Abstract
In mouse tooth development, the roots of the first lower molar develop after crown formation to form 2 cylindrical roots by post-natal day 5. This study compared the morphogenesis and cellular events of the mesial-root-forming (MRF) and bifurcation-forming (BF) regions, located in the mesial and center of the first lower molar, to better define the developmental mechanisms involved in multi-rooted tooth formation. We found that the mesenchyme in the MRF showed relatively higher proliferation than the bifurcation region. This suggested that spatially regulated mesenchymal proliferation is required for creating cylindrical root structure. The mechanism may involve the mesenchyme forming a physical barrier to epithelial invagination of Hertwig’s epithelial root sheath. To test these ideas, we cultured roots in the presence of pharmacological inhibitors of microtubule and actin polymerization, nocodazole and cytochalasin-D. Cytochalasin D also inhibits proliferation in epithelium and mesenchyme. Both drugs resulted in altered morphological changes in the tooth root structures. In particular, the nocodazole- and cytochalasin-D-treated specimens showed a loss of root diameter and formation of a single-root, respectively. Immunolocalization and three-dimensional reconstruction results confirmed these mesenchymal cellular events, with higher proliferation in MRF in multi-rooted tooth formation.
Keywords: tooth development, molar, tooth root, odontogenesis, cell proliferation, histochemistry
Introduction
Tooth crown development requires epithelial-mesenchymal interactions that are mediated by multiple growth and transcription factors (Thesleff and Mikkola, 2002; Tummers and Thesleff, 2009). At the completion of the crown, the outer and inner enamel epithelia of the cervical loops proliferate apically, giving rise to the epithelial extension called HERS (Hertwig’s epithelial root sheath). The pattern of HERS growth correlates with the numbers, lengths, and shapes of the roots (Huang et al., 2009; Sakano et al., 2013). HERS is a transient structure and disappears when tooth root formation is complete (Hosoya et al., 2008). Over the past few decades, far more molecular work has been devoted to crown formation as compared with root patterning. For example, cellular dynamics (proliferation, apoptosis, and migration) have been examined, primarily during crown formation (Shigemura et al., 1999; Peterková et al., 2003; Lee et al., 2009). The morphogenetic and developmental mechanisms involved in multi-root development are poorly understood (Ishikawa et al., 2012).
The traditional view of root bifurcation is that 2 tongue-like epithelia grow toward each other to form a diaphragm (Nanci, 2013). Apical growth of HERS forms 2 epithelial tubes closing off the apical foramen. The development of the root after bifurcation is similar to that of single-rooted teeth (Nanci, 2008). It was hypothesized that the invagination patterns of HERS to form a multi-rooted structure are comparable with those of other structures undergoing branching morphogenesis, such as hair and mammary glands (Mikkola and Millar, 2006). However, to date, a detailed examination of the morphological and cellular mechanisms in multi-rooted tooth development has not been carried out.
Here we exposed developing molars that form 2 roots to 2 drugs that interfere with several cell behaviors. Cytochalasin-D (CD) inhibits cytoskeletal formation and prevents cell locomotion, phagocytosis, and lamellipodia formation. Nocodazole (NC) prevents microtubule polymerization (Zegers et al., 1998) and inhibits cell proliferation. Cytochalasin D has been used in the past to regulate feather bud spacing in chicken embryos (Kim et al., 2005). Nocodazole has been recently used to study midline epithelial seam formation in the palate (Kitase and Shuler, 2013).
In this study, we describe histogenesis and cell proliferation in the mesial-root-forming (MRF) and bifurcation-forming (BF) regions in developing teeth. In addition, loss-of-function experiments with pharmacological inhibitors were carried out in organ cultures to test the cellular mechanisms required for multi-rooted formation.
Materials & Methods
All experiments were performed according to the guidelines of the Kyungpook National University, School of Dentistry, Intramural Animal Use and Care Committee.
Animals
Newborn mice were obtained from time-mated pregnant ICR mice. The day of delivery was designated post-natal day 0 (PN0). The first lower molars at PN3, 5, and 8 were used for all histological analyses and for organ culture experiments.
