Abstract
Arsenic originates from both geochemical and numerous anthropogenic activities. Exposure of the general public to significant levels of arsenic is widespread. Arsenic is a well-documented human carcinogen. Long-term exposure to high levels of arsenic in drinking water have been linked to bladder, lung, kidney, liver, prostate, and skin cancer. Among them, lung cancer is of great public concern. However, little is known about how arsenic causes lung cancer and few studies have considered effects in normal human lung cells. The purpose of this study was to determine the cytotoxicity and genotoxicity of arsenic in human primary bronchial fibroblast and epithelial cells. Our data show that arsenic induces a concentration-dependent decrease in cell survival after short (24 h) or long (120 h) exposures. Arsenic induces concentration-dependent but not time-dependent increases in chromosome damage in fibroblasts. No chromosome damage is induced after either 24 h or 120 h arsenic exposure in epithelial cells. Using neutral comet assay and gamma-H2A.X foci forming assay, we found that 24 h or 120 h exposure to arsenic induces increases in DNA double strand breaks in both cell lines. These data indicate that arsenic is cytotoxic and genotoxic to human lung primary cells but lung fibroblasts are more sensitive to arsenic than epithelial cells. Further research is needed to understand the specific mechanisms involved in arsenic-induced genotoxicity in human lung cells.
Keywords: Arsenic, Genotoxicity, Chromosome aberration, DNA double strand breaks, human lung fibroblasts, human lung epithelial cells
1. Introduction
Arsenic (As) is an abundant naturally occurring element found in earth crust [1]. It is also released into the environment from human activities such as mining, electronics manufacturing and farming. As a result, high arsenic levels can occur in ground water and food raising health concerns for millions of people worldwide. In 2001, the United States Environmental Protection Agency (EPA) revised its drinking water standard for arsenic from 50 ug/l to 10 ug/l to better protect people from the adverse effects of long-term arsenic exposure [2]. However, millions of people worldwide are still exposed to arsenic at concentrations greater than 50 ug/l in drinking water [3, 4].
Arsenic has been classified as group 1 human carcinogen by the International Agency for Research on Cancer (IARC). Studies show that chronic inorganic arsenic exposure leads to the development of lung, skin, liver, kidney and urinary bladder cancers [5]. Among these cancers, lung cancer is a major public health concern due to its high incidence rate and mortality [6].
Arsenic was first found associated with lung cancer in smelter workers exposed to arsenic via inhalation [7, 8]. A significant dose-response relationship between the ingestion of inorganic arsenic in drinking water and increased lung cancer risks was found in Bangladesh [4], Taiwan [9, 10], and Chile [11]. A recent study reported that even after high arsenic exposure level (11-335 ug/l) had been reduced for decades, lung cancer risk were still high in the exposed population [12]. Evidence also shows that even moderate concentrations of arsenic (less than 7.5 ppm) significantly impact lung cancer incidence, suggesting non-occupational exposures or lower levers of environmental exposure to arsenic should also be of concern with respect to lung cancer [13].
Finding an animal model to study arsenic-induced lung cancer has been difficult. While some studies found higher lung cancer rates in arsenic-exposed animals, others show negative results [5]. These negative results may be due a variety of factors including low animal numbers, low doses or short exposure durations [7]. By contrast, most lung cell culture studies support the conclusion that arsenic is a lung carcinogen. The ability of inorganic arsenic to induce malignant cell transformation has been demonstrated in several human lung epithelial cell lines [14-16]. The mechanism of arsenic-induced lung cancer is uncertain. Several hypotheses have generally been proposed including genotoxicity, induction of oxidative stress and inhibition of DNA repair [17]. Among these, the genotoxic mode of action is of high interest but has been under studied in human lung cells [18-21]. Only two studies considered arsenic genotoxicity in human lung cells. They found arsenic induces DNA single strand breaks and DNA-protein crosslinks in human fetal lung fibroblasts [22, 23]. Studies of the impact of arsenic on chromosomes in human lung cells have not yet been considered, despite the importance of chromosomes as a subcellular target in carcinogenesis. Thus, this study assesses the ability of arsenic to induce chromosomal aberrations and DNA double strand breaks in primary human lung cells.
2. Materials and Methods
2.1. Chemicals and Reagents
Sodium metaarsenite, demecolcine, and potassium chloride (KCl) were purchased from Sigma (St. Louis, MO). Giemsa stain was purchased from Biomedical Specialties Inc. (Santa Monica, CA). Crystal violet, methanol and acetone were purchased from J.T. Baker (Phillipsburg, NJ). Dulbecco's minimal essential medium and Ham's F-12 medium (D-MEM/F-12) were purchased from Mediatech Inc. (Herndon, VA). BEGM bronchial epithelial cell growth medium and supplements were purchased from Lonza (Allendale, NJ). Cosmic calf serum (CCS) was purchased from Hyclone, (Logan, UT). Gurr's buffer, trypsin/EDTA, sodium pyruvate, penicillin/streptomycin, and L-glutamine were purchased from Invitrogen Corporation (Grand Island, NY). Tissue culture dishes, flasks and plasticware were purchased from Corning Inc. (Acton, MA).
2.2. Cells and Cell Culture
We chose to use normal primary human bronchial fibroblasts, NHBF (Clonetics), and normal primary human bronchial epithelial cells, NHBE, (Lonza) for these studies. The cell cycle time for both cell lines is 22-24 h. These cells are isolated from normal donor airway located above the bifurcation of the lungs. NHBF were cultured in a 50:50 mix of Dulbecco's minimal essential medium and Ham's F-12 medium plus 15% cosmic calf serum, 1% L-glutamine, 0.1 mM sodium pyruvate and 1% penicillin/streptomycin. NHBE were cultured in serum-free BEGM bronchial epithelial cell growth medium with supplements. All cells were maintained in a 37°C, humidified incubator with 5% CO2. Cells were routinely checked for mycoplasma contamination.
2.3. Chemical Preparation and Treatment
Solutions of sodium arsenite were prepared by weighing out the desired amount of NaAsO2, and dissolving it in double distilled water and then sterile-filtering it through a 10 ml syringe with a 0.2 um filter. Dilutions were made for appropriate treatment concentrations. Cells were seeded in culture dishes, and allowed to grow for 48 h to enter log growth phase. The cells were then treated for 24 h or 120 h with NaAsO2.
