Abstract
Efforts to develop a vaccine for ricin toxin are focused on identifying highly immunogenic, safe, and thermostable recombinant derivatives of ricin’s enzymatic A subunit (RTA). As a means to guide vaccine design, we have embarked on an effort to generate a comprehensive neutralizing and non-neutralizing B cell epitope map of RTA. In a series of previous studies, we identified three spatially distinct linear (continuous), neutralizing epitopes on RTA, as defined by monoclonal antibodies (mAbs) PB10 (and R70), SyH7, and GD12. In this report we now describe a new collection of 19 toxin-neutralizing mAbs that bind non-linear epitopes on RTA. The most potent toxin-neutralizing mAbs in this new collection, namely WECB2, TB12, PA1, PH12 and IB2 each had nanamolar (or sub-nanomolar) affinities for ricin and were each capable of passively protecting mice against a 5–10×LD50 toxin challenge. Competitive binding assays by surface plasmon resonance revealed that WECB2 binds an epitope that overlaps with PB10 and R70; TB12, PA1, PH12 recognize epitope(s) close to or overlapping with SyH7’s epitope; and GD12 and IB2 recognize epitopes that are spatially distinct from all other toxin-neutralizing mAbs. We estimate that we have now accounted for ~75% of the predicted epitopes on the surface of RTA and that toxin-neutralizing mAbs are directed against a very limited number of these epitopes. Having this information provides a framework for further refinement of RTA mutagenesis and vaccine design.
1. INTRODUCTION
Ricin toxin, derived from the seeds of the castor bean plant (Ricinus communis), belongs to the so-called type II family of ribosome-inactivating proteins (RIPs). In its mature form, ricin is a 64 kDa globular glycoprotein composed of a 34 kDa enzymatic subunit (RTA) joined by a single disulfide bond to a 32 kDa binding subunit (RTB). RTB promotes ricin’s attachment, endocytosis and retrograde trafficking from early endosomes to the endoplasmic reticulum (ER) by way of the trans-Golgi network (TGN) [1]. In the lumen of the ER, protein disulfide isomerase (PDI) reduces the disulfide bond joining the two toxin subunits, after which RTA is retrotranslocated across the ER membrane and into the cytoplasm where it refolds [2,3]. RTA’s RNA N-glycosidase activity exerts its effect on the highly conserved adenosine residue (A2662) within the sarcin-ricin loop (SRL) of eukaryotic 28s ribosomal RNA, thereby rendering ribosomes inactive [4]. The crystal structure of RTA suggests that it consists of three somewhat arbitrary folding domains (FD), corresponding to residues 1–117 (FD 1), 118–210 (FD 2), and 211–267 (FD 3) (Figure 1). RTA’s active site constitutes a shallow pocket formed at the interface of the three domains [5,6]. Five residues, Tyr80, Tyr123, Glu177, Arg180, and Trp211, within or near the active site cleft are essential for RTA’s enzymatic activity [6–8].
Figure 1. Location of known B cell epitopes on RTA.

Depiction of RTA highlighting the FDs and immunodominant regions described by O’Hara et al. [19]. Shown are the approximate locations of neutralizing (triangles) and non-neutralizing (circles) linear B cell epitopes.
RTA has been of interest to the biomedical research community for decades: historically for its use as an immunotoxin [9] and more recently because of efforts to develop a ricin toxin subunit vaccine [10,11]. The two most advanced ricin subunit vaccine candidates are RiVax and RVEc. RiVax is a recombinant form of RTA that carries two point mutations (V76M, Y80A) [12,13]. The Y80A mutation attenuates RTA’s RNA N-glycosidase activity, while the V76M mutation eliminates RTA’s intrinsic capacity to elicit vascular leak syndrome (VLS) [12,13]. RVEc is a truncated derivative of RTA (herein referred to as ΔRTA) that lacks FD3 (residues 199–267) as well as a small hydrophobic loop in FD1 (residues 34–43) [14,15]. Results from Phase I clinical trials of RiVax and RVEc indicate that the two vaccines are safe and immunogenic, but only marginally effective at eliciting long-lasting toxin-neutralizing antibodies, which are considered the primary determinant of protective immunity [16].
For that reason, we are pursuing efforts to rationally design derivatives of RTA that are both more immunostimulatory and more thermostable [17]. However, these efforts are being conducted in the absence of a complete understanding of the B cell epitopes on RTA that are important in eliciting toxin neutralizing antibodies. At this time we cannot predict whether the introduction of specific point mutations or deletions in RTA will interfere with the potency (i.e., capacity to elicit toxin-neutralizing antibodies) of the resulting antigens. In 2010, we reported a partial B cell epitope map of RTA based on a collection of monoclonal antibodies (mAbs) directed against linear epitopes on RTA [18,19]. Based on the results of that study, we estimated that toxin-neutralizing antibodies constitute only a fraction (<10%) of the total antibodies elicited by Ricin toxoid (or RiVax) immunization, possibly because toxin-neutralizing B cell epitopes are rare. By subjecting immune sera from RiVax-immunized mice and rabbits to RTA pepscan analysis, we identified six (I–VI) immunodominant (ID) linear regions on RTA. By generating mAbs specific to 5 of the 6 ID regions, we identified neutralizing mAbs directed against epitopes within regions II and IV, whereas non-neutralizing mAbs tended to cluster within regions I, V and VI (Figure 1). The neutralizing mAbs from this original collection included PB10 (residues 97–108) in ID region II, and GD12 (residues 163–174) and SyH7 (residues 187–198) and in ID region [20]IV.
