Significance
We describe the X-ray structures of the same pentameric ligand-gated ion channel (pLGIC) in both its liganded or ligand-free conformations. This provides the molecular basis for understanding the opening and closing (gating mechanism) of these key players in the fast transmission of chemical signals at synapses. As described with classical allosteric proteins, the tertiary changes of the subunits are linked together through the quaternary constraint by a marked reorganization of the interfaces between subunits and the associated binding pockets and cavities. The closed form displays a cavity that may allow a better understanding of the mechanism of action of pharmacological effectors of pentameric ligand-gated ion channels and the rational design of new modulators.
Keywords: X-ray crystallography, allostery, signal transduction, cys-loop receptor
Abstract
Pentameric ligand-gated ion channels mediate fast chemical transmission of nerve signals. The structure of a bacterial proton-gated homolog has been established in its open and locally closed conformations at acidic pH. Here we report its crystal structure at neutral pH, thereby providing the X-ray structures of the two end-points of the gating mechanism in the same pentameric ligand-gated ion channel. The large structural variability in the neutral pH structure observed in the four copies of the pentamer present in the asymmetric unit has been used to analyze the intrinsic fluctuations in this state, which are found to prefigure the transition to the open state. In the extracellular domain (ECD), a marked quaternary change is observed, involving both a twist and a blooming motion, and the pore in the transmembrane domain (TMD) is closed by an upper bend of helix M2 (as in locally closed form) and a kink of helix M1, both helices no longer interacting across adjacent subunits. On the tertiary level, detachment of inner and outer β sheets in the ECD reshapes two essential cavities at the ECD–ECD and ECD–TMD interfaces. The first one is the ligand-binding cavity; the other is close to a known divalent cation binding site in other pentameric ligand-gated ion channels. In addition, a different crystal form reveals that the locally closed and open conformations coexist as discrete ones at acidic pH. These structural results, together with site-directed mutagenesis, physiological recordings, and coarse-grained modeling, have been integrated to propose a model of the gating transition pathway.
Pentameric ligand-gated ion channels (pLGICs) are a superfamily of membrane receptors that mediate fast chemical transmission of nerve signals in the central and peripheral nervous system (1). These allosteric receptors couple neurotransmitter (agonist) binding in the extracellular domain (ECD) to the opening of the ionic pore located in the transmembrane domain (TMD). There are two classes in pLGIC, with either cationic channels (acetylcholine — nAChR — and 5HT3 receptors) or anionic ones (glycine and GABA receptors). The molecular understanding of their allosteric transitions is a central issue in the pharmacology of pLGICs, as it would allow the rational design of novel orthosteric and allosteric ligands. Recently reported full-length structures of several prokaryotic and eukaryotic members of the family have provided significant insights into the conserved architecture of these receptors (2–5). Nevertheless, little is known about the structural events that link the binding/unbinding of agonist to the opening/closure of the channel gate. To understand the gating mechanism, the structures of the same receptor in different allosteric states are needed at atomic resolution. Here we report two crystal structures of wild-type GLIC, a proton-gated bacterial ion channel from Gloeobacter violaceus (6) at two different pHs, above and below pH50.
GLIC structure was initially solved at acid pH in its open conformation (4), and later in a locally closed (LC) conformation (7) displayed by six different mutants with little variations. The LC conformation shares several structural features with the open form but shows a closed pore as a result of a concerted bending of its M2 helices. Independently, recent progress has been made in understanding the structural mechanism of modulation by effectors such as general anesthetics (8) or ethanol (9) and the permeation process (10), using the same bacterial model system. Two other atomic X-ray structures of members of the pLGIC family have been described: an ion channel from the bacteria Erwinia chrysantemi (ELIC), in a closed pore conformation (2) and a eukaryotic glutamate-activated chloride channel (GluCl) in an open pore conformation similar to GLIC’s (5). However, the lack of strong sequence identity between the available structures prevents us from reliably deriving a mechanism of the gating process because it is impossible to disentangle sequence effects from functionally relevant conformational changes.
Functional studies indicate that pLGIC can exist, in addition to an open and a resting form, in several closed forms, including desensitized and intermediate forms (11). In this study, we report a X-ray structure of GLIC at neutral pH in an apparently “closed/resting-state.” The resolution is moderate (4.35 Å), but noncrystallographic averaging over the four copies of the pentamer present in the asymmetric unit results in a much better electron density map than suggested by the nominal resolution. At this neutral pH the pore is closed by a tilt of the upper part of helix M2, as in the LC form, with an additional hinge movement of the beginning of helix M1. This is very different from what is seen in ELIC. At the same time, the ECD has undergone significant structural changes that lead to a profound rearrangement of both the ECD–ECD and ECD–TMD interfaces. Strikingly, the large structural variations observed in the four copies of the pentamer at pH 7 imply a rather flat energy landscape in the absence of ligand, whereas a crystal form grown at pH 4, also presented here, shows that the LC form coexists with the open form, thus suggesting an energy landscape made of two wells in the presence of the ligand.
Results
Structural Variability and Intrinsic Fluctuations at Neutral pH.
