Abstract
Adults recovered from anorexia nervosa (AN) have altered reward modulation within striatal limbic regions associated with the emotional significance of stimuli, and executive regions concerned with planning and consequences. We hypothesized that adolescents with AN would show similar disturbed reward modulation within the striatum and the anterior cingulate cortex, a region connected to the striatum and involved in reward-guided action selection. Using functional magnetic resonance imaging, twenty-two adolescent females (10 restricting-type AN, 12 healthy volunteers) performed a monetary guessing task. Time series data associated with monetary wins and losses within striatal and cingulate regions of interest were subjected to a linear mixed effects analysis. All participants responded more strongly to wins versus losses in limbic and anterior executive striatal territories. However, AN participants exhibited an exaggerated response to losses compared to wins in posterior executive and sensorimotor striatal regions, suggesting altered function in circuitry responsible for coding the affective context of stimuli and action selection based upon these valuations. As AN individuals are particularly sensitive to criticism, failure, and making mistakes, these findings may reflect the neural processes responsible for a bias in those with AN to exaggerate negative consequences.
Keywords: Anorexia Nervosa, functional magnetic resonance imaging, reward, striatum, cingulate
1. Introduction
Anorexia nervosa (AN) typically has an onset during adolescence in females and is characterized by emaciation, an intense fear of gaining weight despite being underweight, and disturbed body image (American Psychiatric Association 2000). Genetic heritability accounts for ~50–80% of the risk of developing AN and contributes to the neurobiological factors underlying this illness (Kaye et al. 2009). There are few effective treatments that reverse core symptoms. Consequently, AN often has a chronic and relapsing course, with the highest death rate of any psychiatric illness.
Individuals with restricting-type AN tend to refuse food, are anhedonic, and find little in life that is rewarding aside from the pursuit of weight loss. Such behaviors have raised the possibility that there are intrinsic disturbances of reward or pleasure (Wagner et al. 2007), perhaps related to altered striatal dopamine function (Frank and Kaye 2005). The role of dopamine in reward processing is well established (Schultz 2006). Human neuroimaging studies show that a highly interconnected network of brain areas, including the dopaminergic midbrain and striatum as well as cortical regions such as the frontal lobes and amygdala, are involved in reward processing of both primary (i.e., pleasurable tastes) and secondary (i.e., money) reinforcers (O’Doherty 2004). Through a feed-forward series of nonreciprocal connections, dopamine-mediated information progresses from the limbic (ventral) to executive (dorsal central) to sensorimotor areas of the striatum (Martinez et al. 2003). The ventral limbic neural circuit, which includes, among other regions, the anterior ventral striatum and ventral anterior cingulate cortex (ACC), is necessary for identifying rewarding and emotionally significant stimuli and for generating affective responses to these stimuli (Phillips et al. 2003). A dorsal executive function neural circuit, which includes the dorsal caudate and dorsal ACC, is thought to modulate selective attention, planning, and effortful regulation of affective states. The sensorimotor circuit includes the posterior putamen and motor cingulate; this circuit is involved with movement and stimulus-response habits (Yin and Knowlton 2006). Together, these circuits code stimulus-reward value, maintain representations of predicted future reward and future behavioral choice, and transform decisions into motor output, thereby integrating and evaluating reward prediction to guide decisions. Striatal dysfunction may contribute to many behaviors seen in AN, including altered reward and affect, decision-making, executive control, and decreased food ingestion (Kaye et al. 2009).
Prior studies by our group have shown that adults recovered from AN (Wagner et al. 2007) exhibit a failure to differentiate feedback valence in ventral striatal regions and an exaggerated response to both reward and punishment in dorsal executive regions in a simple monetary choice feedback task (Delgado et al. 2000) relative to healthy comparison women. Findings in recovered adult AN may be confounded by years of malnutrition or treatment; alternatively, they may be traits of the disorder. The current study investigated AN when ill during adolescence. A replication of altered striatal response to reward and punishment in ill adolescent AN using the same monetary choice task previously employed in recovered adult AN (Wagner et al. 2007) would provide support for the notion that altered limbic and executive striatal processes may be trait related.
Recent studies have shown that adults with AN have structural (Friederich et al. 2012) and functional (Zastrow et al. 2009) alterations within the cingulate, a region that is strongly interconnected with the striatum (Haber and Knutson 2010) and may be subdivided based upon its response to feedback valence (Liu et al. 2011). Notably, Delgado et al. (Delgado et al. 2005) reported rostral ACC response in healthy volunteers to a probabilistic cue period as well as to feedback in a variant of the task presented herein. As voxelwise analyses of the monetary choice task have also reported anterior cingulate activation (Delgado et al. 2000; May et al. 2004; Wagner et al. 2007), we have included this area as a region of interest.
2. Methods
2.1. Participants
Twelve adolescent females aged 12–18 and meeting DSM-IV criteria for a diagnosis of restricting-type AN within six months of study participation and experiencing their first anorectic episode were recruited from local eating disorder (ED) treatment programs. Twelve age-matched healthy comparison adolescent (CA) females were recruited through local advertisements. Axis I diagnoses were made by clinicians with expertise in ED in children and adolescents; assessments included the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) (Sheehan et al. 2010) and a modified Module H (ED diagnosis) from the Structural Clinical Interview for DSM-IV Axis I Disorders (First et al. 1996) that included additional questions to further define ED characteristics. Exclusion criteria for all participants included: past history of alcohol or drug abuse or dependence three months prior to study; medical or neurologic concerns; and any condition contraindicative to MRI. Two participants with AN were on low-dose psychotropic medication (olanzapine). The CA and their first-degree relatives had no history of an ED. The study was conducted according to the Institutional Review Board regulations of the University of California, San Diego. Participants under the age of 18 gave written informed assent, and their parents gave written informed consent; participants aged 18 gave written informed consent. Participants completed other diagnostic and clinical assessments (Beck Depression Inventory [BDI], Temperament Character Inventory [TCI], State-Trait Anxiety Inventory [STAI], Yale-Brown Cornell Eating Disorder Scale) described elsewhere (Wagner et al. 2006), as well as the Similarities and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (Wecshler 1999) and the Reading subtest of the Wide Range Achievement Test Revision 4 (Wilkinson and Robertson 2006). Participants completed the Wisconsin Card Sorting Test (WCST) (Heaton 2005) at a separate session occurring, on average, 27 days (range: 1 – 102 days) prior to the imaging session. At the end of imaging procedures, one adolescent with AN was excluded due to equipment failure, and a second participant with AN was excluded due to motion artifact. Between group comparisons of assessment scores were performed using Student’s t-tests and assumed unequal variance. Effect sizes were computed as the standardized mean difference using Hedges’ g so as to account for bias caused by small sample size (Hedges 1985).
