Abstract
Connexin 37 (Cx37) suppresses cell proliferation when expressed in rat insulinoma (Rin) cells, an effect also manifest in vivo during vascular development and in response to tissue injury. Mutant forms of Cx37 with nonfunctional channels but normally localized, wild-type carboxy termini are not growth suppressive. Here we determined whether the carboxy-terminal (CT) domain is required for Cx37-mediated growth suppression and whether the Cx37 pore-forming domain can be replaced with the Cx43 pore-forming domain and still retain growth-suppressive properties. We show that despite forming functional gap junction channels and hemichannels, Cx37 with residues subsequent to 273 replaced with a V5-epitope tag (Cx37–273tr*V5) had no effect on the proliferation of Rin cells, did not facilitate G1-cell cycle arrest with serum deprivation, and did not prolong cell cycle time comparably to the wild-type protein. The chimera Cx43*CT37, comprising the pore-forming domain of Cx43 and CT of Cx37, also did not suppress proliferation, despite forming functional gap junctions with a permselective profile similar to wild-type Cx37. Differences in channel behavior of both Cx37–273tr*V5 and Cx43*CT37 relative to their wild-type counterparts and failure of the Cx37-CT to interact as the Cx43-CT does with the Cx43 cytoplasmic loop suggest that the Cx37-CT and pore-forming domains are both essential to growth suppression by Cx37.
Keywords: connexin, gap junction channel, growth suppression, protein interactome, endothelium
gap junction channels, by providing a pathway for the intercellular diffusion of metabolites and signaling molecules, coordinate tissue functions such as contraction, secretion, and growth. These dodecameric channels are composed of 1 or more of the 21 members of the mammalian connexin (Cx) gene family (48), each member of which forms intercellular channels able to support, at a minimum, electrotonic signaling. In addition to this shared function, connexin-specific deletion and replacement studies (26, 41, 45) suggest connexin-specific roles in tissue function and development (1, 14, 15, 24, 52). Since most cell types express multiple members of this gene family, clearly not all functions of the gene family are readily accomplished by expression of a single Cx. However, unknown are the critical properties of the individual connexins that make them indispensible for normal cell- and tissue-specific functions.
In addition to forming intercellular gap junction channels, connexins support cell function through formation and regulated function of transmembrane channels, known as connexins or hemichannels, and through protein-protein interactions between, principally, the carboxy-terminal (CT) domain of the protein with itself and other regions of the connexin protein as well as with regulatory kinases, phosphatases, cell cycle regulators, scaffolding components, and other proteins (51). All three modes of connexin function, through gap junction channels, hemichannels, and protein-protein interactions, have been implicated in connexin-dependent growth regulation.
Relative to the 21 members of the mammalian connexin gene family, the mechanisms underlying growth suppression by Cx43 have been most thoroughly studied. Expression of this connexin has no detectable effect on the proliferation of rat insulinoma (Rin) cells (6) or, in some cases, HeLa cells (35), but suppresses the proliferation of other tumor and nontumorigenic cell lines, including the breast cancer cell line MDA-MB-231 (8), C6 glioma cells (17, 19), primary astrocytes (17), and keratinocytes (31). In some of these cell types, function of the Cx43 channel appears to be necessary for growth suppression (19), whereas in other cell types channel function is not necessary (8, 17, 31, 55). In these latter cell types, expression of the CT domain by itself is often sufficient for the growth-suppressive effect conferred by the full-length Cx43.
Given that Cx43 is not universally growth suppressive and that the mechanism of growth suppression differs in a tissue specific manner, it stands to reason that for a single cell type, connexin-mediated growth suppression can be connexin specific (6, 19, 35). Many cell types in the body do not express Cx43 in their differentiated state but “revert” to Cx43 when stressed into proliferation. One prominent example of this is adult arterial endothelium where normally Cx37 and Cx40 are expressed, but when diseased or injured Cx43 expression is often upregulated and Cx37 expression downregulated (2, 28, 46, 53, 54). That Cx37, Cx40, and Cx43 have different functions in the arterial endothelium, with Cx37 serving a growth-suppressive role, is supported by recently published data (14) showing augmented angiogenesis and arteriogenesis following ischemic injury in Cx37-deficient compared with wild-type or Cx40-deficient animals (15, 16).
The goal of the current study was to determine whether the CT region of Cx37 is necessary for Cx37 to mediate growth suppression in Rin cells and whether the growth-suppressive function of this domain is retained when associated with a functional pore domain from another connexin. We showed previously that unlike Cx40 and Cx43, Cx37 profoundly slows cell cycle progression and proliferation of Rin cells (6), an effect that requires a functional channel and for which localization of the CT to gap junction plaques is not sufficient (20, 21). Here, using an inducible expression system, we show that despite forming functional gap junction channels and hemichannels, Cx37 with residues subsequent to 273 replaced with a V5 tag (Cx37–273tr*V5) had no effect on the proliferation of Rin cells, did not facilitate G1 arrest with serum deprivation, and did not prolong cell cycle time comparably to the wild-type protein. In addition, we show that the chimera Cx43*CT37 also did not mediate growth suppression, despite forming junctions with permselective properties similar to wild-type Cx37. Finally, by assessing the ability of the Cx37-CT to modify the function of the pore of Cx43 or to interact with the cytoplasmic loop (CL) domain of Cx43 in a manner comparable to the Cx43CT, we show that properties specific to the Cx37 pore-forming domain along with the Cx37-CT are necessary for Cx37-mediated growth suppression.
MATERIALS AND METHODS
Cell culture.
Rin1046–38 cells were maintained in RPMI medium (Sigma Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS; Gemini Bio Products, West Sacramento, CA) and passed (0.25% trypsin; diluted 1:5) weekly. iRin cells, which stably express the pTET-ON transcription factor (6), were maintained in RPMI with 10% Fetal Plex serum (Gemini Bio Products) and G418 (300 μg/ml; Life Technologies, Grand Island, NY). iRin cells with inducible expression of wild-type Cx37 (iRin37), Cx37- 273tr*V5 (iRin37tr), or Cx43*CT37 (iRin43*CT37) were maintained in RPMI with 10% Fetal Plex, G418 (300 μg/ml), and hygromycin (100 μg/ml; Life Technologies). Induction of protein expression was stimulated by addition of 2 μg/ml doxycycline for all experiments unless otherwise indicated.
Mutant plasmid construction, transfection, and clone isolation.
Cx37–273tr*V5 was amplified from mCx37, previously inserted into the BamI and NotI sites of the pTRE2-hygro plasmid (6), using a 5′-forward primer corresponding to pTRE2 vector sequence (forward primer 5′-CGCCTGGAGACGCCATCC-3′) and 3′-reverse primer whose sequence included residues complementary to Cx37, V5, a stop codon and an NheI restriction site (5′-CTAGCTAGCCTACGTAGAATCGAGACCGAGGAGAGGGTTAGGGATAGGCTTACCGGGTCCCTCGCCCAT-3′). After amplifying the correct sequence, it was digested with BamHI/NheI and ligated into the pTRE2hygro vector (Clontech, Mountain View, CA) to generate pTRE2h-mCx37-273tr*V5. The sequence was confirmed at the University of Arizona UAGC Sequencing Facility. iRin cells were stably transfected with pTRE2h-mCx37-273tr*V5 using lipofectamine (Life Technologies) according to the manufacturer's instructions. Cells resistant to 100 μg/ml hygromycin were isolated (iRin37tr) and dilution cloned, and two clones (2E5 and 1B3) were randomly selected for further study.
