Abstract
Objective
The optimal management of mitral regurgitation (MR) in patients with cardiomyopathy is controversial. Mini-MVR may limit post-operative morbidity and mortality by minimizing recurrent MR. We hypothesized that minimally-invasive fibrillating mitral valve replacement (mini-MVR) with complete chordal sparing would offer a low mortality and halt left ventricular (LV) remodeling in patients with severe cardiomyopathy and severe MR.
Methods and Results
Between 1/06 - 8/09, 65 patients with LVEF ≤ 35% underwent mini-MVR. Demographic, echocardiographic, and clinical outcomes were analyzed.
Results
Operative mortality compared to Society for Thoracic Surgery (STS)-predicted mortality was 6.2 versus 6.6%; 5.6 versus 7.4% among patients with LVEF ≤ 20%; and 8.3 versus 17.9% among patients with STS-predicted mortality of ≥ 10%. At median follow-up of 17 months there was no recurrent MR or change in LV dimensions or LVEF, but there was a decrease (p = 0.02) in right ventricular systolic pressure (RVSP). At the first post-operative visit and longest follow-up, NYHA class decreased from 3.0 ± 0.6 to 1.7 ± 0.7 and 2.0 ± 1.0, respectively (both p < 0.0001). Patients with LVEF ≤ 20% and LVEDD ≥ 6.5cm were more likely to meet a composite of death, transplant, or LV assist device insertion (p = 0.046).
Conclusions
Mini-MVR is safe in advanced cardiomyopathy, and resulted in no recurrent MR, stabilization of LVEF and LV dimensions, and a decrease in RVSP. This mini-MVR fibrillating technique can be used to address severe MR in patients with advanced cardiomyopathy.
Keywords: Mitral valve, surgery, cardiomyopathy, echocardiography
Introduction
The optimal management of patients with advanced heart failure and severe mitral regurgitation (MR) remains controversial. Up to 50% of patients with chronic heart failure have significant MR and worsening severity confers a proportional decrease in survival(1). Decreasing the severity of MR has been shown to decrease congestive heart failure (CHF) symptoms and improve quality of life(2). Although the benefit of preserving the sub-valvular apparatus during mitral valve surgery has been established, the relative superiority among mitral valve annuloplasty, repair, and replacement remains an open question.
In early studies, mitral valve replacement (MVR) was associated with higher surgical risk compared to mitral valve repair (MV repair); however, surgical outcomes may be similar in high-risk populations with advanced heart failure (3). In patients with functional MR from cardiomyopathy the recurrence of moderate or greater MR approaches 30% within one year after MV repair, (4, 5) which may explain in part the lack of survival benefit in patients with advanced cardiomyopathy undergoing MV repair and annuloplasty(6).
We have adopted a technique for MVR with anterior/posterior chordal sparing through a 5-cm right antero-lateral thoracotomy without aortic cross-clamp (Mini-MVR). Previous work by our group has shown that this technique is associated with low surgical mortality in patients with severe MR and a wide range of left ventricular (LV) function(7). In patients with LV dysfunction and severe MR, combining a minimally-invasive approach with preservation of the sub-valvular apparatus during mitral valve replacement may minimize adverse LV remodeling and maximize post-operative functional status. We hypothesized that this technique is durable and offers favorable perioperative morbidity and mortality compared to Society for Thoracic Surgery-predicted rates in patients with left ventricular ejection fraction (LVEF) ≤ 35% with advanced heart failure.
METHODS
Study Design
This study was approved by the Vanderbilt University Institutional Review Board (IRB# 101741). Between 1/06-8/09, 65 patients with LVEF ≤ 35% underwent anterior/posterior chordal sparing mini-MVR under cold fibrillatory arrest and without aortic cross-clamp. All patients had symptomatic heart failure with at least moderate (more than 2+) mitral regurgitation. Operative mortality was defined as death within 30 days of surgical procedure. In-hospital mortality was defined as death during the same admission as the surgical procedure. All causes of mitral regurgitation were included. Patients with endocarditis, congenital diseases, or concomitant mitral stenosis were excluded from the analysis.
Selection Rationale
The procedure had become our standard approach for mitral valve surgery in patients with or without cardiomyopathy in the absence of concomitant aortic valve disease or coronary artery disease (CAD) involving the left main or left anterior descending arteries. This approach is also contraindicated if aortic insufficiency is greater than moderate. For patients with concomitant CAD and mitral disease, if a lesion in the left circumflex artery or right coronary artery is amenable to percutaneous coronary intervention, we precede to stent the coronary artery lesion and then perform a minimally-invasive valve surgery. For CAD involving the left main or the left anterior descending artery this approach is contraindicated.
