In this pilot clinical trial, disulfiram was administered to HIV-infected patients on antiretroviral therapy to determine the drug's effect on the latent reservoir. Reservoir size did not change; however, some participants experienced transient increases in viremia. Disulfiram deserves further study as an antilatency agent.
Keywords: HIV-1 latent reservoir, disulfiram, latency-reversing agents
Abstract
Background. Transcriptionally silent human immunodeficiency virus type 1 (HIV-1) DNA persists in resting memory CD4+ T cells despite antiretroviral therapy. In a primary cell model, the antialcoholism drug disulfiram has been shown to induce HIV-1 transcription in latently infected resting memory CD4+ T cells at concentrations achieved in vivo.
Methods. We conducted a single-arm pilot study to evaluate whether 500 mg of disulfiram administered daily for 14 days to HIV-1–infected individuals on stable suppressive antiretroviral therapy would result in reversal of HIV-1 latency with a concomitant transient increase in residual viremia or depletion of the latent reservoir in resting memory CD4+ T cells.
Results. Disulfiram was safe and well tolerated. There was a high level of subject-to-subject variability in plasma disulfiram levels. The latent reservoir did not change significantly (1.16-fold change; 95% confidence interval [CI], .70- to 1.92-fold; P = .56). During disulfiram administration, residual viremia did not change significantly compared to baseline (1.53-fold; 95% CI, .88- to 2.69-fold; P = .13), although residual viremia was estimated to increase by 1.88-fold compared to baseline during the postdosing period (95% CI, 1.03- to 3.43-fold; P = .04). In a post hoc analysis, a rapid and transient increase in viremia was noted in a subset of individuals (n = 6) with immediate postdose sampling (HIV-1 RNA increase, 2.96-fold; 95% CI, 1.29- to 6.81-fold; P = .01).
Conclusions. Administration of disulfiram to patients on antiretroviral therapy does not reduce the size of the latent reservoir. A possible dose-related effect on residual viremia supports future studies assessing the impact of higher doses on HIV-1 production. Disulfiram affects relevant signaling pathways and can be safely administered, supporting future studies of this drug.
(See the Editorial Commentary by Tolstrup on pages 891–2.)
Combination antiretroviral therapy (ART) has dramatically altered the natural history of human immunodeficiency virus type 1 (HIV-1) infection for most infected individuals with access to treatment [1]. ART reduces plasma HIV-1 RNA to below the limit of clinical detection. It was initially hoped that the virus could be eradicated with 2–3 years of effective ART treatment [2]; however, a latent form of HIV-1 exists in vivo. Stably integrated, transcriptionally silent viral genomes persist in long-lived resting memory CD4+ T cells [3–7]. The stability of this latent reservoir is the major barrier to eradication of HIV-1 [4, 7, 8], requiring patients to remain on ART indefinitely. Given the concern for adverse effects of ART, as well as the financial burden of treatment and need for adherence, strategies to eliminate the latent reservoir have become an urgent research priority.
One eradication strategy that has attracted significant attention involves targeting the latent reservoir through the use of drugs that reverse latency without inducing global T-cell activation [9, 10]. This strategy is based on the hypothesis that cells in which latency has been reversed will be targeted by cytolytic CD8+ T cells or will die by viral cytopathic effects [11]. Previous attempts to target latently infected cells by inducing global T-cell activation have proven too toxic for use in humans [12–14]. Subsequent research has focused on identifying compounds that will induce HIV-1 gene expression in latently infected resting CD4+ T cells without activating the cell itself [15]. To this end, several in vitro models have been described that appear to recapitulate the phenotype of HIV-1 latency in resting CD4+ T cells [16–18]. We have described one such model that makes use of Bcl-2–transduced primary CD4+ T cells [16] and performed a high-throughput screen to identify compounds that induce viral gene expression without triggering cellular activation [19]. One hit from this screen was disulfiram, a US Food and Drug Administration (FDA)–approved drug used to treat alcoholism [20]. Disulfiram (bis [diethylthiocarbamoyl] disulphide) inhibits aldehyde dehydrogenase, resulting in an increased concentration of acetaldehyde when alcohol is consumed [21]. This leads to an unpleasant systemic reaction that serves as a deterrent to alcohol consumption [22, 23]. Disulfiram has been in clinical use for several decades [24] and has a well-characterized safety profile [25, 26].