The chimera Cx43*CT37, centered around valine 236 (common to both proteins), was constructed from two PCR fragments. The first fragment was made by amplifying the sequence encoding amino acid residues 236–333 of Cx37 using a 3′-reverse primer [Primer I] from the pTRE2 vector sequence (5′-CATTCTAAACAACACCCTG-3′) and a 5′-forward primer [Primer II] with sequence identical to mCx43 and mCx37 (5′-TTCTTCAAGGGCGTCAGCCGGGAGATAAAG-3′). The second fragment was made by amplifying the sequence encoding amino acid residues 1–235 of mCx43 also from pTRE2 vector, using the 5′-forward primer [Primer III] from the pTRE2 vector sequence (forward primer 5′-CGCCTGGAGACGCCATCC-3′) and a 3′-reverse primer [Primer IV] whose sequence is complementary to Primer II including mCx37 and Cx43 (5′-CTCCCGGCTGACGCCCTTGAAGAAGACATA-3′). These two PCR products were purified and subsequently used in a PCR reaction with Primer I and Primer III to yield the full chimera sequence. The amplified product was digested with BamHI/NotI and ligated into the pTRE2hygro vector to generate pTRE2h-Cx43*CT37. Sequence was confirmed at the University of Arizona Genetics Core Sequencing Facility. iRin cells were stably transfected with pTRE2h-Cx43*CT37 using lipofectamine (Life Technologies) according to the manufacturer's instructions. Cells resistant to 100 μg/ml hygromycin were isolated (iRin43*CT37) and dilution cloned, and two clones (3C2 and 3A3) were selected for further study (the 3A3 clone was lost before characterization was completed).
Immunoblotting.
Total protein and triton insoluble protein were isolated as previously described (6). Westerns were run on 12% precast SDS-PAGE gels (Bio-Rad, Hercules, CA) and transferred onto nitrocellulose (Bio-Rad). Cx37 antibody (αCx37–18264) was a gift of Dr. Alex Simon, used at 1:5,000 (44). V5 antibody (Life Technologies) was used at 1:5,000 dilution. Positope (Life Technologies) was loaded at 5 μl/lane as a positive control for the V5 antibody. Molecular weight marker (5 μl/lane) is MagicMarkXP (Life Technologies). Primary antibody on the blots was revealed using anti-rabbit HRP secondary antibody (for detecting Cx37; GE Lifescience) and anti-mouse HRP secondary antibody (for detecting V5 antibody; Promega).
Relative expression levels were determined by comparing expression levels in total protein to a standard curve generated by loading in separate gel lanes 0.25, 0.5, 1, and 2 pmol of GST*Cx37 fusion protein (44) or 25, 50, 100, 200, and 500 fmol of Positope. A known amount of cell lysate total protein (20 or 50 μg) was loaded for each sample. After immunoblotting, signal intensity in each lane was determined from an identically sized region of interest whose size was defined by the largest band. Sample intensity (after subtracting background) was plotted as a function of sample content (μmol or fmol loaded), and the data were fit by linear regression; the intensity of bands from cell samples was compared with the standard curve to evaluate moles of Cx37 expressed per microgram of total cell protein.
Immunofluorescence.
Cells were plated onto glass coverslips and induced for 24 h with 2 μg/ml of doxycycline. Cells were washed, fixed, and stained with ToPro3 (Invitrogen) to reveal nuclei and either V5 antibody (1:200) or Cx37 antibody (1:200), as appropriate. Cy3 anti-mouse or anti-rabbit (Jackson Immunoresearch) secondary antibody (1:200) was used to localize primary antibodies and confocal images acquired as previously described (21).
Hemichannel assay.
Cells were plated at low density (4 × 104 cells/well of a 6-well plate) in a small volume of media and left for ∼1 h to adhere to the plate. Additional medium, with or without doxycycline, was then added to each well and the cells incubated for 24–48 h before use. After this induction time (24 or 48 h for iRin37, 48 h for iRin37tr), cells were rinsed twice with media containing 10% serum (FetalPlex), twice with “external” solution (see below), and three times (30 s each) with external solution containing 5 mM EGTA {producing a low free Ca2+ concentration ([Ca2+])}. Three reservoirs were created (20) in each well, and 800 μl of dye solution (containing 0.64 mg/ml NBD-m-TMA and 0.125 mg/ml rhodamine dextran in external solution containing no Ca2+ nor EGTA) were carefully added to each reservoir so as not to disturb the cells within. Plates were placed on ice and covered with aluminum foil to prevent loss of fluorescence signal. After 15 min the dye solution was carefully removed, the reservoir boundary removed, and the cells gently rinsed three times with media containing 10% serum. Finally, the cells were rinsed twice with external solution (normal [Ca2+]) and visualized. The total number of live cells (those excluding rhodamine dextran) and NBD-m-TMA positive/rhodamine dextran negative cells were counted in three fields of view in each reservoir. The number of NBD-m-TMA-positive and rhodamine dextran-negative cells (considered to have active hemichannels) was expressed as a percentage of the total number of live cells in the field.
Electrophysiology.
Cells were plated at low density on coverslips and induced with doxycycline 24 h before use. Coverslips were mounted in a custom-made chamber, and the cells were bathed in “external” solution (containing in mmol/l: 142.5 NaCl, 4 KCl, 1 MgCl2, 5 glucose, 2 sodium pyruvate, 10 HEPES, 15 CsCl, 10 TEA-Cl, 1 BaCl2, and 1 CaCl2 pH 7.2, and osmolarity of 330 mosmol/l). Pairs of cells were identified (using phase contrast optics; Olympus IMT2 inverted microscope, Olympus, Center Valley, PA), and junctional conductance and single channel behavior were evaluated using dual whole cell voltage-clamp techniques with Axopatch1D amplifiers and pClamp software (Molecular Devices, Sunnyvale, CA). Electrodes (5–10 MΩ) were fabricated from 1.2 mm filament glass (AM systems, Everett, WA) using a Sutter Instruments (Novato, CA) puller. Electrodes were filled with a solution containing (in mmol/l): 124 KCl, 14 CsCl, 9 HEPES, 9 EGTA, 0.5 CaCl2, 5 glucose, 9 TEA-Cl, 3 MgCl2, and 5 Na2ATP, pH 7.2 and osmolarity of 326 mosmol/l. Junctional conductance was determined from the current required to maintain a coupled cell at 0 mV while pulsing the opposite cell to +10 mV. Single channel conductances were evaluated in the nonpulsed cell during application of a transjunctional voltage difference (Vj) of ± 25 mV (polarity relative to nonpulsed cell), a Vj value close to the reported V0 (± 28 mV; Ref. 40) for Cx37 where the activity of only 50% of channels has been altered by the applied Vj. When necessary to visualize the activity of individual channels, junctional conductance was reduced using halothane (7). Data were acquired at 2 kHz through a 4-pole Bessel Filter set at 1 kHz. Recordings were further filtered at 50 Hz during analysis using pClamp software. For construction of amplitude histograms, transitions between regions of the current trace stable for 50 ms or longer were measured in pClamp (Molecular Devices). Transition amplitudes for each cell pair were binned in 10-pS bins, and the relative frequency of each bin was determined; mean bin frequency across multiple cell pairs is plotted. For construction of all points histograms, the conductance of every point in the filtered record was calculated and placed into the appropriate 10-pS bin between 0 and 440 pS; the absolute number of points in each bin was then plotted as a function of the bin conductance.
Proliferation assay.
Cells were plated initially at 3,125 cells/cm2 in six-well plates. Half of the wells in each plate received doxycycline (2 μg/ml) on day 0, and the other three wells did not. Medium was replaced, with or without doxycycline as appropriate, every 2 days. Cells were counted on a Nexcelom Bioscience cell counter (Lawrence, MA) or by hemocytometer every 3 days for 15 days. The number of cells in the three wells for each time point was averaged and percent as a function of the number plated on day zero calculated and plotted. The natural log of these values was also calculated and plotted on semi-log paper to assess rate of growth (slope of the regression fit).
Serum deprivation and cell cycle analysis.
