Abstract
Variants of the Bach2 gene are linked to vitiligo, celiac disease and type I diabetes, but the underlying immunological mechanisms are unknown. Here, we demonstrate that Bach2 plays crucial roles in maintaining T cell quiescence, and governing the differentiation, activation, and survival of foxp3+ Treg cells. Bach2-deficient T cells display spontaneous activation and produce elevated levels of TH1/TH2 type cytokines. Without Bach2, Treg cells exhibit diminished foxp3 expression, depleted numbers, hyper-activation, enhanced proliferation and profound loss of competitive fitness in vivo. Mechanistically, reduced survival of Bach2-deficient Treg cells was associated with reduced Bcl-2 and Mcl-1 levels and elevated Bim:Bcl-2 ratio. Additionally, Bach2 deficiency induced selective loss of Helios− foxp3+ Treg cells and a Treg cell transcriptome skewed towards the TH1/TH2 effector program at the expense of the Treg program. In vitro experiments confirmed that Bach2: (1) is indispensable for TCR/TGF-β-induced foxp3 expression and (2) mitigates aberrant differentiation of Treg cells by repression of the competing Gata3-driven TH2 effector program. Importantly, perturbations in the differentiation of induced Treg cells was linked to a fatal TH2 type chronic inflammatory lung disease in Bach2-deficient mice. Thus, Bach2 enforces T cell quiescence, promotes the development and survival of Treg lineage, restrains aberrant differentiation of Treg cells and protects against immune -mediated diseases.
Introduction
Self-tolerance, the inability to elicit or sustain an adaptive immunologic response against a self-antigen, is a critical feature of the adaptive immune system (1–3). Multiple diverse mechanisms are necessary for the establishment and maintenance of self-tolerance, and their individual or collective failure may lead to life-threatening autoimmune disease (2–4). The mechanisms of self-tolerance can be broadly classified as recessive or dominant (2, 4). Recessive mechanisms include clonal deletion of immature self-reactive T cells in the thymus, and functional inactivation/anergy and apoptosis of mature auto-reactive T cells in the periphery. Dominant tolerance is primarily mediated by a subset of CD4 T cells termed regulatory T (Treg) cells that express the signature transcription factor foxp3. These Treg cells not only protect against autoimmunity, they restrain immune responses to foreign antigens in order to limit inflammation and immune-mediated tissue damage (5). Loss-of-function mutations in the foxp3 gene result in Treg cell deficiency, loss of self-tolerance, altered adaptive immune responses, and the development the devastating autoimmune diseases IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked)in people and scurfy mice (6, 7).
Treg cells are a heterogeneous population and have commonly been classified as either natural (nTreg) or peripherally derived (pTreg) cells according to the site at which they acquire their regulatory functions (1, 8). Both classes emerge from CD4 T cells that have successfully navigated thymus-dependent recessive mechanisms of self-tolerance. The development of the nTreg cell lineage proceeds in the thymus and this class yields the majority of Treg cells in the secondary lymphoid organs and peripheral tissues. In contrast, the pTreg cells develop from conventional CD4 T cells, which have disseminated to peripheral tissues such as the gut, and their development proceeds within those tissues under the influence of the local inflammatory and immunological milieu (1, 8). The ability of pTreg cells to differentiate in peripheral tissues greatly augments the regulatory capacity of the nTreg cells.
Regardless of origin, normal Treg cell development and acquisition of regulatory function are dependent on the induction and sustained expression of foxp3 (9–11). Therefore, foxp3 has been touted as a lineage-specifying master regulator for the establishment and maintenance of the Treg cell transcription program. However, there is mounting evidence that foxp3 alone might be insufficient for the induction and/or maintenance of the full spectrum of Treg cell characteristics and signature genes (12–14). Genome-wide gene expression profiling and computational network inference studies have suggested that the full induction of the Treg cell transcription program is dependent upon combinatorial association of foxp3 with a “quintet” of functionally redundant transcription factors such as IRF4, Eos, Lef1, Gata1 and Satb1 (12). Several additional transcription factors such as Bach2, Blimp1, Maf, Tcf1, and Xbp1 are also predicted to influence the Treg cell gene signature. Further characterization of these additional molecules and their role in the development and maintenance of the Treg cell transcriptional program is necessary for understanding the biology of these important cells, and may yield potential targets for the therapeutic interventions in cases where their critical regulatory functions fail.
Genome-wide analysis of foxp3 target genes has suggested that Bach2 is likely a target gene for foxp3, and foxp3 is predicted to down-regulate Bach2 in both thymic and peripheral Treg cells (10, 15). Bach2 was initially characterized as a B cell-specific transcriptional repressor tasked with opposing plasma cell differentiation and the maintenance of B cell identity (16–18), but evidence now suggests that it is also expressed in T cells. Importantly, in Treg cells, Bach2 has now been identified as a target gene for the transcription factor FoxO1 (19), and Fu et al. predicted that Bach2 expression would potentially influence 14 genes within the Treg signature (12). Importantly, in humans, genome-wide association studies have linked Bach2 gene variants to immune-mediated diseases such as celiac disease, vitiligo and Type I diabetes (20–22). Despite this, the actual role of Bach2 in T cell homeostasis, Treg cell differentiation or maintenance is yet to be determined. In this manuscript, we report that Bach2 plays a vital role in regulating the homeostasis of conventional T cells and foxp3+ Treg cells by cell intrinsic mechanisms. Specifically, we show that Bach2 imparts competitive fitness to foxp3+ Treg cells and represses the activation and proliferation of foxp3+ Treg cells. Further, we find that full induction of foxp3 and TGF-β-induced differentiation of Treg cells requires Bach2. Mechanistically, Bach2 is an integral member of the transcriptional network that promotes the differentiation of Helios− foxp3+ Treg cells by supporting the Treg transcriptional program at the expense of the effector T cell program. In the absence of Bach2, altered T cell homeostasis, and perturbations in the development of induced Treg cells led to a fatal TH2 type immune-mediated disease.
Materials and Methods
Mice
Bach2 KO mice on the B6 background were a kind gift from K. Igarashi (Tohoku University, Sendai, Japan) (18). Littermate WT mice were used as controls. C57BL/6 (B6) and C57BL/6 Ly5.1+ mice were purchased from the National Cancer Institute or The Jackson Laboratory (Bar Harbor, ME). All mice were housed in specific-pathogen-free conditions in the animal facilities at the University of Wisconsin-Madison (Madison, WI). Experiments were conducted in accordance with the approved protocols of the institutional animal care committee.
