Within American biomedical research, as supported primarily by the National Institutes of Health (NIH) since the post–World War II era, it has been considered axiomatic that investments in discovery research will ultimately lead to the development of novel approaches to diagnostics and therapeutics that will improve the health and well-being of citizens of the United States and the broader world. I reflect here in a historical context on how one such series of investments in discovery has resulted in the emergence of the first licensed human gene therapy product in Europe, namely Glybera.1,2,3 Glybera (alipogene tiparvovec) is a recombinant adeno-associated virus (rAAV) vector with AAV2 inverted terminal repeats (ITRs) encapsidated into AAV1 capsids (so-called rAAV2/1, or, for this purpose, rAAV1), which, when administered intramuscularly, can result in expression of sufficient levels of lipoprotein lipase (LPL) to be considered a safe and effective treatment of LPL deficiency. It was licensed in the European Union in November 2012, 47 years after the near simultaneous discovery of AAV in laboratories at the NIH Bethesda campus and at the University of Pittsburgh.4,5,6,7,8 I trace key milestones in the progress from the discovery of the virus, to its use as a platform for an approved therapeutic product (Figure 1).
Figure 1.
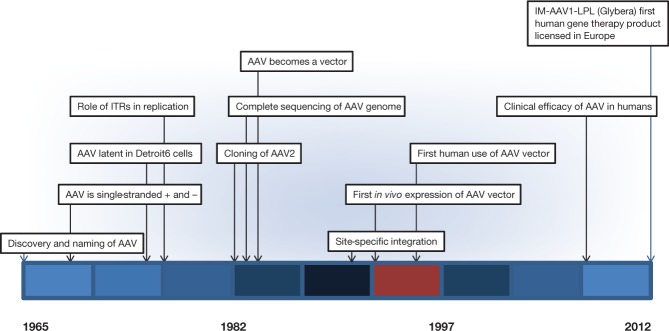
Time line of major milestones in AAV biology and vectorology. The 47 years between the discovery of adeno-associated virus (AAV) and the licensure of Glybera are depicted in three intervals, each approximately 15 years long (1965–1982, 1982–1997, and 1997–2012), with each individually marked segment representing approximately 5 years. IM, intramuscular; ITR, inverted terminal repeat.
The discovery
The emergence of electron microscopy (EM) in the 1960s revolutionized many aspects of biomedical research but perhaps none more so than the discipline of virology. The identification of a simian virus contaminating poliovirus vaccines led to widespread use of EM to examine preparations of partially purified viruses of all types.9,10 This practice led to the discovery of a number of “satellite viruses” that had the ability to replicate efficiently in the presence of other viruses. The application of this process to adenovirus preparations led two groups—one led by M. David Hoggan at the Laboratory of Infectious Diseases of the National Cancer Institute of the NIH Bethesda campus, the other by Robert W. Atchison of the Department of Epidemiology and Microbiology in the Graduate School of Public Health at the University of Pittsburgh—to identify small DNA viruses (20–25 nm in diameter) contaminating cultures of simian and human adenoviruses.7,8 These particles were proven to be antigenically distinct from adenoviruses and therefore not simply degradation products of the latter. Furthermore, Atchison deduced that “replication of the particles in cell culture was obtained only when they were inoculated simultaneously with adenoviruses. This suggests that these adenovirus-associated particles behave as defective viruses.”8
From that point on, the terms “adenovirus-associated virus” and “adeno-associated virus” were both used for a period of time, with the shorter version ultimately being accepted by the International Committee on Taxonomy of Viruses (ICTV) (http://ictvonline.org). Shortly after their discovery by EM, Neil Blacklow, an infectious disease physician at the NIH working with Hoggan, identified AAV in fresh human tissues from infants and children with adenovirus-induced diarrheal illnesses.4,5,6 The coinfection by AAV did not substantially alter the clinical course of these infections but showed a trend toward less severe symptoms in coinfected patients. Thus, the concept of AAV being a nonpathogenic virus began to emerge. In fact, a series of subsequent studies showed a tendency of AAV to inhibit the tumorigenicity of other viruses, such as oncogenic adenoviruses, papillomaviruses, and herpesviruses, a property that has yet to be fully explained or explored. Needless to say, the innocuousness of a virus can diminish somewhat the public health demand for its study.11,12,13,14,15 Subsequent study of AAV thus focused not on its clinical importance but rather on the fascinating question of how a virus expressing only two major genes could possibly function.
