Abstract
Hepatocytes are metabolically active cells of the liver that play an important role in the biosynthesis of proteins including α1-antitrypsin. Mutations in the α1-antitrypsin gene can lead to protein misfolding, polymerization/aggregation and retention of protein within the endoplasmic reticulum of hepatocytes. The intracellular accumulation of α1-antitrypsin aggregates can lead to liver disease and increased likelihood of developing hepatocellular carcinomas. Of note, only ~10% of individuals with α1-antitrypsin-deficiency develop severe liver disease suggesting that there are other genetic and/or environmental factors that determine disease outcome. The nematode, C. elegans, is a powerful genetic model organism to study molecular aspects of human disease. In this review, we discuss the functional similarities between the intestinal cells of C. elegans and human hepatocytes and how a C. elegans model of α1-antitrypsin-deficiency can be used as a tool for identifying genetic modifiers and small molecule drugs.
1. Introduction
The liver is comprised of many specialized cell types including parenchymal (hepatocytes) and non-parenchymal (endothelial, kupffer, ito, pitt) cells (Jungermann, 1989). Hepatocytes are metabolically active cells of the liver responsible for the synthesis of a large number of proteins and lipids for distribution (Selden et al., 1999). Hepatocytes are organized into plates with serial apical and basolateral poles. The apical poles of front-facing and adjacent hepatocytes form a continuous network of bile canaliculi that is in contact with the external environment, into which the bile is secreted. The basal membrane (that is in contact with the blood) called the sinusoidal pole, secretes various components into the circulation, and is responsible for the uptake of recycled biliary salts. This polarity gives the hepatocyte a special cell shape and architecture (Decaens et al., 2008). The specialized metabolic functions of these cells will be discussed in greater detail in the next section.
One of the most prevalent genetic diseases affecting hepatocytes is AT-deficiency. AT-deficiency affects ~1 in every 2,000–5,000 individuals of Northern European and North American descent (de Serres, 2002). It is one of three most common genetic disorders among Caucasians and one of the most common genetic diseases requiring liver transplantation in children (Perlmutter, 2002, Rudnick and Perlmutter, 2005). In the classical form of AT-deficiency, a point mutation in AT (substitution of lysine for glutamic acid at residue 342, commonly known as the “Z” mutation) alters protein folding and causes spontaneous protein polymerization/aggregation. ATZ aggregates/polymers accumulate in the endoplasmic reticulum (ER) of hepatocytes which may lead to hepatic fibrosis and increased susceptibility to hepatocellular carcinomas (Eriksson et al., 1986, Rudnick and Perlmutter, 2005). In addition, the retention of ATZ in the ER causes significant (85–90%) reduction in circulating AT (Hidvegi et al., 2010, Hidvegi et al., 2005). This decrease in anti-protease potential leads to proteolytic digestion of lung connective tissue, resulting in chronic obstructive pulmonary disease (COPD) and emphysema.
2. Cell origin and plasticity
Hepatocytes, along with biliary epithelial cells are derived from the embryonic endoderm, whereas stromal cells, stellate cells, kupffer cells and blood vessels are all of mesodermal origin (liver development, including regeneration, is reviewed in (Zorn, 2008), hepatocyte development is reviewed in (Kanamura et al., 1990)). In C. elegans the 4-cell stage blastomere EMS is induced by polarization to two daughters E and MS. The E blastomere then gives rise to the endoderm, and in turn gives rise to the intestine, whereas the MS daughter cell produces the mesoderm (and eventual pharynx and body wall muscle)(C. elegans intestinal development is reviewed in (McGhee, 2007)). The mammalian liver arises from a further differentiation and specialization of the foregut, whereas the simpler C. elegans retains the specialized liver functions within the intestinal cells.
3. Functions
Hepatocytes maintain the organism’s energy supply by regulating glucose release, producing ketone bodies, catabolizing amino acids, removing ammonia (formed from amino acid breakdown) by synthesizing urea, processing dietary triglycerides and fatty acids from adipocytes. Hepatocytes also have important biosynthetic and biodegradative functions such as metabolism of phospholipids and cholesterol, synthesis/degradation of plasma proteins (albumin, transferrin, clotting factors), formation of bile from cholesterol for digestion, and protection against xenobiotics by transformation and removal via urine and bile. Hepatocytes are also a major control station of the endocrine system, maintaining levels of circulating hormones, and synthesizing and secreting humoral factors (Jungermann and Katz, 1989, Protzer et al., 2012).