2.2. Experimental design
Participants performed a monetary guessing task during fMRI (Delgado et al. 2000; Wagner et al. 2007). The participant was instructed to guess whether the hidden number was less than or greater than 5. The card had an unknown value ranging from 1 to 9, with the number 5 excluded. The participant won $2.00 for each correct guess, lost $1.00 for each incorrect guess, and lost $0.50 if she failed to make a guess in the allotted time period. At the trial onset, a question mark appeared inside the card for 1 sec as a cue that the participant should make a guess. Following this, the hidden number was displayed for 500 ms, followed by a colored arrow for 500 ms, indicating a win (green up arrow), a loss (red down arrow), or a failure to respond (yellow down arrow). All visual information occurred during the first 2 sec of each trial followed by an intertrial interval of 12 sec. Participants were instructed that the computer selected numbers randomly, and that there was no way to know what number would be revealed. In reality, the paradigm randomly preselected a trial as a win or loss, and presented to the participant a number that was congruent with the outcome. A total of four blocks, each containing 26 trials (13 wins, 13 losses), was presented. Participants were compensated $25 upon completing the task.
2.3. MRI
Imaging data were collected with a 3T Signa Excite scanner (GE Medical Systems). FMRI was performed with gradient-recalled echoplanar imaging (TR=2000 ms, TE=30 ms, flip angle=80°, 64 × 64 matrix, ASSET factor=2, 40 2.6-mm ascending interleaved axial slices with a 0.4-mm gap, 186 volumes) (Kwong et al. 1992; Ogawa et al. 1992). The first four volumes of each run were discarded so as to discount T1 saturation. EPI-based field maps were acquired for correcting susceptibility-induced geometric distortions. A high resolution T1-weighted image (SPGR, TI=600 ms, TE=min full, flip angle=8°, 256 × 192 matrix, 170 1.2 mm contiguous slices) was obtained for subsequent spatial normalization.
2.4. Definition of anatomical regions of interest
2.4.1. Striatal regions of interest (ROIs)
Striatal ROIs were defined a priori based on known functional distinctions (Martinez et al. 2003). For the limbic circuit, the nucleus accumbens represented the ‘classic’ limbic striatum, although the ‘limbic’ striatum extends beyond this region (Haber and Knutson 2010). The executive (AKA cognitive) regions included the putamen anterior to the anterior commissure and both the anterior and posterior caudate nucleus, divided at the anterior commissure. The sensorimotor circuit included the putamen posterior to the anterior commissure. Consistent with Martinez (Martinez et al. 2003), some subtle anatomical distinctions within the striatum were neglected; for example, while the caudate is considered largely executive, a small dorsolateral portion is part of the sensorimotor circuit. The putamen has a small dorsolateral part rostral to the anterior commissure that is sensorimotor, whereas a ventromedial part of the putamen caudal to the anterior commissure is both executive and limbic. Given the resolution of BOLD fMRI images and the standard application of spatial smoothing, these ROIs were meant to serve as probabilistic functional distinctions rather than absolute representations of functional striatal subdivisions.
2.4.2. Cingulate ROIs
The anterior cingulate was based on the Harvard-Oxford atlas and was further divided into three subregions (Yucel et al. 2003). The rostral anterior cingulate, known to project to the limbic striatum (Haber and Knutson 2010), was distinguished from the cognitive zone of the dorsal anterior cingulate by drawing a 45 degree line from the anterior commissure. The cognitive zone of the dorsal anterior cingulate, which projects to executive striatal and prefrontal regions, was defined from this line to a line vertical to the anterior commissure. Finally, the motor zone of the dorsal anterior cingulate, which projects to the sensorimotor cortical areas as well as the putamen, was defined as the remaining portion of the original anterior cingulate mask.
2.5. Statistical analysis
Functional images were preprocessed and analyzed using Analysis of Functional NeuroImages (AFNI) software (Cox 1996) and R statistical packages (http://www.r-project.org). EPI images were motion-corrected and aligned to high-resolution anatomical images. Time points with isolated head movements not corrected by coregistration were censored from the statistical analysis. Statistical analyses were performed using a general linear model (GLM), with individual events modeled using AFNI’s TENT function. The TENT function is a piecewise linear spline that estimates an impulse response function and provides a method for visualizing and comparing the approximate shape of the hemodynamic response function. A canonical HRF model was run separately for presenting ROI results as barplots. Event-related signals for the win and loss conditions, calculated as beginning with the win or loss onset time and continuing at 2 sec intervals for 14 sec, were included as regressors of interest. Six motion parameters (translations and rotations) were included as covariates of no interest. Within-voxel auto-correlations of time-series data were controlled with AFNI’s 3dREMLfit. Given the potential for ventricular widening and sulcal atrophy due to malnutrition in the group with AN, registration to the MNI-152 atlas was performed using FMRIB’s Non-linear Image Registration Tool (FNIRT), part of FSL (http://fsl.fmrib.ox.ac.uk/fsl/). Functional data were scaled to percent signal change (PSC) and smoothed with a 4 mm FWHM Gaussian kernel. The PSC map for each individual was visually inspected for outliers before inclusion in group analyses.