Growth in understanding of the AAV life cycle
In 1969, a team at the National Institute of Allergy and Infectious Diseases demonstrated that AAV is a single-stranded DNA virus, utilizing an isotopic labeling method (Figure 2).16 One of the authors of that article, Kenneth I. Berns, subsequently made a series of pivotal discoveries with regard to the AAV life cycle, including description of its persistent infection in cultured cells, a hairpin self-priming mechanism for AAV DNA replication, and, later, the ability of AAV2 to preferentially integrate within what is now termed the AAVS1 locus on human chromosome 19 (refs. 17–24). Interest in AAV biology increased as the novel mechanisms of DNA replication (including replication of the terminal repeats), messenger RNA (mRNA) splicing, and translation of the various versions of the AAV Rep and Cap proteins were elucidated.25,26,27,28,29,30,31,32,32,33,34,35,36,37,38,39,40,41,42 During this time, the laboratories of Berns at the University of Florida and Cornell Weill Medical College, Nicholas Muzyczka at the University of Florida and Stony Brook University, and Barrie Carter at the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) published many of the notable findings.
Figure 2.
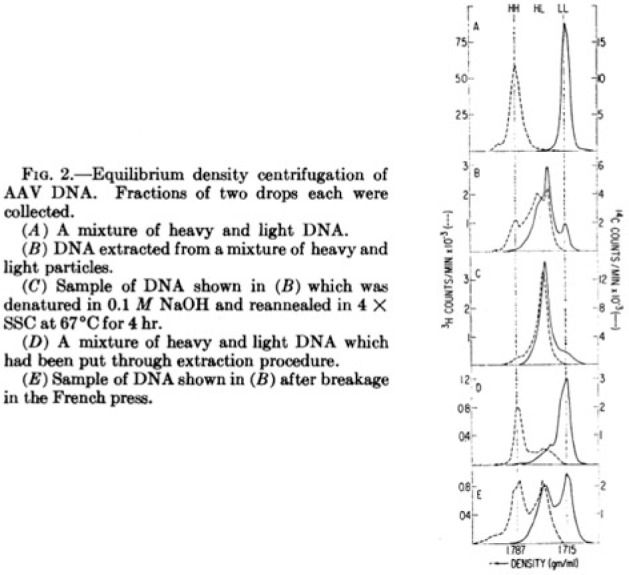
Figure from a 1969 publication on adeno-associated virus (AAV) biology. The illustration depicts an AAV's unusual property of being packaged as single-stranded DNA with equal proportions of plus and minus sense strands. Such studies spurred interest in AAV long before the advent of human gene therapy. Reprinted with permission from ref. 16.
From “not quite a virus” to “quite an amazing vector”
The secondary structure of the AAV ITRs made it technically difficult to clone AAV sequences into a bacterial plasmid. This hurdle was overcome in parallel efforts by the teams of Muzyczka (including R. Jude Samulski) at the University of Florida and Barrie Carter at the NIDDK.43,44,45 This facilitated the complete DNA sequencing of the AAV2 genome in 1983 by Arun Srivastava,46 working in the Berns laboratory at the University of Florida. In addition to making studies of the molecular biology of AAV2 more tractable, the cloning of AAV DNA facilitated the engineering of recombinant AAV2 (rAAV2) vectors, which were likewise nearly simultaneously proven to be viable for gene transfer in mammalian cells in culture by the Muzyczka and Carter laboratories in 1984.14,47 Suddenly AAV was no longer a novelty but rather a potentially useful tool in the emerging field of gene therapy. Early rAAV vectors were hampered by the contamination of preparations with wild-type AAV, a problem that was overcome by Samulski and Thomas Shenk, with their development of non-overlapping plasmid constructs, including a proviral vector plasmid comprising AAV2-ITRs flanking the gene cargo and a helper plasmid expressing Rep and Cap, in trans.48,49 The development of this relatively wild-type free system enabled rAAV2 production that was sufficiently efficient for testing in vivo gene transfer in animal models.
Early in vitro use of rAAV and the first use in humans
In the late 1980s and early 1990s, efforts by the Cystic Fibrosis Foundation (CFF) pushed forward the first in vivo gene therapy experiments in animals and humans for both recombinant adenovirus (rAd) and rAAV vectors. In the case of rAAV2, the packaging limit of the vector (approximately 5 kb) limited the size of the promoter cassette that could be used for expression of the cystic fibrosis transmembrane regulator (CFTR), which has a coding sequence of 4.4 kb (ref. 50). The use of the AAV2 p5 promoter and the subsequent discovery of cryptic promoter activity from elements within the AAV2-ITR enabled the packaging of CFTR vectors.51 These vectors were initially used to overcome the CF defect in airway epithelial cells in culture, and then to express CFTR mRNA and protein in vivo in the rabbit airway after endobronchial instillation with a fiber-optic bronchoscope.51,52,53 Studies in both cultured airway cells and in the airways of rabbits and rhesus monkeys indicated that rAAV2-CFTR persisted for at least six months in airway cells as an episomal element.54,55,56 Based on the observed safety in these studies, rAAV2-CFTR was used for the first human gene therapy in November 1995 in the nose and bronchus of an adult with CF.57 A series of extensions of that original trial, and follow-up trials in the sinuses of CF patients were also completed.58,59,60,61,62,63,64
Shortly after the report of rAAV2-CFTR gene transfer in the airways, a rAAV2–tyrosine hydroxylase vector was used for in vivo expression in the brains of nonhuman primates as a preclinical approach to therapy for Parkinson's disease, and rAAV2–epo and rAAV2–factor IX vectors were demonstrated to be effective for sustained expression of erythropoietin in muscle to stimulate red blood cell production in anemia and factor IX in liver for hemophilia.65,66,67,68 The ability of rAAV2 vectors to express factor IX was subsequently demonstrated in patients as well.69
Early evidence of bioactivity in humans
Within the first 10 years of AAV use in humans, trials of rAAV2 and rAAV1 vectors were undertaken for CF, hemophilia B, Batten's disease, Canavan's disease, and α1-antitrypsin (AAT) deficiency.57,60,61,62,63,,69,70,71,72,73,74,75,76,77,78,79 In vivo expression at low levels was demonstrated in each of these trials. Vector persistence and expression were directly related to the life span of the transduced cells. Trials in the airways generally showed a duration of expression in the range of 60 days, consistent with the life span of airway epithelial cells, whereas trials in the central nervous system and in the uninjured muscle and liver showed longer-term duration: up to several years in some cases. Throughout this period, data on the safety of rAAV vectors began to accumulate.