Technique
The technique has been previously described(7). In brief, following general anesthesia and endotracheal intubation, a pacing Swan-Ganz pulmonary artery catheter, a transesophageal echocardiogram (TEE) probe and external defibrillator (ZOLL Medical Corporation, Chelmsford,MA) are placed. A 5 cm right antero-lateral thoracotomy is performed through the fourth intercostal space. If the descending aorta is free of atheroma greater than grade III, the femoral artery is cannulated using a 16 or 18 French straight cannula (Edwards Life sciences, Irvine, CA, USA). Otherwise axillary cannulation is preferred.
The femoral vein is cannulated with a 28 French venous return cannula (Cardiovations, Inc., CA, USA), and patients are placed on cardiopulmonary bypass (CPB) with vacuum-assisted drainage. Fibrillatory arrest is induced by cooling the patients to 28°C. The left atrium is immediately opened in the atrioventricular groove. Carbon dioxide is continuously insufflated into the chest throughout the procedure to displace intracardiac air and a left atrial sump sucker is used to maintain a clear operative field. The anterior and posterior leaflet chordae were preserved for all patients included in this study. The anterior leaflet is divided in the middle and the two created edges are re-attached to the annulus, thus reattaching all the chordal support of the anterior leaflet. Toward the end of the procedure, the patient is rewarmed and the left atrial appendage is oversewn. Careful examination for air using TEE in deep trendelenburg is performed, while strict air evacuation using CO2 and deairing using a LV vent through the valve is used before complete rewarming and defibrillation. Therefore, if the patient spontaneously cardioverts the mitral valve is kept incompetent to prevent air ejection. The mitral valve is kept incompetent and cardioversion is accomplished with the Zoll pads before completing the left atrial closure.
Echocardiographic Analysis
Echocardiograms were performed at Vanderbilt University on either an iE33 (Philips, Amsterdam, Netherlands) or Acuson (Siemens, Mountain View, California) cart as part of routine clinical care. Echocardiographic data was reviewed by two experienced physicians (L.A.M. and K.B.C.). Quantitative analysis was performed on echocardiograms of adequate quality according to guidelines published by the American Society of Echocardiography (ASE)(8). LV volumes were measured using the method-of-discs from orthogonal apical views. Severity of mitral valve regurgitation was graded using the proximal isovelocity area method, vena contracta, and pulmonary vein Doppler characteristics. Right ventricular systolic pressure was estimated using the simplified Bernoulli equation according to ASE guidelines.
Statistical Analysis
Continuous data are expressed as mean ± standard deviation. Unpaired, two-tailed Student’s t-test and Mann Whitney U-test were used to measure differences in continuous variables between groups according to specifications. Categorical variables were compared between groups using the chi-square test. Event free survival was defined as freedom from a combined end point of death, heart transplantation or transplant listing, or left ventricular assist device (LVAD) insertion. Survival curves were constructed using the Kaplan-Meier method and survival differences compared using the log-rank test. Logistic regression was used to estimate the impact of various parameters. The Society of Thoracic Surgery (STS) estimated rates of mortality and morbidity were calculated using the online STS calculator.(9) Based on previous work from our group, we pre-specified subgroup analysis in high-risk group with either LVEF ≤ 20% or STS-predicted operative mortality > 10%.(10) A p value of < 0.05 was considered statistically significant. Observed and predicted mortality rates were compared using the Wilcoxon signed-rank test. Statistical analyses were performed using Prism 5.0 software (Graph Pad Software Inc, La Jolla, CA) and SPSS 20 software (SPSS Inc, Chicago, IL).
RESULTS
Patients
Sixty-five patients (74% male, 26% female) with a mean age of 65 ± 10 years underwent mini-MVR. Six patients (9%) underwent concurrent tricuspid valve repair or replacement and 10 (15%) underwent percutaneous coronary intervention ≤ 6 hours of mini-MVR (hybrid revascularization). Clinical characteristics, including comorbidities, are shown in Table 1. The acuity of the study cohort was high as evidenced by a mean pre-operative New York Heart Association (NYHA) class of 3.0 ± 0.6 and a high prevalence of diabetes mellitus, chronic renal disease, cerebrovascular disease, and coronary artery disease. In addition, 37% of operations were performed under urgent or emergent circumstances (n = 1 emergent, n = 23 urgent). Urgent operations were defined as a patient presenting with heart failure requiring hospitalization and undergoing MVR on the same admission.
