Abstract
Objective
This study is the first in a series investigating the relationship between autonomic nervous system dysfunction and chronic cerebrospinal venous insufficiency in multiple sclerosis patients. We screened patients for the combined presence of the narrowing of the internal jugular veins and symptoms of autonomic nervous system dysfunction (fatigue, cognitive dysfunction, sleeping disorders, headache, thermal intolerance, bowel/bladder dysfunction) and determined systolic and diastolic blood pressure responses to balloon angioplasty.
Methods
The criteria for eligibility for balloon angioplasty intervention included ≥50% narrowing in one or both internal jugular veins, as determined by the magnetic resonance venography, and ≥3 clinical symptoms of autonomic nervous system dysfunction. Blood pressure was measured at baseline and post-balloon angioplasty.
Results
Among patients who were screened, 91% were identified as having internal jugular veins narrowing (with obstructing lesions) combined with the presence of three or more symptoms of autonomic nervous system dysfunction. Balloon angioplasty reduced the average systolic and diastolic blood pressure. However, blood pressure categorization showed a biphasic response to balloon angioplasty. The procedure increased blood pressure in multiple sclerosis patients who presented with baseline blood pressure within lower limits of normal ranges (systolic ≤105 mmHg, diastolic ≤70 mmHg) but decreased blood pressure in patients with baseline blood pressure above normal ranges (systolic ≥130 mmHg, diastolic ≥ 80 mmHg). In addition, gender differences in baseline blood pressure subcategories were observed.
Discussion
The coexistence of internal jugular veins narrowing and symptoms of autonomic nervous system dysfunction suggests that the two phenomena may be related. Balloon angioplasty corrects blood pressure deviation in multiple sclerosis patients undergoing internal jugular vein dilation. Further studies should investigate the association between blood pressure deviation and internal jugular veins narrowing, and whether blood pressure normalization affects Patient's clinical outcomes.
Keywords: Balloon angioplasty, high pressure baroreceptors, blood pressure deviation, CCSVI, dysautonomia, gender differences
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS). The disease is relapsing or progressive, with multiple pathologies, including demyelination and axonal injury and loss, leading to deficits of motor, and neurocognitive function.1,2 It is the most common neurological disease in young adults3 and has heterogeneous clinical and radiographic manifestations.4 The exact etiology of MS is unknown. However, it is thought that genetic5 and epigenetic6 factors interact with the environmental factors for determining the initiation and the progression of MS.
Among environmental factors, infectious agents, especially Epstein Barr virus, have been studied extensively for their role in triggering MS through molecular mimicry.7,8 Another environmental factor, thought to be involved in the etiology of MS, is the latitude, which in turn is related to the degree of exposure to ultraviolet sunlight, catalyzing the production of 1,25-dihydroxyvitamin D3 in the skin.9,10
A newer theory of the vascular etiology of MS is the chronic cerebrospinal venous insufficiency (CCSVI). This theory, put forward by Zamboni, proposes that CCSVI is caused by impaired cerebrospinal fluid (CSF) flow dynamics and lower brain perfusion. These anomalies are associated with venous reflux and congestion, leading in turn to iron deposition and neuroinflammation. The impairments in the principal pathways of extracranial venous drainage mostly affect the internal jugular veins (IJVs), the vertebral, and the azygos veins.11,12
Although, there are no absolute uniform criteria, and the sensitivity to detect IJV's narrowing varies with the method of measurement,13 using two of the five duplex ultrasound criteria for CCSVI diagnosis, Zamboni group reported CCSVI in all MS patients and none in the controls.11,14 Two additional studies confirmed Zamboni's findings, but the rate of CCSVI was lower among MS patients and was higher among control groups.15,16 However, these studies were all accompanied with methodological bias.17
A more recent multi-center study showed the prevalence of CCSVI to be 86% among MS patients, although marked differences in these percentages were observed among centers.18 In addition, the prevalence of CCSVI was higher among secondary progressive MS patients, correlating with MS disease severity, as indicated by the EDSS scores.18
Nevertheless, the validity of Zamboni's theory was challenged by the results of a study showing a lack of association between CCSVI and cerebral blood flow dynamics; similar cerebral blood flow was registered between MS patients and control groups.19 In addition, MRI studies did not show differences in IJV morphology, reflux, or proportion of blood leaving the brain between MS patients and control subjects.20 Furthermore, 3T magnetic resonance venography (MRV), phase contrast angiography, and multi-phase 3D contrast-enhanced MRI angiography analyses of the intracranial and extracranial neck veins did not show significant differences between the drainage patterns of MS patients and control subjects, and no venous backflow was observed in the two groups.21
In addition, MS patients present with heterogeneous pattern of autonomic dysfunction (ANS). Symptoms of ANS dysfunction include heart rate and blood pressure (BP) abnormalities during rest and during standing, headache, micturation, sudomotor, and gastrointestinal disturbances.22,23 Furthermore, ANS dysregulation has been shown to contribute to cognitive dysfunction,24 fatigue,25 and sleep disorders,26 which are often observed in MS patients.
