Abstract
The degree of differentiation in human cancers generally reflects the degree of malignancy, with the most undifferentiated cancer being also the highest grade and the most aggressive. High-grade serous ovarian carcinomas (HGSOC) are poorly differentiated and fast-growing malignancies. The molecular mechanisms underlying the poor differentiation of HGSOC has not been completely characterized. Evidence suggests that miRNA, miR are dysregulated in HGSOC. Therefore, we focused on those miRNAs that are relevant to tumor differentiation. Expression profiling of miRNAs in HGSOC, indicated miR-106a and its family members were significantly upregulated. Upregulation of miR-106a was further validated by real-time reverse transcriptase PCR (qRT-PCR) and miRNA in situ hybridization in a large cohort of HGSOC specimens. Overexpression of miR-106a in benign and malignant ovarian cells significantly increased the cellular proliferation rate and expanded the side-population fraction. In particular, SKOV3 cells with miR-106a overexpression had significantly higher tumor initial/stem cell population (CD24- and CD133-positive cells) than control SKOV3 cells. Among many miR-106a predicated target genes, p130 (RBL2), an retinoblastoma (Rb) tumor suppressor family member, was not only confirmed as a specific target of miR-106a but also related to tumor growth and differentiation. The importance of mir-106a and RBL2 was further demonstrated in vivo, in which, SKOV3 cells overexpressing miR-106a formed poorly differentiated carcinomas and had reduced RBL2 levels. To our knowledge, this is the first study of miR-106a mediating proliferation and tumor differentiation in HGSOC.
Implications
The current study suggests that the RB tumor suppressor pathway is a critical regulator of growth and differentiation in HGSOC.
Introduction
Ovarian cancer is a deadly disease. According to the American Cancer Society, approximately 21,000 women developed ovarian cancer and approximately 15,000 women died from this disease in the United States in 2011. Despite great efforts in clinical and medical research, the overall survival rate of ovarian cancer has not changed in the past 50 years (1). Histologic and molecular heterogeneity has complicated our understanding of the tumor biology and its behavior and has limited the treatment options for this deadly disease. For example, high-grade serous ovarian carcinomas (HGSOC), accounting for up to 60% of ovarian epithelial carcinoma, are genetically and histologically heterogeneous tumors. According to The Cancer Genomic Atlas (TCGA), HGSOC can be further divided into four subtypes defined as Differentiated, Immunoreactive, Mesenchymal, and Proliferative based on the different molecular signatures from global gene and miRNA profiles (2).
The degree of differentiation of human cancers is used to score the degree of malignancy, with the most undifferentiated cancer being the highest grade and most aggressive. Although the exact mechanisms in controlling HGSOC differentiation are probably complex and remain to be fully characterized, the TCGA study indicates that a subset of gene and miRNA are associated with tumor differentiation (2). miRNAs are a group of small noncoding RNAs that are significantly dysregulated in ovarian epithelial carcinoma (3). Furthermore, the miRNA signature can be used for tumor classification and for grading differentiation (4). In addition to the findings of altered miRNA expression in association with aggressive tumor growth in HGSOC (3), one of the major functional roles of miRNAs in ovarian carcinogenesis is likely to be associated with tumor stem/initiation cell regulation and tumor differentiation (5–8). In our global miRNA profiling analysis in early and late stages of HGSOC, we found that miR-106a and its family members were significantly upregulated. miR-106 family members, as the key players in stem cell self-renewal, are highly induced during the early stages of cell reprogramming (9, 10). Most importantly, estrogen receptor-α (ER-α) target protein c-MYC binds the miR-106a-363 promoter in an estrogen-dependent manner and upregulates miR-106a in breast cancer cell lines (11). miR-106a is known to function in tumor-initiating cells and regulate tumor differentiation through the retinoblastoma (Rb) pathway and cell cycle and FoxO signaling in many solid carcinomas (10–12).
In this study, we want to determine whether miR-106a overexpression is associated with tumorigenesis of HGSOC. We investigated the functional and oncogenic roles of miR-106a both in vivo and in vitro. We characterized that RBL2, a tumor suppressor gene commonly downregulated in HGSOC, is the specific target gene of miR-106a. Our findings suggested that miR-106a can specifically repress expression of the retinoblastoma family member RBL2 and miR-106a overexpression results in rapid tumor growth and poor differentiation.
Materials and Methods
Patients and tissue samples
This retrospective study included 117 cases of high-grade serous carcinoma (HG-PSC) (Table 1) and 30 samples of fallopian tube tissue. All cases were collected from Northwestern Memorial Hospital (Chicago, IL) between 2007 and 2012. Fresh-frozen tissue from 30 cases of HGSOC and matched normal fallopian tube tissue were also collected for the study. The study was approved by the Northwestern University (Chicago, IL) Institutional Review Board. The archived tissue sections were collected and prepared for tissue microarray (TMA) as previously described (13).
Table 1.
Clinical characteristic of 117 ovarian carcinomas
| Title | Parameters | Scale and range |
|---|---|---|
| Age | Range | 34–90 |
| Mean ± SD | 58.8 ± 10.7 | |
| Median | 59 | |
| Tumor type | PSC—high grade | 100 |
| PSC—low grade | 3 | |
| Non-PSC | 14 | |
| Tumor stage | I | 0 |
| II | 0 | |
| IIIA | 3 | |
| IIIB | 5 | |
| IIIC | 91 | |
| IV | 18 | |
| Lymph node metastases | None | 70 |
| Pelvic | 12 | |
| Periaortic | 4 | |
| Both | 33 | |
| Follow-up | Alive with disease | 24 |
| Death | 71 | |
| Disease Free | 22 |
Cell lines and cell cultures
Normal OSE (ovarian surface epithelia) cell line T29 and normal FTSE (fallopian tube secretory epithelia) cell line FT0E187 were generated and originally tested in Dr. Liu’s laboratory. The nature of these cell lines were described in previous publications (14, 15). T29 and FTE187 cells were maintained in cell culture medium consisting of 1:1 Medium199 (Sigma-Aldrich) and MCDB105 medium (Sigma-Aldrich) with 10% heat-inactivated FBS (USA Scientific) and 10 ng/mL EGF (Sigma-Aldrich). Ovarian cancer cell lines SKOV3, HEY, OV-90, and OVCAR-3 were purchased from American Type Culture Collection. SKOV3 cells were cultured in McCoy’s 5A modified medium (Gibco, Invitrogen). HEY, OV-90, and OVCAR-3 cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco, Invitrogen). All media contained 10% FBS (Gibco, Invitrogen), 100 μg/mL penicillin, and 100 μg/mL streptomycin. All cells were cultured in a humidified atmosphere of 5% CO2 at 37°C.
