Abstract
The association between dermatomyositis (DMS) and various types of malignancies has been reported in several studies, with an estimated frequency of 20-25%. DMS may precede, accompany or follow the diagnosis of malignancy. In the present report, we have discussed three cases of dermatomyositis associated with malignancy. In the first case, DMS preceded the diagnosis of gastric adenocarcinoma while in the second and third cases, it followed the diagnosis of ductal carcinoma of the breast and transitional cell carcinoma of the bladder respectively. In all three patients, cutaneous and musculoskeletal features of DMS showed very good response to the treatment, irrespective of the course of malignancy.
Keywords: Dermatomyositis, ductal carcinoma of breast, gastric adenocarcinoma, transitional cell carcinoma of bladder
INTRODUCTION
Dermatomyositis (DMS) is a rare inflammatory disease with musculocutaneous manifestations. Despite few reports to the contrary, DMS is widely accepted as a definite risk factor for internal malignancy, with rates varying from 10% to 50%.[1] The type of malignancy also varies considerably with sex and geographic location, with ovarian and breast carcinoma predominating in women and lung cancer in men, worldwide.[2] Asian studies have reported nasopharyngeal carcinoma as the most common type of malignancy associated with DMS.[3,4] With reports of varied types of carcinomas associated with DMS, malignancy screening can be time consuming and expensive, but should be mandatory.
CASE REPORTS
Case 1
A 36-year-old male presented with complaints of itchy red lesions over the periorbital area, forehead and malar area associated with photosensitivity since 3 months, painless swelling around both eyes since 1 month and pain over the shoulder and hip region, nasal twang of voice and difficulty in deglutition since 2 days. Cutaneous examination of the patient revealed periorbital erythema, edema and hyperpigmentation [Heliotrope rash, Figure 1a and b]. Similar erythema and hyperpigmentation were also present on the neck, chest, shoulders and back [Shawl sign, [Figure 1c and d]. Erythematous hyperpigmented papules were present on the knuckles and both elbows [Gottron's papules, Figure 1e and f]. Musculoskeletal examination revealed proximal muscle weakness of shoulder and hip girdles. Skin biopsy from the erythematous lesion on the forehead revealed atrophic epidermis, focal basal cell vacuolar degeneration, dermal edema, mild perivascular lymphocytic infiltrate and abundant mucin in the dermis [Figure 2a and b]. Blood investigations of the patient revealed raised erythrocyte sedimentation rate (ESR), elevated titers of muscle enzymes (LDH and CPK-MM) and electromyography (EMG) showing first-degree muscle disease, confirming the diagnosis of dermatomyositis. Rhinolaryngoscopy of the patient revealed articular defects of vocal cords due to bulbar muscle palsy [detailed investigations are mentioned in Tables 1 and 2]. The patient was simultaneously investigated for underlying malignancy. Barium swallow of the patient showed esophageal motility disorder, computed tomography (CT) scan of the abdomen revealed thickening in the pyloric region, causing mild luminal compromise, esophago-gastro-duodenoscopy (OGDscopy) showed presence of gastric ulcer at the pyloric antrum and biopsy from the gastric ulcer confirmed the diagnosis of poorly differentiated adenocarcinoma of the stomach [Figure 2c and d]. The patient was diagnosed as DMS associated with poorly differentiated gastric adenocarcinoma. The patient received oral prednisolone 1.5 mg/kg/day with low potent topical corticosteroids and sunscreen. The patient's skin lesions and muscle symptoms showed excellent response [Figure 3a–f] to the treatment with reduction in the titers of muscle enzymes [Table 3]. After clinical improvement, prednisolone was tapered gradually and the patient was referred to an oncologist for management of gastric adenocarcinoma. Unfortunately, the patient was lost to follow-up.
Figure 1.