In addition, venous dilation angioplasty to correct venous obstruction, although controversial, has been shown to have an acceptable safety profile27,28 and clinical efficacy28,29 in MS patients. This intervention has been shown to also improve indicators of ANS dysfunction, including fatigue and heat intolerance in 77% of patients after a 6-month follow-up.30 Similarly, the correction of extracranial IJVs and azygos veins stenosis/occlusion led to 68% and 53.0% improvements in the clinical and psychological components of the MS impact scale (MSIS) scores, respectively, at 1 month post-treatment.28 The physiological and psychological components of the MSIS include symptoms of ANS dysfunction, such as bladder and bowel dysfunction, depression, fatigue, and sleep disorders, which were all improved post-endovascular intervention.28
Furthermore, the rate of annual relapse was reduced post-treatment, and the EDSS scores, recorded 2 years after endovascular intervention, were lower than EDSS scores recorded before treatments.29 These results collectively suggest that endovascular intervention of MS patients could improve ANS function and slow the progression of the disease.
We hypothesized that CCSVI might be linked the symptoms of ANS dysfunction31 and monitored patients for the combined presence of IJVs narrowing and symptoms of ANS dysfunction (fatigue, cognitive dysfunction, sleeping disorders, headache, thermal intolerance, bowel/bladder dysfunction). In addition, we examined BP responses to balloon angioplasty (BA) in MS patients who underwent IJVs dilation.
Methods
The study involved MS patients who visited the Endovascular Clinic (Synergy Health concepts, Newport Beach, CA) between 2011 and 2012. Patients were screened for the combined presence of IJVs narrowing and symptoms of ANS dysfunction. BA was offered to patients who were diagnosed having ≥50% narrowing in one or both IJVs as confirmed by MRV (Siemens 3T TRIO scanner, Siemens Medical Solutions USA, Inc. 51 Valley Stream Parkway, Malvern, PA), which was based on the Haacke protocol (http://www.ms-mri.com/docs/ms-report-interpretation-sep-10-10-emh-9pm.pdf), and by the documentation of three or more symptoms of ANS dysfunction, which were assessed using CCSVI symptom sheet. Based on these criteria, 195 patients were eligible to undergo the BA procedure.
Indicators of ANS dysfunction included fatigue (measured by Modified Fatigue Impact Scale), cognitive impairment (measured by Perceived Deficits Questionnaire), and bladder and bowel dysfunction (measured by Bladder Control Scale and Bowel Control Scale, respectively). Sleeping disorder, headache upon awakening, and thermal intolerance were assessed with customized questions using a 1–5 scale for consistency.
Patients with hypercoaguable state and pregnant patients were excluded from the study. A single measurement of systolic and diastolic BP was performed manually (by a sphygmomanometer) in sitting position, 24 hours before (baseline) and 24 hours post-IJV BA. BP measurements were performed at the same time ± 2 hours during the day, in a room temperature which was tightly regulated at 70℃, due to thermal sensitivity of the population involved.
The study was approved by the Biomed IRB and consent was obtained from each patient before the day of surgery.
Statistical analysis
All statistical analyses were performed using SPSS 14.0 for Windows (SPSS Institute, Chicago, Illinois, USA). Repeated ANOVA, co-varied for age and disease duration, was used to examine the differences in systolic and diastolic BP before and after BA. ANOVA, co-varied for age, disease duration, and the use of anti-hypertensive drugs, measured the differences in baseline BP between males/females, and between RRMS/chronic progressive (CP)MS. We also categorized the BP variable and investigated the relationship between this variable and other indicators of ANS dysfunction using logistic regression and Kendall Tau, incorporating relevant covariates.