Lymphocyte isolation and flow cytometry
Mononuclear cells from thymus and spleen were prepared using standard techniques. Lamina propria (LP) lymphocytes were isolated from the entire small intestine. Following excision of the Peyer’s patches, small intestine was washed, cut into pieces and incubated in HBSS/HEPES Bicarbonate buffer containing 15.4 mg/ml dithioerythritol at 37°C for 30 min. After washes, the LPs were dissociated in type I collagenase solution at 37°C for 50 min. LP lymphocytes were purified on a percoll gradient. Cells isolated from various tissues were stained with antibodies diluted in staining buffer (2% BSA in PBS). Fluorochrome-labeled anti-CD4 (RM4-5), anti-CD8 (53-6.7), anti-CD25 (PC61.5), anti-GITR (DTA-1), anti-CD44 (IM7), anti-CD62L (MEL-14), anti-CD69 (H1.2F3), anti-CD127 (A7R34), anti-KLRG1 (2F1), anti-CD45.2 (Ly5.2, 104), anti-IFN-γ (XMG1.2), anti-TNF-α (MP6-XT22), anti-IL-4 (BVD4-1D11), anti-IL-13 (eBio13A), anti-Ki67 (B56), anti-Foxp3 (FJK-16S), anti-CTLA4 (UC10-4B9), anti-Bcl-2 (3F11), anti-Gata3 (L50-823) and T-bet (eBio4B10) antibodies were purchased from BD Biosciences (San Jose, CA) or e-Bioscience (San Diego, CA). The anti-granzyme B (GB11) antibody was purchased from Invitrogen (Grand Island, NY). Anti-Bim and anti-Tcf1 (C63D9) were purchased from Cell Signaling (Danvers, MA). Anti-Helios (22F6) antibody was purchased from BioLegend (San Diego, CA), and anti-Blimp1 (3H2-E8) antibody was purchased from Novus Biologicals (Littleton, CA). Intracellular foxp3 was stained using the Foxp3 Staining Kit (eBioscience). For intracellular cytokine staining, cells were fixed, permeabilized with Cytofix/Cytoperm (BD Biosciences) following cell surface staining. All samples were acquired with FACSCalibur or LSR II (BD Bioscience). Data were analyzed with FlowJo software (TreeStar, Ashland, OR).
Quantitative RT-PCR and DNA microarray
CD4 T cells were purified from spleen of WT or Bach2 KO mice by negative selection using MACS (Miltenyi Biotec, Auburn, CA). To obtain Treg cells, purified CD4 T cells were stained with anti-CD4, anti-CD25 and anti-GITR, and sorted using a FACSAria II instrument (BD Biosciences). RNAs from purified cells were extracted by RNeasy kit (Qiagen, Valencia, CA), and cDNA was synthesized with the Superscript III reverse transcription kit (Invitrogen). qPCR reactions were done with the Power SYBR Green PCR Master Mix (Applied Biosystems, Grand Island, NY), and data were collected by Applied Biosystems 7300 Real-Time PCR System. Equivalent amount of cDNA (as determined by 18S rRNA measurements) were amplified in 40 cycles of PCR. For microarray analysis, Gene Expression Center at University of Wisconsin-Madison performed BeadChip Mouse Ref-8 V 2.0 (Illumina, San Diego, CA) using 200ng of total RNA extracted as previously described. Gene expression profile was analyzed by using GeneSpring software (Agilent, Santa Clara, CA). The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (GEO) and are accessible through GEO Series accession number GSE52337 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE52337).
Generation of mixed bone marrow chimeras
For generating mixed bone marrow chimeras, bone marrow cells (BMCs) were collected by flushing the marrow from the femurs and tibias from WT and Bach2 KO mice with RPMI 1640 media. A 1:2 mixture of BMCs from WT (Ly5.1; 5 × 106 BM cells) and WT (Ly5.2; 10 × 106 BM cells) or Bach2 KO (Ly5.2; 10 × 106 BM cells) mice were adoptively transferred into lethally irradiated (800 rads) B6/Ly5.1 mice. Bone marrow-reconstituted B6 mice were treated with neomycin (0.025 mg/ml) and polymyxin B (0.013 mg/ml; Sigma-Aldrich, St. Louis, MO) in drinking water for up to 8 weeks, and reconstitution of the lymphoid system by the donor BMCs was assessed at 8 weeks.
In vitro Treg cell differentiation
Splenic CD4+ CD25− GITR− T cells sorted by flow cytometry were used at a density of 2 × 105 cells per well in flat-bottom 96-well plate pre-coated with anti-CD3 (2μg/ml). Cells were cultured for 72 h with IL-2 (100 U/ml) in the presence or absence of different concentrations of recombinant human TGF-β (Peptro Tech, Rocky Hill, NJ). Foxp3 expression was assessed by flow cytometry.
Histopathology
Representative tissue sections were fixed in 10% neutral buffered formalin. The fixed tissues were paraffin embedded and 5-micron-thick sections were routinely stained with hematoxylin and eosin. Photomicrographs were acquired with cellSens software (Olympus). The GNU Image Manipulation Program version 2.8.4 was utilized to improve uniformity of brightness and contrast between individual images, and to remove adjacent lung sections from Figures 6A and 6B.
Figure 6. Bach2 KO mice develop lung pathology.
B6 WT and Bach2 KO mice were sacrificed and examined by H&E stain for lung immune-pathology. (A) WT mouse, 15x original magnification. Normal lung with large caliber bronchioles and pulmonary vessels located centrally (stars), and well-defined small-caliber and terminal airways and alveoli located peripherally (arrowheads). (B) Bach2 KO mouse, 15x original magnification. Lung with eosinophilic crystalline pneumonia (ECP) exhibiting regional loss of alveolar spaces resulting from densely cellular alveolar exudates and interstitial infiltrates (arrowheads). (C) Bach2 KO mouse, 200x original magnification. Lung with ECP: Multiple alveolar spaces are filled with aggregates of hypereosinophilic inflammatory cells (stars). Infiltrates of eosinophils, histiocytes, and plasma cells expand the alveolar walls (arrows). Plasma cells and eosinophils encircle a pulmonary vein (arrowhead) (D) Bach2 KO mouse, 600x original magnification. Lung with ECP, affected alveolus: The densely cellular alveolar exudate is primarily composed of tightly packed variably sized macrophages containing intracytoplasmic hypereosinophilic acicular crystals(star). Eosinophils expand the adjacent interstitum (arrow). (E) Bach2 KO mouse, 600x original magnification. Lung with ECP: A medium-caliber pulmonary vein (star) is encircled by a cuff of plasma cells (arrowheads) admixed with fewer eosinophils and small lymphocytes. (F) Bach2 KO mouse, 600x original magnification. Densely cellular aggregates of eosinophils, plasma cells, and lymphocytes distrupt and fill an airway in a 7 month-old mouse with severe concurrent multisystemic eosinophilic and plasmacytic inflammation. (G) mRNAs of indicated cytokines in WT and Bach2 KO lung lysates were quantitated by qPCR.
Statistical analyses
Data statistics were determined using SigmaPlot software. Student’s two-tailed t-test was used to calculate the statistical significance of differences between groups, and significance was defined at p < 0.05. Error bars represent SEM.
