Abstract
Chronic N-methyl-D-aspartate receptor (NMDAR) blockade with high affinity competitive and uncompetitive antagonists can lead to seizure exacerbation, presumably due to an imbalance in glutamatergic and GABAergic transmission. Acute administration of the moderate affinity NMDAR antagonist memantine in vivo has been associated with pro- and anticonvulsive properties. Chronic treatment with memantine can exacerbate seizures. Therefore, we hypothesized that chronic memantine treatment would increase glutamatergic and decrease GABAergic transmission, similar to high affinity competitive and uncompetitive antagonists. To test this hypothesis, organotypic hippocampal slice culture were treated for 17–21 days with memantine and then subjected to electrophysiological recordings. Whole-cell recordings from dentate granule cells revealed that chronic memantine treatment slightly, but significantly increased sEPSC frequency, mEPSC amplitude and mEPSC charge transfer, consistent with minimally increased glutamatergic transmission. Chronic memantine treatment also increased both sIPSC and mIPSC frequency and amplitude, suggestive of increased GABAergic transmission. Results suggest that a simple imbalance between glutamatergic and GABAergic neurotransmission may not underlie memantine’s ictogenic properties. That said, glutamatergic and GABAergic transmission were assayed independently of one another in the current study. More complex interactions between glutamatergic and GABAergic transmission may prevail under conditions of intact circuitry.
1. Introduction1
N-methyl-D-aspartate receptors (NMDAR) are one of the three ligand-gated ion channels that mediate glutamatergic transmission. NMDAR activation is required for normal brain function, but too much NMDAR activation is associated with pathophysiology in numerous neurological disorders, including epilepsy, brain trauma, stroke, depression, and neurodegenerative disease (Dingledine et al., 1990; Lipton, 2007). Chronic NMDAR blockade with high-affinity competitive and uncompetitive (pore-blocking) antagonists leads to increased glutamatergic synapse connectivity, increased ionotropic glutamate receptor expression and function, increased glutamatergic transmission, and reduced GABAAR-mediated charge transfer (Bausch et al., 2006; Bear et al., 1990; Cline et al., 1987; He et al., 2013; Lin and Constantine-Paton, 1998; McKinney et al., 1999; Muir and Lees, 1995; O’Brien et al., 1998; Parsons et al., 1999; Rao and Craig, 1997). This imbalance between increased glutamatergic and decreased GABAergic transmission results in increased neuronal excitability and is thought to underlie the paradoxical seizure exacerbation that can occur following chronic NMDAR blockade (Bausch et al., 2006; Sveinbjornsdottir et al. 1993; Wang and Bausch, 2004).
Memantine is a moderate-affinity uncompetitive NMDAR antagonist currently used clinically to treat Alzheimer’s disease and dementia. Such NMDAR antagonists were postulated to more specifically target areas of brain pathology because of transient use- and voltage-dependent block and faster open channel dissociation kinetics at elevated glutamate concentrations associated with excitotoxicity and seizures. Thus, moderate-affinity uncompetitive NMDAR antagonists held promise for treatment against numerous neurological disorders, including epilepsy (Chen et al., 1992; Lipton, 1993; Rogawski, 1993; Parsons et al., 1995, 1999). However, although acute memantine administration in in vivo epilepsy models showed anticonvulsive properties, proconvulsive effects also have been reported at 20–60 mg/kg doses (Meldrum et al., 1986; Loscher, 1998; Mareš and Mikulecká, 2009). Chronic treatment with a clinically relevant concentration of memantine also exacerbated seizure-like events (SLE) in an in vitro organotypic hippocampal slice culture model of epileptogenesis (Wang and Bausch, 2004). These finding suggest that memantine can enhance neuronal excitability under epileptogenic conditions. Accordingly, the memantine patient information insert carries an advisory for patients with a seizure history. While chronic memantine treatment was shown previously to increase NMDAR binding sites in aged animals (Bresink et al., 1995), the mechanism(s) underlying memantine’s proconvulsant and seizure exacerbating properties following chronic treatment under epileptogenic conditions remain unknown. In this study, we examined chronic memantine-induced changes in glutamatergic and GABAAR-mediated neurotransmission in the same in vitro model of deafferentation-induced epileptogenesis used previously to document seizure exacerbation. Based upon results from our previous study (Wang and Bausch, 2004) and the ability of increased glutamatergic and decreased GABAergic transmission to increase neuronal excitability and exacerbate seizures, we hypothesized that chronic memantine treatment would increase glutamatergic and decrease GABAergic neurotransmission.
2. Methods
2.1. Organotypic hippocampal slice cultures
Organotypic hippocampal slice cultures were used as a model of deafferentation-induced epileptogenesis (for review see Bausch, 2009) and were prepared from postnatal day 10–11 Sprague-Dawley rat pups (Taconic, Germantown, NY) using the Stoppini method (Stoppini et al., 1991) as described previously (Bausch et al., 2006; He et al., 2012, 2013; Wang and Bausch, 2004). All treatment of animals complied with National Institutes of Health, Department of Defense and institutional guidelines and was approved by the Uniformed Services University Institutional Animal Care and Use Committee. All efforts were made to reduce animal discomfort, reduce animal numbers and use alternatives to in vivo techniques. Slice cultures were treated with vehicle or memantine hydrochloride (1 μM; Tocris Cookson) from 0 day in vitro (DIV) through the end of the culture period (17–21 DIV); medium was changed three times/week. Memantine (1 μM; ~IC50 at −100 to −70 mV; see Parsons et al., 1999) was chosen because memantine is relatively selective for NMDAR at concentrations up to 1 μM (Parsons et al., 1999; but see Johnson and Kotermanski, 2006) and slightly less than 1 μM memantine is found in serum and cerebrospinal fluid following administration of clinically tolerated doses (Kornhuber and Quack, 1995). Concentration-response analyses were not conducted due to the small chronic memantine-induced effects at 1 μM (see Results) and questionable clinical relevance at higher concentrations. Data presented herein for vehicle-treated hippocampal slice cultures were published previously (He et al., 2012, 2013) and constitute a shared control group to reduce animal numbers. Despite disparate publication dates, all cultures treated with vehicle and memantine were always studied concurrently under identical experimental conditions.