Another finding from that study was that the vast majority (>90%) of RTA-specific mAbs are directed against non-linear (or discontinuous/conformational) epitopes [19]. As these mAbs likely reflect the composition of the polyclonal antibody (Ab) response to RTA-based vaccines we deemed it important to better define the location of the nonlinear, toxin-neutralizing epitopes. Towards this end, we have now produced a collection of almost 20 RTA-specific murine mAbs. In depth characterization and competitive binding assays of the most potent toxin-neutralizing mAbs within this collection reveals that they recognize epitopes that overlap with PB10’s (residues 97–108) or SyH7’s (residues 187–198) epitopes. These data suggest that neutralizing B cell epitopes are confined to a few, relatively small areas on the surface of RTA and that preserving the integrity of these regions in vaccine antigens is of paramount importance.
2. Material and Methods
2.1 Chemicals, biological reagents and cell lines
Ricin (Ricinus communis agglutinin II), RTA, and RTB were purchased from Vector Laboratories (Burlingame, CA). ΔRTA, which carries deletions of residues 34–43 and 199–267 was kindly provided by Ralph Tammariello and Dr. Leonard Smith, USAMRIID (Fort Detrick, MD) [10]. Recombinant RTA (rRTA) and rRTA point mutants (R193A/R235A, G212E, P95L/E145K, R213A/R258A, R189A/R234A) were a gift from Drs. Nilgun Tumer and Xiao-Ping Li (Rutgers University, New Brunswick, NJ). RiVax and RiVax point mutants (V18P or S89T) were obtained from Drs. Justin Thomas and Russ Middaugh (University of Kansas, Lawrence, KS). ΔRTA was biotinylated using the Sulfo-NHS-LC-Biotin kit (Pierce, Rockford, IL). Ricin, ΔRTA and biotinylated derivatives were dialyzed against phosphate buffered saline (PBS) at 4°C in 10,000 MW cutoff Slide-A-Lyzer dialysis cassettes (Pierce) prior to use in cytotoxicity and mouse challenge studies. GlutaMax™, fetal bovine serum (FBS) and goat serum were purchased from Gibco-Invitrogen (Carlsbad, CA). A ClonaCell HY™ kit for hybridoma production was purchased from STEMCELL Technologies (Vancouver, BC, Canada). Pre-cast polyacrylamide gels, Laemmli sample buffer and nitrocellulose membrane (0.45μm pore size) were obtained from Bio-Rad Laboratories (Hercules, CA). Unless noted otherwise, all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Vero, and the murine myeloma cell line P3X63.Ag8.653 were purchased from the American Type Culture Collection (Manassas, VA). Cell culture media were prepared by the Wadsworth Center’s media services facility. Cell lines and hybridomas were maintained in a humidified incubator at 37°C with 5% CO2.
2.2 Mouse strains, animal care, immunizations and B cell hybridomas
Female BALB/c mice approximately 8–10 weeks of age were purchased from Taconic Labs (Hudson, NY). Animals were housed under conventional, specific pathogen-free conditions and were treated in compliance with the Wadsworth Center’s Institutional Animal Care and Use Committee (IACUC) guidelines. For hybridoma production, female BALB/c mice were primed intraperitoneally (i.p) with ricin toxoid (RT; 50 μg per mouse in 0.4 ml PBS) on day 0, and then boosted with RT (50 μg) on days 9, 19 and 33. RT was produced as described previously [21]. Three days after the third boost with RT, mice were euthanized, and total splenocytes were fused with the myeloma cell line P3X63.Ag8.653, as described [19,22]. MAbs WECB2, PA1, TB12, PH12 and IB2 were purified using IEX and protein G chromatography under endotoxin-free conditions by the Wadsworth Center protein expression core.
2.3 ELISAs, RTA peptide arrays and Western blots
ELISAs and RTA peptide arrays were performed essentially as described [10,19]. Briefly, Nunc Maxisorb F96 microtiter plates (ThermoFisher Scientific, Pittsburgh, PA) were coated overnight with RTA, RTB, recombinant RTA (rRTA), RiVax, ricin (0.1 μg/well) or rRTA or RiVax point mutants (1 μg/well, where indicated) in PBS (pH 7.4) before being treated with mAbs of interest. Horseradish peroxidase (HRP)-labeled goat anti-mouse IgG-specific polyclonal antibodies or HRP-labeled goat anti-mouse IgG isotype-specific antibodies (SouthernBiotech, Birmingham, AL) were used as secondary reagents. The ELISA plates were developed using the colorimetric detection substrate 3,3′,5,5′-tetramethylbenzidine (TMB; Kirkegaard & Perry Labs, Gaithersburg, MD) and were analyzed with a SpectroMax 250 spectrophotometer, with Softmax Pro 5.2 software (Molecular Devices, Sunnyvale, CA). The RTA peptide array used in this study consisted of either forty-four 12-mers, each overlapping its neighbor by 6 amino acids or twenty nine 18-mers, each overlapping its neighbors by 9 amino acids, collectively spanning the RTA sequence [10. Western blots were performed as previously described using 1ug/ml of each mAb (PH12, TB12, PA1 WECB2) as the primary mAbs {Neal, 2011 #3933,19].
2.4 Vero cell cytotoxicity assays
Vero cell cytotoxicity assays were performed essentially as described [18,19]. Vero cells were trypsinized, adjusted to ~5×104 cells per ml, and seeded (100 μl/well) into white 96-well plates (Corning Life Sciences, Corning, NY), and incubated for 24 hr at 37°C + 5% CO2. Vero cells were then treated with ricin (0.01 μg/ml), ricin:mAb mixture, or medium alone (negative control) for 2 hr. The cells were washed to remove non-internalized toxin or toxin:mAb mixture, and were then incubated for 48 hr. Cell viability was assessed using CellTiter-GLO reagent (Promega, Madison, WI). All treatments were performed in triplicate, and 100% viability was defined as the average value obtained from wells in which cells were treated with medium only. Toxin-neutralizing activity (TNA) was reported as the molar ratio of toxin: mAb required to inhibit ricin cytotoxicity in 50% of the treated cells (IC50).
