Significance
A prominent floral display may increase attractiveness to pollinators but also the risk of damage from herbivores. Here, we show experimentally that differences in the relative strength of interactions with grazers and pollinators could explain variation in selection on floral display among natural populations of an insect-pollinated primrose. In addition, we demonstrate that differences in selection translate into rather rapid changes in the genetic composition of local plant populations. The results indicate that interactions with mutualists and antagonists can drive adaptive differentiation not only across broad geographic scales but also among populations across relatively short distances.
Keywords: adaptive evolution, divergent selection, floral trait, herbivory, natural selection
Abstract
Spatial variation in the direction of selection drives the evolution of adaptive differentiation. However, few experimental studies have examined the relative importance of different environmental factors for variation in selection and evolutionary trajectories in natural populations. Here, we combine 8 y of observational data and field experiments to assess the relative importance of mutualistic and antagonistic interactions for spatial variation in selection and short-term evolution of a genetically based floral display dimorphism in the short-lived perennial herb Primula farinosa. Natural populations of this species include two floral morphs: long-scaped plants that present their flowers well above the ground and short-scaped plants with flowers positioned close to the ground. The direction and magnitude of selection on scape morph varied among populations, and so did the frequency of the short morph (median 19%, range 0–100%; n = 69 populations). A field experiment replicated at four sites demonstrated that variation in the strength of interactions with grazers and pollinators were responsible for among-population differences in relative fitness of the two morphs. Selection exerted by grazers favored the short-scaped morph, whereas pollinator-mediated selection favored the long-scaped morph. Moreover, variation in selection among natural populations was associated with differences in morph frequency change, and the experimental removal of grazers at nine sites significantly reduced the frequency of the short-scaped morph over 8 y. The results demonstrate that spatial variation in intensity of grazing and pollination produces a selection mosaic, and that changes in biotic interactions can trigger rapid genetic changes in natural plant populations.
Spatial variation in the intensity of biotic interactions is an integral part of the geographic mosaic model of coevolution (1, 2), and may result in divergent selection and the maintenance of genetic variation in traits influencing the strength and outcome of interactions (3, 4). However, few studies have presented quantitative estimates of spatiotemporal variation in selection on traits influencing the outcome of biotic interactions across more than a handful of populations. In plants, variation in the composition of the mutualist and antagonist assemblages may result in spatially varying selection on morphology, phenology, and life-history traits (e.g., 5–12). Of particular interest are traits such as floral display that may be subject to conflicting selection from mutualists and antagonists, and where the magnitude and direction of net selection should depend on the relative strength of these interactions (13–20).
Experimental manipulation of environmental conditions is a powerful approach to identify agents of selection and to determine the evolutionary consequences of changes in the selection regime (21, 22). Experimental manipulation of pollen deposition (6, 23, 24) and interactions with herbivores (25–28) can be used to assess the roles of pollinators and herbivores for patterns of selection. Conflicting selection on floral traits by pollinators and herbivores have been inferred in many systems (15, 19, 20), but no study has simultaneously manipulated the intensity of both interactions to determine their relative importance for spatiotemporal variation in selection on plant traits. There is also a lack of studies experimentally examining the importance of biotic interactions for the evolutionary trajectories of natural plant populations (28, 29).
Here, we combine long-term observational data and field experiments to examine causes and consequences of spatial and temporal variation in selection on floral display in the rosette-forming, short-lived, perennial herb Primula farinosa. This species offers an ideal system to examine the outcome of conflicting selection by mutualists and antagonists. It is dimorphic for scape length, with a long-scaped morph displaying the umbellate inflorescence well above the soil surface and a short-scaped morph with the inflorescence very close to the ground. The segregation of scape morphs in controlled crosses is consistent with scape morph being determined by a single biallelic locus with a dominant allele coding for short scape (SI Text, SI Segregation of Scape Morphs in Crosses and Table S1). This difference in floral display affects interactions with both pollinators and antagonists. In previous studies, we have shown that seed production in the long-scaped morph is less likely to be limited by pollen availability (14, 30, 31), whereas the short-scaped morph is less frequently attacked by seed predators (14, 18, 32, 33). The inflorescence of the long-scaped morph should also have a higher probability of being damaged by grazers compared with that of the short-scaped morph. These interactions influence plant fitness largely via fruit production, which is a key fitness component of the study species and straightforward to quantify. In P. farinosa populations in the study area, plant mortality is high, overall fitness is strongly influenced by successful seedling recruitment (34), and total seed production is significantly correlated with number of intact mature fruits produced (r = 0.838, n = 442).