The C. elegans intestinal cell not only has a role in the digestion and absorption of ingested nutrients but also performs many of the essential specialized functions of the mammalian hepatocyte. Most aspects of lipid biology are conserved in C. elegans, with lipid synthesis and modification occurring primarily in the intestine. Lipoprotein secretion and circulation are also conserved (Branicky et al., 2010), as is bile acid synthesis and secretion (Liu et al., 2012). C. elegans like mammals have two sites of beta-oxidation of fatty acids, the mitochondria and peroxisomes, the latter being abundant in the nematode intestine (McGhee, 2007). Even xenobiotic metabolism is conserved and occurs within the intestinal cells of the nematode (Lindblom and Dodd, 2006). Mammals store fat in adipocytes or as lipid droplets in stellate cells of the liver. In C. elegans, fat is stored in lipid droplets within the intestinal cell. Interestingly, C. elegans are auxotrophic for sterols as they lack enzymes for de novo synthesis, thus unlike mammals, cholesterol must be obtained from dietary sources (Branicky, Desjardins, 2010). The similarities between hepatocytes and the intestinal cells of C. elegans suggest that the nematode intestine may be a suitable organ in which to model and study cellular aspects of AT-deficiency.
4. Associated pathologies
Individuals homozygous for ATZ are at risk for liver disease. Liver injury associated with AT homozygosity involves a gain-of-toxic-function mechanism whereby retention of the mutant, aggregated ATZ molecule in the ER, triggers a series of events that are eventually hepatotoxic. The strongest evidence for a gain-of-function mechanism comes from studies in which mice, transgenic for mutant human ATZ, develop liver injury and hepatocellular carcinoma associated with the histopathologic hallmark of the human condition; periodic acid-Schiff-positive, diastase-resistant intracellular globules containing insoluble ATZ aggregates (Carlson et al., 1989) Because there are normal levels of anti-elastases in these mice, as directed by endogenous genes, the liver disease cannot be attributed to loss-of-function. Thus, the liver disease associated with AT deficiency is similar in pathogenesis to a growing list of diseases in which tissue injury results from an aggregated mutant protein. Most notable among these are the neurodegenerative diseases including Alzheimer disease, prion diseases, Parkinsonism, amyotrophic lateral sclerosis, FENIB (familial encephalopathy with neuroserpin inclusion bodies) and Huntington disease as well as an expanding number of serpinopathies (Carrell and Lomas, 2002).
Landmark nationwide prospective newborn screening studies by Sveger in Sweden document an extraordinary variation in the phenotypic expression of liver disease among homozygotes (Sveger, 1976, 1988). Only 10–15% of homozygotes developed clinically significant liver disease over the first 20 years of life (Sveger, 1976, 1988, Sveger and Eriksson, 1995). The data indicate that other genetic modifiers and/or environmental factors predispose a subgroup of homozygotes to liver disease, or protect the remainder of the affected population from liver disease.
The idea that alterations in the quality control apparatus of the ER could constitute genetic modifiers of the liver disease led to subsequent studies to elucidate the mechanisms of quality control relevant to ATZ. Two pathways are responsible for its degradation. The ubiquitin-proteasome pathway plays an essential role in the disposal of soluble forms of ATZ and the autophagic-lysosomal response is critical for disposal of insoluble ATZ (Kamimoto et al., 2006, Kruse et al., 2006a, b, Qu et al., 1996). Studies using cell line and transgenic mouse model systems with inducible expression showed that accumulation of ATZ in the ER evokes a very distinct set of protective responses that include activation of NFκB, mitochondrial and ER caspases, autophagy and suppression of cell proliferation (Hidvegi, Schmidt, 2005, Kamimoto, Shoji, 2006, Rudnick et al., 2004). Although each of these pathways could theoretically be altered by a putative genetic modifier, this concept has not been extensively validated experimentally and specific genes that modify the incidence and severity of tissue damage in AT deficiency have not yet been identified.