2.5 Passive protection studies in mice
To determine the protective capacity of the mAbs found to be very effective at neutralizing ricin in vitro, mAbs WECB2, PA1, TB12, PH12, R70 (50 μg/mouse), IB2 (40 μg/mouse), and GD12 (10 μg/mouse) were diluted into endotoxin-free PBS and then administered in a final volume of 0.4 ml to female BALB/c mice (8–10 weeks old) by i.p injection. Twenty-four hr later, the mice were injected with 5 or 10×LD50 of ricin, as indicated in the figure legends. Survival was monitored over a 3-day period. In addition, hypoglycemia was used as a surrogate marker of intoxication [18,23].
2.6 mAb affinity measurements and competition analysis
MAb affinity determinations and competitive binding assays were determined by surface plasmon resonance (SPR) using a BIAcore 3000 (GE Healthcare) instrument, as described [19].
2.7 Statistical analysis and software
Statistical analysis was carried out with GraphPad Prism 5 (GraphPad Software, San Diego, CA). The significance level threshold was set at α = 0.05. PyMOL (The PyMOL Molecular Graphics System, Version 1.3. Schrödinger, LLC) was used to model B cell epitopes on ricin with Protein Data Bank (PDB) accession 2AAI from the Research Collaboratory for Structural Bioinformatics (RCSB). ElliPro [24] and Discotope [25] were accessed through the Immune Epitope Database (IEDB) portal (www.iedb.org) [26].
3.0 RESULTS
3.1 Identification of RTA-specific mAbs
In an effort to identify additional mAbs that bind unique epitopes on RTA, we produced and screened a collection of B cell hybridomas generated from ricin toxoid-immunized mice. We identified 19 hybdridomas that secreted RTA-specific immunoglobulin G (IgG) mAbs, the majority of which were of the IgG1 subclass (Table 1). These mAbs were deemed RTA-specific based on their reactivity by ELISA with ricin holotoxin and RTA, but not with RTB (Figure 2; data not shown). None of the mAbs were reactive with 12-mer or 18-mer RTA peptide arrays (data not shown), indicating that they recognize “non-linear” epitopes and are distinct from the previously described RTA-specific neutralizing (e.g., R70, PB10, GD12, and SyH7) and non-neutralizing (e.g., FGA12, SB1 and BD7) mAbs, which all bound RTA-specific peptides [19]. The top four most potent toxin-neutralizing mAbs (see below) PH12, TB12, PA1 and WECB2, did react by Western blot with ricin holotoxin and RTA in their denatured and reduced forms, suggesting that the mAbs recognize epitopes that are not strictly discontinuous in nature (Figure 3).
Table 1.
Characterization of anti-RTA mAbs
| mAb | Isotype | TNA (IC50)e | Reactivity with ΔRTA | KD [M]g |
|---|---|---|---|---|
| WECB2a | IgG1 | 1: 2 | + | 5.3 × 10−10 |
| TB12a | IgG1 | 1: 2 | + | 1.3 × 10−10 |
| PA1a | IgG1 | 1: 2 | +f | 5.8 × 10−11 |
| PB10b | IgG2b | 1:3 | + | 4 × 10−8 |
| PH12a | IgG1 | 1: 4 | + | 3.1 × 10−10 |
| PH1a | IgG1 | 1: 4 | + | |
| ED2a | IgG1 | 1: 6 | − | |
| SyH7b | IgG1 | 1:7 | +f | 2 × 10−8 |
| WECH1a | IgG1 | 1: 9 | − | |
| GD12c | IgG1 | 1:11 | + | 2.9 × 10−9 |
| IB2a | IgG1 | 1: 13 | − | 6.7 × 10−10 |
| PC11a | IgG2a | 1:13 | + | |
| R70c,d | IgG1 | 1:21 | + | 3.2 × 10−9 |
| CF3a | IgG1 | 1: 21 | +f | |
| RPC6a | IgG1 | 1:32 | + | |
| TD11a | IgG1 | 1: 43 | + | |
| PF8a | IgG2a | 1: 53 | + | |
| BA10a | IgG1 | 1: 53 | + | |
| JD4a | IgG1 | 1: 77 | − | |
| PH11a | IgG1 | 1: 107 | + | |
| BB6a | IgG1 | 1:128 | + | |
| JD7a | IgG1 | 1:513 | + | |
| M+MD5a | IgG1 | 1:1070 | − | |
| FGA12b | IgG1 | - | − | 4.1 × 10−8 |
| SB1b | IgG2a | - | − | 1.1 × 10−8 |
| BD7b | IgG1 | - | − | 1.5 × 10−7 |
mAbs originally described in
this study,
[19],
[18], or
[28].
Total Neutralizing Activity (TNA) is measured as the molar ratio of toxin: mAb required to inhibit ricin cytotoxicity in 50% ofcted with biotinylated ΔRTA tethered through an avidin “bridge”.
determined using a ricin coated CM5 chip. mAbs that react with RTA peptides are highlighted in red
Figure 2. RTA reactivity of mAbs.
