Abstract
The clinical success of allogeneic T-cell therapy for cancer relies on the selection of antigens that can effectively elicit antitumor responses with minimal toxicity toward nonmalignant tissues. While minor histocompatibility antigens (MiHA) represent promising targets, broad expression of these antigens has been associated with poor responses and T-cell dysfunction that may not be prevented by targeting MiHA with limited expression. In this study, we hypothesized that antitumor activity of MiHA-specific CD8 T cells after allogeneic bone marrow transplant (BMT) is determined by the distribution of antigen relative to the site of tumor growth. To test this hypothesis, we utilized the clinically relevant male-specific antigen HY and studied the fate of adoptively transferred, HY-CD8+ T cells (HY-CD8) against a HY-expressing epithelial tumor (MB49) and pre-B cell leukemia (HY-E2APBX ALL) in BMT recipients. Transplants were designed to produce broad HY expression in nonhematopoietic tissues (female → male BMT, [F>M]), restricted HY expression in hematopoietic tissues (male → female BMT, [M>F]) tissues, and no HY tissue expression (female → female BMT, [F>F]). Broad HY expression induced poor responses to MB49 despite sublethal GVHD and accumulation of HY-CD8 in secondary lymphoid tissues. Antileukemia responses, however, were preserved. In contrast, restriction of HY expression to hematopoietic tissues restored MB49 responses but resulted in a loss of antileukemia responses. We concluded that target alloantigen expression in the same compartment of tumor growth impairs CD8 responses to both solid and hematologic tumors.
INTRODUCTION
The curative potential of allogeneic hematopoietic stem cell transplantation (AlloHSCT) lies partly in the reactivity of donor T-cells against mismatched recipient antigens. The infusion of allogeneic T-cells is a potent form of immunotherapy and has been shown to cure relapsed leukemia [1,2]. Obstacles to the success of this approach have included weak responses to solid tumors [3,4] and certain leukemias [5] as well as challenges separating graft-versus-host (GVHD) from graft-versus-tumor (GVT) effects [6]. The success of this approach requires prudent selection of immunogenic target antigens that produce effective antitumor responses while sparing immune-mediated damage to host tissues.
Minor histocompatibility antigens (miHA) are promising therapeutic targets because they do not require identification of tumor-specific epitopes, and may be less susceptible to tolerance [7]. Targeting MiHA with adoptively transferred T-cells has been shown, preclinically, to eradicate solid and hematologic malignancies [8,9]. Further, durable GVT responses have been generated, with relative sparing of GVHD, by immunizing donors against miHA prior to adoptive transfer [10]. A potential pitfall of this approach, however, is the generation of T-cell dysfunction produced by broad expression of minor antigens in alloHSCT recipients. Indeed, expression of a target MiHA in non-hematopoietic tissues has been shown to substantially reduce the efficacy of adoptively transferred T-cells against solid tumors [11]. Proposed mechanisms for this phenomenon include chronic and inefficient antigen presentation [12] blockade of functional CD8 memory [13], induction of donor T-cell apoptosis [14], and upregulation of negative costimulatory molecules such as PD-1 [15]. While these studies have established the influence of minor antigen distribution on the potency of adoptively transferred T-cells, the relationship between tissue antigen expression, site of tumor growth and T-cell function has not been directly studied in a single antigen system.
In the present study, we adoptively transferred miHA-specific T-cells into alloHSCT recipients mismatched at the male-specific minor antigen HY, expressed on either nonhematopoietic or hematopoietic tissues, and assessed their antitumor efficacy against an HY-expressing epithelial tumor and leukemia. We selected HY as a target antigen because it is endogenous, MHC-restricted, and has been implicated in clinically significant GVHD and GVT effects [16,17]. We demonstrate that broad HY expression produces poor responses to solid tumors that can be improved with hematopoietic restriction of HY. Antileukemia responses, however, are lost with hematopoietic restriction of HY and preserved with broad expression, suggesting that the proximity of target antigen expression to the site of tumor growth predicts the efficacy of adoptively transferred T-cells during alloHSCT.
MATERIALS AND METHODS
Mice
C57BL/6 (B6) CD45.1 (H-2b congenic) mice were purchased directly from the National Cancer Institute-Frederick Animal Production Program (Frederick, MD) via the Jackson Laboratories (Bar Harbor, ME). MataHari (B6 Rag1−/−, CD8+ TCRTg, H2-Db-restricted, CD45.2+) mice [18] were used to generate T-cells for adoptive transfer and purchased from the NIAID-Taconic repository (Rockville, MD). All animals were cared for under an animal use protocol reviewed and approved by the National Cancer Institute Laboratory Animal Safety Program-Animal Care and Use Committee (Bethesda, MD).
T cell-depleted BMT
Bone marrow cells were flushed from the tibias and fibulas of female or male B6 CD45.1+ mice using 10% complete mouse media (CMM; RPMI 1640 with 10% heat-inactivated fetal calf serum, 1% nonessential amino acids, 1% sodium pyruvate, 1% penicillin/streptomycin, 1% l-glutamine (Invitrogen, Carlsbad, CA) and 1% N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid buffer (Sigma-Aldrich, St. Louis, MO), passed through a 70-um nylon filter, and erythrocyte depleted using ACK lysis buffer (Lonza Walkersville, Walkersville, MD). T-cells were depleted from donor marrow using anti-CD3 microbeads through automated magnetic cells sorting (AutoMACS, Miltenyi Biotec, Auburn, CA). 3.5 × 106 T-cell depleted marrow was injected in serum-free RPMI via tail vein injection into recipients that were administered lethal irradiation at a dose of 1100 cGy (137 Cs source) on the same day. Female or male B6 CD45.1+ HSCT recipients were weighed twice every 7 days. Clinical monitoring for GVHD including observation for skin changes, fur changes, hunched posture and mobility was performed daily according to an established scoring system [19]. We have previously demonstrated GVHD in additional target organs, including the liver, in male recipients of HY-specific CD8 donor lymphocyte infusion [20].
Adoptive transfer and detection of HY- specific CD8+ T cells
Single cell suspensions of splenocytes from naïve female MataHari donors were prepared and 1 × 107 splenic CD8+ T cells were intravenously injected on Day 7. The delayed adoptive transfer platform was selected to allow engraftment of donor antigen presenting cells and leukemic cells [21]. Detection of HY-CD8 was performed by flow cytometry in the spleen and bone marrow of mice either euthanized at specific weekly timepoints or for disease-related morbidity by gating on live, CD8a+CD45.2+ cells. This was a reliable gating strategy because splenocytes from HY-TCR transgenic donors contain negligible numbers of CD8a+ myeloid cells and donor CD8 reconstituted from the T-cell depleted transplant would not express the congenic marker CD45.1. Absolute numbers of HY-CD8 were determined per 100,000 live-gated events. If more than 100,000 events were collected, the absolute number of cells was normalized to 100,000 by the proportion [(#CD8a+CD45.2+/total live cells = (x/100,0000)].
Ex vivo sorting and HY peptide stimulation of HY- specific CD8+ T cells
Single cell suspensions of bone marrow were prepared as described above and stained with fluorochrome-conjugated anti-CD8a and CD45.2 antibodies to identify adoptively transferred HY-CD8 for flow cytometry-based sorting. Live, CD8a+CD45.2+ were sorted in PBS/0.1% BSA in sterile conditions on an LSRII flow cytometer (BD Biosciences). Immediately after sorting, proliferation to HY-peptide stimulation was measured using carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution and flow cytometry. Sorted cells were washed twice in PBS/0.1% BSA, labeled with 5 uM CFSE (Molecular Probes, Eugene, OR) for 10 minutes at 37°C, placed in culture at a concentration of 1×106 cells/ml CMM and stimulated with 100 uM UTY peptide (Class I Dominant HY peptide, WMHHNMDLI) for 72 hours at 37°C. Cells were then harvested, washed twice in FACS buffer and prepared for flow cytometry.
Dendritic cell vaccination
Dendritic cells (DC’s) used for vaccines were cultured from male B6 bone marrow as previously described [22]. DC’s were activated with 4 µg/mL anti-CD40 on Day 8, collected within 24 hours of activation, resuspended in serum-free media and intraperitoneally injected at a dose of 1×105 cells/recipient at the time of adoptive CD8+ T cell transfer.