Documentation of clinical efficacy in four diseases
The first true breakthrough in clinical efficacy in humans was made in 2008, with the emergence of data from three parallel trials of rAAV2-RPE65 gene therapy in patients with Leber's congenital amaurosis (LCA; Figure 3), an autosomal recessive disease resulting in functional blindness due to an inability to recycle retinoids in the visual cycle.80,81,82,83,84,85,86,87,88,89,90 Each of these studies showed that a single subretinal injection of rAAV2-RPE65 resulted in long-term objective improvements in sensitivity to light and subjective improvements in vision. The gene therapy also was safe. Follow-up studies of patients in these early cohorts showed persistent improvements for several years.
Figure 3.
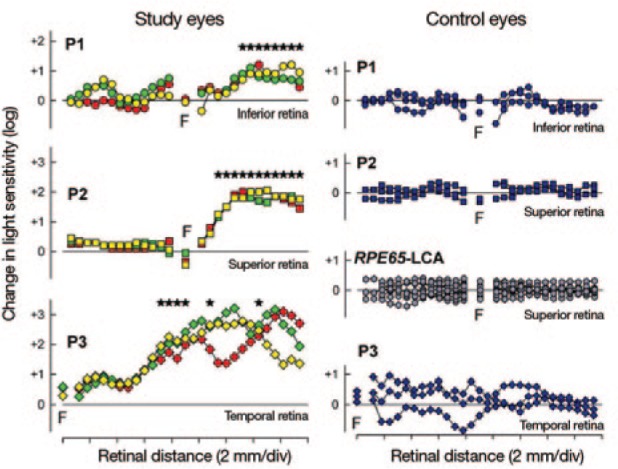
Evidence of efficacy in a human gene therapy trial. The trial used a rAAV2-RPE65 vector to treat patients with the autosomal recessive blinding disorder Leber's congenital amaurosis (LCA). The several-log10-fold change in light sensitivity in the injected portions of the treated retinas (indicated by stars) of three patients (P1, P2, P3) indicates benefit to visual function in these patients. On the opposite side of the panel are the light-sensitivity results from the contralateral eyes (P1, P2, P3) and from an untreated patient (RPE65-LCA). Reprinted with permission from ref. 80.
Shortly after the publication of the LCA data, additional examples of clinical efficacy in rAAV gene therapy trials began to emerge. Hemophilia B trials progressed with the use of an rAAV–factor IX vector delivered systemically to achieve liver delivery.91 Patients in this trial were treated with oral prednisolone because of an elevation of liver enzymes, but after this treatment, factor IX levels stabilized and patients demonstrated a marked reduction in the need for recombinant protein replacement. Several trials were performed with an rAAV2–aromatic amino acid decarboxylase (rAAV2-AADC) vector in both patients with Parkinson's disease and those with congenital AADC deficiency.92,93 The clinical effects in infants with AADC deficiency were most remarkable, showing significant improvements in gross motor development, including the ability to walk by 16 months in one patient, a milestone not previously observed in AADC-deficient infants without treatment.
The emergence of Glybera
AAV serotype 2 was the best studied of the AAV serotypes in the preclinical gene therapy period, and it was the first serotype developed into a vector. The ability to cross-package or pseudotype vector genomes with AAV2-ITRs into capsids of other serotypes was demonstrated in the late 1990s.94,95,96,97 As long as the AAV2-Rep gene was supplied in trans, an AAV2-ITR–flanked genome could be packaged into any other AAV serotype capsid. Studies of the first six AAV serotypes demonstrated that AAV1 capsids were more effective than AAV2 for gene transfer into skeletal muscle, whereas AAV5 and AAV6 capsid vectors were more effective for gene transfer into the murine airway.95,96,97 This property would later be expanded to encompass more than 100 serotypes and genomic variants of AAV that were found to be naturally occurring in humans and nonhuman primates (Figure 4).98,99,100
Figure 4.