Reactivity of the neutralizing mAbs with RTA in different contexts was determined by ELISA; A) all mAbs and B) those that did not recognize ΔRTA. In panel A the mAbs are ordered from most potent mAb to least potent mAb from left to right of the graph based on data presented in Table 1. The cumulative OD450 value (y-axis) was defined as the total reactivity of each form of RTA with (0.1 μg/well each in panel A and 1 μg/well each in panel B) with each mAb (0.5 μg/well; x-axis). RiVax is a rRTA with two point mutations; Y80A/V76M. rRTA* indicates that rRTA was coated on the plate at 1 μg/well. RiVax* indicates that RiVax was coated on the plate at 0.1 μg/well. Biotinylated ΔRTA was captured on the plate via an avidin bridge.
Figure 3. Western blot analysis of reactivity of select mAbs with ricin, RTA and RTB.
The reactivity of MAbs (A) PH12, (B)TB12, (C)PA1 and (D) WECB2 with ricin holotoxin (H), RTA (A) and RTB (B) under non-denatured or denatured (boiled) and/or reduced conditions (β-mercaptoethanol;BME) was evaluated by Western Blot. All the mAbs recognized ricin holotoxin (filled arrowhead) and RTA but not RTB (open arrowhead) under all conditions tested.
3.2 Toxin-neutralizing activity of RTA-specific mAbs
Using the Vero cell cytotoxicity assay described in the Materials and Methods, we determined the ability of each mAb to neutralize ricin in vitro. Each mAb (at a range of concentrations) was mixed with a fixed concentration of ricin, incubated for 1 hr, and then applied to Vero cells. Cell viability was assessed 48 hr later. We found that the molar ratios of toxin to antibody required to achieve an IC50 ranged from approximately 1:2 to 1:1070, suggesting that some of these mAbs are among the most potent toxin-neutralizing mAbs identified to date (Table 1). The most potent neutralizing mAbs had relatively high affinities for ricin, as exemplified by TB12 and PA1, whose dissociation constants (KD) as determined by Biacore analysis were 1×10-10 M and 6×10−11 M, respectively (Table 1).
To evaluate the capacity of the top four neutralizing mAbs to function in vivo, groups of BALB/c mice were passively administered WECB2, TB12, PA1, or PH12 approximately 24 hr prior to being challenged systemically with 10x or 5×LD50 of ricin. We also included IB2 in these studies as we recently reported that it recognizes a unique epitope on RTA that resides near the RTA-RTB interface [20]. Following challenge, mice were monitored for the onset of hypoglycemia, a quantitative indicator of ricin intoxication, and mortality (Table 2). As controls, groups of mice were treated with one of two known protective mAbs, R70 or GD12.
Table 2.
Capacity of RTA mAbs to passively protect mice against ricin challenge.
| Blood glucose (mg/dL)
| |||||
|---|---|---|---|---|---|
| mAb | 0 hr | 24 hr | 48 hr | 72 hr | Survival |
| WECB2b | 112 ± 11 | 82 ± 5 | 68 ± 10 | 83 ± 19 | 4/4g |
| TB12b | 116 ± 13 | 84 ± 2 | 70 ± 21 | 86 ± 9 | 4/4g |
| PA1b | 105 ± 3 | 60 ± 6 | 42 ± 12 | 70 ± 33 | 3/4g |
| PH12b | 118 ± 9 | 85 ± 7d | 80 ± 11e | 86 ± 10 | 4/4g |
| R70b | 117 ± 16 | 73 ± 6 | 44 ± 10 | 67 ± 22 | 4/4g |
| Noneb | 113 ± 11 | 14 ± 3 | NAf | NAf | 0/5 |
|
| |||||
| IB2c | 121 ± 9 | 55 ± 7 | 55 ± 12 | 88 ± 7 | 5/5 |
| GD12c | 116 ± 16 | 56 ± 17 | 56 ± 13 | 77 ± 10 | 5/5 |
| Nonec | 99 ± 8 | 39 ± 29 | NAf | NAf | 0/5 |
values are mean ± standard deviations.
Animals were challenged with 10LD50 bor 5LD50 cof ricin. PH12-treated animals had significantly higher blood glucose levels as compared to the R70-treated group of animals, as determined by ANOVA with Tukeys posttest;
P<0.05, q= 4.83,
P<0.01, q= 6.03.
NA, not applicable; all animals were sacrificed or found dead prior to this time point.
Survival rate not significantly different by Fisher’s Exact Test
As expected, the sham (no antibody treatment) mice succumbed to ricin intoxication within 48 h, whereas mice treated with R70 or GD12 survived toxin challenge. Mice that had received WECB2, TB12, PH12, PA1 or IB2 also survived ricin challenge, with the exception of one mouse in the PA1-treated group that was euthanized due to the severity of hypoglycemia and overt signs of discomfort. Statistical analysis revealed that PH12-treated mice had significantly higher blood glucose levels at 24 h and 48 h post ricin challenge than did R70-treated mice (Table 2), suggesting PH12 is in fact more potent than R70, which is considered the gold standard in the field [19].
3.3 Epitope localization of neutralizing mAbs with RTA deletion and point mutants
As a strategy to tentatively localize the epitopes recognized by the newly isolated neutralizing mAbs, we examined their reactivity with ΔRTA, which lacks FD3. Of the 19 neutralizing mAbs tested, twelve reacted with ΔRTA while seven did not (Table 1; Figure 2A). However, two of the seven mAbs, PA1 and CF3 that were unable to recognize ΔRTA directly bound to an ELISA plate did recognize ΔRTA when the antigen was tethered via a biotin-avidin bridge (Figure 2B). As an additional strategy for epitope identification, we evaluated the reactivity of the 19 neutralizing mAbs with a collection of RTA point mutants. Overall, mAb binding was unaffected by any of the single or double RTA point mutations, with the exception of TB12 and M+MD5 (Figure 4). TB12 failed to recognize RTA carrying a G212E mutation. M+MD5 did not recognize RTA carrying a R193A and R235A mutation or RiVax, which carries a V76M and Y80A mutation. Overall, these data demonstrate that the epitopes of neutralizing mAbs are distributed across RTA’s three FDs, although the targets of the most potent neutralizing mAbs seem to be primarily confined to FD1 and FD2.