Histology and Immunohistochemistry
Histochemical staining was carried out as described (Sohn et al., 2012). Adjacent sections were used for immunohistochemistry. Monoclonal antibodies to detect Ki67 (Thermo-Scientific, CA, USA), pan-Cytokeratin (Thermo-Scientific), BrdU (Zymed, CA, USA), or PCNA (Proliferating Cell Nuclear Antigen; Neo-Markers, TX, USA) were used. Tissue sections were developed in 3,3′-diaminobenzidine (DAB) and counterstained with Harris’ modified hematoxylin. Anti-pan-cytokeratin antibodies stained all epithelia, including HERS. Positive reactions are captured by microscopy (DM2500, LEICA, Germany).
Counting the Ki67-positive Cells
At least 15 first molars were used to quantify the Ki67-positive cells, and 8 sections were chosen at random. The absolute number and percentage of proliferating mesenchymal cells were examined in a designated 100 µm2 area. The data are expressed as the mean ± SD. A Student t test was used to analyze the significance of difference (p < .05).
In vitro Cultivations and Pharmacological Inhibitors
First mandibular molars were dissected at PN3 (Fujiwara et al., 2005). Tooth germs were placed into roller bottles containing DMEM, 10% FBS, and 1% penicillin-streptomycin in a CO2 incubator. Media were changed daily. The bottles were rotated at a speed of 20 rpm and were flushed with a mixture of 50% O2, 45% N2, and 5% CO2 (Kim et al., 2009). Cytochalasin-D (Sigma, USA) and nocodazole (Sigma, USA) were added to the media of the experimental samples (Kim et al., 2005). The optimal concentrations for both cytochalasin-D and nocodazole were determined based on the morphology of the cultured explants after the tests at various concentrations (0.1 ~ 10 µg/mL). Subsequently, the critical concentrations that showed no cytotoxicity were determined to be 1 µg/mL. A 0.1% quantity of DMSO was used as the carrier control.
Three-dimensional Reconstruction
Pan-CKs immunostained sections were photographed with a DM2500 microscope (Leica). Two first mandibular molars (biological replicates) were reconstructed from each specimen (n = 6 per stage). Reconstruction software was downloaded from http://synapses.clm.utexas.edu/tools/reconstruct/reconstruct.stm (August 20, 2007). Each 7-µm section of the tooth root was photographed, and the software was used to align the images automatically and manually.
Results
Tooth Root Morphogenesis
To understand the morphological changes in tooth root formation, we examined the MRF and BF regions in serial frontal sections of PN3, 5, and 8 first molars (Figs. 1A-1F′′). At PN3 and PN5, the first invagination of HERS into the mesenchyme was observed, with a vertical extension in the root-forming region and a horizontally positioned extension in the BF region (Figs. 1A′′, B′′, D′′, E′′). The condensed mesenchyme separating the ends of HERS was noticeable in the root-forming regions at PN5 (Fig. 1B, Appendix Fig. 1). At PN8, in the BF region, both the lingual and buccal HERS were almost in contact (Fig. 1F′′). The distance between each end part of the lingual and buccal HERS was measured after pan-CKs immunostaining (Fig. 1G). From PN3 to PN8, the distance separating the ends of the pan-CKs-positive regions decreased (PN3, 153.9 ± 9.8 µm; PN8, 18.2 ± 4.7 µm). With apical closure of the mesial root, the distances between the 2 ends of the HERS also decreased (PN3, 256.9 ± 15.4 µm; PN8, 168.2 ± 8.6 µm).
Figure 1.
Morphogenesis of mouse lower molar tooth root. (A-F) The tooth root and BF regions were designated and examined by HE staining at PN3, PN5, and PN8. The boxed area in (B) demarcates the condensed mesenchymal cells. (A′-F′) Pan-CK-positively stained epithelial cells, including HERS, were visualized at higher magnifications (A′′-F′′). (G) The distances between the end parts of the buccolingual pan-CK-positive epithelial cells were measured (n = 10). MRF, mesial-root-forming region; BF, bifurcation-forming region; mc, mesenchymal condensation. Scale bars: 100 μm (A, A′, B, B′, C, C′, D, D′, E, E′, F, F′), 50 μm (A′′, B′′, C′′, D′′, E′′, F′′).
