Abstract
We report the enantioselective functionalization of allylic C-H bonds in terminal alkenes by a strategy involving the installation of a temporary functional group at the terminal carbon atom by C-H bond functionalization, followed by diversification of this intermediate with a broad scope of reagents. The method consists of a one-pot sequence of palladium-catalyzed allylic C-H bond oxidation under neutral conditions to form linear allyl benzoates, followed by iridium-catalyzed allylic substitution. This overall transformation forms a variety of chiral products containing a new C-N, C-O, C-S or C-C bond at the allylic position in good yield with high branched-to-linear selectivity and excellent enantioselectivity (ee ≤ 97%). The broad scope of the overall process results from separating the oxidation and functionalization steps; by doing so, the scope of nucleophile encompasses those sensitive to direct oxidative functionalization. The high enantioselectivity of the overall process is achieved by developing an allylic oxidation that occurs without acid to form the linear isomer with high selectivity. These allylic functionalization processes are amenable to an iterative sequence leading to (1,n)-functionalized products with catalyst-controlled diastereo- and enantioselectivity. The utility of the method in the synthesis of biologically active molecules has been demonstrated.
Introduction
The stereochemical complexity of medicinally important compounds is increasing, and recent studies have suggested that compounds containing increased numbers of sp3 carbon centers are more successful through clinical trials.1 Although C-H bond functionalization reactions have the potential to alter the strategies by which these compounds are prepared,2 a major challenge encountered when developing C-H bond functionalization reactions is the control of absolute and relative stereochemistry.2m,3
Likewise, a major challenge facing the development of CH bond functionalization reactions is limited reaction scope. Reactions involving both catalyzed and uncatalyzed alkyl, aryl and acyl substitution reactions occur with broad scope and are used, therefore, widely in medicinal chemistry to build or embellish the core of biologically active compound.4 Yet the same broad scope does not apply to C-H bond functionalization reactions. The functionalization of an aryl C-H bond located ortho to a strong directing group does occur with a range of reagents and oxidants,2c,2l,5 but the functionalization of other classes of C-H bonds do not.
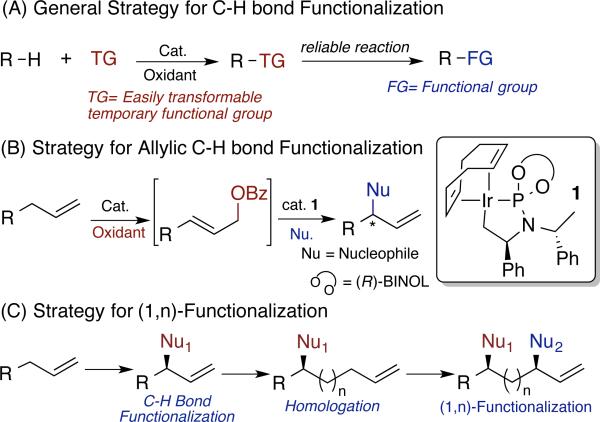
One approach to create a C-H bond functionalization that occurs with broad scope is to combine one C-H bond functionalization reaction with a subsequent step that creates diversity from the initial product of C-H bond functionalization (Figure 1, A). For greatest efficiency, the C-H bond functionalization and subsequent transformation should be conducted in the same reaction vessel without purification of the intermediate. The strength of this approach is illustrated by the borylation of aromatic C-H bonds. In this case, one reliable, iridium-catalyzed C-H borylation reaction leads to an intermediate that can be converted to biaryls, alkylarenes, haloarenes, arylamines, aryl ethers, aryl nitriles, and fluoroalkylarenes, among other products.2k,5b,6
Figure 1.
General Strategy for Aliphatic or Allylic C-H bond Functionalization
We sought to develop a related strategy for enantioselective functionalizations of aliphatic C-H bonds. To develop a general strategy for enantioselective C-H bond functionalization reactions, the introduction of various carbon and heteroatom nucleophiles must be accomplished with control of the configuration - both absolute and relative – of any new stereogenic center generated by the C-H bond functionalization reaction.
Iridium-catalyzed allylic substitution has become one of the most general reactions that form new carbon-carbon or carbon-heteroatom bonds with control of the absolute configuration of an aliphatic stereocenter.7 In this reaction many types of carbon, nitrogen, oxygen, and sulfur nucleophiles react with an allylic electrophile to form products in which the configuration of the new stereo-center is controlled by the catalyst, rather than existing stereogenic centers in the substrate.7e,8
If the electrophile for such substitution reactions could be prepared by a C-H bond oxidation process that is compatible with the substitution reaction, then a strategy for the synthesis of common structural types by C-H activation would result (Figure 1, B). However, the development of such a method for C-H bond oxidation is challenging. As described in more detail below, such a reaction most form linear allylic esters with high linear-to-branched selectivity under neutral conditions without excess of oxidant, and such allylic oxidation reactions are unknown. Published conditions for allylic oxidation are incompatible with most catalysts for subsequent transformations of the oxidation products.
However, if such a combination of oxidation and asymmetric substitution were developed, then an opportunity would be created to use a sequence of allylic functionalization and homologation reactions to form diols, amino alcohols, and diamines, as well as products with a new alkyl or sulfur-containing group. In these reactions the functionality and configurations would be programmed by the identity of the reagent and configuration of the catalyst. This strategy is depicted in Figure 1, (C). The new functional groups could be located at nearly any position along an alkyl chain.