Antibodies, histologic and immunohistochemical staining analysis
Antibodies used for immunohistochemical staining included anti-RBL2 (sc-317; Santa Cruz Biotechnology), anti-p53 (Dako), anti-Ki-67 (cell proliferation marker; Neomarkers), anti-CD133(HPA004922; Sigma-Aldrich), and anti-CD24-PE (ebioscience). Formalin-fixed and paraffin-embedded tissues were sectioned at 4 μm. Tissue slides were deparaffinized in xylene and rehydrated in a graded series of ethanol. Antigen retrieval was performed by heat-induced epitope retrieval as previously described (16). All immunohistochemical staining procedures were performed on a Ventana Nexus automated system. Slides were blocked with 1.5% normal goat serum for 30 minutes at room temperature and then incubated overnight at 4°C with primary antibodies (RBL2, p53, Ki-67, and CD133) in a humid chamber. Staining was detected with I-View 3,3′-diaminobenzidine (DAB) detection system.
miRNA in situ hybridization
In situ hybridization was performed as previously described (17). Formalin-fixed and paraffin-embedded tissues were sectioned at 4 μm. Hybridization was performed with DIG (digoxigenin-dUTP)-labeled, locked nucleic acid (LNA)–based probes specific for human miR-106a and U6 (Exiqon). Hybridizations were performed overnight at 55°C after the addition of 50 nmol/L of miR-106a and 20 nmol/L of U6 (Exiqon) diluted by hybridization solution (BIO-CHAIN Institute Inc.). After stringency wash (50% formamide and 2× SSC) at hybridization temperature, slides were blocked with 2% normal goat serum for 60 minutes at room temperature and then incubated with anti-DIG antibody (1:800; Roche Diagnostics) overnight at 4°C. Alkaline phosphatase activity was detected using BM Purple AP Substrate (Roche Diagnostics). In situ hybridization results were quantified according to the intensity of staining and were normalized by U6.
RNA isolation and quantitative RT-PCR
Total RNA was isolated using the mirVana RNA Isolation Kit following the manufacturer’s instructions (Ambion) and cDNA was synthesized by reverse transcription of 1μg of total RNA with M-MLV Reverse Transcriptase (Promega). MirVana quantitative reverse transcriptase PCR (qRT-PCR) primers and the mirVana qRT-PCR Detection Kit (Ambion) were used to test miR-106a and miR-106b expression. SYBR Green real-time RT-PCR (Applied Biosystems) was used to detect the expression of RBL2. Small nuclear RNA U6 was used as an internal control.
Lentiviral vector construction, viral production, and infection
To generate a miR-106a expression vector, a 522-bp fragment containing pre-miR-106a was cloned into pGIPZ lentiviral vector. RBL2 short hairpin RNA (shRNA) in pGIPZ was purchased from Open Biosystems. Lentivirus-expressed miR-106a or shRBL2 was produced in HEK293T cells packaged by pMD2G and psPAX2. For stable infection, 1 × 105 cells were plated in 6-well plates along with 2 mL of medium without antibiotics. After overnight incubation, the medium was removed and replaced with 1 mL/well of Opti-MEM Reduced-Serum Medium containing lentivirus and 8 μg/mL of polybrene. Next, 50 μL of concentrated lentiviral particles were added to each well. Twenty-four hours later, fresh medium containing 2 μg/mL of puromycin was added to each well. Fresh medium containing puromycin was replaced every 3 to 4 days. Single colonies were obtained after 3 weeks of puromycin selection.
Oligonucleotide transfection
MiRIDIAN miR-106a inhibitor (Dharmacon, Inc) was transfected 50 nmol/L using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Transfection efficiency was monitored using BLOCK-iT Fluorescent Oligo (Invitrogen). Forty-eight hours after transfection, cells were harvested and analyzed for miR-106a and RBL2 expression. The harvested transfected cells were also plated for cellular proliferation assay.
Anchorage-independent colony formation
Anchorage-independent cell growth in soft agar was performed in triplicate with cells suspended in 3 mL of a low-density (0.75 × 104 cells) culture medium containing 0.3% agar (USB Corporation) and seeded onto a base layer of 3 mL of 0.6% agar bed in 60-mm tissue culture dishes. After 2 to 4 weeks (based on different cell lines), colonies were stained, photographed, and scored.
Matrigel invasion assay
Matrigel-coated chambers (BD Biosciences) containing 8-μm pores were used for the assays. Briefly, 2.5 × 105 cells were seeded into the upper chambers (coated in Matrigel) in certain mediums containing 0.5% FBS. The lower chamber of the Transwell was filled with culture media containing 10% FBS as a chemoattractant. The chambers were incubated at 37°C for 16 to 72 hours depending on the different cell lines. Noninvaded cells on the top of the Transwell were scraped off with a cotton swab. Successfully translocated cells were fixed by 10% formalin, stained with 0.1% crystal violet for 30 minutes, and counted under a light microscope.
Cellular proliferation assay
The WST-1 (Roche) was used for cell proliferation assay according to the manufacturer’s instructions. Cells were seeded in 96-well plates in triplicate at densities of 2 × 103 cells per well. Cell proliferation was monitored at different times (1–5 days). After incubation at designated times, 10 μL of WST-1 was added to each well and then incubated for 4 hours at 37°C. Absorbance of the samples was measured by an ELISA plate reader with a test wavelength at 450 nm and a reference wavelength at 630 nm.
3′-UTR of RBL2 vector construction and luciferase reporter assay
The 3′-untranslated region (UTR) of RBL2 containing two putative miR-106a-binding sites (513bp) was amplified by PCR and cloned into psiCHECK2 vector (Promega) using the XhoI and NotI sites. The psiCHECK2-RBL2-Mut construct containing the mutations located at the miR-106a-binding sites was generated by overlap extension PCR and cloned into the psiCHECK2 again (18). For the luciferase assay, T29-Vector and T29-miR-06a cells were seeded at 1 × 104 cells per well in a 96-well plate for 24 hours before transfection. Cells were transfected with 100 ng of psiCHECK2 control, psi-CHECK2-RBL2, and psiCHECK2-RBL2-Mut. Twenty-four hours after transfection, luciferase activity was measured using the Dual-Glo Luciferase Assay System (Promega). Renilla luciferase activity was normalized to corresponding firefly luciferase activity and plotted as a percentage.
