical conidia, 38-40 × 3.5 μm, 3-septate (Saccardo & Saccardo 1906). Xenoseptoria is however distinct from Septoria s. str. in forming pycnidia with multiple papillate necks, and having conidiogenous cells that are mono- or polyphialidic.
Clade 35: Vrystaatia
Vrystaatia Quaedvlieg, W.J. Swart, Verkley & Crous, gen. nov. MycoBank MB804448.
Etymology: Named after the Free State Province in South Africa, “Vrystaat” in Afrikaans, where this fungus was collected.
Foliicolous. Conidiomata black, globose, pycnidial with central, dark brown ostiolar area, substomatal on host, erumpent in culture; wall of 6-8 layers of pale brown textura angularis; exuding cirrhus of orange conidia. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity of conidioma, globose to ampulliform, rarely allantoid, hyaline, smooth; with prominent periclinal thickening, or proliferating several times percurrently near apex, giving rise to macro- and microconidia. Macroconidia solitary, hyaline, smooth, guttulate, subcylindrical to narrowly obclavate or acicular, apex obtuse to subobtuse, base truncate to long obconically truncate, conidia widest at or just above basal septum, transversely euseptate. Microconidia hyaline, smooth, aseptate, pear-shaped to globose or ellipsoid, apex obtuse, base truncate.
Type species: Vrystaatia aloeicola Quaedvlieg, Verkley, W.J. Swart & Crous.
Vrystaatia aloeicola Quaedvlieg, Verkley, W.J. Swart & Crous, sp. nov. MycoBank MB804449. Figs 77, 78.
Fig. 77.
Macro- and microconidia and conidiogenous cells of Vrystaatia aloeicola (CBS 135107). Scale bars = 10 μm.
Fig. 78.
Vrystaatia aloeicola (CBS 135107). A. Conidiomata sporulating on PDA, with characteristic orange conidial cirrhi. B-D. Conidiogenous cells. E, F. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Aloe.
On sterile Carex leaves on WA. Conidiomata black, globose, pycnidial with central, dark brown ostiolar area, substomatal on host, erumpent in culture; wall of 6-8 layers of pale brown textura angularis; exuding cirrhus of orange conidia. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity of conidioma, globose to ampulliform, rarely allantoid, hyaline, smooth, 5-12 × 4-6 μm; with prominent periclinal thickening, or proliferating several times percurrently near apex, 2-2.5 μm diam, giving rise to macro- and microconidia. Macroconidia solitary, hyaline, smooth, guttulate, subcylindrical to narrowly obclavate or acicular, apex obtuse to subobtuse, base truncate to long obconically truncate, conidia widest at or just above basal septum, (1-)3-septate, (30-)40-52(-65) × (2.5-) 3(-3.5) μm. Microconidia hyaline, smooth, aseptate, pear-shaped to globose or ellipsoid, apex obtuse, base truncate, 4-6 × 3-3.5 μm.
Culture characteristics: On MEA colonies spreading fast, with moderate aerial mycelium and smooth, even margin, reaching 30 mm diam after 2 wk; surface with concentric zones of umber and apricot; reverse umber, produces brown exudates; on PDA round lobate margins, lacking aerial mycelium, reaching 20 mm diam after 2 wk, surface fuscous-black to greyish-sepia for younger mycelium, reverse fuscous-black to greyish-sepia for younger mycelium; on OA round, lobate, lacking aerial mycelium, reaching 30 mm diam after 2 wk, surface vinaceous-grey, reverse greyish sepia.
Specimen examined: South Africa, Orange Free State, Bloemfontein, Free State National Botanical Garden, on dead leaf tips of Aloe maculata (Aloaceae), 7 May 2012, P.W. Crous & W.J. Swart (holotype CBS H-21313, culture ex-type CBS 135107 =CPC 20617).
Notes: Vrystaatia is distinct from Septoria s. str. in that it has phialidic conidiogenous cells that proliferate percurrently or with prominent periclinal thickening, and form macro- as well as microconidia in culture, which is not typical of Septoria. Rhabdospora aloetica was described from stems of Aloe sp. in Portugal, with aseptate conidia, 12-16 × 1.5 μm (Saccardo & Saccardo 1906); it is likely this is an asexual morph of Diaporthe. As far as we could establish, no septoria-like fungi have thus far been described from Aloe.
Clade 36: Setophoma
Setophoma Gruyter, Aveskamp & Verkley, Mycologia 102: 1077. 2010.
Conidiomata pycnidial, solitary to confluent, superficial or submerged in agar, globose to subglobose, setose, with papillate ostioles, honey to olivaceous to olivaceous-black, with 2-7(-11) layers of pseudoparenchymatal cells. Conidiogenous cells hyaline, monophyalidic. Conidia aseptate, ellipsoidal to subcylindrical to subfusoid, guttulate.
Type species: S. terrestris (H.N. Hansen) Gruyter, Aveskamp & Verkley, Mycologia 102: 1077. 2010.
Setophoma chromolaenae Quaedvlieg, Verkley, R.W. Barreto & Crous, sp. nov. MycoBank MB804450. Figs 79, 80.
Fig. 79.
Conidia and conidiogenous cells of Setophoma chromolaenae (CBS 135105). Scale bar = 10 μm.
Fig. 80.
Setophoma chromolaenae (CBS 135105). A. Conidiomata forming on OA. B, C, F. Conidiomata with setae. D, E. Conidiogenous cells. G. Conidia. Scale bars: B = 22 μm, C, F = 45 μm, all others = 10 μm.
Etymology: Named after the host genus from which it was collected, Chromolaena.
Conidiomata pycnidial, brown, globose, separate, erumpent, up to 90 μm diam; outer surface covered in brown setae, up to 80 μm long, brown, thick-walled, 3-5 μm diam, 1-4-septate, with slight apical taper to obtuse apex; conidial wall of 3-6 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity, ampulliform, hyaline, smooth, 4-6 × 3-6 μm, with prominent periclinal thickening at apex. Conidia hyaline, smooth, subcylindrical, somewhat narrowly ellipsoid when old, with two prominent guttules at ends, (4.5-)5-6 (-7) × (2-)2.5(-3) μm.
Culture characteristics: On MEA spreading, with sparse aerial mycelium, folded surface, margin smooth, lobate; surface umber with patches of apricot and dirty white, reverse ochreous. On PDA surface iron-grey, reverse olivaceous-grey. On OA surface iron-grey, surrounded by orange to apricot diffuse pigment layer in agar; reaching 55 mm diam after 2 wk.
Specimen examined: Brazil, Rio de Janeiro, Fazenda Santa Rosa, Ponte das Laranjeiras, on leaves of Chromolaena odorata (Asteraceae), 6 Apr. 2010, R.W. Barreto (holotype CBS H-21314, culture ex-type CBS 135105 =CPC 18553).
Note: Setophoma chromolaenae is phylogenetically distinct from S. sacchari and S. terrestris, the two other species presently known from the genus (de Gruyter et al. 2010).
Clade 37: Coniothyrium (Coniothyriaceae)
Coniothyrium Corda, Icon. Fung. (Prague) 4: 38. 1840.
Mycelium immersed, consisting of septate, hyaline to brown, branched hyphae. Conidiomata pycnidial, separate, globose, pale to dark brown, immersed, unilocular, thin-walled; wall of brown, thick-walled textura angularis. Ostiole circular, central, papillate or not. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining the inner cavity, phialidic, annellidic, indeterminate, discrete, doliiform to cylindrical, hyaline to pale brown, smooth, several annellations at apex. Conidia subcylindrical, spherical, ellipsoid or broadly clavate, brown, thick-walled, 0(-1)-euseptate, smooth to verruculose, apex obtuse, base truncate, at times with minute marginal frill (Sutton 1980).
Type species: C. palmarum Corda, Icon. Fung. (Prague) 4: 38. 1840.
Coniothyrium sidae Quaedvlieg, Verkley, R.W. Barreto & Crous, sp. nov. MycoBank MB804451. Figs 81, 82.
Fig. 81.
Conidia and conidiogenous cells of Coniothyrium sidae (CBS 135108). Scale bar = 10 μm.
Fig. 82.
Coniothyrium sidae (CBS 135108). A-E. Conidiomata forming in culture, showing setae. F, G. Conidiogenous cells. H. Conidia. I-K. Asci and ascospores. Scale bars: B, D, E = 100 μm, all others = 10 μm.
Etymology: Named after the host genus from which it was collected, Sida.
Conidiomata pycnidial, globose, immersed becoming erumpent, up to 200 μm diam; wall consisting of 3-4 layers of subhyaline to pale brown textura angularis. Ostiole central, papillate, dark brown, up to 30 μm diam, surrounded by a whorl of brown setae, smooth, thick-walled, 4-8-septate, straight to curved, tapering to subobtuse apices, up to 130 μm long, 5-8 μm diam at base. Conidiogenous cells hyaline, smooth, lining the inner cavity, ampulliform to globose, 4-7 × 4-6 μm; apex with prominent periclinal thickening. Conidia solitary, hyaline, smooth, aseptate, granular (in Shear’s medium, prominently guttulate in lactic acid), fusoid-ellipsoidal, straight to slightly curved, apex obtuse, base truncate to bluntly rounded, (9-)10-12(-13) × (2.5-)3 μm. Ascomata developing after several weeks on MEA, separate, pseudothecial, erumpent, uniloculate, papillate, brown, up to 300 μm diam; wall of 4-8 layers of brown textura angularis. Asci fasciculate, 8-spored, short papillate, hyaline, smooth, subcylindrical, bitunicate, with well-developed apical chamber, 2 μm diam, 55-65 × 8-11 μm. Ascospores bi- to triseriate, brown, smooth, guttulate, straight to slightly curved, (3-)5-septate, apical cell obtusely rounded, basal cell somewhat elongated and subobtuse; in ascospores that are 4-septate, the second cell from the apex is markedly swollen, in 5-septate ascospores the third cell from the apex is markedly swollen, (18-)20-24(-26) × (4-)5(-5.5) μm. Pseudoparaphyses hyaline, smooth, intermingled among asci, anastomosing, cellular, constricted at septa, up to 80 μm long, 2-4 μm diam.