MB49 tumor challenge
The MB49 cell line was originally provided by Dr. Edmund Lattime (Robert Wood Johnson Medical School, New Brunswick, NJ). MB49 is a chemically-induced uroepithelial carcinoma that expresses the male-specific HY minor antigen [23] and is B6-derived. MB49 was maintained in standard sterile culture conditions (37°C, 5% CO2) in CMM. Exponentially growing tumor cells were harvested with trypsin and injected at a dose of 1×106 cells per recipient into the subcutaneous tissue of the left flank. Tumors were measured in two dimensions (length x width) twice every 7 days by digital caliper. Approximate spherical volumes were calculated for each measurement according to (L/2) x (W/2) x (L + W/4) x (4π/3). Mice were euthanized with C02 when tumor diameters ≥ 2.0 cm or tumor-related morbidity developed according to institutional protocols. If a mouse was euthanized or found dead, the most recent tumor measurement was carried forward for the remainder of the experiment.
Pre-B cell acute lymphoblastic leukemia challenge
A male, GFP-conjugated, B6-derived murine pre-B cell acute lymphoblastic leukemia cell line carrying the human E2a-PBX1 transgene by MMLV insertion as previously described [24] were generously provided by Dr. Janet Bijil (Centre de Recherche de l’Hopital Maisonneuve-Rosemont, Montreal, Quebec, Canada). Cells were maintained in culture at 37°C and 5% CO2, harvested on Day 3 in an early exponential growth phase and prepared as single cell suspensions. 5×105 leukemia cells were infused by tail vein injection in serum-free RPMI at the time of BMT. Monitoring for leukemia-related morbidity including hind leg paralysis, wasting, abdominal distention and poor movement was performed daily. Leukemia burden in the bone marrow and spleen was measured by flow cytometry in animals euthanized at specific time points or for leukemia-associated morbidity. Animals with clinical findings suggestive of leukemia morbidity but with no GFP+ cells in the marrow were censored from leukemia survival analysis.
HY-specific immunogenicity of male E2a-PBX ALL by Interferon gamma ELISA
HY-specific immunogenicity of male E2APBX-ALL was established by coculturing male E2a-PBX ALL, female E2a-PBX ALL with positive control male dendritic cells with HY-CD8 for 72 hours at a stimulator:responder ratio of 1 (1×106):1 (1×106). Concentrations of IFN-y (pg/mL) from in vitro co-cultures were measured by ELISA (Quantikine IFN-y, R&D Systems) in triplicate according to the manufacturer’s protocol and read at 450 nm on a microplate reader (BioRad, Hercules, CA).
Flow cytometry
FACS analysis for surface molecule expression was performed on an LSR II Fortessa flow cytometer (BD Biosciences, Hunt Valley, MD). The monoclonal antibodies fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein complex and cyanine 5.5 (PerCP-Cy 5.5), allophycocyanin (APC), and Pacific Blue (PacBlue)-conjugated anti-mouse B220, CD4, CD8a, CD44, CD45.2, CD62L, PD-1 and Tim-3. Compensation controls and immunoglobulin isotype controls were generated for each experiment using fresh splenocytes.
Statistical analysis
Statistical tests were performed using GraphPad Prism version 4.0b for MacIntosh (GraphPad Software, San Diego, CA). For tumor volume measurements, the last tumor volume recorded for each mouse at the time of death was used in calculations of tumor volume at each time point after death. Kaplan-Meier survival curves were created and analyzed using the Wilcoxon rank test to analyze curves. Significant differences between groups in clinical scores, in vivo T-cell enumeration and in vitro assays were determined by unpaired Student t-test. P-values less than 0.05 were considered significant.
Results
Broad HY expression in nonhematopoietic tissues, expands adoptively transferred HY-CD8 in secondary lymphoid tissues and produces GVHD
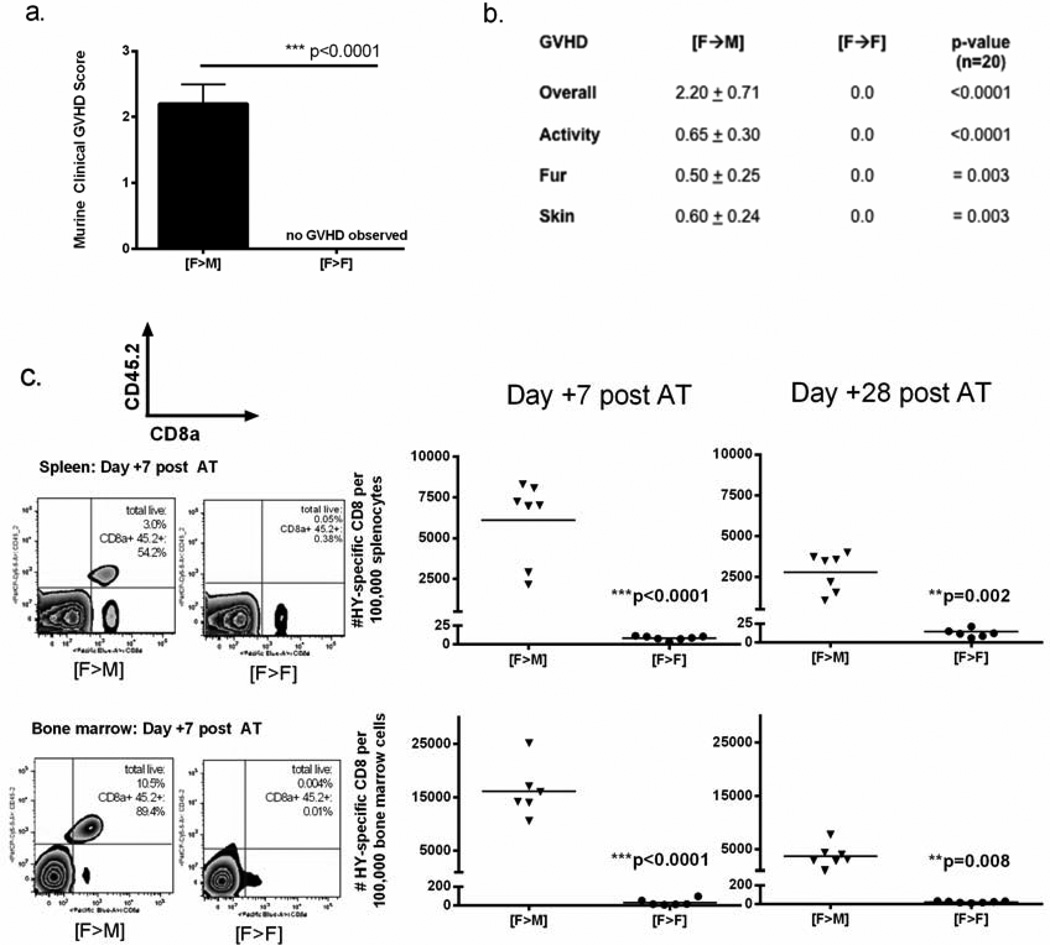
We first established whether HY mismatch was sufficient to produce clinically significant alloreactivity in our model. Critical to this objective was the ability to enumerate and track antigen-specific CD8 as a function of HY tissue distribution in alloHSCT recipients with sublethal GVHD. While HY mismatch has been associated with lethal GVHD when HY-CD8 are infused at the time of alloHSCT, [25] we have produced milder, sublethal alloreactivity that allows for quantitative analysis of T-cell responses using a delayed infusion platform [26]. In this study, B6 male and females received T-cell depleted B6 female BMT ([F—M] and [F→F] respectively) on Day 0, followed by adoptive transfer of 1×107 MataHari (HY-CD8) on Day 7. Clinical GVHD scoring and enumeration of HY-CD8 was performed through Day +35–42 post-BMT. As expected, adoptive transfer of HY-CD8 produced a nonlethal, clinical GVHD in males as defined by clinical score (Figure 1 A). HY-directed GVHD in this model manifested primarily with skin changes (scaling, denuded areas), fur changes (ruffling, patchy loss) and decreased activity (Figure 1B) consistent with other murine models of single minor antigen-mismatch alloHSCT [20,27. Broad expression of HY in [F→M] recipients resulted in the accumulation of large numbers of, CD8a+ CD45.2+ HY-CD8, in both the spleen and bone marrow (Figure 1C). As expected, syngeneic female controls with no tissue expression of HY had low but detectable numbers of HY-CD8. Four weeks after adoptive transfer, significantly higher numbers of HY-CD8 could still be detected in [F→M] recipients compared to their [F→F] counterparts (Figure 1D).