As previously described (6), cells were plated at 106 cells/100-mm plate and plates were divided into two groups, one of which was switched to medium without serum 24 h after plating, the other of which continued in 10% serum. For both groups, 72 h after plating, cells were treated with 2 μg/ml doxycycline to induce Cx37 expression. Cells were either harvested for analysis of cell cycle position by fluorescence-activated cell sorting (FACS) 24 h after introduction of doxycycline (0 time point on graphs), or the medium in all plates was switched to medium containing both doxycycline and 10% serum. Cells from these latter plates were harvested for FACS based cell cycle analysis 4, 10, 24, 48, 72, and 96 h after reintroduction of serum. Cells were harvested and processed for FACS analysis of cell cycle position as previously described (6).
Circular dichroism spectroscopy.
Circular dichroism (CD) experiments were performed using a Jasco J-815 spectrophotometer (Easton, MD) fitted with a Peltier temperature control system. The CD spectra for the Cx37-CT residues 270–333 was recorded in 1× PBS (pH 7.4 and 5.8). For each sample, five scans (wavelength range: 300–190 nm; response time: 1 s; scan rate: 50 nm/min; bandwidth 1.0 nm) were collected using a 0.01-cm quartz cell and processed using Spectra Analysis (Jasco). Each spectrum is shown as the mean residue ellipticity (deg·cm2·dmol−1) as a function of wavelength and average of five scans. All spectra were corrected by subtracting the solvent spectrum. Protein concentrations were determined using a NanoDrop 1000 (Thermo Scientific, Wilmington, DE).
Nuclear magnetic resonance.
All nuclear magnetic resonance (NMR) data were acquired at 7°C using a 600-MHz Varian INOVA NMR Spectrometer (International Equipment Trading, Vernon Hills, IL) outfitted with a cryo-probe at the NMR Facility of the University of Nebraska Medical Center. NMR spectra were processed and phased using NMRPipe and NMRDraw and analyzed using NMRView (http://spin.niddk.nih.gov/NMRPipe). Gradient-enhanced two-dimensional 15N-HSQC experiments were acquired with 1,024 complex points in the direct dimension and 256 complex points in the indirect dimension. Sweep widths were 10,000 Hz in the 1H dimension and 2,430.6 Hz in the 15N dimension.
Statistics.
Unless specified otherwise, all comparisons were made using Student's t-test, two-tailed, unequal variance, with α set at 0.05.
RESULTS
We showed previously that a functional channel is necessary for Cx37-mediated growth suppression and, further, that proper localization of the wild-type CT when associated with a nonfunctional gap junction pore is insufficient for Cx37-mediated growth suppression (20, 21). To determine whether, in addition to channel function, the CT of Cx37 is necessary for Cx37-mediated growth suppression, we truncated Cx37 at position P273, replacing residues 274–333 with a V5 tag (Fig. 1A). The CT sequence thus removed from Cx37 contains the serine and tyrosine residues with the highest probability of phosphorylation as predicted by multiple programs (www.cbs.dtu.dk/services/NetPhos/ and http://gps.biocuckoo.org/). We also tested the sufficiency in mediating growth suppression of the CT of Cx37 when associated with a functional pore domain other than Cx37 by expressing a chimera of the pore-forming domain of Cx43 (residues M1-V236) and CT domain of Cx37 (residues V236-V333), creating Cx43*CT37 (Fig. 1A).
Fig. 1.
Cx37–273tr*V5 and Cx43*CT37 are inducibly expressed by iRin cells. A: schematic of Cx37, Cx37–273tr*V5 and Cx43*CT37 sequences. Sequence shown in black in Cx37 (left) was replaced with sequence encoding V5 to make Cx37–273tr*V5 (middle: black residues from V5 sequence). Cx43*CT37 (right) included the first 236 amino acids of Cx43 (black) and the last 97 amino acids of Cx37 (gray). B: 2 clonal populations of iRin37tr cells, 1B3 and 2E5, express a V5 positive protein of the correct predicted mass at apparently low (1B3) or high levels (2E5). C: multiple clones of iRin43*CT37 express Cx43*CT37 at apparently different levels, with apparently high expression in the 3C2 clone. D: iRin37tr-2E5 clone does not express Cx37–273tr*V5 in the absence of doxycycline induction (Dox− lane), but near maximal expression was induced by 2 μg/ml doxycycline at both 24 and 48 h. E: 3C2 clone of iRin43*CT37 cells was also maximally induced by 2 μg/ml doxycycline, but this clone had greater expression at 48 than 24 h. D and E: both proteins were detected in the triton insoluble fraction (TX lane). Blots were probed with V5 or Cx37 antibody; 30 or 50 μg of total cell protein (for iRin37tr and iRin43*CT37, respectively) were loaded in all lanes except the TX lane where all the triton insoluble protein from an equivalent aliquot of total cell protein was loaded; Mr indicates lane loaded with molecular mass markers; arrowheads indicate the protein of interest and “ns” marks a nonspecific band sometimes detected in total protein from Rin cells.
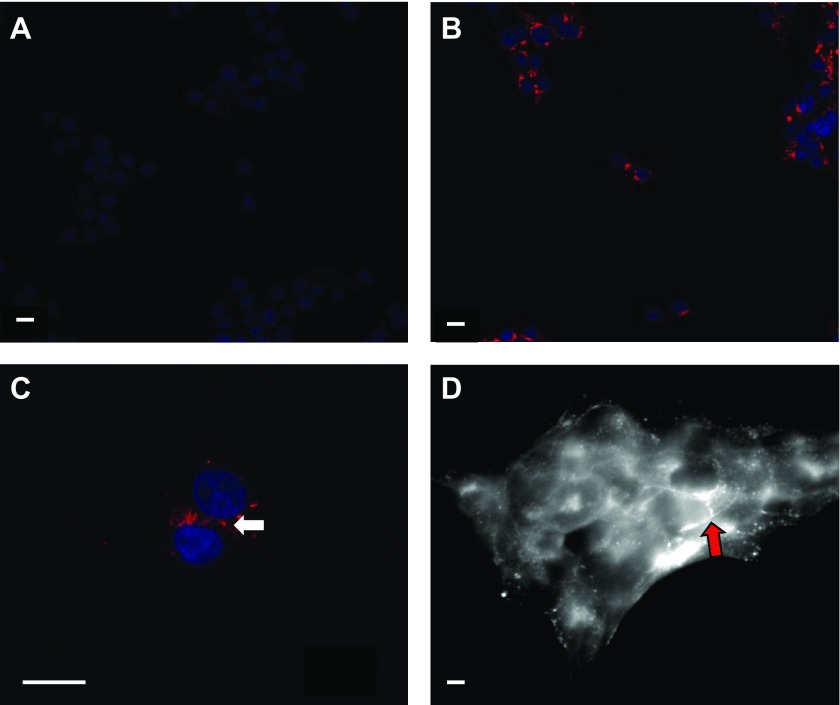
iRin37tr clones expressed a V5-positive protein of appropriate mass (∼30 kDa; Fig. 1B) and iRin43*CT37 clones expressed Cx37-positive protein of appropriate mass (∼37 kDa; Fig. 1C). Nondoxycycline-induced iRin37–273tr*V5–2E5 cells did not detectably express Cx37–273tr*V5, but these cells appeared to express near-maximally following 24 or more hours in the presence of 2 μg/ml doxycycline (Fig. 1D). The 3C2 clone of iRin43*CT37 was similarly induced to near-maximal expression by 2 μg/ml doxycycline at 24 and 48 h (Fig. 1E). Both Cx37–273tr*V5 and Cx43*CT37 were also readily detected in the triton-insoluble fraction (Fig. 1, D and E), consistent with their possible localization to gap junction plaques. Both proteins were observed at cell-cell appositions as well as in the perinuclear region (Fig. 2), as previously shown for the Cx37, Cx37-T154A, and Cx37-C61,65A proteins (6, 20, 21).
Fig. 2.