Culture characteristics: Colonies erumpent, spreading, moderate aerial mycelium even, lobate margins. On MEA surface olivaceous-grey, reverse umber. On OA suface olivaceous-grey with diffuse umber pigment in agar. On PDA surface and reverse olivaceous-grey.
Specimen examined: Brazil, Rio de Janeiro, Nova Friburgo, Riograndina, along roadside on Sida sp. (Malvaceae), 24 Feb. 2008, R.W. Barreto (holotype CBS H-21315, culture ex-type CPC 19602 = RWB 866 = CBS 135108).
Note: De Gruyter et al. (2013) placed several phoma-like species with a similar morphology in the genus Coniothyrium, to which C. sidae is allied. Of interest is the paraphaeosphaeria-like sexual morph that developed in culture, which is newly linked here to Coniothyrium. The genus Paraphaeosphaeria is linked to Paraconiothyrium (Verkley et al. 2004).
Clade 38: Xenobotryosphaeria
Xenobotryosphaeria Quaedvlieg, Verkley & Crous, gen. nov. MycoBank MB804452.
Etymology: Resembling the genus Botryosphaeria, but distinct.
Ascomata brown, globose, smooth, ostiolate, superficial on stems; wall of 3-4 layers of brown textura angularis. Asci clavate, hyaline, smooth, short stipitate, fasciculate, bitunicate, thin-walled, apical chamber not visible, 6-8-spored. Ascospores multiseriate, hyaline, smooth and thin-walled, granular, broadly ellipsoid, ends obtuse, aseptate. Pseudoparaphyses not seen.
Type species: Xenobotryosphaeria calamagrostidis Quaedvlieg, Verkley & Crous.
Xenobotryosphaeria calamagrostidis Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804453. Figs 83, 84.
Fig. 83.
Ascospores and asci of Xenobotryosphaeria calamagrostidis (CBS 303.71). Scale bars = 10 μm.
Fig. 84.
Xenobotryosphaeria calamagrostidis (CBS 303.71). A, C. Ascomata forming in culture. E, G. broken wall with asci. B, D, F. Asci. H. Ascospores. Scale bars: C = 45 μm, all others = 10 μm.
Etymology: Named after the host genus from which it was collected, Calamagrostis.
On Anthriscus stem. Ascomata brown, globose, smooth, superficial on stems, ostiolate, up to 180 μm diam; wall of 3-4 layers of brown textura angularis. Asci clavate, hyaline, smooth, short stipitate, fasciculate, bitunicate, thin-walled, apical chamber not visible, 6-8-spored, 60-80 × 30-40 μm. Ascospores multiseriate, hyaline, smooth and thin-walled, granular, broadly ellipsoid, ends obtuse, aseptate, (17-)18-20(-24) × (11-)12-13(-14) μm. Pseudoparaphyses not seen.
Culture characteristics: Colonies flat, spreading, with sparse to no aerial mycelium. On PDA surface and reverse dirty white; on MEA concolorous with agar; on OA pale pink on surface.
Specimen examined: Italy, Bergamo Vigolo, on Calamagrostis sp. (Poaceae), 20 Jun. 1967, G.A. Hedjaroude (holotype CBS H-21316, culture ex-type CBS 303.71).
Notes: Hedjaroude (1968) studied the specimen (ETH 7131; as Phaeosphaeria silvatica), but obviously the incorrect fungus was cultivated, as X. calamagrostidis is quite distinct from P. silvatica, which has cylindrical-fusoid, brown, 6-8-septate ascospores, 18-18 × 4-5 μm. Xenobotryosphaeria is reminiscent of genera in the Botryosphaeriales, but is phylogenetically distinct (Crous et al. 2006, Phillips et al. 2008, Liu et al. 2012). It also resembles species of Muyocopron (Muyocopronaceae), but the latter genus differs in that it has circular, flattened ascomata, as well as prominent pseudoparaphyses, which are absent in Xenobotryosphaeria.
Clade 39: Phoma
Note: See Aveskamp et al. (2010), de Gruyter et al. (2009, 2013).
Clade 40: Acicuseptoria
Acicuseptoria Quaedvlieg, Verkley & Crous, gen. nov. MycoBank MB804454.
Etymology: Acicu- from acicular (conidia), and Septoria = septoria-like.
Conidiomata pycnidial, erumpent, brown, globose, with central ostiole, exuding a cream conidial mass; wall consisting of 3-6 layers of thin, brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, smooth, ampulliform; proliferating inconspicuously and percurrently at apex, or simply appearing holoblastic. Conidia solitary, hyaline, granular, acicular, straight to gently curved, tapering towards apex that is acutely rounded, base truncate, transversely euseptate.
Type species: Acicuseptoria rumicis Quaedvlieg, Verkley & Crous.
Acicuseptoria rumicis Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804455. Fig. 85.
Fig. 85.
Acicuseptoria rumicis (CBS 522.78). A. Conidiomata sporulating in culture. B-E. Conidiogenous cells. F, G. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Rumex.
On sterile Carex leaves on WA. Conidiomata pycnidial, erumpent, brown, globose, up to 300 μm diam, with central ostiole, exuding a cream conidial mass; wall consisting of 3-6 layers of thin, brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, smooth, ampulliform, 7-15 × 5-7 μm; proliferating inconspicuously and percurrently at apex, or simply appearing holoblastic. Conidia solitary, hyaline, granular, acicular, straight to gently curved, tapering towards apex that is acutely rounded, base truncate, 1.5-2 μm diam, up to 8-septate, (32-)40-60(-70) × 2(-2.5) μm.
Culture characteristics: Colonies lobate, flat with little appressed, white aerial mycelium. On MEA surface olivaceous-grey, reverse umber. On OA suface olivaceous-grey. On PDA surface and reverse olivaceous-grey.
Specimen examined: France, Haute Savoie, Mt. Beaudin, 2000 m alt., stem of Rumex alpinus (Polygonaceae), Oct. 1978, H.A. van der Aa (holotype CBS H-18163, culture ex-type CBS 522.78).
Notes: Acicuseptoria rumicis was originally deposited as Septoria rumicum, but is distinct from the latter in having acicular, narrower conidia. Acicuseptoria is distinct from Septoria s. str. in having acicular conidia.
Clade 41: Stagonospora
Stagonospora (Sacc.) Sacc., Syll. Fung. (Abellini) 3: 445. 1884.
Description: See above.
Type species: S. paludosa (Sacc. & Speg.) Sacc., Syll. Fung. (Abellini) 3: 453. 1884.
Stagonospora duoseptata Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804459. Figs 86, 87.
Fig. 86.
Conidia and conidiogenous cells of Stagonospora duoseptata (CBS 135093). Scale bars = 10 μm.
Fig. 87.

Stagonospora duoseptata (CBS 135093). A. Conidiomata forming in culture. B, C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Etymology: Named after the fact that conidia are 2-septate.
On sterile Carex leaves on WA. Conidiomata dark brown, immersed, subepidermal, pycnidial, globose, up to 400 μm diam, exuding a short, hyaline cirrhus of conidia; wall of 3-4 layers of medium brown textura angularis. Conidiophores hyaline, smooth, lining inner cavity, 0-1-septate, subcylindrical, 10-20 × 4-5 μm. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, subcylindrical to ampulliform or doliiform, 6-8 × 3-4 μm; phialidic with several apical percurrent proliferations. Conidia hyaline, smooth, thin-walled, granular, fusoid-ellipsoidal, 2-septate, with septa 4-6 μm inwards from both obtuse conidial ends; conidia widest in middle, (18-)20-23(-25) × (5-)6(-7) μm.
Culture characteristics: Colonies on PDA flattened, circular with lobate edges, and fine grey aerial mycelium, surface mouse-grey, reverse olivaceous-black, after 14 d, 4 cm diam; on MEA after 14 d, 4.5 cm diam; on OA similar to MEA.
Specimen examined: Netherlands, Nijmegen, de Duffelt, on leaves of a Carex acutiformis (Cyperaceae), 29 Jul. 2012, W. Quaedvlieg (holotype CBS H-21321, culture ex-type CBS 135093 =S618).
Notes: Stagonospora duoseptata is distinct from other species occurring on Carex in that it has fusoid-ellipsoidal, 2-septate conidia, (18-)20-23(-25) × (5-)6(-7) μm, with septa positioned 4-6 μm inwards from its obtuse conidial ends. Stagonospora biseptata (occurring on Carex lanuginosa, Wisconsin, USA) has conidia that are larger, (35-)40-50(-55) × (2-)10-11(-13) μm (Greene 1961).
Stagonospora paludosa (Sacc. & Speg.) Sacc., Syll. Fung. (Abellini) 3: 453. 1884. Figs 88, 89.
Fig. 88.
Conidia and conidiogenous cells of Stagonospora paludosa (CBS 135088). Scale bars = 10 μm.
Fig. 89.

Stagonospora paludosa (CBS 135088). A, B. Conidiomata forming in culture. C, D. Conidiogenous cells. E, F. Conidia. Scale bars: B = 400 μm, all others = 10 μm.
Basionym: Hendersonia paludosa Sacc. & Speg., Michelia 1(no. 3): 353. 1878.
On sterile Carex leaves on WA. Conidiomata black, immersed, subepidermal, pycnidial, globose, up to 400 μm diam, exuding a short, hyaline cirrhus of conidia; wall of 3-4 layers of medium brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, ampulliform to doliiform, 5-10 × 5-10 μm; tapering at apex with prominent periclinal thickening or 1-2 inconspicuous percurrent proliferations visible at apex. Conidia hyaline, smooth, thin-walled, granular, or each cell with a large central guttule, subcylindrical to fusoid, apex subobtusely to obtusely rounded, base truncate (4-7 μm diam), (6-)7-8-septate (becoming constricted at septa with age), (45-)55-63(-65) × (9-)10-11 μm.