We report the discovery of a sequence comprising C-H bond oxidation and asymmetric allylic substitution. The two steps involve a palladium-catalyzed oxidation of alkenes under neutral conditions to form linear allylic benzoates, and iridium-catalyzed allylic substitution to form products containing new C-O, C-N, C-C and C-S bonds with control over absolute and relative configuration of the stereogenic centers in the product. In contrast to recently published asymmetric allylic functionalizations,3o the reactants in our process are common nucleophiles lacking protecting groups and substituents that modulate their reactivity. This process forms products containing new functionalities at stereogenic centers located 1,3-, 1,4-, 1,5- or even further removed from each other, and the absolute and relative configuration of all new stereo-centers are fully controlled by the catalytic process. In addition to creating new building blocks from simple alkenes, this work introduces a strategy for aliphatic substrates to combine C-H bond functionalization with diversification of the intermediate through reliable catalytic chemistry.
Results and Discussion
The development of a process involving the combination of palladium-catalyzed allylic oxidation and iridiumcatalyzed allylic substitution of the oxidation product confronts several challenges that would be met by development of novel conditions for allylic C-H oxidation. First the oxidation step must generate the terminal allylic ester with high selectivity. The branched impurity would reduce the enantioselectivity of the overall process because iridium-catalyzed allylic substitution of the branched ester leads to racemic product at full conversion. Second, the oxidant must be completely consumed in the first step. Remaining oxidant would deactivate the iridium catalyst in the second step. Third, the allylic ester must be generated in a neutral medium if a second catalyst is to be added to convert the oxidation product to a range of different products. The iridium catalyst for asymmetric allylic substitution (1, Figure 1, B) is not stable to acidic media. This issue is a particular concern because most oxidation processes are conducted in acidic medium, and palladium-catalyzed allylic oxidations that have been published recently with oxygen or quinone as the oxidant require acid for converting the oxygen to hydrogen peroxide or the quinone to the hydroquinone. Thus, we first needed to develop a highly selective catalyst for allylic oxidation to form the terminal allylic ester with high selectivity without excess of oxidant under neutral conditions.
A. Development of an allylic oxidation under neutral conditions to form linear products
With these challenges in mind, our initial studies focused on developing conditions for allylic oxidation to form linear allylic esters under conditions lacking acid or oxidant that would interfere with the allylic substitution process. Prior methods for allylic acetoxylation based on early reports from Tsuji9 and Akermark,10 form linear products, but most of these reactions are conducted with excess of acid (often as solvent) or oxidant, or both.11
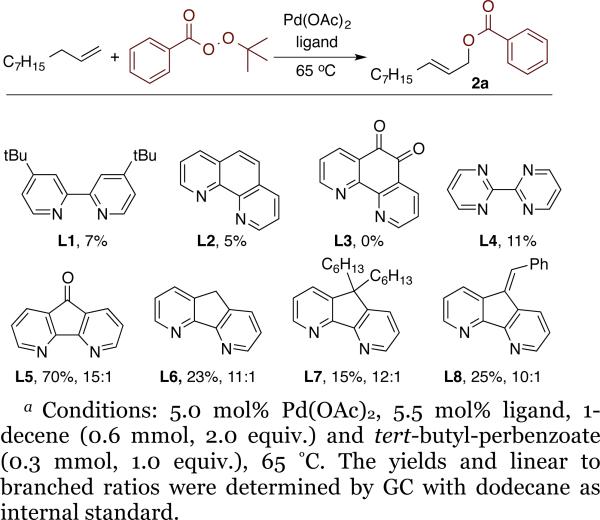
To avoid these acidic conditions with excess oxidant, we investigated the reactions of alkenes with peresters, which would serve as the oxidant and could create an appropriate leaving group for the allylic substitution reaction. Copper-catalyzed allylic oxidation with peresters, such as tert-butyl perbenzoate (also known as the Kharasch-Sosnovsky reaction) has been studied extensively.12 However, this reaction forms branched allylic esters as the major isomer and, therefore, is not suitable for the envisioned allylic functionalization sequence.3g,12b,12c,13 Palladium catalysts also have been used in combination with peresters for oxidation reactions,14 but this combination has not been used for the oxidation of allylic C-H bonds. Thus we sought to develop a palladium catalyzed C-H oxidation with tert-butyl perbenzoate as the oxidant and source of the benzoate group that would form the linear allylic ester.
The combination of Pd(OAc)2 and bidentate nitrogen ligands were tested as catalyst for the benzoylation of 1-decene with tert-butylperbenzoate (Chart 1). The activity and regioselectivity was highest in neat alkene with a 2:1 ratio of alkene to oxidant. Reactions conducted with palladium complexes of tert-butylbipyridine (L1), phenanthroline (L2), 1,10-phenanthroline-5,6-dione (L3) and 2,2′-bipyrimidine (L4) provided low yields of oxidation product 2a (7%, 5%, 0% and 11% respectively). The ligand 4,5-diazafluorenone (L5) was reported by Stahl to form the products of allylic oxidation with O2 as reagent in acetic acid with good linear-to-branched selectivity.11a Thus, we tested this catalyst system for the reaction with tert-butyl perbenzoate as oxidant. Important for the implementation of the functionalization and diversification strategy, this reaction occurred in 70% yield, with a 15:1 linear-to-branched ratio.
