Abstract
Objective
Whether febrile status epilepticus (FSE) produces hippocampal sclerosis (HS) and temporal lobe epilepsy (TLE) has long been debated. Our objective is to determine if FSE produces acute hippocampal injury that evolves to HS.
Methods
FEBSTAT and two affiliated studies prospectively recruited 226 children aged 1 month to 6 years with FSE and controls with simple febrile seizures. All had acute MRIs and follow-up MRIs were obtained at approximately 1 year later in the majority. Visual interpretation by two neuroradiologists informed only of subject age was augmented by hippocampal volumetrics, analysis of the intra-hippocampal distribution of T2 signal, and apparent diffusion coefficients.
Results
Hippocampal T2 hyperintensity, maximum in Sommer's sector, occurred acutely after FSE in 22 of 226 children in association with increased volume. Follow-up MRIs obtained on 14 of the 22 with acute T2 hyperintensity showed HS in 10 and reduced hippocampal volume in 12. In contrast, follow-up of 116 children without acute hyperintensity showed abnormal T2 signal in only 1 (following another episode of FSE). Furthermore, compared to controls with simple febrile seizures, FSE subjects with normal acute MRIs had abnormally low right to left hippocampal volume ratios, smaller hippocampi initially and reduced hippocampal growth.
Interpretation
Hippocampal T2 hyperintensity after FSE represents acute injury often evolving to a radiological appearance of HS after one year. Furthermore, impaired growth of normal appearing hippocampi after FSE suggests subtle injury even in the absence of T2 hyperintensity. Longer follow-up is needed to determine the relationship of these findings to TLE.
INTRODUCTION
Febrile seizures (FSs) are common, occurring in 2–4% of children1. Brief FSs are considered benign1 but prolonged FSs are associated with subsequent epilepsy2. Febrile status epilepticus (FSE) occurs in 5–9% of children with a first FS3, accounting for 25% of pediatric status epilepticus4, with 25,000 to 30,000 FSE cases annually in the US. Whether prolonged FSs cause hippocampal sclerosis (HS) and temporal lobe epilepsy (TLE) has been debated for over 50 years5.
The FEBSTAT study (Consequences of Prolonged Febrile Seizures in Childhood) is prospectively examining outcomes of FSE6. Smaller studies of FSE have reported varied outcomes from HS to subtle asymmetries 7–11. We hypothesized that definite hippocampal T2 hyperintensity immediately following FSE would predict development of HS6. We have reported baseline visual magnetic resonance imaging (MRI) findings, semiology, EEG and virology from FEBSTAT6, 12–14. Here we analyze acute hippocampal abnormalities and their early evolution on follow-up MRIs in children with FSE from FEBSTAT and two companion prospective cohorts compared to children with a first simple febrile seizure (SFS) who underwent similar imaging protocols.
METHODS
Subjects
FSE was defined as a FS lasting 30 minutes or longer or repetitive FSs, lasting at least 30 min without regaining alertness15. Cohort eligibility and procedures have been described6, 12. All sites obtained Institutional Review Board approval and informed consent. As initial MRIs were obtained after confirming eligibility and obtaining informed consent, children with preexisting MRI abnormalities were included. The 226 children aged 1 month to 6 years with FSE 6 were derived from three prospective studies (see Supplementary Fig 1); 191 were from the FEBSTAT cohort12, 23 from the Duke FEBSTAT pilot study11, and 12 from the Columbia first FS study16. Also from the Columbia study, we obtained 38 children with SFSs who had normal initial MRIs and 1 year follow-up MRIs16 to serve as controls for FSE children with normal initial MRIs. In FSE cases, 67% of acute scans were done within 3 days of FSE and 88% within 7 days. Of 22 children with definite hippocampal T2 hyperintensity on acute MRIs, 17 were from FEBSTAT, 4 from Duke and 1 from Columbia.