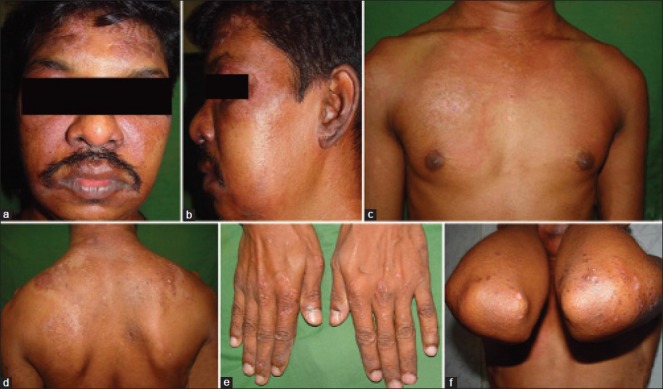
Cutaneous examination of case 1. (a and b) Heliotrope rash and periorbital edema, (c) “V” sign, (d) “Shawl” sign and (e and f) Gottron's papules
Figure 2.
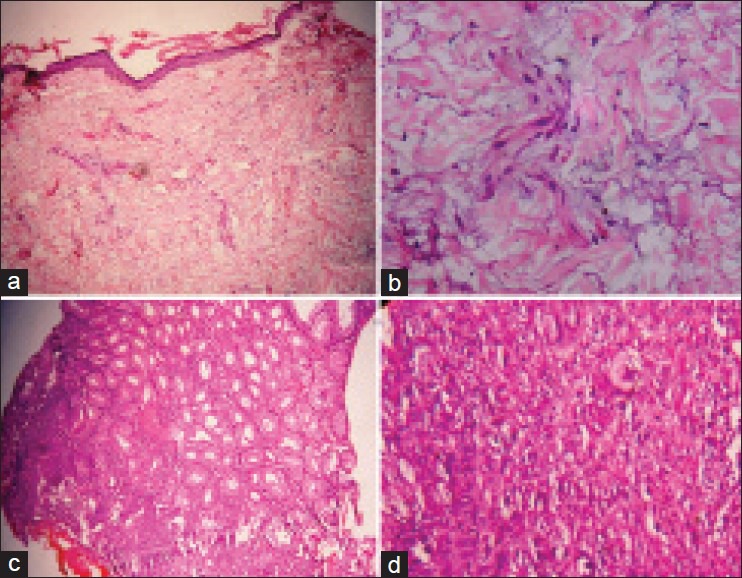
Case 1: (a) Skin biopsy, 100×; hematoxylin and eosin (H and E) stain: Atrophic epidermis, focal basal cell vacuolar degeneration, dermal edema, mild perivascular lymphocytic infiltrate and abundant mucin in the dermis. (b) Skin biopsy, 400×, H and E stain: Dermal edema and abundant mucin in the dermis. (c) Biopsy from gastric ulcer, 100×; H and E stain: Normal gastric glands and transition zone showing malignancy. (d) Biopsy of gastric ulcer, 400×; H and E stain: Tumor cells mainly arranged in solid sheets with paucity of glandular structure and mitotic activity suggestive of poorly differentiated adenocarcinoma
Table 1.
Laboratory investigations of all 3 cases

Table 2.
Special investigations in cases

Figure 3.
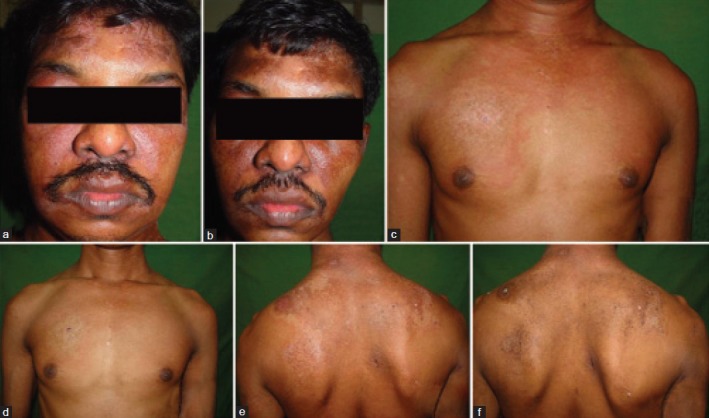
Improvement in skin lesions of dermatomyositis after 2 weeks of treatment with oral steroids. (a) Heliotrope rash and periorbital edema before treatment. (b) Resolution of lesions after treatment. (c) Erythema over neck, chest and arms before treatment. (d) Resolution of the erythema over neck, chest and arms after treatment. (e) Erythematous and poikilodermatous lesions on the back and shoulders (Shawl sign) before treatment. (f) Resolution of erythematous lesions on the back, leaving behind dyspigmentation after treatment
Table 3.