2.4. Cytotoxicity Assays
Cytotoxicity was determined by a clonogenic survival assay measuring the reduction in plating efficiency in treatment groups relative to controls as previously described [24]. There were four dishes per treatment group and each experiment was repeated at least three times.
2.5. Chromosome abnormality
Cells were prepared for chromosomal analysis as previously described [24]. Cells were analyzed for chromosome structure and numerical aberrations, centromere spreading, premature anaphase, and premature centromere division. At least 100 metaphases per data point were analyzed in each experiment. Each experiment was repeated at least three times.
2.6. Neutral Comet Assay
DNA double strand breaks were measured using a gel electrophoresis assay (comet assay) under neutral conditions based on our published method [25]. Briefly, the cell suspension was mixed with low-melting agarose (Trevigen) and spread on a 2 well CometSlide (Trevigen). The cells were then lysed in prechilled lysis solution (Trevigen) at 4°C followed by digesting with proteinase-K (Amresco, Solon, OH) for 2 h at 37°C. Next, cells were electrophoresed in freshly prepared electrophoresis buffer (300 mM sodium acetate, 100 mM Tris, pH 9.0) at 4°C. All the steps described above were conducted under a reduced light level to prevent spurious DNA damage. Slides were then immersed in DNA precipitation solution (1 M ammonium acetate in ethanol) for 30 minutes at room temperature, air-dried and stained with SYBR Green. Comet images were captured using an Olympus fluorescence microscope equipped with a digital video camera and analyzed with the Comet Assay IV image analysis system (Perceptive Inc, UK). The tail intensity was the measurement used to quantify DNA double strand breaks. 100 nuclei were analyzed for each concentration.
2.7. Immunofluorescence for gamma-H2A.X foci formation
We also used gamma-H2AX foci to detect the presence of arsenic-induced DNA double strand breaks based on our published methods [25]. Briefly, cells were grown on 8 well chamber slides. After treatment, the cells were fixed in 4% paraformaldehyde for 10 min, permeablilized with 0.2% Triton X-100 for 5 min and blocked with goat serum (Jackson Immunolaboratories) for 1 h. Cells were then incubated with anti-gamma-H2A.X antibody (Cell Signaling) at 4°C overnight and followed by incubating with AlexaFluor 488-conjugated IgG secondary antibody for 1 h. Nuclei were counterstained with DAPI. The slides were mounted and viewed with an Olympus laser scanning confocal microscope (LSCM) using a 100X objective. Images of the same experiment were obtained using the same LCSM parameters (brightness, contrast, pinhall, etc.) and analyzed with Image J software. 100 nuclei per each treatment concentration were analyzed. Cells with more than 5 foci were considered double strand break positive.
2.8.Statistics
Values were expressed as the mean ± SEM (standard error of the mean) of triplicate experiments. The Student's t-test was used to calculate p-values to determine the statistical significance of difference in means for each pair of concentrations. A 95% confidence interval for the difference in means of each pair of concentrations was constructed based on the Student's t distribution.
3. Results
3.1. Cytotoxicity of arsenic
Arsenic induces a concentration-dependent decrease in relative survival in both human lung fibroblasts and epithelial cells. Specifically, 24 h exposure to concentration of 0.5, 1, 5 and 10 uM sodium arsenite reduced relative survival to 72, 64, 54 and 35% in lung fibroblasts, respectively; and 110, 102, 89 and 59%, respectively, in lung epithelial cells (Figure 1A). Arsenic also induced a time-dependent decrease in relative survival in both cell lines. 120 h exposure to concentrations of 0.5, 1, 5 and 10 uM sodium arsenite reduced relative survival to 65, 57, 31 and 11 % in lung fibroblasts, respectively, and 92, 88, 63 and 0.7 % in lung epithelial cells, respectively (Figure 1B). These data indicate that sodium arsenite is more cytotoxic to human lung fibroblasts than lung epithelial cells (Table 1).
Figure 1. Cytotoxicity of Sodium Arsenite in Primary Human Lung Cells.
This figure shows that sodium arsenite induces a time- and concentration-dependent decrease in relative cell survival in human lung cells. Cells were treated with sodium arsenite for 24 h or 120 h. A) Sodium arsenite decreases relative cell survival in human lung fibroblasts. B) Sodium arsenite decreases relative cell survival in human lung epithelial cells. Data represent four independent experiments ± standard error of mean. *Statistically different from control (p<0.05). ** Statistically different from control (p<0.005).
Table 1. The Spectrum of Arsenic-induced Chromosome Aberration in Primary Human Bronchial Fibroblasts.
| Arsenic Concentration (uM) |
Chromatid Break & Gap |
Isochromatid Break & Gap |
Acentric Fragment |
Double Minute |
Total Damage |
|---|---|---|---|---|---|
| 24 h treatment | |||||
|
| |||||
| 0 | 9.7±1.5 | 0.7±0.3 | 0.0±0.0 | 0.3±0.3 | 10.7±1.8 |
| 0.5 | 11.7±3.2 | 0.3±0.3 | 0.0±0.0 | 0.0±0.0 | 12.0±3.4 |
| 1 | 12.3±2.6 | 0.7±0.7 | 0.0±0.0 | 0.7±0.3 | 13.7±3.0 |
| 5 | 22.7±7.3 | 1.7±1.2 | 0.0±0.0 | 0.7±0.7 | 26.2±7.0 |
| 10 | 47.0±7.1 | 2.3±1.9 | 0.0±0.0 | 2.7±0.9 | 52.0±4.9 |
| 120 h treatment | |||||
|
| |||||
| 0 | 6.0±1.2 | 0.3±0.3 | 0.0±0.0 | 0.0±0.0 | 6.3±1.5 |
| 0.5 | 8.7±1.2 | 0.7±0.7 | 0.0±0.0 | 0.0±0.0 | 9.3±1.9 |
| 1 | 9.0±1.2 | 1.7±0.9 | 0.3±0.3 | 0.7±0.7 | 11.7±2.3 |
| 5 | 14.0±1.0 | 1.3±0.7 | 0.0±0.0 | 1.0±0.6 | 16.3±1.2 |
| 10 | 14.0±0.8 | 1.0±0.0 | 2.0±1.6 | 0.0±0.0 | 17.0±1.0 |
No chromatid exchange, ring, dicentric and fragmented chromosome were observed.