Culture characteristics: Colonies on PDA flat, circular, with grey aerial mycelium, reverse olivaceous-black to buff at the margins, after 14 d, 8.5 cm diam; on MEA umbonate, round, with appressed, grey aerial mycelium, with white patches; OA similar to PDA, but reverse buff with iron-grey patches at the outer region.
Specimens examined: Italy, on Carex riparia (Cyperaceae), Feb. 1878, holotype (presumably lost). Netherlands, Utrecht, Veenendal, de Blauwe Hel, Carex acutiformis (Cyperaceae), 23 Jul. 2012, W. Quaedvlieg (neotype designated here CBS H-21317, culture ex-type S601 = CBS 135088) (MBT175339).
Notes: For more than a century, Stagonospora was confused with Septoria. The introduction of molecular techniques around the turn of the century made it possible to definitively establish that Stagonospora was not linked to Septoria, and that it in fact clusters with other important plant pathogenic genera like Phoma and Leptosphaeria in the Pleosporales (Cunfer & Ueng 1999, Solomon et al. 2006). The type of Stagonospora (S. paludosa) was recollected from a Carex during this study and phylogenetic analyses showed that this species clustered separately from most other known “Stagonospora” spp. (mostly isolated from Poaceae), but together with several other Stagonospora species that were also collected from Carex. This led to the conclusion that Stagonospora s. str. was limited to Carex, and that other commercially important stagonospora-like species on Poaceae (e.g. S. avenae and S. nodorum) in fact belonged to different genera.
Stagonospora perfecta Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804458. Figs 90, 91.
Fig. 90.
Conidia, conidiogenous cells and ascus with ascospores of Stagonospora perfecta (CBS 135099). Scale bars = 10 μm.
Fig. 91.
Stagonospora perfecta (CBS 135099). A. Conidiomata forming in culture. B. Ascomata forming in culture. C-F, I, J. Asci and pseudoparaphyses. G, H. Conidiogenous cells. K. Conidia. Scale bars: B = 300 μm, all others = 10 μm.
Etymology: Named after the fact that both sexual and asexual morphs of the fungus developed in culture.
On sterile Carex leaves on SNA. Ascomata developing on SNA, solitary, globose, brown, erumpent, up to 300 μm diam, with central ostiole; wall of 3-4 layers of brown textura angularis. Pseudoparaphyses intermingled among asci, hyaline, smooth, guttulate, multi-septate, constricted at septa, branched, hyphal-like, 4-6 μm diam, filling entire cavity. Asci stipitate, hyaline, smooth, clavate to fusoid-ellipsoidal, bitunicate, with prominent apiculus, 1.5-2.5 μm diam, 8-spored, 45-100 × 12-18 μm. Ascospores hyaline, smooth, 3- to multi-seriate in ascus, fusoid-ellipsoidal with median septum, prominently constricted at septum, tapering towards subobtuse apices, with 1-2 large guttules per cell, thin-walled, widest just above septum in upper cell, (20-)23-25(-27) × (5-)6-7(-8) μm. Conidiomata up to 300 μm diam, brown, immersed, subepidermal, pycnidial, subglobose with central ostiole, exuding crystalline to creamy conidial mass; wall of 2-3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, ampulliform to doliiform or subcylindrical, with several percurrent proliferations near apex, 5-12 × 4-6 μm. Conidia hyaline, smooth, thin-walled, subcylindrical to narrowly fusoid-ellipsoidal, with obtuse apex and bluntly rounded base, 2-3-septate, slightly constricted at septa, with 1-2 large guttules per cell, (19-)25-29(-32) × (6-)7(-8) μm.
Culture characteristics: Colonies on PDA flattened, convex, circular, with white aerial mycelium, surface fuscous-black, reverse iron-grey to black, after 14 d, 8.5 cm diam; on MEA surface fuscous-black, reverse olivaceous-black; on OA surface isabelline, reverse fuscous-black.
Specimen examined: Netherlands, Limburg, Weert, Moerselpeel, on leaves of Carex acutiformis (Cyperaceae), Sep. 2012, W. Quaedvlieg (holotype CBS H-21320, culture ex-type CBS 135099 =S656).
Notes: Stagonospora perfecta is the first species with a confirmed sexual state in the genus Stagonospora. Of interest is the fact that it is didymella-like, rather than phaeosphaeria-like in morphology, which also explains it clustering in the Didymellaceae. Morphologically S. perfecta resembles S. vitensis (18-32 × 4-6 μm, 2-3(-4)-septate; Ellis & Ellis 1997), but conidia are wider. Stagonospora perfecta is closely related to S. pseudovitensis, though in the latter conidia are slightly longer, more fusoid-ellipsoidal in shape, and lack a sexual morph in culture.
Stagonospora pseudocaricis Quaedvlieg, Verkley, Gardiennet & Crous, sp. nov. MycoBank MB804456. Figs 92, 93.
Fig. 92.

Conidia of Stagonospora pseudocaricis (CBS 135132). Scale bar = 10 μm.
Fig. 93.

Stagonospora pseudocaricis (CBS 135132). A. Conidiomata forming in culture. B, C. Conidia. Scale bars = 10 μm.
Etymology: Named after the species that it resembles, Stagonospora caricis.
On sterile Carex leaves on WA. Conidiomata black, immersed, subepidermal, pycnidial, globose, up to 400 μm diam, exuding a short, hyaline cirrhus of conidia; wall of 3-4 layers of medium brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, ampulliform to doliiform, 5-9 × 5-8 μm; tapering at apex with prominent periclinal thickening or 1-2 inconspicuous percurrent proliferations visible at apex. Conidia hyaline, smooth, thin-walled, granular, or each cell with a large central guttule, subcylindrical to fusoid, apex subobtusely to obtusely rounded, base truncate, (5-)6(-7)-septate, (35-)42-48(-50) × (6-)7-8 μm.
Culture characteristics: Colonies on PDA flat, circular, with appressed, grey aerial mycelium, surface sepia, reverse olivaceous-black to buff, after 14 d, 8.5 cm diam; on MEA umbonate, round, with appressed, grey aerial mycelium with white patches, surface greyish sepia, reverse fuscous-black to olivaceous-black; OA similar to PDA.
Specimens examined: France, Foncegrive, Rive de la Venelle, on Carex acutiformis (Cyperaceae), Oct. 2012, A. Gardiennet (holotype CBS H-21318, culture ex-type CBS 135132 =S610); ibed., S609 = CBS 135414).
Note: Conidia of S. pseudocaricis closely resemble those of S. caricis (25-45 × 4-8 μm, 5-7-septate; Ellis & Ellis 1997), but are longer.
Stagonospora pseudovitensis Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804457. Figs 94, 95.
Fig. 94.
Conidia and conidiogenous cells of Stagonospora pseudovitensis (CBS 135094). Scale bars = 10 μm.
Fig. 95.

Stagonospora pseudovitensis (CBS 135094). A. Conidiomata forming in culture. B, C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Etymology: Named after the species that it resembles, Stagonospora vitensis.
On sterile Carex leaves on WA. Conidiomata black, immersed, subepidermal, pycnidial, globose with central ostiole, up to 180 μm diam; wall of 3-4 layers of pale brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, ampulliform to doliiform, 5-7 × 4-5 μm; tapering at apex with inconspicuous periclinal thickening or percurrent proliferation. Conidia hyaline, smooth, thin-walled, granular, subcylindrical with obtuse apex and truncate to bluntly rounded base, 3-4 μm diam, 3-septate, with large central guttule in each cell, (25-)28-33(-36) × (6-)7(-8) μm.
Culture characteristics: Colonies on PDA flat, circular, aerial mycelium consisting of some grey tufts, surface pale mouse-grey, reverse olivaceous-black, after 14 d, 8.5 cm diam; on MEA similar to PDA, but with appressed, white aerial mycelium, and with some grey tufts; OA similar to MEA, but reverse olivaceous-grey.
Specimens examined: Netherlands, Veenendaal, de Blauwe Hel, on leaves of Carex acutiformis (Cyperaceae), 23 Jul. 2012, W. Quaedvlieg (holotype CBS H-21319, culture ex-type CBS 135094 =S620); ibed., S602.
Note: Conidia of S. pseudovitensis differ from that of S. vitensis (18-32 × 4-6 μm, 2-3(-4)-septate; Ellis & Ellis 1997), by having consistently 3-septate, wider conidia.
Stagonospora uniseptata Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804460. Figs 96, 97.
Fig. 96.
Conidia and conidiogenous cells of Stagonospora uniseptata (CBS 135090). Scale bars = 10 μm.
Fig. 97.
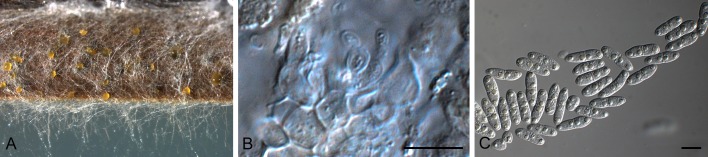
Stagonospora uniseptata (CBS 135090). A. Conidiomata sporulating in culture. B. Conidiogenous cells. C. Conidia. Scale bars = 10 μm.
Etymology: Named after the fact that conidia are 1-septate.
On sterile Carex leaves on WA. Conidiomata up to 150 μm diam, black, immersed, subepidermal, pycnidial, globose with central ostiole, exuding yellow conidial masses; wall of 3-4 layers of red-brown textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, hyaline, smooth, aggregated, lining the inner cavity, ampulliform to subcylindrical, 5-8 × 3-4 μm, with percurrent proliferation at apex. Conidia hyaline, smooth, thin-walled, fusoid-ellipsoidal, with obtuse apex and truncate to bluntly rounded base (2 μm diam), medianly 1-septate, prominently constricted at septum, straight to irregularly curved, widest in middle of either apical or basal cell, granular, including yellow-green reflective guttules, (13-)16-20(-22) × (5-)5.5(-6) μm.
