Abstract
Disruption in nerve growth factor (NGF) signaling via trkA receptors compromises the integrity of the basal forebrain (BF) cholinergic system, yielding cognitive, specifically attentional, impairments in Alzheimer’s disease (AD). Although normal aging is considered a risk factor for AD, the mechanisms underlying the selective vulnerability of the aging cholinergic system to trkA disruption is not clear. The levels of proNGF, a proneurotrophin that possesses higher affinity for p75 receptors, increase in aging. The present study was designed to test the hypothesis that cholinergic and attentional dysfunction in aged rats with reduced BF trkA receptors occurs due to the overactivation of endogenous proNGF signaling. We employed a viral vector that produced trkA shRNA to suppress trkA receptors in the corticopetal cholinergic neurons of aged rats. BF trkA suppression impaired animals’ performance on signal trials in both the sustained attention task (SAT) and the cognitively-taxing distractor version of SAT (dSAT) and these deficits were normalized by chronic intracerebroventricular administration of proNGF antibody. Moreover, depolarization-evoked ACh release and the density of cortical cholinergic fibers were partially restored in these animals. However, SAT/dSAT scores reflecting overall performance did not improve following proNGF blockade in trkA knockdown rats due to impaired performance in non-signal trials. Sustained proNGF blockade alone did not alter baseline attentional performance but produced moderate impairments during challenging conditions. Collectively, our findings indicate that barring proNGF-p75 signaling may exert some beneficial effects on attentional capacities specifically when BF trkA signaling is abrogated. However, endogenous proNGF may also possess neurotrophic effects and blockade of this proneurotrophin may not completely ameliorate attentional impairments in AD and potentially hinder performance during periods of high cognitive load in normal aging.
Keywords: aging, acetylcholine, proNGF, attention, Alzheimer’s disease
1. INTRODUCTION
Basal forebrain (BF) cholinergic neurons located in the nucleus basalis of Meynert (nBM) and the substantia innominata (SI) project to all cortical areas and layers throughout the brain. Substantial evidence suggests that the integrity of cortical cholinergic inputs is necessary for normal attentional performance, and that such performance robustly activates cortical acetylcholine (ACh) release (McGaughy et al., 1996; Everitt and Robbins, 1997; Passetti et al., 2000; Arnold et al., 2002; Dalley et al., 2004; Sarter and Parikh, 2005; Parikh et al., 2007). Attentional impairments constitute the core components of global cognitive decline observed in Alzheimer’s disease (AD; Perry and Hodges, 1999). Moreover, the cortical cholinergic input system undergoes extensive degeneration in AD that correlates with the severity of cognitive symptoms and disease duration (Counts et al., 2004, Mesulam, 2004, Counts and Mufson, 2005). Aging is a well-recognized risk factor for AD. Although the BF cholinergic system is highly vulnerable in aging (Casu et al., 2002), the contribution of age in cholinergic and cognitive decline associated with AD is not well defined.
BF cholinergic neurons require nerve growth factor (NGF) for trophic support (Mobley et al., 1986; Oosawa et al., 1999; Sofroniew et al., 2001). NGF-mediated signaling via a high-affinity tropomyosin-related kinase A (trkA) receptor is crucial for the development, maturation and function of these neurons (Li et al., 1995; Fagan et al., 1997; Huang and Reichardt, 2003). NGF also acts on another non-specific neurotrophin receptor, p75, which belongs to the tumor necrosis receptor family, and induces apoptotic signaling via a coreceptor, sortilin (Chao, 2003; Schor, 2005; Volosin et al., 2006; Clewes et al., 2008). Postmortem studies supported the hypothesis that disruption of trkA receptor function and possibly an imbalance between trkA/p75 signaling may contribute to the degeneration of BF cholinergic neurons leading to cognitive decline in AD (Mufson et al., 2000; Counts et al., 2004; Counts and Mufson, 2005; Mufson et al., 2008). However, recent studies utilizing conditional mutants show that region-specific deletion of the trkA gene does not affect the survival of BF cholinergic neurons (Sanchez-Ortiz et al., 2012) and cognitive performance of young and middle-aged animals (Müller et al., 2012). Moreover, we previously demonstrated that chronic suppression of BF trkA receptors produces cholinergic and attentional deficits in aged but not young rats (Parikh et al., 2013). Collectively, these findings indicate that aging interacts with preexisting abnormalities in trophic signaling to trigger cholinergic and cognitive decline as observed in AD.
NGF is secreted in the central nervous system as proNGF precursor that is synthesized as 25 and 32 kDa isoforms (Fahnestock et al., 2004; Bruno and Cuello, 2006). ProNGF undergoes proteolytic cleavage either intracellularly by proprotein convertases or extracellularly by plasmin to produce mature NGF (Edwards et al., 1988; Seidah et al., 1996; Bruno and Cuello, 2006). The ratio of proNGF to NGF increases in normal aging and other pathological conditions, and this effect presumably occurs as a consequence of disruption in cleavage mechanisms (Hempstead, 2009). ProNGF possesses a higher affinity for p75 receptors and may trigger apoptosis by activating this receptor (Nykjaer et al., 2004). In vitro studies demonstrated that this proneurotrophin exerts neurotoxic effects on sympathetic and BF neurons isolated from aging rodents (Al-Shawi et al., 2007; 2008). Moreover, acute hippocampal injection of proNGF in aged rats produced atrophy of septal cholinergic neurons (Fortress et al., 2011). However the consequences of age-related accumulation of endogenous proNGF on cholinergic signaling and attentional capacities have remained unknown. Here we examined the effects of chronic intracerebroventricular (i.c.v.) infusions of proNGF antibody (proNGF Ab) on cholinergic function in normal and BF trkA-suppressed aged rats. We employed a viral vector-based RNA interference strategy to knockdown trkA receptors specifically in the nBM/SI region of the BF (Parikh et al., 2013). This strategy was adopted to model decreases in trkA but not p75 receptors that is observed in AD patients (Counts et al., 2004), as well as to circumvent behavioral confounds produced by trkA reduction elsewhere in the nervous system (Holtzman et al., 1995; Luther and Birren, 2009). Our results demonstrate that proNGF blockade provides a partial rescue of cholinergic and attentional deficits in aged rats with lower levels of BF trkA receptors. Moreover, endogenous proNGF is not detrimental to the aging cholinergic system provided trophic signaling remains intact.
2. EXPERIMENTAL PROCEDURES
2.1. Animals
Male Wistar rats (retired breeders; 10-12 months) were acquired from Charles River Laboratories (Malvern, PA, USA). The animals were housed in a temperature- and humidity- controlled facility with a 12-hour light/dark cycle (lights “on”: 7:00AM) and had free access to food and water. Rats were maintained until 20 months of age following which training in an operant attentional task was initiated (see behavioral procedures as described in section 2.2). Water access to animals was restricted to a 10-min period in the home cage following each behavioral session. Operant training took place 6 days/week. On non-training days, water access was increased to 30-min. Rats were individually housed and food was available ad- libitum throughout the behavioral training and testing. The experiments were conducted in accordance with the National Institute of Health guidelines and were approved by the Institutional Animal Care and Use Committee, as well as the Institutional Biosafety Committee, at Temple University.
2.2. Behavioral training and testing
2.2.1. Apparatus
Rats were trained in operant chambers encased in sound-attenuating boxes each containing a fan to provide ventilation and low-level background noise (Med Associates Inc., St. Albans, VT). Each chamber was equipped with two retractable levers, a central panel consisting of three panel lights (2.8 W each), a liquid receptacle attached to a water dispenser, and a house light (2.8 W) located on the rear wall. All events including the signal delivery, lever presentations, and water dispense were transmitted using programs written in Medstate notation via SmrtCtrl™ interface running through MED-PC software on a Dell Optiplex 960 computer.
2.2.2. erant sustained attention task (SAT)
Partially water-deprived rats were trained in operant sustained attention task (SAT) as described previously (Demeter et al., 2008; St Peters et al., 2011; Parikh et al., 2013). Briefly, rats were initially autoshaped on a FR-1 schedule of reinforcement to attain the lever press response and subsequent reward (0.02 mL water). To deter a side bias, lever presses on the dominant lever (i.e. the lever with ≥5 presses) ceased to be reinforced until the discrepancy was reduced. Once the rats made 120 lever presses within a session, they were moved to the next phase of training, which required discrimination between signal (illumination of the central panel light for 1 s) and non-signal (no illumination) events. Each event was followed by the presentation of two levers 2 s later; levers remained extended for 4 s or until a lever press occurred. If no response was made during the 4 s lever presentation, an omission was recorded and the intertrial interval (ITI; 12±3 s) was reinstated. On signal trials, a left lever press was scored as a “hit” and rewarded; an incorrect response (depression of the right lever) was deemed a “miss”. During nonsignal trials, a right lever press was scored as a “correct rejection” and reinforced, while a left lever press was considered a “false alarm”. The animals were not rewarded for incorrect responses. The presentation of signal and nonsignal trials were pseudo-randomized. Half of the animals in a group were trained with the reverse set of rules.