The molecular mechanism of in vitro disulfiram-induced HIV-1 latency reactivation is unclear. Disulfiram undergoes a complex metabolism [26] with the downstream metabolite N,N-diethylthiolcarbamate sulfoxide (DETC-MeSO) primarily responsible for aldehyde dehydrogenase inhibition and resultant clinical effect [27]. In contrast, reactivation of latent HIV-1 in vitro occurs only with the parent compound and first metabolite, diethyldithiocarbamic acid (DDTC) [19]. Subsequent metabolites, including diethyldithiocarbamate methyl ester (DDTC-Me), induce no appreciable HIV-1 reactivation [19]. A recent report found that intracellular depletion of the phosphate and tensin homolog (PTEN) protein by disulfiram led to upregulation of the Akt signaling pathway, resulting in HIV-1 gene transcription in the U1 HIV-1–infected monocyte cell line [28]. This potential molecular mechanism of disulfiram activity is under further investigation.
We describe a pilot trial in which we administered 500 mg of disulfiram daily for 14 days to HIV-1–infected patients who had suppression of viremia on ART to determine whether this compound could reactivate latent HIV-1 from resting memory CD4+ T cells. This FDA-approved dose was selected to achieve in vivo concentrations of disulfiram and its metabolites comparable to concentrations that resulted in latency reversal activity in vitro [19]. The safety of higher doses is unknown. We hypothesized that disulfiram treatment would be safe and would result in a transient increase in residual viremia due to virus release from latently infected resting CD4+ T cells and a measureable decline in the size of the latent reservoir.
METHODS
Participants
We conducted an open-label, single-arm, pilot clinical trial at Johns Hopkins Hospital (JHH) and the University of California, San Francisco (UCSF). Inclusion criteria included age >18 years, use of a Department of Health and Human Services–recommended ART regimen continuously for a minimum of 18 months, >90% adherence as determined by self-report, maintenance of undetectable plasma viral load using standard commercial assays (<50 copies RNA/mL) for the previous 12 months, and a CD4+ T-cell count >200 cells/µL for 24 weeks prior to enrollment. Participants had to agree to abstain from alcohol during the 2-week period of disulfiram administration and the subsequent 2 weeks.
Exclusion criteria included the presence of an alcohol use disorder; use of any drug formulation containing alcohol or medications involved in clinically important drug interactions with disulfiram; serious illness requiring hospitalization in the 3 months prior to enrollment; severe myocardial or coronary artery disease; history of psychosis, peripheral neuropathy, seizure disorder or hypothyroidism; evidence of clinically active hepatitis with aspartate aminotransferase (AST) or alanine transaminase (ALT) serum concentrations >3 times the upper limit of normal; treatment with immunomodulatory drugs in the previous 16 weeks; pregnancy or breastfeeding; and allergy to rubber or thiuram derivatives. The protocol was approved by the institutional review boards of both institutions participating in the trial.
Study Design
Potential participants underwent an initial screening visit followed by 2–3 pretreatment visits after enrollment, during which blood was obtained for baseline measurement of residual viremia using a highly sensitive quantitative real-time reverse transcriptase polymerase chain reaction assay (the single-copy assay [SCA]) as previously described [29]. Safety laboratory tests including chemistry and liver function profiles and complete blood counts were obtained weekly during disulfiram administration and at every visit before and after the disulfiram administration period. At day –14, a large peripheral blood sample (180 mL) was obtained to measure the frequency of latently infected resting memory CD4+ T cells using a previously described quantitative viral outgrowth assay [30].
Beginning on day 0, subjects received a directly observed oral dose of 500 mg of disulfiram. Disulfiram was administered daily for 14 days under direct observation on weekdays and by participant administration on weekends. Participants were evaluated at every visit for potential adverse events using a standardized questionnaire and a detailed face-to-face interview with a study investigator. All antiretroviral medications were continued throughout the trial, with medication adherence determined by self-report. Beginning at day 0, residual viremia was measured every 2 days (Monday, Wednesday, and Friday) for 3 weeks using the SCA. SCA was performed at every subsequent study visit for an additional 9 weeks. Plasma samples were also used to quantify disulfiram concentrations. A second 180-mL blood sample was obtained at week 12 for a posttreatment measurement of replication-competent HIV-1 in resting memory CD4+ T cells (Figure 1).
Figure 1.