Table 1.
Pre-operative Demographics and Comorbidities of Entire Cohort
| Variables | Patients (n=65) |
|---|---|
| Age (years) | 64.9 ± 10.4 |
| Female | 17 (26%) |
| Medications (%) | |
| Beta-blocker | 80 |
| ACEI/ARB | 74 |
| MRA | 45 |
| Diuretic | 86 |
| Digoxin | 49 |
| NYHA Class | 3.0 ± 0.6 |
| II | 12 (19%) |
| III | 38 (58%) |
| IV | 15 (23%) |
| Rhythm (%) | |
| Sinus Rhythm | 49 |
| Atrial Fibrillation | 22 |
| Paced | 26 |
| Other | 3 |
| Biventricular Pacing (pre-operative, %) | 12 |
| QRS Duration (ms), excluding paced patients | 115±25 |
| Brain Natriuretic Peptide Level (pg/mL), n = 24 | 935±925 |
| Creatinine ≥ 1.5 mg/dL | 13 (20%) |
| Hemodialysis | 1 (1.5%) |
| Chronic Obstructive Pulmonary Disease | 19 (29%) |
| Diabetes | 28 (43%) |
| Hypertension | 48 (74%) |
| Dyslipidemia | 19 (29%) |
| Cardiogenic shock | 1 (1.5%) |
| Previous cerebrovascular accident | 13 (20%) |
| Urgent surgery | 23 (35%) |
| Emergent surgery | 1 (2%) |
| Concomitant coronary artery disease | 46 (71%) |
| Unstable angina | 6 (9.0%) |
| Congestive heart failure | 53 (82%) |
| Inotropic support | 4 (6.0%) |
| Low cardiac output syndrome | 1 (1.5%) |
| Pre-operative right ventricular dysfunction | 16 (25%) |
| Preoperative intra-aortic balloon pump | 1 (1.5%) |
| Previous cardiac operation | |
| Prior isolated coronary artery bypass grafting |
32 (49%) |
| Prior mitral valve surgery | 3 (4.5%) |
| Other | 3 (4.5%) |
| Previous percutaneous coronary intervention (≤ 6 hours) |
10 (15%) |
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; NYHA = New York Heart Association
Operative characteristics and post-operative complications are shown in Table 2. The majority of patients (81%) had secondary MR due to ischemic cardiomyopathy or dilated cardiomyopathy. The nine (14%) patients with myxomatous MV disease underwent MVR because the mitral valve anatomy was not amenable to MV repair. All patients had an attempt to repair first, and were felt to need a replacement after failure to reconstruct valve competency. There was no perivalvular leak in any patient immediately post-operatively by transesophageal echocardiography. Two patients (3%) experienced post-operative renal failure and one patient (1.5%) had a cerebrovascular accident. In-hospital mortality was 2.0% and operative (30 day) mortality was 6%.
Table 2.
Operative Characteristics and Post-operative Complications
| Variable | Patients |
|---|---|
| Mitral regurgitation etiology | |
| Ischemic | 43 (66%) |
| Functional | 10 (15%) |
| Myxomatous | 9 (14%) |
| Other | 3 (5%) |
|
| |
| Type of Prosthesis | |
| Bioprosthesis | 62 (95%) |
| Mechanical | 3 (5%) |
|
| |
| Fibrillatory arrest time (min.) | 86.9 ± 24.1 |
| Cardiopulmonary bypass time (min.) | 120.4 ± 33.0 |
| Operating room time (min.) | 274.2 ± 54.6 |
| Time of Intubation (hours) | 17.3 ± 19.6 |
| Length of intensive care unit stay (days) | 4.3 ± 3.7 |
| Length of stay (days) | 8.6 ± 4.1 |
| Total transfusions (units) | 2.0 ± 3.0 |
| Post-operative low cardiac output syndrome | 5 (8%) |
| New myocardial infarction | 1 (2%) |
| Reoperation for bleeding | 2 (3%) |
| Post-op cerebrovascular accident | 1 (2%) |
| Post-op acute renal failure | 2 (3%) |
| Renal failure requiring hemodialysis | 0 (0%) |
| Post-op transient ischemic attack | 1 (2%) |
| Tracheostomy | 1 (2%) |
| New atrial fibrillation | 8 (12%) |
| Wound infection | 0 (0%) |
| Permanent pacemaker | 7 (11%) |
| In-hospital mortality | 1 (2%) |
NYHA class at the first post-operative visit (median 1.5 months, [IQR 1.1, 1.8]) decreased from 3.0 ± 0.6 to 1.7 ± 0.7 (p < 0.0001). At the longest available follow-up (median 40.0 months, [IQR 19.3, 50.3]), NYHA class decreased from 3.0 ± 0.7 to 2.0 ± 1.0 (p < 0.0001).