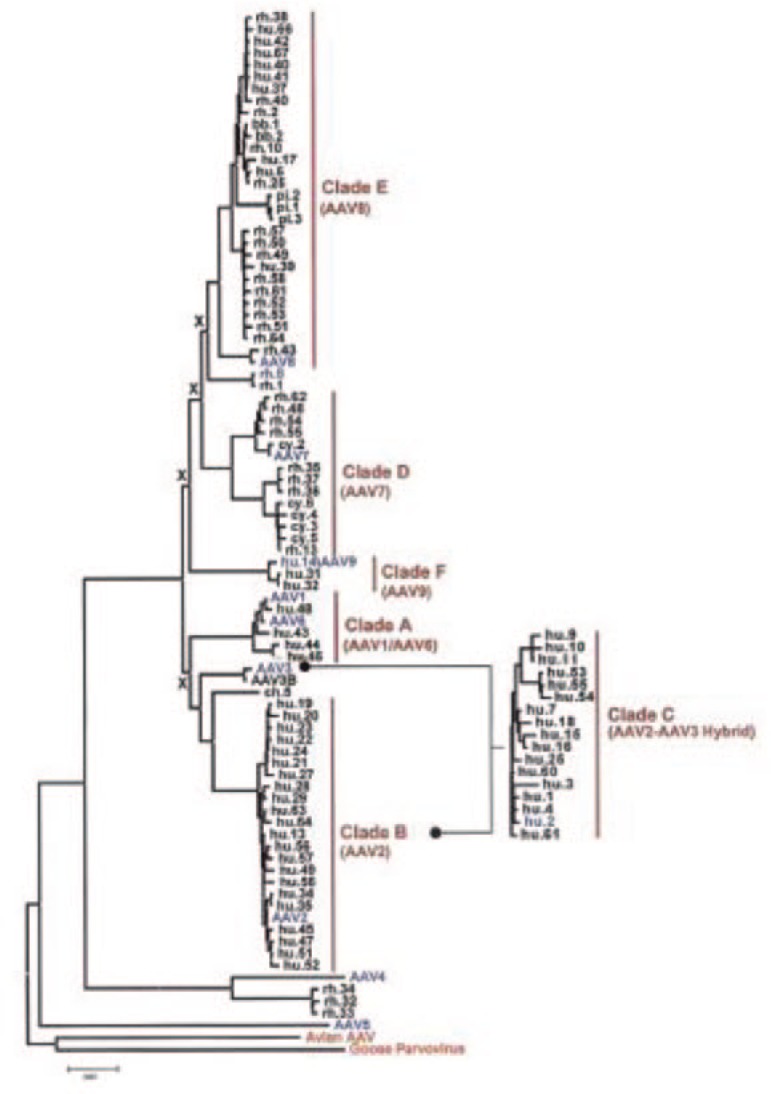
The biodiversity of primate-derived adeno-associated virus (AAV) genomic variants. The variants (shown within clades here), which were identified in in the landmark 2004 article by Gao et al.,98 may provide many new potential capsid vehicles for therapeutic gene transfer in the future. Reprinted with permission from ref. 98.
The ability of rAAV vectors to express gene products from skeletal muscle had initially been exploited with rAAV2 vectors expressing factor IX or AAT in humans. In the early 2000s, trials of intramuscular rAAV1-AAT and rAAV1-LPL were initiated.1,2,3,77 The AAT trials were an attempt to replace the second most abundant serum protein in patients with a defect in this gene, and, although persistent transgene expression was readily demonstrated, the dose was insufficient to reach a therapeutic threshold. Meanwhile the rAAV1-LPL vector resulted in a lowering of the serum cholesterol levels in patients with LPL deficiency and ultimately in a reduction in the recurrent pancreatitis that is observed as a consequence of hyperlipidemia in these patients. On the basis of these data, rAAV1-LPL was licensed under the trade name Glybera in November 2012, 47 years after the discovery of AAV and 17 years after the first use of rAAV2-CFTR in humans.
Lessons learned from the AAV story
Apart from being the vector class to become the first licensed gene therapy product in the Western world, the story of AAV provides a number of potentially useful lessons. The first is that the study of AAV throughout more than half of its history was purely for the sake of scientific curiosity. As has been the case with basic science generally, and the stance of NIH more specifically, the early study of AAV was hypothesis-driven basic science rather than goal-directed therapeutic development. A practical use for AAV was not envisioned in any way until the early 1980s. Had curiosity not driven the scientists of the time to study the peculiar life cycle of this nonpathogenic virus, the world might not have learned of this potentially important platform for treatment of human diseases.