We documented variation in scape morph frequencies among 69 populations and asked the following questions (1): Does selection on scape length vary among populations and years? We quantified selection on scape morph in about 40 populations in each of 2 y, and in five populations across 5 y (2). What are the drivers of variation in selection on scape morph? We documented the relationship between grazing intensity and selection on scape morph, and with a field experiment, we tested the hypothesis that spatial variation in grazing pressure and pollination intensity cause among-population variation in selection on scape morph (3). Do among-population differences in selection result in different evolutionary trajectories? We used observational data to examine whether changes in scape morph frequencies were correlated with estimates of selection on scape morph, and an 8-y field experiment to test whether the exclusion of grazers resulted in a reduced frequency of the short-scaped morph.
Results
Morph Frequency Variation.
The proportion of the short-scaped morph varied from zero to 100% (median 19%) among 69 P. farinosa populations within a 4 × 10-km large area on the island Öland, southeastern Sweden (Fig. 1).
Fig. 1.
(Lower) Frequency distribution of the proportion of the short-scaped morph in 69 populations of P. farinosa on Öland, southeastern Sweden in 2001. (Upper) The photos show the long scaped morph (Left) and the short-scaped morph (Right). Photo: J.Å.
Spatiotemporal Variation in Selection on Floral Display.
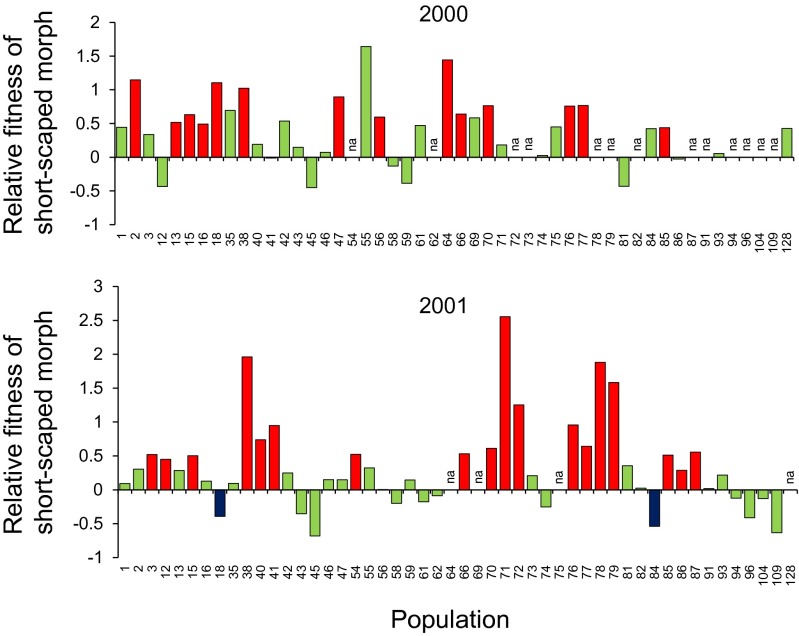
The relative fitness of the two scape morphs varied from a twofold advantage of the long-scaped morph to a 13-fold advantage of the short-scaped morph among the populations for which fecundity data were available from 2000 and/or 2001 (Fig. 2; significant scape morph × population interaction in mixed-model ANOVA of variation in number of mature fruits produced, 2000, χ2 = 23.1, df = 1, P < 0.0001; 2001, χ2 = 23.1, df = 1, P < 0.0001, Table S2). Contrasts indicated that fitness was significantly higher in the short-scaped morph in 14 of 37 populations in 2000, and in 18 of 46 populations in 2001; the reverse was true in 2 populations in 2001 (Fig. 2). The overall higher fecundity of the short-scaped morph was mainly the result of morph-specific differences in fruit set (number of mature fruits per flower). The relative fitness of the short-scaped morph was strongly correlated with its relative fruit set (number of mature fruits per flower) in both years (2000, r = 0.92, n = 37; 2001, r = 0.95, n = 46), and weakly to moderately correlated with differences in relative number of flowers (2000, r = 0.14; 2001, r = 0.59; Figs. S1 and S2).
Fig. 2.
Relative fitness of the short-scaped morph (ln[mean number of intact mature fruits produced by short-scaped plants divided by mean number of intact mature fruits produced by long-scaped plants]) in P. farinosa populations on Öland, southeastern Sweden in 2000 (n = 37 populations) and 2001 (n = 46 populations). Populations in which the difference in fruit production between scape morphs was statistically significant (according to contrasts in mixed-model ANOVA) are indicated in red (S > L) and blue (S < L); statistically nonsignificant differences are indicated in green.
Five of the populations were sampled over 5 y. In this set of populations, the relative fitness of the of the two scape morphs varied among years, and this variation was not synchronous across populations (Fig. 3). The effect of the scape morph × population × year interaction on number of intact mature fruits was statistically significant in a mixed-model ANOVA (χ2 = 14.6, df = 1, P < 0.001), whereas effects of two-way interactions or main effects were not significant (P > 0.05). The mean relative fitness of the short-scaped morph over the 5-y period was positive in all five populations (range 0.047–0.485, n = 5) indicating selection favoring the short morph, but the direction of selection on scape morph varied among years in four of the populations (Fig. 3).