In order to better account for morphometric variation (e.g., due to enlarged ventricular space in some participants with AN) and constrain extracted fMRI signal to gray matter tissue, we segmented each individual’s anatomical image into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) volumes using FMRIB’s Automated Segmentation Tool (FAST). ROIs applied to the fMRI data were constrained to exclude CSF or WM but retain GM. The mean PSC within each ROI for the events of interest at each timepoint (TR) was subjected to linear mixed effects (LMEs) analysis in R. Participant was treated as a random effect, with diagnosis (AN, CA) as the between-group factor, and condition (win, loss) and TR (0–7 TR) as within-group factors. Corrections for multiple comparisons used false discovery rate (FDR) control at q=0.05 (Benjamini and Hochberg 1995). Effect size was computed with Hedges’ g. A multiple regression approach was used, with age as a covariate to control for potential age-related confounds. Pearson product-moment correlation coefficients using the mean PSC and behavioral measures of interest, log transformed to reduce the influence of outliers, were computed to explore potential correlations. We also performed an exploratory voxelwise analysis. To guard against false positives, Monte-Carlo simulations using 3dClustSim indicated that clusters larger than 14 voxels (112 μL) at a threshold of p<0.05 (with a peak voxel of p<0.005) were considered significant.
Whole-brain voxelwise Huber robust regressions (Huber 1964) were conducted in R to examine the relationship between gray matter volume and task-related brain activation within the group with AN. The regression coefficients and their corresponding t values were split into positive and negative relationships with brain activity. Simulations using 3dClustSim determined clusters greater than 14 voxels at a threshold of p<0.05 (uncorrected) were considered significant.
3. Results
3.1. Demographics and clinical assessments
Individuals within the AN and CA groups were of similar age and intelligence (Table 1), but as expected, participants with AN had lower BMI (range: 13.1 – 18.2) and elevated measures of core ED symptoms compared with the CA group. There were some performance differences on the WCST: patients with AN (mean ± SD: 14.9 ± 7.0) committed more perseverative errors than CA (7.0 ± 2.1), indicating that the group with AN was less adaptive to cognitive shifts than the control group, t(10.3)=3.1, p=0.006, g=1.3. There was no difference between groups in the number of categories completed.
Table 1.
Clinical and demographic characteristics.
| AN (n=10) | CA (n=12) | Between Group Comparisons | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | df | t | p | Hedges’g | |
| Age (years) | 16.2 (1.8) | 15.4 (1.6) | 18.3 | 1.1 | 0.3 | 0.4 |
| Duration of illness (months) | 31.1 (26.1) | - | - | - | - | - |
| BMI | 16.4 (1.4) | 21.5 (1.7) | 20.0 | −7.6 | <0.0001 | −3.1 |
| IBW | 82.2 (6.2) | 105.8 (6.5) | 19.6 | −8.7 | <0.0001 | −3.6 |
| Age at menarche (years)a | 12.9 (1.5) | 12.2 (1.2) | 14.7 | 1.1 | 0.3 | 0.5 |
| BDIb | 19.6 (10.3) | 0.4 (0.9) | 8.1 | 5.6 | 0.0005 | 2.3 |
| Drive for thinness (EDI-2)c | 12.1 (6.6) | 0.0 (0.0) | 8.0 | 5.5 | 0.0006 | 2.3 |
| Body dissatisfaction (EDI-2)c | 10.6 (9.0) | 0.3 (0.9) | 8.1 | 3.4 | 0.009 | 1.4 |
| Perfectionism (EDI-2)d | 9.0 (6.3) | 3.8 (2.7) | 10.4 | 2.3 | 0.04 | 0.9 |
| Harm avoidance (TCI)e | 22.1 (7.3) | 10.2 (6.9) | 16.9 | 3.8 | <0.0001 | 1.6 |
| Trait anxiety (STAI)b | 55.0 (11.0) | 24.4 (4.2) | 9.9 | 7.9 | <0.0001 | 3.2 |
| WASI - Similarities | 55.8 (8.5) | 59.3 (8.6) | 19.4 | −1.0 | 0.3 | −0.4 |
| WASI - Matrix Reasoning | 54.2 (5.1) | 54.5 (4.7) | 18.6 | −0.1 | 0.9 | −0.1 |
| WCST - Perseverative Errorsf | 14.9 (7.0) | 7.0 (2.1) | 8.1 | 3.1 | 0.006 | 1.3 |
| WCST - Categories Completedf | 4.9 (1.8) | 5.9 (0.3) | 7.3 | −1.6 | 0.2 | −0.7 |
| WRAT4 - Reading | 68.8 (23.7) | 76.9 (21.5) | 18.5 | −0.8 | 0.4 | −0.3 |
Note: BDI: Beck Depression Inventory; BMI: body mass index; EDI: Eating Disorders Inventory; IBW:ideal body weight; STAI: State-Trait Anxiety Inventory; TCI: Temperament and Character Inventory; WASI: Wechsler Abbreviated Scale of Intelligence; WCST: Wisconsin Card Sorting Test; WRAT4: Wide Range Achievement Test Revision 4.
One AN, one CA were pre-menarche and were excluded from this measure;
two AN and one CA were missing responses for this measure;
one AN was missing responses on this measure;
one CA was missing responses on this measure;
one AN and one CA were missing responses on this measure;
two AN and two CA were missing responses for this measure, and one CA was excluded due to extreme scores.
3.2. Volumetric analysis
The groups did not differ on overall intracranial vault (ICV) volume after controlling for age. The group with AN (715,518 ± 54,606 mm3) showed smaller GM volume relative to CA (732,050 ± 47,085 mm3) after controlling for the effects of age and ICV volume, t(18)=2.4, p=0.03, g=0.4. The group with AN (348,642 ± 31,211 mm3) also had greater CSF volume relative to CA (317,164 ± 20,020 mm3), t(18)=2.2, p<0.05, g=1.1. White matter volume did not differ significantly between groups. Age approached significance as a predictor for GM (t(18)=−1.9, p=0.08, g=0.6) and CSF (t(18)=1.8, p=0.095, g=0.6) volumes.