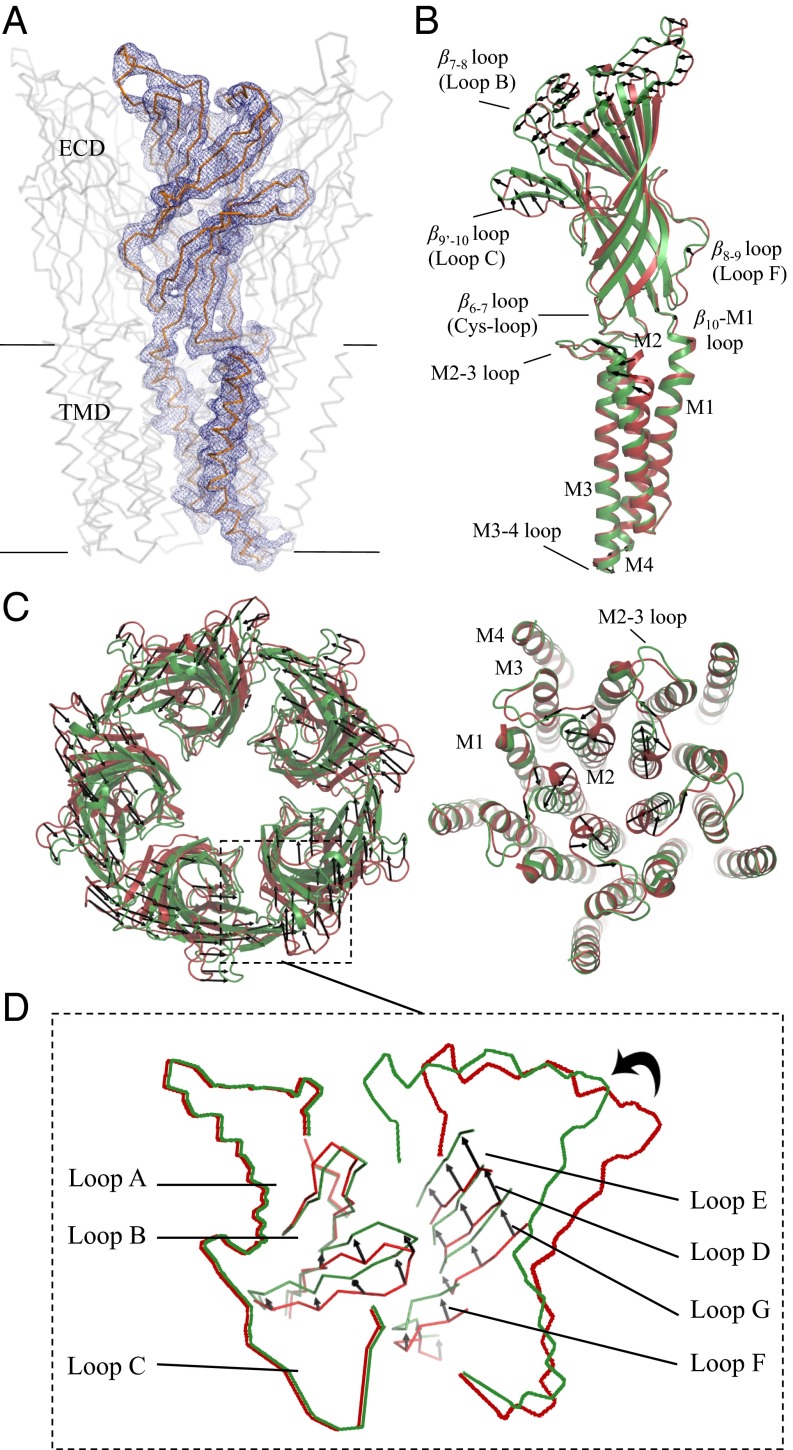
To get structural information on the resting state of GLIC, crystals were grown at neutral pH, which initially diffracted to 8.5 Å. An elaborate dehydrating cryoprotection protocol and extensive screening of the resulting crystals allowed us to collect diffraction data at 4.35 Å (SI Appendix, Table S1). The crystals belong to the space group P21 with four pentamers (about 6,200 residues) in the asymmetric unit and a packing environment different from the one seen in the pH 4 structure (SI Appendix, Fig. S1). Noncrystallographic symmetry (NCS) averaging over the 20 chains in the asymmetric unit together with the use of B-factor sharpening (12) significantly improved the quality of the initial electron density map (Fig. 1A and SI Appendix, Fig. S2) and allowed the unambiguous reconstruction of the entire main chain of the receptor, except for six out of 20 loop C (SI Appendix, Fig. S3), and 65% of the side chains (Fig. 1A) with good refinement statistics (SI Appendix, Table S1).
Fig. 1.
Superimposition of the pH 4 and pH 7 GLIC structures. (A) The Cα trace of a full-length monomer of GLIC is represented in gray. The blue mesh is the 2mFo–DFc NCS-averaged electron density map contoured at a level of 1.5 σ. (B) Side view of a full-length monomer; superimposition of the open pH 4 (green) and closed pH 7 (red) GLIC structures; the black arrows show the direction of the motion observed from the closed form to the open form. (C) Upper view of ECDs. (D) Upper view of TMDs. (E) Enlarged view of the orthosteric agonist binding site at the interface.
We observe that the TMD has lower B factors than the ECD (SI Appendix, Fig. S4), which displays a large intrinsic structural flexibility (Movie S1) and significant deviations from C5 symmetry with a root-mean-square deviation (rmsd) of 1.2 ± 0.2 Å in the ECD compared with 0.2 Å in the TMD (SI Appendix, Fig. S5). The superimposition of the 20 different ECD structures of the pH 7 form shows that most of the variations on the tertiary level come from the loops: the β1–β2 loop and the region between β2 and β5 (except for loop A), as well as β6–β7 (Cys loop), β8–β9 (loop F), and β9’–β10 (loop C), are more flexible than the rest of the molecule (SI Appendix, Fig. S6A). However, the most striking differences between the four pentamers come from their quaternary structure: the rmsd between the six pairs of the four copies of the pH 7 pentamer is within 0.8–1.1 Å, much larger than the rmsd of the open (Protein Data Bank ID code 4HFI) pentamer rotated upon itself using C5 symmetry (0.25 Å). We will return to a more detailed analysis of these structural fluctuations and their functional significance after the description of the quaternary changes between the neutral and acidic pH forms.
Quaternary Changes Between pH 4 and 7 in the ECD.