Fig. 3.
Spatiotemporal variation in relative fitness of the short-scaped morph in five P. farinosa populations sampled over 5 y.
Agents of Selection.
The short-scaped morph was less damaged by grazers than was the long-scaped morph, and the relative fitness of the short morph was positively related to grazing intensity. The proportion of plants that had their inflorescence grazed was 5.4 times higher in the long-scaped morph in 2000 (20.9% vs. 3.9%, paired test, t = 6.3, P < 0.0001, n = 37 populations), and 4.6 times higher in 2001 (18.2% vs. 3.9%, t = 4.4, P < 0.0001, n = 46 populations). The relative fitness of the short-scaped morph was positively related to the grazing intensity (quantified as the proportion of long-scaped plants grazed) in 2000 (linear regression coefficient, b = 1.193, P = 0.007, R2 = 0.17, n = 37 populations), in 2001 (b = 2.164, P < 0.0001, R2 = 0.59, n = 46 populations), and in the five populations sampled over 5 y (b = 1.767, P = 0.010, R2 = 0.22, n = 25 population × year combinations).
A field experiment replicated in four other populations demonstrated that interactions with mammalian grazers and insect pollinators explained almost all variation in selection on scape morph. For unmanipulated control plants, the relative fitness of the short-scaped morph varied from -0.580 to 1.679 among populations (i.e., from a 1.7-fold advantage of the long-scaped morph to a 5.3-fold advantage of the short-scaped morph; Fig. 4A). Outside exclosures, the proportion of plants whose inflorescence was removed by grazers varied among populations (range 0.12–0.63; F3,7 = 40.6, P < 0.0001), was between 1.5 and 4.0 times higher in the long- than in the short-scaped morph (median 2.7 times higher; effect of morph, F1,7 = 44.1, P = 0.0003; population × morph interaction, F3,7 = 9.5, P = 0.0074), but was not affected by supplemental hand-pollination (P > 0.27). Exclusion of mammalian grazers reduced the relative fitness of the short-scaped morph (ANOVA, F1,9 = 9.5, P = 0.01), whereas hand-pollination increased the relative fitness of the short-scaped morph (F1,9 = 5.7, P = 0.04; the grazing × pollination treatment interaction was not statistically significant, F1,9 = 0.6, P = 0.46; Fig. 4 B and C). Among plants protected from grazers and provided with a surplus of pollen, there was no significant selection on scape morph (Fig. 4D). The proportion of fruits consumed by seed predators was generally low (median 6%), varied among populations (F3,25 = 7.6, P = 0.0009) and scape morphs (F1,25 = 9.5, P = 0.0049), but was not affected by experimental treatments (P > 0.18).
Fig. 4.
Effects of grazer exclusion and supplemental hand-pollination on relative fitness of the short-scaped morph of P. farinosa in a field experiment conducted in four populations: (A) control, (B) grazers excluded, (C) plants receiving supplemental hand-pollination, and (D) grazers excluded and plants receiving supplemental hand-pollination. Population × treatment combinations for which the 95% confidence interval of relative fitness (estimated through bootstrapping 1000 times) did not overlap zero are indicated.
Evolution of Morph Frequencies.
Both observational and experimental data indicated that selection on scape morph result in rather rapid changes in morph frequencies. Scape morph frequencies were monitored in 24 of the 69 populations from 2006 to 2012, and over this 6-y period the short-scaped morph tended to increase in 19 populations and decrease in five (significant population × year interaction in ANCOVA; F23,120 = 2.7, P = 0.0002). Across all 24 populations, the short-scaped morph increased by on average 1% per year. In five populations observed over 8 y, the mean relative fitness of the short-scaped morph 2000–2004 was positively correlated with the change in the proportion of the short-scaped morph over the period 2000–2007 (Spearman rank correlation rs = 0.90, P = 0.037, n = 5; change in morph frequency quantified as the regression of proportion short-scaped plants on year). Removal of mammalian grazers from exclosures established in nine populations caused the mean proportion of the short morph to decrease from 0.48 to 0.36 between 2004 and 2012 (paired t test, t = 3.2, df = 8, P = 0.013), whereas in adjacent control plots, it remained essentially the same (0.50 vs. 0.48, t = 0.1, df = 8, P = 0.891; Fig. 5).
Fig. 5.
Change in the proportion of the short-scaped morph from 2004 to 2012 in control plots and exclosures established in nine populations of P. farinosa. Difference in change between the two treatments tested with paired t test.
Discussion
This study identified interactions with pollinators and grazers as major drivers of spatial variation in the direction of selection on floral display in P. farinosa, and showed that differences in selection regimes translated into differences in the evolutionary trajectories of local populations. The experimental results suggest that grazing avoidance is a major factor favoring the short-scaped morph whereas pollinator attraction favors the long-scaped morph, and that shifts in the relative strength of these interactions change the direction of selection and trigger rapid changes in the genetic composition of local populations.