2.2. Electrophysiological recordings and analysis
Whole-cell recordings of dentate granule cell membrane properties and postsynaptic currents (PSCs) were acquired after >20 min memantine/medium washout and analyzed as described previously (Bausch et al,. 2006; He et al., 2012, 2013). Dentate granule cells were chosen because granule cells are thought to restrict invasion of pathological hyperexcitability into the hippocampus (Behr et al., 1996; Behr et al., 1998; Collins et al., 1983) and our previous results showed that chronic memantine treatment exacerbated seizure-like activity involving granule cells (Wang and Bausch 2004). Briefly, recordings were conducted in a submerged recording chamber perfused (2–3 ml/min) with artificial cerebrospinal fluid (aCSF) [(in mM): 124 NaCl, 4.9 KCl, 1.2 KH2PO4, 2.4 MgSO4, 2.5 CaCl2, 25.6 NaHCO3, and 10 glucose, equilibrated with 95% O2, 5% CO2] at room temperature to minimize seizure-like events (Bausch et al. 2006; Bausch and McNamara 2004; 2000). Tetrodotoxin (TTX, 1 μM; Sigma), D(−)-2-amino-5-phosphonopentanoic acid (D-APV, 50 μM; Tocris Cookson, Ellisville, MO), bicuculline methiodide (BMI, 10 μM; Tocris Cookson), and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM; Tocris Cookson) were diluted immediately prior to use and acutely applied by bath superfusion. Recording pipettes were filled with (in mM): K-gluconate 125, KCl 13, HEPES 10, EGTA 10, MgATP 2 (pH 7.2 with KOH). QX-314 bromide (5 mM; Tocris Cookson) was added to the pipette solution immediately prior to use in experiments recording spontaneous excitatory PSCs (sEPSCs). Action potential and membrane properties were collected using current-clamp recordings within 2 min. of establishing whole-cell configuration. PSCs were recorded using voltage-clamp; the membrane potential was clamped at −70 mV and recordings were excluded if the RMP was more positive than −50 mV or series resistance was >15 MΩ or varied >15%. MiniAnalysis software (Synaptosoft Inc., Fort Lee, NJ) was used for analyses of PSCs and generation of cumulative probability plots as described previously (He et al., 2012, 2013). Briefly, cumulative probability plots were generated from PSCs pooled from all cells. PSC detection threshold was set at 8 pA, amplitude was measured at peak negativity, rise and decay times were measured from 10–100% and 100-10%, respectively. All putative PSCs were then evaluated manually for shape and decay. Investigators were blinded to experimental groupings for all data analyses. All sEPSCs, mEPSCs, and mIPSCs from each cell were compiled according to treatment group and compilations used to generate cumulative probability plots. Spontaneous IPSCs were treated similarly, except due to their very high numbers in some cells, up to 80 sIPSCs from each cell were selected at a fixed sampling interval to generate cumulative probability plots for sIPSC amplitude, charge transfer, rise time, and decay time. Most statistical analyses were performed with Sigma Stat software (SPSS Inc., Chicago, Illinois). Significance was defined as P ≤ 0.05. Cumulative probability distributions were tested for significance using a two-tailed Kolmogorov-Smirnov test using MiniAnalysis software; significance was defined as P ≤ 0.025.