Echocardiographic Data
Pre-operative echocardiographic data are shown in Table 3. Mean pre-operative LVEF was 25 ± 7% and 20 patients (31%) had LVEF ≤ 20%. Post-operative echocardiographic data are available in a subset of patients (n = 36; Table 3). At mean follow-up of 19.1 ± 16.4 months, there was no difference in LVEF, LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), or LV mass index. There was a significant decrease in right ventricular systolic pressure (RVSP) from 49.9 ± 16.2 to 41.7 ± 13.7 mmHg (p = 0.02) and a trend toward a decrease in left atrial (LA) dimension (p = 0.07). Demographic, clinical, and pre-operative echocardiographic data for patients who did and did not receive a follow-up echocardiogram were compared. There were no significant differences in pre-operative NYHA class, LV size or function, or RVSP (Supplemental Table 1).
Table 3.
Echocardiographic Measurements
| Variable | Pre-operative | Post-operative | p value |
|---|---|---|---|
| Ejection Fraction, % (n = 44) | 24.8 ± 7.0 (range 8-35) |
25.3 ± 13.8 | 0.84 |
| Left Ventricular End-diastolic Dimension, cm (n = 36) |
6.4 ± 0.9 | 6.2 ± 1.1 | 0.19 |
| Left Ventricular End-systolic Dimension, cm (n = 36) |
5.4 ± 0.9 | 5.3 ± 1.4 | 0.62 |
| Left Ventricular Mass Index, g/m2 (n= 36) |
156.5 ± 43.0 | 145.5 ± 49.7 | 0.13 |
| Right Ventricular Systolic Pressure, mmHg (n =25) |
49.9 ± 16.2 | 41.7 ± 13.7 | 0.02 |
| Right Ventricular Size, cm (n = 19) | 3.5 ± 0.9 | 3.4 ± 0.7 | 0.28 |
| Left Atrial Size, cm (n =34) | 5.0 ± 0.8 | 4.8 ± 0.7 | 0.07 |
At a mean follow-up of 17 months, none of the patients (0/36) had recurrent MR graded greater than trace and there were no perivalvular leaks. The presence of pulmonary hypertension graded moderate or greater decreased from 53% to 19% (p < 0.001).
Mortality and Morbidity Analysis
After a median follow-up time of 35.2 months (IQR 25.5, 50.3), 25 patients (36%) met the composite endpoint of death (n=21), LVAD insertion, heart transplantation, or United Network for Organ Sharing listing for heart transplant. Operative mortality (< 30 days) occurred in 4 patients (6%). Four patients underwent heart transplantation or UNOS listing during the study period. Only 1 patient (1.5%) required pre-operative and 1 different patient (1.5%) required intra-operative balloon pump support.
Observed operative mortality for the entire cohort compared to STS-predicted mortality was 6.2 versus 6.6% ( p < 0.001; Figure 1). Observed versus expected operative mortality was 5.6 versus 7.4% among patients with LVEF ≤ 20% (p = 0.001) and 8.3 versus 17.9% among patients with STS-predicted mortality of ≥ 10% (p = 0.03).
Figure 1.
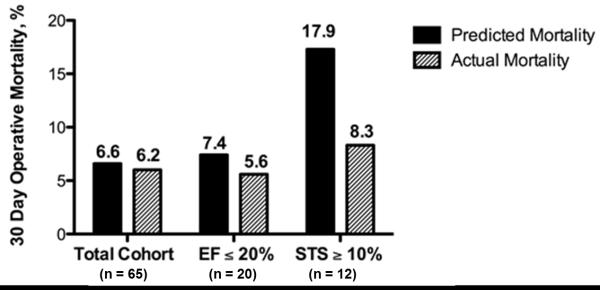
Observed versus predicted operative mortality for the entire cohort of patients, for patients with a LVEF ≤ 20%, and for patients with STS predicted mortality ≥10%. EF = ejection fraction; STS = Society of Thoracic Surgery. Using the Wilcoxon signed-rank test, observed operative survival was significantly better than STS predicted survival for the entire cohort (p < 0.001), patients with EF ≤ 20% (p = 0.001), and patients with STS predicted mortality ≥ 10% (p = 0.03).