MRI Sequences
The pulse sequences on GE and equivalent on Siemens 1.5 T MRI systems were: coronal oblique (perpendicular to the hippocampal axis) T2 weighted imaging, fast spin echo sequence, TR=4500, TE=96–105, ETL=7–8 (echo train length), 20 cm × 15 cm FOV, 3mm slice, 0mm gap, 256×256 matrix and 4 NEX; coronal oblique diffusion weighted imaging (DWI), diffusion weighted echo-planar sequence, TR/TE=6000/76–105, partial Fourier, 22cm FOV, 4mm slice, 1mm gap, 128(frequency)×64(phase) matrix, diffusion sensitizing gradients in AP, SI, LR, b=1000; and T1-weighted imaging 3D coronal fast spoiled gradient-echo sequence, TR/TE/flip = 12/5/20° to 30°, (full echo), 20cm FOV, 1.5 mm slice, 124 slices, 256×192 matrix, 2 NEX. Apparent diffusion coefficient (ADC) maps were calculated from DWI using conventional methods
Visual Analysis of Hippocampal Abnormalities
Hippocampal abnormality was visually assessed by two experienced neuroradiologists (JAB, SC) informed of subject age but blinded to clinical details and type of MRI (acute or follow-up)12. MRIs of SFSs from the Columbia study16 were interspersed with FSE MRIs and identically reviewed by the same central readers. Hippocampal T2 signal (T2Score) was rated from 0–4 (0=normal, 1=equivocal, 2=mildly abnormal T2 signal on one or more slices, 3=moderately abnormal, 4= markedly abnormal throughout hippocampus). For purposes of this analysis, only T2Scores of 2 or greater were considered a definite signal abnormality. Six equivocal hippocampi (T2Score=1) were thereby excluded from the analysis. The radiologic criteria for HS were definite hippocampal atrophy and T2Score of 2 or more12, 17. Discordant readings were conferenced for consensus. Agreement on hippocampal T2Scores (normal vs. equivocal vs. abnormal), the primary outcome of visual readings, was excellent (kappa= 0.80; 95%CI 0.61–0.98).
Volumetric Measurements
Hippocampal regions of interest (ROIs) were traced (SnAP:IRIS18) using conventional boundaries19 by a trained observer (YX) blinded to clinical data. Slices posterior to and including the anterior commissure were summed for hippocampal volume. Right to left (Rt/Lt) volume ratios represent the volume of the right divided by the left hippocampus. A geometry phantom scanned after FEBSTAT subjects indicated small (median 2.8%, IQR 2.1–3.5) corrections used to adjust volumes in analyses that included only FEBSTAT subjects since phantoms were not used for the other cohorts. Consistency of volumetric measurements was assessed using the intraclass correlation coefficient (ICC)20. Ten MRIs randomly selected every two months from 33 FEBSTAT subjects across the age range were analyzed independently (YX, DVL). ICCs were 0.95 and 0.97 for the left and right hippocampi, respectively.
T2 Signal Intensity and Diffusion Measurements
Analysis of T2 intensity and ADC maps was performed by observers (YX, WAG) blinded to clinical data. T2 intensity was measured on coronal sections through the middle of the hippocampal body where sector boundaries are easily delineated21. Each section was subdivided into 8 equal pie slice shaped ROIs (AnalyzeTM, Mayo Clinic, Rochester, MN), and a circular ROI with a radius 1/3 of the radius of the pie centered over the hilus was drawn (Fig 2 Insert). For all hippocampi, mean T2 signal intensities for each ROI were normalized to ipsilateral thalamic intensity. Control values were then subtracted from the values of hyperintense hippocampi yielding a number representing the increase over normal T2 signal level for each ROI in affected hippocampi. Two subjects whose initial MRIs met the radiological criteria for HS were excluded to limit the analysis to acutely injured hippocampi. Hippocampal ADCs were obtained by averaging ADCs of ROIs drawn on coronal ADC maps excluding all non-hippocampal voxels. Not all hippocampi had usable DWIs due to technical issues or subjects being imaged prior to routine use of DWI.
Figure 2.
Increased T2 on Acute and Follow-Up MRIs. Inset - ROIs overlying hippocampal sectors, SS = Sommer's Sector, Sub =subiculum. Plot shows increase in T2 intensity by hippocampal sector in the acute (filled squares; N=21) and follow-up (open squares; N=13) MRIs. On acute scans, the mean (± St Dev) increase in T2 signal across all three Sommer's sector ROIs was 0.141 (± 0.136) vs. 0.057 (±0.099) for all other sectors (t-test; p<0.001). Solid and dashed lines = means over SS and Non-SS sectors at Acute and Follow-Up, respectively. Bars = 95% Confidence Intervals.
Controls for Analyses
We selected controls appropriate for each analysis (Supplementary Table 1). Controls for growth of hyperintense hippocampi had FSE and normal baseline MRIs. For analysis of Rt/Lt volume ratios and hippocampal growth in FSE subjects with normal baseline MRIs, controls were 38 children with SFSs and normal MRIs from the Columbia study. For ADC evolution after FSE and for T2 signal distribution in hyperintense hippocampi, controls with FSE and normal hippocampal signal were used. For each analysis, controls were matched by age, gender, hippocampal lateralization and latency from FSE to baseline MRI.