After attaining 70% correct responses to signal and non-signal trials for three consecutive days, animals progressed to the final stage training, during which the duration of signals was decreased to 25, 50, or 500 ms. Moreover, the ITI was reduced to 9±3s and the house light remained illuminated throughout the session. These events are known to constrain rats’ behavior for continuous monitoring of the central panel (Demeter et al., 2008). Each behavioral session consisted of a pseudo-randomized sequence of 81 signal (27 per signal duration) and 81 nonsignal trials (total 162 trials). Sessions were divided into three blocks of 54 trials (27 signal trials and 27 non-signal trials) with each signal type presented 9 times per block. Stable criterion performance was characterized by signal duration-dependent hit rates, ≥ 70% hits to 500 ms signals, ≥70% correct rejections, and a relatively low number of omissions (<10% of all trials; equal distribution among trial types) for at least 3 consecutive sessions. At this stage, animals were exposed to a distractor session (dSAT) that involved the presentation of distractors (flashing house light @ 0.5 Hz) in the second block. This procedure was adopted to minimize the novelty effects of distractors while evaluating the effects of trkA suppression and/or proNGF blockade on performance under challenging conditions. Following the dSAT session, rats resumed training on SAT and were prepared for stereotaxic surgeries (see section 2.4) after retaining criterion.
2.2.3. Performance Measures
The total numbers of hits, misses, correct rejections, false alarms and omissions were recorded for the entire behavioral session. Each session was analyzed in terms of the relative number of hits (h = hits/hits+misses) for each signal length, and relative number of correct rejections (cr = correct rejections/correct rejections + false alarms). The overall measure of attentional performance was calculated as performance score (SAT/dSAT score) using the formula: (h – fa)/[2(h + fa) – (h + fa)2] as described previously (St. Peters et al., 2011); fa represented the relative number of false alarms. SAT/dSAT scores vary from +1.0 to −1.0; a score of +1.0 indicated that all responses on signal and non-signal trials were correct, 0 indicated the inability to discern signal from non-signal events, and −1.0 indicated that all responses were misses and false alarms. Performance measures were also calculated for each block of 54 trials. Response latencies were determined for hits, misses, correct rejections and false alarms separately, and clustered into performance on signal and non-signal trials for analysis.
2.3. Adeno-associated viral vectors (AAV vectors)
Recombinant AAV vectors expressing either trkA shRNA (sequence targeting 403 position; AAV-trkA) or shRNA against the functional control gene luciferase (AAV-luc) were constructed and packaged into an AAV helper free system (University of Iowa Vector Core Facility). These vectors utilized a synthetic inhibitory BIC-derived RNA (SIBR) expression plasmid to guide the expression of both the miRNA against the targeted gene and green fluorescent protein (GFP) from a single transcriptional unit (Chung et al., 2006; Taylor et al., 2008; Dickson et al., 2010). We previously validated the use of AAV-SIBR vectors for in vivo trkA suppression (see Parikh et al., 2013 for details on AAV-plasmid construction, screening and validation). Prior to use, AAV vectors were desalted in Hyclone buffer (University of Iowa Vector Core) using a dialysis procedure. Briefly, the vectors were pipetted into Slide-A-Lyzer MINI Dialysis Cassette (Thermo Fisher Scientific, Hudson, NH, USA) under sterile conditions. Each cassette was wrapped in a dialysis float and placed in approximately 15mL of Hyclone buffer with gentle stirring at 4°C. The vectors underwent three-1h dialysis sessions and buffer was replaced every hour. At the completion of dialysis, vectors were removed from the cassette and placed in sterile vials for in vivo infusions.
2.4 Stereotaxic Surgeries and experimental design
At the time of surgery, rats were aged 23.61 ± 0.54 months. All surgeries were performed under aseptic conditions. The animals were anesthetized with isoflurane (4-5% for induction and 1-2% maintenance dose) using an anesthesia machine (Surgivet, Dublin, OH, USA). Rats were mounted on a stereotaxic frame (Model 962; David Kopf Instruments, Tujunga, CA, USA) and the head was positioned into the head frame using ear bars. An isothermal deltaphase pad (Braintree Scientific, Braintree, MA, USA) was used to consistently maintain animals’ body temperature at 37°C throughout the surgical procedure. Ophthalmic ointment was applied to lubricate animals’ eyes. Animals’ heads were shaved using clippers, and iodine tincture was applied to clean the skin. A 3 cm incision was made in the midline of the scalp. AAV-luc (control) or AAV-trkA vector (titer: 0.7-2.8 × 1013 vg/mL; 2μL/hemishere) was bilaterally infused into the nBM/SI region of the BF (coordinates from Bregma: AP: −1.3 mm, ML: ±2.5 mm, and DV: −7.0 mm below dura). Infusions were made using a 10-μL Hamilton syringe at a rate of 1 μL/min and the needle remained in place for an additional 4 min to allow complete diffusion of virus particles.
For prolonged i.c.v. infusions of proNGF Ab, osmotic minipumps (Alzet model 1004; DURECT Corporation, Cupertino, CA, USA) were filled with rabbit monoclonal anti-proNGF antibody (EMD Millipore, Billerica, MA, USA; 1:20 dilution) in sterile phosphate-buffered saline (PBS; 0.01 M). These pumps were connected to the brain infusion cannula (DURECT Corporation) via a catheter to deliver antibody solution at a rate of 0.11 μL/hr for 4 weeks. For control infusions, pumps were filled with the vehicle (PBS). Following AAV vector infusions, osmotic minipumps were inserted into the subcutaneous pocket of the midscapular area created toward the animals’ back. The infusion cannula was inserted into the right lateral ventricle (AP: −0.3 mm, ML: −1.5 mm, and DV: −3.5 mm). Dental cement was applied on and around the cannula and secured with three bone screws to keep the structure stable. After the cement dried, the skin above the skull was then sutured with sterile sutures. Triple antibiotic cream was applied to the area surrounding the dental cement. Moreover, animals were given injections of an antibiotic (Baytril) and an analgesic (Buprenorphine) to combat any infection and minimize pain.
A schematic illustration of the experimental design is shown in Figure 1. Animals trained to criterion at SAT were randomly assigned into four groups (AAV-luc + vehicle, AAV-trkA + vehicle, AAV-luc + proNGF Ab, and AAV-trkA + proNGF Ab; N = 8/group). Following surgery, rats were allowed to recover for a period of 72 h and then placed back on task for SAT/dSAT testing. SAT performance was monitored for 4 weeks as AAV vectors require 3-4 weeks to produce maximal in vivo infection and knockdown of trkA receptors (Parikh et al., 2013). As the major objective of the study was to determine the impact of chronic blockade of endogenous proNGF on attentional capacities and cholinergic signaling in aged rats with intact or reduced trkA receptors, osmotic minipumps remained implanted in vector-infused animals for four weeks. Animals were subjected to a dSAT session on the last day of testing and then prepared for electrochemical recordings followed by postmortem brain examination for the cannula/microelectrode placement, trkA knockdown and the expression of cholinergic marker choline-acetyltransferase (ChAT) as described below. For final behavioral analysis, SAT data from the last 3 sessions prior to the dSAT testing procedure were averaged. Behavioral data for the dSAT testing session were analyzed separately.
Figure 1.

Schematic representation of the experimental design. After attaining criterion on SAT (see Section 2 Experimental Procedures for details), rats underwent stereotaxic surgeries for infusions of AAV vectors expressing either trkA shRNA (AAV-trkA) or shRNA for the functional control gene luciferase (AAV-luc; control vector) into the basal forebrain (BF). Simultaneously, mini-osmotic pumps connected to brain infusion cannula were implanted subcutaneously for chronic intracranial administration of either proNGF antibody (Ab) or vehicle (0.01M PBS) into the right ventricle. Animals were randomly assigned into four groups (AAV-luc + vehicle, AAV-trkA + vehicle, AAV-luc + proNGF Ab, and AAV-trkA + proNGF Ab). After recovery from surgery, rats were placed back on task up to 4 weeks for SAT/dSAT testing. At the completion of behavioral testing, animals were anesthetized to conduct electrochemical recordings of cholinergic transmission, following which they were perfused for immunohistochemical analysis.
2.5. Electrochemical recordings of cholinergic transmission
After the completion of behavioral testing, rats were prepared for electrochemical recordings of depolarization-evoked ACh release using choline-sensitive microelectrodes as described earlier (Parikh et al., 2004, 2010, 2013). In brief, ceramic-based microelectrode arrays (Quanteon, Nicholasville, KY, USA) consisting of four rectangular platinum recording sites arranged side by side in pairs were coated with choline oxidase (Sigma-Aldrich, St. Louis, MO, USA). The enzyme was immobilized to the bottom pair of recording sites while the upper pair was coated with bovine serum albumin and served as sentinel channels. The electrode channels were electroplated with m-PD (m-phenylenediamine) to enhance the selectivity of choline against electroactive analytes such as ascorbic acid (AA), dopamine (DA) and uric acid. Choline sensitivity and selectivity was tested for all electrodes by conducting an in vitro calibration. The following calibration criterion was followed for subsequent use of electrodes for in vivo recordings: choline sensitivity >3 pA/μM, limit of detection (LOD) < 500 nM; selectivity ratio for choline:AA >50, linear response for choline concentration R2 > 0.98, and DA response <3pA.