Figure 4. Reactivity of mAbs with RTA point mutants.

Reactivity of neutralizing mAbs with RTA point mutants was determined by ELISA. On the x-axis, the mAbs (0.5 μg/well) are ordered from most potent (left) to least potent (right). The cumulative OD450 values (y-axis) are defined as the total reactivity of RTA, rRTA, or rRTA point mutants (1.0 μg/well) with each mAb (0.5 μg/well; x-axis). * indicates that RiVax coating was done at 0.1 μg/well.
3.4 Competitive binding assays for epitope localization
We next performed competitive binding assays by SPR, in which WECB2, PA1, TB12, PH12, and IB2 were assessed for the capacity to interfere with each other, as well as with previously identified neutralizing mAbs of known epitope specificity (i.e., PB10, GD12 and SyH7). The results of this analysis indicated that there are at least four spatially distinct neutralizing epitopes (or clusters of epitopes) on RTA (Tables 3, 4; Figure 5), as described below.
Table 3.
mAb competition for binding ricin
| 2° mAb Injected | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 1° mAb Injected | Group 1 | Group 2 | Group 3 | Group 4 | ||||||||
|
| ||||||||||||
| WECB2 | PB10 | PA1 | TB12 | PH12 | SyH7 | IB2 | FGA12 | SB1 | BD7 | GD12 | ||
|
| ||||||||||||
| Group 1 | WECB2 | * | 22 | 83 | 69 | 87 | 63 | 66 | * | * | * | 70 |
| PB10 | 21 | * | * | * | * | 86a | 71 | * | 39a | 43a | 60a | |
|
| ||||||||||||
| Group 2 | PA1 | 62 | 53 | * | 12 | 17 | 0 | 79 | * | * | * | 52 |
| TB12 | 60 | 66 | 33 | * | 29 | 4 | 70 | * | * | * | 44 | |
| PH12 | 58 | 67 | 12 | 3 | * | 11 | 70 | * | * | * | 62 | |
| SyH7 | 96 | 75a | 53 | 37 | 32 | * | * | * | 79a | 100a | 100a | |
|
| ||||||||||||
| Group 3 | IB2 | * | 71b | 79 | 70 | 70 | 63 | * | 0b | 35 | 68b | 71 |
|
| ||||||||||||
| Group 4 | GD12 | 96 | 43a | * | * | * | 88a | * | * | 0a | 0a | * |
Table 4.
Neutralizing mAbs bind 4 distinct regions of RTA
| Cluster | mAbs |
|---|---|
| 1 | PB10, R70, WECB2 |
| 2 | SyH7, PA1, TB12, PH12 |
| 3 | IB2, FGA12* |
| 4 | GD12 |
mAbs that bind linear epitopes are underlined.
non-neutralizing mAb
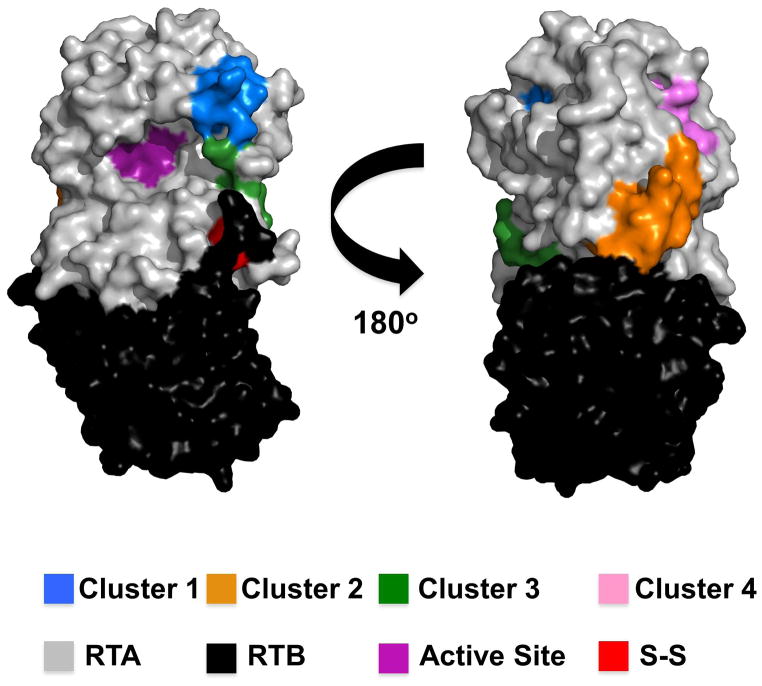
Figure 5. Neutralizing B cell epitope clusters on RTA.
PyMOL surface representation of ricin holotoxin depicting the four distinct clusters of neutralizing B cell epitopes on RTA that are described in this study. The clusters are color-coded consistent with Table 4. Ricin’s subunits, RTA and RTB, are colored grey and black, respectively. RTA’s active site is colored in magenta, and the cysteine residues that form the disulfide bond linking RTA to RTB are colored red.
Epitope cluster 1
The binding of WECB2 to ricin was reduced by ~80% when the toxin was pre-bound with PB10, but was largely unaffected by the other mAbs tested, including GD12, SyH7, and IB2 (Table 3). Conversely, the binding of PB10 to ricin was greatly affected by WEBC2, but not the other neutralizing mAbs. These data suggest that WECB2 recognizes an epitope that is adjacent to or overlapping with PB10’s epitope, which encompasses residues N97-F108 [19,27].
