Abstract
Symptomatic cerebellar slump (CS) and external hydrocephalus (EH) are amongst the rarer complications of foramen magnum decompression (FMD) for Chiari I malformation (CM). CS typically presents with delayed onset headache related to dural traction or with neurological deficit offsetting the benefit of FMD. EH, consisting of ventriculomegaly along with subdural fluid collection(s) (SFCs), has been related to cerebrospinal fluid egress from a tiny breach in an otherwise intact arachnoid. We describe the case of a 21-year-old man with CM and syringomyelia who presented with impaired gag, spastic quadriparesis, and raised intracranial pressure 1 week following an uneventful FMD during which the arachnoid had been widely fenestrated. Magnetic resonance imaging (MRI) showed an infratentorial SFC, dilated aqueduct and triventriculomegaly, features of CS, and a residual but resolving syrinx. His symptoms resolved following a high pressure ventriculo-peritoneal shunt. At a 6-month follow-up visit, he was asymptomatic and demonstrated partial resolution of the syrinx, with no recurrence of the SFC. The unusual features in the clinical course of this patient were an atypical CS syndrome presenting with concomitantly resolving syringomyelia, and the development of EH after a wide arachnoidal fenestration. This is the first case in indexed literature describing such a combination of unusual postoperative complications of a FMD. A hypothesis is presented to explain the clinico-radiological findings of the case.
Keywords: external hydrocephalus, cerebellar slump, foramen magnum decompression, Chiari I malformation
Introduction
Foramen magnum decompression (FMD) is believed to be the most effective treatment for Chiari I malformation (CM) with or without syringomyelia. The frequently reported complications of this procedure include cerebrospinal (CSF) fluid leak,1,2) pseudomeningocele,2) and meningitis.1) Unusual complications include external hydrocephalus (EH) i.e., the presence of ventriculomegaly in association with subdural fluid collections (SFC), symptomatic cerebellar slump (CS), pseudotumour cerebri,3) posterior cerebral artery infarct,3) negative pressure pulmonary edema,4) tethering of the cervico-medullary junction,5) and remote cerebellar hemorrhage.6) We report a first-of-its-kind case wherein two of these unusual complications (EH and CS) occurred in atypical settings following a normal sized FMD: the EH occurred after wide arachnoid fenestration and with an enlarged rather than compressed aqueduct, and symptomatic CS occurred with concomitantly resolving syringomyelia, rather than with persisting or worsening syringomyelia. A mechanism is proposed to clarify the peculiar findings in the case.
Case Report
A 21-year-old man presented with tightness in all limbs, difficulty in swallowing, and holocranial headache with multiple episodes of vomiting a week after undergoing a FMD for CM with syringomyelia. He was initially managed elsewhere empirically with acetazolamide, with no improvement in symptomatology. There was no history of fever, CSF leak, or any swelling at the operated site. On reviewing his symptoms at first admission, it was noted that he had presented with cough-induced headaches and paresthesias in all limbs for a duration of 2 years. He had no neurological deficits at that time and his symptoms had improved following FMD. Examination revealed new findings of an impaired single breath count, papilledema, bilaterally impaired gag reflex, spasticity and grade 4 strength in all limbs. There were no signs of meningitis. The wound was healthy, with no pesudomeningocele.