Cell Proliferation is Relatively Low in the Mesenchyme of the Bifurcation
At PN3 and PN5, before root development, the strong positive localization patterns of Ki67 were examined in the mesenchyme, between both HERS structures, in the MRF regions compared with those of the BF region (Figs. 2A-2H, Appendix Fig. 2). Strong Ki67 signal in HERS was detected in the MRF and BF regions at PN3 (Figs. 2B, 2D, Appendix Fig. 2). Interestingly, the proportion of Ki67-positive mesenchymal cells in the MRF region was almost double those in the BF region at both PN3 and PN5 (Fig. 2I). Similar mesenchymal proliferation patterns were observed in BrdU-stained specimens (Appendix Fig. 3). Proliferation patterns with Ki67 immunostaining in HERS were similar to previous reports showing that the outer enamel epithelium was more highly proliferative than the inner (Appendix Fig. 4; Sakano et al., 2013). There were no apoptotic cells in the epithelium or mesenchyme of the root, as reported previously (data not shown; Lungová et al., 2011).
Figure 2.

Localization patterns of Ki67, a cell proliferation marker, during tooth root development. (A-H) Ki67-positive cells were detected in the dental papillae and HERS in a specific spatiotemporal manner. (A, E) Mesenchymal cells with Ki67-positive staining, located between the HERS, are observed mainly in the MRF region at PN3 and PN5. (I) Ratio of Ki67-positive cell numbers to the whole-cell numbers were examined in the 100-μm2 areas (n = 10). MRF, mesial-root-forming region; BF, bifurcation-forming region. Scale bars, 50 μm (A-H).
Pharmacological Inhibitors during in vitro Cultivations
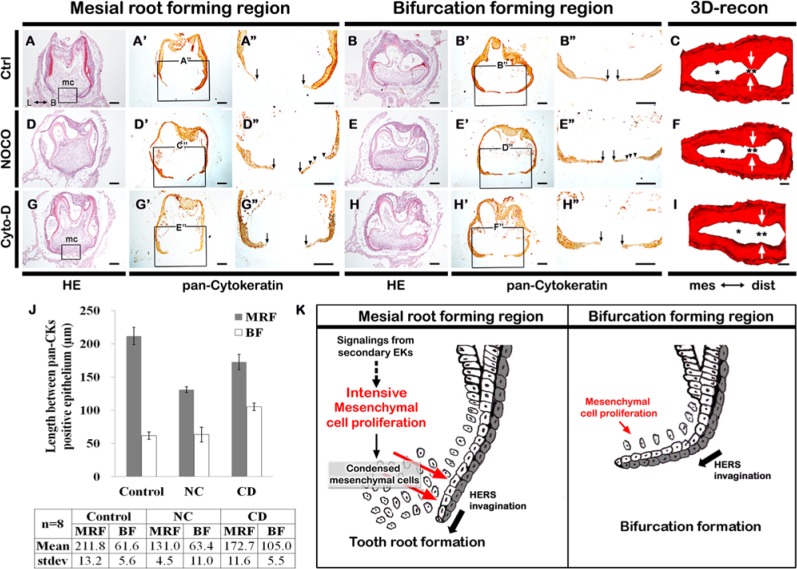
The DMSO-treated specimens, the vehicle controls, developed normally, showing that the organ culture system was promoting HERS extension (Figs. 3A-3B, 3G-3H, 4A, 4B; Appendix Fig. 5). The control specimens showed Ki67 and PCNA localization patterns similar to those of in vivo tooth root development (Figs. 3A, 3B, 3G, 3H; Appendix Fig. 6). After treatment with 1 µg/mL of NC, a decrease in the number of Ki67- and PCNA-positive mesenchymal cells between the HERS was observed (Figs. 3C, 3D, 3I, 3J). In contrast, the number of Ki67- and PCNA-positive cells in HERS increased (Figs. 3C′, 3D′, 3I′, 3J′) and the root sheath was more deeply invaginated into the mesenchyme of the MRF region (Figs. 4D-4D′′). In addition, most specimens showed discontinuity of HERS in both the MRF and BF regions after the NC treatments (Figs. 4D′′, 4E′′). After inhibiting actin filament polymerization by treatment with 1 µg/mL CD treatment, the Ki67 and PCNA localization patterns were unchanged compared with those of the control (Figs. 3E, 3F, 3K, 3L). However, ectopic thickening of the epithelial ends was observed (Figs. 4G′′, 4H′′). These knobs of epithelium were correlated with a significant increase in the gap between the extensions of HERS in the BF region (Figs. 3L′, 4H′′’).