Western blotting
Cells were lysed and protein concentration was determined using the BCA Assay Kit (Thermo Scientific). Protein samples were separated by SDS-PAGE and electrotransferred onto polyvinylidene fluoride membrane. The membrane was incubated with specific primary antibodies. Rabbit anti-pRb2/ p130 (sc-317; Santa Cruz Biotechnology), mouse anti-p21 (sc-6246; Santa Cruz Biotechnology), and mouse anti-β-actin (Sigma-Aldrich) were tested. Proteins of interest were detected with horseradish peroxidase–conjugated secondary antibodies and were developed using the enhanced chemiluminescence (ECL)–enhanced luminol reagent (PerkinElmer Inc.).
Flow cytometry analysis
For side-population analysis, cells (106/mL) were incubated in prewarmed DMEM containing 2% FBS (Gibco, Invitrogen). Cells were stained with 5μg/mL Hoechst 33342 alone or in combination with 50 μmol/L verapamil (Sigma). The cell wells were mixed and placed in the 37°C water bath for exactly 90 minutes. The tubes were mixed several times during incubation. At the end of incubation, cells were centrifuged at 4°C and resuspended in cold PBS with 2% FBS. Propidium iodide (Sigma) solution was added to a final concentration of 2 μg/mL and cells were then analyzed in fluorescence-activated cell sorting (FACS) with UV laser (BD Biosciences).
To evaluate cell-cycle distribution, 1 × 106 cells were fixed in 4 mL of cold 70% ethanol at −20°C overnight. After centrifugation at 1,000 × g for 10 minutes, cell pellets were incubated with 0.5 mL of PBS containing 100 μg/mL RNase and 5μg/mL propidium iodide at 37°C for 30 minutes. Cell-cycle distribution was analyzed by measuring DNA content using FACS (Fluorescence Activated Cell Sorting). For the CD24 and CD133 staining and FACS analysis, 2 × 106 cells were incubated with CD24 and CD133-PE (MACS® Technology Miltenyi Biotech) for 30 minutes at 4°C in the dark. After the washing steps, labeled cells were analyzed by flow cytometry (BD Biosciences).
Nude mice xenografts
Female nude mice (6–8-week old) were purchased from National Cancer Institute (NCI, Frederick National Lab). For xenograft experiments, equal numbers (5 × 106) of SKOV3-Vector and SKOV3-miR-106a cells were harvested and resuspended in 0.1 mL of PBS for subcutaneous injection. Six mice were used for each group and each mouse received two injections. Tumor volume was calculated with the use of the following formula: tumor volume (in mm3) = a × b2 × 0.52, where a is the longest diameter, b is the shortest diameter, and 0.52 is a constant to calculate the volume of an ellipsoid. Tumor sizes were measured weekly, and mice were observed up to 5 weeks. The mouse was euthanized when a tumor reached 1.5 cm in diameter. All tumors for each group were excised, fixed in 10% formalin, and subjected to routine histologic examination and immunostaining of RBL2 and Ki-67.
Statistical analysis
All data are presented as mean and SEs in triplicate. Student t test was used for comparisons between two groups of experiments, and one-way ANOVA was used for comparisons among three or more groups. P < 0.05 was considered statistically significant.
Results
miR-106a and its family members are overexpressed in high-grade ovarian serous carcinoma
miRNA dysregulation is common in HGSOC and many miRNAs are associated with the tumorigenesis of HGSOC (19). However, miRNA expression analyses from most studies were obtained from snap-frozen tissue samples. The tumor and stromal ratio cannot be well characterized. To get a better view of miRNA expression in relatively pure normal and tumor epithelia, we collected tissue samples by microdissection from normal fallopian tube, serous tubal intraepithelial carcinoma (STIC), and HGSOC (invasive) in five cases and obtained global miRNA expression using the HTG (HTG Molecular Diagnostics, Inc.) platform (17). We found that miR-106 and its family members were significantly over-expressed in HGSOC (Supplementary Table S1). miRNA profile analysis in five cases revealed that HGSOC showed more than a 4-fold increase in miR-106a expression over control fallopian tube (Fig. 1C and Supplementary Table S1).
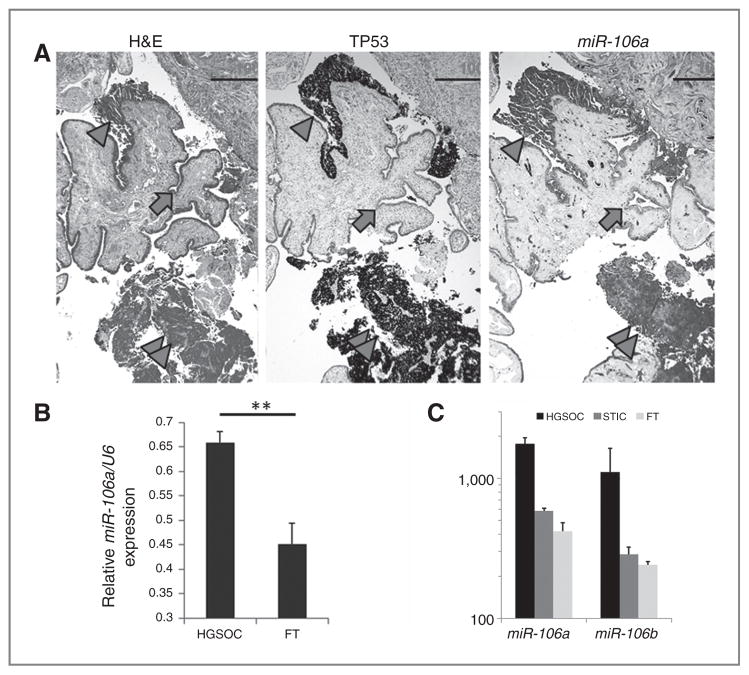
Figure 1.
Expression analysis of miR-106a in ovarian carcinoma. A, photomicrographs illustrate consecutive sections of normal fallopian tube (FT) epithelium (arrow), STIC (arrow head), and invasive high-grade serous carcinoma (double arrow heads) by hematoxylin and eosin stain (H&E, left), Immunostain for P53 (middle) and miRNA in situ hybridization for miR-106a (right). Strong immunoreactivity for P53 represents the accumulation of the mutant P53 in STIC and invasive high-grade serous carcinoma. B, the histobars represent the mean values of relative miR-106a expression in 117 HGSOC and 30 fallopian tube (normalized by RNA loading control of U6) by miRNA in situ hybridization. C, the relative miR-106a and miR-106b expression detected by miRNA profiling analysis (N = 5) in normal fallopian tube (light gray), STIC (dark gray), and HGSOC (black). **, P < 0.01.