3.2. Genotoxicity of Arsenic
Next, we investigated the genotoxicity of arsenic in both cell lines. We considered chromosomal aberrations as a measure of large-scale DNA damage and DNA double strand breaks as a measure of damage on a finer scale [26]. We considered both structural and numerical aberrations for chromosome damage. For structural aberrations, we measured the frequency of damaged cells as percent of metaphases with damage, and the extent of damage within cells as the total aberrations per 100 metaphases. The difference between these two measurements indicates an increasing number of cells with multiple aberrations. Sodium arsenite induced a concentration-dependent increase in both the percent of metaphases with damage and the total aberrations per 100 metaphases in lung fibroblasts but not in lung epithelial cells (Figure 2A-D, Table 1). In epithelial cells, 24 h exposure to 10 uM and 120 h exposure to 5 uM sodium arsenite induced cell cycle arrest and no metaphases were observed. No significant difference on mitotic index in other concentrations compare to control was observed.
Figure 2. Sodium Arsenite Induces Chromosome Damage in Primary Human Lung Cells.
This figure shows that sodium arsenite induces a concentration-dependent increase in chromosome damage in fibroblasts but not in epithelial cells. Cells were treated with sodium arsenite for 24 h or 120 h. A) Chromosome damage in fibroblasts after 24 h or B) 120 h treatment. C) Chromosome damage in epithelial cells after 24 h and D) 120 h treatment. NM: No metaphase. Data represent the chromosome damage found in 100 metaphases and are the means of three independent experiments ± standard error of mean. *Statistically different from control (p<0.05). ** Statistically different from control (p<0.005).
Numerical aberrations manifested as aneuploidy, polyploidy and tetraploidy. Cells were grouped as previously described [27] based on chromosome number into diploid (46 chromosomes), hypodiploid (<46 chromosomes), hyperdiploid (between 47 and 91 chromosomes), and tetraploid (92 chromosomes). Chronic exposure to arsenic induced aneuploidy in human lung fibroblasts (Figure 3). In particular, 5 and 10 uM sodium arsenite increased hyperdiploid metaphases in fibroblasts after 120 h exposure (Figure 3C). Increases in tetraploidy was only found in cells exposed to 5 uM sodium arsenite for 120 h. 24 h or 120 h exposure to arsenic did not induce aneuploidy in epithelial cells.
Figure 3. Arsenic Induces Aneuploidy in Primary Human Lung Fibroblasts.
This figure shows that chronic exposure to sodium arsenite increases in aneuploidy in fibroblasts. A) Percent of metaphases with aneuploidy. B) Percent of metaphases that are diploid, hypodiploid, hyperdiploid, or tetraploid after 24 h or C) 120 h exposure to sodium arsenite. Data represent three independent experiments ± standard error of mean.*Statistically different from control (p<0.05).
Chromosomal aberrations and aneuploidy can result from a defective mitotic checkpoint [28, 29]. Thus, we investigated whether arsenic induces mitotic abnormalities in fibroblasts. We found that exposure to sodium arsenite induced premature centromere division and premature anaphase in lung fibroblasts indicating spindle assembly checkpoint bypass occurred (Figure 4A, B) [27]. Premature centromere division was defined as a cell in which at least one chromosome was still attached to its sister chromatid and at least one chromosome was completely separated from its sister chromatid (Figure 4C-2). Premature anaphase was defined as cells in which all of the sister chromatids were completely separated from each other (Figure 4C-3). Centromere spreading was defined as a cell in which centromere separated in at least one chromosome (Figure 4C-4).
Figure 4. Sodium Arsenite Induces Mitotic Abnormalities in Primary Human Lung Fibroblasts.
This figure shows that sodium arsenite induces premature centromere division and premature anaphase in fibroblasts. Cells were treated with sodium arsenite for 24 or 120 h. A) Percent of metaphases with mitotic abnormalities after 24 h exposure or B) 120 h exposure in lung fibroblasts. C) Representative pictures of normal metaphase (1), premature centromere division (2), premature anaphase (3) and centromere spreading (4). Data represent three independent experiments ± standard error of mean.*Statistically different from control (p<0.05). ** Statistically different from control (p<0.005).
The increased structural chromosomal aberrations suggest that arsenic induced DNA double strand breaks in these cells. DNA double strand breaks are dangerous lesions with severe consequences for cell survival, genomic stability and carcinogenesis [30]. Therefore, we tested the ability of arsenic to induce these lesions. We found that sodium arsenite induced a slight increase in DNA double strand breaks based on the comet assay, presented as tail intensity after 24 h but induced a higher level of damage after 120 h exposure in lung fibroblasts. Specifically, 0.5, 1, 5 and 10 uM sodium arsenite induced increases in tail intensity of 0.2, 1.7, 1.9, and 3.2 compared to control after 24 h exposure, respectively and 4.9, 3.7, 7.0 and 9.8 tail intensity compared to control after 120 h exposure, respectively in human lung fibroblasts (Figure 5A). We found that sodium arsenite also induced an increase in double strand breaks in lung epithelial cells. 24 h exposure induced similar level of DNA lesions in epithelial cells as in fibroblasts but less lesions than that in fibroblasts after 120 h exposure (Figure 5).
Figure 5. Sodium Arsenite Induces DNA Double Strand Breaks in Primary Human Lung Cells.

This figure shows that sodium arsenite induces an increase in comet tail intensity measured by neutral comet assay. A) Comet tail intensity after 24 or 120 h sodium arsenite treatment in lung fibroblasts. Control values of 7 for 24 h treatment and 8 for 120 h treatment were subtracted from treated values. B) Comet tail intensity after 24 or 120 h sodium arsenite treatment in lung epithelial cells. Control values of 7 for 24 h treatment and 4 for 120 h treatment were subtracted from treated values. C) Photomicrographs of human lung fibroblasts (top) and epithelial cells (bottom) following 0 and 1 uM sodium arsenite treatment using neutral micro gel eletrophoresis. One hundred cells per data point were analyzed in each experiment. Data represent an average of at least three independent experiments ± standard error of mean.*Statistically different from control (p<0.05).