Figure 3.
Treatments with the pharmacological inhibitors during in vitro cultivations at PN3 for 4 days. (A-F) Ki67 and (G-L) PCNA immunostaining after 4 days’ cultivation at PN3. Arrows indicate the bulbous ends of HERS in the CD-treated specimens. All scale bars are 50 μm.
Figure 4.
Morphogenesis of in vitro cultivated lower molar tooth. (A, B) DMSO control specimens show morphogenesis similar to that of normal tooth root development. (D, E) The nocodazole-treated specimens show a narrow distance between both sides of the HERS tissues in the MRF (single asterisk) but a normal gap in the BF (double asterisk) regions. The arrowheads indicate HERS discontinuities. (G, H) After cytochalasin-D treatment, the histology and localization patterns of pan-CK-positive epithelial cells showed much wider gaps between the end margins of both HERS tissues with the aggregated structures in MRF and BF regions. (C, F, I) Three-dimensional reconstruction after the immunostaining against pan-CKs. (J) The distances between the HERS were measured. (K) Summary and schematic diagram of tooth root and bifurcation morphogenesis. (C, F, I) The epithelial tissues in the tooth root, which show positive staining of the pan-CKs, were reconstructed. Red indicates the dental papillae facing the epithelium. All scale bars are 100 μm.
Morphological changes caused by the pharmacological inhibitors were defined by computer-aided 3D reconstruction of cytokeratin-stained epithelium (Figs. 4C, 4F, 4I, Appendix Fig. 4). The control specimen showed normal morphogenesis of the tooth root (Fig. 4C). In contrast, the medial roots of NC-treated specimens were narrower in diameter compared with the control medial root (single asterisk, Fig. 4F). The bifurcation was unaffected in the NC-treated teeth (double asterisks, Fig. 4F). However, elsewhere in the root sheath, NC resulted in some discontinuities in the epithelium (arrowheads). In contrast to the subtle effects of NC, CD completely ablated the bifurcation (Fig. 4I). The measurements of the buccal-lingual distance between the HERS confirmed these morphological alterations (Fig. 4J).
Discussion
Based on our morphological observations, the differential invagination patterns of HERS between the MRF and BF regions were correlated with localized, highly proliferative condensations of mesenchymal cells, which were detected only in the nascent medial-root-forming regions. These similar mesenchymal-epithelial interactions were examined in other branching organ, mammary gland (Mikkola and Millar, 2006). In normal molars at PN3 and 5, double the percentage of Ki67-positive cells was observed in the diaphragm on the mesial root as compared with those in the bifurcation. These results suggest that pulp proliferation may induce epithelial cell migration at PN3 and 5.
The roller cultivation system used here overcame the disadvantages of the Trowell’s culture system, in which peripheral regression of tissues occurs from exposure to the medium-gas interphase (Tummers et al., 2007). A previous study reported that mouse molar root development was initiated at PN3 through IGF-1 signaling (Fujiwara et al., 2005). In this study, the mandibles were dissected and the lower molars were cultivated in situ for 4 days at PN3 for examination of the detailed morphological changes in tooth root formation. During the in vitro cultivation carried out in the present study over longer periods of time, roots developed sufficiently for the bifurcation to be seen. This unique culture system allowed us to detect root phenotypes in the NC- and CD-treated specimens. There are several possible explanations for the change in morphology in the chemically treated molars. The first is a direct effect on the mesenchyme. In NC-treated teeth, there were significantly fewer condensed mesenchymal cells in the MRF regions. Thus, the physical barrier to HERS posed by the mesenchyme is absent, allowing HERS to invaginate toward the center of the tooth. These changes would lead to a thinner mesial root. The second prediction is that since the bifurcation already has relatively low mesenchymal proliferation, the addition of NC will not change the shape or direction of HERS. Indeed, we observed slight thinning of the mesial root with no change in the bifurcation. The second explanation for the NC phenotypes is that there is an effect on the epithelium. A large part of root formation depends on differential proliferation of the outer and inner enamel epithelium (Yamamoto et al., 2004; Sakano et al., 2013). It is certainly possible that NC led to decreased epithelial proliferation. The organ culture system, by its nature, exposes all tissues to the chemical; therefore, effects on multiple cell types will occur, either directly or indirectly.