Cx37–273tr*V5 and Cx43*CT37 expression by Rin cells. Cx37–273tr*V5 (red) is not detected in iRin37tr cells in the absence of doxycycline induction (A) and is detected perinuclearly (B) as well as at points of cell-cell contact (C) following doxycycline induction. Blue marks the nuclei. D: Cx43*CT37 is expressed perinuclearly as well as in appositional membranes in iRin43*CT37 cells. Scale bar = 10 μm in all frames.
To verify that the iRin37tr and iRin43*CT37 cell lines expressed their respective proteins at levels previously shown to be sufficient for Cx37 to suppress growth (6, 20, 21), we next quantified Cx37–273tr*V5 expression in the 2E5 and 1B3 clones and Cx43*CT37 expression in the 3C2 clone. As shown and summarized in Fig. 3, all three clones expressed at levels comparable to Cx37 expression by iRin37 cells (2.2 and 8.4 fmol/μg total cell protein for the 1B3 and 2E3 clones of iRin37tr, respectively, and 7 fmol/μg total cell protein for the iRin43*CT37 cells).
Fig. 3.
Cx37–273tr*V5 and Cx43*CT37 expression levels are comparable. A: Western blot of Positope and total protein isolated from the 1B3 and 2E5 clones of iRin37tr cells. Different amounts of Positope were loaded in each lane as indicated (in fmol), and 20 μg of total protein were loaded for each clonal cell line. Mass of Mr markers is indicated at left. Positope runs at 53 kDa whereas Cx37–273tr*V5 runs at ∼30 kDa. B: optical density of bands (from A) plotted as a function of the amount of loaded Positope for 25–200 fmol, the range encompassing the band densities for 20 μg of total protein isolated from the iRin37tr clones. Standard curve was fit by linear regression, R2 = 0.994, intercept −2.08 and slope 1.799 AU/fmol. C: Western blot of Cx37-GST fusion protein and total protein isolated from the 3C2 clone of iRin43*CT37 cells. Different quantities of the fusion protein were loaded in each lane as indicated (in pmol); 50 μg of total protein were loaded for the 3C2 clone. Mass of Mr markers is indicated on the left. Full-length Cx37-GST fusion protein runs at ∼37 kDa (an internal cut-site within GST-portion of the protein resulted in the lower mass bands). D: optical density of Cx37-GST (all bands in a lane) and molar quantity of the loaded fusion protein were linearly related (R2 = 0.99; intercept −4; slope 700 AU/pmol). The 3C2 clone expressed within the linear range of the standard curve.
We next determined whether Cx37–273tr*V5-expressing Rin cells were growth suppressed. Proliferation over 15 days of iRin37 and in each of the iRin37tr clones was evaluated in three experiments, each done in triplicate. Shown in Fig. 4A are the combined results of the six experiments performed on the iRin37tr clones and three iRin37 experiments conducted in parallel. Doxycycline-induced iRin37 cells failed to obviously increase in number over the 15-day period (significantly different from noninduced iRin37 cells; P < 0.01). In contrast, doxycycline-induced iRin37tr cells, noninduced iRin37tr cells, and noninduced iRin37 cells steadily increased in number over the 15-day analysis period. Proliferation rates of the induced and noninduced iRin37tr clones were not different (Fig. 4, A, inset). Doubling times for induced and noninduced iRin37tr cells (either clone) were calculated as previously described (6) and found not to differ, averaging 1.9 days. This value is significantly shorter than the 9 days previously reported for Cx37-expressing cells (6). These results demonstrate that Cx37–273tr*V5 did not prolong cell cycle time when expressed in iRin cells.
Fig. 4.
Cx37–273tr*V5 does not suppress proliferation of iRin cells nor confer on these cells sensitivity to growth factors. A: with doxycycline induction, proliferation of iRin37 cells was slowed significantly (closed vs. open circles: *P < 0.01, 3 experiments each in triplicate). In contrast, proliferation of iRin37tr cells (data from 2E5 and 1B3 clones combined; each with 3 experiments done in triplicate) was unaffected by doxycycline induction (n.s.: closed vs. open squares). Proliferation rates (slopes of lines in inset) for the 2 iRin37tr clones induced or not with 2 μg/ml doxycycline (dox) were not different: 1B3, Dox− 0.39 ± 0.05; 1B3, Dox+ 0.37 ± 0.042; 2E5, Dox− 0.33 ± 0.15; 2E5, and Dox+ 0.34 ± 0.05. Points correspond to the actual data, lines are from the best fit of each data set. B: serum deprivation for 72 h, with doxycycline added for the terminal 24 h, resulted in significant (P < 0.001, n = 10 for serum deprived and nonserum deprived) accumulation iRin37 cells in G1. In contrast, similar treatment of iRin37tr cells resulted in a significant (P < 0.001; n = 11 for serum deprived and n = 6 for nonserum deprived) decrease in G1 cells. This decrease in G1 cells was also observed in similarly treated iRin parental cells (points before x-axis break in C) suggesting this reduction is not specific to expression of Cx37–273tr*V5. C: restoration of 10% serum to iRin37 cells (at time 0 in C) released these cells from G1 arrest (n = 10) and had no distinct effect on iRin37tr or iRin cells (n = 3 for each cell line). †Significant difference between serum exposed and deprived Cx37wt cells or Cx37tr cells; *Significant difference between cells expressing Cx37wt vs. Cx37tr.
In addition to exerting a cell cycle-prolonging effect in iRin cells, Cx37 expression confers on these cells sensitivity to serum deprivation (6). To determine whether the CT domain is necessary for this effect of Cx37 expression, cell cycle position was analyzed for iRin37tr cells that had been exposed for 72 h to medium containing 0 or 10% serum, with doxycycline present for the last 24 of the 72 h. Whereas serum deprivation resulted in an accumulation of Cx37-expressing iRin37 cells in G1 (6), comparable treatment of iRin37tr cells resulted in a significant decrease in the percentage of cells in G1 (Fig. 4B). In addition, whereas the percentage of cells in the S and G2 phases decreased with serum deprivation in Cx37-expressing iRin37 cells (6), the percentage of iRin37tr cells in these phases of the cell cycle was, if anything, increased with serum deprivation (Cx37tr S phase: 10% serum 16.79 ± 1.33, 0% serum 18.56 ± 1.48, n.s.; and G2 phase: 10% serum 7.43 ± 1.1, 0% serum 10.46 ± 1.08, n.s). Comparison of the recovery from serum deprivation in these and the parental cell line (iRin) revealed that iRin37tr cells did not differ from the iRin cells that do not detectably express connexins (Fig. 4C). These data, along with the proliferation data, indicate that the CT domain is necessary for Cx37-mediated growth suppression, cell cycle arrest, and sensitivity to serum (growth factor) deprivation.
Since our previous studies demonstrated that a functional channel is necessary for Cx37 to mediate growth suppression (20, 21), we next determined whether iRin37tr cells (2E5 clone) formed functional gap junctions and hemichannels. Figure 5A shows that pairs of iRin37tr cells were electrically coupled at levels comparable to iRin37 cell pairs. Further, both Cx37 and Cx37–273tr*V5 formed functional hemichannels (Fig. 5B). These results indicate that unlike Cx37, Cx37tr did not exert a growth-suppressive effect on Rin cells despite forming functional channels.
Fig. 5.
Cx37–273tr*V5 forms functional gap junction channels and hemichannels. A: mean junctional conductance [Gj; measured by dual whole cell voltage clamp with transjunctional voltage difference (Vj) = 10 mV] of 30 iRin37 and 8 iRin37tr-2E5 cells was similar (P > 0.35, 3.4 ± 0.7 and 2.5 ± 0.8 nS, respectively). B: hemichannel-mediated dye uptake in iRin37 and iRin37tr-2E5 cells doxycyline induced or not for 24 or 48 h. Total cell no. (n), fields visualized (f), no. of experiments (N) for each data set were as follows: iRin37 (24 h, Dox: n = 1,069, f = 33, N = 11; 24 h, Dox+: n = 574, f = 26, N = 10; 48 h, Dox−: n = 521, f = 10, N = 3; 48 h, Dox+: n = 286, f = 9, N = 3); iRin37tr (48 h, Dox−: n = 409, f = 17, N = 6; and 48 h, Dox−: n = 295, f = 18, N = 6). *Significantly different from noninduced cells (P ≤ 0.05).