Culture characteristics: Colonies on PDA appressed, circular, with short, greyish-white aerial mycelium, surface fusous-black, reverse olivaceous-black to hazel, after 14 d, 8.5 cm diam; on MEA surface hazel, reverse cinnamon; on OA with patches of white aerial mycelium, surface isabelline, reverse olivaceous to fuscous-black.
Specimens examined: Netherlands, Nijmegen, de Duffelt, on leaves of a Carex acutiformis (Cyperaceae), 29 Jul. 2012, W. Quaedvlieg, (holotype CBS H-21322, culture ex-type CBS 135090 =S611); ibed., S607, S608 = CPC 22151 and CPC 22150.
Notes: Of the Stagonospora and Septoria species occurring on Carex, Stagonospora uniseptata is most similar to Septoria caricis (conidia 20-35 × 2.5-3 μm, 1-septate; Ellis & Ellis 1997), but distinct in that conidia are shorter and wider.
Clade 42: Corynespora
Corynespora Güssow, Z. PflKrankh. PflPath. PflSchutz 16: 10. 1906.
Mycelium immersed or superficial. Stroma present in some species. Setae and hyphopodia absent. Conidiophores macronematous, mononematous, straight or flexuous, unbranched, brown or olivaceous brown, smooth. Conidiogenous cells monotretic, integrated, terminal, percurrent, cylindrical or doliiform. Conidia solitary or catenate, dry, acrogenous, simple, obclavate, rarely cylindrical, subhyaline, pale to dark brown or olivaceous brown or straw-coloured, euseptate or distoseptate, smooth, rarely verruculose (Ellis 1971).
Type species: C. mazei Güssow, Consp. Regni Veget. (Leipzig) 16: 13. 1906. [= C. cassiicola (Berk. & M.A. Curtis) C.T. Wei, Mycol. Pap. 34: 5. 1950.]
Corynespora leucadendri Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804461. Figs 98, 99.
Fig. 98.
Conidia and conidiogenous loci of Corynespora leucadendri (CBS 135133). Scale bar = 10 μm.
Fig. 99.

Corynespora leucadendri (CBS 135133). A-C. Conidiogenous cells giving rise to conidia. D. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Leucadendron.
On MEA and PDA after 2 wk. Mycelium consisting of creeping, branched, septate, hyaline, smooth, 3-4(-5) μm diam hyphae that become brown close to conidiophores; stroma lacking. Conidiophores subcylindrical, erect, medium brown, 100-300 μm tall, 4-6(-7) μm diam, thick-walled, transversely multiseptate, with several swollen nodes of conidiophore rejuvenation (up to 12 μm diam). Conidiogenous cells terminal, cylindrical, medium brown, smooth, ends swollen or not, central locus somewhat darkened or inconspicuous, 15-40 × 5-6(-7) μm. Conidia medium brown, obclavate to subcylindrical, straight to slightly curved, thick-walled, (3-)4-6(-10)-distoseptate, basal locus thickened, darkened, protruding, 2-3 μm diam, (35-)70-110(-170) × (6-)7-8(-11) μm.
Culture characteristics: Colonies erumpent, spreading with moderate aerial mycelium and smooth, even margin; reaching 25 mm diam after 2 wk. On MEA surface dirty white, reverse cinnamon. On PDA surface dirty white, reverse buff to rosy buff with diffuse rosy buff pigment. On OA surface dirty white with diffuse rosy buff pigment in agar.
Specimen examined: South Africa, Western Cape Province, Helderberg Nature Reserve, from the leaves of Leucadendron sp. (Proteaceae), 14 Aug. 2000, S. Lee (holotype CBS H-21323, culture ex-type CBS 135133 =CPC 19345).
Notes: This species was not treated by Marincowitz et al. (2008), and presently no species of Corynespora are known from Leucadendron. Furthermore, based on conidial morphology, none of the species treated by Ellis (1971, 1976) resemble C. leucadendri, nor is it similar to any Corynespora sequence presently deposited in GenBank. For these reasons we thus introduce C. leucadendri as a new taxon.
Clade 43: Setoseptoria
Setoseptoria Quaedvlieg, Verkley & Crous, gen. nov. MycoBank MB804462.
Etymology: Named after its conidiomata which are septoria-like, but setose.
Conidiomata pycnidial, brown, immersed, globose with central ostiole, somewhat papillate, apical erumpent part at times with brown, verruculose to warty setae; wall of 6-8 layers of brown textura angularis; inner layer of 6-10 layers of hyaline textura angularis. Conidiophores lining the inner cavity, reduced to conidiogenous cells, or with one supporting cell. Conidiogenous cells hyaline, smooth, subcylindrical to doliiform; apical region with several inconspicuous percurrent proliferations, or with periclinal thickening; collarette inconspicuous, or prominent, flared. Conidia hyaline, smooth, becoming somewhat olivaceous and verruculose in older cultures, subcylindrical, tapering in apical part to obtuse or subobtuse apex, base truncate, transversely euseptate, straight to somewhat curved, mostly with one large central guttule per cell, older conidia becoming constricted at septa, disarticulating into phragmospores.
Type species: Setoseptoria phragmites Quaedvlieg, Verkley & Crous.
Setoseptoria phragmitis Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804463. Fig. 100.
Fig. 100.

Setoseptoria phragmitis (CBS 114802). Conidioma sporulating in culture. B. Setae. C, D. Conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Phragmites.
On sterile Carex leaves on WA. Conidiomata pycnidial, brown, immersed, globose with central ostiole, up to 30 μm diam, somewhat papillate, up to 200 μm diam, apical erumpent part at times with brown, verruculose to warty setae; wall of 6-8 layers of brown textura angularis; inner layer of 6-10 layers of hyaline textura angularis. Conidiophores lining the inner cavity, reduced to conidiogenous cells, or with one supporting cell. Conidiogenous cells hyaline, smooth, subcylindrical to doliiform, 7-12 × 3-4 μm; apical region with several inconspicuous percurrent proliferations, or with periclinal thickening; collarette inconspicuous, or prominent, flared. Conidia hyaline, smooth, becoming somewhat olivaceous and verruculose in older cultures, subcylindrical, (1-)3-septate, (19-)25-35(-38) × (3.5-)4 μm, tapering in apical part to obtuse or subobtuse apex, base truncate, 1.5-2.5 μm diam, straight to somewhat curved, mostly with one large central guttule per cell, older conidia becoming constricted at septa, disarticulating into phragmospores.
Culture characteristics: Colonies on PDA umbonate, round, fluffy grey white aerial mycelium on the younger parts with longer grey blackish tufts on the older parts, surface olivaceous-black to buff at the younger mycelium, reverse olivaceous-black at the older parts to buff at the younger mycelium, after 14 days 6 cm diam; on MEA similar toPDA but after 14 d, 7 cm diam; on OA similar to PDA.
Specimens examined: hong Kong, Mai Po Mangrove, from the leaves of Phragmites australis (Poaceae), 12 Mar. 1998, K.D. Hyde (holotype CBS H-21324, culture ex-type CBS 114802 =HKUCC 2689); ibid., 3 Feb. 2000, K.D. Hyde (CBS 114966 =HKUCC 6029).
Notes: Setoseptoria needs to be compared to Dearnessia and Trichoseptoria (see above). The genus Trichoseptoria is poorly known, and details about its conidiogenesis is lacking, and thus it cannot be compared until it has been recollected. Setoseptoria is distinct from Dearnessia in that it has conidiogenous cells with prominent percurrent proliferation, and conidia that tend to become olivaceous and verruculose in older cultures, and disarticulate into phragmospores. Several Septoria species have been described from Phragmites, including S. phragmitis (conidia 20-30 × 1.5-2 μm), S. arundinacea (conidia 6-7-septate, 60-70 × 5-6 μm), S. curva (conidia 14-20 × 3.5-4.5 μm), and S. graminum (conidia multiseptate, 55-75 × 1-1.3 μm), all of which appear to differ from Setoseptoria phragmitis based on its conidial morphology.
Clade 44: Septorioides
Septorioides Quaedvlieg, Verkley & Crous, gen. nov. MycoBank MB804464.
Etymology: Resembling the genus Septoria.
Foliicolous. Conidiomata black, unilocular, globose, flattened, opening by means of irregular rupture; wall consisting of 6-10 layers of dark brown textura irregularis to angularis, exuding a crystal conidial mass. Paraphyses intermingled among conidiophores, hyaline, cylindrical, branched at base, septate with obtuse ends. Microconidia hyaline, smooth, cylindrical, mostly straight, apex obtuse, base truncate. Conidiophores reduced to conidiogenous cells or with a supporting cell. Conidiogenous cells lining the inner cavity in basal layer, hyaline, smooth, subcylindrical to ampulliform, giving rise to macro- and microconidia. Spermatia formed in conidiomata, cylindrical, hyaline, smooth, straight to curved. Macroconidia hyaline, smooth, guttulate, subcylindrical, straight to irregularly curved, tapering in apical cell to subobtuse apex, base truncate, transversely euseptate.
Type species: Septorioides pini-thunbergii (S. Kaneko) Quaedvlieg, Verkley & Crous.
Septorioides pini-thunbergii (S. Kaneko) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804465. Fig. 101.
Fig. 101.

Septorioides pini-thunbergii (CBS 473.91). A. Colony sporulating on PDA. B. Spermatia. C-E. Conidiogenous cells. F. Conidia. Scale bars = 10 μm.
Basionym: Septoria pini-thunbergii S. Kaneko, Trans. Mycol. Soc. Japan 30(4): 463. 1989.