The specimens treated with CD, which inhibits cytoskeletal formation and prevents cell locomotion, displayed a distinct phenotype compared with those treated with NC. The tips of HERS became thicker and bulbous. While we did not quantify the number of proliferating cells from a qualitative point of view, there was stronger staining of Ki67 and PCNA at the bulbous ends of HERS. The accumulation of cells suggested that migration is inhibited in CD-treated teeth. Fitting the hypothesis of a block to migration, CD teeth were unable to form a furcation, and only a single root formed.
The molecular signaling taking place between the mesenchyme and epithelium during root formation has been investigated in several mouse knockout studies. Several of these studies have conditionally targeted genes in the dentin and pulp mesenchyme, and rootless teeth develop. The conditional deletion of Beta-catenin and Tgfbr2 in odontoblasts resulted in rootless teeth or abnormal root morphology, indicating that Wnt and Tgf signaling is involved (Kim et al., 2013; Wang et al., 2013; Zhang et al., 2013). These studies also illustrate that signals from the dental mesenchyme affect HERS and thus support our hypothesis that the mesenchyme is the driver of single- vs. multi-rooted tooth patterning. We also acknowledge that epithelial signals such as Fgf10 could also be important for establishing HERS in the first place (Yokohama-Tamaki et al., 2006). Other systemic factors such as growth hormone levels are involved in root length but not in whether 1 or 2 roots form (Smid et al., 2007).
Acknowledgments
We thank Professor Joy M. Richman for critical reading of the manuscript.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korea government (MEST) (no. 2008-0062282). This research was supported by Kyungpook National University Research Fund, 2012.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Fujiwara N, Tabata MJ, Endoh M, Ishizeki K, Nawa T. (2005). Insulin-like growth factor-I stimulates cell proliferation in the outer layer of Hertwig’s epithelial root sheath and elongation of the tooth root in mouse molars in vitro. Cell Tissue Res 320:69-75 [DOI] [PubMed] [Google Scholar]
- Hosoya A, Kim JY, Cho SW, Jung HS. (2008). BMP4 signaling regulates formation of Hertwig’s epithelial root sheath during tooth root development. Cell Tissue Res 333:503-509 [DOI] [PubMed] [Google Scholar]
- Huang X, Bringas P, Jr, Slavkin HC, Chai Y. (2009). Fate of HERS during tooth root development. Dev Biol 334:22-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Ida-Yonemochi H, Nakakura-Ohshima K, Ohshima H. (2012). The relationship between cell proliferation and differentiation and mapping of putative dental pulp stem/progenitor cells during mouse molar development by chasing BrdU-labeling. Cell Tissue Res 348:95-107 [DOI] [PubMed] [Google Scholar]
- Kim JY, Cho SW, Song WC, Lee MJ, Cai J, Ohk SH, et al. (2005). Formation of spacing pattern and morphogenesis of chick feather buds is regulated by cytoskeletal structures. Differentiation 73:240-248 [DOI] [PubMed] [Google Scholar]
- Kim JY, Lee MJ, Cho KW, Lee JM, Kim YJ, Kim JY, et al. (2009). Shh and ROCK1 modulate the dynamic epithelial morphogenesis in circumvallate papilla development. Dev Biol 325:273-280 [DOI] [PubMed] [Google Scholar]
- Kim TH, Bae CH, Lee JC, Ko SO, Yang X, Jiang R, et al. (2013). β-catenin is required in odontoblasts for tooth root formation. J Dent Res 92:215-221 [DOI] [PubMed] [Google Scholar]
- Kitase Y, Shuler CF. (2013). Microtubule disassembly prevents palatal fusion and alters regulation of the E-cadherin/catenin complex. Int J Dev Biol 57:55-60 [DOI] [PubMed] [Google Scholar]