Timing of trial events. After enrollment, participants underwent several baseline measurements of residual viremia using a highly sensitive quantitative real-time reverse transcriptase polymerase chain reaction assay (the single-copy assay). Five hundred milligrams of disulfiram was administered daily from day 0 to day 13. A 180-mL blood sample was obtained at day –14 and again on day 84 to estimate the frequency of latently infected cells in peripheral blood using a limiting dilution co-culture method. Safety laboratory tests including a complete blood count and metabolic panel were drawn weekly before, during, and 2 weeks after disulfiram administration, and monthly thereafter. Abbreviation: DSF, disulfiram.
Disulfiram Mass Spectrometry
An ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) assay was developed to measure disulfiram concentrations in plasma. The UPLC-MS system consisted of a Dionex Ultimate 3000 UPLC system coupled to a TSQ Vantage Triple Stage Quadrupole mass spectrometer (Thermo Fisher Scientific). A deuterated analogue (d20-disulfiram; deuterated at 20 positions on the molecule) was used as the internal standard. The analytes were extracted via protein precipitation and were chromatographically separated on a Phenomenex Kinetex column (phase C18; diameter, 2.1 mm × 50 mm; particle size, 1.7 µm; pore size, 100A°) using mobile phases consisting of (1) water, 0.1% formic acid and (2) acetonitrile, 0.1% formic acid delivered at a flow rate of 400 µL/minute. The resolved analytes were detected by mass spectrometry in selected reaction monitoring mode under negative electrospray ionization using the following transitions: disulfiram m/z 297.2/116.1 and d20-disulfiram m/z 317.2/126.1. The assay was linear from 15 ng/mL to 6400 ng/mL of disulfiram with an r2 of 0.996 (SD 0.001). Interday and intraday precision ranged from 1.8% to 6.3% and 1.2% to 5.8%, respectively, whereas the accuracies were 95.4% to 104%, and 93.6% to 105%, respectively.
Biostatistical Analysis
Maximum likelihood estimation of rates of latently infected resting CD4+ T cells was carried out using the NLMIXED procedure in SAS version 9.2 (SAS Institute, Cary, North Carolina), with Wald 95% confidence intervals (CIs) calculated for log IUPM (infectious units per million) and then back-transformed to the IUPM scale. For cases with no positive co-cultures, the estimated IUPM was zero and an upper 95% confidence bound was calculated as 3/(total number of cells tested) [31]. We modeled the effect of the postdisulfiram time point (vs predisulfiram) on log IUPM across all participants, using the primary data from each co-culture of whether it was positive or negative and how many cells it contained. This model was fit by maximum likelihood in the NLMIXED procedure in SAS, and included parameters for each participant's baseline log IUPM to account for the matched pre–post nature of the data within individuals. Additional models allowed the postdisulfiram effect to differ for those who ever had a detectable disulfiram level compared to those who did not. The software provided Wald P values and 95% CIs. We back-transformed estimates and CIs to fold-effects on IUPM. We performed sensitivity analyses by excluding particular wells that had probability <.001 given the estimated IUPM.
We modeled residual viremia measured by SCA using negative binomial regression, with a random intercept to account for within-person correlation, again using the SAS NL mixed procedure. To prevent very large values from dominating the analyses, we set SCA values >56 to equal 56, which was the 97th percentile of all observed values. We initially fit a model with one parameter for how viremia during disulfiram administration differed from the baseline period and one parameter for how it differed postdisulfiram compared to baseline. We then fit models that examined a number of possible refinements: allowing viremia 2 hours after the first dose (measured at JHH) to differ from viremia at other times during disulfiram administration; allowing viremia during and after administration to differ depending on whether disulfiram was ever detected in any the patient's blood specimens; and allowing viremia during administration to be influenced by the concurrently measured blood level of disulfiram. We chose the primary model for presentation as the simplest one for which all further refinements had a P value >.05.
RESULTS
Study Participants and Safety Outcomes
We enrolled 16 participants (11 at JHH, 5 at UCSF; Table 1). The median CD4+ T-cell count and percentage at the time of enrollment were 609 cells/μL (range, 224–1168 cells/μL; interquartile range [IQR], 366 cells/μL) and 30% (range, 12.6%–42.7%; IQR, 11%), respectively. The median time of viral suppression (<50 copies/mL) was 79 months (range, 16–162 months; IQR, 79 months). ART regimens for 8 participants combined 2 nucleoside reverse transcriptase inhibitors (NRTIs) with the nonnucleoside reverse transcriptase inhibitor efavirenz, and regimens for the other 6 patients combined 2 NRTIs with a ritonavir-boosted protease inhibitor. Two participants were taking regimens that included agents from >2 antiretroviral drug classes. One dropped out of the study after completing 12 days of disulfiram therapy.