Chart 1.
Effect of Ligands on Palladium Catalyzed benzoylation of Allylic C-H Bondsa
Reactions conducted with the catalysts formed from Pd(OAc)2 and derivatives of diazafluorenone ligands L6, L7 and L8 gave lower yields of 2a than those conducted with L5. Thus, catalyst derived from Pd(OAc)2 and 4,5-diazafluoreneone was used for further studies on the development of asymmetric allylic C-H functionalization with tert-butyl perbenzoate as oxidant.
B
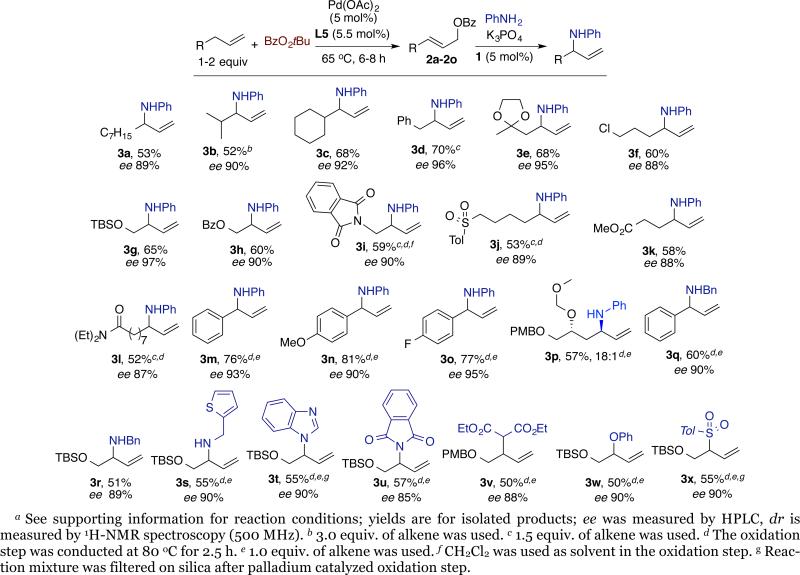
Compatibility and scope of the strategy for asymmetric allylic C-H bond functionalization and iridium-catalyzed amination reactions. If the iridium catalyzed allylic amination would occur directly on the material generated from the allylic oxidation, then a simple process involving first adding the palladium catalyst and oxidant, followed by adding the iridium catalyst and nucleophile, would lead to a range of allylic functionalization products. To consume the benzoic acid product from the substitution reactions with neutral nucleophiles, a base is needed. Thus, we added K3PO4 as base with these nucleophiles. Under these conditions, the reaction with aniline as nucleophile resulted in complete conversion of 2a. The final amination product (3a) was isolated in 53% overall yield for the two steps and 89% ee (Chart 2). The scope of alkenes that undergo the allylic amination sequence is revealed by the data in Chart 2. In addition to the linear 1-decene, branched terminal alkenes underwent the asymmetric allylic amination in good yields and excellent enantioselectivity (3b-3d). The reaction sequence was tolerant of a broad range of functional groups that could undergo further derivatization. Alkenes containing ketal and chloride functional groups were well tolerated, affording the corresponding aromatic amines 3e and 3f, respectively. Alkenes containing silyl ether and benzoate functional groups reacted to afford 1,2 amino alcohol derivatives 3g and 3h respectively. The C-H bond functionalization sequence was also suitable for the synthesis of the chiral 1,2 diamine 3i. Functional groups containing weakly acidic protons, such as sulfones, esters and amides, were also tolerated, and the sequence provided functionalized alkenes 3j-3l. Finally, allylarenes bearing electron-donating or electron-withdrawing groups formed the corresponding amines in good yields with excellent regioselectivity and stereoselectivity (3m-3o).
Chart 2.
Scope of the Alkenes and nucleophiles in the Allylic C-H bond Functionalizationa
Because the iridium catalyst controls the configuration of the new stereocenter, 7e,8 the allylic substitution provides a method to control the diastereoselectivity of the amination of chiral, non-racemic alkenes. For example, the chiral alkene (ee > 99%) containing a MOM-protected alcohol (MOM=methoxy methyl) reacted to form the substitution product 3p in 57% yield with 18:1 diastereoselectivity.
Because the oxidation and functionalization steps occur sequentially, the process occurs with a range of nucleophiles that would be incompatible with direct oxidative functionalization reactions. The data in Chart 2 reveal the broad scope of this asymmetric allylic functionalization process with a variety of nitrogen, oxygen, carbon and sulfur nucleophiles. For example, the protocol we developed occurred with alkylamines and nitrogen heterocycles to form the allylamine and N-allyl heteroarene derivatives 3q-3t in good isolated yields with excellent enantioselectivity. Potassium phthalimide also reacted to give the corresponding amination product 3u in similar yield to that obtained with the other nitrogen nucleophiles. In addition, sodium dimethyl malonate (carbon-), sodium phenoxide (oxygen-) and sodium p-toluenesulfinate (sulfur) nucleophiles reacted to form products 3v-3x containing new C-C, C-O and C-S bonds at the stereogenic center.