Associated with needle blight, or isolated as endophyte. On PDA. Conidiomata black, unilocular, globose, flattened, up to 400 μm diam, opening by means of irregular rupture; wall consisting of 6-10 layers of dark brown textura irregularis to angularis, exuding a crystal conidial mass. Paraphyses intermingled among conidiophores, hyaline, cylindrical, branched at base, septate with obtuse ends, 2-2.5 μm diam, up to 80 μm long. Microconidia hyaline, smooth, cylindrical, mostly straight, apex obtuse, base truncate, 5-15 × 2-2.5 μm. Conidiophores reduced to conidiogenous cells or with a supporting cell. Conidiogenous cells lining the inner cavity in basal layer, hyaline, smooth, subcylindrical to ampulliform, 10-15 × 4-6 μm, giving rise to macro- and microconidia. Spermatia formed in conidiomata, cylindrical, hyaline, smooth, straight to curved, 3-7 × 2 μm. Macroconidia hyaline, smooth, guttulate, subcylindrical, straight to irregularly curved, tapering in apical cell to subobtuse apex, base truncate, (60-)70-80(-110) × 3.5(-4) μm, (1-)3-6(-10)-septate.
Specimen examined: Japan, Akita Prefecture, Tenno-cho, on needles of Pinus thunbergii (Pinaceae), Aug. 1984, S. Kaneko & Y. Zinno, culture ex-type of Septoria pini-thunberghii (CBS 473.91).
Note: Septorioides is distinguished from Septoria by having conidiomata that open by means of an irregular split (acervular), and having paraphyses intermingled among its conidiophores. Septorioides pini-thunbergii was originally described from blighted needles of Pinus thunbergii in Japan (Kaneko et al. 1989). It was also recently isolated as endophyte from needles of P. densiflora in Korea (Yoo & Eom 2012).
Clade 45: Phlyctema
Phlyctema Desm., Ann. Sci. Nat., Sér. 3, 8: 16. 1847.
= Allantozythia Höhn., Mykol. Unters. 3: 322. 1923.
Mycelium immersed, branched, septate, hyaline. Conidiomata eustromatic, immersed, erumpent, sporodochial, separate, yellowish brown, pulvinate, circular, unilocular but convoluted, thick-walled; wall of textura angularis, darker brown and thicker-walled at base than at the sides. Ostiole absent, dehiscence by irregular rupture. Conidiophores hyaline, septate, branched irregularly, cylindrical to filiform, formed from the wall lining the conidiomata. Conidiogenous cells enteroblastic, phialidic, integrated or discrete, determinate, hyaline, with minute collarette and periclinal thickening. Conidia hyaline, aseptate, fusiform, eguttulate, straight to slightly curved or irregular (Sutton 1980).
Type species: P. vagabunda Desm., Ann. Sci. Nat., Bot., Sér. 3, 8: 16. 1847.
Notes: Phlyctema is characterised by having eustromatic, convulated, pulvinate to sporodochial conidiomata, branched, hyaline conidiophores, and phialidic conidiogenous cells that give rise to hyaline, aseptate, fusiform, straight to curved conidia. The genus has more than 80 names, and is in need of revision.. The type species is linked to a sexual morph known as Neofabraea alba (Verkley 1999).
Phlyctema vincetoxici Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804466. Figs 102, 103.
Fig. 102.
Conidia and conidiogenous cells of Phlyctema vincetoxici (CBS 123727). Scale bar = 10 μm.
Fig. 103.

Phlyctema vincetoxici (CBS 123727). A. Colonies forming on OA. B, C. Conidiogenous cells. D. Conidia. Scale bars = 10 μm.
Etymology: Named after the host genus from which it was collected, Vincetoxicum.
Conidiomata immersed, separate, eustromatic, unilocular, convulated, opening by irregular rupture, becoming acervular to sporodochial, up to 450 μm diam; wall of 3-6 layers of brown textura angularis; outer surface covered in brown, warty hyphae. Conidiophores hyaline, smooth, subcylindrical, lining the inner layer, branched, 1-4-septate, 15-50 × 4-5 μm. Conidiogenous cells phialidic, hyaline, smooth, subcylindrical to cymbiform or doliiform, with apical periclinal thickening and minute, non-flaring collarette, 7-18 × 3.5-5 μm. Conidia hyaline, smooth, guttulate, aseptate, fusiform, curved, tapering to subobtuse apex and truncate base, (27-)33-37(-40) × 3(-3.5) μm.
Culture characteristics: Colonies on PDA flat, circular, with sparse, white aerial mycelium, surface dark-brick, reverse greyish sepia, after 14 d, 7 cm diam; on MEA undulate, lacking aerial mycelium, after 14 d, 6 cm diam; on OA flat, circular, lacking aerial mycelium, after 14 d, 8.5 cm diam.
Specimen examined: Czech Republic, Moravia, Podyji National Park, Masovice, Klinka area, on leaves of Vincetoxicum officinale (Asclepiadaceae), 17 Sep. 2008, G. Verkley (holotype CBS H-21325, culture ex-type CBS 123727 =V6015.2).
Notes: No species of Phlyctema has thus far been described on Vincetoxicum. Septoria vincetoxici (conidia 30-50 × 1-1.5 μm; Saccardo 1884) has somewhat longer, narrower conidia. Phlyctema vincetoxici was found sporulating in leaf spots showing numerous hypophyllous teleospore sori of the rust fungus Cronartium flaccidum (identified by H.A. van der Aa).
Clade 46: Kirstenboschia
Kirstenboschia Quaedvlieg, Verkley & Crous, gen. nov. MycoBank MB804467.
Etymology: Kirstenbosch National Botanical Garden is one of the most acclaimed botanical gardens of the world, set against the foot of Cape Town’s Table Mountain. With more than 7000 plant species, it has also proven to be a source of numerous undescribed fungal species. Kirstenbosch was established in 1913, and to celebrate its centenary (2013), the fungal genus Kirstenboschia is named after this beautiful garden.
Foliicolous. Conidiomata erumpent, sporodochial, separate, with slightly raised outer margin of 3-10 layers of textura intricata. Conidiophores lining the inner cavity, hyaline, smooth, septate, subcylindrical, branched below and above. Conidiogenous cells terminal and lateral, hyaline, smooth, ampulliform to subcylindrical, proliferating sympodially, apical loci truncate, at times appearing subdenticulate. Conidia solitary, hyaline, scolecosporous, smooth, granular, thin-walled, acicular to narrowly obclavate with subobtuse apex and truncate to long obconically truncate base, 3-septate, irregularly curved.
Type species: K. diospyri Quaedvlieg, Verkley & Crous.
Kirstenboschia diospyri Quaedvlieg, Verkley & Crous, sp. nov. MycoBank MB804468. Figs 104, 105.
Fig. 104.
Conidia and conidiogenous cells of Kirstenboschia diospyri (CBS 134911). Scale bars = 10 μm.
Fig. 105.

Kirstenboschia diospyri (CBS 134911). A. Conidiomata forming in culture. B, C. Conidiogenous cells. D. Conidia. Scale bars: A = 300 μm, all others = 10 μm.
Etymology: Named after the host genus from which it was collected, Diospyros.
Conidiomata erumpent, sporodochial, up to 300 μm diam, separate, appearing creamy to pale yellow when sporulating on SNA with barley leaves, with slightly raised outer margin of 3-10 layers of textura intricata. Conidiophores lining the inner cavity, hyaline, smooth, 0-4-septate, subcylindrical, branched below and above, 5-15 × 2-4 μm. Conidiogenous cells 5-10 × 2-3 μm, terminal and lateral, hyaline, smooth, ampulliform to subcylindrical, proliferating sympodially, apical loci truncate, at times appearing subdenticulate, 1 μm diam. Conidia solitary, hyaline, scolecosporous, smooth, granular, thin-walled, acicular to narrowly obclavate with subobtuse apex and truncate to long obconically truncate base, 3-septate, irregularly curved, (40-)60-70(-75) × (1.5-)2 μm.
Culture characteristics: Colonies on PDA erumpent, with moderate aerial mycelium, and smooth, lobate margin; surface and reverse dirty white. On OA dirty white with diffuse brown pigment in agar. On MEA surface folded, irregular, strongly erumpent, dirty white, reverse sienna.
Specimen examined: South Africa, Western Cape Province, Kirstenbosch Botanical Garden, on leaves of Diospyros whyteana (Ebenaceae), 9 Aug. 2011, P.W. Crous (holotype CBS H-21326, culture ex-type CBS 134911 =CPC 19869).
Note: Kirstenboschia is distinguished from Septoria s. str. and allied genera based on its distinctive, sporodochial conidiomata, and conidiogenous cells that proliferate sympodially, but at times are subdenticulate.
Clade 47: Phlogicylindrium
Phlogicylindrium Crous, Summerb. & Summerell, Fungal Diversity 23: 340. 2006.
Foliicolous. Conidiomata synnematal to sporodochial, pale brown. Macroconidiophores arising from a brown stroma of 3-6 layers of textura angularis, giving rise to subcylindrical, hyaline (dark brown at the base), smooth, frequently branched conidiophores, 0-2(-6)-septate. Macroconidiogenous cells hyaline, smooth, subcylindrical, proliferating sympodially and percurrently near apex. Macroconidia hyaline, smooth, subcylindrical, transversely septate, apex obtusely rounded, base truncate, slightly curved. Microconidia formed in acervular conidiomata together with macroconidia. Microconidiophores intermingled among macroconidiophores, hyaline, smooth, subcylindrical, branched, 1-4-septate. Microconidiogenous cells terminal and lateral, hyaline, smooth, ampulliform, phialidic, solitary or in penicillate clusters. Microconidia hyaline, smooth, hamate, curved, apex subobtuse, base truncate, widest in upper third, aseptate (Summerell et al. 2006).
Type species: P. eucalypti Crous, Summerb. & Summerell, Fungal Diversity 23: 340. 2006.
Phlogicylindrium eucalyptorum Crous, Fungal Planet 20. 2007. Figs 106, 107.
Fig. 106.
Macro- and microconidia and conidiogenous cells of Phlogicylindrium eucalyptorum (CBS 111689). Scale bars = 10 μm.