Table 1.
Participant Baseline Characteristics
| Participant No. | Age | Sex | Race/Ethnicity | Duration of Viral Suppressiona | ART Regimen | Screening CD4 Count (%)b |
|---|---|---|---|---|---|---|
| 7150 | 24 | M | AA | 16 | FTC/TDF/EFV | 524 (27) |
| 7151 | 55 | M | W | 87 | FTC/TDF/DRV/r | 275 (31) |
| 7152 | 43 | M | W | 45 | FTC/TDF/EFV | 644 (39) |
| 7153 | 37 | M | AA | 138 | 3TC/ABC/EFV | 1157 (39) |
| 7154 | 46 | M | W | 150 | FTC/TDF/NVP/FPV/r | 613 (28) |
| 7155 | 52 | M | W | 101 | FTC/TDF/EFV | 886 (43) |
| 7156 | 57 | M | W | 162 | FTC/TDF/DRV/r | 1168 (35) |
| 7157 | 38 | M | AA | 53 | 3TC/ABC/EFV | 604 (28) |
| 7158 | 46 | M | W | 79 | 3TC/ABC/ATV/r | 953 (42) |
| 7160 | 44 | M | H | 24 | 3TC/ABC/ATV/r | 224 (13) |
| 7161 | 48 | F | AA | 37 | ABC/3TC/DRV/r | 871 (28) |
| 2006 | 60 | M | W | 178 | 3TC/TDF/ATV/r | 561 (29) |
| 2135 | 53 | M | W | 78 | FTC/TDF/EFV | 749 (42) |
| 2428 | 48 | M | W | 13 | FTC/TDF/EFV | 511 (21) |
| 2432 | 57 | M | W | 26 | FTC/TDF/EFV | 503 (34) |
| 3037 | 52 | M | AA | 58 | 3TC/ABC/TDF/ZDV/ETV/DRV/r | 504 (23) |
Abbreviations: 3TC, lamivudine; AA, African American; ABC, abacavir; ART, antiretroviral therapy; ATV/r, atazanavir boosted with ritonavir; DRV/r, darunavir boosted with ritonavir; EFV, efavirenz; ETV, etravirine; FPV/r, fosamprenavir boosted with ritonavir; FTC, emtricitabine; H, Hispanic; NVP, nevirapine; TDF, tenofovir; W, non-Hispanic white; ZDV, zidovudine.
a Consecutive months of documented viral load (plasma HIV-1 RNA) suppression below limit of clinical detection on ART.
b Absolute CD4+ T-cell count measured in cells/μL.
Disulfiram was safe and well tolerated in all participants. Observed adverse events during the study were consistent with grades I and II toxicity. One participant had a single detectable viral load measured by a standard commercial assay (620 copies/mL) at a postdisulfiram time point that returned to an undetectable level (<50 copies/mL) at next study visit and remained undetectable for the trial duration. All other participants maintained undetectable viral loads as measured by commercial viral load assays throughout the trial. No substantial changes in CD4+ T-cell count or percentage were observed in any participant for the duration of the trial.
Effect of Disulfiram Administration on the Frequency of Latently Infected Cells
The size of the latent reservoir from each participant was measured by limiting dilution co-culture assay [30] 2 weeks before and 10 weeks after disulfiram administration (Figure 2). There was little change in the geometric mean frequency of latently infected cells 10 weeks after disulfiram administration compared to baseline (postdisulfiram fold-effect = 1.16; 95% CI, .70–1.92; P = .56). A majority of participants had a latent reservoir size within the previously described dynamic range of this assay [8].
Figure 2.