C. Short synthesis of biologically active molecules
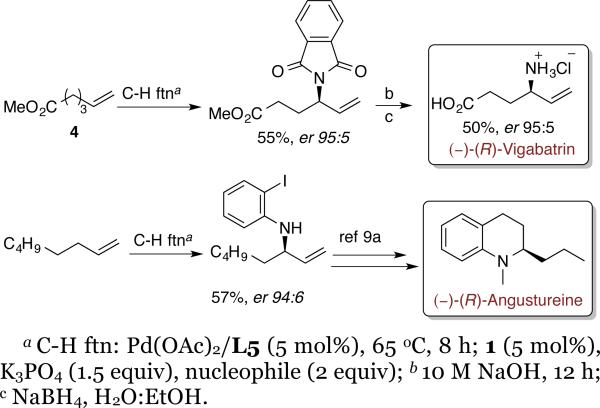
By exploiting this asymmetric allylic functionalization, we were able to conduct short, asymmetric syntheses of two biologically active compounds (-)-(R)-vigabatrin and (-)-(R)-angustureine from simple commercially available alkenes, in part to assign the absolute configuration of the functionalization products.
(-)-(R)-vigabatrin was synthesized from unsaturated ester 4 using the (R, R, R)-1 enantiomer of the catalyst, followed by basic workup and removal of phthalimide protecting group (Scheme 1). Similarly, (-)-(R)-angustureine was synthesized from 1-octene using 2-iodoaniline as nucleophile and (R, R, R)-1 as catalyst. The N-allylamine product was subjected to the previously reported sequence of hydroboration, intramolecular Suzuki-Miyaura cross-coupling, and alkylation to provide (-)-angustureine.8a The relative configuration of the product and iridium catalyst shows that the allylic substitution step of the functionalization sequence occurs with the same stereoselectivity as previously reported substitutions of allylic carbonates.7c,15 Previously reported mechanistic studies from our group on iridium catalyzed allylic substitution reactions7d,16 have shown that the reaction occurs by a highly diastereoselective oxidative addition of an allylic benzoate to an iridium complex 1, followed by attack of the nucleophile onto the allyl ligand from the side opposite to the metal for all of the nucleophiles in the current study. This nucleophilic attack is a faster than the η3-η1-η3 isomerization of the allyl complex.
Scheme 1.
Synthesis of biologically active molecules
D. Iterative C-H bond functionalization: Synthesis of (1,n)-functionalized chiral fragments
To extend the utility of this process to the introduction of multiple functional groups with control of absolute and relative configurations, we developed an iterative sequence of C-H bond functionalizations and homologations to prepare (1,n)-functionalized alkenes (Figure 1, C). In this sequence, the composition of the new functionality and the configuration of each new stereogenic center depends on the reagent and configuration of the catalyst, respectively. Various strategies have been reported for creating (1,2), (1,3) or (1,4) functionalization in molecules. Prominent examples include addition reactions to alkenes, such as dihydroxylation or halogenation reactions (1,2-functionalization), aldol reactions, 1,3 di-polar additions (1,3-functionalization), and metal-catalyzed 1,4 conjugate addition reactions (1,4-functionalization), respectively. However, no method has been reported to prepare (1,n)-functionalized molecules with high diastereoselectivity and enantioselectivity containing a range of installed functional groups and programmed configurations by C-H bond functionalization.
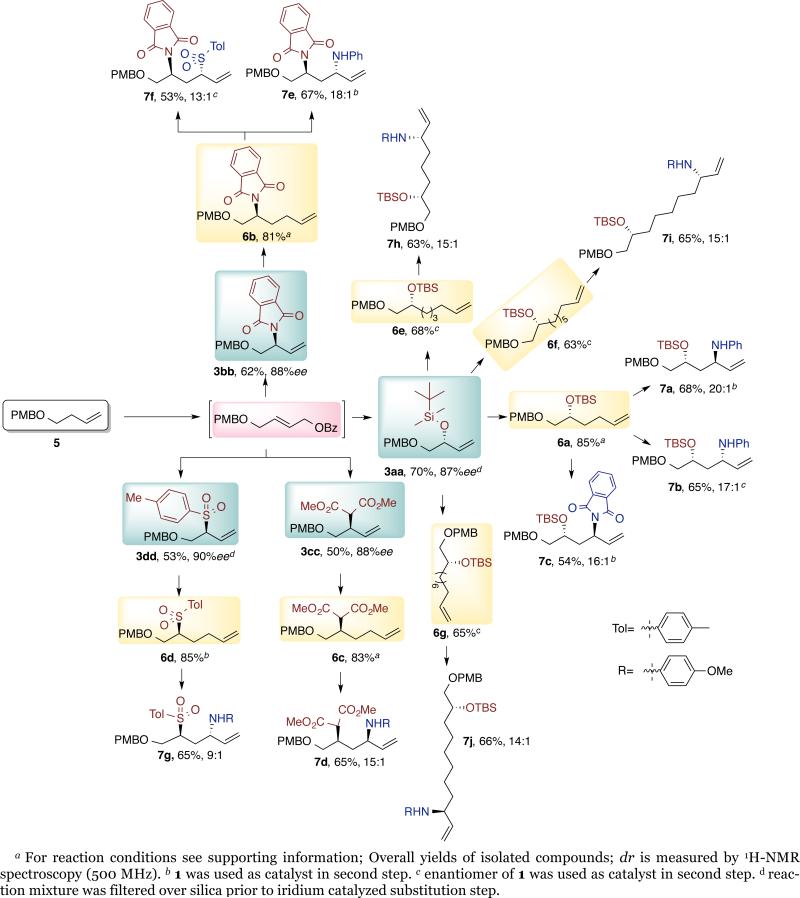
To demonstrate this potential, we converted the simple PMB-protected but-3-en-1-ol (5) to a wide range of tri-functional products with controlled functionality and configuration. These results are summarized in Chart 3.