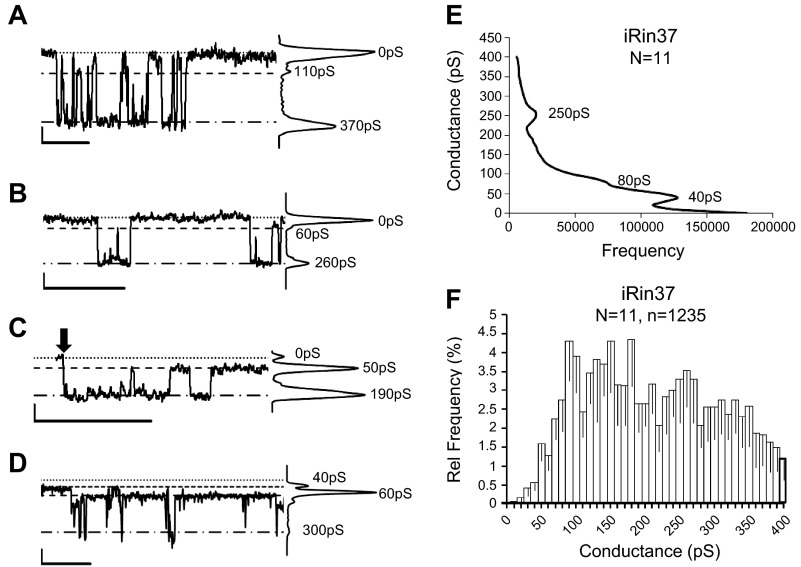
The results from the iRin37tr cells suggest that function of the channel itself is not sufficient and that the CT domain is also necessary for Cx37-mediated growth suppression. The CT domain of connexins is known to regulate channel function (conductance, gating, selective permeability) at least in part by interacting with the pore-forming domains of the channel. Therefore, we next determined whether behavior of truncated Cx37 channels differed from full length Cx37 channels, which would indicate regulation by the CT of Cx37 pore function. Consistent with our previous report (6), the Cx37 channel displays complex behavior, occupying several open states stably. In an effort to better understand this complex behavior, we examined a total of 24 min of record (Vj = 25 mV) in 11 cell pairs and constructed all points histograms of 57 shorter segments (2–5 s each, 5 min total). Figure 6 shows several of these multisecond traces as well as relative frequency and all points histograms from the compiled data. The Cx37 channel transitions from the closed to fully open state of ∼370 pS (Fig. 6A) as well as from the closed to a 260- to 280-pS substate (Fig. 6B). Other open states, e.g., 190 and 50 pS, were also frequently observed, as were transitions between these states (Fig. 6, C and D). Figure 6, E and F, shows the all points and relative frequency histograms, respectively, compiled from 11 cell pairs. The relative frequency histogram revealed no obvious peaks, consistent with the channel transitioning between all of the observed open states with approximately similar frequency. The all points histogram suggests the Cx37 channel spends the bulk of its time (at Vj = ± 25 mV) in the closed or 40- to 50-pS substate, consistent with the frequency of records like that shown in Fig. 6D, where it appears multiple channels residing in the 40- to 50-pS substate were open for long stretches of the record. The closed state and the 40- to 50-pS and 250-pS substates were evident in the compiled all points histogram, but the fully open state was not.
Fig. 6.
Cx37 displays complex channel behavior in Rin cells. A–D: segments of single channel records and corresponding all points histogram from 3 of the 11 studied cell pairs (B and C from same cell pair). Note the presence of multiple stable open states within and between cell pairs. Vj = 25 mV, scale bars correspond to 1 s and 2 pA. Arrow, when present, indicates beginning of pulse. E: compiled all points histogram from the 11 cell pairs (5-min total record time; sampling frequency 2 kHz). Cx37 channels spent the bulk of their time in the closed state (0 pS), with 40- and 250-pS subconductance states also evident; note that the fully open state was not visited sufficiently frequently or for a long enough duration to be evident in the all points histogram. F: relative frequency of 1,235 observed transition amplitudes in the 11 cell pairs.
In contrast to the behavior of the wild-type Cx37 channel, the truncated channel behavior was less complex (Vj = ±25 mV). The truncated channel existed stably in the fully open state, ∼350 pS, and a 30- to 80-pS substate (Fig. 7, A and B), with frequent transitions between these states (Fig. 7D). Less frequently, channels were observed to visit intermediate conductance states (Fig. 7C). The all points histogram compiled from 20 min of record derived from seven cell pairs (Fig. 7D) shows the truncated channel spent the bulk of its time in the 30- to 50-pS substate configuration, but also sufficient time in the fully open state that this conductance level was evident in the histogram. Interestingly, the closed state was less prevalent than the 30- to 50-pS substate for Cx37–273tr*V5, and the 250-pS conductance level detected for the wild-type Cx37 channel was absent for the Cx37–273tr*V5 channel. Figure 7F shows the relative frequency difference plot, wherein Cx37–273tr*V5 relative event frequencies (Fig. 7E) were subtracted from the corresponding relative event frequencies for Cx37. This difference plot shows that the wild-type channel visited the lower conductance substates more frequently than the truncated channel, which visited the fully open state more frequently. These data strongly suggest that the CT domain of Cx37 influences the tendency of the channel to open as well as the stability and conductance of the adopted open state configuration.
Fig. 7.
Behavior of Cx37tr channels is less complex than wild-type Cx37 channels. A–C: segments of single channel record and corresponding all points histogram from 2 of 7 cell pairs (A and B from same cell pair). The fully open state is frequently observed in the truncation mutant, and fewer substates were evident than in wild-type Cx37. Vj = 25 mV; scale bars correspond to 1 s and 2 pA. Arrow indicates beginning of pulse. D: compiled all points histogram from the 7 cell pairs (20 min total record time; sampling frequency 2 kHz). Cx37tr channels spent the bulk of their time in a residual state (30 or 50 pS), but the fully open channel is also evident. E: relative frequency of observed transition amplitudes in 6 of 7 cell pairs. To be included in this histogram, transition had to be preceded and followed by current stability of 50 ms or longer. Note the preponderance of transitions in the 300- to 400-pS range, corresponding to transitions from closed to fully open or from the 30- to 50-pS substate to fully open. F: difference plot wherein relative frequency of transitions in each bin for the truncated channel were subtracted from the relative frequency of transitions in each corresponding bin for the wild-type channels. Transitions to and from the fully open state were far more common for the Cx37tr than Cx37 channel.
Our previously published data indicated that Cx37-mediated growth suppression requires a functional channel (20, 21); the current data set indicates that the CT domain is also necessary for Cx37-mediated growth suppression, possibly as a regulator of channel function. For Cx43, regulation by the CT of channel function (permselectivity, gating, channel open state) involves interaction of the CT with the pore-forming domain to include the CL (3, 5, 10, 12, 13, 23, 30, 38, 39, 42). In recently published work, we showed that the channels formed by Cx43*CT37 and Cx43tr were similar. Here, we used a peak fitting program (Origin) to fit the single population of channel events in each event histogram and verified that the mean unitary conductance for Cx43*CT37 (99 ± 16 pS) and Cx43M257 (105 ± 12 pS) was not different, suggesting the Cx37 CT is unable to regulate the Cx43 pore in a manner similar to the Cx43CT. Interestingly, the permselective (permeability vs. conductance) profile of junctions formed by Cx43*CT37 was indistinguishable from that of wild-type Cx37 (13). We therefore next determined whether the CT of Cx37 retained growth-suppressive function when attached to a pore-forming domain with permselective properties similar to Cx37. Despite the many similarities between iRin43*CT37 and iRin37 cells [comparable expression levels (Fig. 3, C and D), similar protein localization (Fig. 2D), and similar permselective properties (13)], Cx43*CT37 expression did not slow the proliferation of Rin cells comparably to Cx37 expression. Instead iRin43*CT37 cells proliferated at rates comparable to their noninduced counterparts and to noninduced iRin37 and iRin37tr cells and induced iRin37tr cells (Fig. 8A).