Results
Table 1 presents the demographics and the characteristics of MS patients who were diagnosed as having both IJVs narrowing and symptoms of ANS dysfunction. Among MS patients who were screened, 91% identified as having both IJVs with flow obstruction and three or more symptoms of ANS dysfunction. The average age for patients was 47.8 ± 10.8 years with significant difference between males (50.2 ± 11.0 years) and females (46.5 ± 10.5 years, p = 0.03), and disease duration was 12.5 ± 9.2 years. Most of patients were those who refused drug treatment for their symptoms; therefore, the number of patients who used disease modifying therapies (DMTs) was relatively low (7.7%). Among patients, 151 were relapsing remitting (RR) MS and 44 were chronic progressive (CP) (23 secondary progressive (SP) and 21 primary progressive (PP)).
Table 1.
MS patients' demographics and characteristics. Age and disease duration are presented as the mean ± SD.
| Patient's characteristics | |
|---|---|
| Total (n) | 195 (69 M–35%) |
| age (years) | 47.8 ± 10.8 (25–77) |
| Male | 50.2 ± 11.0 (26–77) |
| Female | 46.5 ± 10.5 (25–77) |
| p Value | 0.03 |
| disease duration (years) | 12.5 ± 9.2 (2–60) |
| Male | 13.6 ± 10.4 (2–60) |
| Female | 11.9 ± 8.4 (2–41) |
| p Value | 0.32 |
| RRMS | 151 (77.4%) |
| CPMS (SPMS+PPMS) | 44 (22.5%) |
| Drugs' use | |
| DMTs | 15 (7.7%) |
| Anti-hypertensives | 11 (5.6%) |
| Symptoms of autonomic dysfunction | n (%) |
| Cognition | 124 (63.5%) |
| Fatigue | 151 (77.4%) |
| headache | 82 (42.0%) |
| Sleep disorder | 113 (57.9%) |
| Thermal intolerance | 144 (73.8%) |
| Bladder | 149 (76.4%) |
| Bowel | 149 (76.4%) |
CP: chronic progressive; DMT: disease modifying therapies; MS: multiple sclerosis; n: number of subjects; RRM: relapsing remitting; PP: primary progressive; SP: secondary progressive. p ≤ 0.05 is considered significant.
The percentages of MS patients who presented with headache upon waking were significantly lower (42%) than those who presented with cognitive dysfunction (63.5%), fatigue (77.4%), sleeping disorders (57.9%), thermal intolerance (73.8%), and bowel/bladder dysfunction (76.4%) (p values < 0.0001). In addition, the percentages of patients with cognitive dysfunction were lower than percentages of patients with fatigue, thermal intolerance, and bowel/bladder dysfunction (p values from 0.001 to 0.03). The percentages of patients with sleeping disorders were lower than patients with fatigue, thermal intolerance, and bladder/bowel dysfunction (p values from 0.0001 to 0.0005).
Table 2 and Figure 1(a) present the average systolic BP and Table 2 and Figure 1(b) present the diastolic BP before and after BA in 195 patients as well as the minimum and the maximum percent changes in these variables. Among MS patients, the systolic and diastolic BP ranged between 87 and 215 mmHg and from 52 to 109 mmHg, respectively.
Table 2.
The effects of BA on systolic and diastolic BP deviations. The presented data are the mean ± SD. p Values are corrected for confounding factors, including age, disease duration, and use of anti-hypertensive drugs.
| Systolic BP (mmHg) | n | Pre-BA (mmHg) | Post-BA (mmHg) | p value | min/max change (%) |
|---|---|---|---|---|---|
| ≤105>150 | 195 | 122.8 ± 19.4 | 120.0 ± 17.6 | 0.03 | (−63.7/+51.6) |
| ≤105 | 34 | 98.5 ± 4.5 | 103.9 ± 13.7 | 0.03 | (−63.7/+24.5) |
| 106–120 | 65 | 113.7 ± 4.1 | 115.0 ± 12.8 | 0.4 | (−32.1/+17.6) |
| 121–130 | 41 | 125.5 ± 2.8 | 124.0 ± 12.8 | 0.4 | (−21.5/+20.3) |
| 131–140 | 25 | 134.5 ± 2.8 | 130.0 ± 12.0 | 0.07 | (−19.3/+19.1) |
| 141–150 | 16 | 145.1 ± 3.1 | 130.1 ± 14.0 | <0.001 | (−3.0/+26.1) |
| >150 | 14 | 169.8 ± 17.8 | 141.2 ± 25.4 | <0.001 | (−1.9/+51.6) |
| Diastolic BP (mmHg) | |||||
| ≤70>100 | 195 | 81.4 ± 11.1 | 79.3 ± 11.3 | 0.009 | (−36.5/+52.1) |
| ≤70 | 39 | 66.0 ± 4.4 | 71.5 ± 8.0 | <0.001 | (−36.5/+14.5) |
| 71–80 | 54 | 76.1 ± 2.9 | 77.1 ± 9.0 | 0.4 | (−31.6/+24.3) |
| 81–90 | 60 | 85.6 ± 2.8 | 79.5 ± 10.3 | <0.001 | (−13.3/+51.2) |
| 91–100 | 36 | 94.8 ± 2.5 | 88.4 ± 12.0 | 0.001 | (−22.8/+52.1) |
| >100 | 6 | 106.3 ± 2.1 | 93.8 ± 4.5 | 0.003 | (+5.7/+50.8) |
BA: balloon angioplasty; BP: blood pressure.