3. Results
3.1. Chronic memantine treatment differentially altered sEPSCs and mEPSCs
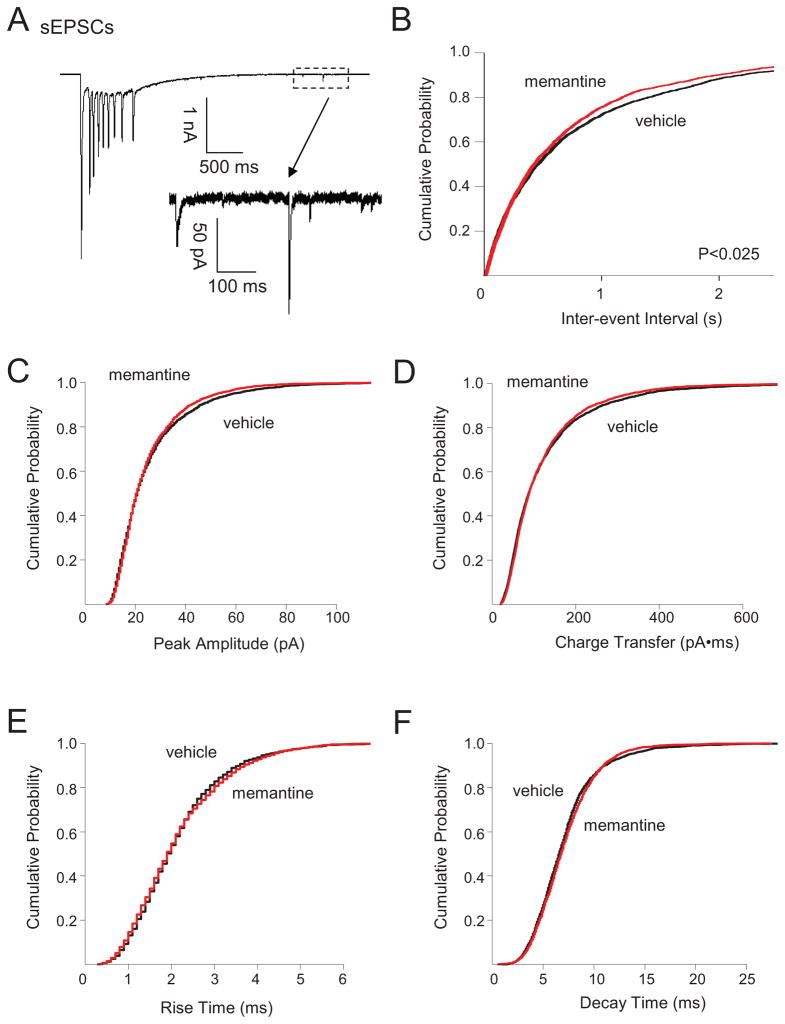
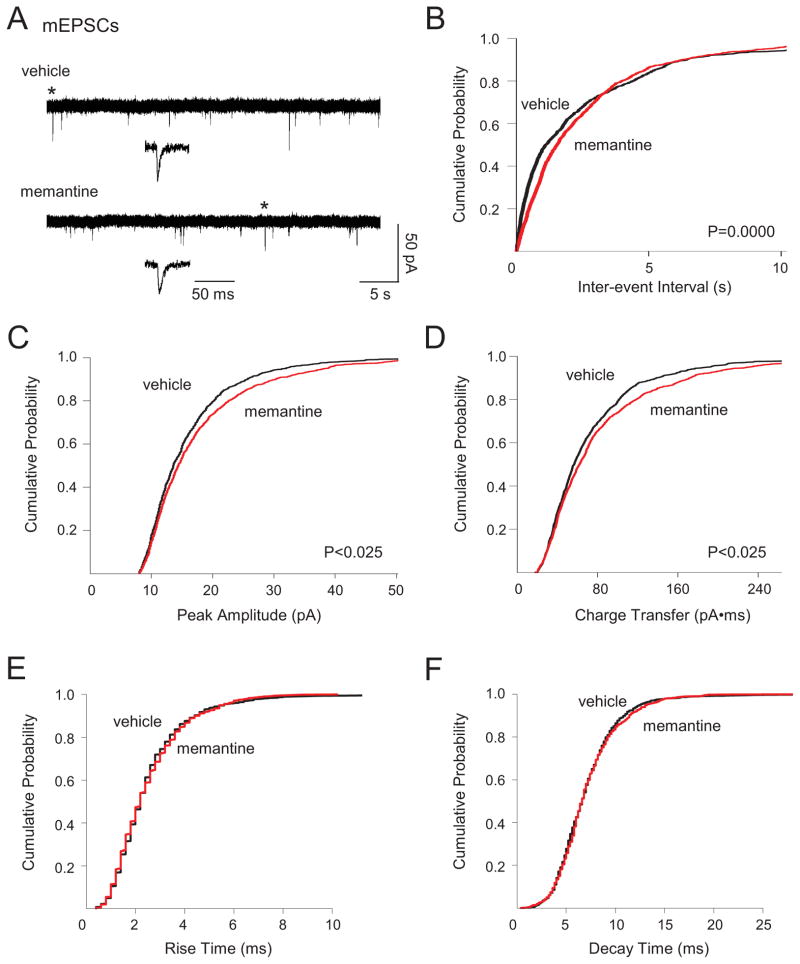
To begin to assess chronic memantine-induced changes in glutamatergic circuits, we first recorded sEPSCs in individual dentate granule cells. We found previously that sEPSCs in granule cells from vehicle-treated organotypic hippocampal slice cultures could be separated into two distinct populations based upon amplitude. Large amplitude sEPSCs (>2 nA, sEPSClarge) were characterized by low frequency, long duration, and multiple large peaks (Fig. 1A, top; Table 1) and represented the synaptic component of epileptiform bursts in single granule cells. Small amplitude sEPSCS (<300 pA, sEPSCsmall) were characterized by higher frequency, shorter decay, and a single peak (Fig. 1A, bottom) (Bausch and McNamara, 2004; He et al., 2013). Chronic memantine treatment did not significantly affect sEPSClarge measures (Table 1) or sEPSCsmall amplitude, charge transfer, or kinetics (Fig. 1C–F), but did elicit a very slight, but significant increase in sEPSCsmall frequency (Fig. 1B). We next recorded action potential-independent miniature EPSCs (mEPSCs) in dentate granule cells to examine changes in glutamatergic transmission at individual synapses. Chronic memantine treatment slightly, but significantly decreased mEPSC frequency (Fig. 2B). Chronic memantine also slightly, but significantly increased mEPSC amplitude and charge transfer (Fig. 2C, D, respectively), but did not affect mEPSC kinetics (Fig. 2E, F).
Fig. 1.