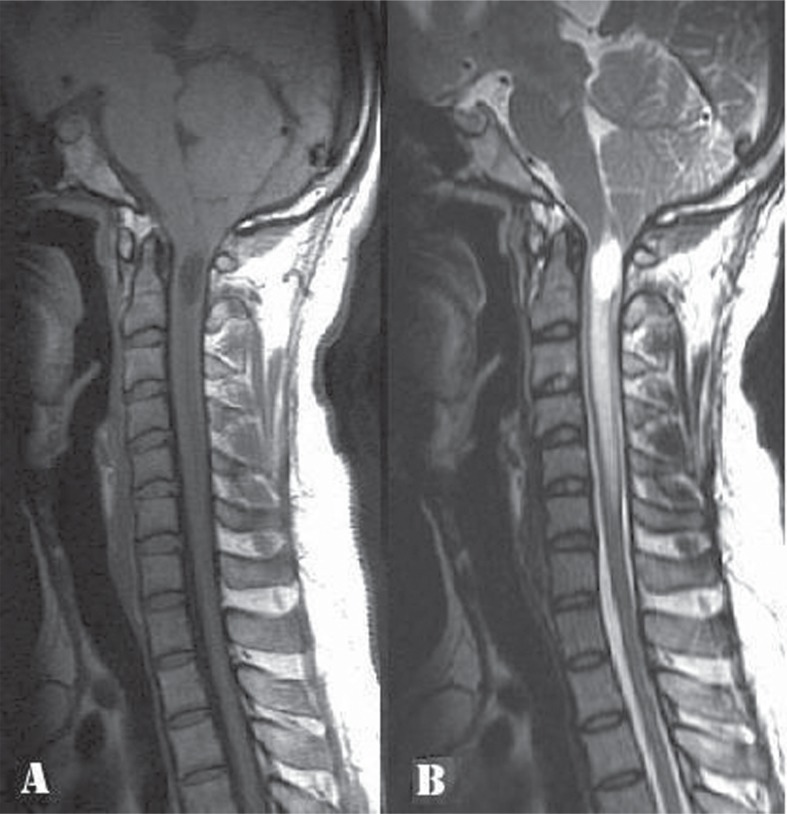
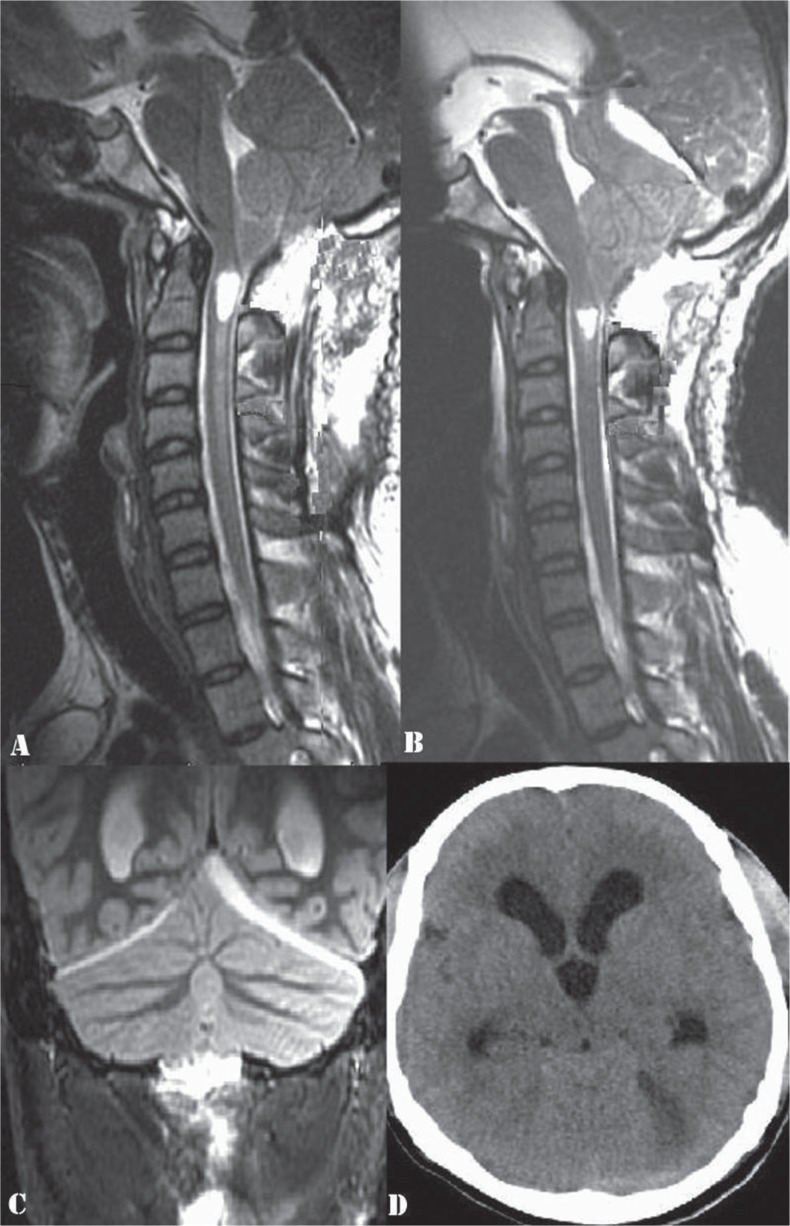
His MRI images of the craniovertebral junction and cervical spine at the first admission, in the postoperative period, and at the second admission were compared. His first MRI (Fig. 1) had showed evidence of tonsillar herniation upto the posterior arch of C1, a syrinx in the upper cervical cord, and edema extending down upto C6 level. There was no hydrocephalus. X-rays had ruled out any associated bony anomaly. A day after the FMD, the MRI showed significant reduction in the cord edema and persistence of the syrinx (Fig. 2A). MRI at the time of the neurological deterioration a week after the FMD showed complete resolution of the cord edema and reduction in the size of the syrinx (Fig. 2B). The cranio-caudal sagittal dimension of the cerebellum appeared to be diminished compared to the previous MRIs, and the caudal cerebellum appeared to be impacted at the cervicomedullary junction. The dorsal aspect of this slumped cerebellum was flush against the lower brainstem, obliterating the fourth ventricular outlet. These findings were not seen in the immediate postoperative MRI (Fig. 2A). With a dura-plasty in place, there was no significant artificial cistern magna, and no evidence of cerebellar hemispheric ptosis into the normal sized (2.5 × 3 cm) craniectomy. There were additional findings of an infratentorial subdural hygroma (Fig. 2B–D), a dilated aqueduct (Fig. 2B), and triventriculomegaly with periventricular lucency. The diagnosis was thus that of CS syndrome and EH in the setting of resolving syringomyelia. In view of his clinical findings of brainstem dysfunction and failure of conservative treatment, the options of an endoscopic third ventriculostomy versus a ventriculoperitoneal (VP) shunt were considered. A high pressure VP shunt was inserted, avoiding the risk of overdrainage and upward herniation. The shunt was done soon after the second admission, a week after the FMD.
Fig. 1.
