Abstract
Ghrelin is an orexigenic hormone produced mainly by the gastrointestinal system and the brain. Much evidence also indicates a role for ghrelin in sleep and thermoregulation. Further, ghrelin was recently implicated in immune system modulation. Administration of bacterial lipopolysaccharide (LPS) induces fever, anorexia, and increased non-rapid-eye movement sleep (NREMS) and these actions are mediated primarily by proinflammatory cytokines. Ghrelin reduces LPS-induced fever, suppresses circulating levels of proinflammatory cytokines and reduces the severity and mortality of various models of experimental endotoxemia. In the present study, we determined the role of intact ghrelin signaling in LPS-induced sleep, feeding, and thermoregulatory responses in mice. Sleep-wake activity was determined after intraperitoneal, dark onset administration of 0.4, 2 and 10 µg LPS in preproghrelin knockout (KO) and wild-type (WT) mice. In addition, body temperature, motor activity and changes in 24-hour food intake and body weight were measured. LPS induced dose-dependent increases in NREMS, and suppressed rapid-eye movement sleep, electroencephalographic slow-wave activity, motor activity, food intake and body weight in both Ppg KO and WT mice. Body temperature changes showed a biphasic pattern with a decrease during the dark period followed by an increase in the light phase. The effects of the low and middle doses of LPS were indistinguishable between the two genotypes. Administration of 10 µg LPS, however, induced significantly larger changes in NREMS and wakefulness amounts, body temperature, food intake and body weight in the Ppg KO mice. These findings support a role for ghrelin as an endogenous modulator of inflammatory responses and a central component of arousal and feeding circuits.
Introduction
Activation of the immune system by systemic microbial infection triggers a coordinated set of adaptive behavioral changes collectively called sickness behavior. The acute phase response is characterized by these behavioral adaptations and changes in immune, neurologic, endocrine, metabolic functions including decreased food intake, social withdrawal, lethargy as well as altered sleep-wake activity. Bacterial lipopolysaccharide (LPS), a heat-stable biologically active component of the outer membrane of Gram-negative bacteria, is a well-characterized and widely used model of experimental endotoxemia (reviewed in Alexander and Rietschel, 2001). Peripheral administration of LPS induces a broad range of biological effects such as fever, anorexia, increased non-rapid-eye movement sleep (NREMS) and decreased rapid-eye movement sleep (REMS) in humans (Mullington et al., 2000), rabbits (Krueger et al., 1986, Kimura et al., 1994), rats (Kapás et al., 1998) and mice (Opp and Toth, 1998, Morrow and Opp, 2005, Nadjar et al., 2013). Numerous studies demonstrate that LPS-induced sickness behavior is mediated primarily by proinflammatory cytokines such as interleukin 1 beta (IL1β), IL6, and tumor necrosis factor alpha (TNFα) acting in the brain or on vagal cytokine receptors to modulate brain cytokine expression (Krueger et al., 1994, Singh and Jiang, 2004).
Ghrelin is a brain-gut peptide hormone implicated in various physiological processes including food intake and sleep-wake activity (reviewed in Szentirmai and Kapás, 2012). For example, sleep deprivation increases plasma and hypothalamic ghrelin levels in rats (Bodosi et al., 2003). Intracerebroventricular (icv) and hypothalamic microinjections of ghrelin increase wakefulness, suppress non-rapid-eye-movement sleep (NREMS) and rapid-eye-movement sleep (REMS) in rats (Szentirmai et al., 2006, Szentirmai et al., 2007). Icv injections of ghrelin increase wakefulness and suppress sleep, while systemic administration of the peptide fails to alter sleep-wake activity in mice (Szentirmai, 2012). Ghrelin receptor knockout mice exhibit attenuated responses to arousal-promoting stimuli such as fasting and exposure to a new environment (Esposito et al., 2012). Preproghrelin knockout (Ppg KO) mice have slightly altered sleep-wake activity at thermoneutral ambient temperature and normal food anticipatory activity (Szentirmai et al., 2007, Szentirmai et al., 2010) but display severe sleep and thermoregulatory impairments when challenged with fasting in a cold environment (Szentirmai et al., 2009).
Recent evidence indicates a role for ghrelin in modulating the activity of the immune system. Ghrelin and the ghrelin receptor (growth hormone secretagogue receptor, GHS-R) are expressed in human T lymphocytes and monocytes (Dixit et al., 2009). Ghrelin inhibits the expression and production of IL1β, IL6, and TNFα by activated human T cells and monocytes (Dixit et al., 2009). Ghrelin reduces LPS-induced increases in serum levels of TNFα, IL1β and IL6, partially, through the activation of the vagus nerve in rats and mice (Wang et al., 2009, Wu et al., 2007). Ghrelin treatment attenuates LPS-induced fever (Soriano et al., 2011), reduces the severity and mortality of various models of experimental endotoxemia and sepsis (Chang et al., 2003, Chorny et al., 2008, Gonzales-Rey et al., 2006, Wu et al., 2007). Furthermore, ghrelin improves bacterial clearance from peritoneal fluid in vivo and shows bactericidal properties against Escherichia coli in vitro (Chorny et al., 2008). Serum ghrelin levels, after LPS treatment, are elevated (Chang et al., 2003, Wang et al., 2009) or suppressed (Basa et al., 2003, Hataya et al., 2003, Wang et al., 2006) depending on the time of the measurement.
In the present study we determined the role of intact ghrelin signaling on LPS-induced sleep, feeding, and thermoregulatory responses in mice. We report that after a high dose of LPS challenge Ppg KO mice display augmented hypothermia and NREMS and suppressed REMS and food intake compared to wild-type (WT) controls. These findings further support a role for ghrelin in sleep-wake activity, thermoregulation and immune responses.
Methods
a. Animals
Male, 5–6 months old Ppg KO [originally named as Ghrelin −/− mice, (Sun et al., 2003)] and wild-type (WT) mice were used in the experiments. Breeding pairs of ghrelin KO and WT mice with a C57BL6J/129SvEv genetic background, backcrossed to C57BL6J for 10 generations, were generated and given as a generous gift by Drs. Roy G. Smith and Yuxiang Sun at Baylor College of Medicine (Houston, TX), and further bred and kept in a non-SPF animal facility at Washington State University. Each mouse used in the experiments was genotyped (Transnetyx, Cordova, TN). Procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Protocol (ASAF # 3512 and 3948) was approved by the Institutional Animal Care and Use Committee at Washington State University.