Fig. 107.
Phlogicylindrium eucalyptorum (CBS 111689). A. Colony on OA. B, E. Microcyclic conidiation with macro- and macroconidia. C. Macroconidiogenous cells. D. Microconidia. F. Macroconidia. Scale bars = 10 μm.
On OA. Conidiomata synnematal to sporodochial, pale brown up to 300 μm diam. Macroconidiophores arising from a brown stroma of 3-6 layers of textura angularis, giving rise to subcylindrical, hyaline (dark brown at the base), smooth, frequently branched conidiophores, 0-2(-6)-septate, 15-25(-45) × 3-4 μm. Macroconidiogenous cells hyaline, smooth, subcylindrical, 10-15 × 2-4 μm, proliferating sympodially and percurrently near apex. Macroconidia hyaline, smooth, subcylindrical, 1(-3)-septate, apex obtusely rounded, base truncate, slightly curved, (27-)40-50(-55) × 2-2.5(-3) μm. Microconidia formed in acervular conidiomata together with macroconidia. Microconidiophores intermingled among macroconidiophores, hyaline, smooth, subcylindrical, branched, 1-4-septate, 20-40 × 2-2.5 μm. Microconidiogenous cells terminal and lateral, hyaline, smooth, ampulliform, phialidic, 5-16 × 2-2.5 μm, solitary or in penicillate clusters of up to 3. Microconidia hyaline, smooth, hamate, curved, apex subobtuse, base truncate, widest in upper third, aseptate, (16-)17-20(-24) 1.5(-2) μm.
Specimens examined: Australia, Victoria, Otway Ranges, (near Gellibrand), latitude: -38.568412, longitude: 143.539586, elevation: 175 m, on leaves of Eucalyptus globulus (Myrtaceae), Sep. 2005, I. Smith, holotype CBS H-19771, cultures ex-type CPC 12429 = CBS 120221; New South Wales, on leaves of E. nitens, 22 Nov. 1996, P.W. Crous (CBS 111689 =CPC 1547 = STE-U 1547).
Notes: The present strain represents the second collection of this fungus. Isolates from this collection formed a microconidial state not observed in the type (Crous et al. 2007c), and novel for species of Phlogicylindrium.
DISCUSSION
The main question considered in the present study was: what is Septoria? To address this we included 370 isolates representing 170 species, sampled from six continents. Furthermore, we also generated several phylogenetic datasets based on partial sequences of the ITS, LSU, Btub, RPB2 and EF-1α loci. In the final analysis, it was clear that Septoria is a well-defined genus and phylogenetic clade, with pycnidial, ostiolate conidiomata, conidiophores reduced to conidiogenous cells that proliferate sympodially, and hyaline, filiform conidia with transverse eusepta, fitting the original concept of Sutton (1980). However, when host material has been incubated for a while, several pycnidial species tend to form acervuli (also not clearly defined when studied in culture on normal agar media), and conidiogenous cells could have a combination of sympodial and percurrent proliferation (as observed in Pseudocercospora; Crous et al. 2013).
The present study, including that of Verkley et al. (2013) defined an additional 15 genera that were formerly treated as “septoria” in the widest sense. Although it has recently been shown that Phoma is a generic complex representing many morphologic and phylogenetic genera (Aveskamp et al. 2010, de Gruyter et al. 2010, 2013), this was not expected to also be the case for Septoria. Furthermore, many of the septoria-like genera discussed earlier in this paper are presently still not known from sequence, and thus their phylogeny remains to be resolved, meaning that they could add futher entities to the list of acknowledged septoria-like genera.
Although Septoria s. str. is a genus in the Mycosphaerellaceae (Capnodiales), several of the septoria-like genera clustered outside this family. Species of Septoria are morphologically conserved, and in the past many taxa were identified based on host, which has been shown to be unreliable (see Verkley et al. 2013), as several taxa have wide host ranges. Another complication revealed in the present study is that many septoria-like genera cluster in different phylogenetic clades, but have still retained the Septoria morphological characters, which means that as in Phoma, future identifications in this complex will also have to rely on DNA sequence data to support morphological conclusions.
The genus Stagonospora has always been separated from Septoria on the basis that Septoria has conidiogenous cells with sympodial proliferation, whereas in Stagonospora they proliferate percurrently. As shown in the present study, however, conidiogenesis is far too broad a feature to define all genera that express these modes of proliferation in their conidiogenous cells. Stagonospora, which is based on S. paludosa, was epitypified in this study, and shown to cluster apart from Septoria s. str. Another major surprise lies in the fact that Septoria nodorum blotch, caused by “Stagonospora” nodorum, clusters in a distinct genus, unrelated to Stagonospora s. str., and also separate from Phaeosphaeria s. str. A repercussion from these findings is the fact that the common cereal pathogens, which are neither Stagonospora, Septoria, Phaeosphaeria or Leptosphaeria (see de Gruyter et al. 2013), now have to be accommodated in a new genus, Parastagonospora. Furthermore, it appears that Stagonospora s. str. occurs on Poaceae, but has thus far only been confirmed from Carex, though further sampling will undoubtedly extend the host range of this genus. Parastagonospora is thus a novel, distinct stagonospora-like genus, which has sexual morphs that are phaeosphaeria-like in morphology, thus quite unlike those of Stagonospora s. str., which are more didymella-like in morphology.
The genus Phaeosphaeria is based on P. oryzae (asexual morph Phaeoseptoria oryzae), for which we could designate an epitype in this study. Furthermore, we also recollected the type species of Phaeoseptoria, P. papayae, for which we also designated an epitype. As expected, Phaeoseptoria clusters with Phaeosphaeria, for which we choose the name of the sexual morph, Phaeosphaeria, on the basis that it is clearly resolved, and well established in literature. In contrast, Phaeoseptoria has in recent years become a muddled concept harbouring unrelated coelomycetes with pigmented conidia.
Obtaining a culture of Cytostagonospora martiniana clarified the phylogenetic position of the genus as distinct from Septoria, resolving the difference of opinion between von Arx (1983), who regarded it as synonym of Septoria, versus Sutton (1980), who retained it as separate genus. Of interest is the unique mode of conidiogenesis, ranging from holoblastic sympodial to polyphialides with periclinal thickening to percurrent proliferation. It should be noted, however, that although this is a distinct genus, C. mariniana is not the type of Cytostagonospora, and C. photiniicola (occurring on Photinia serrulata, Austria) will have to be recollected to confirm that these two fungi are congeneric.
The genus Phloeospora (based on P. ulmi) has for long been assumed to be a synonym of Septoria based on morphology. It is thus good to finally see it resolved as separate phylogenetic lineage, which is also supported morphologically based on its acervular conidiomata and conidiogenous cells with prominent percurrent proliferation. In spite of resolving 21 genera, several lineages remain unresolved, and are simply treated as “septoria-like” awaiting the recollection of additional material.
It is surprising that so many of the cereal pathogens actually have a confused taxonomy. Eyespot disease of wheat, formely treated as Tapesia (Ramulispora asexual states), was shown to represent a distinct genus Oculimacula (Helgardia asexual states) (Crous et al. 2003), while Quaedvlieg et al. (2011) determined that Septoria tritici blotch, caused by “Septoria” tritici, is in fact better accommodated in a new genus, Zymoseptoria, which appears to be restricted to members of Poaceae. The present study also resolved the phylogenetic position of Septoria nodorum blotch, which proved to not represent a member of Septoria, Stagonospora, or Phaeosphaeria, but to represent a distinct genus, described here as Parastagonospora. Clearly more attention should be directed towards resolving the taxonomy of the pathogens of agricultural crops of major economic importance in future, as these findings also have implications for genomic studies, where organisms from different genera, and even families get compared to one another, and new evolutionary hypotheses are proposed on the assumption that these taxa are congeneric. To clarify the taxonomy of well-known plant pathogens, however, many species will have to be recollected, and epitypified, so that authentic cultures and DNA barcodes will become available to fix the genetic application of these names.
General conclusions
The genus Septoria is defined by having pycnidial to acervular conidiomata, and hyaline conidiophores that give rise to conidiogenous cells that proliferate sympodially and percurrently, forming hyaline, filiform conidia with transverse eusepta. Many species have wide host ranges, and host occurrence should not be used as primary character for identification (see Verkley et al. 2013, this issue). Although species of Septoria and several of the novel genera introduced here have mycosphaerella-like sexual states, the name Mycosphaerella is restricted to the genus Ramularia, and is unavailable for species of Septoria and related genera.
Acknowledgments
We thank the technical staff, Arien van Iperen (cultures), and Marjan Vermaas (photographic plates), for their invaluable assistance. The research leading to these results has received funding from the European Community’s Seventh Framework Program (FP7/2007-2013)/grant agreement no. 226482 (Project: QBOL - Development of a new diagnostic tool using DNA barcoding to identify quarantine organisms in support of plant health) by the European Commission under the theme “Development of new diagnostic methods in support of Plant Health policy” (no. KBBE-2008-1-4-01). Special thanks also go to Dr Ellen van Agtmaal who prepared the line drawings included in this paper from photomicrographs and published materials (Sutton 1980) using Adobe Photoshop CS3.