To confirm miR-106a overexpression in STIC and HGSOC, we conducted miRNA in situ hybridization for miR-106a (Exiquon; see Materials and Methods) in a large cohort of HGSOC (N = 117) (Table 1) and control fallopian tube (N = 30). As illustrated in Fig. 1A, both STIC and invasive HGSOC showed high levels of miR-106a expression, whereas normal fallopian tube epithelial cells showed lower miR-106a expression. By a semiquantitative analysis, we found that miR-106a expression was significantly higher in HGSOC (0.68 ± 0.0532) than in normal fallopian tube (0.46 ± 0.1109; P < 0.01; Fig. 1B). However, miR-106a expression detected by miRNA in situ hybridization is at best for a semiquantitation, and may not reflect the actual level of miR-106a expression.
miR-106a overexpression promotes ovarian cancer cell proliferation in vitro
To investigate the oncogenic properties of miR-106a in HGSOC, we prepared and established cell lines with stable miR-106a overexpression in normal fallopian tube (FTE187), ovarian surface (T29) epithelial cell lines, and ovarian cancer (SKOV3 and HEY) cell lines (Supplementary Fig. S1A). We found that introducing miR-106a overexpression resulted in a significant increase of anchorage-independent growth in both normal (T29) and malignant (SKOV3) cell lines (Supplementary Fig. S1B; P < 0.05) in soft agar assay. miR-106a overexpression could also slightly enhance tumor cell migration in Matrigel (Supplementary Fig. S1C). miR-106a overexpression significantly enhanced cell proliferation in normal fallopian tube epithelial cells (FTE187; Fig. 2A). miR-106a overexpression could also promote cell proliferation in ovarian cancer cell lines (Fig. 2A). Cell-cycle analysis by flow cytometry showed that miR-106a overexpression led to an increase in the percentage of cells at S and G2–M phase for both FTE187 and SKOV3 cells (Fig. 2B and C).
Figure 2.
miR-106a promotes normal and malignant ovarian/ fallopian tube epithelial cell proliferation. A, growth curves illustrate the significant differences in cell proliferation from day 2 to 5 in normal fallopian tube secretory epithelial cell line (FTE187; left) and ovarian cancer cell line (SKOV3; right) with and without miR-106a overexpression (see Materials and Methods). B and C, cell-cycle analysis by the cell flow cytometer reveals that a significant increase of S-phase cell population in FTE187 and in SKOV3 with miR-106a overexpression (C) in comparison with those without miR-106a overexpression (B; P < 0.05).
SKOV3 cells with miR-106a overexpression slightly enhanced invasion through Matrigel (Supplementary Fig. S1), but did not promote apoptosis based on Annexin V–PI staining (data not shown). When treated with different doses of paclitaxel and cisplatin, SKOV3 cell lines with miR-106a overexpression were significantly more resistant to chemotherapy (Supplementary Fig. S2). The findings suggest that miR-106a can promote cell proliferation and confer chemoresistance.
miR-106a increases the yield of putative cancer stem cells
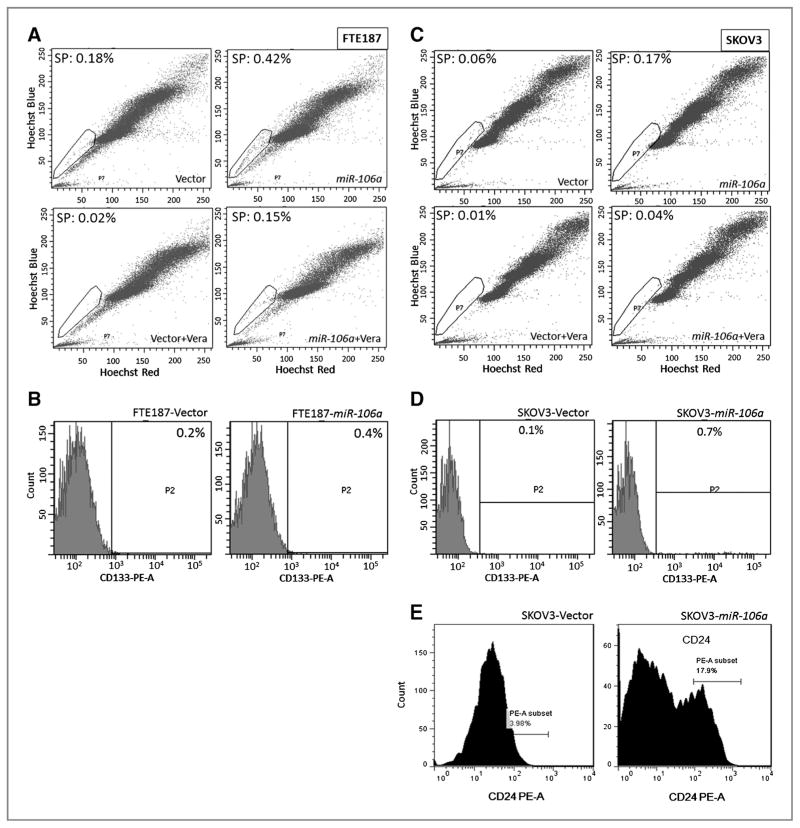
We next measured the proportion of cell population in cells with and without miR-106a overexpression, respectively. Side-population cells were quantitated by Hoechst 33342 staining and flow cytometry in FTE187 and SKOV3 (Fig. 3). Benign and malignant ovarian cancer cells that were transduced with miR-106a showed more than 2-fold increase in the size of side-population, when compared with cells transfected with the control vectors. The findings can be repeated in another two cell lines (Supplementary Fig. S4). To further evaluate whether miR-106 overexpression promotes the formation of ovarian tumor-initiating cells, we examined the ratio of ALDH1, CD133, and CD24-positive and -negative cell populations by flow cytometry analysis. CD133 is considered as stem cell marker (20). As shown in Fig. 3B and D, both normal (FTE187) and malignant (SKOV3) ovarian cell lines with miR-106a overexpression had slightly increased frequency of CD133-positive cells. CD24 expression level was also increased from 3.98% to 17.9% in SKOV3-miR-106a cells as compared with SKOV3-Vector cells (Fig. 3E). No significant change of ALDH1 by miR-106a overexpression was seen (data not shown). Our findings suggested that miR-106a may promote ovarian cancer stem cell formation.