Figure 1. Adoptively transferred HY-specific CD8 T-cells expand and produce GVHD in allogeneic males.
Lethally irradiated B6 male mice received 5×106 T-cell (CD3) depleted marrow on Day 0, followed by 1×107 HY-specific CD8 by tail vein injection on Day +7. Clinical GVHD scoring was performed once weekly. (A) Composite GVHD scores on Day +60 posttransplant were consistent with sublethal GVHD, particularly manifested as (B) changes in skin integrity, fur ruffling and posture/activity, compared with no clinical signs of GVHD in syngeneic female recipients of female BMT. GVHD scoring data represent 4 independent experiments with 6–10 mice/group. (C) HY-specific CD8 were enumerated from spleen and bone marrow of transplant recipients by flow cytometry 7 and 28 days after adoptive transfer (Day +14 and +35 post-BMT) by gating on live, CD8a+CD45.2+ cells. Representative flow plots (left panel) and total numbers of CD8a+CD45.2+ cells per 100,000 splenocytes (right panel, top) or bone marrow cells (left panel, bottom) are shown.
Adoptively transferred HY-CD8 expand to broad HY tissue expression but do not control a HY-expressing solid tumor
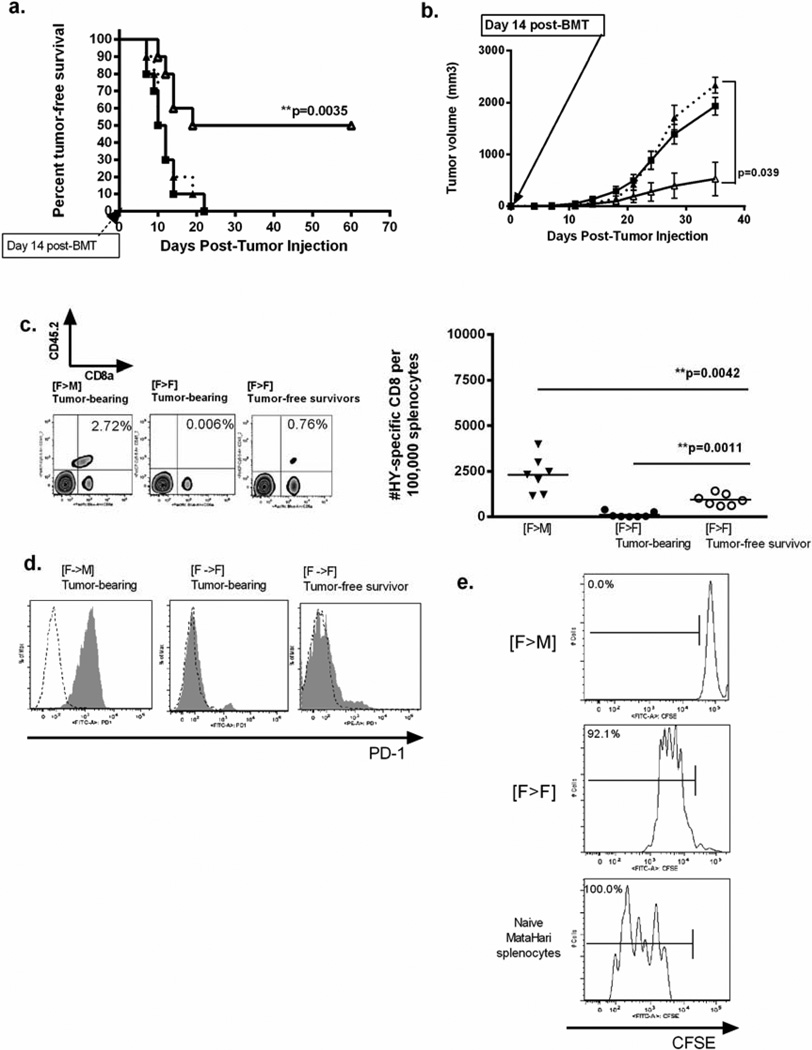
Although vaccination against miHA has been shown to cure solid tumors, we have previously observed reduced tumor-specific vaccine responses when alloreactivity generated against minor antigens are not shared by the tumor [20, 26]. In the present study, we wished to determine how CD8 function is affected by alloreactivity produced by an antigen shared by the tumor. Again, [F→M] and [F→F] T-cell depleted BMT recipients were given 10 × 10e6 HY-CD8 on Day +7, followed by subcutaneous injection with MB49 , an immunogenic [28] HY-expressing uroepithelial tumor, on Day +14. [F→M] that did not receive HY-specific adoptive transfer served as controls. All [F→M] mice developed rapidly-growing tumors, with 100% tumor-associated mortality by Day +35 post-tumor injection (Day+42 post BMT) (Figure 2A–B). This survival curve was statistically identical to [F→M] who did not receive HY-CD8. [F→F] recipients of HY-CD8 adoptive transfer, however, demonstrated preserved antitumor responses with 55 ± 3.7% tumor free survival (p< 0.0035). [F-→F] that did developed tumors did so at a significantly slower rate (p= 0.039) and had longer overall survival, suggesting improved tumor control (Figure 2B).
Figure 2. Adoptively transferred HY-specific CD8 cannot control MB49 in male recipients.
Experimental [F→M] and control [F→F] B6 mice received T-cell depleted B6 female bone marrow on Day 0, followed by adoptive transfer of HY-CD8 on Day +7. They were then challenged with 1×105 MB49, an HY-expressing epithelial tumor, subcutaneously on Day +14. Tumor-free survival was followed daily (A), and tumor volumes measured in 2 dimensions twice weekly (B). [F→M] recipients (closed square, solid line) had poor tumor control, similar to [F→F] controls that did not receive HY-CD8 (dotted line, closed triangle). [F→F] recipients of HY-CD8 preserved immune responses. Enumeration of adoptively transferred HY-CD8 in spleen as live, CD8a+CD45.2+ cells 14 days after adoptive transfer showed significant accumulation of HY-CD8 in [F→M] despite poor tumor control, compared to [F→F] tumor bearing (closed circle) or tumor-free recipients. (D) Expression of PD-1 (gray shaded) compared to IgG2k isotype controls (black dotted) was measured on live, CD8+CD45.2+ HY-specific CD8 on Day +14 post BMT (at the time of MB49 injection) by flow cytometry. Expansion data and PD-1 histograms are representative of at least 4 independent experiments with 6–10 mice/group. (E) Live, CD8+CD45.2+ cells were collected by flow sorting on Day +14 post-BMT and restimulated in vitro with the Class I immunodominant HY peptide (UTY). Proliferation was measured by CFSE dilution at 72 hours. Naïve female MataHari (HY-specific) splenocytes served as controls.
Enumeration of CD8+CD45.2+ HY-CD8 in the spleen on Day +21 post-tumor injection (Day +42 post-BMT) was performed on [F→M], [F→F] who developed tumor and [F→F] tumor-free survivors in a cohort not included in survival analysis. This timepoint was selected because it represented both the point of accelerating tumor growth and robust HY-CD8 accumulation in the GVHD studies. Significantly more HY-CD8 accumulated in the spleen (Figure 2C, representative flow plots left panel, total number of HY-CD8 right panel) of [F→M] recipients, compared to syngeneic [F→F] recipients that developed tumor (p=0.0042). [F→F] tumor-free survivors had significantly higher numbers of HY-CD8 than their tumor-bearing counterparts, suggesting that adoptively transferred HY-CD8 could expand in response to MB49 and were not deleted in the short term. Interestingly, 100% of HY-CD8 recovered from the spleen of [F→M] recipients expressed PD-1, while [F→F] did not express PD-1 regardless of whether they were able to control tumor (Figure 2D).