In vivo recordings were conducted from the prelimbic region of the medial prefrontal cortex (PFC) from urethane-anesthetized animals placed in a stereotaxic apparatus. Dental cement and the infusion cannula were carefully removed and the skull was thoroughly cleaned with sterile saline prior to microelectrode implantation. Single barrel glass capillaries were pulled using a micropipette puller to attain a tip diameter of ~15 μm and attached to the microelectrodes so that the tip of the capillaries were positioned between the enzyme-coated and sentinel channels. The spacing between the capillaries and microelectrodes were kept at 70-100 μm. Glass capillaries were filled with KCl (70 mM) that passed through a sterile syringe filter (0.22 μm). The microelectrode/capillary structure was lowered into the right medial PFC (AP: +3.0 mm, ML: −0.7 mm, DV: −2.7-3.0 mm) using a microdrive (MO-10, Narishige International, East Meadow, NY, USA). An Ag/AgCl reference electrode prepared from a miniature silver wire was implanted in the rostral cortical region of the contralateral hemisphere. Amperometric recordings were conducted at 2 Hz by applying a fixed potential of +0.7V and data was digitized using a FAST-16 potentiostat (Quanteon). Background currents were stabilized for 60 min following which brief pulses of potassium (200 nL) were locally applied to produce rapid depolarization of prefrontal cholinergic terminals. These pulses were applied at 2-10 psi every 2 min through the capillaries via a PTFE tubing connected to a picospritzer (ALA Scientific Instruments, Farmingdale, NY, USA). Choline signals were analyzed with respect to peak signal amplitudes. Self-referencing was adopted to eliminate any artifacts on enzyme- coated channels due to background noise levels or drug application by subtracting currents from sentinel channels.
2.6. Histology and Immunohistochemistry
At the completion of the electrochemical recording session, the animals were transcardially perfused using 100 mL of ice-cold 0.1 M PBS followed by 300 mL of 4% paraformaldehyde (PFA; pH 7.4-7.6). The brains were removed, postfixed overnight in PFA and then transferred to 30% sucrose (in 0.1 M PBS) for 72 h. Coronal sections (50μm) were taken on a freezing microtome (SM2000R, Leica, Wetzlar, Germany) and the slices were stored in cryoprotectant solution (15% glucose, 30% ethylene glycol, and 0.04% sodium azide in 0.05 M PBS) at −20 °C until further processing. To verify the placement of infusion cannula into the right ventricle and microelectrode into the right PFC, brain sections were mounted onto gelatin-coated slides and stained with cresyl violet.
2.6.1. GFP/ChAT and trkA immunostaining
Brain slices from the BF (nBM/SI region) were stained and processed to assess transduction efficiency of AAV vectors by conducting GFP/ChAT double immunostaining (Parikh et al., 2013). Serial sections randomly selected from the rostral-caudal axis of BF (0.84mm – 1.94 mm) were thawed and rinsed in 0.05 M Tris-buffered saline (TBS). Following 1 h blocking in 10% donkey serum, sections were incubated in goat anti-ChAT antibody (EMD Millipore, 1:200 dilution) overnight at 4°C on a shaker. The sections were washed (3 × 5 min) in TBS containing 1% triton X 100 (TBST) and incubated with 1:250 diluted rhodamine-conjugated donkey anti-goat IgG (Jackson Immunoresearch Laboratories Inc., Westgrove, PA, USA) for 2 h. After 4×10 min washes in TBST, the sections were mounted on gelatin-coated slides and coverslipped with Prolong-Gold antifade reagent (Life Technologies, Grand Island, NY). ChAT-immunostained sections were also used for the morphometric analysis of BF cholinergic neurons (see section 2.6.3). BF trkA immunostaining was performed on sections parallel to those used for GFP/ChAT immunostaining. Briefly, sections were blocked in 10% goat serum and incubated with rabbit anti-trkA antibody (1:1000 dilution; EMD Millipore) overnight. Following washes in TBST, sections were incubated with biotinylated goat anti-rabbit for 2 hrs. The staining was developed by incubating the sections in streptavidin-HRP followed by 3-3’-diaminobenzidine (DAB) and nickel chloride. Stained sections were mounted on gelatin-coated slides, air dried, dehydrated and coverslipped with DPX.
2.6.2. Presynaptic ChAT density
ChAT fibers were examined in the medial PFC to determine the impact of manipulations on cortical cholinergic terminal density. For this analysis, serial coronal sections (AP: +3.2 – +2.8 mm) that alternate with those taken for the placement of the microelectrode were used to sample cholinergic fibers from the prelimbic region in which ACh release was measured (above). Additionally, the density of cholinergic processes was also assessed in the cingulate and infralimbic regions of the PFC. Free-floating sections were incubated in TBS containing 0.03% H2O2 to block endogenous peroxidase for 30 min. Following blockade in 10% donkey serum, the sections were incubated with goat anti-ChAT (1:100) overnight. Sections were rinsed in TBST and then incubated in biotinylated donkey anti-goat IgG for 2 h. Peroxidase staining was developed using streptavidin-HRP and DAB.
2.6.3. Image analysis
All sections were analyzed using a Leica brightfield/fluorescent microscope (DM4000B) equipped with DFC 425C digital camera and Leica Application Suite software (Leica Microsystems Inc.). BF sections were evaluated for infection efficiency by determining the expression of GFP and ChAT in nBM/SI region as described previously (Parikh et al., 2013). Briefly, images were captured using Image Overlay Module from both green and red filters simultaneously at 400x magnification from 3 representative fields in each hemisphere. One hundred BF neurons (50/hemisphere) were analyzed for each animal to determine the proportion of GFP-positive neurons that colocalize with ChAT-expressing neurons. The efficacy of AAV vector was evaluated by analyzing BF trkA expression. Images were acquired at 400x magnification in the brightfield mode and processed for semi-quantitative analysis of trkA pixel density in the analyzed area (325 × 245 μm) using NIH Image J. All images were converted to the binary mode and threshold levels were adjusted to enhance the visibility of cell bodies. The density of trkA receptors was expressed as the percentage of trkA-immunopositive pixels in the analyzed area. Average trkA densities were based on analyses from three sections per animal.
Morphometric analysis of BF cholinergic neurons was conducted by measuring the cross-sectional area of soma using NIH Image J. ChAT-immunopositive cells from nBM/SI region labeled with rhodamine were randomly selected from both hemispheres. Cell borders were marked and the area of the cell was automatically calculated by the program based on the length (in pixels) and area (pixel2) of the user-defined border. The cross-sectional area was converted from pixel2 to μm2 based on the scaling at 400x magnification (12.09 pixels/μm). Fifty cells were analyzed from three sections per animal. Prefrontal ChAT density was analyzed from cortical layers III/V using a grid counting procedure (St Peters et al., 2011; Parikh et al., 2013). Images were captured with a 40x objective and threshold levels were adjusted to maximize visualization of ChAT-immunopositive fibers using a macro created in Adobe Photoshop CS4. Fiber counts were made in the area of 40,000 μm2 using 2500 μm2 grids. ChAT-positive counts were based on the average of counts from three sections per animal.
2.7. Statistical analysis
Statistical analyses were performed using SPSS/PC+ version 21.0 (IBM-SPSS, Armonk, NY, USA). Behavioral data for average hits, correct rejections, SAT/dSAT scores and total omissions were analyzed using one-way ANOVAs to compare group differences. Mixed design repeated measure ANOVAs were conducted on behavioral measures with block (3-levels) or signal duration (3-levels) as the within-subject variable and manipulation (4 levels) as the between-subject variable. Data acquired from amperometric recordings, regarding ACh release, and immunohistochemical examination, which included GFP/ChAT colocalization, BF trkA density, cholinergic cell cross-sectional area and ChAT fiber density, were analyzed using one-way ANOVAs. Correlations between cholinergic fiber density and SAT and dSAT scores were conducted using Pearson’s r. All post hoc comparisons were made using Fisher’s least significant difference (LSD) test. A cut-off p value of 0.05 was considered statistically significant. Exact p values were reported as recommended by Greenwald et al. (1996).
3. RESULTS
3.1. Vector efficiency, trkA knockdown and proNGF blockade
Recombinant AAV vectors expressing either trkA or luc shRNA were infused into the nBM/SI region to target cortically-projecting BF cholinergic neurons. The tissues were analyzed postmortem following the completion of behavioral and electrochemical recording studies four weeks later. As both vectors contained a reporter gene, GFP, the extent of virus spread and infection efficiency was captured by visualizing the expression of GFP and its colocalization with ChAT-positive neurons. Figure 2A,B illustrates the diffusion of AAV-luc vector in the BF region. In general, virus spread varied from 1.5mm – 1.8mm along the rostral-caudal axis of the BF region, encompassing nBM/SI region along the dorsomedial and ventral wall of globus pallidus. A representative coronal section depicting colocalization of GFP in the cholinergic neurons from nBM/SI region is shown (Figure 2C-E). Quantitative analysis from sampled brain slices indicated that on average 68±3% of cholinergic neurons were infected with the AAV vector. One-way ANOVA indicated that there were no significant differences in the infection efficiency between groups (F3,31=1.34, p = 0.28). Additionally, the analysis of trkA immunostaining in parallel sections showed significantly reduced trkA receptor expression in aged rats infused with AAV-trkA vector as compared to those infused with AAV luc vector (F1,30 = 21.92, p < 0.001; Figure 2F-H). These data confirm the in vivo efficacy of AAV vector to chronically suppress BF trkA receptors as demonstrated in our previous study (Parikh et al., 2013).
Figure 1.