Figure 3.
miR-106a overexpression increases stem cell-like (side-population, SP) population. The side-population, major population of normal (FTE187; A) and malignant (SKOV3; C) cell lines were analyzed by flow cytometer (see Materials and Methods). Circled areas represent the side-populations. Overall, a 2- to 4-fold increase of side-population cells were observed in four cell lines with miR-106a overexpression. CD133 and CD24 expression were counted by flow cytometry in FTE187 (B) and in SKOV3 (D and E). Cells with miR-106a overexpression significantly increase CD24- and CD133-positive cell population in comparison with those without miR-106a overexpression (P < 0.05).
miR-106a specifically represses RBL2 expression in ovarian cancer
Previous studies revealed that miR-106 and its family member can specifically repress CDKN1A (p21) expression through targeting at its 3′-UTR (21, 22). Repression of p21 may partially explain the mitogenic role of miR-106a in ovarian cancer. However, little is known about the role of miR-106a in tumor differentiation. We found that RBL2 is one of the predicted target genes of miR-106a. RBL2 is a member of the Rb gene family, and is involved in cell-cycle regulation, tumor differentiation, and stem cell self-renewal (23). Previous studies (24) from global gene expression showed that higher expression of miR-106a or its family members is associated with lower RBL2 expression (23). To investigate whether RBL2 is the specific target of miR-106a, we examined and characterized the molecular interaction between miR-106a and RBL2. Among four ovarian cancer cell lines, three (HEY, OVCAR3, and OV-90) showed high levels of endogenous miR-106a expression and OV-90 had the highest miR-106a (Fig. 4A).
Figure 4.
Tumor suppressor gene RBL2 is specifically targeted by miR-106a. A, RT-PCR analysis reveals an inverse correlation of endogenous RBL2 with miR-106a expression in normal (FTE187 and T29) and malignant (SKOV3, HEY, OV-90, and OVCAR3) cell lines. U6 is used as an RNA loading control. B, two predicted binding sites of the miR-106a family in RBL2 3′-UTR and their sequence. C, histobars illustrate the relative luciferase expression in T29 cells cotransfected with wild-type and mutant miR-106a–binding sites with (gray bars) and without (black bars) miR-106a overexpression. Vector stands for luciferase transfection without RBL2 3′-UTR. D, Western blot analysis reveals that introducing stable miR-106a overexpression in FTE187 cells results in significant reduction of RBL2 and P21 (well known target gene of miR-106a, used as positive control). miR-106a and U6 expression are shown below. Histobars represent the average levels of RBL2 and P21 expression with and without miR-106a expression. *, P < 0.05; **, P < 0.01. E, blocking RBL2 expression promotes cell proliferation as did with miR-106a overexpression. F, miR-106a–targeted gene expression was further validated by blocking miR-106a expression with anti-miR-106a in OV-90 cells; blocking miR-106a expression inhibits cell proliferation compared with control cells.
By computer software analyses (PicTar, TargetScan, and miRBase), we found that RBL2 had two very conservative sites complementary to miR-106a “seed” sequence (Fig. 4B). The luciferase expression constructs containing 1.35 kb of wild-type and mutant (replacement of four nucleotides in “seed” sequence of miR-106a-binding sites) 3′-UTR sequence of RBL2 cDNA were respectively generated. Cotransfection of miR-106a with wild-type RBL2 3′-UTR construct resulted in at least 2-fold reduction (P < 0.05) in luciferase activity in T29 cells, but no change of luciferase expression was noted when cotransfected with mutant RBL2 3′-UTR construct (Fig. 4C). RT-PCR and Western blot analyses showed that transient transfection of miR-106a (Dharmacon; see Materials and Methods) in FTE187 cells resulted in a significant reduction of RBL2 expression at mRNA (not shown) and protein level (Fig. 4D). As a control, p21 expression was also determined. These results indicate that RBL2 can be specifically repressed by miR-106a (Fig. 4C and D).
To investigate whether miR-106a–mediated RBL2 down-regulation was associated with cell proliferation, we prepared ovarian cancer cell lines with stable miR-106a overexpression and constant inhibition of RBL2 expression (shRBL2), respectively (Fig. 4E). We found that introducing miR-106a overexpression or inhibiting RBL2 expression significantly enhanced HEY cell proliferation (Fig. 4E). This finding could be reproduced in another ovarian cancer cell line OV-90 (data not shown). Anti-miR-106a treatment in OV-90 cells restored RBL2 expression and prohibited tumor cell growth (Fig. 4F). These findings suggest that miR-106a enhances cell proliferation by negatively regulating the tumor suppressor gene RBL2.
miR-106a overexpression enhances tumor growth and dedifferentiation in xenografts
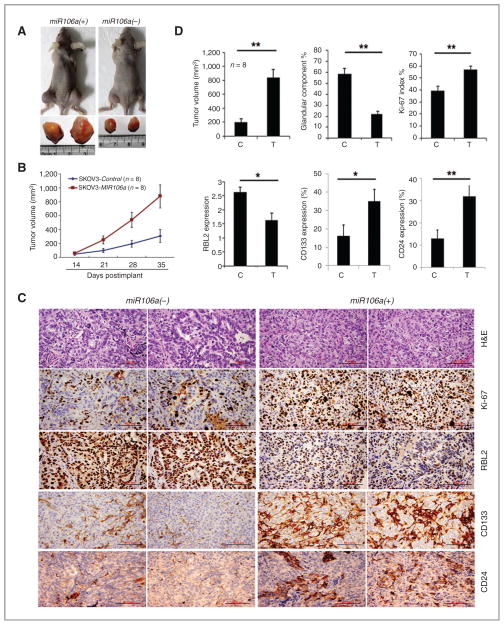
To examine whether miR-106a overexpression promotes ovarian cancer growth in vivo, we inoculated ovarian cancer cell line SKOV3 with and without miR-106a overexpression in nude mice (N = 8 for each group). Tumor size began to show significant difference 3 weeks after implantation, and reached a peak tumor size of 870 mm3 for SKOV3-miR-106a, and only 220 mm3 for SKOV3-Vector (P < 0.01; Fig. 5A and B). In addition to difference in tumor size, the two groups also differed in the degree of tumor differentiation. In tumor sections of SKOV3-vector, more than 60% of the tumor showed glandular differentiation, characterized by well-formed epithelial glands with an orientated cell border and moderate nuclear atypia (Fig. 5C and Supplementary Fig. S5). Immunostaining for Ki-67 showed a proliferative index of 40% (Fig. 5D) and a low rate of tumor necrosis (data not shown). Immunostaining revealed a strong and diffuse immunoreactivity for RBL2 in almost all tumor cells (Fig. 5C and D). In contrast, tumors formed by SKOV3 cells with miR-106a overexpression showed a mostly solid growth pattern (glandular and solid ratio of 1:4). SKOV3-miR-106a cells tended to be larger with a higher nuclear to cytoplasmic ratio and were more poorly differentiated than the SKOV3-vector cells (Fig. 5C). The Ki-67 index in SKOV3-miR-106a tumors was nearly 60% (Fig. 5D) with brisk mitoses and extensive tumor necrosis. As expected, a lower immunoreactivity for RBL2 (relative immunointensity was 1.5 in comparison with 2.7 for SKOV3-vector; Fig. 5C). Immunostaining revealed a significant increase of CD133- and CD24-positive cells in SKOV3-miR-106a tumor (Fig. 5C and D and Supplementary Fig. S3). In summary, we found that the xenografts of SKOV3-miR-106a showed larger tumor masses with high proliferation index, increased tumor stem cell population, and poor differentiation. These findings suggested that miR-106a overexpression is associated with an aggressive ovarian cancer growth and dedifferentiation through negative regulation of tumor suppressor gene RBL2.