Other Studies
The FEBSTAT cohort had EEGs and virology studies done for HHV6 and HHV7 at baseline. DNA was also banked at Coriell6. Details are in the online files (Supplementary Results).
Statistics
Results of statistical analysis (DCH, EB, CL) are presented as mean and standard deviation. A mixed effect model with subject-specific random intercept was used to analyze hippocampal growth, adjusting for age and gender22. All analyses were conducted using SAS 9.1 (SAS Institute, Cary, NC, U.S.A). Tests of the fixed effects were performed when the mixed model indicated a difference between the two groups or between the acute and follow-up time points. A Bonferroni approach was used to correct for the multiplicity of tests. Multivariate regression models examined effects of other risk factors on hippocampal volume (Supplementary Results).
RESULTS
Hippocampal T2 Signal in Acute MRIs
Definite abnormal hippocampal signal occurred in 22 (9.7%) of 226 FSE children. Fifteen were right-sided, 6 were left-sided and one was bilateral. On visual inspection, the most intense T2 signal appeared in CA1 and the prosubiculum coinciding with Sommer's sector23, 24 located in the lateral inferior aspect of the hippocampal body (Fig 1). This was confirmed by measurement of T2 signal intensity in all acutely hyperintense hippocampi (Fig 2). Contralateral hippocampal T2 intensity was not increased.
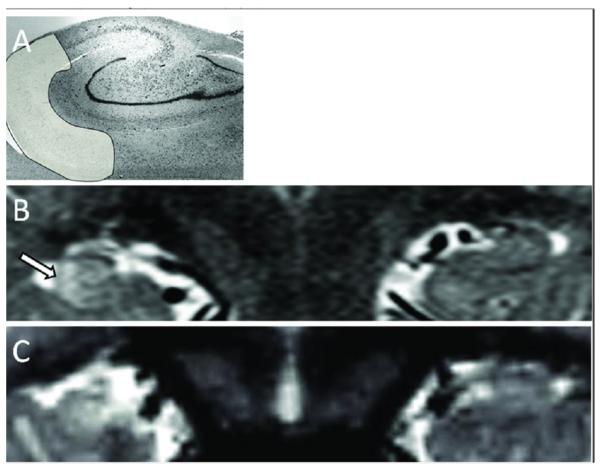
Figure 1.
Increased Hippocampal T2 Signal Following FSE. A) Nissl stain of hippocampal body outlining area of Sommer's sector (Courtesy G. Mathern). B) Acutely swollen and hyperintense right hippocampus of a 13 month old male 3 days after a 120 min long episode of FSE. Note the right hippocampus is larger and has increased T2 signal most prominent in Sommer's sector (Arrow). C) Follow up MRI 6 months later of same child showing the right hippocampus now smaller and the T2 signal increase is persistent but no longer maximum in Sommer's sector.
Nine subjects with hippocampal hyperintensity also had other MRI abnormalities described previously12; 4 had hippocampal malrotation (HIMAL) and 5 had extrahippocampal abnormalities (Supplementary Results, Additional Abnormalities and Table 2).
Quantitative Acute Hippocampal Volumes and ADCs
In the 21 cases with unilateral abnormal hippocampal signal, hyperintense hippocampi were larger than contralateral hippocampi in 13 (61.9%). Excluding three subjects with preexisting small hippocampi described below, mean volume of hyperintense hippocampi was greater than contralateral hippocampi (2273 ± 600.7 vs. 2111 ± 533.6 mm3; Paired t-test; p=0.02; N=18).
Two subjects had visually small and hyperintense hippocampi meeting our radiologic criteria of HS12. These hippocampi, imaged two days following FSE, were also small by volumetric measurement. Another child had a small schizencephalic hemisphere and a small hyperintense ipsilateral hippocampus.
ADC maps were available on 13 of 22 subjects with hyperintense hippocampi after excluding the 2 subjects with HS and the one subject with bilateral hyperintensity. The mean ADC of the hyperintense hippocampi (1.01 × 10−3 mm2/s ± 8.35 × 10−5; N=13) was lower than that of the matching contralateral hippocampi (1.05 × 10−3 mm2/s ± 9.90 × 10−5; N=13) but this difference was not statistically significant (Paired t-test; p=0.06).