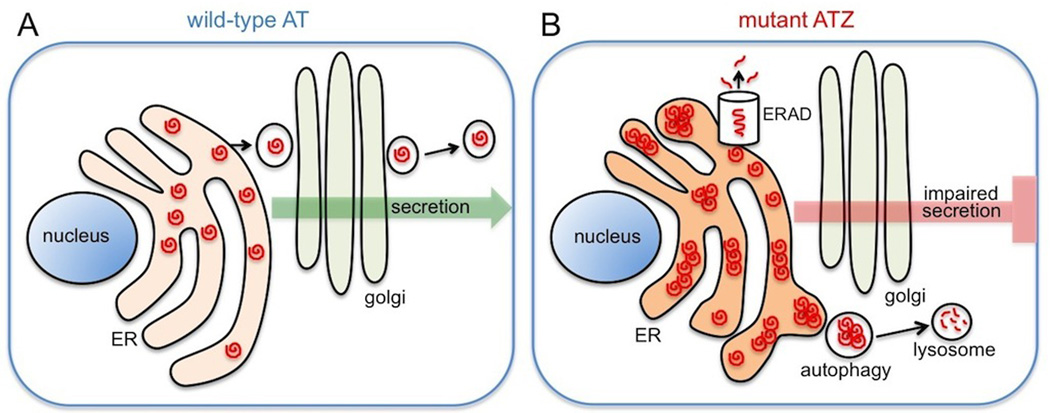
A major advantage of using C. elegans is the ability to perform powerful forward and reverse genetic analyses. A model of AT-deficiency that faithfully recapitulates the pathologies associated with the disease should be a useful tool for identifying genetic factors that influence disease outcome. A transgenic model of AT-deficiency has recently been developed by expressing wild-type and mutant (Z) human AT in the intestine of C. elegans ((Gosai et al., 2010)). As observed in human hepatocytes, wild-type AT was efficiently secreted by the intestinal cells. In contrast, secretion of mutant ATZ was impaired and significant intracellular accumulation of ATZ was observed (Fig. 1). These results demonstrated that the cellular pathologies associated with ATZ accumulation in human hepatocytes could be reproduced in C. elegans and provided validation for the use of the C. elegans model for investigating genetic and chemical modifiers that alter ATZ accumulation.
Figure 1.
Schematic of C. elegans intestinal cells showing the different fate of wild-type ATM and mutant ATZ proteins. Wild-type ATM travels through the ER-golgi secretory pathway and is efficiently secreted out of the cell (A). In contrast mutant ATZ protein forms polymers/aggregates within the ER and fails to be secreted (B).
Currently, full organ transplantation is the only completely effective therapy available for liver disease associated with AT-deficiency. Several strategies that might ameliorate phenotypes associated with the disease are currently being considered. Glycerol and phenyl butyric acid (PBA), have been shown to elicit chemical chaperone activity in model cell lines and PBA mediated a substantial increase in serum levels of ATZ in the mouse model of AT deficiency (Burrows et al., 2000). Another strategy involves screening for small molecules that would fit into a small hydrophobic cavity on the surface of the ATZ molecule and therein reduce its polymerogenic properties (Parfrey et al., 2003). Also promising is the potential use of liver-directed gene transfer of transcription factor EB (TFEB) a master regulator of lysosomal function and autophagy. This technology has recently been used successfully in the PiZ transgenic mouse, resulting in a dramatic reduction in hepatic ATZ, liver apoptosis and fibrosis (Pastore et al., 2013). An unbiased high-throughput screen for drugs that ameliorate the accumulation of mutant ATZ has recently been described using C. elegans (Gosai, Kwak, 2010). The screen identified a number of FDA-approved drugs that significantly reduced the intracellular accumulation of ATZ. Studies suggest that some of these drugs function by enhancing ATZ clearance by activating autophagy. Studies to determine the efficacy of these drugs in reversing the phenotypes associated with AT-deficiency in mammalian systems are currently underway.
In summary, hepatocytes and intestinal cells of C. elegans share remarkable functional similarities. Cellular pathologies associated with AT-deficiency in the human hepatocytes can be successfully modeled in the intestinal cells of C. elegans. The C. elegans model was then used to conduct a high-throughput small molecule screen for drugs that alleviate misfolded protein accumulation. Modeling aspects of hepatocyte-related diseases in the C. elegans intestine provides an elegant approach for investigating complex genetic diseases and should enhance our understanding of disease pathogenesis and the development of effective new therapies.