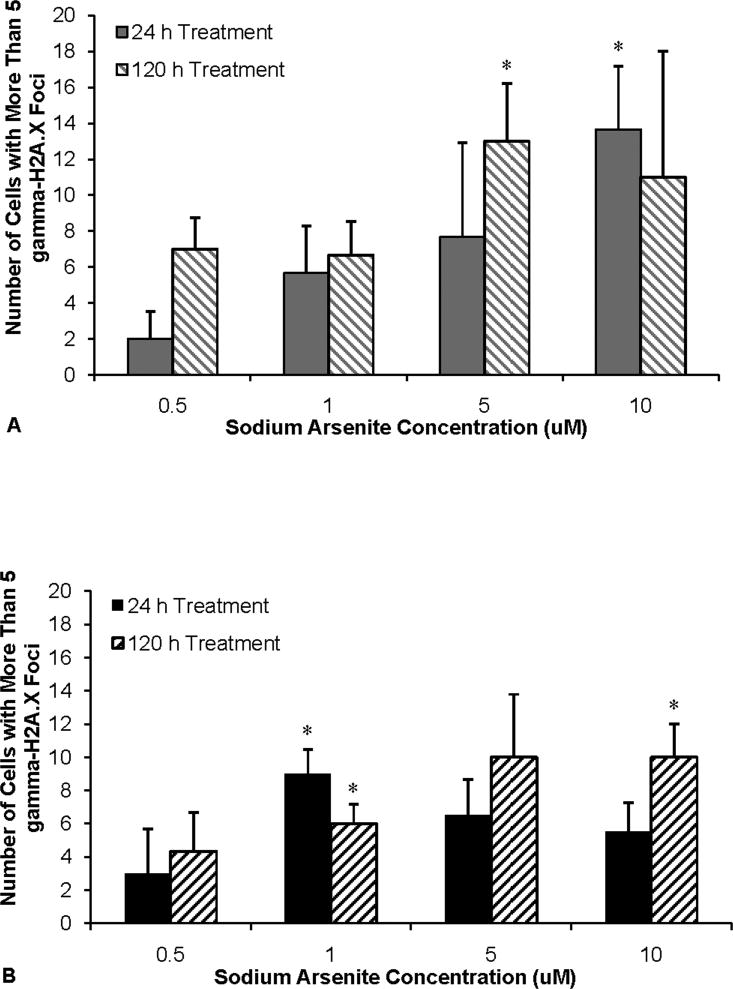
Arsenic also induced gamma-H2A.X foci formation, further confirming the formation of DNA double strand breaks. For example, concentrations of 0.5, 1, 5 and 10 uM sodium arsenite induced 2, 6, 8 and 14 percent of cells with more than 5 gamma-H2A.X foci compare to control after 24 h exposure, respectively and 7, 7, 13 and 11 percent of cells with more than 5 gamma-H2A.X foci after 120 h exposure, respectively, in fibroblasts (Figure 6A). These data suggest that arsenic is able to induce DNA double strand breaks. We found that sodium arsenite also induced an increase in gamma-H2A.X foci formation in lung epithelial cells. Specifically, 24 h exposure to concentrations of 0.5, 1, 5 and 10 uM sodium arsenite induced 3, 9, 7 and 6 percent of cells with more than 5 gamma-H2A.X foci compare to control, respectively and 120 h exposure induced 4, 6, 10 and 10 percent of cells with more than 5 gamma-H2A.X foci, respectively in epithelial cells (Figure 6B).
Figure 6. Sodium Arsenite Induces Gamma-H2A.X Foci Formation in Primary Human Lung Cells.
This figure shows that sodium arsenite induces an increase in DNA double strand breaks measured as gamma-H2A.X foci formation. A) Quantification of gamma-H2A.X foci after 24 or 120 h treatment with sodium arsenite in lung fibroblasts. Control values of 17% of cells with more than 5 gamma-H2A.X foci for 24 h treatment and 6% for 120 h treatment were subtracted from treated values. B) Quantification of gamma-H2A.X foci after 24 or 120 h treatment with sodium arsenite in lung epithelial cells. Control values of 14% of cells with more than 5 gamma-H2A.X foci for 24 h treatment and 15% for 120 h treatment were subtracted from treated values. C) Photomicrographs of gamma-H2A.X foci (green) in human lung fibroblasts (top) and epithelial cells (bottom) treated with sodium arsenite. Nuclei were stained with DAPI (blue). One hundred cells per data point were analyzed. Foci numbers were counted with ImageJ program. Data represent an average of at least three independent experiments ± standard error of mean. *Statistically different from control (p<0.05).
4. Discussion
Lung is a major target for arsenic carcinogenesis in humans by both ingestion and inhalation exposure [31]. However, few studies consider human lung cells and the carcinogenic mode of action of arsenic in lung is unknown. Those studies that did consider arsenic in human lung cells mostly used human lung cancer cells or immortalized lung cells as a model system [12, 32-35]. Arsenic is thought to induce oxidative stress and inhibit DNA damage repair. Cancer cells can have different antioxidative systems and repair activity [36] and have shown arsenic-resistance due to altered expression of transporter proteins [36]. Some methods of immortalization inactivate important genes and affect arsenic uptake [37-40]. Thus, while these studies provide useful information towards understanding arsenic's mechanism, it is important to understand how arsenic affects normal human lung cells. Accordingly, we investigated the genotoxic effects of inorganic arsenic in normal primary human bronchial fibroblasts and epithelial cells.
We found arsenic to be cytotoxic across a concentration range of 0.5-10 uM. These findings are consistent with previous studies that used the MTT assay to evaluate arsenic cytotoxicity in human lung fibroblasts and epithelial cells, although different cell types showed different sensitivity to arsenic exposure [36, 41-43, Table 2]. For example, one study showed that 4 uM sodium arsenite significantly decreased the population of viable bronchial epithelial cells [41].
Table 2. Summary of Arsenic Toxic Effect in Primary Human Lung Cells.
| Effect | Primary Human Lung Fibroblasts* |
Primary Human Lung Epithelial cells* |
||
|---|---|---|---|---|
|
|
|
|||
| 24 h | 120 h | 24 h | 120 h | |
| Cytotoxicity | 0.5-10 uM | 0.5-10 uM | 5, 10 uM | 5, 10 uM |
| Chromosome Damage | 5, 10 uM | 5, 10 uM | N | N |
| Aneuploidy | N | 10 uM | N | N |
| Mitotic Abnormality | 10 uM | 5, 10 uM | N | N |
| Comet Tail Intensity | 10 uM | 0.5, 5 uM | 1, 10 uM | 1, 10 uM |
| Gamma-H2A.X Foci | 10 uM | 5 uM | 1 uM | 1, 10 uM |
N: No effect
Concentrations show statistical difference from control at each time point.
Other studies reported that arsenic trioxide decreases cell viability at higher concentrations (>10 uM) in lung fibroblasts and an immortalized epithelial cell line, BEAS2B [42, 43]. Our data further support the observation of the difference between fibroblast and epithelial cells with the clonogenic survival assay.