Poor survival, rapid tumor growth and PD-1 expression suggested that adoptively transferred CD8 became dysfunctional in [F→M] mice where HY was broadly expressed. To begin to investigate whether HY-CD8 could respond to HY independent of the allogeneic environment, HY-CD8 were isolated by flow sorting by flow sorting live, CD8a+CD45.2+ splenocytes on Day +21 (Day+14 post-adoptive transfer). An increased HY-CD8 dose of 5×107 was utilized to improve the sorted yield. The timepoint selected corresponded to the day of MB49 injection in tumor challenge studies. Sorted HY-CD8 and naive MataHari control splenocytes were placed in culture, stimulated ex vivo with the Class I immunodominant HY peptide UTY, and proliferation measured after 72 hours by CFSE dilution. Under these conditions, both HY-CD8 recovered from [F→F] recipients and naïve HY-CD8 controls proliferated, while HY-CD8 from [F→M] recipients, did not (Figure 2E).
HY-expressing dendritic cell vaccination cannot restore functionality of HY-CD8 expanded by broad HY expression
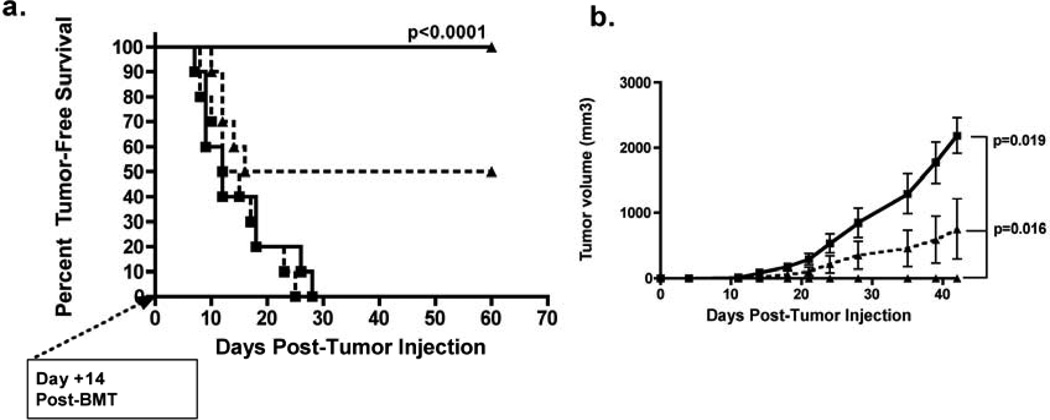
To determine whether professional HY antigen presentation could overcome poor immune responses in [F→M] recipients , 1 ×105 anti-CD40 activated male DCs were injected intraperitoneally at the time of HY-CD8 adoptive transfer on Day +7, followed by MB49 challenge on Day +14. DC vaccination completely prevented tumor growth in [F→F] recipients (100% tumor-free survival, Figure 3A), while all of the [F→M] developed progressive tumors. As expected, survival curves in unvaccinated controls receiving HY-CD8 were consistent with those shown in Figure 2B.
Figure 3. HY-expressing dendritic cell vaccination cannot restore antitumor responses in [F→M] recipients of HY-specific CD8 who express HY in nonhematopoietic tissues.
Lethally irradiated female or male B6 recipients were transplanted with TCD female B6 BM on Day 0, 1×107 HY-CD8 on Day +7 and 1×105 CD40-activated male dendritic cells were injected intraperitoneally also on Day +7.. AlloHSCT recipients were then followed for (A) tumor-free survival and (B) tumor growth in [F→F] recipients (closed triangle, solid line) and [F→M] recipients (closed square, solid line), with rapidly growing tumors (closed square, solid line). Unvaccinated [F>M] (closed square, dotted line) and [M>F] (closed triangle, dotted line) who received HY-CD8 served as controls. Survival and tumor volume curves are representative of 3 independent experiments with 8–10 mice/group.
Restriction of HY to hematopoietic tissues preserves functionality of adoptively transferred HY-CD8 but does not prevent PD-1 expression
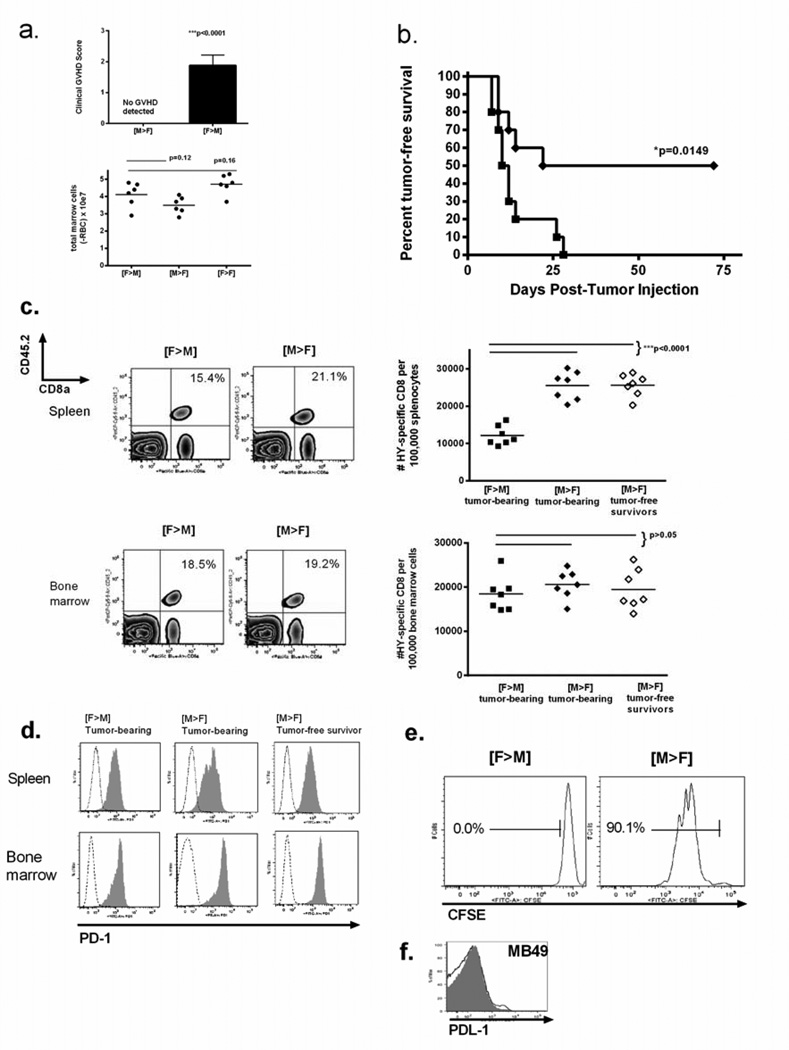
To determine whether restricted expression of HY could improve antitumor responses, we transplanted T-cell depleted B6 male marrow into female B6 mice [M→F] and adoptively transferred HY-CD8 on Day +7 as described before. No systemic GVHD was observed in [M→F] recipients of HY-CD8 (Figure 4A, top panel) and marrow cellularity was similar at Day +60 to [F→M] (Figure 4A, bottom panel), excluding an early immune-mediated aplasia.
Figure 4. Hematopoietic restriction of HY preserves responses to MB49 despite PD1 expression on adoptively transferred HY-specific CD8.
To restrict HY to hematopoietic tissues, lethally irradiated female B6 mice received T-cell depleted B6 male [M→F] BMT on Day 0 followed by 1×107 HY-specific CD8 on Day +7. AlloHSCT recipients were (A) clinically graded for GVHD and total bone marrow cells recorded after erythrocyte lysis to assess overall marrow cellularity. (B) Recipients were next challenged with MB49 on Day +14 and followed for tumor-free survival in [F→M] (closed square) and [M→F] (closed diamond) groups. Data shows a single experiment representative of 3 independent experiments with 8–12 mice/group. (C) Accumulation of HY-CD8 was measured by flow cytometry as live, CD8a+CD45.2+ cells 14 days after adoptive transfer via representative contour plots (left panels) and absolute numbers per 100,000 splenocytes (right, top panel) or bone marrow cells (right, bottom panel). (D) PD-1 expression was measured by flow cytometry on HY-CD8 in the spleen and bone marrow of [M→F] and [F→M] recipients 14 days after adoptive transfer. Histograms depict live, CD8a+CD45.2+PD1+ cells (gray shaded) compared to IgG2k isotype controls (dashed line). Data shown are representative of 3 independent experiments. (E) To verify functionality, HY-CD8 were sorted from [F→M] and [M→F] ex vivo 14 days after adoptive transfer, restimulated with UTY peptide, and proliferation measured by CFSE dilution after 72 hours. (F) Expression of PDL-1 (B7:H1), the ligand for PD-1, was measured by flow cytometry in MB49 prior to subcutaneous injection (gray shaded) and at Day +28 in animals that succumbed to tumor-related mortality and shown in this representative histogram.