3.3. Behavioral analysis
The mean reaction time for patients with AN (503.0 ± 50.0 ms) did not differ from CA (500.8 ± 65.1 ms), t(19.9)=−0.09, p=0.9, g=0.04. The number of high value guesses for the group with AN (53.9 ± 8.6) did not differ from CA (53.9 ± 5.9), t(15.4)= −0.01, p=1.0, g=0.0. Similarly, the number of low value guesses for participants with AN (45.8 ± 9.8) did not differ from CA (48.0 ± 6.7), t(15.4)= −0.6, p=0.6, g=−0.25. Comparisons of error rates in the group with AN (4.3 ± 4.2) and CA (2.1 ± 2.5) were not significant, t(14.01)=1.5, p=0.2, g=0.6.
There was a trend for adolescents with AN to make the same choice over multiple trials (0.5 ± 0.1) relative to CA (0.5 ± 0.1), t(18.97)= −1.9, p=0.07, g=0.8. We therefore examined whether participants were more likely to make the same choice as one previously rewarded (Win-Stay) or more likely to select the opposite choice in the case of a previous loss (Lose-Switch). The group with AN showed a tendency to employ a Win-Stay strategy (0.3 ± 0.1) relative to CA (0.2 ± 0.1), t(19.9)=1.8, p=0.09, g=0.7. The use of a Lose-Switch strategy in individuals with AN (0.3 ± 0.1) did not differ from CA (0.3 ± 0.1), t(17.9)= −0.7, p=0.5, g=−0.31.
3.4. ROI analyses
There was a main effect of condition detected in several of the striatal and cingulate ROIs. However, there were also several ROIs with a significant group by condition interaction, suggesting regional differences in how the two groups processed reward and punishment, as detailed below and summarized in Figure 1. The outcome of other main effects and interactions, most of which were not significant, are presented in the supplemental material (Table S1).
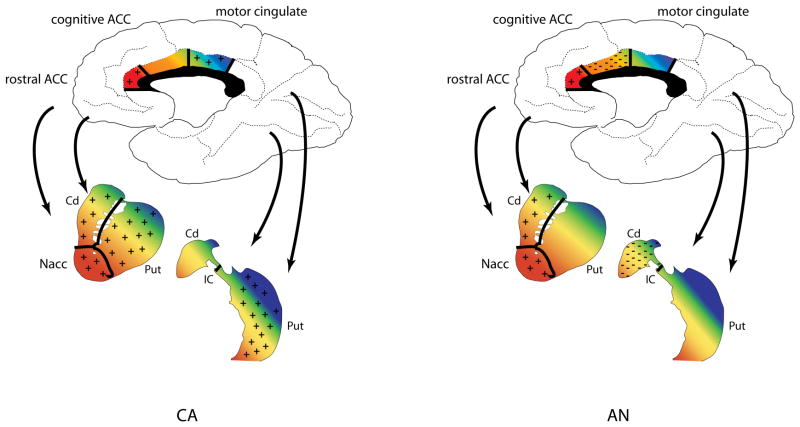
Figure 1.
Overview of striatal and cingulate regions of interest (ROI) projections and response patterns in left) CA and right) AN. Black lines within the ROIs demonstrate approximate boundaries of each ROI. Colors indicate functional connectivity between subregions of the cingulate with the striatum, with reddish areas mainly associated with limbic function, orange/yellow mainly with executive function, and green/blue mainly with motor function. Monetary wins, being appetitive, were considered rewarding; monetary losses were considered punishment, as they represented the loss of an appetitive stimulus following an incorrect choice. + symbol: region responded more strongly to wins than to losses; − symbol: region responded more strongly to losses than to wins; ACC: anterior cingulate cortex; AN: ill adolescent females with anorexia nervosa; CA: healthy comparison adolescent females; Cd: caudate; IC: internal capsule; Nacc: nucleus accumbens; Put: putamen.
3.4.1. Limbic system
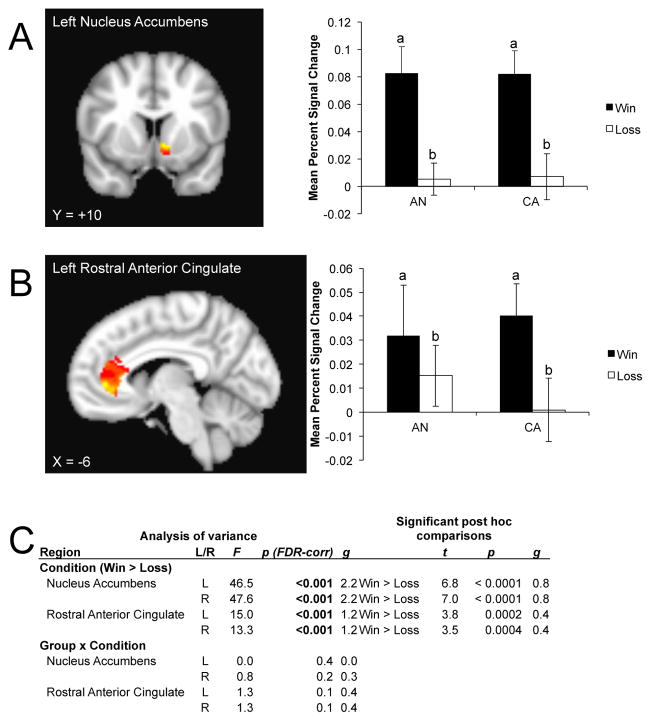
All limbic ROIs showed a main effect of condition (Figure 2). Within the bilateral nucleus accumbens, post hoc analyses indicated a stronger response to reward relative to punishment for all participants. This was also seen within the bilateral rostral ACC. No group x condition interaction was detected.
Figure 2.