Chart 3.
Iterative C-H Functionalization: Synthesis of (1,n)-Functionalized Alkenes
Mono-functionalized compounds derived from 5 were prepared by the C-H bond functionalization sequence described above with tert-butyldimethylsilanol, potassium phthalimide, sodium dimethyl malonate and sodium 4-methylbenzenesulfinate, as the nucleophiles to form products 3aa-3dd. These products were homologated to the (1,n,m) alkene intermediates 6a-6g by hydroboration with 9-BBN followed by Suzuki coupling with n-bromoalk-1-enes, in excellent yields.
Various trifunctional building blocks (Chart 3, 7a-7j) containing a (1,3)-, (1,5)-, (1,7)-, and (1,8)-relationship between two stereogenic centers were then formed from alkenes (6a-6g) in good yield. The diastereoselectivities of these reactions were high, and the relative configurations were controlled by the catalyst. For example, both diastereomers of a silyl protected enantioenriched amino alcohol (7a and 7b) were prepared using catalyst 1 and the enantiomer of 1 respectively, with dr values similar to the er values of the reactions of achiral alkenes.8b,8c The chiral alkene prepared from malonate addition was further functionalized with the aromatic amine in high diastereoselectivity (7d). A chiral 1,3 diamine containing different substituents at nitrogen also formed with high diastereoselectivity (7e). The 1,3 amino-sulfones, which could serve as a precursor to amino-sulfone ligands, were prepared with similarly high diastereoselectivity (7f and 7g). Alkenes (6e-6g) provided (1,4), (1,5), and (1,6)-functionalized fragments 7h-7j in excellent diastereo- and enantioselectivity simply by choosing the appropriate chain length of the 1-bromoalkene.
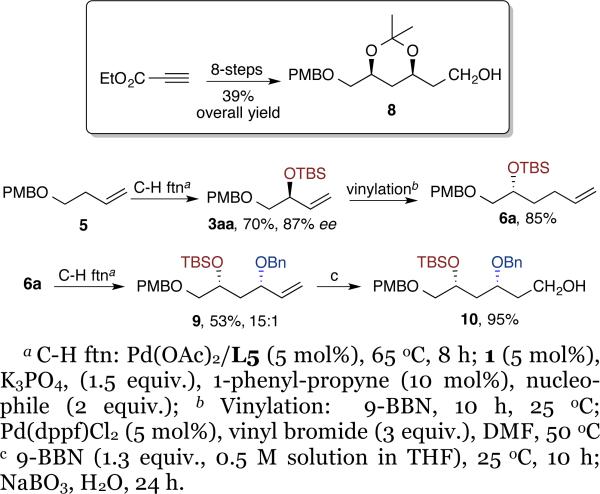
This iterative sequence also was followed to prepare chiral fragment 8 that was used in the synthesis of spongistatin (Scheme 2).17 The 1,3,4 triol derivative 9 was prepared from 5 in excellent diastereo and enantioselectivity (53%, 15:1, 99% ee). Treatment of 9 with 9-BBN, followed by oxidation formed alcohol 10 in 95% yield. By this iterative method we installed three different protecting groups in triol 9, thus providing selective access to each hydroxyl functional group for further transformations. Moreover, the catalyst controlled the configuration of each stereogenic center, thus allowing access to all possible stereoisomers of this fragment simply by changing the choice of the enantiomer of the catalyst without changing the protocol or the starting substrate.
Scheme 2.
Synthesis of chiral fragment of spongistatin
Conclusion
In conclusion, a new approach to asymmetric C-H bond functionalization of allylic C-H bonds in unactivated terminal alkenes to install a broad range of functional groups has been developed. This process comprises a one-pot sequence involving a palladium catalyzed allylic C-H oxidation system with a perester as both reagent and oxidant, thereby occurring under neutral conditions, and an iridium-catalyzed allylic substitution of the product that occurs with broad scope and programmed stereochemistry to form the branched, chiral products containing new C-C, C-N, C-O and C-S bonds in high yield with excellent enantioselectivity (ee up to 97%). An iterative C-H bond functionalization is also reported that can be used to form a wide range of enantioenriched (1,n)-functionalized products, including used in the synthesis of spongistatin, (-)-(R)-vigabatrin and (-)-(R)-angustureine, from trivial alkene starting materials.
This work underscores the value of developing C-H bond functionalization reactions that form common synthetic intermediates under conditions appropriate for subsequent direct diversification by a second catalytic process. Few examples of C-H bon functionalization reactions occurring in this fashion have been reported. The borylation of arene C-H bonds2k,5b,6 is one example of a process that forms the functionalized intermediate without side products that interfere with subsequent transformations.
C-H bond oxidation by P450 enzymes18 creates a functional group that can be converted to a halogen or can be used for subsequent Mitsunobu reactions, but the products are formed in a medium that is incompatible with the second step. The development of additional C-H bond functionalization reactions that form common synthetic intermediates will be the focus of future studies in our laboratory.