Improvement in the muscle enzymes

Case 2
A 65-year-old married female, operated case of carcinoma of the right breast (in situ ductal carcinoma), presented with complaints of sudden appearance of swelling and redness over the right breast since 2 weeks. The swelling was associated with appearance of red-colored minimally itchy lesions on the forehead, cheeks, nape of neck retroauricular area and pinna since 6 days. The patient also developed generalized swelling all over the body since 2 days with difficulty in raising her arms above the head and getting up from the squatting position. Cutaneous examination of the patient revealed violaceous erythema over the periorbital area, forehead, malar area, chin ([Figure 4a], pre- and postauricular area [Figure 4b and c] and both the breasts [Figure 4d and e]. Systemic examination of the patient revealed generalized edema and musculoskeletal system examination revealed proximal muscle weakness affecting the limb girdles. Laboratory investigations of the patient revealed raised ESR and markedly elevated muscle enzymes (LDH, CPK-MM) [Tables 1 and 2]. Electromyography showed presence of myopathic denervation consistent with polymyositis, while a muscle biopsy from the quadriceps muscles revealed inflammatory myositis. Skin biopsy from the erythematous lesion revealed atrophic epidermis, focal basal cell vacuolar degeneration and dermal edema [Figure 4f], suggesting the diagnosis of dermatomyositis. The patient was simultaneously investigated for the recurrence of operated ductal carcinoma of the breast and metastasis. Ultrasonography of the right breast showed edema of the skin and subcutaneous tissue with fluid collection in the subcutaneous tissue without evidence of any mass. Positron emission tomography (PET) scan of the whole body did not reveal recurrence of previous malignancy or metastasis. The patient was diagnosed as dermatomyositis associated with treated ductal carcinoma of the breast and was initially started on oral prednisolone 1.5 mg/kg/day. But, in the absence of satisfactory improvement, subcutaneous injection methotrexate 15 mg/week was added. The patient showed excellent improvement in skin lesions and muscle function within 2 weeks [Figure 5a–f] with reduction in titers of muscle enzymes [Table 3]. The dose of oral steroids and methotrexate was tapered slowly without recurrence of the disease, leading to complete discontinuation of the treatment at the end of 4 months.
Figure 4.
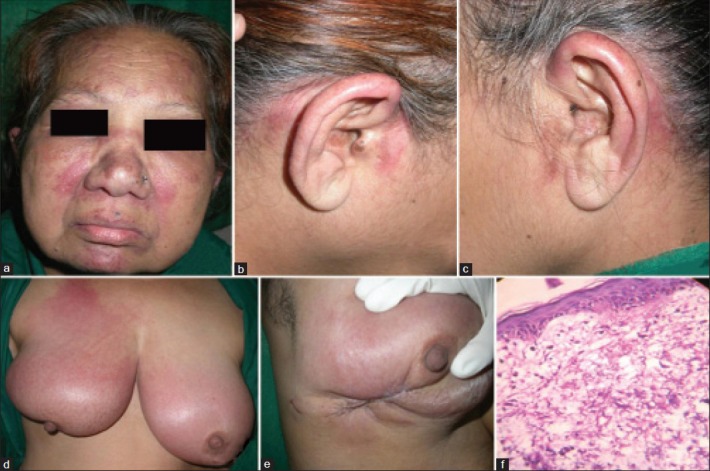
(a) Cutaneous examination of case 2 showing violaceous erythema over the periorbital area (heliotrope rash), forehead, malar area and chin. (b and c) Erythematous plaques on the pinnae and pre- and postauricular areas. (d) Erythema and edema of both the breast, more on the right side than on the left. (e) Scar of previous surgery on the right breast surrounded by erythema. (f) Skin biopsy from the erythematous lesion on the forehead. 400×, Hematoxylin and eosin showing atrophic epidermis, focal basal cell vacuolar degeneration and dermal edema
Figure 5.