Fig. 8.
Cx43*CT37 expression has no effect on Rin cell proliferation, possibly due to absence of interaction between Cx37-CT and Cx43-CL. A: exponential growth rate (evident from slopes of the regression lines) for iRin cells was significantly (P < 0.0001) slowed by expression of Cx37 but not Cx43*CT37 or Cx37–273tr*V5 (open symbols, gray solid lines represent no doxycycline; filled symbols, dashed lines represent doxycycline-stimulated cells). Points correspond to the means of corresponding data, lines represent the best fit of each data set. [iRin37: N ≥ 12 (each experiment in triplicate) for each time point; iRin43*CT37: N = 3, each in triplicate; iRin37tr: N = 6 data from 2E5 and 1B3 clones combined]. B: circular dichroism shows Cx37-CT is disordered at both pH 7.4 and 5.8. C: 15N-HSQC spectra were collected for the 15N-Cx43-CL in the presence (red) and absence (black) of unlabeled Cx37-CT. The 2 spectra were entirely coincident, indicating no interaction between these proteins.
To determine whether this failure of the Cx37-CT to exert a growth-suppressive effect when associated with the Cx43 pore-forming domain might reflect its inability to regulate (interact with) the Cx43 pore, we next determined whether the Cx37-CT was able to interact with the Cx43-CL in a manner comparable to the interaction of Cx43-CT and CL (5, 10, 23). We used CD and NMR conformational analysis of the Cx37-CT to detect possible interaction with 15N-Cx43-CL. Figure 8B shows that like the CTs of Cx40 and Cx43, Cx37-CT is an intrinsically disordered protein (5). However, unlike the CTs of Cx40 and Cx43, Cx37-CT failed to interact with the Cx43-CL (Fig. 8C). Together the electrophysiological and NMR data indicate a lack of interaction between the Cx43-CL and Cx37-CT and suggest that, despite similar permselective profiles, the Cx37-CT requires other properties specific to the Cx37 pore domain for its growth-suppressive function.
DISCUSSION
We showed previously that Cx37, but not Cx40 or Cx43, potently suppresses the proliferation of Rin cells (6), increasing cell cycle time by fourfold and inducing G1-cell cycle arrest when the cells are deprived of growth factors (6). A similar role appears to be served by Cx37 in the in vivo arterial vasculature, suppressing angiogenesis, arteriogenesis, and vasculogenesis (14). Contrary to expectation (25), the growth-suppressive effect of Cx37 expression in Rin cells requires a functional channel; a properly localized wild-type CT associated with a nonfunctional pore domain is not sufficient for Cx37-mediated growth suppression (20, 21). Here we show that in the absence of the CT, the functional Cx37 pore domain is not sufficient to support Cx37-mediated growth suppression. Instead, the Cx37 growth-suppressive effect appears to require the Cx37-CT and an associated pore-forming domain with properties not provided by the Cx43 pore-forming domain.
The current data show that, unlike wild-type Cx37, expression of Cx37–273tr*V5 had no impact on proliferation of iRin cells and did not confer on these cells sensitivity to growth factors, suggesting that the CT is necessary for Cx37-mediated growth suppression. The necessity for the CT could reflect its regulatory control of channel or nonchannel functions. Our data show that function of Cx37–273tr*V5 gap junction channels differed considerably from wild-type Cx37. The conductances of the fully open channels formed by each protein were comparable (∼300–350 pS), but wild-type Cx37 channels preferred the closed state and a 40- to 50-pS subconductance state. A 250-pS subconductance state was also observed, but the fully open state was visited infrequently and briefly, such that this state was not detected in all points histograms of channel activity compiled from multiple minutes of data. In contrast, Cx37–273tr*V5 gap junction channels resided preferentially in a 40- to 50-pS substate (rather than the closed state) and spent sufficient time in the fully open state (350 pS) that this state was detected in all points histograms compiled from multiple minutes of data. Similar differences between truncated and full length proteins have been reported for both Cx43 (37) and Cx40 (3). The differences in Cx37tr*V5 and Cx37 channel behavior demonstrate an important role for the CT domain in regulating channel function, but offer no insight into whether regulated channel function is causative or incidental to the Cx37 growth-suppressive function.
We previously reported that the gap junctions formed by the Cx43*CT37 chimera display a permselectivity profile indistinguishable from wild-type Cx37 channels (12, 13). Despite incorporation of a wild-type Cx37-CT and formation of gap junction channels with a permselective profile similar to Cx37 gap junctions (13), expression of this chimera failed to suppress the proliferation of iRin cells. That Cx43*CT37 forms channels and junctions with properties (conductance, permselectivity) distinct from wild-type Cx43 and similar to truncated Cx43 suggests that the CT of Cx37 does not interact with the Cx43 pore-forming domain in a manner comparable to the CT of Cx43 (as previously suggested, see Ref. 13). Lack of interaction between the CT of Cx37 and the pore-forming domain of Cx43 is further supported by the NMR data presented herein, which show that the Cx37-CT does not interact with the CL of Cx43, a component of the pore-forming domain of Cx43 essential to its regulated channel function. Consequently, failure of the chimera to suppress Rin cell proliferation (despite the presence of wild-type Cx37-CT in junctions with permselective properties very similar to wild-type Cx37) suggests that interaction of the Cx37-CT with the Cx37 pore-forming domain is necessary for Cx37-mediated growth suppression. Altogether our data suggest that interaction between the CT and pore-forming domains of Cx37 may be essential to Cx37 adopting a conformation conducive to its growth-suppressive function. The significance of this conformation appears not to be regulation of channel/junctional permselectivity, although that may occur, but rather the conformation of the CT itself, which may support, or not, interaction with critical growth regulatory proteins.
Connexins are broadly recognized to serve a growth regulatory function, but the mechanistic basis for growth regulation remains poorly defined. The best studied connexin in this context is Cx43 (25). The proliferation of numerous tumor-derived cell lines and primary cells derived from various tissues is suppressed (to varying degrees) when those cells express or are induced to express Cx43 (8, 17, 19, 31). Cx43 appears to serve a similar function in vivo, contributing to regulated growth, differentiation, and tumor suppression in many tissues (11, 33, 47). Mechanisms contributing to Cx43-mediated growth regulation are tissue- and cell-type-specific and involve channel-dependent and/or channel-independent functions of the protein, both of which are defined by unique sequences and functions of the protein CT domain. In many tissues, as they acquire their differentiated state, Cx43 is replaced by other connexins; however, injury and disease frequently result in downregulation of the alternate connexin and upregulation of Cx43. Remaining unclear are the properties of the downregulated connexin (e.g., Cx37) that make it better suited to tissue homeostasis and differentiated function and apparently less well suited than Cx43 to support of regulated growth in an injury or disease setting.
Cx37 is prominently expressed by endothelial cells of the arterial vasculature (22). In adult animals, endothelial cells must be nonproliferative until challenged by injury or disease, when they need to participate in vascular repair by angiogenic and arteriogenic mechanisms. Cx37 is downregulated and Cx43 upregulated in such settings (18, 29, 32, 46, 54). Studies comparing vascular development and function in Cx37−/− or Cx40−/− mice to wild-type mice indicate that in the endothelium Cx43 is growth permissive, supporting a regulated response to stress and injury that is essential to vascular repair, whereas Cx37 is growth suppressive, supporting the nonproliferative homeostatic function of the differentiated vessel wall (2, 14, 15, 46, 53). However, few studies have focused on the properties of Cx37 and Cx43 that make them uniquely suited to their specific roles. The data presented herein and previously (6, 14, 15) suggest a unique, growth-suppressive role for Cx37 in the vascular wall that relies on properties of the Cx37-CT specifically supported by the Cx37 pore domain.