Surgery
The body weights of the WT and KO mice at the time of surgery were 32.2 ± 0.6 g and 32.8 ± 0.7 g, respectively. During surgery, mice were anesthetized with intraperitoneal (ip) injection of ketamine-xylazine mixture (87 and 13 mg/kg, respectively). The animals were implanted with cortical electroencephalographic (EEG) electrodes, placed over the frontal and parietal cortices, and electromyographic (EMG) electrodes in the dorsal neck muscles. The EEG and EMG electrodes were connected to a pedestal fixed to the skull with dental cement. Temperature-sensitive transmitters were implanted in the abdominal cavity for telemetry temperature and activity recordings. Mice were allowed to recover from surgery for at least 10 days before baseline recordings started. During the recovery and experimental periods, all mice were housed in individual recording cages located in a sound-attenuated environmental chamber at a constant, thermoneutral ambient temperature (29 ± 1°C) and controlled light–dark cycles [12–12 h, lights on: Zeitgeber time (ZT0) at 5 am]. Food and water were available ad libitum throughout the experiments. The animals were fed with regular lab chow (Harlan Teklad, Product No. 8640); fat, proteins, and carbohydrates provided 17%, 29%, and 54% of calories, respectively.
Experiment
After recovery from surgery, mice were handled daily to habituate them to the experimental procedures. On the baseline day, the animals were injected with isotonic saline (ip, 0.1 ml/10 g body weight). On the test day, LPS from Escherichia coli serotype 0111:B4 (Sigma, St. Louis, MO) was dissolved in isotonic NaCl and was injected ip in the same volume. Three doses of LPS, 0.4 (n = 8 for Ppg WT, n = 7 for Ppg KO), 2 (n = 7 for Ppg WT, n = 8 for Ppg KO), and 10 µg/mouse (n = 10 for Ppg WT, n = 8 for Ppg KO) were tested. Separate groups of animals were used for each dose of LPS. All injections were performed 5–10 min prior to the onset of the dark phase. Recordings were carried out for 24 h after the injections.
Sleep-wake recordings and analyses
Recording cables connected the animals to commutators, which were further routed to Grass Model15 Neurodata amplifier system (Grass Instrument Division of Astro-Med, Inc., West Warwick, RI). The high-pass and low-pass filters for EEG signals were 0.5 and 30.0 Hz, respectively. The EMG signals were filtered with low and high cut-off frequencies at 100 and 10,000 Hz, respectively. The outputs from the amplifiers were fed into an analog-digital converter (digitized at 256 Hz) and collected by computer (SleepWave software, Biosoft Studio, Hersey, PA). Sleep-wake states were scored visually off-line in 10-s segments according to the following criteria. Non-rapid-eye-movement sleep (NREMS): high-voltage EEG delta waves (0.5–4 Hz) and decreased muscle tone; rapid-eye-movement sleep (REMS): predominant EEG theta activity (6–8 Hz) and lack of muscle tone with occasional muscle twitches; wakefulness (W): low-voltage EEG activity, and varying levels of increased muscle activities. Time spent in W, NREMS and REMS was calculated in 2- and 12-h blocks. EEG power data from each artifact free 10-s segment were subjected to off-line spectral analysis by fast Fourier transformation. EEG power data in the range of 0.5 to 4.0 Hz during NREMS were used to compute EEG slow-wave activity (SWA). EEG SWA data were normalized for each animal by using the average EEG SWA across 24 h on the baseline day as 100. The results were averaged in 2-h bins.
Telemetry recordings
Core body temperature and locomotor activity were recorded by Mini Mitter telemetry system (Philips Respironics, Bend, OR). Temperature and activity values were collected every 1 and 10 min, respectively, throughout the experiment and were averaged into 2- and 12-h time blocks. Activity values were normalized for each animal by using the average activity across 24 h on the baseline day as 100.
d. Statistics
Time spent in wakefulness, NREMS and REMS, as well as, EEG SWA, body temperature and motor activity were calculated in 2- and 12-hour blocks. Three-way mixed ANOVA was performed separately for each dose of LPS across 24 h on the 12-h data (independent measure: genotype, repeated measures: time and treatment). When ANOVA indicated significant effects, Student-Newman-Keuls test was used as the post hoc test for all experiments. NREMS, REMS and wake episode numbers and average episode durations were calculated for the 12-h dark period and comparisons were made between genotypes by performing Student’s t-tests. Changes in daily food intake and body weight were analyzed by using Student’s t-tests. An α-level of P < 0.05 was considered to be significant. Detailed statistical results are in Table 1.
Table 1.