Epitope cluster 2
The abilities of PA1, TB12, and PH12 to bind ricin were all affected (by varying degrees) by SyH7 (Table 3), suggesting that they bind epitopes overlapping with, or adjacent to, residues E187-S198 [19]. For example, PA1 completely prevented the binding of SyH7 to ricin, indicating that those two mAbs likely recognize closely overlapping epitopes. However, SyH7 only partially blocked PA1 binding to ricin, suggesting that PA1’s “footprint” on RTA exceeds that of SyH7’s. TB12 and PH12 also competitively inhibited SyH7 but less effectively than PA1, suggesting they recognize epitopes partially encompassing residues E187-S198.
Epitopes 3 and 4
The binding of IB2 and GD12 to ricin were not affected by any of the other mAbs tested, demonstrating that these two mAbs recognize epitopes that are spatially or structurally distinct from other known epitopes. As noted previously, GD12 recognizes a linear epitope spanning RTA residues 163–174 [18]. While the exact epitope recognized by IB2 is not known, we speculated in a recent report that it may be situated in the N-terminus near the cysteine residues that forms a disulfide bridge with RTB [20].
Overall, these data suggest that neutralizing (linear and non-linear) B cell epitopes on RTA are limited to a few, relatively small regions on the surface of RTA (Table 4).
4.0 DISCUSSION
In an effort to better define the regions of RTA that are responsible for eliciting protective immunity to ricin, in this report we have characterized a collection of mAbs that recognize non-linear B cell epitopes on RTA. As these mAbs failed to react with RTA peptide arrays, they were, by definition, distinct from the neutralizing mAbs we previously described, including PB10, GD12, and SyH7 [18,19]. The most potent toxin-neutralizing mAbs in this new collection, namely WECB2, TB12, PA1, PH12, and IB2 each had nanamolar (or sub-nanomolar) affinities for ricin and were each capable of passively protecting mice against a 5–10×LD50 toxin challenge. The most important finding of this study, however, is that WECB2, TB12, PA1, and PH1 likely bind near to previously described neutralizing linear B cell epitopes on RTA. Competition analysis indicated that WECB2 binds an epitope that overlaps with PB10, while TB12, PA1, and PH12 recognize epitope(s) similar to or overlapping with SyH7. The other two mAbs, GD12 and IB2, apparently recognize spatially distinct epitopes. As will be discussed below, we estimate that we have now accounted for ~75% of the predicted B cell epitopes on the surface of RTA and that toxin-neutralizing epitopes constitute a relatively small amount of surface area on RTA. This information provides a framework for further refinement of RTA mutagenesis and vaccine design.
The results in this study reaffirm the importance of immunodominant α-helix B (corresponding to residues 97–109) as a target of toxin-neutralizing antibodies. The first mAb R70 (UNIVAX 70) identified against this epitope was described by Lemley and colleagues [28], while Lebeda and colleagues recognized that α-helix B is highly conserved within the RIPs and is likely a universal target for toxin-inactivating antibodies [29]. Since those initial reports we and others have described additional R70-like mAbs, including 23D7 [30], PB10 [19] and 6C2 [31]. We now add WECB2 to that list. Using a phage-displayed peptide library-based approach, we defined in high resolution the specific resides within α-helix B that are recognized by R70 and PB10 [27]. It will be interesting to determine WECB2’s exact footprint on RTA, as it is even more potent than R70 and PB10 at neutralizing ricin. Remarkably, despite the importance of α-helix B in eliciting protective immunity to ricin, it remains unknown how antibodies against this region of RTA ultimately interfere with ricin’s cytotoxic activities.
Monoclonal Abs PA1, TB12 and PH12 likely target epitopes within the vicinity of SyH7’s epitope, which is situated within an arginine-rich region (residues 187–204) that forms an exposed, positively charged patch on the “backside” of RTA that has been proposed to interact with ribosomal stalk proteins [32,33]. Binding of SyH7 to ricin was almost completely abolished when either PA1, TB12 and PH12 were prebound to the toxin, whereas the converse was not true; SyH7 only partially blocked PA1, TB12 and PH12 binding to ricin. The observed binding hierarchy is not due to differences in affinities for ricin, as all four mAbs have more of less similar dissociation constants, but possibly differences in relative “footprints” on the subunit’s surface.
The two remaining mAbs that were included in this study, IB2 and GD12, bind epitopes distinct from all other mAbs in our collection. Based on several lines of evidence, we postulate that IB2’s epitope is situated near the disulfide bond that links RTA and RTB [20]. We recently reported that IB2 interferes with PDI-mediated reduction of ricin in vitro, although we have not shown that IB2 actually traffics to the ER in complex with ricin. GD12’s epitope is situated within α-helix E (residues T163-M174), which runs through the core of FD2 and terminates with two residues (E177 and R180) involved in RTA’s catalytic activity. GD12 recognizes one of the few known human B cell epitopes on RTA, first identified by pepscan analysis of serum from Hodgkin’s lymphoma patients treated with RTA immunotoxin [34]. Again, it is unknown how GD12 actually inactivates ricin toxin, although GD12 could theoretically distort the active site from a distance.
We estimate that our collection of neutralizing and non-neutralizing mAbs target epitopes that cover roughly 50% of the surface of RTA [18,19,33] and approximately 75% of the total number of predicted B cell epitopes, based on programs like Emini Surface Accessibility, Discotope [25] and ElliPro [24] (Figure 6). If our estimates are correct, then neutralizing B cell epitopes may indeed comprise very little “real estate” on the surface of RTA. It is therefore imperative that these neutralizing B cell epitopes be preserved when (re)designing the next generation of ricin toxin subunit vaccines.