Follow-up MRIs
Follow-up MRIs were available on 139 FSE subjects. Of these, 130 were technically adequate for evaluation of initial and final T2 signal with follow-up intervals between 1 month and 2.5 years (Median 1.07 years; IQR 1.00 – 1.25). Among the 130 subjects, 116 had initially normal hippocampal signal and only one developed abnormal signal on follow-up after a second episode of FSE; none had radiologic evidence of HS.
Of the 22 with abnormal hippocampal signal, 14 returned for follow-up MRI, some of which had additional abnormalities (Supplementary Results, Additional Abnormalities and Supplementary Table 2). Ten of the 14 (71.4%) met visual criteria for HS on follow-up including one who had HS on the initial MRI. Volumetric measurement indicated volume loss was more frequent than HS, with 13 of 15 (87%) hyperintense hippocampi in 12 of 14 (85.7%) subjects showing decreased volume, including both hippocampi of the subject with bilateral hyperintensity (Fig 3A). Although 4 subjects with follow-up MRIs had HIMAL in addition to hyperintensity, HIMAL alone does not produce hippocampal shrinkage (Supplementary Results).
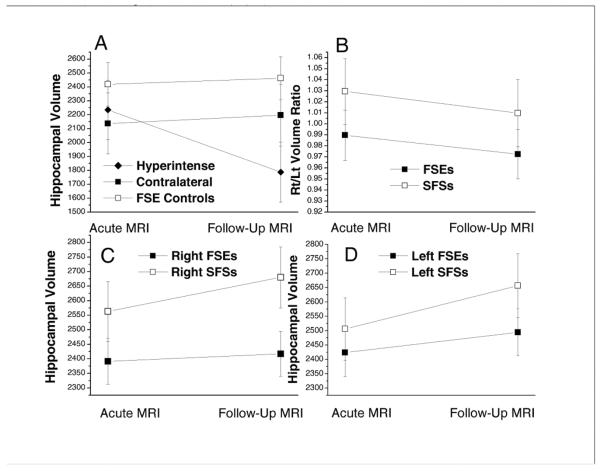
Figure 3.
Hippocampal Volumes from Acute and Follow-up MRIs. A) Hyperintense hippocampi (Hyperintense; N=15) vs. hippocampi contralateral to them (Contralateral; N=13) and control hippocampi from FSE subjects with normal acute MRIs (FSE Controls; N=30). The number of hyperintense hippocampi is greater than the number of contralateral hippocampi because one subject had bilateral hyperintensity. Note that the hyperintense hippocampi lost approximately 20% of volume between the initial and follow up MRIs, whereas the contralateral and FSE control hippocampal volumes increased. The mixed linear model indicated that, at follow-up MRI. the mean volume of hyperintense hippocampi was less than the controls (p<0.0001) and the contralaterals (p=0.0001). At the baseline MRI, the mean volumes of the groups were not significantly different. B) Rt/Lt volume ratios of FSE subjects with normal acute MRIs were less than SFS subjects with normal acute MRIs (mean estimated Rt/Lt volume ratios of 0.98 vs. 1.03 respectively; F-test p=0.02). Rt/Lt ratios did not change significantly from baseline to follow-up MRIs (F test p=0.10). C) Right hippocampal volumes of FSE were less than SFS group by 171 and 262 mm3 at the acute and follow-up MRIs respectively (Bonferroni adjusted p=0.03 for acute and p <0.001 for follow-up). D) Left hippocampal volumes of FSE were less than SFS subjects by 82 and 162 mm3 at the acute and follow-up MRIs although these differences did not attain significance (Bonferroni adjusted p=0. 90 for acute and p= 0.07 for follow-up). From the acute to follow-up MRI, the volumes of both sides increased for SFS subjects (Bonferroni adjusted left side: p = 0.03; right side: p = 0.11). For FSE subjects, the slope of growth was flatter and volume change was not significant on either side (left side: p = 0.74; right side: p = 1.00). Bars are 95% confidence intervals. For B, C and D the groups were identical with FSEs (N=59) and SFS controls (N=38).
It was unlikely that the severity of the initial insult differed in those with and without follow-up because there were no significant differences between these groups for febrile status duration and hippocampal T2Scores, or in the incidence of focal status and EEG abnormalities. However, in the 22 with hippocampal hyperintensity, the presence of multiple conditions affecting the well being, such as developmental delay, epilepsy or recurrent status, seemed to increase the likelihood of follow-up (Supplementary Results: Proportion with Follow-Up). For instance, of the 22 with hippocampal hyperintensity, 5 developed epilepsy early, mostly not TLE, and all returned for follow-up.