Compared with the open form, a concerted quaternary reorganization is observed in the ECD of the pH 7 structure, whereupon the separate monomeric domains have undergone rigid-body motions with respect to the C5 symmetry axis (SI Appendix, Fig. S7A). The ECD–ECD interface is reduced in the neutral pH conformation by about 180 Å2 and the ECD–TMD interface by about 50 Å2 (SI Appendix, Table S2). Meanwhile the solvent exposure is increased by about 80 Å2 per monomer in the pore when it opens. The resulting effect is best described in terms of a differential radial (blooming) and tangential (twist) motions (Fig. 1 B and C) at different heights above the membrane plane (SI Appendix, Fig. S8A). These motions are also revealed by coarse-grained Normal Mode Analysis (NMA) (SI Appendix, Fig. S8B): the third lowest frequency mode of the neutral pH form shows a combination of blooming and twist and accounts for 47% of the difference vectors between the two forms, whereas the pH 4 form has two separate modes (again twist and bloom, but separated) that account for, respectively, 22% and 25% of the transition. These modes have already been described as an important component of the allosteric transition of pLGICs, on the basis of NMA on only one form (13, 14) or by using ELIC as a model of the closed form (4). Here, however, the relative contribution of the blooming motion is markedly different. The remaining part of the transition is explained by more local modes of higher frequency that account for the closure of the pore (Fig. 1 B and D).
Comparing each pair of adjacent monomers of the pH 7 GLIC structure after superimposition of only one monomer reveals that the position of the next-neighbor ECD monomers differ by 1.32 ± 0.6 Å (SI Appendix, Fig. S9) compared with 0.31 ± 0.16 Å in GLIC open state (pH 4), further illustrating the greater flexibility in the neutral pH structure than in the open structure of GLIC (Movie S1). This plasticity in the ECD–ECD interactions was already suggested by earlier studies on the isolated GLIC–ECD that crystallizes as a hexamer (15). Having at our disposal a structural ensemble composed of 20 different dimer interfaces, we asked whether the conformational changes of one monomer correlate with those of the neighboring subunit. Pair-wise cross-correlation analysis reveals that the tip of loop C follows the rigid-body motion of the inner β sheet of the neighboring monomer, together with an interaction between the Cys loop and loop F (SI Appendix, Fig. S9). In the pH 4 form, loop C elicits more contacts with loop B of the same subunit than in the pH 7 form, through a network of interactions between the side chains of Arg77, Arg133, and Glu181 that is disrupted in the neutral pH form. Superimposition of the two dimers observed at pH 4 and pH 7 using all C-alpha atoms shows that the rearrangement of the interface of the orthosteric pocket (Fig. 1E) involves the sliding (translation) of the complementary subunit by about one interstrand distance, highlighting the importance of subunit interface plasticity in this allosteric transition. As a consequence, the change from pH 7 to pH 4 results in a constriction of the orthosteric pocket (SI Appendix, Fig. S10) and a better defined structure of loop C. To analyze the potential functional significance of the fluctuations in the neutral pH crystal form, we calculated the principal components (PCs) of the corresponding covariance matrix, averaged over the 20 monomers. We then projected the difference vectors between the acidic and neutral pH forms onto the first PC. For the subunit–subunit fluctuations, the first PC has an overlap of 0.88 with the quaternary transition in the ECD (SI Appendix, Fig. S11). This is consistent with a very powerful idea in statistical physics (16) that states that the fluctuations of an unperturbed system prefigure the response of this system to an external perturbation (here the binding of the ligand). Strikingly, we also found that the first PC of the monomer fluctuations in the pH 7 form has an overlap of 0.75 with the tertiary transition in the ECD. We now describe these tertiary transitions.
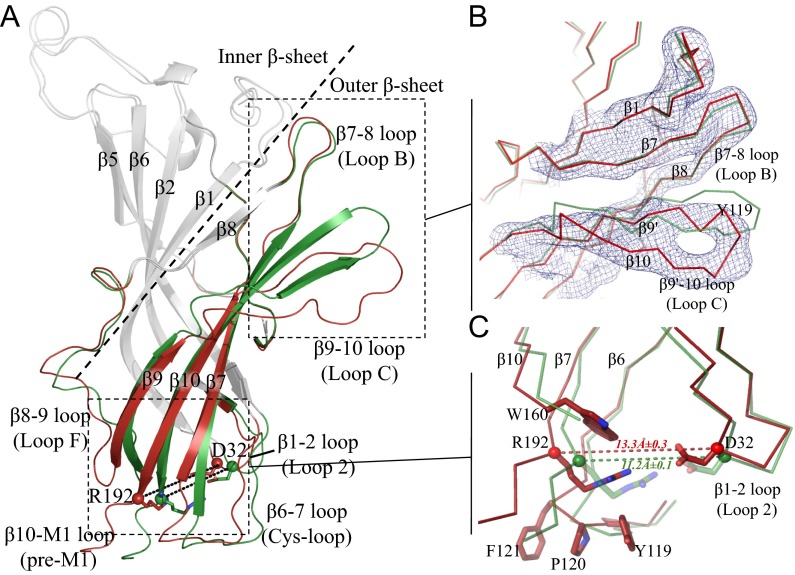
Tertiary Changes Between pH 4 and pH 7 in the ECD.
Like other members of pLGICs and acetylcholine binding proteins (AChBPs), the ECD of GLIC adopts a highly conserved Ig-like β sandwich composed of an inner and an outer β sheet, whose connecting loops play a critical role in channel function (1). Concerning the loops, the rmsd of each neutral pH monomer with the acidic pH form (SI Appendix, Fig. S6B) indicates that both loop C and loop F significantly differ in the two forms, whereas differences in the β1–β2 loop and the region between β2 and β5 are less pronounced (Figs. 1B and 2A). This is consistent with the changes of both loop C and loop F occurring in the agonist-bound form, as described in both AChBP and other pLGICs (17, 18). In addition, there are some differences in the Cys loop and β10–M1 (pre-M1) regions, which play a critical role at the ECD–TMD interface.
Fig. 2.
Tertiary reorganization observed in an ECD monomer. (A) The structural elements that differ between the pH 4 and pH 7 ECD GLIC structures are shown in green for pH 4 and in red for pH 7 GLIC. Other parts of the structures are in gray. Residues Asp32 and Arg192 are shown as sticks. The dashed line delineates the inner and outer β sheets. (B) Enlarged view of the orthosteric agonist binding site illustrating the detachment of loop C from loop B. The blue mesh is the 2mFo–DFc NCS-averaged electron density map contoured at a level of 1.5 σ. (C) The interface between the ECD and the TMD at the level of the β1–β2, β6–β7, and β10–M1 loops. The dashed line indicates the distance between the Cα atoms of Asp32 and Arg192.