Figure 6. Known and predicted B cell epitopes on RTA.
PyMOL surface representation of ricin depicting known and predicted B cell epitopes on RTA. ElliPro and Discotope (available through the IEDB) were used to predict B cell epitopes on RTA. The colored shading corresponds to the following: dark orange, known epitopes but not predicted; marine blue, predicted and validated epitopes; light orange, predicted but not yet validated. Ricin’s subunits, RTA and RTB, are colored grey and black, respectively. RTA’s active site is colored in magenta, and the cysteine residues that form the disulfide bond linking RTA to RTB are colored red.
Supplementary Material
HIGHLIGHTS.
Produced new collection of monoclonal antibodies against ricin toxin’s A subunit
Top five monoclonal antibodies passively protected mice from ricin challenge
Localized epitopes recognized by top five monoclonal antibodies
These results provide basis for rationale design of RTA vaccines
Acknowledgments
We would like to thank Jianzhong Tang and Mark Meola of the Wadsworh Center’s Cell culture and media core facility for maintaining a number of hybridomas for us, and Dr. Karen Chave and Dan Milich of the Wadsworth Center’s Protein Expression core facility for monoclonal antibody purification. We gratefully acknowledge Drs. Nilgun Tumer and Xiao-Ping Li (Rutgers University), Drs. Justin Thomas and Russ Middaugh (University of Kansas), as well as Ralph Tammariello and Dr. Leonard Smith (USAMRIID) for providing RTA point and deletion mutants for the purpose of epitope mapping. Finally, we would like to thank Dr. Robert Brey at Soligenix, Inc. for his useful suggestions and discussions. This work was supported by grant AI097688 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sandvig K, Skotland T, van Deurs B, Klokk TI. Retrograde transport of protein toxins through the Golgi apparatus. Histochem Cell Biol. 2013 doi: 10.1007/s00418-013-1111-z. [DOI] [PubMed] [Google Scholar]
- 2.Spooner RA, Hart PJ, Cook JP, Pietroni P, Rogon C, Hohfeld J, et al. Cytosolic chaperones influence the fate of a toxin dislocated from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2008;105:17408–17413. doi: 10.1073/pnas.0809013105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spooner RA, Lord JM. How Ricin and Shiga Toxin Reach the Cytosol of Target Cells: Retrotranslocation from the Endoplasmic Reticulum. Curr Top Microbiol Immunol. 2012;357:19–40. doi: 10.1007/82_2011_154. [DOI] [PubMed] [Google Scholar]
- 4.Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxins on eukaryotic ribosomes. J Biol Chem. 1987;262:5908–5912. [PubMed] [Google Scholar]
- 5.Katzin BJ, Collins EJ, Robertus JD. Structure of ricin A-chain at 2. 5 A. Proteins. 1991;10:251–259. doi: 10.1002/prot.340100309. [DOI] [PubMed] [Google Scholar]
- 6.Rutenber E, Katzin BJ, Ernst S, Collins EJ, Mlsna D, Ready MP, et al. Crystallographic refinement of ricin to 2. 5 A. Proteins. 1991;10:240–250. doi: 10.1002/prot.340100308. [DOI] [PubMed] [Google Scholar]
- 7.Monzingo AF, Robertus JD. X-ray analysis of substrate analogs in the ricin A-chain active site. J Mol Biol. 1992;227:1136–1145. doi: 10.1016/0022-2836(92)90526-p. [DOI] [PubMed] [Google Scholar]
- 8.Rutenber E, Robertus JD. Structure of ricin B-chain at 2. 5 A resolution. Proteins. 1991;10:260–269. doi: 10.1002/prot.340100310. [DOI] [PubMed] [Google Scholar]
- 9.Thrush GR, Lark LR, Clinchy BC, Vitetta ES. Immunotoxins: an update. Annu Rev Immunol. 1996;14:49–71. doi: 10.1146/annurev.immunol.14.1.49. [DOI] [PubMed] [Google Scholar]
- 10.O’Hara JM, Brey RN, Mantis NJ. Comparative Efficacy in Mice of Two Lead Candidate Ricin Toxin A Subunit (RTA) Vaccine. Clinical and Vaccine Immunology. 2013 doi: 10.1128/CVI.00098-13. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smallshaw JE, Vitetta ES. Ricin Vaccine Development. Curr Top Microbiol Immunol. 2012;357:259–272. doi: 10.1007/82_2011_156. [DOI] [PubMed] [Google Scholar]
- 12.Smallshaw JE, Firan A, Fulmer JR, Ruback SL, Ghetie V, Vitetta ES. A novel recombinant vaccine which protects mice against ricin intoxication. Vaccine. 2002;20:3422–3427. doi: 10.1016/s0264-410x(02)00312-2. [DOI] [PubMed] [Google Scholar]
- 13.Smallshaw JE, Ghetie V, Rizo J, Fulmer JR, Trahan LL, Ghetie MA, et al. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol. 2003;21:387–391. doi: 10.1038/nbt800. [DOI] [PubMed] [Google Scholar]
- 14.McLain DE, Horn TL, Detrisac CJ, Lindsey CY, Smith LA. Progress in Biological Threat Agent Vaccine Development: A Repeat-Dose Toxicity Study of a Recombinant Ricin Toxin A-Chain (rRTA) 1-33/44-198 Vaccine (RVEc) in Male and Female New Zealand White Rabbits. Int J Toxicol. 2011 doi: 10.1177/1091581810396730. [DOI] [PubMed] [Google Scholar]
- 15.McLain DE, Lewis BS, Chapman JL, Wannemacher RW, Lindsey CY, Smith LA. Protective Effect of Two Recombinant Ricin Subunit Vaccines in the New Zealand White Rabbit Subjected to a Lethal Aerosolized Ricin Challenge: Survival, Immunological Response and Histopathological Findings. Toxicol Sci. 2011 doi: 10.1093/toxsci/kfr274. [DOI] [PubMed] [Google Scholar]