Spatial variation in the direction of selection should contribute to the maintenance of adaptive genetic variation among populations, and may in combination with gene flow also promote the maintenance of genetic variation within populations (cf. 35). In P. farinosa, selection favored the short-scaped morph over the long-scaped morph in many populations, but the reverse was true in some populations. Gene flow among P. farinosa populations subject to divergent selection may thus contribute to the maintenance of the dimorphism in floral display within populations. In addition, previous experiments have demonstrated negative frequency-dependent selection on scape morph mediated by pollinators within large patchy populations (18). Both variable selection among populations and frequency-dependent selection within populations may thus promote the persistence of the scape-length polymorphism in this system.
The field experiment identified pollinators and grazers as major agents of selection on floral display in P. farinosa, and showed that variation in net selection on scape length was largely the result of differences in the relative strength of selection mediated by these mutualists and antagonists. This study assessed the causes of selection on floral display by experimentally manipulating the interaction intensities with both mutualists and antagonists. Differential pollination success favored the long-scaped morph, whereas differential grazing damage favored the short-scaped morph. Pollinator-driven selection for tall plants has been demonstrated experimentally in previous studies of P. farinosa (14, 30, 31) and other animal-pollinated plants (e.g., 36, 37), and is likely to be related to the capacity of pollinators to detect inflorescences of different height and their willingness to forage close to the ground. The advantage of a long scape during pollination was counteracted by higher probability of grazing damage. Because grazers in this area remove the vegetation only above ca. 1–5 cm, the inflorescence of the short-scaped morph runs a lower risk of being eaten than does that of the long-scaped morph. Grazer-mediated selection for short scape is consistent with positive correlations between plant stature and risk of grazing damage in other systems (19), and the prevalence of prostrate growth forms in areas subject to high grazing pressure (38). In line with previous studies (18, 32), the long-scaped morph lost a higher proportion of fruits to seed predators compared with the short-scaped morph in the field experiment. However, in the experiment differential seed predation was not large enough to outweigh the advantage of the long-scaped morph when grazers were excluded (Fig. 4B), or to result in a clear advantage of the short-scaped morph when plants were pollinated by hand and grazers excluded (Fig. 4D). Taken together, the results suggest that among-population variation in direction and strength of selection on scape morph to a large extent can be attributed to spatial variation in the balance between selection exerted by pollinators and grazers.
It is likely that the fitness advantage of the short-scaped morph observed in many populations would be less strong if also male reproductive success had been considered. Flower production did not differ between scape morphs, except in one population (population 81 in 2001; Fig. S1). Because seed predation and most grazing damage occurs only after flowering, these interactions should influence relative male reproductive success very little, and interactions with pollinators are, if anything, likely to favor the long morph also through male function.
We cannot completely rule out that the scape morphs differ also in other components of fitness. However, available evidence suggests that selection on scape morph mainly acts through differential reproductive success. Consistent with common-garden experiments (39), no significant difference between scape morphs in mortality of flowering plants (Table S3), or in age at first reproduction [mean = 1.2 y after germination for both long-scaped (n = 195), and short-scaped plants (n = 55)] was recorded in a demographic study conducted in three populations in the study area (34).
A key finding of the present study was that differences in selection regime were associated with rapid changes in the genetic composition of natural populations and in field experiments. Changes in morph frequencies in natural populations across 8 y were correlated with the mean relative fitness of the short-scaped morph over a 5-y period. Moreover, exclusion of grazers increased the relative fitness of the long-scaped morph and had after 8 y resulted in a 10% decrease in the proportion of the short-scaped morph. High mortality and fast development from seedling to flowering individual in P. farinosa populations in the study area should have promoted the rapid changes in morph frequencies. In three populations monitored for 7 y (34), mortality among vegetative and reproductive plants combined ranged from 9.9% to 100% (median 61.9%; n = 18 annual transitions). Of seedlings surviving to the reproductive stage, 85% had begun flowering the year after germination (n = 250), and all had done so by the third year after germination (data extracted from the study reported in ref. 34). Geographic variation in biotic interactions has been hypothesized to drive population differentiation in plant traits influencing interaction strength and outcome (2, 12, 40). The present study provides experimental evidence for a causal link between selection mediated by biotic agents and microevolutionary change in natural plant populations.