p ≤ 0.05 is considered significant.
Figure 1.
The effects of balloon angioplasty (BA) on systolic (a) and diastolic (b) blood pressure (BP) deviations. The presented data is the mean ± SEM. p values are corrected for confounding factors, including age, disease duration, and use of anti-hypertensive drugs. Asterisks indicate significance; p ≤ 0.05 is considered significant.
BA reduced significantly both systolic (122.8 ± 19.4 mmHg vs. 120.0 ± 17.6 mmHg, p = 0.03) and diastolic (81.4 ± 11.1 mmHg vs. 79.3 ± 11.3 mmHg, p = 0.009) BP. In addition, changes (Δ) in systolic (r = 0.468, p <0.0001) and diastolic (r = 0.508, p < 0.0001) BP correlated positively with the baseline systolic and diastolic BP, respectively.
Subsequently, we categorized systolic and diastolic BP from low to high, adding 10–15 mmHg to each subsequent level, allowing adequate number of patients in each subcategory for optimal power analysis. The lower ranges of systolic (≤105 mmHg) and diastolic (≤70 mmHg) BP is an approximation of the average BP in 195 patients minus one standard deviation.
The BP categorization showed a biphasic response to BA. At the lower ranges of ≤105 mmHg systolic BP, BA intervention led to a significant rise in systolic BP from 98.5 ± 4.5 mmHg to 103.9 ± 13.7 mmHg, p = 0.03. BA had no significant effects on systolic BP ranging from 106 to 130 mmHg. However, at higher ranges of systolic BP of 131–140 mmHg, 141–150 mmHg, and systolic BP >150 mmHg, BA reduced BP from 134.5 ± 2.8 mmHg to 130.0 ± 12.0 mmHg, p = 0.07, from 145.1 ± 3.1 mmHg to 130.1 ± 14.0 mmHg, p < 0.001, and from 169.0 ± 17.8 mmHg to 141.2 ± 25.4 mmHg, p < 0.001, respectively (Table 2 and Figure 1(a)).
In addition, at the lower ranges of diastolic BP of ≤70 mmHg, BA led to a significant increase in diastolic BP from 66.0 ± 4.4 mmHg to 71.5 ± 8.0 mmHg, p < 0.001, but had no significant effects on diastolic BP ranging from 71 to 80 mmHg. However, at higher ranges of diastolic BP of 81–90 mmHg, 91–100 mmHg, and >100 mmHg, BA reduced diastolic BP from 85.6 ± 2.8 mmHg to 79.5 ± 10.3 mmHg, p < 0.001, from 94.8 ± 2.5 mmHg to 88.4 ± 12.0 mmHg, p = 0.001, and from 118.5 ± 29.7 mmHg to 93.8 ± 4.5 mmHg, p = 0.003, respectively (Table 2 and Figure 1(b)).
Gender stratification
Gender stratification, corrected for differences in age, disease duration, and the use of anti-hypertensive drugs, showed significantly higher systolic BP in males as compared to their female counterparts (130.1 ± 19.9 mmHg vs. 118.8 ± 18.0 mmHg, p = 0.001). Similarly, the diastolic BP was higher for males than for females (83.3 ± 10.5 mmHg vs. 80.4 ± 11.3 mmHg, p = 0.08). After BA, the differences in the systolic (126.2 ± 15.9 mmHg vs. 116.6 ± 17.5 mmHg, p < 0.001) and diastolic (81.8 ± 9.6 mmHg vs. 77.9 ± 12.0 mmHg, p = 0.02) BP between the two groups remained significant.