Statistical results: The effects of 0.4, 2 and 10 µg lipopolysaccharide (LPS) on non-rapid-eye movement sleep (NREMS), rapid-eye movement sleep (REMS), wakefulness and slow-wave activity (SWA) of the electroencephalogram. Df: degree of freedom, Treatm.: Treatment, Gen.: Genotype
| 0.4 µg | 2 µg | 10 µg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | ||
| NREMS | ||||||||||
| Treatm. | 1,13 | 11.7 | P ≤ 0.05 | 1,13 | 18.4 | P ≤ 0.05 | 1,16 | 66.7 | P ≤ 0.05 | |
| Gen. | 1,13 | 0.6 | n.s. | 1,13 | 1.1 | n.s. | 1,16 | 1.5 | n.s. | |
| Treatm. × Gen. | 1,13 | 1.2 | n.s. | 1,13 | 1.8 | n.s. | 1,16 | 2.4 | n.s. | |
| Time × Gen. | 1,13 | 0.5 | n.s. | 1,13 | 0.7 | n.s. | 1,16 | 1.1 | n.s. | |
| Treatm. × Time | 1,13 | 29.7 | P ≤ 0.05 | 1,13 | 91.3 | P ≤ 0.05 | 1,16 | 166.9 | P ≤ 0.05 | |
| Treatm. × Time × Genotype | 1,13 | 2.9 | n.s. | 1,13 | 0 | n.s. | 1,16 | 5.8 | P ≤ 0.05 | |
| REMS | ||||||||||
| Treatm. | 1,13 | 2.6 | n.s. | 1,13 | 31.3 | P ≤ 0.05 | 1,16 | 36.2 | P ≤ 0.05 | |
| Gen. | 1,13 | 1.9 | n.s. | 1,13 | 0.1 | n.s. | 1,16 | 0.3 | n.s. | |
| Treatm. × Gen. | 1,13 | 0 | n.s. | 1,13 | 0.7 | P ≤ 0.05 | 1,16 | 1.3 | n.s. | |
| Time × Gen. | 1,13 | 0.2 | n.s. | 1,13 | 5.3 | n.s. | 1,16 | 0 | n.s. | |
| Treatm. × Time | 1,13 | 1.4 | n.s. | 1,13 | 0.4 | n.s. | 1,16 | 4 | n.s. | |
| Treatm. × Time × Genotype | 1,13 | 1.3 | n.s. | 1,13 | 1.5 | n.s. | 1,16 | 0.1 | n.s. | |
| Wakefulness | ||||||||||
| Treatm. | 1,13 | 9.5 | P ≤ 0.05 | 1,13 | 22.7 | P ≤ 0.05 | 1,16 | 60.2 | P ≤ 0.05 | |
| Gen. | 1,13 | 1.2 | n.s. | 1,13 | 0.6 | n.s. | 1,16 | 1.7 | n.s. | |
| Treatm. × Gen. | 1,13 | 1.2 | n.s. | 1,13 | 1.2 | n.s. | 1,16 | 2.1 | n.s. | |
| Time × Gen. | 1,13 | 0.3 | n.s. | 1,13 | 2.9 | n.s. | 1,16 | 1.2 | n.s. | |
| Treatm. × Time | 1,13 | 30.8 | P ≤ 0.05 | 1,13 | 64.3 | P ≤ 0.05 | 1,16 | 135.7 | P ≤ 0.05 | |
| Treatm. × Time × Genotype | 1,13 | 2.5 | n.s. | 1,13 | 0 | n.s. | 1,16 | 4.7 | P ≤ 0.05 | |
| SWA | ||||||||||
| Treatm. | 1,13 | 1.7 | n.s. | 1,13 | 22.8 | P ≤ 0.05 | 1,16 | 44.7 | P ≤ 0.05 | |
| Gen. | 1,13 | 4.6 | n.s. | 1,13 | 2.5 | n.s. | 1,16 | 1.6 | n.s. | |
| Treatm. × Gen. | 1,13 | 4.4 | n.s. | 1,13 | 2.5 | n.s. | 1,16 | 1.6 | n.s. | |
| Time × Gen. | 1,13 | 0.8 | n.s. | 1,13 | 0 | n.s. | 1,16 | 0 | n.s. | |
| Treatm. × Time | 1,13 | 3.9 | n.s. | 1,13 | 15.6 | P ≤ 0.05 | 1,16 | 18.7 | P ≤ 0.05 | |
| Treatm. × Time × Genotype | 1,13 | 1.3 | n.s. | 1,13 | 1.2 | n.s. | 1,16 | 0.1 | n.s. | |
| Body temperature | ||||||||||
| Treatm. | 1,13 | 12.2 | P ≤ 0.05 | 1,13 | 22.6 | P ≤ 0.05 | 1,16 | 8.83 | P ≤ 0.05 | |
| Gen. | 1,13 | 0.1 | n.s. | 1,13 | 0.6 | n.s. | 1,16 | 1.6 | n.s. | |
| Treatm. × Gen. | 1,13 | 5.8 | P ≤ 0.05 | 1,13 | 0.5 | n.s. | 1,16 | 1.9 | n.s. | |
| Time × Gen. | 1,13 | 0.1 | n.s. | 1,13 | 0.6 | n.s. | 1,16 | 0 | n.s. | |
| Treatm. × Time | 1,13 | 2.6 | n.s. | 1,13 | 21.1 | P ≤ 0.05 | 1,16 | 2.9 | P ≤ 0.05 | |
| Treatm. × Time × Genotype | 1,13 | 0.5 | n.s. | 1,13 | 2.3 | n.s. | 1,16 | 4.9 | P ≤ 0.05 | |
| Motor activity | ||||||||||
| Treatm. | 1,13 | 20.9 | P ≤ 0.05 | 1,13 | 61.1 | P ≤ 0.05 | 1,16 | 66.7 | P ≤ 0.05 | |
| Gen. | 1,13 | 0.1 | n.s. | 1,13 | 0.1 | n.s. | 1,16 | 5.2 | P ≤ 0.05 | |
| Treatm. × Gen. | 1,13 | 0.1 | n.s. | 1,13 | 0.1 | n.s. | 1,16 | 5.2 | P ≤ 0.05 | |
| Time × Gen. | 1,13 | 0.8 | n.s. | 1,13 | 2.1 | n.s. | 1,16 | 0.1 | n.s. | |
| Treatm. × Time | 1,13 | 18.5 | P ≤ 0.05 | 1,13 | 88.1 | P ≤ 0.05 | 1,16 | 80.8 | P ≤ 0.05 | |
| Treatm. × Time × Genotype | 1,13 | 0.1 | n.s. | 1,13 | 0.5 | n.s. | 1,16 | 1.3 | n.s. | |
Results
Both Ppg KO and WT mice exhibited normal diurnal rhythms of sleep-wake activity, body temperature, and motor activity under baseline conditions (data not shown). The baseline parameters, including sleep, body temperature, locomotor activity, body weight and food intake were not significantly different between the two genotypes confirming previous findings (Sun et al., 2003, Szentirmai et al., 2007).