The field experiment identified grazers and pollinators as major agents of selection, suggesting that factors governing the relative abundances of mutualists and antagonists are likely to influence the evolutionary trajectories of P. farinosa populations. The results are consistent with a large literature indicating the importance of interactions with pollinators (41, 42) and herbivores (5, 43) for the evolution of plant traits, but they also provide a striking example of how land use can influence the evolutionary dynamics of plant populations. Deer and moose may graze P. farinosa, but cows, horses, and sheep are currently the main grazers in the study area. The grazing pressure in the alvar grasslands is thus largely determined by management decisions. The observation of selection favoring the short-scaped morph in many populations and the increase in the proportion of short-scaped plants in most of the 24 populations monitored over 6 y is likely the result of the recent increase in grazing pressure in the area. Grazing intensity has increased considerably during the last 15 y. The current high grazing pressure is thus a relatively recent feature of the area, which may explain why the long-scaped morph still dominates in most populations. The experimental results imply that land use policies may strongly influence the selection regime and evolutionary trajectories in seminatural grasslands, and illustrate the need to consider the effects of management not only for species conservation but also for the maintenance of adaptive genetic variation.
A comprehensive understanding of selection requires that both the targets and agents of selection are identified, and the genetic basis of adaptive variation documented (4, 22). Here we have experimentally both identified the drivers of spatial variation in selection among natural populations and demonstrated how this variation translates into changes in the genetic composition of local populations. Divergent selection on floral display in the primrose studied was largely explained by spatial variation in the relative strength of interactions with mutualist pollinators and antagonist grazers, and manipulation of the selection regime resulted in rather rapid changes in scape morph frequency. The results indicate that interactions with mutualists and antagonists can drive adaptive differentiation not only across broad geographic scales (2, 11, 12), but also among plant populations across relatively short distances. This kind of information is fundamental to link environmental heterogeneity to mosaic selection and adaptive evolution.
Materials and Methods
Study System.
P. farinosa L. (Primulaceae) is a hermaphroditic, self-incompatible, distylous perennial herb found primarily in moist meadows on calcareous ground (44). It is distributed in Europe from central Sweden and Scotland to central Spain and Bulgaria (45). On the island Öland, off the southeastern Swedish coast, it occurs as two distinct scape morphs (46). The long-scaped morph produces a 3- to 30-cm scape with a smooth surface, whereas the short-scaped morph produces a 0- to 3-cm short, thick and striated scape. In the population survey conducted in 2001, we determined the scape length of 2,442 plants at fruit maturation, and the overlap in scape length was small between plants classified as short- and long-scaped, respectively. Ninety-nine percent of all long-scaped plants had a scape length of 30 mm or longer (mean ± SD, 101.4 ± 36.9 mm, n = 1318), whereas 99% of all short-scaped plants were shorter than 30 mm (9.7 ± 6.7 mm, n = 1124; Fig. S3). Flowers are arranged in an umbel at the top of the scape, and flowering takes place in May. In the study area, butterflies (especially Pyrgus malvae) and solitary bees (especially Osmia bicolor) are the main pollinators. The tortricid moth, Falseuncaria ruficiliana, is the main seed predator, and wild (deer and moose) and domesticated (cattle, sheep, and horses) grazers may consume the entire inflorescence.
Morph Frequency Variation.
In 2001, we documented the proportion of long- and short-scaped plants in 69 P. farinosa populations in a 4 × 10 km large area in the northern part of the Great Alvar on Öland, southeastern Sweden. A population was operationally defined as a group of plants that was isolated from its closest conspecific by at least 50 m. In populations that included fewer than 200 flowering plants, the scape morph was recorded for all plants. In larger populations, we recorded the number of long- and short-scaped plants in transects established across the population. Sample sizes ranged from 4 to 408 (median = 207).
Selection on Scape Morph and Changes in Scape Morph Frequencies in Natural Populations.
In populations with at least 10 flowering plants of each morph, we determined the relative fitness of the two morphs. We marked up to 30 short-scaped and 50 long-scaped plants during flowering in each of 37 populations in 2000 (2,576 plants in total) and 46 populations in 2001 (2,833 plants in total). At fruit maturation, we recorded the number of flowers produced and the number of intact mature fruits (not consumed by grazers or the seed predator) of all marked plants. In addition to intact fruits and fruits damaged by the seed predator, the pedicels and the dry remains of flowers that did not initiate fruit development are still present on the plants at the time of fruit maturation, making it possible determine total flower production. In five populations, flower and fruit production were estimated in the same manner over a period of 5 y (2000−2004) by marking a different set of flower-producing plants each year. We used mixed-model ANOVA to examine the effects of scape morph (fixed factor), population (random factor), and year (random factor; multiyear study only) on the number of intact mature fruits produced. The statistical significance of random factors was determined by using as test statistic the difference between the 2 log likelihood of the full model and that of a model from which the random factor of interest had been removed (47). To determine whether estimates of selection on scape morph were correlated with changes in morph frequencies over time, the frequency of the short-scaped morph in the five populations with estimates of fruit production from 5 y was monitored each year from 2000 until 2007. To determine whether any consistent change in scape morph frequencies could be observed across a wider sample of populations, we annually recorded scape morph frequencies in large permanent plots in 24 populations from 2006 to 2012 (sample sizes for individual population × year combinations ranged from 2 to 3,057, median 290). The plots ranged in size from about 100 m2 to 2000 m2 and included all or the majority of plants in each population. Trends in scape-morph frequency change were identified by regressing the proportion of the short-scaped morph (weighted by the square-root of the sample size) on year in a model that also included population and the population × year interaction as independent variables. In the study populations, scape morph varied independently of style morph. We had information about style morph for 71% of the plants scored in 2000 (n = 2,576), and for 42% of the plants scored in 2001 (n = 2,833). Preliminary analysis indicated no significant effect of style morph or its interactions with scape morph and population on fruit production (Table S4), and style morph was therefore not included in the final analysis. For each population and year, we quantified the relative fitness of the short-scaped morph as ln(mean number of intact mature fruits produced by short-scaped plants divided by mean number of intact mature fruits produced by long-scaped plants). Positive values indicate a selective advantage of the short-scaped morph, whereas negative values indicate the reverse. To quantify relative number of flowers per plant and relative fruit set (proportion of flowers developing into intact mature fruits) of the two scape morphs in each population, we similarly calculated the natural log of the ratio of the means for the short- and long-scaped morphs.