Limbic regions of interest showing a significant main effect of condition (win > loss). For each region, the left panel shows the region of interest mask overlaying the voxelwise analysis for visualization purposes; the right panel shows the mean percent signal change calculated across the entire region of interest. A) Left nucleus accumbens; B) left rostral anterior cingulate. Hot colors indicate voxels reflecting a greater response to wins relative to losses within the regions of interest. C) Analysis of variance showing a significant main effect of condition and the post hoc comparisons revealing directionality of the effect within limbic areas. The group x condition interaction was not significant. Where different, lowercase letters on the barplot indicate a significant difference of mean percent signal for the main effect of win > loss. AN: ill adolescent females with anorexia nervosa; CA: control adolescent females; FDR: false discovery rate.
3.4.2. Executive system
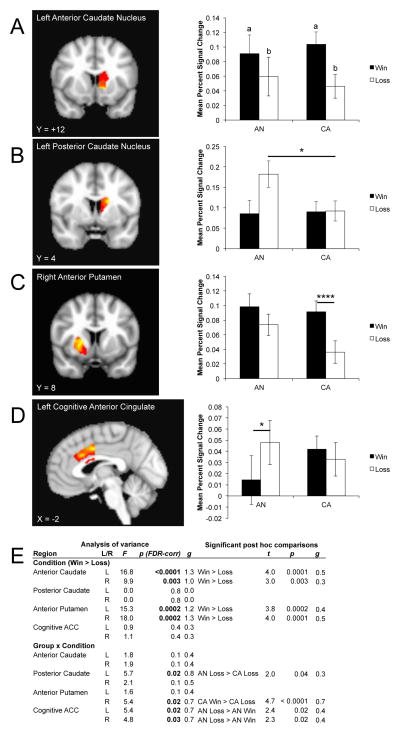
There was a dissociation between how the anterior and posterior regions of the executive ROIs responded to monetary feedback. Both the bilateral anterior caudate and anterior putamen demonstrated a main effect of condition; post hoc analyses indicated that for both regions (Figure 3) participants responded more strongly to reward than to punishment. There was also an interaction within the right anterior putamen, with CA responding more strongly to wins than losses. The left posterior caudate also showed a group by condition interaction: the group with AN was significantly more responsive to punishment than the CA group. The right posterior caudate was similar, but did not reach statistical significance. The bilateral cognitive ACC demonstrated a group by condition effect driven by a greater response to punishment relative to reward in participants with AN. The right cognitive ACC suggested a tendency for the group with AN to respond more strongly to punishment than CA.
Figure 3.
Anatomical location and mean percent signal change for executive regions of interest demonstrating either a main effect of condition or a group x condition interaction. A) Left anterior caudate nucleus; B) left posterior caudate nucleus; C) right anterior putamen; D) left cognitive anterior cingulate cortex. Hot colors indicate voxels reflecting a greater response to wins relative to losses (or the group x condition interaction if the main effect of condition was not significant) within the regions of interest. E) Table of significant analysis of variance main effects, interactions, and post hoc comparisons. Where different, lowercase letters on the barplot indicate a significant difference of mean percent signal for the main effect of win > loss. AN: ill adolescent females with anorexia nervosa; CA: control adolescent females; FDR: false discovery rate. *p < 0.05; ****p < 0.0001
3.4.3. Sensorimotor system
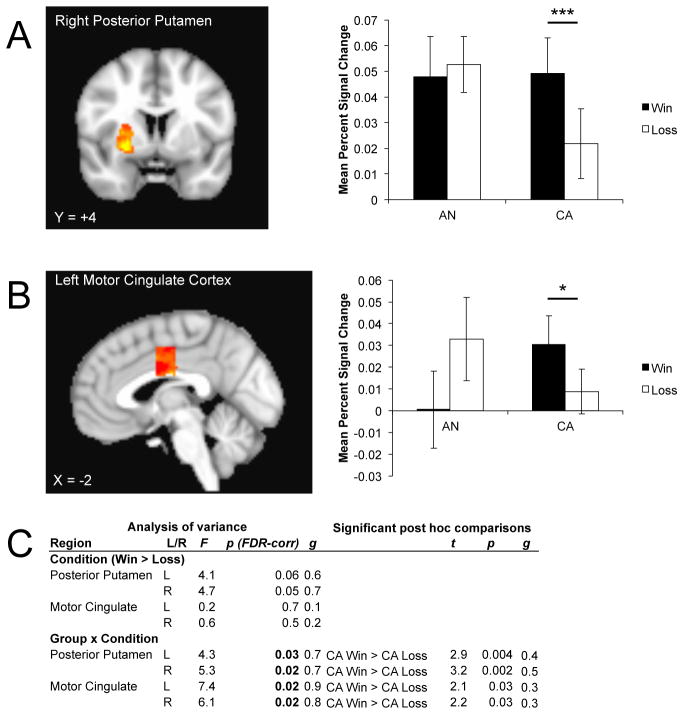
The bilateral posterior putamen showed a trend for condition (Figure 4). There was also a group by condition effect, driven by a greater response to reward relative to punishment in CA. The participants with AN did not differentiate between conditions, although there was a trend for the ill group, relative to CA, to respond to punishment in the right posterior putamen. The bilateral motor cingulate also showed a group by condition interaction; as seen in the posterior putamen, this was related to a greater response to reward relative to punishment only in the CA.
Figure 4.
Anatomical location and mean percent signal change within sensorimotor regions of interest demonstrating a group by condition interaction for A) right posterior putamen; B) left motor cingulate. Hot colors indicate voxels reflecting a greater response to the group x condition interaction within the regions of interest. C) Analysis of variance reported a lack of a main effect of condition within sensorimotor areas. The group x condition interaction, however, was significant within the bilateral posterior putamen and motor cingulate. Only significant post hoc comparisons are shown. AN: ill adolescent females with anorexia nervosa; CA: control adolescent females; FDR: false discovery rate. *p < 0.05; ***p < 0.005.
3.5. Voxelwise analysis
Voxelwise analyses revealed a main effect of group (Table S2) and condition (Table S3). There were also several significant interaction effects, including group by condition (Table S4). These are presented in the supplement.
3.6. Relationships to other measures
We conducted separate exploratory correlations between ROI BOLD response and the log transforms of harm avoidance, anxiety, WCST perseverative errors, and BMI for each group. None were significant after correction for multiple comparisons. Several comparisons, presented in the supplement, were significant at uncorrected p values.