Figure 5.
miR-106a overexpression leads to faster growth and poor differentiation of tumor massin xenografts of nude mice. A, photographs illustrate an example of larger tumor masses in xenografts of SKOV3 cell lines with miR-106a overexpression, in comparison with the tumors without miR-106a overexpression. B, growth curves for tumor volumes in xenografts of nude mice with SKOV3 cell line with and without miR-106a overexpression (each consists of 8 mice), measured from day 14 to 35. C, photomicrographs illustrate the histology (top), Ki-67, RBL2, CD133, and CD24 expression (by immunohistochemistry stain, middle and lower) in xenografts of SKOV3 cell lines with [miR-106a (+)] and without [miR-106a (−)] miR-106a overexpression. SKOV3 tumors without miR-106a overexpression show well-differentiated tumor growth, characterized by mostly glandular growth pattern, low Ki-67 index (low cell proliferation rate), and high level of RBL2 expression. Although SKOV3 tumors with miR-106a overexpression show poorly differentiated and solid growth patterns, high Ki-67 index and lower RBL2 expression. D, the statistical analysis of mean (wide histobars) and standard errors (small T-bars) between tumors with (T) and without miR-106 (C) overexpression in the order of tumor volume,% of glandular component, Ki-67 index, RBL2, CD133, and CD24 expression. *, P < 0.05; **, P < 0.01.
Expressions of miR-106a and its target genes in HGSOC
miR-106a and its family members were found to be significantly overexpressed in HGSOC by our chip-based miRNA profiling analysis in formalin-fixed and paraffin-embedded tissue sections (Fig. 1). Previous study showed that RBL2 is downregulated in ovarian carcinoma (24). We confirmed that RBL2 is specifically targeted by miR-106a (Fig. 4). To further investigate whether miR-106a and miR-106b–mediated downregulation of RBL2 occurs in HGSOC, we examined their expressions in 30 HGSOC tumor tissues and eight normal fallopian tube tissues by real-time RT-PCR with RNA loading controls of U6 and β-actin. miR-106a and miR-106b expressions were significantly higher in HGSOC (32.44 ± 10.22 and 36.18 ± 11.79, respectively) than in fallopian tube [8.10 ± 0.87 and 12.05 ± 1.69, respectively (Fig. 6A and B)]. In comparison, fallopian tube had relatively higher levels of RBL2 expression (14.04 ± 2.41) than HGSOC (3.81 ± 1.00; P < 0.01; Fig. 6C). Further case-matched analysis revealed that the expression of miR-106a and b was negatively correlated with that of RBL2 (r = −0.12—0.23; Fig. 6D). All these findings support that miR-106a/b overexpression and RBL2 down-regulation in HGSOC are the crucial molecular events that are associated with tumor dedifferentiation.
Figure 6.
miR-106a, miR-106b, and RBL2 expression in HGSOCs and fallopian tube. Real-time RT-PCR analysis of miR-106a (A), miR-106b (B), and RBL2 (C) expression in individual panels and combined comparison (D). The relative expression of each gene was normalized by either U6 (for miRNA) or actin (for RBL2).
Discussion
Altered miRNA expression is commonly observed in ovarian cancer (2, 25). miRNAs that are associated with specific phenotypes of ovarian cancer have been studied extensively, including DNA damage response (miR-182; ref. 17), epithelial–mesenchymal transition (miR-200; ref. 26), p53 dysfunction (miR-34; ref. 27), cell proliferation and initiation (let-7; ref. 28), tumor survival (miR-214; ref. 29), and so on. Here, we demonstrated that miR-106 is overexpressed in ovarian cancer and leads to the down-regulation of tumor suppressor RBL2. Thus, many miRNAs are involved in the various aspects of ovarian carcinogenesis. miR-106 functions as an Onco-miR and has been found to be associated with carcinogenesis in many carcinomas (24, 30–33). miR-106a and family members were significantly overexpressed in ovarian carcinoma (Supplementary Table S1; refs. 34–36). In this study, we found that miR-106a was significantly overexpressed in both early and late stage of HGSOC (Fig. 1). Tumors with miR-106a over-expression exhibited a higher proliferation rate and larger tumor size than the control tumors (Figs. 2 and 5). SKOV3 cells with miR-106a overexpression showed more paclitaxel resistance than the control tumor cells (Supplementary Fig. S2). This is consistent with a recent study, in which miR-106a overexpression is significantly associated with paclitaxel resistance both in ovarian cancer cell lines and in primary human ovarian cancer (37). Moreover, upregulated miR-106a expression enriched the population of tumor-initiating cells, as reflected by the expanded side-population and CD133-positive cell population (Fig. 3 and Supplementary Fig. S3). In the xenografts of nude mice, we noted that tumors formed from SKOV3 cells with miR-106a overexpression were not only larger in size, but were also more poorly differentiated, and showed a significantly higher proliferation index than tumors from control SKOV3 cells (Fig. 5). All these findings prompted us to search for the specific target genes of miR-106a that are associated with ovarian cancer. As illustrated in Figs. 4 and 5, we found that RBL2 is specifically repressed by miR-106a, and that its dysregulation is closely related to the aggressiveness of ovarian cancer both in vitro and in vivo.
It appeared that miR-106a overexpression can confer two prominent oncogenic features to ovarian cancer cells, the enhancement of cell proliferation (Figs. 2, 3, and 5; Supplementary Fig. S1) and the promotion of tumor-initiating cell expansion and dedifferentiation (Figs. 4 and 5; Supplementary Fig. S5). The mitogenic function of miR-106a can be best explained by its repression of cell-cycle checkpoint protein p21 (Fig. 4D), as reported in previous studies (22), and of tumor suppressor RBL2 identified in this study (Fig. 4E). Therefore, miR-106a and its family members may serve as good targets in reducing tumor growth.