Representative Procedure for allylic C-H functionalization
To a 4 ml vial containing a magnetic stirbar, Pd(OAc)2 (3.3 mg, 5 mol%) and L5 (3 mg, 5.5 mol%) were added, followed by 25.0 μL of DCM. The reaction mixture was then stirred for 15 min at room temperature. The solvent was then removed under vacuum, and dodacane (25.0 μL) and 1-decene (0.6 or 0.3 mmol) were added followed by tert-butyl perbenzoate (58.27 mg, 0.3 mmol). The vial was sealed with a cap containing a PTFE septum and then heated at 65 °C for 8 h (monitored by 1H-NMR for consumption of oxidant). The vial was kept at high vacuum for 3-4 h to remove volatile materials and brought into the glove box. The reaction mixture was dissolved in 0.5 ml of dry toluene. To the resulting solution K3PO4 (95.5 mg, 0.45 mmol) and the aniline (2.0 mmol) were added, followed by a solution of iridium catalyst 1 (13.0 mg, 5 mol%) in 0.5 ml of dry toluene. The resulting he reaction mixture was stirred at 25 °C until the linear benzoyl ester was fully consumed, as determined by GC or TLC. The crude reaction mixture was then treated with 2 ml of EtOAc and extracted with brine. The solvent was evaporated from the organic layer, and the product was purified by flash column chromatography on silica gel eluting with a mixture of hexane and ethyl acetate (99:1).
Supplementary Material
ACKNOWLEDGMENT
We thank the NIH (GM-55382 to JFH) and the Swiss National Science Foundation (SNSF) (PBGEP2_139830 to AS) for financial support and Johnson-Matthey for Pd(OAc)2 and [Ir(COD)Cl]2.
Footnotes
Supporting Information
Experimental procedures and analytical data. This material is available free of charge via the internet at http://pubs.acs.org
The authors declare no competing financial interests.
REFERENCES
- 1.Lovering F, Bikker J, Humblet C. J. Med. Chem. 2009;52:6752. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 2.a White MC. Science. 2012;335:807. doi: 10.1126/science.1207661. [DOI] [PubMed] [Google Scholar]; b Yamaguchi J, Yamaguchi AD, Itami K. Angew. Chem., Int. Ed. 2012;51:8960. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]; c Song GY, Wang F, Li XW. Chem. Soc. Rev. 2012;41:3651. doi: 10.1039/c2cs15281a. [DOI] [PubMed] [Google Scholar]; d Ramirez TA, Zhao BG, Shi Y. Chem. Soc. Rev. 2012;41:931. doi: 10.1039/c1cs15104e. [DOI] [PubMed] [Google Scholar]; e Neufeldt SR, Sanford MS. Acc. Chem. Res. 2012;45:936. doi: 10.1021/ar300014f. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Roizen JL, Harvey ME, Du Bois J. Acc. Chem. Res. 2012;45:911. doi: 10.1021/ar200318q. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Kuhl N, Hopkinson MN, Wencel-Delord J, Glorius F. Angew. Chem., Int. Ed. 2012;51:10236. doi: 10.1002/anie.201203269. [DOI] [PubMed] [Google Scholar]; h Campbell AN, Stahl SS. Acc. Chem. Res. 2012;45:851. doi: 10.1021/ar2002045. [DOI] [PMC free article] [PubMed] [Google Scholar]; i McMurray L, O'Hara F, Gaunt MJ. Chem. Soc. Rev. 2011;40:1885. doi: 10.1039/c1cs15013h. [DOI] [PubMed] [Google Scholar]; j Boorman TC, Larrosa I. Chem. Soc. Rev. 2011;40:1910. doi: 10.1039/c0cs00098a. [DOI] [PubMed] [Google Scholar]; k Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem. Rev. 2010;110:890. doi: 10.1021/cr900206p. [DOI] [PubMed] [Google Scholar]; l Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624. doi: 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Giri R, Shi BF, Engle KM, Maugel N, Yu JQ. Chem. Soc. Rev. 2009;38:3242. doi: 10.1039/b816707a. [DOI] [PubMed] [Google Scholar]; n Bergman RG. Nature. 2007;446:391. doi: 10.1038/446391a. [DOI] [PubMed] [Google Scholar]; o Sun CL, Li BJ, Shi ZJ. Chem. Rev. 2011;111:1293. doi: 10.1021/cr100198w. [DOI] [PubMed] [Google Scholar]