There are multiple serines and tyrosines in the Cx37-CT with a predicted probability of at least 90% for phosphorylation by growth factor-activated kinases. hCx37-CT also contains an endothelial nitric oxide synthase interaction site, revealed by cross-linking and surface plasmon resonance approaches (39), and this interaction site is distinct from a polymorphic site at position 319 (resulting in either a serine or proline). Kumari et al. (27) characterized the channel conductance and voltage-dependent gating properties of the two polymorphic forms as well as the epitope tagged and untagged truncated forms of hCx37 (including a frame shift mutant). They saw no differences in channel open state conductances between polymorphs and any of the truncated versions, although the V0 for voltage-dependent gating was broadened in the truncated forms. Although these authors expressed the hCx37 polymorphs and mutants in rat insulinoma cells, the cell line differed from that used in the current study and possible effects of hCx37 expression on proliferation of those cells was not examined. In contrast to the results of Kumari et al., Deroutte et al. (9) found that the conductance and ATP permeability of the polymorphic forms differ, and Morel et al. (36) showed that the 319P form uniquely slows the proliferation of HeLa and SK-HEP-1 cells, possibly due to unique targeting of the serine at position 321 by GSK-3. Since the hCx37 319S polymorph was not growth suppressive in these cell types, it seems likely that the growth-suppressive mechanisms activated by hCx37–319P in HeLa and SK-HEP-1 cells would differ from those activated by mCx37 (with serine at position 319) in Rin cells. Certainly the presence of a proline instead of a serine would be expected to alter the conformation of the Cx37-CT, possibly revealing or concealing growth regulatory sites otherwise not available. Perhaps a proline at this site induces a conformational change similar to what phosphorylation at this serine would do, potentially triggering in a gate-keeper fashion (49) phosphorylation at other sites that influence the Cx37 growth regulatory properties. It will be interesting to determine whether phosphorylation at serine 319 (or possibly substitution of aspartate at this residue) modulates phosphorylation at S321 to effect growth suppression by hCx37, as proposed (36).
Numerous studies have been conducted on the regulatory impact of phosphorylation of residues in Cx43, residues that sequence alignment programs predict align with or are in close proximity to the serine residues in Cx37 missing in Cx37–273tr*V5, including in Cx37 (Cx43): S275 (S282), S302 (S328–330), S325 (S365), and S328 (S368) (for review, see Ref. 34). Future studies will need to address the importance of these putative phosphorylation sites to growth suppression by Cx37. Certainly the consensus view in the extant literature supports the contention that Cx37 is a phosphoprotein, the phosphorylation or dephosphorylation of which likely associates with proliferation (32, 36), apoptosis (43), inflammatory response (2, 46, 50, 53), and coronary artery disease and atherosclerosis (4, 28, 54).
In summary, we show that the CT domain of Cx37 is required for Cx37-mediated suppression of rat insulinoma cell proliferation. We also show that the Cx37-CT regulates the function of the Cx37 channel, presumably by interacting with one or more regions of the pore-forming domain. Finally, we show that the pore-forming domain of Cx43 cannot replace that of Cx37 in supporting growth suppression by the Cx37-CT, possibly because the Cx37-CT does not interact with the CL of Cx43. Thus it appears that Cx37-CT interaction with the functional Cx37-pore domain is required for growth suppression by this protein.
GRANTS
These studies were supported by National Institutes of Health Grants 5R01-HL-058732 (to J. M. Burt) and 5R01-GM-072631 (to P. L. Sorgen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.K.N. and J.M.B. conception and design of research; T.K.N. and P.L.S. performed experiments; T.K.N., P.L.S., and J.M.B. analyzed data; T.K.N., P.L.S., and J.M.B. interpreted results of experiments; T.K.N., P.L.S., and J.M.B. prepared figures; T.K.N., P.L.S., and J.M.B. drafted manuscript; T.K.N., P.L.S., and J.M.B. edited and revised manuscript; T.K.N., P.L.S., and J.M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ek Vitorin for thoughtful suggestions and occasional assistance during the course of these experiments and Dr. Jennifer Fang for occasional technical assistance.
REFERENCES
- 1.Alcolea S, Jarry-Guichard T, de Bakker J, Gonzalez D, Lamers W, Coppen S, Barrio L, Jongsma H, Gros D, Van Rijen H. Replacement of connexin40 by connexin45 in the mouse: impact on cardiac electrical conduction. Circ Res 94: 100–109, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Angelillo-Scherrer A, Fontana P, Burnier L, Roth I, Sugamele R, Brisset A, Morel S, Nolli S, Sutter E, Chassot A, Capron C, Borgel D, Saller F, Chanson M, Kwak BR. Connexin 37 limits thrombus propensity by downregulating platelet reactivity. Circulation 124: 930–939, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Anumonwo JM, Taffet SM, Gu H, Chanson M, Moreno AP, Delmar M. The carboxyl terminal domain regulates the unitary conductance and voltage dependence of connexin40 gap junction channels. Circ Res 88: 666–673, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Boerma M, Forsberg L, Van Zeijl L, Morgenstern R, De Faire U, Lemne C, Erlinge D, Thulin T, Hong Y, Cotgreave IA. A genetic polymorphism in connexin 37 as a prognostic marker for atherosclerotic plaque development. J Intern Med 246: 211–218, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Bouvier D, Spagnol G, Chenavas S, Kieken F, Vitrac H, Brownell S, Kellezi A, Forge V, Sorgen PL. Characterization of the structure and intermolecular interactions between the connexin40 and connexin43 carboxyl-terminal and cytoplasmic loop domains. J Biol Chem 284: 34257–34271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burt JM, Nelson TK, Simon AM, Fang JS. Connexin 37 profoundly slows cell cycle progression in rat insulinoma cells. Am J Physiol Cell Physiol 295: C1103–C1112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burt JM, Spray DC. Volatile anesthetics block intercellular communication between neonatal rat myocardial cells. Circ Res 65: 829–837, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Conklin CM, Bechberger JF, MacFabe D, Guthrie N, Kurowska EM, Naus CC. Genistein and quercetin increase connexin43 and suppress growth of breast cancer cells. Carcinogenesis 28: 93–100, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Derouette JP, Desplantez T, Wong CW, Roth I, Kwak BR, Weingart R. Functional differences between human Cx37 polymorphic hemichannels. J Mol Cell Cardiol 46: 499–507, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Duffy HS, Sorgen PL, Girvin ME, O'Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC. pH-dependent intramolecular binding and structure involving Cx43 cytoplasmic domains. J Biol Chem 277: 36706–36714, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Dyce PW, Norris RP, Lampe PD, Kidder GM. Phosphorylation of serine residues in the C-terminal cytoplasmic tail of connexin43 regulates proliferation of ovarian granulosa cells. J Membr Biol 245: 291–301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ek-Vitorin JF, Burt JM. Quantification of gap junction selectivity. Am J Physiol Cell Physiol 289: C1535–C1546, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Ek-Vitorin JF, Burt JM. Structural basis for the selective permeability of channels made of communicating junction proteins. Biochim Biophys Acta 1828: 51–68, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang JS, Angelov SN, Simon AM, Burt JM. Cx37 deletion enhances vascular growth and facilitates ischemic limb recovery. Am J Physiol Heart Circ Physiol 301: H1872–H1881, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang JS, Angelov SN, Simon AM, Burt JM. Cx40 is required for, and Cx37 limits, postischemic hindlimb perfusion, survival and recovery. J Vasc Res 49: 2–12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang JS, Angelov SN, Simon AM, Burt JM. Compromised regulation of tissue perfusion and arteriogenesis limit, in an AT1R-independent fashion, recovery of ischemic tissue in Cx40−/− mice. Am J Physiol Heart Circ Physiol 304: H816–H827, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu CT, Bechberger JF, Ozog MA, Perbal B, Naus CC. CCN3 (NOV) interacts with connexin43 in C6 glioma cells: possible mechanism of connexin-mediated growth suppression. J Biol Chem 279: 36943–36950, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed [see comments]. Circ Res 83: 636–643, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Goldberg GS, Bechberger JF, Tajima Y, Merritt M, Omori Y, Gawinowicz MA, Narayanan R, Tan Y, Sanai Y, Yamasaki H, Naus CC, Tsuda H, Nicholson BJ. Connexin43 suppresses MFG-E8 while inducing contact growth inhibition of glioma cells. Cancer Res 60: 6018–6026, 2000 [PubMed] [Google Scholar]