4. Discussion
This is the first study to use positive and negative monetary feedback to examine reward processing in adolescents ill with AN. Relative to CA, participants with AN demonstrated an anterior to posterior transition from normal to abnormal response patterns within both the striatum and cingulate. Specifically, participants with AN showed normal responses in limbic and anterior executive striatal territories by responding more strongly to wins versus losses. However, in posterior executive and sensorimotor striatal regions, the group with AN tended to show an abnormal pattern, with an exaggerated response to losses compared to wins. These results may be consistent with data showing that adult (ill and recovered) individuals with AN are particularly sensitive to punishment (Jappe et al. 2011). In clinical terms, individuals with AN tend to be overly sensitive to negative consequences, such as criticism, making mistakes, or the thought of failure. The findings in this study may reflect the neural processes responsible for such behavior.
4.1. Limbic system
Both CA and participants with AN demonstrated an increased response to reward and a decreased response to punishment within the nucleus accumbens and the rostral anterior cingulate. Similar findings in the anteroventral striatum have been reported in healthy adults (Delgado et al. 2000; Wagner et al. 2007), children, and adolescents (May et al. 2004) and is in keeping with its general role in reward processing (O’Doherty 2004). The findings in the ill adolescent group, however, contrast with those for recovered AN adults (Wagner et al. 2007), who failed to show distinctions between reward and punishment in the anteroventral striatum. The reason for the normal limbic response in the adolescents ill with AN and abnormal limbic response in adults recovered from AN is not known. While other studies show that adults with AN have abnormal ventral striatum function (Cowdrey et al. 2011), this is the first study involving adolescents ill with AN and using a guessing paradigm of monetary wins and losses, raising the possibility that this finding may be related to differences due to recovery status and/or developmental stage of participants.
4.2. Executive system
While both CA and AN participants showed an enhanced response for reward relative to punishment in the anterior caudate and anterior putamen, participants with AN demonstrated an enhanced response for losses in posterior regions, particularly the posterior caudate, whereas CA did not differentiate outcome valence in this region. Differences between anterior and posterior striatal functional activity have been reported during instrumental learning or Pavlovian conditioning (Mattfeld et al. 2011) in healthy adults and may differentiate between encoding valence (anterior striatum) and association learning/feedback monitoring (posterior striatum). An interesting question in AN is whether this represents excessive activation to loss cues, or a failure to inhibit this region successfully. Participants with AN also demonstrated a greater response to punishment relative to reward within the cognitive ACC. The anterior cingulate plays an important role in conflict detection, and this may extend to encoding effort (Botvinick et al. 2004). Given that inflexibility and set-shifting impairment are consistent findings in AN (Tchanturia et al. 2012), including the AN participants in this study, the altered response in anterior cingulate may therefore reflect not only impaired outcome monitoring, but also impaired representation of task difficulty. In comparison, Wagner and colleagues (Wagner et al. 2007) reported that the anterior caudate differentiated between wins and losses for both adults recovered from AN and matched comparison women, but the recovered group exhibited greater peak activations overall. Because the Wagner study did not investigate other striatal subregions or cingulate ROIs, a direct comparison of findings is not possible.
4.3. Sensorimotor system
The posterior putamen and motor cingulate both showed a preference for wins relative to losses for CA; other studies of healthy volunteers performing this task did not report activation differences (Delgado et al. 2000; May et al. 2004). With its strong connectivity with skeletomotor regions (Yin and Knowlton 2006) the putamen has been associated with motor performance and habit learning. Recent data suggest that both the associative and habit learning systems, despite operating independently, may be engaged simultaneously, if not competitively (Balleine 2009; Yin and Knowlton 2006). While the task does not involve learning, it is possible that the healthy adolescents were nonetheless attempting to associate responses with a favorable outcome. In contrast to CA, the group with AN showed no significant differences in response to reward or punishment within either the posterior putamen or motor cingulate. Adults suffering from AN have shown impaired procedural learning (Lawrence 2003) and have been hypothesized to have altered habit learning (Tchanturia et al. 2012). That is, once a stimulus-outcome pairing has been learned, individuals with AN have difficulty replacing the stimulus-outcome association. The inability of this group to distinguish feedback type may be related to poor procedural learning/enhanced reliance upon habit performance.
Overall, the distinct responses within subzones of the striatum reflect input from similar zones within the cingulate and prefrontal cortex (Figure 1). As part of the limbic pathway, the ventral striatum, which includes the nucleus accumbens, ventromedial caudate, and rostroventral putamen, receives projections from the rostral ACC and orbitofrontal cortex. For executive processes, the cognitive ACC and dorsal PFC project to the dorsal striatum (i.e., anterior caudate) (Haber and Knutson 2010). Finally, the posterior striatum receives projections from the motor cingulate, as well as ventrolateral PFC, inferior temporal regions, primary sensory and motor areas, and the posterior supplementary motor area (Yin and Knowlton 2006). These areas work together to compare potential options, choose the option most valued, and present the option so an action might be promoted for its acquisition (Haber and Knutson 2010). These actions support the striatum’s role in both habit learning and goal-directed learning (Yin and Knowlton 2006), with the anterior striatum involved in reward based learning and the posterior striatum implicated in visual stimulus-response learning and feedback monitoring. Humans and animals use reward and punishment to respond appropriately to stimuli and learn from experience (Schultz 2006). While performing our feedback-based task, both groups showed a similar preference to reward relative to punishment in limbic regions. However, this difference was less apparent in the executive and motor regions among participants ill with AN. These data support the possibility that individuals with AN have altered action-outcome learning. While adolescents with AN may have normal reward expectancies, the greater response to loss within the posterior executive areas of caudate for the group with AN but not CA suggests altered learning. Although punishment can be used for stimulus-response learning (Mattfeld et al. 2011), it is typically less salient when rewards are available. This suggests that individuals with AN may be more sensitive to punishment compared to reward in terms of stimulus-response learning.