The second lesson is more difficult to interpret. It seems ironic that a vector based on a virus discovered on the NIH campus and first made into a vector on the same campus supported by NIH funding, as well as the first one used in animals and humans on NIH-funded projects, would emerge as a licensed therapy in the Netherlands rather than in the United States through the use of a production protocol also developed at the NIH. Did this occur because the regulatory environment in Europe was more open to innovations in gene therapy? Was it because of a more favorable investment environment there? Or was it the determined will of investigators at the sponsoring company, UniQure, or its predecessor, Amsterdam Molecular Therapeutics, that resulted in this outcome? The ongoing history of this field may provide clearer answers to these questions. In the meantime, gene therapy researchers and investors alike should be invigorated by this development and continue to strive to exploit this platform to benefit more patients, with a greater diversity of diseases, who have been waiting so long for this field to mature. In addition, the NIH, as well as the investigators funded by them, should be lauded for their prescience and commitment to basic scientific research.
Acknowledgments
Many thanks to Xandra O. Breakefield for encouraging the writing of this piece and for her helpful editorial suggestions.
References
- Burnett JR., and, Hooper AJ. Alipogene tiparvovec, an adeno-associated virus encoding the Ser(447)X variant of the human lipoprotein lipase gene for the treatment of patients with lipoprotein lipase deficiency. Curr Opin Mol Ther . 2009;11:681–691. [PubMed] [Google Scholar]
- Bryant LM, Christopher DM, Giles AR, Hinderer C, Rodriguez JL, Smith JB.et al. (2013Lessons learned from the clinical development and market authorization of Glybera Hum Gene Ther Clin Dev 2455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S. Endgame: Glybera finally recommended for approval as the first gene therapy drug in the European Union. Mol Ther. 2012;20:1831–1832. doi: 10.1038/mt.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow NR, Hoggan MD., and, Rowe WP. Isolation of adenovirus-associated viruses from man. Proc Natl Acad Sci USA. 1967;58:1410–1415. doi: 10.1073/pnas.58.4.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow NR, Hoggan MD, Kapikian AZ, Austin JB., and, Rowe WP. Epidemiology of adenovirus-associated virus infection in a nursery population. . Am J Epidemiol. 1968;88:368–378. doi: 10.1093/oxfordjournals.aje.a120897. [DOI] [PubMed] [Google Scholar]
- Blacklow NR, Hoggan MD., and, Rowe WP. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst. 1968;40:319–327. [PubMed] [Google Scholar]
- Hoggan MD, Blacklow NR., and, Rowe WP. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci USA. 1966;55:1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison RW, Casto BC., and, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Horvath BL., and, Fornosi F. Excretion of SV-40 virus after oral administration of contaminated polio vaccine. Acta Microbiol Acad Sci Hung. 1964;11:271–275. [PubMed] [Google Scholar]
- Katz SL. Efficacy, potential and hazards of vaccines. N Engl J Med. 1964;270:884–888. doi: 10.1056/NEJM196404232701707. [DOI] [PubMed] [Google Scholar]
- Katz E., and, Carter BJ. Effect of adeno-associated virus on transformation of NIH 3T3 cells by ras gene and on tumorigenicity of an NIH 3T3 transformed cell line. Cancer Res. 1986;46:3023–3026. [PubMed] [Google Scholar]
- de la Maza LM., and, Carter BJ. Inhibition of adenovirus oncogenicity in hamsters by adeno-associated virus DNA. J Natl Cancer Inst. 1981;67:1323–1326. [PubMed] [Google Scholar]
- Coker AL, Russell RB, Bond SM, Pirisi L, Liu Y, Mane M.et al. (2001Adeno-associated virus is associated with a lower risk of high-grade cervical neoplasia. Exp Mol Pathol 7083–89. [DOI] [PubMed] [Google Scholar]
- Hermonat PL. Adeno-associated virus inhibits human papillomavirus type 16: a viral interaction implicated in cervical cancer. Cancer Res. 1994;54:2278–2281. [PubMed] [Google Scholar]
- Hermonat PL, Plott RT, Santin AD, Parham GP., and, Flick JT. Adeno-associated virus Rep78 inhibits oncogenic transformation of primary human keratinocytes by a human papillomavirus type 16-ras chimeric. Gynecol Oncol. 1997;66:487–494. doi: 10.1006/gyno.1997.4789. [DOI] [PubMed] [Google Scholar]
- Rose JA, Berns KI, Hoggan MD., and, Koczot FJ. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci USA. 1969;64:863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski RJ, Zhu X, Xiao X, Brook JD, Housman DE, Epstein N.et al. (1991Targeted integration of adeno-associated virus (AAV) into human chromosome 19 EMBO J 103941–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AK, Hoggan MD, Hauswirth WW., and, Berns KI. Integration of the adeno-associated virus genome into cellular DNA in latently infected human Detroit 6 cells. J Virol. 1980;33:739–748. doi: 10.1128/jvi.33.2.739-748.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns KI, Hauswirth WW, Fife KH., and, Lusby E.1979Adeno-associated virus DNA replication Cold Spring Harb Symp Quant Biol 43Pt 2): 781–787. [DOI] [PubMed] [Google Scholar]
- Berns KI., and, Hauswirth WW. Adeno-associated viruses. Adv Virus Res. 1979;25:407–449. doi: 10.1016/s0065-3527(08)60574-6. [DOI] [PubMed] [Google Scholar]