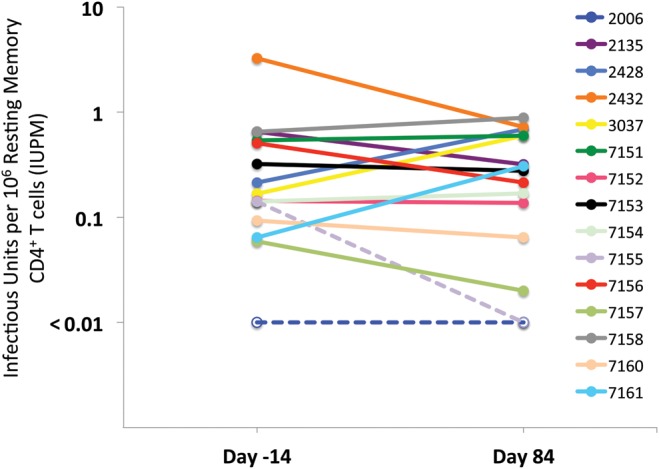
Effect of disulfiram (DSF) on latent reservoir size. No substantial change in the frequency of latently infected cells was observed 10 weeks after disulfiram administration compared to baseline as measured by limiting dilution co-culture assay (post-DSF fold-effect = 1.16; 95% confidence interval, .70–1.92; P = .56). *Dashed lines/open circles represent 3 co-culture assays in which no infected cells were identified (these co-cultures had 12.5 million to 27.5 million cells assayed). Abbreviation: IUPM, infectious units per million.
Effect of Disulfiram Administration and Disulfiram Plasma Concentration on Residual Viremia
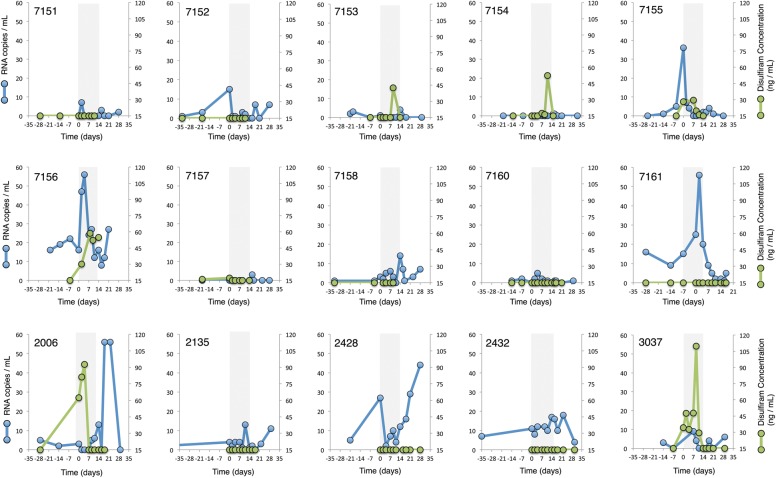
Residual viremia was measured by SCA in plasma samples obtained at enrollment, days –14, –7, 0, 2, 4, 7, 9, 11, 14, 16, and 18, and at weeks 3, 4, 8, and 12. Individual plasma virus and disulfiram concentrations through week 4 are shown in Figure 3. Our initial model estimated that residual viremia averaged 1.53-fold higher during disulfiram administration than during the predisulfiram baseline period (95% CI, .88- to 2.69-fold; P = .13) and averaged 1.93-fold higher postdisulfiram than baseline (95% CI, 1.04- to 3.57-fold; P = .039).
Figure 3.
Individual single-copy assay and disulfiram (DSF) plasma concentration results. The gray bar (between days 0 and 13) represents the period of directly observed DSF administration. Half of Johns Hopkins Hospital participants (numbers 7151–7161) had an increase in viremia within several hours of the first DSF dose, but viremia during DSF administration after that time averaged only slightly higher than baseline and was not statistically significant. Six of 15 participants had detectable DSF plasma concentrations during the dosing interval. The lower limit of detection of DSF plasma concentrations using mass spectrometry was 15 ng/mL.
Participants at JHH (n = 10) received disulfiram 2 hours prior to plasma sampling at each visit. We observed an increase in residual viremia after the first dose of disulfiram (day 0) in several participants. Residual viremia on day 0 was estimated to average 3.81-fold higher than the predisulfiram baseline (95% CI, 1.43- to 10.18-fold; P = .01). Average residual viremia for the remainder of the disulfiram dosing interval did not differ significantly from baseline (1.30-fold increase; 95% CI, .74- to 2.27-fold; P = .33). Table 2 shows our model, which takes into account these early increases in viremia.
Table 2.