Subsequently, we calculated the percentages of males and females within lower limits of systolic (≤105 mmHg) and diastolic (≤70 mmHg) BP, males and females who had systolic and diastolic BP within 106–130 mmHg ranges, and 71–80 mmHg ranges, and males and females who had systolic and diastolic BP >130 mmHg and >80 mmHg, respectively. These categories were based on BP ranges which showed significant or near significant changes post-BA.
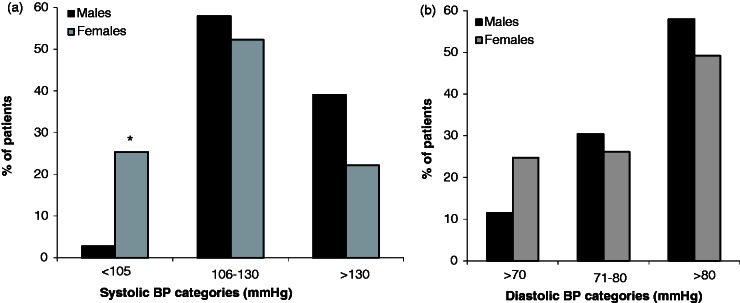
A smaller percentage of males showed systolic (2.8% vs. 25.4%, p = 0.001) and diastolic (11.5% vs. 24.8%, p = 0.13) BP at lower normal ranges than females. However, a higher percentage of males had systolic BP >130 mmHg (39.1% vs. 22.2%, p = 0.07) and diastolic BP >80 mmHg (58.0% vs. 49.2%). In addition, the prevalence of thermal intolerance was higher among females than among males (p = 0.02) (Figure 2(a) and (b)).
Figure 2.
Gender differences in systolic (a) and diastolic (b) blood pressure (BP). The figure presents the percentage of males and females MS patients with BP lower than normal ranges (≤105 mmHg for systolic BP and ≤70 mmHg for diastolic BP), within normal ranges (106–130 mmHg for systolic BP and 70–80 mmHg for diastolic BP), and above normal ranges (>130 mmHg for systolic BP and >80 mmHg for diastolic BP). Asterisks indicate significance; p ≤ 0.05 is considered significant.
Stratification by disease stage
Patients' stratification to CPMS and RRMS patients (corrected for age, disease duration, and the use of antihypertensive drugs) showed a tendency toward a higher baseline systolic (123.4 ± 22.7 mmHg vs. 122.6 ± 18.4 mmHg) but no differences in diastolic (81.2 ± 12.4 mmHg vs. 81.5 ± 10.7 mmHg) BP in CPMS patients as compared to RRMS patients. BA procedure widened the differences in systolic (121.4 ± 18.6 mmHg vs. 119.6 ± 17.2 mmHg) and diastolic (80.8 ± 12.6 mmHg vs. 78.9 ± 10.9 mmHg) BP among groups, but these differences remained statistically non-significant. However, the prevalence of fatigue (p = 0.02), cognitive dysfunction (p = 0.011), and sleeping disorders (p = 0.042) were higher in CPMS patients than in RRMS patients.
Correlation studies
Non-parametric correlation studies using Kendall Tau showed a relationship between systolic BP and sleeping disorders (r = 0.163, p = 0.02). In addition, post-BA changes (Δ) in diastolic BP correlated with cognitive dysfunction (r = 0.215, p = 0.002), the latter in turn correlated with fatigue (r = 0.152, p = 0.03) and headache (r = 0.212, p = 0.003), and headache correlated with sleeping disorders (r = 0.136, p = 0.05). Bowel dysfunction correlated strongly with dysfunction of the bladder (r = 1.0, p = 0.00), but these two symptoms did not show relationship with either BP or with other symptoms of ANS dysfunction (Table 3).
Table 3.
Correlation between BP and ANS dysfunction symptoms. p ≤ 0.05 is considered significant.
| Variables | Correlation coefficient | p value |
|---|---|---|
| Systolic BP/sleeping disorders | 0.163 | 0.02 |
| Diastolic BP/cognitive dysfunction | 0.215 | 0.002 |
| Cognitive dysfunction/fatigue | 0.152 | 0.03 |
| Cognitive dysfunction/headache | 0.212 | 0.003 |
| Sleeping disorders/headache | 0.136 | 0.05 |
| Bowel/bladder dysfunction | 1 | 0 |
ANS: autonomic nervous system; BP: blood pressure.