- 3.a El-Qisiari AK, Qaseer HA, Henry PM. Tetrahedron Lett. 2002;43:4229. [Google Scholar]; b Clark JS, Roche C. Chem. Commun. 2005:5175. doi: 10.1039/b509678b. [DOI] [PubMed] [Google Scholar]; c Zhou ZN, Andrus MB. Tetrahedron Lett. 2012;53:4518. [Google Scholar]; d Hoang VDM, Reddy PAN, Kim TJ. Organometallics. 2008;27:1026. [Google Scholar]; e Eames J, Watkinson M. Angew. Chem., Int. Ed. 2001;40:3567. doi: 10.1002/1521-3773(20011001)40:19<3567::AID-ANIE3567>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]; f Malkov AV, Pernazza D, Bell M, Bella M, Massa A, Teply F, Meghani P, Kocovsky P. J. Org. Chem. 2003;68:4727. doi: 10.1021/jo034179i. [DOI] [PubMed] [Google Scholar]; g Andrus MB, Zhou ZN. J. Am. Chem. Soc. 2002;124:8806. doi: 10.1021/ja026266i. [DOI] [PubMed] [Google Scholar]; h Chai Z, Rainey TJ. J. Am. Chem. Soc. 2012;134:3615. doi: 10.1021/ja2102407. [DOI] [PubMed] [Google Scholar]; i Li QA, Yu ZX. Angew. Chem. 2011;50:2144. doi: 10.1002/anie.201005215. [DOI] [PubMed] [Google Scholar]; j Collet F, Lescot C, Dauban P. Chem. Soc. Rev. 2011;40:1926. doi: 10.1039/c0cs00095g. [DOI] [PubMed] [Google Scholar]; k Covell DJ, White MC. Angew. Chem., Int. Ed. 2008;47:6448. doi: 10.1002/anie.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Nishioka Y, Uchida T, Katsuki T. Angew. Chem., Int. Ed. 2013;52:1739. doi: 10.1002/anie.201208906. [DOI] [PubMed] [Google Scholar]; m Trost BM, Thaisrivongs DA, Donckele JE. Angew. Chem., Int. Ed. 2012;52:1523. doi: 10.1002/anie.201207870. [DOI] [PubMed] [Google Scholar]; n Davies HML, Morton D. Chem. Soc. Rev. 2011;40:1857. doi: 10.1039/c0cs00217h. [DOI] [PubMed] [Google Scholar]; o Bao HL, Tambar UK. J. Am. Chem. Soc. 2012;134:18495. doi: 10.1021/ja307851b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a Hartwig JF. Organotransition metal chemistry : from bonding to catalysis. University Science Books; Sausalito, Calif: 2010. [Google Scholar]; b Tsuji J. In: Palladium reagents and catalysts : new perspectives for the 21st century. 2nd ed. Wiley J, editor. Chichester, West Sussex; Hoboken, NJ: 2004. [Google Scholar]; c Buchwald SL. Adv. Synth. Catal. 2004;346:1524. doi: 10.1002/adsc.201200398. [DOI] [PMC free article] [PubMed] [Google Scholar]; d March J. Advanced organic chemistry: reactions, mechanisms, and structure. McGraw-Hill; New York: 1968. [Google Scholar]
- 5.a Leow D, Li G, Mei TS, Yu JQ. Nature. 2012;486:518. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Dai HX, Yu JQ. J. Am. Chem. Soc. 2012;134:134. doi: 10.1021/ja2097095. [DOI] [PubMed] [Google Scholar]; c Wang CM, Chen H, Wang ZF, Chen JA, Huang Y. Angew. Chem., Int. Ed. 2012;51:7242. doi: 10.1002/anie.201203230. [DOI] [PubMed] [Google Scholar]; d Rousseau G, Breit B. Angew. Chem., Int. Ed. 2011;50:2450. doi: 10.1002/anie.201006139. [DOI] [PubMed] [Google Scholar]; e Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a Robbins DW, Hartwig JF. Angew. Chem., Int. Ed. 2013;52:933. doi: 10.1002/anie.201208203. [DOI] [PubMed] [Google Scholar]; b Partridge BM, Hartwig JF. Org. Lett. 2013;15:140. doi: 10.1021/ol303164h. [DOI] [PubMed] [Google Scholar]; c Ye YD, Sanford MS. J. Am. Chem. Soc. 2012;134:9034. doi: 10.1021/ja301553c. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Robbins DW, Hartwig JF. Org. Lett. 2012;14:4266. doi: 10.1021/ol301570t. [DOI] [PubMed] [Google Scholar]; e Hartwig JF. Acc. Chem. Res. 2012;45:864. doi: 10.1021/ar200206a. [DOI] [PubMed] [Google Scholar]; f Hartwig JF. Chem. Soc. Rev. 2011;40:1992. doi: 10.1039/c0cs00156b. [DOI] [PubMed] [Google Scholar]
- 7.a Liu WB, Xia JB, You SL. Top. Curr. Chem. 2012;38:155. [Google Scholar]; b Tosatti P, Nelson A, Marsden SP. Org. Biomol. Chem. 2012;10:3147. doi: 10.1039/c2ob07086c. [DOI] [PubMed] [Google Scholar]; c Hartwig JF, Pouy MJ. Top. Organometal. Chem. 2011;34:169. [Google Scholar]; d Hartwig JF, Stanley LM. Acc. Chem. Res. 2010;43:1461. doi: 10.1021/ar100047x. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Bartels B, Garcia-Yebra C, Rominger F, Helmchen G. Eur. J. Inorg. Chem. 2002:2569. [Google Scholar]; f Takeuchi R, Kashio M. Angew Chem Int Edit. 1997;36:263. [Google Scholar]; g Polet D, Alexakis A, Tissot-Croset K, Corminboeuf C, Ditrich K. Chem.-Eur. J. 2006;12:3596. doi: 10.1002/chem.200501180. [DOI] [PubMed] [Google Scholar]