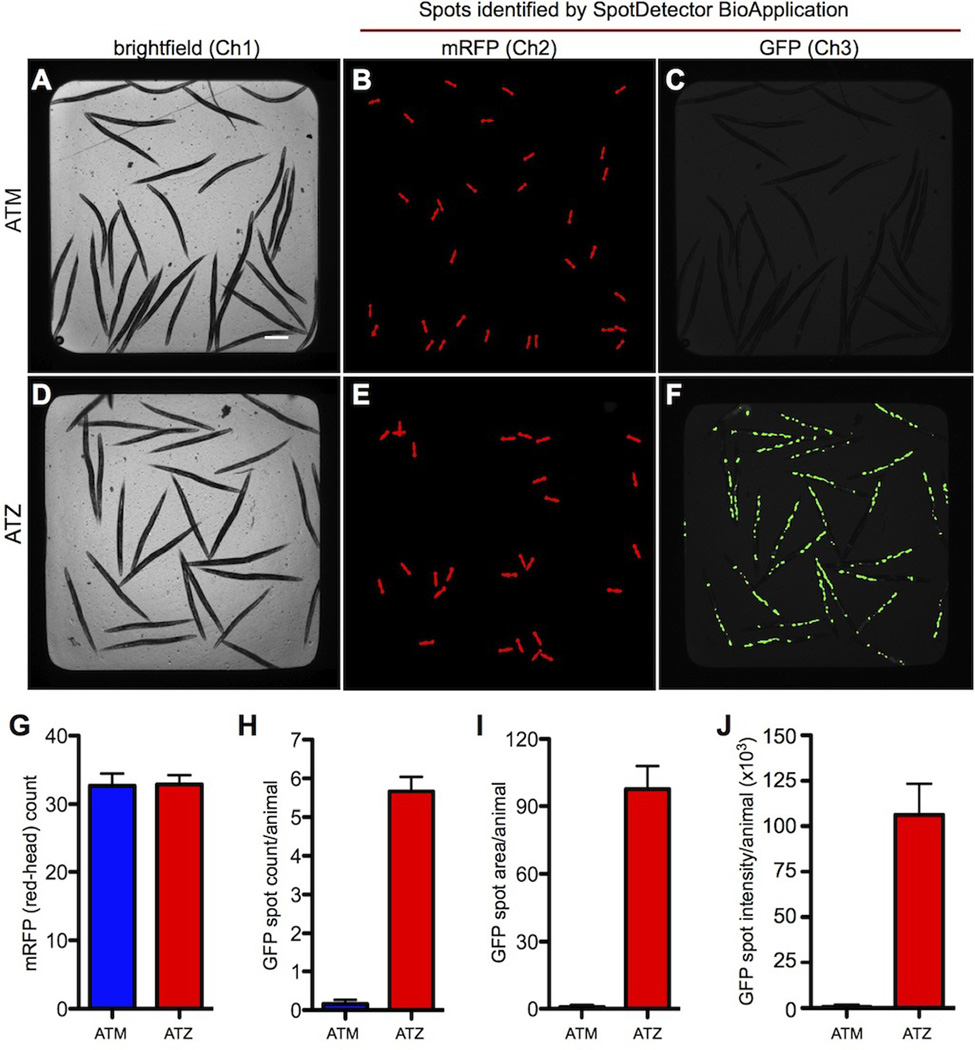
Figure 2. High-content assay for the analysis of transgenic animals expressing the wild-type (ATM) and mutant (ATZ) forms of human α1-antitrypsin (AT) fused to GFP.
(Gosai, Kwak, 2010) Brightfield (A and D) and fluorescent (B–C and E–F) images of animals expressing sGFP::ATM (upper panel) and sGFP::ATZ (lower panel). Note the accumulation of GFP-positive, protein aggregates in (F) that is lacking in (C). Bar graphs showing the average number of transgenic animals in each well (G) and the average number (H), area (I) and intensity (J) of the protein aggregates detected within the intestinal cells of animals expressing wild-type ATM (blue) and mutant ATZ (red). Figure reproduced from (Gosai, Kwak, 2010).