Compared with the open form, we observe a tertiary structural change of the outer β sheet (Fig. 2A) that is characterized by the loss of the hydrogen bonding pattern typical of a β strand in β9’ and β10 strands, accompanied by a vertical shift of loop C (Fig. 2B). The relative movement of the inner and outer β sheets causes the loss of a conserved salt bridge between residues Asp32 in the β1–β2 loop and Arg192 in β10–M1 loop, as well as a redistribution of the network of hydrophobic interactions between the β1–β2 loop, loop F, the pre-M1, and the Cys loop (Fig. 2C). The disruption of the D32–R192 salt bridge by site-directed mutagenesis has been shown to drastically reduce the open–closed probability of the channel in nAChR and other pLGICs (19, 20). The β sheet’s movement was further analyzed to identify rigid body parts and the rotation axis that relates them (SI Appendix, Fig. S7B). It is necessary to distinguish between lower outer β sheet and the rest—namely, the upper outer β sheet and the inner β sheet. The rotation axis that describes their relative movement goes through the hydrophobic core of the ECD, reminiscent of, but not identical to the one determined by the superposition of alpha (ligand-bound) and nonalpha (unbound) subunit(s) structures obtained by cryo-EM on the muscle nAChR (21).
Upon rotation of the inner β sheet with respect to the lower outer β sheet, these two β sheets detach from each other, at the level of the ECD–TMD interface. This detachment creates a cavity involving 12 residues belonging to the Cys loop, the pre-M1, and loop F. These residues delineate a hydrophobic pocket in the core of the ECD (SI Appendix, Fig. S12), close to its interface with the TMD, and display a high level of conservation within the superfamily (22). In particular, the pocket is bordered on one side by two small hydrophobic residues, Cys27 and Leu30, and on the side of loop F (Leu157) by a larger aromatic residue, either a conserved Phe/Tyr in the anionic subfamily or Trp160 in the cationic one. Interestingly, the latter Trp residue is part of the conserved GEW sequence motif (SI Appendix, Fig. S13), which has been shown to be implicated in the binding of regulatory Ca2+ ions (23). Strikingly, this region has also been identified as a binding site for other regulatory divalent ions in ELIC (24), as well as a Bromoform molecule (25).
Changes in the TMD.
In the pH 7 structure the radius of the transmembrane pore is not compatible with (hydrated) ion flow and is thus in a nonconductive conformation (SI Appendix, Fig. S14). This is due to a concerted bending of the upper part of the M2 helices of all five subunits that obstructs the pore by forming a tightly packed bundle, along with a revolving motion of the M2–3 loop that is similar to what is observed in the LC conformation (7) (Fig. 1D). However, there are some differences with the LC form, especially a 10° hinge motion of the N terminus of M1 occurring at the level of a conserved proline residue (Pro204 in GLIC). This hinge motion could be used, in conjunction with the rotation described above, as collective variables to analyze the transition pathway. In the pLGIC family this canonical proline in the top of M1 disrupts the normal α-helix hydrogen bonding network (26). The critical role of this portion of M1 in gating, along with M2, is supported by the role previously assigned to the main-chain carbonyl oxygen at position n-4 to this proline for binding allosteric modulators such as ivermectin in GluCl (5) and ethanol in GLIC (9). These movements of M1 and M2 result in a very different TMD–TMD interface, where now M2 helix interacts more closely with the other subunit through helix M1 rather than helix M2, in the open form (Fig. 1D and 4C).
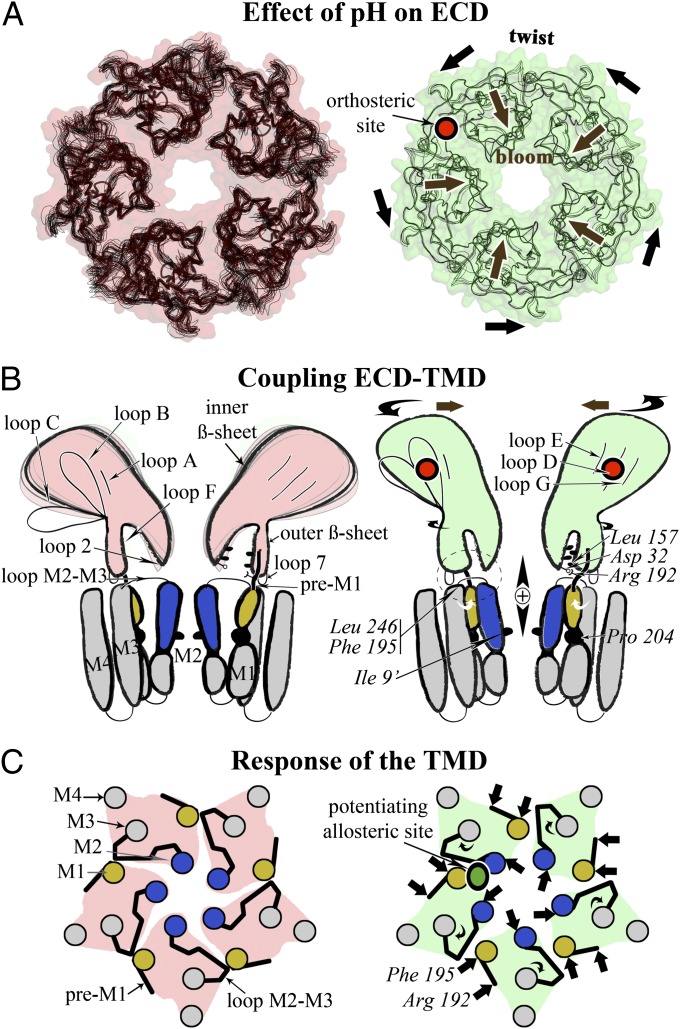
Fig. 4.