Discussion
This study screened MS patients for the co-existence of ANS dysfunction symptoms and CCSVI. Among MS patients who presented in the clinic between 2011 and 2012, 91% were identified as having both IJVs narrowing and symptoms of ANS dysfunction, suggesting a strong association between the two phenomena. This conclusion is further supported by the results of studies showing that endovascular treatment improves indicators of ANS dysfunction, including bladder and bowel dysfunction, depression, fatigue, sleep disorders, and heat intolerance.28–30
BA treatment also led to BP reduction in hypertensive subjects. Our results are consistent with studies showing that, dilation of the internal carotid arteries of patients with atheromatous stenosis results in the stimulation of baroreceptors and an increase in cardiovagal activity, leading to a reduction in BP lasting up to 24 hours.32
However, unlike the arterial system, which is equipped with high pressure baroreceptors, the venous system is equipped with low pressure baroreceptors. These baroreceptors have the function of adjusting blood volume via sensing the central venous pressure. Nevertheless, BA has the potential to activate arterial high pressure baroreceptors, depending on the degree of changes in central venous pressure.33
In addition, the response to BA was biphasic depending on baseline BP levels and was affected by the degree and direction of the BP deviation. BA led to an increase in BP, in those patients who presented with BP below normal ranges, but decreased in those patients who had BP above normal ranges. The changes in BP were more significant when BP was above normal ranges than below it. Our results suggest that BA's effect on BP depends on the setting of the baroreceptors and on the degree of BP deviations from the normal ranges.
However, the baroreceptors' response to changes in venous pressure may be short-lived, as these receptors usually reset to previous value after 24–48 hours. Future studies should measure long-term effects of BA and determine whether BA-induced IJV dilation has effects on variables other than baroreflece's activation, and whether IJVs restenosis is accompanied by recurrent BP deviations.
Furthermore, the percentages of female patients presenting with BP in the lower limits of the normal were significantly higher than males. In contrast, higher percentages of males presented with BP values near and above the normal ranges. This pattern was more pronounced for the systolic, as compared to diastolic BP, suggesting that MS patients have ANS imbalance related to the functional activities of both sympathetic and parasympathetic ANS, the nature of which may be gender-dependent. Regardless of the type, this imbalance may be among factors contributing to the occurrence of CCSVI.
Gender differences in the cardiac ANS tone has not been studied in MS patients. However, consistent with our results, spectral power analysis of heart rate variability in healthy controls show that women have higher cardiovagal input, whereas men demonstrate a higher sympathetic activity, both at baseline and in response to external stressors.34,35 Furthermore, estrogen has been shown to affect cardiovascular ANS function, leading to lower BP in females.36
Since sympathetic37,38 and parasympathetic39,40 ANS have a role in the regulation of inflammatory and neurodegenerative processes, differences in the functional activities of these two systems may explain gender differences in the pathological processes observed in MS patients.41,42 Nevertheless, future studies should investigate possible gender differences in CCSVI occurrence and the contribution of steroid hormones to this phenomenon.
The ANS dysfunction symptoms were higher for fatigue and bladder/bowel dysfunction and lower for headache and sleeping disorders, suggesting differences in the occurrences of ANS dysfunction symptoms in MS patients.43 The degree to which the three branches of the ANS (sympathetic, parasympathetic, and enteric system) contribute to the observed clinical symptoms is unknown. However, fatigue has been shown to be related to sympathetic ANS dysfunction,44 whereas thermal intolerance seems to stem from the parasympathetic dysfunction.45 The absence of correlation between bowel/bladder dysfunction and BP, or between bowel/bladder dysfunction and other indicators of ANS dysfunction suggest that these two clinical symptoms may have separate causes.
It should be noted that authors are engaged in additional studies, aiming to show the close association between CCSVI and ANS dysfunction.