Chronic memantine treatment increased sEPSC frequency. Spontaneous EPSCs were recorded in aCSF containing BMI (10 μM) and pipette solution containing QX-314 (5 mM) to block action potentials. Spontaneous EPSCs from all cells were used to generate cumulative probability plots and included 2507 sEPSCs for vehicle and 2240 sEPSCs for memantine (114–429 sEPSCs/cell, vehicle; 44–473, memantine). A. Traces show representative (top) large-amplitude sEPSC (sEPSCslarge) and (bottom) small amplitude sEPSCs (sEPSCssmall, expanded from the boxed area in the top trace). Cumulative probability plots show B. minimally increased sEPSCssmall frequency, but no change in sEPSCssmall C. amplitude, D. charge transfer, E. rise time or F. decay time in granule cells from slice cultures treated chronically with memantine compared to vehicle. Number of granule cells/slice cultures is: vehicle, n=11; memantine, n=9. A two-tailed Kolmogorov-Smirnov test was used to assess significance. Data presented for vehicle-treated granule cells are from He et al, 2013 and constitute a shared control group to reduce animal numbers.
Table 1.
sEPSCslarge
| Treatment | n | Frequency (Hz) | Amplitude (pA) | Duration (ms) |
|---|---|---|---|---|
| vehicle | 11 | 0.026 ± 0.003 | −5110 ± 531 | 1874 ± 70 |
| memantine | 9 | 0.033 ± 0.005 | −4706 ± 317 | 1770 ± 204 |
Means ± SEM. n, the number of granule cells/hippocampal slice cultures. Data presented for vehicle-treated granule cells are from He et al, 2013, and represent a shared control group to reduce animal numbers.
Fig. 2.
Chronic memantine treatment altered mEPSCs. Miniature EPSCs were recorded in aCSF containing BMI (10 μM) and TTX (1 μM). Miniature EPSCs from all cells were used to generate cumulative probability plots and included 1004 mEPSCs for vehicle and 809 mEPSCs for memantine (7–192 mEPSCs/cell, vehicle; 9–132, memantine). A. Traces show representative mEPSCs recorded in granule cells from cultures treated chronically with vehicle (top) or memantine (bottom). Traces in insets are an expanded time scale of individual mEPSCs marked by * in the traces immediately above the insets. Cumulative probability plots show decreased mEPSC B. frequency, increased C. amplitude, and D. charge transfer, but no change in mEPSC E. rise time or F. decay time in granule cells from slice cultures treated chronically with memantine compared to vehicle. Vertical scale bar in A bottom, right applies to all traces in A. Horizontal scale bar in A bottom, right applies to long traces in A; that in A inset, to all traces in insets. Number of granule cells/slice cultures is: vehicle, n=18; memantine, n=20. A two-tailed Kolmogorov-Smirnov test was used to assess significance. Data presented for vehicle-treated granule cells are from He et al, 2013 and constitute a shared control group to reduce animal numbers.
3.2. Chronic memantine increased both sIPSC and mIPSC
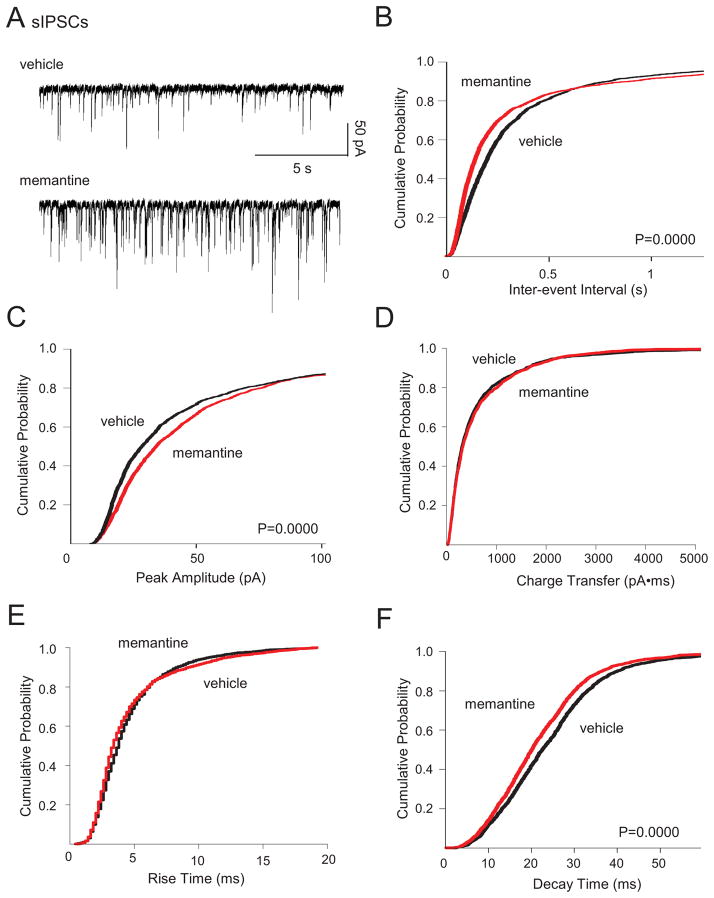
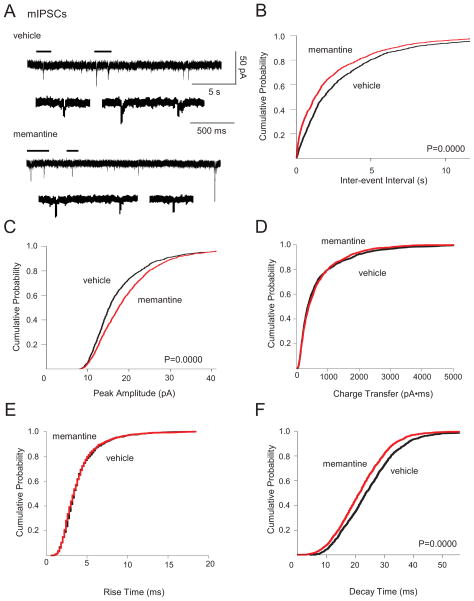
We next documented chronic memantine-induced changes in GABAA receptor-mediated neurotransmission by recording spontaneous inhibitory PSCs (sIPSCs) and miniature inhibitory PSCs (mIPSCs) in individual dentate granule cells. Chronic memantine treatment increased sIPSC and mIPSC frequency and amplitude (Fig. 3A–C, 4A–C, respectively). Chronic memantine also decreased sIPSC and mIPSC decay time (Fig. 3F and Fig. 4A, F, respectively), without affecting sIPSC or mIPSC charge transfer or rise time (Fig. 3D, E and Fig. 4D, E, respectively).