- Lee DS, Park JT, Kim HM, Ko JS, Son HH, Gronostajski RM, et al. (2009). Nuclear factor I-C is essential for odontogenic cell proliferation and odontoblast differentiation during tooth root development. J Biol Chem 284:17293-17303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungová V, Radlanski RJ, Tucker AS, Renz H, Míšek I, Matalová E. (2011). Tooth-bone morphogenesis during postnatal stages of mouse first molar development. J Anat 218:699-716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola ML, Millar SE. (2006). The mammary bud as a skin appendage: unique and shared aspects of development. J Mammary Gland Biol Neoplasia 11:187-203 [DOI] [PubMed] [Google Scholar]
- Nanci A. (2013). Ten Cate’s Oral histology: development, structure, and function. 8th ed. St. Louis, MO: Elsevier, pp. 89-91 [Google Scholar]
- Peterková R, Peterka M, Lesot H. (2003). The developing mouse dentition: a new tool for apoptosis study. Ann NY Acad Sci 1010:453-466 [DOI] [PubMed] [Google Scholar]
- Sakano M, Otsu K, Fujiwara N, Fukumoto S, Yamada A, Harada H. (2013). Cell dynamics in cervical loop epithelium during transition from crown to root: implications for Hertwig’s epithelial root sheath formation. J Periodontal Res 48:262-267 [DOI] [PubMed] [Google Scholar]
- Shigemura N, Kiyoshima T, Kobayashi I, Matsuo K, Yamaza H, Akamine A, et al. (1999). The distribution of BrdU- and TUNEL-positive cells during odontogenesis in mouse lower first molars. Histochem J 31:367-377 [DOI] [PubMed] [Google Scholar]
- Smid JR, Rowland JE, Young WG, Coschigano KT, Kopchick JJ, Waters MJ. (2007). Mouse molar dentin size/shape is dependent on growth hormone status. J Dent Res 86:463-468 [DOI] [PubMed] [Google Scholar]
- Sohn WJ, Ji YR, Kim HS, Gwon GJ, Chae YM, An CH, et al. (2012). Rgs19 regulates mouse palatal fusion by modulating cell proliferation and apoptosis in the MEE. Mech Dev 129:244-254 [DOI] [PubMed] [Google Scholar]
- Thesleff I, Mikkola M. (2002). The role of growth factors in tooth development. Int Rev Cytol 217:93-135 [DOI] [PubMed] [Google Scholar]
- Tummers M, Thesleff I. (2009). The importance of signal pathway modulation in all aspects of tooth development. J Exp Zool B Mol Dev Evol 312B:309-319 [DOI] [PubMed] [Google Scholar]
- Tummers M, Yamashiro T, Thesleff I. (2007). Modulation of epithelial cell fate of the root in vitro. J Dent Res 86:1063-1067 [DOI] [PubMed] [Google Scholar]
- Wang Y, Cox MK, Coricor G, Macdougall M, Serra R. (2013). Inactivation of Tgfbr2 in Osterix-Cre expressing dental mesenchyme disrupts molar root formation. Dev Biol 382:27-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Cho SW, Kim EJ, Kim JY, Fujiwara N, Jung HS. (2004). Developmental properties of the Hertwig’s epithelial root sheath in mice. J Dent Res 83:688-692 [DOI] [PubMed] [Google Scholar]
- Yokohama-Tamaki T, Ohshima H, Fujiwara N, Takada Y, Ichimori Y, Wakisaka S, et al. (2006). Cessation of Fgf10 signaling, resulting in a defective dental epithelial stem cell compartment, leads to the transition from crown to root formation. Development 133:1359-1366 [DOI] [PubMed] [Google Scholar]
- Zegers MM, Zaal KJ, van IJzendoorn SC, Klappe K, Hoekstra D. (1998). Actin filaments and microtubules are involved in different membrane traffic pathways that transport sphingolipids to the apical surface of polarized HepG2 cells. Mol Biol Cell 9:1939-1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Yang G, Wu X, Xie J, Yang X, Li T. (2013). Disruption of Wnt/β-catenin signaling in odontoblasts and cementoblasts arrests tooth root development in postnatal mouse teeth. Int J Biol Sci 9:228-236 [DOI] [PMC free article] [PubMed] [Google Scholar]