Study limitations
Although the results of this study pave the way for additional investigation of the relationship between ANS dysfunction and CCSVI, one should point out study limitations. First, the available BP data were limited to patients who were positive for IJVs narrowing. Therefore, it was not possible to determine the relationship between BP deviation and the presence of IJVs narrowing. Second, there was no patient follow-up to verify whether changes in BP persist beyond 24 hours, and whether these changes beneficially influence long term patient's clinical outcomes.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet 2002; 359: 1221–1231. [DOI] [PubMed] [Google Scholar]
- 2.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005; 58: 840–846. [DOI] [PubMed] [Google Scholar]
- 3.Charil A, Filipi M. Inflammatory demyelination and neurodegeneration in early multiple sclerosis. J Neurol Sci 2007; 259: 7–15. [DOI] [PubMed] [Google Scholar]
- 4.De Stefano N, Narayanan S, Francis GS, et al. Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch Neurol 2001; 58: 65–70. [DOI] [PubMed] [Google Scholar]
- 5.Willer CJ, Dyment DA, Risch NJ, et al. Twin concordance and sibling recurrence rates in multiple sclerosis. Proc Natl Acad Sci U S A 2003; 100: 12877–12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burrell AM, Handel AE, Ramagopalan SV, et al. Epigenetic mechanisms in multiple sclerosis and the major histocompatibility complex (MHC). Discov Med 2011; 11: 187–196. [PubMed] [Google Scholar]
- 7.Virtanen JO, Jacobson S. Viruses and multiple sclerosis. CNS Neurol Disord Drug Targets 2012; 11: 528–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang HL, Jacobsen H, Ikemizu S, et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol 2002; 3: 940–943. [DOI] [PubMed] [Google Scholar]
- 9.Westberg M, Feychting M, Jonsson F, et al. Occupational exposure to UV light and mortality from multiple sclerosis. Am J Ind Med 2009; 52: 353–357. [DOI] [PubMed] [Google Scholar]
- 10.van der Mei IA, Ponsonby AL, Dwyer T, et al. Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case-control study. BMJ 2003; 327: 316–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamboni P, Galeotti R, Menegatti E, et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2009; 80: 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamboni P. The big idea: iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis. J R Soc Med 2006; 99: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doepp F, Wurfel JT, Pfueller CF, et al. Venous drainage in multiple sclerosis: a combined MRI and ultrasound study. Neurology 2011; 77: 1745–1751. [DOI] [PubMed] [Google Scholar]
- 14.Zamboni P, Menegatti E, Galeotti R, et al. The value of cerebral Doppler venous haemodynamics in the assessment of multiple sclerosis. J Neurol Sci 2009; 282: 21–27. [DOI] [PubMed] [Google Scholar]
- 15.Al-Omari MH, Rousan LA. Internal jugular vein morphology and hemodynamics in patients with multiple sclerosis. Int Angiol 2010; 29: 115–120. [PubMed] [Google Scholar]
- 16.Simka M, Kostecki J, Zaniewski M, et al. Extracranial Doppler sonographic criteria of chronic cerebrospinal venous insufficiency in the patients with multiple sclerosis. Int Angiol 2010; 29: 109–114. [PubMed] [Google Scholar]
- 17.Zecca C, Gobbi C. Chronic cerebrospinal venous insufficiency (CCSVI) and multiple sclerosis (MS): a critical review. CNS Neurol Disord Drug Targets 2011; 10: 757–761. [DOI] [PubMed] [Google Scholar]
- 18.Bastianello S, Romani A, Viselner G, et al. Chronic cerebrospinal venous insufficiency in multiple sclerosis: clinical correlates from a multicentre study. BMC Neurol 2011; 11: 132–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doepp F, Paul F, Valdueza JM, et al. No cerebrocervical venous congestion in patients with multiple sclerosis. Ann Neurol 2010; 68: 173–183. [DOI] [PubMed] [Google Scholar]
- 20.Sundstrom P, Wahlin A, Ambarki K, et al. Venous and cerebrospinal fluid flow in multiple sclerosis: a case-control study. Ann Neurol 2010; 68: 255–259. [DOI] [PubMed] [Google Scholar]
- 21.Wattjes MP, van Oosten BW, de Graaf WL, et al. No association of abnormal cranial venous drainage with multiple sclerosis: a magnetic resonance venography and flow-quantification study. J Neurol Neurosurg Psychiatry 2011; 82: 429–435. [DOI] [PubMed] [Google Scholar]