Results
Bach2 controls peripheral Tcell quiescence
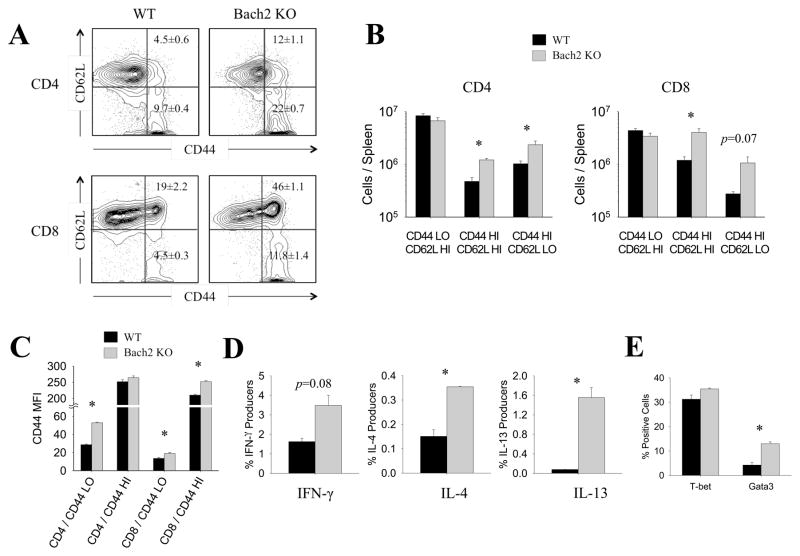
Our laboratory reanalyzed previously published T cell microarray data to identify candidate transcription factors predicted to impact T cell differentiation, and found strong evidence that Bach2 mRNA was expressed in T cells (23, 24). Additionally, Bach2 gene variants have been linked to autoimmune diseases including celiac disease, vitiligo and Type I diabetes (20–22). We investigated the extent to which Bach2 was required for peripheral T cell homeostatic maintenance using Bach2-deficient (Bach2 KO) mice. A striking increase in the percentages and total numbers of CD4 and CD8 T cells with the activated/memory (CD44HI) phenotype occurred in spleens of Bach2 KO mice, as compared to those in wild type (WT) mice (Fig. 1A and 1B). Notably, Bach2 deficiency increased the numbers of CD4 and CD8 T cells with the activated (CD44HI/CD62LHI) and effector (CD44HI/CD62LLO) phenotypes. Interestingly, regardless of their activation status (naïve/CD44LO versus activated/CD44HI), Bach2-deficient CD4 and CD8 T cells expressed higher levels of cell surface CD44 per individual cell, than their WT counterparts (Fig. 1C). Notably, a larger fraction of the Bach2 KO CD4 T cells produced readily detectable levels of TH1 (IFN-γ) and TH2 (IL-4 and IL-13) type cytokines (Fig. 1D). The enhanced productions of TH1 and TH2 cytokines by Bach2 KO CD4 T cells were consistent with increased levels of the effector lineage-specific transcription factors, T-bet and Gata3 respectively (Fig. 1E). Collectively, data in Fig. 1 suggested that Bach2 promotes T cell quiescence by repressing the activation and differentiation of TH1/TH2 effector CD4 T cells under normal homeostatic conditions.
Figure 1. Bach2 regulates peripheral T cell homeostasis.
Splenocytes were collected from naïve WT and Bach2 KO mice. (A–C) Activation of T cells was analyzed by staining with anti-CD44 and anti-CD62L. (A) Frequencies of naïve versus activated CD4 and CD8 T cells. (B) Numbers of naïve versus activated CD4 and CD8 T cells were calculated per spleen. (C) Median fluorescence intensity (MFI) of CD44 for each subset is plotted. (D) Splenocytes were stimulated for 5 hrs in vitro with PMA and ionomycin in the presence of Brefeldin A, and cytokine production was measured by intracellular staining. (E) Expression of transcription factors such as T-bet and Gata3 was assessed by flow cytometry following intracellular staining. Data are representative of two independent experiments. *p < 0.05
Bach2 regulates the homeostasis of foxp3+ regulatory T cells
Treg cells have been previously reported by the Rudensky group to exhibit dynamic developmental alterations in Bach2 expression (10, 15), and the Benoist group subsequently predicted that Bach2 expression can potentially influence the transcriptional signature of Treg cells (12). Therefore, we next investigated whether the dysregulated T cell homeostasis resulting from Bach2 deficiency extended to alterations in the number and phenotype of foxp3+ Treg cells in the thymus and spleen. As illustrated in Fig. 2A, we identified a small but reproducible and statistically significant reduction in the percentages (1.3-fold, p<0.06) and total number(1.7-fold, p<0.045) of foxp3+ Treg cells in the thymus of Bach2 KO mice. Further, these thymic Treg cells exhibited an activated phenotype characterized by elevated levels of CD25, CD44 and GITR expression. Bach2 deficiency did not, however, affect Treg cell expression of CTLA4 in the thymus (Fig. 2B). In contrast to the less dramatic thymic Treg cell alterations, Bach2 deficiency resulted in a marked reduction in the frequency and number of Treg cells in the spleen (Fig. 2C). The frequencies of splenic Treg cells in Bach2 KO mice were only 57% of those exhibited by WT mice. Further, Foxp3+ Treg cells in spleen of Bach2 KO mice displayed enhanced levels of CD44, CTLA4, GITR, KLRG1, CD127 (the IL-7 receptor), Granzyme B and Blimp1 a phenotype that is considered as prototypical for effector Treg (eTreg) cells (Fig. 2D, 2E and 2I) (25).
Figure 2. Bach2 controls the homeostasis of regulatory T cells.
Spleens and thymus were collected from naïve WT and Bach2 KO mice (over 9 weeks old), and analyzed for Treg cells by flow cytometry. (A) Frequency and number of thymic Treg cells. (B) Phenotypic analysis of thymic foxp3+ Treg cells. (C) Frequency and numbers of splenic Treg cells. (D) Phenotypic analysis of splenic foxp3+ Treg cells. (E) Surface expression of KLRG1 and intracellular expression of granzyme B were analyzed for foxp3+ Treg cells by flow cytometry. (F) Bcl-2 and Bim expression was assessed by intracellular staining in splenic foxp3+ Treg cells and foxp3− conventional CD4 T cells; numbers indicates MFI of Bcl-2. (G) Treg (CD4+ CD25+ GITR+) cell apoptosis was measured by Annexin V staining directly ex vivo. (H) Ki67 staining on thymic and splenic foxp3+ Treg cells. (I) Expression levels of foxp3, blimp1 and Tcf1 on thymic (upper panel) and splenic (lower panel) foxp3+ Treg cells were assessed by flow cytometry. (J) Treg cells from mLN nodes and small intestinal LP were quantified by flow cytometry. Data are representative of two independent experiments. *p < 0.05
Next, we characterized the expression of Bcl-2, Bim, Ki67 and Annexin V in Treg cells from Bach2 KO mice to investigate whether the diminished number of Treg cells resulted from defects in cell survival and/or proliferation. The balance between Bcl-2 and Bim is believed to be important for survival of Treg cells (26). We found that the levels of Bcl-2 in Bach2 KO Treg cells were dramatically lower than in WT Treg cells (Fig. 2F), and the resulting Bim: Bcl-2 ratios were substantially increased in Bach2 KO Treg cells (Fig. 2F). The mRNA levels for the anti-apoptotic molecule Mcl-1 were also lower in Bach2 KO Treg cells (Fig. 3A) (27). Additionally, higher percentages of Treg cells from Bach2 KO mice exhibited the apoptotic annexin V+ phenotype (Fig. 2G). To examine the effect of Bach2 deficiency on the proliferation of foxp3+ Treg cells, we compared the cell cycle status of WT and Bach2 KO Treg cells by staining for the proliferative marker Ki67. Bach2 deficiency augmented the frequency of Ki67+ Treg cells in the thymus and spleen (Fig. 2H). In spleen, while only 20% of WT Treg cells expressed Ki67, 50–60% of Treg cells were Ki67+ in the Bach2 KO mice. The increased Ki67+ cells among Bach2 KO foxp3+ Treg cells suggested that Bach2 exerts anti-proliferative effects. It is also possible that increased proliferation of Bach2 KO Tregs might reflect compensatory proliferation that occurs under conditions where Treg cells’ maintenance is compromised (28). The intestinal tract is an anatomical location that is critical for the peripheral induction of Treg cells. To assess whether Bach2 regulates accumulation of Treg cells in the intestines, we quantified foxp3+ Treg cells in the mesenteric lymph nodes (mLNs) and lamina propria (LP). Interestingly, there was a striking reduction in the frequencies of CD4 T cells in the LP (Fig. 2J) but not in the mLNs (not shown) of Bach2 KO mice. Consistent with other tissues examined, the percentages of Tregs in mLNs (p<0.0004) and LP (p<0.002) were significantly reduced in Bach2 KO mice (Fig. 2J). Taken together, data in Fig. 2 suggested that Bach2 plays a non-redundant role in Treg cell homeostasis in lymphoid and non-lymphoid tissues by regulating the activation, generation and maintenance of Treg cells.