Single-Copy Assay Kinetics
| SCA Time Point | Fold Changea | % Change | 95% CI | P Value |
|---|---|---|---|---|
| DSF first dose (day 0)b | 3.81 | 281.30 | 42.8–917.7 | .01 |
| DSF dosing interval (days 1–13)c | 1.30 | 29.90 | −25.7 to 127.4 | .33 |
| Post-DSF interval (days 14–84)d | 1.88 | 87.90 | 2.8–243.2 | .04 |
Abbreviations: CI, confidence interval; DSF, disulfiram; SCA, single-copy assay.
a Fold-change and percentage change compare average SCA values with the pre-DSF baseline SCA estimate of 2.2 copies/mL (95% CI, .9–5.4).
b Day 0 SCA comparison performed with Johns Hopkins Hospital (JHH) SCA values only (n = 10) because University of California, San Francisco subjects did not undergo plasma sampling immediately after day 0 DSF dose.
c JHH SCA values only (n = 10).
d Data for all subjects included (n = 15).
Subject-to-Subject Variability in Disulfiram Exposure
We measured plasma levels of disulfiram by mass spectrometry. There was substantial and unexplained variability in disulfiram concentrations that did not appear to be predicted by treatment regimen (Figure 2). Six of 15 subjects had detectable plasma disulfiram concentrations at some point during the 2-week dosing interval (subjects 7153, 7154, 7155, 7156, 2006, 3037 shown in Figure 3; lower limit of detection 15 ng/mL). There was a nonsignificant trend suggesting higher average viremia in these 6 subjects compared to those in whom disulfiram concentrations were not detected (estimated difference, 0.47-fold; 95% CI, .20- to 1.10-fold, P = .077). Comparing postdisulfiram average viremia to the predisulfiram baseline showed an estimated 2.96-fold increase over baseline among these 6 subjects (95% CI, 1.29- to 6.81-fold; P = .01) compared to those without detectable disulfiram (1.39-fold; 95% CI, .69- to 2.79-fold; P = .33; difference detected vs not detected, 2.13-fold; 95% CI, .85- to 5.4-fold; P = .10).
DISCUSSION
We conducted a pilot clinical trial in which we administered the FDA-approved drug disulfiram for 14 days to HIV-1–infected individuals on ART to evaluate the safety and efficacy of this intervention as a means to perturb the HIV-1 latent reservoir. Disulfiram was well tolerated by all participants. The size of the latent reservoir, measured by a well-validated in vitro viral outgrowth assay, [30] did not decrease after the intervention. We observed only a small and not statistically significant average change in residual viremia during disulfiram treatment compared to baseline. In a post hoc analysis limited to 10 subjects with frequent sampling, we observed an unexpected rapid and transient increase in plasma viremia. Disulfiram exposure varied substantially among subjects.
There is much interest in understanding the kinetics of the later stages of viral replication, including proviral gene transcription, translation, viral budding, and release in resting CD4+ T cells. A recently published study in which a single dose of the histone deacetylase inhibitor (HDACi) vorinostat was administered to 8 HIV-1–infected patients with viral suppression estimated a mean 4.8-fold increase in cell-associated HIV-1 RNA within 4–7 hours of drug administration [32]. Similar data have been presented by Lewin and colleagues with vorinostat [33]. Levin and Tolstrup recently reported rapid increases in plasma viremia after exposure to the HDACi panobinostat in an ongoing phase 1/2 clinical trial (NCT01680094) [34]. In an in vitro study assessing the impact of various antilatency drugs on the kinetics of HIV-1 RNA production, the impact of disulfiram was more rapid and transient than vorinostat [35], an observation consistent with the data presented here.
Disulfiram appeared to have no effect on the size of the latent reservoir as measured by quantitative in vitro viral outgrowth. The mechanism of action of disulfiram in inducing proviral transcription is not currently understood. Disulfiram may induce HIV-1 transcription by activating the Akt signaling pathway, as has been recently described in cell line models of HIV-1 latency [28]. Alternatively, it is possible that even potent and sustained reversal of latency may not affect the reservoir in a durable manner. One important tenet of the “shock and kill” HIV-1 eradication strategy that makes use of latency-reversing agents targeting the reservoir is that virus-producing cells will be cleared by the immune system or will be eliminated by viral cytopathic effects following viral reactivation. However, it appears that reversing latency without T-cell activation may not be sufficient to kill latently infected CD4+ T cells [36]. The study presented here suggests that “shock and kill” strategies with drugs such as disulfiram will likely require another step to prime the immune system to clear virus-producing resting memory CD4+ T cells.