Cholinergic targeting of AAV vectors, trkA knockdown and proNGF blockade. (A) Schematic representation of the AAV vector infusion site. AAV vectors expressing shRNA either for trkA (AAV-trkA) or functional control protein luciferase (AAV-luc) were infused into the nucleus basalis of Meynert/substantia innominata (nBM/SI) region of the basal forebrain (BF; highlighted in green). This region contains major cholinergic cell groups that arise from the ventromedial wall of the globus pallidus (GP) and project throughout the cortex (LV: lateral ventricle; IC: internal capsule; CP: caudate putamen). (B) Coronal section depicting the infected BF neurons expressing GFP in the nBM/SI region from an AAV-luc-infused rat. The transduction efficiency of AAV vectors was confirmed by GFP/ChAT immunohistochemistry at the completion of the study. A representative coronal section depicting the GFP-expressing neurons (C; green), ChAT-positive neurons (D; red) and colocalization of GFP and ChAT (E; merged images) from a sample area in the BF is shown. White arrowheads show double-labelled neurons. BF trkA immunoreactivity from representative sections taken from animals infused with AAV-luc (F) and AAV-trkA (G) vector (black arrows point to trkA-positive neurons). (H) Infusion of viral vector expressing trkA shRNA produced profound reduction in trkA immunoreactivity in the nBM/SI region (***, p < 0.001). Besides AAV infusions, animals also received chronic i.c.v infusions of either the vehicle or proNGF antibody (Ab). (I) A representative micrograph of a Nissl-stained section showing the cannula placement (see black arrows for cannula tract) in the right ventricle. (J) Western blots depicting the specificity of proNGF antibody to detect 26 and 32 kDa isoforms of rat proNGF (left). These isoforms were not detected when the antibody was incubated with proNGF blocking peptide (right).
Brain proNGF activity was blocked by infusing a rabbit anti-proNGF antibody into the cerebral ventricles for 4 weeks via osmotic minipumps. A representative Nissl-stained section depicting the tract of the guide cannula into the right lateral ventricle is shown in Figure 2I. The efficacy of the antibody to produce proNGF blockade in rat tissues was evaluated by western blotting. Frontal cortices from a pilot aged animal were isolated and homogenized in HEPES buffer containing a cocktail of protease inhibitors. Proteins (25 μg/well) were separated on 4-15% Tris HCl polyacrylamide gels and transferred onto PVDF membranes. Incubation of membranes with rabbit proNGF antibody (1:1000) overnight produced two distinct bands of proNGF isoforms at 26 kDa and 32 kDa respectively (Figure 2J). On the other hand, proNGF bands were not visible when the membrane was incubated overnight with the antibody in combination with a proNGF blocking peptide (Neuromics, Edina, MN, USA), confirming that the proNGF antibody used in this study specifically binds to rat proNGF.
3.2. Attentional performance in aged rats prior to surgeries
Aged rats acquired criterion to SAT performance in 85.58 ± 4.85 training sessions. To ensure that no differences in baseline performance were present before surgical manipulations and allocation of animals into four treatment groups, one-way ANOVAs were conducted on three-day averages of primary behavioral measures. There were no significant group differences for hits (F3,28=0.570; p = 0.639), correct rejections (F3,28 = 1.350; p = 0.278), SAT score (F3,28 = 0.0574; p = 0.637), or omissions (F3,28 = 1.222; p = 0.344). Therefore, the rats’ attentional performance remained similar in all groups prior to the infusion of viral vectors and implantation of the osmotic pump for chronic intracranial infusions.
3.3. Chronic proNGF blockade and SAT performance
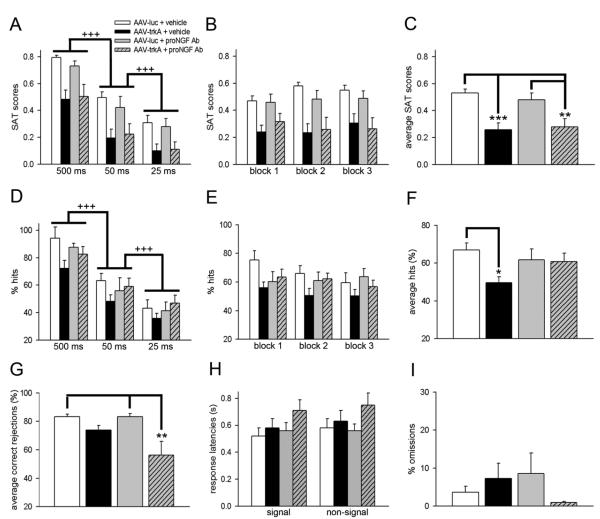
In terms of overall performance, SAT scores varied significantly between each signal duration (F2,56 = 114.63; p < 0.001; see Figure 3A for multiple comparisons) but did not differ across blocks (F2,56 = 0.53; p = 0.59; Figure 3B). Mixed factor ANOVAs failed to show either a signal x manipulation (F6,56 = 0.38; p = 0.89) or block x manipulation (F6,56 = 1.33; p = 0.26) interaction. Analysis of average SAT scores revealed significant group differences (F3,28 = 9.54; p < 0.001). Multiple comparisons indicated that trkA suppression impaired overall performance in both vehicle- and proNGF Ab-infused groups. As noted in Figure 3C, the SAT scores in these groups barely remained above chance levels (0.2), reflecting that trkA knockdown significantly impaired the rats’ ability to accurately discern between signal and non-signal trials, and proNGF blockade did not restore this ability. As the reduction in the index of overall attentional performance could either reflect decreases in hits (detection of signals in signal trials) or increases in false alarms (claims for signals in non-signal trials), we further analyzed performance of animals in signal and non-signal trials, respectively
Figure 3.
Effects of trkA suppression on attentional performance in the presence and absence of proNGF blockade. All data are Mean ± SEM. (A-C) The index of overall attentional performance expressed as SAT scores. SAT performance was signal duration-dependent but did not differ by block. In general, trkA knockdown rats displayed lower SAT scores for each signal and block, as well as, for the entire session. Bar charts depicting the proportion of hits collapsed by signal-duration (D) and by block (E). Hit rates displayed a similar pattern as SAT scores in terms of the significant main effect of signal. TrkA knockdown aged rats infused with vehicle displayed lower hit rates for each signal duration reflecting that attentional impairments in these animals primarily occurred due to the inability to detect signals. Correct responses on signal trials were not affected by block and displayed no interaction with the manipulation (see section 3.3). (F) Hits averaged over the entire SAT session indicate that sustained proNGF blockade normalized impaired performance on signal trials in trkA knockdown rats. (G) Average correct rejections remained unaffected by either trkA suppression or proNGF blockade but declined when the two manipulations were combined. (H) Response latencies for signal and non-signal trials remained unaffected with any manipulation. (I) Omissions remained relatively low (< 10%) and there were no differences between groups . (main effect of signal: +++ p < 0.001; post hoc comparisons: * p < 0.05; ** p < 0.01; *** p < 0.001).
Hit rates remained signal-duration dependent for all animals (main effect of signal: F2,56 = 122.92; p < 0.001). The proportion of correct responses on signal trials remained highest with the 500 ms stimulus duration and declined gradually with lower signal durations (500 ms vs. 50 ms: p < 0.001; 50 ms vs. 25 ms: p < 0.001; Figure 3D). Although there was a significant manipulation effect presumably due to lower hit rates, in general, for AAV-trkA + vehicle group, this effect did not interact with signal duration (main effect of group: F3,28 = 3.35; p = 0.03; signal × manipulation interaction: F6,56 = 0.94; p = 0.46). These data are in agreement with previous studies that suggest that detection of signal in SAT remain signal-duration dependent and are not affected by continuous task practice (McGaughy et al., 1996; McGaughy and Sarter, 1998). The ability to sustain performance on signal trials throughout the duration of the task was assessed by analyzing data in blocks of 54 trials (Figure 3E). Mixed factor ANOVAs show neither an effect of block nor block x manipulation interaction on hit rate (main effect of block: F2,56 = 3.18; p = 0.07; block x manipulation interaction: F6,56 = 1.20; p = 0.33) reflecting that trkA suppression in the absence or presence of proNGF Ab did not affect performance based on task duration. Average hits during SAT sessions differ significantly between groups (F3,28 = 2.80; p = 0.05; Figure 3F). Post hoc analysis indicated that average hits were lower in AAV-trkA + vehicle group as compared to AAV-luc + vehicle group (LSD: p = 0.02). However, proNGF blockade did not affect this behavioral measure in trkA-suppressed aged rats reflecting normalization of performance on signal trials (p = 0.86 vs. AAV-luc + vehicle). Average hits also remained unaffected in AAV-luc + proNGF group (p = 0.44). Interestingly, the analysis of performance on non-signal trials measured as average correct rejections also displayed a significant group effect (F3,28 = 5.64; p = 0.004). This effect was observed primarily due to lower correct rejections in the AAV-trkA + proNGF Ab group (for multiple comparisons, see Figure 3G). The performance on non-signal trials remained unaffected in trkA knockdown + vehicle group (p = 0.18 vs AAV-luc + vehicle). These data indicate that reduced SAT scores manifesting attentional deficits (as discussed above) primarily occurred as a consequence of disruption in signal processing in trkA knockdown aged rats. In contrast, lower SAT scores in AAV-trkA + proNGF Ab primarily occur as a consequence of higher false alarms or lower correct rejections. The response latencies for signal and non-signal trials in AAV-luc + vehicle rats were 0.52 ±0.06 s and 0.59 ± 0.07 s, respectively. None of the manipulations affected this measure (signal trials: F3,28 = 1.51; p = 0.29; non-signal trials: F3,28 = 1.41; p = 0.26; Figure 3H). The rate of omissions remained substantially low in all groups and was not affected by any manipulation (F3,28 = 1.05; p = 0.39; Figure 3I). These data reflect that motivation to perform and attain goals was neither affected by trkA nor proNGF signaling in aged rats.