A sequential model for the activation of pLGICs. (A) The reduced flexibility in the ECD when going from the pH 7 form (Left, pink) to the pH 4 form (Right, green) is illustrated with superimposed atomic models. The quaternary associated twist and bloom motions are indicated with black arrows. The binding site of the agonist is shown in red. (B) Changes of the ECD–TMD interface in both states are shown in a schematic way. Residues shown experimentally to be involved in these interactions are indicated. The top of M2 helix is in blue, and the N terminus of M1 is in yellow. (C) Schematic view from the top and down the C5 axis of the TMD conformation in both states. The binding site for a positive modulator (anesthetics) is shown in green.
Critical Residues at the ECD–TMD Interface.
In the GLIC open conformation the C-terminal end of the M2–3 loop interacts with pre-M1 through hydrophobic interactions including side chains of Phe195 and Met252. These interactions are lost in the GLIC neutral pH structure due to the tilt of pre-M1 following the tertiary structural change in the ECD; in this process, Phe195 Cα position changes, whereas Met252 does not (SI Appendix, Fig. S15). We tested the F195A mutant by electrophysiology and observed a marked loss of function, despite normal expression in the membrane, suggesting that this aromatic side chain contributes to the gating (Table 1 and SI Appendix, Fig. S16). The mutants Y194A and M252A are comparatively almost wild-type like (SI Appendix, Fig. S16 and Table 1).
Table 1.
Electrophysiology of GLIC mutants, showing the pH50, Hill-slope (nH), and lower and upper bound of Imax found for n replicate oocytes (measured from at least three separate injections)
| Construct | pH50 | nH | Imax, µA | n |
| GLIC WT | 5.3 ± 0.2 | 1.29 ± 0.06 | −1.8<>−7.5 | 7 |
| D32E | 4.6 ± 0.1 | 1.6 ± 0.2 | −0.4<>−1.1 | 4 |
| D32N | ≤4.0 | 2 ± 0.6 | −0.4<>−1.7 | 7 |
| D32N/R192Q | ND | ND | −0.2<>−0.3 | 4 |
| L157A | ND | ND | −0.1<>−0.2 | 4 |
| Y194A | 5.2 ± 0.3 | 1.7 ± 0.3 | −3.7<>−7.8 | 4 |
| F195A | ND | ND | −0.4<>−1.0 | 3 |
| L246A | ND | ND | −0.1<>−0.3 | 3 |
| M252A | 5.3 ± 0.4 | 1.2 ± 0.2 | −1.9<>−7.8 | 4 |
ND represents nondeterminable pH50 as currents only appeared at pH 4, usually with very low amplitudes, and no dose–response curve could be recorded. Because fits for D32N did not plateau and resulted in many incomplete curves, the pH50 is not certain at 4.0 ± 0.1 and listed as ≤4.0.
In the open conformation Leu246 interacts with the ECD through a hydrophobic cleft formed by the side chains of Phe116 and Tyr119 from the β6–β7 loop and the backbone of the β1–β2 loop (upward binding mode), whereas it interacts with the TMD through a hydrophobic cleft formed upon the relative detachment of M2 and M3 helices in the neutral pH conformation (downward binding mode). We generated the L246A mutation and observed a striking loss-of-function, as expected (Table 1). It should be noted that this position is highly conserved within the family (SI Appendix, Fig. S13) and site-directed mutagenesis experiments performed on various pLGICs yield either strong gain or loss-of-function phenotypes (7).
The loss of the Asp32–Arg192 salt bridge in the pH 7 form, together with the redistribution of hydrophobic interactions between the β1–β2 loop, loop F, the pre-M1, and the β6–β7 loop (Cys loop), locally destabilize the β1–β2 and β6–β7 loops, thus discouraging the upward binding mode of Leu246. We performed two mutants to probe the importance of the D32–R192 interaction for stabilizing the open form: D32N has a significantly shifted pH50, whereas the double-mutant D32N–R192Q has a marked loss-of-function (SI Appendix, Fig. S16 and Table 1).
Finally, there is a shift of the backbone in the loop F region that causes a change of environment for Leu157 from a hydrophobic one to a more exposed one. We thus constructed the mutant L157A and tested it by electrophysiology; a marked loss of function was observed, as predicted (SI Appendix, Fig. S16 and Table 1). Overall there is a very good agreement between the predicted phenotypes of the designed mutants and the observed ones.
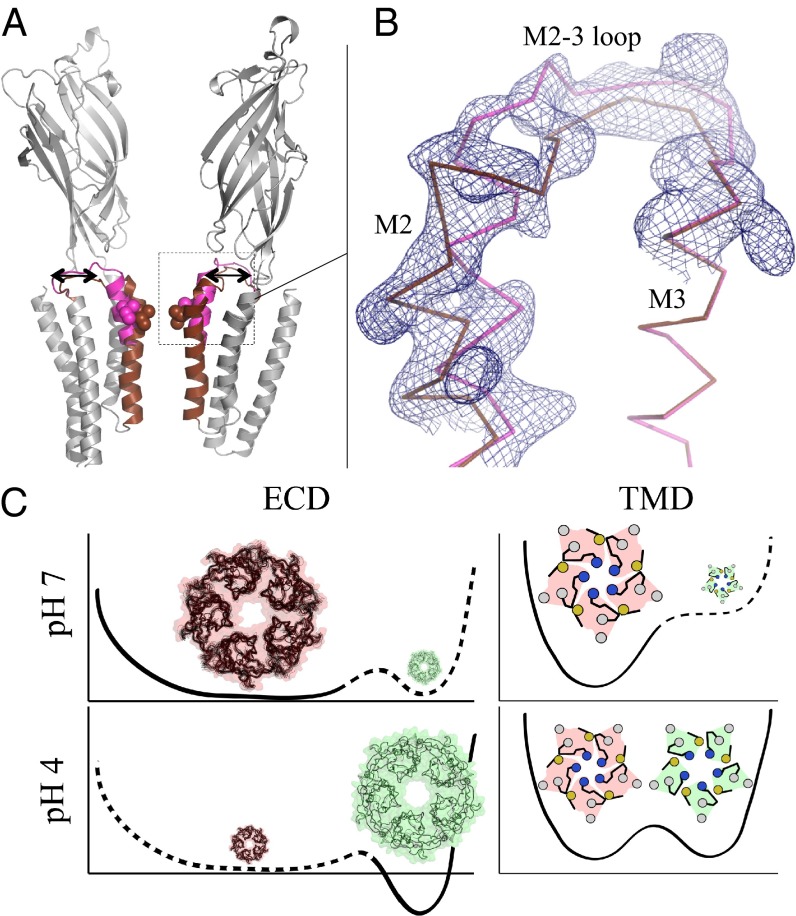
Open and LC Forms of GLIC Coexist in Crystals at pH 4.