The effects of LPS on sleep-wake activity and EEG SWA in Ppg KO and WT mice
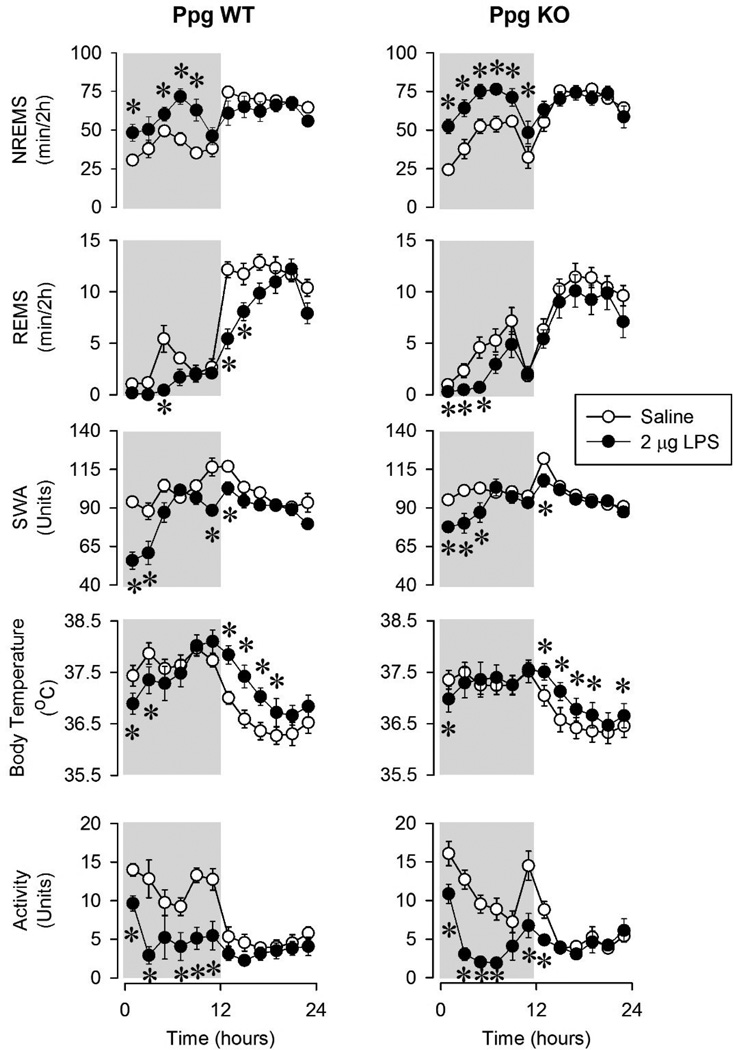
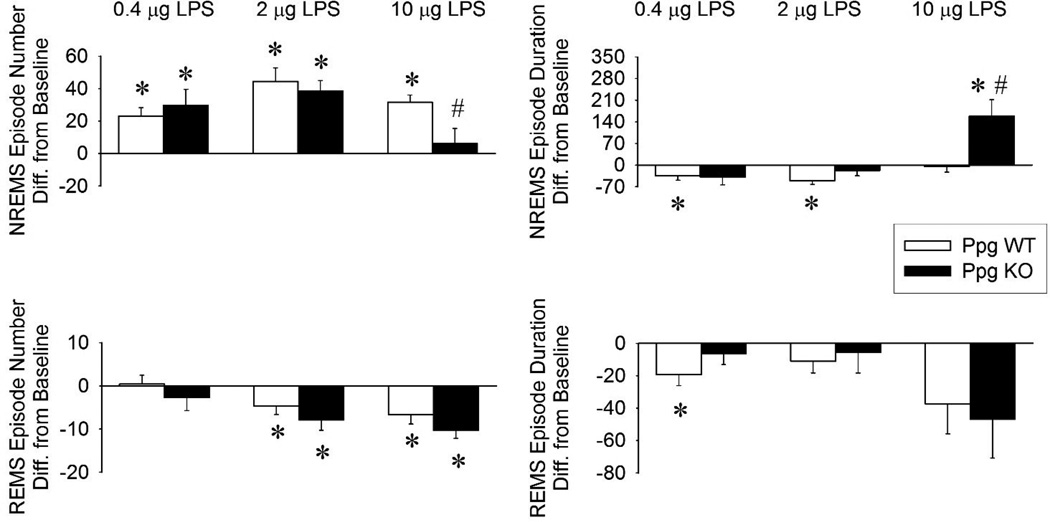
Intraperitoneal injection of LPS at dark onset induced significant, dose-dependent increases in NREMS accompanied by the suppression of wakefulness (data not shown) and REMS in both Ppg KO and WT mice (Figures 1,2,3 and Tables 1 and 2). The lowest dose, 0.4 µg LPS, increased NREMS in the first 4 hours after injection in both genotypes. The increase in NREMS was due to the increased number of NREMS episodes in both genotypes (Figure 4). The average duration of NREMS episodes was slightly, albeit significantly, shorter in the WT mice. There was a tendency towards attenuated REMS in the Ppg KO mice but statistical analysis revealed no significant difference in the sleep responses between Ppg KO and WT mice. EEG SWA was not significantly different from baseline in either genotypes.
Figure 1.
Non-rapid-eye-movement sleep (NREMS), rapid-eye-movement sleep (REMS), electroencephalogram slow-wave activity (SWA), body temperature and motor activity in wild-type (WT) and preproghrelin knockout mice (Ppg KO). Open symbols: saline injection, solid symbols: 0.4 µg lipopolysaccharide (LPS) administered intraperitoneally (ip). Data were analyzed in 2-h time blocks. Time “0”: time of the injections at ZT12. Dark shaded areas: dark period. Error bars: standard error. Asterisks denote significant differences between control and treatment days (post hoc Student-Newman-Keuls test).
Figure 2.
NREMS, REMS, EEG SWA, body temperature and motor activity in WT and Ppg KO mice after saline injection (open symbols) and 2 µg LPS (solid symbols) administration. See legends to Figure 1 for details.
Figure 3.
NREMS, REMS, EEG SWA, body temperature and motor activity in WT and Ppg KO mice after vehicle injection (open symbols) and 10 µg LPS (solid symbols) administration. See legends to Figure 1 for details.
Table 2.
The amount of wakefulness (min), NREMS (min), REMS (min), EEG SWA, body temperature and motor activity after systemic injections of saline and 0.4, 2 and 10 µg LPS.