4.4. Clinical implications
From a clinical standpoint, these findings may have critical relevance. It is well known that it is difficult to engage AN patients in treatment or get them to change their behaviors. Our findings are consistent with clinical observations that AN are highly sensitive to punishment (Jappe et al. 2011), and this bias is likely to interfere with motivation or ability to learn from experience. That is, ill AN individuals tend to perceive their actions as incorrect or flawed and are highly sensitive to criticism, rather than being able to appropriately proportion reward and punishment in order to learn from experience. That the altered response to feedback occurred in regions associated with cognitive control and habit performance supports the clinical observation of enhanced inhibitory control as well as difficulty in changing behavior (Tchanturia et al. 2012). AN adults are less tolerant of uncertainty and are perfectionistic, with an overemphasis on self-imposed standards (Lampard et al. 2011). An over-reliance on executive brain circuits involved in linking action to outcome may therefore constitute an attempt at “strategic” (as opposed to hedonic) means of responding to reward stimuli (Kober et al. 2010). These data add further support to the hypothesis (Kaye et al. 2009) that AN individuals have an imbalance between ventral limbic and dorsal executive circuits. Such insights may be valuable in developing more effective treatment interventions for specific AN traits. More studies are needed to further explore this apparent dissociation within striatal and cingulate zones.
An important consideration is the impact of stage of brain development on our findings. During adolescence, the motivational and cognitive control systems develop at different rates, with limbic projections to the PFC developing earlier than frontostriatal projections (Casey and Jones 2010); this is supported by evidence showing the adolescent ventral striatum is more responsive to rewards than in adults. In comparison, executive regions and their connectivity continue to develop in later adolescence; this parallels the development of AN symptoms. Clinically, individuals with restricting-type AN tend to display greater inhibitory control than healthy comparison participants (Tchanturia et al. 2012), and this sub-type has been distinguished from binge-purge adolescent groups in a go-nogo fMRI task (Lock et al. 2011). Longitudinal designs may aid the interpretation of the apparent shift in limbic and executive response as a function of neurodevelopment and illness status. Malnutrition in AN is known to be associated with changes in brain structure and metabolism (Castro-Fornieles et al. 2007), and thus could contribute to our findings. With these potential influences in mind, our methodological approach carefully constrained ROIs to gray matter and fMRI signal, reviewing appropriate application for each participant in an effort to avoid confounds of atrophy due to malnutrition on the BOLD signal.
4.5. Limitations
This study is limited by its modest sample size; however, the patient sample was homogeneous for the restricting subtype. This is a strength over most imaging studies of AN, which often do not differentiate patient subtypes, as it permits a more careful consideration of the relationship of specific symptoms with activation differences. Although one aim of this study was to determine whether ill AN exhibited similar functional response patterns to recovered AN, this was not meant to be a direct comparison. Most fMRI studies have focused on recovered adults and therefore may be confounded by the long-term effects of malnutrition or treatment, making it difficult to draw broad conclusions and, importantly, to understand the effects of a disorder that typically initiates in adolescence (Klein and Walsh 2003). Further studies are needed to determine if recovered adolescent AN share similar neural responses with ill AN. Another concern is the high comorbidity of depression in AN and the significant difference between cohorts on BDI scores. Children and adolescents with pediatric major depressive disorder (Forbes et al. 2006) have demonstrated altered ventral striatal activity to reward which was found to be inversely correlated with depression severity. While it is possible that depressive symptoms may have contributed to our findings, this is unlikely, as BDI scores did not correlate with any of the regions of interest in this study. Studies have also shown positive relationships between dorsal caudate function and both anxiety (Wagner et al. 2007) and harm avoidance (Frank and Kaye 2005) in individuals recovered from AN. Due to our modest sample size, we could not detect significant relationships between regions of interest and anxiety or harm avoidance in the adolescent patient group, although we did find potential relationships with WCST perseveration errors that showed directional differences between groups at uncorrected p levels. Additional studies with larger sample sizes would permit a more detailed exploration of these potential relationships. Finally, although efforts were made to minimize the influence of various treatments by recruiting adolescents from our treatment program, which employs the Maudsley Family Based Treatment method, two participants were on a low-dose regimen (2.5 mg or less) of olanzapine, a serotonin and dopamine antagonist. In healthy volunteers, a single dose of olanzapine has been shown to dampen the response to reward in the ventral striatum (Abler et al. 2007). The sample of AN participants on olanzapine was too small to analyze findings. Moreover, AN did not differ from CW in terms of reward and punishment response within the nucleus accumbens, and the exclusion of these two participants did not alter the significance of our findings. While we cannot be certain, it does not appear that olanzapine use in these two participants produced an appreciable effect in this study. Additional studies examining various treatment effects in a larger sample would better address this limitation.
4.6. Conclusions
This is the first study, to our knowledge, to show altered response to monetary reward processing in adolescents ill with AN compared to healthy adolescents. The results suggest that, while limbic striatal regions tend to respond to wins in a similar fashion as seen in healthy adolescents, there is an exaggerated response to losses in the posterior striatal areas. These results support the notion that individuals with AN may be sensitive to punishment, and that this may be an underlying trait of the disorder. Additional studies are needed to further examine whether this sensitivity to punishment has implications for patients’ with AN action-outcome learning, and whether this is a stable trait post-recovery.