- 16.Vitetta ES, Smallshaw JE, Schindler J. A Small Phase IB Clinical Trial of an Alhydrogel-Adsorbed Recombinant Ricin Vaccine (RiVax) Clin Vaccine Immunol. 2012 doi: 10.1128/CVI.00381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas JC, O’Hara JM, Hu L, Gao FP, Joshi SB, Volkin DB, et al. Effect of single-point mutations on the stability and immunogenicity of a recombinant ricin A chain subunit vaccine antigen. Human vaccines & immunotherapeutics. 2013;9:740–748. doi: 10.4161/hv.22998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neal LM, O’Hara J, Brey RN, 3rd, Mantis NJ. A monoclonal immunoglobulin G antibody directed against an immunodominant linear epitope on the ricin A chain confers systemic and mucosal immunity to ricin. Infect Immun. 2010;78:552–561. doi: 10.1128/IAI.00796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Hara JM, Neal LM, McCarthy EA, Kasten-Jolly JA, Brey RN, 3rd, Mantis NJ. Folding domains within the ricin toxin A subunit as targets of protective antibodies. Vaccine. 2010;28:7035–7046. doi: 10.1016/j.vaccine.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Hara JM, Mantis NJ. Neutralizing Monoclonal Antibodies against Ricin’s Enzymatic Subunit Interfere with Protein Disulfide Isomerase-Mediated Reduction of Ricin Holotoxin In Vitro. J Immunol Methods. 2013 doi: 10.1016/j.jim.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neal LM, McCarthy EA, Morris CR, Mantis NJ. Vaccine-induced intestinal immunity to ricin toxin in the absence of secretory IgA. Vaccine. 2011;29:681–689. doi: 10.1016/j.vaccine.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yermakova A, Vance DJ, Mantis NJ. Sub-Domains of Ricin’s B Subunit as Targets of Toxin Neutralizing and Non-Neutralizing Monoclonal Antibodies. PLoS One. 2012;7:e44317. doi: 10.1371/journal.pone.0044317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pincus SH, Eng L, Cooke CL, Maddaloni M. Identification of hypoglycemia in mice as a surrogate marker of ricin toxicosis. Comp Med. 2002;52:530–533. [PubMed] [Google Scholar]
- 24.Ponomarenko J, Bui HH, Li W, Fusseder N, Bourne PE, Sette A, et al. ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC bioinformatics. 2008;9:514. doi: 10.1186/1471-2105-9-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haste Andersen P, Nielsen M, Lund O. Prediction of residues in discontinuous B-cell epitopes using protein 3D structures. Protein Sci. 2006;15:2558–2567. doi: 10.1110/ps.062405906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vita R, Zarebski L, Greenbaum JA, Emami H, Hoof I, Salimi N, et al. The immune epitope database 2. 0. Nucleic Acids Res. 2010;38:D854–862. doi: 10.1093/nar/gkp1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vance DJ, Mantis NJ. Resolution of two overlapping neutralizing B cell epitopes within a solvent exposed, immunodominant alpha-helix in ricin toxin’s enzymatic subunit. Toxicon. 2012;60:874–877. doi: 10.1016/j.toxicon.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemley PV, Amanatides P, Wright DC. Identification and characterization of a monoclonal antibody that neutralizes ricin toxicity in vitro and in vivo. Hybridoma. 1994;13:417–421. doi: 10.1089/hyb.1994.13.417. [DOI] [PubMed] [Google Scholar]
- 29.Lebeda FJ, Olson MA. Prediction of a conserved, neutralizing epitope in ribosome-inactivating proteins. Int J Biol Macromol. 1999;24:19–26. doi: 10.1016/s0141-8130(98)00059-2. [DOI] [PubMed] [Google Scholar]
- 30.Mantis NJ, McGuinness CR, Sonuyi O, Edwards G, Farrant SA. Immunoglobulin A antibodies against ricin A and B subunits protect epithelial cells from ricin intoxication. Infect Immun. 2006;74:3455–3462. doi: 10.1128/IAI.02088-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai J, Zhao L, Yang H, Guo H, Fan K, Wang H, et al. Identification of a novel functional domain of ricin responsible for its potent toxicity. J Biol Chem. 2011;286:12166–12171. doi: 10.1074/jbc.M110.196584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XP, Kahn PC, Kahn JN, Grela P, Tumer NE. Arginine residues on the opposite side of the active site stimulate the catalysis of ribosome depurination by ricin A chain by interacting with the P-protein stalk. J Biol Chem. 2013 doi: 10.1074/jbc.M113.510966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Hara JM, Yermakova A, Mantis NJ. Immunity to ricin: fundamental insights into toxin-antibody interactions. Curr Top Microbiol Immunol. 2012;357:209–241. doi: 10.1007/82_2011_193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castelletti D, Fracasso G, Righetti S, Tridente G, Schnell R, Engert A, et al. A dominant linear B-cell epitope of ricin A-chain is the target of a neutralizing antibody response in Hodgkin’s lymphoma patients treated with an anti-CD25 immunotoxin. Clin Exp Immunol. 2004;136:365–372. doi: 10.1111/j.1365-2249.2004.02442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




 25%
25%
 25–50%
25–50%
 >50%
>50%