Preoperative cervical spine magnetic resonance imaging (MRI), sagittal sections: A: T1 weighted image (WI) and B: T2 WI, showing tonsillar herniation up to the C1 posterior arch level, a syrinx in the upper cervical cord and edema in the cord extending down up to C6 level.
Fig. 2.
A: Postoperative cervical spine MRI done one day after the FMD-T2WI, sagittal section, showing a persisting syrinx and significant reduction in the cord edema. B: MRI cervical spine-T2WI, sagittal section, done a week after the FMD, showing complete resolution of the cord edema and reduction in the size of the syrinx. There is cerebellar slump as evidenced by decreased cranio-caudal sagittal dimension of the cerebellum as compared to that in Figs. 1B and 2A and impaction of the caudal cerebellum at the cervicomedullary junction. The aqueduct is dilated. C: MRI brain-T2 WI, coronal section, demonstrating an infratentorial subdural hygroma on the left side. D: Plain CT brain, axial section, showing triventriculomegaly with periventricular lucency and the left sided infratentorial subdural hygroma. WI: weighted image, MRI: magnetic resonance imaging, FMD: foramen magnum decompression, CT: computed tomography.
Findings at his first surgery were reviewed. It was noted that following a 2.5 × 3 cm sized FMD, excision of C1 posterior arch and durotomy, the arachnoid had been widely opened, precluding the need for a re-exploratory surgery recommended in arachnoid-sparing cases that develop EH.4) There had been no subarachnoid adhesions at the fourth ventricular outlet, and the surgical field had been relatively bloodless, ruling out blood, debris, or arachnoid adhesions to be causative of the ensuing hydrocephalus. A duraplasty had been done with an artificial dural substitute.
His symptoms of raised intracranial pressure and brainstem dysfunction improved following the VP shunt. There was improvement in his single breath count, tone, and strength in limbs and his IXth/Xth function. A follow-up MRI after 6 months showed further resolution of the syrinx, well decompressed ventricles, reversal of the CS, and resolution of the infratentorial SDH (Fig. 3).
Fig. 3.