| WT | ||||||
| Baseline | 0.4 µg LPS | Baseline | 2 µg LPS | Baseline | 10 µg LPS | |
| WAKE (min) | 664.23 ± 11.7 | 634.8 ± 11.4 | 691.1 ± 10.3 | 625.7 ± 26 | 624.5 ± 16.3 | 493.4 ± 34.2 |
| NREMS (min) | 681.17 ± 11.3 | 715.9 ± 10.0 | 651.2 ± 20.1 | 717.2 ± 51.6 | 736.8 ± 24.6 | 898.7 ± 45.5 |
| REMS (min) | 94.60 ± 4.1 | 89.3 ± 3.8 | 87 ± 3.1 | 60.8 ± 4.8 | 78.7 ± 9.5 | 47.9 ± 12 |
| EEG SWA (Units) | 100 ± 0 | 104.9 ± 1.1 | 100 ± 0 | 86.6 ± 3.5 | 100 ± 0 | 87.9 ± 3.5 |
| Body temperature (°C) | 37 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.2 | 37.3 ± 0.2 | 36.8 ± 0.2 | 36.9 ± 0.1 |
| Activity (Unit) | 100 ± 0 | 78.2 ± 5.5 | 100 ± 0 | 56.1 ± 8.6 | 100 ± 0 | 60.4 ± 10.4 |
| Ppg KO | ||||||
| Baseline | 0.4 µg LPS | Baseline | 2 µg LPS | Baseline | 10 µg LPS | |
| WAKE (min) | 699 ± 19.2 | 636.9 ± 21.5 | 684.8 ± 27.5 | 579.6 ± 35.4 | 597.1 ± 26 | 405 ± 55.7 |
| NREMS (min) | 653.1 ± 16.2 | 720.6 ± 22.6 | 672.6 ± 29.8 | 798.3 ± 43 | 762.9 ± 26 | 1001.5 ± 60.8 |
| REMS (min) | 87.9 ± 5.1 | 82.5 ± 3.4 | 81.6 ± 3.7 | 62.1 ± 9.4 | 79.9 ± 2.6 | 34.7 ± 6.3 |
| EEG SWA (Units) | 100 ± 0 | 98.8 ± 2.8 | 100 ± 0 | 92.3 ± 2.4 | 100 ± 0 | 82.4 ± 2.4 |
| Body temperature (°C) | 36.9 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.2 | 37.1 ± 0.2 | 36.8 ± 0.1 | 36.7 ± 0.2 |
| Activity (Unit) | 100 ± 0 | 78.8 ± 7.6 | 100 ± 0 | 55.5 ± 7.8 | 100 ± 0 | 29.6 ± 6.0 |
Figure 4.
Changes in the number and duration of NREMS and REMS episodes during the 12-h dark period after LPS administrations in Ppg KO (black bars) and WT (white bars) mice. Error bars: standard error. Asterisks denote significant differences between control and treatment days (paired t-test). #: significant differences between genotypes (Student t-test).
The middle dose of LPS elicited similar but more pronounced changes in sleep (Figure 2, Tables 1 and 2). NREMS during the first 12 hours after injection was increased by 104. ± 25.8 min in the WT mice and 131.1 ± 13.9 min in the Ppg KO mice. These increases in NREMS were due to the increase in frequency of NREMS episodes in both KO and WT mice. The 2 µg LPS injection suppressed REMS in both genotypes, mainly during the first half of the dark period but also in the first 4 hours of the light phase in the WT animals. The animals had decreased amounts of REMS due to fewer REMS episodes in the dark period. EEG SWA was significantly suppressed in the first half of the dark phase in both genotypes. There was no significant differences between the two genotypes in the sleep responses after 2 µg LPS.
The highest LPS dose, 10 µg, elicited the largest, longest-lasting sleep changes in both genotypes (Figure 3, Tables 1 and 2). NREMS amounts during the dark period increased by 141.6 ± 18 min in the WT mice and by 208 ± 19 min in the Ppg KO animals. The changes in NREMS were significantly different between the two genotypes. Changes in NREMS architecture after 10 µg LPS were significantly different between Ppg KO and WT mice. Thus, LPS induced sleep fragmentation in WT mice as indicated by the increased number of NREMS episodes and shorter NREMS episode durations. In Ppg KO mice, NREMS was more consolidated as evidenced by the fewer and longer NREMS episodes during the dark phase compared to WT mice and their own baseline values. In the light phase, NREMS amounts returned to baseline. Wakefulness (data not shown) during the dark phase was decreased in both Ppg KO and WT mice but it was significantly more attenuated in the Ppg KO mice compared to WT mice (191 ± 24 min in KOs vs 130.7 ± 17.6 min in WTs; Student t-test, p < 0.05). REMS was suppressed by the 10µg LPS injections in both genotypes during the dark and light phases. WT mice lost 30.8 ± 9.8 min of REMS while KO mice had 48 ± 8.8 min less REMS time during the 24 hour period after the injection. This decrease was due to significantly fewer numbers of REMS episodes in both groups of mice. EEG SWA was suppressed by LPS administration in both genotypes but the effects were significantly longer-lasting in the Ppg KO compared to WT mice (Student t-test, p < 0.05).
The effects of LPS on body temperature, motor activity, food intake and body weight in Ppg KO and WT mice
The lowest dose of LPS had no effect on body temperature in WT mice (Figure 1). In the Ppg KO mice, body temperature increased during the light phase. After the middle dose of LPS, there was a tendency towards decreased body temperature in the dark period followed by a significant increase during the light phase in both genotypes (Figure 2). There was no significant difference in the responses of Ppg KO and WT mice after the 2 µg dose of LPS. Changes in body temperature after the 10 µg dose of LPS showed a bi-phasic pattern (Figure 3). Body temperature was suppressed during the dark phase in both genotypes. Ppg KO mice, however, showed a significantly augmented hypothermic response after the LPS injection. Three of the Ppg KO mice developed at least one ~ 4–5°C hypothermic bout during the dark phase after LPS injection. None of the WT mice showed similar drops in body temperature after LPS treatment. During the light phase, body temperature increased in both genotypes.
Motor activity was suppressed after all three doses of LPS injections in both genotypes (Figures 1,2, and 3). Motor activity was suppressed in the WT mice only during the dark periods after each dose of LPS. The 10 µg dose of LPS suppressed motor activity in the Ppg KO mice during both the dark and light phases. This change was significantly different between the WT and Ppg KO mice.