Figure 3. Bach2 regulates the balance of the effector and Treg transcription programs.
Whole genome transcriptional profiles from WT and Bach2 KO CD4+ CD25+ GITR+ splenic Tregs were analyzed by DNA microarray. (A) Scatter plot represents comparison of normalized expression values in WT versus Bach2 KO. (B and C) Expression of genes associated with TH1, TH2, TH17 or Treg functions in WT and Bach2 KO mice is presented as Box Whisker plots (B) and heat maps(C).
To determine whether the reduced foxp3+ Treg cell number in Bach2 KO mice was associated with dysregulated foxp3 expression, we compared the level of foxp3 expressed per Treg cell in WT and Bach2 KO mice. Notably, the expression of foxp3 protein was markedly reduced in Bach2 KO Treg cells in the spleen, but not in the thymus (Fig. 2I). This prominent feature was consistently documented in Bach2 KO mice that are > 9–10 weeks of age. A recent study has predicted Tcf1 and Blimp1 to be important transcription factors (other than foxp3) that can potentially influence the expression of several genes constituting the Treg signature (12). Compared to WT Treg cells, Bach2 KO Treg cells displayed severely reduced Tcf1 expression, whereas Blimp1 levels were elevated (Fig. 2I). Based on these data, we inferred that Bach2 promotes the Treg cell transcriptional program by regulating the levels of foxp3, Tcf1 and Blimp1.
Bach2 governs the balance of the effector and Treg transcription programs during Treg cell differentiation
To understand the transcriptional basis for the altered Treg cell homeostasis in Bach2 KO mice, we compared the transcriptomes of CD4+CD25+GITR+ WT and Bach2 KO Treg cells. The scatter plot in Fig. 3A illustrates the altered expression of multiple genes that are known to regulate Treg cell development and/or homeostasis. First, the expressions of gata1 and lef1 that are members of the quintet of transcription factors that promote Treg differentiation were substantially reduced in Bach2 KO Treg cells (12). Likewise, Bach2 deficiency resulted in reduced levels of foxo1 (29, 30), id3 (31) and rara (32, 33), which are known to play prominent roles in the development of Tregs. Signaling molecules tnfsf4 and tnfrsf4 (encodes OX40L and OX40, respectively) inhibit foxp3 expression in Treg cells (34, 35), and interestingly, their expressions were elevated in Bach2 KO Treg cells. The elevated levels of prdm1 (encodes Blimp1) and irf4 along with itgae (CD103) and klrg1 are consistent with the activated or effector phenotype of Tregs in Bach2 KO mice (Fig. 2E and 2I) (25, 36). Additionally, genes like pde3b (10) and satb1 (37) that are typically expressed at low levels in Tregs were further decreased in Bach2 KO Treg cells. Bach2 deficiency was associated with diminished expression of pro-survival molecules bcl2 and mcl1, which might explain the enhanced apoptosis of Bach2 KO Treg cells (27). These data suggested that Bach2 might promote Treg cell homeostasis by activating genes that control their differentiation and survival.
The relative dominance of the various competing effector transcription programs versus the foxp3-centric Treg cell transcription program is a key determinant factor in the differentiation and stability of the Treg cells. Therefore, we investigated whether Bach2 deficiency affected the competing TH1, TH2 or the TH17 transcriptional program in Treg cells (37). As illustrated in Fig. 3B and 3C, genes associated with TH1 were up-regulated in Bach2 KO Treg cells and included the signature transcription factor tbx21 (encodes T-bet), ifng and il12rb2. Much more impressive was the marked skewing of the transcriptional profile towards the TH2 lineage in Bach2 KO Treg cells; the expression of the TH2 lineage-defining transcription factor gata3 along with TH2 type cytokines genes including il4, il5, il6 and il13 were increased in Bach2 KO Treg cells. Although the expression of rorc (encodes Ror-γt) was decreased (Fig. 3B and 3C), other genes associated with TH17 were largely unaltered in the absence of Bach2. On the basis of these data, we inferred that Bach2 might enable the differentiation of Treg cells by repression of the competing TH1 and TH2 effector transcriptional program.
A cell-intrinsic role for Bach2 in regulating the homeostasis of conventional and foxp3+ Treg cells
To assess whether Bach2 regulated the development and/or survival of foxp3+ Tregs by cell-intrinsic mechanism(s), we generated mixed bone marrow (BM) chimeras by reconstituting lethally irradiated WT/Ly5.1 mice with a mixture of BM cells from WT/Ly5.1 and WT/Ly5.2 or Bach2 KO/Ly5.2 mice. Control chimeras (CCs) were reconstituted with a mixture of BM cells from WT/Ly5.1 and WT/Ly5.2 mice while the experimental chimeras (ECs) were derived by reconstitution of WT/Ly5.1 mice with BM cells from WT/Ly5.1 and Bach2 KO/Ly5.2 mice. Eight weeks after reconstitution, the percentages of activated cells were greater amongst Bach2 KO CD4 and CD8 T cells, as compared to their WT counterparts (Fig. 4A). Additionally, in the thymus and spleen of CCs, the percentages of foxp3+ Treg cells among WT/Ly5.1 CD4 T cells were slightly higher than among WT/Ly5.2 CD4 T cells (Fig. 4B). In striking contrast, the percentages of foxp3+ Treg cells among Bach2 KO/Ly5.2 CD4 T cells in the thymus and spleen of ECs were 5-6-fold lower, as compared to the WT/Ly5.1 CD4 T cells in the same mouse (Fig. 4B). Direct comparison between WT/Ly5.2 CD4 T cells in CCs with Bach2 KO/Ly5.2 CD4 T cells in the ECs also showed 3-6-fold reduction in the percentages of foxp3+ Treg cells among Bach2 KO CD4 T cells (Fig. 4B). Thus, Bach2 KO foxp3+ Treg cells displayed loss of competitive fitness to develop and/or persist in mixed BM chimeras. The Bach2 KO Treg cells in ECs expressed more Blimp1, and a greater percentage of Bach2 KO foxp3+ Treg cells were Ki67+ and expressed higher levels of CD44 and CD69, as compared to their WT/Ly5.2 or WT/Ly5.1 counterparts (Fig. 4C). Thus, the increased proliferation and the effector phenotype (CD44HI/CD69HI/Blimp1HI) of Bach2 KO foxp3+ Treg cells are consequential to cell-intrinsic loss of Bach2. By extension, these data imply that Bach2 might repress the activation and proliferation of effector foxp3+ Treg cells by cell-intrinsic mechanism(s). And, loss of Bach2 might result in premature activation, proliferation, and perhaps apoptosis of effector foxp3+ Treg cells. Another notable finding was that Bach2 KO foxp3+ Treg cells expressed significantly lower levels of foxp3 (p<0.00003), in comparison to WT Treg cells (Fig. 4D). In summary, data in Fig. 4 provide compelling evidence that: (1) Bach2 promotes T-cell quiescence by T-cell-intrinsic mechanisms; (2) in the absence of Bach2, Treg cells fail to develop and/or persist in a competitive environment; (3) Bach2 restrains the activation and proliferation of Treg cells; (4) Bach2 is required for full induction of foxp3 in Treg cells by cell-intrinsic mechanism(s).