T2 signal intensity no longer appeared greatest in Sommer's sector on follow-up MRIs (Fig 1C). Measurements (Fig 2) confirmed this visual impression with mean signal increase in Sommer's sector of 0.048 (± 0.088) compared to 0.062 (± 0.095) in the remaining sectors (t-test; p=0.5).
Both acute and follow-up ADCs were available on 7 subjects with hyperintense hippocampal signal. The mean ADC had increased by 3.7% (± 12.8), whereas over the same interval, the mean hippocampal ADC had decreased by 11% (± 12.4) in 14 matched FSE controls (t-test; p = 0.02) as expected in normally maturing brain25.
Previous studies of FSE report that hippocampi appearing normal acutely become increasingly asymmetric on follow-up, suggesting subtle injury8, 9. To detect subtle effects in our cohort, we selected from our FSE and SFS subjects only those with completely normal acute MRIs and available follow-up MRIs obtained between 6 and 24 months after the initial seizures, yielding 59 FSE and 38 SFS cases. We analyzed hippocampal volumes and volume ratios in these cases using a linear mixed model, controlling for age and gender. Subsequent t-tests were performed on the fixed effects to compare the simple main effects of SFS and FSE subjects at acute and follow-up MRI. The mean Rt/Lt volume ratio estimated from the model was significantly less in FSE subjects than in SFSs (Fig 3B). Mean right and left hippocampal volumes of the FSE group were less than the SFS group on acute and follow-up MRIs and FSE hippocampi appeared to grow more slowly (Fig 3C, D).
Although it is too early to determine the ultimate incidence and types of epilepsy in this cohort, at the time of these follow-up MRIs, only 16 of 226 children had developed epilepsy (7.1%). Three have clinical Dravet's syndrome and normal MRIs. The great majority of the epilepsy is not TLE which is not surprising given the prolonged latency of TLE following FSE26, 27. Developmental delay, determined on enrollment using the Bayley Scales of Infant Development, was also uncommon and found in only 31 of 226 with FSE (13.7%) and in 4 of 22 with hippocampal hyperintensity (18.2%).
Other Risk Factors Influencing Hippocampal Volume Change After FSE
In the 59 FSE cases with visually normal baseline MRIs, we examined the effect of age at FSE, duration, focality, focal slowing or attenuation on baseline EEG and presence of HHV-6 or HHV-7 viremia on change in hippocampal volume (Supplementary Results). On multivariate analysis, hippocampal volume change was affected by age with younger age showing more growth, but only in the left hippocampus and HHV6/HHV7 viremia, with those with viremia showing more growth on both sides, even after adjusting for age. We were unable to assess these factors in hippocampi with abnormal signal because essentially all these cases had volume loss
DISCUSSION
It is clear from FEBSTAT12 and other studies7, 28–30 that FSE can result in acute hippocampal injury visible on MRI. This report demonstrates, furthermore, that the increased hippocampal signal is maximal in Sommer's sector acutely and that many of the affected hippocampi lose volume, meeting radiologic criteria for HS17. Additionally, even in our subjects with normal acute MRIs, subsequent decreased hippocampal growth suggests a subtle acute hippocampal injury.
Evidence for cytotoxic edema was found in the combination of abnormal signal in Sommer's sector, reflecting the known vulnerability of that sector to seizures23, 31 increased hippocampal volume, and a trend for reduced ADCs. Although acutely increased ADCs were found in one study of FSE, those hippocampi did not have visibly increased T2 signal32 as our subjects did. On follow-up, T2 hyperintensity was no longer maximal in Sommer's sector and this change in distribution, along with reduced volume and increasing ADCs may reflect chronic changes of neuronal loss and gliosis in Sommer's sector as described by others23, 24, 33. The combined MRI findings of increased hippocampal signal and atrophy seen here are reliable indicators of HS17.
The appearance consistent with HS on initial MRI of two of our subjects was unexpected and supports the suggestion that HS might occasionally precede FSE made by others using retrospective analysis34, 35.
Reliable biomarkers for TLE following FSE will be essential to identify children at risk once antiepileptogenic intervention becomes available36, 37. Little data is available for evaluation of such markers in humans, although important biomarker studies in animal models of FSE38 exist. Presently, we can assess biomarkers for HS only, as latency from FSE to the appearance of TLE can be ≥10 years26, 27. However, in the setting of FSE, it seems that T2 hyperintensity at baseline both greatly increases the risk for developing HS and is a prerequisite for appearance of HS at one year after FSE. Finally, our estimate of the proportion with HS must be tempered by consideration of the limited sample size.