- Kotin RM., and, Berns KI. Organization of adeno-associated virus DNA in latently infected Detroit 6 cells. Virology. 1989;170:460–467. doi: 10.1016/0042-6822(89)90437-6. [DOI] [PubMed] [Google Scholar]
- Kotin RM, Siniscalco M, Samulski RJ, Zhu XD, Hunter L, Laughlin CA.et al. (1990Site-specific integration by adeno-associated virus Proc Natl Acad Sci USA 872211–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotin RM, Menninger JC, Ward DC., and, Berns KI. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics. 1991;10:831–834. doi: 10.1016/0888-7543(91)90470-y. [DOI] [PubMed] [Google Scholar]
- Kotin RM, Linden RM., and, Berns KI. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992;11:5071–5078. doi: 10.1002/j.1460-2075.1992.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RO, Samulski RJ., and, Muzyczka N. In vitro resolution of covalently joined AAV chromosome ends. Cell. 1990;60:105–113. doi: 10.1016/0092-8674(90)90720-y. [DOI] [PubMed] [Google Scholar]
- Snyder RO, Im DS., and, Muzyczka N. Evidence for covalent attachment of the adeno-associated virus (AAV) Rep protein to the ends of the AAV genome. J Virol. 1990;64:6204–6213. doi: 10.1128/jvi.64.12.6204-6213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RO, Im DS, Ni T, Xiao X, Samulski RJ., and, Muzyczka N. Features of the adeno-associated virus origin involved in substrate recognition by the viral Rep protein. J Virol. 1993;67:6096–6104. doi: 10.1128/jvi.67.10.6096-6104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im DS., and, Muzyczka N. Factors that bind to adeno-associated virus terminal repeats. J Virol. 1989;63:3095–3104. doi: 10.1128/jvi.63.7.3095-3104.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im DS., and, Muzyczka N. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell. 1990;61:447–457. doi: 10.1016/0092-8674(90)90526-k. [DOI] [PubMed] [Google Scholar]
- Im DS., and, Muzyczka N. Partial purification of adeno-associated virus Rep78, Rep52, and Rep40 and their biochemical characterization. J Virol. 1992;66:1119–1128. doi: 10.1128/jvi.66.2.1119-1128.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Brister JR, Im DS., and, Muzyczka N. Characterization of the adeno-associated virus Rep protein complex formed on the viral origin of DNA replication. Virology. 2003;313:364–376. doi: 10.1016/s0042-6822(03)00340-4. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zolotukhin I, Im DS., and, Muzyczka N. Biochemical characterization of adeno-associated virus Rep68 DNA helicase and ATPase activities. J Virol. 1999;73:1580–1590. doi: 10.1128/jvi.73.2.1580-1590.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyöstiö SR, Owens RA, Weitzman MD, Antoni BA, Chejanovsky N., and, Carter BJ. Analysis of adeno-associated virus (AAV) wild-type and mutant Rep proteins for their abilities to negatively regulate AAV p5 and p19 mRNA levels. J Virol. 1994;68:2947–2957. doi: 10.1128/jvi.68.5.2947-2957.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempe JP., and, Carter BJ. Alternate mRNA splicing is required for synthesis of adeno-associated virus VP1 capsid protein. J Virol. 1988;62:3356–3363. doi: 10.1128/jvi.62.9.3356-3363.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin JD, Tal J., and, Carter BJ. Negative and positive regulation in trans of gene expression from adeno-associated virus vectors in mammalian cells by a viral Rep gene product. Mol Cell Biol. 1986;6:2884–2894. doi: 10.1128/mcb.6.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens RA., and, Carter BJ. In vitro resolution of adeno-associated virus DNA hairpin termini by wild-type Rep protein is inhibited by a dominant-negative mutant of Rep. J Virol. 1992;66:1236–1240. doi: 10.1128/jvi.66.2.1236-1240.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens RA, Weitzman MD, Kyöstiö SR., and, Carter BJ. Identification of a DNA-binding domain in the amino terminus of adeno-associated virus Rep proteins. J Virol. 1993;67:997–1005. doi: 10.1128/jvi.67.2.997-1005.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman MD, Kyöstiö SR, Carter BJ., and, Owens RA. Interaction of wild-type and mutant adeno-associated virus (AAV) Rep proteins on AAV hairpin DNA. J Virol. 1996;70:2440–2448. doi: 10.1128/jvi.70.4.2440-2448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempe JP., and, Carter BJ. Regulation of adeno-associated virus gene expression in 293 cells: control of mRNA abundance and translation. J Virol. 1988;62:68–74. doi: 10.1128/jvi.62.1.68-74.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay FT, de la Maza LM., and, Carter BJ. Parvovirus RNA transcripts containing sequences not present in mature mRNA: a method for isolation of putative mRNA precursor sequences. Proc Natl Acad Sci USA. 1979;76:625–629. doi: 10.1073/pnas.76.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton A, Palumbo P., and, Berns KI. Expression from the adeno-associated virus p5 and p19 promoters is negatively regulated in trans by the Rep protein. J Virol. 1989;63:4450–4454. doi: 10.1128/jvi.63.10.4450-4454.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns KI, Kotin RM., and, Labow MA. Regulation of adeno-associated virus DNA replication. Biochim Biophys Acta. 1988;951:425–429. doi: 10.1016/0167-4781(88)90116-9. [DOI] [PubMed] [Google Scholar]