When [M→F] were challenged with MB49 at Day +14 post-BMT, tumor-free survival was 52.5 ± 4.9% (Figure 4B), similar to [F>F] recipients shown in Figure 2A. Enumeration of CD8a+CD45.2+ cells in [F>M], [M>F], and [F>F] from spleen and bone marrow at the time of tumor challenge (7 days after adoptive transfer, 14 days post-BMT) revealed a similar accumulation of HY-CD8 in these tissues (Figure 4C). However, 21 days following tumor challenge (Day +42 post-BMT), there were significantly higher numbers of HY-CD8 in the spleen of [M→F], compared to [F→M] recipients, regardless of their ability to control tumor (Figure 4C, top panel). Interestingly, numbers of HY-CD8 in the bone marrow remained high in all groups, and were not statistically different between groups (Figure 4C, bottom panel).
Unexpectedly, as shown in figure 4D, PD-1 was uniformly expressed on CD8a+CD45.2+ HY-CD8 recovered from the spleen and bone marrow of tumor-bearing [F→M], tumor-bearing [M→F] and tumor-free [M→F] (Day 21 post-tumor challenge, Day +42 post-BMT), suggesting that PD-1 expression did not correlate with the ability to reject tumor. To determine the ability of adoptively transferred HY-CD8 to respond to HY independent of the allogeneic environment, live, CD8a+CD45.2+ bone marrow cells were flow sorted in a separate experiment 14 days after adoptive transfer, placed in culture, stimulated with UTY for 72 hours and proliferation measured by CFSE dilution. CD8a+CD45.2+ cells from [M→F] bone marrow did proliferate, while those sorted from [F>M] bone marrow did not (Figure 4E) indicating that bone marrow expression of HY impaired the functionality of HY specific T cells. PDL-1 (B7:H1), the ligand for PD-1, was not expressed on MB49 either prior to injection or on established tumors in mice (Figure 4F). Collectively, these results suggest that PD1 expression on T cells and PD-1 ligation was not required for loss of HY-CD8 tumor control, and that PD-1 expression may instead represent a nonspecific activation marker, perhaps due to T-cell activating allogeneic effects.
HY expression on hematopoietic tissues results in poor antileukemia responses, despite continued expansion of HY-CD8 in the bone marrow
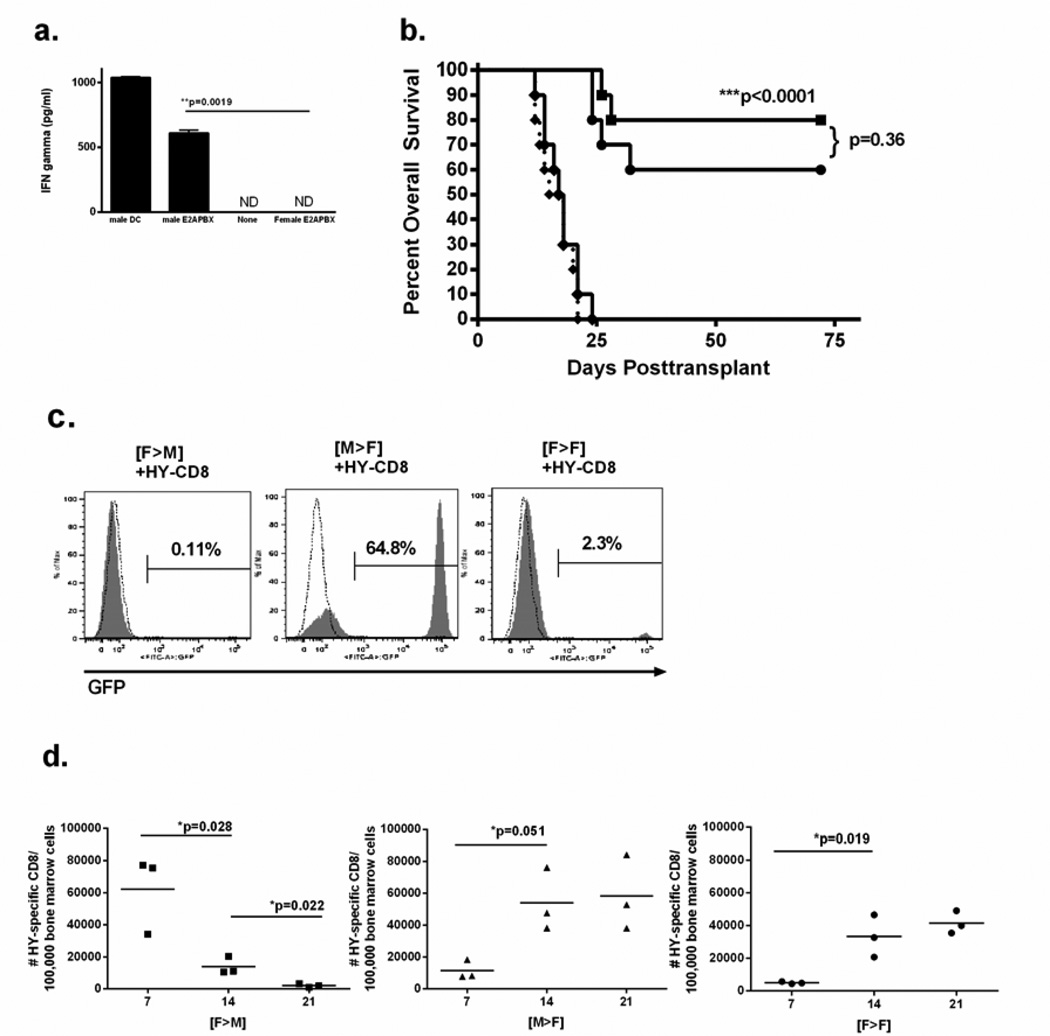
We next determined whether hematopoietic restriction of HY could also preserve antileukemia responses against a GFP-conjugated, murine pre-B cell acute lymphoblastic leukemia derived from male mice carrying the human E2A-PBX transgene (E2APBX-ALL) [24]. To establish that this male-derived E2APBX-ALL cell line expressed immunologically significant HY, we cocultured HY-CD8 for 72 hours with male or female E2APBX-ALL in a 1:1 ratio and measured interferon-gamma production by ELISA. Coculture of HY-CD8 with female E2APBX-ALL did not produce detectable interferon gamma levels, while coculture with male E2APBX-ALL resulted in substantial interferon gamma production at 72 hours (607 ± 26.1 pg/ml, p=0.0019, Figure 5A). This confirmed HY-specific immunogenicity of the male E2APBX-ALL cells for incorporation into the alloHSCT model.
Figure 5. Hematopoietic restriction of HY does not preserve antileukemia responses, which are restored with broad expression of HY.
(A) Immunogenicity of a murine, male, GFP-conjugated pre-B cell ALL (E2APBX-ALL) was determined by coculturing 1×106 male vs female E2APBX with 1×10e6 MataHari splenocytes and measuring interferon gamma production by ELISA at 48 hours. Male dendritic cells and naïve MataHari splenocytes alone served as positive and negative controls, respectively. (B) Overall survival was measured after 1×105 E2APBX-ALL cells were incorporated into T-cell depleted intravenous grafts into ([F—M] solid square), ([M→F], solid diamond) and ([F→F], solid circle) recipients on Day 0, followed by adoptive transfer of HY-CD8 on Day +7. [F→F] who did not receive HY-CD8 (dotted line) served as negative controls. (C), The quantity of GFP+ cells in the bone marrow was assessed by flow cytometry to measure leukemia burden either at time of death or Day+72 posttransplant. (D) Number of bone marrow-infiltrating HY-CD8 (live, CD8+CD45.2+) on Day +7, +14 and +21 following T-cell depleted transplant and coinjection (intravenously) of 1×105 E2APBX-ALL cells. Data shown are representative of 3 independent experiments.