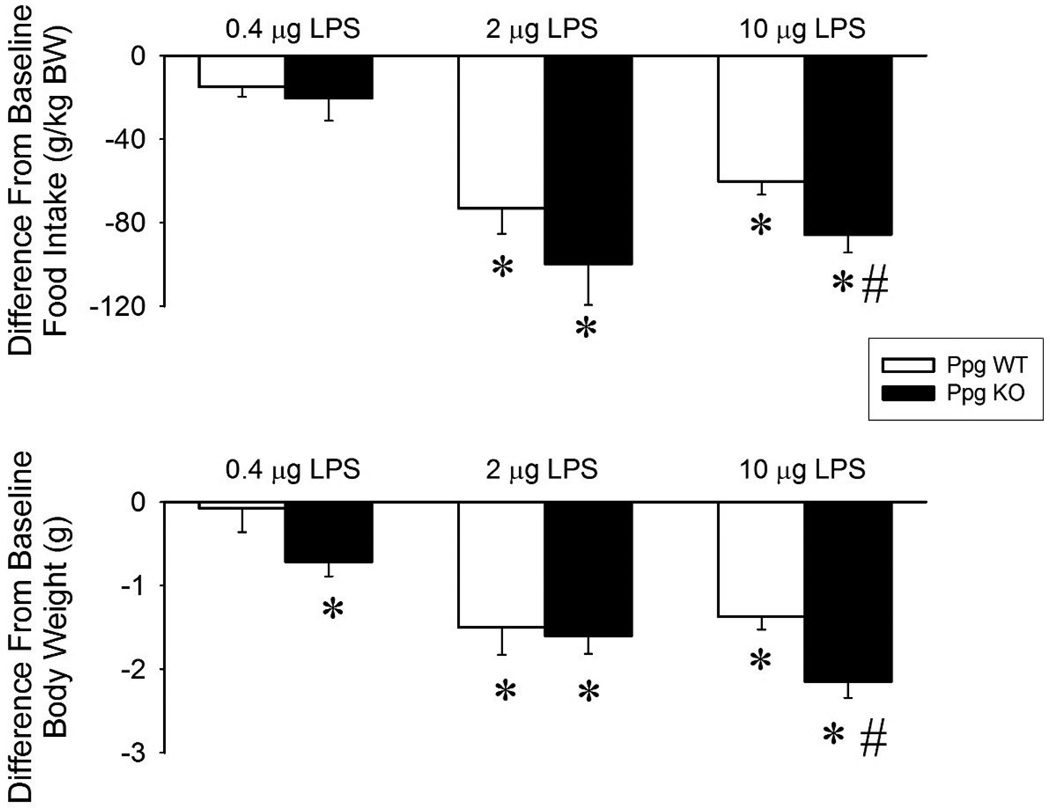
The lowest dose of LPS did not have any effect on the food intake or body weight in WT mice (Figure 5). While food intake was unchanged in the Ppg KO mice, body weight decreased significantly after the 0.4 µg dose of LPS. The middle and highest doses of LPS suppressed food intake and body weight in both WT and Ppg KO mice. The changes after 10 µg LPS were significantly larger in the Ppg KO mice compared to controls.
Figure 5.
Changes in daily food intake (top panel) and body weight (bottom panel) after 0.4, 2 and 10 µg LPS injections in WT (white bars) and Ppg KO mice (black bars). Asterisks: significant differences between control and treatment days (paired t-test). #: significant difference between genotypes (Student t-test).
Discussion
Results presented herein confirm previous findings that systemic administration of LPS promotes NREMS and suppresses REMS in mice (Toth and Opp, 2001, Morrow and Opp, 2005, Nadjar et al., 2013). Other facets of the acute phase response accompany these LPS-induced changes in sleep including decreases in EEG SWA, motor activity, body temperature, food intake and body weight in both WT and Ppg KO mice. Further, in Ppg KO mice, amplified sleep, anorectic and hypothermic responses to LPS challenge occur compared to WT mice. Collectively, these data support a role for ghrelin as an endogenous modulator of acute phase responses, including sickness behavior, and the corresponding arousal and feeding circuits.
Current results are also consistent with those previously reported to the extent that: a) The amounts of baseline NREMS and REMS are similar in WT and Ppg KO mice (Szentirmai et al., 2007). b) Systemic injection LPS has robust, immediate effects on sleep-wake activity in mice (Morrow and Opp, 2005, Toth and Opp, 2001, Nadjar et al., 2013). c) The lowest and middle doses of LPS increase sleep fragmentation as indicated by the increase in the number of NREMS episodes and decrease in the average duration of NREMS episodes (Lancel et al, 1995, Kapás et al., 1998). d) LPS suppresses REMS during the initial 12 hours post-injection. e) The decreases in REMS are due to decreases in the number of REMS episodes. Previously, similar LPS-induced suppression of REMS was reported in rabbits (Krueger et al., 1986, Toth and Krueger, 1989), rats (Lancel et al., 1995) and mice (Morrow and Opp, 2005, Toth and Opp, 2001).
The highest LPS dose, 10 µg, elicits larger increases in NREMS in the Ppg KO mice compared to controls. Additionally, the 10 µg LPS dose increases the duration of NREMS episodes while the number of the individual episodes is unaltered in the Ppg KO animals, indicating sleep consolidation rather than sleep fragmentation in the KO mice. These amplified sleep-promoting effects of LPS in Ppg KO animals are consistent with the proposed arousal-promoting role of the ghrelin system (reviewed in Szentirmai and Kapás 2012). Central injection of ghrelin stimulates wakefulness (Szentirmai et al., 2006, Szentirmai et al., 2007, Szentirmai 2012) and mice that lack ghrelin signaling show attenuated responses to arousal-inducing stimuli (Esposito et al., 2012). Proinflammatory cytokines are posited to mediate responses to LPS challenge. For instance, peripheral administration of LPS induces the synthesis and secretion of IL1β and TNFα (Galanos and Freudenberg, 1993). Both IL1β and TNFα enhance NREMS and suppress REMS (Kapás et al, 1992, Kubota et al., 2000, Opp and Toth, 1998). Further, ghrelin reduces LPS-induced increases in serum levels of TNFα and IL1β in rats and mice (Wang et al., 2009, Wu et al., 2007). The exacerbated sleep effects in the Ppg KO mice may indicate that in the absence of ghrelin signaling there are enhanced TNFα and IL1β responses and a corresponding enhancement of the acute phase response to LPS challenge.
Changes in EEG SWA in response to LPS or during the course of acute bacterial or viral infections differ among species and also depend on the route of infection and the type of pathogen. In humans, both increases in (Mullington et al., 2000) and unaltered EEG SWA (Pollmächer et al., 1993) are observed after endotoxin administration. In rabbits, biphasic responses are reported; initial EEG SWA increases are followed by EEG SWA suppression (Toth and Krueger, 1989). In rats, similar decreases in NREMS intensity occur in response to LPS (Kapás et al., 1998, Lancel et al., 1995). In mice, dark onset administration of LPS induces decreases in EEG delta power during NREMS (Toth and Opp, 2001, Morrow and Opp, 2005). In our experiment, EEG SWA is suppressed after LPS treatment and these results are consistent with prior murine-LPS studies. Shortened sleep epochs, that is increased sleep fragmentation, diminishes EEG SWA (Trachsel et al., 1988). Increased sleep fragmentation in response to LPS may be one of the factors that lead to suppressed EEG SWA. However, additional factors likely affect EEG SWA because in the Ppg KO animals, the highest dose of LPS did not enhance sleep fragmentation while the EEG SWA-suppressing effects were significantly longer and more-pronounced than in the WT mice.