Figure 4. Bach2 regulates the homeostasis of Treg cells by cell-intrinsic mechanisms.
Mixed bone marrow chimeras were generated by the transfer of a mixture of bone marrow cells from WT/Ly5.1 and WT/Ly5.2 or Bach2 KO/Ly5.2 into lethally irradiated WT/Ly5.1 mice. While control chimeras were reconstituted by a mixture of WT/Ly5.2 and WT/Ly5.1 BM cells, experimental chimeras were reconstituted by the mixture of Bach2 KO/Ly5.2 and WT/Ly5.1 BM cells. At least eight weeks after reconstitution, thymus and spleens were harvested, and analyzed for Treg cells. (A) Spleens were harvested, and T cells were analyzed for their activation status. Cells are gated on WT/Ly5.2 (control chimera) and Bach2 KO/Ly5.2 (experimental chimera) for direct comparison. Frequencies and numbers of naïve versus activated CD4 and CD8 T cells were assessed and calculated per spleen. (B) Frequencies of foxp3+ Treg cells among the gated cells in both thymus and spleen were measured by flow cytometry. (B and C) Surface markers and Treg cell markers on WT/Ly5.2 (control chimera) and Bach2 KO/Ly5.2 (experimental chimera) Treg cells were measured by flow cytometry. (C) Frequency of Ly5.2+ Treg cells positive for CD44, CD69 and Ki67 and Blimp1 MFI. (D) MFIs of foxp3 for Ly5.2+ WT and Bach2 KO Treg cells. Data are representative of two independent experiments.* p < 0.05.
Bach2 promotes the induction of foxp3 and generation of regulatory T (iTreg) cells by repressing the effector transcription program
Analyses of Bach2 KO mice and mixed bone marrow chimeras clearly demonstrated a vital role for Bach2 in determining the competitive fitness of foxp3+ Treg cells. It was of interest to investigate whether Bach2 regulated the development and/or persistence of subsets of Treg cells. The expression level of Helios, an Ikaros transcription factor, has been used to define subsets of Treg cells in the periphery (38). Intriguingly, we found that the percentages and numbers of Helios+ foxp3+ Treg cells were not significantly different in spleens of WT and Bach2 KO mice (Fig. 5A). However, in Bach2 KO mice, the frequency (3-fold, p<0.02) and numbers (5-fold, p<0.03) of splenic Helios− foxp3+ Treg cells were dramatically reduced, as compared to those in WT mice. These data suggested that that Bach2 deficiency selectively impaired the development and/or survival of the Helios− foxp3+ Treg cells in the periphery (Fig. 5A).
Figure 5. Bach2 is required for the induction of foxp3 and differentiation of iTregs.
(A) Splenocytes from WT and Bach2 KO mice were stained with anti-CD4, anti-foxp3 and anti-Helios. Frequencies and numbers of Helios+ and Helios− Tregs are displayed. (B–F) Conventional CD4 T cells (CD25− GITR−) from WT and Bach2 KO mice were purified by FACS, and stimulated with anti-CD3 in the presence or absence of recombinant human TGF-β for 72 hr. (B) Representative contour plots depict foxp3 expression following in vitro Treg differentiation. Bar graph displays frequencies of foxp3+ iTregs with or without anti-CD3 in the presence of indicated concentrations of TGF-β. (C) MFIs of foxp3 and GITR for foxp3+ iTregs from WT and Bach2 KO are plotted. (D–E) RNA from cells cultured for iTreg cell differentiation was reverse transcribed. Relative levels of Treg signature transcription factors (D), CD4 T cell lineage-specific transcription factors (E) and cytokines (F) were quantified by quantitative RT-PCR. Data are representative of two independent experiments. *p < 0.05
Next, we asked whether the in vitro induction of foxp3 and generation/differentiation of foxp3+ iTreg cells requires Bach2. T cell receptor stimulation of naïve conventional WT CD4 T cells in the presence of TGF-β readily induced foxp3 expression in 26% of the stimulated WT conventional CD4 T cells (Fig. 5B). Remarkably, conventional CD4 T cells from Bach2 KO mice exhibited a profound impairment in foxp3 expression and differentiation into foxp3+ iTreg cells. Antigen receptor stimulation and exposure to TGF-β induced foxp3 only in 3% of the Bach2 KO conventional CD4 T cells (Fig. 5B). Moreover, iTreg cells that were derived from Bach2 KO CD4 T cells expressed low levels of foxp3 on a per cell basis (Fig. 5C). These data strongly suggested that Bach2 plays an essential role in the induction of foxp3 and generation of iTreg cells by cell-intrinsic mechanism(s).