Abnormal hippocampal growth has been reported after FSE even in hippocampi with an initially normal appearing MRI8, 9. Therefore we analyzed the growth of hippocampi appearing normal at baseline. The Rt/Lt volume ratios of our FSE subjects with normal MRIs were abnormally low, but did not change from the baseline to follow-up, whereas SFS controls had a mean Rt/Lt ratio >1 as expected39. In addition, hippocampal volumes and growth were greater in subjects with SFS compared to those with FSE. The decreased right hippocampal volume of the FSE hippocampi and the predominance of hyperintensity on the right suggest asymmetrric hippocampal vulnerability present before FSE as has been suggested previously40. Finally, we looked for predictors of slowed growth in hippocampi appearing normal at baseline. Although baseline EEGs correlate with baseline imaging abnormalities13, they did not predict hippocampal volume change over the first year. FS duration is clearly an important predictor of T2 signal abnormalities, as our SFS controls had no signal abnormalities12. However, among our FSE cases differences in duration did not seem to affect baseline or one-year hippocampal findings12. We cannot explain why HHV6/HHV7 viremia cases showed more growth. It remains to be seen whether slowed hippocampal growth alone predicts HS.
Our study has limitations. Without MRIs prior to FSE, ADCs and volumetrics must provide evidence that hippocampal abnormalities seen are acute. Although abnormal hippocampal signal following FSE evolved to radiological HS over a year, there is no pathological confirmation. Follow-up MRIs are not available on all subjects for a variety of reasons, chief among them parental concern about a sedated MRI in a child who while at risk, was perceived to be doing well at this time. This problem was expected and the study was powered based on a 70% follow-up rate at one year. In the group with abnormal hippocampal signal, multiple additional abnormalities were more likely to be associated with follow-up. Whether these abnormalities, such as developmental delay or epilepsy increased the likelihood of HS cannot be determined from our limited sample. However, children with known severe prior abnormalities were excluded from FEBSTAT6. Finally, given the limitations inherent in imaging of children requiring sedation, full MRI protocols were not always obtainable.
In conclusion, T2 hyperintensity following FSE often evolved to visual appearance of HS on MRI10, 11, 17. Even children with FSE and a normal MRI had smaller hippocampi and reversed Rt/Lt hippocampal volume ratios compared to the SFS group. This may reflect subtle abnormalities in hippocampal development that predispose to FSE. Furthermore, compared to SFS controls, visually normal hippocampi of FSE subjects grew more slowly, suggesting that they sustained an insult. As the latency of TLE following FSE is usually prolonged26, 27, longer follow-up, now in progress, is needed to determine the final clinical outcomes in this cohort.
Supplementary Material
ACKNOWLEDGEMENTS
Funding/Support: Supported by NINDS grant NS43209 (P.I. S. Shinnar, M.D.,Ph.D) and NICHD grant 36867 (PI: D.C. Hesdorffer PhD).
Footnotes
Additional Contributions: We acknowledge the helpful contributions of other members of the FEBSTAT Team: Jennifer Ayala BA, Mootoo Chunasamy RT, Sharon Katz REEG-T, Ronda L. Facchini PhD, James Hannigan RT, Ann Mancini MA, Maryana Sigalova MA, Erica Weiss MA (Montefiore Medical Center and Jacobi Medical Center, Albert Einstein College of Medicine, Bronx, NY); Veronica J. Hinton PhD, (Columbia University School of Medicine, New York, NY); Melanie Bonner PhD, Karen M. Cornett BS MT, William Gallentine DO, Elizabeth Rende RN MSN CPNP, James Voyvodic PhD (Duke University Medical Center, Durham, NC); Joanne Andy RT, Terrie Conklin RN, Susan Grasso MD, Diane James R-EEG T, Susan Landers RT, Virginia Van de Water PhD (Eastern Virginia Medical School, Children's Hospital of the King's Daughters, Norfolk, VA); John Curran MD, Aaliyah Hamidullah MS, Andrew Kim MD, Julie Renaldi PhD, Diana Umanzor, (Lurie Children's Hospital, Northwestern University Feinberg School of Medicine, Chicago, IL); Tanya Bazemore REEG-T, James Culbert PhD, Kathryn O'Hara* RN, Syndi Seinfeld DO, Jean Snow RT-R (Virginia Commonwealth University, Richmond, VA); Brian J Bush MS MIT, Sreedevi Chandrasekaran, Lori L Davis, Christiane Rogers, Cynthia Shier Sabo MS (International Epilepsy Consortium at Virginia Commonwealth University, Richmond VA); Joan Conry MD (Childrens National Medical Center, Washington DC); Tracy Glauser MD (Cincinnati Children's Hospital, Cincinnati OH); Jeffrey L Noebels MD PhD (Baylor College of Medicine, Houston, TX).