Fig. 3.
Chronic memantine treatment increased sIPSCs. Spontaneous IPSCs were recorded in aCSF containing D-APV (50 μM) and CNQX (10 μM). IPSCs from all cells were used to generate cumulative probability plots and included 1524 sIPSCs for vehicle and 1587 sIPSCs for memantine (30–80 sIPSCs/cell, vehicle; 57–80, memantine). A. Traces show representative sIPSCs recorded in granule cells from cultures treated chronically with vehicle (top) or memantine (bottom). Cumulative probability plots show increased sIPSC B. frequency and C. amplitude; no change in sIPSC D. charge transfer or E. rise time; and decreased F. decay time in granule cells from slice cultures treated chronically with memantine compared to vehicle. Scale bars in A bottom, right apply to all traces in A. Number of granule cells/slice cultures is: vehicle, n=21; memantine, n=21. A two-tailed Kolmogorov-Smirnov test was used to assess significance. Data presented for vehicle-treated granule cells are from He et al, 2012 and constitute a shared control group to reduce animal numbers.
Fig. 4.
Chronic memantine treatment increased mIPSCs. Miniature IPSCs were recorded in aCSF containing D-APV (50 μM), CNQX (10 μM), and TTX (1 μM). IPSCs from all cells were used to generate cumulative probability plots and included 1058 mIPSCs for vehicle and 1260 mIPSCs for memantine (17–92 mIPSCs/cell, vehicle; 16–260, memantine). A. Traces show representative mIPSCs recorded in granule cells from cultures treated chronically with vehicle (top) or memantine (bottom). Lower traces are expanded time scales of periods marked by horizontal lines above the upper traces. Cumulative probability plots show increased mIPSC B. frequency and C. amplitude; no change in sIPSC D. charge transfer or E. rise time; and decreased F. decay time in granule cells from slice cultures treated chronically with memantine compared to vehicle. Vertical scale bar in A top, right applies to all traces in A. Horizontal scale bar in A top, right applies to all upper traces in A; that in A vehicle, expanded to all lower expanded traces in A. Number of granule cells/slice cultures is: vehicle, n=22; memantine, n=21. A two-tailed Kolmogorov-Smirnov test was used to assess significance. Data presented for vehicle-treated granule cells are from He et al, 2012 and constitute a shared control group to reduce animal numbers.
3.3. RMP was slightly more negative following chronic memantine treatment
Lastly, we measured chronic memantine-induced changes in action potential and membrane properties in individual dentate granule cells because these properties could influence PSC measures. Comparison of chronic memantine treatment to vehicle showed no differences in granule cell input resistance, action potential threshold or number of action potentials fired at threshold or in response to 200 pA input. However, resting membrane potential (RMP) was slightly, but significantly more negative following chronic memantine treatment (Table 3).
4. Discussion
4.1. Summary of results
To our knowledge, this is the first report demonstrating alterations in glutamatergic transmission, GABAergic transmission and resting membrane potential following chronic memantine treatment. We originally hypothesized that chronic memantine exposure would increase glutamatergic and decrease GABAergic neurotransmission. In regard to glutamatergic transmission alone, the very small chronic memantine-induced increase in sEPSCsmall frequency suggests minimally increased excitability in glutamatergic circuits synapsing onto dentate granule cells, while decreased mEPSC frequency suggests decreased excitatory synapse number and/or glutamate release probability at individual synapses. Increased mEPSC amplitude and charge transfer suggest increased glutamate receptor function. The discrepancy between increased mEPSC and unaltered sEPSC amplitudes may reflect a differential effect of chronic memantine on action potential-independent and action potential-dependent neurotransmitter vesicle pools (Fredj and Burrone, 2009). Regardless, these latter findings, are consistent with our hypothesis that chronic memantine treatment would increase glutamatergic transmission. That said, the slightly more negative RMP following chronic memantine treatment would reduce relative action potential threshold and thereby may negate the slightly increased glutamatergic transmission when placed in the context of circuit excitability. GABAergic transmission also may modulate excitatory circuit dynamics. In terms of GABAergic transmission alone, chronic memantine-induced increases in sIPSC and mIPSC frequency and amplitude suggest increased excitability in GABAergic circuits synapsing onto dentate granule cells, increased GABAergic synapse number or probability of release and increased GABAA receptor function, respectively. Increased GABAergic transmission following chronic memantine treatment stands in direct contrast to our original hypothesis and suggests that a simple imbalance between glutamatergic and GABAergic neurotransmission may not underlie memantine’s ictogenic properties. That said, glutamatergic and GABAergic transmission were assayed independently of one another in our current study. More complex interactions between glutamatergic and GABAergic transmission may prevail under conditions of intact circuitry.