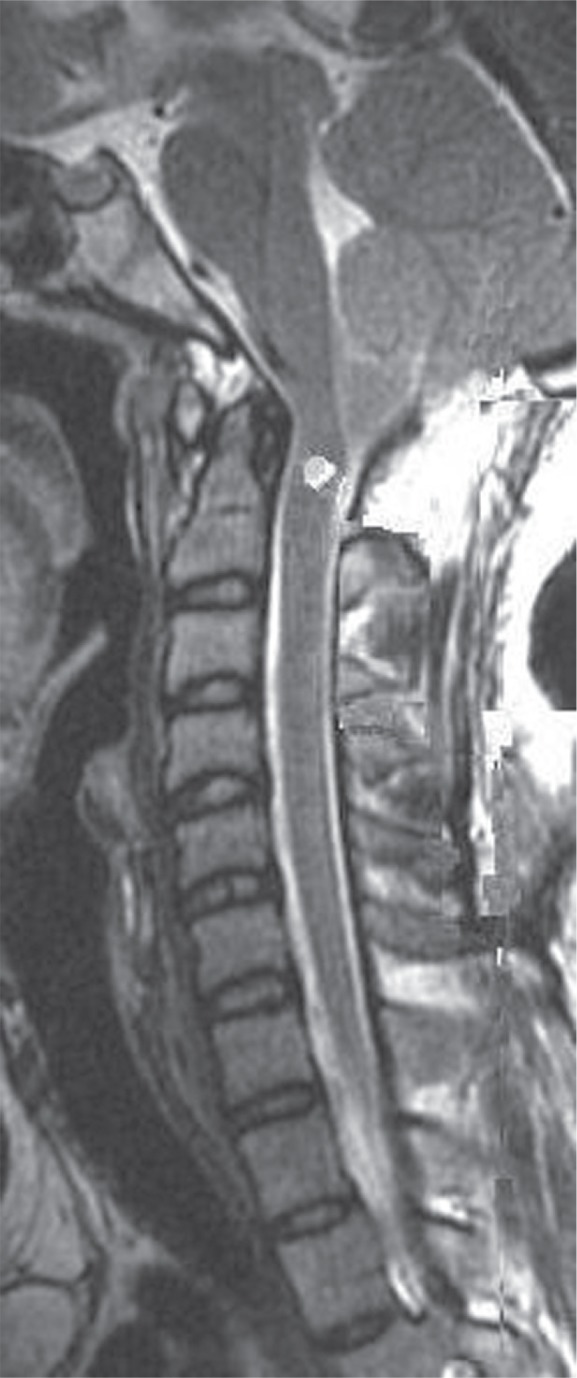
Follow-up magnetic resonance imaging cervical spine, T2 WI, sagittal section, showing further resolution of the syrinx as compared to that in Figs. 1 and 2. There is resolution of the cerebellar slump and of the infratentorial subdural hygroma. WI: weighted image.
Discussion
Asymptomatic descent or slump of the cerebellum has been surprisingly found to occur more commonly than ascent of the same following decompressive craniocervical surgery for CM.7) In a majority of cases, CS is not severe enough to cause recurrence of the erstwhile craniospinal pressure dissociation. This almost universal phenomenon has been ascribed to factors such as a pre-existing tight posterior fossa, effects of gravity, and previous weakening of the neural structures by gradually progressive tonsillar herniation.7) A few cases go on to develop symptomatic CS or cerebellar ptosis, commonly in the presence of a craniectomy that is too large for the specific patient.7) A generally accepted upper limit is 4 × 4 cm. It is suggested that enough bone be left to provide support to the greatest diameter of the cerebellar hemisphere as seen on a sagittal magnetic resonance (MR) image.8) Higher rates of symptomatic CS have been reported in patients with durotomy without duraplasty.9) In some patients, the type of material used for dural grafting has also been implicated.8) Presentation of symptomatic CS is typically with delayed onset, intractable, and dural traction-related headaches that differ from the typical CM related cough headaches. By re-establishing dorsal adherence to the brainstem, CS can also obstruct CSF flow at the cervicomedullary junction and offset the desired effects of FMD. This can present as persistence or worsening of the associated syringomyelia and its symptoms. VP shunts or syringo-peritoneal shunts have been tried with variable results in treating symptomatic CS. Partial suboccipital cranioplasty, the currently recommended treatment, helps in alleviating the headache by supporting the cerebellum.8) In patients with CSF flow obstruction, an intradural exploration is additionally recommended in helping reverse the obstruction and facilitating resolution of syringomyelia.