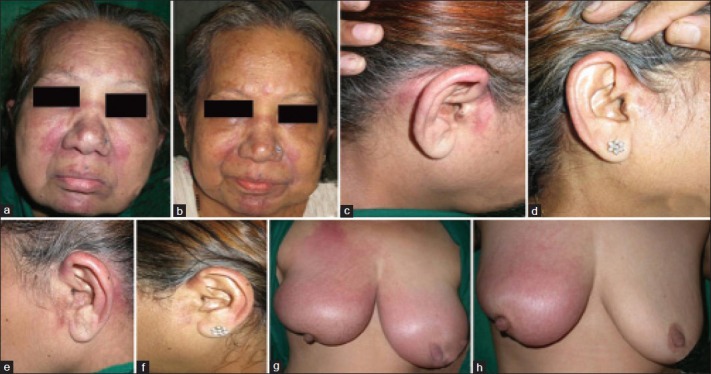
(a) Heliotrope rash, periorbital edema and erythematous lesions on the forehead, malar area and chin before treatment. (b) After treatment. (c) Erythema over the right pinna and pre- and postauricular areas before treatment. (d) After treatment. (e) Erythema over the left pinna and pre- and postauricular areas before treatment before treatment. (f) After treatment. (g) Erythema and edema over both the breasts before treatment. (h) After treatment
Case 3
A 58-year-old married male presented with complaints of swelling and redness of the periorbital area, face, trunk and upper extremities since 2 months, difficulty in raising limbs and getting up from squatting position since 15 days and nasal twang of voice and nasal regurgitation of the food since 1 day. The patient was apparently alright 3 months ago, when he developed increased frequency of micturition, urgency and reddish discoloration of urine lasting for 3-4 days. The patient was investigated and diagnosed to have high-grade transitional cell carcinoma of the urinary bladder with extension to prostate on biopsy of the prostate. Cutaneous examination of the patient revealed confluent macular violaceous erythema and edema of the periorbital area, forehead and malar area [Figure 6a] with erythematous rash on the neck, chest [Figure 6b] and back. Systemic examination of the patient revealed generalized edema and musculoskeletal system examination revealed weakness of the muscles of limb girdles.
Figure 6.
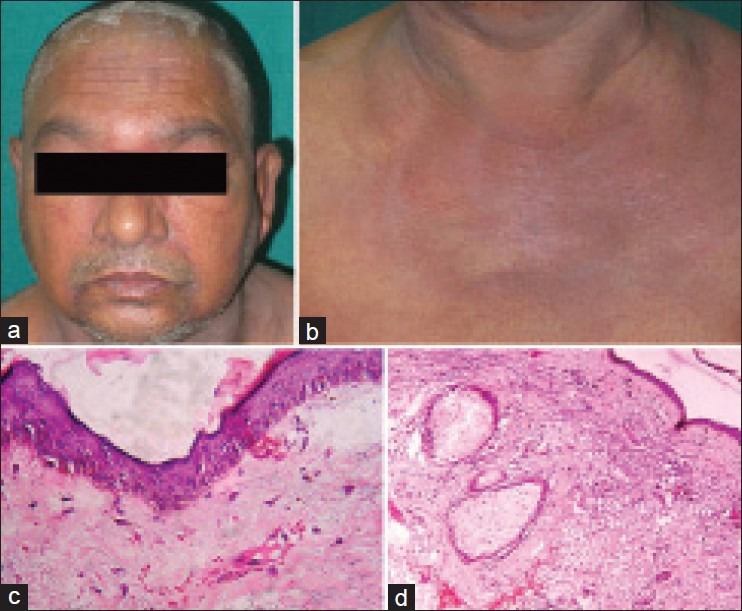
(a) Cutaneous examination of case 3: Heliotrope rash and periorbital edema. (b) Erythema over the “V” area of the neck (i.e., “v” sign) and upper chest. (c) Skin biopsy, 400×, hematoxylin and eosin (H and E): Showing atrophic epidermis, basal cell vacuolar degeneration, dermal edema and perivascular inflammatory infiltrate. (d) Skin biopsy, 400×, H and E: Showing atrophic epidermis, basal vacuolar degeneration, dermal edema and perivascular inflammatory infiltrate with abundant mucin in the dermis
Laboratory investigations of the patient revealed raised ESR, positive antinuclear antibody test (ANA 4+) and markedly elevated muscle enzymes (LDH, CPK-MM). Electromyography showed presence of myopathic denervation consistent with polymyositis, while a muscle biopsy from the quadriceps muscles revealed inflammatory myositis. Urine examination of the patient showed 30-40 RBCs/hpf, 8 to 15 transitional epithelial cells and 3-5 pus cells/hpf [Tables 1 and 2]. Skin biopsy from the violaceous erythematous lesion reveled atrophic epidermis, basal cell vacuolar degeneration, dermal edema and