GLIC fused to a C-terminal tag made of 10 histidines (GLICHis10) was produced in insect cells, purified, and crystallized at pH 4. This resulted in a P212121 crystal form (SI Appendix, Fig. S1), distinct from the previously described C2 crystal form that contains the open structure of GLIC (4), and this structure was solved at 3.35 Å resolution (SI Appendix, Table S1). Surprisingly, the electron density reveals that both the open and the LC conformations coexist in the unit cell (Fig. 3A). Indeed, neither the open form alone nor the LC form alone fully satisfied the diffraction data, as strong peaks in the Fourier difference map clearly indicated the occurrence of more than one conformation of the upper half of M2 helices and the M2–M3 loops (SI Appendix, Fig. S17). After incorporation of these two alternative conformations in the model, it was found they were sufficient to explain the data (there was no unexplained density left in the Fo–Fc map). In a homopentamer, the LC and open forms are exclusive from each other, as neighboring chains displaying different (open and LC) conformations would sterically clash. It is therefore likely that the crystal contains a mixture of pentamers adopting either the open or the LC conformations.
Fig. 3.
GLIC open and LC forms coexist at pH 4. (A) Ribbon representation of the GLIC receptor showing the equilibrium of the M2 helices and M2–M3 loops corresponding to the coexisting LC (brown) and open (magenta) conformations in GLICHis10 structure. The rest of the structure is colored in gray. The receptor is viewed from the side, and the two front subunits are removed for clarity. (B) Enlarged view showing the 2mFo–DFc electron density map surrounding the M2 helix and the M2–M3 loop contoured at a level of 1 σ (blue mesh). (C) Energy landscape inferred from the pH 7 crystal form (Upper, pink) and the pH 4 form (Lower, green) described here, for each domain (TMD and ECD).
To summarize, the unique structure of GLIC at low pH with the LC and open forms coexisting as discrete forms in a crystal suggests that both conformations are in equilibrium when the ECD is in an agonist-bound conformation, displaying fluctuations that are significantly different in nature from the ones observed in the resting state (Fig. 3B).
Gating Transition Pathway: A Plausible Model.
Previous attempts to model the gating transition assume that ELIC is a good model for the resting form of GLIC (27–29). Here we provide a model based on the crystal structure of the two end points of the transition of the same pLGIC. To generate physically plausible trajectories we used iENM (30), a coarse-grained method based on a simplified energy landscape with two harmonic wells centered on the start and end points of the transition. It was found to predict the expected sequence of structural events on hemoglobin (31). It was used here to generate plausible coarse-grained (CA atoms only) trajectories between GLICpH7, GLICLC, and GLICOpen (Movies S2 and S3). Analysis of these trajectories reveals that the conformational changes at the tertiary and quaternary level happen roughly at the same pace (SI Appendix, Fig. S18A): the ECD transitions first, and the TMD follows (SI Appendix, Fig. S18B). Three main events can be distinguished, involving the sequential transition of three groups: (i) the inner and outer β sheets, most of their connecting loops, and the pre-M1; (ii) the Cys loop, loop M2–3, and the most extracellular part of the transmembrane helices; and (iii) the rest of the TMD located below the level of the conserved Proline in M1. The corresponding motions—in the reference frame of the full-length pentamer—are (i) an unblooming of the ECD, whose amplitude decreases as the twisting motion increases, resulting in the reshaping of the ECD–ECD interfaces; (ii) initiation of channel opening at the level of the hydrophobic gate in the transmembrane pore; and (iii) rigid-body rotation of the TMD, corresponding to the end of the twisting motion (Fig. 4).
When transitions from GLICpH7 toward either GLICLC or GLICopen are compared, they appear to share the same initial conformational changes (SI Appendix, Fig. S18C): it is only when the ECD has experienced about 70% of its transition that both trajectories split apart and the TMD either stays closed or opens up. The gating mechanism that emerges is thus concerted. It starts at the extracellular tip and propagates toward the cytoplasmic end. This predicted sequence of gating events is consistent with a wealth of experimental results collected on the nicotinic receptor eukaryotic homolog (32), even though details of the scenario might differ from one member of the family to another.
Discussion
The structural reorganization observed upon pH activation in GLIC can now be analyzed in view of numerous functional studies accumulated over decades of research on other pLGICs. Upon activation, we observe a contraction of the orthosteric site and a more extensive interface between neighboring subunits. This observation is in line with many studies on AChBP structures that correlate agonist and antagonist binding to a contraction or an expansion of the orthosteric site, respectively (17, 33). However, both the amplitude and the mechanism of this expansion/contraction in GLIC differ from what is usually described in AChBP structures, especially because there is no quaternary rearrangement in the latter structures, whereas there is a profound one in GLIC. It should also be mentioned that, based on the structure of muscle nAChR obtained by electron microscopy (21), a closure of loops B and C was also described and suggested to occur upon activation, as observed by comparison of alpha versus nonalpha subunits. Judging from our structures, a tighter association of loops A and B, which are located at the junction of the inner and outer β sheets, with loop C, whose tip remains attached to the complementary subunit in both forms, may indeed constitute one of the keys of the local reorganization due to the agonist binding.
The pore conformation observed in the neutral form of GLIC is consistent with molecular dynamics (MD) studies of the TMD alone of GLIC (34), which showed closing events of the pore involving the bending of the top of helix M2. Furthermore, crosslinking M2 and M3 in their upper parts as in the open form (and not as in the resting form) results in a marked gain-of-function phenotypes (7). Also, it is striking that the M2–M1–M2 interface specific to the pH 4 form coincides with the recently identified binding site of a positive modulator (9) (Fig. 4).