Next, we sought to decipher the transcriptional basis for the impaired foxp3 expression in Bach2 KO CD4 T cells in response to TGF-β. Specifically we asked whether Bach2 deficiency dysregulated the expression of foxp3 and/or the “quintet” of transcription factors that govern the differentiation of Treg cells (12). As illustrated in Fig. 5D, the expression of foxp3 was reduced, but the levels of gata1 were particularly elevated in Bach2 KO CD4 T cells. While the levels of ikzf4 (encodes Eos) and lef1 were mildly altered in Bach2 KO CD4 T cells, loss of Bach2 elevated the expression of ikzf2 (encodes Helios) and xbp1. We also interrogated whether loss of Bach2 resulted in aberrant induction of the helper T cell effector program at the expense of the Treg cell differentiation. Notably, concomitant with impaired expression of foxp3, the levels of TH2 type effector lineage-specification factor gata3, and prdm1 were strongly induced in Bach2 KO CD4 T cells (Fig. 5D and 5E). Strikingly, enhanced gata3 expression was associated with a dramatic induction (>1,000-fold) of IL-4 in Bach2 KO CD4 T cells (Fig. 5F). Interestingly, in the absence of Bach2, the levels of IFN-γ were also noticeably elevated with minimum alterations in the induction of tbx21. IL-10 expression by eTreg cells requires Blimp1 (25), and consistent with this report, enhanced Blimp1 levels was associated with markedly elevated levels of IL-10 mRNA in Bach2 KO CD4 T cells (Fig. 5F). The elevated expression of Blimp1 in the absence of Bach2 is likely a sequel to loss of Bach2-mediated repression of Blimp1. As compared to augmented expressions of IL-4, IFN-γ and IL-10, Bach2 deficiency resulted in less impressive alterations in the levels of IL-17. Taken together, data in Fig. 5 provide compelling evidence that Bach2 plays an essential role in the TGF-β-induced expression of foxp3 and in vitro differentiation of Treg cells. Additionally, these data suggested that Bach2 represses the effector TH1/TH2 transcription program and promotes the commitment of CD4 T cells to the Treg program. This inference is consistent with a recent report that came out when this manuscript was in preparation (39).
Bach2 KO mice develop a fatal lung disease
Strikingly, we observed an exceptionally high incidence of eosinophilic crystalline pneumonia (ECP) and eosinophilic diseases in our colony of Bach2 KO mice. Through opportunistic postmortem sampling of lung tissue, the characteristic histologic lesions of ECP were first identifiable in some Bach2 KO mice at 8 weeks of age. Beyond 8 weeks, Bach2 KO mice exhibited a progressive age-dependent increase in morbidity and mortality. Some Bach2 KO mice exhibited moderate respiratory distress and hunched posture by 4 months of age, and by 7–8 months of age nearly all Bach2 KO mice displayed severe respiratory distress necessitating humane euthanasia.
Compared to normal histology of WT lungs (Fig. 6A), ECP lung lesions were present in only a few foci in younger subclinical mice, while the lesions were widespread in lungs of older clinically affected Bach2 KO mice (Fig. 6B). Histologically, there was disruption of the alveolar architecture by intra-alveolar aggregates of large macrophages that had hypereosinophilic acicular intracytoplasmic crystals (Fig. 6D), which are primarily composed of YM1 protein (also referred to as chitinase 3-like 3 crystals; Chi313), a secretory product of pulmonary macrophages (40, 41). Notably, the genesis of YM1 protein-containing crystals in ECP and the Charcott-Leyden-like crystals (found in the lungs of human asthmatic patients) are both products of dysregulated TH2 type immunity in the lungs (41, 42). Affected alveoli were lined by type II pneumocytes, and the interstitium was expanded by lymphoplasmacytic, eosinophilic, and histiocytic infiltrates (Fig. 6C). In the most severe cases, some airways were also filled with dense aggregates of eosinophils (Fig. 6F). Pulmonary veins in affected lung sections were often encircled by dense cuffs of plasma cells admixed with fewer eosinophils and lymphocytes Additionally, atypical plasma cells exhibiting marked cytomegaly, and karyomegaly were a repeated finding in the perivascular cuffs of the most severely affected mice (Fig. 6E). Although ECP was the most common histologic lesion in the Bach2 KO mice, the most severely affected mice exhibited concurrent multi-systemic plasmacytic and eosinophilic inflammation. Consistent with the pattern of lung lesions, the expressions of IL-4 and IL-13 in particular and IL-17A levels were markedly elevated in the lungs of Bach2 KO mice (Fig. 6G). Taken together, the nature of the lung pathology along with elevated levels of IL-4 and IL-13 in the affected lungs are highly reminiscent of TH2 type immunity-driven chronic pulmonary inflammation. It should be noted that identical TH2 type chronic lung inflammation and ECP occur in mice that are selectively deficient for iTreg cells (41). Therefore, the fatal lung inflammation in Bach2 KO mice is likely a sequel to perturbations in the development of iTreg cells that are tasked to suppress chronic TH2 type lung inflammation.
Discussion
It is abundantly clear that Bach2 plays a pivotal role as a transcriptional repressor in B cells by opposing the differentiation of plasma cells from activated B cells. Since Bach2 is considered primarily as a B cell-specific transcriptional factor, its role in T cells remains largely unexplored. In this manuscript, we ascribe crucial regulatory roles for Bach2 in the homeostasis of T cells. Specifically, we document that Bach2 is an integral member of the transcriptional network that enforces quiescence of mature T cells and regulates the competitive fitness, activation, and differentiation of foxp3+ Treg cells. These findings have clinical implications because we also find that Bach2 deficiency results in fatal lung disease in mice and Bach2 variants are implicated in the genesis of autoimmune diseases in humans (20–22).
First, we show that in the global absence of Bach2, T cells display a spontaneously activated phenotype and produce inflammatory cytokines. Studies using mixed bone marrow chimeras suggest that Bach2 promotes the quiescence of T cells, at least in part by cell-intrinsic mechanisms (Fig. 4A). The intrinsic mechanisms of Bach2-mediated regulation of naïve T cell quiescence include repression of transcription factors such as T-bet, Blimp1 (not shown) and Gata3 that drive the effector program in T cells. By repression of these transcription factors, Bach2 might mitigate the aberrant and premature activation of peripheral T cells in response to homeostatic cues. Prominently, activated/effector CD4 T cells in Bach2 KO mice display a highly skewed TH2 type polarity, which accompanied a modest elevation of the TH1 type T cells. The skewing towards the TH2 type in Bach2 KO mice is not restricted to conventional CD4 T cells since activation of CD4 T cells under Treg-inducing conditions (with TGF-β) also induces high levels of IL-4 and Gata3. Thus, Bach2-deficient T cells have an increased propensity to differentiate into TH2 effector cells, which is likely linked to the loss of Bach2-dependent repression of Gata3 expression; when this manuscript was in preparation, it was reported that Bach2 might directly repress the induction of Gata3, at least in iTreg T cells (39).
In addition to the maintenance of T cell quiescence, Bach2 plays a vital role in maintaining the size of the Treg cell niche in the thymus and periphery; loss of Bach2 led to a substantive reduction in the number of foxp3+ Treg cells in the thymus and periphery. Our data suggest that regulation of Treg cell homeostasis by Bach2 is multifactorial and occurs by at least three mechanisms that are not mutually exclusive.
First, Bach2 confers competitive fitness for foxp3+ Treg cells in thymus and periphery, by cell intrinsic mechanisms that promote the differentiation and/or survival of foxp3+ nTreg cells. One mechanism by which Bach2 might confer competitive fitness for foxp3+ nTreg cells is by enhancing their survival because Bach2 KO Treg cells express diminished levels of the anti-apoptotic Bcl-2 and Mcl-1, which are known to promote the viability of Treg cells (26, 27). The control of Treg cell survival by Bach2 deficiency might include potential repression of cellular Bcl-2 and Mcl-1 expressions, resulting from elevated levels of Blimp1 (25).