perivascular inflammatory infiltrate with abundant mucin in the dermis [Figure 6c and d]. Ultrasonography of the abdomen and pelvis revealed 1 cm × 3.2 cm-sized mass at the bladder base, involving the prostate and suspected to be transitional cell carcinoma of the bladder. CT scan of the abdomen reveled ill-defined, mildly enhancing lesion at the bladder base extending into the prostate which on biopsy and immunohistochemistry confirmed the diagnosis of high grade transitional cell carcinoma. Rhinolaryngoscopy of the patient showed bulbar palsy. The patient was diagnosed as DMS associated with transitional cell carcinoma of the bladder and received oral prednisolone 1.5 mg/kg/day and subcutaneous injection methotrexate 15 mg/week in view of severity of skin and muscle involvement. Skin lesions of the patient showed complete resolution after 2 weeks of the treatment [Figure 7a–d], but muscle function recovery was monitored by fall in titers of muscle enzymes [Table 3]. Unfortunately, the patient died of sudden cardiac arrest while awaiting the surgery.
Figure 7.
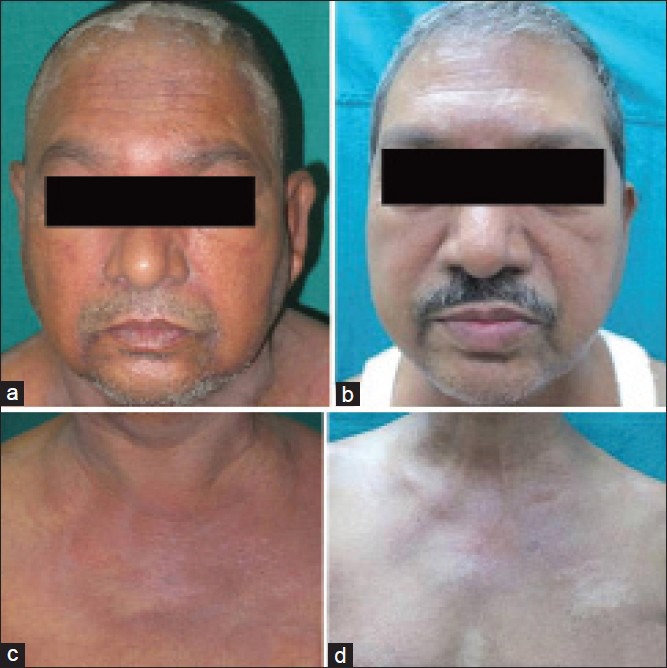
(a) Cutaneous examination of case 3: Heliotrope rash and periorbital edema before treatment. (b) Resolution of heliotrope rash and periorbital edema after 2 weeks of treatment. (c) Erythema over the “v” area of the neck (i.e., “V” sign) and upper chest. (d) Resolution of erythema over the “v” area of the neck and upper chest after 2 weeks of treatment
DISCUSSION
DMS is a connective tissue disease with characteristic cutaneous manifestations, and it is well known to be associated with various types of malignancies. The first suggestion that DMS was associated with malignancy was made by Sterz in 1916, and numerous cases have since been reported.[5] It is widely accepted as a definite risk factor for internal malignancy, with rates varying from 10% to 50% in different studies.[1]
The malignancy types vary in each country. Hill et al. reported DMS in strong association with ovarian, lung, gastric, colorectal and pancreatic malignancy in several Northern European countries, whereas Wong reported that nasopharyngeal carcinoma was as high as 75% in Hong Kong[6] and Ang et al. reported a rate of 50% in Singapore.[7] On the other hand, in Japan, gastric, lung, breast and ovarian malignancy were commonly associated with DMS (in order of frequency). Asian studies have shown nasopharyngeal carcinoma as the most common association.[3,4]
Other malignancies associated with DMS included those of gastric, liver, cervical lymph node metastases of unknown origin, cholangial, esophageal, colonic, laryngeal, renal, tongue and lymphoma.