In addition, recent experimental studies on GLIC, obtained by other groups, are also in line with the gating mechanism derived from the GLIC X-ray structures presented here. Indeed, the surface cysteine accessibility method (35), as well as site-directed spin labeling and electron paramagnetic resonance spectroscopy (36, 37), revealed that activation involves an outward translational movement of the tip of M2 helices, whereas the lower part of these helices remains relatively immobile. The tilting of the top of helix M2 was also invoked in a recent paper as the primary mechanism for the closure of the pore (36), in a manner similar to what we observe in the neutral pH form of GLIC. Therefore, we conclude that the neutral pH GLIC structure presented here possesses all of the structural features expected from the resting-state form. We stress that the closure of the pore in GLIC pH 7 is different from the one observed in ELIC in which M2 and M3 move as a block and M4 appears decoupled from the rest of the TMD. Actually, agonist binding in ELIC is accompanied by no significant structural change (38, 39), as would be expected for a desensitized form. Further studies will be needed to see if a crystal structure of GLIC can also be solved in such a conformation.
We note that other known allosteric systems (40), including membrane receptors (41) also display a marked structural flexibility in the resting state and that some models of the voltage-gated ion channels postulate diffusion in a flat energy landscape for this form (42). In the large-scale allosteric transition of adenylate kinase, it was recently found that the conformational basin of the closed form is much larger than previously assumed and wider than in the open state (43), as inferred here (Fig. 3). This would also imply a large entropy in the number of preexisting transition pathways, which was predicted to lower the free-energy barrier of allosteric transitions (44). Furthermore, the analysis of the trends of the fluctuations of the resting forms in GLIC explains a large part of the transition toward the open form, as predicted by linear response theory (16). On the other hand, the coexistence of the LC and open forms in the same crystal as discrete states fits well with the allosteric scheme postulated long ago for these receptors (1, 45). Here we provide a plausible pathway based on a coarse-grained model (30). More detailed models of the transition using all-atoms models will be needed to understand the exact role of pH in the transition and will be reported elsewhere. The validation of these possible pathways will require experimental data such as those available on nAChR (32).
Materials and Methods
GLIC and GLICHis10 were expressed and purified as in ref. 8. Crystals underwent a special dehydration protocol before cryocooling. Electrophysiology recordings were performed by expressing GLIC and its variants in Xenopus laevis oocytes as in ref. 10. Full methods are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank L. Malherbe for preparing some of the mutants. We thank J. Gouge for help in data collection, M. Prevost for sharing data on mutants, and G. Bricogne (Global Phasing) for sharing expertise on refinement in Buster. We thank the Platform for Protein Expression of the Proteopole in the Institut Pasteur (respectively, J. Bellalou and S. Petres) as well as the staff of synchrotron beamlines in both ESRF (Grenoble) and Soleil (Orsay). We gratefully acknowledge the financial support of ANR (Grant NicoChimera) and DARRI (Institut Pasteur). L.S. was funded by a postdoctoral Roux fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The crystallography, atomic coordinates, and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4NPP and 4NPQ).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314997111/-/DCSupplemental.
References
- 1.Corringer PJ, et al. Structure and pharmacology of pentameric receptor channels: From bacteria to brain. Structure. 2012;20(6):941–956. doi: 10.1016/j.str.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452(7185):375–379. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- 3.Hilf RJ, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457(7225):115–118. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- 4.Bocquet N, et al. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457(7225):111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- 5.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474(7349):54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bocquet N, et al. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature. 2007;445(7123):116–119. doi: 10.1038/nature05371. [DOI] [PubMed] [Google Scholar]
- 7.Prevost MS, et al. A locally closed conformation of a bacterial pentameric proton-gated ion channel. Nat Struct Mol Biol. 2012;19(6):642–649. doi: 10.1038/nsmb.2307. [DOI] [PubMed] [Google Scholar]
- 8.Nury H, et al. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2011;469(7330):428–431. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- 9.Sauguet L, et al. Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat Commun. 2013;4:1697. doi: 10.1038/ncomms2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauguet L, et al. Structural basis for ion permeation mechanism in pentameric ligand-gated ion channels. EMBO J. 2013;32(5):728–741. doi: 10.1038/emboj.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lape R, Plested AJ, Moroni M, Colquhoun D, Sivilotti LG. The α1K276E startle disease mutation reveals multiple intermediate states in the gating of glycine receptors. J Neurosci. 2012;32(4):1336–1352. doi: 10.1523/JNEUROSCI.4346-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholls RA, Long F, Murshudov GN. Low-resolution refinement tools in REFMAC5. Acta Crystallogr D Biol Crystallogr. 2012;68(Pt 4):404–417. doi: 10.1107/S090744491105606X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertaccini EJ, Lindahl E, Sixma T, Trudell JR. Effect of cobratoxin binding on the normal mode vibration within acetylcholine binding protein. J Chem Inf Model. 2008;48(4):855–860. doi: 10.1021/ci700456s. [DOI] [PubMed] [Google Scholar]
- 14.Taly A, et al. Normal mode analysis suggests a quaternary twist model for the nicotinic receptor gating mechanism. Biophys J. 2005;88(6):3954–3965. doi: 10.1529/biophysj.104.050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nury H, et al. Crystal structure of the extracellular domain of a bacterial ligand-gated ion channel. J Mol Biol. 2010;395(5):1114–1127. doi: 10.1016/j.jmb.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Ikeguchi M, Ueno J, Sato M, Kidera A. Protein structural change upon ligand binding: Linear response theory. Phys Rev Lett. 2005;94(7):078102. doi: 10.1103/PhysRevLett.94.078102. [DOI] [PubMed] [Google Scholar]