For the leukemia challenge study, 1×105 male E2APBX-ALL were injected intravenously at the time of T cell depleted BMT on Day 0 into [F→M], [M→F] and [F→F] recipients, followed by adoptive transfer of 1×107 HY-CD8 on Day +7. GFP+ leukemia burden in the bone marrow was assessed postmortem by flow cytometry. In contrast to the solid tumor model, [M→F] recipients suffered 100% leukemia-associated mortality by Day +25 post-BMT, at a rate identical to [M→F] recipients that did not receive HY-CD8 adoptive transfer (Figure 5B). [F→M] recipients, on the other hand, had 82.1 + 5.8% leukemia-free survival. As in the solid tumor model, [F→F] recipients also preserved antileukemia responses after adoptive transfer of HY-CD8, with 62 + 5.2% survival. Leukemia-associated mortality in [M→F] recipients was characterized by massive splenomegaly and a high burden (64.8 + 9.7%) of GFP+ leukemia in the bone marrow (Figure 5C) measured by flow cytometry at the time of death. [F→M] survivors had no or very low levels of detectable leukemia on Day +72 post-BMT (0.11 ± 0.83%), while [F→F] recipients had low levels of leukemia, with 2.2 ± 1.7% GFP+ cells in the marrow (Figure 5C). Achievement of leukemia control by Day +21 in [F→M] recipients was associated with a contraction of the HY-CD8 population in the bone marrow (Figure 5D) compared to persistence of HY-CD8 in [M→F] with poor leukemia control. In contrast to the solid tumor model, leukemia challenge was sufficient to expand HY-CD8 in [F→F] recipients, though to a lesser degree.
Restoration of leukemia control by broad HY expression is associated with a loss of PDL-1 expression on leukemia cells, despite continued expression of PD-1 on marrow-infiltrating HY-CD8
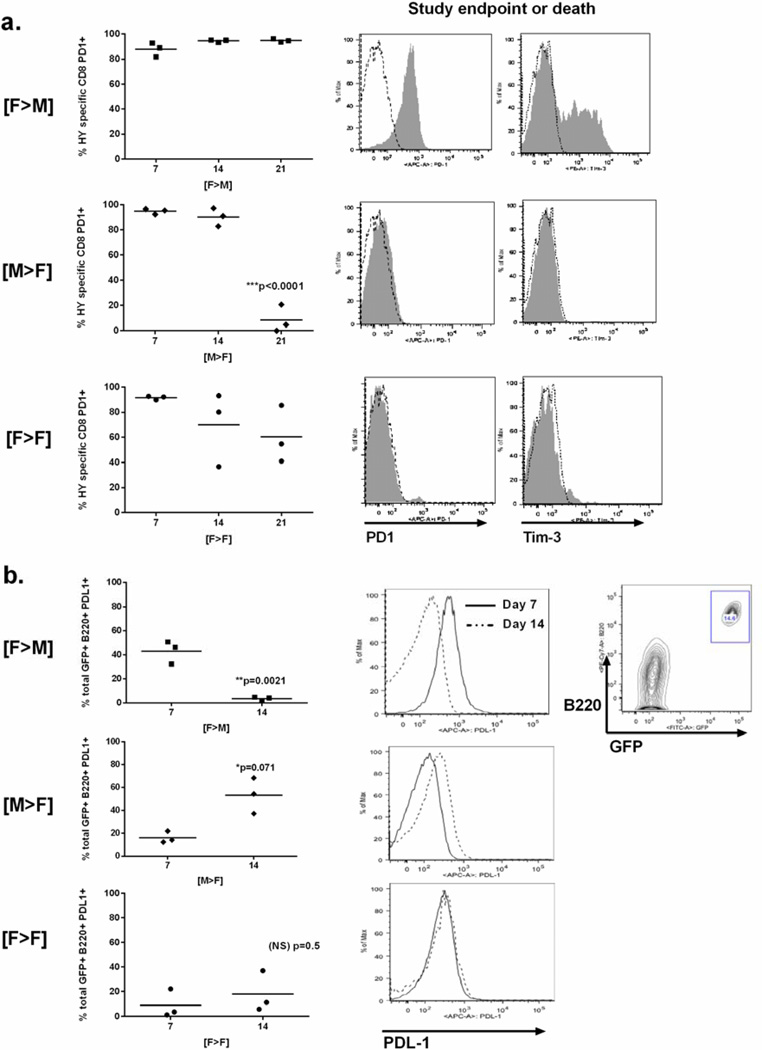
Next, we determined the PD-1 expression status of bone marrow infiltrating HY-CD8 following leukemia challenge from the time of engraftment (Day +7) to the time of either leukemia progression (in [M→F]) or control (in [F→M] and [F→ F]). Despite good antileukemia control and contraction of the HY-CD8 pool in [F→M] recipients, nearly 100% of HY-CD8 expressed PD-1 (Figure 6A, top row), and 43.7 ± 4.8 of PD-1+ cells coexpressed the negative costimulatory receptor Tim-3, required for PD-1 induced CD8 exhaustion in some models [29]. In [M→F] recipients where leukemia control is poor, HY-CD8 maintained high levels of PD-1 expression (Figure 6A, center row) until the time of leukemia-associated death. Unexpectedly, by this time, HY-CD8 had lost their PD-1 expression and did not coexpress Tim-3. [F→F] recipients expressed PD-1 in a high percentage of their cells early in engraftment (Day +7). Although some [F→F] lost PD-1 expression by Day +21, this did not correlate with loss or gain of leukemia control (data not shown) as in the setting of HY allogeneic mismatch.
Figure 6. Expression of PDL-1 on leukemia cells, but not PD-1 on HY-CD8, correlates with improved leukemia control when HY is broadly expressed.
(A). The percent of HY-specific CD8 (live, CD8+CD45.2) that were also PD1+ was measured by flow cytometry at Day +7, +14 and +21 following T-cell depleted transplant into [F>M], [M>F] and [F>F] recipients who simultaneously received 1×105 E2APBX-ALL cells on Day 0. PD-1 and Tim-3 expression (gray shaded) compared to IgG2k isotype controls was measure on live, CD8+CD45.2+ cells from the bone marrow was measured by flow cytometry at either study endpoint or at the time of death (left panels). Data shown are representative of 2 independent experiments. (B) Percentage of total GFP+B220+ E2APBX-ALL expressing PDL1 (right panels) was measured by flow cytometry on Day +7, +14 and +21 post-transplant and leukemia challenge (center panels). Representative histograms comparing PDL-1 fluorescence intensity on Day +7 (dotted lines) and +14 (solid lines) are also shown. A contour plots showing the gating strategy for identification of live, B220+GFP+ HY-E2APBX-ALL in the bone marrow is shown to the far right. Data shown are representative of 2 independent experiments.
Finally, we determined whether PD-1 ligand (PDL-1, or B7:H1) expression on HY-E2APBX leukemia cells correlated with maintenance or loss of PD-1 expression on HY-CD8. In two independent experiments, we observed that as leukemia control is achieved in [F→M] recipients, PDL-1 expression on live HY-E2APBX leukemia cells is lost despite continued expression of PD-1 on HY-CD8. Conversely, as leukemia control is lost in [M→F] recipients, the number of HY-E2APBX leukemia cells expressing PDL-1 increases despite loss of PD1 expression on HY-CD8.