4.2. Comparison with the NMDAR antagonist, D-APV
We previously reported no spontaneous seizure-like activity in organotypic hippocampal slice cultures treated chronically with vehicle or the high-affinity competitive antagonist, D-APV in physiological recording buffer. However, multiple recurrent seizure-like events were recorded in 40% of chronic memantine-treated cultures under identical conditions. Following GABAA receptor blockade, chronic D-APV-treated cultures exhibited a 3% and 47% increase in seizure number and duration, respectively compared to vehicle. In contrast, chronic memantine-treated cultures showed a 72% increase in seizure number and an 82% increase in total seizure duration (Wang and Bausch, 2004). Therefore, we anticipated that chronic memantine treatment would elicit similar and perhaps enhanced changes in glutamatergic transmission, GABAergic transmission and intrinsic granule cell membrane properties compared to those reported previously following chronic D-APV treatment (Bausch et al., 2006; He et al., 2012, 2013). However, chronic memantine increased sEPSC frequency and mEPSC amplitude and charge transfer to a lesser degree than previously reported for chronic D-APV. Furthermore, chronic D-APV increased sEPSClarge duration and peak number as well as sEPSCsmall amplitude and charge transfer (He et al., 2013), but chronic memantine did not significantly affect these measures. Thus, chronic memantine increased glutamatergic transmission onto dentate granule cells to a lesser extent than previously reported for chronic D-APV treatment. Even greater discrepencies between chronic D-APV and memantine treatment were apparent in GABAergic transmission and intrinsic granule cell properties. While chronic D-APV modestly reduced sIPSC and mIPSC measures (Bausch et al., 2006; He eat al., 2012), chronic memantine increased both sIPSCs and mIPSCs. Chronic memantine slightly reduced resting membrane potential, but chronic D-APV treatment resulted in a slightly more negative action potential threshold. Reasons for these disparate results, despite targeting the same receptor, are unclear. Findings that chronic memantine increased sEPSC and mEPSC measures to a lesser degree than previously reported for chronic D-APV suggest that glutamatergic function was increased in proportion to the degree of NMDAR inhibition. That said, the degree of NMDAR inhibition cannot explain differences between the two NMDAR antagonists on GABAergic transmission and membrane properties and degree of inhibition is not the sole differential property between memantine and D-APV. Unlike D-APV, memantine is a voltage- and use-dependent blocker, can become trapped in the ligand-gated channel, and has rapid dissociation kinetics (for review, see Parsons et al., 2007). Other differences that may play a role in memantine’s differential effects compared to D-APV include memantine’s greater propensity to target extrasynaptic over synaptic NMDAR (Xia et al., 2010) and its affinity for α7-containing nicotinic acetylcholine receptors (Aracava et al., 2005) and 5-HT3 receptors (Rammes et al., 2001) at the clinically relevant concentration used in this study.
4.3. Role of GABAergic transmission on seizures
On a simplistic level, chronic memantine-induced increases in GABAergic transmission onto dentate granule cells coupled with relatively small changes in excitatory transmission and intrinsic membrane properties predict an overall decrease in neuronal excitability, rather than seizure exacerbation. However, the role(s) of GABAergic transmission in the epileptic brain are complex and there is ample precedent for opposite effects on GABAergic transmission than predicted by simple models. In general, GABAA receptor-mediated excitation, which occurs during periods of high activity, and GABAA receptor-mediated synchronization of excitatory circuits have been reported and postulated to explain GABA-mediated seizure exacerbation (for review see Avoli and de Curtis, 2011). Consistent with this idea, chronic memantine is the only NMDAR antagonist that both a) elicited spontaneous SLE in the presence of GABAA receptor-mediated transmission and b) increased GABAergic transmission in the dentate gyrus (Wang and Bausch, 2004; Bausch et al., 2006; He et al., 2012). Conversely, NMDAR are highly expressed in hippocampal GABAergic interneurons (Monyer et al., 1994; Moriyoshi et al., 1991), which raises the possibility that altered IPSCs occurred secondary to chronic memantine-induced alterations in glutamatergic inputs onto GABAergic interneurons to achieve a homeostatic balance between interneuron input and output. In intact circuits these homeostatic changes may negate one another and result in no overall change in dentate granule cell inhibition. However, neither of these possibilities fully explain the further SLE exacerbation when GABAA receptor-mediated transmission was blocked following chronic memantine treatment (Wang and Bausch, 2004). One possibility arises from the facts that chronic memantine-induced seizure exacerbation was documented at near physiological recording temperatures (Wang and Bausch, 2004), while PSCs in the current study were recorded at room temperature. Temperature plays an important role in ion permeability and pump function, neurotransmitter release and reuptake, resting membrane potential, firing rates, neuronal synchronization, and circuit dynamics. Thus, the balance of glutamatergic and GABAergic transmission may be different at near physiological temperatures and under these conditions, chronic memantine-induced increases in glutamatergic transmission may be more pronounced and contribute to seizure exacerbation. Conversely, chronic memantine-induced alterations in alternate, non-granule cell containing neuronal circuits may be responsible for memantine’s seizure exacerbating properties. These non-granule cell contributions normally may be under GABAergic control and become unmasked following GABAA receptor blockade. Indeed, GABAergic transmission has been shown to differentially facilitate and control synaptic transmission and synchronization in a distinct hippocampal formation circuit-dependent manner (Gafurov and Bausch, 2013). Thus, the contributions of GABAergic transmission to memantine’s paradoxical seizure exacerbation remain to be determined.