Several predictive factors of associated malignancy have been described over the years, with the age of the patient being the most important.[8] The incidence is higher in patients with age more than 40 years[9] and male sex.[3,10] Other factors include raised ESR,[11] skin lesions,[12] muscle biopsy findings[13] and muscle enzyme levels,[3,14] cutaneous necrosis,[10] interstitial lung diseases,[3] amyopathic dermatomyositis,[10] muscle biopsy findings,[13] constitutional symptoms, rapid onset of disease and Raynaud's phenomenon.[14]
Malignancy was often detected within the first year of onset of DMS. Zang et al. studied 115 cases of DMS with malignancy, in which malignancy preceded dermatomyositis in 14 cases, was diagnosed concurrently in 22 patients and in another 79 patients it appeared within 3 years after the DMS was diagnosed. Among them, malignancy was detected in 66 patients in the first year, 11 patients in the second year and only two patients in the third year.[9]
The exact incidence of DMS with the three malignancies reported in our cases is not available, but breast malignancy has been increasingly reported in association with DMS in females,[2] and there are isolated case reports of DMS associated with gastric and transitional cell carcinoma of the bladder. Two patients of DMS with transitional cell carcinoma of the bladder had presented with amyopathic dermatomyositis.[15]
DMS associated with malignancy is generally more resistant to corticosteroid and cytotoxic therapies than idiopathic myositis.[16] Dermatomyositis activity reflects the state of malignancy, and some reports have described improvement of DMS without immunosuppressive drugs after the resection of the malignancy.[16,17,18] The overall survival rate was considerably worse when compared with other forms of myositis.[19]
The unique features of our cases include appearance of associated undiagnosed gastric malignancy in a young patient of DMS in the first case and occurrence of DMS after diagnosis and treatment of underlying malignancy in the second case. Few important observations drawn from our cases are that the possibility of DMS should be kept in mind in patients of malignancy even if the malignancy is treated. Underlying malignancy should be ruled out even in young patients, as seen with our first case.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ghate J, Katsambas A, Augerinou G, Jorizzo JL. Review article: A therapeutic update on dermatomyositis/polymyositis. Int J Dermatol. 2000;39:81–7. doi: 10.1046/j.1365-4362.2000.00851.x. [DOI] [PubMed] [Google Scholar]
- 2.Airio A, Pukkala E, Isomaki H. Elevated cancer incidence in patients with dermatomyositis: A population based study. J Rheumatol. 1995;22:1300–3. [PubMed] [Google Scholar]
- 3.Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: A case-control study. Br J Dermatol. 2001;144:825–31. doi: 10.1046/j.1365-2133.2001.04140.x. [DOI] [PubMed] [Google Scholar]
- 4.Leow YH, Goh CL. Malignancy in adult dermatomyositis. Int J Dermatol. 1997;36:904–7. doi: 10.1046/j.1365-4362.1997.00190.x. [DOI] [PubMed] [Google Scholar]