Patients with LVEF ≤ 20% and LVEDD ≥ 6.5cm (n=7) were significantly more likely to meet the composite endpoint and thus represented a particularly high-risk group of patients for this procedure (Log-rank 0.046, Figure 2). A logistic regression analysis was used to test the impact of various factors on the composite outcome. In univariate analysis, ORs for the composite outcome were 2.0 for LVESD (p = 0.03), 5.1 for creatinine > 1.5 (p = 0.02), 7.7 for NYHA class 3 (p = 0.06) and 14.7 for NYHA class 4 (p = 0.02). Although the small sample size (n=65 patients) limited the ability to use a multivariate approach, results yielded evidence supporting the impact of these measures on the composite outcome.
Figure 2.
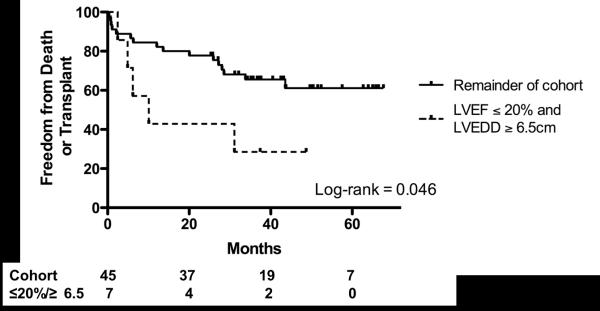
Freedom from Death or Transplant in patients with LVEF ≤ 20% and LVEDD ≥ 6.5cm.
Discussion
Major Findings
Minimally-invasive mitral valve replacement with bileaflet chordal sparing using a fibrillating technique is safe, and provides durable freedom from recurrent MR in patients with advanced cardiomyopathy. This approach further confers a sustained improvement in functional class in patients with advanced cardiomyopathy. Operative mortality in our cohort was lower than that predicted by the Society for Thoracic Surgery risk calculator, and this was especially apparent when the STS estimated risk was ≥ 10%. As expected, at mean 17 months follow-up, there were no patients with MR graded higher than trace, and no patients had perivalvular leaks. There was no progression of LV dilation or decrement in LVEF, and stabilization of LV size and function was accompanied by a significant decrease in RVSP estimated by Doppler echocardiography.
Mitral Valve Surgery in Advanced Heart Failure
The best approach to MR in patients with severe LV dysfunction is unknown. Bolling et al(11) published the first report of clinical and echocardiographic improvement after mitral valve surgery in patients with advanced cardiomyopathy. They described a cohort of 16 patients undergoing mitral valve annuloplasty (MVA) with preservation of the chordal apparatus. At 8 month follow-up, there was a decrease in NYHA functional class (3.9 ± 0.3 to 1.7 ± 0.5, p < 0.001) in surviving patients and one year survival was 75% with no operative deaths. These findings were later validated by the same group in an expanded cohort of 48 patients, although rates of recurrent MR at follow-up were not reported(12). In a large, retrospective study, Wu et al compared outcomes between patients with MR and severe LV dysfunction who underwent MVA (surgical management) and medical management in operative candidates(6). They found no survival benefit with surgical management. Recent data published by the Mayo Clinic showed that the specifics of mitral valve repair versus replacement did not seem to affect survival in this group of patients, while patients associated comorbidities seem to be the major driver of survival for this population. Another large study of patients undergoing MV surgery for ischemic MR and associated ischemic cardiomyopathy found that reoperation rates were higher after MV repair, whereas valve related complications were similar between MVR and MV repair(13), while no survival benefits was observed comparing MVR and MV repair in propensity-matched populations.