REFERENCES
- 1.Febrile Seizures. National Institute of Health; Bethesda, MD: 1980. Febrile seizures: Consensus development conference summary. [Google Scholar]
- 2.Shinnar S. Febrile Seizures and Mesial Temporal Sclerosis. Epilepsy Curr. 2003;3:115–8. doi: 10.1046/j.1535-7597.2003.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hesdorffer DC, Benn EK, Bagiella E, et al. Distribution of febrile seizure duration and associations with development. AnnNeurol. 2011;70:93–100. doi: 10.1002/ana.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shinnar S, Pellock JM, Moshe SL, et al. In whom does status epilepticus occur: age-related differences in children. Epilepsia. 1997;38:907–14. doi: 10.1111/j.1528-1157.1997.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 5.Mathern GW, Adelson PD, Cahan LD, Leite JP. Hippocampal neuron damage in human epilepsy: Meyer's hypothesis revisited. ProgBrain Res. 2002;135:237–51. doi: 10.1016/s0079-6123(02)35023-4. [DOI] [PubMed] [Google Scholar]
- 6.Hesdorffer DC, Shinnar S, Lewis DV, et al. Design and Phenomenology of the FEBSTAT Study. Epilepsia. 2012;53:1471–80. doi: 10.1111/j.1528-1167.2012.03567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott RC, Gadian DG, King MD, et al. Magnetic resonance imaging findings within 5 days of status epilepticus in childhood. Brain. 2002;125:1951–9. doi: 10.1093/brain/awf202. [DOI] [PubMed] [Google Scholar]
- 8.Farrow TD, Dickson JM, Grunewald RA. A six-year follow-up MRI study of complicated early childhood convulsion. PediatrNeurol. 2006;35:257–60. doi: 10.1016/j.pediatrneurol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Scott RC, King MD, Gadian DG, et al. Hippocampal abnormalities after prolonged febrile convulsion: a longitudinal MRI study. Brain. 2003;126:2551–7. doi: 10.1093/brain/awg262. [DOI] [PubMed] [Google Scholar]
- 10.Provenzale JM, Barboriak DP, VanLandingham K, et al. Hippocampal MRI signal hyperintensity after febrile status epilepticus is predictive of subsequent mesial temporal sclerosis. AJR Am J Roentgenol. 2008;190:976–83. doi: 10.2214/AJR.07.2407. [DOI] [PubMed] [Google Scholar]
- 11.Vanlandingham KE, Heinz ER, Cavazos JE, Lewis DV. MRI evidence of hippocampal injury after prolonged, focal febrile convulsions. AnnNeurol. 1998;43:413–26. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- 12.Shinnar S, Bello JA, Chan S, et al. MRI abnormalities following febrile status epilepticus in children: The FEBSTAT Study. Neurology. 2012;79:871–7. doi: 10.1212/WNL.0b013e318266fcc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordli DR, Moshe SL, Shinnar S, et al. Acute EEG Findings in Children with Febrile Status Epilepticus: Results of the FEBSTAT Study. Neurology. 2012;79:1–7. doi: 10.1212/WNL.0b013e3182759766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein LG, Shinnar S, Hesdorffer DC, et al. Human herpesvirus 6 and 7 in febrile status epilepticus: The FEBSTAT Study. Epilepsia. 2012;53:1481–8. doi: 10.1111/j.1528-1167.2012.03542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maytal J, Shinnar S. Febrile status epilepticus. Peds. 1990;86:611–6. [PubMed] [Google Scholar]
- 16.Hesdorffer DC, Chan S, Tian H, et al. Are MRI-detected brain abnormalities associated with febrile seizure type? Epilepsia. 2008;49:765–71. doi: 10.1111/j.1528-1167.2007.01459.x. [DOI] [PubMed] [Google Scholar]
- 17.Jackson GD, Berkovic SF, Tress BM, et al. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurol. 1990;40:1869–75. doi: 10.1212/wnl.40.12.1869. [DOI] [PubMed] [Google Scholar]
- 18.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–28. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–50. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 20.Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 21.Duvernoy HM. The Human Hippocampus. 1st ed. Springer-Verlag; Berlin: 1998. [Google Scholar]
- 22.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