Field Experiment.
To assess experimentally the importance of interactions with grazers and pollinators for among-population variation in selection on scape morph and in scape morph frequencies, we established a field experiment in 2004. In each of nine P. farinosa populations located in the middle and southern parts of the Great Alvar on Öland, we established two plots, to which one of two experimental treatments was randomly assigned (exclosure vs. control). The size of the experimental plots varied, depending on the density and shape of the local population, from 70 m2 to 960 m2 (median 158 m2). Exclosures were fenced to exclude large mammalian grazers (120-cm tall fence with mesh size of 15 × 15 cm), and no damage from grazers was recorded inside exclosures during the experiment. Scape morph frequencies in control plots and exclosures were documented during flowering in 2004 and in 2012 by determining the scape morph of all flower-producing plants. In 2006, we added a hand-pollination treatment to the experimental design in four populations. In these populations, we marked up to 60 long- and 60 short-scaped plants in each experimental plot; half of the plants of each scape morph were randomly assigned to supplemental hand-pollination, while the remaining plants served as open-pollinated controls. Because pollinating short-styled (thrum) plants by hand without damaging the flower is difficult and very time consuming, only long-styled (pin) plants were included in the hand-pollination experiment. Populations were visited regularly during the flowering period to ensure that all flowers on plants in the pollination treatment received supplemental hand-pollination with compatible pollen during the period of stigma receptivity. At fruit maturation, we scored the number of flowers, the number of fruits damaged by grazers and seed predators, and the number of intact mature fruits produced by each plant and calculated the relative fitness of the short-scaped morph in the four treatment combinations of each population as described above. We used ANOVA to examine the effects of population, grazing treatment (exclosure vs. control), pollination treatment (supplemental hand-pollination vs. open pollination), and the grazing × pollination treatment interaction on relative fitness of the short-scaped morph (n = 16), and to determine the effects of population, scape morph, pollination treatment, and the population × scape morph interaction on the proportion of plants outside exclosures whose inflorescence was removed by grazers (n = 16), and the effects of population, scape morph, grazing treatment, and pollination treatment on proportion of fruits consumed by the seed predator (analysis conducted on means calculated for each population × scape morph × grazing treatment × pollination treatment combination; n = 32). Less than half of the plants were subject to supplemental hand-pollination in the experiment conducted in 2006, and to minimize the effects of the experimental hand-pollination on the relative fitness of the two scape morphs in the experiment, fruits produced by hand-pollinated plants were removed from the plots. The proportion of hand-pollinated plants varied from 17% to 31% (median 27%; n = 4) in the control, and from 7% to 38% (median 30%) in the exclosures in the 2006 experiment. The hand-pollination treatment was conducted only in a single year, and should therefore have had a minimal impact on the evolution of scape morph frequencies in the experiment.
Supplementary Material
Acknowledgments
We thank Camille Madec, Didrik Vanhoenacker, Tove von Euler, and a large number of dedicated field assistants for help with data collection, and Martin Breed for R-script. This study was financially supported by grants from Formas and the Swedish Research Council (to J.Å. and J.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301421110/-/DCSupplemental.