Supplementary Material
Acknowledgments
Supported by NIH grants R21-MH086017, R01-MH042984-17A1, R01-MH042984-18S1, and the Price Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abler B, Erk S, Walter H. Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharm (Berl) 2007;191:823–833. doi: 10.1007/s00213-006-0690-y. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association; Association AP, editor. Diagnostic & Statistical Manual of Mental Disorders: DSM:VI-TR. 4. Washington, DC: 2000. [Google Scholar]
- Balleine B. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199:43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statist Soc B-Methodological. 1995;57 [Google Scholar]
- Botvinick M, Cohen J, Carter C. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Casey B, Jones R. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolsc Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. quiz 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Fornieles J, Bargallo N, Lazaro L, Andres S, Falcon C, Plana M, Junque C. Adolescent anorexia nervosa: cross-sectional and follow-up frontal gray matter disturbances detected with proton magnetic resonance spectroscopy. J Psychtr Res. 2007;41:952–958. doi: 10.1016/j.jpsychires.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Cowdrey F, Park R, Harmer C, McCabe C. Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol Psych. 2011;70:736–743. doi: 10.1016/j.biopsych.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Delgado M, Miller M, Inati S, Phelps E. An fMRI study of reward-related probability learning. Neuroimage. 2005;24:862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Delgado M, Nystrom L, Fissel C, Noll D, Fiez J. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Users guide for the structured clinical interview for DSM-IV Axis I disorders- research version (SCID-I, version 2.0, February 1996 FINAL VERSION) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Forbes EM, CJ, Siegele C, Labdouceur C, Ryan N, Carter C, Birmaher B, Axelson D, Dahl R. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G, Kaye WH. Positron emission tomography studies in eating disorders: multireceptor brain imaging, correlates with behavior and implications for pharmacotherapy. Nuc Med & Biol. 2005;32:755–761. doi: 10.1016/j.nucmedbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Friederich H, Walther S, Bendszus M, Biller A, Thomann P, Zeigermann S, Katus T, Brunner R, Zastrow A, Herzog W. Grey matter abnormalities within cortico-limbic-striatal circuits in acute and weight-restored anorexia nervosa patients. Neuroimage. 2012;59:1106–1113. doi: 10.1016/j.neuroimage.2011.09.042. [DOI] [PubMed] [Google Scholar]
- Haber S, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharm. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R. A Manual of the Wisconsin Card Sorting Test. Odessa, Florida: Psychological Assessment Resources; 2005. [Google Scholar]
- Hedges L. Statistical Methods for Meta-Analysis. Orlando FL: Academic Press; 1985. [Google Scholar]
- Huber P. Robust Estimation of Location Parameter. Annals of Mathematical Stats. 1964;35 [Google Scholar]
- Jappe L, Frank G, Shott M, Rollin M, Pryor T, Hagman J, Yang T, Davis E. Heightened sensitivity to reward and punishment in anorexia nervosa. Int J Eat Disord. 2011;44:317–324. doi: 10.1002/eat.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye W, Fudge J, Paulus M. New insight into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- Klein D, Walsh B. Eating disorders. Int Rev Psychiatry. 2003;15:205–216. doi: 10.1080/0954026031000136839. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross E, Weber J, Mischel W, Hart C, Ochsner K. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K, Belliveau J, Chesler D, Goldberg I, Weisskoff R, Poncelet B, Kennedy D, Hoppel B, Cohen M, Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampard A, Byrne S, McLean N, Fursland A. Avoidance of affect in the eating disorders. Eat Behav. 2011;12:90–93. doi: 10.1016/j.eatbeh.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Lawrence A. Impaired visual discrimination learning in anorexia nervosa. Appetite. 2003;20:85–89. doi: 10.1016/s0195-6663(02)00138-1. [DOI] [PubMed] [Google Scholar]
- Liu X, Hariston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci & Bibehav Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J, Garrett A, Beenhakker J, Reiss A. Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. Am J Psychiatry. 2011;168:55–64. doi: 10.1176/appi.ajp.2010.10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang D, Huang Y, Cooper T, Kegeles LS, Zarahn E, Abi-Dargham A, Haber SN, Laurelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. Journal of Cerebral Blood Flow and Metabolism. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Mattfeld A, Gluck M, Stark C. Functional specialization within the striatum along both the dorsal/ventral and anterior/posterior axes during associative learning via reward and punishment. Learn Mem. 2011;18:703–711. doi: 10.1101/lm.022889.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS. Event-related functional magnetic resonance imaging of reward-related brain circuity in children and adolescents. Biol Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- O’Doherty J. Reward representations and reward related learning in the human brain: insights from neuroimaging. Science. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank D, Menon R, Ellermann J, Kim S, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M, Drevets WR, SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psych. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Ann Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Sheehan K, Shytle RJJ, Bannon Y, Rogers J, Milo M, Stock S, Wilkinson B. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) J Clin Psychiatry. 2010;71:313–326. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- Tchanturia K, Davies H, Roberts M, Harrison A, Nakazato M, Schmidt U, Treasure J, Morris R. Poor cognitive flexibility in eating disorders: examining the evidence using the Wisconsin Card Sorting Task. PLoS One. 2012;7:e28331. doi: 10.1371/journal.pone.0028331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Aizenstein H, Venkatraman M, Fudge J, May J, Mazurkewicz L, Frank G, Bailer UF, Fischer L, Nguyen V, Carter C, Putnam K, Kaye WH. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry. 2007;164:1842–1849. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- Wagner A, Barbarich N, Frank G, Bailer U, Weissfeld L, Henry S, Achenbach S, Vogel V, Plotnicov K, McConaha C, Kaye W, Wonderlich S. Personality traits after recovery from eating disorders: Do subtypes differ? International Journal of Eating Disorders. 2006;39:276–284. doi: 10.1002/eat.20251. [DOI] [PubMed] [Google Scholar]
- Wecshler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Pearson; 1999. [Google Scholar]
- Wilkinson G, Robertson G. Wide Range Achievement Test 4 Professional Manual. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- Yin H, Knowlton B. The role of the basal ganglia in habit formation. Nature Neuroscience Rev. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yucel M, Wood S, Fornito A, Riffkin J, Velakoulis D, Pantelis C. Anterior cingulate dysfunction: implications for psychiatric disorders? J Psychiatry Neurosci. 2003;28:350–354. [PMC free article] [PubMed] [Google Scholar]
- Zastrow A, Kaiser SSC, Walthe S, Herzog W, Tchanturia K, Belger A, Weisbrod M, Treasure J, Friederich H. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. Am J Psychiatry. 2009;166:608–616. doi: 10.1176/appi.ajp.2008.08050775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.