Second, diminished levels of foxp3 in Bach2 KO Treg cells might reduce the stability of the Treg cell transcription program and increase the plasticity of these cells in the periphery. Direct ex vivo analysis of the transcriptomes of peripheral Treg cells from Bach2 KO mice suggest that the balance of the Treg and the effector transcription programs is skewed in the absence of Bach2, in favor of the latter. In addition to the reduced expression of Treg cell-associated signature genes, there was a concomitant elevation in the levels of the genes linked to the TH1/TH2 effector programs in Bach2 KO Treg cells. These data suggest that Bach2 likely favors the Treg development by inducing the Treg transcriptional program and repression of the competing TH1/TH2 effector programs. This idea is consistent with a report from the Flavell group, which shows that attenuated foxp3 expression in Treg cells results in lymphadenopathy and a fatal TH2 type immunopathology driven by aberrant differentiation of Treg cells into TH2 effector cells (9). Other groups also have demonstrated that pro-inflammatory cytokines such as IL-4, IL-21 and IFN-γ produced by effector/memory T cells strongly suppress foxp3 induction, and the effect is reversed by retinoic acid (RA) signaling (32, 33). Interestingly, according to our transcriptome analysis, the expression of RA receptor alpha is much decreased in Bach2 KO Treg cells in addition to increased levels of IL-4, IL-21 and IFN-γ. Therefore, the augmented TH1/TH2 immune environment in the Bach2 KO mice and the reduced expression of RA receptor might also dampen foxp3 levels and impede the development and/or maintenance of foxp3+ Treg cells in the periphery. In our transcriptome analysis, it should be noted that WT and Bach2 KO Treg cells are likely exposed to different inflammatory milieu before isolation. Hence, it is possible that some of the alterations in gene expression in Bach2 KO Treg cells are induced by the inflammatory environment, independent of Bach2 deficiency. This caveat can be addressed by isolating WT and Bach2 KO Tregs from bone marrow chimeric mice, but small number of Bach2 KO Treg cells in these mice precludes such analysis. Alternatively, we are developing an inducible system of Bach2 ablation in foxp3+ cells that would largely mitigate the confounding effects of environment on gene expression in Treg cells.
Third, it is possible that the in vivo development of foxp3+ iTregs might occur less effectively without Bach2. Indeed, we find that Helios− foxp3+ Treg cells are reduced in Bach2 KO mice and in vitro induction of foxp3 and Tregs from Bach2 KO CD4 T cells is severely impaired. Activation of Bach2 KO CD4 T cells with anti-CD3 and TGF-β results in aberrant induction of cells that express IL-4, IL-13 and Gata3, instead of foxp3. These data again suggest that Bach2 promotes the dominance of the Treg transcriptional program over the competing TH1/TH2 effector transcriptional program during differentiation of iTreg cells by cell-intrinsic mechanisms. Additionally, the cell extrinsic dysregulated immune environment in Bach2 KO mice might oppose the differentiation of foxp3+ iTregs. Based on the data presented in this manuscript we propose that Bach2 promotes the differentiation and/or survival of both thymus- and peripherally-derived Treg cells.
Further, in Bach2 KO mice and bone marrow chimeric mice, the residual foxp3+ Treg cells uniformly displayed an activated phenotype that is reminiscent of eTreg cells (25). Thus, similar to its role in conventional T cells, Bach2 might promote quiescence of foxp3+ Treg cells by opposing their activation and proliferation in the thymus and periphery. Blimp1 and IRF4 facilitate the maturation of eTreg cells (25), and hyper-induction of Blimp1 in Bach2 KO Treg cells might enhance the induction of eTregs.
Importantly, altered immune homeostasis in Bach2 KO mice invariably resulted in fatal lung disease, characterized by ECP and eosinophilic inflammation. ECP is widely recognized as a progressive and fatal idiopathic disease syndrome in mice that occurs with increased frequency in the C57BL/6 from which the Bach2 KO mice are derived, and frequencies up to 80–100% in certain strains such as moth-eaten and 129S4/SvJae (43, 44). The pulmonary crystals of ECP are predominantly composed of YM1 protein, a secretory product of pulmonary macrophages, which have been alternatively activated under the influence of TH2-associated cytokines IL-4 and IL-13 (40, 41). The pathologic role of these characteristic YM1 crystals in ECP remains unknown, however it is likely that their production by alveolar macrophages, particularly to the degree observed in ECP, is a sentinel of pathologic TH2 immune dysregulation. The occurrence of systemic eosinophilic inflammation also is strongly indicative of aberrant TH2 immunity and chronic allergic inflammation in Bach2 KO mice. Indeed, the levels of IL-4 and IL-13 are strikingly elevated in the lungs of Bach2 KO mice. What is the immunologic mechanism underlying the pathogenesis of lung disease in Bach2 KO mice? Intriguingly, identical chronic inflammatory lung disease characterized by ECP and obstructive airways also develops spontaneously in mice that are deficient for iTreg cells, but not for nTreg cells (41). As in the Bach2 KO mice, the primary immunologic lesion in the lungs of iTreg cell-deficient mice is the hyper-induction of TH2 cytokines. Therefore, it is highly likely that defective development of iTreg cells that mitigate the development of TH2 type inflammatory lung pathology underlies the fatal pulmonary disease in Bach2 KO mice. Thus, Bach2 plays a non-redundant role in the development of iTregs that protect against chronic inflammatory pulmonary disease, which has significant implications in the pathogenesis of diseases such as asthma in humans (40, 42). Although Bach2 KO mice die of pulmonary pathology, inflammatory disease is evident in other tissues including the uterus and the intestines. The lesions in the intestines of Bach2 KO mice are consistent with a diagnosis of multifocal lymphoplasmacytic and eosinophilic enteritis (not shown). It is worth noting that intestinal inflammation in Bach2 KO mice is associated with diminished numbers of Treg cells in the LP and mLNs. Studies by Roychoudhuri et al. have shown that the development of intestinal inflammatory disease in Bach2 KO mice can be mitigated by providing WT Treg cells (39), which suggests that Bach2 protects against immune pathology by promoting dominant immune regulatory mechanisms including the development and survival of Treg cells.
In conclusion, our studies have identified a novel role for Bach2 in the transcriptional regulation of T cell quiescence, Treg cell differentiation and homeostasis in vivo. These findings have provided crucial insights into the molecular regulation of immune homeostasis, and suggest a molecular and cellular basis for the pathogenesis for TH2 type immune-mediated lung disease. Additionally, the findings in this manuscript have implications in understanding the pathogenesis of immune-mediated diseases of humans such as celiac disease, vitiligo and Type I diabetes, which have been linked to Bach2 gene variants.
Acknowledgments
This work was supported by PHS grants from the National Institutes of Health (AI48785 and AI101976) to Dr. M. Suresh. David G. Gasper was supported by a training grant from the National Institutes of Health (T32OD010423).