The pharmacokinetics and pharmacodynamics of disulfiram appear to be highly variable among subjects. Up to 50% will not have a disulfiram-ethanol reaction with a 250-mg dose [37]. For some individuals, doses of 500 mg are insufficient to instigate this reaction [37]. A formal study of the elimination kinetics of disulfiram found marked intersubject variability in plasma levels of disulfiram and its metabolites [25]. A separate study identified a 600-fold variation in disulfiram plasma concentrations among subjects [38]. The mechanism for this variability remains unknown. Using highly sensitive mass spectrometry, we also found substantial subject-to-subject variability in drug exposure, and could detect plasma disulfiram concentrations in only 6 of 15 participants. These measurements take into account only the parent compound; mass spectrometry assays for downstream metabolites may illuminate intersubject disulfiram pharmacokinetics and are in development for future studies. These participants had a significant “post-drug” increase in low-level viremia that was sustained over the 2 months following the disulfiram dosing interval, and also demonstrated a nonsignificant trend toward decrease in the size of the latent reservoir, suggesting that higher exposures to the drug in vivo may have more pronounced and prolonged effects on HIV-1 production.
In summary, this trial attempted to safely translate in vitro discoveries affecting the latent reservoir into initial in vivo analysis. Disulfiram was safe and well tolerated, but did not appear to significantly perturb the latent reservoir. The apparent exposure-response effect observed in this study highlights significant intersubject variability in disulfiram pharmacokinetics and suggest that higher doses of disulfiram might be more effective. It is also possible that combining disulfiram with other latency-reversing agents will have a more pronounced effect on the reservoir, and the favorable safety profile of disulfiram provides support for such combination approaches.
Notes
Acknowledgments. We are indebted to the trial participants for their courage, dedication, and altruism. We are grateful to Morrie Faiman for contributions regarding disulfiram pharmacology; Joseph Wong for assistance with viral quantification methods; Janice Clements for input on nonhuman primate models of HIV-1 latency; and Joel Gallant, Joe Cofrancesco, and Emily Richie for patient referrals.
Disclaimer. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins Institute for Clinical and Translational Research (ICTR), National Center for Advancing Translational Sciences (NCATS), or National Institutes of Health (NIH). None of these funding sources played any role in study design, collection, analysis or interpretation of data, writing of the manuscript, or decisions regarding manuscript submission.
Financial support. This work was supported by an ARCHE Collaborative Research Grant from the Foundation for AIDS Research, with additional support from Martin Delaney CARE and DARE Collaboratories (NIH grant numbers AI096113 and 1U19AI096109), the National Institute of Allergy and Infectious Diseases (K24 AI069994), the UCSF/Gladstone Center for AIDS Research (P30 AI027763), the UCSF Clinical and Translational Science Institute (UL1 RR024131), the Johns Hopkins Center for AIDS Research, the NIH (grant 43222 to R. F. S.), the Howard Hughes Medical Institute (to R. F. S.) and the Center for AIDS Prevention Studies (P30 MH62246). This work was also supported by the Johns Hopkins ICTR, which is funded in part by grant number UL1 TR 000424-06 from NCATS, a component of the NIH, and NIH Roadmap for Medical Research.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Moore RD, Keruly JC, Gebo KA, Lucas GM. An improvement in virologic response to highly active antiretroviral therapy in clinical practice from 1996 through 2002. J Acquir Immune Defic Syndr. 2005;39:195–8. [PubMed] [Google Scholar]
- 2.Perelson AS, Essunger P, Cao Y, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–91. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 3.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 4.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 5.Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94:13193–7. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 7.Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–90. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 8.Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–8. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 9.Trono D, Van Lint C, Rouzioux C, et al. HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science. 2010;329:174–80. doi: 10.1126/science.1191047. [DOI] [PubMed] [Google Scholar]