References
- 1.Thompson JN. The Coevolutionary Process. Chicago: Univ Chicago Press; 1994. [Google Scholar]
- 2.Thompson JN. The Geographic Mosaic of Coevolution. Chicago: Univ Chicago Press; 2005. [Google Scholar]
- 3.Schluter D. The Ecology of Adaptive Radiation. Oxford, UK: Oxford Univ Press; 2000. [Google Scholar]
- 4.Mitchell-Olds T, Willis JH, Goldstein DB. Which evolutionary processes influence natural genetic variation for phenotypic traits? Nat Rev Genet. 2007;8(11):845–856. doi: 10.1038/nrg2207. [DOI] [PubMed] [Google Scholar]
- 5.Marquis RJ. The selective impact of herbivores. In: Fritz RS, Simms EL, editors. Plant Resistance to Herbivores and Pathogens. Chicago: Univ Chicago Press; 1992. pp. 301–325. [Google Scholar]
- 6.Galen C. Rates of floral evolution: Adaptation to bumblebee pollination in an alpine wildflower, Polemonium viscosum. Evolution. 1996;50(1):120–125. doi: 10.1111/j.1558-5646.1996.tb04478.x. [DOI] [PubMed] [Google Scholar]
- 7.Nuismer SL, Thompson JN, Gomulkiewicz R. Coevolutionary clines across selection mosaics. Evolution. 2000;54(4):1102–1115. doi: 10.1111/j.0014-3820.2000.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 8.Herrera CM, Castellanos MC, Medrano M. In: Geographical context of floral evolution: Towards an improved research programme in floral diversification. Ecology and Evolution of Flowers. Harder LD, Barrett SCH, editors. New York: Oxford Univ Press; 2006. pp. 278–294. [Google Scholar]
- 9.Elzinga JA, et al. Time after time: Flowering phenology and biotic interactions. Trends Ecol Evol. 2007;22(8):432–439. doi: 10.1016/j.tree.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Siepielski AM, Benkman CW. Conflicting selection from an antagonist and a mutualist enhances phenotypic variation in a plant. Evolution. 2010;64(4):1120–1128. doi: 10.1111/j.1558-5646.2009.00867.x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson B, Alexandersson R, Johnson SD. Evolution and coexistence of pollination ecotypes in an African Gladiolus (Iridaceae) Evolution. 2010;64(4):960–972. doi: 10.1111/j.1558-5646.2009.00880.x. [DOI] [PubMed] [Google Scholar]
- 12.Benkman CW, Smith JW, Maier M, Hansen L, Talluto MV. Consistency and variation in phenotypic selection exerted by a community of seed predators. Evolution. 2013;67(1):157–169. doi: 10.1111/j.1558-5646.2012.01736.x. [DOI] [PubMed] [Google Scholar]
- 13.Ehrlén J. Risk of grazing and flower number in a perennial plant. Oikos. 1997;80(3):428–434. [Google Scholar]
- 14.Ehrlén J, Käck S, Ågren J. Pollen limitation, seed predation and scape length in Primula farinosa. Oikos. 2002;97(1):45–51. [Google Scholar]
- 15.Strauss SY, Irwin RE. Ecological and evolutionary consequences of multispecies plant-animal interactions. Annu Rev Ecol Evol Syst. 2004;35:435–466. [Google Scholar]
- 16.Sandring S, Riihimäki M-A, Savolainen O, Ågren J. Selection on flowering time and floral display in an alpine and a lowland population of Arabidopsis lyrata. J Evol Biol. 2007;20(2):558–567. doi: 10.1111/j.1420-9101.2006.01260.x. [DOI] [PubMed] [Google Scholar]
- 17.Parachnowitsch AL, Caruso CM. Predispersal seed herbivores, not pollinators, exert selection on floral traits via female fitness. Ecology. 2008;89(7):1802–1810. doi: 10.1890/07-0555.1. [DOI] [PubMed] [Google Scholar]
- 18.Toräng P, Ehrlén J, Ågren J. Mutualists and antagonists mediate frequency-dependent selection on floral display. Ecology. 2008;89(6):1564–1572. doi: 10.1890/07-1283.1. [DOI] [PubMed] [Google Scholar]
- 19.Gómez JM, Perfectti F, Bosch J, Camacho JPM. A geographic selection mosaic in a generalized plant-pollinator-herbivore system. Ecol Monogr. 2009;79(2):245–263. [Google Scholar]
- 20.Armbruster WS, Lee J, Baldwin BG. Macroevolutionary patterns of defense and pollination in Dalechampia vines: Adaptation, exaptation, and evolutionary novelty. Proc Natl Acad Sci USA. 2009;106(43):18085–18090. doi: 10.1073/pnas.0907051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reznick DA, Bryga H, Endler JA. Experimentally induced life history evolution in a natural population. Nature. 1990;346(6282):357–359. [Google Scholar]
- 22.MacColl ADC. The ecological causes of evolution. Trends Ecol Evol. 2011;26(10):514–522. doi: 10.1016/j.tree.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Fishman L, Willis JH. Pollen limitation and natural selection on floral characters in the yellow monkeyflower, Mimulus guttatus. New Phytol. 2008;177(3):802–810. doi: 10.1111/j.1469-8137.2007.02265.x. [DOI] [PubMed] [Google Scholar]