The importance of ghrelin for thermoregulation is demonstrated by the strikingly different body temperature responses to LPS between WT and Ppg KO mice. Mice respond to systemic LPS treatment with an initial hypothermia followed by prolonged rise in body temperature (Wang et al., 1997, Morrow and Opp, 2005). We observed similar changes in body temperature in the WT mice after all three doses of LPS. The magnitude of the hypothermic response to 10 µg LPS is, however, greatly amplified in the Ppg KO mice compared to WT mice. These data, together with our previous observations showing the Ppg KO mice develop hypothermia when fasted in a cold environment, indicate that ghrelin signaling is essential for thermoregulation (Szentirmai et al., 2009).
Bacterial infection and LPS treatment are associated with anorexia (reviewed in Langhans, 1996). Current results are consistent with the earlier reports in that the middle and high doses of LPS suppress 24 hour food intake in both Ppg KO and WT mice. Herein we extend those findings to demonstrate the involvement of ghrelin signaling; the anorexic responses are greater in the Ppg KO than in WT mice. As mentioned, synthesis and release of TNFα, IL1β and IL6 are part of the acute phase response to LPS. IL1β and TNFα also reduce food intake in rats and mice (reviewed in Kelley et al., 2003) and thus likely mediate the anorectic effects of bacterial products. Ghrelin reduces LPS-induced TNFα, IL1β and IL6 (Wang et al., 2009, Wu et al., 2007). It is reasonable to propose that the larger effects of LPS on food intake and body weight occurring in the Ppg KO mice are due to lack of ghrelin’s cytokine suppressing actions.
Collectively, current findings clearly indicate that ghrelin signaling is involved in the development of the acute phase response to LPS including modulation of sickness behavior. We posit that in the Ppg KO mice, the lack of ghrelin signaling disrupts ghrelin-mediated counter regulatory control mechanisms involved in the sleep, thermoregulatory and anorectic responses to LPS.
Acknowledgements
We thank the excellent technical help of Richard Brown and Jacob Pellinen. We thank Drs. Roy G. Smith and Yuxiang Sun for providing breeding pairs of preproghrelin KO and WT mice. This work was supported by Washington State University Faculty Seed Grant to ÉS and The National Institutes of Health grant numbers NS025378 and HD036520 to JMK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin. Res. 2001;7:167–202. [PubMed] [Google Scholar]
- 2.Basa NR, Wang L, Arteaga JR, Heber D, Livingston EH, Tache Y. Bacterial lipopolysaccharide shifts fasted plasma ghrelin to postprandial levels in rats. Neurosci. Lett. 2003;343:25–28. doi: 10.1016/s0304-3940(03)00312-4. [DOI] [PubMed] [Google Scholar]
- 3.Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1071–R1079. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 4.Chang L, Zhao J, Yang J, Zhang Z, Du J, Tang C. Therapeutic effects of ghrelin on endotoxic shock in rats. Eur. J Pharmacol. 2003;473:171–176. doi: 10.1016/s0014-2999(03)01972-1. [DOI] [PubMed] [Google Scholar]
- 5.Chorny A, Anderson P, Gonzalez-Rey E, Delgado M. Ghrelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria. J Immunol. 2008;180:8369–8377. doi: 10.4049/jimmunol.180.12.8369. [DOI] [PubMed] [Google Scholar]
- 6.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esposito M, Pellinen J, Kapás L, Szentirmai E. Impaired wake-promoting mechanisms in ghrelin receptor-deficient mice. Eur. J Neurosci. 2012;35:233–243. doi: 10.1111/j.1460-9568.2011.07946.x. [DOI] [PubMed] [Google Scholar]
- 8.Galanos C, Freudenberg MA. Mechanisms of endotoxin shock and endotoxin hypersensitivity. Immunobiology. 1993;187:346–356. doi: 10.1016/S0171-2985(11)80349-9. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–1720. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Hataya Y, Akamizu T, Hosoda H, Kanamoto N, Moriyama K, Kangawa K, Takaya K, Nakao K. Alterations of plasma ghrelin levels in rats with lipopolysaccharide-induced wasting syndrome and effects of ghrelin treatment on the syndrome. Endocrinology. 2003;144:5365–5371. doi: 10.1210/en.2003-0427. [DOI] [PubMed] [Google Scholar]
- 11.Kapás L, Hansen MK, Chang HY, Krueger JM. Vagotomy attenuates but does not prevent the somnogenic and febrile effects of lipopolysaccharide in rats. Am. J Physiol. 1998;274:R406–R411. doi: 10.1152/ajpregu.1998.274.2.R406. [DOI] [PubMed] [Google Scholar]
- 12.Kapás L, Hong L, Cady AB, Opp MR, Postlethwaite AE, Seyer JM, Krueger JM. Somnogenic, pyrogenic, and anorectic activities of tumor necrosis factor-alpha and TNF-alpha fragments. Am. J. Physiol. 1992;263:R708–R715. doi: 10.1152/ajpregu.1992.263.3.R708. [DOI] [PubMed] [Google Scholar]
- 13.Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav. Immun. 2003;17(Suppl 1):S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 14.Kimura M, Toth LA, Agostini H, Cady AB, Majde JA, Krueger JM. Comparison of acute phase responses induced in rabbits by lipopolysaccharide and double-stranded RNA. Am J Physiol. 1994;267:R1596–R1605. doi: 10.1152/ajpregu.1994.267.6.R1596. [DOI] [PubMed] [Google Scholar]
- 15.Krueger JM, Kubillus S, Shoham S, Davenne D. Enhancement of slow-wave sleep by endotoxin and lipid A. Am. J. Physiol. 1986;251:R591–R597. doi: 10.1152/ajpregu.1986.251.3.R591. [DOI] [PubMed] [Google Scholar]
- 16.Krueger JM, Toth LA, Floyd R, Fang J, Kapas L, Bredow S, Obal F., Jr Sleep, microbes and cytokines. Neuroimmunomodulation. 1994;1:100–109. doi: 10.1159/000097142. [DOI] [PubMed] [Google Scholar]
- 17.Kubota T, Kushikata T, Fang J, Krueger JM. Nuclear factor-kappaB inhibitor peptide inhibits spontaneous and interleukin-1beta-induced sleep. Am. J Physiol Regul. Integr. Comp Physiol. 2000;279:R404–R413. doi: 10.1152/ajpregu.2000.279.2.R404. [DOI] [PubMed] [Google Scholar]