3.5. dSAT performance
Attentional performance under distracting conditions recruit top-down mechanisms involving the PFC to counteract loss of stimulus control and maintain goal-directed behavior (Sarter et al., 2005; Sarter et al., 2006; St Peters et al., 2011). The effects of endogenous proNGF blockade on top-down modulation of attention in the normal and trkA-suppressed aged rats was assessed using the dSAT procedure that involved the presentation of distracters in block 2 of the SAT. As expected, the dSAT scores differed significantly across blocks (F2,56 = 16.41; p < 0.001); overall dSAT performance declined in the distractor block as compared to pre- and post-distractor blocks (LSD: p < 0.001 block 2 vs. block 1; p < 0.001 block 2 vs. block 3; Figure 4A). These data indicate that presentation of distractors through a flashing houselight did indeed tax rats’ attentional capacities. A main effect of manipulation on dSAT scores was observed primarily due to impaired performance of rats infused with AAV-trkA (F3,28=10.29; p < 0.001; see Figure 4A and average dSAT scores in Figure 4B); however, this effect did not interact with block (F6,56 = 0.48; p = 0.81) indicating that the distractor challenge affected the overall performance in all animals irrespective of manipulation. As shown in Figure 4B, post hoc comparisons on average dSAT scores indicated that trkA knockdown impaired dSAT performance in both vehicle- and proNGF Ab-infused rats. As hit rates recovered following proNGF blockade in trkA suppressed animals, lower dSAT scores indicate detrimental performance on non-signal trials (see below). There was a trend for lower dSAT scores in AAV-luc + proNGF animals (p = 0.1 vs. AAV-luc + vehicle) that presumably were a consequence of reduced hit rates but not correct rejections.
Figure 4.
Attentional performance under challenging conditions. All data are Mean ± SEM. Behavioral testing in dSAT involved the presentation of distractors (flashing house light @ 0.5 Hz) in the second block. (A) The presentation of distractors reduced dSAT scores in block 2 as compared to blocks 1 and 3 in all animals. Moreover, dSAT scores remained lower in all blocks in trkA-suppressed rats, and this effect was also emphasized in average dSAT performance (B). (C) The performance in signal trials differed significantly across blocks; hit rates in the distractor and post-distractor blocks declined in all groups. Bar charts depict hit rates for each signal duration (D) and averaged over the entire session (E). dSAT performance in signal trials was signal duration-dependent and this effect interacted with the manipulation. Multiple comparisons indicated that hit rates remained significantly lower for all signal lengths in AAV-trkA + vehicle group. Moreover, proNGF blockade restored this decline in trkA-suppressed rats. However, the correct responses on signals with shorter durations also remained lower in animals with intact trkA receptors but infused with proNGF Ab. Average hit rates exhibited a similar pattern with lower performance in AAV-trkA + vehicle and AAV-luc + proNGF Ab groups, respectively. (F) The performance in non-signal trials dropped for all groups in the distractor block and recovered in the post-distractor block. Correct rejections remained lower throughout the dSAT session in trkA knockdown rats infused with proNGF Ab. These data indicate that lower dSAT scores observed in these animals were mainly attributed to higher false alarms as hit rates were not affected. (LSD: * p < 0.05; ** p < 0.01; *** p < 0.001)
The proportion of hits during dSAT differed significantly across block (F2,56 = 6.46; p = 0.002) but this effect did not interact with the manipulation (block x manipulation: F6,56 = 0.39; p = 0.88). As portrayed in Figure 4C, the performance on signal trials in block 1 was significantly better in all groups as compared to the performance in block 2 (p = 0.012) and block 3 (p = 0.003), respectively. Although dSAT scores were higher in the post-distractor block as opposed to the distractor block, hit rates did not recover or even worsened, indicating that attentional performance following the distractor challenge improved presumably due to better performance in non-signal trials (see below). Similar to the analysis of SAT performance, the proportion of hits remained signal-dependent in the dSAT task (F2,56 = 162.09; p < 0.001). Moreover, this effect interacted with the manipulation (signal x manipulation: F6,56 = 3.10; p = 0.01). To determine the source of this interaction, separate one-way ANOVAs were applied for each signal length. These analyses yielded significant group differences in hit rates for all signal durations (500 ms: F3,28 = 4.85; p = 0.008; 50 ms: F3,28 = 4.19; p = 0.01; 25 ms: F3,28 = 3.28; p = 0.03). Signal performance remained significantly lower in AAV-trkA + vehicle group as compared to AAV-luc + vehicle group (see post hoc tests in Figure 4D). Moreover, hit rates for the highest and lowest signal duration in the AAV-trkA + proNGF Ab group remained significantly higher than trkA knockdown rats infused with vehicle (500 ms: p = 0.02; 25 ms: p = 0.009). On the other hand, proNGF blockade per se reduced hits for lower signal durations (50 ms: p = 0.009; 25 ms: p = 0.1; vs. AAV-luc). Similar trends were also observed when average hits were analyzed (group differences: F3,28 = 4.99; p = 0.007). Chronic trkA suppression significantly lowered overall accuracies on signal trials, and proNGF blockade reversed this effect (see multiple comparisons in Figure 4E). However, chronic i.c.v. infusions of proNGF Ab lowered average hits in rats that received injections of the control vector into the BF (p = 0.02).
Analysis of performance on non-signal trials, measured as the proportion of correct rejections in the dSAT revealed a main effect of block (F2,56 = 20.81; p < 0.001) and a main effect of group (F3,28 = 4.30; p = 0.01) but no interaction (F6,56 = 0.45; p = 0.82). A closer examination of these data revealed that performance in non-signal trials worsened in all groups during the distractor block, presumably due to corresponding increases in false alarms (Figure 4F). However, correct rejections in block 3 normalized to pre-distractor block levels in all animals (means for the main effect: block 1 = 73.60 ± 3.30%; block 3 = 79.90 ± 2.90%). As seen with the analysis of SAT performance, correct rejections in AAV-trkA + proNGF group remained generally lower as compared to the other groups throughout dSAT (Figure 4F).
3.6. Depolarization-evoked cholinergic transients
The detection of attention-capturing cues is mediated by transient (second-based) increases in prefrontal ACh release (Parikh et al., 2007; Parikh and Sarter; 2008; Howe et al., 2013). Cholinergic projections to the PFC are also active under conditions of high attentional load and influence top-down control of signal detection (Kozak et al., 2006; Furey et al., 2008). Therefore, the magnitude of prefrontal cholinergic transients could directly impact attentional capacities. We examined the ability of presynaptic cholinergic terminals in the PFC to produce ACh release following terminal depolarization that on a temporal time scale mimics cholinergic transients. Following the completion of behavioral studies, animals were prepared for electrochemical recordings. Choline-sensitive microelectrodes used in the present experiments (n = 20) exhibited a sensitivity of 7.79 ± 0.76 pA/μM for choline and an LOD of 388.02 ± 37.08 nM. The R2 value calculated for linear increases in choline concentration based on in vitro calibration was 0.993 ± 0.001, and selectivity of choline:AA was 157.63 ± 35.76. As shown in Figure 5A, the local application of brief pulses of potassium produced robust increases in extracellular choline concentration above background levels in all groups. Depolarization-evoked choline signals detected over brief time epochs using amperometry occur as a consequence of rapid hydrolysis of synaptic ACh release by endogenous AChE (Parikh et al., 2004; Parikh and Sarter, 2013). The amplitudes of cholinergic signals differ significantly between groups (F3,24 = 4.48; p = 0.01; Figure 5A, B). Post hoc multiple comparisons indicated that depolarization-evoked ACh release was markedly attenuated by trkA knockdown in aged rats. Although signal amplitudes remained lower in AAV-trkA + proNGF Ab group as compared to AAV-luc group, the difference was not statistically significant (p = 0.06). Chronic proNGF antibody infusions did not alter ACh release in aged rats injected with AAV-luc vector (p = 0.95). These results demonstrate that suppression of cholinergic transients in trkA knockdown aged rats is partially restored with proNGF blockade. Moreover endogenous proNGF does not seem to exert any detrimental effects on the capacity of cholinergic terminals to release phasic ACh.
Figure 5.
Amperometric recordings of prefrontal cholinergic transmission using choline-sensitive microelectrodes. All data are Mean ± SEM. (A) Representative traces of choline signals evoked by local application of potassium in the medial PFC. These signals reflect ACh release and occur as a consequence of rapid hydrolysis of ACh by acetylcholinesterase. The hydrolyzed choline is oxidized by choline oxidase present on the enzyme at a fixed potential to generate changes in current. B) Cholinergic signal amplitudes were significantly lower in trkA knockdown rats infused with vehicle as compared to trkA intact rats. (LSD: ** p < 0.01).