- 10.Choudhary SK, Margolis DM. Curing HIV: pharmacologic approaches to target HIV-1 latency. Annu Rev Pharmacol Toxicol. 2011;51:397–418. doi: 10.1146/annurev-pharmtox-010510-100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeks SG. HIV: shock and kill. Nature. 2012;487:439–40. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- 12.Chun TW, Engel D, Mizell SB, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–5. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 13.Brooks DG, Arlen PA, Gao L, Kitchen CM, Zack JA. Identification of T cell-signaling pathways that stimulate latent HIV in primary cells. Proc Natl Acad Sci U S A. 2003;100:12955–60. doi: 10.1073/pnas.2233345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang FX, Xu Y, Sullivan J, et al. IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. J Clin Invest. 2005;115:128–37. doi: 10.1172/JCI22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coiras M, Lopez-Huertas MR, Perez-Olmeda M, Alcami J. Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat Rev Microbiol. 2009;7:798–812. doi: 10.1038/nrmicro2223. [DOI] [PubMed] [Google Scholar]
- 16.Yang HC, Xing S, Shan L, et al. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest. 2009;119:3473–86. doi: 10.1172/JCI39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyagi M, Pearson RJ, Karn J. Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J Virol. 2010;84:6425–37. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosque A, Planelles V. Studies of HIV-1 latency in an ex vivo model that uses primary central memory T cells. Methods. 2011;53:54–61. doi: 10.1016/j.ymeth.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing S, Bullen CK, Shroff NS, et al. Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J Virol. 2011;85:6060–4. doi: 10.1128/JVI.02033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams E. Effects of alcohol on workers with carbon disulfide. JAMA. 1937;109:1472. [Google Scholar]
- 21.Johansson B. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr Scand Suppl. 1992;369:15–26. doi: 10.1111/j.1600-0447.1992.tb03310.x. [DOI] [PubMed] [Google Scholar]
- 22.Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. JAMA. 1986;256:1449–55. [PubMed] [Google Scholar]
- 23.Tennant FS., Jr Disulfiram will reduce medical complications but not cure alcoholism. JAMA. 1986;256:1489. [PubMed] [Google Scholar]
- 24.Suh JJ, Pettinati HM, Kampman KM, O'Brien CP. The status of disulfiram: a half of a century later. J Clin Psychopharmacol. 2006;26:290–302. doi: 10.1097/01.jcp.0000222512.25649.08. [DOI] [PubMed] [Google Scholar]
- 25.Faiman MD, Jensen JC, Lacoursiere RB. Elimination kinetics of disulfiram in alcoholics after single and repeated doses. Clin Pharmacol Ther. 1984;36:520–6. doi: 10.1038/clpt.1984.213. [DOI] [PubMed] [Google Scholar]
- 26.Petersen EN. The pharmacology and toxicology of disulfiram and its metabolites. Acta Psychiatr Scand Suppl. 1992;369:7–13. doi: 10.1111/j.1600-0447.1992.tb03309.x. [DOI] [PubMed] [Google Scholar]
- 27.Hart BW, Faiman MD. In vivo pharmacodynamic studies of the disulfiram metabolite S-methyl N,N-diethylthiolcarbamate sulfoxide: inhibition of liver aldehyde dehydrogenase. Alcohol Clin Exp Res. 1994;18:340–5. doi: 10.1111/j.1530-0277.1994.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 28.Doyon G, Zerbato J, Mellors JW, Sluis-Cremer N. Disulfiram reactivates latent HIV-1 expression through depletion of the phosphatase and tensin homolog. AIDS. 2013;27:F7–11. doi: 10.1097/QAD.0b013e3283570620. [DOI] [PubMed] [Google Scholar]
- 29.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–6. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304:3–15. doi: 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]
- 31.Eypasch E, Lefering R, Kum CK, Troidl H. Probability of adverse events that have not yet occurred: a statistical reminder. BMJ. 1995;311:619–20. doi: 10.1136/bmj.311.7005.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Archin NM, Liberty AL, Kashuba AD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–5. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliot J, Solomon A, Wightman F. The safety and effect of multiple doses of vorinostat on HIV transcription in HIV+ patients receiving cART [abstract 50LB]. 20th Conference on Retroviruses and Opportunistic Infections,; Atlanta, GA. 2013. [Google Scholar]
- 34.Levin J, Tolstrup M. Cyclic panobinostat (LBH589) dosing in HIV-1 patients: findings from the CLEAR trial. 7th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention,; 30 June–3 July 2013; Kuala Lumpur, Malaysia. [Google Scholar]
- 35.Di Iulio J, Mattis MP, Thorball C. Dynamics of HIV latency and reactivation by transcriptome profiling [abstract 369]. 20th Conference on Retroviruses and Opportunistic Infections,; 3–6 March 2013; Atlanta, GA. [Google Scholar]
- 36.Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brewer C. How effective is the standard dose of disulfiram? A review of the alcohol-disulfiram reaction in practice. Br J Psychiatry. 1984;144:200–2. doi: 10.1192/bjp.144.2.200. [DOI] [PubMed] [Google Scholar]
- 38.Haley TJ. Disulfiram (tetraethylthioperoxydicarbonic diamide): a reappraisal of its toxicity and therapeutic application. Drug Metab Rev. 1979;9:319–35. doi: 10.3109/03602537908993897. [DOI] [PubMed] [Google Scholar]