Discussion
Endogenous minor histocompatibility antigens (miHA) remain ideal targets for adoptive immunotherapy due to their physiologic expression levels compared to antigens generated, or targeted, by exogenous gene modification. Since most miHA are not tumor-specific, we hypothesized that the antitumor reactivity of T-cells to endogenous antigens is directly affected by the relative expression of that antigen in malignant and nonmalignant tissues. The degree to which the pattern of nonmalignant tissue antigen expression impacts the functionality of miHA-directed responses against tumors that might share the antigen directly impacts which candidate antigens should be selected for adoptive therapy in the clinic. The ability of an experimental system to answer these questions preclinically required 1) controlling the degree of nonmalignant tissue miHA expression, 2) availability of T-cells specific for that miHA, and 3) tumors that endogenously express the miHA and are capable of eliciting miHA-specific responses. We devised an experimental system that satisfied these criteria, based on mismatch at the minor alloantigen complex HY.
The male minor antigen complex HY was selected as the model miHA in this system because it has been well-validated as a clinically relevant minor antigen in allogeneic transplant, capable of generating both GVHD and GVL [30]. HY disparity represents a mismatch at multiple genes, rather than a single MHC-associated locus, and thus may represents a more physiologic, and translatable, approach to antigen mismatch than artificially gene deficient mice [31]. In addition, HY is among the few miHA in which the immunodominant peptide complex is known, in mice and in humans, for which we, and others, have generated clinically significant immune responses against tumors that endogenously express HY [18,28]. Although we recognize that, due to the sex-specific expression of HY, there are clinical scenarios in which our data cannot be directly extrapolated, for example, the occurrence of a male/HY-expressing leukemia in a female recipient, these scenarios were required as experimental controls to isolate the key determinants of antigen-specific T-cell responses as a function of minor antigen distribution. Our observations based on this model, for example, that hematopoietic restriction of a miHA may induce local CD8 dysfunction and may not represent an ideal target antigen, can certainly be extrapolated to the selection of other miHA complexes being considered for therapeutic use.
We specifically observed that broad expression of HY in nonhematopoietic tissues produces poor responses to an HY-expressing solid tumor and is associated with significant accumulation of adoptively transferred HY-specific CD8 in secondary lymphoid tissues that express PD-1. This was not surprising, given the growing body of evidence that chronic, high levels of viral [32–34] or alloantigen [35] presentation can produce T-cell dysfunction. This observation is also consistent with reports by Meunier and colleagues [11], that broad tissue expression of minor antigens can generate large numbers of dysfunctional CD8 that are more susceptible to apoptosis. While our data show that these cells persist in relatively stable numbers 4 weeks following adoptive transfer, the fact that they cannot respond to repeat antigen stimulation ex vivo suggests a more terminal dysfunction that is not entirely dependent on the allogeneic environment. Further, our model indicates that professional HY antigen presentation in the form of an activated DC vaccine cannot overcome these effects, an observation that has clinical relevance for the incorporation of antigen-specific cancer vaccines into alloHSCT regimens.
Targeting hematopoietically restricted minor antigens has been proposed as an ideal strategy to optimize graft-versus-tumor (GVT) effects while sparing systemic GVHD [7, 36]. Asakura and colleagues have shown that GVT effects are augmented when target minor antigens are hematopoietically restricted, and can reverse poor responses induced by T-cells exposed to broadly expressed minor antigens [14]. In addition, Li and colleagues have demonstrated that adoptive transfer of memory CD8 from donors vaccinated against a restricted miHA can enhance GVT effects [10]. On this basis, we reasoned that hematopoietic restriction of HY might also improve poor HY-CD8 responses to MB49. Indeed, responses to MB49 were partially restored and HY-CD8 retained the ability to proliferate to HY ex vivo, despite continued expression of PD-1. We were able to preserve CD8 activity to a restricted miHA without prior donor vaccination, suggesting that prudent selection of minor antigens alone may maximize the benefit of adoptively transferred allogeneic T-cells without additional manipulations to the donor.
Restoration of responses to MB49 led us to question whether restriction of HY to hematopoietic tissues would also confer superior antileukemia responses. However, GVT effects were profoundly reduced in this setting despite continued presence of HY-CD8 in the bone marrow and loss of PD-1 expression, compared to superior responses when HY was broadly expressed. We therefore concluded that expression of a shared minor antigen in the same tissue compartment as the tumor (nonhematopoietic for solid tumors, hematopoietic for leukemia) predicts poor GVT responses. Conversely, minor antigens expressed outside the primary tumor compartment (hematopoietic for solid tumors, nonhematopoietic for leukemia) produced durable GVT responses.
In our model, this location-specific pattern of antigen expression was the primary determinant of antitumor CD8 activity, rather than degree of clinical GVHD, expansion of adoptively transferred T-cells or expression of PD-1. Although PD-1 has most recently been associated with T-cell dysfunction and immune regulation, it has also been described as a marked of T-cell activation [37,38] and we would expect HY-specific CD8 to be activated in the setting of HY-directed alloreactivity. The fact that PD-1 is not expressed on CD8+ T cells in the setting of poor antileukemia responses suggests that PD-1 biology may be uniquely modulated when alloimmunity is localized to a leukemia-bearing marrow environment.
Teshima and colleagues recently observed that PD-1 blockade partially reverses GVL suppression induced by epithelial expression of alloantigen and subsequent CD8 exhaustion and apoptosis [14]. These work is complemented by the observation, by WD Schlomchik and colleagues, that PD-1 expression is increased on effector memory (but not central memory) CD8 capable of tissue homing and expansion but not sustained GVHD or interferon production, suggesting a repertoire-independent functional defect in these cells, transgenic for a ubiquitously expressed minor antigen. [39]. While we also observe alloantigen-specific CD8 dysfunction associated with epithelial expression of HY in the solid tumor model, our leukemia model suggests that epithelial expression of alloantigen partially preserves GVL effects and PD-1 expression is insufficient to produce a dysfunctional phenotype.
Our leukemia challenge model indicates that continued presence of PDL-1 on lymphoblastic leukemia cells produces loss of leukemia control when the target miHA is hematopoietically restricted, an approach that was sufficient to reverse epithelial alloantigen-induced CD8 dysfunction in the solid tumor model. We therefore contend that blockade of PDL-1, alone or in conjunction with PD-1 blockade, would be required to completely reverse GVL suppression in this setting, and studies are ongoing to address this effect. Studies of PDL-1 blockade have been limited in preclinical ALL studies and represent the next logical step in understanding this phenomenon.
Further, we demonstrate a loss of PDL-1 expression on leukemia cells, despite ongoing expression of PD-1 on miHA-specific CD8, in the setting of broad antigen expression. It is intriguing to consider whether specific signals, from alloreactive CD8, the local environment, or both, may be capable of modulating PD-1/PDL-1 interactions [29,40] to abrogate immune escape. Our observations regarding the differential effects of alloantigen distribution, and CD8 reactivity, could be particularly useful in identifying critical “molecular switches” in this interaction that could be used to augment the efficacy of miHA-targeted adoptive therapy for acute lymphoblastic leukemia while avoiding exogenous gene modification of T-cells.
Acknowledgements
The authors thank Crystal Mackall and Daniel Fowler for their critical review of the data and manuscript drafts. The GFP-conjugated male E2APBX-ALL cell line was generously provided by Janet Bijil, Centre de Recherche de l’Hopital Maisonneuve-Rosemont, Montreal, Quebec, Canada. This work was supported by the NIH-T32 Training Grant “Laboratory Training in Pediatric Hematology-Oncology”, Principal Investigator Donald Small, Johns Hopkins University. The authors have no relevant financial conflicts or interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement: The authors have no financial conflicts of interest to declare.