Cell Facts.
Hepatocytes are the main cell type of the liver that account for approximately 70–80% of the hepatic mass
Hepatocytes perform multiple functions including synthesis of proteins, cholesterol, phospholipids and metabolism of fat, glucose and xenobiotics
AT-deficiency is one of the most common genetic disorders affecting hepatocytes
The intestine of C. elegans shares remarkable similarities with the human liver and performs many of the specialized functions of hepatocytes
A model of AT-deficiency in C. elegans may facilitate identification of genetic modifiers and could accelerate development of novel therapies
ACKNOWLEDGMENTS
This work was supported by grants from The Hartwell Foundation, and the National Institutes of Health (DK079806, DK081422, DK084512, DK096990). Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- Branicky R, Desjardins D, Liu JL, Hekimi S. Lipid transport and signaling in Caenorhabditis elegans. Dev Dyn. 2010;239:1365–1377. doi: 10.1002/dvdy.22234. [DOI] [PubMed] [Google Scholar]
- Burrows JA, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc Natl Acad Sci U S A. 2000;97:1796–17801. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JA, Rogers BB, Sifers RN, Finegold MJ, Clift SM, DeMayo FJ, et al. Accumulation of PiZ alpha 1-antitrypsin causes liver damage in transgenic mice. J Clin Invest. 1989;83:1183–1190. doi: 10.1172/JCI113999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell RW, Lomas DA. Alpha1-antitrypsin deficiency--a model for conformational diseases. N Engl J Med. 2002;346:45–53. doi: 10.1056/NEJMra010772. [DOI] [PubMed] [Google Scholar]
- de Serres FJ. Worldwide racial and ethnic distribution of alpha1-antitrypsin deficiency: summary of an analysis of published genetic epidemiologic surveys. Chest. 2002;122:1818–1829. doi: 10.1378/chest.122.5.1818. [DOI] [PubMed] [Google Scholar]
- Decaens C, Durand M, Grosse B, Cassio D. Which in vitro models could be best used to study hepatocyte polarity? Biol Cell. 2008;100:387–398. doi: 10.1042/BC20070127. [DOI] [PubMed] [Google Scholar]
- Eriksson S, Carlson J, Velez R. Risk of cirrhosis and primary liver cancer in alpha 1-antitrypsin deficiency. N Engl J Med. 1986;314:736–739. doi: 10.1056/NEJM198603203141202. [DOI] [PubMed] [Google Scholar]
- Gosai SJ, Kwak JH, Luke CJ, Long OS, King DE, Kovatch KJ, et al. Automated high-content live animal drug screening using C. elegans expressing the aggregation prone serpin alpha1-antitrypsin Z. PLoS One. 2010;5:e15460. doi: 10.1371/journal.pone.0015460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidvegi T, Ewing M, Hale P, Dippold C, Beckett C, Kemp C, et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329:229–232. doi: 10.1126/science.1190354. [DOI] [PubMed] [Google Scholar]
- Hidvegi T, Schmidt BZ, Hale P, Perlmutter DH. Accumulation of mutant alpha1-antitrypsin Z in the endoplasmic reticulum activates caspases-4 and -12, NFkappaB, and BAP31 but not the unfolded protein response. J Biol Chem. 2005;280:39002–39015. doi: 10.1074/jbc.M508652200. [DOI] [PubMed] [Google Scholar]
- Jungermann K. [Regulation of liver functions by autonomic hepatic nerves] Naturwissenschaften. 1989;76:547–559. doi: 10.1007/BF00462861. [DOI] [PubMed] [Google Scholar]
- Jungermann K, Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989;69:708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- Kamimoto T, Shoji S, Hidvegi T, Mizushima N, Umebayashi K, Perlmutter DH, et al. Intracellular inclusions containing mutant alpha1-antitrypsin Z are propagated in the absence of autophagic activity. J Biol Chem. 2006;281:4467–4476. doi: 10.1074/jbc.M509409200. [DOI] [PubMed] [Google Scholar]
- Kanamura S, Kanai K, Watanabe J. Fine structure and function of hepatocytes during development. J Electron Microsc Tech. 1990;14:92–105. doi: 10.1002/jemt.1060140204. [DOI] [PubMed] [Google Scholar]