- 22.Haensch CA, Jorg J. Autonomic dysfunction in multiple sclerosis. J Neurol 2006; 253: I3–I9. [DOI] [PubMed] [Google Scholar]
- 23.Flachenecker P. Autonomic dysfunction in Guillain-Barre syndrome and multiple sclerosis. J Neurol 2007; 254: II96–II101. [DOI] [PubMed] [Google Scholar]
- 24.Heesen C, Koehler G, Gross R, et al. Altered cytokine responses to cognitive stress in multiple sclerosis patients with fatigue. Mult Scler 2005; 11: 51–57. [DOI] [PubMed] [Google Scholar]
- 25.Flachenecker P, Rufer A, Bihler I, et al. Fatigue in MS is related to sympathetic vasomotor dysfunction. Neurology 2003; 61: 851–853. [DOI] [PubMed] [Google Scholar]
- 26.Ferini-Strambi L, Smirne S. Cardiac autonomic function during sleep in several neuropsychiatric disorders. J Neurol 1997; 244: S29–S36. [DOI] [PubMed] [Google Scholar]
- 27.Petrov I, Grozdinski L, Kaninski G, et al. Safety profile of endovascular treatment for chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Endovasc Ther 2011; 18: 314–323. [DOI] [PubMed] [Google Scholar]
- 28.Hubbard D, Ponec D, Gooding J, et al. Clinical improvement after extracranial venoplasty in multiple sclerosis. J Vasc Interv Radiol 2012; 23: 1302–1308. [DOI] [PubMed] [Google Scholar]
- 29.Salvi F, Bartolomei I, Buccellato E, et al. Venous angioplasty in multiple sclerosis: neurological outcome at two years in a cohort of relapsing-remitting patients. Funct Neurol 2012; 27: 55–59. [PMC free article] [PubMed] [Google Scholar]
- 30.Kostecki J, Zaniewski M, Ziaja K, et al. An endovascular treatment of chronic cerebro-spinal venous insufficiency in multiple sclerosis patients – 6 month follow-up results. Neuro Endocrinol Lett 2011; 32: 557–562. [PubMed] [Google Scholar]
- 31.Sternberg Z. Autonomic dysfunction: a unifying multiple sclerosis theory, linking chronic cerebrospinal venous insufficiency, vitamin D(3), and Epstein-Barr virus. Autoimmun Rev 2012; 12: 250–259. [DOI] [PubMed] [Google Scholar]
- 32.Mangin L, Medigue C, Merle JC, et al. Cardiac autonomic control during balloon carotid angioplasty and stenting. Can J Physiol Pharmacol 2003; 81: 944–951. [DOI] [PubMed] [Google Scholar]
- 33.Zoller RP, Mark AL, Abboud FM, et al. The role of low pressure baroreceptors in reflex vasoconstrictor responses in man. J Clin Invest 1972; 51: 2967–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mann MC, Exner DV, Hemmelgarn BR, et al. Impact of gender on the cardiac autonomic response to angiotensin II in healthy humans. J Appl Physiol 2012; 112: 1001–1007. [DOI] [PubMed] [Google Scholar]
- 35.Liao D, Barnes RW, Chambless LE, et al. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability--the ARIC study. Atherosclerosis Risk in Communities. Am J Cardiol 1995; 76: 906–912. [DOI] [PubMed] [Google Scholar]
- 36.Liu CC, Kuo TB, Yang CC. Effects of estrogen on gender-related autonomic differences in humans. Am J Physiol Heart Circ Physiol 2003; 285: H2188–H2193. [DOI] [PubMed] [Google Scholar]
- 37.Elenkov IJ, Wilder RL, Chrousos GP, et al. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 2000; 52: 595–638. [PubMed] [Google Scholar]
- 38.Sanders VM, Straub RH. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav Immun 2002; 16: 290–332. [DOI] [PubMed] [Google Scholar]
- 39.Czura CJ, Tracey KJ. Autonomic neural regulation of immunity. J Intern Med 2005; 257: 156–166. [DOI] [PubMed] [Google Scholar]
- 40.Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cell Mol Life Sci 2004; 61: 2322–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelfrey CM, Cotleur AC, Lee JC, et al. Sex differences in cytokine responses to myelin peptides in multiple sclerosis. J Neuroimmunol 2002; 130: 211–223. [DOI] [PubMed] [Google Scholar]
- 42.Kezele IB, Arnold DL, Collins DL. Atrophy in white matter fiber tracts in multiple sclerosis is not dependent on tract length or local white matter lesions. Mult Scler 2008; 14: 779–785. [DOI] [PubMed] [Google Scholar]
- 43.Kodounis A, Stamboulis E, Constantinidis TS, et al. Measurement of autonomic dysregulation in multiple sclerosis. Acta Neurol Scand 2005; 112: 403–408. [DOI] [PubMed] [Google Scholar]
- 44.Niepel G, Bibani RH, Vilisaar J, et al. Association of a deficit of arousal with fatigue in multiple sclerosis: effect of modafinil. Neuropharmacology 2013; 64: 380–388. [DOI] [PubMed] [Google Scholar]
- 45.Feinberg M. The problems of anticholinergic adverse effects in older patients. Drugs Aging 1993; 3: 335–348. [DOI] [PubMed] [Google Scholar]