3.7. BF cholinergic neurons and prefrontal ChAT density
The impact of sustained proNGF blockade on the morphology of BF cholinergic neurons and the density of cholinergic processes in the cortex was examined by ChAT immunohistochemistry. As depicted in Figure 6A,B, in general, neuronal cross-sectional area remained lower in aged rats infused with AAV-trkA as compared to rats infused with the control vector. However, this effect did not differ significantly between groups (F3,28 = 1.39; p = 0.26). The density of cholinergic fibers in the prelimbic region of the PFC markedly declined in the AAV-trkA + vehicle group (F3,26 = 3.10; p = 0.04; LSD: p = 0.01 vs. AAV-luc + vehicle; Figure 6D,E). Similar trends were also noted in the cingulate and infralimbic regions (cingulate: F3,24 = 3.21; p = 0.04; infralimbic: F3,24 = 4.70; p = 0.01; see post hoc tests in Figure 6E). These findings are in agreement with our previous study that shows reduction in trkA receptors affect the integrity of cortical cholinergic processes in aged rats (Parikh et al., 2013). ProNGF blockade minimized the loss of cholinergic fibers in trkA-suppressed animals in both prelimbic and cingulate regions; ChAT fiber counts in these animals did not differ with the control group (prelimbic: p = 0.45; cingulate: p = 0.06 vs. AAV-luc + vehicle). The density of cholinergic terminals remained marginally higher in AAV-trkA + proNGF Ab group as compared to AAV-trkA + vehicle group in the infralimbic region; yet it was significantly lower than AAV-luc animals infused with vehicle (p = 0.01). Infusion of proNGF Ab surprisingly reduced the density of cholinergic fibers in aged rats with intact trkA receptors (all p < 0.04 vs. AAV-luc + vehicle; Figure 6E). Prelimbic ChAT-positive fiber counts positively correlated with performance on signal trials in both SAT and dSAT (SAT: Pearson’s r = 0.38, p = 0.03; dSAT: Pearson’s r = 0.52; p = 0.002). These data correspond to previous studies that show integrity of prefrontal cholinergic terminals is critical for the optimization of both bottom-up and top-down signal processing (Dalley et al., 2004; Newman and McGaughy, 2008).
Figure 6.
Cell size of BF cholinergic neurons and cortical cholinergic fiber density. All data are Mean ± SEM. (A) Representative coronal sections depicting ChAT-immunoreactive nBM neurons (marked by white arrowheads) from the sampled areas. The cholinergic soma and dendrites appeared to be shrunken in trkA knockdown rats, though the cross-sectional area did not differ significantly between groups (B). (C-E) ChAT-positive fibers in the medial PFC of control (AAV-luc) and trkA knockdown (AAV-trkA) aged rats that received chronic i.c.v infusions with either vehicle or proNGF Ab. (C) Schematic illustration of the sampling areas for ChAT fiber counts from regions of prelimbic (PrL), cingulate (Cg) and infralimbic (IL) cortex. Counting frames are shown as black squares. (D) Representative photographs illustrate ChAT-immunostained fibers from analyzed prefrontal regions. (E) Bar charts depicting ChAT fiber counts from all treatment groups. The density of cholinergic fibers declined significantly in BF trkA-suppressed rats infused with vehicle as compared to rats with intact trkA receptors in all prefrontal regions. Such a reduction in ChAT fiber density was not observed in AAV-trkA + proNGF animals in both prelimbic and cingulate regions, respectively. However, sustained proNGF blockade per se reduced prefrontal cholinergic processes. (LSD: * , ** p < 0.05, 0.01).
4. Discussion
Although the greatest risk factor for AD is advancing age, normal aging per se does not robustly affect the integrity of cortical cholinergic inputs and their ability to support attentional functions (Sarter and Bruno, 1998; Burk et al., 2002; Parikh and Sarter, 2010). We previously demonstrated that knockdown of NGF-trkA receptors produced by targeting BF cholinergic neurons with an AAV vector expressing trkA shRNA in aged rats make these neurons highly vulnerable and leads to disruption in cholinergic signaling and attentional impairments (Parikh et al., 2013). As cortical and hippocampal proNGF levels are known to be elevated in normal aging and AD (Peng et al., 2004; Pedraza et al., 2005; Al-Shawi et al., 2007; Bruno et al., 2009; Terry et al., 2011), and this proneurotrophin exerts neurotoxic effects by activating the p75-sortilin receptor complex (Chao, 2003; Clewes et al., 2008; Sobottka et al., 2008), we hypothesized that sustained blockade of proNGF signaling would rescue cholinergic and attentional deficits in aged rats with reduced BF trkA receptors.
Here we report that chronic i.c.v. infusion of proNGF Ab in trkA knockdown aged rats rescued performance deficits in signal trials in both the SAT and the attentionally-taxing version of SAT (dSAT). Moreover, depolarization-evoked ACh release and the density of ChAT-positive fibers in the PFC were partially restored in these animals. However, proNGF blockade in trkA-suppressed animals drastically increased the rate of false alarms. A similar trend in performance was observed when a distractor challenge was presented to these animals. SAT performance of aged rats with intact BF trkA receptors that received proNGF Ab infusions did not differ from vehicle-infused rats. While the hit rates of these animals remained lower during the distractor sessions, correct rejections did not differ from the controls. The blockade of endogenous proNGF alone did not affect the amplitudes of potassium-evoked cholinergic signals in aged control rats.
Activation of cortical cholinergic inputs is necessary for the optimization of the signal detection process. Previous studies have shown that deafferentation of the BF corticopetal projection system in animals performing SAT drastically reduced correct responses in signal trials but spared the ability to respond correctly on non-signal trials (McGaughy et al., 1996, 1999; McGaughy and Sarter, 1998). The cholinergic lesion-induced impairments in attentional performance do not result from the disruption of primary sensory mechanisms but rather a dysfunctional detection process (Sarter et al., 2005). Moreover, phasic (second-based) increases in ACh release in the PFC foster attentional performance by switching from a default response mode into the detection mode (Parikh et al., 2007; Parikh and Sarter, 2008; Howe et al., 2013). Cortical cholinergic inputs also recruit prefrontal efferent circuitry to mediate top-down effects under conditions of higher attentional load and the magnitude of ACh release vary as a function of demands on attention (Kozak et al., 2006; Sarter et al., 2006; St Peters et al., 2011). Impairments in the SAT and dSAT performance observed in trkA knockdown aged rats infused with vehicle in the present study occur primarily due to lower hit rates but not correct rejections. These findings coupled with the suppression of depolarization-evoked phasic cholinergic transients, shrinkage of cholinergic cell soma and reduced prefrontal cholinergic terminal density correspond with the view that abrogated trkA signaling in aging compromises the cortical cholinergic input system leading to the disruption of signal detection and attentional capacities (Parikh et al., 2013). Restoration of hit rates following proNGF Ab infusions in trkA- silenced rats both during SAT and dSAT indicates that age-related accumulation of proNGF may be responsible for the disruption of cholinergic signaling leading to impairments in signal-driven cholinergic modulation of detection. These data are in agreement with prior studies that show hippocampal infusions of proNGF in rodents inhibit neurogenesis and produce cholinergic atrophy (Fortress et al., 2011; Guo et al., 2013).
However, augmented proNGF signaling in normal aging does not seem to drastically impact cholinergic function as attentional capacities and cholinergic signaling remained unaffected in aged rats with intact trkA receptors despite increases in cortical proNGF and p75 expression (Parikh et al., 2013). Moreover, in the present study, proNGF blockade alone neither bolstered SAT performance nor increased the capacity of cholinergic terminals to release ACh in aged rats. Thus our findings do not completely align with prior studies that demonstrate neurotoxic effects of exogenously applied proNGF either in vitro or in vivo under normal conditions (Sobottka et al., 2008; Song et al., 2010; Fortress et al., 2011; Guo et al., 2013). There could be two possibilities for this discrepancy. ProNGF is shown to produce loss of neurons isolated from aged animals in a dose-dependent manner (Al-Shawi et al., 2008). Therefore, it may very well be the case that cholinergic atrophy occurs only at higher concentrations of proNGF which are not usually observed in normal aged animals. The second possibility is that the recombinant form of proNGF utilized in these studies is a cleavage-resistant form that binds specifically to p75 receptors and promotes apoptosis (Lee et al., 2001), whereas the endogenous form of proNGF could still bind to trkA receptors with low affinity and exert neurotrophic effects (Fahnestock et al., 2004).
It is noteworthy that despite improvement in hit rates, proNGF blockade did not improve SAT scores in trkA knockdown rats due to their poor performance in non-signal trials; these animals displayed lower correct rejections and proportionately higher false alarms (claims for signals in the absence of signals). Likewise, the dSAT scores remained below chance levels in both distractor and post-distractor blocks due to impaired performance in non-signal trials. These effects could not be attributed to side (lever) bias or adoption of a different (riskier) strategy as the animals’ hit rates remained signal-duration dependent and similar to control aged rats. Increases in false alarms or false detections occur mostly due to a failure in switching from the processing of signal to a default state and reflect a dysfunctional executive process (Miner et al., 1997; Sarter et al., 2005). The cellular basis for such cognitive deficits observed in trkA knockdown rats that received proNGF Ab is not entirely clear and remains somewhat speculative. The development of BF GABAergic neurons is regulated by proNGF-p75 signaling via a non-cell autonomous mechanism (Lin et al., 2007) or potentially through a cell autonomous mechanism (Formaggio et al., 2011). Moreover, these non-cholinergic corticopetal neurons are known to mediate the executive aspects of attention (Burk and Sarter, 2001). Therefore, it is conceivable that disruption in the ability to switch from signal trials to non-signal trials occurred primarily due to blockade of proNGF signaling and consequent dysfunction in the BF GABAergic system. However, proNGF blockade per se did not affect SAT scores. Perhaps, the executive process that regulates switching between signal and non-signal trials requires a coordinated activity of both BF cholinergic and GABergic neurons. As the cholinergic signaling was only partially restored following proNGF blockade in trkA-silenced rats, higher false alarms observed in these animals plausibly occurred as a consequence of dysregulated cholinergic-GABAergic interactions. Depolarization-evoked ACh release did not decrease in trkA intact rats that received proNGF Ab infusions, and therefore, the interactions between corticopetal cholinergic and non-cholinergic projection systems were not affected.