Remodeling after Mitral Surgery in Advanced Heart Failure
The effect of mitral valve surgical technique on LV remodeling is also unclear. In secondary MR from dilated cardiomyopathy, the cardiomyopathic process persists after mitral valve surgery resulting in progressive LV remodeling, further dilation of the mitral annulus, an increase in mitral regurgitation. This creates subsequent increased incidence of recurrent MR. Even with undersizing during MV repair, the posterior leaflet is tethered and post-operative MV competence after annuloplasty continues to be altered. Restricting annular dilation by MVR may therefore limit recurrent MR, perhaps promoting positive long-term remodeling in patients with very low LVEF. Although mitral valve annuloplasty does eliminate or significantly reduce MR at the time of surgery in most patients, there is a high rate of recurrent MR at medium to long-term follow up(4, 14, 15). McGee et al(5) found that although high-grade (3 or 4+) MR was uncommon immediately postoperatively, the degree of MR increased rapidly during the first 6 months and then became relatively stable. Overall, 28% of patients had 3 or 4+ MR at 6 months follow-up. Others showed an incidence of moderate or greater MR of 29% at three years follow-up after MV repair and the degree of recurrent MR correlated with decline in postoperative EF in this cohort (16). High rates of recurrent MR after MV repair and/or MVA may contribute to the failure of these procedures to provide a survival benefit. Therefore, MVR, which in our cohort is associated with no recurrent MR, may represent an attractive alternative to MV repair and/or MVA in this population. MVR provides a durable solution to the treatment of secondary MR, but initial attempts that did not preserve the chordal apparatus were associated with a postoperative decline in left ventricular systolic function(17). However, conservation of the sub-valvular apparatus during MVR is associated with improved post-operative LVEF and decreased LV volume(18).
Outcomes after Mitral Valve Surgery
Several studies have shown higher operative mortality after MVR when compared with MV repair (19-21). Our operative mortality and post-operative morbidity compare favorably with STS-predicted rates and rates from recent studies comparing outcomes after MV repair and MVR(19, 20). Other recent studies suggest that survival in high-risk populations such as ours may be similar between MVR and MV repair (13, 15, 20). We believe that the preservation of both leaflet chordae is essential in maintaining the left ventricular geometry and function. Moreover, avoiding aortic cross-clamping and administration of cardioplegia is essential, especially in patients with LVEF <20% and poor right ventricular (RV) function. Although speculative, hypothermic fibrillatory arrest, in our opinion, proves to be a superior myocardial protection method for patients with severe LV or RV dysfunction. This technique keeps the heart decompressed by opening the left atrium immediately upon fibrillation. Thus, the LV is not stressed by volume; the LV cannot be allowed to distend, which increases intracardiac pressure and reduces coronary perfusion pressure. Fibrillatory arrest has been associated with lower lactate accumulation and higher coronary flow compared to cross-clamping with cardioplegic arrest, which may confer added myocardial protection(22). On the other hand, Gammie et al recently showed in a large study of less-invasive mitral valve operations that fibrillating heart techniques are associated with a three-fold higher risk of perioperative stroke (23). However, our group recently described a cohort (n = 504) of patients undergoing mitral valve surgery with the fibrillating technique described herein, and we did not observe a significant risk of stroke. Among the highest risk patients in that study (STS predicted mortality ≥ 10%), only 1/47 (2%) experienced a perioperative stroke (10).
Post-operative functional status is perhaps the most important metric of operative success. Mini-MVR in our cohort resulted in a significant decrease in NYHA class at both short and intermediate-term follow-up. Studies examining functional class after MV repair have shown similar improvements in post-operative functional status(12, 24). To our knowledge, our study represents the longest follow-up functional class data of any cohort of patients with advanced heart failure after minimally-invasive fibrillating mitral valve replacement. Given the durability of the mini-MVR technique in eliminating MR, sustained functional class improvement may be more likely after MVR compared to MV repair.
No randomized trial data exist comparing surgical versus medical management in patients with LV dysfunction and severe mitral regurgitation. In patients undergoing MV surgery, there are no randomized data comparing surgical techniques, though data from large randomized studies are forthcoming from the Cardiothoracic Surgery Network. Our data demonstrate that a minimally-invasive fibrillating surgical approach to MVR can be performed safely and has favorable long-term outcomes with respect to functional status and LV remodeling. Our results of stabilization of LVEF and LV dimensions after chordal sparing MVR are similar to earlier studies(25) and expand on those data by showing that this can be accomplished safely in a large cohort of patients with severe LV dysfunction.
Our cohort is too small to draw conclusions about the efficacy of this technique among different etiologies of MV disease or in comparison to other techniques. However, these data warrant prospective evaluation of this technique and consideration of a randomized trial comparing this technique to conventional mitral valve repair in patients with advanced heart failure.