- Kruse KB, Brodsky JL, McCracken AA. Autophagy: an ER protein quality control process. Autophagy. 2006a;2:135–137. doi: 10.4161/auto.2.2.2388. [DOI] [PubMed] [Google Scholar]
- Kruse KB, Brodsky JL, McCracken AA. Characterization of an ERAD gene as VPS30/ATG6 reveals two alternative and functionally distinct protein quality control pathways: one for soluble Z variant of human alpha-1 proteinase inhibitor (A1PiZ) and another for aggregates of A1PiZ. Mol Biol Cell. 2006b;17:203–212. doi: 10.1091/mbc.E04-09-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom TH, Dodd AK. Xenobiotic detoxification in the nematode Caenorhabditis elegans. J Exp Zool A Comp Exp Biol. 2006;305:720–730. doi: 10.1002/jez.a.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Desjardins D, Branicky R, Agellon LB, Hekimi S. Mitochondrial oxidative stress alters a pathway in Caenorhabditis elegans strongly resembling that of bile acid biosynthesis and secretion in vertebrates. PLoS Genet. 2012;8:e1002553. doi: 10.1371/journal.pgen.1002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee JD. The C. elegans intestine. WormBook. 2007:1–36. doi: 10.1895/wormbook.1.133.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfrey H, Mahadeva R, Ravenhill NA, Zhou A, Dafforn TR, Foreman RC, et al. Targeting a surface cavity of alpha 1-antitrypsin to prevent conformational disease. J Biol Chem. 2003;278:33060–33066. doi: 10.1074/jbc.M302646200. [DOI] [PubMed] [Google Scholar]
- Pastore N, Blomenkamp K, Annunziata F, Piccolo P, Mithbaokar P, Maria Sepe R, et al. Gene transfer of master autophagy regulator TFEB results in clearance of toxic protein and correction of hepatic disease in alpha-1-anti-trypsin deficiency. EMBO Mol Med. 2013;5:397–412. doi: 10.1002/emmm.201202046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter DH. Liver injury in alpha1-antitrypsin deficiency: an aggregated protein induces mitochondrial injury. J Clin Invest. 2002;110:1579–1583. doi: 10.1172/JCI16787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12:201–213. doi: 10.1038/nri3169. [DOI] [PubMed] [Google Scholar]
- Qu D, Teckman JH, Omura S, Perlmutter DH. Degradation of a mutant secretory protein, alpha1-antitrypsin Z in the endoplasmic reticulum requires proteasome activity. J Biol Chem. 1996;271:22791–22795. doi: 10.1074/jbc.271.37.22791. [DOI] [PubMed] [Google Scholar]
- Rudnick DA, Liao Y, An JK, Muglia LJ, Perlmutter DH, Teckman JH. Analyses of hepatocellular proliferation in a mouse model of alpha-1-antitrypsin deficiency. Hepatology. 2004;39:1048–1055. doi: 10.1002/hep.20118. [DOI] [PubMed] [Google Scholar]
- Rudnick DA, Perlmutter DH. Alpha-1-antitrypsin deficiency: a new paradigm for hepatocellular carcinoma in genetic liver disease. Hepatology. 2005;42:514–521. doi: 10.1002/hep.20815. [DOI] [PubMed] [Google Scholar]
- Selden C, Khalil M, Hodgson HJ. What keeps hepatocytes on the straight and narrow? Maintaining differentiated function in the liver. Gut. 1999;44:443–446. doi: 10.1136/gut.44.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveger T. Liver disease in alpha1-antitrypsin deficiency detected by screening of 200,000 infants. N Engl J Med. 1976;294:1316–1321. doi: 10.1056/NEJM197606102942404. [DOI] [PubMed] [Google Scholar]
- Sveger T. The natural history of liver disease in alpha 1-antitrypsin deficient children. Acta Paediatr Scand. 1988;77:847–851. doi: 10.1111/j.1651-2227.1988.tb10767.x. [DOI] [PubMed] [Google Scholar]
- Sveger T, Eriksson S. The liver in adolescents with alpha 1-antitrypsin deficiency. Hepatology. 1995;22:514–517. doi: 10.1002/hep.1840220221. [DOI] [PubMed] [Google Scholar]
- Zorn AM. Liver development. 2008 [Google Scholar]