- 23.Sommer W. Erkrankung des Ammonshornes als aetiologisches moment der epilepsie. Archiv Psychiatrie Nervenkrankh. 1880;10:631–75. [Google Scholar]
- 24.Mathern GW, Babb TL, Armstrong DL. Hippocampal sclerosis. In: Engel JJ, Pedley TA, editors. Epilepsy: A comprehensive textbook. 1 ed. Lippincott - Raven; Philadelphia: 1998. pp. 133–56. [Google Scholar]
- 25.Hermoye L, Saint-Martin C, Cosnard G, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 26.French JA, Williamson PD, Thadani VM, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. AnnNeurol. 1993;34:774–80. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 27.Mathern GW, Pretorius J, Babb TL. Influence of the type of initial precipitating injury and at what age it occurs on the course and outcome in patients with temporal lobe seizures. Journal of Neurosurgery. 1995;82:220–7. doi: 10.3171/jns.1995.82.2.0220. [DOI] [PubMed] [Google Scholar]
- 28.Farina L, Bergqvist C, Zimmerman RA, et al. Acute diffusion abnormalities in the hippocampus of children with new-onset seizures: the development of mesial temporal sclerosis. Neuroradiology. 2004;46:251–7. doi: 10.1007/s00234-003-1122-x. [DOI] [PubMed] [Google Scholar]
- 29.Sokol DK, Demyer WE, Edwards-Brown M, et al. From swelling to sclerosis: acute change in mesial hippocampus after prolonged febrile seizure. Seizure. 2003;12:237–40. doi: 10.1016/s1059-1311(02)00195-4. [DOI] [PubMed] [Google Scholar]
- 30.Tanabe T, Hara K, Shimakawa S, et al. Hippocampal damage after prolonged febrile seizure: one case in a consecutive prospective series. Epilepsia. 2011;52:837–40. doi: 10.1111/j.1528-1167.2010.02958.x. [DOI] [PubMed] [Google Scholar]
- 31.Meldrum B. First Alfred Meyer Memorial Lecture: Epileptic brain damage: A consequence and a cause of seizures. Neuropathology and Applied Neurobiology. 1997;23:185–202. [PubMed] [Google Scholar]
- 32.Scott RC, King MD, Gadian DG, et al. Prolonged febrile seizures are associated with hippocampal vasogenic edema and developmental changes. Epilepsia. 2006;47:1493–8. doi: 10.1111/j.1528-1167.2006.00621.x. [DOI] [PubMed] [Google Scholar]
- 33.Londono A, Castillo M, Lee YZ, Smith JK. Apparent diffusion coefficient measurements in the hippocampi in patients with temporal lobe seizures. AJNR AmJ Neuroradiol. 2003;24:1582–6. [PMC free article] [PubMed] [Google Scholar]
- 34.Davies KG, Hermann BP, Dohan FC, Jr., et al. Relationship of hippocampal sclerosis to duration and age of onset of epilepsy and childhood febrile seizures in temporal lobectomy patients. Epilepsy Res. 1996;24:119–26. doi: 10.1016/0920-1211(96)00008-3. [DOI] [PubMed] [Google Scholar]
- 35.Bower SP, Kilpatrick CJ, Vogrin SJ, et al. Degree of hippocampal atrophy is not related to a history of febrile seizures in patients with proved hippocampal sclerosis. J NeurolNeurosurgPsychiatry. 2000;69:733–8. doi: 10.1136/jnnp.69.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomes WA, Shinnar S. Prospects for imaging-related biomarkers of human epileptogenesis: a critical review. BiomarkMed. 2011;5:599–606. doi: 10.2217/bmm.11.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galanopoulou AS, Moshe SL. In search of epilepsy biomarkers in the immature brain: goals, challenges and strategies. BiomarkMed. 2011;5:615–28. doi: 10.2217/bmm.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClelland S, Dube CM, Yang J, Baram TZ. Epileptogenesis after prolonged febrile seizures: Mechanisms, biomarkers and therapeutic opportunities. NeurosciLett. 2011;497:155–62. doi: 10.1016/j.neulet.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Utsunomiya H, Takano K, Okazaki M, Mitsudome A. Development of the temporal lobe in infants and children: Analysis by MR based volumetry. American Journal of Neuroradiology. 1999;20:717–23. [PMC free article] [PubMed] [Google Scholar]
- 40.Grunewald RA, Farrow T, Vaughan P, et al. A magnetic resonance study of complicated early childhood convulsion. J NeurolNeurosurgPsychiatry. 2001;71:638–42. doi: 10.1136/jnnp.71.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.