Hydrocephalus following FMD, seen in around 6% of the cases,10) has been reported to occur due to meningitis, arachnoid adhesions, scarring or blood/debris causing obstruction at the fourth ventricular outlet, blood causing blockage of the arachnoid villi or impaired absorption of CSF,10,11) or as recently reported, due to the formation of infratentorial SFCs that cause aqueductal compromise.10) Although the term “external hydrocephalus” was initially reserved for extracerebral fluid collections in the presence of raised intracranial pressure in infants,12) there have been reports of a similar condition occuring in adults.13,14) Review of indexed literature yielded 10 previous reports of symptomatic EH, i.e., a combination of infra- or supratentorial SFCs and internal hydrocephalus complicating FMD.11,14–18) Nine of these 10 cases were arachnoid-sparing FMDs, and a tiny breach at the arachnoid-dural interface was held responsible for the development of high-pressure subdural collection(s). Torquing or compression of the aqueduct by the infratentorial SFC was implicated to be the cause of the hydrocephalus in these cases. In one case where the arachnoid was widely opened in the first instance, blood contamination of CSF was suggested to cause impaired CSF absorption in various compartments and the ensuing EH.11) Treatment options of EH include antiedema measures, temporary, or permanent ventricular shunting and re-exploratory posterior fossa surgery to ensure wide opening of the arachnoid19) or to clip the same with the dura at the point of CSF egress.13)
Considering the above background, there were two unusual features in our case. First, the symptoms of brainstem compression in our patient were found to be secondary to CS per se, and not to progression of syringomyelia. CS has not been documented so far to present with neurological deficit in the setting of resolving syringomyelia, as alluded to earlier. In that sense, the CS syndrome in our case was atypical. As an explanation for the findings in our case, we invoke Williams' hypothesis related to the pathogenesis of syringomyelia. According to this hypothesis, CSF inflow from the 4th ventricle through the central canal causes syringomyelia in Chiari malformation. In our case, the CS induced compression at the cranio-cervical junction would have resulted in obstruction of CSF communication between the 4th ventricle and the central canal, with ensuing reduction of CSF inflow into the syrinx and decrease in syrinx size. The simultaneous obstruction of the 4th ventricle outlet would have resulted in the development of obstructive hydrocephalus.
Review of the recent insights into the theories of syringomyelia provides an alternative clarification in our case. Syringomyelia in CM is now postulated to be due to impaired absorption of extracellular fluid into the intramedullary venous channels, a phenomenon that is regulated by the compliance of the spinal CSF and thence of the posterior spinal cord veins.20) We hypothesize that in our case, duraplasty afforded improvement in spinal CSF and venous compliance, and this eventually manifested in the resolution of the syrinx even though there was concomitant mechanical obstruction at the cervicomedullary junction by the slumped cerebellum.
The second unusual feature in our case, documented for the first time in literature, is the fact that EH after a FMD had occurred with demonstrable evidence of an enlarged, rather than compromised, aqueduct. This indicated the presence of an obstruction at the fourth ventricular outlet that was severe enough to offset any effect that the SFC may have had on the aqueduct. We postulate that a generous drainage of CSF subsequent to a wide opening of the arachnoid probably led to the development of the SFC, and to the symptomatic CS that in turn may have caused outlet obstruction and hydrocephalus. Hence, a true arachnoid-sparing FMD may not have resulted in the development of either the SFC or the symptomatic CS.
Conclusion
In this first-of-its-kind report, the authors describe an atypical CS syndrome that presented with brainstem compression and associated EH in the setting of resolving syringomyelia following FMD for CM.