It was intriguing that proNGF blockade in aged rats with intact trkA receptors also produced moderate impairments in animals’ ability to perform under conditions of high attentional load. Such impairments in dSAT performance were mostly associated with reduced correct responses in cognitively-taxing signal trials (i.e. trials with shorter signal duration) presumably in the distractor and post-distractor block but not with performance in non-signal trials (see Results in section 3.4). However, the lack of effect on depolarization-evoked ACh release in the PFC suggests that these effects were not related to proNGF-induced aberrations in phasic cholinergic signaling. On the other hand, the density of prefrontal ChAT-positive fibers was lower in these animals. Although ChAT fiber density was used as an index of presynaptic cholinergic terminal density, it is possible that reduced cholinergic fibers observed in our study mostly reflected reduced ChAT expression that remained below the threshold levels required for semi-quantitative image analysis and not the loss of terminals. As uptake of choline via the high-affinity choline transporter (CHT), not ChAT activity, is the rate limiting step for ACh synthesis and release (Ferguson and Blakely, 2004; Sarter and Parikh, 2005), reduced ChAT expression may not necessarily indicate reduced ability of presynaptic terminals to release ACh. Considering this scenario, it is likely that CHT function was not affected by proNGF blockade. This interpretation may explain the disparity between the electrochemical recording and the immunohistochemistry data, but it does not account for the moderate impairments in dSAT performance observed following proNGF blockade in rats infused with the control vector. As discussed above, the recovery from the detrimental effects of the distractor requires increased top-down control from the PFC and this may be associated with sustained increases in prefrontal cholinergic transmission. It has been suggested that tonic (minute-based) increases in ACh release acts to suppress the processing of distractors and amplify detection processing under conditions of higher attentional load (Demeter and Sarter, 2013). Although, the effects of proNGF blockade on tonic cholinergic transmission were not investigated in this study, there remains a possibility that reduced proNGF signaling affected the tonic component of ACh release. This speculation reinforces the role of endogenous proNGF signaling in exerting neurotrophic effects presumably via its low affinity trkA receptors as suggested earlier (Fahnestock et al., 2004; Buttigieg et al., 2007; Masoudi et al., 2009).
In conclusion, our studies demonstrate that barring proNGF from binding to p75 receptors and activating death-inducing signaling cascades may exert some beneficial effects on the cholinergic system and attentional capacities specifically when trkA receptor levels are reduced. This finding has major implications for mild cognitive impairment and AD which are associated with trkA downregulation, cholinergic cytopathology and cognitive dysfunction. However, blocking proNGF signaling may not completely ameliorate attentional impairments in these age-related pathologies and potentially hinder attentional performance during periods of high cognitive load in normal aging. Further studies are warranted to delineate mechanisms that mediate apoptotic versus neurotrophic roles of proNGF and how they impact cholinergic signaling in aging and pathological aging.
HIGHLIGHTS.
proNGF blockade restored hit rates in aged trkA-knockdown rats
Overall attentional performance did not improve with proNGF blockade
proNGF antibody infusions impaired performance under distracting conditions
Endogenous proNGF is not detrimental for cholinergic function in normal aging
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute on Aging (AG0292592) and the Rosalinde and Arthur Gilbert Foundation/American Federation for Aging Research. We thank Dr. David L. Turner (University of Michigan) for the initial design and construction of AAV-SIBR vectors. We also thank Avery Zucco and Cameron Pollock for assisting us with behavioral training and immunohistochemistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Al-Shawi R, Hafner A, Chun S, Raza S, Crutcher K, Thrasivoulou C, Simons P, Cowen T. ProNGF, sortilin, and age-related neurodegeneration. Ann N Y Acad Sci. 2007;1119:208–215. doi: 10.1196/annals.1404.024. [DOI] [PubMed] [Google Scholar]
- Al-Shawi R, Hafner A, Olsen J, Olson J, Chun S, Raza S, Thrasivoulou C, Lovestone S, Killick R, Simons P, Cowen T. Neurotoxic and neurotrophic roles of proNGF and the receptor sortilin in the adult and ageing nervous system. Eur J Neurosci. 2008;27:2103–2114. doi: 10.1111/j.1460-9568.2008.06152.x. [DOI] [PubMed] [Google Scholar]
- Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience. 2002;114:451–460. doi: 10.1016/s0306-4522(02)00292-0. [DOI] [PubMed] [Google Scholar]
- Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Natl Acad Sci U S A. 2006;103:6735–6740. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno MA, Leon WC, Fragoso G, Mushynski WE, Almazan G, Cuello AC. Amyloid beta- induced nerve growth factor dysmetabolism in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68:857–869. doi: 10.1097/NEN.0b013e3181aed9e6. [DOI] [PubMed] [Google Scholar]
- Burk JA, Herzog CD, Porter MC, Sarter M. Interactions between aging and cortical cholinergic deafferentation on attention. Neurobiol Aging. 2002;23:467–477. doi: 10.1016/s0197-4580(01)00315-3. [DOI] [PubMed] [Google Scholar]
- Burk JA, Sarter M. Dissociation between the attentional functions mediated via basal forebrain cholinergic and GABAergic neurons. Neuroscience. 2001;105:899–909. doi: 10.1016/s0306-4522(01)00233-0. [DOI] [PubMed] [Google Scholar]
- Buttigieg H, Kawaja MD, Fahnestock M. Neurotrophic activity of proNGF in vivo. Exp Neurol. 2007;204:832–835. doi: 10.1016/j.expneurol.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Casu MA, Wong TP, De Koninck Y, Ribeiro-da-Silva A, Cuello AC. Aging causes a preferential loss of cholinergic innervation of characterized neocortical pyramidal neurons. Cereb Cortex. 2002;12:329–337. doi: 10.1093/cercor/12.3.329. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chung KH, Hart CC, Al-Bassam S, Avery A, Taylor J, Patel PD, Vojtek AB, Turner DL. Polycistronic RNA polymerase II expression vectors for RNA interference based on BIC/miR-155. Nucleic Acids Res. 2006;34:e53. doi: 10.1093/nar/gkl143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewes O, Fahey MS, Tyler SJ, Watson JJ, Seok H, Catania C, Cho K, Dawbarn D, Allen SJ. Human ProNGF: biological effects and binding profiles at TrkA, P75NTR and sortilin. J Neurochem. 2008;107:1124–1135. doi: 10.1111/j.1471-4159.2008.05698.x. [DOI] [PubMed] [Google Scholar]
- Counts SE, Mufson EJ. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol Exp Neurol. 2005;64:263–272. doi: 10.1093/jnen/64.4.263. [DOI] [PubMed] [Google Scholar]
- Counts SE, Nadeem M, Wuu J, Ginsberg SD, Saragovi HU, Mufson EJ. Reduction of cortical TrkA but not p75(NTR) protein in early-stage Alzheimer's disease. Ann Neurol. 2004;56:520–531. doi: 10.1002/ana.20233. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Demeter E, Sarter M. Leveraging the cortical cholinergic system to enhance attention. Neuropharmacology. 2013;64:294–304. doi: 10.1016/j.neuropharm.2012.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter E, Sarter M, Lustig C. Rats and humans paying attention: cross-species task development for translational research. Neuropsychology. 2008;22:787–799. doi: 10.1037/a0013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson HM, Zurawski J, Zhang H, Turner DL, Vojtek AB. POSH is an intracellular signal transducer for the axon outgrowth inhibitor Nogo66. J Neurosci. 2010;30:13319–13325. doi: 10.1523/JNEUROSCI.1324-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH, Selby MJ, Garcia PD, Rutter WJ. Processing of the native nerve growth factor precursor to form biologically active nerve growth factor. J Biol Chem. 1988;263:6810–6815. [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Garber M, Barbacid M, Silos-Santiago I, Holtzman DM. A role for TrkA during maturation of striatal and basal forebrain cholinergic neurons in vivo. J Neurosci. 1997;17:7644–7654. doi: 10.1523/JNEUROSCI.17-20-07644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M, Yu G, Michalski B, Mathew S, Colquhoun A, Ross GM, Coughlin MD. The nerve growth factor precursor proNGF exhibits neurotrophic activity but is less active than mature nerve growth factor. J Neurochem. 2004;89:581–592. doi: 10.1111/j.1471-4159.2004.02360.x. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Blakely RD. The choline transporter resurfaces: new roles for synaptic vesicles? Mol Interv. 2004;4:22–37. doi: 10.1124/mi.4.1.22. [DOI] [PubMed] [Google Scholar]
- Formaggio E, Dalfini AC, Fazzini F, Fumagalli G, Chiamulera C. GABAergic neurons expressing p75 in rat substantia innominata and nucleus basalis. Mol Cell Neurosci. 2011;46:625–632. doi: 10.1016/j.mcn.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Buhusi M, Helke KL, Granholm AC. Cholinergic Degeneration and Alterations in the TrkA and p75NTR Balance as a Result of Pro-NGF Injection into Aged Rats. J Aging Res. 20112011:460543. doi: 10.4061/2011/460543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV, Drevets WC. Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology. 2008;33:913–923. doi: 10.1038/sj.npp.1301461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald AG, Gonzalez R, Harris RJ, Guthrie D. Effect sizes and p values: what should be reported and what should be replicated? Psychophysiology. 1996;33:175–183. doi: 10.1111/j.1469-8986.1996.tb02121.x. [DOI] [PubMed] [Google Scholar]