The cytogenetic effect of arsenic exposure is strongly associated with its carcinogenesis and has been used as a biomarker for internal indicators of environmental or occupational exposures [19, 44]. Populations exposed to high levels of arsenic in drinking water show a significant increase in the frequency of chromatid and isochromatid deletions in lymphocytes and of micronuclei in oral and urinary epithelial cells [19]. Cell culture studies show that arsenic induces micronucleus, chromosome aberrations and sister chromatid exchange in lung cancer cells and other cell types [45-47]. Very few studies have been done with respect to arsenic-induced chromosome aberrations in normal human lung cells. We found that, in lung fibroblasts, 24 h or 120 h exposure to arsenic induces mainly chromatid gaps and breaks which is consistent with a previous study in skin fibroblasts [48]. In contrast, arsenic did not induce chromosome aberrations in lung epithelial cells (Table 1). One study did report that chronic exposure to sodium arsenite (3.8 uM) induced a 4.8-fold increase in micronuclei in h-TERT immortalized human small airway epithelial cells [14]. However, in that study, the clastogenic effect was observed after 28 weeks of exposure, much longer than our 120 h exposure. Our data also support findings with other cell types. Studies showed that arsenic induces chromatid breaks in Chinese hamster lung fibroblasts and both structural and numerical chromosome aberrations in human peripheral blood lymphocytes [49, 50]. Increased chromosomal aberrations were also found in arsenic-exposed individuals with skin lesions [51].
We found that 120 h exposure to 5 uM sodium arsenite induces aneuploidy in fibroblasts. This data is consistent with studies in other types of human cells [32, 52]. Studies have shown that arsenic disrupts microtubule assembly and spindle formation in human lymphocytes [47] and induces centrosome amplification in BEAS-2B cells [32]. These results indicate that the induction of centrosome and mitotic abnormalities might be mechanisms that lead to arsenic-induced aneuploidy. In this study, we also found that arsenic induces mitotic abnormalities exhibited as premature centromere division and premature anaphase in lung fibroblasts indicating arsenic causes spindle checkpoint bypass [27]. The underlying mechanism is uncertain. One study reported that arsenic can alter the localization of chromosomal passenger protein Aurora B and spindle checkpoint proteins, BUBR1 and MAD2 [53]. The loss of Aurora B could lead to diminishing of BUBR1 and MAD2 which result in failure of inhibiting anaphase-promoting complex leading to spindle checkpoint bypass [27, 28, 54]. This is the first study to investigate arsenic-induced aneuploidy in human lung epithelial cells. Consistent with the effect on chromosome structure aberration, arsenic did not induce aneuploidy in lung epithelial cells.
Studies have shown that arsenic induces DNA lesions such as oxidative DNA damage, DNA strand breaks and DNA crosslinks [55-58]. Among these DNA lesions, DNA double strand breaks are one of the most dangerous DNA lesions in cells [59]. Their repair is more difficult than that of other types of DNA damage because both strands are broken. Incorrect repair can lead to the loss or amplification of genomic material and ultimately tumorigenesis. Only few studies investigated arsenic-induced DNA double strand breaks [22, 60, 61]. One earlier study showed that arsenic induces DNA double strand breaks and DNA-protein crosslinks in human fetal lung fibroblasts [22]. They observed double strand breaks at a similar concentration range (1-5 uM) as ours. Our data are also consistent with studies using other cell type such as, Chinese hamster ovary cells [60] and CGL-2 cells [61]. Our study is the first to show that arsenic induces DNA double strand breaks in lung epithelial cells. The mechanism of double strand break formation by arsenic is not fully understood. One hypothesis is that arsenic induces oxidative DNA damage and inhibits DNA damage repair leading to larger numbers of damaged DNA to collapse replication forks forming double strand breaks [60].
Arsenic is also known to cause skin and bladder cancers. When we compared our data to that in human bladder and skin cells, we found that the cytotoxicity patterns for sodium arsenite are similar in all cell types [41, 62-64]. Despite the use of different assays, arsenic-induced cytotoxicity in normal human lung fibroblasts and epithelial cells in our study is higher than that in bladder and skin cells with the MTT reduction assay in the literature [57, 64-67]. This is consistence with Stybolo's observation that arsenic is more cytotoxic to lung cells than to bladder and skin cells [41]. However, human urothelial carcinoma cells appear more sensitive to arsenic cytotoxicity [68] than normal lung epithelial cells. Similarly to our findings, studies have shown that arsenic causes chromosome aberrations in human skin and urothelial cells, presented as an increased induction of micronuclei [44, 51, 69]. Sodium arsenite was also found to cause DNA double strand breaks in human skin cells exposed to 1 uM arsenic for 15 weeks [70]. Although arsenic-induced DNA double strand breaks have not been reported in human bladder cells, it has been shown that arsenic causes DNA damage in the alkaline comet assay which measure mainly single strand breaks [57, 68, 71].
Our study is the first observation that primary human lung fibroblasts are more sensitive to arsenic-induced cytotoxicity and genotoxicity than primary human lung epithelial cells (Table 2). The underlying explanation is uncertain, but it may be due to a difference in arsenic uptake. Cellular uptake of arsenic is believed to be cell type sensitive and fibroblasts may accumulate higher amounts of arsenic within the cell [71]. Another possibility may involve differences in cellular biomethylation activity which could lead to differences in accumulating and methylating arsenic to MMA and DMA [61, 71-73].
5. Conclusions
Although numerous of epidemiology studies have linked arsenic exposure to lung cancer, very few studies have investigated the toxic effect and mechanism in normal human lung cells. This study is one of the few studies that focus on human primary bronchial fibroblast and epithelial cells. In sum, we found that lung fibroblasts are more sensitive to arsenic cytotoxicity than epithelial cells. Arsenic induces DNA double strand breaks in both lung fibroblasts and epithelial cells but only induces chromosome aberrations including chromosome breaks, aneuploidy and mitotic abnormalities, in fibroblasts. This study produced some of the first data describing the effects of arsenic in its target cells. Our data will result in important new information about lung cancer and arsenic-induced carcinogenesis. Further research will focus on investigating the difference in the uptake of arsenic or in cellular biomethylation activity in these two cell types and investigating the specific mechanisms involved in arsenic-induced genotoxicity.
Highlights.
Arsenite is more cytotoxic to primary human lung fibroblasts than lung epithelial cells.