- Samulski RJ, Berns KI, Tan M., and, Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc Natl Acad Sci USA. 1982;79:2077–2081. doi: 10.1073/pnas.79.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski RJ, Srivastava A, Berns KI., and, Muzyczka N. Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell. 1983;33:135–143. doi: 10.1016/0092-8674(83)90342-2. [DOI] [PubMed] [Google Scholar]
- Laughlin CA, Tratschin JD, Coon H., and, Carter BJ. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene. 1983;23:65–73. doi: 10.1016/0378-1119(83)90217-2. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Lusby EW., and, Berns KI. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983;45:555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin JD, West MH, Sandbank T., and, Carter BJ. A human parvovirus, adeno-associated virus, as a eucaryotic vector: transient expression and encapsidation of the procaryotic gene for chloramphenicol acetyltransferase. Mol Cell Biol. 1984;4:2072–2081. doi: 10.1128/mcb.4.10.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski RJ, Chang LS., and, Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol. 1987;61:3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski RJ, Chang LS., and, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte TR, Solow R, Owens RA, Afione S, Zeitlin PL., and, Carter BJ. Gene expression from adeno-associated virus vectors in airway epithelial cells. Am J Respir Cell Mol Biol. 1992;7:349–356. doi: 10.1165/ajrcmb/7.3.349. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Afione SA, Solow R, Drumm ML, Markakis D, Guggino WB.et al. (1993Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter J Biol Chem 2683781–3790. [PubMed] [Google Scholar]
- Egan M, Flotte T, Afione S, Solow R, Zeitlin PL, Carter BJ.et al. (1992Defective regulation of outwardly rectifying Cl– channels by protein kinase A corrected by insertion of CFTR Nature 358581–584. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Afione SA, Conrad C, McGrath SA, Solow R, Oka H.et al. (1993Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector Proc Natl Acad Sci USA 9010613–10617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte TR, Afione SA., and, Zeitlin PL. Adeno-associated virus vector gene expression occurs in nondividing cells in the absence of vector DNA integration. Am J Respir Cell Mol Biol. 1994;11:517–521. doi: 10.1165/ajrcmb.11.5.7946381. [DOI] [PubMed] [Google Scholar]
- Afione SA, Conrad CK, Kearns WG, Chunduru S, Adams R, Reynolds TC.et al. (1996In vivo model of adeno-associated virus vector persistence and rescue J Virol 703235–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CK, Allen SS, Afione SA, Reynolds TC, Beck SE, Fee-Maki M.et al. (1996Safety of single-dose administration of an adeno-associated virus (AAV)-CFTR vector in the primate lung Gene Ther 3658–668. [PubMed] [Google Scholar]
- Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, Rosenstein B.et al. (1996A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease Hum Gene Ther 71145–1159. [DOI] [PubMed] [Google Scholar]
- Moss RB, Rodman D, Spencer LT, Aitken ML, Zeitlin PL, Waltz D.et al. (2004Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial Chest 125509–521. [DOI] [PubMed] [Google Scholar]
- Aitken ML, Moss RB, Waltz DA, Dovey ME, Tonelli MR, McNamara SC.et al. (2001A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease Hum Gene Ther 121907–1916. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Moran ML, Messner AH, Daifuku R, Conrad CK, Reynolds T.et al. (1998A phase I/II study of tgAAV-CF for the treatment of chronic sinusitis in patients with cystic fibrosis Hum Gene Ther 9889–909. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Messner AH, Moran ML, Daifuku R, Kouyama K, Desch JK.et al. (1999Safety and biological efficacy of an adeno-associated virus vector–cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus Laryngoscope 1092 Pt 1): 266–274. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Nepomuceno IB, Messner AH, Moran ML, Batson EP, Dimiceli S.et al. (2002A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies Hum Gene Ther 131349–1359. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Zeitlin PL, Reynolds TC, Heald AE, Pedersen P, Beck S.et al. (2003Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)–CFTR vector in adult cystic fibrosis patients: a two-part clinical study Hum Gene Ther 141079–1088. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Schwiebert EM, Zeitlin PL, Carter BJ., and, Guggino WB. Correlation between DNA transfer and cystic fibrosis airway epithelial cell correction after recombinant adeno-associated virus serotype 2 gene therapy. Hum Gene Ther. 2005;16:921–928. doi: 10.1089/hum.2005.16.921. [DOI] [PubMed] [Google Scholar]
- Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D.et al. (1997Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors Nat Genet 16270–276. [DOI] [PubMed] [Google Scholar]
- During MJ, Samulski RJ, Elsworth JD, Kaplitt MG, Leone P, Xiao X.et al. (1998In vivo expression of therapeutic human genes for dopamine production in the caudates of MPTP-treated monkeys using an AAV vector Gene Ther 5820–827. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O'Malley KL.et al. (1994Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain Nat Genet 8148–154. [DOI] [PubMed] [Google Scholar]
- Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF.et al. (1999Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors Nat Med 5 64–70. [DOI] [PubMed] [Google Scholar]