References
- 1.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.von Boehmer H, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol. 2010;11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- 5.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 7.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 8.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 10.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 12.Fu W, Ergun A, Lu T, Hill JA, Haxhinasto S, Fassett MS, Gazit R, Adoro S, Glimcher L, Chan S, Kastner P, Rossi D, Collins JJ, Mathis D, Benoist C. A multiply redundant genetic switch ‘locks in’ the transcriptional signature of regulatory T cells. Nat Immunol. 2012;13:972–980. doi: 10.1038/ni.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Samstein RM, Arvey A, Josefowicz SZ, Peng X, Reynolds A, Sandstrom R, Neph S, Sabo P, Kim JM, Liao W, Li MO, Leslie C, Stamatoyannopoulos JA, Rudensky AY. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151:153–166. doi: 10.1016/j.cell.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 16.Kallies A, Nutt SL. Bach2: plasma-cell differentiation takes a break. EMBO J. 2010;29:3896–3897. doi: 10.1038/emboj.2010.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muto A, Ochiai K, Kimura Y, Itoh-Nakadai A, Calame KL, Ikebe D, Tashiro S, Igarashi K. Bach2 represses plasma cell gene regulatory network in B cells to promote antibody class switch. EMBO J. 2010;29:4048–4061. doi: 10.1038/emboj.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muto A, Tashiro S, Nakajima O, Hoshino H, Takahashi S, Sakoda E, Ikebe D, Yamamoto M, Igarashi K. The transcriptional programme of antibody class switching involves the repressor Bach2. Nature. 2004;429:566–571. doi: 10.1038/nature02596. [DOI] [PubMed] [Google Scholar]
- 19.Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, Peng M, Chan P, Ma Q, Mo Y, Meijer D, Zhao K, Rudensky AY, Atwal G, Zhang MQ, Li MO. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin Y, Birlea SA, Fain PR, Ferrara TM, Ben S, Riccardi SL, Cole JB, Gowan K, Holland PJ, Bennett DC, Luiten RM, Wolkerstorfer A, van der Veen JP, Hartmann A, Eichner S, Schuler G, van Geel N, Lambert J, Kemp EH, Gawkrodger DJ, Weetman AP, Taieb A, Jouary T, Ezzedine K, Wallace MR, McCormack WT, Picardo M, Leone G, Overbeck A, Silverberg NB, Spritz RA. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676–680. doi: 10.1038/ng.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubois PC, Trynka G, Franke L, Hunt KA, Romanos J, Curtotti A, Zhernakova A, Heap GA, Adany R, Aromaa A, Bardella MT, van den Berg LH, Bockett NA, de la Concha EG, Dema B, Fehrmann RS, Fernandez-Arquero M, Fiatal S, Grandone E, Green PM, Groen HJ, Gwilliam R, Houwen RH, Hunt SE, Kaukinen K, Kelleher D, Korponay-Szabo I, Kurppa K, MacMathuna P, Maki M, Mazzilli MC, McCann OT, Mearin ML, Mein CA, Mirza MM, Mistry V, Mora B, Morley KI, Mulder CJ, Murray JA, Nunez C, Oosterom E, Ophoff RA, Polanco I, Peltonen L, Platteel M, Rybak A, Salomaa V, Schweizer JJ, Sperandeo MP, Tack GJ, Turner G, Veldink JH, Verbeek WH, Weersma RK, Wolters VM, Urcelay E, Cukrowska B, Greco L, Neuhausen SL, McManus R, Barisani D, Deloukas P, Barrett JC, Saavalainen P, Wijmenga C, van Heel DA. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet. 2010;42:295–302. doi: 10.1038/ng.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plagnol V, Howson JM, Smyth DJ, Walker N, Hafler JP, Wallace C, Stevens H, Jackson L, Simmonds MJ, Bingley PJ, Gough SC, Todd JA. Genome-wide association analysis of autoantibody positivity in type 1 diabetes cases. PLoS Genet. 2011;7:e1002216. doi: 10.1371/journal.pgen.1002216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, Suresh M. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol. 2012;188:4305–4314. doi: 10.4049/jimmunol.1103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, Belz GT, Smyth GK, Busslinger M, Nutt SL, Kallies A. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Szymczak-Workman AL, Gravano DM, Workman CJ, Green DR, Vignali DA. Preferential control of induced regulatory T cell homeostasis via a Bim/Bcl-2 axis. Cell Death Dis. 2012;3:e270. doi: 10.1038/cddis.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, Humblet-Baron S, Schonefeldt S, Herold MJ, Hildeman D, Strasser A, Bouillet P, Lu LF, Matthys P, Freitas AA, Luther RJ, Weaver CT, Dooley J, Gray DH, Liston A. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:959–965. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 29.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 30.Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch’en IL, Stockmann C, Katayama CD, Hedrick SM. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maruyama T, Li J, Vaque JP, Konkel JE, Wang W, Zhang B, Zhang P, Zamarron BF, Yu D, Wu Y, Zhuang Y, Gutkind JS, Chen W. Control of the differentiation of regulatory T cells and T(H)17 cells by the DNA-binding inhibitor Id3. Nat Immunol. 2011;12:86–95. doi: 10.1038/ni.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolting J, Daniel C, Reuter S, Stuelten C, Li P, Sucov H, Kim BG, Letterio JJ, Kretschmer K, Kim HJ, von Boehmer H. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med. 2009;206:2131–2139. doi: 10.1084/jem.20090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, Killeen N, Ishii N, Li XC. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beyer M, Thabet Y, Muller RU, Sadlon T, Classen S, Lahl K, Basu S, Zhou X, Bailey-Bucktrout SL, Krebs W, Schonfeld EA, Bottcher J, Golovina T, Mayer CT, Hofmann A, Sommer D, Debey-Pascher S, Endl E, Limmer A, Hippen KL, Blazar BR, Balderas R, Quast T, Waha A, Mayer G, Famulok M, Knolle PA, Wickenhauser C, Kolanus W, Schermer B, Bluestone JA, Barry SC, Sparwasser T, Riley JL, Schultze JL. Repression of the genome organizer SATB1 in regulatory T cells is required for suppressive function and inhibition of effector differentiation. Nat Immunol. 2011;12:898–907. doi: 10.1038/ni.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roychoudhuri R, Hirahara K, Mousavi K, Clever D, Klebanoff CA, Bonelli M, Sciume G, Zare H, Vahedi G, Dema B, Yu Z, Liu H, Takahashi H, Rao M, Muranski P, Crompton JG, Punkosdy G, Bedognetti D, Wang E, Hoffmann V, Rivera J, Marincola FM, Nakamura A, Sartorelli V, Kanno Y, Gattinoni L, Muto A, Igarashi K, O’Shea JJ, Restifo NP. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis. Nature. 2013;498:506–510. doi: 10.1038/nature12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013;31:317–343. doi: 10.1146/annurev-immunol-032712-095906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoenerhoff MJ, Starost MF, Ward JM. Eosinophilic crystalline pneumonia as a major cause of death in 129S4/SvJae mice. Vet Pathol. 2006;43:682–688. doi: 10.1354/vp.43-5-682. [DOI] [PubMed] [Google Scholar]
- 44.Ward JM, Yoon M, Anver MR, Haines DC, Kudo G, Gonzalez FJ, Kimura S. Hyalinosis and Ym1/Ym2 gene expression in the stomach and respiratory tract of 129S4/SvJae and wild-type and CYP1A2-null B6, 129 mice. Am J Pathol. 2001;158:323–332. doi: 10.1016/S0002-9440(10)63972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]