Disclaimer: The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Miller JS, Warren EH, van den Brink MR, et al. NCI First International Workshop on the Biology, Prevention and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Biology Underlying Recurrence of Disease following Allogeneic HSCT. Biol Blood Marrow Transplant. 2010;16(5):565–586. doi: 10.1016/j.bbmt.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 3.Warlick ED, DeFor T, Blazar BR, et al. Successful remission rate and survival after lymphodepleting chemotherapy and donor lymphocyte infusion for relapsed hematologic malignancies postallogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:480–486. doi: 10.1016/j.bbmt.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett D, Fish JD, Grupp SA. Autologous and allogeneic cellular therapies for high-risk pediatric solid tumors. Pediatr Clin North Am. 2010;57:47–66. doi: 10.1016/j.pcl.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matte-Martone C, Venkatesan S, Tan HS, et al. Graft-versus-leukemia (GVL) against mouse blast-crisis chronic myelogenous leukemia (BCML) and chronic-phase chronic myelogenous leukemia (CP-CML): shared mechanisms of T cell killing but programmed death ligands render CP-CML, and not BC-CML GVL resistant. J Immunol. 2011;15:1653–1663. doi: 10.4049/jimmunol.1100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 7.Feng X, Kwok MH, Younes H, Brickner AG. Targeting minor histocompatibility antigens in graft versus tumor or graft versus leukemia responses. Trends Immunol. 2008;29:624–632. doi: 10.1016/j.it.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meunier MC, Delisle JS, Bergeron J, Rineau V, Baron C, Perreault C. T cells targeted against a single minor histocompatibility antigen can cure solid tumors. Nat Med. 2005;11:1222–1229. doi: 10.1038/nm1311. [DOI] [PubMed] [Google Scholar]
- 9.Fontaine P, Roy-Proulx G, Knafo L, Baron C, Roy DC, Perreault C. Adoptive transfer of minor histocompatibility antigen-specific T lymphocyte eradicates leukemia cells without causing graft-versus-host disease. Nat Med. 2001;7:789–794. doi: 10.1038/89907. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Matte-Martone C, Zheng H, et al. Memory T cells from minor histocompatibility antigen-vaccinated and virus-immune donors improve GVL and immune reconstitution. Blood. 2011;118:5965–5976. doi: 10.1182/blood-2011-07-367011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meunier MC, Roy-Proulx G, Labrecque N, Perreault C. Tissue distribution of target antigen has a decisive influence on the outcome of adoptive cancer immunotherapy. Blood. 2003;101:766–770. doi: 10.1182/blood-2002-04-1032. [DOI] [PubMed] [Google Scholar]
- 12.Zuniga EI, Harker JA. T-cell exhaustion due to persistent antigen: Quantity not quality? Eur J Immunol. 2012;42:2285–2289. doi: 10.1002/eji.201242852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flutter B, Edwards N, Fallah-Arani F, et al. Nonhematopoietic antigen blocks memory programming of alloreactive CD8+ T cells and drives their eventual exhaustion in mouse models of bone marrow transplantation. J Clin Invest. 2010;120:3855–3868. doi: 10.1172/JCI41446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asakura S, Hashimoto D, Takashima S, et al. Alloantigen expression on non-hematopoietic cells reduces graft-versus-leukemia effects in mice. J Clin Invest. 2010;120:2370–2378. doi: 10.1172/JCI39165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q, Munger MR, Veenstra RG, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YH, Faaij CM, van Halteren AG, et al. In situ detection of HY-specific T cells in acute graft-versus-host disease-affected male skin after sex-mismatched stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:381–387. doi: 10.1016/j.bbmt.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 17.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplant. Blood. 2004;103:347–352. doi: 10.1182/blood-2003-07-2603. [DOI] [PubMed] [Google Scholar]
- 18.Valjuskikh A, Lantz O, Celli S, Matzinger P, Heeger PS. Cross-primed CD8(+) T-cells mediate graft rejection via a distinct effector pathway. Nat Immunol. 2002;3:844–851. doi: 10.1038/ni831. [DOI] [PubMed] [Google Scholar]
- 19.Cooke KR, Kozbik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 88:3230–3239. [PubMed] [Google Scholar]
- 20.Capitini CM, Nasholm NM, Duncan BB, et al. Graft-versus-host disease impairs vaccine responses through decreased CD4+ and CD8+ T cell proliferation and increased perforin-mediated CD8+ T cell apoptosis. J Immunol. 2013;190:1351–1359. doi: 10.4049/jimmunol.1200391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu YF, Gavrilescu LC, Betancur M, Lazarides K, Klingemann H, Van Etten RA. Distinct graft-versus-leukemic stem cell effects of early or delayed donor leukocyte infusions in a mouse chronic myeloid leukemia model. Blood. 2012;119:273–284. doi: 10.1182/blood-2011-01-331009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fry T, Shand J, Milliron M, Tasian S, Mackall C. Antigen loading of DC’s with irradiated apoptotic tumor cells induces improved antitumor immunity compared with other approaches. Cancer Immunol Immunother. 2009;58:1257–1264. doi: 10.1007/s00262-008-0638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall C. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bijl J, Savageau M, Thompson A, Savageau G. High incidence of proviral integrations in the Hoxa locus in a new model of E2a-PBX1-induced B-cell leukemia. Genes Dev. 2005;19:224–233. doi: 10.1101/gad.1268505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toubai T, Tawara I, Sun Y, et al. Induction of acute GVHD by sex-mismatched H-Y antigens in the absence of functional radisensitive host hematopoietic-derived antigen presenting cells. Blood. 2012;119:3844–3853. doi: 10.1182/blood-2011-10-384057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capitini CM, Herby S, Milliron M, Anver M, Mackall CL, Fry TJ. Bone marrow deficient in IFN-y selectively reverses GVHD-associated immunosuppression and enhances a tumor-specific GVT effect. Blood. 2009;113:5002–5009. doi: 10.1182/blood-2008-11-187385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy P, Negrin R, Hill GR. Mouse models of bone marrow transplantation. Biol Blood Marrow Transplant. 2008;14:129–135. doi: 10.1016/j.bbmt.2007.10.021. Erratum in: Biol Blood Marrow Transplant. 2008;14: 1317–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melchionda F, McKirdy MK, Medeiros P, Fry TJ, Mackall CL. Escape from immune surveillance does not result in tolerance to tumor-associated antigens. J Immunother. 2004;27:329–338. doi: 10.1097/00002371-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Norde WJ, Hobo W, van der Voort R, Dolstra H. Coinhibitory molecules in hematologic malignancies: targets for therapeutic intervention. Blood. 2012;120:728–736. doi: 10.1182/blood-2012-02-412510. [DOI] [PubMed] [Google Scholar]
- 30.Hobo W, Broen K, van der Velden WJ, et al. Association of disparities in known minor histocompatibility antigens with relapse-free survival and graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:274–282. doi: 10.1016/j.bbmt.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perreault C. In search of immunodominant minor histocompatibility antigens. Biol Blood Marrow Transplant. 2013;19:171–172. doi: 10.1016/j.bbmt.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Velu V, Kannanganat S, Ibegbu C, et al. Elevated expression levels of inhibitory receptor programmed death-1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81:5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richter K, Brocker T, Oxenius A. Antigen amount dictates CD8(+) T-cell exhaustion during chronic viral infection irrespective of the type of antigen presenting cell. Eur J Immunol. 2012;42:2290–2304. doi: 10.1002/eji.201142275. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed R. Restoring function in exhausted CD8 T-cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 35.Wang XN, Haniffa MA, Holtick U, et al. Regulatory T-cell suppression of CD8+ T-cell mediated graft-versus-host reaction requires their presence during priming. Transplantation. 2009;88:188–197. doi: 10.1097/TP.0b013e3181ac14ce. [DOI] [PubMed] [Google Scholar]
- 36.Warren EH, Fujii N, Akatsuka Y, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T-cells specific for minor histocompatibility antigens. Blood. 2010;115:3869–3878. doi: 10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hokey DA, Johnson BF, Smith J, et al. Activation drives PD-1 expression during vaccine-specific proliferation following lentivirus infection in macaques. Eur J Immunol. 2008;38:1435–1445. doi: 10.1002/eji.200737857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown KE, Freeman GJ, Wherry EJ, Sharpe AH. Role of PD-1 in regulating acute infections. Curr Opin Immunol. 2010;22:397–401. doi: 10.1016/j.coi.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juchem WK, Anderson BE, Zhang E, et al. A repertoire-independent and cell-intrinsic defect in murine GVHD induction by effector memory T cells. Blood. 2011;118:6209–6219. doi: 10.1182/blood-2011-01-330035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norde WJ, Maas F, Hobo W, et al. PD-1/PDL-1 interactions contribute to functional T-cell impairment in patients who relapse with cancer after allogeneic stem cell transplantation. Cancer Res. 2011;71:5111–5122. doi: 10.1158/0008-5472.CAN-11-0108. [DOI] [PubMed] [Google Scholar]