Limitations
Our study is limited by its single-center experience and relatively small sample size; however, this is the largest cardiomyopathy cohort reported using this surgical technique. To maximize operative success, this procedure should be performed by surgeons who have had specific training in this technique; however, once this training has been obtained, it can be performed in a wide variety of clinical settings. No multivariate analysis was feasible due to few events. The decision to operate on these patients was made by the individual surgeon based on clinical judgment. There may have been other patients deemed non-operative candidates, for whom we do not have outcome data. However our cohort includes patients with significant comorbidities. Only a subset of our cohort had post-operative echocardiogram data available. In order to address the potential for selection bias in this group, we showed that there were no differences in pre-operative functional class or echocardiographic parameters between those who did and did not undergo repeat echocardiography.
Our series does not include a control group of patients who underwent mitral valve repair because replacement through a minimally invasive fibrillating approach is the preferred treatment in patients with severe functional and ischemic MR and advanced cardiomyopathy in whom mitral valve surgery is indicated. We recognize this as a significant limitation of our study, but our data suggest that mini-MVR results in no recurrent MR and outcomes similar to other series of high risk patients undergoing mitral valve repair. The use of bioprostheses was heavily favored in our cohort given the relatively limited life expectancy of patients with advanced cardiomyopathy.
Conclusions
Our data show that a fibrillating mini-MVR with complete chordal sparing can be performed safely in patients with advanced cardiomyopathy, as it compares to published and STS-predicts rates of operative mortality. In our cohort, this procedure resulted in no recurrent MR, stabilization of LVEF and LV dimensions, and a decrease in RV systolic pressure. In addition, there was a sustained improvement of at least one functional class at 30 months follow-up.
Future studies examining the best treatment options for patients with significant MR and LV dysfunction should consider this surgical approach as a viable treatment option.
Supplementary Material
Supplementary Table 1. Demographics and Comorbidities of Cohorts with and without Repeat Echocardiogram
Acknowledgements
None
Footnotes
Disclosures: none
REFERENCES
- 1.Mehra MR, Reyes P, Benitez RM, Zimrin D, Gammie JS. Surgery for severe mitral regurgitation and left ventricular failure: what do we really know? J Card Fail. 2008 Mar;14(2):145–50. doi: 10.1016/j.cardfail.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Acker MA, Bolling S, Shemin R, et al. Mitral valve surgery in heart failure: insights from the Acorn Clinical Trial. J Thorac Cardiovasc Surg. 2006 Sep;132(3):568–77. 77 e1–4. doi: 10.1016/j.jtcvs.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 3.Di Salvo TG, Acker MA, Dec GW, Byrne JG. Mitral valve surgery in advanced heart failure. J Am Coll Cardiol. 2010 Jan 26;55(4):271–82. doi: 10.1016/j.jacc.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 4.Hung J, Papakostas L, Tahta SA, et al. Mechanism of recurrent ischemic mitral regurgitation after annuloplasty: continued LV remodeling as a moving target. Circulation. 2004 Sep 14;110(11 Suppl 1):II85–90. doi: 10.1161/01.CIR.0000138192.65015.45. [DOI] [PubMed] [Google Scholar]
- 5.McGee EC, Jr., Gillinov AM, Blackstone EH, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004;128(6):916. doi: 10.1016/j.jtcvs.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 6.Wu AH, Aaronson KD, Bolling SF, Pagani FD, Welch K, Koelling TM. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol. 2005 Feb 1;45(3):381–7. doi: 10.1016/j.jacc.2004.09.073. [DOI] [PubMed] [Google Scholar]
- 7.Umakanthan R, Petracek MR, Leacche M, et al. Minimally invasive right lateral thoracotomy without aortic cross-clamping: an attractive alternative to repeat sternotomy for reoperative mitral valve surgery. J Heart Valve Dis. 2010 Mar;19(2):236–43. [PubMed] [Google Scholar]
- 8.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003 Jul;16(7):777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 9.Online STS Risk Calculator.