References
- 1). Menezes AH: Chiari I malformations and hydromyelia—complications. Pediatr Neurosurg 17: 146– 154, 1991–1992. [DOI] [PubMed] [Google Scholar]
- 2). Perrini P, Benedetto N, Tenenbaum R, Di Lorenzo N: Extra-arachnoidal cranio-cervical decompression for syringomyelia associated with Chiari I malformation in adults: technique assessment. Acta Neurochir (Wien) 149: 1015– 1022; discussion 1022–1023, 2007. [DOI] [PubMed] [Google Scholar]
- 3). Furtado SV, Visvanathan K, Reddy K, Hegde AS: Pseudotumor cerebri: as a cause for early deterioration after Chiari I malformation surgery. Childs Nerv Syst 25: 1007– 1012, 2009. [DOI] [PubMed] [Google Scholar]
- 4). Hirano Y, Sugawara T, Sato Y, Sato K, Omae T, Sasajima T, Mizoi K: Negative pressure pulmonary edema following foramen magnum decompression for Chiari malformation type I. Neurol Med Chir (Tokyo) 48: 137– 139, 2008. [DOI] [PubMed] [Google Scholar]
- 5). Vergani F, Nicholson C, Jenkins A: Tethering of the cervicomedullary junction with central cord oedema after foramen magnum decompression for Chiari malformation. Br J Neurosurg 25: 327– 329, 2011. [DOI] [PubMed] [Google Scholar]
- 6). Kaneko T, Koyanagi I, Murakami T: Remote cerebellar hemorrhage after foramen magnum decompression surgery for Chiari I malformation—case report. Neurol Med Chir (Tokyo) 51: 134– 136, 2011. [DOI] [PubMed] [Google Scholar]
- 7). Duddy MJ, Williams B: Hindbrain migration after decompression for hindbrain hernia: a quantitative assessment using MRI. Br J Neurosurg 5: 141– 152, 1991. [DOI] [PubMed] [Google Scholar]
- 8). Holly LT, Batzdorf U: Management of cerebellar ptosis following craniovertebral decompression for Chiari I malformation. J Neurosurg 94: 21– 26, 2001. [DOI] [PubMed] [Google Scholar]
- 9). Barkovich AJ, Sherman JL, Citrin CM, Wippold FJ: MR of postoperative syringomyelia. AJNR Am J Neuroradiol 8: 319– 327, 1987. [PMC free article] [PubMed] [Google Scholar]
- 10). Zakaria R, Kandasamy J, Khan Y, Jenkinson MD, Hall SR, Brodbelt A, Pigott T, Mallucci CL: Raised intracranial pressure and hydrocephalus following hindbrain decompression for Chiari I malformation: a case series and review of the literature. Br J Neurosurg 26: 476– 481, 2012. [DOI] [PubMed] [Google Scholar]
- 11). Perrini P, Rawlinson A, Cowie RA, King AT: Acute external hydrocephalus complicating craniocervical decompression for syringomyelia-Chiari I complex: case report and review of the literature. Neurosurg Rev 31: 331– 335, 2008. [DOI] [PubMed] [Google Scholar]
- 12). Andersson H, Elfverson J, Svendsen P: External hydrocephalus in infants. Childs Brain 11: 398– 402, 1984. [DOI] [PubMed] [Google Scholar]
- 13). Cardoso ER, Schubert R: External hydrocephalus in adults. Report of three cases. J Neurosurg 85: 1143– 1147, 1996. [DOI] [PubMed] [Google Scholar]
- 14). Elton S, Tubbs RS, Wellons JC, Blount JP, Grabb PA, Oakes WJ: Acute hydrocephalus following a Chiari I decompression. Pediatr Neurosurg 36: 101– 104, 2002. [DOI] [PubMed] [Google Scholar]
- 15). Bahuleyan B, Menon G, Hariharan E, Sharma M, Nair S: Symptomatic posterior fossa and supratentorial subdural hygromas as a rare complication following foramen magnum decompression for Chiari malformation Type I. J Neurosurg 114: 510– 513, 2011. [DOI] [PubMed] [Google Scholar]
- 16). Filis AK, Moon K, Cohen AR: Symptomatic subdural hygroma and hydrocephalus following Chiari I decompression. Pediatr Neurosurg 45: 425– 428, 2009. [DOI] [PubMed] [Google Scholar]
- 17). Marshman LA, Benjamin JC, Chawda SJ, David KM: Acute obstructive hydrocephalus associated with infratentorial subdural hygromas complicating Chiari malformation type I decompression. Report of two cases and literature review. J Neurosurg 103: 752– 755, 2005. [DOI] [PubMed] [Google Scholar]
- 18). Suzuki F, Kitagawa T, Takagi K, Nozaki K: Subacute subdural hygroma and presyrinx formation after foramen magnum decompression with duraplasty for Chiari type 1 malformation. Neurol Med Chir (Tokyo) 51: 389– 393, 2011. [DOI] [PubMed] [Google Scholar]
- 19). Bahl A, Murphy M, Thomas N, Gullan R: Management of infratentorial subdural hygroma complicating foramen magnum decompression: a report of three cases. Acta Neurochir (Wien) 153: 1123– 1128, 2011. [DOI] [PubMed] [Google Scholar]
- 20). Koyanagi I, Houkin K: Pathogenesis of syringomyelia associated with Chiari type 1 malformation: review of evidences and proposal of a new hypothesis. Neurosurg Rev 33: 271– 284; discussion 284–285, 2010. [DOI] [PubMed] [Google Scholar]