- 24.Sandring S, Ågren J. Pollinator-mediated selection on floral display and flowering time in the perennial herb Arabidopsis lyrata. Evolution. 2009;63(5):1292–1300. doi: 10.1111/j.1558-5646.2009.00624.x. [DOI] [PubMed] [Google Scholar]
- 25.Mauricio R, Rausher MD. Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution. 1997;51(5):1435–1444. doi: 10.1111/j.1558-5646.1997.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 26.Stinchcombe JR, Rausher MD. Diffuse selection on resistance to deer herbivory in the ivyleaf morning glory, Ipomoea hederacea. Am Nat. 2001;158(4):376–388. doi: 10.1086/321990. [DOI] [PubMed] [Google Scholar]
- 27.Gómez JM. Herbivory reduces the strength of pollinator-mediated selection in the Mediterranean herb Erysimum mediohispanicum: Consequences for plant specialization. Am Nat. 2003;162(2):242–256. doi: 10.1086/376574. [DOI] [PubMed] [Google Scholar]
- 28.Agrawal AA, Hastings AP, Johnson MTJ, Maron JL, Salminen J-P. Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science. 2012;338(6103):113–116. doi: 10.1126/science.1225977. [DOI] [PubMed] [Google Scholar]
- 29.Turley NE, et al. Contemporary evolution of plant growth rate following experimental removal of herbivores. Am Nat. 2013;181(Suppl 1):S21–S34. doi: 10.1086/668075. [DOI] [PubMed] [Google Scholar]
- 30.Ågren J, Fortunel C, Ehrlén J. Selection on floral display in insect-pollinated Primula farinosa: Effects of vegetation height and litter accumulation. Oecologia. 2006;150(2):225–232. doi: 10.1007/s00442-006-0509-x. [DOI] [PubMed] [Google Scholar]
- 31.Vanhoenacker D, Ågren J, Ehrlén J. Spatio-temporal variation in pollen limitation and reproductive success of two scape morphs in Primula farinosa. New Phytol. 2006;169(3):615–621. doi: 10.1111/j.1469-8137.2005.01615.x. [DOI] [PubMed] [Google Scholar]
- 32.Vanhoenacker D, Ågren J, Ehrlén J. Spatial variability in seed predation in Primula farinosa: Local population legacy versus patch selection. Oecologia. 2009;160(1):77–86. doi: 10.1007/s00442-009-1287-z. [DOI] [PubMed] [Google Scholar]
- 33.Vanhoenacker D, Ågren J, Ehrlén J. Non-linear relationship between intensity of plant-animal interactions and selection strength. Ecol Lett. 2013;16(2):198–205. doi: 10.1111/ele.12029. [DOI] [PubMed] [Google Scholar]
- 34.Toräng P, Ehrlén J, Ågren J. Linking environmental and demographic data to predict future population viability of a perennial herb. Oecologia. 2010;163(1):99–109. doi: 10.1007/s00442-009-1552-1. [DOI] [PubMed] [Google Scholar]
- 35.Lenormand T. Gene flow and the limits to natural selection. Trends Ecol Evol. 2002;17(4):183–189. [Google Scholar]
- 36.O'Connell LM, Johnston MO. Male and female pollination success in a deceptive orchid, a selection study. Ecology. 1998;79(4):1246–1260. [Google Scholar]
- 37.Sletvold N, Ågren J. Pollinator-mediated selection on floral display and spur length in the orchid Gymnadenia conopsea. Int J Plant Sci. 2010;171(9):999–1009. [Google Scholar]
- 38.Diaz S, Noy-Meir I, Cabido M. Can grazing response of herbaceous plants be predicted from simple vegetative traits? J Appl Ecol. 2001;38(3):497–508. [Google Scholar]
- 39.Toräng P, Ehrlén J, Ågren J. Habitat quality and among-population differentiation in reproductive effort and flowering phenology in the perennial herb Primula farinosa. Evol Ecol. 2010;24(4):715–729. [Google Scholar]
- 40.Zangerl AR, Berenbaum MR. Increase in toxicity of an invasive weed after reassociation with its coevolved herbivore. Proc Natl Acad Sci USA. 2005;102(43):15529–15532. doi: 10.1073/pnas.0507805102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fægri K, van der Pijl L. The Principles of Pollination Ecology. Oxford, UK: Pergamon; 1979. [Google Scholar]
- 42.Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annu Rev Ecol Evol Syst. 2004;35:375–403. [Google Scholar]
- 43.Núñez-Farfán J, Fornoni J, Valverde PL. The evolution of resistance and tolerance to herbivores. Annu Rev Ecol Evol Syst. 2007;38:541–566. [Google Scholar]
- 44.Hambler DJ, Dixon JM. Primula farinosa L. J Ecol. 2003;91(4):694–705. [Google Scholar]
- 45.Tutin TG, Heywood VH, Burges NA, Valentine DH, editors. Flora Europaea. Vol 3. Cambridge, UK: Cambridge Univ Press; 1972. [Google Scholar]
- 46. Lagerberg T (1948) Vilda växter i Norden (Natur och Kultur, Stockholm, Sweden), 2nd Ed.
- 47.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Inst; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.