Arsenite induces chromosome aberrations in primary human lung fibroblasts but not in epithelial cells
Exposure to arsenite induces DNA double strand breaks in both primary human lung fibroblasts and epithelial cells
Acknowledgments
We would like to thank Christy Gianios, Jr. for technology support. This work is supported by NIEHS grants R15ES021587 (H.X.), ES016893 (J.P.W.) and the Maine Center for Toxicology and Environmental Health at the University of Southern Maine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.US Environmental Protection Agency. Inorganic Arsenic, TEACH Chemical Summary. 2007 [Google Scholar]
- 2.Environmental Protection Agency. National primary drinking water regulations; arsenic and clarifications to compliance and new source contaminants monitoring; Final rule. Fed Reg. 2001;66:6976–7066. [Google Scholar]
- 3.Chan PC, Huff J. Arsenic carcinogenesis in animals and in humans:Mechanistic, experimental and epidemiological evidence. Environ Carcino Ecotox Rev. 1997;15:83–122. [Google Scholar]
- 4.Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ. 2000;78:1093–103. Review. [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. 2011;123:305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Some Metals and Metallic Compounds. Lyon: International Agency for Research on Cancer. 1980;23:39–141. [PubMed] [Google Scholar]
- 8.Martinez VD, Becker-Santos DD, Vucic EA, Lam S, Lam WL. Induction of human squamous cell-type carcinomas by arsenic. J Skin Cancer. 2011:454157. doi: 10.1155/2011/454157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CJ, Chuang YC, Lin TM, Wu HY. Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: high-arsenic artesian well water and cancers. Cancer Res. 1985;45:5895–5899. [PubMed] [Google Scholar]
- 10.Wu MM, Kuo TL, Hwang YH, Chen CJ. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989;130:1123–1132. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- 11.Ferreccio C, González C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology. 2000;11:673–679. doi: 10.1097/00001648-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Steinmaus CM, Ferreccio C, Romo Acevedo J, Yuan Y, Cortes S, Marshall G, Moore LE, Balmes JR, Liaw J, Golden T, Smith AH. Drinking water arsenic in northern Chile: high cancer risks 40 years after exposure cessation. Cancer Epidemiol Biomarkers Prev. 2013;22:623–630. doi: 10.1158/1055-9965.EPI-12-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putila JJ, Guo NL. Association of arsenic exposure with lung cancer incidence rates in the United States. PLoS One. 2011;6:e25886. doi: 10.1371/journal.pone.0025886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen G, Calaf GM, Partridge MA, Echiburú-Chau C, Zhao Y, Huang S, Chai Y, Li B, Hu B, Hei TK. Neoplastic transformation of human small airway epithelial cells induced by arsenic. Mol Med. 2008;14:2–10. doi: 10.2119/2007-00090.Wen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang Q, Pan J, Wang X, Zhang Z, Chen F, Shi X. Reduced reactive oxygen species-generating capacity contributes to the enhanced cell growth of arsenic-transformed epithelial cells. Cancer Res. 2010;70:5127–5135. doi: 10.1158/0008-5472.CAN-10-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stueckle TA, Lu Y, Davis ME, Wang L, Jiang BH, Holaskova I, Schafer R, Barnett JB, Rojanasakul Y. Chronic occupational exposure to arsenic induces carcinogenic gene signaling networks and neoplastic transformation in human lung epithelial cells. Toxicol Appl Pharmacol. 2012;261:204–216. doi: 10.1016/j.taap.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitchin KT. Recent advances in arsenic carcinogenesis: modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol. 2001;172:249–261. doi: 10.1006/taap.2001.9157. Review. [DOI] [PubMed] [Google Scholar]
- 18.Dulout FN, Grillo CA, Seoane AI, Maderna CR, Nilsson R, Vahter M, Darroudi F, Natarajan AT. Chromosomal aberrations in peripheral blood lymphocytes from native Andean women and children from northwestern Argentina exposed to arsenic in drinking water. Mutat Res. 1996;370:151–158. doi: 10.1016/s0165-1218(96)00060-2. [DOI] [PubMed] [Google Scholar]
- 19.Gonsebatt ME, Vega L, Salazar AM, Montero R, Guzmán P, Blas J, Del Razo LM, García-Vargas G, Albores A, Cebrián ME, Kelsh M, Ostrosky-Wegman P. Cytogenetic effects in human exposure to arsenic. Mutat Res. 1997;386:219–228. doi: 10.1016/s1383-5742(97)00009-4. [DOI] [PubMed] [Google Scholar]
- 20.Bau DT, Wang TS, Chung CH, Wang AS, Wang AS, Jan KY. Oxidative DNA adducts and DNA-protein cross-links are the major DNA lesions induced by arsenite. Environ Health Perspect. 2002;110:753–756. doi: 10.1289/ehp.02110s5753. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kesari VP, Kumar A, Khan PK. Genotoxic potential of arsenic at its reference dose. Ecotoxicol Environ Saf. 2012;80:126–131. doi: 10.1016/j.ecoenv.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Dong JT, Luo XM. Arsenic-induced DNA-strand breaks associated with DNA-protein crosslinks in human fetal lung fibroblasts. Mutat Res. 1993;302:97–102. doi: 10.1016/0165-7992(93)90010-s. [DOI] [PubMed] [Google Scholar]
- 23.Mourón SA, Golijow CD, Dulout FN. DNA damage by cadmium and arsenic salts assessed by the single cell gel electrophoresis assay. Mutat Res. 2001;498:47–55. doi: 10.1016/s1383-5718(01)00266-2. [DOI] [PubMed] [Google Scholar]
- 24.Wise JP, Sr, Wise SS, Little JE. The cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human lung cells. Mutat Res. 2002;517:221–229. doi: 10.1016/s1383-5718(02)00071-2. [DOI] [PubMed] [Google Scholar]