- 17.Hansen SB, et al. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 2005;24(20):3635–3646. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourne Y, et al. Structural determinants in phycotoxins and AChBP conferring high affinity binding and nicotinic AChR antagonism. Proc Natl Acad Sci USA. 2010;107(13):6076–6081. doi: 10.1073/pnas.0912372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee WY, Sine SM. Principal pathway coupling agonist binding to channel gating in nicotinic receptors. Nature. 2005;438(7065):243–247. doi: 10.1038/nature04156. [DOI] [PubMed] [Google Scholar]
- 20.Purohit P, Auerbach A. Acetylcholine receptor gating at extracellular transmembrane domain interface: The “pre-M1” linker. J Gen Physiol. 2007;130(6):559–568. doi: 10.1085/jgp.200709857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346(4):967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Dellisanti CD, Hanson SM, Chen L, Czajkowski C. Packing of the extracellular domain hydrophobic core has evolved to facilitate pentameric ligand-gated ion channel function. J Biol Chem. 2011;286(5):3658–3670. doi: 10.1074/jbc.M110.156851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galzi JL, Bertrand S, Corringer PJ, Changeux JP, Bertrand D. Identification of calcium binding sites that regulate potentiation of a neuronal nicotinic acetylcholine receptor. EMBO J. 1996;15(21):5824–5832. [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann I, Marabelli A, Bertozzi C, Sivilotti LG, Dutzler R. Inhibition of the prokaryotic pentameric ligand-gated ion channel ELIC by divalent cations. PLoS Biol. 2012;10(11):e1001429. doi: 10.1371/journal.pbio.1001429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spurny R, et al. Multisite binding of a general anesthetic to the prokaryotic pentameric Erwinia chrysanthemi ligand-gated ion channel (ELIC) J Biol Chem. 2013;288(12):8355–8364. doi: 10.1074/jbc.M112.424507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.England PM, Zhang Y, Dougherty DA, Lester HA. Backbone mutations in transmembrane domains of a ligand-gated ion channel: implications for the mechanism of gating. Cell. 1999;96(1):89–98. doi: 10.1016/s0092-8674(00)80962-9. [DOI] [PubMed] [Google Scholar]
- 27.Nury H, et al. One-microsecond molecular dynamics simulation of channel gating in a nicotinic receptor homologue. Proc Natl Acad Sci USA. 2010;107(14):6275–6280. doi: 10.1073/pnas.1001832107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calimet N, et al. From the cover: A gating mechanism of pentameric ligand-gated ion channels. Proc Natl Acad Sci USA. 2013;110(42):E3987–E3996. doi: 10.1073/pnas.1313785110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng W, Auerbach A. Decrypting the sequence of structural events during the gating transition of pentameric ligand-gated ion channels based on an interpolated elastic network model. PLOS Comput Biol. 2011;7(1):e1001046. doi: 10.1371/journal.pcbi.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tekpinar M, Zheng W. Predicting order of conformational changes during protein conformational transitions using an interpolated elastic network model. Proteins. 2010;78(11):2469–2481. doi: 10.1002/prot.22755. [DOI] [PubMed] [Google Scholar]
- 31.Tekpinar M, Zheng W. Coarse-grained and all-atom modeling of structural states and transitions in hemoglobin. Proteins. 2013;81(2):240–252. doi: 10.1002/prot.24180. [DOI] [PubMed] [Google Scholar]
- 32.Auerbach A. Thinking in cycles: MWC is a good model for acetylcholine receptor-channels. J Physiol. 2012;590(Pt 1):93–98. doi: 10.1113/jphysiol.2011.214684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celie PH, et al. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron. 2004;41(6):907–914. doi: 10.1016/s0896-6273(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhu F, Hummer G. Pore opening and closing of a pentameric ligand-gated ion channel. Proc Natl Acad Sci USA. 2010;107(46):19814–19819. doi: 10.1073/pnas.1009313107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parikh RB, Bali M, Akabas MH. Structure of the M2 transmembrane segment of GLIC, a prokaryotic Cys loop receptor homologue from Gloeobacter violaceus, probed by substituted cysteine accessibility. J Biol Chem. 2011;286(16):14098–14109. doi: 10.1074/jbc.M111.221895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velisetty P, Chalamalasetti SV, Chakrapani S. Conformational transitions underlying pore opening and desensitization in membrane-embedded Gloeobacter violaceus ligand-gated ion channel (GLIC) J Biol Chem. 2012;287(44):36864–36872. doi: 10.1074/jbc.M112.401067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velisetty P, Chakrapani S. Desensitization mechanism in prokaryotic ligand-gated ion channel. J Biol Chem. 2012;287(22):18467–18477. doi: 10.1074/jbc.M112.348045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spurny R, et al. Pentameric ligand-gated ion channel ELIC is activated by GABA and modulated by benzodiazepines. Proc Natl Acad Sci USA. 2012;109(44):E3028–E3034. doi: 10.1073/pnas.1208208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez-Gutierrez G, et al. Mutations that stabilize the open state of the Erwinia chrisanthemi ligand-gated ion channel fail to change the conformation of the pore domain in crystals. Proc Natl Acad Sci USA. 2012;109(16):6331–6336. doi: 10.1073/pnas.1119268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velyvis A, Yang YR, Schachman HK, Kay LE. A solution NMR study showing that active site ligands and nucleotides directly perturb the allosteric equilibrium in aspartate transcarbamoylase. Proc Natl Acad Sci USA. 2007;104(21):8815–8820. doi: 10.1073/pnas.0703347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Granier S, Kobilka B. A new era of GPCR structural and chemical biology. Nat Chem Biol. 2012;8(8):670–673. doi: 10.1038/nchembio.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goychuk I, Hänggi P. Ion channel gating: A first-passage time analysis of the Kramers type. Proc Natl Acad Sci USA. 2002;99(6):3552–3556. doi: 10.1073/pnas.052015699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potoyan DA, Zhuravlev PI, Papoian GA. Computing free energy of a large-scale allosteric transition in adenylate kinase using all atom explicit solvent simulations. J Phys Chem B. 2012;116(5):1709–1715. doi: 10.1021/jp209980b. [DOI] [PubMed] [Google Scholar]
- 44.Itoh K, Sasai M. Entropic mechanism of large fluctuation in allosteric transition. Proc Natl Acad Sci USA. 2010;107(17):7775–7780. doi: 10.1073/pnas.0912978107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Changeux JP. 50 years of allosteric interactions: The twists and turns of the models. Nat Rev Mol Cell Biol. 2013;14(12):819–829. doi: 10.1038/nrm3695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.