REFERENCES
- Aptroot A. (2006). Mycosphaerella and its anamorphs. 2, Conspectus of Mycosphaerella. CBS Biodiversity Series 5 CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands: [Google Scholar]
- Arx JA von. (1983). Mycosphaerella and its anamorphs. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen Series C-Biological and Medical Sciences 86(1): 15–54 [Google Scholar]
- Arzanlou M, Crous PW. (2006). Phaeosphaeriopsis musae. Fungal Planet 9 CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands: [Google Scholar]
- Aveskamp M, Gruyter H de, Woudenberg J, Verkley G, Crous PW. (2010). Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Studies in Mycology 65: 1–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes I, Crous PW, Wingfield BD, Wingfield MJ. (2004). Multigene phylogenies reveal that red band needle blight of Pinus is caused by two distinct species of Dothistroma, D. septosporum and D. pini. Studies in Mycology 50: 551–565 [Google Scholar]
- Beach WS. (1919). Biologic specialization in the genus Septoria. American Journal of Botany 6: 1–32 [Google Scholar]
- Bedlan G. (2011). Septoria juliae sp. nov. - a new Septoria species on Nerium oleander. Journal für Kulturpflanzen 63: 430–431 [Google Scholar]
- Bissett J. (1982). Stagonospora avenae. Fungi Canadenses 239 National Mycological Herbarium, Biosystematics Research Institute, Agriculture Canada, Ottawa: [Google Scholar]
- Braun U. (1990). Taxonomic problems of the Ramularia / Cercosporella complex. Studies in Mycology 32: 65–75 [Google Scholar]
- Braun U. (1995). A monograph of Cercosporella, Ramularia and allied genera (Phytopathogenic Hyphomycetes). Vol. 1 IHW-Verlag, Eching: [Google Scholar]
- Braun U. (1998). A monograph of Cercosporella, Ramularia and allied genera (Phytopathogenic Hyphomycetes). Vol. 2 IHW-Verlag, Eching: [Google Scholar]
- Câmara MPS, Ramaley AW, Castlebury LA, Palm ME. (2003). Neophaeosphaeria and Phaeosphaeriopsis, segregates of Paraphaeosphaeria. Mycological Research 107: 516–522 [DOI] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556 [Google Scholar]
- Cheewangkoon R, Crous PW, Hyde KD, Groenewald JZ, To-anan C. (2008). Species of Mycosphaerella and related anamorphs on Eucalyptus leaves from Thailand. Persoonia 21: 77–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu O. (1984). Taxonomic revision of Septoria-like fungi parasitic on Betulaceae. Transactions of the British Mycological Society 83: 383–398 [Google Scholar]
- Crous PW. (1998). Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21 APS Press, MN, USA: [Google Scholar]
- Crous PW, Braun U. (2003). Mycosphaerella and its anamorphs: 1. Names published in Cercospora and Passalora. CBS Biodiversity Series 1 CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands: [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. (2007a). Mycosphaerella is polyphyletic. Studies in Mycology 58: 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Hunter GC, Wingfield MJ, Verkley GJM, et al. (2013). Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Schubert K, Groenewald JZ. (2007b). Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Ferreira FA, Sutton BC. (1997). A comparison of the fungal genera Phaeophleospora and Kirramyces (coelomycetes). South African Journal of Botany 63: 111–115 [Google Scholar]
- Crous PW, Groenewald JZ, Gams W. (2003). Eyespot of cereals revisited: ITS phylogeny reveals new species relationships. European Journal of Plant Pathology 109: 841–850 [Google Scholar]
- Crous PW, Groenewald JZ, Smith IW. (2007c). Phlogicylindrium eucalyptorum. Fungal Planet 20 CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands: [Google Scholar]
- Crous PW, Kang JC, Braun U. (2001). A phylogenetic redefinition of anamorph genera in Mycosphaerella based on ITS rDNA sequence and morphology. Mycologia 93: 1081–1101 [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, et al. (2009a). Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Shivas RG, Wingfield MJ, Summerell BA, Rossman AY, et al. (2012a). Fungal Planet description sheets 128-127. Persoonia 29: 138–153 23105159 [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, et al. (2006). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Groenewald JZ. (2009b). Novel species of Mycosphaerellaceae and Teratosphaeriaceae. Persoonia 23: 119–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, et al. (2009c). Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Tanaka K, Summerell BA, Groenewald JZ. (2011). Additions to the Mycosphaerella complex. IMA Fungus 2: 49–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Christensen M, Castañeda-Ruiz RF, Groenewald JZ. (2012b). How important are conidial appendages? Persoonia 28: 126–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (2009d). CBS Laboratory Manual Series 1 CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands: [Google Scholar]
- Crous PW, Wingfield MJ, Park RF. (1991). Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628–632 [Google Scholar]
- Cunfer BM. (2000). Stagonospora and Septoria diseases of barley, oat, and rye. Canadian Journal of Plant Pathology 22: 332–348 [Google Scholar]
- Cunfer BM, Ueng PP. (1999). Taxonomy and identification of Septoria and Stagonospora species on small-grain cereals. Annual Review of Phytopathology 37: 267–284 [DOI] [PubMed] [Google Scholar]
- Deighton FC. (1987). New species of Pseudocercospora and Mycovellosiella, and new combinations into Pseudocercospora and Phaeoramularia. Transactions of the British Mycological Society 88: 365–391 [Google Scholar]
- Demaree JB, Wilcox MS. (1943). The fungus causing the so-called “Septoria leaf-spot disease” of raspberry. Phytopathology 33: 986–1003 [Google Scholar]
- Desmazières JBHJ. (1847). Quatorzième notice sur les plantes cryptogames récemment découvertes en France. Annales des Sciences Naturelles Botanique, Série 3, 8: 9-37, 172-193 [Google Scholar]
- Donk MA. (1964). Nomina conervana proposita. Deuteromycetes. Regnum Vegetable 34: 7–15 [Google Scholar]
- Dyko BJ, Sutton BC. (1979). Two new and unusual deuteromycetes. Transactions of the British Mycological Society 72: 411–417 [Google Scholar]
- Ellis MB. (1971). Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, UK: [Google Scholar]
- Ellis MB. (1976). More Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, UK: [Google Scholar]
- Ellis MB, Ellis JP. (1997). Microfungi on Land Plants - An Identification Handbook. Richmond Publishing, England: [Google Scholar]
- Eriksson OE. (1967). On graminicolous pyrenomycetes from Fennoscandia I, II, Ill. Phragmosporous and scolecosporous species. Arkiv för Botanik Series 2, 6: 381–440 [Google Scholar]
- Evans HC. (1984). The genus Mycosphaerella and its anamorphs Cercoseptoria, Dothistroma and Lecanosticta on pines. Mycological Papers 153: 1–102 [Google Scholar]
- Eyal Z, Sharen AL, Prescott JM, Ginkel M van. (1987). The Septoria diseases of wheat: concepts and methods of disease management. Mexico, DF, CIMMYT: [Google Scholar]
- Farr DF. (1991). Septoria species on cornus. Mycologia 83: 611–623 [Google Scholar]
- Farr DF. (1992). Species of Septoria on the Fabaceae, subfamily Faboidae, tribe Genistae. Sydowia 44: 13–31 [Google Scholar]
- Farr DF, Rossman AY. (2013). Fungal Databases, Systematic Mycology and Microbiology Laboratory, ARS, USDA: Retrieved May 3, 2013, from http://nt.ars-grin.gov/fungaldatabases/ [Google Scholar]
- Feau N, Hamelin RC, Bernier L. (2006). Attributes and congruence of three molecular data sets: Inferring phylogenies among Septoria related species from woody perennial plants. Molecular Phylogenetics and Evolution 40: 808–829 [DOI] [PubMed] [Google Scholar]
- Ferreira FA. (1989). Patologia Florestal. Principais Doenças Florestais No Brasil. Sociedade de Investigaçoes Florestais, Viçosa, MG, Brasil: [Google Scholar]
- Frank J, Crous PW, Groenewald JZ, Oertel B, Hyde KD, et al. (2010). Microcyclospora and Microcyclosporella: novel genera accommodating epiphytic fungi causing sooty blotch on apple. Persoonia 24: 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara M. (2002). Three Phaeosphaeria species and Paraphaeosphaeria michotii isolated from Phragmites leaves in Osaka, Japan. Mycoscience 43: 275–382 [Google Scholar]
- Golzar H, Wang C. (2012). First report of Phaeosphaeriopsis glaucopunctata as the cause of leaf spot and nectrosis on Ruscus aculeatus in Australia. Australasian Plant Disease Notes 7: 13–15 [Google Scholar]
- Goodwin SB. (2004). Minimum phylogenetic coverage: An additional criterion to guide the selection of microbial pathogens for initial genomic sequencing efforts. Phytopathology 94: 800–804 [DOI] [PubMed] [Google Scholar]
- Greene HC. (1961). Notes on Wisconsin parasitic fungi. XXVII. Wisconsin Academy of Sciences, Arts and Letters 50: 141–161 [Google Scholar]
- Groenewald JZ, Nakashima C, Nishikawa J, Shin H-D, Park J-H, et al. (2013). Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology 75: 115–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruyter J de, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW. (2010). Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102: 1066–1081 [DOI] [PubMed] [Google Scholar]
- Gruyter J de, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW. (2013). Redisposition of Phoma-like anamorphs in Pleosporales. Studies in Mycology 75: 1–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98 [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, et al. (2011). The Amsterdam Declaration on Fungal Nomenclature. IMA Fungus 2: 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755 [DOI] [PubMed] [Google Scholar]
- Inácio CA, Dianese JC. (1998). Some foliicolous fungi on Tabebuia species. Mycological Research 102: 695–708 [Google Scholar]
- Jørstad I. (1965). Septoria and septoroid fungi on dicotyleones in Norway. Oslo University Press, Oslo: [Google Scholar]