- 18.Lancel M, Crönlein J, Müller-Preuss P, Holsboer F. Lipopolysaccharide increases EEG delta activity within non-REM sleep and disrupts sleep continuity in rats. Am. J Physiol. 1995;268:R1310–R1318. doi: 10.1152/ajpregu.1995.268.5.R1310. [DOI] [PubMed] [Google Scholar]
- 19.Langhans W. Bacterial products and the control of food ingestive behavior: Clinical implications. Nutrition. 1996;12:303–315. doi: 10.1016/s0899-9007(96)80052-9. [DOI] [PubMed] [Google Scholar]
- 20.Morrow JD, Opp MR. Diurnal variation of lipopolysaccharide-induced alterations in sleep and body temperature of interleukin-6-deficient mice. Brain Behav. Immun. 2005;19:40–51. doi: 10.1016/j.bbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Mullington J, Korth C, Hermann DM, Orth A, Galanos C, Holsboer F, Pollmächer T. Dose-dependent effects of endotoxin on human sleep. Am. J Physiol Regul. Integr. Comp Physiol. 2000;278:R947–R955. doi: 10.1152/ajpregu.2000.278.4.R947. [DOI] [PubMed] [Google Scholar]
- 22.Nadjar A, Blutstein T, Aubert A, Laye S, Haydon P. Astrocyte-derived adenosine modulates increased sleep pressure during inflammatory response. Glia. 2013;61:724–731. doi: 10.1002/glia.22465. [DOI] [PubMed] [Google Scholar]
- 23.Opp MR, Toth LA. Somnogenic and pyrogenic effects of interleukin-1beta and lipopolysaccharide in intact and vagotomized rats. Life Sci. 1998;62:923–936. doi: 10.1016/s0024-3205(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 24.Pollmächer T, Schreiber W, Gudewill S, Vedder H, Fassbender K, Wiedemann K, Trachsel L, Galanos C, Holsboer F. Influence of endotoxin on nocturnal sleep in humans. Am. J Physiol. 1993;264:R1077–R1083. doi: 10.1152/ajpregu.1993.264.6.R1077. [DOI] [PubMed] [Google Scholar]
- 25.Soriano RN, Nicoli LG, Carnio EC, Branco LG. Exogenous ghrelin attenuates endotoxin fever in rats. Peptides. 2011;32:2372–2376. doi: 10.1016/j.peptides.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Stengel A, Goebel M, Wang L, Reeve JR, Jr, Tache Y, Lambrecht NW. Lipopolysaccharide differentially decreases plasma acyl and desacyl ghrelin levels in rats: potential role of the circulating ghrelin-acylating enzyme GOAT. Peptides. 2010;31:1689–1696. doi: 10.1016/j.peptides.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol. Cell Biol. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szentirmai É. Central but not systemic administration of ghrelin induces wakefulness in mice. PLoS. One. 2012;7:e41172. doi: 10.1371/journal.pone.0041172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szentirmai É, Kapás L. Ghrelin Regulation of Sleep, Circadian Clock and Body Temperature. In: Thorner M, Smith RG, editors. Ghrelin in Health and Disease. Vol. 2012. Springer Science and Business Media; 2012. pp. 149–180. [Google Scholar]
- 31.Szentirmai E, Hajdu I, Obal F, Jr, Krueger JM. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res. 2006a;1088:131–140. doi: 10.1016/j.brainres.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 32.Szentirmai E, Hajdu I, Obal F, Jr, Krueger JM. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res. 2006b;1088:131–140. doi: 10.1016/j.brainres.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 33.Szentirmai E, Kapás L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am. J Physiol Regul. Integr. Comp Physiol. 2007;292:R575–R585. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- 34.Szentirmai E, Kapás L, Sun Y, Smith RG, Krueger JM. Spontaneous sleep and homeostatic sleep regulation in ghrelin knockout mice. Am. J Physiol Regul. Integr. Comp Physiol. 2007;293:R510–R517. doi: 10.1152/ajpregu.00155.2007. [DOI] [PubMed] [Google Scholar]
- 35.Szentirmai É, Kapás L, Sun Y, Smith RG, Krueger JM. The preproghrelin gene is required for the normal integration of thermoregulation and sleep in mice. Proc Natl Acad Sci U. S. A. 2009;106:14069–14074. doi: 10.1073/pnas.0903090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szentirmai É, Kapás L, Sun Y, Smith RG, Krueger JM. Restricted feeding-induced sleep, activity, and body temperature changes in normal and preproghrelin-deficient mice. Am. J Physiol Regul. Integr. Comp Physiol. 2010;298:R467–R477. doi: 10.1152/ajpregu.00557.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toth LA, Krueger JM. Effects of microbial challenge on sleep in rabbits. FASEB J. 1989;3:2062–2066. doi: 10.1096/fasebj.3.9.2663582. [DOI] [PubMed] [Google Scholar]
- 38.Toth LA, Opp MR. Cytokine- and microbially induced sleep responses of interleukin-10 deficient mice. Am. J Physiol Regul. Integr. Comp Physiol. 2001;280:R1806–R1814. doi: 10.1152/ajpregu.2001.280.6.R1806. [DOI] [PubMed] [Google Scholar]
- 39.Trachsel L, Schreiber W, Holsboer F, Pollmächer Endotoxin enhances EEG alpha and beta power in human sleep. Sleep. 1994;17:132–139. doi: 10.1093/sleep/17.2.132. [DOI] [PubMed] [Google Scholar]
- 40.Trachsel L, Tobler I, Borbely AA. Electroencephalogram analysis of non-rapid eye movement sleep in rats. Am. J Physiol. 1988;255:R27–R37. doi: 10.1152/ajpregu.1988.255.1.R27. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Ando T, Dunn AJ. Effect of homologous interleukin-1, interleukin-6 and tumor necrosis factor-alpha on the core body temperature of mice. Neuroimmunomodulation. 1997;4:230–236. doi: 10.1159/000097341. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St-Pierre DH, Tache Y. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am. J. Physiol Gastrointest. Liver Physiol. 2006;291:G611–G620. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- 43.Wang W, Bansal S, Falk S, Ljubanovic D, Schrier R. Ghrelin protects mice against endotoxemia-induced acute kidney injury. Am. J Physiol Renal Physiol. 2009;297:F1032–F1037. doi: 10.1152/ajprenal.00044.2009. [DOI] [PubMed] [Google Scholar]
- 44.Wu R, Dong W, Zhou M, Zhang F, Marini CP, Ravikumar TS, Wang P. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am. J Respir. Crit Care Med. 2007;176:805–813. doi: 10.1164/rccm.200604-511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]