4.4. Conclusions
Overall, although our documented chronic memantine-induced increases in glutamatergic and GABAergic neurotransmission and more negative RMP were significant, they were relatively small. These rather limited changes may explain in part why memantine can exacerbate seizures in susceptible individuals, but otherwise is generally well-tolerated clinically.
Table 2.
Membrane Properties
| Treatment | RIN (MΩ) | RMP (mV) | Action Potential
|
||
|---|---|---|---|---|---|
| Threshold (mV) | Number at Threshold | Number at 200 pA | |||
| Granule cell | |||||
| vehicle | 149 ± 7 (50) | −58.5 ± 0.2 (50) | −36.4 ± 0.3 (50) | 2.0 (50) | 6.0 (50) |
| memantine | 133 ± 5 (58) | −59.7 ± 0.3 (58)* | −36.4 ± 0.2 (58) | 2.0 (58) | 5.0 (58) |
Means ± SEM (RMP, RIN, action potential Threshold); medians (action potential numbers).
Abbreviations: RIN, input resistance; RMP, resting membrane potential. The number of neurons/hippocampal slice cultures is indicated in parentheses.
p<0.01, two-tailed t-test, different than vehicle. Data presented for vehicle-treated granule cells are from He et al., 2013, and represent a shared control group to reduce animal numbers.
Highlights.
Chronic memantine treatment slightly increased glutamatergic transmission
Chronic memantine treatment increased GABAergic transmission
Chronic memantine treatment elicited more negative resting membrane potential
Acknowledgments
We thank Dr. Li-Rong Shao for helpful discussions. Data presented for vehicle-treated granule cells were published previously (He et al., 2012, 2013) and constitute a shared control group to reduce animal numbers. Work was supported by the Defense Brain and Spinal Cord Injury Program, Congressionally Directed Medical Research Programs award W81XWH-04-1-0065/PR030035 and National Institute of Neurological Disorders and Stroke grant NS045964 to SBB and a Uniformed Services University Intramural Student Research award to SH. Funding sources had no involvement in: study design; data collection, analysis, or interpretation; or manuscript preparation. The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense or Uniformed Services University.
Footnotes
Abbreviations: aCSF, artificial cerebrospinal fluid; BMI, bicuculline methiodide; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; D-APV, D(−)-2-amino-5-phosphonopentanoic acid; GABA, γ-aminobutyric acid; mEPSC, miniature excitatory postsynaptic current; mIPSC, miniature inhibitory postsynaptic current; NMDAR, N-methyl-D-aspartate receptors; PSC, postsynaptic current; RMP, resting membrane potential; sEPSC, spontaneous excitatory postsynaptic current; sEPSClarge, large amplitude spontaneous excitatory postsynaptic current; sEPSCsmall, small amplitude spontaneous excitatory postsynaptic current; sIPSC, spontaneous inhibitory postsynaptic current; SEM, standard error of the mean; TTX, tetrodotoxin
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aracava Y, Pereira EF, Maelicke A, Albuquerque EX. Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons. J Pharmacol Exp Ther. 2005;312:1195–1205. doi: 10.1124/jpet.104.077172. [DOI] [PubMed] [Google Scholar]
- Avoli M, de Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol. 2011;95:104–132. doi: 10.1016/j.pneurobio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausch SB. Organotypic hippocampal slice cultures as a model of limbic epileptogenesis. In: Baraban SC, editor. Neuromethods: Innovations in Epilepsy Research (Neuromethods) 40. Humana Press; New York: 2009. pp. 183–201. [Google Scholar]
- Bausch SB, He S, Petrova Y, Wang XM, McNamara JO. Plasticity of both excitatory and inhibitory synapses is associated with seizures induced by removal of chronic blockade of activity in cultured hippocampus. J Neurophysiol. 2006;96:2151–2167. doi: 10.1152/jn.00355.2006. [DOI] [PubMed] [Google Scholar]
- Bausch SB, McNamara JO. Contributions of mossy fiber and CA1 pyramidal cell sprouting to dentate granule cell hyperexcitability in kainic acid-treated hippocampal slice cultures. J Neurophysiol. 2004;92:3582–3595. doi: 10.1152/jn.01028.2003. [DOI] [PubMed] [Google Scholar]
- Bausch SB, McNamara JO. Synaptic connections from multiple subfields contribute to granule cell hyperexcitability in hippocampal slice cultures. J Neurophysiol. 2000;84:2918–2932. doi: 10.1152/jn.2000.84.6.2918. [DOI] [PubMed] [Google Scholar]
- Bear MF, Kleinschmidt A, Gu QA, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr J, Gloveli T, Gutierrez R, Heinemann U. Spread of low Mg2+ induced epileptiform activity from the rat entorhinal cortex to the hippocampus after kindling studied in vitro. Neurosci Lett. 1996;216:41–44. doi: 10.1016/0304-3940(96)13019-6. [DOI] [PubMed] [Google Scholar]
- Behr J, Lyson KJ, Mody I. Enhanced propagation of epileptiform activity through the kindled dentate gyrus. J Neurophysiol. 1998;79:1726–1732. doi: 10.1152/jn.1998.79.4.1726. [DOI] [PubMed] [Google Scholar]
- Bresink I, Danysz W, Parsons CG, Tiedtke P, Mutschler E. Chronic treatment with the uncompetitive NMDA receptor antagonist memantine influences the polyamine and glycine binding sites of the NMDA receptor complex in aged rats. J Neural Transm. 1995;10:11–26. doi: 10.1007/BF02256626. [DOI] [PubMed] [Google Scholar]