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A.et al. (2000Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector Nat Genet 24257–261. [DOI] [PubMed] [Google Scholar]
- McPhee SW, Janson CG, Li C, Samulski RJ, Camp AS, Francis J.et al. (2006Immune responses to AAV in a phase I study for Canavan disease J Gene Med 8577–588. [DOI] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR.et al. (2003AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B Blood 1012963–2972. [DOI] [PubMed] [Google Scholar]
- Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N.et al. (2008Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA Hum Gene Ther 19463–474. [DOI] [PubMed] [Google Scholar]
- Crystal RG, Sondhi D, Hackett NR, Kaminsky SM, Worgall S, Stieg P.et al. (2004Clinical protocol. Administration of a replication-deficient adeno-associated virus gene transfer vector expressing the human CLN2 cDNA to the brain of children with late infantile neuronal ceroid lipofuscinosis Hum Gene Ther 151131–1154. [DOI] [PubMed] [Google Scholar]
- Leone P, Shera D, McPhee SW, Francis JS, Kolodny EH, Bilaniuk LT.et al. (2012Long-term follow-up after gene therapy for Canavan disease Sci Transl Med 4165ra163.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson C, McPhee S, Bilaniuk L, Haselgrove J, Testaiuti M, Freese A.et al. (2002Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain Hum Gene Ther 131391–1412. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Trapnell BC, Humphries M, Carey B, Calcedo R, Rouhani F.et al. (2011Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results Hum Gene Ther 221239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT.et al. (2009Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy Proc Natl Acad Sci USA 10616363–16368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantly ML, Spencer LT, Humphries M, Conlon TJ, Spencer CT, Poirier A.et al. (2006Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults Hum Gene Ther 171177–1186. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Brantly ML, Spencer LT, Byrne BJ, Spencer CT, Baker DJ.et al. (2004Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults Hum Gene Ther 1593–128. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ.et al. (2008Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics Proc Natl Acad Sci USA 10515112–15117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L.et al. (2008Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial Hum Gene Ther 19979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K.et al. (2008Effect of gene therapy on visual function in Leber's congenital amaurosis N Engl J Med 3582231–2239. [DOI] [PubMed] [Google Scholar]
- Testa F, Maguire AM, Rossi S, Pierce EA, Melillo P, Marshall K.et al. (2013Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital amaurosis type 2 Ophthalmology 1201283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC.et al. (2012AAV2 gene therapy readministration in three adults with congenital blindness Sci Transl Med 4120ra15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL.et al. (2010Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration Mol Ther 18643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F.et al. (2009Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial Lancet 3741597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J.et al. (2008Safety and efficacy of gene transfer for Leber's congenital amaurosis N Engl J Med 3582240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ.et al. (2012Gene therapy for Leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years Arch Ophthalmol 1309–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL.et al. (2009Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year Hum Gene Ther 20999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL.et al. (2009Vision 1 year after gene therapy for Leber's congenital amaurosis N Engl J Med 361725–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC.et al. (2011Adenovirus-associated virus vector–mediated gene transfer in hemophilia B N Engl J Med 3652357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwu WL, Muramatsu S, Tseng SH, Tzen KY, Lee NC, Chien YH.et al. (2012Gene therapy for aromatic l-amino acid decarboxylase deficiency Sci Transl Med 4134ra61.. [DOI] [PubMed] [Google Scholar]
- Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K.et al. (2010A phase I study of aromatic l-amino acid decarboxylase gene therapy for Parkinson's disease Mol Ther 181731–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SE, Jones LA, Chesnut K, Walsh SM, Reynolds TC, Carter BJ.et al. (1999Repeated delivery of adeno-associated virus vectors to the rabbit airway J Virol 739446–9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Chirmule N, Berta SC, McCullough B, Gao G., and, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ., and, Walsh CE. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X.et al. (2002Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity J Virol 76791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X.et al. (2004Clades of adeno-associated viruses are widely disseminated in human tissues J Virol 786381–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ.et al. (2003Adeno-associated viruses undergo substantial evolution in primates during natural infections Proc Natl Acad Sci USA 1006081–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]