- 10.Petracek MR, Leacche M, Solenkova N, et al. Minimally invasive mitral valve surgery expands the surgical options for high-risks patients. Ann Surg. 2011 Oct;254(4):606–11. doi: 10.1097/SLA.0b013e3182300399. [DOI] [PubMed] [Google Scholar]
- 11.Bolling SF, Deeb GM, Brunsting LA, Bach DS. Early outcome of mitral valve reconstruction in patients with end-stage cardiomyopathy. J Thorac Cardiovasc Surg. 1995;109(4):676. doi: 10.1016/S0022-5223(95)70348-9. [DOI] [PubMed] [Google Scholar]
- 12.Bolling SF, Pagani FD, Deeb GM, Bach DS. Intermediate-Term Outcome Of Mitral Reconstruction In Cardiomyopathy. J Thorac Cardiovasc Surg. 1998;115(2):381. doi: 10.1016/S0022-5223(98)70282-X. [DOI] [PubMed] [Google Scholar]
- 13.Maltais S, Schaff HV, Daly RC, et al. Mitral regurgitation surgery in patients with ischemic cardiomyopathy and ischemic mitral regurgitation: factors that influence survival. J Thorac Cardiovasc Surg. 2011 Nov;142(5):995–1001. doi: 10.1016/j.jtcvs.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 14.Crabtree TD, Bailey MS, Moon MR, et al. Recurrent Mitral Regurgitation and Risk Factors for Early and Late Mortality After Mitral Valve Repair for Functional Ischemic Mitral Regurgitation. Ann Thorac Surg. 2008;85(5):1537–43. doi: 10.1016/j.athoracsur.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 15.Chan V, Ruel M, Mesana TG. Mitral valve replacement is a viable alternative to mitral valve repair for ischemic mitral regurgitation: a case-matched study. Ann Thorac Surg. 2011 Oct;92(4):1358–65. doi: 10.1016/j.athoracsur.2011.05.056. discussion 65-6. [DOI] [PubMed] [Google Scholar]
- 16.Tahta SA, Oury JH, Maxwell JM, Hiro SP, Duran CM. Outcome after mitral valve repair for functional ischemic mitral regurgitation. J Heart Valve Dis. 2002 Jan;11(1):11–8. discussion 8-9. [PubMed] [Google Scholar]
- 17.Schuler G, Peterson KL, Johnson A, et al. Temporal response of left ventricular performance to mitral valve surgery. Circulation. 1979;59(6):1218–31. doi: 10.1161/01.cir.59.6.1218. [DOI] [PubMed] [Google Scholar]
- 18.Hennein HA, Swain J, McIntosh CL, Bonow RO, Srone CD, Clark RE. Comparative assessment of chordal preservation versus chordal resection during mitral valve replacement. Journal of Thoracic and Cardiovascular Surgery. 1990;99(5):828–37. [PubMed] [Google Scholar]
- 19.Magne J, Girerd N, Sénéchal M, et al. Mitral repair versus replacement for ischemic mitral regurgitation comparison of short-term and long-term survival. Circulation. 2009;120(SUPPL. 1):S104–S11. doi: 10.1161/CIRCULATIONAHA.108.843995. [DOI] [PubMed] [Google Scholar]
- 20.Micovic S, Milacic P, Otasevic P, et al. Comparison of valve annuloplasty and replacement for ischemic mitral valve incompetence. The heart surgery forum. 2008;11(6):E340–5. doi: 10.1532/HSF98.20081087. [DOI] [PubMed] [Google Scholar]
- 21.Al-Radi OO, Austin PC, Tu JV, David TE, Yau TM, Bolling S. Mitral repair versus replacement for ischemic mitral regurgitation. Annals of Thoracic Surgery. 2005;79(4):1260–7. doi: 10.1016/j.athoracsur.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 22.Grotte GJLF, Kay HR, Fallon JT, Austen WG, Buckley MJ. Effect of ventricular fibrillation and potassium induced arrest on myocardial recovery in hypothermic hearts. Surgical Forum. 1980;31:296–8. [Google Scholar]
- 23.Gammie JS, Zhao Y, Peterson ED, O’Brien SM, Rankin JS, Griffith BP. J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery. Less-invasive mitral valve operations: trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg. 2010 Nov;90(5):1401–8. 10 e1. doi: 10.1016/j.athoracsur.2010.05.055. discussion 8-10. [DOI] [PubMed] [Google Scholar]
- 24.De Bonis M, Lapenna E, La Canna G, et al. Mitral valve repair for functional mitral regurgitation in end-stage dilated cardiomyopathy: role of the “edge-to-edge” technique. Circulation. 2005 Aug 30;112(9 Suppl):I402–8. doi: 10.1161/CIRCULATIONAHA.104.525188. [DOI] [PubMed] [Google Scholar]
- 25.Yun KL, Sintek CF, Miller DC, et al. Randomized trial comparing partial versus complete chordal-sparing mitral valve replacement: effects on left ventricular volume and function. J Thorac Cardiovasc Surg. 2002 Apr;123(4):707–14. doi: 10.1067/mtc.2002.121048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Demographics and Comorbidities of Cohorts with and without Repeat Echocardiogram