- Chen HS, Pellegrini JW, Aggarwal SK, Lei SZ, Warach S, Jensen FE, Lipton SE. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: Therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT, Debski EA, Constantine-Paton M. N-methyl-D-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci USA. 1987;84:4342–4345. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RC, Tearse RG, Lothman EW. Functional anatomy of limbic seizures: focal discharges from medial entorhinal cortex in rat. Brain Res. 1983;280:25–40. doi: 10.1016/0006-8993(83)91170-8. [DOI] [PubMed] [Google Scholar]
- Dingledine R, McBain CJ, McNamara JO. Excitatory amino acid receptors in epilepsy. Trends Pharmacol Sci. 1990;11:334–338. doi: 10.1016/0165-6147(90)90238-4. [DOI] [PubMed] [Google Scholar]
- Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci. 2009;12:751–758. doi: 10.1038/nn.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafurov B, Bausch SB. GABAergic transmission facilitates ictogenesis and synchrony between CA3, hilus, and dentate gyrus in slices from epileptic rats. J Neurophysiol. 2013;110:441–455. doi: 10.1152/jn.00679.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Shao LR, Rittase WB, Bausch SB. Increased Kv1 channel expression may contribute to decreased sIPSC frequency following chronic inhibition of NR2B-containing NMDAR. Neuropsychopharmacology. 2012;37:1338–1356. doi: 10.1038/npp.2011.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Shao LR, Wang Y, Bausch SB. Synaptic and extrasynaptic plasticity in glutamatergic circuits involving dentate granule cells following chronic N-methyl-D-aspartate receptor inhibition. J Neurophysiol. 2013;109:1535–1547. doi: 10.1152/jn.00667.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Kotermanski SE. Mechanism of action of memantine. Curr Opin Pharmacol. 2006;6:61–67. doi: 10.1016/j.coph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Quack G. Cerebrospinal fluid and serum concentrations of the N-methyl-D-aspartate (NMDA) receptor antagonist memantine in man. Neurosci Lett. 1995;195:137–139. doi: 10.1016/0304-3940(95)11785-u. [DOI] [PubMed] [Google Scholar]
- Lin SY, Constantine-Paton M. Suppression of sprouting: An early function of NMDA receptors in the absence of AMPA/kainate receptor activity. J Neurosci. 1998;18:3725–3737. doi: 10.1523/JNEUROSCI.18-10-03725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SE. Neuroprotective effects of memantine. Reply Neurology. 1993;43:1054–1055. doi: 10.1212/wnl.43.5.1054. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- Mareš P, Mikulecká A. Different effects of two N-methyl-D-aspartate receptor antagonists on seizures, spontaneous behavior, and motor performance in immature rats. Epilepsy & Behavior. 2009;14:32–39. doi: 10.1016/j.yebeh.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Loscher W. Pharmacology of glutamate receptor antagonists in the kindling model of epilepsy. Prog Neurobiol. 1998;54:721–741. doi: 10.1016/s0301-0082(97)00092-0. [DOI] [PubMed] [Google Scholar]
- McKinney RA, Luthi A, Bandtlow CE, Gahwiler BH, Thompson SM. Selective glutamate receptor antagonists can induce or prevent axonal sprouting in rat hippocampal slice cultures. Proc Natl Acad Sci USA. 1999;96:11631–11636. doi: 10.1073/pnas.96.20.11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS, Turski L, Schwarz M, Czuczwar SJ, Sontag KH. Anticonvulsant action of 1,3-dimethyl-5-aminoadamantane. Pharmacological studies in rodents and baboon, Papio papio. Naunyn Schmiedebergs Arch Pharmacol. 1986;332:93–97. doi: 10.1007/BF00633204. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Muir KW, Lees KR. Clinical experience with excitatory amino acid antagonist drugs. Stroke. 1995;26:503–513. doi: 10.1161/01.str.26.3.503. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Quack G, Bresink I, Baran L, Przegalinski E, Kostowski W, Krzascik P, Hartmann S, Danysz W. Comparison of the potency, kinetics and voltage-dependency of open channel blockade for a series of uncompetitive NMDA antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology. 1995;34:1239–1258. doi: 10.1016/0028-3908(95)00092-k. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Stöffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Rammes G, Rupprecht R, Ferrari U, Zieglgänsberger W, Parsons CG. The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neurosci Lett. 2001;306:81–84. doi: 10.1016/s0304-3940(01)01872-9. [DOI] [PubMed] [Google Scholar]
- Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. Therapeutic potential of excitatory amino acid antagonists: channel blockers and 2,3-benzodiazepines. Trends Pharmacol Sci. 1993;14:325–331. doi: 10.1016/0165-6147(93)90005-5. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Sveinbjornsdottir S, Sander JWAS, Upton D, Thompson PJ, Patsalos PN, Hirt D, Emre M, Lowe D, Duncan JS. The excitatory amino acid antagonist D-CPP-ene (SDZ EAA-494) in patients with epilepsy. Epilepsy Research. 1993;16:165–174. doi: 10.1016/0920-1211(93)90031-2. [DOI] [PubMed] [Google Scholar]
- Wang XM, Bausch SB. Effects of distinct classes of N-methyl-D-aspartate receptor antagonists on seizures, axonal sprouting and neuronal loss in vitro: suppression by NR2B-selective antagonists. Neuropharmacology. 2004;47:1008–1020. doi: 10.1016/j.neuropharm.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Xia P, Chenm HS, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]