- 5.Sterz G. Polymyositis. Berliner Klinische Wochenschrift (Clinical Berlin Weekly) 1916;53:489. [Google Scholar]
- 6.Wong KO. Dermatomyositis: A clinical investigation of twenty-three cases in Hong Kong. Br J Dermatol. 1969;81:544–7. doi: 10.1111/j.1365-2133.1969.tb16031.x. [DOI] [PubMed] [Google Scholar]
- 7.Ang P, Sugeng MW, Chua SH. Classical and amyopathic dermatomyositis seen at the National Skin Centre of Singapore: A 3-year retrospective review of their clinical characteristics and association with malignancy. Ann Acad Med Singapore. 2000;29:219–23. [PubMed] [Google Scholar]
- 8.Yamauchi K, Kogashiwa Y, Nagafuji H, Kohno N. Head and neck cancer with dermatomyositis: A report of two clinical cases. Int J Otolaryngol 2010. 2010 doi: 10.1155/2010/401825. 401825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Jiang SP, Huang L. Dermatomyositis and malignancy: A retrospective study of 115 cases. Eur Rev Med Pharmacol Sci. 2009;13:77–80. [PubMed] [Google Scholar]
- 10.Eapen BR. Malignancy in dermatomyositis: A Bayesian Belief Network approach. Indian J Dermatol Venereol Leprol. 2007;73:445. [Google Scholar]
- 11.Amerio P, Girardelli CR, Proietto G, Forleo P, Cerritelli L, Feliciani C, et al. Usefulness of erythrocyte sedimentation rate as tumor marker in cancer associated dermatomyositis. Eur J Dermatol. 2002;12:165–9. [PubMed] [Google Scholar]
- 12.Gallais V, Crickx B, Belaich S. Prognostic factors and predictive signs of malignancy in adult dermatomyositis. Ann Dermatol Venereol. 1996;123:722–6. [PubMed] [Google Scholar]
- 13.Wakata N, Kurihara T, Saito E, Kinoshita M. Polymyositis and dermatomyositis associated with malignancy: A 30-year retrospective study. Int J Dermatol. 2002;41:729–34. doi: 10.1046/j.1365-4362.2002.01648.x. [DOI] [PubMed] [Google Scholar]
- 14.Sparsa A, Liozon E, Herrmann F, Ly K, Lebrun V, Soria P, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: A study of 40 patients. Arch Dermatol. 2002;138:885–90. doi: 10.1001/archderm.138.7.885. [DOI] [PubMed] [Google Scholar]
- 15.Apaydin R, Gül U, Bahadir S, Siviloglu C, Ofluoglu I. Dermatomyositis without muscle weakness associated with transitional cell carcinoma of the bladder. J Eur Acad Dermatol Venereol. 2002;16:172–4. doi: 10.1046/j.1468-3083.2002.00392_2.x. [DOI] [PubMed] [Google Scholar]
- 16.Carsons S. The association of malignancy with rheumatic and connective tissue diseases. Semin Oncol. 1997;24:360–72. [PubMed] [Google Scholar]
- 17.Takahashi F, Tsuta K, Nagaoka T, Miyamoto H, Saito Y, Amano H, et al. Successful resection of dermatomyositis associated with thymic carcinoma: Report of a case. Surg Today. 2008;38:245–8. doi: 10.1007/s00595-007-3601-x. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita K, Hosokawa M, Hirohashi S, Arimura Y, Endo T, Denno R, et al. Epstein-Barr virus-associated gastric cancer in a patient with dermatomyositis. Intern Med. 2001;40:96–9. doi: 10.2169/internalmedicine.40.96. [DOI] [PubMed] [Google Scholar]
- 19.András C, Ponyi A, Constantin T, Csiki Z, Szekanecz E, Szodoray P, et al. Dermatomyositis and polymyositis associated with malignancy: A 21-year retrospective study. J Rheumatol. 2008;35:438–44. [PubMed] [Google Scholar]