- Jørstad I. (1967). Septoria and Septoroid fungi on Gramineae in Norway. Oslo University Press, Oslo: [Google Scholar]
- Kaneko S, Fujioka H, Zinno Y. (1989). A new species of Septoria on Japanese black pine. Transactions of the Mycological Society of Japan 30: 463–466 [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht SC, Crous PW, Groenewald JZ, Tewoldemedhin YT, Marasas WFO. (2011). Diaporthaceae associated with root rot of maize. IMA Fungus 2: 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Mel’nik V, Taylor JE, Crous PW. (2004). Diversity of saprobic hyphomycetes on Proteaceae and Restionaceae from South Africa. Fungal Diversity 17: 91–114 [Google Scholar]
- Li HY, Sun GY, Zhai XR, Batzer JC, Mayfield DA, et al. (2012). Dissoconiaceae associated with sooty blotch and flyspeck on fruits in China and the United States. Persoonia 28: 113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JK, Phookamsak R, Mingkhuan M, Wikee S, Li YM, et al. (2012) Towards a natural classification of Botryosphaeriales. Fungal Diversity 57: 149–210 [Google Scholar]
- Liu Y, Whelen S, Hall B. (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molecular Biology and Evolution 16: 1799–1808 [DOI] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, Wingfield MJ. (2010). Species concepts in Calonectria (Cylindrocladium). Studies in Mycology 66: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marincowitz S, Crous PW, Groenewald JZ, Wingfield MJ. (2008). Microfungi occurring on Proteaceae in the fynbos. CBS Biodiversity Series 7 CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands: [Google Scholar]
- Mason-Gamer RJ, Kellogg EA. (1996). Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Systematic Biology 45: 524–545 [Google Scholar]
- McDonald MC, Razavi M, Friesen TL, Brunner PC, McDonald BA. (2012). Phylogenetic and population genetic analyses of Phaeosphaeria nodorum and its close relatives indicate cryptic species and an origin in the Fertile Crescent. Fungal Genetics and Biology 49: 882–895 [DOI] [PubMed] [Google Scholar]
- Michaelides T, Morgan DP, Doster MA. (1995). Foliar and fruit fungal diseases. In: Pistachio Production (Ferguson L, ed). Center for Fruit and Nut Crop Research and Information, Pomology Department, University of California, Davis CA.: 148–159 [Google Scholar]
- Monod M. (1983). Monographie taxonomique des gnomoniacées ascomycètes de l’ordre des Diaporthales. Sydowia 9: 1–315 [Google Scholar]
- Nag Raj TR. (1993). Coelomycetous anamorphs with appendage-bearing conidia. Mycologue Publications, Waterloo, Ontario: [Google Scholar]
- Niekerk JM van, Groenewald JZ, Verkley GJM, Fourie PH, Wingfield MJ, Crous PW. (2004). Systematic reappraisal of Coniella and Pilidiella, with specific reference to species occurring on Eucalyptus and Vitis in South Africa. Mycological Research 108: 283–303 [DOI] [PubMed] [Google Scholar]
- Nylander JAA. (2004). MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre Uppsala University 2: 1–2 [Google Scholar]
- O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC. (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences of the United States of America 95: 2044–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrak F. (1957). Über die auf Aconitum vorkommenden Arten der gattung Septoria. Sydowia 11: 375–379 [Google Scholar]
- Petrak F, Sydow H. (1927). Die Gattungen der Pyrenomyzeten, Sphaeropsideen und Melanconieen. 1. Teil. Die phaeosporen Sphaeropsideen und die Gattung Macrophoma. Repertorium specierum novarum regni vegetabilis, Beihefte Bd 42 [Google Scholar]
- Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, et al. (2008). Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21: 29–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest MJ. (2006). Fungi of Australia: Septoria. ABRS, Canberra: CSIRO publishing, Melbourne, Australia; [Google Scholar]
- Punithalingam E. (1976). Septoria chrysanthemella. CMI Descriptions of Pathogenic Fungi and Bacteria 669 Commonwealth Mycological Institute, Kew, UK: [Google Scholar]
- Quaedvlieg W, Groenewald JZ, de Jesús Yáñez-Morales M, Crous PW. (2012). DNA barcoding of Mycosphaerella species of quarantine importance to Europe. Persoonia 29: 101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Kema GHJ, Groenewald JZ, Verkley GJM, Seifbarghi S, et al. (2011). Zymoseptoria gen. nov.: a new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia 26: 57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RW. (1970). A mycological colour chart. CMI and British Mycological Society; Kew, UK: [Google Scholar]
- Rogers DP. (1949). Nomina conservanda proposita and nomina confusa - Fungi: Nomina conservanda. Farlowia 3: 425–493 [Google Scholar]
- Saccardo PA. (1884). Sylloge Fungorum: Sylloge Sphaeropsidearum et Melanconiearum 3: 542 Padova, Italy: [Google Scholar]
- Saccardo PA. (1895). Sylloge Fungorum: Supplementum Universale, Pars III 11: 542 Padova, Italy: [Google Scholar]
- Saccardo PA, Saccardo D. (1906). Sylloge Fungorum: Supplementum Universale, Pars VII 18: 1–828 Padova, Italy: [Google Scholar]
- Saccardo PA, Trotter A. (1913). Sylloge Fungorum: Supplementum Universale, Pars IX 22: 1–1612 Padova, Italy: [Google Scholar]
- Shin HD. (1995). New fungal diseases of economic resource plants in Korea (II). Korean Journal of Plant Pathology 11: 120–131 [Google Scholar]
- Shin HD, Sameva EF. (2002). Taxonomic notes on the genus Septoria in Korea (II). Mycotaxon 83: 287–300 [Google Scholar]
- Shin HD, Sameva EF. (2004). Septoria in Korea. National Institute of Agricultural Science and Technology, Republic of Korea: [Google Scholar]
- Shoemaker RA, Babcock CE. (1989). Phaeosphaeria. Canadian Journal of Botany 67: 1500–1599 [Google Scholar]
- Sivanesan A. (1984). The Bitunicate Ascomycetes and their anamorphs. J. Cramer, Vaduz, Germany: [Google Scholar]
- Solomon PS, Lowe RGT, Tan KC, Waters ODC, Oliver RP. (2006). Stagonospora nodorum: cause of stagonospora nodorum blotch of wheat. Molecular Plant Pathology 7: 147–156 [DOI] [PubMed] [Google Scholar]
- Stukenbrock EH, Quaedvlieg W, Javan-Nikhah M, Zala M, Crous PW, McDonald BA. (2012). Zymoseptoria ardabilia and Z. pseudotritici, two progenitor species of the septoria tritici leaf blotch fungus Z. tritici (synonym: Mycosphaerella graminicola). Mycologia 104: 1397–1407 [DOI] [PubMed] [Google Scholar]
- Summerell BA, Groenewald JZ, Carnegie AJ, Summerbell RC, Crous PW. (2006). Eucalyptus microfungi known from culture. 2. Alysidiella, Fusculina and Phlogicylindrium genera nova, with notes on some other poorly known taxa. Fungal Diversity 23: 323–350 [Google Scholar]
- Sutton B, Pollack F. (1974). Microfungi on Cercocarpus. Mycopathologia 52: 331–351 [DOI] [PubMed] [Google Scholar]
- Sutton BC, Pascoe IG. (1987). Septoria species on Acacia. Transactions of the British Mycological Society 89: 521–532 [Google Scholar]
- Sutton BC, Pascoe IG. (1989). Some Septoria species on native Australian plants. Studies in Mycology 31: 177–186 [Google Scholar]
- Sutton BC, Swart HJ. (1986). Australian leaf-inhabiting fungi XXIII. Colletogloeum species and similar fungi on Acacia. Transactions of the British Mycological Society 87: 93–102 [Google Scholar]
- Sutton BC. (1964). Coelomycetes III. Annellolacinia gen. nov., Aristastoma, Phaecytostroma, Seimatosporium, etc. Mycological Papers 64 Commonwealth Mycological Institute, Kew, UK: [Google Scholar]
- Sutton BC. (1977). Coelomycetes VI. Nomenclature of generic names proposed for Coelomycetes. Mycological Papers 141 Commonwealth Mycological Institute, Kew, UK: [Google Scholar]
- Sutton BC. (1980). The coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. Commonwealth Mycological Institute, Kew, UK: [Google Scholar]
- Teterevnikova-Babayan DN. (1987). Fungi of the genus Septoria in the U.S.S.R. Akademiya Nauk Armyanskoi SSR, Yerevan: [Google Scholar]
- Verkley GJM. (1999). A monograph of the genus Pezicula and its anamorphs. Studies in Mycology 44: 1–180 [Google Scholar]
- Verkley GJM, Priest MJ. (2000). Septoria and similar coelomycetous anamorphs of Mycosphaerella. Studies in Mycology 45: 123–128 [Google Scholar]
- Verkley GJM, Crous PW, Groenewald JZ, Braun U, Aptroot A. (2004a). Mycosphaerella punctiformis revisited: morphology, phylogeny, and epitypification of the type species of the genus Mycosphaerella (Dothideales, Ascomycota). Mycological Research 108: 1271–1282 [DOI] [PubMed] [Google Scholar]
- Verkley GJM, Silva da M, Wicklow DT, Crous PW. (2004b). Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Studies in Mycology 50: 323–335 [Google Scholar]
- Verkley GJM, Starink-Willemse M, van Iperen A, Abeln ECA. (2004c). Phylogenetic analyses of Septoria species based on the ITS and LSU-D2 regions of nuclear ribosomal DNA. Mycologia 96: 558–571 [PubMed] [Google Scholar]
- Verkley GJM, Quaedvlieg W, Shin HD, Crous PW. (2013). A new approach to species delimitation in Septoria. Studies in Mycology 75: 213–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield EM. (1940). Nomina generica conservanda. Transactions of the British Mycological Society 24: 282–293 [Google Scholar]
- Walker J, Sutton BC, Pascoe IG. (1992). Phaeoseptoria eucalypti and similar fungi on Eucalyptus, with description of Kirramyces gen. nov. (Coelomycetes). Mycological Research 96: 911–924 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego, California: 315–322 [Google Scholar]
- Wingfield MJ, De Beer ZW, Slippers B, Wingfield BD, Groenewald JZ, et al. (2012). One fungus, one name promotes progressive plant pathology. Molecular Plant Pathology 13: 604–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J-J, Eom A-H. (2012). Molecular identification of endophytic fungi isolated from needle leaves of conifers in Bohyeon Mountain, Korea. Mycobiology 40: 231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Crous PW, Schoch CL, Hyde KD. (2012). Pleosporales. Fungal Diversity 53: 1–221 [DOI] [PMC free article] [PubMed] [Google Scholar]