- Guo J, Wang J, Zhang Z, Yan J, Chen M, Pang T, Zhang L, Liao H. proNGF inhibits neurogenesis and induces glial activation in adult mouse dentate gyrus. Neurochem Res. 2013;38:1695–1703. doi: 10.1007/s11064-013-1071-7. [DOI] [PubMed] [Google Scholar]
- Hempstead BL. Commentary: Regulating proNGF action: multiple targets for therapeutic intervention. Neurotox Res. 2009;16:255–260. doi: 10.1007/s12640-009-9054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Kilbridge J, Li Y, Cunningham ET, Lenn NJ, Clary DO, Reichardt LF, Mobley WC. TrkA expression in the CNS: evidence for the existence of several novel NGF- responsive CNS neurons. J Neurosci. 1995;15:1567–1576. doi: 10.1523/JNEUROSCI.15-02-01567.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Berry AS, Francois J, Gilmour G, Carp JM, Tricklebank M, Lustig C, Sarter M. Prefrontal cholinergic mechanisms instigating shifts from monitoring for cues to cue-guided performance: converging electrochemical and fMRI evidence from rats and humans. J Neurosci. 2013;33:8742–8752. doi: 10.1523/JNEUROSCI.5809-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex. 2006;16:9–17. doi: 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- Li Y, Holtzman DM, Kromer LF, Kaplan DR, Chua-Couzens J, Clary DO, Knüsel B, Mobley WC. Regulation of TrkA and ChAT expression in developing rat basal forebrain: evidence that both exogenous and endogenous NGF regulate differentiation of cholinergic neurons. J Neurosci. 1995;15:2888–2905. doi: 10.1523/JNEUROSCI.15-04-02888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PY, Hinterneder JM, Rollor SR, Birren SJ. Non-cell-autonomous regulation of GABAergic neuron development by neurotrophins and the p75 receptor. J Neurosci. 2007;27:12787–12796. doi: 10.1523/JNEUROSCI.3302-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther JA, Birren SJ. p75 and TrkA signaling regulates sympathetic neuronal firing patterns via differential modulation of voltage-gated currents. J Neurosci. 2009;29:5411–5424. doi: 10.1523/JNEUROSCI.3503-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoudi R, Ioannou MS, Coughlin MD, Pagadala P, Neet KE, Clewes O, Allen SJ, Dawbarn D, Fahnestock M. Biological activity of nerve growth factor precursor is dependent upon relative levels of its receptors. J Biol Chem. 2009;284:18424–18433. doi: 10.1074/jbc.M109.007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain- lesioned rats. Psychopharmacology (Berl) 1999;144:175–182. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Sustained attention performance in rats with intracortical infusions of 192 IgG-saporin-induced cortical cholinergic deafferentation: effects of physostigmine and FG 7142. Behav Neurosci. 1998;112:1519–1525. doi: 10.1037//0735-7044.112.6.1519. [DOI] [PubMed] [Google Scholar]
- Mesulam M. The cholinergic lesion of Alzheimer's disease: pivotal factor or side show? Learn Mem. 2004;11:43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- Miner LA, Ostrander M, Sarter M. Effects of ibotenic acid-induced loss of neurons in the medial prefrontal cortex of rats on behavioral vigilance: evidence for executive dysfunction. J Psychopharmacol. 1997;11:169–178. doi: 10.1177/026988119701100210. [DOI] [PubMed] [Google Scholar]
- Mobley WC, Rutkowski JL, Tennekoon GI, Gemski J, Buchanan K, Johnston MV. Nerve growth factor increases choline acetyltransferase activity in developing basal forebrain neurons. Brain Res. 1986;387:53–62. doi: 10.1016/0169-328x(86)90020-3. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer's disease: therapeutic implications. Expert Rev Neurother. 2008;8:1703–1718. doi: 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Ma SY, Cochran EJ, Bennett DA, Beckett LA, Jaffar S, Saragovi HU, Kordower JH. Loss of nucleus basalis neurons containing trkA immunoreactivity in individuals with mild cognitive impairment and early Alzheimer's disease. J Comp Neurol. 2000;427:19–30. doi: 10.1002/1096-9861(20001106)427:1<19::aid-cne2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Müller M, Triaca V, Besusso D, Costanzi M, Horn JM, Koudelka J, Geibel M, Cestari V, Minichiello L. Loss of NGF-TrkA signaling from the CNS is not sufficient to induce cognitive impairments in young adult or intermediate-aged mice. J Neurosci. 2012;32:14885–14898. doi: 10.1523/JNEUROSCI.2849-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, McGaughy J. Cholinergic deafferentation of prefrontal cortex increases sensitivity to cross-modal distractors during a sustained attention task. J Neurosci. 2008;28:2642–2650. doi: 10.1523/JNEUROSCI.5112-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Oosawa H, Fujii T, Kawashima K. Nerve growth factor increases the synthesis and release of acetylcholine and the expression of vesicular acetylcholine transporter in primary cultured rat embryonic septal cells. J Neurosci Res. 1999;57:381–387. [PubMed] [Google Scholar]
- Parikh V, Howe WM, Welchko RM, Naughton SX, D'Amore DE, Han DH, Deo M, Turner DL, Sarter M. Diminished trkA receptor signaling reveals cholinergic-attentional vulnerability of aging. Eur J Neurosci. 2013;37:278–293. doi: 10.1111/ejn.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Ji J, Decker MW, Sarter M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J Neurosci. 2010;30:3518–3530. doi: 10.1523/JNEUROSCI.5712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Sarter M, Bruno JP. Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci. 2004;20:1545–1554. doi: 10.1111/j.1460-9568.2004.03614.x. [DOI] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- Parikh V, Sarter M, Koob G. Cognitive decline in laboratory animals: models, measures, and validity. In: Thompson RF, LeMoal M, editors. Encyclopedia of Behavioral Neuroscience. Vol. 1. Elsevier; Amsterdam: 2010. pp. 294–301. [Google Scholar]
- Parikh V, Sarter M. Forebrain cholinergic systems and cognition: New insights based on rapid detection of choline spikes using enzyme-based biosensors. In: Dale N, Marinesco S, editors. Neuromethods: Springer Protocols. Vol. 80. Humana Press Inc; New York: 2013. pp. 257–277. [Google Scholar]
- Passetti F, Dalley JW, O'Connell MT, Everitt BJ, Robbins TW. Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur J Neurosci. 2000;12:3051–3058. doi: 10.1046/j.1460-9568.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- Pedraza CE, Podlesniy P, Vidal N, Arévalo JC, Lee R, Hempstead B, Ferrer I, Iglesias M, Espinet C. Pro-NGF isolated from the human brain affected by Alzheimer's disease induces neuronal apoptosis mediated by p75NTR. Am J Pathol. 2005;166:533–543. doi: 10.1016/S0002-9440(10)62275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol. 2004;63:641–649. doi: 10.1093/jnen/63.6.641. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer's disease. A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. Pt 3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ortiz E, Yui D, Song D, Li Y, Rubenstein JL, Reichardt LF, Parada LF. TrkA gene ablation in basal forebrain results in dysfunction of the cholinergic circuitry. J Neurosci. 2012;32:4065–4079. doi: 10.1523/JNEUROSCI.6314-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Age-related changes in rodent cortical acetylcholine and cognition: main effects of age versus age as an intervening variable. Brain Res Brain Res Rev. 1998;27:143–156. doi: 10.1016/s0165-0173(98)00003-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci. 2005;6:48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- Schor NF. The p75 neurotrophin receptor in human development and disease. Prog Neurobiol. 2005;77:201–214. doi: 10.1016/j.pneurobio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Benjannet S, Pareek S, Savaria D, Hamelin J, Goulet B, Laliberte J, Lazure C, Chrétien M, Murphy RA. Cellular processing of the nerve growth factor precursor by the mammalian pro-protein convertases. Biochem J. 1996;314:951–960. doi: 10.1042/bj3140951. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobottka B, Reinhardt D, Brockhaus M, Jacobsen H, Metzger F. ProNGF inhibits NGF-mediated TrkA activation in PC12 cells. J Neurochem. 2008;107:1294–1303. doi: 10.1111/j.1471-4159.2008.05690.x. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Song W, Volosin M, Cragnolini AB, Hempstead BL, Friedman WJ. ProNGF induces PTEN via p75NTR to suppress Trk-mediated survival signaling in brain neurons. J Neurosci. 2010;30:15608–15615. doi: 10.1523/JNEUROSCI.2581-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Peters M, Demeter E, Lustig C, Bruno JP, Sarter M. Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J Neurosci. 2011;31:9760–9771. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Chung KH, Figueroa C, Zurawski J, Dickson HM, Brace EJ, Avery AW, Turner DL, Vojtek AB. The scaffold protein POSH regulates axon outgrowth. Mol Biol Cell. 2008;19:5181–5192. doi: 10.1091/mbc.E08-02-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Kutiyanawalla A, Pillai A. Age-dependent alterations in nerve growth factor (NGF)-related proteins, sortilin, and learning and memory in rats. Physiol Behav. 2011;102:149–157. doi: 10.1016/j.physbeh.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ. Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J Neurosci. 2006;26:7756–7766. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]