This is the first study to show that SKOV3 cells with miR-106 overexpression produces poorly differentiated or dedifferentiated carcinomas in xenografts of nude mice (Fig. 5). It is emerging that miR-106 and its family members regulate stem cell differentiation (11, 23). For example, the expression of miR-106b~25 cluster is high in self-renewing adult Neural Stem/Progenitor Cells and but is low when cells are stimulated to undergo differentiation (10). miR-106 clusters are one of a few miRNAs that are highly induced in cells that regained pluripotency (9). In transgenic mice, overexpression of the miR-106 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells by targeting rbl2 (38). All these findings suggest that miR-106 plays an important role in controlling cell proliferation and differentiation. However, the role of miR-106a in tumor cell differentiation has not been well characterized or studied. In this study, we demonstrated that miR-106 promotes cell proliferation and inhibits differentiation.
Regulation of miR-106 cluster in ovarian cancer remains unclear. Recent study by Thangavel and colleagues (39) in breast cancer revealed that activation of Rb pathway suppresses miR-106 cluster expression, whereas knockdown Rb expression or Rb-deficient cells can rescue or enhance miR-106 cluster expression. These findings provide another layer of evidence that miR-106a and its family members are critical in regulation of Rb function and loss of Rb pathway in most ovarian cancer may be responsible for miR-106a overexpression.
RBL2, a member of the retinoblastoma family of proteins, is significantly downregualted in ovarian cancer (24). RBL2 is capable of repressing E2F4 target genes as a part of the DREAM repressor complex (40). P130 (RBL2/RB2), a major E2F component and pocket protein, actively regulates the ES cell proliferation (41) and likely functions through regulation of DNA methyltransferase (Dnmt) expression (42). The RBL2/p130-E2F4 protein complex inhibits the transcription of multiple genes that are required for cell-cycle progression and induces cell-cycle arrest at G0–G1, which is required for induction of cell differentiation in cells of many tissues in vivo and various cell lineages in vitro (43). The functional role of RBL2 characterized so far may best explain the tumor phenotype identified in xenografts of SKOV3 tumor in this study. It is not surprising that well-differentiated SKVO3 tumors (mostly consisting of well-formed glandular formation) have high RBL2 expression and poorly differentiated tumors (solid and highly mitotic activity) have low RBL2 expression (Fig. 5). On the basis of these findings, we propose that miR-106–mediated downregulation of RBL2 may represent one of the major molecular events contributing to the aggressiveness of HGSOC.
Supplementary Material
Acknowledgments
The authors thank Mrs. Bella Shamaltsuyeva of NU pathologic core laboratory for her excellent work in immunohistochemistry.
Grant Support
This study was partially supported by awards from NIH NCI (R21CA167038), The National Basic Research Program of China (973 Program, 2011CB966201 and 2012CB944701), and The National Natural Science Foundation of China (grant no. 81171897). Shandong Province Foundation of outstanding Young Scientists (grant no. BS2010YY043).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Molecular Cancer Research Online (http://mcr.aacrjournals.org/).
Authors’ Contributions
Conception and design: Z. Liu, J. Liu, J.-J. Wei
Development of methodology: Z. Liu, X. Zhang, J.-J. Wei
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): Z. Liu, X. Zhang, J.-J. Wei
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Z. Liu, E. Gersbach, X. Zhang, P. Lee, C. Shao, J.-J. Wei
Writing, review, and/or revision of the manuscript: Z. Liu, E. Gersbach, P. Lee, J. Liu, C. Shao, J.-J. Wei
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): E. Gersbach, R. Dong, C. Shao, J.-J. Wei
Study supervision: B. Kong, J.-J. Wei
Conducting some experiments: X. Xu
References
- 1.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Volinia S, Bonome T, Calin GA, Greshock J, Yang N, et al. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:7004–9. doi: 10.1073/pnas.0801615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 5.Cheng W, Liu T, Wan X, Gao Y, Wang H. MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. FEBS J. 2012;279:2047–59. doi: 10.1111/j.1742-4658.2012.08589.x. [DOI] [PubMed] [Google Scholar]
- 6.Nam EJ, Lee M, Yim GW, Kim JH, Kim S, Kim SW, et al. MicroRNA profiling of a CD133(+) spheroid-forming subpopulation of the OVCAR3 human ovarian cancer cell line. BMC Med Genomics. 2012;5:18. doi: 10.1186/1755-8794-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu CX, Xu M, Tan L, Yang H, Permuth-Wey J, Kruk PA, et al. MicroRNA miR-214 regulates ovarian cancer cell stemness by targeting p53/ Nanog. J Biol Chem. 2012;287:34970–8. doi: 10.1074/jbc.M112.374611. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol. 2009;10:116–25. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Yang CS, Nakashima K, Rana TM. Small RNA-mediated regulation of iPS cell generation. EMBO J. 2011;30:823–34. doi: 10.1038/emboj.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peck B, Schulze A. A role for the cancer-associated miR-106b~25 cluster in neuronal stem cells. Aging. 2011;3:329–31. doi: 10.18632/aging.100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brett JO, Renault VM, Rafalski VA, Webb AE, Brunet A. The microRNA cluster miR-106b~25 regulates adult neural stem/ progenitor cell proliferation and neuronal differentiation. Aging. 2011;3:108–24. doi: 10.18632/aging.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catela Ivkovic T, Aralica G, Cacev T, Loncar B, Kapitanovic S. miR-106a overexpression and pRB downregulation in sporadic colorectal cancer. Exp Mol Pathol. 2012;94:148–54. doi: 10.1016/j.yexmp.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Wei JJ, Wu J, Luan C, Yeldandi A, Lee P, Keh P, et al. HMGA2: a potential biomarker complement to P53 for detection of early-stage high-grade papillary serous carcinoma in fallopian tubes. Am J Surg Pathol. 2010;34:18–26. doi: 10.1097/PAS.0b013e3181be5d72. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Yang G, Thompson-Lanza JA, Glassman A, Hayes K, Patterson A, et al. A genetically defined model for human ovarian cancer. Cancer Res. 2004;64:1655–63. doi: 10.1158/0008-5472.can-03-3380. [DOI] [PubMed] [Google Scholar]