- 20.Good ME, Ek-Vitorin JF, Burt JM. Extracellular loop cysteine mutant of cx37 fails to suppress proliferation of rat insulinoma cells. J Membr Biol 245: 369–380, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good ME, Nelson TK, Simon AM, Burt JM. A functional channel is necessary for growth suppression by Cx37. J Cell Sci 124: 2448–2456, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haefliger JA, Nicod P, Meda P. Contribution of connexins to the function of the vascular wall. Cardiovasc Res 62: 345–356, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Hirst-Jensen BJ, Sahoo P, Kieken F, Delmar M, Sorgen PL. Characterization of the pH-dependent interaction between the gap junction protein connexin43 carboxyl terminus and cytoplasmic loop domains. J Biol Chem 282: 5801–5813, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kanady JD, Dellinger MT, Munger SJ, Witte MH, Simon AM. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev Biol 354: 253–266, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kardami E, Dang X, Iacobas DA, Nickel BE, Jeyaraman M, Srisakuldee W, Makazan J, Tanguy S, Spray DC. The role of connexins in controlling cell growth and gene expression. Prog Biophys Mol Biol 94: 245–264, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development 127: 4179–4193, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Kumari SS, Varadaraj K, Valiunas V, Ramanan SV, Christensen EA, Beyer EC, Brink PR. Functional expression and biophysical properties of polymorphic variants of the human gap junction protein connexin37. Biochem Biophys Res Commun 274: 216–224, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol 22: 225–230, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Kwak BR, Pepper MS, Gros DB, Meda P. Inhibition of endothelial wound repair by dominant negative connexin inhibitors. Mol Biol Cell 12: 831–845, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lampe PD, Tenbroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol 149: 1503–1512, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. The tumor-suppressive function of Connexin43 in keratinocytes is mediated in part via interaction with caveolin-1. Cancer Res 70: 4222–4232, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Larson DM, Wrobleski MJ, Sagar GD, Westphale EM, Beyer EC. Differential regulation of connexin43 and connexin37 in endothelial cells by cell density, growth, and TGF-β1. Am J Physiol Cell Physiol 272: C405–C415, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Maass K, Ghanem A, Kim JS, Saathoff M, Urschel S, Kirfel G, Grummer R, Kretz M, Lewalter T, Tiemann K, Winterhager E, Herzog V, Willecke K. Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol Biol Cell 15: 4597–4608, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marquez-Rosado L, Solan JL, Dunn CA, Norris RP, Lampe PD. Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim Biophys Acta 1818: 1985–1992, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mesnil M, Krutovskikh V, Piccoli C, Elfgang C, Traub O, Willecke K, Yamasaki H. Negative growth control of HeLa cells by connexin genes: connexin species specificity. Cancer Res 55: 629–639, 1995 [PubMed] [Google Scholar]
- 36.Morel S, Burnier L, Roatti A, Chassot A, Roth I, Sutter E, Galan K, Pfenniger A, Chanson M, Kwak BR. Unexpected role for the human Cx37 C1019T polymorphism in tumour cell proliferation. Carcinogenesis 31: 1922–1931, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Moreno AP, Chanson M, Elenes S, Anumonwo J, Scerri I, Gu H, Taffet SM, Delmar M. Role of the carboxyl terminal of connexin43 in transjunctional fast voltage gating. Circ Res 90: 450–457, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Moreno AP, Saez JC, Fishman GI, Spray DC. Human Connexin43 gap junction channels: regulation of unitary conductances by phosphorylation. Circ Res 74: 1050–1057, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Pfenniger A, Derouette JP, Verma V, Lin X, Foglia B, Coombs W, Roth I, Satta N, Dunoyer-Geindre S, Sorgen P, Taffet S, Kwak BR, Delmar M. Gap junction protein Cx37 interacts with endothelial nitric oxide synthase in endothelial cells. Arterioscler Thromb Vasc Biol 30: 827–834, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reed KE, Westphale EM, Larson DM, Wang HZ, Veenstra RD, Beyer EC. Molecular cloning and functional expression of human connexin37, an endothelial cell gap junction protein. J Clin Invest 91: 997–1004, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scherer SS, Xu YT, Nelles E, Fischbeck K, Willecke K, Bone LJ. Connexin32-null mice develop demyelinating peripheral neuropathy. Glia 24: 8–20, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Seki A, Duffy HS, Coombs W, Spray DC, Taffet SM, Delmar M. Modifications in the biophysical properties of connexin43 channels by a peptide of the cytoplasmic loop region. Circ Res 95: e22-e28, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Seul KH, Kang KY, Lee KS, Kim SH, Beyer EC. Adenoviral delivery of human connexin37 induces endothelial cell death through apoptosis. Biochem Biophys Res Commun 319: 1144–1151, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Simon AM, Chen H, Jackson CL. Cx37 and Cx43 localize to zona pellucida in mouse ovarian follicles. Cell Commun Adhes 13: 61–77, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature 385: 525–529, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Simon AM, McWhorter AR, Chen H, Jackson CL, Ouellette Y. Decreased intercellular communication and connexin expression in mouse aortic endothelium during lipopolysaccharide-induced inflammation. J Vasc Res 41: 323–333, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Sirnes S, Bruun J, Kolberg M, Kjenseth A, Lind GE, Svindland A, Brech A, Nesbakken A, Lothe RA, Leithe E, Rivedal E. Connexin43 acts as a colorectal cancer tumor suppressor and predicts disease outcome. Int J Cancer 131: 570–581, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res 62: 228–232, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Solan JL, Marquez-Rosado L, Sorgen PL, Thornton PJ, Gafken PR, Lampe PD. Phosphorylation at S365 is a gatekeeper event that changes the structure of Cx43 and prevents down-regulation by PKC. J Cell Biol 179: 1301–1309, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaiyapuri S, Jones CI, Sasikumar P, Moraes LA, Munger SJ, Wright JR, Ali MS, Sage T, Kaiser WJ, Tucker KL, Stain CJ, Bye AP, Jones S, Oviedo-Orta E, Simon AM, Mahaut-Smith MP, Gibbins JM. Gap junctions and connexin hemichannels underpin hemostasis and thrombosis. Circulation 125: 2479–2491, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vinken M, Decrock E, De VE, Ponsaerts R, D'hondt C, Bultynck G, Ceelen L, Vanhaecke T, Leybaert L, Rogiers V. Connexins: sensors and regulators of cell cycling. Biochim Biophys Acta 1815: 13–25, 2011 [DOI] [PubMed] [Google Scholar]
- 52.White TW. Unique and redundant connexin contributions to lens development. Science 295: 319–320, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, Chanson M, Goodenough DA, Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med 12: 950–954, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Yeh HI, Lu CS, Wu YJ, Chen CC, Hong RC, Ko YS, Shiao MS, Severs NJ, Tsai CH. Reduced expression of endothelial connexin37 and connexin40 in hyperlipidemic mice: recovery of connexin37 after 7-day simvastatin treatment. Arterioscler Thromb Vasc Biol 23: 1391–1397, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Zhang YW, Kaneda M, Morita I. The gap junction-independent tumor-suppressing effect of connexin 43. J Biol Chem 278: 44852–44856, 2003 [DOI] [PubMed] [Google Scholar]