- 25.Xie H, Wise SS, Holmes AL, Xu B, Wakeman TP, Pelsue SC, Singh NP, Wise JP., Sr Carcinogenic lead chromate induces DNA double-strand breaks in human lung cells. Mutat Res. 2005;586:160–172. doi: 10.1016/j.mrgentox.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wise SS, Holmes AL, Qin Q, Xie H, Katsifis SP, Thompson WD, Wise JP., Sr Comparative genotoxicity and cytotoxicity of four hexavalent chromium compounds in human bronchial cells. Chem Res Toxicol. 2010;23:365–372. doi: 10.1021/tx900363j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wise SS, Holmes AL, Xie H, Thompson WD, Wise JP., Sr Chronic exposure to particulate chromate induces spindle assembly checkpoint bypass in human lung cells. Chem Res Toxicol. 2006;19:1492–1498. doi: 10.1021/tx0601410. [DOI] [PubMed] [Google Scholar]
- 28.Holmes AL, Wise SS, Pelsue SC, Aboueissa AM, Lingle W, Salisbury J, Gallagher J, Wise JP., Sr Chronic exposure to zinc chromate induces centrosome amplification and spindle assembly checkpoint bypass in human lung fibroblasts. Chem Res Toxicol. 2010;23:386–395. doi: 10.1021/tx900360w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. Review. [DOI] [PubMed] [Google Scholar]
- 30.van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2001;2:196–206. doi: 10.1038/35056049. Review. [DOI] [PubMed] [Google Scholar]
- 31.Smith AH, Ercumen A, Yuan Y, Steinmaus CM. Increased lung cancer risks are similar whether arsenic is ingested or inhaled. J Expo Sci Environ Epidemiol. 2009;19:343–348. doi: 10.1038/jes.2008.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao WT, Lin P, Cheng TS, Yu HS, Chang LW. Arsenic promotes centrosome abnormalities and cell colony formation in p53 compromised human lung cells. Toxicol Appl Pharmacol. 2007;225:162–170. doi: 10.1016/j.taap.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Andrew AS, Mason RA, Memoli V, Duell EJ. Arsenic activates EGFR pathway signaling in the lung. Toxicol Sci. 2009;109:350–357. doi: 10.1093/toxsci/kfp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker AM, Stevens JJ, Ndebele K, Tchounwou PB. Arsenic trioxide modulates DNA synthesis and apoptosis in lung carcinoma cells. Int J Environ Res Public Health. 2010;7:1996–2007. doi: 10.3390/ijerph7051996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mass MJ, Wang L. Arsenic alters cytosine methylation patterns of the promoter of the tumor suppressor gene p53 in human lung cells: a model for a mechanism of carcinogenesis. Mutat Res. 1997;386:263–277. doi: 10.1016/s1383-5742(97)00008-2. [DOI] [PubMed] [Google Scholar]
- 36.Miao ZF, Chang EE, Tsai FY, Yeh SC, Wu CF, Wu KY, Wang CJ, Tsou TC. Increased aquaglyceroporin 9 expression disrupts arsenic resistance in human lung cancer cells. ToxicolIn Vitro. 2009;23:209–216. doi: 10.1016/j.tiv.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Albright CD, Jones RT, Hudson EA, Fontana JA, Trump BF, Resau JH. Transformed human bronchial epithelial cells (BEAS-2B) alter the growth and morphology of normal human bronchial epithelial cells in vitro. Cell Biol Toxicol. 1990;6:379–398. doi: 10.1007/BF00120804. [DOI] [PubMed] [Google Scholar]
- 38.Willey JC, Broussoud A, Sleemi A, Bennett WP, Cerutti P, Harris CC. Immortalization of normal human bronchial epithelial cells by human papillomaviruses 16 or 18. Cancer Res. 1991;51:5370–5377. [PubMed] [Google Scholar]
- 39.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, Milchgrub S, Smith AL, Souza RF, Gilbey L, Zhang X, Gandia K, Vaughan MB, Wright WE, Gazdar AF, Shay JW, Minna JD. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 40.Wise SS, Holmes AL, Wise JP., Sr Particulate and soluble hexavalent chromium are cytotoxic and genotoxic to human lung epithelial cells. Mutat Res. 2006;610:2–7. doi: 10.1016/j.mrgentox.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- 42.Park WH, Kim SH. Arsenic trioxide induces human pulmonary fibroblast cell death via the regulation of Bcl-2 family and caspase-8. MolBiol Rep. 2012;39:4311–4318. doi: 10.1007/s11033-011-1218-z. [DOI] [PubMed] [Google Scholar]
- 43.Wang F, Shi Y, Yadav S, Wang H. p52-Bcl3 complex promotes cyclin D1 expression in BEAS-2B cells in response to low concentration arsenite. Toxicology. 2010;29:12–18. doi: 10.1016/j.tox.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh P, Basu A, Singh KK, Giri AK. Evaluation of cell types for assessment of cytogenetic damage in arsenic exposed population. Mol Cancer. 2008;7 doi: 10.1186/1476-4598-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez VD, Buys TP, Adonis M, Benítez H, Gallegos I, Lam S, Lam WL, Gil L. Arsenic-related DNA copy-number alterations in lung squamous cell carcinomas. Br J Cancer. 2010;103:1277–1283. doi: 10.1038/sj.bjc.6605879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larramendy ML, Popescu NC, Dipaolo JA. Induction by inorganic metal salts of sister chromatid exchanges and chromosome aberrations in human and syrian hamster cell strains. Environ Mol Mutagen. 2006;3:597–606. [Google Scholar]
- 47.Kligerman AD, Tennant AH. Insights into the carcinogenic mode of action of arsenic. Toxicol Appl Pharmacol. 2007;222:281–288. doi: 10.1016/j.taap.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Oya-Ohta Y, Kaise T, Ochi T. Induction of chromosomal aberrations in cultured human fibroblasts by inorganic and organic arsenic compounds and the different roles of glutathione in such induction. Mutat Res. 1996;357:123–129. doi: 10.1016/0027-5107(96)00092-9. [DOI] [PubMed] [Google Scholar]
- 49.Sinha D, Bhattacharya RK, Siddiqi M, Roy M. Amelioration of sodium arsenite-induced clastogenicity by tea extracts in Chinese hamster v79 cells. J Environ Pathol Toxicol Oncol. 2005;24:129–140. doi: 10.1615/jenvpathtoxoncol.v24.i2.60. [DOI] [PubMed] [Google Scholar]