- 15.Shan W, Mercado-Uribe I, Zhang J, Rosen D, Zhang S, Wei J, et al. Mucinous adenocarcinoma developed from human fallopian tube epithelial cells through defined genetic modifications. Cell Cycle. 2012;11:2107–13. doi: 10.4161/cc.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahajan A, Liu Z, Gellert L, Zou X, Yang G, Lee P, et al. HMGA2: a biomarker significantly overexpressed in high-grade ovarian serous carcinoma. Mod Pathol. 2010;23:673–81. doi: 10.1038/modpathol.2010.49. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Liu J, Segura MF, Shao C, Lee P, Gong Y, et al. MiR-182 overexpression in tumourigenesis of high-grade serous ovarian carcinoma. J Pathol. 2012;228:204–15. doi: 10.1002/path.4000. [DOI] [PubMed] [Google Scholar]
- 18.Wei JJ, Wu X, Peng Y, Shi G, Olca B, Yang X, et al. Regulation of HMGA1 expression by microRNA-296 affects prostate cancer growth and invasion. Clin Cancer Res. 2011;17:1297–305. doi: 10.1158/1078-0432.CCR-10-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creighton CJ, Hernandez-Herrera A, Jacobsen A, Levine DA, Mankoo P, Schultz N, et al. Integrated analyses of microRNAs demonstrate their widespread influence on gene expression in high-grade serous ovarian carcinoma. PLoS OnE. 2012;7:e34546. doi: 10.1371/journal.pone.0034546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Guo X, Chang DY, Rosen DG, Mercado-Uribe I, Liu J. CD133 expression associated with poor prognosis in ovarian cancer. Mod Pathol. 2012;25:456–64. doi: 10.1038/modpathol.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li B, Shi XB, Nori D, Chao CK, Chen AM, Valicenti R, et al. Down-regulation of microRNA 106b is involved in p21-mediated cell cycle arrest in response to radiation in prostate cancer cells. Prostate. 2011;71:567–74. doi: 10.1002/pros.21272. [DOI] [PubMed] [Google Scholar]
- 22.Gibcus JH, Kroesen BJ, Koster R, Halsema N, de Jong D, de Jong S, et al. MiR-17/106b seed family regulates p21 in Hodgkin’s lymphoma. J Pathol. 2011;225:609–17. doi: 10.1002/path.2958. [DOI] [PubMed] [Google Scholar]
- 23.Trompeter HI, Abbad H, Iwaniuk KM, Hafner M, Renwick N, Tuschl T, et al. MicroRNAs MiR-17, MiR-20a, and MiR-106b act in concert to modulate E2F activity on cell cycle arrest during neuronal lineage differentiation of USSC. PLoS ONE. 2011;6:e16138. doi: 10.1371/journal.pone.0016138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Andrilli G, Masciullo V, Bagella L, Tonini T, Minimo C, Zannoni GF, et al. Frequent loss of pRb2/p130 in human ovarian carcinoma. Clin Cancer Res. 2004;10:3098–103. doi: 10.1158/1078-0432.ccr-03-0524. [DOI] [PubMed] [Google Scholar]
- 25.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 26.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corney DC, Hwang CI, Matoso A, Vogt M, Flesken-Nikitin A, Godwin AK, et al. Frequent downregulation of miR-34 family in human ovarian cancers. Clin Cancer Res. 2010;16:1119–28. doi: 10.1158/1078-0432.CCR-09-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shell S, Park SM, Radjabi AR, Schickel R, Kistner EO, Jewell DA, et al. Let-7 expression defines two differentiation stages of cancer. Proc Natl Acad Sci U S A. 2007;104:11400–5. doi: 10.1073/pnas.0704372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang H, Kong W, He L, Zhao JJ, O’Donnell JD, Wang J, et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–33. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 30.Bellan C, De Falco G, Tosi GM, Lazzi S, Ferrari F, Morbini G, et al. Missing expression of pRb2/p130 in human retinoblastomas is associated with reduced apoptosis and lesser differentiation. Invest Ophthalmol Vis Sci. 2002;43:3602–8. [PubMed] [Google Scholar]
- 31.Caputi M, Groeger AM, Esposito V, De Luca A, Masciullo V, Mancini A, et al. Loss of pRb2/p130 expression is associated with unfavorable clinical outcome in lung cancer. Clin Cancer Res. 2002;8:3850–6. [PubMed] [Google Scholar]
- 32.Leoncini L, Bellan C, Cossu A, Claudio PP, Lazzi S, Cinti C, et al. Retinoblastoma-related p107 and pRb2/p130 proteins in malignant lymphomas: distinct mechanisms of cell growth control. Clin Cancer Res. 1999;5:4065–72. [PubMed] [Google Scholar]
- 33.Milde-Langosch K, Goemann C, Methner C, Rieck G, Bamberger AM, Loning T. Expression of Rb2/p130 in breast and endometrial cancer: correlations with hormone receptor status. Br J Cancer. 2001;85:546–51. doi: 10.1054/bjoc.2001.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14:2690–5. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- 35.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 36.Wyman SK, Parkin RK, Mitchell PS, Fritz BR, O’Briant K, Godwin AK, et al. Repertoire of microRNAs in epithelial ovarian cancer as determined by next generation sequencing of small RNA cDNA libraries. PLoS ONE. 2009;4:e5311. doi: 10.1371/journal.pone.0005311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huh JH, Kim TH, Kim K, Song JA, Jung YJ, Jeong JY, et al. Dysregulation of miR-106a and miR-591 confers paclitaxel resistance to ovarian cancer. Br J Cancer. 2013;109:452–61. doi: 10.1038/bjc.2013.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarro A, Marrades RM, Vinolas N, Quera A, Agusti C, Huerta A, et al. MicroRNAs expressed during lung cancer development are expressed in human pseudoglandular lung embryogenesis. Oncology. 2009;76:162–9. doi: 10.1159/000201569. [DOI] [PubMed] [Google Scholar]
- 39.Thangavel C, Boopathi E, Ertel A, Lim M, Addya S, Fortina P, et al. Regulation of miR106b cluster through the RB pathway: mechanism and functional targets. Cell Cycle. 2013;12:98–111. doi: 10.4161/cc.23029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litovchick L, Sadasivam S, Florens L, Zhu X, Swanson SK, Velmurugan S, et al. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell. 2007;26:539–51. doi: 10.1016/j.molcel.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Becker KA, Stein JL, Lian JB, van Wijnen AJ, Stein GS. Establishment of histone gene regulation and cell cycle checkpoint control in human embryonic stem cells. J Cell Physiol. 2007;210:517–26. doi: 10.1002/jcp.20903. [DOI] [PubMed] [Google Scholar]
- 42.Sinkkonen L, Hugenschmidt T, Berninger P, Gaidatzis D, Mohn F, Artus-Revel CG, et al. MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nat Struct Mol Biol. 2008;15:259–67. doi: 10.1038/nsmb.1391. [DOI] [PubMed] [Google Scholar]
- 43.Petrov NS, Zhidkova OV, Zenin VV, Rozanov Iu M, Popov BV. The cell cycle regulator p130 and beta-catenin form a complex in mesenchymal stem cells. Tsitologiia. 2011;53:107–15. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.