- 8.a Satyanarayana G, Pflasterer D, Helmchen G. Eur. J. Org. Chem. 2011:6877. [Google Scholar]; b Kim D, Lee JS, Kong SB, Han H. Angew. Chem., Int. Ed. 2013;52:4203. doi: 10.1002/anie.201209112. [DOI] [PubMed] [Google Scholar]; c Kim D, Reddy S, Singh OV, Lee JS, Kong SB, Han H. Org. Lett. 2013;15:512. doi: 10.1021/ol3033237. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji J, Sakai K, Nagashima H, Shimizu I. Tetrahedron Lett. 1981;22:131. [Google Scholar]
- 10.Hansson S, Heumann A, Rein T, Akermark B. J. Org. Chem. 1990;55:975. [Google Scholar]
- 11.a Campbell AN, White PB, Guzei IA, Stahl SS. J. Am. Chem. Soc. 2010;132:15116. doi: 10.1021/ja105829t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Thiery E, Aouf C, Belloy J, Harakat D, Le Bras J, Muzart J. J. Org. Chem. 2010;75:1771. doi: 10.1021/jo902587u. [DOI] [PubMed] [Google Scholar]; c Liu GS, Wu YC. Top. Curr. Chem. 2010;292:195. doi: 10.1007/128_2009_16. [DOI] [PubMed] [Google Scholar]
- 12.a Kharasch MS, Sosnovsky G. J. Am. Chem. Soc. 1958;80:756. [Google Scholar]; b Beckwith ALJ, Zavitsas AA. J. Am. Chem. Soc. 1986;108:8230. [Google Scholar]; c Rawlinson Dj, Sosnovsk G. Synth.-Int. J. Methods. 1972:1. [Google Scholar]
- 13.a Gooßen LJ, Rodríguez N, Gooßen K. Angew. Chem., Int. Ed. 2008;47:3100. doi: 10.1002/anie.200704782. [DOI] [PubMed] [Google Scholar]; b Malkov AV, Baxendale IR, Bella M, Langer V, Fawcett J, Russell DR, Mansfield DJ, Valko M, Kocovsky P. Organometallics. 2001;20:673. [Google Scholar]; c Bayardon J, Sinou D, Guala M, Desimoni G. Tetrahedron-Asymmetr. 2004;15:3195. [Google Scholar]
- 14.a Wei Y, Yoshikai N. Org. Lett. 2011;13:5504. doi: 10.1021/ol202229w. [DOI] [PubMed] [Google Scholar]; b Liu XZ, Hii KK. J. Org. Chem. 2011;76:8022. doi: 10.1021/jo201164m. [DOI] [PubMed] [Google Scholar]; c Giri R, Liang J, Lei JG, Li JJ, Wang DH, Chen X, Naggar IC, Guo CY, Foxman BM, Yu JQ. Angew. Chem., Int. Ed. 2005;44:7420. doi: 10.1002/anie.200502767. [DOI] [PubMed] [Google Scholar]; d Tsuji J, Nagashima H. Tetrahedron. 1984;40:2699. [Google Scholar]; e Bird C, Booth BL, Haszeldine RN, Neuss GRH, Smith MA, Flood A. J. Chem. Soc., Dalton Trans. 1982:1109. [Google Scholar]; f Yin GY, Wu YC, Liu GS. J. Am. Chem. Soc. 2010;132:11978. doi: 10.1021/ja1030936. [DOI] [PubMed] [Google Scholar]; g Vickers CJ, Mei TS, Yu JQ. Org. Lett. 2010;12:2511. doi: 10.1021/ol1007108. [DOI] [PubMed] [Google Scholar]
- 15.a Helmchen G. In: In Iridium Complexes in Organic Synthesis. Oro LA, Claver C, editors. Wiley-VCH; Weinheim, Germany: 2009. p. 211. [Google Scholar]; b Miyabe H, Takemoto Y. Synlett. 2005:1641. [Google Scholar]; c Takeuchi R, Kezuka S. Synthesis-Stuttgart. 2006:3349. [Google Scholar]
- 16.Madrahimov ST, Hartwig JF. J. Am. Chem. Soc. 2012;134:8136. doi: 10.1021/ja212217j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a Ball M, Gaunt MJ, Hook DF, Jessiman AS, Kawahara S, Orsini P, Scolaro A, Talbot AC, Tanner HR, Yamanoi S, Ley SV. Angew. Chem., Int. Ed. 2005;44:5433. doi: 10.1002/anie.200502008. [DOI] [PubMed] [Google Scholar]; b Gaunt MJ, Jessiman AS, Orsini P, Tanner HR, Hook DF, Ley SV. Org. Lett. 2003;5:4819. doi: 10.1021/ol035849+. [DOI] [PubMed] [Google Scholar]; c Gaunt MJ, Hook DF, Tanner HR, Ley SV. Org. Lett. 2003;5:4815. doi: 10.1021/ol035848h. [DOI] [PubMed] [Google Scholar]
- 18.a Narayan ARH, Sherman DH. Science. 2013;339:283. doi: 10.1126/science.1233324. [DOI] [PubMed] [Google Scholar]; b Meinhold P, Peters MW, Chen MMY, Takahashi K, Arnold FH. Chembiochem. 2005;6:1765. doi: 10.1002/cbic.200500261. [DOI] [PubMed] [Google Scholar]; c Fasan R, Chen MM, Crook NC, Arnold FH. Angew. Chem., Int. Ed. 2007;46:8414. doi: 10.1002/anie.200702616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.