- 50.Tiwari H, Rao MV. Curcumin supplementation protects from genotoxic effects of arsenic and fluoride. Food Chem Toxicol. 2010;48:1234–1238. doi: 10.1016/j.fct.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh P, Basu A, Mahata J, Basu S, Sengupta M, Das JK, Mukherjee A, Sarkar AK, Mondal L, Ray K, Giri AK. Cytogenetic damage and genetic variants in the individuals susceptible to arsenic-induced cancer through drinking water. Int J Cancer. 2006;118:2470–2478. doi: 10.1002/ijc.21640. [DOI] [PubMed] [Google Scholar]
- 52.Ramírez P, Eastmond DA, Laclette JP, Ostrosky-Wegman P. Disruption of microtubule assembly and spindle formation as a mechanism for the induction of aneuploid cells by sodium arsenite and vanadium pentoxide. Mutat Res. 1997;386:291–298. doi: 10.1016/s1383-5742(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki T, Miyazaki K, Kita K, Ochi T. Trivalent dimethylarsenic compound induces histone H3 phosphorylation and abnormal localization of Aurora B kinase in HepG2 cells. Toxicol Appl Pharmacol. 2009;241:275–282. doi: 10.1016/j.taap.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 54.Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li D, Morimoto K, Takeshita T, Lu Y. Arsenic induces DNA damage via reactive oxygen species in human cells. Environ Health Prev Med. 2001;6:27–32. doi: 10.1007/BF02897306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong JT, Luo XM. Arsenic-induced DNA-strand breaks associated with DNA-protein crosslinks in human fetal lung fibroblasts. Mutat Res. 1993;302:97–102. doi: 10.1016/0165-7992(93)90010-s. [DOI] [PubMed] [Google Scholar]
- 57.Dopp E, von Recklinghausen U, Hartmann LM, Stueckradt I, Pollok I, Rabieh S, Hao L, Nussler A, Katier C, Hirner AV, Rettenmeier AW. Subcellular distribution of inorganic and methylated arsenic compounds in human urothelial cells and human hepatocytes. Drug Metab Dispos. 2008;36:971–979. doi: 10.1124/dmd.107.019034. [DOI] [PubMed] [Google Scholar]
- 58.Qin XJ, Hudson LG, Liu W, Ding W, Cooper KL, Liu KJ. Dual actions involved in arsenite-induced oxidative DNA damage. Chem Res Toxicol. 2008;21:1806–1813. doi: 10.1021/tx8001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–254. doi: 10.1038/85798. Review. [DOI] [PubMed] [Google Scholar]
- 60.Ying S, Myers K, Bottomley S, Helleday T, Bryant HE. BRCA2-dependent homologous recombination is required for repair of Arsenite-induced replication lesions in mammalian cells. Nucleic Acids Res. 2009;37:5105–5113. doi: 10.1093/nar/gkp538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yih LH, Hsueh SW, Luu WS, Chiu TH, Lee TC. Arsenite induces prominent mitotic arrest via inhibition of G2 checkpoint activation in CGL-2 cells. Carcinogenesis. 2005;26:53–63. doi: 10.1093/carcin/bgh295. [DOI] [PubMed] [Google Scholar]
- 62.Chai CY, Huang YC, Hung WC, Kang WY, Chen WT. Arsenic salt-induced DNA damage and expression of mutant p53 and COX-2 proteins in SV-40 immortalized human uroepithelial cells. Mutagenesis. 2007;22:403–408. doi: 10.1093/mutage/gem035. [DOI] [PubMed] [Google Scholar]
- 63.Drobná Z, Waters SB, Devesa V, Harmon AW, Thomas DJ, Stýblo M. Metabolism and toxicity of arsenic in human urothelial cells expressing rat arsenic (+3 oxidation state)-methyltransferase. Toxicol Appl Pharmacol. 2005;207:147–159. doi: 10.1016/j.taap.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao R, Hou Y, Zhang Q, Woods CG, Xue P, Fu J, Yarborough K, Guan D, Andersen ME, Pi J. Cross-regulations among NRFs and KEAP1 and effects of their silencing on arsenic-induced antioxidant response and cytotoxicity in human keratinocytes. Environ Health Perspect. 2012;120:583–589. doi: 10.1289/ehp.1104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sandoval M, Morales M, Tapia R, del Carmen Alarcón L, Sordo M, Ostrosky-Wegman P, Ortega A, López-Bayghen E. p53 response to arsenic exposure in epithelial cells: protein kinase B/Akt involvement. Toxicol Sci. 2007;99:126–140. doi: 10.1093/toxsci/kfm153. [DOI] [PubMed] [Google Scholar]
- 66.Bredfeldt TG, Kopplin MJ, Gandolfi AJ. Effects of arsenite on UROtsa cells: low-level arsenite causes accumulation of ubiquitinated proteins that is enhanced by reduction in cellular glutathione levels. Toxicol Appl Pharmacol. 2004;198:412–418. doi: 10.1016/j.taap.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 67.Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- 68.Wang TC, Jan KY, Wang AS, Gurr JR. Trivalent arsenicals induce lipid peroxidation, protein carbonylation, and oxidative DNA damage in human urothelial cells. Mutat Res. 2007 Feb 3;615(1-2):75–86. doi: 10.1016/j.mrfmmm.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Moore LE, Smith AH, Hopenhayn-Rich C, Biggs ML, Kalman DA, Smith MT. Micronuclei in exfoliated bladder cells among individuals chronically exposed to arsenic in drinking water. Cancer Epidemiol Biomarkers Prev. 1997;6:31–36. [PubMed] [Google Scholar]
- 70.Li Y, Ling M, Xu Y, Wang S, Li Z, Zhou J, Wang X, Liu Q. The repressive effect of NF-kappaB on p53 by mot-2 is involved in human keratinocyte transformation induced by low levels of arsenite. Toxicol Sci. 2010;116:174–182. doi: 10.1093/toxsci/kfq109. [DOI] [PubMed] [Google Scholar]
- 71.Dopp E, von Recklinghausen U, Diaz-Bone R, Hirner AV, Rettenmeier AW. Cellular uptake, subcellular distribution and toxicity of arsenic compounds in methylating and non-methylating cells. Environ Res. 2010;110:435–442. doi: 10.1016/j.envres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 72.Lee TC, Ho IC, Lu WJ, Huang JD. Enhanced expression of multidrug resistance-associated protein 2 and reduced expression of aquaglyceroporin 3 in an arsenic-resistant human cell line. JBiol Chem. 2006;281:18401–18407. doi: 10.1074/jbc.M601266200. [DOI] [PubMed] [Google Scholar]
- 73.Tatum FM, Hood RD. Arsenite uptake and metabolism by rat hepatocyte primary cultures in comparison with kidney- and hepatocyte-derived rat cell lines. Toxicol Sci. 1999;52:20–25. doi: 10.1093/toxsci/52.1.20. [DOI] [PubMed] [Google Scholar]